Abstract
Mitochondrial reactive oxygen species (mtROS) generated continuously under physiological conditions have recently emerged as critical players in the regulation of immune signaling pathways. In this study we have investigated the regulation of antiviral signaling by increased mtROS production in plasmacytoid dendritic cells (pDCs), which, as major producers of type I interferons (IFN), are the key coordinators of antiviral immunity. The early phase of type I IFN production in pDCs is mediated by endosomal Toll-like receptors (TLRs), whereas the late phase of IFN response can also be triggered by cytosolic retinoic acid-inducible gene-I (RIG-I), expression of which is induced upon TLR stimulation. Therefore, pDCs provide an ideal model to study the impact of elevated mtROS on the antiviral signaling pathways initiated by receptors with distinct subcellular localization. We found that elevated level of mtROS alone did not change the phenotype and the baseline cytokine profile of resting pDCs. Nevertheless increased mtROS levels in pDCs lowered the TLR9-induced secretion of pro-inflammatory mediators slightly, whereas reduced type I IFN production markedly via blocking phosphorylation of interferon regulatory factor 7 (IRF7), the key transcription factor of the TLR9 signaling pathway. The TLR9-induced expression of RIG-I in pDCs was also negatively regulated by enhanced mtROS production. On the contrary, elevated mtROS significantly augmented the RIG-I-stimulated expression of type I IFNs, as well as the expression of mitochondrial antiviral-signaling (MAVS) protein and the phosphorylation of Akt and IRF3 that are essential components of RIG-I signaling. Collectively, our data suggest that increased mtROS exert diverse immunoregulatory functions in pDCs both in the early and late phase of type I IFN responses depending on which type of viral sensing pathway is stimulated.
Abbreviations: 7-AAD, 7-aminoactinomycin-D; AMA, Antimycin-A; IFN, interferon; IRF, interferon regulatory factor; MAVS, mitochondrial antiviral-signaling; mtROS, mitochondrial reactive oxygen species; pDCs, plasmacytoid dendritic cells; PI3K, phosphatidylinositol-3-kinase; RIG-I, retinoic-acid-inducible gene-I; RLR, RIG-I-like receptor; ROS, reactive oxygen species; TLR, Toll-like receptor
Keywords: Plasmacytoid dendritic cell, Mitochondrial ROS, Type I interferon, Endosomal TLR signaling, RIG-I signaling, Antiviral response
Graphical abstract
Highlights
-
•
MtROS have versatile role in fine-tuning the type I IFN responses of pDCs.
-
•
MtROS affect the TLR- and RIG-I-mediated type I IFN responses in pDCs oppositely.
-
•
MtROS decrease the TLR-induced early phase of type I IFN production in pDCs.
-
•
MtROS enhance the RIG-I-mediated late phase of type I IFN production in pDCs.
1. Introduction
Under oxidative stress conditions the intracellular levels of reactive oxygen species (ROS) are elevated that by damaging different biological targets such as proteins, lipids or DNA can lead to a variety of pathologies. Nevertheless, functioning as signaling molecules, endogenous ROS can contribute to the maintenance of redox balance and thus to the physiological functions of the cells [1]. Although multiple mechanisms can generate ROS within the cells in vivo, the mitochondrial electron transport chain is assumed to be the major source of endogenous ROS [2]. In the respiratory chain electrons are passed through a series of protein complexes (labeled Complex I–IV) via redox reactions. As a natural process, some electrons leave the electron transport chain and react with molecular oxygen leading to the formation of mitochondrial ROS (mtROS), the natural byproducts of oxidative metabolism. Mitochondrial ROS have two faces: on the one hand, they maintain a favorable redox balance [3], on the other hand, contribute to the pathogenesis of various oxidative stress-related diseases (reviewed in [4]). Mitochondrial Complexes I-III are the major sources of mtROS production: Complexes I and II release mtROS into the matrix, whereas Complex III can produce them both into the matrix and the intermembrane space of mitochondria [2], [5]. Mitochondrial ROS can easily penetrate from the intermembrane space to the cytosol and act as signaling molecules via directly modifying the activity of cell-signaling components, thus influencing the outcome of signal transduction pathways [6], [7].
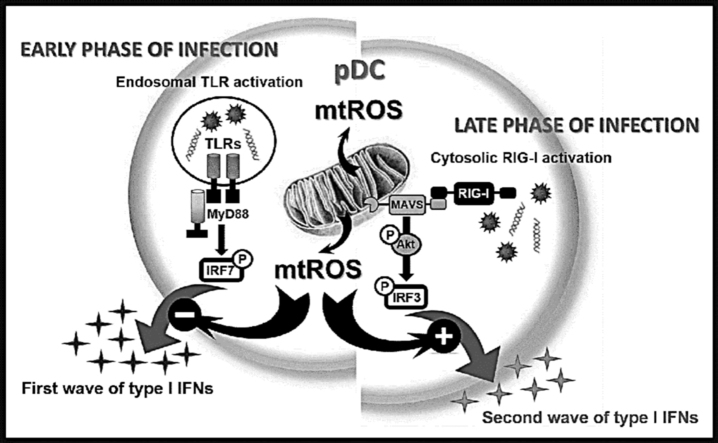
An extensive body of literature indicates the involvement of mtROS in multiple signaling pathways (reviewed in [3]) including antiviral responses (reviewed in [8]), which is in the focus of our present study. However, the putative regulatory role of mtROS on signaling induced by antiviral sensors of plasmacytoid dendritic cells (pDCs) has not been investigated yet. Plasmacytoid DCs are the key regulators of antiviral immunity and as professional type I interferon (IFN) producing cells, they secrete 200–1000 times more type I IFNs than other leukocytes or IFN-producing tissue cells (e.g. reactive epithelium) in response to viruses [9]. The robust type I IFN production of pDCs is critical in the clearance of certain acute viral infections as shown by the depletion of pDCs that leads to impaired type I IFN secretion and persistence of viral infection [10].
Viruses are very heterogeneous in structure, genomic composition, and replication strategy; therefore, multiple sensors are required to detect their presence in various cellular compartments. The initial recognition of virus-derived nucleic acids and the first wave of type I IFN production are mainly mediated by endosomal Toll-like receptor (TLR) 7 and 9 in pDCs that are selectively expressed in these cells. In the early phase of viral infection pDCs circulating in blood or locating in secondary lymphoid tissues engulf non-infectious viral particles or apoptotic bodies from infected cells and produce high amount of type I IFNs that is independent of intrinsic viral replication in pDCs [11]. Later on, when pDCs leave the bloodstream and accumulate at the site of infection, they might become infected by viruses that requires the detection of viral replication intermediates by cytosolic receptors such as retinoic acid-inducible gene-I (RIG-I)-like helicases [12]. We have previously demonstrated that in contrast with conventional dendritic cells and macrophages [13], RIG-I is not expressed in resting pDCs, but it can be upregulated by endosomal TLR stimulation [14]. Based on our results we proposed a model where TLRs orchestrate the first phase of type I IFN production whereas RIG-I participates only in the late phase of antiviral responses by boosting the production of type I IFNs in pDCs.
Based on the unique expression of the above mentioned viral sensors in pDCs, we aimed to investigate the possible regulatory role of mtROS on the antiviral signaling pathways initiated by receptors localized to different cellular compartments. To this end, we studied the potential impact of elevated mtROS levels on the early phase of type I IFN production mediated by endosomal TLRs and on the late phase of type I IFN secretion induced by the cytosolic RIG-I sensor in pDCs.
2. Materials and methods
2.1. Cell line
In most of our experiments we used the human plasmacytoid dendritic cell line GEN2.2 [49] (kindly provided by Dr. Joel Plumas and Dr. Laurence Chaperot, Research and Development Laboratory, French Blood Bank Rhône-Alpes, Grenoble, France), which is deposited with the CNCM (French National Collection of Microorganism Cultures) under the number CNCMI-2938. GEN2.2 cells were grown on a layer of mitomycin C (Sigma-Aldrich, St. Louis, MO, USA)-treated murine MS5 feeder cells (Cat. no. ACC 441, Leibniz Institute DSMZ-German Collection of Microorganisms and Cell Cultures, Braunschweig, Germany) in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Life Technologies Corporation, Carlsbad, CA, USA), 100 U/ml penicillin, 100 ng/ml streptomycin (both from Sigma-Aldrich) and 5% non-essential amino acids (Life Technologies Corporation). For experiments, the GEN2.2 cells were removed from the feeder layer and seeded on 24-well plates at a concentration of 5 × 105 cells/500 μl in complete RPMI 1640 medium (Sigma-Aldrich). Cell lines were grown and incubated at 37 °C in 5% CO2 humidified atmosphere.
2.2. Isolation and culturing of primary human pDCs
Primary pDCs were isolated from human heparinized leukocyte-enriched buffy coats, which were obtained from healthy blood donors drawn at the Regional Blood Center of Hungarian National Blood Transfusion Service (Debrecen, Hungary) in accordance with the written approval of the Director of the National Blood Transfusion Service and the Regional and Institutional Ethics Committee of the University of Debrecen, Faculty of Medicine (Debrecen, Hungary). Peripheral blood mononuclear cells (PBMCs) were isolated using Ficoll-Paque (GE Healthcare, Uppsala, Sweden) density gradient centrifugation. Plasmacytoid DCs were then purified from PBMCs by positive selection using the human CD304 (BDCA-4/Neuropilin-1) MicroBead Kit (Miltenyi Biotec, Bergish Gladbach, Germany), according to the manufacturer's protocol. After separation on VarioMACS magnet, the purity of isolated pDCs was > 96% as confirmed by flow cytometry.
Freshly isolated pDCs were cultured in 48-well cell culture plates at a density of 5 × 105 cells/ml in RPMI 1640 medium (Sigma-Aldrich) supplemented with 10% heat-inactivated FBS (Life Technologies Corporation), 2 mM L-glutamine, 100 U/ml penicillin, 100 ng/ml streptomycin (all from Sigma-Aldrich), and 50 ng/ml recombinant human IL-3 (Peprotech EC, London, UK). During treatments cells were incubated at 37 °C in a humidified atmosphere containing 5% CO2.
2.3. Induction of elevated level of mtROS
To induce elevated mtROS generation, cells were treated with Antimycin-A (AMA; Sigma-Aldrich). To detect mtROS production GEN2.2 cells were stained with 5 µM MitoSox™ Red mitochondrial superoxide indicator (Life Technologies Corporation) according to the manufacturer's recommendations. The MitoSox™ Red loaded cells were exposed to increasing concentrations of AMA (0.1, 0.2, 0.5, 1 and 2 µg/ml; Sigma-Aldrich) for 6 h. In parallel experiments cells were also treated with MitoTEMPO (300 µM, Sigma-Aldrich), a mitochondria-targeted antioxidant, which is a specific scavenger of mtROS. MitoTEMPO was added 1 h prior to or along with the AMA treatment. Following treatments the fluorescence intensity of MitoSox™ Red was measured at 580 nm with FACS Calibur flow cytometer (Becton Dickinson, Franklin Lakes, NJ, USA) and data were analyzed by FlowJo software (Treestar, Ashland, OR, USA). The viability of the AMA-exposed cells was assessed by 7-aminoactinomycin-D (7-AAD; 10 µg/ml; Sigma-Aldrich) staining using flow cytometric analysis.
2.4. Cell stimulation
For activation, cells were exposed to the TLR9 agonist CpG-A (ODN 2216, 1 μM; Cat. no. HC4037 from Hycult Biotech, Uden, The Netherlands) in the absence or presence of AMA (0.5 μg/ml; Sigma-Aldrich) for 6 h. The optimal concentration of AMA was chosen based on preliminary experiments performed on GEN2.2 cells.
To induce RIG-I expression, cells were incubated with 0.25 μM CpG-A for 16 h. Thereafter cells were washed, re-seeded in fresh, complete RPMI 1640 medium and stimulated with 5′ triphosphate-double stranded RNA (5′ppp-dsRNA, Cat. no. tlrl-3prna from InvivoGen, San Diego, CA, USA), a specific agonist of RIG-I, complexed with the transfection reagent LyoVec™ (InvivoGen), according to the manufacturer's recommendations. Briefly, 25 μl of the 5′ppp-dsRNA-LyoVec complex containing 1 μg/ml working concentration of the RIG-I ligand was added to the cells for the indicated time period in all experiments. In parallel experiments cells were exposed to 0.5 μg/ml AMA (Sigma-Aldrich) in combination with 5′ppp-dsRNA.
2.5. Flow cytometry
After 24 h of treatments the phenotypical analysis of the cells was performed by flow cytometry using FITC-labeled monoclonal antibodies against CD40 and CD80 (Cat. no. 334306 and 305206 both from BioLegend, San Diego, CA, USA), PE-labeled CD86 (Cat. no. FAB141P from R&D System, Minneapolis, MN, USA), HLA-DQ (Cat. no. 318106 from BioLegend) and isotype-matched control antibodies (all from BioLegend). Fluorescence intensities were measured with FACS Calibur (Becton Dickinson) and data were analyzed with FlowJo software (TreeStar).
2.6. ELISA
Cell culture supernatants were harvested after 6 h of treatments and the concentrations of TNF-α, IL-6 and IL-8 were measured with the BD OptEIA human ELISA kits (BD Biosciences, San Diego, CA, USA). The pre-coated human IFN-α ELISA kit was purchased from PBL InterferonSource (Piscataway, NJ, USA). Assays were performed according to the manufacturer's instructions. Absorbance measurements were carried out by a Synergy HT microplate reader (Bio-Tek Instruments, Winooski, VT, USA) at 450 nm.
2.7. Quantitative real time PCR
Total RNA was isolated from 5 × 105 cells using TRI reagent (Molecular Research Center, Inc., Cincinnati, OH, USA). Total RNA (1 μg) was treated with DNase I (Thermo Fisher Scientific, Waltham, MA, USA) to exclude amplification of genomic DNA then reverse transcribed into cDNA using the High Capacity cDNA RT Kit of Applied Biosystems (Carlsbad, CA, USA). The cDNA products were diluted at a ratio of 1:2, then amplified using Dream Taq DNA Polymerase (Thermo Fisher Scientific) and IFNA1 (Assay ID Hs. PT.49a.3184790.g, Integrated DNA Technologies, Coralville, IA, USA) gene-specific assay according to the manufacturer's instructions. Quantitative PCR was performed using the ABI StepOne Real-Time PCR System (Applied Biosystems) and cycle threshold values were determined using the StepOne v2.1 Software (Applied Biosystems). The relative amount of mRNA (2−ΔCt) was obtained by normalizing to the cyclophilin house keeping gene in each experiment.
2.8. Western blotting
For Western blotting 5 × 105 cells were lysed in Laemmli buffer and then the protein extracts were resolved by SDS-PAGE using 10% polyacrylamide gels and electro-transferred to nitrocellulose membranes (Bio-Rad Laboratories GmbH, Munich, Germany). Non-specific binding sites were blocked with 5% non-fat dry milk (or 5% BSA in case of phospho-IRF3) diluted in TBS Tween buffer (50 mM Tris, 0.5 M NaCl, 0.05% Tween-20, pH 7.4). Membranes were probed with the following primary antibodies: anti-RIG-I (Cat. no. 4520, Cell Signaling, Danvers, MA, USA), anti-phospho-IRF7 (S477; Cat. No. 12390, Cell Signaling), anti-IRF7 (Cat. no. 4920, Cell Signaling), anti-phospho-IRF3 (S386; Cat. no. ab76493, Abcam, Cambridge, UK), anti-IRF3 (Cat. no. 4302, Cell Signaling), anti-phospho-Akt (S473; Cat. no. AF887, R&D System), anti-Akt1 (Cat. no. sc-5298, Santa Cruz Biotechnology, Heidelberg, Germany), anti-mitochondrial antiviral-signaling (MAVS; Cat. no. 3993, Cell Signaling) and anti-beta-actin (Cat. no. sc-47778, Santa Cruz Biotechnology). The bound primary antibodies were detectedwith anti-mouse or anti-rabbit horseradish peroxidase-conjugated secondary antibodies (GE Healthcare, Little Chalfont, Buckinghamshire, UK) at a dilution of 1:10,000 and were visualized by the ECL system using SuperSignal West Pico or Femto chemiluminescent substrates (Thermo Scientific, Rockford, IL, USA) and X-ray film exposure. Densitometric analysis of immunoreactive bands was performed using the Kodak 1D Image Analysis Software version 3.6 (Kodak Digital Science Imaging, Eastman Kodak Company, New Haven, CT, USA). To ensure equal protein loading after staining with phospho-IRF7, phospho-IRF3 and phospho-Akt specific antibodies the membranes were stripped and re-probed with anti-IRF7, anti-IRF3 and anti-Akt1 antibodies, respectively. Beta-actin served as loading control for RIG-I and MAVS.
2.9. Statistical analysis
Data from the different treatment groups were analyzed by Student's unpaired t-test or ANOVA, followed by Bonferroni post hoc analyses for least-significant differences. Data analysis was performed with GraphPad Prism v.6. software (GraphPad Software Inc., La Jolla, CA, USA). Differences were considered to be statistically significant at p < 0.05.
3. Results
3.1. AMA treatment increases mtROS production in GEN2.2 cells that can be prevented by pre-treatment with the antioxidant MitoTEMPO
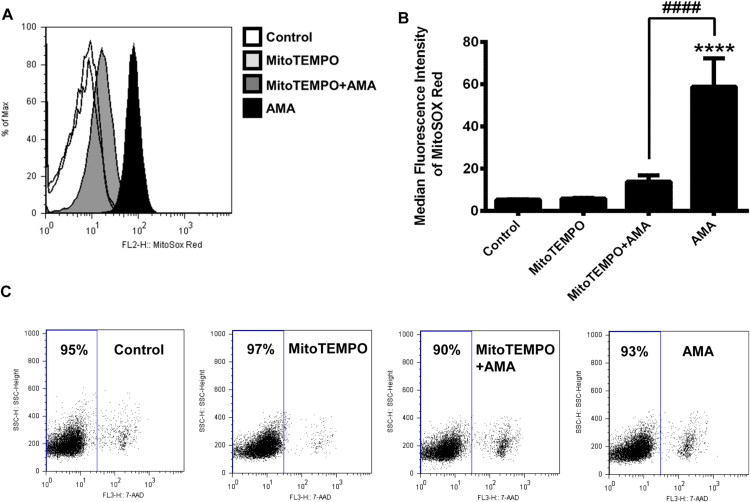
The availability of human pDCs is limited due to their low frequency in peripheral blood [9]. To overcome this limitation, in most of our experiments we have used the human plasmacytoid dendritic cell line GEN2.2, which is functionally similar to primary pDCs [15]. In the first step we have investigated the sensitivity of GEN2.2 cells to AMA, an inhibitor of mitochondrial electron transport chain that we used to increase mtROS generation in the cells. AMA binding to the quinone reduction site of the cytochrome bc1 Complex (Complex III) inhibits the transfer of electrons and subsequently leads to the release of ROS into the mitochondrial matrix and to the intermembrane space [5]. First we have determined the optimal concentration of AMA that increases the level of mtROS consistently without affecting cell viability. To this end, GEN2.2 cells were loaded with MitoSox™ Red mitochondrial superoxide indicator and then exposed to increasing concentration of AMA (as described in Materials and Methods) for 6 h. Although, the intensity of MitoSox™ Red fluorescence increased in a dose dependent-manner, we observed a significant decrease in cell viability when reaching the AMA concentration of 1 μg/ml (data not shown). However, exposure to 0.5 µg/ml of AMA resulted in a substantial and consistent increase in MitoSox™ Red fluorescence intensity (Fig. 1A, B), while did not affect cell viability assessed by 7-AAD staining (Fig. 1C). Thus, to mimic the elevated production of mtROS in GEN2.2 cells induced by metabolic changes or different stress signals under in vivo conditions AMA was used at 0.5 µg/ml concentration in all our further experiments.
Fig. 1.
Generation of elevated level of mtROS in GEN2.2 cells without affecting the cell viability. Cells were loaded with MitoSox™ Red mitochondrial superoxide indicator and then treated with AMA (0.5 μg/ml) for 6 h to increase the production of mtROS. As a control AMA was used in combination with MitoTEMPO (300 μM) that limits mtROS accumulation. MitoTEMPO was added 1 h prior to and along with the AMA treatments. The fluorescence intensity of MitoSox™ Red dye correlates with the level of mtROS generated in the cells. The changes in fluorescence intensities of MitoSox™ Red dye were monitored by flow cytometry. A representative histogram (A) and the means ± SD (B) of eight independent experiments are shown. The percentage of dead cells was determined by 7-AAD staining. (C) Dot plots are representatives of eight independent experiments. ****p < 0.0001 vs. control, ####p < 0.0001 vs. AMA. AMA: Antimycin-A.
To verify that the increased fluorescence intensity of MitoSox™ Red was due to the AMA-induced mtROS production we have repeated our measurements in the presence of the mitochondria-targeted antioxidant MitoTEMPO [16]. Pre-treatment of cells with MitoTEMPO and its parallel administration with AMA almost completely abolished the AMA-induced mtROS generation in GEN2.2 cells. These results indicate that the increased MitoSox™ Red fluorescence was indeed due to mtROS production triggered by the inhibition of Complex III activity and not to other factors (Fig. 1A, B).
3.2. Increased levels of mtROS do not alter significantly the CpG-A-induced phenotypical changes of GEN2.2 cells but diminish their pro-inflammatory cytokine and chemokine release
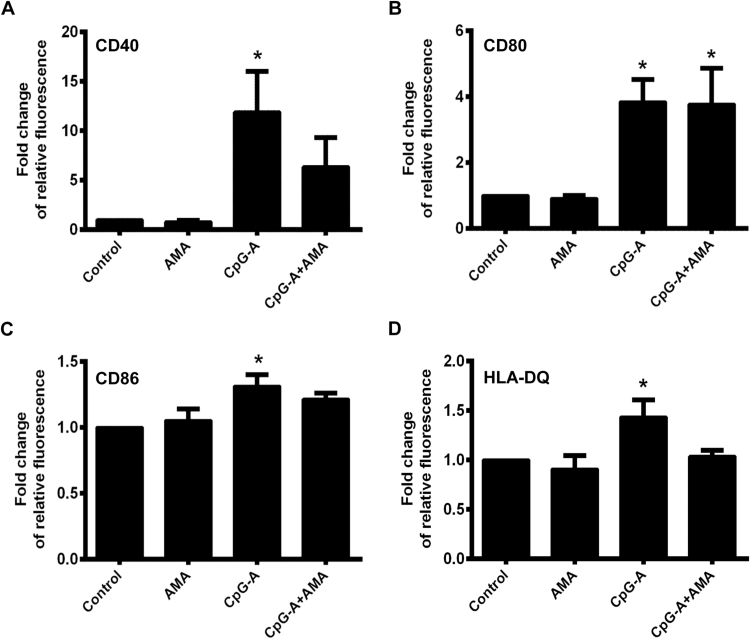
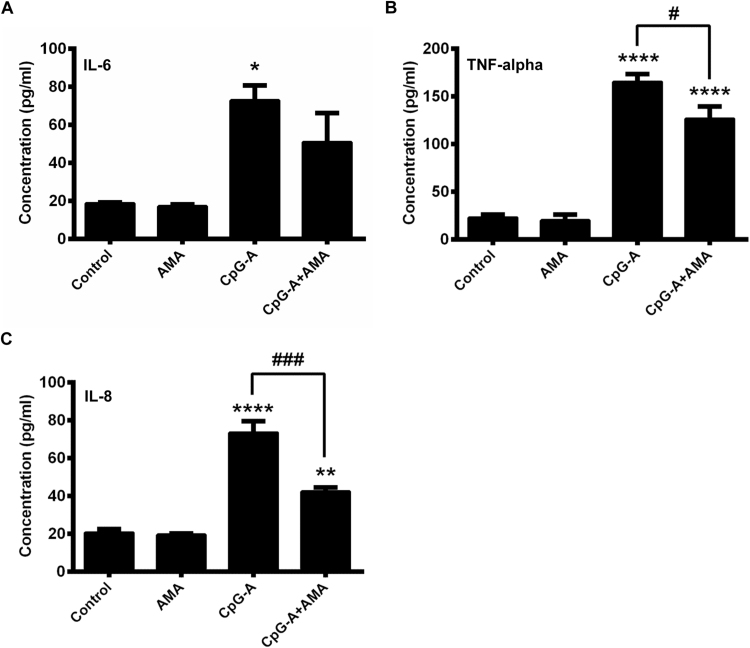
In the next step we investigated the effects of elevated mtROS production on the phenotype and pro-inflammatory cytokine and chemokine secretion of pDCs treated with CpG-A, a synthetic oligonucleotide with unmethylated CpG motifs mimicking viral DNA [17]. This type of CpG oligonucleotides is a potent activator of TLR9 and can result in robust type I IFN production in pDCs [18]. Non-activated and CpG-A-activated pDCs were exposed to AMA and changes in the expression of cell surface markers were monitored by flow cytometry, whereas the amount of secreted IL-6, TNF-alpha and IL-8 proteins in the cell culture supernatants was measured by ELISA. Specifically, we investigated the expression of the CD40, CD80 and CD86 costimulatory molecules and the MHC class II family protein HLA-DQ that represents the activation and subsequent maturation of dendritic cells [19]. Our results show that the AMA-induced production of mtROS does not affect cell surface expression of the above mentioned molecules indicating that the elevated level of mtROS alone does not lead to the activation and the maturation of pDCs (Fig. 2A–D). Nevertheless, co-stimulation of pDCs with AMA and the TLR9 agonist CpG-A, which significantly increases the cell surface expression of all examined molecules when applied alone, resulted in a moderate but not significant decrease in the expression of CD40, CD86 and HLA-DQ (Fig. 2A, C, D), whereas did not affect the expression of CD80 (Fig. 2B). Treatment of the GEN2.2 cells with AMA alone does not affect their basal cytokine and chemokine secretion, whereas it decreases significantly the TLR9-ligand induced production of TNF-alpha and IL-8 (Fig. 3B, C). In levels of IL-6 we could not observe significant changes upon AMA treatment of TLR9-stimulated cells (Fig. 3A). Based on our data, elevated production of mtROS tends to inhibit TLR9-induced activation of pDCs and its effects are more apparent on cytokine and chemokine release than on phenotypic changes.
Fig. 2.
Phenotypical analysis of AMA-exposed GEN2.2 cell. Cells were treated with AMA (0.5 μg/ml) and CpG-A (1 μM) separately and in combination or were left untreated. Following 6 h of stimulation the expression levels of CD40 (A), CD80 (B), CD86 (C) and HLA-DQ (D) cell surface proteins were analyzed by flow cytometry. Relative fluorescence intensity values were calculated using the respective isotype-matched control antibodies. The bars represent fold changes compared to the untreated control and data are expressed as the mean ± SD of four independent experiments. *p < 0.05 vs. control. AMA: Antimycin-A.
Fig. 3.
Pro-inflammatory cytokine and chemokine secretion of GEN2.2 cells exposed to AMA. Cells were stimulated with AMA (0.5 μg/ml) and CpG-A (1 μM) separately and in combination. After 6 h cell culture supernatants were collected and the levels of IL-6 (A), TNF-alpha (B) and IL-8 (C) proteins were assessed by ELISA. Data are presented as means ± SD of four independent experiments. *p < 0.05, **p < 0.01, ****p < 0.0001 vs. control, #p < 0.05, ###p < 0.001 vs. CpG-A. AMA: Antimycin-A.
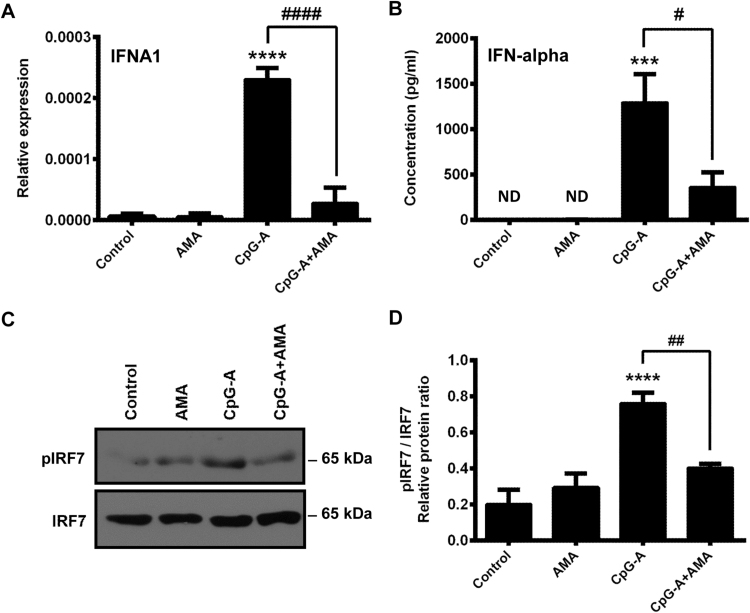
3.3. Mitochondrial ROS suppress the CpG-A-induced production of IFN-α via blocking IRF7 phosphorylation in GEN2.2 cells
The unique capability of pDCs is the massive production of type I IFNs in response to a wide range of viruses [9]. Therefore, in our next experiments we aimed to reveal the impact of elevated mtROS levels on type I IFN production of pDCs. Previously we have described that the type I IFN production of pDCs occurs in two waves. Endosomal TLRs mediate the initial activation of the cells, whereas cytosolic RIG-I receptors induced by TLR activation can be engaged in the second wave of type I IFN responses [14]. First we investigated how increased mtROS affect the initial wave of type I IFN production induced by endosomal TLR activation. Resting or TLR9-stimulated GEN2.2 cells were treated with AMA and 6 h after stimulation the expression of IFNA1 gene and the production of IFN-α were measured. We found that administration of AMA does not affect the baseline expression of type I IFN genes (Fig. 4A), and we could not detect IFN-α production in either unstimulated or in AMA-treated cell (Fig. 4B), indicating that the elevated level of mtROS alone does not induce type I IFN production of pDCs. However, the CpG-A-induced expression of type I IFNs was significantly decreased at both mRNA and protein levels when cells were activated in the presence of AMA (Fig. 4A, B).
Fig. 4.
AMA effects on the production of type I IFNs and phosphorylation of IRF7 in TLR9-activated GEN2.2 cells. Cells were stimulated with the TLR9 agonist CpG-A (1 μM) in the presence or absence of AMA (0.5 μg/ml). After 6 h of stimulation the mRNA expression of IFNA1 was measured by real-time PCR (A). The level of secreted IFN-α cytokine was determined by ELISA (B). Cells were stimulated for 30 min as described above then the levels of the phosphorylated (p-IRF7) and native form of IRF7 were determined by Western blotting (C, D). Bars represent the means ± SD of three individual experiments (A, B, D) and a representative blot is shown (C). ***p < 0.001, ****p < 0.0001 vs. control, #p < 0.05, ##p < 0.01, ####p < 0.0001 vs. CpG-A. AMA: Antimycin-A, ND: not determined.
Activation of the endosomal TLR9 receptor leads to the phosphorylation of the IRF7 transcription factor through the MyD88-dependent signaling pathway resulting in the production of type I IFNs [20]. To analyze the molecular mechanism of the observed inhibitory effects of elevated mtROS on TLR9-mediated signal transduction, we have investigated the impact of AMA treatment on the activity of IRF7. We have found that AMA alone does not alter the phosphorylation of IRF7 in resting cells, whereas it decreases this process in TLR9-stimulated pDCs (Fig. 4C, D). Our data indicate that the negative regulatory effect of mtROS on the phosphorylation of IRF7 in the TLR9 signaling pathway may be responsible for the diminished production of type I IFNs in pDCs.
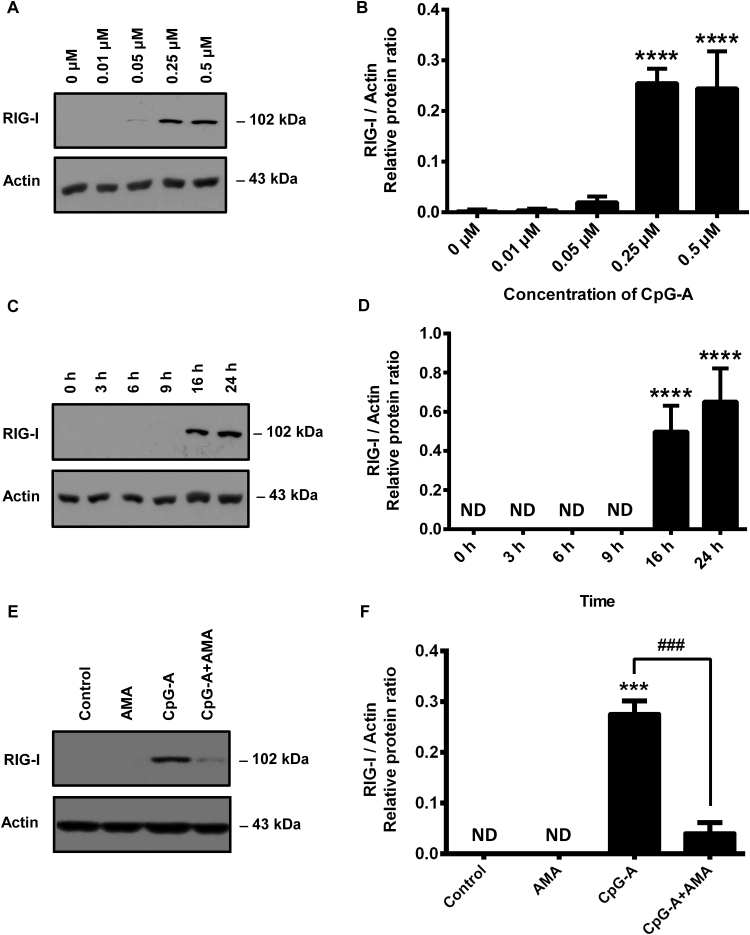
3.4. Elevated mtROS level decreases the CpG-A induced expression of RIG-I
The second wave of type I IFN responses can be mediated by cytosolic RIG-like helicases only after their expression is upregulated by endosomal TLR activation in pDCs [14]. To investigate the effect of elevated mtROS level on RIG-I-mediated type I IFN production of pDCs, first, we have determined the lowest concentration of CpG-A which still provides a robust induction of RIG-I in GEN2.2 cells. Based on our dose-response curve obtained by using Western blot, we have found that 0.25 μM concentration of CpG-A can boost the expression of RIG-I (Fig. 5A, B). Significant RIG-I expression was induced after 16 h of stimulation with CpG-A that was only slightly further increased by 24 h (Fig. 5C, D). Therefore, to induce RIG-I expression GEN2.2 cell were treated with 0.25 μM of CpG-A for 16 h in our subsequent experiments.
Fig. 5.
Dose- and time-dependent induction of cytosolic RIG-I receptor in GEN2.2 cells and the expression of RIG-I in AMA-exposed cells. In order to induce the cytosolic expression of RIG-I GEN2.2 cells were treated with increasing concentration of the specific TLR9 ligand, CpG-A (ranging from 0.01 to 0.5 μM). After 16 h the presence of RIG-I was detected in the cell lysates by Western blotting (A, B). To evaluate the time-dependent induction of RIG-I, GEN2.2 cells were exposed to 0.25 μM of CpG-A then the expression of RIG-I was measured in different time points (C, D). Finally cells were exposed to 0.25 μM of CpG-A in the presence or absence of AMA (0.5 μg/ml) or left untreated, for 16 h. Then cells were lysed and the protein level of RIG-I was assessed (E, F). Representative blots (A, C, E) and the means ± SD of four independent experiments (B, D, F) are shown. ***p < 0.001, ****p < 0.0001 vs. controls, ###p < 0.001 vs. CpG-A. AMA: Antimycin-A, ND: not determined.
Next, we have studied the effect of elevated mtROS levels on the TLR9-induced expression of RIG-I. We have found that RIG-I is not expressed in untreated or AMA-treated cells; however, the presence of AMA interfered with the TLR9-induced expression of RIG-I significantly (Fig. 5E, F). These data together suggest that elevated levels of mtROS inhibit both the early wave of type I IFN responses and the endosomal TLR-induced expression of RIG-I in pDCs.
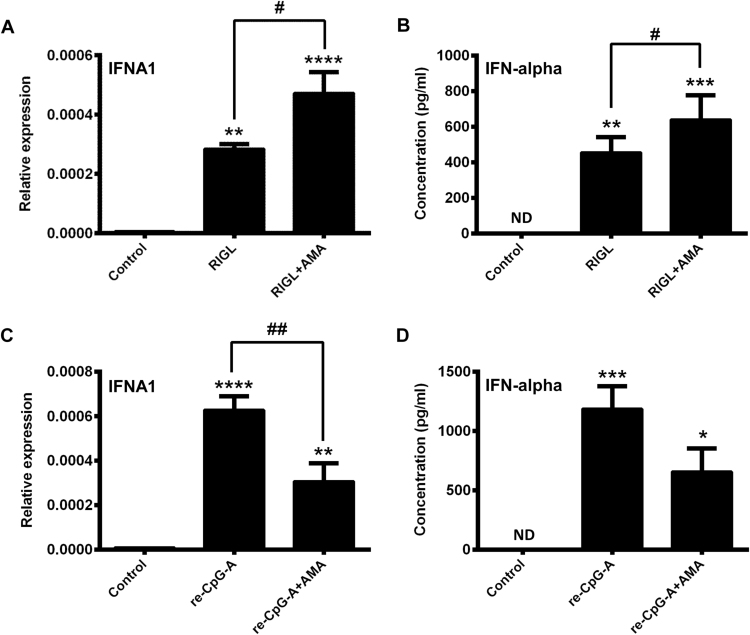
3.5. Increased level of mtROS enhances type I IFN production in RIG-I-activated GEN2.2 cells whereas decreases it in CpG-A re-stimulated cells
Next we analyzed how the elevated level of mtROS might influence the second wave of type I IFN production triggered by RIG-I. As we have shown previously resting primary pDCs [14] as well as resting GEN2.2 cells (Fig. 5A–F) do not express RIG-I at the protein level. Therefore, we provoked the upregulation of RIG-I in GEN2.2 cells using the TLR9 ligand CpG-A as described previously (Fig. 5A–D). After 16 h of stimulation the cell supernatants were removed and after thorough washing steps the cells were harvested and re-seeded in fresh medium as described in the Materials and Methods. After treatment of pDCs with 5′ppp-dsRNA, a synthetic RIG-I ligand, which mimics replicating viral RNA intermediates, type I IFN production was determined by Q-PCR and Western blotting.
We have found that GEN2.2 cells are able to response to 5′ppp-dsRNA treatment and produce type I IFNs when pre-activated by TLR9 ligand (Fig. 6A, B). These data imply that similar to primary pDCs, TLR9-mediated signals induce the expression of functional RIG-I in GEN2.2 cells. Interestingly, addition of AMA together with 5′ppp-dsRNA, the specific RIG-I-ligand, augmented the RIG-I-elicited type I IFN production (Fig. 6A, B). These data demonstrate that the endosomal TLR-mediated early phase is inhibited (Fig. 4A, B), whereas the RIG-I-mediated late phase of type I IFN production is intensified by the elevated mtROS levels.
Fig. 6.
RIG-I and TLR9 induced type I IFN production in CpG-A-pre-conditioned GEN2.2 cells in the presence of AMA. Cells were pre-treated with CpG-A (0.25 μM) for 16 h to induce the cytosolic expression of RIG-I. Following thorough washing steps cells were re-exposed to the specific RIG-I ligand 5′ppp-dsRNA (RIGL, 1 μg/ml) in the presence or absence of AMA (0.5 μg/ml). The IFNA1 mRNA expression level was determined by real-time PCR after 3 h (A) and IFN-α protein level was assessed by ELISA after 6 h (B). In parallel experiments the re-activation of the cells was carried out with high dose of CpG-A (1 μM) in combination or not with AMA (0.5 μg/ml). IFNA1 mRNA (C) and IFN-α protein levels (D) were measured as in (A) and (B), respectively. Data are presented as means ± SD of four individual experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control, #p < 0.05 vs. RIGL and ##p < 0.01 vs. CpG-A. AMA: Antimycin-A, ND: not determined.
To exclude the possibility that the opposing regulatory effects of increased mtROS on TLR9- and RIG-I-mediated cellular responses are due to the different activation state of pDCs at the time of mtROS induction, in parallel experiments, GEN2.2 cells were activated with a small dose of CpG-A (0.25 µM) then re-stimulated with a higher dose of CpG-A (1 µM) in the presence or absence of AMA (Fig. 6C, D). A negative regulatory role of elevated mtROS on type I IFN production was also found when pDCs were re-stimulated by endosomal TLR ligand and not by RIG-I-specific ligand (Fig. 6C, D). These observations suggest that regulatory effects of increased mtROS on type I IFN responses of pDCs are signaling pathway specific but are independent of the activation state of the cells.
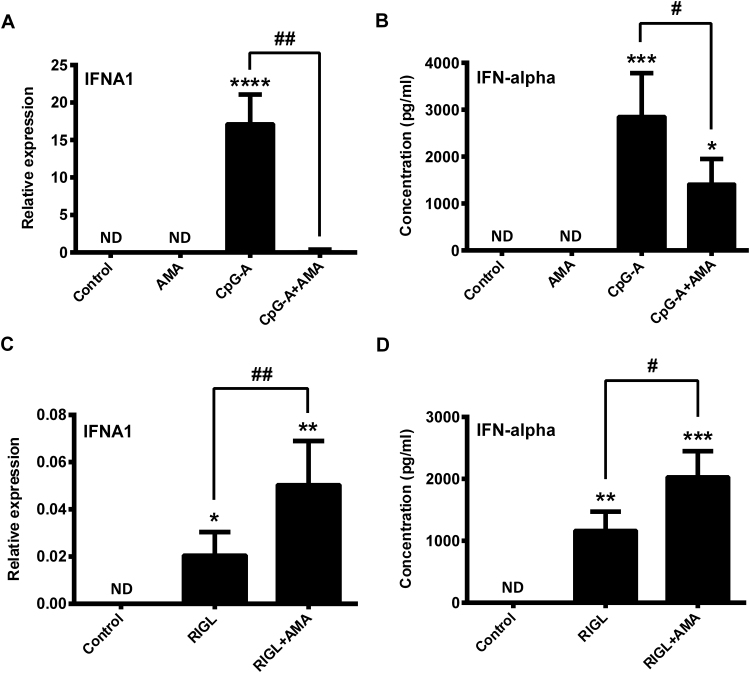
3.6. Elevated level of mtROS has similar effects on the first and second wave of type I IFN responses in primary human pDCs as in GEN2.2 cells
To validate the opposing modulatory role of mtROS in the regulation of type I IFN production by pDCs, we isolated primary human pDCs from peripheral blood of healthy donors and performed the same experiments as on GEN2.2 cells. We found that similarly to GEN2.2 cells, AMA treatment significantly inhibited the TLR9-induced IFN-α production (Fig. 7A, B), whereas significantly increased the RIG-I-initiated IFN-α secretion in primary human pDCs (Fig. 7C, D). These results further support our observations that mtROS have a versatile regulatory function in the coordination of the early and late phase of type I IFN responses in human pDCs.
Fig. 7.
Effects of AMA treatments on the first and second waves of type I IFN production in primary human pDCs. Freshly isolated primary pDCs were stimulated with the TLR9 agonist CpG-A (1 μM) in the presence or absence of AMA (0.5 μg/ml). After 6 h of stimulation the mRNA expression of IFNA1 was measured by real-time PCR (A) and the level of secreted IFN-α cytokine was determined by ELISA (B). To investigate the effects of AMA on the second wave of type I IFN responses, primary pDCs were pre-treated with CpG-A for 16 h then after thorough washing steps cells were re-exposed to 5′ppp-dsRNA (RIGL, 1 μg/ml) in the presence or absence of AMA (0.5 μg/ml). After 3 h the IFNA1 mRNA expression level was determined by real-time PCR (C) and after 6 h IFN-α protein level was assessed by ELISA (D). Data are presented as means ± SD of three independent experiments. *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001 vs. control, #p < 0.05 vs. CpG-A or RIGL and ##p < 0.01 vs. CpG-A or RIGL. AMA: Antimycin-A, ND: not determined.
3.7. Increased level of mtROS positively regulates the expression and the phosphorylation of the key signaling molecules of the RIG-I signaling cascade
In the last set of experiments, we have investigated the expression level and the phosphorylation state of the key signaling components in the RIG-I pathway to assess their relation to the positive regulatory role of enhanced mtROS production.
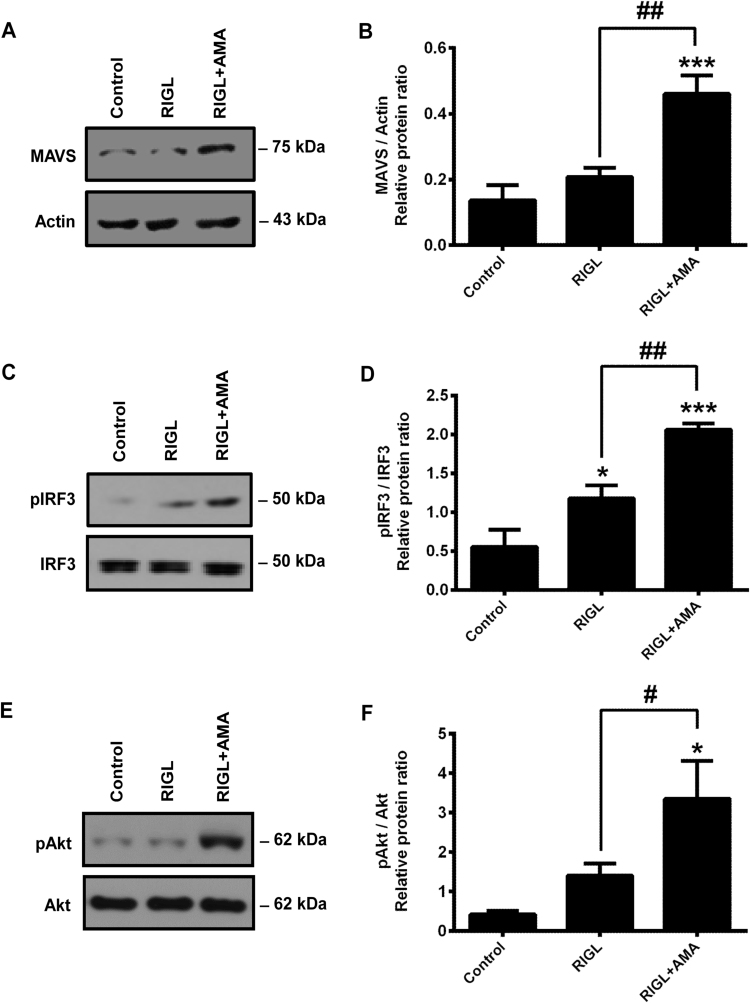
RIG-I expression in GEN2.2 cells was induced as described above and then cells were activated with 5′ppp-dsRNA in the presence or absence of AMA. Following treatments, we have analyzed the expression of MAVS (also known as IPS-1 or Cardif) adaptor protein, and the phosphorylation of IRF3 in cell lysates by Western blotting. MAVS protein level was similar in control and 5′ppp-dsRNA stimulated cells; however, it was increased significantly by co-treatment with 5′ppp-dsRNA and AMA (Fig. 8A, B). Furthermore, we have also found that the RIG-I-induced IRF3 phosphorylation was significantly higher upon co-treatment of pDCs with RIG-I ligand and AMA compared to treatment with 5′ppp-dsRNA only (Fig. 8C, D).
Fig. 8.
Analysis of signaling events in RIG-I-activated GEN2.2 cells in the presence or absence of AMA. Cytosolic RIG-I expression was induced in GEN2.2 by pre-treatment with CpG-A (0.25 μM) for 16 h then cells were stimulated with the specific RIG-I ligand 5′ppp-dsRNA (RIGL, 1 μg/ml) in the presence or absence of AMA (0.5 μg/ml). After 2 h of stimulation the expression of the MAVS adaptor protein (A, B) and after 30 min of stimulation the phosphorylation of IRF3 (C, D) and Akt (E, F) were determined by Western blotting. Representative blots (A, C, E) and the means ± SD of four independent experiments (B, D, F) are shown. *p < 0.05, **p < 0.01, ***p < 0.001 vs. control, #p < 0.05, ##p < 0.01 vs. RIGL. AMA: Antimycin-A.
Previous publications have revealed that the phosphatidylinositol-3-kinase (PI3K)/Akt signaling pathway is upregulated upon viral infection and required for the complete activation and phosphorylation of IRF3 [21]. Furthermore, activation of PI3K/Akt signaling has already been shown to be regulated in a redox-sensitive manner [22]. Based on these observations we have analyzed the activation of Akt in the presence of elevated mtROS levels. Our data demonstrate that stimulation of pDCs with 5′ppp-dsRNA tends to increase the phosphorylation of Akt; however, the changes are not significant compared to the control (Fig. 8E, F). Nevertheless, co-exposure of the cells to 5′ppp-dsRNA and AMA resulted in a significant increase in Akt phosphorylation compared both to the control and to the cells stimulated with 5′ppp-dsRNA only (Fig. 8E, F). Based on our results enhanced mtROS level exerts a direct, activating effect on RIG-I signaling and contributes to the RIG-I induced type I IFN production of pDCs.
4. Discussion
Mitochondria-derived ROS are continuously generated under normal conditions and at physiological levels they function as second messengers in a broad range of signaling pathways, thus exert beneficial effects on several cellular processes [4]. Nevertheless, the increased production of mtROS has been associated with multiple pathologies such as cancer, hypertension, atherosclerosis, diabetes, autoimmune disorders [4] or age-related diseases [23]. Over the past decades a myriad of studies also demonstrated the critical role of mtROS in cellular signaling pathways and innate immune responses including antiviral and antibacterial immunity [24], [25], [26]. In this study we aimed to characterize the role of mtROS in the TLR- and RIG-I-mediated type I IFN responses in human pDCs that play a pivotal role in antiviral immunity.
Various exogenous and endogenous stimuli, including immunoreceptor ligation, cytokine stimulation, hypoxia and infections can increase mtROS levels. Besides other pathogens, a number of viruses cause oxidative stress to the host cells to manipulate signaling pathways and promote their replication [27], [28], [29]. Infections by blood-borne hepatitis viruses, human immunodeficiency virus, influenza A, Epstein-Barr virus, respiratory syncytial virus, and other viruses can increase ROS production or decrease cytosolic antioxidant levels (reviewed in [30]). Increased ROS production might contribute to alterations in mitochondrial functions and dysfunctional mitochondria, in turn, can contribute to exacerbate oxidative stress [31]. Furthermore, certain viral proteins are able to directly [32] or indirectly [33] trigger enhanced release of ROS from mitochondria. Not only replication of viruses in the host cells but also sensing of viral components and damage-associated molecular pattern (DAMP) molecules by immune cells via pattern recognition receptors can boost mtROS generation. It has been demonstrated that stimulation of cell surface TLRs (TLR1, TLR2 and TLR4) but not of endosomal TLRs (TLR3, TLR7, TLR8 and TLR9) resulted in increased mtROS production through TNF-receptor associated factor 6 (TRAF6) and evolutionarily conserved signaling intermediate in Toll pathway (ECSIT) signaling [34]. When TRAF6 and ECSIT were depleted, macrophages produced significantly less mtROS that resulted in decreased bactericidal activity [34]. Plasmacytoid DCs are considered to be a unique dendritic cell subset that exhibit a distinct TLR repertoire and rely mainly on the endosomal TLR7 and TLR9 receptors to sense viral infections. However, recent studies have reported that pDCs from healthy subjects also express TLR2 on the cell surface [35], [36], which recognizes a wide array of viral proteins after forming heterodimers with TLR1 or TLR6 [37]. Virus-infected cells release high mobility group box 1 (HMGB1), which functions as an extracellular signaling and DAMP molecule [38]. It has been shown that HMGB1 binds to at least five different cell surface receptors including receptor for advanced glycation endproducts (RAGE) [39] expressed by pDCs [40]. Ligand-RAGE interaction generates oxidative stress via activation of NADPH oxidase and amplification of mtROS production (reviewed in [41]). These findings indicate that several mechanisms can be responsible for enhanced mtROS production in pDCs at the early phase of viral infection.
Previously, we have shown that exogenously added ROS impact the cellular responses of primary human pDCs to microbial stimulus. Most importantly, a low dose of hydrogen peroxide (H2O2) inhibited the TLR7-induced phenotypic activation, cytokine production, and the T-cell-activating capacity of pDCs [42]. Our recent results show that similarly to H2O2, mtROS decrease the TLR9-induced type I IFN secretion of both GEN2.2 cells and primary pDCs. Thus, our results suggest that endosomal TLR-mediated antiviral responses of human pDCs are greatly sensitive to ROS and reduced when exposed to either exogenous or endogenous sources of ROS.
Previously, it was thought that plasmacytoid DCs preferentially use the TLR system rather than RIG-I-like receptors (RLR) for the detection of viral infections contrary to conventional dendritic cells or macrophages [43]. However, we have recently found that RIG-I also contributes to viral recognition and subsequent antiviral immune responses of pDCs [14]. In resting human pDCs, RIG-I is expressed at a very low level, but its expression is greatly upregulated by stimulating the cells with TLR7 or TLR9 ligands [14]. Thus, our previous results imply that upon recognition of viral nucleic acids the early type I IFN response depends on TLR-mediated signals, whereas the second wave of type I IFN production can be mediated by RLR signaling as well. After sensing viral nucleic acids, TLR7 and TLR9 receptors activate NF-κB and IRF7 in a MyD88-dependent manner, whereas RLRs interact with the MAVS adaptor protein leading to activation of the NF-κB, IRF3 and IRF7 transcription factors [44]. Similar to TLRs, the signaling of cytosolic RLRs is also regulated via mtROS. This was explored by the use of autophagy defective Atg5-/- mouse fibroblasts and macrophages, which accumulate dysfunctional mitochondria as well as mitochondria-associated MAVS and exhibit elevated mtROS levels [45]. Atg5-/- cells also possess increased RLR signaling, type I IFN secretion and resistance to vesicular stomatitis virus (VSV) infection that can be blocked by antioxidant treatment [45]. Our current findings that mtROS have a positive impact on the TLR-induced RIG-I pathway in pDCs, are in line with these previous results showing that mtROS potentiate RLR signaling [45]. Thus, our results further support the idea that mtROS are important second messengers in the RIG-I-MAVS signaling pathway during viral infection. A recent study concluded that mtROS modulate the DNA virus-triggered but not the RNA virus-initiated innate immune responses negatively in a murine model [46]. Inhibition of electron transport chain decreased the Herpes simplex virus (HSV)- but not the Sendai virus-induced production of cytokines indicating that mitochondrial oxidative stress modulates DNA-driven innate immune responses, whereas RIG-I, which recognizes Sendai virus is insensitive to mtROS [46]. In addition, murine peritoneal macrophages treated with AMA showed diminished CCL5 and IFN-β production upon stimulation of TLR2, TLR4, TLR9 and DNA receptors but not of RLRs [24]. Moreover, pre-treatment with inhibitors of the electron transport chain reduced the HSV-2-induced IκBα phosphorylation and IRF3 nuclear translocation in human THP1 macrophages and murine peritoneal cells. By contrast, our findings demonstrate increased IRF3 activation upon AMA treatment of RIG-I-stimulated pDCs and we suppose that the varying results might be explained by cell type specificities. Since RIG-I might also signal through IRF7 we have also measured its phosphorylation at serine 477 following treatments; however, we could not detect any differences (data not shown). Therefore, we suggest that mtROS promote the RIG-mediated type I IFN production through enhancing the activation of IRF3 in human pDCs.
Despite the identification of many signaling partners linking MAVS to the IRF and NF-κB routes the regulating mechanisms of MAVS in virus-induced signaling are poorly understood. A recent study revealed that PI3K and Akt play a pivotal role for the RIG-I mediated signaling via association with MAVS [21]. Inhibition of PI3K and Akt blocked IRF3 activation and IFN-β expression induced by the RIG-I agonist short polyinosinic-polycytidylic acid (polyI:C) or Sendai virus in macrophages. Further, it was shown that the pleckstrin homology domain of Akt interacts with the CARD domain of MAVS and the regulatory unit of PI3K also associates with it. These results suggest that Akt and MAVS provide a signaling platform for activating downstream signaling pathways upon viral infection. Moreover, a recent study indicates that the release of mtROS into the cytoplasm induces ROS-dependent phosphorylation of Akt [47]. In line with these reported findings we observed that RIG-I stimulation is associated with the augmentation of Akt phosphorylation, that could be further increased by AMA treatment. When investigating MAVS protein levels we could detect an mtROS-dependent but RIG-I-independent upregulation of the protein level. These observations correlate with previous findings showing that oxidative-stress can lead to MAVS oligomerization and subsequent activation independently of RIG-I helicases [48]. Based on these findings we suggest that one of the mechanisms whereby mtROS support the RIG-I mediated signaling pathway is the modulation of MAVS activity, however, this hypothesis requires further clarification.
Plasmacytoid DCs are the major sources of type I IFNs upon viral infections; however, the excessive production of type I IFNs is not always beneficial, as it can result in uncontrolled inflammation and destruction of healthy tissues [43]. In this regard, control of the TLR-mediated systemic IFN responses by circulating pDCs during the early phase of viral infection may prevent excessive tissue damage. At the late stages of infection pDCs redistribute to the infected peripheral tissues where they can be exposed to elevated levels of ROS produced by inflammatory cells (reviewed in [26]) and can be infected by replicating viruses leading to cytosolic recognition of viral RNA by RIG-I, which results in the second wave of type I IFN production in pDCs. Of note, the RIG-I mediated second phase of IFN production is much lower compared to the TLR-initiated first phase of IFN secretion and is boosted by mtROS as supported by our results.
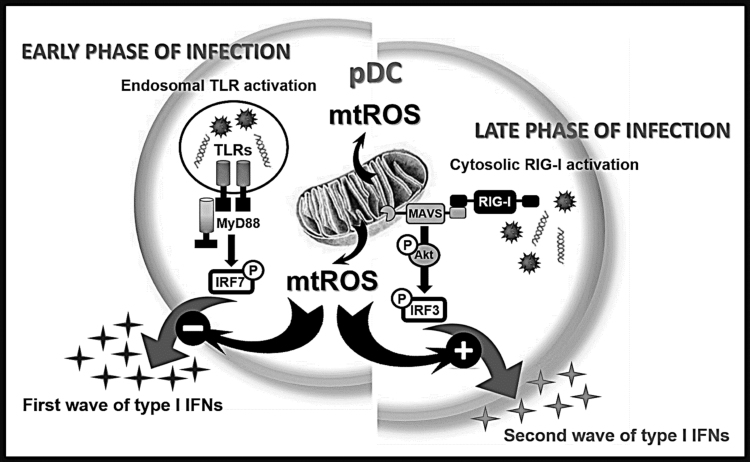
In conclusion, we propose a model where mtROS impact the TLR-induced first wave of type I IFN responses negatively, whereas affect the RIG-I-mediated second wave of type I IFN production positively (Fig. 9). The opposing effect of mtROS on the TLR- and RLR-mediated signaling pathway reflects the versatile role of mtROS in fine-tuning the type I IFN mediated innate immune responses by pDCs. Further characterization of this spatio-temporal regulation of signaling pathways by mtROS might expand our knowledge to improve drugs targeting mtROS-dependent molecules for the treatment of inflammatory diseases.
Fig. 9.
Overview of the effects of elevated level of mtROS on the endosomal TLR-, and cytosolic RIG-I-mediated pDC activation. Upon infection cells are affected by many various exogenous and endogenous stress factors leading to higher respiratory activity of the mitochondria and subsequent increase in mtROS production in the cells. We found that the elevated level of mtROS has a negative impact on the first wave of type I IFN responses mediated by endosomal TLRs whereas it has a positive effect on the second wave of type I IFN production guided by cytosolic RIG-I receptor in pDCs. Thus, our data indicate opposing regulatory role of mtROS depending on the receptor-context in pDCs.
Conflict of interest
The authors declare that they have no conflict of interest.
Acknowledgements
GEN2.2 cells used in this study were generously provided by Joel Plumas and Laurence Chaperot of Research and Development Laboratory, EFS Rhônes-Alpes, 29 Av Maquis du Gresivaudan, BP 35, 38701 La Tronche, France. This work was supported by the National Research, Development and Innovation Office (NKFIH, PD 115776 and PD_16 120887 to K.P.; K 125337 to A.B.), the Romanian Ministry of Education, Executive Agency for Higher Education, Research, Development and Innovation Funding (PNCDI II, 119/2014 to A.Sz., A.L. and A.B) and the US National Institute of Allergic and Infectious Diseases (AI062885 to I.B.). The work was also supported by GINOP-2.3.2-15-2016-00050 project (T.B. and A.B). The project is co-financed by the European Union and the European Regional Development Fund. K.P. was also supported by the János Bolyai Research Scholarship from the Hungarian Academy of Sciences.
References
- 1.Schieber M., Chandel N.S. ROS function in redox signaling and oxidative stress. Curr. Biol. 2014;24(10):R453–R462. doi: 10.1016/j.cub.2014.03.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Turrens J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003;552(Pt 2):335–344. doi: 10.1113/jphysiol.2003.049478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shadel G.S., Horvath T.L. Mitochondrial ROS signaling in organismal homeostasis. Cell. 2015;163(3):560–569. doi: 10.1016/j.cell.2015.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li X., Fang P., Mai J., Choi E.T., Wang H., Yang X.F. Targeting mitochondrial reactive oxygen species as novel therapy for inflammatory diseases and cancers. J. Hematol. Oncol. 2013;6:19. doi: 10.1186/1756-8722-6-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muller F.L., Liu Y., Van Remmen H. Complex III releases superoxide to both sides of the inner mitochondrial membrane. J. Biol. Chem. 2004;279(47):49064–49073. doi: 10.1074/jbc.M407715200. [DOI] [PubMed] [Google Scholar]
- 6.Rhee S.G., Bae Y.S., Lee S.R., Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci. Stke. 2000;(53) doi: 10.1126/stke.2000.53.pe1. (pe1) [DOI] [PubMed] [Google Scholar]
- 7.Finkel T. From sulfenylation to sulfhydration: what a thiolate needs to tolerate. Sci. Signal. 2012;5(215) doi: 10.1126/scisignal.2002943. (p. pe10) [DOI] [PubMed] [Google Scholar]
- 8.Pourcelot M., Arnoult D. Mitochondrial dynamics and the innate antiviral immune response. FEBS J. 2014;281(17):3791–3802. doi: 10.1111/febs.12940. [DOI] [PubMed] [Google Scholar]
- 9.Siegal F.P., Kadowaki N., Shodell M., Fitzgerald-Bocarsly P.A., Shah K., Ho S., Antonenko S., Liu Y.J. The nature of the principal type 1 interferon-producing cells in human blood. Science. 1999;284(5421):1835–1837. doi: 10.1126/science.284.5421.1835. [DOI] [PubMed] [Google Scholar]
- 10.Lande R., Gilliet M. Plasmacytoid dendritic cells: key players in the initiation and regulation of immune responses. Ann. N. Y Acad. Sci. 2010;1183:89–103. doi: 10.1111/j.1749-6632.2009.05152.x. [DOI] [PubMed] [Google Scholar]
- 11.Baranek T., Dalod M. How opportunistic agents benefit from viral infections: the plasmacytoid dendritic cell connection. Cell Host Microbe. 2008;4(4):305–307. doi: 10.1016/j.chom.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Kumagai Y., Kumar H., Koyama S., Kawai T., Takeuchi O., Akira S. Cutting Edge: TLR-dependent viral recognition along with type I IFN positive feedback signaling masks the requirement of viral replication for IFN-{alpha} production in plasmacytoid dendritic cells. J. Immunol. 2009;182(7):3960–3964. doi: 10.4049/jimmunol.0804315. [DOI] [PubMed] [Google Scholar]
- 13.Fullam A., Schroder M. DExD/H-box RNA helicases as mediators of anti-viral innate immunity and essential host factors for viral replication. Biochim. Biophys. Acta. 2013;1829(8):854–865. doi: 10.1016/j.bbagrm.2013.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Szabo A., Magyarics Z., Pazmandi K., Gopcsa L., Rajnavolgyi E., Bacsi A. TLR ligands upregulate RIG-I expression in human plasmacytoid dendritic cells in a type I IFN-independent manner. Immunol. Cell Biol. 2014;92(8):671–678. doi: 10.1038/icb.2014.38. [DOI] [PubMed] [Google Scholar]
- 15.Di Domizio J., Blum A., Gallagher-Gambarelli M., Molens J.P., Chaperot L., Plumas J. TLR7 stimulation in human plasmacytoid dendritic cells leads to the induction of early IFN-inducible genes in the absence of type I IFN. Blood. 2009;114(9):1794–1802. doi: 10.1182/blood-2009-04-216770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dikalova A.E., Bikineyeva A.T., Budzyn K., Nazarewicz R.R., McCann L., Lewis W., Harrison D.G., Dikalov S.I. Therapeutic targeting of mitochondrial superoxide in hypertension. Circ. Res. 2010;107(1):106–116. doi: 10.1161/CIRCRESAHA.109.214601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kadowaki N., Ho S., Antonenko S., Malefyt R.W., Kastelein R.A., Bazan F., Liu Y.J. Subsets of human dendritic cell precursors express different toll-like receptors and respond to different microbial antigens. J. Exp. Med. 2001;194(6):863–869. doi: 10.1084/jem.194.6.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kerkmann M., Costa L.T., Richter C., Rothenfusser S., Battiany J., Hornung V., Johnson J., Englert S., Ketterer T., Heckl W., Thalhammer S., Endres S., Hartmann G. Spontaneous formation of nucleic acid-based nanoparticles is responsible for high interferon-alpha induction by CpG-A in plasmacytoid dendritic cells. J. Biol. Chem. 2005;280(9):8086–8093. doi: 10.1074/jbc.M410868200. [DOI] [PubMed] [Google Scholar]
- 19.Hellman P., Eriksson H. Early activation markers of human peripheral dendritic cells. Hum. Immunol. 2007;68(5):324–333. doi: 10.1016/j.humimm.2007.01.018. [DOI] [PubMed] [Google Scholar]
- 20.Bao M., Liu Y.J. Regulation of TLR7/9 signaling in plasmacytoid dendritic cells. Protein Cell. 2013;4(1):40–52. doi: 10.1007/s13238-012-2104-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yeon S.H., Song M.J., Kang H.R., Lee J.Y. Phosphatidylinositol-3-kinase and Akt are required for RIG-I-mediated anti-viral signalling through cross-talk with IPS-1. Immunology. 2015;144(2):312–320. doi: 10.1111/imm.12373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakanishi A., Wada Y., Kitagishi Y., Matsuda S. Link between PI3K/AKT/PTEN pathway and NOX proteinin diseases. Aging Dis. 2014;5(3):203–211. doi: 10.14336/AD.2014.0500203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Marchi S., Giorgi C., Suski J.M., Agnoletto C., Bononi A., Bonora M., De Marchi E., Missiroli S., Patergnani S., Poletti F., Rimessi A., Duszynski J., Wieckowski M.R., Pinton P. Mitochondria-ros crosstalk in the control of cell death and aging. J. Signal Transduct. 2012:329635. doi: 10.1155/2012/329635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg S.E., Sena L.A., Chandel N.S. Mitochondria in the regulation of innate and adaptive immunity. Immunity. 2015;42(3):406–417. doi: 10.1016/j.immuni.2015.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Koshiba T., Yasukawa K., Yanagi Y., Kawabata S. Mitochondrial membrane potential is required for MAVS-mediated antiviral signaling. Sci. Signal. 2011;4(158) doi: 10.1126/scisignal.2001147. (p. ra7) [DOI] [PubMed] [Google Scholar]
- 26.Schwarz K.B. Oxidative stress during viral infection: a review. Free Radic. Biol. Med. 1996;21(5):641–649. doi: 10.1016/0891-5849(96)00131-1. [DOI] [PubMed] [Google Scholar]
- 27.Ivanov A.V., Valuev-Elliston V.T., Ivanova O.N., Kochetkov S.N., Starodubova E.S., Bartosch B., Isaguliants M.G. Oxidative stress during HIV infection: mechanisms and consequences. Oxid. Med Cell Longev. 2016:8910396. doi: 10.1155/2016/8910396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin X., Wang R., Zou W., Sun X., Liu X., Zhao L., Wang S., Jin M. The influenza virus H5N1 infection can induce ROS production for viral replication and host cell death in A549 cells modulated by human Cu/Zn superoxide dismutase (SOD1) overexpression. Viruses. 2016;8(1) doi: 10.3390/v8010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Machida K., Cheng K.T., Lai C.K., Jeng K.S., Sung V.M., Lai M.M. Hepatitis C virus triggers mitochondrial permeability transition with production of reactive oxygen species, leading to DNA damage and STAT3 activation. J. Virol. 2006;80(14):7199–7207. doi: 10.1128/JVI.00321-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anand S.K., Tikoo S.K. Viruses as modulators of mitochondrial functions. Adv. Virol. 2013:738794. doi: 10.1155/2013/738794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Bacha T., Da Poian A.T. Virus-induced changes in mitochondrial bioenergetics as potential targets for therapy. Int. J. Biochem. Cell Biol. 2013;45(1):41–46. doi: 10.1016/j.biocel.2012.09.021. [DOI] [PubMed] [Google Scholar]
- 32.Li Y., Boehning D.F., Qian T., Popov V.L., Weinman S.A. Hepatitis C virus core protein increases mitochondrial ROS production by stimulation of Ca2+ uniporter activity. FASEB J. 2007;21(10):2474–2485. doi: 10.1096/fj.06-7345com. [DOI] [PubMed] [Google Scholar]
- 33.McGuire K.A., Barlan A.U., Griffin T.M., Wiethoff C.M. Adenovirus type 5 rupture of lysosomes leads to cathepsin B-dependent mitochondrial stress and production of reactive oxygen species. J. Virol. 2011;85(20):10806–10813. doi: 10.1128/JVI.00675-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.West A.P., Brodsky I.E., Rahner C., Woo D.K., Erdjument-Bromage H., Tempst P., Walsh M.C., Choi Y., Shadel G.S., Ghosh S. TLR signalling augments macrophage bactericidal activity through mitochondrial ROS. Nature. 2011;472(7344):476–480. doi: 10.1038/nature09973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hernandez J.C., Arteaga J., Paul S., Kumar A., Latz E., Urcuqui-Inchima S. Up-regulation of TLR2 and TLR4 in dendritic cells in response to HIV type 1 and coinfection with opportunistic pathogens. AIDS Res. Hum. Retrovir. 2011;27(10):1099–1109. doi: 10.1089/aid.2010.0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Torres S., Hernandez J.C., Giraldo D., Arboleda M., Rojas M., Smit J.M., Urcuqui-Inchima S. Differential expression of toll-like receptors in dendritic cells of patients with dengue during early and late acute phases of the disease. PLoS Negl. Trop. Dis. 2013;7(2):e2060. doi: 10.1371/journal.pntd.0002060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oliveira-Nascimento L., Massari P., Wetzler L.M. The role of TLR2 in infection and immunity. Front. Immunol. 2012;3:79. doi: 10.3389/fimmu.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tang D., Kang R., Zeh H.J., 3rd, Lotze M.T. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011;14(7):1315–1335. doi: 10.1089/ars.2010.3356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rauvala H., Rouhiainen A. RAGE as a receptor of HMGB1 (Amphoterin): roles in health and disease. Curr. Mol. Med. 2007;7(8):725–734. doi: 10.2174/156652407783220750. [DOI] [PubMed] [Google Scholar]
- 40.Dumitriu I.E., Baruah P., Bianchi M.E., Manfredi A.A., Rovere-Querini P. Requirement of HMGB1 and RAGE for the maturation of human plasmacytoid dendritic cells. Eur. J. Immunol. 2005;35(7):2184–2190. doi: 10.1002/eji.200526066. [DOI] [PubMed] [Google Scholar]
- 41.Daffu G., del Pozo C.H., O'Shea K.M., Ananthakrishnan R., Ramasamy R., Schmidt A.M. Radical roles for RAGE in the pathogenesis of oxidative stress in cardiovascular diseases and beyond. Int. J. Mol. Sci. 2013;14(10):19891–19910. doi: 10.3390/ijms141019891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pazmandi K., Magyarics Z., Boldogh I., Csillag A., Rajnavolgyi E., Bacsi A. Modulatory effects of low-dose hydrogen peroxide on the function of human plasmacytoid dendritic cells. Free Radic. Biol. Med. 2012;52(3):635–645. doi: 10.1016/j.freeradbiomed.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Swiecki M., Colonna M. The multifaceted biology of plasmacytoid dendritic cells. Nat. Rev. Immunol. 2015;15(8):471–485. doi: 10.1038/nri3865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Koshiba T. Mitochondrial-mediated antiviral immunity. Biochim. Biophys. Acta. 2013;1833(1):225–232. doi: 10.1016/j.bbamcr.2012.03.005. [DOI] [PubMed] [Google Scholar]
- 45.Tal M.C., Sasai M., Lee H.K., Yordy B., Shadel G.S., Iwasaki A. Absence of autophagy results in reactive oxygen species-dependent amplification of RLR signaling. Proc. Natl. Acad. Sci. USA. 2009;106(8):2770–2775. doi: 10.1073/pnas.0807694106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gonzalez-Dosal R., Horan K.A., Paludan S.R. Mitochondria-derived reactive oxygen species negatively regulates immune innate signaling pathways triggered by a DNA virus, but not by an RNA virus. Biochem. Biophys. Res. Commun. 2012;418(4):806–810. doi: 10.1016/j.bbrc.2012.01.108. [DOI] [PubMed] [Google Scholar]
- 47.Feng B., Ye C., Qiu L., Chen L., Fu Y., Sun W. Mitochondrial ROS release and subsequent Akt activation potentially mediated the anti-apoptotic effect of a 50-Hz magnetic field on FL cells. Cell. Physiol. Biochem. 2016;38(6):2489–2499. doi: 10.1159/000445599. [DOI] [PubMed] [Google Scholar]
- 48.Buskiewicz I.A., Montgomery T., Yasewicz E.C., Huber S.A., Murphy M.P., Hartley R.C., Kelly R., Crow M.K., Perl A., Budd R.C., Koenig A. Reactive oxygen species induce virus-independent MAVS oligomerization in systemic lupus erythematosus. Sci. Signal. 2016;9(456) doi: 10.1126/scisignal.aaf1933. (p. ra115) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chaperot L., Blum A., Manches O., Lui G., Angel J., Molens J.P., Plumas J. Virus or TLR agonists induce TRAIL-mediated cytotoxic activity of plasmacytoid dendritic cells. J. Immunol. 2006;176(1):248–255. doi: 10.4049/jimmunol.176.1.248. [DOI] [PubMed] [Google Scholar]