Abstract
The structures of 12 new “enantiomeric”-like abyssomicin metabolites (abyssomicins M–X) from Streptomyces sp. LC-6-2 are reported. Of this set, the abyssomicin W (11) contains an unprecedented 8/6/6/6 tetracyclic core, while the bicyclic abyssomicin X (12) represents the first reported naturally occurring linear spirotetronate. Metabolite structures were determined based on spectroscopic data and X-ray crystallography, and Streptomyces sp. LC-6-2 genome sequencing also revealed the corresponding putative biosynthetic gene cluster.
Graphical abstract
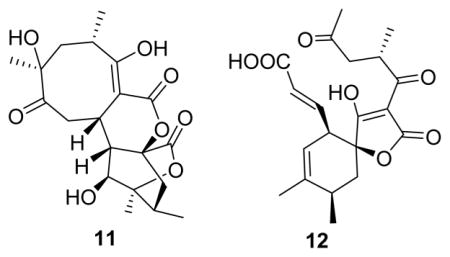
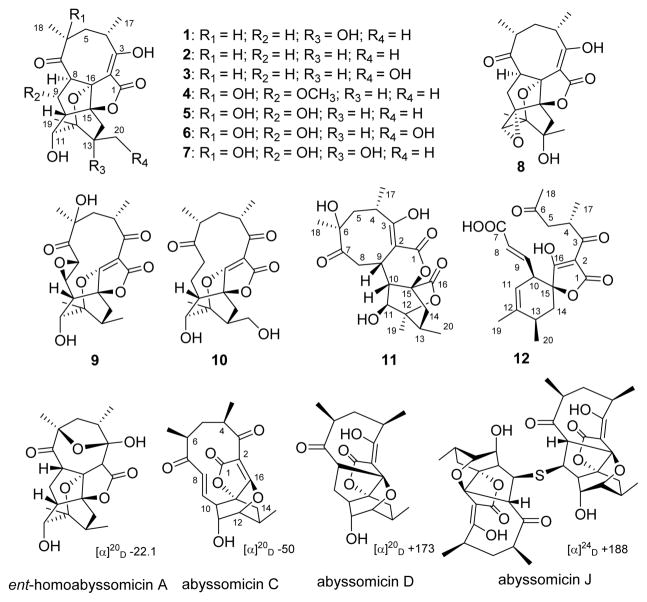
Class I spirotetronate polyketides contain a signature spirotetronate moiety within a macrocycle of variable size, the smallest of which are C11 macrocyclic abyssomicins (Figures 1 and S1).1–7 Abyssomicins B–D from a marine-associated Verrucosispora were the first reported abyssomicins,1 with a number of additional analogues since reported from Verrucosispora and Streptomyces species.8–13 Abyssomicin C was the first natural product reported to inhibit the formation of p-aminobenzoate (p-ABA), a key folic acid precursor in bacteria. Within this context, abyssomicin C was found to be an irreversible inhibitor of bacterial amino-4-deoxychorismate (ADC) synthase via an ADC synthase Cys-263 thiol/abyssomicin C-9 enone hetero-Michael addition.1,9,14,15 Abyssomicin C and the dimeric abyssomicin J display potent anti-Gram-positive bacteria activity, where abyssomicin J was noted as a putative oxidatively activated prodrug.1,14,16–18 Spirotetronate biosynthesis also features novel stereoselective [4 + 2] cycloaddition macrocyclization reactions, as exemplified by abyssomicin, versipelostatin, and pyrroindomycin biosynthetic studies (Figure S1).19–21 As part of an effort to explore the potential of actinomycetes associated with underground coal mine fires in Appalachia as a source for new natural products and/or biocatalysts,22–31 herein we report the discovery of 12 new abyssomicin analogues (M–X, 1–12) as metabolites of the Lotts Creek coal fire-affliated isolate Streptomyces sp. LC-6-2 (Figure 1). Distinct from archetypical abyssomicins’ absolute configuration, abyssomicins M–X (1–12) displayed global stereochemical features reminiscent of the unique “enantiomeric” ent-homoabyssomicins A and B.13 In addition, abyssomicin W (11) highlights an 8/6/6/6 tetracyclic core, while the bicyclic abyssomicin X (12) is the first reported naturally occurring linear spirotetronate.
Figure 1.
New metabolites (1–12) isolated from Streptomyces sp. LC-6-2 and representative prototypical abyssomicin comparators.
RESULTS AND DISCUSSION
Ten actinomycete strains were purified from second-generation plates deriving from a soil sample collected from the Lotts Creek coal fire (Perry County, Kentucky, United States). Preliminary LC-HRMS analyses of the Streptomyces sp. LC-6-2 crude extract revealed a set of potentially novel secondary metabolites based on an AntiBase 201432 database comparison. Scale-up fermentation (10 L) of Streptomyces sp. LC-6-2, followed by extraction, fractionation, and various chromatographic methods (Scheme S1), gave 12 new compounds: abyssomicins M (1, yield: 0.46 mg/L), N (2, yield: 0.41 mg/L), O (3, yield: 2.21 mg/L), P (4, yield: 0.83 mg/L), Q (5, yield: 4.05 mg/L), R (6, yield: 1.08 mg/L), S (7, yield: 1.14 mg/L), T (8, yield: 0.45 mg/L), U (9, yield: 3.02 mg/L), V (10, yield: 0.54 mg/L), W (11, yield: 0.40 mg/L), and X (12, yield: 1.83 mg/L).
Structure Elucidation
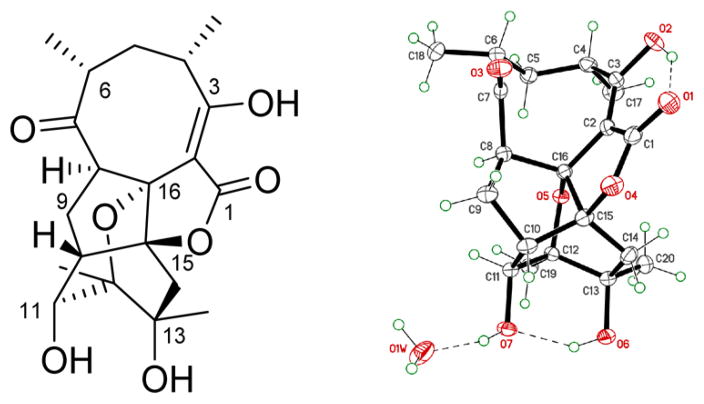
The molecular formula of 1 (C20H26O7), based on determined HRESIMS, was consistent with 20 carbons (including four methyls, three methylenes, five methines, one double bond, one ketone, one carboxyl, and four quaternary carbons) observed via 13C and 1H NMR (Tables 1 and 3) and HSQC. Two COSY spin systems were established as CH3-17/CH-4/CH2-5/CH-6/CH3-18 and H-8/H-9/H-10/H-11/11-OH (Figure S2). Further analysis of 1D/2D NMR data revealed a close relationship between 1 and abyssomicin D,1 with structural divergence at C-12 and C-13 (Figure 2). The corresponding C-12 methyl (CH3-19) at 1 was established via HMBC (correlations from CH3-19 to C-11, C-12, and C-13 and from H-11 to C-19), while the 13-OH of 1 was established based on the C-13 chemical shift and HMBC (correlations from 13-OH to C-12, C-13, and C-20 in DMSO-d6). The relative configuration of 1, established by NOESY (Figure S3), highlighted the facial orientation of H-4 and H-6 to oppose that of H-8, H-11, CH3-17, CH3-18, CH3-19, and CH3-20 based on NOESY (key correlations: H-4/H-6, CH3-17/H-5b, H-5b/H-8, H-8/CH3-18, H-8/H-9a, H-9a/H-11, H-11/CH3-19, and CH3-19/CH3-20). Interestingly, the specific rotation of 1 ([α]25D −126.7) was similar to that of ent-homoabyssomicin A (Figure 1, one of only two reported abyssomicins with “enantiomeric” configuration).13 Subsequent single-crystal X-ray crystallography (CDCC accession number 1495049; Figure 2 and Table S1) established the absolute configuration of 1 as 4S,6R,8R,10S,11S,12R,13R,15S,16R. On basis of this cumulative analysis, 1 was identified as a new abyssomicin analogue and thereby designated abyssomicin M (where the use of “micin” reflects early precedent in contrast to a Streptomyces point of origin).
Table 1.
13C NMR (100/125 MHz) Data for Compounds 1–4 and 8–12
| no. | 1a | 2c | 3a | 4a | 8c | 9a | 10a | 11a | 12b |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 173.1, C | 174.9, C | 173.2, C | 171.5, C | 173.4, C | 168.9, C | 169.5, C | 169.2, C | 176.8, C |
| 2 | 99.0, C | 98.8, C | 98.8, C | 106.4, C | 98.4, C | 100.2, C | 99.0, C | 96.6, C | 96.9, C |
| 3 | 179.5, C | 180.7, C | 178.9, C | 186.5, C | 180.9, C | 199.0, C | 199.7, C | 180.7, C | 201.2, C |
| 4 | 40.3, CH | 40.4, CH | 39.9, CH | 40.0, CH | 40.7, CH | 40.5, CH | 42.2, CH | 30.0, CH | 38.1, CH |
| 5 | 33.7, CH2 | 34.2, CH2 | 33.1, CH2 | 38.3, CH2 | 32.8, CH2 | 40.3, CH2 | 38.4, CH2 | 46.1, CH2 | 47.5, CH2 |
| 6 | 48.1, CH | 48.1, CH | 47.7, CH | 77.8, C | 48.5, CH | 79.7, C | 45.9, CH | 78.0, C | 210.9, C |
| 7 | 210.7, C | 210.2, C | 210.5, C | 209.1, C | 209.5, C | 207.2, C | 211.6, C | 215.3, C | 170.2, C |
| 8 | 58.5, CH | 59.7, CH | 58.5, CH | 66.1, CH | 53.2, CH | 48.3, CH | 34.4, CH2 | 43.9, CH2 | 122.6, CH |
| 9 | 27.2, CH2 | 27.6, CH2 | 26.8, CH2 | 77.1, CH | 36.2, CH2 | 55.9, CH | 19.4, CH2 | 27.2, CH | 149.8, CH |
| 10 | 50.3, CH | 49.7, CH | 48.8, CH | 50.6, CH | 60.5, C | 45.9, CH | 44.6, CH | 45.3, CH | 47.8, CH |
| 11 | 81.3, CH | 80.9, CH | 78.9, CH | 72.2, CH | 64.8, CH | 67.0, CH | 74.8, CH | 71.7, CH | 120.8, CH |
| 12 | 76.0, C | 78.5, C | 77.6, C | 76.8, C | 75.4, C | 88.6, C | 88.5, C | 84.9, C | 141.0, C |
| 13 | 73.7, C | 27.7, CH | 35.2, CH | 26.7, CH | 86.5, C | 27.6, C | 35.0, C | 28.0, CH | 32.5, CH |
| 14 | 44.2, CH2 | 33.8, CH2 | 29.4, CH2 | 33.6, CH2 | 38.7, CH2 | 34.7, CH2 | 33.4, CH2 | 40.6, CH2 | 36.2, CH2 |
| 15 | 86.9, C | 87.4, C | 87.2, C | 80.7, C | 95.2, C | 78.0, C | 77.8, C | 76.7, C | 86.0, C |
| 16 | 84.4, C | 85.7, C | 84.8, C | 87.0, C | 90.0, C | 179.4, C | 184.6, C | 171.5, C | 199.4, C |
| 17 | 19.6, CH3 | 19.9, CH3 | 19.4, CH3 | 18.8, CH3 | 21.0, CH3 | 17.3, CH3 | 16.5, CH3 | 17.2, CH3 | 18.0, CH3 |
| 18 | 19.3, CH3 | 19.8, CH3 | 19.0, CH3 | 28.1, CH3 | 20.0, CH3 | 25.7, CH3 | 19.3, CH3 | 28.0, CH3 | 30.1, CH3 |
| 19 | 17.6, CH3 | 20.9, CH3 | 20.8, CH3 | 20.6, CH3 | 15.5, CH3 | 18.6, CH3 | 19.1, CH3 | 19.0, CH3 | 21.3, CH3 |
| 20 | 26.0, CH3 | 16.1, CH3 | 61.5, CH2 | 15.9, CH3 | 20.5, CH3 | 16.0, CH3 | 60.2, CH2 | 16.0, CH3 | 19.5, CH3 |
| 9-OCH3 | 56.9, CH3 |
Measured in DMSO-d6.
Measured in CD3OD.
Measured in CDCl3.
Table 3.
1H NMR (400/500 MHz) Data for Compounds 1–4, 11, and 12 (δH in ppm, multi, J in Hz)
| no. | 1a | 2c | 3a | 4a | 11a | 12b |
|---|---|---|---|---|---|---|
| 4 | 2.41, m | 2.48, md | 2.40, m | 2.43, md | 3.26, m | 3.94, m |
| 5 | 1.60, m 2.63, dd (12.0) |
1.61, m 2.78, dd (12.2) |
1.58, m 2.65, dd (12.4) |
1.64, dd (5.6, 12.6) 2.88, dd (12.6) |
1.54, m (2H) | 2.40, md 2.97, dd (8.3, 17.0) |
| 6 | 2.13, m | 2.35, md | 2.12, m | |||
| 8 | 3.57, t (9.0) | 3.29, t (8.7) | 3.48, md | 3.15, me | 1.76, t (11.6) 3.29, m |
5.69, d (15.7) |
| 9 | 1.57, m 2.04, m |
1.58, m 2.36, md |
1.51, m 2.00, m |
4.20, dd (3.8, 7.8) | 4.05, m | 7.04, dd (8.6, 15.7) |
| 10 | 2.49, m | 2.50, md | 2.32, m | 2.41d | 2.39, br s | 2.90, br s |
| 11 | 3.88, d (6.8) | 3.74, br s | 3.61, d (4.5) | 3.96, br s | 3.62, br s | 5.23, br s |
| 13 | 2.36, md | 2.35, m | 2.22, m | 2.44d | 2.39d | |
| 14 | 1.67, d (14.5) 2.34, d (14.6) |
1.14, dd (4.6, 12.9) 2.43, md |
1.19, m 2.15, d (13.3) |
0.93,m 2.07, t (12.5) |
1.43, m 2.45, md |
1.72, m (2H) |
| 17 | 1.23, d (7.1) | 1.35, d (7.0) | 1.24, d (6.7) | 1.14, d (7.2) | 1.04, d (6.3) | 1.05, d (6.7) |
| 18 | 1.01, d (7.3) | 1.08, d (7.3) | 1.00, d (7.2) | 1.09, s | 1.16, s | 2.12, s |
| 19 | 1.27, s | 1.21, s | 1.16, s | 1.06, s | 1.28, s | 1.77, s |
| 20 | 1.10, s | 0.91, d (6.2) | 3.14, 3.51, md | 0.76, d (6.6) | 0.93, d (5.5) | 1.10, d (6.9) |
| 3-OH | 10.96, s | 11.26, s | 10.98, s | 13.63, s | ||
| 6-OH | 5.68, s | |||||
| 11-OH | 6.36, d (7.1) | 5.66, d (4.7) | 6.11, d (4.4) | |||
| 13-OH | 5.76, s | |||||
| 20-OH | 4.42, br s | |||||
| 9-OCH3 | 3.16, se |
Measured in DMSO-d6.
Measured in CD3OD.
Measured in CDCl3.
Signals overlapped.
Figure 2.
Structure of abyssomicin M (1).
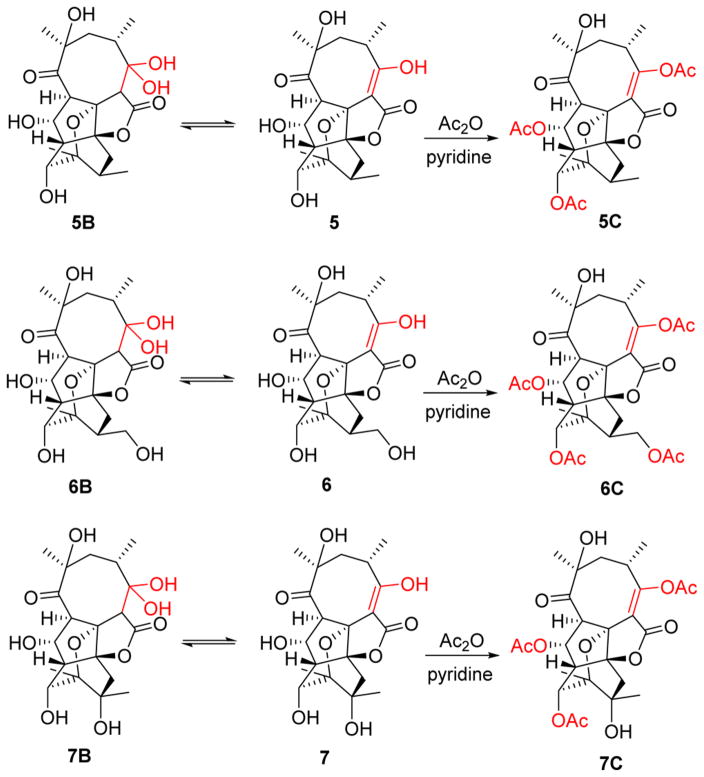
Compounds 2–8 were determined to be variations (deoxy, hydroxy, methoxy, epoxy) of 1 via HRESIMS and 1D/2D NMR (Tables 1–5, Figure S2 and S3) and also displayed opposite specific rotation that of the archetypical abyssomicins. They were thereby designated as new abyssomicins N–T (2–8). Intriguingly, equilibrium mixtures of the C-2/C-3 enol and C-3 gem-diol (hydrate) of compounds 5–7 were observed, where subsequent per-acetylation using acetic anhydride and pyridine led to single products for structure elucidation (Figure 4).
Table 5.
1H NMR (400/500 MHz) Data for Compounds 7, 7B, 7C, 8, 9, and 10 (δH in ppm, multi, J in Hz)
| no. | 7a | 7Ba | 7Cb | 8b | 9a | 10a |
|---|---|---|---|---|---|---|
| 2 | 3.29, s | |||||
| 4 | 2.55, m | 2.20, m | 2.72, m | 2.47c | 2.64c | 3.16, m |
| 5 | 1.78, dd (5.7, 15.3) 2.73, dd (13.6) |
1.95, dd (8.6, 13.1) 2.20, m |
2.04, m 2.86, dd (11.9) |
1.53, m 2.92, dd (12.2) |
1.58, dd (5.5, 16.0) 2.18, dd (1.8, 16.0) |
1.34c 1.81, m |
| 6 | 2.47c | 2.60d | ||||
| 8 | 3.18, d (8.0) | 2.99, d (7.8) | 3.67, d (8.4) | 3.23, dd (7.0, 12.2) | 3.93, d (1.7) | 2.63d |
| 9 | 4.52, m | 4.22, m | 5.46, dd (4.1, 8.4) | 1.85, dd (7.0, 13.1) 2.65, t (13.1) |
3.15, dd (2.1, 3.0) | 1.77, m 2.01, dd (3.5, 10.7) |
| 10 | 2.43, d (4.0) | 2.41, d (4.5) | 3.10, d (4.1) | 2.93, t (3.0) | 2.21 | |
| 11 | 4.29, d (7.6) | 4.22, br s | 5.44, br s | 3.01, s | 3.34, d (2.6) | 3.86, t (5.4) |
| 13 | 2.47, m | 2.40e | ||||
| 14 | 1.59, d (14.5) 2.27, d (14.5) |
2.04, d (14.7) 2.26 d (14.4) |
1.91, d (15.2) 2.47, d (15.2) |
1.42, d (11.8) 2.78, d (11.8) |
1.30, dd (3.4, 12.6) 2.67c |
1.35c 2.40e |
| 17 | 1.16, d (7.2) | 1.13, d (6.7) | 1.27, d (7.1) | 1.29, d (7.0) | 1.06, d (6.5) | 0.96, d (6.8) |
| 18 | 1.10, s | 1.22, s | 1.31, s | 1.13, d (7.2) | 1.36, s | 0.98, d (7.1) |
| 19 | 1.29, s | 1.22, s | 1.38, s | 1.46, s | 1.45, s | 1.49, s |
| 20 | 1.07, s | 1.15, s | 1.29, s | 1.45, s | 0.94, d (7.2) | 3.24, 3.53, m |
| 3-OH | 10.89, s | 5.96, s | 11.46, s | |||
| 6-OH | 4.93, s | 4.93, s | ||||
| 9-OH | 5.29, d (4.7) | 5.31, d (4.7) | ||||
| 11-OH | 6.34, d (7.6) | 6.39, d (7.3) | 5.96, d (5.6) | |||
| 13-OH | 5.80, s | 5.72, s | ||||
| 20-OH | 4.73, t (5.2) |
Measured in DMSO-d6.
Measured in CDCl3.
Signals overlapped.
Figure 4.
Equilibrium mixtures and acetylation of 5–7.
In a similar manner, HRESIMS and 1D/2D NMR (Tables 1 and 5, Figures S2 and S3) revealed 9 as a C-11-deacetylated analogue of ent-homoabyssomicin B.13 Compound 10 shared the 9 tetracyclic abyssomicin core but was distinguished by the presence of an additional 20-OH (reminiscent of 3 and 6) and the lack of the 6-OH and C-8/C-9-epoxide. Thus, 9 and 10 were named abyssomicins U and V.
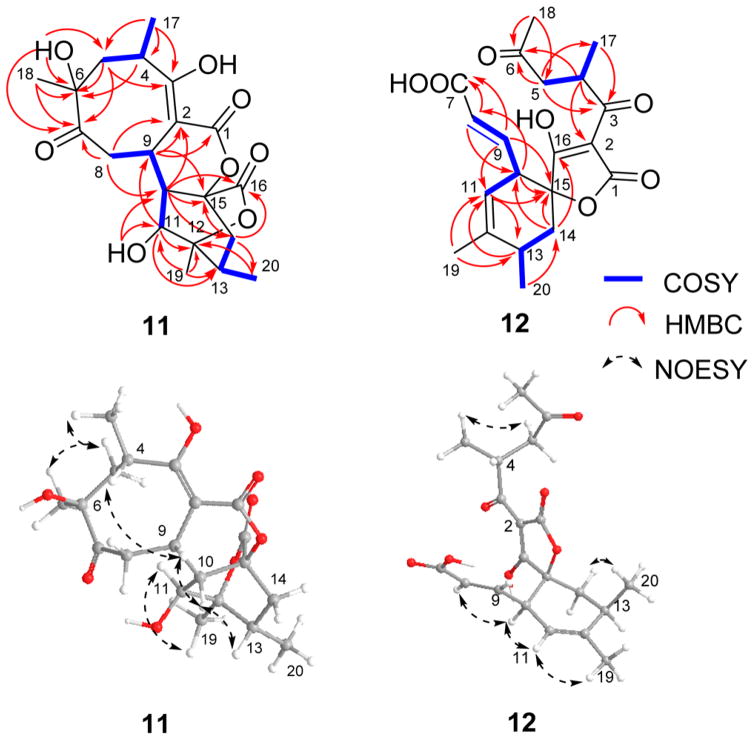
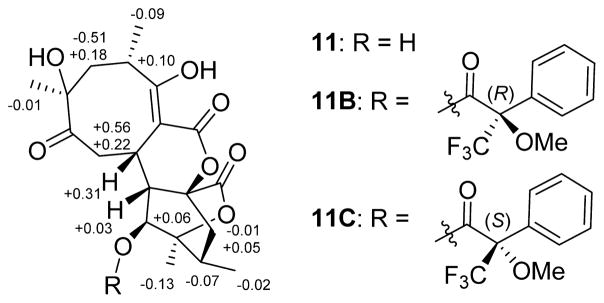
Compound 11 (C20H26O8) also displayed common abyssomicin UV and NMR signatures with putative structural divergence at C-2, C-9, and C-16 (Tables 1 and 3). Specifically, C-16 in 11 displayed both a unique 13C chemical shift (δC 171.5) and distinct HMBC correlations with H-10 (δH 2.39) and H2-14 (δH 1.43, 2.45), consistent with a lack of C-2/C-16 bond and the presence of a novel lactone (Figure 1). Additional key evidence included HMBC correlations from H-9 (δH 4.05) to C-1, C-2, and C-3 and from H2-8 to C-2, C-7, C-9, and C-10, which established the C-2/C-9 bond. The similarities in optical rotation and NOE correlations among 11 and 1 implicated relative stereochemical conservation among shared substructures (Figure 3). Treatment of 11 with (S)- and (R)-MTPA chlorides gave the C-11 mono-MTPA esters (11B and 11C, Figure 5), respectively, as a basis for C-11 stereochemical determination.33,34 The calculated H-11 Δδ values (Figure 5) supported an S-configuration consistent with that of 1. These cumulative analyses established 11 (abyssomicin W) as an 8/6/6/6 tetracyclic system where the C-2/C-16 disconnection and C-2/C-9 connection are unique among abyssomicins reported to date.
Figure 3.
1H,1H–COSY, key HMBC, and NOESY correlations for compounds 11 and 12.
Figure 5.
ΔδS–R values for MTPA esters of 11.
Compound 12 (C20H24O7) also displayed common abyssomicin NMR signatures with clear C-6 and C-7 structural divergence and the presence of two additional double bonds (Tables 1 and 3). 13C and 1H NMR and HMBC (Figure 3) established the nature of the two double bonds [(E)-Δ8,9 and (Z)-Δ11,12] and the unprecedented loss of the C-6/C-7 bond with concomitant formation of the C-6 ketone and C-7 carboxylate. The relative configuration of 12 was also established by NOESY (Figure 3). As a novel ring-open abyssomicin congener and the first reported naturally occurring linear spirotetronate, 12 was designated as abyssomicin X.
Prior abyssomicin SAR studies highlighted the presence of the C-8/C-9 α,β-unsaturated carbonyl as the key reactive abyssomicin pharmacophore.18 Consistent with their lack of this reactive moiety, compounds 1–12 were inactive at or below 60 and 10 μM in standard antimicrobial and cancer cell line cytotoxicity assays, respectively.
DISCUSSION
In summary, metabolic profiling of the coal mine fire isolate Streptomyces sp. LC-6-2 led to the discovery of a set of 12 abyssomicin analogues, highlighting the first reported example of a bacterial strain capable of producing a broad array of abyssomicins that share global stereochemical features with the unique “enantiomeric” ent-homoabyssomicins A and B.13 Among this set, the bicyclic abyssomicin X (12) and tetracyclic abyssomicin W (11) also notably expand the structural diversity of abyssomicin-associated scaffolds reported to date. From a biosynthetic perspective, the existence of both abyssomicins and ent-abyssomicins raises questions with respect to the fundamental drivers of stereoselectivity in abyssomicin ring formation as well as the corresponding impact of substrate stereochemical configuration on subsequent downstream tailoring reactions. Comparison of the newly discovered abyssomicins M–X (1–12) with previously reported abyssomicins suggests some global conservation among putative biosynthetic late-stage tailoring reactions including 9-O-methylation in 4 (also observed in abyssomicins E8 and L11) and 6-OH substitution in 4–7, 9, and 11 (also observed in abyssomicins E8 and ent-homoabyssomicin B13). Consistent with the newly discovered abyssomicins M–X (1–12), whole genome sequencing of Streptomyces sp. LC-6-2 revealed a single putative abyssomicin biosynthetic gene cluster (Figure S4 and Table S2) and thereby sets the stage for future gene locus validation and biosynthetic interrogation.
EXPERIMENTAL SECTION
General Experimental Procedures
Optical rotation was recorded on a Jasco DIP-370 digital polarimeter (Jasco, Easton, MD, USA). UV spectra were recorded on an Ultrospec 8000 spectrometer (GE, Pittsburgh, PA, USA). All NMR data were recorded at 500 or 400 MHz for 1H and 100 MHz for 13C with Varian Inova NMR spectrometers (Agilent Technologies, Santa Clara, CA, USA). LC-MS was conducted with an Agilent 6120 Quadrupole MSD mass spectrometer (Agilent Technologies) equipped with an Agilent 1200 Series Quaternary LC system and an Eclipse XDB-C18 column (150 × 4.6 mm, 5 μm). HRESIMS spectra were recorded on an AB SCIEX Triple TOF 5600 system (AB Sciex, Framingham, MA, USA). Single-crystal X-ray diffraction was measured on a Bruker-Nonius X8 Proteum. HPLC analyses were performed on an Agilent 1260 system equipped with a photodiode array (PDA) detector and a Phenomenex C18 column (250 × 4.6 mm, 5 μm; Phenomenex, Torrance, CA, USA). Semipreparative HPLC separation was performed on a Varian Prostar 210 HPLC system (Agilent) equipped with a PDA detector 330 using a Supelco DiscoveryBio wide pore C18 column (250 × 21.2 mm, 10 μm; flow rate, 8 mL/min; wavelength, 254 nm; Sigma-Aldrich, St. Louis, MO, USA). A Nanodrop 2000c UV–vis spectrophotometer (Thermo Scientific, Waltham, MA, USA) was used to measure concentration and purity of DNA. Sephadex LH-20 (25–100 μm) was purchased from GE Healthcare (Little Chalfont, United Kingdom). C18-functionalized silica gel (40–63 μm) was purchased from Material Harvest Ltd. (Cambridge, United Kingdom). Amberlite XAD16N resin (20–60 mesh) was purchased from Sigma-Aldrich. TLC silica gel plates (60 F254) were purchased from EMD Chemicals Inc. (Darmstadt, Germany). All solvents and reagents used were of ACS grade or greater, used without further purification, and purchased from Pharmco-AAPER (Brookfield, CT, USA), Sigma-Aldrich, TCI America (Tokyo, Japan), or Alfa-Aesar (Ward Hill, MA, USA) unless otherwise noted.
Isolation of Streptomyces sp. LC-6-2
The soil sample was collected from the Lotts Creek coal fire, Perry County, KY. The isolation of strain LC-6-2 followed previously reported protocols.25,27
DNA Extraction, Genome Sequencing, and Analyses
A single colony of Streptomyces sp. LC-6-2 from M2 agar (glucose, 4.0 g/L; yeast extract, 4.0 g/L; malt extract, 10.0 g/L; CaCO3, 2.0 g/L; agar, 18.0 g/L) growth was used to inoculate in a 250 mL baffled flask with 50 mL of M2 broth (glucose, 4.0 g/L; yeast extract, 4.0 g/L; malt extract, 10.0 g/L; CaCO3, 2.0 g/L). After 3 days of incubation at 28 °C with 200 rpm agitation, cells were recovered via centrifugation (5000g for 15 min at 4 °C) and used for genomic DNA isolation using an UltraClean microbial DNA isolation kit (Mo Bio Laboratories, CA, USA). DNA quality and quantity were assessed using gel electrophoresis and absorbance. The resultant DNA solution was subjected to massively parallel sequencing using the MiSeq sequencer (Illumina, San Diego, CA) at the University of Kentucky Advanced Genetic Technologies Center (UK-AGTC). Sequences were assembled using Newbler v.2.9 (Roche Diagnostics, Indianapolis, IN, USA) with gap fill-in and verification accomplished via polymerase chain reaction and amplicon sequencing to give the final draft genome (6.9 Mb, 78× coverage, 952 scaffolds with a mean size of 7954.8 bp, 72.6% GC percentage). The abs gene cluster was identified by BLAST searching, and manual annotation was done using Geneious 6.1.8 software35 and BLAST search tools (http://blast.ncbi.nlm.nih.gov/Blast.cgi). Protein functions were assigned through database comparison with the BLAST search tools (Figure S4 and Table S1). The sequence of the putative abs biosynthetic gene cluster has been deposited at GenBank under accession number KY432814.
Phylogeny of Streptomyces sp. LC-6-2
The partial 16S rRNA gene fragment was amplified using universal primers (27F, 5′-AGAGTTTGATCMTGGCTCAG-3′; 1492R, 5′-GGTTACCTT-GTTACGACTT-3′)36 and Advantage GC2 polymerase (Clontech, Mountain View, CA, USA), and the desired PCR-amplified product was isolated using the QIAquick gel extraction kit (Qiagen, Valencia, CA, USA). The amplified fragment (1307 bp) displayed 98% identity (BLAST search) to the 16S rRNA gene sequence of Streptomyces echinatus strain NBRC 12763. The sequence of 16S rRNA has been deposited in the NCBI nucleotide database with the accession number KT204465.
Fermentation, Extraction, Isolation, and Purification
Streptomyces sp. LC-6-2 was cultivated on M2 agar plates at 28 °C for 7 days. Chunks of the corresponding agar with bacterial growth were added to three 250 mL Erlenmeyer flasks, each containing 50 mL of medium SGG (soluble starch, 10.0 g/L; glucose, 10.0 g/L; glycerol, 10.0 mL/L; corn steep powder, 2.5 g/L; peptone, 5.0 g/L; yeast extract, 2.0 g/L; NaCl, 1.0 g/L; CaCO3, 3.0 g/L). After 3 days of incubation at 28 °C with 200 rpm agitation, the seed cultures were used to inoculate 100 flasks (250 mL), each containing 100 mL of medium SGG. The fermentation (10 L total) was continued for 7 days at 28 °C with 200 rpm agitation. The obtained culture broth was centrifuged at 3000g for 30 min (4 °C). The biomass (mycelium) was extracted with MeOH (3 × 500 mL), and then the recovered organics were evaporated in vacuo at 40 °C to yield 10.8 g of crude extract. The supernatant was mixed with 3% (w/v) XAD-16 resin and stirred overnight, followed by filtration. The resin was washed with H2O (3 × 600 mL) and then extracted with MeOH until the eluant was colorless. The MeOH extract was subsequently evaporated to afford 21.1 g of crude extract. Both extracts (obtained from the biomass and supernatant) revealed a similar metabolite profile based on HPLC and TLC analyses and were therefore combined (31.9 g).
As highlighted in Scheme S1, the combined crude extract was subjected to HP-20SS resin column chromatography (8 × 40 cm, 800 g) eluted with a gradient of aqueous CH3CN (10–100%) to yield 12 fractions, A–L. Fraction C (0.8 g) was subjected to a Sephadex LH-20 column (4 × 100 cm, 2 mL/min, MeOH) to obtain three subfractions, C1–C3. Subfraction C2 (0.6 g) was further purified by semi-preparative HPLC (20–40% aqueous CH3CN over 25 min) to yield compound 12 (7.8 mg, retention time: 16.2 min) as a colorless, amorphous powder. Fraction D (0.2 g) was purified by semi-preparative HPLC (10–45% aqueous CH3CN over 30 min) to yield compounds 12 (10.5 mg, retention time: 16.2 min) and 6 (10.8 mg, retention time: 12.1 min) as colorless, amorphous powders. Fraction E (0.6 g) was subjected to a Sephadex LH-20 column (4 × 100 cm, 2 mL/min, MeOH) to obtain three subfractions, E1–E3. Subfraction E2 (0.28 g) was further purified by semipreparative HPLC (20–35% aqueous CH3CN over 30 min) to yield compound 7 (11.4 mg, retention time: 15.5 min) as a colorless, amorphous powder. Fraction F (1.3 g) was subjected to a Sephadex LH-20 column (4 × 100 cm, 2 mL/min, MeOH) to obtain three subfractions, F1–F3. Subfraction F2 (0.3 g) was further purified by semipreparative HPLC (10–55% aqueous CH3CN over 40 min) to yield compound 10 (5.4 mg, retention time: 14.1 min) as a colorless, amorphous powder. Fraction G (0.3 g) was purified by semipreparative HPLC (20–50% aqueous CH3CN over 30 min) to yield compounds 3 (22.1 mg, retention time: 17.9 min) and 5 (30.0 mg, retention time: 19.0 min) as colorless, amorphous powders. Fraction H (0.2 g) was purified by semi-preparative HPLC (25–50% aqueous CH3CN over 35 min) to yield compounds 5 (14.0 mg, retention time: 19.0 min) and 9 (10.2 mg, retention time: 17.4 min) as colorless, amorphous powders. Fraction I (0.3 g) was purified by semipreparative HPLC (25–50% aqueous CH3CN over 35 min) to yield compounds 1 (8.1 mg, retention time: 21.7 min), 4 (6.3 mg, retention time: 22.3 min), 5 (10.5 mg, retention time: 19.0 min), 8 (1.5 mg, retention time: 22.8 min), and 9 (18.2 mg, retention time: 17.4 min) as colorless, amorphous powders. Fraction J (0.3 g) was purified by semipreparative HPLC (27–52% aqueous CH3CN over 35 min) to yield compounds 1 (4.6 mg, retention time: 21.7 min), 4 (8.3 mg, retention time: 22.3 min), 8 (3.0 mg, retention time: 22.8 min), 9 (12.0 mg, retention time: 17.4 min), and 11 (4.0 mg, retention time: 19.6 min) as colorless, amorphous powders. Fraction K (0.1 g) was purified by semipreparative HPLC (35–70% aqueous CH3CN over 30 min) to yield compound 2 (4.1 mg, retention time: 25.8 min) as a colorless, amorphous powder.
Abyssomicin M (1): white, amorphous powder; [α]25D −126.7 (c 0.8, MeOH); UV (MeOH) λmax (log ε) 264 (3.00) nm; 13C and 1H NMR data, see Tables 1 and 3; (+)-ESIMS m/z 379.2 [M + H]+, 396.2 [M + NH4]+; (−)-ESIMS m/z 377.2 [M − H]−, 413.1 [M + Cl]−; (+)-HRESIMS m/z 379.1756 [M + H]+ (calcd for C20H27O7, 379.1757), 396.2020 [M + NH4]+ (calcd for C20H30O7N, 396.2022).
Abyssomicin N (2): white, amorphous powder; [α]25D −82.5 (c 0.3, MeOH); UV (MeOH) λmax (log ε) 261 (2.40) nm; 13C and 1H NMR data, see Tables 1 and 3; (+)-ESIMS m/z 363.2 [M + H]+, 380.2 [M + NH4]+, 385.2 [M + Na]+; (−)-ESIMS m/z 361.1 [M + Cl]−, 397.1 [M + Cl]−; (+)-HRESIMS m/z 363.1798 [M + H]+ (calcd for C20H27O6, 363.1808), 385.1626 [M + Na]+ (calcd for C20H26O6Na, 385.1627).
Abyssomicin O (3): white, amorphous powder; [α]25D −94.5 (c 0.4, MeOH); UV (MeOH) λmax (log ε) 263 (2.25) nm; 13C and 1H NMR data, see Tables 1 and 3; (+)-ESIMS m/z 379.1 [M + H]+; (−)-ESIMS m/z 377.1 [M − H]−; (+)-HRESIMS m/z 379.1747 [M + H]+ (calcd for C20H27O7, 379.1757), 396.2009 [M + NH4]+ (calcd for C20H30O7N, 396.2022), 401.1565 [M + Na]+ (calcd for C20H26O7Na, 401.1575).
Abyssomicin P (4): white, amorphous powder; [α]25D −76.3 (c 0.7, MeOH); UV (MeOH) λmax (log ε) 264 (1.89) nm; 13C and 1H NMR data, see Tables 1 and 3; (+)-ESIMS m/z 409.2 [M + H]+; (−)-ESIMS m/z 443.1 [M + Cl]−; (+)-HRESIMS m/z 431.1665 [M + Na]+ (calcd for C21H28O8Na, 431.1682).
Abyssomicin Q (5): white, amorphous powder; [α]25D −52.7 (c 1.0, MeOH); UV (MeOH) λmax (log ε) 264 (1.73) nm; 13C and 1H NMR data, see Tables 2 and 4; (+)-ESIMS m/z 395.2 [M + H]+, 811.3 [2 M + Na]+; (−)-ESIMS m/z 429.1 [M + Cl]−, 439.1 [M + HCOO]−; (+)-HRESIMS m/z 395.1694 [M + H]+ (calcd for C20H27O8, 395.1706), 417.1510 [M + Na]+ (calcd for C20H26O8Na, 417.1525).
Table 2.
13C NMR (100/125 MHz) Data for Compounds 5, 5B, 5C, 6, 6B, 6C, 7, 7B, and 7C
| no. | 5a | 5Ba | 5Cb,d | 6c | 6Bc | 6Cb | 7a | 7Ba | 7Cb |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 172.7, C | 171.7, C | NDe | 177.3, C | 175.6, C | 164.3, C | 172.2, C | 171.3, C | 164.5, C |
| 2 | 98.1, C | 54.3, CH | 114.2, C | 99.7, C | 55.9, CH | 113.9, C | 97.9, C | 54.4 CH | 110.2,qC |
| 3 | 179.9, C | 106.4, C | 166.7, C | 182.7, C | 108.3, C | 167.7, C | 180.1, C | 106.4, C | 167.8, C |
| 4 | 35.8, CH | 42.3, CH | 36.5, CH | 37.5, CH | 43.7, CH | 36.5, CH | 35.9, CH | 42.4, CH | 36.6, CH |
| 5 | 37.9, CH2 | 36.3, CH2 | 39.2, CH2 | 39.6, CH2 | 38.3, CH2 | 38.9, CH2 | 38.1, CH2 | 36.3, CH2 | 39.2, CH2 |
| 6 | 77.6, C | 80.2, C | 78.2, C | 79.6, C | 82.6, C | 78.1, C | 77.7, C | 80.2, C | 78.3, C |
| 7 | 208.8, C | 200.3, C | 206.4, C | 210.1, C | 201.9, C | 206.6, C | 208.7, C | 200.1, C | 206.9, C |
| 8 | 66.9, CH | 68.3, CH | 63.0, CH | 68.7, CH | 70.1, CH | 63.5, CH | 66.4, CH | 67.7, CH | 64.0, CH |
| 9 | 66.9, CH | 67.6, CH | 70.2, CH | 68.9, CH | 70.1, CH | 73.8, CH | 67.0, CH | 67.7, CH | 70.3, CH |
| 10 | 53.5, CH | 52.8, CH | 49.6, CH | 55.4, CH | 54.4, CH | 49.2, CH | 54.4, CH | 53.5, CH | 50.8, CH |
| 11 | 71.9, CH | 71.7, CH | 73.8, CH | 74.7, CH | 74.5, CH | 70.0, CH | 74.8, CH | 74.4, CH | 75.9, CH |
| 12 | 78.0, C | 77.6, C | 77.3, C | 79.3, C | 78.8, C | 81.2, C | 75.2, C | 75.1, C | 73.1, C |
| 13 | 26.8, CH | 27.0, CH | 28.9, CH | 36.6, CH | 36.8, CH | 33.7, CH | 73.1, C | 73.1, C | 76.7, C |
| 14 | 33.7, CH2 | 31.7, CH2 | 34.2, CH2 | 30.6, CH2 | 28.7, CH2 | 29.6, CH2 | 44.0, CH2 | 42.4, CH2 | 45.1, CH2 |
| 15 | 84.5, C | 83.1, C | 81.6, C | 86.5, C | 84.6, C | 76.0, C | 84.0, C | 82.7, C | 81.7, C |
| 16 | 84.5, C | 83.7, C | 84.1, C | 86.8, C | 85.8, C | 84.3, C | 83.7, C | 83.2, C | 83.6, C |
| 17 | 19.0, CH3 | 11.2, CH3 | 17.5, CH3 | 19.9, CH3 | 12.2, CH3 | 17.9, CH3 | 18.9, CH3 | 11.3, CH3 | 17.5, CH3 |
| 18 | 27.2, CH3 | 22.6, CH3 | 27.7, CH3 | 28.0, CH3 | 22.9, CH3 | 27.4, CH3 | 27.1, CH3 | 22.6, CH3 | 27.7, CH3 |
| 19 | 20.4, CH3 | 20.6, CH3 | 20.8, CH3 | 21.6, CH3 | 21.2, CH3 | 20.8, CH3 | 17.3, CH3 | 17.4, CH3 | 17.3, CH3 |
| 20 | 15.8, CH3 | 15.9, CH3 | 16.0, CH3 | 63.8, CH2 | 63.3, CH2 | 64.2, CH2 | 25.6, CH3 | 25.6, CH3 | 25.7, CH3 |
Measured in DMSO-d6.
Measured in CDCl3.
Measured in CD3OD.
Obtained from HSQC and HMBC.
ND: not detected.
Table 4.
1H NMR (400/500 MHz) Data for Compounds 5, 5B, 5C, 6, 6B, and 6C (δH in ppm, multi, J in Hz)
| no. | 5a | 5Ba | 5Cb | 6c | 6Bc | 6Cb |
|---|---|---|---|---|---|---|
| 2 | 3.41, s | 3.56, s | ||||
| 4 | 2.58, m | 2.25, m | 2.71, m | 2.71, m | 2.38d | 2.72, m |
| 5 | 1.81, dd (5.4, 15.2) 2.84, dd (12.6) |
1.99, m 2.32d |
2.00, m 2.92, d (15.1) |
1.91, dd (5.6, 15.2) 2.94, dd (12.3) |
2.08, m 2.39d |
1.99, dd (5.4, 15.6) 2.88, dd (12.2) |
| 8 | 3.18, d (8.0) | 3.00, d (7.3) | 3.63, d (7.8) | 3.23, d (8.2) | 3.00, d (7.9) | 3.61, d (8.5) |
| 9 | 4.52, dd (3.8, 8.0) | 4.22, dd (5.2, 7.3) | 5.46, dd (3.9, 8.0) | 4.75, dd (4.2, 8.2) | 4.39, dd (4.8, 7.9) | 5.18, s |
| 10 | 2.30d | 2.26, m | 2.97, d (3.4) | 2.45, m | 2.43, m | 3.00, d (3.9) |
| 11 | 4.16, br s | 4.11, br s | 5.21, br s | 4.20, br s | 4.21, br s | 5.46,dd (4.2, 8.5) |
| 13 | 2.30d | 2.30d | 2.33, m | 2.54, m | 2.54, m | 2.60, m |
| 14 | 0.86, dd, 4.4, 12.7 2.27d |
1.23e 2.32d |
1.14, m 2.46, t (12.8) |
1.32d 2.26 |
1.58, m 2.32d |
1.37, dd (5.6, 14.5) 2.40, t (11.5) |
| 17 | 1.23e | 1.22e | 1.29, d (7.0) | 1.31d | 1.28e | 1.27, d (7.1) |
| 18 | 1.14, s | 1.26, s | 1.31, s | 1.24, s | 1.35, s | 1.31, s |
| 19 | 1.14, s | 1.08, s | 1.22, s | 1.32, s | 1.27, se | 1.31, s |
| 20 | 0.82, d (5.7) | 0.92, d (5.6) | 0.97, d (6.6) | 3.37, t (9.2, 9.8) 3.72, dd (5.8, 10.0) |
3.51, t (9.2, 10.0) 3.79, dd (5.8, 10.0) |
3.86, t (9.1, 9.7) 4.30, dd (6.0, 10.7) |
| 3-OH | 10.93, s |
Measured in DMSO-d6.
Measured in CDCl3.
Measured in CD3OD.
Signals overlapped.
Abyssomicin R (6): white, amorphous powder; [α]25D −10.0 (c 0.2, MeOH); UV (MeOH) λmax (log ε) 258 (1.16) nm; 13C and 1H NMR data, see Tables 2 and 4; (+)-ESIMS m/z 411.1 [M + H]+, 428.2 [M + NH4]+; (−)-ESIMS m/z 445.1 [M + Cl]−, 455.1 [M + HCOO]−; (+)-HRESIMS m/z 411.1654 [M + H]+ (calcd for C20H27O9, 411.1655), 428.1922 [M + NH4]+ (calcd for C20H30O9N, 428.1921).
Abyssomicin S (7): white, amorphous powder; [α]25D −37.6 (c 0.4, MeOH); UV (MeOH) λmax (log ε) 262 (3.05) nm; 13C and 1H NMR data, see Tables 2 and 5; (+)-ESIMS m/z 411.1 [M + H]+, 428.2 [M + NH4]+; (−)-ESIMS m/z 409 [M − H]−, 445.0 [M + Cl]−, 455.1 [M + HCOO]−; (+)-HRESIMS m/z 411.1658 [M + H]+ (calcd for C20H27O9, 411.1655).
Abyssomicin T (8): white, amorphous powder; [α]25D −87.6 (c 0.4, MeOH); UV (MeOH) λmax (log ε) 262 (3.72) nm; 13C and 1H NMR data, see Tables 1 and 5; (+)-ESIMS m/z 377.1 [M + H]+; (−)-ESIMS m/z 375.1 [M − H]−, 411.1 [M + Cl]−; (+)-HRESIMS m/z 394.1837 [M + NH4]+ (calcd for C20H28O7N, 394.1866), 399.1385 [M + Na]+ (calcd for C20H24O7Na, 199.1420).
Abyssomicin U (9): white, amorphous powder; [α]25D +107.7 (c 0.7, MeOH); UV (MeOH) λmax (log ε) 245 (4.60) nm; 13C and 1H NMR data, see Tables 1 and 5; (+)-ESIMS m/z 393.2 [M + H]+; (−)-ESIMS m/z 437.2 [M + HCOO]−; (+)-HRESIMS m/z 393.1518 [M + H]+ (calcd for C20H25O8, 393.1549), 415.1344 [M + Na]+ (calcd for C20H24O8Na, 415.1369).
Abyssomicin V (10): white, amorphous powder; [α]25D +87.7 (c 0.3, MeOH); UV (MeOH) λmax (log ε) 243 (3.21) nm; 13C and 1H NMR data, see Tables 1 and 5; (+)-ESIMS m/z 379.1 [M + H]+; (−)-ESIMS m/z 377.2 [M − H]−, 413.1 [M + Cl]−; 423.2 [M + HCOO]–; (+)-HRESIMS m/z 379.1752 [M + H]+ (calcd for C20H27O7, 379.1757), 401.1570 [M + Na]+ (calcd for C20H26O7Na, 401.1576).
Abyssomicin W (11): white, amorphous powder; [α]25D −50.0 (c 0.4, MeOH); UV (MeOH) λmax (log ε) 270 (1.28) nm; 13C and 1H NMR data, see Tables 1 and 3; (+)-ESIMS m/z 412.2 [M + NH4]+; (−)-ESIMS m/z 429.1 [M + Cl]−, 439.2 [M + HCOO]−; (+)-HRESIMS m/z 412.1955 [M + NH4]+ (calcd for C20H30O8N, 412.1971), 806.3593 [2 M + NH4]+ (calcd for C40H56O16N, 806.3599).
Abyssomicin X (12): white, amorphous powder; [α]25D +135.0 (c 0.7, MeOH); UV (MeOH) λmax (log ε) 229 (4.96), 268 (4.58) nm; 13 C and 1H NMR data, see Tables 1 and 3; (+)-ESIMS m/z 377.1 [M + H]+, 394.2 [M + NH4]+; (−)-ESIMS m/z 375.1 [M − H]−; (+)-HRESIMS m/z 377.1600 [M + H]+ (calcd for C20H25O7, 377.1600), 394.1865 [M + NH4]+ (calcd for C20H28O7N, 394.1866).
Mosher Ester Analysis.32,33
Compound 11 (1 mg) was dissolved in 500 μL of DCM and 8 μL of pyridine, to which were then added sequentially dimethylaminopyridine (1 mg) and (S)-MTPA-Cl (20 μL). The reaction mixture was stirred at room temperature overnight and subsequently quenched via the addition of two drops of H2O. The corresponding mono-(R)-MTPA ester derivative (11B, 1 mg) was purified via semipreparative HPLC (25–50% aqueous CH3CN over 30 min). The mono-(S)-MTPA ester derivative 11C (1 mg) was made by using (R)-MTPA-Cl (20 μL) following the same protocol above. The calculated H-11 Δδ values (Figure 5) supported an S-configuration consistent with that of 1.
11-(R)-MTPA-abyssomicin W (11B)
1H NMR (CDCl3, 500 MHz) δH 13.72 (1H, s), 5.24 (1H, d, J = 5.7 Hz, H-11), 3.95 (1H, m, H-9), 3.34 (1H, dd, J = 4.2, 11.9 Hz, H-8a), 3.03 (1H, m, H-4), 2.37 (1H, br s, H-10), 2.28–2.35 (4H, overlapped, H-5a, H-8b, H-13, H-14a), 1.89 (1H, t, J = 13.1 Hz, H-5b), 1.73 (1H, dd, J = 7.5, 9.9 Hz, H-14b), 1.33 (3H, s, H-18), 1.26 (3H, s, H-19), 1.18 (3H, d, J = 6.2 Hz, H-17), 1.03 (3H, d, J = 4.9 Hz, H-20); (+)-HRESIMS m/z + 628.2368 [M + NH4] (calcd for C30H37F3NO10, 628.2370).
11-(S)-MTPA-abyssomicin W (11C)
1H NMR (CDCl3, 500 MHz) 5.18 (1H, d, J = 5.3 Hz, H-11), 3.64 (1H, m, H-9), 2.93 (1H, br s, H-4), 2.79 (1H, m, H-5a), 2.54 (1H, dd, m, H-8a), 2.34–2.36 (3H, overlapped, H-10, H-13, H-14a), 2.10 (1H, t, J = 6.9 Hz, H-8b), 1.71 (1H, m, H-5b), 1.68 (1H, m, H-14b), 1.39 (3H, s, H-19), 1.34 (3H, s, H-18), 1.29 (3H, d, J = 6.5 Hz, H-17), 1.05 (3H, d, J = 5.5 Hz, H-20); (+)-HRESIMS m/z 628.2352 [M + NH4]+ (calcd for C30H37F3NO10, 628.2370).
Acetylation of 5–7
Compounds 5, 6, and 7 (3 mg each) were treated with acetic anhydride (0.2 mL) and pyridine (0.2 mL) at room temperature overnight. Drying under N2, dissolving in MeOH (200 mL), and purifying via semipreparative HPLC (25–60% aqueous CH3CN over 35 min) yielded compounds 5C, 6C, and 7C.
3,9,11-Triacetylabyssomicin abyssomicin Q (5C): white, amorphous powder; 13C and 1H NMR data, see Tables 2 and 4; (+)-ESIMS m/z 538.2 [M + NH4]+, 503.2 [(M − H2O) + H)]+; (−)-ESIMS m/z 555.1 [M + Cl]–; (+)-HRESIMS m/z 503.1895 [M + H – H2O]+ (calcd for C26H31O10, 503.1917), 543.1815 [M + Na]+ (calcd for C26H32O11Na, 543.1842), 1063.3723 [2 M + Na]+ (calcd for C52H64O22Na, 1063.3787).
3,9,11,20-Tetraacetylabyssomicin abyssomicin R (6C): white, amorphous powder; 13C and 1H NMR data, see Tables 2 and 4; (+)-HRESIMS m/z 596.2399 [M + NH4]+ (calcd for C28H38NO13, 596.2343).
3,9,11-Triacetylabyssomicin abyssomicin S (7C): white, amorphous powder; 13C and 1H NMR data, see Tables 2 and 5; (+)-HRESIMS m/z 559.1777 [M + Na]+ (calcd for C26H32O12Na, 559.1791).
X-ray Crystallography
Colorless bulk crystals of 1 were obtained in acetone/H2O (2:1). X-ray diffraction data was collected at 90.0(2) K on a Bruker-Nonius X8 Proteum diffractometer with graded-multilayer focused Cu K(alpha) X-rays. Raw data were integrated, scaled, merged, and corrected for Lorentz–polarization effects using the APEX2 package.37 Corrections for absorption were applied using SADABS.38 The structure was solved by direct methods (SHELXT39) and refined against F2 by weighted full-matrix least-squares (SHELXL-201440). Hydrogen atoms were found in difference maps but subsequently placed at calculated positions and refined using a riding model. Non-hydrogen atoms were refined with anisotropic displacement parameters. The final structure model was checked using an R-tensor41 and by Platon/checkCIF.42 Crystallographic data for the structure of 1 have been submitted to the Cambridge Crystallographic Data Centre as supplementary publication CCDC 1495049 (see Figure 2 and Table S1). Copies of these data can be obtained free of charge via www.ccdc.cam.ac.uk/conts/retrieving.html (or from the Cambridge Crystallographic Data Centre, 12 Union Road, Cambridge CB2 1EZ, UK; fax: +44-(0)1223-336033 or e-mail: deposit@ccdc.cam.ac.uk).
Antibacterial, Antifungal, and Cancer Cell Line Viability Assays
Antibacterial (Staphylococcus aureus ATCC 6538, Micrococcus luteus NRRL B-287, Escherichia coli NRRL B-3708, Salmonella enterica ATCC 10708), antifungal (Saccharomyces cerevisiae ATCC 204508), and cell line cytotoxicity (non-small-cell lung A549) assays were accomplished in triplicate following our previously reported protocols.27,29
Supplementary Material
Acknowledgments
This work was supported by National Institutes of Health grants R01 GM115261 (J.S.T.) and T32 DA016176 (Y.Z.), the University of Kentucky College of Pharmacy, the University of Kentucky Markey Cancer Center, and the National Center for Advancing Translational Sciences (UL1TR001998).
Footnotes
Notes
The authors declare the following competing financial interest(s): JST is a co-founder of Centrose (Madison, WI).
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acs.jnat-prod.7b00108.
Workup isolation scheme; 1D/2D NMR and HRMS spectra of 1–12; and crystal data (PDF)
References
- 1.Bister B, Bischoff D, Strobele M, Riedlinger J, Reicke A, Wolter F, Bull AT, Zahner H, Fiedler HP, Sussmuth RD. Angew Chem, Int Ed. 2004;43:2574–2576. doi: 10.1002/anie.200353160. [DOI] [PubMed] [Google Scholar]
- 2.Keller-Schierlein W, Muntwyle R, Pache W, Zähner H. Helv Chim Acta. 1969;52:127–142. [Google Scholar]
- 3.Ding WD, Williams DR, Northcote P, Siegel MM, Tsao R, Ashcroft J, Morton GO, Alluri M, Abbanat D, Maiese WM, Ellestad GA. J Antibiot. 1994;47:1250–1257. doi: 10.7164/antibiotics.47.1250. [DOI] [PubMed] [Google Scholar]
- 4.Kellerjuslen C, King HD, Kuhn M, Loosli HR, Pache W, Petcher TJ, Weber HP, Vonwartburg A. J Antibiot. 1982;35:142–150. doi: 10.7164/antibiotics.35.142. [DOI] [PubMed] [Google Scholar]
- 5.Park HR, Furihata K, Hayakawa Y, Shin-ya K. Tetrahedron Lett. 2002;43:6941–6945. [Google Scholar]
- 6.Lacoske MH, Theodorakis EA. J Nat Prod. 2015;78:562–575. doi: 10.1021/np500757w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vieweg L, Reichau S, Schobert R, Leadlay PF, Süssmuth RD. Nat Prod Rep. 2014;31:1554–1584. doi: 10.1039/c4np00015c. [DOI] [PubMed] [Google Scholar]
- 8.Niu XM, Li SH, Gorls H, Schollmeyer D, Hilliger M, Grabley S, Sattler I. Org Lett. 2007;9:2437–2440. doi: 10.1021/ol0705999. [DOI] [PubMed] [Google Scholar]
- 9.Keller S, Nicholson G, Drahl C, Sorensen E, Fiedler HP, Sussmuth RD. J Antibiot. 2007;60:391–394. doi: 10.1038/ja.2007.54. [DOI] [PubMed] [Google Scholar]
- 10.Igarashi Y, Yu LK, Miyanaga S, Fukuda T, Saitoh N, Sakurai H, Saiki I, Alonso-Vega P, Trujillo ME. J Nat Prod. 2010;73:1943–1946. doi: 10.1021/np100292h. [DOI] [PubMed] [Google Scholar]
- 11.Wang Q, Song FH, Xiao X, Huang P, Li L, Monte A, Abdel-Mageed WM, Wang J, Guo H, He WN, Xie F, Dai HQ, Liu MM, Chen CX, Xu H, Liu M, Piggott AM, Liu XT, Capon RJ, Zhang LX. Angew Chem, Int Ed. 2013;52:1231–1234. doi: 10.1002/anie.201208801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leon B, Navarro G, Dickey BJ, Stepan G, Tsai A, Jones GS, Morales ME, Barnes T, Ahmadyar S, Tsiang M, Geleziunas R, Cihlar T, Pagratis N, Tian Y, Yu H, Linington RG. Org Lett. 2015;17:262–265. doi: 10.1021/ol503349y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Abdalla MA, Yadav PP, Dittrich B, Schuffler A, Laatsch H. Org Lett. 2011;13:2156–2159. doi: 10.1021/ol103076y. [DOI] [PubMed] [Google Scholar]
- 14.Riedlinger J, Reicke A, Zahner H, Krismer B, Bull AT, Maldonado LA, Ward AC, Goodfellow M, Bister B, Bischoff D, Süssmuth RD, Fiedler HP. J Antibiot. 2004;57:271–279. doi: 10.7164/antibiotics.57.271. [DOI] [PubMed] [Google Scholar]
- 15.Keller S, Schadt HS, Ortel I, Sussmuth RD. Angew Chem, Int Ed. 2007;46:8284–8286. doi: 10.1002/anie.200701836. [DOI] [PubMed] [Google Scholar]
- 16.Nicolaou KC, Harrison ST. Angew Chem, Int Ed. 2006;45:3256–3260. doi: 10.1002/anie.200601116. [DOI] [PubMed] [Google Scholar]
- 17.Nicolaou KC, Harrison ST. J Am Chem Soc. 2007;129:429–440. doi: 10.1021/ja067083p. [DOI] [PubMed] [Google Scholar]
- 18.Nicolaou KC, Harrison ST, Chen JS. Synthesis. 2009;2009:33–42. doi: 10.1055/s-0028-1083259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Byrne MJ, Lees NR, Han LC, van der Kamp MW, Mulholland AJ, Stach JE, Willis CL, Race PR. J Am Chem Soc. 2016;138:6095–6098. doi: 10.1021/jacs.6b00232. [DOI] [PubMed] [Google Scholar]
- 20.Hashimoto T, Hashimoto J, Teruya K, Hirano T, Shin-Ya K, Ikeda H, Liu HW, Nishiyama M, Kuzuyama T. J Am Chem Soc. 2015;137:572–575. doi: 10.1021/ja510711x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tian Z, Sun P, Yan Y, Wu Z, Zheng Q, Zhou S, Zhang H, Yu F, Jia X, Chen D, Mandi A, Kurtan T, Liu W. Nat Chem Biol. 2015;11:259–265. doi: 10.1038/nchembio.1769. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Reynolds AR, Elshahawi SI, Shaaban KA, Ponomareva LV, Saunders MA, Elgumati IS, Zhang Y, Copley GC, Hower JC, Sunkara M, Morris AJ, Kharel MK, Van Lanen SG, Prendergast MA, Thorson JS. Org Lett. 2015;17:2796–2799. doi: 10.1021/acs.orglett.5b01203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang X, Shaaban KA, Elshahawi SI, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J Antibiot. 2014;67:571–575. doi: 10.1038/ja.2014.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shaaban KA, Wang X, Elshahawi SI, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J Nat Prod. 2013;76:1619–1626. doi: 10.1021/np400308w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang X, Elshahawi SI, Shaaban KA, Fang L, Ponomareva LV, Zhang Y, Copley GC, Hower JC, Zhan CG, Kharel MK, Thorson JS. Org Lett. 2014;16:456–459. doi: 10.1021/ol4033418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shaaban KA, Singh S, Elshahawi SI, Wang X, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J Antibiot. 2014;67:223–230. doi: 10.1038/ja.2013.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang X, Shaaban KA, Elshahawi SI, Ponomareva LV, Sunkara M, Zhang Y, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. J Nat Prod. 2013;76:1441–1447. doi: 10.1021/np400231r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shaaban KA, Singh S, Elshahawi SI, Wang X, Ponomareva LV, Sunkara M, Copley GC, Hower JC, Morris AJ, Kharel MK, Thorson JS. Nat Prod Res. 2014;28:337–339. doi: 10.1080/14786419.2013.855932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shaaban KA, Saunders MA, Zhang Y, Tran T, Elshahawi SI, Ponomareva LV, Wang X, Zhang J, Copley GC, Sunkara M, Kharel MK, Morris AJ, Hower JC, Tremblay MS, Prendergast MA, Thorson JS. J Nat Prod. 2017;80:2–11. doi: 10.1021/acs.jnatprod.6b00948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang X, Zhang Y, Ponomareva LV, Qiu Q, Woodcock R, Elshahawi SI, Chen X, Zhou Z, Hatcher BE, Hower JC, Zhan C, Parkin S, Kharel MK, Voss SR, Shaaban KA, Thorson JS. Angew Chem, Int Ed. 2017;56:2994–2998. doi: 10.1002/anie.201612447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Elshahawi SI, Cao H, Shaaban KA, Ponomareva LV, Subramanian T, Farman ML, Spielmann HP, Phillips GN, Jr, Thorson JS, Singh S. Nat Chem Biol. 2017;13:366–368. doi: 10.1038/nchembio.2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laatsch H. AntiBase 2014: The Natural Compound Identifier. Wiley-VCH; Weinheim: 2014. [Google Scholar]
- 33.Dale JA, Mosher HS. J Am Chem Soc. 1973;95:512–519. [Google Scholar]
- 34.Hoye TR, Jeffrey CS, Shao F. Nat Protoc. 2007;2:2451–2458. doi: 10.1038/nprot.2007.354. [DOI] [PubMed] [Google Scholar]
- 35.Kearse M, Moir R, Wilson A, Stones-Havas S, Cheung M, Sturrock S, Buxton S, Cooper A, Markowitz S, Duran C, Thierer T, Ashton B, Meintjes P, Drummond A. Bioinformatics. 2012;28:1647–1649. doi: 10.1093/bioinformatics/bts199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lane DJ. In: Nucleic Acid Techniques in Bacterial Systematics. Stackebrandt E, Goodfellow M, editors. John Wiley and Sons Ltd; New York: 1991. pp. 115–147. [Google Scholar]
- 37.Bruker. APEX2. Bruker-AXS; Madison WI, USA: 2006. [Google Scholar]
- 38.Krause L, Herbst-Irmer R, Sheldrick GM, Stalke D. J Appl Crystallogr. 2015;48:3–10. doi: 10.1107/S1600576714022985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheldrick GM. Acta Crystallogr. 2015;A71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sheldrick GM. Acta Crystallogr. 2015;C71:3–8. doi: 10.1107/S2053273314026370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Parkin S. Acta Crystallogr, Sect A: Found Crystallogr. 2000;A56:157–162. doi: 10.1107/s010876739901497x. [DOI] [PubMed] [Google Scholar]
- 42.Spek AL. Acta Crystallogr, Sect D: Biol Crystallogr. 2009;D65:148–155. doi: 10.1107/S090744490804362X. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.