Abstract
Multiple studies have assessed parity as a risk factor for lung cancer but results have been inconclusive. We searched MEDLINE (through August, 2010) and the Institute of Scientific Information Web of Knowledge database (through April, 2011) to identify studies investigating the association of parity with lung cancer and allowing the calculation of dose-response trends using a linear model. Between-study heterogeneity was assessed using Cochran’s Q statistic and the I2 index. Summary per-child relative risks (RRs) with their 95% confidence interval (CI) were estimated using random effects meta-analysis. Sixteen eligible studies (8095 lung cancer patients; 350,295 unaffected individuals) provided data for meta-analysis. There was significant between-study heterogeneity (p<0.001; I2=73%). The summary per livebirth RR was 0.98 (95% CI, 0.95–1.02), indicating no effect of parity on lung cancer risk. Results were consistent in case-control (n=11), RR=0.99 (95% CI, 0.94–1.04), and cohort studies (n=5), RR=0.97 (95% CI, 0.92–1.03). Studies not including small-cell lung cancer patient found a borderline protective effect of parity, RR=0.94 (95% CI, 0.88–1.00). In contrast, no effect was observed in studies including small-cell lung cancer patients, RR=1.00 (95% CI, 0.98–1.03); p for difference=0.05. Overall, there was little evidence of a dose-response relationship between increasing number of livebirths and lung cancer; however, studies have produced heterogeneous results. Future studies should include analyses in well-defined histological disease subgroups.
Keywords: parity, lung cancer, meta-analysis, parity, systematic review, women’s health
Introduction
In 2010 more than 100,000 women were diagnosed with lung cancer in the USA; the disease is the most frequent cause of cancer-related death among women in the USA and the second most frequent cause world-wide [1, 2]. Although lung cancer mortality among men has reached a plateau or is declining in developed countries, it continues to increase among women in developing countries [3, 4]. The identification of environmental exposures predisposing to the development of lung cancer, such as tobacco consumption, environmental tobacco smoke and asbestos, are among the greatest successes of epidemiology and explain the bulk of the population incidence of lung cancer [5–7]. Yet, lung cancer arises in never smokers and a complex interplay of genetic and hormonal factors is believed to modify the effect of environmental carcinogens on disease initiation and progression [7].
It has been hypothesized that women may be more susceptible to the carcinogenetic effects of tobacco and that lung cancer in women may be biologically and clinically different from disease in men [8, 9]. Case-control studies in the 1990’s suggested that, for the same amount of tobacco exposure, women may be at increased risk for lung cancer compared to men [10]. Several large cohort studies failed to confirm this association and the issue remains controversial [11–15]. Regardless of whether women have an increased susceptibility to the carcinogenetic effects of smoking, lung cancer in women appears to have a different natural history compared to men, with several studies demonstrating superior survival for women when adjusting for disease stage, histology and treatment [9]. Women are also more likely to develop adenocarcinoma, a histological subtype with weaker associations with tobacco smoking [16]. Additional evidence suggesting that sex-related factors contribute to lung cancer carcinogenesis comes from studies demonstrating a familial aggregation of cancers of the reproductive system among relatives of female lung cancer patients [17, 18] as well as the increased lung cancer risk among female survivors of reproductive organ cancers [19–22]. In addition, lung tissue, both non-cancerous and tumor-derived, appears to express hormonal receptors suggesting that it may be responsive to hormonal stimuli [23–26].
Taken together, these observations suggest that hormonal factors may influence lung cancer pathogenesis and have motivated epidemiological studies investigating the association of hormonal and endocrine factors with lung cancer. Among the different exposures that have been investigated, parity (the number of livebirths in a woman’s lifetime), is likely less prone to recall bias and misclassification, and – in recent analyses – has been found to be inversely associated with lung cancer risk [27–29]. Many of the studies investigating the parity-lung cancer association are underpowered to detect moderate effect sizes and contradictory results have been reported, ranging from strongly protective effects [27, 28] to substantial increases in the risk of the disease with increasing parity [30]. To further investigate the association between parity and lung cancer risk and to identify potential sources of between-study heterogeneity we conducted a systematic review and dose-response meta-analysis of the relevant studies.
Methods
Literature search and eligibility criteria
We searched the MEDLINE database (through Pubmed, from inception to August 31st, 2010) to identify studies reporting on epidemiological investigations of the association between parity (defined as the total number of live-births) and lung cancer occurrence.
We used combinations of key words related to the exposure (such as “parity”, “pregnancy”, “livebirth”) and the outcome of interest (“lung cancer”, “pulmonary neoplasm”, “lung adenocarcinoma”), along with a combination of search filters for identifying observational studies. The complete search strategy is available upon request from the authors. We also perused the reference lists of eligible studies and relevant review articles. To increase the yield of our search we used the Institute of Scientific Information (ISI) Web of Knowledge database (last search: April 3rd, 2011) to identify articles citing the studies we considered eligible. We screened the titles and abstracts of the articles citing the originally identified studies to identify additional potentially eligible articles.
Eligible studies had to have an analytic design (case-control, nested case-control, or cohort) and report or allow the calculation of relative risk (RR) estimates (odds ratios, risk ratios, incidence rate ratios or hazard ratios) with their variance across at least three categories of parity, so as to allow estimation of the dose-response relationship between parity and lung cancer occurrence risk (i.e. studies of lung cancer incidence) [31, 32]. Alternatively, we considered studies that directly reported per-child risk estimates with their variance. We only considered studies reporting on at least 20 cases and excluded case reports, case series, comparative studies not using an analytical epidemiologic design, or studies not reporting analyses of primary data (e.g., letters, editorials, narrative reviews). We only considered English-language full text publications. Studies reporting on aero-digestive malignancies other than lung cancer were excluded unless they provided or allowed the calculation of risk estimates separately for lung cancer. We also excluded studies reporting exclusively on lung cancer mortality. When multiple studies pertained to the same or partially overlapping populations, we only considered the report with the longest follow-up (for cohort studies) or the largest number of cases (for case-control studies) from which data were extractable.
Data extraction
For each eligible study, two reviewers (IJD and JKP) independently extracted the following information: author, year of publication, population studied (selection of cases and controls for case-control studies; cohort selection and follow-up methods for cohort studies), settings and location where the study was conducted, relevant dates (including periods of recruitment, exposure, follow-up, and data collection), demographics of participants, outcome and exposure definitions (including lung cancer diagnosis and exposure ascertainment methods), use of matching (and variables used for matching cases and controls), number of cases and controls (for case-control studies) or affected and unaffected individuals (for cohort studies) stratified by parity levels, distribution of different lung cancer histologies in affected individuals, smoking related information, the percentage of women receiving hormone replacement therapy, the duration of follow-up, adjusted and unadjusted (when available) RR estimates (comparing participant groups defined by parity) and their variance (or sufficient statistics to calculate that variance). For all comparisons, the primary analysis used the maximally adjusted RR estimates reported from each study. For all descriptive variables we attempted to capture values separately for cases and controls (unaffected individuals); when such information was not available we recorded information for the overall study population.
Assessment of validity
We considered the following characteristics as being reflective of study validity: definition and measurement of exposure, definition and ascertainment of outcome, participation rates and potential for selection bias, consideration of potential confounders and effect modifiers (such as age and smoking status), methods used to define parity levels (when multiple exposure groups are analyzed), factors used for adjusting RR estimates (with particular focus on the handling of tobacco use-related information, given the strong association with tobacco use and lung cancer development). Regarding model building, we will assess whether a description of the procedure to select the model was provided (i.e., whether any model selection process was described), whether matching variables were entered in the final model (for matched studies). These items are largely consistent with the STrengthening the Reporting of OBservational studies in Epidemiology (STROBE) guidelines [33, 34]. We did not merge these items into a quality score because different scoring methods produce inconsistent results and may introduce bias [35].
Evidence synthesis
To estimate summary dose-response coefficients (i.e., per livebirth RRs) from each study we used the methods proposed by Greenland et al. [31, 32]. Briefly, for studies that reported RR estimates for at least three exposure categories, we used the log-RRs and their variances along with the marginal data for each exposure category (i.e. the number of cases and the number of controls for case-control studies or the number of cases and total person time for cohort studies) to estimate study-specific per-child RR for lung cancer. For each study, we used the group with the lowest number of livebirths as the reference group [31, 32]. For studies not using the category with the lowest number of livebirths as the reference, we used the effective count method proposed by Hamling et al. to recalculate the RR using the stratum with the lowest number of livebirths as the reference [36]. We assessed graphically whether linearity of the log-RR over parity was a plausible assumption.
Between-study heterogeneity of the per-child RR, estimated from each study was assessed with Cochran’s Q statistic and between-study inconsistency was quantified by the I2 statistic [37, 38]. Because of the low power of the Q statistic, we used p<0.1 as the threshold for statistical significance.
We summarized the coefficients across studies using an inverse-variance random effect model [39].
Sensitivity analyses and assessment of bias
It was expected that for some exposure groups open-ended definitions of parity would be provided [40]. For our primary analysis these categories were assigned a value that was equal to their lower limit plus half the width of the adjacent category. To assess whether our results were sensitive to the method of assigning values to open-ended exposure categories, we performed sensitivity analysis using two alternative approaches by assigning to these categories: (a) the value of their lower limit and (b) a value equal to their lower limit plus the width of the adjacent interval. We also explored the effect of excluding open-ended categories on the meta-analysis results. For each sensitivity analysis we re-calculated the dose-response coefficient from each study and then performed random effects meta-analysis across all studies, as described above.
In additional sensitivity analyses, we explored whether a single study influenced the results of the meta-analysis by calculating the summary RR after excluding each of the eligible studies in turn [41]. We also repeated the analysis using a fixed effects model [41, 42].
Subgroup analyses and assessment of small study effects
To explore potential sources of heterogeneity we assessed the following predefined subgroups: study design, participant ethnicity, completeness of histological confirmation, lung cancer histology, specification of parity modeling details, and whether adjustment for important covariates was performed. To estimate the effect of these study-level covariates on the association of parity with lung cancer, we used random effects meta-regression [43, 44].
To explore whether less precise studies reported results that were systematically different from those of more precise studies, we used the Egger regression-based test. This test is often referred to as a test for “publication bias” [45, 46].
Statistical analyses were conducted using Stata version 11.1/SE (Stata Corp., College Station, TX). Statistical significance was defined as a two-sided p-value<0.05 for all tests, except those for heterogeneity. Where applicable, we followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) reporting guidelines [47].
Results
Eligible studies
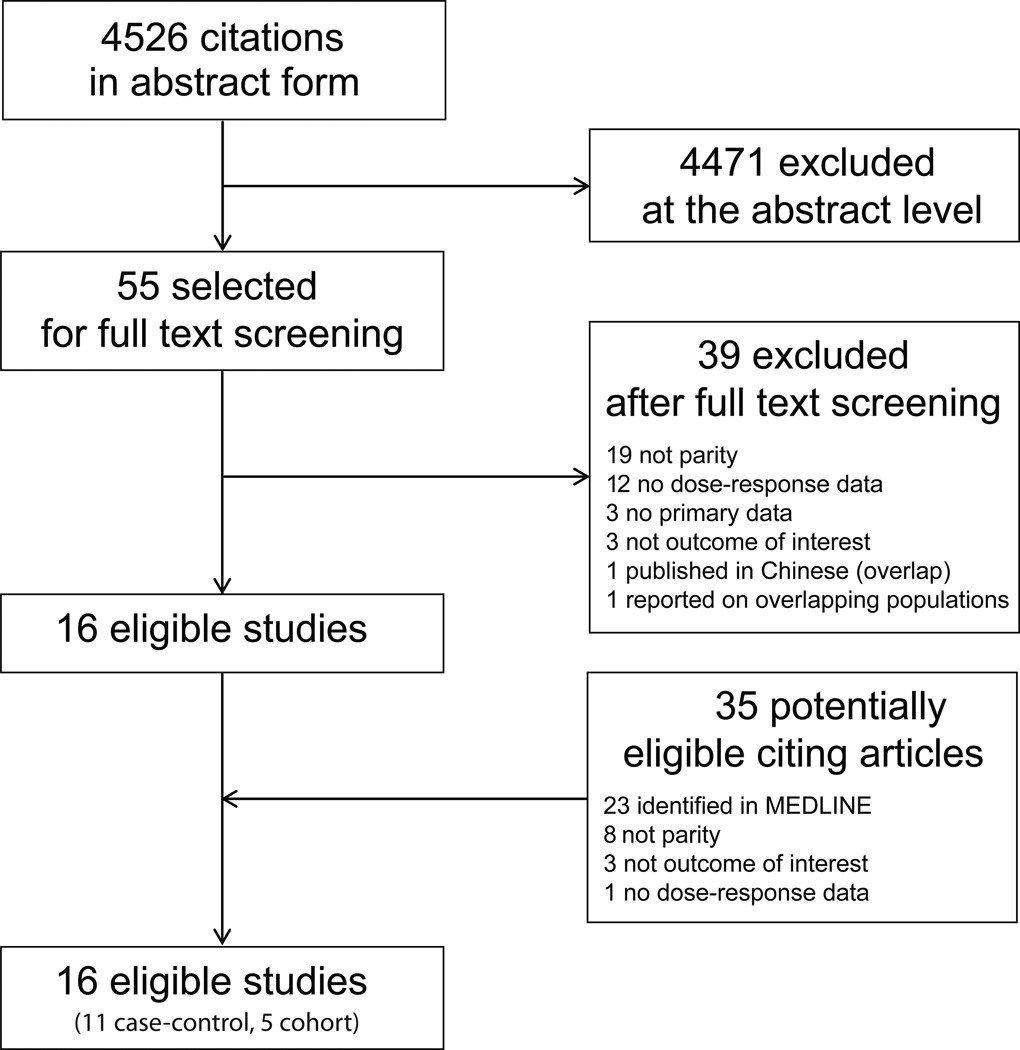
Our literature search retrieved 4526 citations of which, after screening of abstracts and titles, 55 were considered potentially eligible and were reviewed in full text. Of these studies, 39 were excluded (Figure 1) and 16 studies (11 case-control and 5 cohort studies) were considered eligible for inclusion in the meta-analysis. The ISI search retrieved 402 articles citing these 16 studies. After screening titles and abstracts, 35 were considered potentially eligible and were reviewed in full text; however, no additional eligible studies were identified. The characteristics of eligible studies are summarized in Table 1; additional details are presented in Supplementary Table 1.
Figure 1.
Search strategy flow.
Table 1.
Characteristics of eligible studies.a
| Author, year (Country) |
Study design Recruitment period |
Age in yearsb Menopausal status (premenopausal n, %) Hormone replacement therapy (n, %) |
Participants and follow-up information |
Lung cancer histology N (%) |
|---|---|---|---|---|
| Wu-Williams, 1990 (China) (58) |
Case-control (Harbin and Shenyang registries) 1985–1987 |
56 [NR]; 55 [NR] | 964 / 959 (of whom 956 cases and 951 of controls had information on parity) |
Available for 688 of cases; adenocarcinoma 310 (45); squamous-cell 201 (29); small-cell 117 (17); other 66 (10) |
| Taioli, 1994c (USA) (59) |
Case-control NR |
NR | 180 / 303 | Adenocarcinoma 180 (100) |
| Zhou, 2000 (China) (60) |
Case-control (Shenyang city) Cases, 1991–1995; controls, 1988–1989 |
Cases, 57 [NR]; controls, 57 [NR] | 72 / 72 | Adenocarcinoma 72 (100) |
| Seow, 2002 (Singapore) (61) |
Case-control 1996–1998 |
Cases, 65 [12]; controls, 63 [13] | 303 / 763 | Adenocarcinoma 166 (55); squamous-cell 56 (19); small-cell 21 (7); other 60 (20) |
| Brenner, 2003 (China) (62) |
Case-control (Pingliang and Qingyang prefectures) 1994–1998 |
Cases: <45, 21 (19); 45–54, 38 (35); 55–64, 34 (31); >65, 16 (15); controls: <45, 51 (12); 45–54, 162 (37); 55–64, 144 (33); >65, 78 (18) |
109 / 435 (of whom 108 cases and 431 controls had information on parity) |
Available for 38 of 109 cases; small-cell 16 (42); squamous-cell 14 (37); adenocarcinoma 1 (3); not specified 7 (18) |
| Kreuzer, 2003 (Germany) (63) |
Case-control 1990–1996 |
Cases, 60 [NR]; controls, 59 [NR] | 811 / 912 | Small-cell 207 (26); squamous -cell 174 (22); adenocarcinoma 386 (48); other 44 (5) |
| Liu, 2005 (Japan) (64) |
Prospective cohort (JPHC Study) 1990–1994 |
Total cohort, 40–49 years 17,915 (40); 50–59 years 18,318 (41); 60–69 years 8444 (19)d |
Total cohort, n=44,677; total person-years=395,448 years; incident cases, 153 |
Adenocarcinoma 118 (77); other 20 (13); unknown 17 (11) |
| Elliot, 2006 (UK) (30) |
Case-control (nested within the RCGP OCS) |
Mean age at recruitment in the cohort was 29 years. | 162 / 486 | NR |
| Kabat, 2007e (Canada) (65) |
Prospective cohort (NBSS) 1980–1985 |
Cases, 51 [5]; non-cases 49 [6] | Cases, 750; non-cases, 89,062; mean follow-up = 16.1 years |
Squamous-cell 100 (13); adenocarcinoma 355 (47); small-cell 122 (16); large-cell, 49 (7); other/mixed, 102 (14) |
| Schwartz, 2007 (USA) (66) |
Case-control 2001–2005 |
Cases, 60 [9]; controls, 58 [9] | 488 / 498 | Adenocarcinoma 346 (71); squamous-cell 39 (8); large-cell 15 (3); other 83 (17) |
| Weiss, 2008 (China) (67) |
Prospective cohort (SWHS) 1996 – 2000 |
Cases, f 19 (9%) 40–44y; 30 (14%) 45–49y; 25 (11%) 50–54 y; 30 (14%) 55–59 y; 45 (20%) 60–64y; 71 (32%) ≥65y. Total person-time,g 29% 40–44y; 21% 45–49y; 14% 50–54y; 10% 55–59y; 13% 60– 64y; 13% ≥65y |
Cases, 220; non-cases, 71,094; 506,522 total person-years; mean follow-up for cases = 4.1 years |
Available for 168 (76) of cases: adenocarcinoma, 78 (46) |
| Koushik, 2009 (Canada) (68) |
Case-control 1996–1997 |
Cases, 62 [9]; controls, 62 [9] | 422 / 577 (of whom 413 cases and 568 controls had information on parity) |
Adenocarcinoma, 201 (48); squamous- cell, 83 (20); small cell, 73 (17); large cell, 37 (9); other, 28 (7) |
| Seow, 2009 (Singapore) (27) |
Prospective cohort (SCHS) 1993 – 1998 |
Entire cohort at recruitment, 56 [8] | Cases, 298; non-cases, 33,730; mean follow-up = 9.6 years |
Available for 85% of cases: Adenocarcinoma 138 (46) |
| Baik, 2010 (USA) (29) |
Prospective cohort (NuHS) 1984–2006 |
63 [38–87]h | Cases, 1729; total cohort n=107,171 women with 1,590,432 total person-years; follow-up = 22 years |
Histologic confirmation for 1505 cases: adenocarcinoma, 707 (47%); small-cell carcinoma, 271 (18); squamous-cell carcinoma, 256 (17); large-cell carcinoma, 75 (5); unspecified NSCLC, 151 (10); other, 45 (3) |
| Meinhold, 2010 (USA) (57) |
Case-control (MLCS) 1998 – ongoing |
Cases, 66 [58–73]; hospital controls, 64 [56–71]; population controls 67 [61–72]i |
430 / 316 (hospital controls) + 295 (population controls) |
NSCLC, 430 (100) |
| Paulus, 2010 (USA) (28) |
Case-control (LCSS) 1992–2003 |
Cases, 66 [11]; controls, 58 [11] | 1004 / 848 | Adenocarcinoma, 455 (45); squamouscell, 150 (15); bronchioloalveolar, 121 (12); other, 278 (28) |
ATS = American Thoracic Society; CWLCS = Czech Women’s Lung Cancer Study; ICD = International Classification of Diseases; JPHC = Japanese Public Health Center; LCCS = Lung Cancer Susceptibility Study; MLCS = Maryland Lung Cancer Study; MVA = Motor Vehicle Administration; NBSS = National Breast Screening Study; NHS = National Health System; NR = not reported; NSCLC = non-small cell lung cancer; NuHS, Nurses Health Study; RCGP OCS = Royal College of General Practitioners Oral Contraception Study; SD = standard deviation; SEER = Surveillance Epidemiology and End-Results; SCHS = Singapore Chinese Health Study; SWHS: Shanghai Women’s Health Study.
Numbers have been rounded to the nearest integer.
Mean age [SD], unless otherwise indicated.
Some descriptive data extracted from Wynder et al., 1997.
Individuals (%) in each age stratum.
Some descriptive data extracted from Miller et al., 1992.
Number (percentage) of cases in each age stratum.
Percentage of total person-time in each age stratum.
Mean age over follow-up [range].
Median [interquartile range].
Eligible studies had been published between 1990 and 2010, and their enrollment periods ranged from 1980 to 2010. Six studies enrolled predominantly (>80%) white individuals, seven enrolled predominantly East Asian populations and two enrolled mixed populations (one study did not report information on the ethnic distribution of participants). Eleven studies had a case-control design (4945 cases and 6443 controls total) and 5 had a cohort design (3150 cases and 343,852 unaffected individuals total), for a combined sample size of 8095 lung cancer patients and 350,295 controls/unaffected individuals. The median number of lung cancer cases was 363 (inter-quartile range, IQR, 171–781) and the median number of controls/unaffected participants was 806 (IQR, 492-39127).
In the 11 case-control studies mean or median participant age ranged between 51 to 66 years for cases and between 55 and 64 years for controls. In the 3 out of 5 cohort studies that provided relevant information, mean or median age of participants at cohort inception ranged between 29 and 63 years. Lung cancer histology information was reported in 15 of the 16 studies and was available for at least 80% of cancer cases in 10 of them. The proportion of adenocarcinoma histology ranged between 3% and 100%, while the proportion of squamous cell histology ranged between 0 and 37%. Of the 15 studies that provided relevant information, 7 studies reported enrolling at least some patients with small-cell lung cancer and 8 studies did not (of those, two studies enrolled only cases with adenocarcinoma histology). In 15 out of 16 studies that provided relevant information the percentage of ever smokers ranged from 0 to 88% and the proportion of never smokers ranged between 8% and 100% among cases (one study did not report data on tobacco consumption and smoking history information).
Assessment of validity
Our assessment of study validity is summarized in Supplementary Table 2. Most studies used standardized questionnaires and in-person interviews to assess parity and smoking status. Participation rates were reported in 10 of the studies and ranged between 25% and 97%. Seven of these studies had participation rates higher than 80%. The majority of case-control studies were hospital-based (7 out of 11), and one case-control study was nested in an enumerated cohort. Regarding statistical analyses, the model building and selection process were generally not fully reported. One study did not report risk estimates accounting for participant age. Two studies exclusively included never smokers; of the remaining 14 studies, three did not report estimates from analyses accounting for participants’ personal smoking history. Two studies reported estimates for the effect of parity adjusted for other reproductive risk factors.
Among the eight studies that used a matched case-control design, age was the most common matching variable.
Evidence synthesis
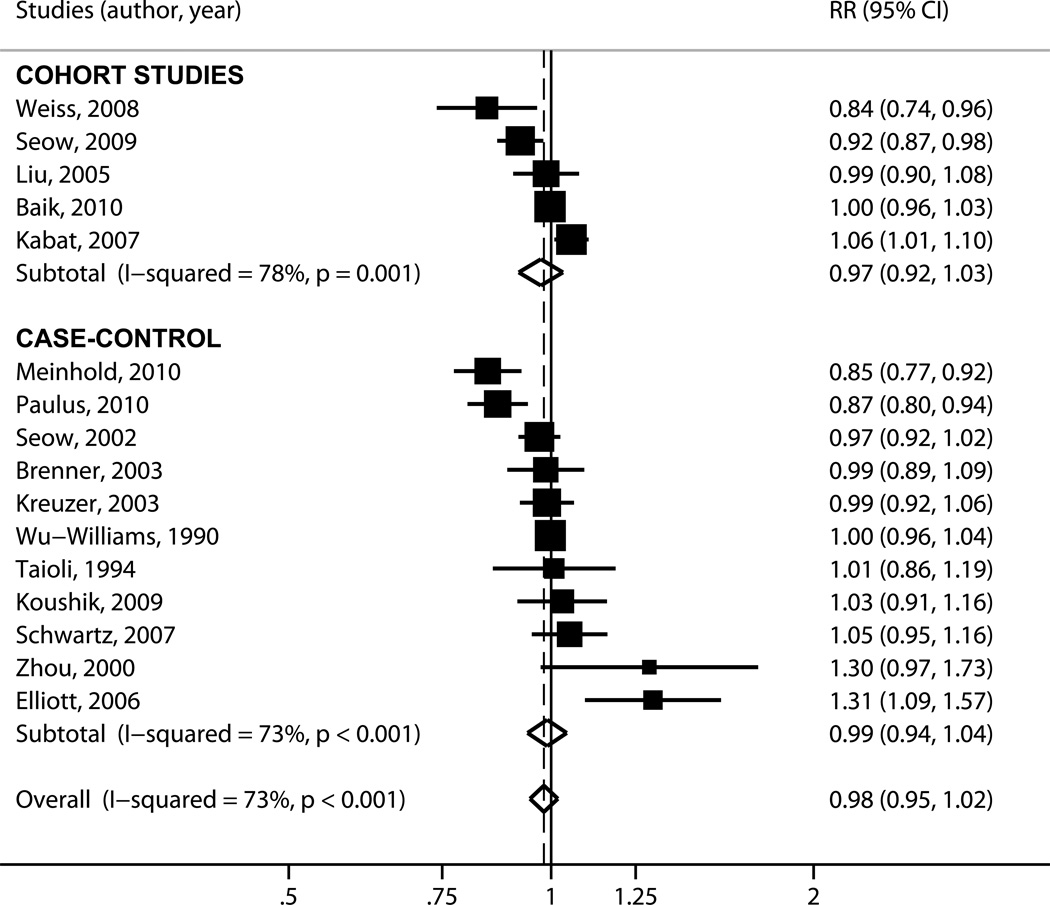
It was deemed that a linear model is appropriate for the data (Supplementary Figure 2). The point estimates of the per-study dose-response coefficients ranged from a protective effect, RR=0.84 (95% CI, 0.74, 0.96) to a strong harmful effect; RR=1.31 (95% CI, 1.09–1.57) per livebirth. Overall, there was substantial between-study heterogeneity (pQ<0.001; I2=73%). The random effects summary RR was 0.98 (95% CI, 0.95–1.02), suggesting that a large effect of parity on lung cancer risk is unlikely (Figure 2).
Figure 2.
Forest plot for the meta-analysis of parity and lung cancer risk. Each study is shown by the point estimate of the RR (square proportional to the weight of each study) and 95% CI for the RR (extending lines); summary RRs and 95% CIs by random effects calculations are depicted as a diamonds. Point estimate values higher than 1 indicate that increasing parity is associated with increased lung cancer risk. Results are stratified by study design (case-control versus cohort) and then studies are listed by their reported per-child relative risk. CI = confidence interval; RR = relative risk.
Subgroup analyses
Heterogeneity remained significant when we examined subgroups stratified by study design, the reporting of histology information or the types of statistical analyses performed (Table 2). Heterogeneity could not be explained by study design: the summary estimates from case-control RR=0.99 (95% CI, 0.94–1.04) and cohort studies, RR=0.97 (95% CI, 0.92–1.03) were almost identical. Excluding studies that did not report estimates from analyses accounting for participants’ personal smoking history did not significantly affect the summary result, RR=0.98 (0.94–1.02; p=0.29). Studies not including tumors of small-cell lung cancer histology suggested a protective effect of parity, RR=0.94 (95% CI, 0.88–1.00; p=0.06), whereas there was no effect among studies that reported including at least some patients with SCLC, RR=1.00 (95% CI, 0.98–1.03; p=0.72). Meta-regression analyses did not identify any study-level characteristic to explain between-study heterogeneity (Table 3) with the exception of tumor histology: studies limited to tumors of NSCLC histology provided estimates of the per-child RR that were lower than those of studies reporting on mixed histologies. The ratio of the RRs was 0.93 (95% CI, 0.86–1.00; p=0.05). We did not perform meta-analyses stratified by smoking status because information was inconsistently reported in the primary studies and any such analyses would have been susceptible to reporting bias.
Table 2.
Results of main and subgroup analyses for the association of parity and lung cancer risk.
| Comparison | Number of studies |
Heterogeneity (pQ; I2) |
OR (95% CI); p-value |
|---|---|---|---|
| All studies | 16 | <0.001; 73% | 0.98 (0.95–1.02); 0.31 |
| Study design | |||
| Case-control | 11 | <0.001; 73% | 0.99 (0.94–1.04); 0.69 |
| Cohort studies | 5 | 0.001; 78% | 0.97 (0.92–1.03); 0.36 |
| Ethnicity | |||
| Non-East Asian | 8 | <0.001; 83% | 0.99 (0.93–1.06); 0.06 |
| East Asians | 7 | 0.04; 54% | 0.97 (0.93–1.01); 0.13 |
| Histology | |||
| Did not report including SCLC | 8 | 0.002; 69% | 0.94 (0.88–1.00); 0.06 |
| Included SCLC | 7 | 0.33; 13% | 1.00 (0.98–1.03); 0.72 |
|
Histological confirmation available for ≥80% |
|||
| No/ NR | 6 | <0.001; 81% | 0.97 (0.89–1.06); 0.49 |
| Yes | 10 | <0.001; 69% | 0.99 (0.95–1.03); 0.58 |
| Details on parity modeling | |||
| No/ unclear | 8 | <0.001; 72% | 1.00 (0.94–1.06); 0.91 |
| Yes | 8 | <0.001; 77% | 0.97 (0.92–1.02); 0.27 |
| Adjustment for smoking | |||
| No | 3 | 0.15; 47% | 1.00 (0.92–1.09); 0.98 |
| Yes | 13 | <0.001; 77% | 0.98 (0.94–1.02); 0.29 |
| Adjustment for age | |||
| No | 1 | NA | 1.00 (0.96–1.03); 0.87 |
| Yes | 15 | <0.001; 75% | 0.98 (0.94–1.02); 0.37 |
CI = confidence interval; NA = not applicable; NR = not reported; OR = odds ratio; SCLC = small-cell lung cancer. For some subgroups, the number of studies does not add up to 16 when studies did not report relevant information.
Table 3.
Meta-regression results for the association of parity with lung cancer risk.
| Covariate assessed in meta-regression | Relative RR (95% CI) | Interaction p-value |
|---|---|---|
| Study design (case-control vs. cohort) | 0.98 (0.87–1.10) | 0.66 |
| Ethnicity (East-Asian vs. non-East Asian) | 0.97 (0.86–1.10) | 0.63 |
| Lung cancer histology (exclusively NSCLC vs. inclusion of SCLC) | 0.93 (0.86–1.00) | 0.05 |
| Histological confirmation available for ≥80% (yes vs. no/NR) | 1.02 (0.91–1.15) | 0.66 |
| Details on parity modeling (reported vs. not) | 0.97 (0.87–1.08) | 0.57 |
| Adjustment for smoking (yes vs. no) | 0.97 (0.83–1.13) | 0.65 |
| Adjustment for age (yes vs. no) | 0.99 (0.81–1.20) | 0.88 |
| Proportion of adenocarcinomas among cases (continuous) | 1.20 (0.91–1.59) | 0.17 |
| Proportion of never smokers among cases (continuous) | 0.95 (0.80–1.13) | 0.56 |
| Proportion of never smokers among non-cases (continuous) | 0.97 (0.72–1.31) | 0.85 |
| Enrollment start year (continuous) | 1.00 (0.99–1.00) | 0.16 |
| Publication year (continuous) | 1.00 (0.99–1.00) | 0.34 |
CI = confidence interval; NR = not reported; NSCLC = non-small cell lung cancer; RR = relative risk; SCLC = small-cell lung cancer. Confidence intervals and p-values were derived from Student’s t-distribution.
Potential for bias and sensitivity analysis
There was no evidence that estimates of the dose-response relationship were systematically different between more precise and less precise studies (Egger test p-value=0.94).
The summary estimate was robust to different methods for assigning exposure levels to the open-ended exposure categories or to the exclusion of these categories: the summary RR always remained close to 1 and the association was non-significant in all cases. In addition, the results remained robust when repeating the meta-analysis by excluding each study in turn and when the summary RR was obtained through fixed effects calculations, RR=0.99 (95% CI, 0.97–1.00; p = 0.11).
Discussion
Given the worldwide increase in lung cancer-related mortality, particularly among women, there is great interest in identifying risk factors that affect disease risk among women. Parity is an interesting candidate exposure because it is reliably assessed using survey methods and recent evidence has suggested that increasing parity may be inversely associated with lung cancer risk. Our systematic review of 16 epidemiological studies investigating the association of parity with lung cancer indicates that any effect of parity is likely to be small. We identified extensive between-study heterogeneity that remained largely unexplained in subgroup and meta-regression analyses. In meta-regression analyses, the only study level characteristic with suggestive evidence that it modified the association between parity and lung cancer was the inclusion of tumor histologies other than NSCLC among cases.
Although the findings regarding histology are intriguing, they need to be interpreted with caution. Given the large number of exploratory analyses that we performed, the borderline p-value and the small magnitude of the interaction effect should at best be considered hypothesis-generating observations [48]. Nonetheless, future studies may benefit from exploring better defined disease subgroups, by limiting analyses to NSCLC or performing subgroup analyses by histology (adenocarcinoma versus other NSCLC) [28].
By its very nature, a meta-analysis inherits all the shortcomings of the constituent studies. We observed extensive clinical and methodological variability among the included studies. For case-control studies not nested within an enumerated cohort, control sampling strategies were quite variable. Because reporting of patient characteristics was often incomplete, comparing analyzed populations across studies is challenging. Further, most studies did not describe how the final regression models were built or how their performance was evaluated. Thus, it is not possible to deduce if there is risk of selective reporting. There was also substantial heterogeneity in the statistical analyses, especially regarding the potential confounders included in the final models, and their functional form.
Because the mean or median age of control participants was relatively low and lung cancer appears most often in ages over 70 years, some outcome misclassification may have ensued. However, lung cancer is a rare disease and it is unlikely that misclassification affect study results substantially, lest explain the between-study heterogeneity. Furthermore, given that any effect of parity is expected to be small, and the fact that age and tobacco smoking are strongly associated with lung cancer risk, there is a high risk of residual confounding, particularly in studies that did not adjust for these factors or for those that adjusted in incomplete ways [49]. Hormonal and reproductive characteristics are associated with differences in smoking behavior in many populations, and other environmental exposures, age or socio-economic status may confound or interact with the effect of parity on lung cancer risk. Particularly in East Asian countries, female sex is associated with exposures such as indoor air pollution from cooking fumes that may further influence the association of parity with lung cancer and deserve consideration as potential confounders [50–53]. Inadequate control for confounding and lack of consideration of potential interaction effects may have influenced the results of individual studies as well as our estimation of the average parity effect [54–56].
In view of recently published studies supporting a protective effect of parity on lung cancer risk [28, 29, 57], along with our finding that tumor histology may modify this association, further research may be warranted. However, it is doubtful whether conducting yet another epidemiological study will help explain the substantial between-study heterogeneity we quantified. Arguably, the best way to explore and explain the between study heterogeneity is through a collaborative re-analysis (meta-analysis) of individual participant data from existing studies after standardizing definitions of the exposures and outcomes of interest and a common, a priori agreed upon analytical plan. Further, a meta-analysis of individual patient data would provide a more definitive estimate of the association between parity and lung cancer risk. It would also offer the opportunity to powerfully address additional hypotheses regarding lung cancer risk, if necessary data are available.
Several limitations need to be considered when interpreting our results. First, we did not have access to the primary data from most of the studies included in the meta-analysis and could not perform additional adjustments for potentially important covariates. Differences in statistical adjustments across studies may account for some of the unexplained between-study heterogeneity [49]. In addition, because the parity distribution in each included study was unavailable, we could not accurately assign an exposure value to open-ended parity categories [40]. This is a common limitation in studies of dose-response relationships based on aggregate data. However, our results remained robust to sensitivity analyses to different methods of assigning exposure values to open ended categories, indicating that the assignment did not have undue influence on our results [41].
In conclusion, our review identified substantial heterogeneity among studies of the effect of parity on lung cancer. Study design features, participant ethnicity or aspects of study quality could not explain this variability. There was some indication that the histology of lung cancer cases affected study results, suggesting that future studies should be limited to – or provide separate results by – specific histological subtypes. A collaborative re-analysis of primary data from the individual studies, after standardizing exposure and outcome definitions and developing a uniform approach for confounding control will help explore and explain the observed between-study differences, and would be in the position to provide a more definitive answer regarding the parity-lung cancer association.
Supplementary Material
Scaterplots of the log-RR of each parity category over parity level for each study included in the meta-analysis. Each log-RR estimate is presented as a black circle, proportional to the number of events in the corresponding parity category. Fitted lines are presented to allow visual assessment of linearity. Studies are arranged by year of publication and then alphabetically.
Acknowledgement
The authors would like to thank Dr. Christina Baik, MD (Fred Hutchinson Cancer Research Center, Seattle, WA) and Dr. Gary Strauss, MD MPH (Tufts Medical Center, Boston, MA) for providing additional details and clarifications from their study on parity and lung cancer risk [29].
This study was supported in part by grant UL1RR025752 from the National Center for Research Resources to Tufts-Clinical Translational Science Institute (IJD, JKP). The content is solely the responsibility of the authors and does not represent the official views of the National Center for Research Resources or the National Institutes of Health. The funders did not participate in the design, conduct, analysis, interpretation of the study, or the decision to submit the manuscript for publication.
Abbreviations
- CI
confidence interval
- IQR
inter-quartile range
- ISI
Institute of Scientific Information
- MOOSE
Meta-analysis of Observational Studies in Epidemiology
- NSCLC
non-small cell lung cancer
- RR
relative risk
- STROBE
STrengthening the Reporting of OBservational studies in Epidemiology
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
None declared.
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010 doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Alberg AJ, Brock MV, Samet JM. Epidemiology of lung cancer: looking to the future. J Clin Oncol. 2005;23:3175–3185. doi: 10.1200/JCO.2005.10.462. [DOI] [PubMed] [Google Scholar]
- 4.Edwards BK, Ward E, Kohler BA, Eheman C, Zauber AG, Anderson RN, Jemal A, Schymura MJ, Lansdorp-Vogelaar I, Seeff LC, van Ballegooijen M, Goede SL, Ries LA. Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer. 2010;116:544–573. doi: 10.1002/cncr.24760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Doll R, Peto R. The causes of cancer: quantitative estimates of avoidable risks of cancer in the United States today. J Natl Cancer Inst. 1981;66:1191–1308. [PubMed] [Google Scholar]
- 6.Vineis P, Alavanja M, Buffler P, Fontham E, Franceschi S, Gao YT, Gupta PC, Hackshaw A, Matos E, Samet J, Sitas F, Smith J, Stayner L, Straif K, Thun MJ, Wichmann HE, Wu AH, Zaridze D, Peto R, Doll R. Tobacco and cancer: recent epidemiological evidence. J Natl Cancer Inst. 2004;96:99–106. doi: 10.1093/jnci/djh014. [DOI] [PubMed] [Google Scholar]
- 7.Alberg AJ, Ford JG, Samet JM. Epidemiology of lung cancer: ACCP evidencebased clinical practice guidelines. Chest. (2nd edition) 2007;132:29S–55S. doi: 10.1378/chest.07-1347. [DOI] [PubMed] [Google Scholar]
- 8.Thomas L, Doyle LA, Edelman MJ. Lung cancer in women: emerging differences in epidemiology, biology, and therapy. Chest. 2005;128:370–381. doi: 10.1378/chest.128.1.370. [DOI] [PubMed] [Google Scholar]
- 9.Patel JD. Lung cancer in women. J Clin Oncol. 2005;23:3212–3218. doi: 10.1200/JCO.2005.11.486. [DOI] [PubMed] [Google Scholar]
- 10.Risch HA, Howe GR, Jain M, Burch JD, Holowaty EJ, Miller AB. Are female smokers at higher risk for lung cancer than male smokers? A case-control analysis by histologic type. Am J Epidemiol. 1993;138:281–293. doi: 10.1093/oxfordjournals.aje.a116857. [DOI] [PubMed] [Google Scholar]
- 11.Bain C, Feskanich D, Speizer FE, Thun M, Hertzmark E, Rosner BA, Colditz GA. Lung cancer rates in men and women with comparable histories of smoking. J Natl Cancer Inst. 2004;96:826–834. doi: 10.1093/jnci/djh143. [DOI] [PubMed] [Google Scholar]
- 12.Thun MJ, Day-Lally CA, Calle EE, Flanders WD, Heath CW., Jr Excess mortality among cigarette smokers: changes in a 20-year interval. Am J Public Health. 1995;85:1223–1230. doi: 10.2105/ajph.85.9.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bach PB, Kattan MW, Thornquist MD, Kris MG, Tate RC, Barnett MJ, Hsieh LJ, Begg CB. Variations in lung cancer risk among smokers. J Natl Cancer Inst. 2003;95:470–478. doi: 10.1093/jnci/95.6.470. [DOI] [PubMed] [Google Scholar]
- 14.Blot WJ, McLaughlin JK. Are women more susceptible to lung cancer? J Natl Cancer Inst. 2004;96:812–813. doi: 10.1093/jnci/djh180. [DOI] [PubMed] [Google Scholar]
- 15.Risch HA, Miller AB. Re: Are women more susceptible to lung cancer? J Natl Cancer Inst. 2004;96:1560. doi: 10.1093/jnci/djh302. author reply 1560–1561. [DOI] [PubMed] [Google Scholar]
- 16.Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, Heath CW., Jr Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst. 1997;89:1580–1586. doi: 10.1093/jnci/89.21.1580. [DOI] [PubMed] [Google Scholar]
- 17.Sellers TA, Potter JD, Folsom AR. Association of incident lung cancer with family history of female reproductive cancers: the Iowa Women's Health Study. Genet Epidemiol. 1991;8:199–208. doi: 10.1002/gepi.1370080306. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz AG, Siegfried JM, Weiss L. Familial aggregation of breast cancer with early onset lung cancer. Genet Epidemiol. 1999;17:274–284. doi: 10.1002/(SICI)1098-2272(199911)17:4<274::AID-GEPI3>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 19.Curtis RE, Hoover RN, Kleinerman RA, Harvey EB. Second cancer following cancer of the female genital system in Connecticut, 1935–82. Natl Cancer Inst Monogr. 1985;68:113–137. [PubMed] [Google Scholar]
- 20.Storm HH, Ewertz M. Second cancer following cancer of the female genital system in Denmark, 1943–80. Natl Cancer Inst Monogr. 1985;68:331–340. [PubMed] [Google Scholar]
- 21.Ewertz M, Mouridsen HT. Second cancer following cancer of the female breast in Denmark, 1943–80. Natl Cancer Inst Monogr. 1985;68:325–329. [PubMed] [Google Scholar]
- 22.Harvey EB, Brinton LA. Second cancer following cancer of the breast in Connecticut, 1935–82. Natl Cancer Inst Monogr. 1985;68:99–112. [PubMed] [Google Scholar]
- 23.Chaudhuri PK, Thomas PA, Walker MJ, Briele HA, Das Gupta TK, Beattie CW. Steroid receptors in human lung cancer cytosols. Cancer Lett. 1982;16:327–332. doi: 10.1016/0304-3835(82)90014-3. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi S, Mizuno T, Tobioka N, Ichimura H, Samoto T, Tanaka H, Masaoka A, Wakabayashi S, Umemura S, Fukuoka H, Nagai H. Sex steroid receptors in diverse human tumors. Gann. 1982;73:439–445. [PubMed] [Google Scholar]
- 25.Beattie CW, Hansen NW, Thomas PA. Steroid receptors in human lung cancer. Cancer Res. 1985;45:4206–4214. [PubMed] [Google Scholar]
- 26.Cagle PT, Mody DR, Schwartz MR. Estrogen and progesterone receptors in bronchogenic carcinoma. Cancer Res. 1990;50:6632–6635. [PubMed] [Google Scholar]
- 27.Seow A, Koh WP, Wang R, Lee HP, Yu MC. Reproductive variables, soy intake, and lung cancer risk among nonsmoking women in the Singapore Chinese Health Study. Cancer Epidemiol Biomarkers Prev. 2009;18:821–827. doi: 10.1158/1055-9965.EPI-08-0892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paulus JK, Asomaning K, Kraft P, Johnson BE, Lin X, Christiani DC. Parity and risk of lung cancer in women. Am J Epidemiol. 2010;171:557–563. doi: 10.1093/aje/kwp441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baik CS, Strauss GM, Speizer FE, Feskanich D. Reproductive factors, hormone, use risk for lung cancer in postmenopausal women, the Nurses' Health Study. Cancer Epidemiol Biomarkers Prev. 2010;19:2525–2533. doi: 10.1158/1055-9965.EPI-10-0450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Elliott AM, Hannaford PC. Use of exogenous hormones by women and lung cancer: evidence from the Royal College of General Practitioners' Oral Contraception Study. Contraception. 2006;73:331–335. doi: 10.1016/j.contraception.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 31.Greenland S, Longnecker MP. Methods for trend estimation from summarized dose-response data, with applications to meta-analysis. Am J Epidemiol. 1992;135:1301–1309. doi: 10.1093/oxfordjournals.aje.a116237. [DOI] [PubMed] [Google Scholar]
- 32.Berlin JA, Longnecker MP, Greenland S. Meta-analysis of epidemiologic dose-response data. Epidemiology. 1993;4:218–228. doi: 10.1097/00001648-199305000-00005. [DOI] [PubMed] [Google Scholar]
- 33.von Elm E, Altman DG, Egger M, Pocock SJ, Gotzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med. 2007;147:573–577. doi: 10.7326/0003-4819-147-8-200710160-00010. [DOI] [PubMed] [Google Scholar]
- 34.Vandenbroucke JP, von Elm E, Altman DG, Gotzsche PC, Mulrow CD, Pocock SJ, Poole C, Schlesselman JJ, Egger M. Strengthening the Reporting of Observational Studies in Epidemiology (STROBE): explanation and elaboration. Ann Intern Med. 2007;147:W163–W194. doi: 10.7326/0003-4819-147-8-200710160-00010-w1. [DOI] [PubMed] [Google Scholar]
- 35.Greenland S, O'Rourke K. On the bias produced by quality scores in meta-analysis, and a hierarchical view of proposed solutions. Biostatistics. 2001;2:463–471. doi: 10.1093/biostatistics/2.4.463. [DOI] [PubMed] [Google Scholar]
- 36.Hamling J, Lee P, Weitkunat R, Ambuhl M. Facilitating meta-analyses by deriving relative effect and precision estimates for alternative comparisons from a set of estimates presented by exposure level or disease category. Stat Med. 2008;27:954–970. doi: 10.1002/sim.3013. [DOI] [PubMed] [Google Scholar]
- 37.Cochran W. The Combination of Estimates from Different Experiments. Biometrics. 1954;10:101–129. [Google Scholar]
- 38.Higgins JP, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21:1539–1558. doi: 10.1002/sim.1186. [DOI] [PubMed] [Google Scholar]
- 39.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 40.Il'yasova D, Hertz-Picciotto I, Peters U, Berlin JA, Poole C. Choice of exposure scores for categorical regression in meta-analysis: a case study of a common problem. Cancer Causes Control. 2005;16:383–388. doi: 10.1007/s10552-004-5025-x. [DOI] [PubMed] [Google Scholar]
- 41.Olkin I. Diagnostic statistical procedures in medical meta-analyses. Stat Med. 1999;18:2331–2341. doi: 10.1002/(sici)1097-0258(19990915/30)18:17/18<2331::aid-sim259>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 42.Poole C, Greenland S. Random-effects meta-analyses are not always conservative. Am J Epidemiol. 1999;150:469–475. doi: 10.1093/oxfordjournals.aje.a010035. [DOI] [PubMed] [Google Scholar]
- 43.Thompson SG, Higgins JP. How should meta-regression analyses be undertaken and interpreted? Stat Med. 2002;21:1559–1573. doi: 10.1002/sim.1187. [DOI] [PubMed] [Google Scholar]
- 44.Knapp G, Hartung J. Improved tests for a random effects meta-regression with a single covariate. Stat Med. 2003;22:2693–2710. doi: 10.1002/sim.1482. [DOI] [PubMed] [Google Scholar]
- 45.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. Bmj. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lau J, Ioannidis JP, Terrin N, Schmid CH, Olkin I. The case of the misleading funnel plot. Bmj. 2006;333:597–600. doi: 10.1136/bmj.333.7568.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283:2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 48.Higgins JP, Thompson SG. Controlling the risk of spurious findings from meta-regression. Stat Med. 2004;23:1663–1682. doi: 10.1002/sim.1752. [DOI] [PubMed] [Google Scholar]
- 49.Egger M, Schneider M, Davey Smith G. Spurious precision? Meta-analysis of observational studies. Bmj. 1998;316:140–144. doi: 10.1136/bmj.316.7125.140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ko YC, Cheng LS, Lee CH, Huang JJ, Huang MS, Kao EL, Wang HZ, Lin HJ. Chinese food cooking and lung cancer in women nonsmokers. Am J Epidemiol. 2000;151:140–147. doi: 10.1093/oxfordjournals.aje.a010181. [DOI] [PubMed] [Google Scholar]
- 51.Ko YC, Lee CH, Chen MJ, Huang CC, Chang WY, Lin HJ, Wang HZ, Chang PY. Risk factors for primary lung cancer among non-smoking women in Taiwan. Int J Epidemiol. 1997;26:24–31. doi: 10.1093/ije/26.1.24. [DOI] [PubMed] [Google Scholar]
- 52.Seow A, Poh WT, Teh M, Eng P, Wang YT, Tan WC, Yu MC, Lee HP. Fumes from meat cooking and lung cancer risk in Chinese women. Cancer Epidemiol Biomarkers Prev. 2000;9:1215–1221. [PubMed] [Google Scholar]
- 53.Yu IT, Chiu YL, Au JS, Wong TW, Tang JL. Dose-response relationship between cooking fumes exposures and lung cancer among Chinese nonsmoking women. Cancer Res. 2006;66:4961–4967. doi: 10.1158/0008-5472.CAN-05-2932. [DOI] [PubMed] [Google Scholar]
- 54.Kinsey T, Jemal A, Liff J, Ward E, Thun M. Secular trends in mortality from common cancers in the United States by educational attainment, 1993–2001. J Natl Cancer Inst. 2008;100:1003–1012. doi: 10.1093/jnci/djn207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Menvielle G, Boshuizen H, Kunst AE, Dalton SO, Vineis P, Bergmann MM, Hermann S, Ferrari P, Raaschou-Nielsen O, Tjonneland A, Kaaks R, Linseisen J, Kosti M, Trichopoulou A, Dilis V, Palli D, Krogh V, Panico S, Tumino R, Buchner FL, van Gils CH, Peeters PH, Braaten T, Gram IT, Lund E, Rodriguez L, Agudo A, Sanchez MJ, Tormo MJ, Ardanaz E, Manjer J, Wirfalt E, Hallmans G, Rasmuson T, Bingham S, Khaw KT, Allen N, Key T, Boffetta P, Duell EJ, Slimani N, Gallo V, Riboli E, Bueno-de-Mesquita HB. The role of smoking and diet in explaining educational inequalities in lung cancer incidence. J Natl Cancer Inst. 2009;101:321–330. doi: 10.1093/jnci/djn513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Albano JD, Ward E, Jemal A, Anderson R, Cokkinides VE, Murray T, Henley J, Liff J, Thun MJ. Cancer mortality in the United States by education level and race. J Natl Cancer Inst. 2007;99:1384–1394. doi: 10.1093/jnci/djm127. [DOI] [PubMed] [Google Scholar]
- 57.Meinhold CL, Berrington de Gonzalez A, Bowman ED, Brenner AV, Jones RT, Lacey JV, Jr, Loffredo CA, Perlmutter D, Schonfeld SJ, Trivers GE, Harris CC. Reproductive and hormonal factors and the risk of nonsmall cell lung cancer. Int J Cancer. 2010 doi: 10.1002/ijc.25434. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Scaterplots of the log-RR of each parity category over parity level for each study included in the meta-analysis. Each log-RR estimate is presented as a black circle, proportional to the number of events in the corresponding parity category. Fitted lines are presented to allow visual assessment of linearity. Studies are arranged by year of publication and then alphabetically.