Keywords: nerve regeneration, prealbumin, hemorrhagic stroke, infection, gastrointestinal hemorrhage, prognostic indicator, prognosis, neural regeneration
Abstract
Serum prealbumin is a recognized marker of malnutrition, but its prognostic role in patients with hemorrhagic stroke remains unclear. In this study, we retrospectively reviewed the records of 105 patients with hemorrhagic stroke admitted to Renmin Hospital of Wuhan University, China, from January to December 2015. We collected demographic and radiological data, and recorded serum prealbumin levels at admission and on days 1, 3, 6, 9, and 14–21. The existence of infections and gastrointestinal hemorrhage, and clinical condition at discharge were also recorded. Serum prealbumin levels during hospitalization were significantly lower in patients with infections compared with those without infections, and also significantly lower in patients with gastrointestinal hemorrhage compared with those without. Serum prealbumin levels at discharge were significantly higher in patients with good recovery than in those with poor recovery. We conclude that regular serum prealbumin measurements in patients with hemorrhagic stroke may be a useful indicator for determining clinical status and prognosis, which may therefore help to guide clinical decision-making.
Introduction
Hemorrhagic stroke is estimated to be the leading cause of disability and the second most common cause of death globally. The global burden of hemorrhagic stroke increased significantly between 1990 and 2010 in terms of the absolute number of people with incident hemorrhagic stroke (47% increase), number of deaths (20% increase), and disability-adjusted life years lost (14% increase) (Hemphill et al., 2015). The presentations of hemorrhagic stroke vary in terms of their severity and mortality, but a third of patients die within 1 month after onset, and most survivors are left with some permanent disability (Schrader et al., 2003; Hemphill et al., 2015). In addition to the direct effects of the initial bleeding event and secondary neurologic complications, patients with aneurysmal subarachnoid or intracerebral hemorrhage are predisposed to other medical complications with direct impacts on outcome, length of hospital and intensive care unit stay, and associated increased care costs (Dennis et al., 2008; Urra et al., 2009; Sharma et al., 2014). Furthermore, patients with hemorrhagic stroke have been shown to experience alterations in the levels of acute phase proteins, an acute surge of sympathetic activity, and changes in vascular tone (Fluri et al., 2012), fluid status and administration, cardiac output, and major organ blood flow, resulting in a hyperdynamic state (Gariballa et al., 1998; Gaudiani et al., 2014).
Infections, gastrointestinal hemorrhage, and fluid and electrolyte disorders are the most frequent medical complications that occur after hemorrhagic stroke (Unosson et al., 1994; Steiner et al., 2014; Codullo et al., 2016). Hemorrhagic stroke-associated infection (HSAI) is one of the major complications and has been shown to worsen the clinical course and outcome of stroke patients (Steiner et al., 2014), while gastrointestinal hemorrhage was observed in 91% of patients undergoing endoscopy within 24 hours following hemorrhagic stroke (Unosson et al., 1994; O’Malley et al., 1995; Ohwaki et al., 2008; Codullo et al., 2016). In experimental studies, HSAI was shown to cause severe inflammatory responses (such as fever, acidosis, and coagulation function disturbance) and lead to further deterioration of the central nervous system. HSAI and gastrointestinal hemorrhage may delay rehabilitation, prolong hospitalization, and even cause death in patients with hemorrhagic stroke (Ingenbleek and Young, 1994; May et al., 2015). However, early detection and intervention may prevent these complications and improve patient prognosis and clinical outcome (Fleming et al., 2007; Rambod et al., 2008; Cheng et al., 2015). The identification of effective biomarkers for the early prediction of HSAI and gastrointestinal hemorrhage may thus be clinically relevant in this context.
Prealbumin is a 55-kDa protein that is synthesized in the liver (Tempel et al., 2015) and serves as a carrier protein for thyroxine and retinol-binding protein. Prealbumin possesses the shortest biological half-life (1–2 days) of all serum proteins (Wartenberg and Mayer, 2010; Davis et al., 2012). Recent studies identified copeptin, procalcitonin, leukocytes, C-reactive protein, interleukin-6, and heart rate variability measured on admission as possible predictors of stroke (Davis et al., 2012; Caccialanza et al., 2013; Kwan et al., 2013b). However, there are currently no relevant interventional measurements for these indicators. Serum prealbumin is a negative acute-phase reactant that decreases during inflammation, malignancies, and liver cirrhosis (Gocmen et al., 2010; Kwan et al., 2013a; Ye et al., 2015; Lin et al., 2017). Importantly, serum prealbumin concentrations fall rapidly as a result of decreased synthesis following reprioritization of the synthesis of acute-phase proteins such as C-reactive protein (Bourguignat et al., 1996; Bramer et al., 2014). Several studies have confirmed that inflammation is associated with decreased serum prealbumin (Ingenbleek and Young, 2002; Kopple et al., 2002; Rambod et al., 2008; Gurlek Gokcebay et al., 2015; Liu et al., 2015). Low serum prealbumin levels have also been associated with malnutrition, impaired functional status, poor outcome, and mortality (Yovita et al., 2004; Chrysostomou et al., 2010). To provide a theoretical basis for clinical treatment, the present investigation aimed to determine the value of serum prealbumin as a meaningful predictor of clinical status and prognosis in patients with hemorrhagic stroke.
Subjects and Methods
Subjects
The study was registered with the Chinese Clinical Trial Register (registration number: ChiCTR-ROC-16008562) and was approved by the Medical Ethics Committee of Renmin Hospital of Wuhan University, Ethical Review (Scientific Research) 2014 (number: 2012LKSZ (010) H).
Consecutive patients with hemorrhagic stroke admitted to Renmin Hospital of Wuhan University, China from January to December 2015 were enrolled in the study. All patients experiencing a new focal or global neurological event were admitted within 48 hours. A diagnosis of hemorrhagic stroke was verified by skull computed tomography (CT) and/or magnetic resonance imaging (MRI) on admission (Hemphill et al., 2015). Patients with transient ischemic attack, ischemic stroke, traumatic brain injury, brain tumor, chronic inflammatory disease, connective tissue or autoimmune diseases, malignant tumor, or deficient liver or renal function were excluded.
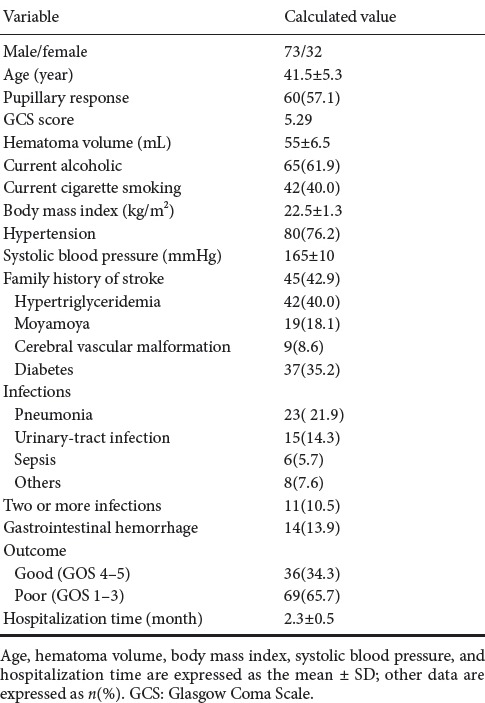
A total of 105 patients with hemorrhagic stroke met the inclusion criteria. The clinical characteristics of the study population are presented in Table 1.
Table 1.
Clinical characteristics of the study population
Detection method
Following admission, all patients received routine treatments and nursing care in accordance with clinical guidelines. Routine blood and biochemical tests, electrocardiograms, and baseline brain CT/MRI scans were performed in all patients on admission. Laboratory investigations for vascular risk factors, brain CT angiography, and a thorough cardiac investigation were also performed.
The severity of the cerebrovascular event was classified according to the Glasgow Coma Scale (GCS) (Hemphill et al., 2015). All patients were assigned to one of two categories using the Glasgow Outcome Scale (GOS) at discharge: good-outcome group (GOS = 4–5) and poor-outcome group (GOS = 1–3).
In addition to collecting demographic and radiological data, we also recorded serum prealbumin levels at admission and on days 3, 6, 9, 14–21. Fasting peripheral venous blood samples were obtained via the median cubital vein or basilic vein, and serum prealbumin was measured in the hospital biochemistry department using an automatic biochemical analyzer (AU5800, Beckman, CA, USA). Serum prealbumin levels ≤ 170 mg/L were defined as abnormal.
The presence of infections or gastrointestinal hemorrhage, and condition at discharge were also recorded. When indicated, infections were validated and identified by infectious-disease specialists.
Statistical analysis
Data are presented as the mean ± SD. Statistical analysis was performed using SPSS for Windows 17.0 software (SPSS, Chicago, IL, USA). A probability value < 0.05 was considered statistically significant. Statistical analyses were performed using chi-square tests for binary and categorical data and Mann-Whitney U tests for continuous variables.
Results
Value of serum prealbumin for predicting infection in hemorrhagic stroke patients
Infections were observed in 51 patients (48.8%), of which pneumonia accounted for 45.1% (n = 23). Serum prealbumin levels were significantly lower in patients with evidence of infection during hospitalization compared with non-infected patients on days 6 and 9 after admission (P = 0.001, P = 0.039, respectively). As shown in Figure 1, serum prealbumin levels in patients with evidence of infection were significantly reduced on day 6, followed by gradual normalization, while serum prealbumin levels in non-infected patients were typically within the normal reference range (200–400 mg/L).
Figure 1.
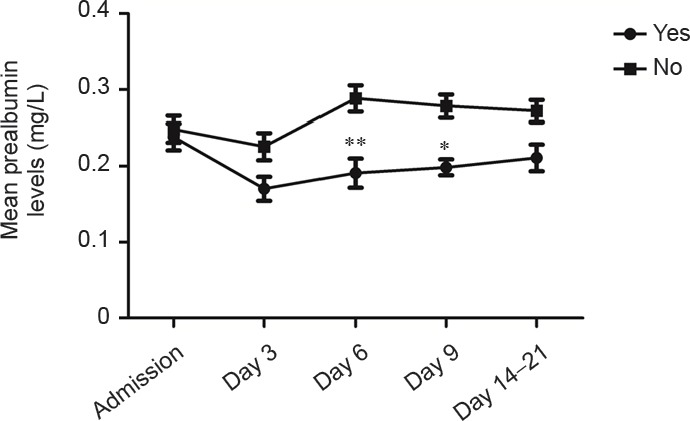
Serum prealbumin levels in hemorrhagic stroke patients with and without evidence of infection.
Infections were observed in 51 patients, and no infections in 54 patients. Data are expressed as the mean ± SD. *P < 0.05, **P < 0.01, vs. no infection (Mann-Whitney U test).
Value of serum prealbumin for predicting gastrointestinal hemorrhage in hemorrhagic stroke patients
Serum prealbumin levels on day 6 after admission were significantly lower in patients with compared with those without gastrointestinal hemorrhage (P = 0.026). As shown in Figure 2, serum prealbumin levels in patients with gastrointestinal hemorrhage fell to a minimum on day 6 after onset, followed by a gradual return to baseline levels. In contrast, serum prealbumin levels in patients without gastrointestinal hemorrhage were significantly higher than average (180.25 mg/L), with a gradually increasing trend.
Figure 2.
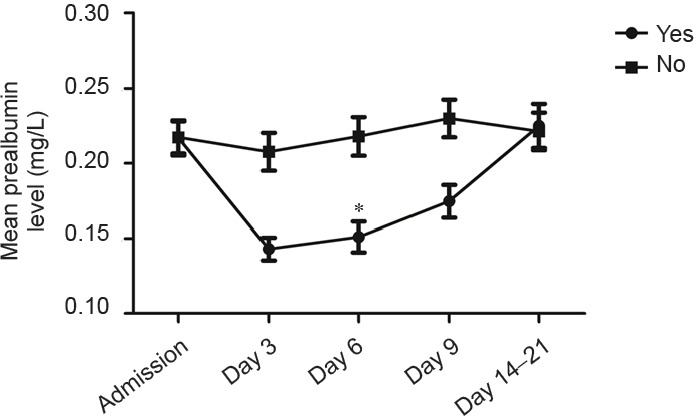
Serum prealbumin levels in hemorrhagic stroke patients with and without gastrointestinal hemorrhage.
Gastrointestinal hemorrhage was observed in 14 patients, and no gastrointestinal hemorrhage in 91 patients. Data are expressed as the mean ± SD. *P < 0.05, vs. no gastrointestinal hemorrhage (Mann-Whitney U test).
Value of serum prealbumin for predicting clinical outcome in hemorrhagic stroke patients
Serum prealbumin levels in patients with poor outcomes at discharge were significantly lower compared with patients with good outcomes on days 3, 6, and 14–21 after admission (P = 0.041, P = 0.02, P = 0.02, respectively). As shown in Figure 3, serum prealbumin levels in patients with good recovery at discharge were higher than average (180.25 mg/L); levels declined to a minimum on day 3 after onset, with a gradual recovery to baseline levels after the sixth day. In contrast, serum prealbumin levels in patients with poor recovery were significantly lower.
Figure 3.
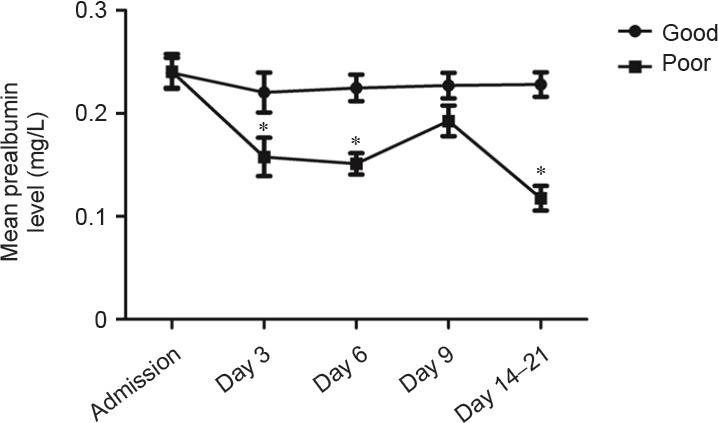
Serum prealbumin levels in hemorrhagic stroke patients with good and poor outcomes.
Good outcome: GOS = 4–5; poor outcome: GOS = 1–3. There were 72 patients with good outcomes and 33 with poor outcomes. Data are expressed as the mean ± SD. *P < 0.05, vs. good clinical outcome (Mann-Whithey U test).
Discussion
Inflammation, infection, trauma, and neoplasms may result in significant changes in plasma concentrations of acute-phase reactive proteins, including C-reactive protein, transferrin, amyloid protein A, and prealbumin. Acute-phase reactive proteins, as markers of clinical inflammation, have been used as important prognostic indicators after surgery and following stroke (Fleming et al., 2007; Rocha et al., 2010; Muangchan et al., 2012). C-reactive protein is commonly used as clinical marker of infection and inflammation (Dalrymple et al., 2013; Upadhyay et al., 2013; Lourenco et al., 2014; Lee et al., 2015; Qin et al., 2015; Gu et al., 2016), and serum prealbumin is a highly sensitive marker of nutrition and survival in dialysis patients (Lin et al., 2011; Chen et al., 2014; Fujii et al., 2014). A variety of inflammatory mediators induce tissue and organ damage during the acute phase response to injury and infection, including damage to liver sinusoidal endothelial cells, resulting in a decline in serum prealbumin synthesis. Decreased serum prealbumin levels have been reported in various diseases, such as kidney injury, systemic inflammation, liver cirrhosis, and brain trauma (Cruse et al., 1992; Hilker et al., 2003; Dziedzic et al., 2006). The results of the present study provide the first evidence for an association between decreased serum prealbumin and risk in acute ischemic stroke patients (Yeun and Kaysen, 1997; Shenkin, 2006).
Hemorrhagic stroke patients demonstrate differing levels of consciousness, commonly associated with an inability to protect the airway and thus effectively discharge respiratory secretions. In addition, the immune response can be impaired in relation to helper T-cell function, lymphokine-activated killer cell cytotoxicity, and other immune-cell dysfunctions (Phang and Aeberhardt, 1996; Chertow et al., 2005). Surgery and indwelling catheters predispose the tissue mucosa to infection. In the present study, infections were observed in 51 patients (65.4%), of which pneumonia accounted for 45.1% (23 patients). There was a significant difference in serum prealbumin levels on days 6 and 9 after admission between patients with and without evidence of infection during hospitalization. Hemorrhagic stroke is associated with a significant increase in basal metabolic rate, and alterations in energy consumption are thus likely to contribute to a rapid prealbumin deficiency. Serum prealbumin levels may therefore serve as an indicator of metabolic and nutritional states during the acute phase of the clinical course. Infection is a major complication of acute stroke, with an incidence rate of 21–62% (Zahuranec et al., 2014), leading to prolonged hospitalization and poorer outcomes. The findings of the current study suggest that low serum prealbumin levels might be an early biomarker of infection risk. Given that serum prealbumin can be detected rapidly and monitored easily, measurement of this marker may help hemorrhagic stroke patients at high risk of infection to be identified and treated appropriately.
Gastrointestinal hemorrhage is an additional co-morbid complication associated with hemorrhagic stroke, mainly secondary to acute post-traumatic stress ulcers (Wang et al., 2009; Sikora Newsome et al., 2015). The present study included 14 patients (14%) with gastrointestinal hemorrhage, and demonstrated a significant correlation between gastrointestinal hemorrhage and serum prealbumin levels. Furthermore, serum prealbumin levels in patients with poor recovery at discharge demonstrated greater variability compared with patients with better outcomes. Monitoring of serum prealbumin levels should thus pay attention to variability from baseline, particularly on post-injury day 3.
In summary, the results of the present study provide the first evidence for correlations between serum prealbumin levels and post-injury infection, gastrointestinal hemorrhage, and clinical outcome at discharge in patients with hemorrhagic stroke. Early detection of serum prealbumin levels during the acute phase of hemorrhagic stroke may therefore reflect the severity and prognosis of the disease, and may provide a rapid guide for appropriate interventions to prevent deterioration. Further research is required to determine if nutritional supplementation might increase serum prealbumin levels and thus reduce the risk of nosocomial infection and gastrointestinal hemorrhage in patients with hemorrhagic stroke.
Footnotes
Funding: This work was supported by the National Natural Science Foundation of China, No. 81571147; an American Heart Association Award, No. 14FTF19970029.
Conflicts of interest: None declared.
Research ethics: The study followed international and national regulations in accordance with the Declaration of Helsinki and relevant ethical principles. Patients and/or their family members volunteered to participate in the study.
Registration: This trial was registered with the Chinese Clinical Trial Register (registration number: ChiCTR-ROC-16008562).
Declaration of patient consent: The authors certify that they have obtained all appropriate patient consent forms. In the form the patients have given their consent for their images and other clinical information to be reported in the journal. The patients understand that their names and initials will not be published and due efforts will be made to conceal their identity, but anonymity cannot be guaranteed.
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Furness S, Norman C, Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- 1.Bourguignat A, Ferard G, Jenny JY, Gaudias J, Kempf I. Diagnostic value of C-reactive protein and transthyretin in bone infections of the lower limb. Clin Chim Acta. 1996;255:27–38. doi: 10.1016/0009-8981(96)06388-7. [DOI] [PubMed] [Google Scholar]
- 2.Bramer D, Hoyer H, Gunther A, Nowack S, Brunkhorst FM, Witte OW, Hoyer D. Study protocol: prediction of stroke associated infections by markers of autonomic control. BMC Neurol. 2014;14:9. doi: 10.1186/1471-2377-14-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Caccialanza R, Palladini G, Klersy C, Cereda E, Bonardi C, Quarleri L, Vadacca G, Albertini R, Merlini G. Serum prealbumin: an independent marker of short-term energy intake in the presence of multiple-organ disease involvement. Nutrition. 2013;29:580–582. doi: 10.1016/j.nut.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 4.Chen D, Bao L, Lu SQ, Xu F. Serum albumin and prealbumin predict the poor outcome of traumatic brain injury. PLoS One. 2014;9:e93167. doi: 10.1371/journal.pone.0093167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cheng V, Inaba K, Haltmeier T, Gutierrez A, Siboni S, Benjamin E, Lam L, Demetriades D. Serum transthyretin is a predictor of clinical outcomes in critically ill trauma patients. Surgery. 2015;158:438–444. doi: 10.1016/j.surg.2015.02.019. [DOI] [PubMed] [Google Scholar]
- 6.Chertow GM, Goldstein-Fuchs DJ, Lazarus JM, Kaysen GA. Prealbumin, mortality, and cause-specific hospitalization in hemodialysis patients. Kidney Int. 2005;68:2794–2800. doi: 10.1111/j.1523-1755.2005.00751.x. [DOI] [PubMed] [Google Scholar]
- 7.Chrysostomou S, Stathakis C, Petrikkos G, Daikos G, Gompou A, Perrea D. Assessment of prealbumin in hemodialysis and renal-transplant patients. J Ren Nutr. 2010;20:44–51. doi: 10.1053/j.jrn.2009.04.001. [DOI] [PubMed] [Google Scholar]
- 8.Codullo V, Cereda E, Klersy C, Cavazzana I, Alpini C, Bonardi C, Turri A, Franceschini F, Caccialanza R, Montecucco C, Caporali R. Serum prealbumin is an independent predictor of mortality in systemic sclerosis outpatients. Rheumatology (Oxford) 2016;55:315–319. doi: 10.1093/rheumatology/kev322. [DOI] [PubMed] [Google Scholar]
- 9.Cruse JM, Lewis RE, Bishop GR, Kliesch WF, Gaitan E. Neuroendocrine-immune interactions associated with loss and restoration of immune system function in spinal cord injury and stroke patients. Immunol Res. 1992;11:104–116. doi: 10.1007/BF02918615. [DOI] [PubMed] [Google Scholar]
- 10.Dalrymple LS, Johansen KL, Chertow GM, Grimes B, Anand S, McCulloch CE, Kaysen GA. Longitudinal measures of serum albumin and prealbumin concentrations in incident dialysis patients: the comprehensive dialysis study. J Ren Nutr. 2013;23:91–97. doi: 10.1053/j.jrn.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davis CJ, Sowa D, Keim KS, Kinnare K, Peterson S. The use of prealbumin and C-reactive protein for monitoring nutrition support in adult patients receiving enteral nutrition in an urban medical center. JPEN J Parenter Enteral Nutr. 2012;36:197–204. doi: 10.1177/0148607111413896. [DOI] [PubMed] [Google Scholar]
- 12.Dennis RA, Johnson LE, Roberson PK, Heif M, Bopp MM, Cook J, Sullivan DH. Changes in prealbumin, nutrient intake, and systemic inflammation in elderly recuperative care patients. J Am Geriatr Soc. 2008;56:1270–1275. doi: 10.1111/j.1532-5415.2008.01789.x. [DOI] [PubMed] [Google Scholar]
- 13.Dziedzic T, Pera J, Klimkowicz A, Turaj W, Slowik A, Rog TM, Szczudlik A. Serum albumin level and nosocomial pneumonia in stroke patients. Eur J Neurol. 2006;13:299–301. doi: 10.1111/j.1468-1331.2006.01210.x. [DOI] [PubMed] [Google Scholar]
- 14.Fleming CE, Saraiva MJ, Sousa MM. Transthyretin enhances nerve regeneration. J Neurochem. 2007;103:831–839. doi: 10.1111/j.1471-4159.2007.04828.x. [DOI] [PubMed] [Google Scholar]
- 15.Fluri F, Morgenthaler NG, Mueller B, Christ-Crain M, Katan M. Copeptin, procalcitonin and routine inflammatory markers-predictors of infection after stroke. PLoS One. 2012;7:e48309. doi: 10.1371/journal.pone.0048309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii T, Yajima R, Takada T, Sutoh T, Morita H, Yamaguchi S, Tsutsumi S, Kuwano H. Serum albumin and prealbumin do not predict recurrence in patients with breast cancer. Anticancer Res. 2014;34:3775–3779. [PubMed] [Google Scholar]
- 17.Gariballa SE, Parker SG, Taub N, Castleden CM. Influence of nutritional status on clinical outcome after acute stroke. Am J Clin Nutr. 1998;68:275–281. doi: 10.1093/ajcn/68.2.275. [DOI] [PubMed] [Google Scholar]
- 18.Gaudiani JL, Sabel AL, Mehler PS. Low prealbumin is a significant predictor of medical complications in severe anorexia nervosa. Int J Eat Disord. 2014;47:148–156. doi: 10.1002/eat.22233. [DOI] [PubMed] [Google Scholar]
- 19.Gocmen H, Ediger D, Uzaslan E, Doganay S, Guney NA, Ege E. The relationships of serum prealbumin levels with parameters that indicate severity of disease and emphysema pattern in patients with stable chronic obstructive pulmonary disease. Eurasian J Med. 2010;42:105–110. doi: 10.5152/eajm.2010.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gu LN, Zhang M, Zhu H, Liu JY. Higher frequency of brain abnormalities in neuromyelitis optica spectrum disorder patients without primary Sjögren's syndrome. Neural Regen Res. 2016;11:1633–1637. doi: 10.4103/1673-5374.193243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gurlek Gokcebay D, Emir S, Bayhan T, Demir HA, Gunduz M, Tunc B. Assessment of nutritional status in children with cancer and effectiveness of oral nutritional supplements. Pediatr Hematol Oncol. 2015;32:423–432. doi: 10.3109/08880018.2015.1065303. [DOI] [PubMed] [Google Scholar]
- 22.Hemphill JC, 3rd, Greenberg SM, Anderson CS, Becker K, Bendok BR, Cushman M, Fung GL, Goldstein JN, Macdonald RL, Mitchell PH, Scott PA, Selim MH, Woo D. Guidelines for the management of spontaneous intracerebral hemorrhage: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2015;46:2032–2060. doi: 10.1161/STR.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 23.Hilker R, Poetter C, Findeisen N, Sobesky J, Jacobs A, Neveling M, Heiss WD. Nosocomial pneumonia after acute stroke: implications for neurological intensive care medicine. Stroke. 2003;34:975–981. doi: 10.1161/01.STR.0000063373.70993.CD. [DOI] [PubMed] [Google Scholar]
- 24.Ingenbleek Y, Young V. Transthyretin (prealbumin) in health and disease: nutritional implications. Annu Rev Nutr. 1994;14:495–533. doi: 10.1146/annurev.nu.14.070194.002431. [DOI] [PubMed] [Google Scholar]
- 25.Ingenbleek Y, Young VR. Significance of transthyretin in protein metabolism. Clin Chem Lab Med. 2002;40:1281–1291. doi: 10.1515/CCLM.2002.222. [DOI] [PubMed] [Google Scholar]
- 26.Kopple JD, Mehrotra R, Suppasyndh O, Kalantar-Zadeh K. Observations with regard to the National Kidney Foundation K/DOQI clinical practice guidelines concerning serum transthyretin in chronic renal failure. Clin Chem Lab Med. 2002;40:1308–1312. doi: 10.1515/CCLM.2002.225. [DOI] [PubMed] [Google Scholar]
- 27.Kwan J, Horsfield G, Bryant T, Gawne-Cain M, Durward G, Byrne CD, Englyst NA. IL-6 is a predictive biomarker for stroke associated infection and future mortality in the elderly after an ischemic stroke. Exp Gerontol. 2013a;48:960–965. doi: 10.1016/j.exger.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Kwan J, Pickering RM, Kunkel D, Fitton C, Jenkinson D, Perry VH, Ashburn AM. Stroke Association Rehabilitation Research Centre (2013b) Impact of stroke-associated infection on long-term survival: a cohort study. J Neurol Neurosurg Psychiatry. 84:297–304. doi: 10.1136/jnnp-2012-302552. [DOI] [PubMed] [Google Scholar]
- 29.Lee JL, Oh ES, Lee RW, Finucane TE. Serum albumin and prealbumin in calorically restricted, nondiseased individuals: a systematic review. Am J Med. 2015;128:1023.e1–22. doi: 10.1016/j.amjmed.2015.03.032. [DOI] [PubMed] [Google Scholar]
- 30.Lin MY, Liu WY, Tolan AM, Aboulian A, Petrie BA, Stabile BE. Preoperative serum albumin but not prealbumin is an excellent predictor of postoperative complications and mortality in patients with gastrointestinal cancer. Am Surg. 2011;77:1286–1289. [PubMed] [Google Scholar]
- 31.Lin SP, Long Y, Chen XH, Lin PY, Jiang HL. STAF score is a new simple approach for diagnosing cardioembolic stroke. Int J Neurosci. 2017;127:261–266. doi: 10.1080/00207454.2016.1185715. [DOI] [PubMed] [Google Scholar]
- 32.Liu M, Yang J, Yu X, Huang X, Vaidya S, Huang F, Xiang Z. The role of perioperative oral nutritional supplementation in elderly patients after hip surgery. Clin Interv Aging. 2015;10:849–858. doi: 10.2147/CIA.S74951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lourenco P, Silva S, Frioes F, Alvelos M, Amorim M, Couto M, Torres-Ramalho P, Guimaraes JT, Araujo JP, Bettencourt P. Low prealbumin is strongly associated with adverse outcome in heart failure. Heart. 2014;100:1780–1785. doi: 10.1136/heartjnl-2014-305747. [DOI] [PubMed] [Google Scholar]
- 34.May CC, Arora S, Parli SE, Fraser JF, Bastin MT, Cook AM. Augmented renal clearance in patients with subarachnoid hemorrhage. Neurocrit Care. 2015;23:374–379. doi: 10.1007/s12028-015-0127-8. [DOI] [PubMed] [Google Scholar]
- 35.Muangchan C, Harding S, Khimdas S, Bonner A, Canadian Scleroderma Research group, Baron M, Pope J. Association of C-reactive protein with high disease activity in systemic sclerosis: results from the Canadian Scleroderma Research Group. Arthritis Care Res (Hoboken) 2012;64:1405–1414. doi: 10.1002/acr.21716. [DOI] [PubMed] [Google Scholar]
- 36.O’Malley T, Langhorne P, Elton RA, Stewart C. Platelet size in stroke patients. Stroke. 1995;26:995–999. doi: 10.1161/01.str.26.6.995. [DOI] [PubMed] [Google Scholar]
- 37.Ohwaki K, Yano E, Nagashima H, Nakagomi T, Tamura A. Impact of infection on length of intensive care unit stay after intracerebral hemorrhage. Neurocrit Care. 2008;8:271–275. doi: 10.1007/s12028-007-9007-1. [DOI] [PubMed] [Google Scholar]
- 38.Phang PT, Aeberhardt LE. Effect of nutritional support on routine nutrition assessment parameters and body composition in intensive care unit patients. Can J Surg. 1996;39:212–219. [PMC free article] [PubMed] [Google Scholar]
- 39.Qin CS, Li XY, Jiang XJ, Feng GK. Micro-inflammatory state and calcification. Zhongguo Zuzhi Gongcheng Yanjiu. 2015;19:4721–4725. [Google Scholar]
- 40.Rambod M, Kovesdy CP, Bross R, Kopple JD, Kalantar-Zadeh K. Association of serum prealbumin and its changes over time with clinical outcomes and survival in patients receiving hemodialysis. Am J Clin Nutr. 2008;88:1485–1494. doi: 10.3945/ajcn.2008.25906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schrader J, Lüders S, Kulschewski A, Berger J, Zidek W, Treib J, Einhäupl K, Diener HC, Dominiak P Acute Candesartan Cilexetil Therapy in Stroke Survivors Study Group. The ACCESS Study: evaluation of Acute Candesartan Cilexetil Therapy in Stroke Survivors. Stroke. 2003;34:1699–1703. doi: 10.1161/01.STR.0000075777.18006.89. [DOI] [PubMed] [Google Scholar]
- 42.Sharma A, Giraddi G, Krishnan G, Shahi AK. Efficacy of serum prealbumin and CRP levels as monitoring tools for patients with fascial space infections of odontogenic origin: A clinicobiochemical study. J Maxillofac Oral Surg. 2014;13:1–9. doi: 10.1007/s12663-012-0376-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Shenkin A. Serum prealbumin: Is it a marker of nutritional status or of risk of malnutrition? Clin Chem. 2006;52:2177–2179. doi: 10.1373/clinchem.2006.077412. [DOI] [PubMed] [Google Scholar]
- 44.Sikora Newsome A, Casciere BC, Jordan JD, Rhoney DH, Sullivan KA, Morbitzer KA, Moore JD, Durr EA. The role of statin therapy in hemorrhagic stroke. Pharmacotherapy. 2015;35:1152–1163. doi: 10.1002/phar.1674. [DOI] [PubMed] [Google Scholar]
- 45.Steiner T, Al-Shahi Salman R, Beer R, Christensen H, Cordonnier C, Csiba L, Forsting M, Harnof S, Klijn CJ, Krieger D, Mendelow AD, Molina C, Montaner J, Overgaard K, Petersson J, Roine RO, Schmutzhard E, Schwerdtfeger K, Stapf C, Tatlisumak T, et al. European Stroke Organisation (ESO) guidelines for the management of spontaneous intracerebral hemorrhage. Int J Stroke. 2014;9:840–855. doi: 10.1111/ijs.12309. [DOI] [PubMed] [Google Scholar]
- 46.Tempel Z, Grandhi R, Maserati M, Panczykowski D, Ochoa J, Russavage J, Okonkwo D. Prealbumin as a serum biomarker of impaired perioperative nutritional status and risk for surgical site infection after spine surgery. J Neurol Surg A Cent Eur Neurosurg. 2015;76:139–143. doi: 10.1055/s-0034-1394188. [DOI] [PubMed] [Google Scholar]
- 47.Unosson M, Ek AC, Bjurulf P, von Schenck H, Larsson J. Feeding dependence and nutritional status after acute stroke. Stroke. 1994;25:366–371. doi: 10.1161/01.str.25.2.366. [DOI] [PubMed] [Google Scholar]
- 48.Upadhyay K, Uniyal A, Guleria R, Prasad D, Sharma A, Pandey R, Mohan A. Serum prealbumin is a useful marker of inflammation and monitoring tool in patients with AECOPD. Chest. 2013;144:708A. [Google Scholar]
- 49.Urra X, Cervera A, Obach V, Climent N, Planas AM, Chamorro A. Monocytes are major players in the prognosis and risk of infection after acute stroke. Stroke. 2009;40:1262–1268. doi: 10.1161/STROKEAHA.108.532085. [DOI] [PubMed] [Google Scholar]
- 50.Wang Q, Ding H, Tang JR, Zhang L, Xu YJ, Yan JT, Wang W, Hui RT, Wang CY, Wang DW. C-reactive protein polymorphisms and genetic susceptibility to ischemic stroke and hemorrhagic stroke in the Chinese Han population. Acta Pharmacol Sin. 2009;30:291–298. doi: 10.1038/aps.2009.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wartenberg KE, Mayer SA. Medical complications after subarachnoid hemorrhage. Neurosurg Clin N Am. 2010;21:325–338. doi: 10.1016/j.nec.2009.10.012. [DOI] [PubMed] [Google Scholar]
- 52.Ye F, Liu J, Yang S, Guo FQ. Higher apolipoprotein B levels are associated with earlier onset of first-ever atherosclerotic stroke. Int J Neurosci. 2015;125:186–190. doi: 10.3109/00207454.2014.951042. [DOI] [PubMed] [Google Scholar]
- 53.Yeun JY, Kaysen GA. Acute phase proteins and peritoneal dialysate albumin loss are the main determinants of serum albumin in peritoneal dialysis patients. Am J Kidney Dis. 1997;30:923–927. doi: 10.1016/s0272-6386(97)90105-0. [DOI] [PubMed] [Google Scholar]
- 54.Yovita H, Djumhana A, Abdurachman SA, Saketi JR. Correlation between anthropometrics measurements, prealbumin level and transferin serum with Child-Pugh classification in evaluating nutritional status of liver cirrhosis patient. Acta Med Indones. 2004;36:197–201. [PubMed] [Google Scholar]
- 55.Zahuranec DB, Lisabeth LD, Sanchez BN, Smith MA, Brown DL, Garcia NM, Skolarus LE, Meurer WJ, Burke JF, Adelman EE, Morgenstern LB. Intracerebral hemorrhage mortality is not changing despite declining incidence. Neurology. 2014;82:2180–2186. doi: 10.1212/WNL.0000000000000519. [DOI] [PMC free article] [PubMed] [Google Scholar]


