Abstract
Adenosine is a neuroprotective agent that modulates neurotransmission and is modulated by other neurotransmitters. Spontaneous, transient adenosine is a recently discovered mode of signaling where adenosine is released and cleared from the extracellular space quickly, in less than three seconds. Spontaneous adenosine release is regulated by adenosine A1 and A2a receptors, but regulation by other neurotransmitter receptors has not been studied. Here, we examined the effect of glutamate and GABA receptors on the concentration and frequency of spontaneous, transient adenosine release by measuring adenosine with fast-scan cyclic voltammetry in the rat caudate-putamen. The glutamate NMDA antagonist, 3-(R-2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid (CPP, 6 mg/kg i.p.), increased the frequency of adenosine transients and the concentration of individual transients but NMDA (agonist, 50 mg/kg, i.p.) did not change the frequency. In contrast, antagonists of other glutamate receptors had no effect on the frequency or concentration of transient adenosine release, including the AMPA antagonist NBQX (15 mg/kg i.p.) and the mGlu2/3 glutamate receptor antagonist LY 341495 (5 mg/kg i.p.). The GABAB antagonist CGP 52432 (30 mg/kg i.p.) significantly decreased the number of adenosine release events while the GABAB agonist baclofen (4 mg/kg i.p.) increased the frequency of adenosine release. The GABAA antagonist bicuculline (5 mg/kg i.p.) had no significant effects on adenosine. NMDA and GABAB likely act presynaptically, affecting the overall cell excitability for vesicular release. The ability to regulate adenosine with NMDA and GABAB receptors will help control the modulatory effects of transient adenosine release.
Keywords: voltammetry, NMDA, GABAB, baclofen, CPP, CGP 52432
Introduction
Adenosine is an important neuromodulator in the brain, regulating neurotransmission. Generally, adenosine has an inhibitory effect on neurotransmission,1 acting through A1 adenosine receptors to depress release. For example, adenosine regulates glutamate, GABA, and dopamine neurotransmission.2,3 Many neurotransmitters also regulate the release of adenosine, revealing a feedback loop between adenosine modulation of neurotransmission and neurotransmitter regulation of adenosine.4–6 The ability of neurotransmitters to regulate adenosine often differs based on brain region and type of adenosine signaling. Our lab has recently discovered a new mode of adenosine signaling: spontaneous, transient release that lasts for only about three seconds.7–10 These spontaneous transients are modulated by A1 receptors 7 and A2a receptors11 in the caudate and prefrontal cortex but regulation by other neurotransmitter receptors has not been studied.
Glutamate is the primary excitatory neurotransmitter and adenosine modulates glutamate release. In the hippocampus, adenosine receptors inhibit glutamate efflux12 through pre-synaptic A1 receptors, while A1-A2a heteromers in glutamatergic terminals13 exert dynamic inhibitory and excitatory control of glutamate neurotransmission.14 Similarly, glutamate can also regulate the release of adenosine. For example, application of the glutamate agonist NMDA evokes adenosine release in cerebellum brain slices15 and decreases neuromodulation due to adenosine acting at A1 receptors.16 The glutamate agonist, AMPA, evokes adenosine release in the basal forebrain and cortex.17 A combination of NMDA and AMPA antagonists decreased high and low frequency stimulated adenosine release in striatal brain slices.18 However, in neostriatal brain slices, A2a agonists inhibited NMDA currents but not AMPA currents.19 Dale’s group found that train-evoked adenosine release is independent of NMDA and AMPA receptors, while single pulse, evoked adenosine is dependent on NMDA and AMPA receptors in the cerebellum.20 Metabotropic glutamate (mGlu) receptors also interact closely with adenosine receptors,21 as A2a and metabotropic glutamate receptors are co-localized pre-synaptically.22 Single pulse adenosine release is also dependent on mGlu4 receptors in cerebellar brain slices.6 Thus, the regulation of adenosine by glutamate receptors depends on the type of adenosine release and brain region.
GABA, the precursor to glutamate, is an inhibitory neurotransmitter that is also regulated by adenosine. During normoxic conditions in the hippocampus, potassium-evoked GABA release is decreased through A1 and A2a receptors, while during ischemia only A2a receptors decrease release.23 Similarly, adenosine suppresses GABA induced currents through A1 receptors in the dorsal horn of rats24 and inhibits evoked GABA release in the hypothalamus.25 The modulatory interactions between adenosine and GABA are bi-directional, as GABA also modulates evoked adenosine release. Single pulse adenosine release is dependent on GABAB receptors in cerebellar brain slices.6 Thus the interplay between adenosine and GABA is dependent on cellular conditions, region, and release type.
Here, we examine the effects of glutamate and GABA receptors to regulate spontaneous, transient adenosine release in the caudate-putamen of anesthetized rats. Administering antagonists of NMDA and GABAB modulated the frequency of spontaneous adenosine release, while antagonists of AMPA, GABAA, and group II metabotropic glutamate receptors had no effect. However, the effects were opposite those expected if NMDA and GABAB receptors caused release; blocking NMDA with CPP caused larger, more frequent adenosine transients while blocking GABAB with CGP 52432 decreased the frequency of adenosine transients. Administering NMDA did not have the opposite effect of the NMDA antagonist, suggesting the receptor is already activated, but baclofen, a GABAB antagonist did increase the frequency of adenosine transients, an effect opposite that of the GABAB agonist. Thus, NMDA and GABAB appear to modulate adenosine through presynaptic mechanisms, a key finding that will enable future studies that control spontaneous, transient release to harness its neuromodulatory properties.
Results and Discussion
Adenosine Detection with Fast-scan Cyclic Voltammetry
Adenosine release was measured rapidly using fast-scan cyclic voltammetry and spontaneous events were monitored in vivo in anesthetized rats. Carbon-fiber microelectrodes were inserted into the caudate-putamen of the rat brain and a waveform continuously applied to electrochemically detect adenosine. Applying the waveform causes a large background current, which is subtracted out; thus fast-scan cyclic voltammetry is a differential technique to measure rapid changes in concentration on a sub-second time scale.26 Adenosine undergoes a set of two electron oxidations27,28 at the microelectrode that results in a current that is proportional to the concentration of adenosine at the electrode.
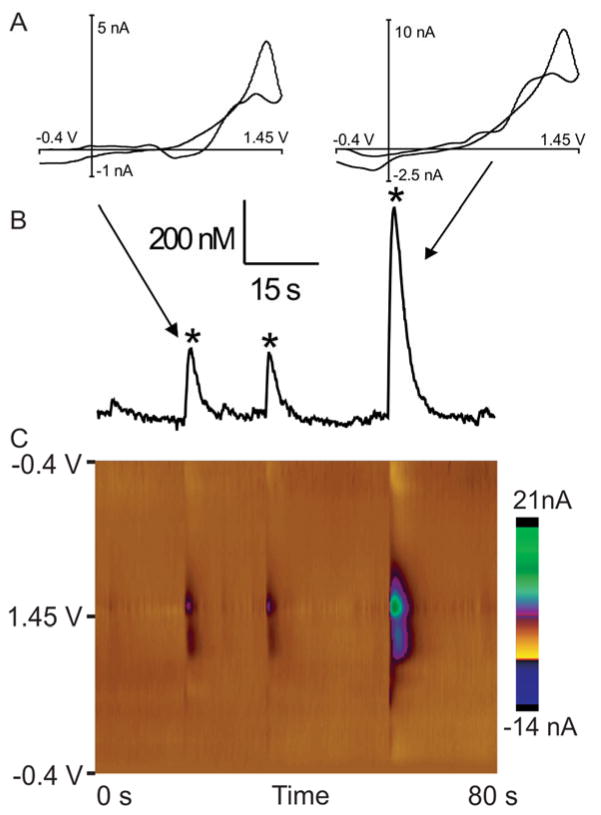
Cyclic voltammograms for adenosine (Fig. 1A) show the characteristic electrochemical fingerprint for adenosine with a primary oxidation peak at 1.4 V and a secondary oxidation peak at 1.0 V. Using principal component analysis, a concentration vs time plot (Fig. 1B) for adenosine is calculated7 and displayed directly above the color plots (Fig. 1C). Three spontaneous adenosine release events are starred. We have also used a recently written computer program to automate the identification of adenosine transients from these traces.29 Three dimensional color plots of fast-scan cyclic voltammetry data are commonly plotted to show changes in analyte oxidation over time. The green/purple circles in the center of the 3-D plot are the primary oxidation peaks, while the green/purple circles directly below at 1.0 V are the secondary oxidation peaks for adenosine. The data show that large adenosine events are released and rapidly cleared from the extracellular space in less than four seconds. All data collection occurred after at least an hour of electrode implantation, in order to minimize any adenosine changes from damage due to insertion, as previous work has found immediate mechanosensitive adenosine release (within seconds) upon electrode movement.9 Experiments were performed in anesthetized animals immobilized in a stereotaxic apparatus so the electrode would not move during the experiment.
Figure 1. Spontaneous, transient adenosine release.
A) Cyclic voltammograms (CV) of spontaneously release adenosine in vivo. The primary oxidation of adenosine occurs at 1.4 V and the secondary peak is at 1.0 V. B) A concentration vs time trace collected in vivo over an 80 second window. The concentrations were calculated from the current using in vitro values with principal components analysis. C) 3-D color plot of three example spontaneous events in vivo. Adenosine oxidation is green/purple.
Control Experiments
To verify that the frequency and concentration of adenosine transients did not change over time, a control experiment was performed with a vehicle injection of DMSO and saline. Adenosine transients in the hour before vehicle were compared to transients in the hour after vehicle administration. There was no significant difference in the number of transients (Fig. S1, n = 6 animals, paired t-test, p = 0.31) or underlying frequency distributions (Fig. S1B, n = 6 animals, KS test, p = 0.74). There was also no significant difference in the concentration of individual adenosine transients (Fig. S1C, n = 250 events pre-drug and 221 events post-vehicle, unpaired t-test, p = 0.71) or the maximum cumulative concentration of transients (Fig. S1D, n = 6 animals, paired t-test, p = 0.11) following DMSO/saline injection. Thus, the frequency and concentration of adenosine transients do not change with time and any changes observed in drug experiments can be attributed to the drug.
Histology was performed to prove the electrode did little damage to the brain. Fig. S2 shows the path of an electrode with its placement in the caudate putamen. The track is not easy to see, proving our electrode does not cause much tissue damage. This medial area of the caudate was chosen because transients have been previously recorded here,7 but the striatum is heterogeneous and adenosine release may vary within the caudate.30
Glutamatergic Regulation of Adenosine Release
NMDA Receptors
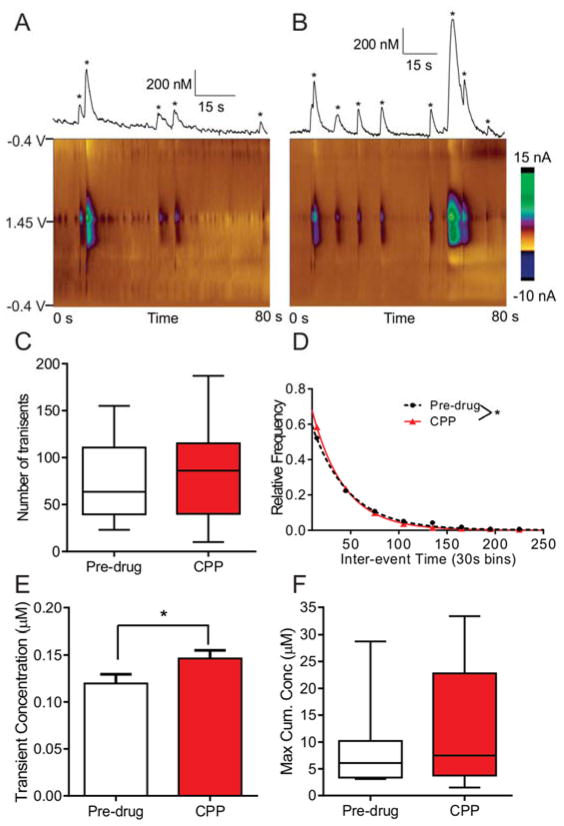
Antagonists were used to test the effect of different receptors to mediate adenosine release. CPP, a selective NMDA receptor antagonist, was injected at 6.25 mg/kg i.p., a dose previously established to be an effective antagonist in the brain.31,32 Example data show the difference between pre-drug (Fig. 2A) and post-CPP transients (Fig. 2B). The color plot and concentration vs time traces for pre-drug data show five transients in an 80 second window, while eight transients are observed in the same time frame following CPP administration. The concentration of some adenosine events also appears larger after CPP. On average, although there was a trend towards more transients released after CPP administration, the number of transients did not significantly increase (Fig. 2C, n = 8 animals, paired t-test, p = 0.28). The box plot shows the median as the middle line, with the colored region being 25–75 % of values and the whiskers showing the range. However, when a histogram is made of the inter-event times, i.e. the time between consecutive transients, and the underlying distributions compared before and after drug administration, there was a significant difference in the frequency of adenosine release (Fig. 2D, n = 8 animals, KS test, p = 0.035). Similarly, the mean inter-event time decreased from 47 ± 2 s to 41 ± 3 s (n = 582 events pre-drug, n = 668 events post-drug, unpaired t-test, p = 0.048). The concentration per transient was calculated using all the transients and the mean concentration increased after CPP (Fig. 2E, n = 590 events pre-drug and 676 events post-drug, unpaired t-test, p = 0.039). To examine the cumulative concentration, the concentration of each transient was added for the one hour pre-drug or one hour CPP time period. Similar to the number of transients, the maximum cumulative concentration shows a trend of increasing after CPP, but the differences were not significant (Fig. 2F, n=8 animals, paired t-test, p = 0.13). The number of transients and cumulative concentrations were averaged per animal while for frequency distributions and concentration per transient, all adenosine transient event data were used. Thus, the larger n using all the transients made it easier to pull out significant differences in frequency and event concentration, but the same trends are observed in the measures that used only one value per animal.
Figure 2. The effect of NMDA antagonist CPP on adenosine release.
A) Example pre-drug release of adenosine. In this 80s time period, 5 adenosine transients (starred) were observed. B) Example adenosine trace after CPP (6.25 mg/kg, i.p.), where 8 transients, many larger in concentration, are now observed. C) Number of transients (n = 8 animals, paired t-test, p = 0.28). For all box plots, the line shows the median, the box the 25–75 % values, and the whiskers the range. D) Inter-event time histogram (30 s bins) pre-drug and after CPP were significantly different (n = 8 animals, KS test, p = 0.035). E) Event concentration was significantly different after CPP (n = 590 pre-drug, 676 post-drug, unpaired t-test, p = 0.039). Error bars are SEM. F) Maximum cumulative concentration is not statistically different (n = 8 animals, paired t-test, p = 0.13).
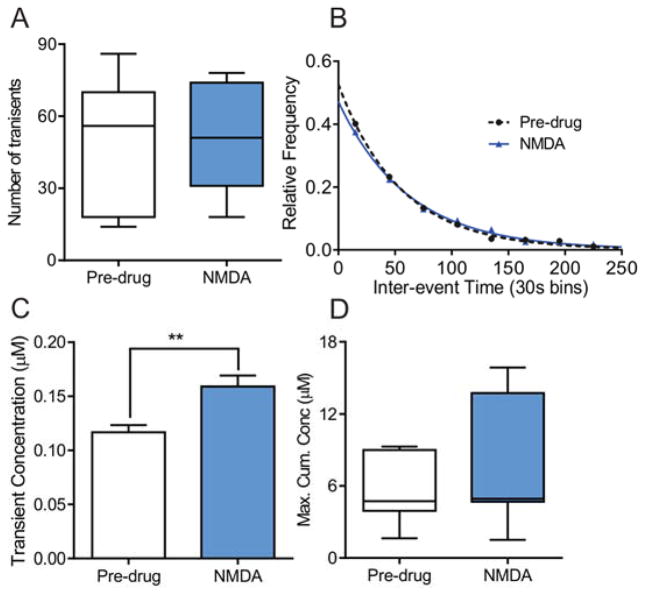
For all antagonists that significantly affected adenosine release, we also administered the agonist to test its effect. Fig. 3 shows the data for NMDA (50 mg/kg i.p.), an NMDA receptor agonist. The dose of 50 mg/kg i.p. was chosen because it is low enough not to cause death or seizures33 but large enough to see effects in the brain34. The number of transients (Fig. 3A, n = 7 animals, paired t-test, p=0.85) did not change with NMDA. Similarly, the mean inter-event times (pre-drug: mean = 70 ± 6 s, n = 325 events; postdrug: mean = 73 ± 5 s, n = 319 events; unpaired t-test, p = 0.85) as well as the distribution of inter-event times (Fig. 3B, n = 7 animals, KS test, p = 0.40) did not change with NMDA. Surprisingly, the average concentration per transient did increase after NMDA (Fig. 3C, n = 347 events pre-drug and n =358 events post-drug, unpaired t-test, p < 0.01), similar to the NMDA antagonist. The cumulative concentration also did not change (Fig. 3D, n = 7 animals, paired t-test, p = 0.23). Thus, while the NMDA antagonist caused a change in frequency, the agonist did not.
Figure 3. The effect of NMDA agonist NMDA on adenosine release.
A) The number of transients before and after NMDA (50 mg/kg, i.p.) was not different (n = 7 animals, paired t-test, p = 0.85). B) The underlying distributions of inter-event times were not different (n = 7 animals, KS test, p = 0.40). C) The event concentration significantly increased after NMDA (n = 347 events pre-drug and 358 events post-drug, unpaired t-test, p < 0.01). D) The maximum cumulative concentration also did not change (n = 7 animals, paired t-test, p = 0.23)
AMPA Receptors
The AMPA receptor is another ionotropic, non-NMDA sensitive glutamate receptor that can mediate adenosine signaling in some brain regions17. NBQX is a selective antagonist of the AMPA receptor that provides potent anti-convulsant protection against seizures at 15 mg/kg i.p.35 so we selected this dose. There were no significant differences in the number of adenosine transients after NBQX (Fig. 4A, n = 5 animals, paired t-test p = 0.99). In addition, the mean inter-event times (pre-drug: mean = 64 ± 4 s, n = 269 events; post-drug: mean = 64 ± 4 s, n = 269 events; unpaired t-test, p = 0.97) and the distribution of inter-event times remained unchanged following NBQX (Fig. 4B, n = 5 animals, KS test, p = 0.38). When examining the concentration of each individual transient, there was no change in concentration for each transient with NBQX (Fig. 4C, n = 274 events pre-drug, n=274 events post-drug, unpaired t-test, p = 0.32,). The maximum cumulative concentration also did not differ (Fig. 3D, n = 5 animals, paired t-test, p = 0.65). In summary, the AMPA antagonist, NBQX, caused no changes in the frequency or concentration of spontaneous, transient adenosine release.
Figure 4. The effect of AMPA antagonist NBQX on transient adenosine release.
A) Number of transients before and after NBQX (15 mg/kg, i.p.) are not different (n = 5 animals, paired t-test, p = 0.99). B) The underlying distributions of inter-event times are not different (n = 5 animals, KS test, p = 0.38). C) The event concentration does not change after NBQX (n = 274 both pre-drug and post-drug, unpaired t-test, p = 0.32). D) The maximum cumulative concentration also does not change (n = 5 animals, paired t-test, p = 0.65)
Metabotropic Glutamate Receptors
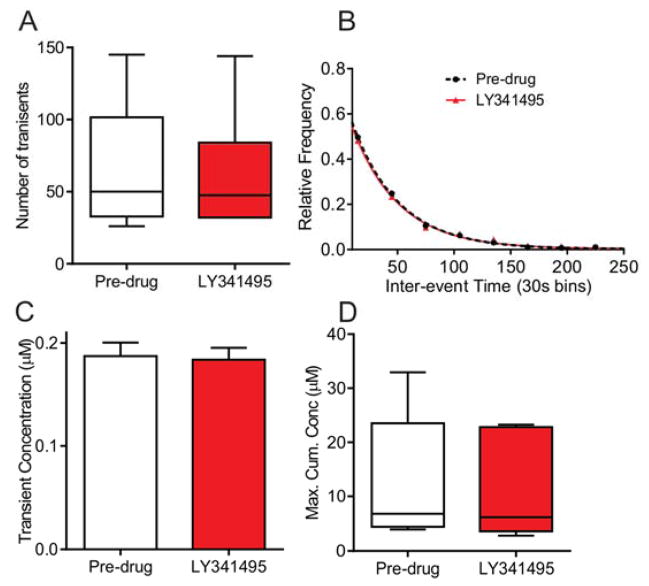
There are multiple metabotropic glutamate receptors and here we investigated group II metabotropic glutamate receptors (mGlu2/3) in vivo as mGlu2/3 have shown pre-synaptic inhibition in the CNS36. The specific mGlu2/3 antagonist, LY 341495, decreased the number of seizures in epileptic mice at 5 mg/kg i.p.37 and thus we chose this dose. The mGlu2/3 antagonist did not alter the number of adenosine transients (Fig. 5A, n = 6 animals, paired t-test, p = 0.33). Similarly, the mean inter-event time (pre-drug: mean = 53±4 s, n = 387 events; post-drug: mean = 56 ± 4 s, n = 361 events; unpaired t-test, p = 0.52) or the underlying frequency distribution did not change following LY 341495 administration (Fig. 5B, n = 6 animals, KS test, p = 0.16). The concentration per transient also did not change after LY 341495 administration (Fig. 5C, n = 393 events pre-drug, n = 367events post-drug, unpaired t-test, p = 0.84) nor did the maximum cumulative concentration change (Fig. 5D, n = 6 animals, paired t-test, p = 0.36). Thus, mGlu2/3 receptors do not regulate the frequency or concentration of spontaneous adenosine release.
Figure 5. The effect of mGlu2/3 receptor antagonist LY 341495 on transient adenosine release.
A) Number of transient release events did not change following mGlu2/3 antagonism with LY 341495 (5 mg/kg, i.p., n = 6 animals, paired t-test, p = 0.33). B) The underlying distribution of inter-event times did not change (n = 6 animals, KS test, p = 0.16). C) The event concentration did not change (n = 393 events pre-drug, 367 events post-drug, unpaired t-test, p = 0.84). D) The maximum cumulative concentration did not change (n = 6 animals, paired t-test, p = 0.36).
Glutamatergic receptor regulation of transient adenosine release
Adenosine and glutamate have a concomitant relationship in the brain, where inhibitory effects of adenosine modulate the effects of glutamate38 and excitatory glutamatergic receptors cause release of adenosine. For example, NMDA application evokes adenosine that acts at inhibitory A1 receptors.39 However, not all studies have shown a regulation of adenosine by glutamate receptors. Electrically-stimulated, transient adenosine release was partially dependent on ionotropic glutamate receptors in the striatum, but was independent of them in the hippocampus, nucleus accumbens, and cortex.30 Similarly, train-evoked adenosine release was also independent of NMDA and AMPA receptors in cerebellar rat brain slices20.
From these previous studies,5,6,18 we hypothesized that if spontaneous transient adenosine release was due to activation of NMDA or AMPA receptors, their antagonists would decrease release. However, neither NMDA nor AMPA receptor antagonists decreased the amount of transient adenosine released. Interestingly, the agonist NMDA did increase the concentration of each release event, providing some evidence that NMDA activation might increase the concentration spontaneous adenosine release.
Instead of finding that ionotropic glutamate antagonists decreased adenosine release, we found the opposite effect: inhibiting NMDA receptors with CPP increased the frequency of transient release events and also increased the concentration per event. This result is surprising since blocking NMDA receptors is generally inhibitory and the observed effect was excitatory. One possible explanation is a feedback loop between adenosine and pre-synaptic NMDA receptors.40 Glutamate and adenosine (or ATP, a precursor of adenosine) could be co-localized in vesicles and activation of pre-synaptic NMDA receptors by glutamate might signal to decrease the frequency of vesicular release, decreasing adenosine as well. Therefore blocking NMDA receptors would increase adenosine release. NMDA antagonists increase the paired-pulse facilitation response for glutamate, by blocking the feedback of glutamate on NMDA receptors.41 In addition, A1 and NMDA autoreceptors are co-localized14,42 and A1 receptors antagonists also increase the frequency of adenosine release.7 NMDA itself did not have the opposite effect on frequency of the NMDA antagonist CPP, which may indicate that NMDA receptors are fully activated at basal conditions and cannot be further activated to decrease the frequency of adenosine release.
In contrast to NMDA antagonist, the AMPA antagonist NBQX had no effect on the frequency of transient adenosine release. Previous research had identified that AMPA receptors regulate adenosine release; for example, AMPA antagonists decreased single pulse stimulated adenosine.5,6 However, other studies found that AMPA receptors had no effect on train-evoked adenosine release in the cerebellum20 and adenosine A2a agonists inhibited NMDA currents but not AMPA currents.19 Our results agree with the latter studies, as NBQX had no effect on the frequency or concentration of spontaneous adenosine transients. Metabotropic glutamate receptors regulate signaling via G-protein coupled receptors. Metabotropic glutamate group II receptors (mGlu2/3) are generally located pre-synaptically and inhibit neurotransmitter release, although they are also localized on cholinergic interneurons in the striatum.43 The mGlu antagonist LY 341495 has an affinity of 14–22 nM for mGlu 2/3 and is less potent for mGlu4 receptors (Ki = 22 μM).44 LY 341495 had no effect on spontaneous adenosine release, indicating that mGlu 2/3 receptors do not regulate adenosine release. Future studies could examine other types of metabotropic glutamate receptors, such as mGlu4 receptors,6 perhaps in brain slices where the blood brain barrier is not an issue.
GABA receptors
GABAA Receptors
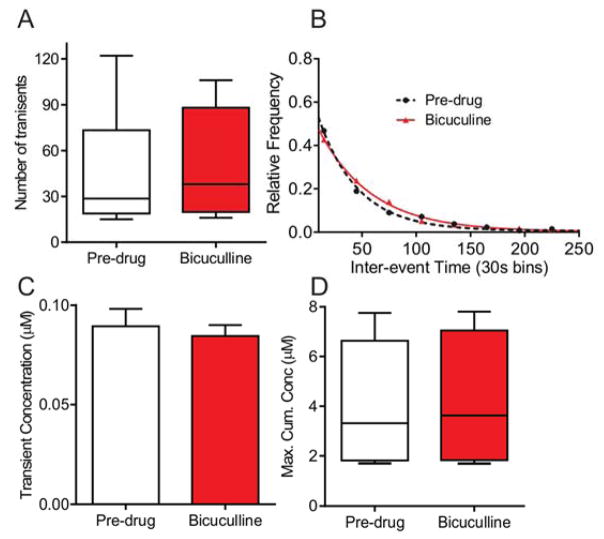
There are two main types of GABA receptors: GABAA receptors which are ionotropic, ligand gated ion channels and GABAB receptors which are metabotropic receptors that indirectly open ion channels. The GABAA antagonist bicuculline did not affect the number of adenosine transients (Fig. 6A, n = 6 animals, paired t-test, p = 0.42). The dose of 5 mg/kg i.p. was chosen because it has been previously used to reverse locomotor effects 45 and did not cause seizures in our rats. Bicuculline also did not change the mean inter-event time (pre-drug: 78 ± 7 s, n = 265 events; post-drug: 70 ± 6 s, n = 295 events; unpaired t-test, p = 0.33) or the underlying frequency distributions of transients (Fig. 6B, n = 6 animals, KS test, p = 0.54). There was no change in either the concentration of each adenosine release event (Fig. 6C, n = 271 events pre-drug, n = 301 events post-drug, unpaired t-test, p = 0.63) or the maximum cumulative adenosine concentration (Fig. 6D, n = 6 animals, paired t-test, p = 0.20). Ionotropic GABAA receptors thus did not regulate transient adenosine release.
Figure 6. The effects of GABAA receptor antagonist bicuculline on spontaneous adenosine release.
A) Number of transients did not change after the GABAA antagonist bicuculline (5 mg/kg, i.p., n = 6 animals, paired t-test, p = 0.42). B) The inter-event histograms were also not different (n = 6 animals, KS test, p = 0.54). C) The event concentration did not change after bicuculline (n = 271 transients pre-drug and 301 post-drug, unpaired t-test, p = 0.63). D) The maximum cumulative adenosine concentration did not change (n = 6 animals, paired t-test, p = 0.20).
GABAB Receptors
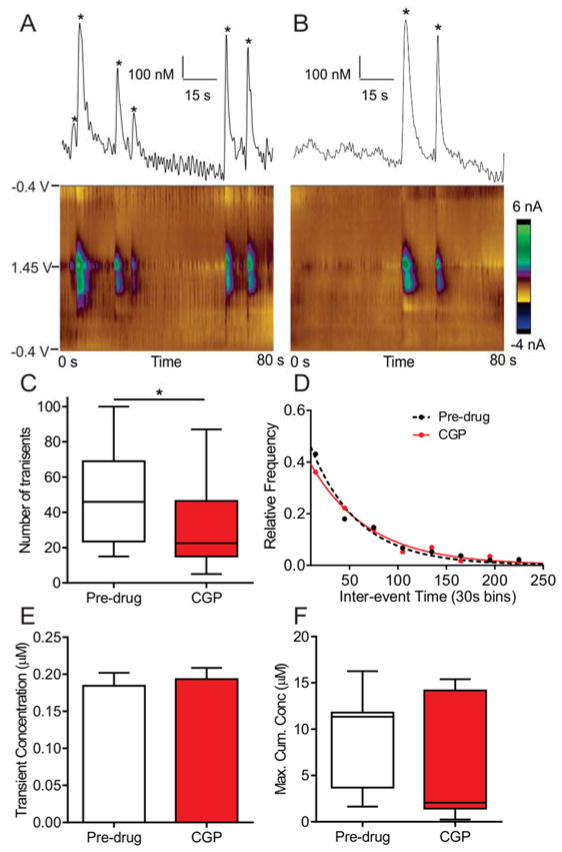
The GABAB antagonist, CGP 52432, has been used previously in brain slices to increase glutamate receptor-dependent adenosine release.6 CGP 52432 has anti-depressant properties at 30 mg/kg in mice,46 thus we used that dose. The example color plots and traces show about 6 transient pre-drug (Fig. 7A) in 80 s, while only 2 transients are observed in the same period of time after CGP 52432 (Fig. 7B). CGP 52432 significantly decreased the number of adenosine transients (Fig. 7C, n = 8 animals, paired t-test, p = 0.0059). The mean inter-event times increased with GABAB antagonist, indicating longer times between consecutive transients, from 64 ± 4 s (n = 383 transients) pre-drug to 89 ± 8 s (n = 270 transients, unpaired t-test, p = 0.046). However, the underlying distributions were not significantly different (Fig. 7D, n = 8 animals, KS test, p = 0.30). The GABAB antagonist CGP 52432 did not significantly change the average adenosine transient concentration (Fig. 7E, n = 391 events pre-drug, n = 278 events post-drug, unpaired t-test, p = 0.72). CGP 52432 did not also significantly decrease the maximum cumulative concentration (Fig. 7F, n = 8 animals, paired t-test, p = 0.26). The GABAB receptor antagonist regulated the number and frequency of adenosine transients, but not the concentration of release.
Figure 7. The effects of GABAB receptor antagonist CGP 52432.
A) Example pre-drug data showing 6 adenosine transients in an 80 s period (starred). B) Example data after the GABAB antagonist CGP 52432 (30 mg/kg, i.p.) in the same rat, where only 2 transients were observed. The magnitude of the transients is similar after CGP 52432. C) The number of transients decreased significantly after CGP 52432 (n = 8 animals, paired t-test, p = 0.0059). D) The underlying distribution for the inter-event time histograms were not significantly different (n = 8 animals, KS test, p = 0.30), however the mean inter-event times are different (64 ± 4 s, n = 383 events pre-drug, 89 ± 8 s, n = 270 events post-drug, unpaired t-test, p = 0.046). E) The GABAB antagonist did not change the average event concentration (n = 391 events pre-drug, 278 events post-drug, unpaired t-test, p = 0.72). F) The maximum cumulative concentration also did not change (n = 8 animals, paired t-test, p = 0.26).
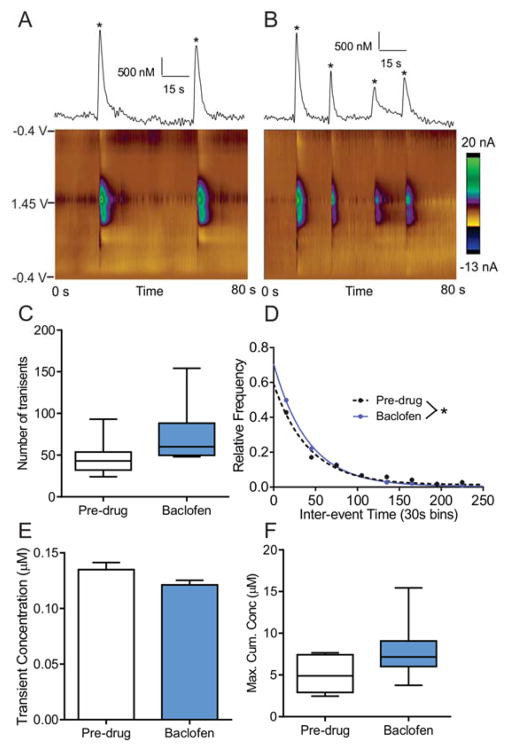
Because the GABAB antagonist had a significant effect on the frequency of spontaneous release, we also administered the GABAB agonist baclofen (4.0 mg/kg, i.p.) to a separate set of animals. The dose of 4.0 mg/kg was chosen because it has been previously used to facilitate behavioral flexibility47 and suppress ethanol self-administration in rats.48 The example color plots and traces show about 2 transients pre-drug (Fig. 8A) in an 80 s window, while 4 transients are observed in the same period of time after baclofen (Fig. 8B). The GABAB agonist has the opposite effect of the antagonist: the mean inter-event time decreased (pre-drug: mean 74 ± 5 s, n=362 events; post-drug: mean =47 ± 2, n = 578 events; unpaired t-test, p < 0.0001) and the distribution of inter-event times significantly changed after baclofen (Fig. 8D, n = 8 animals, K-S test, p = < 0.01). While the number of transients (Fig. 8C, n = 8 animals, paired t-test, p = 0.12) and cumulative concentration (Fig. 8F, n = 8 animals paired t-test, p=0.087) did not significantly change after baclofen, they also trended in the opposite direction as the antagonist, with more transients and a larger cumulative concentration. The amount released per transient was not significantly changed (Fig. 8E, n = 369 events pre-drug, n = 586 events post-drug, unpaired t-test, p = 0.80). Overall, these experiments show that GABAB receptors can be either inhibited or activated to produce opposite effects on the number of adenosine transients.
Figure 8. The effects of GABAB receptor agonist baclofen.
A) Example pre-drug release of adenosine. In this 80 s time period, 2 adenosine transients (starred) were observed. B) Example adenosine trace after baclofen (4.0 mg/kg, i.p.), where 4 transients are observed. C) Number of transients (n = 8 animals, paired t-test, p = 0.12). For all box plots, the line shows the median, the box the 25–75 % values, and the whiskers the range. D) Inter-event time histogram (30 s bins) pre-drug and after baclofen were significantly different (KS test, p < 0.01). E) Event concentration was not significantly different after baclofen (n=369 events pre-drug, 586 events post-drug, unpaired t-test, p = 0.80). Error bars are SEM. F) Maximum cumulative concentration was not statistically different (n = 8 animals, paired t-test, p = 0.087).
Regulation of spontaneous adenosine by GABAergic receptors
GABA is the primary inhibitory neurotransmitter in the brain and GABAB receptors are inhibitory metabotropic receptors. GABAB receptors are located pre- and post-synaptically and are localized on glutamatergic terminals49 and GABAergic terminals.50,51 Previously, GABAB antagonists slightly increased single pulse evoked release, but had little effect on train evoked adenosine release.6 Therefore, we expected that if GABAB receptors had an effect, the antagonist would increase the concentration or frequency of spontaneous adenosine release. The GABAB antagonist, CGP 52432, did not affect concentration but did affect the frequency; however, CGP 52432 had the opposite effect as expected, decreasing the frequency of spontaneous adenosine release. The GABAB agonist baclofen had the opposite effect of the antagonist, increasing the frequency of spontaneous release. Our studies were performed in the striatum, whereas the previous single-spike evoked release was performed in the cerebellum so there are differences by brain region as well as stimulation type. Our results suggest a feedback loop, similar to that observed in the NDMA studies, but of opposite effect (Fig. 9B). Pre-synaptically located GABAB receptors can regulate GABA concentrations52 and if blocking GABAB receptors raised GABA levels, this could cause general inhibition and decrease of adenosine release. The inhibition after GABAB antagonist may lead to fewer action potentials and fewer adenosine release events.
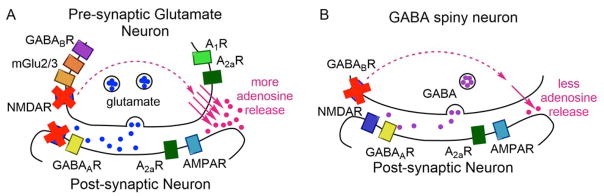
Figure 9. Possible presynaptic mechanisms to regulate spontaneous transient adenosine release.
A) Cartoon of receptor positions on glutamate neurons. Blocking NMDA receptors with CPP increases the frequency of transient adenosine release, likely through a pre-synaptic feedback loop that causes more vesicular release. B) Cartoon of GABA spiny neuron. Blocking GABAB receptors with CGP 52432 decreases the frequency of adenosine release, and the GABAB agonist baclofen increases frequency of release, likely through a feedback loop that increases GABA and general inhibition. GABAB receptors are also located on glutamatergic neurons.
GABAA receptors are ionotropic receptors that gate chloride channels. Although there is no evidence of co-localization of GABA and adenosine receptors, cross-talk between GABAA and A1 receptors has been demonstrated.53 Adenosine decreases the hyperpolarization effects of GABAA through A1 receptors54 and alters the response of GABAA receptors from inhibitory to excitatory after seizures.55 Those studies all focused on the effects of adenosine modulating GABA release. Here, we examined the effect of blocking GABAA receptors on adenosine release and found that the GABAA antagonist, bicuculline, had no effect on transient adenosine release (Fig. 7). Thus, spontaneous adenosine release is not regulated by ionotropic GABAA receptors, only metabotropic GABAB receptors.
Presynaptic regulation of Adenosine by Glutamate and GABA Receptors
The regulation of adenosine release is complex and can vary from brain region to brain region 30,56. Indeed, even the striatum itself is heterogeneous, especially for dopamine and GABA release, and our studies examined regulation of adenosine release in only one location, the medial striatum.57,58 The main result is that blocking NMDA and GABAB regulates spontaneous adenosine release, particularly the frequency of release, in the medial caudate. Adenosine A1 and A2a receptors have been shown to self-regulate spontaneous adenosine release and the mechanism of action is thought to be presynaptic.7,11,53 However, the exact release site of spontaneous adenosine is not known; it could be from neurons or it could be from astrocytes. While NMDA and GABAB antagonists have opposite effects of what might be expected if activating those receptors caused release, the results are consistent with those receptors acting pre-synaptically to control glutamate and GABA concentrations and overall cell excitability (Fig. 9). However, these receptors, particularly NMDA receptors, are localized on many cells, including post-synaptic cells, glial cells, and interneurons. Not all pre-synaptic receptors modulate adenosine release, as the pre-synaptic mGlu2/3 receptors34 had no effect on the frequency of release. Receptors that are primarily localized post-synaptically, like AMPA38 and GABAA49 receptors, did not regulate transient adenosine release. The regulation by pre-synaptic receptors as well as the fast time course of release suggest that spontaneous adenosine release is vesicular,54 although vesicular release could be adenosine or ATP, which is quickly broken down to adenosine.55 Spontaneous adenosine regulates neurotransmission 3 and oxygen levels,11 so antagonists of NMDA or GABAB could be used to modulate adenosine release frequency and thus regulate the rapid modulatory effects of adenosine.
Conclusions
This study shows that spontaneous, transient adenosine release is modulated by glutamate and GABA receptors, specifically NMDA and GABAB receptors. These receptors regulate the frequency of the release through general excitation and inhibition. The frequency of spontaneous, transient adenosine is not dependent on metabotropic glutamate (mGlu2/3), GABAA, or AMPA receptors. The regulation of spontaneous, transient release is different compared to previous studies of electrically-stimulated adenosine release; thus regulation of adenosine release differs by release mode. By understanding which receptors regulate fast acting adenosine, future studies and drug therapies will be able to manipulate and control the rapid modulatory properties of adenosine.
Methods
Chemicals and Drugs
All drugs were administered i.p to rats and purchased from Tocris Biosciences (Ellisville, MO, USA) unless otherwise noted. The NMDA receptor antagonist, CPP (3-((R)-2-Carboxypiperazin-4-yl)-propyl-1-phosphonic acid) was dissolved in 200 μL saline and injected at 6.25 mg/kg. The NMDA receptor agonist, NMDA, was dissolved in 500 μL saline and injected at 50 mg/kg. The AMPA antagonist NBQX (2,3-Dioxo-6-nitro-1,2,3,4-tetrahydrobenzo[f]quinoxaline-7-sulfonamide) was dissolved in saline and DMSO (500 μL each) and injected at 15 mg/kg. The group II metabotropic glutamate receptor antagonist LY 341495 ((2S)-2-Amino-2-[(1S,2S)-2-carboxycycloprop-1-yl]-3-(xanth-9-yl) propanoic acid) was dissolved in saline and DMSO (500 μL) and injected at 5 mg/kg. The GABAB receptor antagonist CGP 52432 (3-[[(3,4-Dichlorophenyl)methyl]amino]propyl] diethoxymethyl)phosphinic acid) was dissolved in 2.5 mL DMSO and 1 mL saline and injected at 30 mg/kg. The GABAB receptor agonist, baclofen, was dissolved in 0.3 mL saline and injected at 4.0 mg/kg. The GABAA receptor antagonist bicuculline was dissolved in 500 μL of saline and injected at 5 mg/kg.
Animal Experiments
All animal experiments were reviewed and approved by the Institutional Animal Care and Use Committee of the University of Virginia. Male Sprague-Dawley rats (Charles River Laboratories, Wilmington, MA, USA) between 250–350 grams anesthetized with urethane (1.5 mg/kg, i.p.), shaved, and placed in a stereotaxic frame. Bupivacaine (250 μL, Sensorcaine, MPF, APP Pharmaceuticals, LLC; Schaumburg, IL, USA) was injected subcutaneously at the surgical site for analgesia prior to incision. Holes were drilled in the skull for the placement of the electrode in the caudate-putamen (in mm from bregma): AP: +1.2, ML: +2.0, DV: –4.5. The Ag/AgCl reference electrode was placed on the contralateral side. Body temperature was regulated with a heating pad and thermistor probe (FHC; Bowdoin, ME, USA). In order to make sure the rat was deeply anesthetized throughout, anesthesia was checked every hour by toe pinch.
A carbon-fiber electrode was placed in the caudate-putamen and equilibrated for at least 30 minutes with the waveform applied. If few transients were seen during that 30 minutes of equilibration (less than 10/hour), the electrode was removed and a new electrode was inserted. After transients were verified, the electrode was then allowed to equilibrate for another 30 min, and then an hour of pre-drug data was collected. A drug was injected i.p and one hour of data was collected after drug injection. While the post-drug period might include a few minutes where the drug is still working into the bloodstream and across the blood brain barrier, there is not a good way to measure pharmacodynamics and most of the post-drug collection should be influenced by the drug. After in vivo testing, electrodes were calibrated in vitro using flow injection analysis with 1.0 μM adenosine in PBS buffer.11
Statistics
All statistics were performed using GraphPad Prism 6 (GraphPad Software Inc., San Diego, CA, USA). The data are presented as mean ± SEM. A Kolmogorov-Smirnov (KS) test was used to determine underlying distributions between inter-event times (time between consecutive transients). A t-test was used to compare means between 2 groups. All data were considered significant at the 95% confidence level. Principal components analysis, with confirmation by an analyst, was used to identify adenosine transients, as previously described 7.
Supplementary Material
Acknowledgments
Funding Sources: NIH R01NS076875 to BJV.
This work was funded by NIH (R01NS076875) to BJV.
Footnotes
Author contributions
MDN and BJV conceived of the project. MDN and YW performed the experiments. MDN, YW, and MG analyzed the data. MDN and BJV wrote the paper.
Notes
The authors declare no competing financial interest.
Associated content
Supporting information: Supplemental methods (electrochemistry) and a supplemental figure (Fig. S1) of vehicle injection are provided. Figure S2 shows histology.
References
- 1.Latini S, Pedata F. Adenosine in the Central Nervous System: Release Mechanisms and Extracellular Concentrations. J Neurochem. 2001;79(3):463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- 2.Ochi M, Shiozaki S, Kase H. Adenosine A2A Receptor-Mediated Modulation of GABA and Glutamate Release in the Output Regions of the Basal Ganglia in a Rodent Model of Parkinson’s Disease. Neuroscience. 2004;127(1):223–231. doi: 10.1016/j.neuroscience.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 3.Ross AE, Venton BJ. Adenosine Transiently Modulates Stimulated Dopamine Release in the Caudate–putamen via A1 Receptors. J Neurochem. 2015;132(1):51–60. doi: 10.1111/jnc.12946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brambilla D, Chapman D, Greene R. Adenosine Mediation of Presynaptic Feedback Inhibition of Glutamate Release. Neuron. 2005;46(2):275–283. doi: 10.1016/j.neuron.2005.03.016. [DOI] [PubMed] [Google Scholar]
- 5.Klyuch BP, Richardson MJE, Dale N, Wall MJ. The Dynamics of Single Spike-Evoked Adenosine Release in the Cerebellum. J Physiol. 2011;589(2):283–295. doi: 10.1113/jphysiol.2010.198986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Klyuch BP, Dale N, Wall MJ. Receptor-Mediated Modulation of Activity-Dependent Adenosine Release in Rat Cerebellum. Neuropharmacology. 2012;62(2):815–824. doi: 10.1016/j.neuropharm.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 7.Nguyen MD, Lee ST, Ross AE, Ryals M, Choudhry VI, Venton BJ. Characterization of Spontaneous, Transient Adenosine Release in the Caudate-Putamen and Prefrontal Cortex. PLoS One. 2014;9(1):e87165. doi: 10.1371/journal.pone.0087165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen MD, Ross AE, Ryals M, Lee ST, Venton BJ. Clearance of Rapid Adenosine Release Is Regulated by Nucleoside Transporters and Metabolism. Pharmacol Res Perspect. 2015;3(6) doi: 10.1002/prp2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ross AE, Nguyen MD, Privman E, Venton BJ. Mechanical Stimulation Evokes Rapid Increases in Extracellular Adenosine Concentration in the Prefrontal Cortex. J Neurochem. 2014;130(1):50–60. doi: 10.1111/jnc.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Adamah-Biassi EB, Almonte AG, Blagovechtchenski E, Grinevich VP, Weiner JL, Bonin KD, Budygin EA. Real Time Adenosine Fluctuations Detected with Fast-Scan Cyclic Voltammetry in the Rat Striatum and Motor Cortex. J Neurosci Methods. 2015;256:56–62. doi: 10.1016/j.jneumeth.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang Y, Venton BJ. Correlation of Transient Adenosine Release and Oxygen Changes in the Caudate-Putamen. J Neurochem. 2016 doi: 10.1111/jnc.13705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fastbom J, Fredholm BB. Inhibition of [3H] Glutamate Release from Rat Hippocam Pal Slices by L-Phenylisopropyladenosine. Acta Physiol Scand. 1985;125(1):121–123. doi: 10.1111/j.1748-1716.1985.tb07698.x. [DOI] [PubMed] [Google Scholar]
- 13.Corsi C, Melani A, Bianchi L, Pedata F. Striatal A2A Adenosine Receptor Antagonism Differentially Modifies Striatal Glutamate Outflow in Vivo in Young and Aged Rats. Neuroreport. 2000;11(11) doi: 10.1097/00001756-200008030-00048. [DOI] [PubMed] [Google Scholar]
- 14.Ciruela F, Casadó V, Rodrigues RJ, Luján R, Burgueño J, Canals M, Borycz J, Rebola N, Goldberg SR, Mallol J, et al. Presynaptic Control of Striatal Glutamatergic Neurotransmission by Adenosine A1–A2A Receptor Heteromers. J Neurosci. 2006;26(7):2080–LP-2087. doi: 10.1523/JNEUROSCI.3574-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hoehn K, Craig CG, White TD. A Comparison of N-Methyl-D-Aspartate-Evoked Release of Adenosine and [3H]norepinephrine from Rat Cortical Slices. J Pharmacol Exp Ther. 1990;255(1):174–LP-181. [PubMed] [Google Scholar]
- 16.Manzoni OJ, Manabe T, Nicoll RA. Release of Adenosine by Activation of NMDA Receptors in the Hippocampus. Science (80- ) 1994;265(5181):2098–LP-2101. doi: 10.1126/science.7916485. [DOI] [PubMed] [Google Scholar]
- 17.Sims RE, Dale N. Activity-Dependent Adenosine Release May Be Linked to Activation of Na+-K+ ATPase: An In Vitro Rat Study. PLoS One. 2014;9(1):e87481. doi: 10.1371/journal.pone.0087481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pajski ML, Venton BJ. Adenosine Release Evoked by Short Electrical Stimulations in Striatal Brain Slices Is Primarily Activity Dependent. ACS Chem Neurosci. 2010;1(12):775–787. doi: 10.1021/cn100037d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wirkner K, Assmann H, Köles L, Gerevich Z, Franke H, Nörenberg W, Boehm R, Illes P. Inhibition by Adenosine A2A Receptors of NMDA but Not AMPA Currents in Rat Neostriatal Neurons. Br J Pharmacol. 2000;130(2):259–269. doi: 10.1038/sj.bjp.0703234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wall MJ, Dale N. Auto-Inhibition of Rat Parallel fibre–Purkinje Cell Synapses by Activity-Dependent Adenosine Release. J Physiol. 2007;581(2):553–565. doi: 10.1113/jphysiol.2006.126417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Agnati LF, Guidolin D, Albertin G, Trivello E, Ciruela F, Genedani S, Tarakanov A, Fuxe K. An Integrated View on the Role of Receptor Mosaics at Perisynaptic Level: Focus on Adenosine A2A, Dopamine D2, Cannabinoid CB1, and Metabotropic Glutamate mGlu5 Receptors. J Recept Signal Transduct. 2010;30(5):355–369. doi: 10.3109/10799893.2010.487492. [DOI] [PubMed] [Google Scholar]
- 22.Bogenpohl JW, Ritter SL, Hall RA, Smith Y. Adenosine A2A Receptor in the Monkey Basal Ganglia: Ultrastructural Localization and Colocalization with the Metabotropic Glutamate Receptor 5 in the Striatum. J Comp Neurol. 2012;520(3):570–589. doi: 10.1002/cne.22751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Saransaari P, Oja SS. GABA Release Modified by Adenosine Receptors in Mouse Hippocampal Slices under Normal and Ischemic Conditions. Neurochem Res. 2005;30(4):467–473. doi: 10.1007/s11064-005-2682-4. [DOI] [PubMed] [Google Scholar]
- 24.Wu L, Li H, Li Y-Q. Adenosine Suppresses the Response of Neurons to Gaba in the Superficial Laminae of the Rat Spinal Dorsal Horn. Neuroscience. 2003;119(1):145–154. doi: 10.1016/s0306-4522(03)00074-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen G, van den Pol AN. Adenosine Modulation of Calcium Currents and Presynaptic Inhibition of GABA Release in Suprachiasmatic and Arcuate Nucleus Neurons. J Neurophysiol. 1997;77(6):3035–LP-3047. doi: 10.1152/jn.1997.77.6.3035. [DOI] [PubMed] [Google Scholar]
- 26.Nguyen MD, Venton BJ. Fast-Scan Cyclic Voltammetry for the Characterization of Rapid Adenosine Release. Comput Struct Biotechnol J. 2015;13:47–54. doi: 10.1016/j.csbj.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dryhurst G. Electrochemistry of Biological Molecules. Academic Press; New York: 1977. p. 185. [Google Scholar]
- 28.Swamy BEK, Venton BJ. Subsecond Detection of Physiological Adenosine Concentrations Using Fast-Scan Cyclic Voltammetry. Anal Chem. 2007;79(2):744–750. doi: 10.1021/ac061820i. [DOI] [PubMed] [Google Scholar]
- 29.Borman RP, Wang Y, Nguyen MD, Ganesana M, Lee ST, Venton BJ. Automated Algorithm for Detection of Transient Adenosine Release. ACS Chem Neurosci. 2016 doi: 10.1021/acschemneuro.6b00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pajski ML, Venton BJ. The Mechanism of Electrically Stimulated Adenosine Release Varies by Brain Region. Purinergic Signal. 2013;9(2):167–174. doi: 10.1007/s11302-012-9343-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lehmann J, Schneider J, McPherson S, Murphy DE, Bernard P, Tsai C, Bennett DA, Pastor G, Steel DJ, Boehm C. CPP, a Selective N-Methyl-D-Aspartate (NMDA)-Type Receptor Antagonist: Characterization in Vitro and in Vivo. J Pharmacol Exp Ther. 1987;240(3):737–LP-746. [PubMed] [Google Scholar]
- 32.Starr BS, Starr MS. Glutamate Antagonists Modify the Motor Stimulant Actions of D1 and D2 Agonists in Reserpine-Treated Mice in Complex Ways That Are Not Predictive of Their Interactions with the Mixed D1/D2 Agonist Apomorphine. J Neural Transm - Park Dis Dement Sect. 1993;6(3):215–226. doi: 10.1007/BF02260924. [DOI] [PubMed] [Google Scholar]
- 33.Leander JD, Lawson RR, Ornstein PL, Zimmerman DM. N-Methyl-D-Aspartic Acid-Induced Lethality in Mice: Selective Antagonism by Phencylidine-like Drugs. Brain Res. 1988;448(1):115–120. doi: 10.1016/0006-8993(88)91107-9. [DOI] [PubMed] [Google Scholar]
- 34.Guertin PA. Role of NMDA Receptor Activation in Serotonin Agonist-Induced Air-Stepping in Paraplegic Mice. Spinal Cord. 2004;42(3):185–190. doi: 10.1038/sj.sc.3101580. [DOI] [PubMed] [Google Scholar]
- 35.Chapman AG, Smith SE, Meldrum BS. The Anticonvulsant Effect of the Non-NMDA Antagonists, NBQX and GYKI 52466, in Mice. Epilepsy Res. 1991;9(2):92–96. doi: 10.1016/0920-1211(91)90018-b. [DOI] [PubMed] [Google Scholar]
- 36.Zhang C, Schmidt JT. Adenosine A1 and Class II Metabotropic Glutamate Receptors Mediate Shared Presynaptic Inhibition of Retinotectal Transmission. J Neurophysiol. 1999;82(6):2947–LP-2955. doi: 10.1152/jn.1999.82.6.2947. [DOI] [PubMed] [Google Scholar]
- 37.Ngomba RT, Biagioni F, Casciato S, Bree EW, Battaglia G, Bruno V, Nicoletti F, van Luijtelaar ELJM. The Preferential mGlu2/3 Receptor Antagonist, LY341495, Reduces the Frequency of Spike–wave Discharges in the WAG/Rij Rat Model of Absence Epilepsy. Neuropharmacology. 2005;49(Supple):89–103. doi: 10.1016/j.neuropharm.2005.05.019. [DOI] [PubMed] [Google Scholar]
- 38.Masino SA, Diao L, Illes P, Zahniser NR, Larson GA, Johansson B, Fredholm BB, Dunwiddie TV. Modulation of Hippocampal Glutamatergic Transmission by ATP Is Dependent on Adenosine A1 Receptors. J Pharmacol Exp Ther. 2002;303(1):356–LP-363. doi: 10.1124/jpet.102.036731. [DOI] [PubMed] [Google Scholar]
- 39.Ferguson AL, Stone TW. Glutamate-Induced Depression of EPSP–spike Coupling in Rat Hippocampal CA1 Neurons and Modulation by Adenosine Receptors. Eur J Neurosci. 2010;31(7):1208–1218. doi: 10.1111/j.1460-9568.2010.07157.x. [DOI] [PubMed] [Google Scholar]
- 40.Petralia RS, Yokotani N, Wenthold RJ. Light and Electron Microscope Distribution of the NMDA Receptor Subunit NMDAR1 in the Rat Nervous System Using a Selective Anti-Peptide Antibody. J Neurosci. 1994;14(2):667–LP-696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Li Y-H, Zhang JWG. Presynaptic NR2B-Containing NMDA Autoreceptors Mediate Glutamatergic Synaptic Transmission in the Rat Visual Cortex. Current Neurovascular Research. 2009:104–109. doi: 10.2174/156720209788185632. [DOI] [PubMed] [Google Scholar]
- 42.Rebola N, Pinheiro PC, Oliveira CR, Malva JO, Cunha RA. Subcellular Localization of Adenosine A1 Receptors in Nerve Terminals and Synapses of the Rat Hippocampus. Brain Res. 2003;987(1):49–58. doi: 10.1016/s0006-8993(03)03247-5. [DOI] [PubMed] [Google Scholar]
- 43.Niswender CM, Conn PJ. Metabotropic Glutamate Receptors: Physiology, Pharmacology, and Disease. Annu Rev Pharmacol Toxicol. 2010;50:295–322. doi: 10.1146/annurev.pharmtox.011008.145533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kingston AE, Ornstein PL, Wright RA, Johnson BG, Mayne NG, Burnett JP, Belagaje R, Wu S, Schoepp DD. LY341495 Is a Nanomolar Potent and Selective Antagonist of Group II Metabotropic Glutamate Receptors. Neuropharmacology. 1998;37(1):1–12. doi: 10.1016/s0028-3908(97)00191-3. [DOI] [PubMed] [Google Scholar]
- 45.Marazioti A, Spyraki C, Thermos K. GABA Antagonists Reverse the Somatostatin Dependent Attenuation of Rat Locomotor Activity. Neuropeptides. 2009;43(3):207–212. doi: 10.1016/j.npep.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 46.Felice D, O’Leary OF, Pizzo RC, Cryan JF. Blockade of the GABAB Receptor Increases Neurogenesis in the Ventral but Not Dorsal Adult Hippocampus: Relevance to Antidepressant Action. Neuropharmacology. 2012;63(8):1380–1388. doi: 10.1016/j.neuropharm.2012.06.066. [DOI] [PubMed] [Google Scholar]
- 47.Beas BS, Setlow B, Bizon JL. Effects of Acute Administration of the GABA(B) Receptor Agonist Baclofen on Behavioral Flexibility in Rats. Psychopharmacology (Berl) 2016;233(14):2787–2797. doi: 10.1007/s00213-016-4321-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Walker BM, Koob GF. The γ-Aminobutyric Acid-B Receptor Agonist Baclofen Attenuates Responding for Ethanol in Ethanol-Dependent Rats. Alcohol Clin Exp Res. 2007;31(1):11–18. doi: 10.1111/j.1530-0277.2006.00259.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kulik Á, Vida I, Luján R, Haas CA, López-Bendito G, Shigemoto R, Frotscher M. Subcellular Localization of Metabotropic GABAB Receptor Subunits GABAB1a/b and GABAB2 in the Rat Hippocampus. J Neurosci. 2003;23(35):11026–LP-11035. doi: 10.1523/JNEUROSCI.23-35-11026.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nisenbaum ES, Berger TW, Grace AA. Depression of Glutamatergic and Gabaergic Synaptic Responses in Striatal Spiny Neurons by Stimulation of Presynaptic GABAB Receptors. Synapse. 1993;14(3):221–242. doi: 10.1002/syn.890140306. [DOI] [PubMed] [Google Scholar]
- 51.Margeta-Mitrovic M, Mitrovic I, Riley RC, Jan LY, Basbaum AI. Immunohistochemical Localization of GABAB Receptors in the Rat Central Nervous System. J Comp Neurol. 1999;405(3):299–321. doi: 10.1002/(sici)1096-9861(19990315)405:3<299::aid-cne2>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi M, Takei H, Yamamoto K, Hatanaka H, Koshikawa N. Kinetics of GABAB Autoreceptor-Mediated Suppression of GABA Release in Rat Insular Cortex. J Neurophysiol. 2012;107(5):1431–LP-1442. doi: 10.1152/jn.00813.2011. [DOI] [PubMed] [Google Scholar]
- 53.Shrivastava AN, Triller A, Sieghart W. GABA(A) Receptors: Post-Synaptic Co-Localization and Cross-Talk with Other Receptors. Front Cell Neurosci. 2011;5:7. doi: 10.3389/fncel.2011.00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li H, Wu L, Li Y-Q. Adenosine Suppresses GABAA Receptor-Mediated Responses in Rat Sacral Dorsal Commissural Neurons. Auton Neurosci. 2004;111(2):71–79. doi: 10.1016/j.autneu.2004.01.004. [DOI] [PubMed] [Google Scholar]
- 55.Ilie A, Raimondo JV, Akerman CJ. Adenosine Release during Seizures Attenuates GABAA Receptor-Mediated Depolarization. J Neurosci. 2012;32(15):5321–LP-5332. doi: 10.1523/JNEUROSCI.5412-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Cunha RA. Different Cellular Sources and Different Roles of Adenosine: A1 Receptor-Mediated Inhibition through Astrocytic-Driven Volume Transmission and Synapse-Restricted A2A Receptor-Mediated Facilitation of Plasticity. Neurochem Int. 2008;52(1–2):65–72. doi: 10.1016/j.neuint.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 57.Tepper J, Tecuapetla F, Koos T, Ibanez-Sandoval O. Heterogeneity and Diversity of Striatal GABAergic Interneurons. FrontNeuroanat. 2010;4:150. doi: 10.3389/fnana.2010.00150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rodriguez M, Morales I, Gomez I, Gonzalez S, Gonzalez-Hernandez T, Gonzalez-Mora JL. Heterogeneous Dopamine Neurochemistry in the Striatum: The Fountain-Drain Matrix. J Pharmacol Exp Ther. 2006;319(1):31–LP-43. doi: 10.1124/jpet.106.104687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.