Abstract
To provide information on the development of a gynecologic oncology training program in a low-resource setting in Kenya.
This is a review of a collaboration between Kenyan and North American physicians who worked together to develop a gynecologic oncology training in Kenya. We review the published data on the increase of cancer incidence in sub-Saharan Africa and outline the steps that were taken to develop this program.
The incidence of cervical cancer in Kenya is very high and is the leading cause of cancer mortality in Kenya. WHO identifies cancer as a new epidemic affecting countries in sub-Saharan Africa. In Kenya, a country of 45 million, there is limited resources to diagnose and treat cancer. In 2009 in western Kenya, at Moi University there was no strategy to manage oncology in the Reproductive Health department. There was only 1 gynecologic oncologists in Kenya in 2009. A collaboration between Canadian and Kenya physicians resulted in development of a gynecologic oncology clinical program and initiation of fellowship training in Kenya. In the past 4 years, five fellows have graduated from a 2 year fellowship training program. Integration of data collection on all the patients as part of this program provided opportunities to do clinical research and to acquire peer reviewed grants.
This is the first recognized fellowship training program in sub-Saharan Africa outside of South Africa. It is an example of a collaborative effort to improve women's health in a low-resource country. This is a Kenyan managed program through Moi University. These subspecialty trained doctors will also provide advice that will shape health care policy and provide sustainable expertise for women diagnosed with a gynecologic cancer.
Highlights
-
•
Cervix cancer incidence and mortality is extremely high in sub-Saharan Africa.
-
•
There is limited medical expertise to manage cancer in Kenya.
-
•
We initiated a gynecologic oncology fellowship training program in Kenya and to date 5 been trained.
-
•
The first structured care model developed for women with gynecologic cancers in Kenya
-
•
The first African gynecologic oncology fellowship training program outside of South Africa
1. The problem
It is well documented that cancer incidence and mortality are increasing more rapidly in low and middle-income (LMIC) compared to high-income countries (Farmer et al., 2010). By 2020 it is predicted that 70% of all new cancers will occur in LMIC (Farmer et al., 2010).
International Agency for Cancer Research, Globocan, WHO and Global Cancer Statistics (Torre et al., 2015, World Health Organization, n.d., International Agency for Research on Cancer, n.d.) estimate that there were approximately 850,000 new cancers in Africa in 2012 and that this number will grow to 1056, 000 by 2020. In sub-Saharan Africa the mortality rate for those diagnosed with a cancer is estimated to be 72%, which is almost double, the rate for developed countries. In addition to a lack of resources, the high mortality rates in sub-Saharan Africa are also related to a lack of awareness among some policy makers and the general public (Jemal et al., 2012). Over the last 30 plus years' in Africa, public health priorities have focused on infectious diseases including HIV/AIDS, tuberculosis and malaria. The WHO has now identified non-communicable diseases including cancer as a new epidemic in sub-Saharan Africa (Jemal et al., 2012). They also identified that there is a severe shortage of health professional in sub-Saharan Africa, which has 24% of the world's health burden and only 3% of the World's Health Care workers (The World Health Report 2006-Working Together for Health).
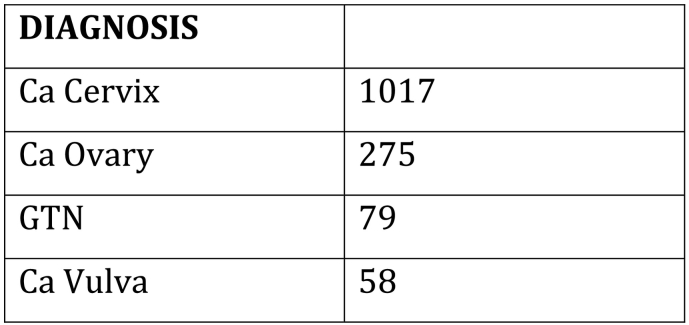
This paper is focused on Kenya, an East African country where the incidence of cervix cancer is high and is the leading cause of cancer mortality in women. The age-adjusted incidence of cervix cancer in Kenya is 40.1 per 100,000 (International Agency for Research on Cancer, n.d.), and the mortality is 21.8 per 100,000 women. In contrast the age-adjusted incidence and mortality in North America is 6.6 and 2.6 respectively (International Agency for Research on Cancer, n.d.). This discrepancy in incidence between these regions is related to a lack of screening, a limited awareness of cancer in the general population and avoidant behavior associated with a fear of cancer and a stigma that is associated with it (Jemal et al., 2012). The high cervical cancer mortality rate in Kenya is related to the fact that most women present with advance stage of their cancer, to limitations in treatment capacity, lack of facilities, lack of medical expertise, and an extreme shortage of radiation therapy (Orang'o et al., 2016b, Abdel-Wahab et al., 2013). In Kenya, a country of 45 million people, there is only 1 functional public radiation machine (cobalt-60) (Grover et al., 2014). This facility is located in Nairobi and its accessibility for the majority of women is extremely limited where patients wait as long as 6–12 months for treatment. Lack of access to radiation influences treatment strategies for cervical cancer patients in low resource settings. Both incidence and mortality are also related to the high incidence of HIV/AIDS in western Kenya (Jemal et al., 2012, Wools-Kaloustian et al., 2006). Other gynecologic cancers also need treatment and include ovarian, vulvar, endometrial cancer and uterine sarcomas. Gestational trophoblastic neoplasia, is another common cancer in western Kenya and can be cured if treated correctly.
In 2009 there were no trained gynecologic oncologists in western Kenya and only one or two in Nairobi who had been trained many years prior to 2009. General gynecologists in Kenya had little understanding of oncology. They may have seen many cancer patients during their training but they never spent much time caring for these women, as almost all were palliative when they presented to the hospital. In western Kenya neither the government nor the hospital, had a strategy for cancer care. At our institution Moi Teaching and Referral Hospital (MTRH) most women diagnosed with cervix cancer presented to the emergency departments with symptoms including bleeding, discharge and pain and almost all had advanced stage 3B or worse disease. Many present with very low hemoglobins and symptoms of anemia. Their treatment consisted of a blood transfusion if necessary, after which they were discharged home. They were offered appointments but no treatment was available and few returned for their appointments.
The problem in Kenya in 2009 was a high incidence and mortality of gynecological cancers and the complete absence of effective screening and treatment in an environment of very limited resources. The gap between patient needs and physician and hospital capability was very large. This paper describes our efforts over the past 8 years to improve this situation and train gynecologist to deliver comprehensive cancer care to women.
2. The solution: development of a gynecologic oncology program
In 2008 the University of Toronto joined the AMPATH (Academic Model Providing Access to Healthcare) organization and became the North American lead in Reproductive Health at Moi University. AMPATH is a consortium of North American universities and medical schools led by Indiana University and in collaboration with Moi University and Moi Teaching and Referral Hospital they provide access to health care in western Kenya whose population is approximately 5 million. Through grants from PEPFAR, USAID and others AMPATH has established a network of clinics that provide HIV care to thousands of people in western Kenya. AMPATH is viewed as being one of the most successful HIV/AIDS programs in Africa (Wools-Kaloustian et al., 2006).
AMPATH's organization enabled the gynecologic oncology program to develop without needing to reinvent the wheel, and without having to navigate different political aspects to delivering healthcare. Gynecologic oncology was able to use and leverage the AMPATH infrastructure in building the cervical cancer-screening program in a similar fashion to how the program in Zambia was developed (Parham et al., 2015).
2.1. Initial phase
In 2009, a cervical cancer screening study was initiated at MTRH to compare Pap smear, testing to VIA (visualization and Inspection with Ascetic acid) (Grover et al., 2014). This study offered screening only to HIV women attending the center for their monthly examination and anti-retroviral medications (Khozaim et al., 2014). In 2008 a gynecologist from MTRH visited the University of Toronto as an observer and the following year a gynecologic oncologist visited MTRH with the objective to teach the gynecologists how to do a LEEP excision on women who were identified with cervical abnormalities during screening in the outpatient clinic.
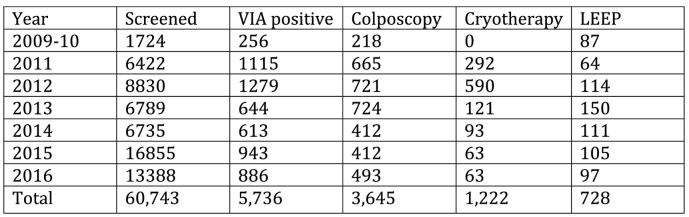
Two observations resulted in clinical changes at MTRH, which in retrospect were the initial steps to developing a gynecologic oncology program. The first was identification that there was no coordinated approach to manage cancer patients at MTRH. This led to a recommendation that the gynecologist start a specific oncology clinic. In that clinic all gynecologic oncology patients were seen by the same two gynecologists, which provided them the opportunity to gain experience through their consistency in following up and consulting on new patients. The second change was to convince the hospital and AMPATH to initiate a cervical cancer-screening clinic that would extend past the completion of the study and include both HIV affected and non-affected women. This required both to commit resources for cervical cancer screening. Over the last 6 years VIA screening has expanded to include more than 60,000 women (Orang'o et al., 2016a) (Fig. 1).
Fig. 1.
Cervical cancer screening.
2.1.1. Surgical training
In the first year they screened 1000 new patients, using VIA, and they began to identify women with early stage (stage 1) cancers that could be treated surgically. Identifying stage 1 cervical cancers was new to these gynecologists something they had not seen and something we did not anticipate in advance. This was a tipping point because no one at MTRH knew how to do a radical hysterectomy and in fact none had even seen one being done.
The two Kenyan gynecologists working in the oncology clinic wanted to learn how to do a radical hysterectomy so that they could treat the women identified with early stage disease. It was an exciting time for them because for the first time they saw an opportunity to cure patients with cervix cancer. Teaching modules were developed by The Society of Gynecologic Oncology of Canada, as power point presentations to address every step of the surgery including management of complications (Elit et al., 2010). Over the course of two weeks, the two designated gynecologists learned how to do a radical hysterectomy and pelvic node dissection and together with their mentor they did 7 procedures over that time period.
The surgical training was very structured. At the completion of each surgical procedure a detailed debriefing was undertaken to review the performance of each of the trainees. This evaluation was very practical and assessed their skill as
-
a.
unable to do the procedure
-
b.
able to complete the procedure with a lot of assistance
-
c.
able to complete the procedure with limited assistance
-
d.
able to complete the procedure independently
The goal was for them to achieve complete independence over the training period. The evaluation included all steps in the surgical procedure including simple ones such as insertion of the foley catheter and patient positioning to far more complex components of the surgery such as identification of the obturator nerve, uterine artery, and freeing the ureter from the parametrium. Initially the two surgeons struggled through the first three procedures, but completed the seventh independently without the mentor. Since that training in 2010, they have successfully completed over 230 abdominal radical hysterectomies between 2010 and December 2016. Fig. 2 is the number of new patients seen in the gynecologic oncology clinic between 2010 and 2016.
Fig. 2.
Gyneconcology clinic data.
Lessons learned included an understanding that the training modules were very valuable teaching aids, taking them through the surgical steps, the anatomy and management of potential complications including injury to the bladder, ureter, bowel and blood vessels. The modules were reviewed before any surgery was undertaken and the two gynecologists had copies of them to review at home. These modules also included a video from a robotic assisted radical hysterectomy (Elit et al., 2010, Elit and Chamberlain Froese, 2015).
The second lesson was the value in training two surgeons together because they helped each other and continued to work together on every surgical case after the mentor left Kenya. This provided each of them the necessary support to jointly maintain and improve their surgical skills.
The readers may be surprised or even doubt the ability to learn how to do a radical hysterectomy in two weeks. The starting point for them is different than for North American training fellows. The Kenyan gynecologists are skilled surgeons. In obstetrics they are often required to deal with extremely complex surgical problems including severe postpartum hemorrhage, ruptured uteri, obstructed labor, and fistulae, which unfortunately are common problems that happen regularly in low resource countries. They have good fundamental surgical skills.
2.1.2. Developing local treatment protocols
At the same time through direction and leadership from AMPATH, in particular from Mathew Strother (Strother et al., 2013), we established a process to develop written clinical algorithms for all gynecologic malignancies in Kenya. Our starting position was to use protocols established in North America and modify them to the Kenyan context. We also wanted protocols that were evidence based. Chemotherapy supply was limited in Kenya and AMPATH established a policy to use it (free of charge for patients) only for disease sites that had written protocols. Therefore, the protocols were necessary and over time proved to be very valuable in establishing effective care in gynecologic oncology. Following the first draft of the protocols we then reviewed the literature to evaluate the evidence for the algorithms we had written. For example, in Kenya, we were limited to cisplatin, cyclophosphamide, etoposide and methotrexate for chemotherapy. We could not develop an ovarian cancer protocol that would necessitate the use of paclitaxel and carboplatin. We used the literature primarily from GOG studies to justify using cisplatin and cyclophosphamide for ovarian cancer and we published a paper on their use in low resource settings (Sterling et al., 2011).
We used studies from Brazil to help us understand the use of neoadjuvant chemotherapy for cervical cancer. In a setting where there is limited access to radiation, neoadjuvant chemotherapy became a strategy to shrink tumors so that they would be small enough to remove surgically. We learned very quickly that some tumors responded quickly and extremely well to neoadjuvant chemotherapy and its use was also extremely effective at improving the symptoms of advanced cervical cancers including bleeding, pain and discharge. Many women with advanced cancers had symptom relief after one dose of cisplatinum.
We have since published the protocol and the results of the use of cisplatin to palliate advanced cervical cancers (Orang'o et al., 2016b).
2.2. Consolidation phase: gynecologic oncology training in kenya
The two Kenyan gynecologists understood that they were only partially trained in oncology and they both wanted more. It was also important to them that they become recognized by their colleagues for having the oncology expertise. It was helpful that they understood that they were only partially trained. Trained gynecologic oncologists understand the complexity of treatment decisions for their patients. Those not trained in oncology sometimes say or think that if a gynecologist can do the surgery they can function as an oncologist. In Africa there may be a temptation to shorten the training to meet the need as fast as possible. In our opinion that will not succeed and in the end it may be more harmful than beneficial.
The two Kenyan gynecologists were both planning to apply to training programs in South Africa. Experience from MTRH and others in sub-Saharan Africa was that trainees who left their home base to obtain training, most often did not return (The World Health Report 2006-Working Together for Health).
This was a second tipping point because it became the stimulus to initiate and to develop a gynecologic oncology fellowship training in Kenya. Initially it was a dream that with a great deal of help from Kenyan leadership in the department of Reproductive Health became a reality. The first steps were to get support from the Department of Reproductive Health at Moi University who would subsequently champion the training through all the various steps required by Moi University. After gaining support from the RH department the Dean of Medicine required us to hold a stakeholders meeting that would include several Deans from Moi University, gynecologists from MTRH and Nairobi, radiation oncology, pathology, radiology and several non-physician women who had no affiliation with the program. This meeting was essential because through it we gained support from the Dean of nursing, from the associated medical groups and from the public. One of the most remarkable aspects of the meeting was when a layperson stood and spoke about how physicians in Kenyan are poor communicators. As a result of her opinion we initiated a course in communication as part of the curriculum. Based on our results in Kenya we subsequently started a communication course for the University of Toronto fellows who evaluated it very highly and recommended that we have it annually. This is an example on how learning in global health goes both ways.
Approval for this training program was required from the post graduate education committee which supervises all residency programs, from the Dean of Medicine, from the Moi University Dean's committee and finally from the senate. Once all the approvals were obtained this program officially became the first medical postgraduate subspecialty-training program at Moi University and the first official university gynecologic oncology-training program in sub-Saharan Africa outside of South Africa. Approval from Moi University was a long process taking 18 months. This was a very valuable step to developing a sustainable training program. The sustainability and credentialing for this training program is the responsibility of Moi University. They have the power and the resources to make the training accountable and at the same time potential trainees want the stamp of approval from the university.
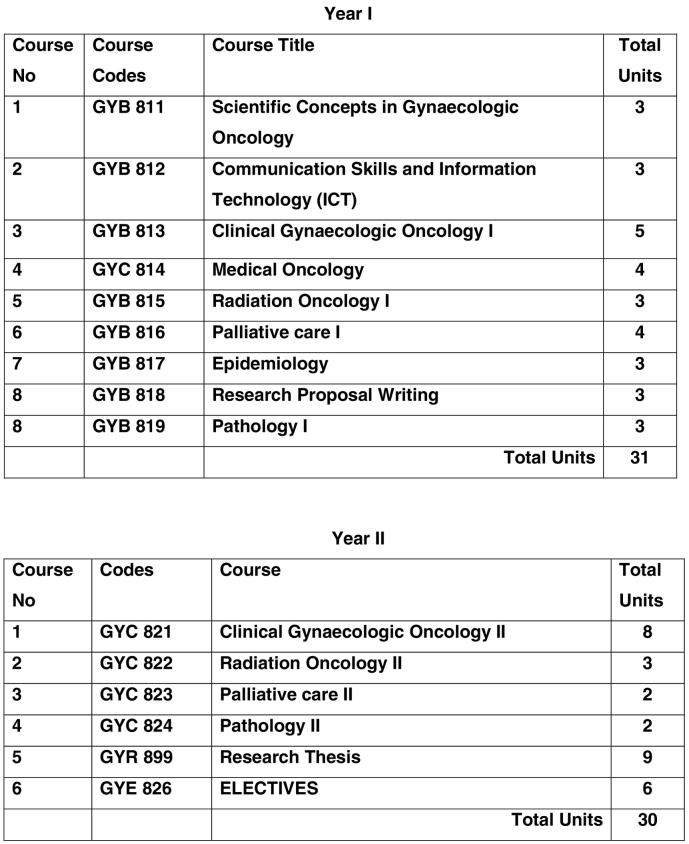
This fellowship training program (Masters degree program) followed all the requirements that have been established for all Moi University medical training programs using the SPICES model of education (Fig. 3). This is a two-year training program with courses for what we call in North America rotations (Fig. 3). It included training in medical oncology at MTRH, in radiation oncology, pathology, palliative care, as well as the bulk of the time in clinical gynecologic oncology. In addition they are required to take a Moi University epidemiology course. They were evaluated at the end of each course. Moi University guidelines also stipulated that the fellows were required to take an exam at the end of their first and second years by an external examiner who had no prior affiliation with the program.
Fig. 3.
Gynecologic oncology curriculum.
The subspecialty-training program in Gynecologic oncology will adopt the SPICES model of Medical Education adopted by Moi University College of Health Sciences that features methods of learning that are Student-centered, Problem-based, Integrated, Community-oriented, Electives and Systematic 9.0
There were some practical barriers to overcome to ensure that we provided both the knowledge and clinical education they required. The first two trainees trained in a setting where there were no full-time faculty on site and we needed to establish a strategy where the fellows would be adequately trained. Several Canadian and Dutch gynecologic oncologists agreed to travel to Kenya, each for two weeks in the first and second years of the program, to provide the education and the clinical training. We established a program where a gynecologic oncologist was in Kenya for two weeks every two months. The mentors would focus on knowledge and clinical training along with reviewing relevant published medical papers. For example, each mentor was given 1–2 chapters from a gynecologic oncology textbook to review with the fellows during their time in Kenya. The mentors would be in the outpatient clinic and would scrub on all cases. The fellows were freed up from their other responsibilities during faculty visits. This program was funded from two funds at The University of Toronto through the generous contributions from the McCarthur and Kimel Family Foundation funds. Expenses as well as a small honorarium were provided to all visiting faculty, and their home institutions considered the time to be academic activity, not holiday time. Each travelling faculty provided their time in kind.
We understood that all the training for a gynecologic oncologist could not happen in Kenya. We had the fellows travel to Canada, as observers for pathology, radiation oncology and palliative care training. This was very successful and fully embraced by faculty at Princess Margaret Hospital. While in Toronto the fellows needed to complete a project for each of the courses and this became part of their evaluation. This experience gave them an opportunity to experience an organized program in gynecologic oncology, which in turn stimulated them to think about how to set up their own program in Kenya.
All courses in Toronto were very valuable but a special mention needs to be made about the palliative care training. The fellows, who had no previous training or mentorship in this aspect of care, were well integrated into the program. They observed how the palliative care physicians delivered bad news and how they spoke to patients about their illnesses and impending death. This experience had an unexpected very beneficial result, which helped change the behavior of these Kenyan doctors. Prior to this training they told us that Kenyans do not like to be told they have cancer and Kenyans do not like to talk about death. For those of us who were clinicians in the 1980′s the same was said of our North American patients. But once trained, the Kenyan physicians started to talk to their patients about their cancer prognosis and about dying. Women and their families in Kenya appreciated the direct and honest discussion about their cancers and they would often thank the doctor for telling them about their prognosis. These gynecologic oncologists now model this behavior to their students, their residents and fellows.
The first two fellows completed their training in September 2014. A third fellow completed his training in 2015 and two more in 2016, for a total of 5. Four of the five graduates stayed in Kenya, 3 at moi University and one in Kisumu to start a new program there. The fifth, is from Uganda, and is the only fully trained gynecologic oncologist in Uganda and she is in Kampala. This program is open to anyone applicant from east Africa. Two more are currently in training. To date the program has only accepted practicing gynecologists, as no one yet has applied to start the program directly out of their residency. Certification of their training comes from Moi University and the department of Reproductive Health. They also have recognition as sub specialist with the Kenyan Medical Practitioners and Dentists Board, their medical regulators in Kenya. As well they are recognized within the Kenyan Obstetrics and Gynecology Society (KOGS).
2.3. Quality evaluation and research
Research has become a significant part of this program. There are several reasons for this. First it is embedded in AMPATH, which focuses a great deal of its activities in research along with clinical care. The initial mentorship of this program includes mentors with a research track record. It was recognized by all, that if we were going to understand the impact of this program, clinical databases needed to be initiated so that clinical care could be evaluated and changed if necessary.
From the start of the radical hysterectomy training we initiated a database to collect prospective data on treatment and treatment outcome for all oncology patients. To do this effectively we hired a full-time data manager to collect and manage the data (Fig. 4, Fig. 5).
Fig. 4.
Grants and awards.
Fig. 5.
Conference presentations.
In retrospect, the databases were very important components to developing a comprehensive program in gynecologic oncology. The data was used for publications and from the recognition gained from publishing this group was able to successfully obtain multiple peer reviewed grants that enabled them to further develop their program through research. There are additional benefits for Kenyans. Academic promotion in Kenya is highly valued and with promotion comes additional income. North America faculty can also benefit for their involvement in research and in many respects research is a win-win-win. The third win is for the patients whose care is monitored carefully through the research, and whose care will be changed over time based on the clinical evaluations that are only possible from clinical databases (Fig. 4).
2.4. Future/vision
The stated vision for the gynecologic oncology fellowship in Kenya is that successful participants would have the expertise and also be recognized within reproductive health to manage all gynecologic oncology patients, to administer a program in gynecologic oncology and to continue to train others.
3. Goal
The goal is to continue to train fellows in gynecologic oncology and to populate the country and possibly even East Africa with expertise. As fellows graduate and begin to work in different areas of Kenya it is hoped that other fellowship training programs will develop.
4. Conclusions
Moi University has a 2-year fellowship-training program in gynecologic oncology. It was developed slowly, and it evolved from a mentoring relationship to a sustainable gynecologic oncology training program. Its success was dependent on the relationships developed between the Canadian and Kenyan physicians. There was and is a mutual respect for each other and an understanding that we have different cultures yet at the same time we shared common goals. It required innovation, to adapt to the circumstances in Kenya that are very different than Canada.
The World Health Organization has recommended a stepwise approach to developing health care initiatives. They recommend that you choose 1 or 2 priorities and focus on them. That was our approach, yet we did not really know it at the time. Most of the credit belongs to the Kenyans, who were driven to improve care for the women in Kenya. They pushed us more than we pushed them and it is for that reason we all succeeded.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Acknowledgements
The authors wish to acknowledge Hellen Muliro who entered and managed the data collection for the gynecologic oncology program. In addition the fellows, Dr. Jane Namugga and Dr. Greg Ganda for their efforts to keep the database up to date.
The authors would like to acknowledge the six gynecologic oncologists who participated as faculty in this program and also acknowledge their university affiliations for their support of this program.
Dr. Joan Murphy University of Toronto, Toronto Ontario
Dr. Barry Rosen University of Toronto, Toronto Ontario
Dr. Allan Covens University of Toronto, Toronto Ontario
Dr. Christopher Giede University of Saskatoon, Saskatoon, Saskatchewan
Dr. Peter Bryson Queens University Kingston Ontario
Dr. Luc lonkhuijzen Amsterdam Medical Center Amsterdam, The Netheralnds
References
- Abdel-Wahab May, Bourque Jean-Marc, Pynda Yaroslav, Izewska Joanna, der Merwe Debbie Van, Zubizarreta Eduardo, Rosenblatt Eduardo. Status of radiotherapy resources in Africa: an international atomic energy analysis. 2013, April;14(4):e168–3175. doi: 10.1016/S1470-2045(12)70532-6. http://www.thelancet.com/journals/lanonc/article/PIIS1470-2045(12)70532-6/fulltext?rss=yes [DOI] [PubMed] [Google Scholar]
- Elit L., Chamberlain Froese L., editors. Women's Health in the Majority World: Issues and Initiatives. Second edition. Nova Science Publishers Inc.; New York: 2015. ISBN: 978-1-63482-454-5 Aug 2015. [Google Scholar]
- Elit L., Rosen B., Jimenez W., Giede C., Cybulka P., Sinesac S., Dodge J., Erdenejargal A., Orang'o E., Bernardini M., Finlayson S., McAlpine J., Miller D. Teaching cervical cancer surgery in low or middle resource countries. Int. J. Gynecol. Cancer. 2010, Dec;20(9):1604–1608. [PubMed] [Google Scholar]
- Farmer P., Frenk J., Knaul F.M., Shulman L.N., Alleyne G., Armstrong L., Atun R., Blayney D., Chen L., Feachem R., Gospodarowicz M., Gralow J., Gupta S., Langer A., Lob-Levyt J., Neal C., Mbewu A., Mired D., Piot P., Reddy K.S., Sachs J.D., Sarhan M., Seffrin J.R. Expansion of cancer care and control in countries of low and middle income: a call to action. Lancet. Oct 2, 2010;376(9747):1186–1193. doi: 10.1016/S0140-6736(10)61152-X. https://www.ncbi.nlm.nih.gov/pubmed/20709386 (Epub 2010 Aug 13) [DOI] [PubMed] [Google Scholar]
- Grover Surbhi, Melody J. Xu, Yeager Alyssa, Rosman Lori, Groen Reinou S., Chackungal Smita, Rodin Danielle, Mangaali Margaret, Nurkic Sommer, Fernandes Annemarie, Lin Lilie L., Thomas Gillian, Tergas Ana I. A systematic review of radiotherapy capacity in low- and middle-income countries. Front Oncol. 2014;4(380) doi: 10.3389/fonc.2014.00380. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4302829/ Published online 2015 Jan 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Agency for Research on Cancer Globocan 2012: Estimated Cancer Incidence and Mortality Worldwide in 2012. http://globocan.iarc.fr/
- Jemal A., Bray F., Forman D., O'Brien M., Ferlay J., Center M., Parkin O.M. Cancer burden in Africa and opportunities for prevention. Cancer. 2012;118:4372–4384. doi: 10.1002/cncr.27410. [DOI] [PubMed] [Google Scholar]
- Khozaim K., Orang'o E., Christoffersen-Deb A., Itsura P., Oguda J., Muliro H., Ndiema J., Mwangi G., Strother M., Cu-Uvin S., Rosen B., Washington S. Successes and challenges of establishing a cervical cancer screening and treatment program in western Kenya. Int. J. Gynaecol. Obstet. 2014 Jan 1;124(1):12–18. doi: 10.1016/j.ijgo.2013.06.035. [DOI] [PubMed] [Google Scholar]
- Orang'o Elkanah Omenge, Wachira Juddy, Asirwa Fredrick Chite, Busakhala Naftali, Naanyu Violet, Kisuya Job, Otieno Grieven, Keter Alfred, Mwangi Ann, Inui Thomas. Factors associated with uptake of visual inspection with acetic acid (VIA) for cervical cancer screening in Western Kenya. PLoS One. 2016;11(6) doi: 10.1371/journal.pone.0157217. http://www.pubpdf.com/pub/27310005/Factors-Associated-with-Uptake-of-Visual-Inspection-with-Acetic-Acid-VIA-for-Cervical-Cancer-Screening Epub 2016 Jun 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orang'o E., Itsura P., Tonui P., Muliro H., Rosen B., van Lonkhuijzen L. Palliative cisplatinum for advanced cervical cancer in a resource poor setting: a case series from Kenya. J. Glob. Oncol. 2016;00 doi: 10.1200/JGO.2016.006411. (American Society of Clinical Oncology) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parham G.P., Mwanahamunta M.H., Kapambwe S., Muwonge R., Bateman A.C., Blevins M. Population-level-scale-up of cervical prevention services in a low resource setting: development, implementation, and evaluation of the cervical cancer prevention program in Zambia. PLos One. 2015 Apr17;10(4) doi: 10.1371/journal.pone.0122169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterling L., van Lonkhuijzen L., Nyangena J., Orango E., Strother M., Busakhala N., Rosen B. Protocol development for ovarian cancer treatment in Kenya: a brief report. Int. J. Gynecol. Cancer. 2011 Feb;21(2):424–427. [PubMed] [Google Scholar]
- Strother R.M., Aswira F.C., Busakhala N.B., Njiru E., Orang'o E., Njuguna F., Skiles J., Carter J., Mega A., Kaspers G.J.L., Rosen B., Krzyzanowska M.K., Washington S., Greist A., Rosmarin A., Loehrer P.J. The evolution of comprehensive cancer care in Western Kenya. J. Cancer Policy. 2013:e25–e30. [Google Scholar]
- The World Health Report 2006-Working Together for Health. www.who.int/whr/2006/ [DOI] [PubMed]
- Torre L.A., Bray F., Siegel R., Ferlay J., Lortet-Treulent J., Jemal A. Global cancer statistics. 2015;65:87–108. doi: 10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- Wools-Kaloustian K., Kimaiyo S., Diero L. Viability and effectiveness of large scale HIV treatment initiatives in sub Saharan Africa: experience from western Kenya. AIDS. 2006;20(1):41–48. doi: 10.1097/01.aids.0000196177.65551.ea. [DOI] [PubMed] [Google Scholar]
- World Health Organization Health Statistics and information Systems. WHO Mortality Database. http://www.who.int/healthinfo/mortality_data/en/