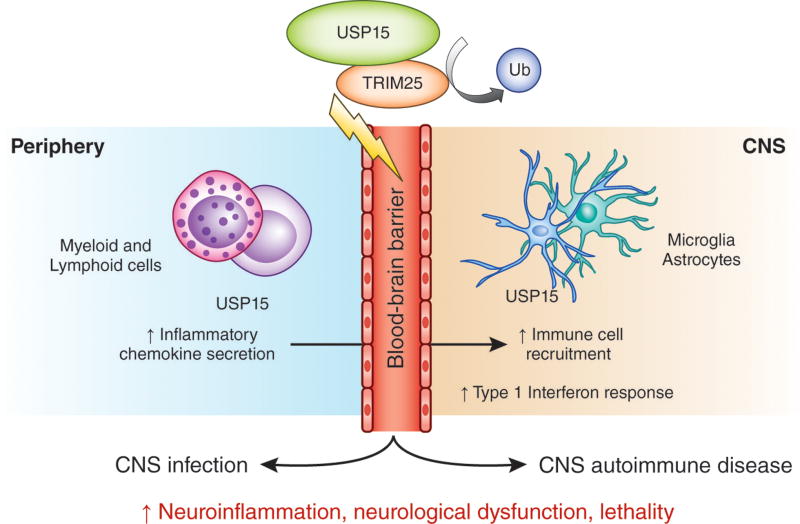
Integrated mechanisms underlying the pathogenesis of infection and autoimmunity reveal fundamental pathways of immune activation and may represent novel targets for multi-indication therapies. Torre et al.1 describe the discovery of the deubiquitinase USP15 as a common mechanism controlling microbial and autoimmune neuroinflammation (Fig. 1). These results point to exciting new directions in understanding how the ubiquitin system may govern organismal susceptibility to neuroinflammatory diseases.
Fig. 1.
USP15-mediated type 1 IFN response is a common pathway regulating infection and autoimmune neuroinflammation. Deubiquitinating enzyme USP15 stabilizes E3 ubiquitin ligase TRIM25 by removal of ubiquitin (Ub). USP15 expression by peripheral and CNS resident cells is linked to immune regulatory functions of USP15 during acute neuroinflammation in experimental cerebral malaria and autoimmune encephalomyelitis. USP15-TRIM25 interaction controls the type 1 IFN response to exacerbate lethality in microbial and autoimmune CNS diseases characterized by BBB dysfunction and immune cell recruitment into the CNS.
Deubiquitinating enzymes (DUBs) catalyze the removal of ubiquitin from other proteins and target molecules, thereby preventing degradation of their targets or altering ubiquitin-dependent signaling in the cell. In addition to their catalytic domains, DUBs have non-catalytic domains that can confer function. Indeed, DUBs are now recognized as candidates for drug discovery due to the specificity of their regulation and action. Ubiquitin-specific proteases (USPs) are the largest DUB gene family and include at least 56 cysteine proteases, including USP15.
Identified in 1999, USP15 is a regulator of transforming growth factor beta (TGF-β) and bone morphogenetic protein (BMP) signaling pathways via deubiquitination of TGF-β receptor I and receptor-activated SMAD proteins2, 3, 4. Other targets of USP15 include E3 ubiquitin ligases, which catalyze protein ubiquitination5, 6, 7, highlighting the intricate feedback loops in the ubiquitin system. In mice, USP15 promotes carcinogenesis, both by stabilizing the TGF-β receptor I4 and repressing the T cell response to cancer cells6, 8. Although no firm genetic link has been established between USP15 and human disease, higher expression of USP15 in human glioblastoma correlates with shorter life expectancy4.
Previously, two conflicting in vitro studies found opposing roles for USP15 in regulating the immune system’s type I interferon (IFN) responses, which protect the organism during viral infection but can have negative consequences in autoimmune conditions. Now, using a combined approached of genome-wide N-ethyl-N-nitrosourea (ENU) mutagenesis and whole-exome sequencing, Torre et al. identified a point mutation in Usp15, introducing a leucine-to-arginine substitution at residue 749 (L749R), that leads to resistance to experimental cerebral malaria (ECM), induced by Plasmodium berghei ANKA (PbA) infection, in mice. The loss-of-function mutant USP15L749R was associated with reduced USP15 protein stability in vitro1. Usp15L749R/+ heterozygous mice showed a moderate increase in survival, but homozygous Usp15L749R mice, similar to Usp15−/− mice, were strongly protected from ECM lethality. ECM-resistant phenotypes have been described in several other ENU-generated mutant mice (for example, ThemisI23N 9), but the Usp15L749R mutant showed protection in both microbial and autoimmune neuroinflammation. Torre et al. report that, in experimental autoimmune encephalomyelitis (EAE), an animal model for multiple sclerosis (MS), Usp15L749R mutant mice are protected from death and have decreased inflammatory gene expression before disease onset. These data generate sufficient interest to motivate future research to characterize the role of USP15 in EAE experimental paradigms with less mortality and study the role of USP15 not only in EAE pre-onset, but also onset, peak and chronic phases of the disease. Future studies can also determine the contribution of USP15 to hallmarks of EAE pathology, such as blood-brain barrier (BBB) disruption, microglia activation, demyelination, and axonal damage10.
How does USP15 inhibit neuroinflammation? Strikingly, USP15 mutation blocked central nervous system (CNS) infiltration of peripherally derived myeloid cells and circulating T cells in ECM. The effects of USP15 were restricted to regulating the innate immune response. Although expression of peripheral helper T cell type I (TH1) cytokines were unaffected in Usp15L749R mutants, the expression of chemoattractant macrophage inflammatory protein 1 was decreased at early disease stages of ECM and EAE. It remains to be determine if the effect of USP15 on CNS immunity is linked to early immune modulation in chemotaxis and/or regulation of BBB integrity9, 11, which is a critical site of host-pathogen interaction in human cerebral malaria. Nonetheless, in Usp15L749R mice challenged with microbial or autoimmune neuroinflammation, downregulation of a type 1 IFN–gene signature correlated with decreased immune cell recruitment in the CNS. RNA sequencing studies, followed by gene-set-enrichment and leading-edge analysis, identified the type I IFN response as the principal USP15-regulated gene signature in the CNS in both ECM and EAE. Indeed, USP15 is a known regulator of innate immune signaling by controlling ubiquitin E3 ligase TRIM25-mediated type 1 IFN response5. Torre et al. validated a functional interaction of a complex of USP15 with TRIM25 in neuroinflammation by showing ECM resistance in double heterozygous Usp15L749R/+ Trim25+/− mice. Furthermore, the IFN-stimulated gene family was a common signature identified in ECM Usp15L749R and Trim25−/− mice.
What are the cellular mechanisms that mediate the effects of USP15? As shown by Torre et al., USP15 was highly expressed in lymphoid and myeloid cells. Bone marrow transplantation experiments showed that the USP15 effect was mediated by cells of the hematopoietic lineage. In the healthy CNS, USPs are expressed by microglia in different brain regions, and can act as negative regulators of microglia activation12. Torre et al. showed increased expression of USP15, as well as TRIM25, in activated human microglia and astrocytes after cytokine stimulation in vitro. Other studies showed USP15 to be a positive or negative regulator of IFN response5, 7, suggesting distinct cell-type-specific down-stream signaling pathways that might be dependent or independent of the DUB activity of USP15. USP15 is ubiquitously expressed, and the mice used in the study were conventional knockouts and mutants. Thus, future studies using conditional deletion of USP15 will address how USP15 signaling in CNS resident cells and peripheral immune cells contribute to neuroinflammation.
The results of the current study inform emerging themes in neuroimmunology for common mechanisms of immune regulation in brain infection and autoimmunity. Risk for developing an autoimmune CNS diseases, such as MS, is determined by a complex interaction of multiple gene variations and environmental factors, including infection13. The extent to which the observed effects of USP15 to viral or bacterial infections or other autoimmune diseases can be generalized will need to be further explored. Given the ubiquitous expression of USP15, it would be interesting to see whether specificity could be achieved by targeting this pathway selectivity in the CNS. However, in the meantime, Torre et al. with a multipronged experimental paradigm, has shed light on common genetic threads that may control susceptibility to both CNS infection and autoimmunity.
References
- 1.Torre S, et al. Nat Immunol. 2016 [Google Scholar]
- 2.Baker RT, et al. Genomics. 1999;59:264–274. doi: 10.1006/geno.1999.5879. [DOI] [PubMed] [Google Scholar]
- 3.Inui M, et al. Nat Cell Biol. 2011;13:1368–1375. doi: 10.1038/ncb2346. [DOI] [PubMed] [Google Scholar]
- 4.Eichhorn PJ, et al. Nat Med. 2012;18:429–435. doi: 10.1038/nm.2619. [DOI] [PubMed] [Google Scholar]
- 5.Pauli EK, et al. Sci Signal. 2014;7:ra3. doi: 10.1126/scisignal.2004577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zou Q, et al. Nat Immunol. 2014;15:562–570. doi: 10.1038/ni.2885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang H, et al. Sci Rep. 2015;5:11220. doi: 10.1038/srep11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zou Q, et al. Cell Rep. 2015;13:2470–2479. doi: 10.1016/j.celrep.2015.11.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torre S, et al. Infect Immun. 2015;83:759–768. doi: 10.1128/IAI.02586-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davalos D, et al. Nat Commun. 2012;3:1227. doi: 10.1038/ncomms2230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nacer A, et al. PLoS Pathog. 2012;8:e1002982. doi: 10.1371/journal.ppat.1002982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldmann T, et al. EMBO J. 2015;34:1612–1629. doi: 10.15252/embj.201490791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gourraud PA, Harbo HF, Hauser SL, Baranzini SE. Immunol Rev. 2012;248:87–103. doi: 10.1111/j.1600-065X.2012.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]