Abstract
A multifunctional fusion protein, IL-13.E13K-D2-NLS, effectively recognizes glioblastoma (GBM) cells and delivers its portion to the cell nucleus. IL-13.E13K-D2-NLS is composed of a cancer cell targeting ligand (IL-13.E13K), specialized cytosol translocation bacterial toxin domain 2 of Pseudomonas exotoxin A (D2) and SV40 T antigen nuclear localization signal (NLS). We have now tested whether we can produce proteins that would serve as a delivery vehicle to lysosomes and mitochondria as well. Moreover, we examined whether IL-13.E13K-D2-NLS can deliver anti-cancer drugs like doxorubicin to their nuclear site of action in cancer cells. We have thus constructed two novel proteins: IL-13.E13K-D2-LLS which incorporates lysosomal localization signal (LLS) of a human lysosomal associated membrane protein (LAMP-1) for targeting to lysosomes and IL-13-D2-KK2, which incorporates a pro-apoptotic peptide (KLAKLAK)2 (KK2) exerting its action in mitochondria. Furthermore, we have produced IL-13.E13K-D2-NLS and IL-13.E13K-D2-LLS versions containing a cysteine for site-specific conjugation with a modified doxorubicin, WP936. We found that single-chain recombinant proteins IL-13.E13K-D2-LLS and IL-13-D2-KK2 are internalized and localized mostly to the lysosomal and mitochondrial compartments, respectively, without major trafficking to cells’ nuclei. We also determined that IL-13.E13K-D2-NLS-cys[WP936], IL-13.E13K-D2-LAMP-cys[WP936] and IL-13-D2-KK2 were cytotoxic to GBM cells overexpressing IL-13RA2, while much less cytotoxic to GBM cell lines expressing low levels of the receptor. IL-13.E13K-D2-NLS-cys[WP936] was the most potent of the tested anti-tumor agents including free WP936. We believe that our receptor-directed intracellular organelle-targeted proteins can be employed for numerous specific and safer treatment applications when drugs have specific intracellular sites of their action.
INTRODUCTION
Glioblastoma (GBM), a high-grade astrocytoma (HGA), is the most prevalent and aggressive primary brain tumor.[1] GBM tumors are very heterogeneous in nature and even the multimodal therapies such as chemotherapy, radiation, surgical resection and electric fields result in poor prognosis with a median survival of ~16 months post-diagnosis.[2–6] We have previously found that interleukin 13 receptor alpha 2 (IL-13RA2) is overexpressed in >70% of GBM tumor specimens.[7–9] We have shown that IL-13RA2 is absent in normal adult tissue except in the testes and that IL-13RA2 biochemical moiety is a tumor-associated receptor.[10,11] Upon binding to IL-13RA2, IL-13 induces receptor-mediated endocytosis.[7] Hence wild type and mutated IL-13 based fusion cytotoxins containing a derivative of Pseudomonas exotoxin A (PE), PE38QQR possess potent anti-tumor activity against GBM tumors in vitro and in vivo.[7,12] The first generation of the cytotoxin demonstrated clinical efficacy.[13,14] An IL-13 mutant, IL-13.E13K binds specifically to IL-13RA2, but it has significantly altered affinity towards the IL-13RA1, a subunit of a normal tissue receptor for IL-13 that is shared with IL-4, IL-13RA1/IL-4A.[12,15] This allows for much more specific targeting of tumor cells vs. normal cells.
Safe delivery of effective anti-tumor agents is the main goal in cancer therapy. Anthracyclins, e.g., doxorubicin are among the most effective therapies against hematologic malignancies and solid tumors.[16–19] However, clinically effective doses of anthracyclins cause myelosuppression, cumulative cardiotoxicity, stomatitis, and extravasation.[20,21] This is because oftentimes only a limited amount of the functional drug reaches tumor cells whereas it also acts on the normal healthy tissues resulting in serious adverse side-effects. Site specific delivery of the anti-cancer agents like doxorubicin focused to tumor cells could reduce its adverse effects. One example is the antibody drug/label conjugates. Monoclonal antibodies (MAb) and their drug conjugates have been successfully implemented in the treatment of solid tumors and blood malignancies.[22–24] This trend has been only accelerated in recent years with the approvals of Adcetris and Kadcyla antibody drug conjugates.[25–28]
Our laboratory has previously developed a single-chain, recombinant proteinaceous agent that recognizes interleukin-13 receptor alpha 2 (IL-13RA2) and delivers the C-terminal portion of the protein to a specific subcellular compartment (e.g., nuclei) of the GBM cells.[29] This was achieved on the basis of specific recognition of tumor cells by IL-13.E13K, internalization of the agent and proteolytic cleavage in the endocytic compartment releasing the C-terminal fragment of the protein into cell cytosol, and then subsequent transport and accumulation in cells’ nuclei facilitated by the nuclear localization signal (NLS).[29–31] The protein is termed IL-13.E13K-D2-NLS and could be used for more selective delivery of chemotherapeutics which the site of action is in the nuclei, like doxorubicin. In the current study, we examined whether IL-13.E13K-D2-NLS has potential to deliver a thiol reactive derivative of doxorubicin, WP936.[32,33] Being that IL-13RA2 is also over-expressed in pancreatic cancer, colorectal cancer, head and neck cancer, melanoma, and breast cancer[34–40] drug conjugates with chemotherapeutics would be suitable for targeting this receptor in solid tumors including GBM. Our current results show that IL-13.E13K-D2-NLS-cys enters the sub-cellular compartment and induces cytotoxicity in the GBM cells when conjugated with a chemotherapeutic, such as WP936.
Similar to NLS, lysosomal localization sequences (LLS) can be used as a potential drug trafficking signal. In the current study we also explored whether our delivery vectors containing a LAMP-1 LLS could distribute fragments of targeted proteinaceous compound to the targeted organellles. Moreover, a synthetic, pro-apoptotic peptide, (KLAKLAK)2 is known to specifically act on the tumor endothelium. (KLAKLAK)2 is a 14-amino-acid amphipathic α-helical peptide.[41] Due to its cationic nature, upon internalization, it selectively disrupts the anionic mitochondrial membrane resulting in a release of cytochrome C from the electron transport chain, and activation of the pro-apoptotic caspase pathway.[42] Others have fused (KLAKLAK)2 with various peptidomimetics such as DPI and MCA205[43] against murine sarcomas, and with RGD-4C against breast carcinoma cells.[42] Employing the same strategy as our other targeted proteinaceous agents, we have developed and constructed IL13-D2-KK2 for targeting IL13RA2-GBM cells. Here, we determined that these novel molecularly targeted and genetically engineered recombinant proteins selectively recognize tumor cells and accumulate in the intra-cellular compartments evoking anti-tumor effect.
METHODS
Cell Culture
GBM cell lines, U87, U-251 MG, LN229 and T98G were obtained from the American Type Culture Collection (Manassas, VA) and grown as recommended. SnB19-asIL-13Rα2 are SnB19 GBM cells transfected with anti-sense IL-13Rα2 gene with subsequently diminished expression of the receptor, in contrast to the empty vector-transfected SnB19-pcDNA cells[29]. The transfected SnB19 cell lines were grown in RPMI 1640 (Hyclone, Logan, UT) with 200 µg/ml of Geneticin. G48a cells were grown and maintained in RPMI 1640 (Lonza, Walkersville, MD) supplemented with glucose, adjusted to 4 gm/liter of media and 10% FCS.
Cloning, production and purification of targeted proteins
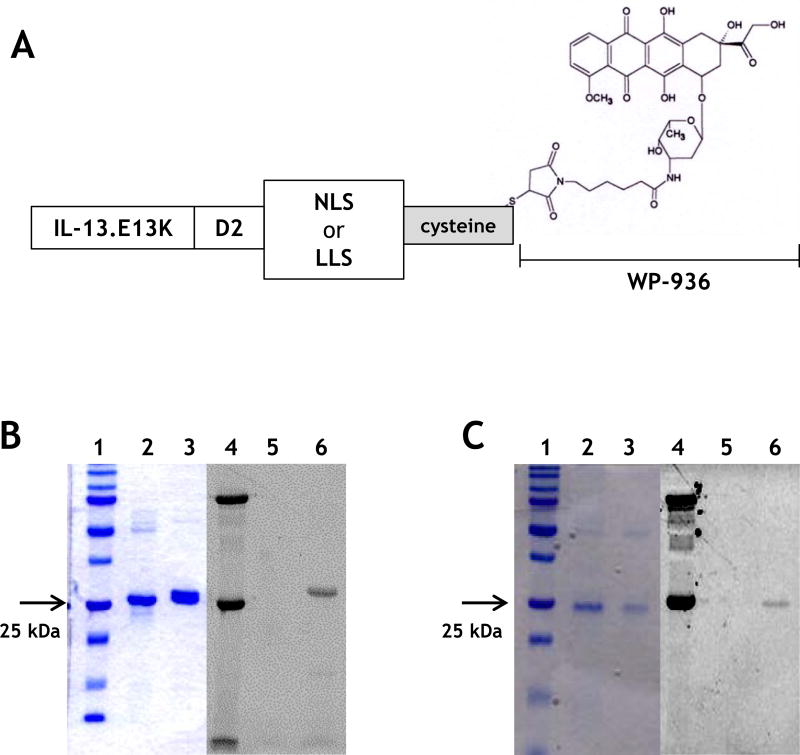
The IL-13 mutant recombinant constructs were made by replacing the wild-type IL-13 sequence from the parent plasmid with the mutant IL-13.E13K sequence. The IL-13-D2 plasmid was engineered by subcloning it from a previously generated IL-13-PE38QQR plasmid. Cysteine residue was added at NLS (NLS-cys) and LLS (LLS-cys) signal peptides C-terminal end to conjugate with a derivative of doxorubicin by forming disulfide bonds (Fig. 2A). A duplex primer cloning strategy was then employed wherein NLS-cys, LLS-cys and (KLAKLAK)2 5′ and 3′ sequence primers were synthesized (Invitrogen) and made into duplex DNA (containing XhoI/BamHI ends) by incubating the primers in favorable annealing conditions. The annealed duplex was then subcloned into the IL-13.E13K-D2 containing plasmid using Xho1/BamH1 at the 3′end to produce IL-13.E13K-D2-NLS-cys or IL-13.E13K-D2-LLS-cys.
NLS-cys primers
5′ - TCG AGT TCA AGT GAC GAT GAA GCC ACA GCG GAC AGC CAA CAT GCT GCA CCT CCG AAG AAA AAG AGA AAA GTA TGT TGA GAG
5′ - GAT CCT CTC AAC ATA CTT TTC TCT TTT TCT TCG GAG GTG CAG CAT GTT GGC TGT CCG CTG TGG CTT CAT CGT CAC TTG AAC
Molecular weight: 27510.3 Theoretical pI: 8.69
LLS-cys primers
5′ - TCG AGT CGC AAA CGC AGC CAT GCG GGC TAT CAG ACC ATT TGC TGA GGG
5′ - G ATC CCC TCA GCA AAT GGT CTG ATA GCC CGC ATG GCT GCG TTT GCG AC
Theoretical pI/Mw: 9.30 / 26118.96
(KLAKLAK)2 duplex primers
5′- TCG AGT AAA CTG GCG AAA CTG GCG AAG AAG CTG GCC AAA CTG GCC AAG TGA GGG
5′- G ATC C CC TCA CTT GGC CAG TTT GGC CAG CTT CTT CGC CAG TTT CGC CAG TTT AC
Theoretical pI/Mw: 9.43 / 26361.43
These recombinant constructs were used to transform DH5α E.coli cells for amplification. All the constructs were sequenced at the DNA sequencing laboratory of the Comprehensive Cancer Center at Wake Forest University and analyzed for their inframe DNA sequence using an automated sequence analyzer prior to protein expression.
Protein expression was performed under the IPTG-inducible T7 promotor in an E. coli protein expression system as previously described.[44] In brief, IL-13.E13K-D2-NLS-cys, IL-13.E13K-D2-LLS-cys and IL-13-D2-KK2 recombinant constructs were expressed in BL21 E.coli respectively and the cells were grown in Luria-broth media supplemented with 100 µg/mL of ampicillin at 37 °C shaker. When A600 of the bacteria culture media reached log phase growth (0.7–0.8), the recombinant protein expression in the cells was induced by addition of IPTG (final concentration 1 mM) and allowed to incubate for a further 90 minutes. The expressed proteins in the inclusion bodies were then denatured using 7 M guanidine (MP Biomedicals, Salon, OH) and reduced with 1, 4-dithiothreitol (Sigma, St. Louis, MO). The reduced proteins were then renatured in a 0.1 M Tris buffer containing arginine/L-glutathione oxidase (Sigma). The protein was further dialyzed and purified by SP sepharose ion exchange liquid chromatography system (GE Healthcare). The purified proteins were subsequently run on SDS-PAGE gels to identify of the isolated proteins. All of the proteins obtained were >90% pure.
Protein conjugation with WP936, a doxorubicin derivative
A solution of protein was diluted to 100 µM in 50 mM Na2HPO4. Tris-(2-carboxyethyl) phosphine (TCEP; Molecular Probes. Eugene, Oregon) was added to a 10 fold molar excess. WP936 was slowly added while mixing to a final concentration of 100 µM and incubated with continuous stirring overnight at 4 °C. Unreacted label was removed by dialysis with PBS. Efficiency of labeling on the single cysteine residue was assessed by UV-VIS spectrophotometry (Nanodrop 1000 spectrophotometer, Thermo).
Determination of conjugation ratio
A calibration was developed to determine the molar conjugation ratio of WP936 to protein (IL-13.E13K-D2-NLS-cys or IL-13.E13K-D2-LLS-cys) based on the absorbance spectrum of the conjugate as previously shown to evaluate the WP936 conjugation and Thermo Scientific technical tip #31.[45,46] The extinction coefficient of doxorubicin is 8030 cm− M− and those of IL-13.E13K-D2-NLS-cys or IL-13.E13K-D2-LLS-cys are 20970 M−cm− and 22460 M−cm− (at 495nm) when assuming all cysteine residues are reduced by forming disulfide bond with doxorubicin.[47]
Colorimetric MTT or MTS/PMS cell viability assay
2–2.5 × 103 U87, G48a, U-251 MG, and Snb19 pcDNA GBM cells, over-expressors of IL-13RA2, T98G, Snb19 A/S RA2 and LN229, which do not over-express the receptor, were plated per well in triplicates for each concentration to be tested. After 24 hrs of incubation at 37 °C for the cells to attach, increasing concentrations of the IL-13.E13K-D2-NLS-cys[WP936] or IL-13.E13K-D2-LLS-cys[WP936] ranging from 0.02–2000 nM in 0.02% F127 (Sigma-Aldrich) were added and the plate was incubated for 48–72 hrs. Cells treated with cyclohexamide were used as controls.
Due to WP936’s overlapped excitation wavelength with MTS/PMS (495nm), MTT assay was employed to analyze cell viability treated with WP936 conjugated proteins. Briefly, after the incubation with WP936 conjugated protein, MTT reagent in phenol red free RPMI 1640 (Gibco, Grand Island, NY) was added to be 1/10 of culture volume. The plate was incubate at 37 °C for up to 4 hrs in a humidified, 5% CO2 atmosphere Equal volume of acidified iso-propanol was added to stop the reaction. The absorbance was measured at 570 nm. Background absorbance at 690 nm was used as a reference wavelength and was subtracted from the absorbance at 570 nm before plotting.
Viability of cells treated with IL-13-D2-KK2 in 0.02% of F127 was measured using the MTS [3-(4, 5-dimethylthiazol-2-yl)-5(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium]/PMS [phenazine methosulfate] dye (Promega, Madison, WI) as per the manufacturer’s instructions. The absorbance was measured at 490 nm. Both were measured using Spectra Max 340 PC (Molecular Devices, Sunnyvale, CA) microplate reader, and data were plotted as percentage of control versus concentration of the agent used.
Direct biotin labeling of IL-13.E13K-D2-LLS-cys and IL-13-D2-KK2
Biotin-XX Microscale Protein Labeling Kit (Invitrogen, Carlsbad, CA) was used to label the proteins as per the manufacturer’s instructions. The biotin-labeled proteins were separated on an SDS-PAGE gel and a Western blot carried out using streptavidin-HRP (Pierce, Rockford, IL) to detect for biotin-labeled proteins. The number of biotin molecules attached to the proteins was determined by the FluoReporter Biotin Quantitation assay kit (Invitrogen) as per the manufacturer’s guidelines. The fluorescent signals were measured using the BMG Optima plate reader (Ortenberg, Germany) and data was plotted as concentration of the standard Biocytin in pmoles versus relative fluorescence units.
Immunofluorescence localization studies on IL-13RA2 positive U-251 MG or U87 cells with biotin-conjugated proteins
1 × 105 U-251 MG or U87 GBM cells were plated on sterilized coverslips per well in a 12-well plate. The wells were plated in duplicates for each time point. After 24 hrs, 1 µM/well of biotin-labeled proteins was added for the indicated incubation time. After incubation, the cells were fixed with 10% buffered formalin for 15 mins at 37 °C and washed 4× with PBS. The cells were then permeabilized with 0.1% Triton-X-100/0.2% BSA-PBS for 1 min at RT, and washed 3 times with 1× PBS. For co-localization studies, anti-Lamp-1 (EMD Millipore Billerica, MA) or anti-ATP synthase (ThermoFisher, Waltham, MA) was applied to the cells for 1 hr at RT followed by the appropriate Alexa-Fluor conjugated secondary antibodies (Molecular Probes, Eugene, OR). Location of the biotin conjugates was detected with anti-streptavidin-555 (Invitrogen) and the nuclei were stained with DAPI (4', 6-diamidino-2-phenylindole) or Topro-3 iodide (Invitrogen, Carlsbad, CA). F-actin staining was performed with Phalloidin-Alexa 488 (Invitrogen).
After wells were washed 3 times with 1× PBS, the coverslips were mounted with Fluoroguard (ScyTek). The coverslips with DAPI stained cells were analyzed under the fluorescent microscope (Olympus, IX70, Center Valley, PA and Olympus Fluoview 1200 confocal microscope) and processed using the Image-Pro plus 5.1 and OlyVIA V2.8 software. The coverslips with Topro-3 iodide stained cells were then observed with LSM 510 Zeiss Confocal Microscope (Cellular Imaging Core, Comprehensive Cancer Center, and Wake Forest University) and the images processed using Zeiss LSM Image Browser (version 4.2).
Immunoblotting
500 ng of each of the recombinant biotin conjugated proteins were loaded onto a 12% SDS-PAGE gel and transferred to a polyvinylidene difluoride (PVDF) membrane (Pierce, Rockford, IL). Blots were blocked with 5% milk-phosphate buffered saline (PBS) for 1 hr at room temperature (RT). Biotin-proteins were detected using streptavidin conjugated with horseradish peroxidase (Thermo Fisher Scientific, Rockford, IL) diluted 1:10000 in blocking buffer. Detection was performed using the ECL plus Western Blotting Detection System (Amersham Biosciences, UK). Membranes were exposed to autoradiographic film Kodak Biomax XR. Films were scanned at 600× dpi and images compiled using Paint Shop X2
Flow cytometry
Cells were treated with 10 nM of the IL-13.E13K ligand and collected at various timepoints (0 to 24 hrs). IL-13RA2-specific primary antibody and anti-IgG-Alexa Fluor 488 secondary antibody (Molecular Probes, Eugene, OR) were used for detection. An IgG isotype was used as a control. The experiments were performed using BD FACS Calibur or Accuri C6 flow cytometers (BD Biosciences).
RESULTS
Persistence of IL-13RA2 on cell surface of U-251 GBM cells during treatment with IL-13.E13K
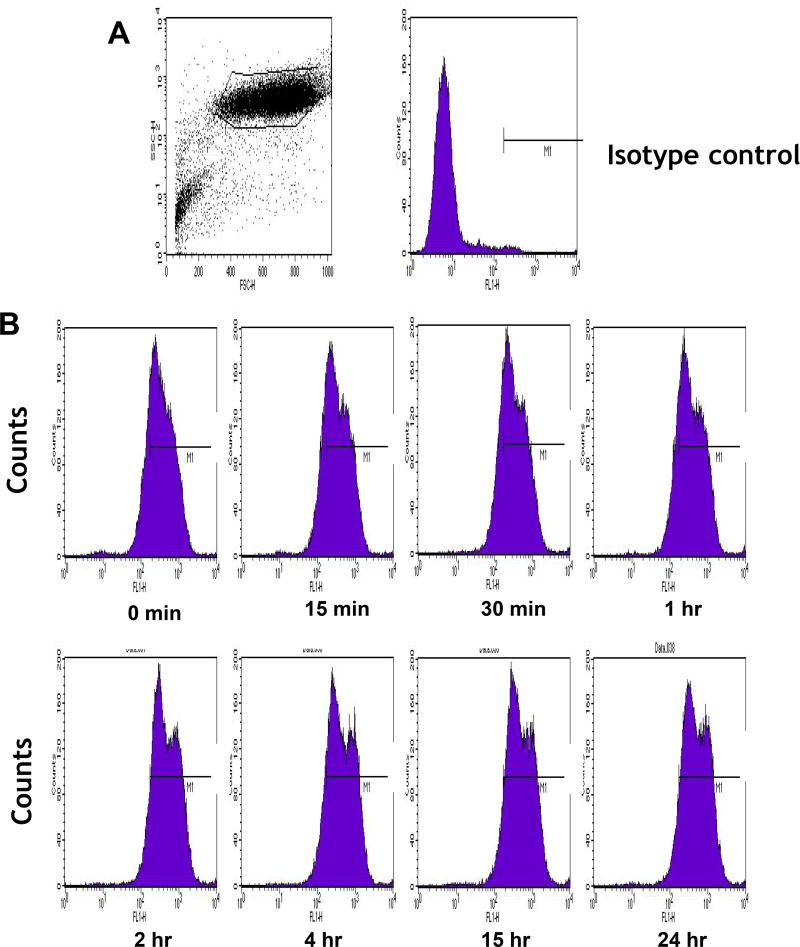
In our multi-level strategy of delivering drugs to their sites of actions in specific intracellular compartments of targeted cancer cells, we effectively exploit IL-13RA2 which is overexpressed in vast majority of patients with GBM.[7,9] To ascertain that the receptor is suitable for long-term delivery of our experimental therapeutics, we treated U-251 GBM cells with 10 nM of IL-13.E13K, which specifically recognizes IL-13RA2, and examined the levels of the receptor up to 24 hrs of treatment by flow cytometry (Fig. 1). The presence of IL-13RA2 on cell surface remained stable and was not down-regulated appreciably in response to treatment with its ligand, IL-13.E13K (Fig. 1B). Hence, the receptor can be suitably utilized for delivery of candidate drugs for a prolonged period of time.
Figure 1.
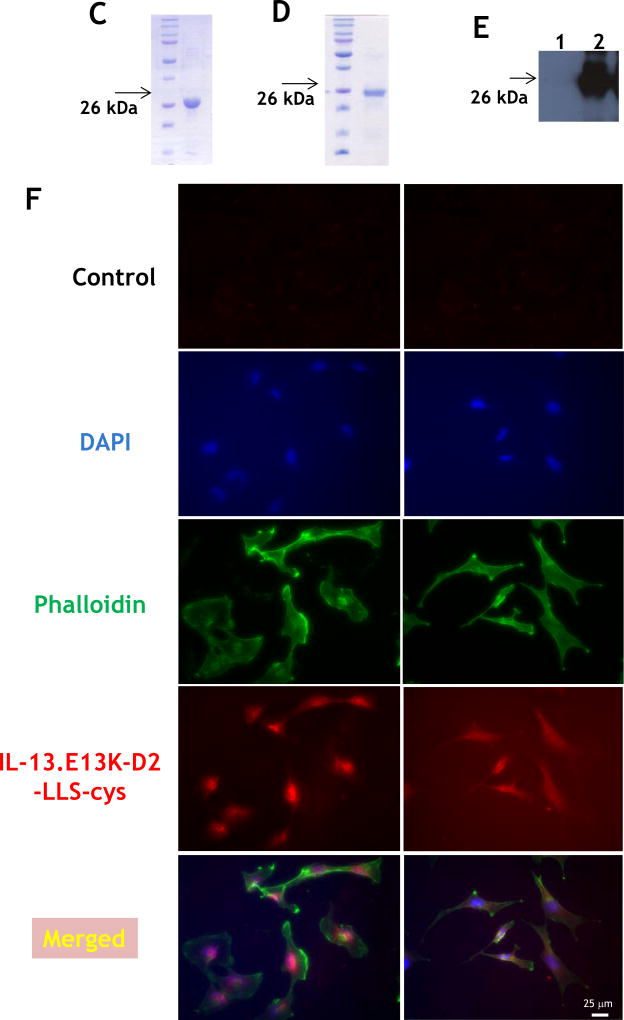
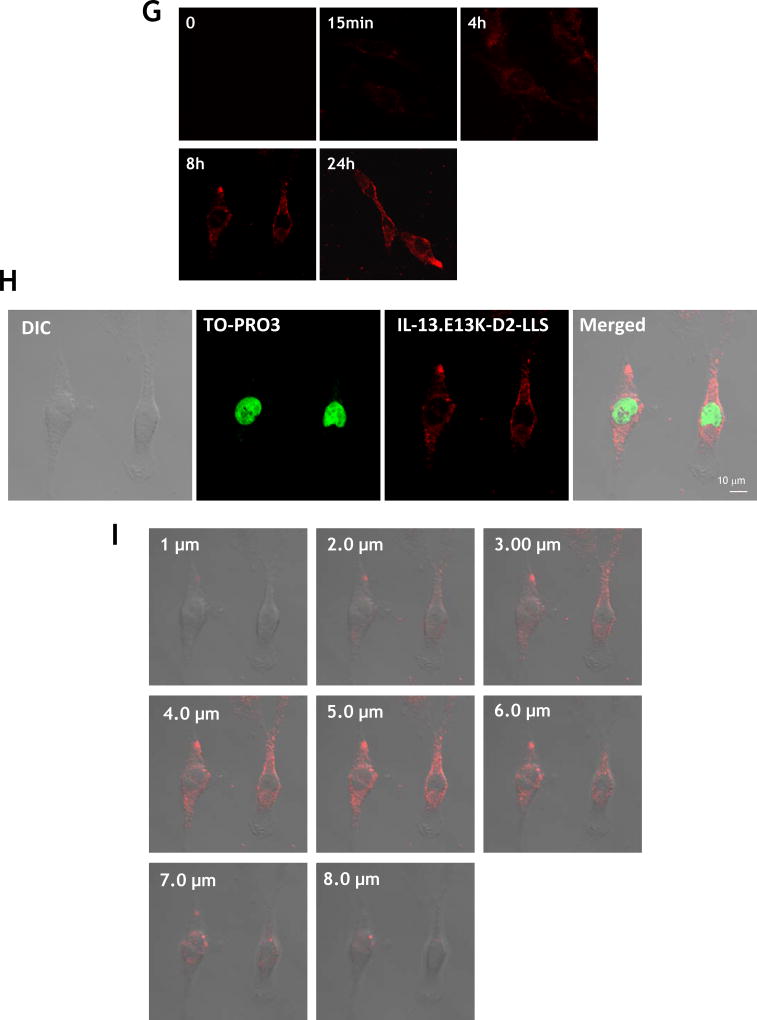
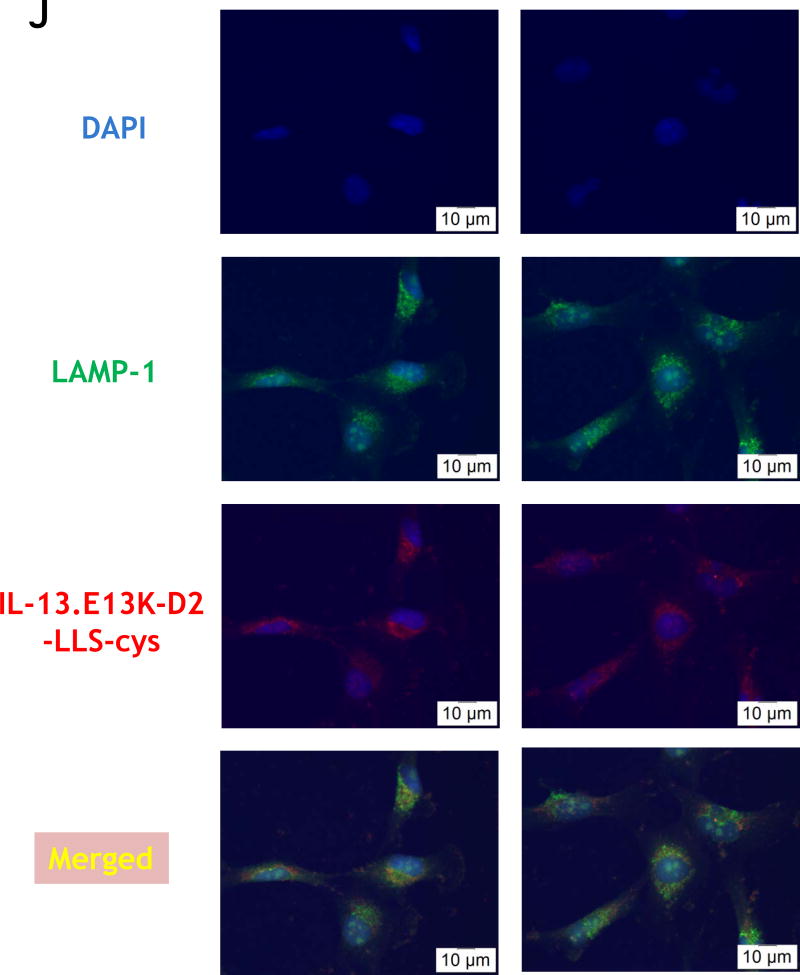
(A,B) Flow cytometry for IL-13RA2 in U-251 GBM cells. Isotype control (A) and (B) receptor detection at various timepoints. Highly purified IL-13.E13K-D2-NLS-cys (C) and IL-13.E13K-D2-LLS-cys (D). (E) Immunoblot of non-biotinylated (lane 1) and biotinylated (lane 2) IL-13.E13K-D2-LLS-cys probed with streptavidin-HRP. (F,G) Internalization of biotinylated IL-13.E13K-D2-LLS-cys (1 µM) in U-251 MG cells. The cells were analyzed using anti-streptavidin Alexa Fluor 555 (red) by fluorescence microscopy. Two different fields are shown in two column panels. (H) U-251-MG cells were treated with biotin-labeled IL-13.E13K-D2-LLS-cys (1 µM) and cells were stained for the nuclei and the protein. DIC, differential interference contrast. (I) Subcellular localization of IL-13.E13K-D2-LLS-cys was monitored using Z-stack analysis. (J) Internalization and intracellular distribution of IL-13.E13K-D2-LLS-cys. U-251 MG cells were incubated for 8 hrs with 1 µM biotin-conjugated IL-13.E13K-D2-LLS-cys (red) with co-staining of LAMP-1 protein (green).
Production and purification of IL-13.E13K-D2-LLS-cys and IL-13.E13K-D2-NLS-cys
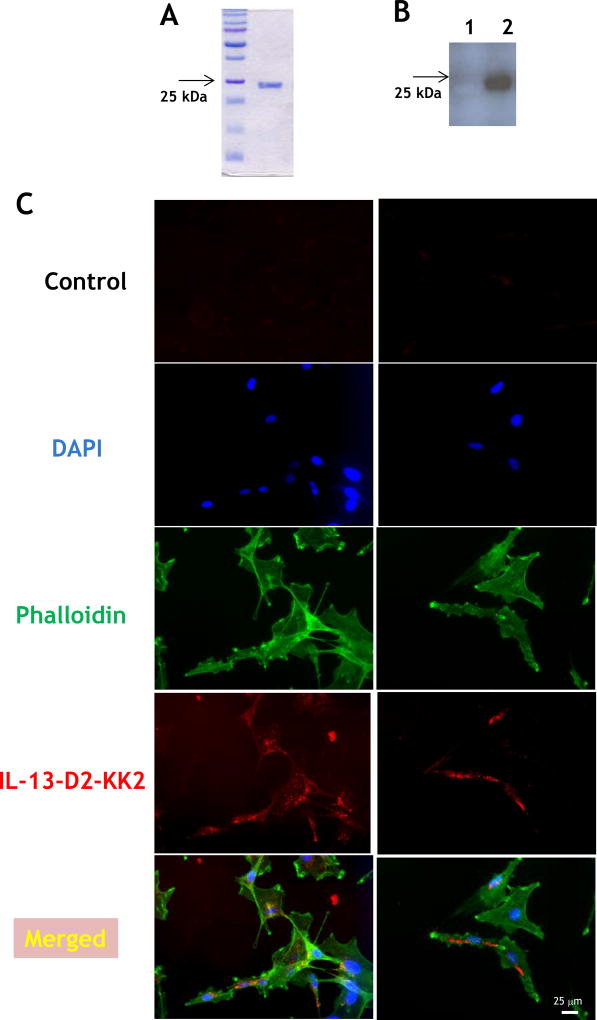
After having successfully developed a recombinant, single-chain protein delivering its C-terminal portion to the nuclei of targeted cells,[29] we have now constructed a recombinant protein for potential delivery of drug loads to the lysosomal compartment of cancer cells over-expressing IL-13RA2. We used lysosome-associated membrane protein 1 (LAMP-1) lysosomal localization signal sequence (LLS) in combination with IL-13.E13K ligand and D2 domain of PE to produce IL-13.E13K-D2-LLS-cys. We also produced IL-13.E13K-D2-NLS-cys for first in class conjugation to a cytotoxic agent, a chemotherapeutic in this case. IL-13.E13K-D2-NLS-cys and IL-13.E13K-D2-LLS-cys were produced as single-chain proteins in E.coli and purified by FPLC (Fig. 1C and D, respectively). The isolated proteins were >90% pure.
Internalization of IL-13.E13K-D2-LLS-cys protein
We biotinylated IL-13.E13K-D2-LLS-cys using biotin-XX sulfosuccinimidyl ester to form a stable biotinylated conjugate protein, which was detected using an immunoblot assay (Fig. 1E). Only the presence of biotinylated IL-13.E13K-D2-LLS-cys protein was demonstrated (Fig. 1E, lane 2), whereas no signal was detected for the IL-13.E13K-D2-LLS-cys non-biotinylated protein (Fig. 1E, lane 1). We then analyzed cell internalization and localization of biotinylated IL-13.E13K-D2-LLS-cys protein (1 µM) using anti-streptavidin Alexa Fluor-555 antibody and fluorescent microscopy (Fig. 1F). We treated U-251 MG GBM cells with biotinylated protein for 4 hrs; cells were also stained with phalloidin for actin labeling. We observed that the biotinylated protein effectively internalized after 4 hrs of incubation (Fig. 1F), which further increased with longer incubation times (8 and 24 hrs, respectively) (Fig. 1G). We also performed confocal microscopy and Z-stack analysis to examine the intracellular localization of the protein. IL-13.E13K-D2-LLS-cys internalized into cells with a clear omission of accumulation in the nuclei (Fig. 1H). The Z-stack analysis confirmed that IL-13.E13K-D2-LLS-cys protein internalized into the U-251 MG cells and is localized primarily in the cytosol and perinuclear regions, but never in the nuclei even after 24 hrs of treatment (Fig. 1I). This is in sharp contrast to the nuclei trafficking of IL-13.E13K-D2-NLS seen previously.[29].
IL-13.E13K-D2-LLS-cys co-localizes with LAMP-1 in GBM cells
We confirmed the binding of biotinylated IL-13.E13K-D2-LLS-cys protein to the lysosomal compartment by determining its localization near LAMP-1. We first treated the U-251 MG cells at various time points (5 min, 4, 8, 12 and 24 hrs) with biotinylated IL-13.E13K-D2-LLS-cys followed by a double immunofluorescence staining against streptavidin and LAMP-1. The double immunofluorescence staining showed that the studied proteins localize frequently to the same regions in cells (Fig. 1J).
Conjugation of targeting proteins with a cytotoxic thiol reactive doxorubicin derivative, WP936
We next examined whether the proteins capable of delivering their portions to two different intracellular compartments of cells would cause cytotoxicity upon conjugation with a cytotoxic agent. IL-13.E13K-D2-NLS-cys was conjugated with thiol reactive doxorubicin derivative WP936[33] using TCEP, (IL-13.E13K-D2-NLS-cys[WP936]) in a way that the exact site of conjugation in both components of this anti-tumor agent is known (Fig. 2A). We also conjugated IL-13.E13K-D2-LLS-cys with WP936 using the same method. The schematic of both drug conjugates is shown in Fig. 2A. The IL-13.E13K-D2-NLS-cys[WP936] and IL-13.E13K-D2-LLS-cys[WP936] conjugates were analyzed using SDS-PAGE gel and Typhoon scan (Fig. 2B and C). Only the successfully WP936-conjugated proteins emitted the detectable fluorescence signals at the expected molecular sizes (Fig. 2B and C).
Figure 2.
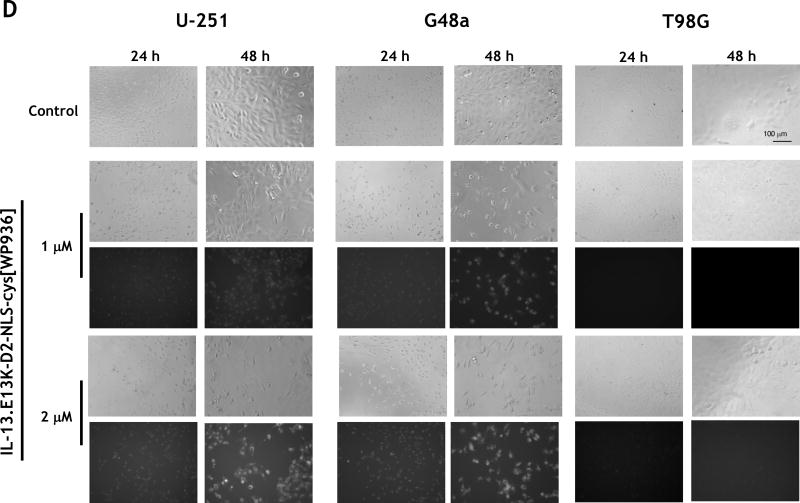
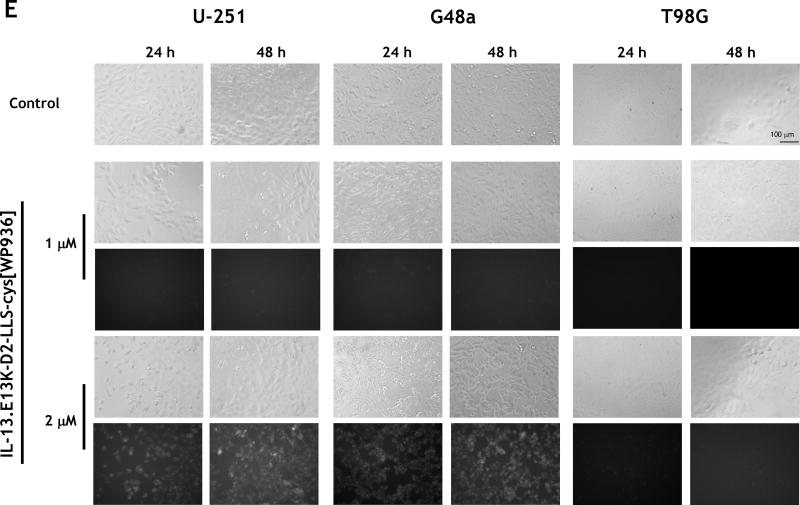
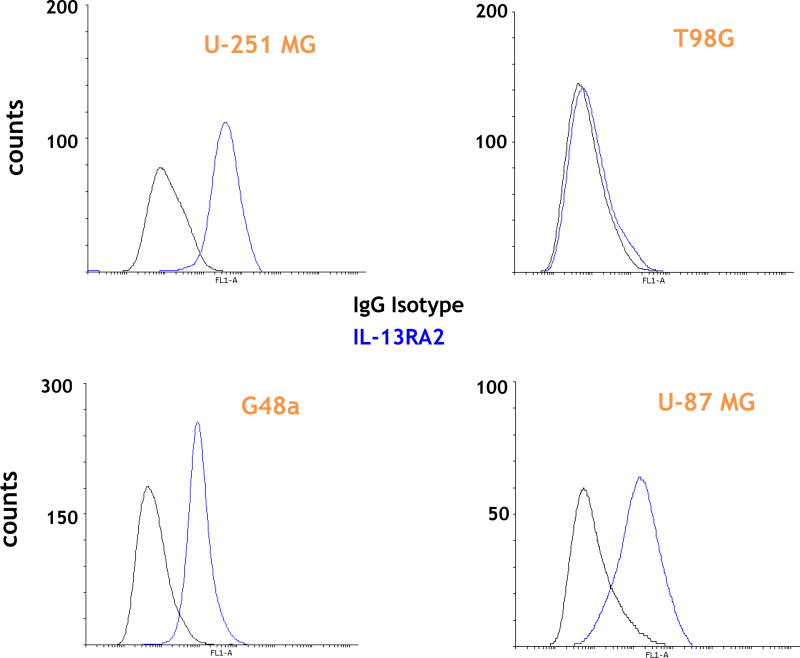
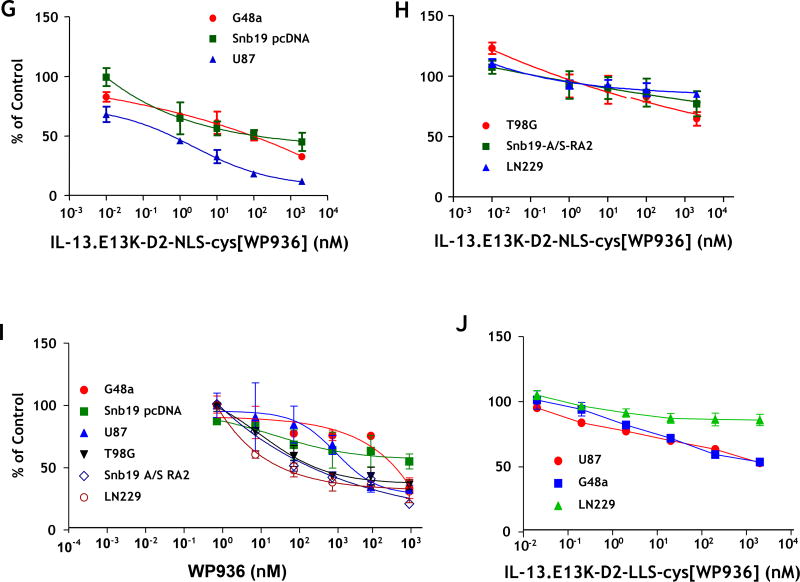
(A) Schemata of IL-13.E13K-D2-NLS-cys[WP936] and IL-13.E13K-D2-LLS-cys[WP936] drug conjugates. (B) Analysis of IL-13.E13K-D2-NLS-cys[WP936] drug conjugate. Coommassie blue stained SDS-PAGE of protein molecular weight standards (lane 1), drug-free IL-13.E13K-D2-NLS-cys (lane 2), and drug-conjugated IL-13.E13K-D2-NLS-cys (lane 3). Typhoon image (lanes 4–6) as in lanes 1–3. (C) Analysis of IL-13.E13K-D2-LLS-cys[WP936] drug conjugate. Coommassie blue stained SDS-PAGE of protein molecular weight standards (lane 1), drug-free (lane 2), and drug-conjugated IL-13.E13K-D2-LLS-cys (lane 3). Typhoon image (lanes 4–6) as in lanes 1–3. (D) Treatment of U-251-MG, G48a and T98G cells with IL-13.E13K-D2-NLS-cys[WP936] drug conjugate. Cells were visualized using phase contrast or fluorescent microscopy after 24 and 48 hrs of treatment. (E) Treatment of U-251-MG, G48a and T98G cells with IL-13.E13K-D2-LLS-cys[WP936] drug conjugate. Cells were visualized using phase contrast microscopy after 24 and 48 hrs of treatment. (F) Flow cytometry of IL-13RA2 expression in GBM cells. (G) Cytotoxic effect of IL-13.E13K-D2-NLS-cys[WP936] in U-87, G48a and SnB19 pcDNA cells GBM cells over-expressing IL-13RA2 and (H) in T98G, SnB19-A/SRA2 and LN229 cells expressing low levels of the receptor. MTT cell viability assay was used. (I) Cytotoxic effect of a modified unconjugated doxorubicin, WP936, in GBM cells. MTT assay was used. (J) Cytotoxicity of IL-13.E13K-D2-LLS-cys[WP936] in GBM cells.
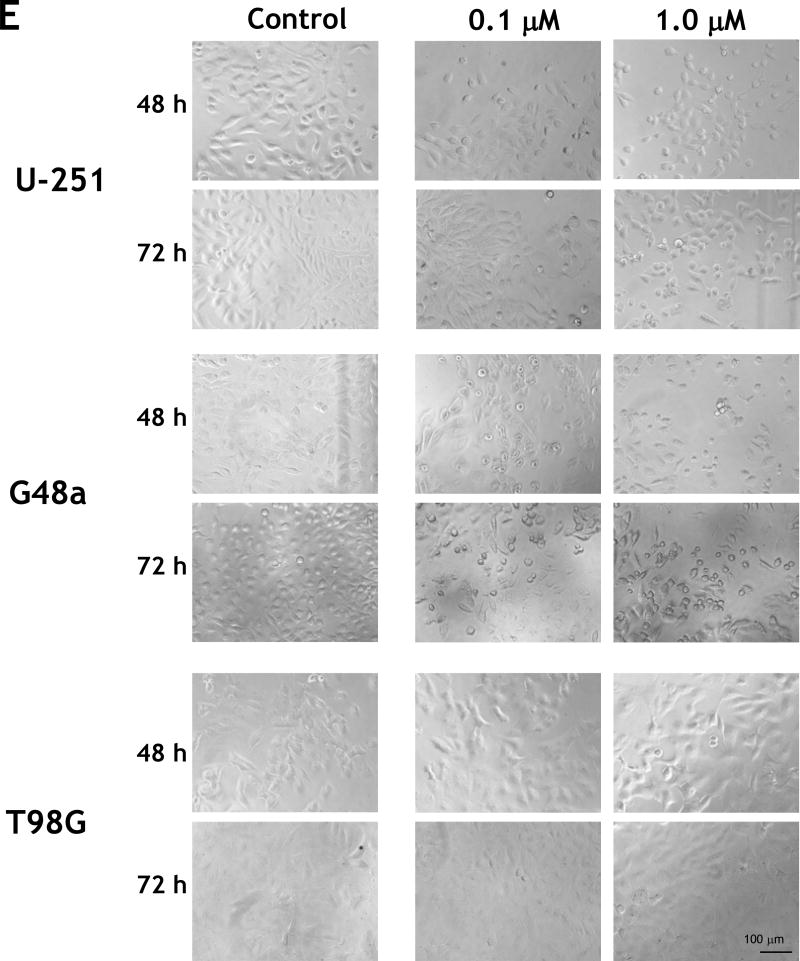
We further analyzed potential cytotoxic effect of IL-13.E13K-D2-NLS-cys[WP936] conjugate on U-251 MG cells that over-express the IL-13RA2. We found that the treatment increased cell rounding/blebbing resembling pro-apoptotic cells and a diminishing number of cells using phase contrast microscopy (Fig. 2D). Fluorescent detection of WP936 showed accumulation of the conjugate in treated cells, which was dose- and time-dependent (Fig. 2D). Similar effects were seen in the G48a GBM cells, also over-expressors of IL-13RA2 (Fig. 2D). In contrast, T98G cells that express low levels of the targeted receptor did not change significantly in response to treatment with the drug conjugate and little of the conjugate’s accumulation was observed in cells even at a 2 µM concentration after 48 hrs (Fig. 2D).
We noted only a slightly present cell rounding/blebbing in the U-251 MG and G48a cells after treatment with 2 µM of IL-13.E13K-D2-LLS-cys[WP936] and even much less at a lower dose (Fig. 2E). However, the conjugate clearly accumulated in the IL-13RA2 over-expressors like U-251 and G48a cells as evidenced by an increase in fluorescence, especially at a higher concentration of the conjugate used (Fig. 2E). No effect of IL-13.E13K-D2-LLS-cys[WP936] on cells was visible nor the accumulation of the conjugate was observed in T98G cells expressing lower amounts of IL-13RA2 (Fig. 2E). Thus, comparatively the conjugate delivering the cytotoxic load of a modified anthracyclin to cells nuclei (Fig. 2D) caused much greater effect in cells than the one directing the drug to lysosomes (Fig. 2E). Cells that are not over-expressing IL-13RA2 do not respond to such a treatment in a readily visible manner (Fig. 2D and E). We have performed additional flow cytometry experiment to ascertain that the T98G cells do not over-express IL-13RA2 while the other cells under study do (Fig. 2F)
WP936 conjugate is more cytotoxic than an unconjugated drug
We next examined the cytotoxicity of IL-13.E13K-D2-NLS-cys[WP936] on a panel of GBM cells using cell viability assay. This included cells over-expressing IL-13RA2 (G48a, SnB19 pcDNA, and U-87) (Fig. 2G) and cells expressing low levels of the receptor (T98G, SnB19-A/S-RA2 and LN229) (Fig. 2H); the SnB19-A/S-RA2 are the cells transfected with an anti-sense IL-13RA2 gene with subsequently diminished expression of the IL-13RA2 receptor, and the SnB19 pcDNA cells are empty-vector transfected.[48] A potent cytotoxic effect was observed in cells over-expressing IL-13RA2 with increasing concentration of IL-13.E13K-D2-NLS-cys[WP936] (Fig. 2G). On the other hand, IL-13.E13K-D2-NLS-cys[WP936] showed no or little cytotoxicity on cell lines expressing low levels of IL-13RA2 (Fig. 2H). We analyzed the cytotoxic effect of unconjugated WP936 in the same panel of GBM cells. All 7 GBM cell lines were treated with unconjugated WP936 and analyzed using MTS-PMS assay. All the cells responded to the drug independent of the status of IL-13RA2, but at significantly higher concentrations from the drug conjugate (Fig. 2I). We next analyzed the cytoxicity of IL-13.E13K-D2-LLS-cys[WP936] conjugate, on IL-13RA2 over-expressors (G48a and U87 cells) and low expressors (LN229 cells). As expected, this WP936 conjugated carrier protein was less active in affecting GBM cells over-expressing the receptor when compared to IL-13.E13K-D2-NLS-cys[WP936] and had no action on receptor-negative cells (Fig. 2J).
Cancer cell targeting fusion protein with pro-apoptotic peptide (KLAKLAK)2
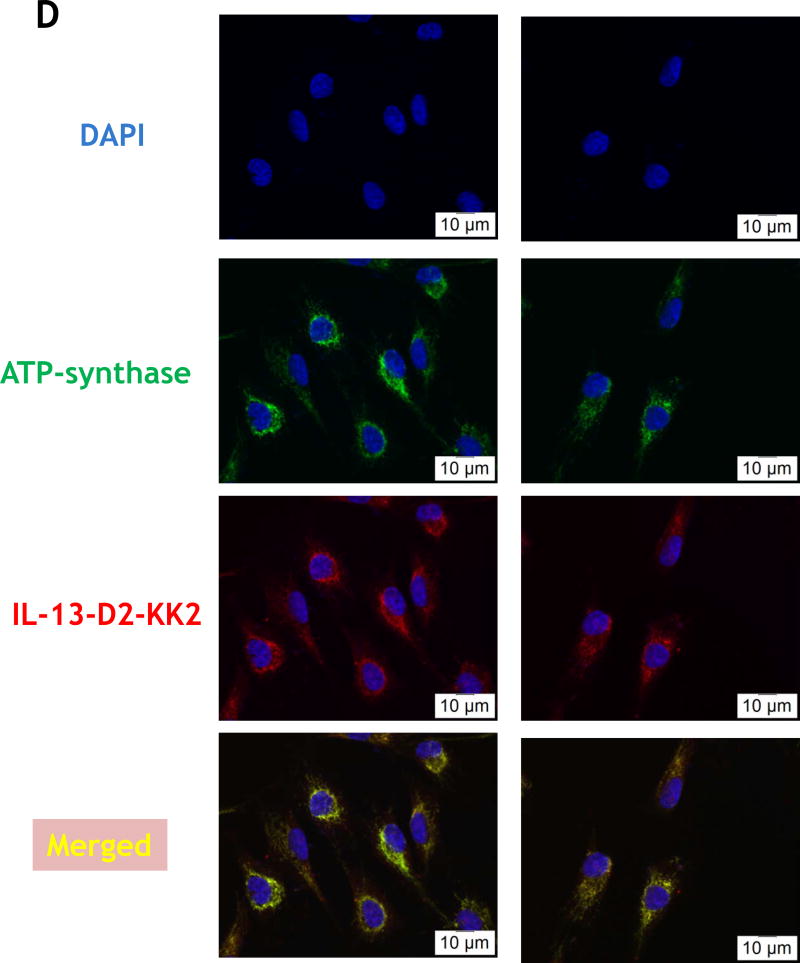
(KLAKLAK)2 is a 14-amino-acid amphipathic α-helical peptide which causes apoptosis in the cancer cell by mitochondrial-dependent apoptosis. Our first step was to design, synthesize and produce IL-13-D2-KK2 protein. IL-13-D2-KK2 protein was produced in BL21 E. coli cells and then highly purified using FPLC (Fig. 3A). Further, we wanted to study the intracellular pathway and subcellular localization of IL-13-D2-KK2 fusion protein. Hence, we biotin-conjugated the IL-13-D2-KK2 at the primary amines, and the biotinylated protein was detected using HRP-streptavidin (Fig. 3B). Biotinylated IL-13-D2-KK2 protein effectively internalized into the U-251 MG cells after 24 hrs of incubation (Fig. 3C).
Figure 3.
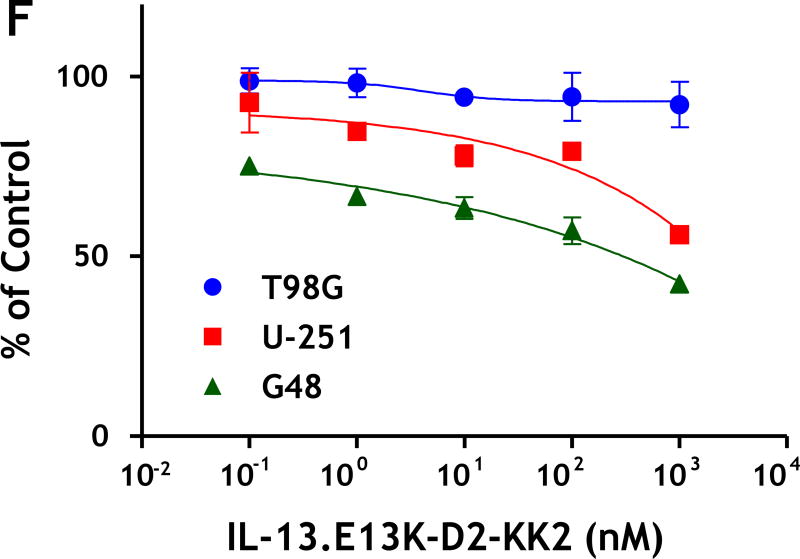
(A) Purified recombinant IL-13-D2-KK2 protein. SDS-PAGE stained with Coommassie blue. (B) Immunoblot of biotinylated IL-13-D2-KK2 probed with Streptavidin-HRP. (C) Cell internalization of biotinylated IL-13-D2-KK2. The protein was analyzed by using anti-streptavidin Alexa Fluor 555 and fluorescent microscopy. (D) U-251 cells were treated with biotinylated IL-13-D2-KK2 for 8 hrs and the co-localization of biotinylated IL-13-D2-KK2 with mitochondrial ATP-synthase enzyme was analyzed by confocal microscopy. (E) IL-13-D2-KK2 effect on GBM cell lines U-251 MG, G48a and T98G analyzed using phase contrast microscopy. (F) GBM cells were treated with IL-13-D2-KK2 for 72 hrs and the cytotoxicity was measured by an MTS assay.
ATP synthase is a large molecular weight enzyme embedded in the inner membrane of mitochondria. We further confirmed the internalization and localization of IL-13-D2-KK2 protein to the mitochondria by determining the co-localization of the protein with ATP synthase. We treated the U-251 MG cells at various time points (15 min, 4, and 8 hrs) with IL-13-D2-KK2 biotinylated protein followed by double immunofluorescence staining against streptavidin and ATP-synthase. The double immunofluorescence staining result suggest that the biotinylated protein is able to localize near mitochondrial membranes of U-251 MG cells as early as 4 hrs after treatment with IL-13-D2-KK2 (Fig. 3D).
We found that both U-251 MG and G48 cells showed pro-apoptotic effects such as cell rounding and blebbing after treatment with IL-13-D2-KK2 protein (Fig. 3C). T98G cells showed no such responses after treatment with the conjugated protein (Fig. 3C). Next we compared the cytotoxicity using the MTT cell viability assay where panel of GBM cell lines were incubated with increasing concentration of IL-13-D2-KK2 protein. We found that after 72 hrs there was an increase in cell cytotoxicity with increasing concentration of the conjugated protein in cell lines overexpressing IL-13RA2 (U-251 and U87), whereas no effect was seen in cells expressing low levels of IL-13RA2 (T98G) (Fig. 3F).
DISCUSSION
In the current study, we designed and successfully constructed novel recombinant proteins, containing IL-13.E13K ligand targeting IL-13RA2, and either lysosomal compartment through lysosomal localization signal of LAMP-1, IL-13.E13K-D2-LLS, or a pro-apoptotic peptide (KLAKLAK)2, IL-13.E13K-D2-KK2, targeting mitochondrial compartment of tumor cells. We demonstrated that single-chain recombinant proteins IL-13.E13K-D2-LLS-cys and IL-13-D2-KK2 specifically recognize GBM cells, are internalized and localized in the vicinity or with the targeted lysosomal and mitochondrial compartments without any detectable protein fragments transport to cells’ nuclei. For effective delivery of the cytotoxic load to tumor cells, we conjugated derivative of doxorubicin WP936 to the c-terminal cysteines introduced into our IL-13.E13K-D2-NLS-cys and IL-13.E13K-D2-LLS-cys fusion proteins. Not only did IL-13.E13K-D2-NLS-cys[WP936] and IL-13.E13K-D2-LLS-cys[WP936] bound to the targeted cells, but they were also able to deliver and accumulate WP936 within cells and their respective subcellular compartments. The cell viability assays showed that IL-13.E13K-D2-NLS-cys[WP936] inhibits growth/kills GBM cells over-expressing the targeted receptor more potently than either WP936 alone or e.g., IL-13.E13K-D2-LLS-cys[WP936].
In our previous study, we had developed a novel strategy based on genetic engineering of proteins and constructed a universal proteinaceous molecule, which recognizes cancer cells and travels intracellularly specifically to the nucleus.[29] This recombinant protein targets IL-13RA2 on GBM cells with a modified receptor ligand, IL-13.E13K and a specialized cytosol translocation bacterial toxin domain D2 of Pseudomonas exotoxin A and consists of a nuclear localization signal from the SV40 T antigen to form the IL-13.E13K-D2-NLS nuclear delivery vector. We documented directly the journey of a designer protein-based vector from the cell surface to the nucleus of GBM cells.[29] This very strategy was successfully utilized in the current study for delivery of proteins specifically to lysosomal and mitochondrial compartments, respectively. We have thus achieved the development of higher multi-specificity order of targeting drug conjugates delivering cytotoxic load in a pre-programmed way into cellular compartments. We have succeeded in targeting the lysosomal compartment of tumor cells. An earlier study investigating the presence of lysosomes in astrocytic brain tumors has suggested high amounts of lysosomes present in GBM tumors.[50] Hence, targeting of the lysosomes in which drugs could be hydrolyzed to active forms could potentially be a promising therapeutic strategy against GBM. In the present study, lysosomal targeting with WP936 served more of a purpose of contrasting control in the experiments, because the anthracyclin exerts its action in the nucleus. However, some degree of hydrolysis, freeing and finding its way to the nucleus by WP936, especially during prolonged incubation and accumulation of drug conjugates in tumor cells, can be expected as our results would attest.
The cellular localization experiments strongly suggest a possibility of attaching various labels/drugs/dyes to our specific targeted proteins for efficient delivery either to nuclei or lysosomes or mitochondria. We had previously discovered a possibility of targeting cancer cells and delivery of peptides/proteins into the cell cytosol using modified PE.[30] Others have also applied this approach using Diphtheria toxin fragments.[51] In a follow up of these original experiments, we were able to show the delivery of our subcellular compartment-directed construct, IL-13.E13K-D2-NLS, to the nucleus. Next, we conjugated IL-13.E13K-D2-NLS with a derivative of doxorubicin named WP-936, to produce IL-13.E13K-D2-NLS-cys[WP936]. A general idea behind making these novel drug conjugates was to specifically recognize and affect GBM cells while reducing the access of a chemotherapeutic to normal tissues. Our data demonstrate significant increase in cytotoxicity with this drug conjugate construct in cells overexpressing IL-13RA2. On the other hand, WP936 conjugation with IL-13.E13K-D2-LLS-cys protein, showed some cytotoxic effect on targeted cells, but less pronounced than by IL-13.E13K-D2-NLS-cys[WP936]. Both of these recombinant proteins demonstrated much lower or no cytotoxicity in tumor cells expressing low levels of IL-13RA2. Therefore, our novel drug conjugate represents a template for development of specific cytotoxic anti-GBM agents. Using a similar approach, a monoclonal antibody linked to the NLS peptide conjugated to the Auger electron emitter was able to recognize cells and cause cytotoxicity.[52,53] More importantly, a lower dose of these protein agent-WP936 conjugates was required to achieve cancer cell cytotoxicity compared to the free WP936 drug. A major potential advantage of these agents would be that a lower dose of the WP936 would be required for chemotherapy, leading to lower systemic load, and likely reduced side-effects than free WP936.
Another emerging targeted drug delivery system in the treatment of cancer is the mitochondrial drug delivery system. (KLAKLAK)2 proapoptotic peptide was found to be cytotoxic to bacteria[41], but it cannot efficiently permeate across eukaryotic plasma membrane and hence exhibits low mammalian cell cytotoxicity. However, due to the similarities between the bacterial cell membrane and eukaryotic mitochondrial membrane, once it enters the cell it causes cytotoxicity by disrupting the mitochondrial membrane. A recent study conducted using KLA, a variant of (KLAKLAK)2 proapoptotic peptide, fused with a linear tumor-penetrating homing peptide iRGD demonstrated that it is internalized into cultured tumor cells.[54] Once inside the cells, the peptide leads to apoptosis through both the mitochondrial-induced apoptotic pathway and the death receptor pathway. Another study showed that the proapoptotic peptide (KLAKLAK)2 conjugated to penetratin led to selective inhibition of tumor cell growth.[55] To promote delivery of (KLAKLAK)2 specifically to the GBM cells, we generated a recombinant protein IL-13-D2-KK2, which targets IL-13RA2. After biotin conjugation and detection with streptavidin, we showed that IL-13-D2-KK2 was effectively internalized into the GBM cells and co-localized with ATP-synthase. We also demonstrated that IL-13-D2-KK2 has cytotoxic properties against a panel of GBM cell lines, which overexpresses IL-13RA2. Hence our construct could potentially be used as a pro-apoptotic anti-cancer therapeutic agent, which will be specific for targeting mitochondria in GBM tumors.
In summary, in this proof-of-principle study we have generated novel multi-specificity fusion recombinant proteins for targeting lysosomal and mitochondrial compartments of IL-13RA2 positive GBM tumor cells. We have also produced first-in-class drug conjugates in which a chemotherapeutic is delivered to the site of its intracellular action. Our drug candidates were able to successfully carry the WP936 cargo to the intracellular compartments of the cell and caused significant cytotoxicity. In addition, we have demonstrated that a specifically delivered proapoptotic peptide caused cytotoxic effect in the GBM cells. Our novel drug conjugates/fusion proteins may thus provide a highly specific and thus safer therapeutic option than the existing ones. We will test these and other chemotherapeutic drug conjugates for their stability, pharmacokinetics and potential to cross the blood-brain tumor barrier. This is in consideration that the conjugates might be delivered systemically in patients. Also, recent advances could allow more efficient penetration of such drugs given systemically through an opening of the blood-brain barrier as reviewed in Rodriguez et al.[56] The drug conjugates of this type can be also administered to brain tumor patients loco-regionally, directly to tumor bed using convection-enhanced delivery[57]. Local administration can be repeated many times and drugs can be infused for prolonged periods of time, which would represent a desirable feature of the treatment plan.[58,59] Future pre-clinical studies will pave the way for clinical evaluation of the conjugates using optimal mean of their administration.
Acknowledgments
Various assays were performed with the help of the Shared Resources of the Cancer Center Support Grant (P30 CA12197) to the Wake Forest Baptist Medical Center Comprehensive Cancer Center: Flow Cytometry, Cell and Viral Vector Laboratory, Tumor Tissue and Pathology, and Cellular Imaging.
This work was supported by an NIH grant CA71745 to WD
Conflict of interest: Patent applications have been filed or patents obtained by Wake Forest University related to the subject of this manuscript. Dr. Waldemar Debinski is a consulting scientific advisor and a shareholder in Targepeutics, Inc.
Footnotes
Preliminary findings related to this work were presented at the 4th Quadrennial Meeting of the World Federation of Neuro-Oncology held in conjunction with the 2013 Scientific Meeting and Education Day of the Society for Neuro-Oncology, San Francisco, 2013.
Clinical trial registration: Not applicable
Criteria for inclusion in the authors’ list: PS has designed and performed several experiments and assembled the first full draft of the manuscript; YAC has designed and performed multiple experiments and contributed with the write ups; HP has proposed, designed and performed several experiments related to KLAK peptide; DH has supervised much of the work, designed and performed several experiments and contributed to the writing of the manuscript; IF has generated the modified chemotherapeutics and contributed to the design of experiments; WP contributed to the overall design of drug conjugates and with the interpretation of the results; WD has provided ideas for most of the drug conjugates, supervised all the work performed and edited the manuscript.
The manuscript includes data that have not been published or are under consideration to be published by another journal.
References
- 1.Stupp R, Taillibert S, Kanner AA, Kesari S, Steinberg DM, Toms SA, et al. Maintenance Therapy With Tumor-Treating Fields Plus Temozolomide vs Temozolomide Alone for Glioblastoma: A Randomized Clinical Trial. JAMA. 2015;314(23):2535–43. doi: 10.1001/jama.2015.16669. [DOI] [PubMed] [Google Scholar]
- 2.Stupp R, Hegi ME, Mason WP, van den Bent MJ, Taphoorn MJ, Janzer RC, et al. Effects of radiotherapy with concomitant and adjuvant temozolomide versus radiotherapy alone on survival in glioblastoma in a randomised phase III study: 5-year analysis of the EORTC-NCIC trial. Lancet Oncol. 2009;10(5):459–66. doi: 10.1016/S1470-2045(09)70025-7. [DOI] [PubMed] [Google Scholar]
- 3.Stupp R, Mason WP, van den Bent MJ, Stupp R, Mason WP, van den Bent MJ, Weller M, Fisher B, Taphoorn MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352:987–96. doi: 10.1056/NEJMoa043330. [DOI] [PubMed] [Google Scholar]
- 4.Chan MD, Tatter SB, Lesser G, Shaw EG. Radiation oncology in brain tumors: current approaches and clinical trials in progress. Neuroimaging Clin N Am. 2010;20(3):401–8. doi: 10.1016/j.nic.2010.04.005. [DOI] [PubMed] [Google Scholar]
- 5.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: a summary. Acta Neuropathol. 2016;131(6):803–20. doi: 10.1007/s00401-016-1545-1. [DOI] [PubMed] [Google Scholar]
- 6.Sanai N, Berger MS. Recent surgical management of gliomas. Adv Exp Med Biol. 2012;746:12–25. doi: 10.1007/978-1-4614-3146-6_2. [DOI] [PubMed] [Google Scholar]
- 7.Debinski W, Gibo DM, Hulet SW, Connor JR, Gillespie GY. Receptor for interleukin 13 is a marker and therapeutic target for human high-grade gliomas. Clin Cancer Res. 1999;5:985–90. [PubMed] [Google Scholar]
- 8.Debinski W. An immune regulatory cytokine receptor and glioblastoma multiforme: an unexpected link. Crit Rev Oncog. 1998;9:255–68. [PubMed] [Google Scholar]
- 9.Wykosky J, Gibo DM, Stanton C, Debinski W. IL-13 Receptor alpha-2, EphA2, and Fra-1 as molecular denominators of high-grade astrocytomas and specific targets for combinatorial therapy. Clin. Cancer Res. 2008;14:199–208. doi: 10.1158/1078-0432.CCR-07-1990. [DOI] [PubMed] [Google Scholar]
- 10.Debinski W, Gibo DM. Molecular expression analysis of restrictive receptor for interleukin 13, a brain tumor-associated cancer/testis antigen. Mol Med. 2000;6:440–9. [PMC free article] [PubMed] [Google Scholar]
- 11.Mintz A, Gibo DM, Slagle-Webb B, Christensen ND, Debinski W. IL-13Rα2 is a Glioma-Restricted Receptor for Interleukin-13. Neoplasia. 2002;4:388–99. doi: 10.1038/sj.neo.7900234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Debinski W, Gibo DM, Obiri NI, Kealiher A, Puri RK. Novel anti-brain tumor cytotoxins specific for cancer cells. Nat Biotechnol. 1998;16:449–53. doi: 10.1038/nbt0598-449. [DOI] [PubMed] [Google Scholar]
- 13.Sampson JH, Archer G, Pedain C, Wembacher-Schröder E, Westphal M, et al. Poor drug distribution as a possible explanation for the results of the PRECISE trial. J Neurosurg. 2010;113:301–9. doi: 10.3171/2009.11.JNS091052. [DOI] [PubMed] [Google Scholar]
- 14.Kunwar S, Chang S, Westphal M, Vogelbaum M, Sampson J, Barnett G, et al. Phase III randomized trial of CED of IL13-PE38QQR vs Gliadel wafers for recurrent glioblastoma. PRECISE Study Group. Neuro Oncol. 2010;12(8):871–81. doi: 10.1093/neuonc/nop054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson J, Debinski W. Mutants of interleukin 13 with altered reactivity toward IL13 receptors. J Biol Chem. 1999;274:29944–50. doi: 10.1074/jbc.274.42.29944. [DOI] [PubMed] [Google Scholar]
- 16.Keizer HG, Pinedo HM, Schuurhuis GJ, Joenje H. Doxorubicin (adriamycin): A critical review of free radical-dependent mechanisms of cytotoxicity. Pharmacol Ther. 1990;47:219–31. doi: 10.1016/0163-7258(90)90088-j. [DOI] [PubMed] [Google Scholar]
- 17.Minotti G, Menna P, Salvatorelli E, Cairo G, Gianni L. Anthracyclines: Molecular Advances and Pharmacologic Developments in Antitumor Activity and Cardiotoxicity. Pharmacol Rev. 2004;56:185–229. doi: 10.1124/pr.56.2.6. [DOI] [PubMed] [Google Scholar]
- 18.Marina NM, Cochrane D, Harney E, Zomorodi K, Blaney S, Winick N, et al. Dose escalation and pharmacokinetics of pegylated liposomal doxorubicin (Doxil) in children with solid tumors: a pediatric oncology group study. Clin Cancer Res. 2002;8:413–8. [PubMed] [Google Scholar]
- 19.Ruggiero A, Rosa GD, Rizzo D, Leo A, Maurizi P, Nisco AD, et al. Myocardial performance index and biochemical markers for early detection of doxorubicin-induced cardiotoxicity in children with acute lymphoblastic leukaemia. Int J Clin Oncol. 2012;18:927–33. doi: 10.1007/s10147-012-0458-9. [DOI] [PubMed] [Google Scholar]
- 20.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65:157–70. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 21.Vejpongsa P, Yeh ETH. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64:938–45. doi: 10.1016/j.jacc.2014.06.1167. [DOI] [PubMed] [Google Scholar]
- 22.Couzin-Frankel J. Breakthrough of the year 2013. Cancer immunotherapy. Science. 2013;20;342(6165):1432–3. doi: 10.1126/science.342.6165.1432. [DOI] [PubMed] [Google Scholar]
- 23.Tuma RS. Enthusiasm for antibody-drug conjugates. J Natl Cancer Inst. 2011;103(20):1493–4. doi: 10.1093/jnci/djr422. [DOI] [PubMed] [Google Scholar]
- 24.Moskowitz CH, Nademanee A, Masszi T, Agura E, Holowiecki J, Abidi MH, et al. Brentuximab vedotin as consolidation therapy after autologous stem-cell transplantation in patients with Hodgkin's lymphoma at risk of relapse or progression (AETHERA): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet. 2015;385(9980):1853–62. doi: 10.1016/S0140-6736(15)60165-9. [DOI] [PubMed] [Google Scholar]
- 25.Younes A, Bartlett NL, Leonard JP, Kennedy DA, Lynch CM, Sievers EL, et al. Brentuximab Vedotin (SGN-35) for Relapsed CD30-Positive Lymphomas. N Engl J Med. 2010;363:1812–21. doi: 10.1056/NEJMoa1002965. [DOI] [PubMed] [Google Scholar]
- 26.Chari RV, Martell BA, Gross JL, Cook SB, Shah SA, Blättler WA, et al. Immunoconjugates containing novel maytansinoids: promising anticancer drugs. Cancer Res. 1992;52:127–31. [PubMed] [Google Scholar]
- 27.Verma S, Miles D, Gianni L, Krop IE, Welslau M, Baselga J, et al. EMILIA Study Group. Trastuzumab emtansine for HER2-positive advanced breast cancer. N Engl J Med. 2012;367(19):1783–91. doi: 10.1056/NEJMoa1209124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Goeij BE, Lambert JM. New developments for antibody-drug conjugate-based therapeutic approaches. Curr Opin Immunol. 2016;40:14–23. doi: 10.1016/j.coi.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 29.Pandya H, Gibo DM, Debinski W. Molecular Targeting of Intracellular Compartments Specifically in Cancer Cells. Genes Cancer. 2010;1:421–33. doi: 10.1177/1947601910375274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pandya H, Debinski W. Toward intracellular targeted delivery of cancer therapeutics: progress and clinical outlook for brain tumor therapy. BioDrugs. 2012;26(4):235–44. doi: 10.2165/11631600-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Debinski W, Obiri NI, Powers SK, Pastan I, Puri RK. Human glioma cells overexpress receptors for interleukin 13 and are extremely sensitive to a novel chimeric protein composed of interleukin 13 and pseudomonas exotoxin. Am Assoc Cancer Res. 1995;1:1253–8. [PubMed] [Google Scholar]
- 32.Raucher D, Bidwell G, III, Priebe W, Fokt I. Thermally-targeted delivery of medicaments including doxorubicin. 20100022466 US Patent.
- 33.Bidwell GL, Fokt I, Priebe W, Raucher D. Development of elastin-like polypeptide for thermally targeted delivery of doxorubicin. Biochem Pharmacol. 2007;73:620–31. doi: 10.1016/j.bcp.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 34.Caput D, Laurent P, Kaghad M, Lelias J-M, Lefort S, Vita N, et al. Cloning and Characterization of a Specific Interleukin (IL)-13 Binding Protein Structurally Related to the IL-5 Receptor α Chain. J Biol Chem. 1996;271:16921–6. doi: 10.1074/jbc.271.28.16921. [DOI] [PubMed] [Google Scholar]
- 35.Donaldson DD, Whitters MJ, Fitz LJ, Neben TY, Finnerty H, Henderson SL, et al. The Murine IL-13 Receptor α2: Molecular Cloning, Characterization, and Comparison with Murine IL-13 Receptor α1. J Immunol. 1998;161:2317–24. [PubMed] [Google Scholar]
- 36.Kornmann M, Kleeff J, Debinski W, Korc M. Pancreatic cancer cells express interleukin-13 and-4 receptors, and their growth is inhibited by Pseudomonas exotoxin coupled to interleukin-13 and-4. Anticancer Res. 1999;19(1A):125–31. [PubMed] [Google Scholar]
- 37.Barderas R, Bartolome RA, Fernandez-Acenero MJ, Torres S, Casal JI. High expression of IL-13 receptor alpha2 in colorectal cancer is associated with invasion, liver metastasis, and poor prognosis. Cancer Res. 2012;72(11):2780–90. doi: 10.1158/0008-5472.CAN-11-4090. [DOI] [PubMed] [Google Scholar]
- 38.Debinski W, Levin RJ, Miner R, Puri RK. Head and neck cancer cell lines express receptor for interleukin 13 and are killed by chimeric proteins composed of interleukin 13 and Pseudomonas exotoxin. 4th International Conference on Head and Neck Cancer; July 28-August 1, 1996; Toronto. [Google Scholar]
- 39.Zhao Z, Wang L, Xu W. IL-13Ralpha2 mediates PNR-induced migration and metastasis in ERalpha-negative breast cancer. Oncogene. 2015;34(12):1596–607. doi: 10.1038/onc.2014.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Beard RE, Abate-Daga D, Rosati SF, Zheng Z, Wunderlich JR, Rosenberg SA, et al. Gene expression profiling using nanostring digital RNA counting to identify potential target antigens for melanoma immunotherapy. Clin Cancer Res. 2013;19(18):4941–50. doi: 10.1158/1078-0432.CCR-13-1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Javadpour MM, Juban MM, Lo WC, Bishop SM, Alberty JB, Cowell SM, et al. De novo antimicrobial peptides with low mammalian cell toxicity. J Med Chem. 1996;39:3107–13. doi: 10.1021/jm9509410. [DOI] [PubMed] [Google Scholar]
- 42.Ellerby HM, Arap W, Ellerby LM, Kain R, Andrusiak R, Rio GD, et al. Anti-cancer activity of targeted pro-apoptotic peptides. Nat Med. 1999;5:1032–8. doi: 10.1038/12469. [DOI] [PubMed] [Google Scholar]
- 43.Mai JC, Mi Z, Kim SH, Ng B, Robbins PD. A proapoptotic peptide for the treatment of solid tumors. Cancer Res. 2001;61:7709–12. [PubMed] [Google Scholar]
- 44.Madhankumar AB, Mintz A, Debinski W. Alanine-scanning mutagenesis of alpha-helix D segment of interleukin-13 reveals new functionally important residues of the cytokine. J Biol Chem. 2002;277:43194–205. doi: 10.1074/jbc.M205047200. [DOI] [PubMed] [Google Scholar]
- 45.https://www.thermofisher.com/us/en/home/life-science/protein-biology/protein-biology-learning-center.html#/legacy=www.piercenet.com
- 46.Dreher MR, Raucher D, Balu N, Michael Colvin O, Ludeman SM, et al. Evaluation of an elastin-like polypeptide-doxorubicin conjugate for cancer therapy. J Control Release. 2003;91:31–43. doi: 10.1016/s0168-3659(03)00216-5. [DOI] [PubMed] [Google Scholar]
- 47.Willner D, Trail PA, Hofstead SJ, King HD, Lasch SJ, Braslawsky GR, et al. (6-Maleimidocaproyl)hydrazone of doxorubicin--a new derivative for the preparation of immunoconjugates of doxorubicin. Bioconjug Chem. 1993;4:521–7. doi: 10.1021/bc00024a015. [DOI] [PubMed] [Google Scholar]
- 48.Pandya H, Gibo DM, Garg S, Kridel S, Debinski W. An interleukin 13 receptor α 2–specific peptide homes to human Glioblastoma multiforme xenografts. Neuro-Oncol. 2012;14:6–18. doi: 10.1093/neuonc/nor141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida M, Muneyuki E, Hisabori T. ATP synthase--a marvellous rotary engine of the cell. Nat Rev Mol Cell Biol. 2001;2(9):669–77. doi: 10.1038/35089509. [DOI] [PubMed] [Google Scholar]
- 50.Jensen SS, Aaberg-Jessen C, Christensen KG, Kristensen B. Expression of the lysosomal-associated membrane protein-1 (LAMP-1) in astrocytomas. Int J Clin Exp Pathol. 2013;6:1294–305. [PMC free article] [PubMed] [Google Scholar]
- 51.Stenmark H, Moskaug JO, Madshus IH, Sandvig K, Olsnes S. Peptides fused to the amino-terminal end of diphtheria toxin are translocated to the cytosol. J Cell Biol. 1991;113:1025–32. doi: 10.1083/jcb.113.5.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Costantini DL, Chan C, Cai Z, Vallis KA, Reilly RM. 111In-Labeled Trastuzumab (Herceptin) Modified with Nuclear Localization Sequences (NLS): An Auger Electron-Emitting Radiotherapeutic Agent for HER2/neu-Amplified Breast Cancer. J Nucl Med. 2007;48:1357–68. doi: 10.2967/jnumed.106.037937. [DOI] [PubMed] [Google Scholar]
- 53.Rosenkranz AA, Vaidyanathan G, Pozzi OR, Lunin VG, Zalutsky MR, Sobolev AS. Engineered modular recombinant transporters: application of new platform for targeted radiotherapeutic agents to alpha-particle emitting 211At. Int J Radiat Oncol Biol Phys. 2008;72:193–200. doi: 10.1016/j.ijrobp.2008.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Qifan W, Fen N, Ying X, Xinwei F, Jun D, Ge Z. iRGD-targeted delivery of a pro-apoptotic peptide activated by cathepsin B inhibits tumor growth and metastasis in mice. Tumor Biol. 2016;37(8):10643–52. doi: 10.1007/s13277-016-4961-x. [DOI] [PubMed] [Google Scholar]
- 55.Alves ID, Carré M, Montero M-P, Castano S, Lecomte S, Marquant R, et al. A proapoptotic peptide conjugated to penetratin selectively inhibits tumor cell growth. Biochim Biophys Acta BBA - Biomembr. 2014;1838:2087–98. doi: 10.1016/j.bbamem.2014.04.025. [DOI] [PubMed] [Google Scholar]
- 56.Rodriguez A, Tatter SB, Debinski W. Neurosurgical Techniques for Disruption of the Blood-Brain Barrier for Glioblastoma Treatment. Pharmaceutics. 2015;7(3):175–87. doi: 10.3390/pharmaceutics7030175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Debinski W, Tatter SB. Convection-enhanced delivery for the treatment of brain tumors. Expert Rev Neurother. 2009;9(10):1519–27. doi: 10.1586/ern.09.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown CE, Alizadeh D, Starr R, Weng L, Wagner JR, Naranjo A, et al. Regression of Glioblastoma after Chimeric Antigen Receptor T-Cell Therapy. N Engl J Med. 2016;375(26):2561–9. doi: 10.1056/NEJMoa1610497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barua NU, Hopkins K, Woolley M, O'Sullivan S, Harrison R, Edwards RJ, et al. A novel implantable catheter system with transcutaneous port for intermittent convection-enhanced delivery of carboplatin for recurrent glioblastoma. Drug Deliv. 2016;23(1):167–73. doi: 10.3109/10717544.2014.908248. [DOI] [PubMed] [Google Scholar]