Abstract
Neurogenesis is currently an area of great interest in neuroscience. It is closely linked to brain diseases, including mental disorders and neurodevelopmental disease. Both embryonic and adult neurogeneses are influenced by glucocorticoids secreted from the adrenal glands in response to a variety of stressors. Moreover, proliferation/differentiation of the neural stem/progenitor cells (NSPCs) is affected by glucocorticoids through intracellular signaling pathways such as phosphoinositide 3-kinase (PI3K)/Akt, hedgehog, and Wnt. Our review presents recent evidence of the impact of glucocorticoids on NSPC behaviors and the underlying molecular mechanisms; this provides important information for understanding the pathological role of glucocorticoids on neurogenesis-associated brain diseases.
Keywords: neural progenitor cells, glucocorticoids, neurogenesis, intracellular signaling pathways
Introduction
Glucocorticoids have been suggested to be involved in several brain diseases associated with stress such as post-traumatic stress disorder (PTSD), anxiety disorders, and major depressive disorder (MDD) (Holsboer et al., 2000; Herbert et al., 2013; Numakawa et al., 2013; Griffin et al., 2014; Raglan et al., 2017). Blood levels of glucocorticoids are increased in response to a variety of environmental stressors and are regulated by the negative feedback loop of the hypothalamic-pituitary-adrenal (HPA) axis (Ising et al., 2005; Owashi et al., 2008). Maternal stress during pregnancy has also been demonstrated to increase the risk of attention deficit hyperactivity disorder (ADHD) and autism spectrum disorder (ASD) in the offspring, through the elevation of glucocorticoid levels (Graignic-Philippeet et al., 2014). The impact of glucocorticoids on neuronal functions, including cell survival and synaptic plasticity in the central nervous system (CNS), has been extensively investigated through in vitro and in vivo studies. Moreover, research has indicated that neural stem/progenitor cells (NSPCs) are an important target of excess glucocorticoids during stress. The resultant dysregulation of neurogenesis may thus be involved in the onset of the brain diseases mentioned above. Furthermore, impaired neurogenesis in the hippocampal region may disrupt the HPA-axis functions because hippocampal neurons play an essential role in the negative feedback regulation of the HPA-axis (Roozendaa et al., 2001; Furay et al., 2008).
This review focuses on the relationship between glucocorticoids and neurogenesis demonstrated by both in vitro and in vivo studies. We have discussed recent evidence concerning altered intracellular signaling and cell phenotypes caused by glucocorticoids in NSPCs.
Adult Neurogenesis and Major Depressive Disorder
There is growing interest in the possible etiological contribution of adult neurogenesis in psychiatric diseases including MDD, bipolar disorder, schizophrenia, anxiety disorders, and PTSD (Schoenfeld and Cameron, 2015; Yun et al., 2016). The onset of MDD has been suggested to be particularly associated with adult neurogenesis in the dentate gyrus of the hippocampus. Several lines of evidence show reduced hippocampal neurogenesis in animal models of MDD caused by social defeat stress, chronic corticosterone (CORT, a murine glucocorticoid) exposure, lipopolysaccharide administration, and unpredictable chronic mild stress (altered bedding, cage tilting, shaking, cage exchange, induced defensive posture, altered light-dark cycle) (Levone et al., 2014; Tang et al., 2016). Various therapeutic interventions for MDD such as antidepressants, electroconvulsive shock, enriched environment, and exercise appear to enhance NSPC proliferation and the survival rate of newborn neurons in the adult hippocampus (Madsen et al., 2000; Santarelli et al., 2003; Jhaetal, 2011; Kiuchi et al., 2012). Human postmortem studies also show fewer granule neurons in the dentate gyrus of unmedicated MDD patients and an increased number of hippocampal NSPCs in MDD patients treated with antidepressants (Boldrini et al., 2009, 2012, 2013). These correlations between depression and reduced neurogenesis in both human and animal models suggest that neurogenesis may play a significant role in the etiology of depression. In order to address this hypothesis, hippocampal irradiation and inducible genetic modification of NSPCs have been utilized to regulate adult neurogenesis in stressed or antidepressant-treated animals. Although there have been conflicting results on the causal contribution of decreased neurogenesis in depressive behaviors, several studies have consistently reported that impaired hippocampal neurogenesis diminishes the effect of antidepressants in rodents (Santarelli et al., 2003; Surget et al., 2008, 2011). In the context of reduced neurogenesis in stressed animals, Hill and colleagues reported that increased survival of adult-born neurons induced by NSPCs-specific deletion of pro-apoptotic gene Bax ameliorated depressive behaviors in CORT-treated mice (Hill et al., 2015). It is of interest that both enhanced and reduced neurogenesis under basal conditions (unstressed and untreated) did not change behavior in animals. These results indicate that the alteration of neurogenesis is necessary but not sufficient to achieve antidepressant effects or cause depressive behaviors (Santarelli et al., 2003; Hill et al., 2015).
Chronically elevated glucocorticoid levels under prolonged stress are the most common biological feature in MDD patients (Figure 1) (Numakawa et al., 2013). Animals chronically treated with CORT at a dose of 20 mg/kg/day for 25 days exhibited depressive behaviors and decreased neurogenesis in the hippocampal dentate gyrus (Sawamoto et al., 2016). Adrenalectomy (surgical removal of the adrenal glands) also prevents depressive behaviors and reduced neurogenesis in chronically stressed murines, suggesting that glucocorticoids are a major mediator of depressive behaviors and impaired neurogenesis under chronic stress (Lehmann et al., 2013). Importantly, the hippocampus negatively regulates HPA-axis activity in response to elevated blood glucocorticoid levels, raising the possibility that impaired hippocampal neurogenesis influences the negative feedback function to regulate HPA-axis activity.
Figure 1.
Glucocorticoid hypothesis of neurodevelopmental disease and depressive disorder.
Possible impacts of stress-induced glucocorticoid secretion from adrenal cortex on adult or embryonic neurogenesis are shown. ADHD: Attention deficient hyperactivity disorder, ASD: autism spectrum disorder; GCs: glucocorticoids. Reference: (1) Wong et al., 2000; (2) Lenze et al., 2011; (3) Sawamoto et al., 2016; (4) Lehmann et al., 2013; (5) Schloesser et al., 2009; (6) Grizenko et al., 2012; (7)Van den Bergh and Marcoen., 2004; (8) Beversdorf et al., 2005; (9) Kinney et al., 2008; (10) Van den Bergh et al., 2008; (11) Khashan et al., 2008; (12) van Os and Selten, 1998; (13) Kanagawa et al., 2006; (14) Noorlander et al., 2014.
Schloesser et al. (2009) reported that suppression of hippocampal neurogenesis led to increased HPA response after exposure to a mild stressor. In the study, NSPCs in the hippocampus were eliminated in transgenic mice using the herpes simplex virus thymidine kinase (HSV-tk) in glial fibrillary acidic protein (GFAP) positive NSPCs by administration of toxins specific to HSV-tk expressing proliferative cells. With X-irradiation of mouse hippocampi, Surget showed that ablation of hippocampal neurogenesis alone did not affect the negative feedback in the HPA-axis; however, it diminished fluoxetine-induced restoration of the hippocampal regulation of HPA-axis activity under chronic stress (Surget et al., 2011). It has also been reported that enhancing newborn neuron survival restored depressive behaviors in mice, without affecting the HPA-axis regulation either at baseline or following CORT treatment (Hill et al., 2015). These conflicting results suggest that the influence of hippocampal neurogenesis on the regulation of HPA-axis activity may vary based on the type of stress and antidepressant used.
Embryonic Neurogenesis and Neurodevelopmental Disorder
Embryonic neurogenesis may be involved in the onset of neurodevelopmental and psychiatric diseases; as such, it is an important target for investigating the impact of stress and glucocorticoids. Growing epidemiological evidence indicates that maternal stress during pregnancy increases the risk of ADHD, depression, schizophrenia, and ASDs in the offspring (Figure 1) (van Os and Selten, 1998; Van den Bergh and Marcoen, 2004; Beversdorf et al., 2005; Kinney et al., 2008; Khashan et al., 2008; Van den Bergh et al., 2008; Grizenko et al., 2012; Graignic-Philippe et al., 2014). Although the mechanisms of how maternal stress affects fetal brain development are not fully understood, excessive glucocorticoid transfer from mother to fetus is proposed as a key factor (Wilcoxon and Redei, 2007; Salomon et al., 2011). Catalytic conversion of glucocorticoid to cortisone by placental 11 beta-hydroxysteroid dehydrogenase type 2 prevents maternal glucocorticoid transfer to the fetus (Reynolds, 2013). Chronic severe stress and prolonged elevation of glucocorticoid levels in maternal serum exceeds catalytic conversion capacity and a considerable quantity of glucocorticoids then reaches the fetus (Reynolds, 2013). Fetal exposure to glucocorticoids can also be induced during therapeutic administration of synthetic glucocorticoids to promote fetal lung maturation. Such treatment is routinely used in obstetrical practice, although some clinical studies have demonstrated its adverse effect on childhood cognition and long-term behavior (Crowther et al., 2007; French et al., 2009; Braun et al., 2013). However, it should be noted that reports regarding the long-term effect of therapeutic glucocorticoid administration are still mixed; thus, further large-scale randomized controlled trials are required (Stutchfield et al., 2013).
Animals exposed to prenatal stress appear to exhibit a variety of behavioral abnormalities, including reduced exploration activity, decreased spatial memory, inability to extinguish conditioned fear memory, increased anxiety, and depressive behaviors (Alonso et al., 1991; Lordi et al., 2000; Schneider et al., 2002; Sundberg et al., 2006; Salomon et al., 2011; Anacker et al., 2013a; Bingham et al., 2013). Interestingly, anxiogenic behavior observed in prenatally-stressed rats (via a combination of restraint, forced swim and elevated platform stress) were ameliorated by maternal adrenalectomy, which was reversed by maternal administration of high-dose CORT (Salomon et al., 2011). In another report, dams receiving both adrenalectomy and CORT administration produced offspring with increased depressive behavior (Wilcoxon and Redei, 2007). Maternal CORT treatment also impaired the ability to extinguish conditioned fear memory, a hallmark of PTSD, in offspring as well as in prenatally stressed rats (Bingham et al., 2013). These studies essentially suggest that increased maternal serum levels of glucocorticoids could cause some behavioral abnormalities in offspring.
Although the impact of glucocorticoids on embryonic neurogenesis is still being investigated, several studies have demonstrated the detrimental effects of perinatal glucocorticoid exposure on neurogenesis. Decreased body weight, hippocampal volume, and number of proliferating cells in the subventricular zone of the lateral ventricle, subgranular zone of the hippocampus, and cortex were observed in rats after administration of dexamethasone (DEX), a synthetic glucocorticoid, at postnatal days 4–7 (Kanagawa et al.,2006). Furthermore, a single administration of DEX on pregnant mice at embryonic day 15.5 resulted in decreased body weight and hippocampal volume, increased apoptotic cells in the hippocampus, and reduced cell proliferation in the subgranular zone of the dentate gyrus in pups (Noorlander et al., 2014). Remarkably, the prenatally DEX-treated mice exhibited a deficit in spatial memory, impaired hippocampal long-term depression, decreased hippocampal neurogenesis, and shortened lifespan in the adult period, indicating the long-lasting impact of prenatal DEX exposure on the CNS after birth (Noorlander et al., 2008).
Glucocorticoid Effect on Neural Stem/Progenitor Cells
To clarify the molecular mechanisms underlying neurogenesis impairment caused by increased glucocorticoids, several in vitro studies have proposed various impacts of glucocorticoids on the cellular system in NSPCs. Endogenous glucocorticoids have two specific receptors: the mineralocorticoid receptor (MR) and the glucocorticoid receptor (GR), both of which can function as transcription factors. As MR has a high affinity for glucocorticoids, it is occupied even at the basal blood glucocorticoid levels (De Kloet et al., 1998). On the other hand, GR has a low affinity to glucocorticoids and is activated in response to increased levels of stress-induced glucocorticoids (De Kloet et al., 1998). Interestingly, MR and GR differentially affect the proliferation and differentiation of NSPCs. Anacker et al. (2013a) demonstrated that a low concentration of cortisol (human endogenous glucocorticoid) enhanced proliferation of human hippocampal progenitor cells and differentiation into astroglias, and suppressed differentiation into neurons, through MR function. Meanwhile, a high concentration of cortisol decreased proliferation of NSPCs and neural differentiation without affecting astroglial differentiation, via activating GR (Anacker et al., 2013a). These results suggest that the basal level of glucocorticoids modulates neurogenesis via MR, while an increased level of glucocorticoids inhibits neurogenesis via GR function. Consistent with this idea, the negative impact of glucocorticoids on neurogenesis via GR activation has been reported in various types of NSPCs (Bose et al., 2010; Samarasinghe et al., 2011; Raciti et al., 2016).
It is well known that ligand binding triggers translocation of cytosolic GR to the nucleus, and subsequently, GR directly binds to the promoter region of target genes or modifies activity of other transcriptional factors (Mitre-Aguilar et al., 2015). In addition to such genomic actions, plasma membrane GR (mGR) rapidly activates intracellular signaling cascades in response to ligand binding, which is known as non-genomic functions (Mitre-Aguilar et al., 2015). Although little is known about the influence of non-genomic pathways of GR on NSPCs, one study has reported a possible contribution of the non-genomic pathway on glucocorticoid-induced suppression of neurogenesis in cultured NSPCs (Samarasinghe et al., 2011). Activation of mGR in cultured NSPCs rapidly stimulated ERK1/2 signaling after DEX treatment in a Caveolin-1 dependent manner, which led to phosphorylation of connexin43. Because connexin43 diminishes gap junction intercellular communication (GJIC) and pharmacological GJIC inhibition is sufficient to suppress the proliferation of NSPCs, the mGR-dependent GJIC inhibition would contribute to NSPCs proliferation (Samarasinghe et al., 2011). Interestingly, the inhibitory action of mGR on NSPC proliferation lasted at least 24 hours, even though DEX exposure was transient (1 hour). These findings imply that proliferative activity of NSPCs can be interrupted by a transient increase of glucocorticoids triggered by stressful events.
In cultured rat embryonic NSPCs, DEX exposure for two days upregulated negative regulators of the cell cycle (p16 and p21) and senescence-related genes (high mobility group 1 and heterochromatin protein 1), and downregulated the mitochondrial genes (NADH dehydrogenase 3 and cytochrome b) concomitant with the suppression of NSPCs proliferation. Importantly, these features were retained for 10 days after ceasing DEX exposure, resembling the long-lasting effect of GR. Because DEX-exposed NSPCs showed decreased methylation of global DNA and expression of DNA methyltransferases, GR-mediated epigenetic events may also contribute to changes in gene expression profiles in daughter cells as a long-lasting influence of glucocorticoid exposure (Bose et al., 2010). This group recently showed that DEX treatment on human induced pluripotent stem cell-derived neuroepithelial-like stem cells exhibited a long-lasting decrease of neural differentiation, with significant downregulation of antioxidants and increased intracellular reactive oxygen species generation. Decreased neural differentiation was counteracted by the antioxidant N-acetyl-cysteine, suggesting that the intracellular redox system affects neural differentiation (Raciti et al., 2016). Importantly, the DEX-induced neural differentiation defect was accompanied by reduced expression of TrkB, a receptor of brain-derived neurotrophic factor (BDNF), which was also reversed by antioxidant treatment. Although the relationship between TrkB expression and neural differentiation was not elucidated in the study, the interference of glucocorticoids with the BDNF/TrkB signaling pathways is of interest given that BDNF is a potent enhancer of neural differentiation and maturation.
Besides transcriptional regulation, alteration of the ubiquitin proteasome system (UPS) is also involved in the inhibitory effect of glucocorticoids on neurogenesis. Rat embryonic NSPCs showed a decrease in cell proliferation after DEX exposure, which was accompanied by the reduction of cyclin D1 levels, a positive regulator of the cell cycle (Sundberg et al., 2006). The increased ubiquitination of cyclin D1 and counteraction of MG132 (an inhibitor of the UPS) reduced DEX effects (on both expression of cyclin D1 and cell proliferation), implying an involvement of the UPS in the DEX-inhibited cell proliferation. Moreover, the authors proposed that baculoviral inhibitor of apoptosis repeat-containing 6 gene (BRUCE/Apollon) is another possible target of DEX-induced UPS alteration in the suppression of NSPCs proliferation (Sippel et al., 2009). Further, it was revealed that DEX increased the expression of the deubiquitinating enzyme Usp8/Ubpy, which decreased BRUCE possibly via the stabilization of BRUCE-targeting ubiquitin-protein ligase Nrdp1. Although the molecular mechanism behind increased cyclin D1 ubiquitination is still not clear, these findings suggest that the alteration of the UPS may be accountable for part of the gene expression mediated by glucocorticoids.
Intrinsic and extrinsic signaling cascades regulate proliferation and differentiation of NSPCs. Several groups, including the authors, have reported impairment of various signaling cascades by glucocorticoid exposure. Gene expression microarray combined with pathway analysis showed that a high-dose of cortisol inhibited TGF-β-SMAD2/3 and hedgehog signaling in human hippocampal progenitor cell lines (Anacker et al., 2013a). These signaling abnormalities were also observed in the hippocampus of adult rats exposed to prenatal stress. In addition, hedgehog signaling activation by smoothened agonist, purmorphamine, canceled the inhibitory effect of cortisol on neuronal differentiation (Anacker et al., 2013a). This supports the possible involvement of hedgehog signaling in neurogenesis regulation. Serum- and glucocorticoid-inducible kinase 1 (SGK1), a direct target gene of GR, has been proposed as a contributor to repression of hedgehog signaling (Anacker et al., 2013b). In human hippocampal progenitor cell lines, an SGK1 inhibitor reversed the cortisol-induced suppression of hedgehog signaling, proliferation and neuronal differentiation. Interestingly, SGK1 potentiated and maintained the cortisol-induced phosphorylation and nuclear translocation of GR even after withdrawal of cortisol, implying a positive feedback role of SGK1 on GR function. This study indicates that SGK1 is one of the candidate factors regulating the long-lasting effects of glucocorticoids. It may mediate hedgehog signaling indirectly, as the SGK1 inhibitor would reverse various GR-mediated phenotypes through repressing its positive feedback action.
Wnt signaling pathways are also involved in self-renewal, expansion, differentiation and maturation of NSPCs in both the developing and adult brain (Bengoa-Vergniory et al., 2015). Thus, their correlation with glucocorticoids is salient. DEX treatment of human NSPCs inhibited both proliferation and neural differentiation of NSPCs, accompanied by increased Dickkopf1 (DKK1, endogenous Wnt-signaling antagonist) levels and reduced levels of canonical Wnt target genes including cyclin D1 and inhibitor of DNA binding 2 (ID2) (Moors et al., 2012). Neutralization with anti-DKK1 antibody antagonized the DEX-induced impairment in proliferation and differentiation of NSPCs. The GR was also shown to bind to the promoter region of DKK1 gene, implying direct regulation of DKK1 expression by GR. Interestingly, DEX-induced alteration of DKK1/Wnt pathway was also reported in osteoblasts in the context of DEX-induced osteoporosis, indicating the importance of glucocorticoid-mediated DKK1/Wnt pathway in a variety of cell populations (Ohnaka et al., 2005).
ERK- and PI3K/Akt-signaling are critical in neuronal events including neurogenesis, as they are pivotal to signaling after stimulation by growth factors (Samuels et al., 2009; Wang et al., 2017). Glucocorticoids exert a negative effect on these signaling cascades in various cell types, including neurons (Sandri et al., 2004; Smith et al., 2005; Horsch et al., 2007; González et al., 2010; Kumamaru et al., 2011). Recently, the authors found that ERK and Akt activities were increased during in vitro differentiation of rat embryonic NSPCs, and this activation was decreased by CORT application (Odaka et al., 2016) (Figure 2). The importance of Akt activation for proper neural differentiation was demonstrated using specific inhibitors for these signaling pathways. A potent PI3K/Akt signaling activator, IGF1, counteracted the CORT-induced suppression of neural differentiation, suggesting its role in impaired neurogenesis. Considering that Wnt signaling inhibition caused a defect in neural differentiation, glycogen synthase kinase-3 beta (GSK-3β) is one of the possible downstream targets of PI3K/Akt signaling. Phosphorylation of GSK-3β by Akt inhibits the enzymatic activity of GSK-3β and results in the prevention of the proteasomal degradation of β-catenin, as is the case with canonical Wnt signaling pathways (Katoh et al., 2006). Indeed, it was demonstrated that glucocorticoid suppressed PI3K/Akt/GSK-3β/β-catenin pathways in osteoblast-like cells (Smith et al., 2005). Moreover, an in vivo study showed that endogenous adult neurogenesis was enhanced by the activation of the PI3K/Akt/GSK-3β system after cerebral ischemia, further supporting an involvement of these pathways in neurogenesis (Kisoh et al., 2016).
Figure 2.

Inhibition of Akt signaling by corticosterone (CORT) exposure in neural stem/progenitor cells (NSPCs).
(A) Schematic representation of experimental procedure. Primary embryonic rat neurospheres were digested and plated on polyethylemine-coated dishes at day –3. After 3 days of expansion of NSPCs in the proliferation medium, the medium was switched to differentiation induction medium at day 0 and cultured until day 7. CORT exposure was performed each day after plating. (B) Robust increase of phosphorylated-Akt (pAkt) level during differentiation. (C) Decreased pAkt levels in NSPCs at day 7 after differentiation induction in the presence of CORT.
Another putative downstream target of PI3K/Akt is mTOR (mammalian target of rapamycin). The stimulation of mTOR complex1 (mTORC1) by the activation of PI3K/Akt subsequently induces activation of p70 ribosomal S6 protein kinases 1/2 and inhibition of eukaryotic initiation factor 4E-bonding proteins, triggering the translational response of the mTOR cascade (Wang et al., 2017). PI3K/Akt/mTOR signaling is essential for normal brain development. Deletion of mTOR in NSPCs disrupted progenitor self-renewal and suppressed neural differentiation, and resulted in microcephaly (Ka et al., 2014; Wang et al., 2017). It is of note that mTOR activity is negatively regulated by GSK-3, implying that Wnt or PI3K/Akt-induced GSK-3β inhibition may enhance mTOR activity (Ka et al., 2014). GSK-3 also negatively regulated hedgehog signaling via targeting a downstream molecule of hedgehog pathway Gli (Pan et al., 2006; Wang et al., 2006). Although further studies are needed, the negative action of glucocorticoids on DKK1/Wnt, PI3K/Akt, and hedgehog signaling appear to synergistically suppress neurogenesis through their crosstalk (Figure 3).
Figure 3.
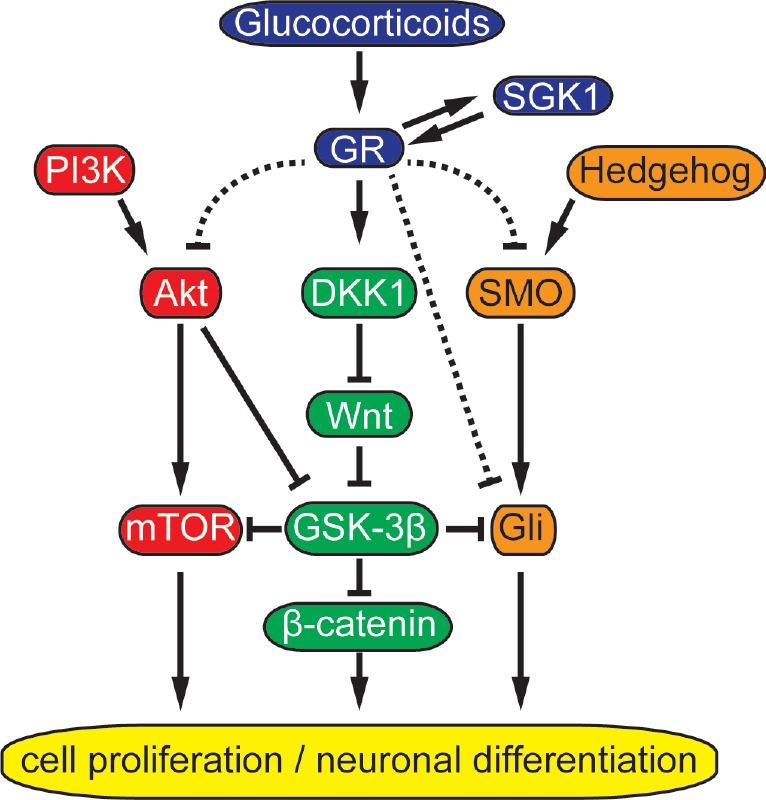
Possible crosstalk of intracellular signaling pathways affected by glucocorticoid exposure.
Blue: Glucocorticoid receptor (GR) signaling; red: major pathway of the phosphoinositide 3-kinase (PI3K)/Akt signaling; green: major pathway of the Wnt signaling; and orange: major pathway of the hedgehog signaling. Arrow head: Positive regulation; T bar: negative regulation; and dotted line: detailed mechanisms were unidentified. DKK1: Dickkopf1; GSK-3β: glycogen synthase kinase-3 beta; mTOR: mammalian target of rapamycin; SGK1: serum- and glucocorticoid-inducible kinase 1; SMO: smoothened.
Downregulation of both PI3K/Akt and ERK pathways by glucocorticoids implies the possible involvement of receptor tyrosine kinases (RTKs, major activators of these signaling pathways). BDNF is one of the most-studied RTK activators as a target of glucocorticoid action. BDNF has multiple functions in both the embryonic and adult brain, such as NSPCs proliferation, differentiation, survival, and synaptic plasticity. Several studies report that exposure to stress and glucocorticoids decreases expression of BDNF in the hippocampus and/or the cortex in rodents (Smith et al., 1995; Schaaf et al., 1997; Dwivedi et al., 2006). Recent studies using the BZ cell line (established by targeted oncogenesis in mouse hippocampus) showed that GR was recruited to a promoter region of the BDNF gene to repress transcription through unidentified transcription factor tethering (Chen et al., 2017). Glucocorticoids also impair BDNF-stimulated intracellular signaling pathways. Generally, BDNF binds to TrkB to activate mainly three intracellular cascades; PI3K/Akt, MAPK/ERK, and PLC-γ pathways (Begni et al., 2017). The authors previously reported that DEX exposure attenuated the interaction of TrkB with Shp2, and subsequently suppressed ERK signaling and BDNF-induced enhancement of synaptic maturation in rat cortical neurons. DEX also inhibited BDNF-induced PLC-γ activation and its regulation of neurotransmitter release in rat cortical neurons (Numakawa et al., 2009). Intracellular interaction between GR and TrkB was also revealed, which is important in the TrkB-PLC-γ interaction. Although inhibitory actions of glucocorticoids on BDNF-stimulated PI3K/Akt signaling in neural cells have not been reported, upregulation of p85α monomer caused by glucocorticoids inhibited Akt activity by competing for the RTK binding site with p110/p85 heterodimers in osteoblasts and myoblasts (Kuo et al., 2012; Zou et al., 2015). Although these mechanisms might be accountable for some glucocorticoid-induced phenotypes in NSPCs, further studies are required to explore the functional crosstalk between glucocorticoids and BDNF in NSPCs.
Future Directions
In this review, we outlined recent evidence on functional interactions between stress hormone glucocorticoids and neurogenesis. Investigating altered cell fate and related intracellular signaling in NSPCs affected by glucocorticoid stress is important to reduce the risk of developmental brain diseases, including mental disorders and neurodegenerative diseases. In spite of differences in cell population and niche, both embryonic and adult NSPCs exhibit similar phenotypes, including suppression of proliferation and neural differentiation by high levels of glucocorticoids. These phenotypic similarities might be attributed to a common molecular mechanism in embryonic and adult NSPCs. Studies on glucocorticoid-related impairments discussed above, however, were revealed by using NSPCs originating from embryos; thus, it should be ascertained if these mechanisms can also be applied in adult NSPCs.
Although GR is believed to be a major contributor of glucocorticoid stress, MR (which have a high affinity receptor for glucocorticoids) act positively on neurogenesis (Anacker et al., 2013a), indicating that the functional balance between GR and MR is critical for glucocorticoid action in neurogenesis. Although genetic manipulation of GR in animals could be a promising tool for investigation, several studies on neurogenesis in GR knockout or knockdown mice have yielded inconsistent results. GR heterozygous mice displayed reduced hippocampal neurogenesis under the stress condition but not the basal condition (Kronenberg et al., 2009). Brain-specific deletion of GR did not affect neurogenesis in hippocampal granule cell layer in the basal condition (Gass et al., 2000). It is difficult to evaluate the influence of GR deletion in neurogenesis using systemic or whole-brain GR-deficient mice, as GR deletion in hippocampal or hypothalamic neurons disrupts the negative feedback loop of the HPA-axis. This in turn causes hypercorticoidism, which can interfere with neurogenesis. Indeed, cell type-specific knockdown of GR in vivo showed different results. For example, viral-mediated knockdown of GR selectively in hippocampal newborn cells resulted in enhancement of neural differentiation and maturation under the basal condition (Fitzsimons et al., 2013). In the future, NSPC-specific manipulation of GR could be a powerful tool for precisely understanding GR-mediated actions in neurogenesis in vivo.
Footnotes
Funding: This study was supported by grants from by Takeda Science Foundation (TN, NA), and from the Grant-in-Aid for Scientific Research(C) (JSPS KAKENHI JP16K06996) (to TN) and JSPS KAKENHI Grant Number 17J04183 (to HO) in the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Conflicts of interest: None declared.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Open peer reviewers: Mariagrazia Grilli, University of Piemonte Orientale, Italy; Claire-Anne Gutekunst, Emory University School of Medicine, USA.
References
- 1.Alonso SJ, Arevalo R, Afonso D, Rodríguez M. Effects of maternal stress during pregnancy on forced swimming test behavior of the offspring. Physiol Behav. 1991;50:511–517. doi: 10.1016/0031-9384(91)90538-y. [DOI] [PubMed] [Google Scholar]
- 2.Anacker C, Cattaneo A, Luoni A, Musaelyan K, Zunszain PA, Milanesi E, Rybka J, Berry A, Cirulli F, Thuret S, Price J, Riva MA, Gennarelli M, Pariante CM. Glucocorticoid-related molecular signaling pathways regulating hippocampal neurogenesis. Neuropsychopharmacology. 2013a;38:872–883. doi: 10.1038/npp.2012.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anacker C, Cattaneo A, Musaelyan K, Zunszain PA, Horowitz M, Molteni R, Luoni A, Calabrese F, Tansey K, Gennarelli M, Thuret S, Price J, Uher R, Riva MA, Pariante CM. Role for the kinase SGK1 in stress, depression, and glucocorticoid effects on hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2013b;110:8708–8713. doi: 10.1073/pnas.1300886110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Begni V, Riva MA, Cattaneo A. Cellular and molecular mechanisms of the brain-derived neurotrophic factor in physiological and pathological conditions. Clin Sci (Lond) 2017;131:123–138. doi: 10.1042/CS20160009. [DOI] [PubMed] [Google Scholar]
- 5.Bengoa-Vergniory N, Kypta RM. Canonical and noncanonical Wnt signaling in neural stem/progenitor cells. Cell Mol Life Sci. 2015;72:4157–4172. doi: 10.1007/s00018-015-2028-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beversdorf DQ, Manning SE, Hillier A, Anderson SL, Nordgren RE, Walters SE, Nagaraja HN, Cooley WC, Gaelic SE, Bauman ML. Timing of prenatal stressors and autism. J Autism Dev Disord. 2005;35:471–478. doi: 10.1007/s10803-005-5037-8. [DOI] [PubMed] [Google Scholar]
- 7.Bingham BC, Rani CS, Frazer A, Strong R, Morilak DA. Exogenous prenatal corticosterone exposure mimics the effects of prenatal stress on adult brain stress response systems and fear extinction behavior. Psychoneuroendocrinology. 2013;38:2746–2757. doi: 10.1016/j.psyneuen.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 8.Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, Arango V. Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology. 2009;34:2376–2389. doi: 10.1038/npp.2009.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boldrini M, Santiago AN, Hen R, Dwork AJ, Rosoklija GB, Tamir H, Arango V, John Mann J. Hippocampal granule neuron number and dentate gyrus volume in antidepressant-treated and untreated major depression. Neuropsychopharmacology. 2013;38:1068–1077. doi: 10.1038/npp.2013.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boldrini M, Hen R, Underwood MD, Rosoklija GB, Dwork AJ, Mann JJ, Arango V. Hippocampal angiogenesis and progenitor cell proliferation are increased with antidepressant use in major depression. Biol Psychiatry. 2012;72:562–571. doi: 10.1016/j.biopsych.2012.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Braun T, Challis JR, Newnham JP, Sloboda DM. Early-life glucocorticoid exposure: the hypothalamic-pituitary-adrenal axis, placental function, and long-term disease risk. Endocr Rev. 2013;34:885–916. doi: 10.1210/er.2013-1012. [DOI] [PubMed] [Google Scholar]
- 12.Bose R, Moors M, Tofighi R, Cascante A, Hermanson O, Ceccatelli S. Glucocorticoids induce long-lasting effects in neural stem cells resulting in senescence-related alterations. Cell Death Dis. 2010;1:e92. doi: 10.1038/cddis.2010.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen H, Lombès M, Le Menuet D. Glucocorticoid receptor represses brain-derived neurotrophic factor expression in neuron-like cells. Mol Brain. 2017;10:12. doi: 10.1186/s13041-017-0295-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crowther CA, Doyle LW, Haslam RR, Hiller JE, Harding JE, Robinson JS ACTORDS Study Group. Outcomes at 2 years of age after repeat doses of antenatal corticosteroids. N Engl J Med. 2007;357:1179–1189. doi: 10.1056/NEJMoa071152. [DOI] [PubMed] [Google Scholar]
- 15.De Kloet ER, Vreugdenhil E, Oitzl MS, Joëls M. Brain corticosteroid receptor balance in health and disease. Endocr Rev. 1998;19:269–301. doi: 10.1210/edrv.19.3.0331. [DOI] [PubMed] [Google Scholar]
- 16.Dwivedi Y, Rizavi HS, Pandey GN. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: differential regulation of specific exons by antidepressants and corticosterone. Neuroscience. 2006;139:1017–1029. doi: 10.1016/j.neuroscience.2005.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fitzsimons CP, van Hooijdonk LW, Schouten M, Zalachoras I, Brinks V, Zheng T, Schouten TG, Saaltink DJ, Dijkmans T, Steindler DA, Verhaagen J, Verbeek FJ, Lucassen PJ, de Kloet ER, Meijer OC, Karst H, Joels M, Oitzl MS, Vreugdenhil E. Knockdown of the glucocorticoid receptor alters functional integration of newborn neurons in the adult hippocampus and impairs fear-motivated behavior. Mol Psychiatry. 2013;18:993–1005. doi: 10.1038/mp.2012.123. [DOI] [PubMed] [Google Scholar]
- 18.French NP, Hagan R, Evans SF, Mullan A, Newnham JP. Repeated antenatal corticosteroids: effects on cerebral palsy and childhood behavior. Am J Obstet Gynecol. 2004;190:588–595. doi: 10.1016/j.ajog.2003.12.016. [DOI] [PubMed] [Google Scholar]
- 19.Furay AR, Bruestle AE, Herman JP. The role of the forebrain glucocorticoid receptor in acute and chronic stress. Endocrinology. 2008;149:5482–5490. doi: 10.1210/en.2008-0642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gass P, Kretz O, Wolfer DP, Berger S, Tronche F, Reichardt HM, Kellendonk C, Lipp HP, Schmid W, Schütz G. Genetic disruption of mineralocorticoid receptor leads to impaired neurogenesis and granule cell degeneration in the hippocampus of adult mice. EMBO Rep. 2000;1:447–451. doi: 10.1093/embo-reports/kvd088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.González R, Ruiz-León Y, Gomendio M, Roldan ER. The effect of glucocorticoids on ERK-1/2 phosphorylation during maturation of lamb oocytes and their subsequent fertilization and cleavage ability in vitro. Reprod Toxicol. 2010;29:198–205. doi: 10.1016/j.reprotox.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Graignic-Philippe R, Dayan J, Chokron S, Jacquet AY, Tordjman S. Effects of prenatal stress on fetal and child development: a critical literature review. Neurosci Biobehav Rev. 2014;43:137–162. doi: 10.1016/j.neubiorev.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 23.Griffin GD, Charron D, Al-Daccak R. Post-traumatic stress disorder: revisiting adrenergics, glucocorticoids, immune system effects and homeostasis. Clin Transl Immunology. 2014;3:e27. doi: 10.1038/cti.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Grizenko N, Fortier ME, Zadorozny C, Thakur G, Schmitz N, Duval R, Joober R. Maternal stress during pregnancy, ADHD symptomatology in children and genotype: gene-environment interaction. J Can Acad Child Adolesc Psychiatry. 2012;21:9–15. [PMC free article] [PubMed] [Google Scholar]
- 25.Han J, Wang B, Xiao Z, Gao Y, Zhao Y, Zhang J, Chen B, Wang X, Dai J. Mammalian target of rapamycin (mTOR) is involved in the neuronal differentiation of neural progenitors induced by insulin. Mol Cell Neurosci. 2008;39:118–124. doi: 10.1016/j.mcn.2008.06.003. [DOI] [PubMed] [Google Scholar]
- 26.Herbert J. Cortisol and depression: three questions for psychiatry. Psychol Med. 2013;43:449–469. doi: 10.1017/S0033291712000955. [DOI] [PubMed] [Google Scholar]
- 27.Hill AS, Sahay A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to reduce anxiety and depression-like behaviors. Neuropsychopharmacology. 2015;40:2368–2378. doi: 10.1038/npp.2015.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Holsboer F. The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology. 2000;23:477–501. doi: 10.1016/S0893-133X(00)00159-7. [DOI] [PubMed] [Google Scholar]
- 29.Horsch K, de Wet H, Schuurmans MM, Allie-Reid F, Cato AC, Cunningham J, Burrin JM, Hough FS, Hulley PA. Mitogen-activated protein kinase phosphatase 1/dual specificity phosphatase 1 mediates glucocorticoid inhibition of osteoblast proliferation. Mol Endocrinol. 2007;21:2929–2940. doi: 10.1210/me.2007-0153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ising M, Künzel HE, Binder EB, Nickel T, Modell S, Holsboer F. The combined dexamethasone/CRH test as a potential surrogate marker in depression. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1085–1093. doi: 10.1016/j.pnpbp.2005.03.014. [DOI] [PubMed] [Google Scholar]
- 31.Jha S, Dong B, Sakata K. Enriched environment treatment reverses depression-like behavior and restores reduced hippocampal neurogenesis and protein levels of brain-derived neurotrophic factor in mice lacking its expression through promoter IV. Transl Psychiatry. 2011;1:e40. doi: 10.1038/tp.2011.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ka M, Condorelli G, Woodgett JR, Km WY. mTOR regulates brain morphogenesis by mediating GSK3 signaling. Development. 2014;141:4076–4086. doi: 10.1242/dev.108282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kanagawa T, Tomimatsu T, Hayashi S, Shioji M, Fukuda H, Shimoya K, Murata Y. The effects of repeated corticosteroid administration on the neurogenesis in the neonatal rat. Am J Obstet Gynecol. 2006;194:231–238. doi: 10.1016/j.ajog.2005.06.015. [DOI] [PubMed] [Google Scholar]
- 34.Katoh M, Katoh M. Cross-talk of WNT and FGF signaling pathways at GSK3beta to regulate beta-catenin and SNAIL signaling cascades. Cancer Biol Ther. 2006;5:1059–1064. doi: 10.4161/cbt.5.9.3151. [DOI] [PubMed] [Google Scholar]
- 35.Khashan AS, Abel KM, McNamee R, Pedersen MG, Webb RT, Baker PN, Kenny LC, Mortensen PB. Higher risk of offspring schizophrenia following antenatal maternal exposure to severe adverse life events. Arch Gen Psychiatry. 2008;65:146–152. doi: 10.1001/archgenpsychiatry.2007.20. [DOI] [PubMed] [Google Scholar]
- 36.Kinney DK, Miller AM, Crowley DJ, Huang E, Gerber E. Autism prevalence following prenatal exposure to hurricanes and tropical storms in Louisiana. J Autism Dev Disord. 2008;38:481–488. doi: 10.1007/s10803-007-0414-0. [DOI] [PubMed] [Google Scholar]
- 37.Kisoh K, Hayashi H, Itoh T, Asada M, Arai M, Yuan B, Tanonaka K, Takagi N. Involvement of GSK-3β phosphorylation through PI3-K/Akt in cerebral ischemia-induced neurogenesis in rats. Mol Neurobiol. 2016 doi: 10.1007/s12035-016-0290-8. doi:10.1007/s12035-016-0290-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kiuchi T, Lee H, Mikami T. Regular exercise cures depression-like behavior via VEGF-Flk-1 signaling in chronically stressed mice. Neuroscience. 2012;207:208–217. doi: 10.1016/j.neuroscience.2012.01.023. [DOI] [PubMed] [Google Scholar]
- 39.Kleiderman S, Gutbier S, Ugur Tufekci K, Ortega F, Sá JV, Teixeira AP, Brito C, Glaab E, Berninger B, Alves PM, Leist M. Conversion of nonproliferating astrocytes into neurogenic neural stem cells: control by FGF2 and interferon-γ. Stem Cells. 2016;34:2861–2874. doi: 10.1002/stem.2483. [DOI] [PubMed] [Google Scholar]
- 40.Kronenberg G, Kirste I, Inta D, Chourbaji S, Heuser I, Endres M, Gass P. Reduced hippocampal neurogenesis in the GR(+/-) genetic mouse model of depression. Eur Arch Psychiatry Clin Neurosci. 2009;259:499–504. doi: 10.1007/s00406-009-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kumamaru E, Numakawa T, Adachi N, Kunugi H. Glucocorticoid suppresses BDNF-stimulated MAPK/ERK pathway via inhibiting interaction of Shp2 with TrkB. FEBS Lett. 2011;585:3224–3228. doi: 10.1016/j.febslet.2011.09.010. [DOI] [PubMed] [Google Scholar]
- 42.Kuo T, Lew MJ, Mayba O, Harris CA, Speed TP, Wang JC. Genome-wide analysis of glucocorticoid receptor-binding sites in myotubes identifies gene networks modulating insulin signaling. Proc Natl Acad Sci U S A. 2012;109:11160–11165. doi: 10.1073/pnas.1111334109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lehmann ML, Brachman RA, Martinowich K, Schloesser RJ, Herkenham M. Glucocorticoids orchestrate divergent effects on mood through adult neurogenesis. J Neurosci. 2013;33:2961–2972. doi: 10.1523/JNEUROSCI.3878-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lenze EJ, Mantella RC, Shi P, Goate AM, Nowotny P, Butters MA, Andreescu C, Thompson PA, Rollman BL. Elevated cortisol in older adults with generalized anxiety disorder is reduced by treatment: a placebo-controlled evaluation of escitalopram. Am J Geriatr Psychiatry. 2011;19:482–490. doi: 10.1097/JGP.0b013e3181ec806c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Levone BR, Cryan JF, O’Leary OF. Role of adult hippocampal neurogenesis in stress resilience. Neurobiol Stress. 2014;1:147–155. doi: 10.1016/j.ynstr.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lordi B, Patin V, Protais P, Mellier D, Caston J. Chronic stress in pregnant rats: effects on growth rate, learning, and memory capabilities of the offspring. Int J Psychophysiol. 2000;37:195–205. doi: 10.1016/s0167-8760(00)00100-8. [DOI] [PubMed] [Google Scholar]
- 47.Madsen TM, Treschow A, Bengzon J, Bolwig TG, Lindvall O, Tingström A. Increased neurogenesis in a model of electroconvulsive therapy. Biol Psychiatry. 2000;47:1043–1049. doi: 10.1016/s0006-3223(00)00228-6. [DOI] [PubMed] [Google Scholar]
- 48.Mitre-Aguilar IB, Cabrera-Quintero AJ, Zentella-Dehesa A. Genomic and non-genomic effects of glucocorticoids: implications for breast cancer. Int J Clin Exp Pathol. 2015;8:1–10. [PMC free article] [PubMed] [Google Scholar]
- 49.Moors M, Bose R, Johansson-Haque K, Edoff K, Okret S, Ceccatelli S. Dickkopf 1 mediates glucocorticoid-induced changes in human neural progenitor cell proliferation and differentiation. Toxicol Sci. 2012;125:488–495. doi: 10.1093/toxsci/kfr304. [DOI] [PubMed] [Google Scholar]
- 50.Noorlander CW, Visser GH, Ramakers GM, Nikkels PG, de Graan PN. Prenatal corticosteroid exposure affects hippocampal plasticity and reduces lifespan. Dev Neurobiol. 2008;68:237–246. doi: 10.1002/dneu.20583. [DOI] [PubMed] [Google Scholar]
- 51.Noorlander CW, Tijsseling D, Hessel EV, de Vries WB, Derks JB, Visser GH, de Graan PN. Antenatal glucocorticoid treatment affects hippocampal development in mice. PLoS One. 2014;9:e85671. doi: 10.1371/journal.pone.0085671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Numakawa T, Adachi N, Richards M, Chiba S, Kunugi H. Brain-derived neurotrophic factor and glucocorticoids: reciprocal influence on the central nervous system. Neuroscience. 2013;239:157–172. doi: 10.1016/j.neuroscience.2012.09.073. [DOI] [PubMed] [Google Scholar]
- 53.Numakawa T, Kumamaru E, Adachi N, Yagasaki Y, Izumi A, Kunugi H. Glucocorticoid receptor interaction with TrkB promotes BDNF-triggered PLC-gamma signaling for glutamate release via a glutamate transporter. Proc Natl Acad Sci U S A. 2009;106:647–652. doi: 10.1073/pnas.0800888106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Odaka H, Numakawa T, Yoshimura A, Nakajima S, Adachi N, Ooshima Y, Inoue T, Kunugi H. Chronic glucocorticoid exposure suppressed the differentiation and survival of embryonic neural stem/progenitor cells: Possible involvement of ERK and PI3K/Akt signaling in the neuronal differentiation. Neurosci Res. 2016;113:28–36. doi: 10.1016/j.neures.2016.07.002. [DOI] [PubMed] [Google Scholar]
- 55.Ohnaka K, Tanabe M, Kawate H, Nawata H, Takayanagi R. Glucocorticoid suppresses the canonical Wnt signal in cultured human osteoblasts. Biochem Biophys Res Commun. 2005;329:177–181. doi: 10.1016/j.bbrc.2005.01.117. [DOI] [PubMed] [Google Scholar]
- 56.Owashi T, Otsubo T, Oshima A, Nakagome K, Higuchi T, Kamijima K. Longitudinal neuroendocrine changes assessed by dexamethasone/CRH and growth hormone releasing hormone tests in psychotic depression. Psychoneuroendocrinology. 2008;33:152–161. doi: 10.1016/j.psyneuen.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 57.Pan Y, Bai CB, Joyner AL, Wang B. Sonic hedgehog signaling regulates Gli2 transcriptional activity by suppressing its processing and degradation. Mol Cell Biol. 2006;26:3365–3377. doi: 10.1128/MCB.26.9.3365-3377.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raciti M, Ong J, Weis L, Edoff K, Battagli C, Falk A, Ceccatelli S. Glucocorticoids alter neuronal differentiation of human neuroepithelial-like cells by inducing long-lasting changes in the reactive oxygen species balance. Neuropharmacology. 2016;107:422–431. doi: 10.1016/j.neuropharm.2016.03.022. [DOI] [PubMed] [Google Scholar]
- 59.Raglan GB, Schmidt LA, Schulkin J. The role of glucocorticoids and corticotropin-releasing hormone regulation on anxiety symptoms and response to treatment. Endocr Connect. 2017;6:R1–R7. doi: 10.1530/EC-16-0100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Reynolds RM. Glucocorticoid excess and the developmental origins of disease: two decades of testing the hypothesis – 2012 Curt Richter Award Winner. Psychoneuroendocrinology. 2013;38:1–11. doi: 10.1016/j.psyneuen.2012.08.012. [DOI] [PubMed] [Google Scholar]
- 61.Roozendaal B, Phillips RG, Power AE, Brooke SM, Sapolsky RM, McGaugh JL. Memory retrieval impairment induced by hippocampal CA3 lesions is blocked by adrenocortical suppression. Nat Neurosci. 2001;4:1169–1171. doi: 10.1038/nn766. [DOI] [PubMed] [Google Scholar]
- 62.Salomon S, Bejar C, Schorer-Apelbaum D, Weinstock M. Corticosterone mediates some but not other behavioural changes induced by prenatal stress in rats. J Neuroendocrinol. 2011;23:118–128. doi: 10.1111/j.1365-2826.2010.02097.x. [DOI] [PubMed] [Google Scholar]
- 63.Samarasinghe RA, Di Maio R, Volonte D, Galbiati F, Lewis M, Romero G, DeFranco DB. Nongenomic glucocorticoid receptor action regulates gap junction intercellular communication and neural progenitor cell proliferation. Proc Natl Acad Sci U S A. 2011;108:16657–16662. doi: 10.1073/pnas.1102821108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Samuels IS, Saitta SC, Landreth GE. MAP’ing CNS development and cognition: an ERKsome process. Neuron. 2009;61:160–167. doi: 10.1016/j.neuron.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, Walsh K, Schiaffino S, Lecker SH, Goldberg AL. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117:399–412. doi: 10.1016/s0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- 67.Sawamoto A, Okuyama S, Yamamoto K, Amakura Y, Yoshimura M, Nakajima M, Furukawa Y. 3,5,6,7,8,3′,4′-heptamethoxyflavone, a citrus flavonoid, ameliorates corticosterone-induced depression-like behavior and restores brain-derived neurotrophic factor expression, neurogenesis, and neuroplasticity in the hippocampus. Molecules. 2016;21:541. doi: 10.3390/molecules21040541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Schaaf MJ, Hoetelmans RW, de Kloet ER, Vreugdenhil E. Corticosterone regulates expression of BDNF and trkB but not NT-3 and trkC mRNA in the rat hippocampus. J Neurosci Res. 1997;48:334–341. [PubMed] [Google Scholar]
- 69.Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schneider ML, Moore CF, Kraemer GW, Roberts AD, DeJesus OT. The impact of prenatal stress, fetal alcohol exposure, or both on development: perspectives from a primate model. Psychoneuroendocrinology. 2002;27:285–298. doi: 10.1016/s0306-4530(01)00050-6. [DOI] [PubMed] [Google Scholar]
- 71.Schoenfeld TJ, Cameron HA. Adult neurogenesis and mental illness. Neuropsychopharmacology. 2015;40:113–128. doi: 10.1038/npp.2014.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Smith E, Frenkel B. Glucocorticoids inhibit the transcriptional activity of LEF/TCF in differentiating osteoblasts in a glycogen synthase kinase-3beta-dependent and -independent manner. J Biol Chem. 2005;280:2388–2394. doi: 10.1074/jbc.M406294200. [DOI] [PubMed] [Google Scholar]
- 73.Smith MA, Makino S, Kvetnansky R, Post RM. Stress and glucocorticoids affect the expression of brain-derived neurotrophic factor and neurotrophin-3 mRNAs in the hippocampus. J Neurosci. 1995;(3 Pt 1):1768–1777. doi: 10.1523/JNEUROSCI.15-03-01768.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stutchfield PR, Whitaker R, Gliddon AE, Hobson L, Kotecha S, Doull IJ. Behavioural, educational and respiratory outcomes of antenatal betamethasone for term caesarean section (ASTECS trial) Arch Dis Child Fetal Neonatal Ed. 2013;98:F195–200. doi: 10.1136/archdischild-2012-303157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sundberg M, Savola S, Hienola A, Korhonen L, Lindholm D. Glucocorticoid hormones decrease proliferation of embryonic neural stem cells through ubiquitin-mediated degradation of cyclin D1. J Neurosci. 2006;26:5402–5410. doi: 10.1523/JNEUROSCI.4906-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Surget A, Saxe M, Leman S, Ibarguen-Vargas Y, Chalon S, Griebel G, Hen R, Belzung C. Drug-dependent requirement of hippocampal neurogenesis in a model of depression and of antidepressant reversal. Biol Psychiatry. 2008;64:293–301. doi: 10.1016/j.biopsych.2008.02.022. [DOI] [PubMed] [Google Scholar]
- 77.Surget A, Tanti A, Leonardo ED, Laugeray A, Rainer Q, Touma C, Palme R, Griebel G, Ibarguen-Vargas Y, Hen R, Belzung C. Antidepressants recruit new neurons to improve stress response regulation. Mol Psychiatry. 2011;16:1177–1188. doi: 10.1038/mp.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tang MM, Lin WJ, Pan YQ, Guan XT, Li YC. Hippocampal neurogenesis dysfunction linked to depressive-like behaviors in a neuroinflammation induced model of depression. Physiol Behav. 2016;161:166–173. doi: 10.1016/j.physbeh.2016.04.034. [DOI] [PubMed] [Google Scholar]
- 79.Van den Bergh BR, Marcoen A. High antenatal maternal anxiety is related to ADHD symptoms, externalizing problems, and anxiety in 8- and 9-year-olds. Child Dev. 2004;75:1085–1097. doi: 10.1111/j.1467-8624.2004.00727.x. [DOI] [PubMed] [Google Scholar]
- 80.Van den Bergh BR, Van Calster B, Smits T, Van Huffel S, Lagae L. Antenatal maternal anxiety is related to HPA-axis dysregulation and self-reported depressive symptoms in adolescence: a prospective study on the fetal origins of depressed mood. Neuropsychopharmacology. 2008;33:536–545. doi: 10.1038/sj.npp.1301450. [DOI] [PubMed] [Google Scholar]
- 81.van Os J, Selten JP. Prenatal exposure to maternal stress and subsequent schizophrenia. The May 1940 invasion of The Netherlands. Br J Psychiatry. 1998;172:324–326. doi: 10.1192/bjp.172.4.324. [DOI] [PubMed] [Google Scholar]
- 82.Wang B, Li Y. Evidence for the direct involvement of {beta}TrCP in Gli3 protein processing. Proc Natl Acad Sci U S A. 2006;103:33–38. doi: 10.1073/pnas.0509927103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wang L, Zhou K, Fu Z, Yu D, Huang H, Zang X, Mo X. Brain development and Akt signaling: the crossroads of signaling pathway and neurodevelopmental diseases. J Mol Neurosci. 2017;61:379–384. doi: 10.1007/s12031-016-0872-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wilcoxon JS, Redei EE. Maternal glucocorticoid deficit affects hypothalamic-pituitary-adrenal function and behavior of rat offspring. Horm Behav. 2007;51:321–327. doi: 10.1016/j.yhbeh.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, Prolo P, Karp B, McCutcheon IE, Geracioti TD, Jr, DeBellis MD, Rice KC, Goldstein DS, Veldhuis JD, Chrousos GP, Oldfield EH, McCann SM, Gold PW. Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A. 2000;97:325–330. doi: 10.1073/pnas.97.1.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yun S, Reynolds RP, Masiulis I, Eisch AJ. Re-evaluating the link between neuropsychiatric disorders and dysregulated adult neurogenesis. Nat Med. 2016;22:1239–1247. doi: 10.1038/nm.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Zou W, Yang S, Zhang T, Sun H, Wang Y, Xue H, Zhou D. Hypoxia enhances glucocorticoid-induced apoptosis and cell cycle arrest via the PI3K/Akt signaling pathway in osteoblastic cells. J Bone Miner Metab. 2015;33:615–624. doi: 10.1007/s00774-014-0627-1. [DOI] [PubMed] [Google Scholar]



