Abstract
1-Methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) is a neurotoxin that selectively damages dopaminergic neurons in the substantia nigra pars compacta and induces Parkinson's like symptoms in rodents. Quercetin (QC) is a natural polyphenolic bioflavonoid with potent antioxidant and anti-inflammatory properties but lacks of clinical attraction due to low oral bioavailability. Piperine is a well established bioavailability enhancer used pre-clinically to improve the bioavailability of antioxidants (e.g., Quercetin). Therefore, the present study was designed to evaluate the neuroprotective potential of QC together with piperine against MPTP-induced neurotoxicity in rats. MPTP (100 μg/μL/rat, bilaterally) was injected intranigrally on days 1, 4 and 7 using a digital stereotaxic apparatus. QC (25 and 50 mg/kg, intragastrically) and QC (25 mg/kg, intragastrically) in combination with piperine (2.5 mg/kg, intragastrically) were administered daily for 14 days starting from day 8 after the 3rd injection of MPTP. On day 22, animals were sacrificed and the striatum was isolated for oxidative stress parameter (thiobarbituric acid reactive substances, nitrite and glutathione), neuroinflammatory cytokine (interleukin-1β, interleukin-6, and tumor necrosis factor-α) and neurotransmitter (dopamine, norepinephrine, serotonin, gamma-aminobutyric acid, glutamate, 3,4-dihydroxyphenylacetic acid, homovanillic acid, and 5-hydroxyindoleacetic acid) evaluations. Bilateral infusion of MPTP into substantia nigra pars compacta led to significant motor deficits as evidenced by impairments in locomotor activity and rotarod performance in open field test and grip strength and narrow beam walk performance. Both QC (25 and 50 mg/kg) and QC (25 mg/kg) in combination with piperine (2.5 mg/kg), in particular the combination therapy, significantly improved MPTP-induced behavioral abnormalities in rats, reversed the abnormal alterations of neurotransmitters in the striatum, and alleviated oxidative stress and inflammatory response in the striatum. These findings indicate that piperine can enhance the antioxidant and anti-inflammatory properties of QC, and QC in combination with piperine exhibits strong neuroprotective effects against MPTP-induced neurotoxicity.
Keywords: nerve regeneration; 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine; Quercetin; Piperine; Parkinson's disease; excitotoxicity; oxido-nitrosative stress; neurotransmitters; neural regeneration
Introduction
Parkinson's disease (PD) is a progressive neurodegenerative hypokinetic movement disorder which results from the selective degeneration of dopaminergic neurons in the substantia nigra pars compacta (SNpc) accompanied by behavioral and motor abnormalities like resting tremor, rigidity, bradykinesia, and postural instability (Fu et al., 2015). The exact etiology of PD is unknown and it is believed to be caused by increased oxidative stress, long term toxin exposure, mitochondrial dysfunction and neurotransmitter imbalance. Numerous bodies of evidences have indicated that neurotransmitter imbalance (i.e., dopamine, norepinephrine (NE), serotonin, gamma-aminobutyric acid (GABA) and glutamate), excitotoxicity, increased cytokine level and advanced age are directly linked to the severity of PD (di Michele et al., 2013). Excessive degeneration of dopaminergic neurons is well correlated with increased reactive oxygen species (ROS) concentration and glutamate hyperactivity (Singh et al., 2016). The treatment of PD is currently a major problem worldwide so there is a need to determine effective compounds that have fewer side effects in order to treat PD either alone or as adjuvant with available therapy. Moreover, a large number of neurotoxins like 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP), 6-hydroxy dopamine (6-OHDA), and rotenone are used in preclinical practice to investigate the pathological background of PD.
MPTP is a widely used neurotoxin to mimic PD in animal models. It can directly inhibit mitochondrial complex I activity, which leads to the death of striatal dopaminergic neurons (Meredith and Rademacher, 2011). Previous reports have demonstrated that MPTP results in striatal dopaminergic destruction and alters GABA and glutamate levels and therefore it is usually used to mimic the clinical practice in idiopathic PD patients (Hajj et al., 2015; Caravaggio et al., 2016).
Quercetin (QC) is a polyphenolic bioflavonoid abundantly found in vegetables, fruits, red wines, and black berries. QC possesses potent antioxidant, anti-inflammatory and neuroprotective properties (Bournival et al., 2009). Structurally, QC contains two pharmacophores in its chain providing optimal capacity to scavenge free radicals (Sandhir and Mehrotra, 2013) and has been shown to protect nerve cells that die in Parkinson's disease (PD) (Ahn and Jeon, 2015). Besides its numerous therapeutic applications, QC has low plasma bioavailability and high tissue distribution as its aglycone moiety immediately undergoes a series of biotransformation reactions and converts into different types of active metabolites upon oral administration (Gonzales et al., 2016). Previous reports have shown the limited penetrability of QC across blood-brain barrier and interpecies variation in bioavailability (Reinboth et al., 2010; Ishisaka et al., 2011). The other drug used in the present study is piperine, a nitrogenous and commercially available compound of plant origin obtained from Piper nigrum and Piper longum. Piperine has been reported to enhance the bioavailability of numerous compounds through diverse mechanisms like reducing biotransformation rate in gut by inhibiting cytochrome P3A (CYP3A) and UDP-GDH and enhancing the blood supply in the enteric vessel due to its local vasodilatory effect (Bhardwaj et al., 2002). Therefore, the present study was designed to investigate the neuroprotective, anti-inflammatory and antioxidant effect of QC in combination with piperine against MPTP-induced neurotoxicity in rats.
Material and Methods
Experimental animals
Forty-five male Wistar rats weighing 250–280 g were housed under standard laboratory conditions (room temperature 22 ± 1°C and relative humidity of 60%) with 12-hour light/dark cycles. These animals were fed with standard diet in accordance with Institutional Animal Ethics Committee (IAEC) guidelines. The experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee (ISFCP/IAEC/CPCSCE/M15/P276/2015) and performed according to Indian National Science Academy (INSA) for the use and care of experimental animals. Rats were randomly divided into five groups containing nine animals in each (n = 9) to avoid variability: control group (normal control, intranigral injection of normal saline), MPTP group (intranigral injection of MPTP), QC 25 mg/kg and 50 mg/kg groups (intranigral injection of MPTP followed by intragastric (i.g.) administration of QC 25 mg/kg and 50 mg/kg), and QC + piperine group (intranigral injection of MPTP followed by i.g. administration of QC 25 mg/kg and i.g. administration of piperine 2.5 mg/kg).
Drugs and treatment schedule
The following drugs were used: MPTP (Sigma-Aldrich Corporation, St. Louis, MO, USA) (100 μg/μL, intranigral administration on days 1, 4 and 7) after dissolving in 0.9% normal saline solution. QC (low dose 25 mg/kg, i.g. and high dose 50 mg/kg, i.g. (Hi Media, Mumbai, India)) and piperine [2.5 mg/kg, i.g. (Sigma-Aldrich Corporation)] were dissolved in sodium carboxy methyl cellulose solution (0.5% v/w). QC alone and in combination with piperine were administered once daily for 14 days in two separate groups, starting from day 8 after the 3rd injection of MPTP. Behavioral parameters were assessed on days 1, 7, 14 and 21, respectively. On day 22, the animals were sacrificed, the striatum was isolated for oxidative stress parameter [thiobarbituric acid reactive substances (TBARS), nitrite, reduced glutathione], pro-inflammatory cytokine [interleukin (IL)-1β, IL-6 and tumor necrosis factor-alpha (TNF-α)] and neurotransmitter [dopamine (DA), NE, 5-hydroxytryptamine (5-HT), gamma-amino butyric acid (GABA), glutamate, 3,4-dihydroxyphenylacetic acid (DOPAC), homovanillic acid (HVA), 5-hydroxyindoleacetic acid (5-HIAA)] evaluations (Figure 1).
Figure 1.
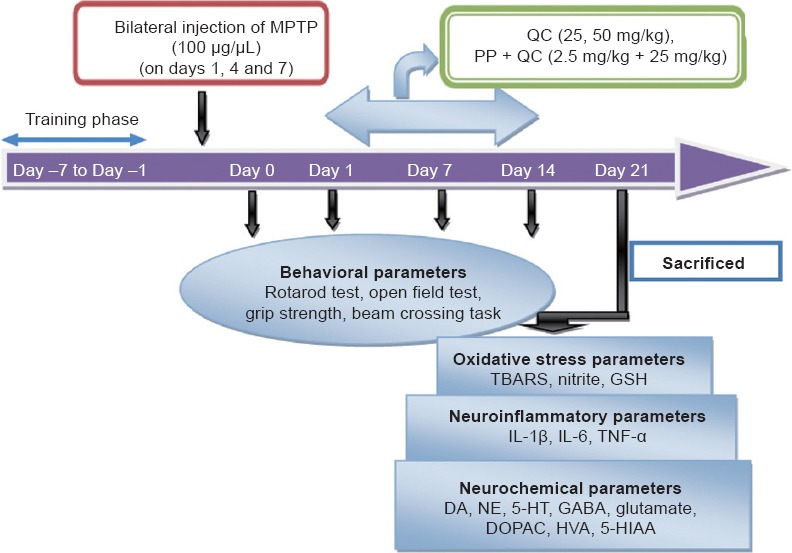
Experimental procedure.
MPTP: 1-Methy-4-phenyl-1,2,3,6-tetrahydropyridine; TBARS: thiobarbituric acid reactive substances; GSH: glutathione; IL-1β: interleukin-1 beta; IL-6: interleukin-6; TNF-α: tumor necrosis factor-alpha; DA: dopamine; NE: norepinephrine; 5-HT: serotonin; GABA; gamma-aminobutyric acid; DOPAC; 3,4-dihydroxyphenylacetic acid; HVA: homovanillic acid; 5-HIAA: 5-hydroxyindoleacetic acid.
MPTP administration
Brain surgery was performed using a stereotaxic apparatus (Stoelting, Wood Dale, IL, USA). Animals were anesthetized with ketamine [80 mg/kg, intraperitoneally (i.p.)] and diazepam (5 mg/kg, i.p.). After exposure of skull surface, cannulas were implanted 2 mm above the SNpc using coordinates (Paxinos and Watson, 2007): anteroposterior (AP): −5.0 mm from bregma; mediolateral (ML): ± 2.1 mm from midline; dorsoventral (DV): −7.7 mm from skull as described by Paxinos and Watson (2007). All groups were intranigrally administered MPTP (100 μg/μL) except the control group in which normal saline (0.9%) was used. All rats after surgery were treated with antibiotic gentamicin (5 mg/kg, i.p.) and were placed back to their home cages.
Measurement of body weight
The percentage change in body weight was calculated according to the formula: Body weight change = (weight of animal on day 1–weight of animal on day 22)/weight of animal on day 1× 100%.
Behavioral assessments
Spontaneous locomotor performance
Spontaneous locomotor activity was assessed by the open field test (Kumar et al., 2011) in an apparatus made up of wooden, rectangular box measuring 100 × 100 × 40 cm3. All animals were provided with 3 minute habituation period before initiating the actual motor activity tasks. The rat's horizontal locomotor activity was recorded as the total number of squares crossed during a 5-minute period as described earlier (Kumar et al., 2011).
Rotarod performance
The Rotarod test apparatus (UgoBasile, Comerio, Italy) was used to analyze the motor balance (latency to fall) and grip performance of rats. All rats were trained before the initiation of experimental procedure. Each rat was placed individually on the rod (diameter 7 cm, length 30 cm, rotating at 25 r/min) for measuring their locomotor ability. The maximum retention (cut off) time on rotarod was 180 seconds (Kumar et al., 2011).
Grip strength measurement
Forelimb grip strength of rats was measured by holding the tail and then the tail was lowered toward the apparatus until it grabbed a mesh with the frontal paws. After holding the platform, the rats were immediately pulled backward with the tail in a horizontal plane. Total beam crossing time and the number of foot errors were recorded in KgF (Karunanithi et al., 2011).
Beam crossing task
Beam crossing task (Karunanithi et al., 2011) was used to measure the gait abnormalities and foot slip counts. The apparatus consisted of a flat wooden beam (length 130 cm and width 1 cm) suspended at a height of 100 cm from the floor to avoid the intentional fall. A black box containing nesting material was placed at the end of beam as a target platform. All animals were previously habituated (trained) with the apparatus for 5 minutes prior to experimentation. The time taken to cross the beam from one point to other end and the number of foot slips were recorded in each trial.
Dissection and homogenization
On day 22, nine animals in each group were further divided into three sub-groups for biochemical estimations, neuroinflammatory cytokine and neurotransmitter analysis immediately after behavioral assessment. All the tested samples were run in duplicate to minimize the statistical errors. After sacrificing the animals, the brains were isolated and preserved at –80°C until analysis. On the experimental day, the brains were removed from the deep freezer and the striatum was isolated, weighed and homogenized in 0.1 M phosphate buffer (pH 7.4). The homogenized striatal suspension solution was then used for oxidative stress, neuroinflammatory cytokine and neurotransmitter estimations.
Measurement of striatal oxidative stress parameters
TBARS values
The level of striatal TBARS as a measure of lipid peroxidation was quantified as described earlier (Wills, 1966). The absorbance of the sample was determined at 532 nm using a spectrophotometer (Shimadzu, UV-1700). The values of TBARS were quantified using the molar extinction coefficient of the chromophore (1.56 × 105/M/cm) expressed as percentage of the normal control group (Kumar et al., 2012).
Nitrite level
The level of nitrite in the striatal tissue homogenate was carried out using Griess reagent in accordance with the method reported by Green et al. (1982). The absorbance of tested sample was measured at 540 nm using a Shimadzu spectrophotometer. The level of nitrite was determined from a sodium nitrite standard curve and expressed as percentage of the normal control group.
Reduced glutathione level
Reduced glutathione (GSH) in the striatal tissue was quantified according to the method of Ellman (1959). Yellow color formed in the tested sample was measured at 412 nm using a Shimadzu spectrophotometer. The results were calculated and expressed as percentage of the normal control group.
Protein level
The protein level in the striatal tissue homogenate was measured according to the Lowry method (Lowry et al., 1951) using Folin phenol reagent. The color change at the end of reaction, indicating presence of protein, was measured at 650 nm using a Shimadzu spectrophotometer. The level of the protein present in the tested sample was calculated from the standard graph.
Striatal proinflammatory cytokines
The striatal levels of pro-inflammatory cytokines IL-1β, IL-6 and TNF-α were quantified using corresponding rat immunoassay kits (KRISHGEN Bio System, Paseo Drive, Brea, CA, USA) according to the method described by Singh et al. (2016).
Striatal neurotransmitter analysis
Catecholamines
Estimation of striatal catecholamines was performed by high performance liquid chromatography using an electrochemical (EC) detector. Striatal levels of catecholamines (DA, norepinephrine, 5-HT and their metabolites DOPAC, HVA and 5-HIAA) were determined according to the method of Patel et al. (2005).
GABA and glutamate estimation
For the quantification of amino acids, striatal homogenate solution was derivatized with o-phthalaldehyde/β-mercaptoethanol (OPA/β-ME) following the method reported earlier (Reinhoud et al., 2013). The quantitative analysis of GABA and glutamate in the striatal tissue sample was performed in accordance with the method of Lasley and Gilbert (2002).
Statistical analysis
All data are presented as the mean ± SEM and analyzed via GraphPad Prism 5.0 software for Windows (GraphPad Software, San Diego, CA, USA). Values were expressed as the mean ± SEM. Two-way analysis of variance (ANOVA) followed by Bonferroni's post hoc test was applied to check the level of significance for behavior results, whereas the biochemical results were analyzed using one-way analysis of variance followed by Tukey's post hoc test. A value of P < 0.05 was considered statistically significant.
Results
Protective effect of QC alone and in combination with piperine on body weight and behavioral changes in MPTP-treated rats
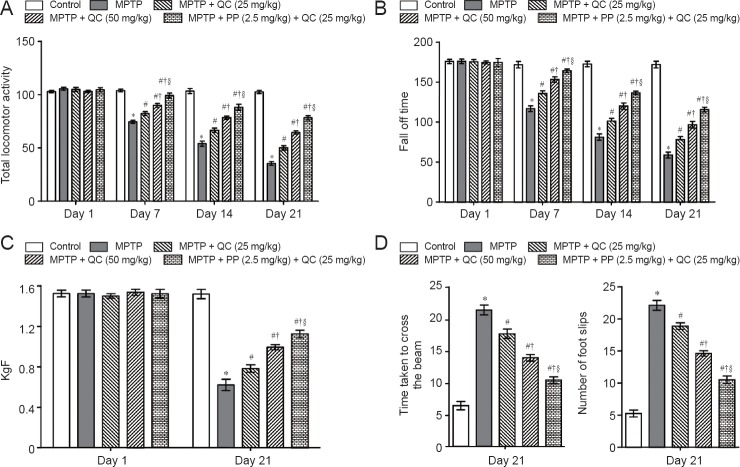
MPTP administered rats showed gradual declines in body weight, locomotor activity, motor coordination and balance, and grip strength at the end of the study (day 21) (P < 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) treatment significantly restored rat's body weight, locomotor activity, motor coordination, and grip strength as compared with administration of MPTP alone (P < 0.05). Further, piperine treatment (2.5 mg/kg) together with low dose QC (25 mg/kg) significantly enhanced its protective effect than treatment with QC alone (P < 0.05) (Figures 2 and 3).
Figure 2.
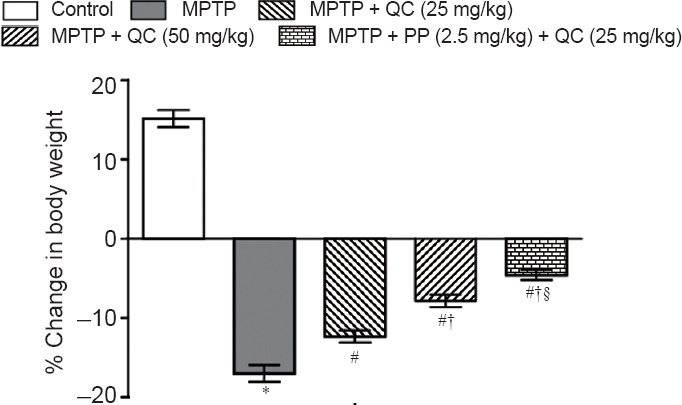
Protective effect of QC alone and its combination with piperine on body weight of MPTP-treated rats.
Body weight change = (weight of animal on day 1– weight of animal on day 22)/weight of animal on day 1 × 100%. Results are expressed as the mean ± SEM and analyzed by two-way analysis of variance followed by Bonferroni's post hoc test. *P < 0.001, vs. control group; #P < 0.05, vs. MPTP group; †P < 0.05, vs. QC 25 mg/kg group; §P < 0.05, vs. QC 50 mg/kg group. QC: Quercetin; MPTP: 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine; PP: piperine.
Figure 3.
Protective effect of QC alone and its combination with piperine (PP) on behavioral changes in MPTP-treated rats.
(A) Total locomotor activity (the number of squares crossed per 5 minutes) assessed by the open field test. (B) Motor coordination and balance assessed by the rotarod test. (C) Grip strength. (D) Motor coordination and balance assessed by the beam walking task. Results are expressed as the mean ± SEM and analyzed by two-way analysis of variance followed by Bonferroni's post hoc test. *P < 0.001, vs. control; #P < 0.05, vs. MPTP; †P < 0.05, vs. MPTP + QC (25 mg/kg); §P < 0.05, vs. MPTP + QC (50 mg/kg). QC: Quercetin; MPTP: 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine.
Protective effect of QC alone and its combination with piperine on oxidative stress in MPTP-treated rats
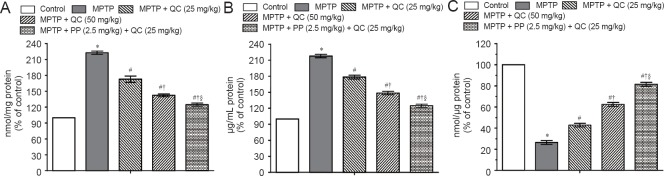
MPTP-treated rats showed raised oxido-nitrosative stress as confirmed by high TBARS and nitrite levels and decreased striatal reduced glutathione level on day 22 (P < 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) treatment significantly and dose-dependently attenuated the increase in TBARS and nitrite levels with decrease in glutathione level as compared with the control group (P < 0.05). Piperine (2.5 mg/kg) together with low dose QC (25 mg/kg) significantly ameliorated the oxido-nitrosative stress as compared with QC alone (25 and 50 mg/kg) due to its enhanced bioavailability (P < 0.05) (Figure 4).
Figure 4.
Protective effect of QC alone and its combination with piperine on oxidative stress in MPTP-treated rats on day 22.
(A) TBARS; (B) nitrite level; (C) reduced glutathione level. Results are expressed as the mean ± SEM. One-way analysis of variance followed by Tukey's post hoc test was used. *P < 0.001, vs. control; #P < 0.05, vs. MPTP; †P < 0.05, vs. MPTP + QC (25 mg/kg); §P < 0.05, vs. MPTP + QC (50 mg/kg). All the tested samples were run in duplicate to minimize the statistical errors. QC: Quercetin; MPTP: 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine.
Protective effect of QC alone and its combination with piperine on neuroinflammatory cytokines in MPTP-treated rats
MPTP-treated rats showed gradually increased striatal proinflammatory cytokines level on day 22 (P < 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) treatment for 14 days substantially and dose-dependently reversed the increases in the levels of TNF-α, IL-1β and IL-6 as compared with MPTP group (P < 0.05). Piperine (2.5 mg/kg) together with QC (25 mg/kg) further improved its protective effect as compared with QC alone (P < 0.05) (Figure 5).
Figure 5.
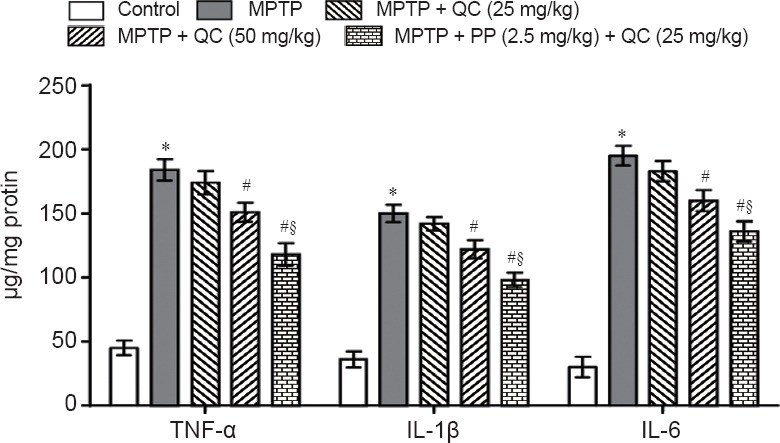
Protective effects of QC alone and its combination with piperine on neuroinflammatory cytokines in MPTP-treated rats on day 22.
Results are expressed as the mean ± SEM and analyzed by one-way analysis of variance followed by Tukey's post hoc test. *P < 0.001, vs. control; #P < 0.05, vs. MPTP; §P < 0.05, vs. MPTP + QC (50 mg/kg). All the tested samples were run in duplicate to minimize the statistical errors. QC: Quercetin; MPTP: 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine; TNF-α: tumor necrosis factor-alpha; IL-1β: interleukin-1beta; IL-6: interleukin-6.
Protective effect of QC alone and its combination with piperine on neurotransmitters in MPTP-treated rats
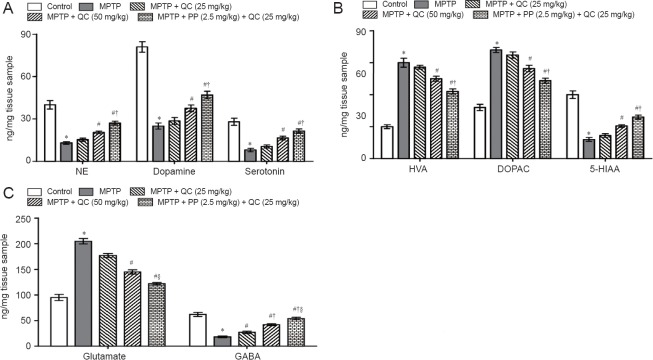
Bilateral infusion of MPTP into SNpc showed significant reduction in the levels of striatal catecholamines (norepinephrine, dopamine and serotonin) at the end of the study (on day 22) (P < 0.01) as compared with the control group. QC (25 and 50 mg/kg) treatment significantly increased catecholamine levels as compared with MPTP control (P < 0.05). Piperine (2.5 mg/kg) together with QC (25 mg/kg) significantly increased striatal catecholamine levels as compared with QC alone (P < 0.01). MPTP treatment resulted in elevated DOPAC and HVA levels as well as decreased striatal 5-HIAA levels. QC (25 and 50 mg/kg) administration significantly attenuated the increased DOPAC and HVA levels and decreased 5-HIAA levels as compared with MPTP control (P < 0.05). Piperine (2.5 mg/kg) together with QC (25 mg/kg) significantly enhanced the protective effect of QC as compared with QC alone (P < 0.05) (Figure 6).
Figure 6.
Protective effect of QC alone and its combination with piperine on striatal levels of neurotransmitters in MPTP-treated rats on day 22.
(A) Catecholamines. (B) Catecholamine metabolites. (C) Glutamate and GABA levels. Results are expressed as the mean ± SEM and analyzed by one-way analysis of variance followed by Tukey's post hoc test. *P < 0.001, vs. control; #P < 0.05, vs. MPTP; †P < 0.05, vs. MPTP + QC (25 mg/kg); §P < 0.05, vs. MPTP + QC (50 mg/kg). All the tested samples were run in duplicate to minimize the statistical errors. QC: Quercetin; MPTP: 1-methy-4-phenyl-1,2,3,6-tetrahydropyridine; NE: norepinephrine; HVA: homovanillic acid; DOPAC: 3,4-dihydroxyphenylacetic acid; 5-HIAA: 5-hydroxyindoleacetic acid; GABA: gamma-aminobutyric acid.
MPTP-treated rats showed gradually decreased striatal GABA level and increased glutamate level at the end of the study (on day 22) (P < 0.001) as compared with normal control rats. QC (25 and 50 mg/kg) significantly and dose-dependently reversed the alteration in striatal GABA and glutamate levels as compared with MPTP control (P < 0.05). Co-administration of piperine (2.5 mg/kg) together with QC (25 mg/kg) dose-dependently increased GABA level and decreased glutamate level than administratin of QC alone (P < 0.05) (Figure 6).
Discussion
The present study evaluated the neuroprotective effect of QC alone and its combination with piperine against MPTP-induced neurotoxicity in rats. Intranigral administration of MPTP to rats led to significant motor deficits, oxidative stress, pro-inflammatory cytokine activation and neurotransmitter alterations. 1-Methyl-4-phenylpyridinium (MPP+) is a neurotoxic metabolite of MPTP, which selectively inhibits complex I (NADH dehydrogenase) of the mitochondrial electron transport chain, decreases ATP production and thereby causes destruction of dopaminergic neurons in SNpc (Hutter et al., 2012). The MPTP-induced behavioral and motor changes in rats are considered to be likely observed in humans suffering from PD as evidenced by bradykinesia, rigidity, and hypokinesia. Treatment with QC alone and in combination with piperine not only improved motor dysfunction but also restored striatal neurotransmitter signalling. These findings indicate that QC when given in combination with piperine could significantly reverse toxin induced motor abnormalities and magnitude of depleted level of nigrostriatal dopamine in rats.
An increasing number of evidence has demonstrated that increased oxidative stress, mitochondrial dysfunction, and neuroinflammation further exacerbate the damage to macromolecules including DNA, proteins, and lipids (Guo et al., 2013). However, degeneration of the membrane lipid yields high intracellular ROS level eventually leading to the loss of membrane integrity and finally dopaminergic neurodegeneration. Moreover, dopaminergic neuron loss is not only due to decreased mitochondrial functionin but also increased level of reactive oxygen species (ROS) and proinflammatory cytokines also play a crucial role. Dopaminergic neurons of nigrostriatal area are more sensitive to oxidative damage as this area is associated with high energy consumption and low antioxidant (GSH) makes them more prone to oxidative damage (Yamaguchi and Shen, 2007). In the present study, administration of QC alone and in combination with piperine significantly decreased ROS as evidenced by elevated GSH level indicating the antioxidant potential of QC. Structurally GSH contains thiol moiety, which tends to plays a pivotal role in preventing oxidative damage and hence acts as a biomarker of oxidative stress in biological systems. It is well implicated that high iron content in the brain makes the substantia nigra more susceptible to PD and is also evidenced by postmortem reports. QC prevents against iron induced striatal toxicity by acting as ion chelator (Lee and Andersen, 2010).
In recent years, the involvement of neuroinflammatory mediators in the nigrostriatal degeneration of dopaminergic neurons in PD has gained increasing attention. Moreover, consistent increase in oxidative stress due to mitochondrial failure after MPTP challenge activates the microglial cells which initiate the inflammatory responses responsible for neuronal dysfunction particularly in the SNpc as evidenced by various experimental models of PD. A postmortem study has also demonstrated that detection of microglial expression in the nigrostriatal region and cerebrospinal fluid particularly including TNF-α, IL-1β and IL-6 suggested the involvement of immune components in PD pathogenesis (Chao et al., 2014). Similarly in the present study, the levels of inflammatory mediators were significantly increased after MPTP treatment, indicating that neuroinflammation plays a crucial role in the neurodegenerative disorders particularly in PD. These results were consistent with clinical findings from a reported study (Wang et al., 2015). In the present study, quercetin significantly attenuated MPTP-induced neuroinflammation.
The imbalance between direct and indirect dopaminergic pathway in the basal ganglia produces uncontrolled involuntary movements in PD due to degeneration of dopaminergic neurons and subsequent alterations in striatal neurotransmitter signaling (Liu et al., 2015; Grosch et al., 2016). In the present study, intranigral administration of MPTP intensively caused regression of nigrostriatal area and significantly altered the level of neurotransmitters like dopamine, NE, serotonin, GABA, glutamate, and their metabolites in striatum. However, this damage is not only due to its direct toxicity but also increased ROS, excessive excitotoxicity and mitochondrial failure are considered to be the prevalence factors responsible for oxidation of catecholamines and their metabolism by monoamine oxidase enzymes. QC does not directly modulate the level of neurotransmitters in the brain but it prevents degeneration of dopaminergic, GABAergic and glutamatergic neurons in striatum and thereby restores their levels in the brain (Bournival et al., 2009). There is evidence that elevation in neurotransmitter level was likely to be attributable to the inhibitory activity of QC on MAO and COMT or due to decreased oxidative stress load on neurons (Chakraborty et al., 2014).
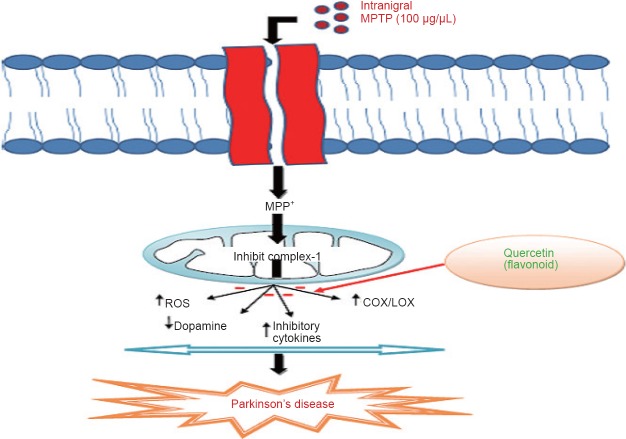
QC, a powerful neuroprotective agent, attenuates the death of striatal neuronal cells and thereby improves motor dysfunction (Karuppagounder et al., 2013). QC is also preclinically reported as a neuroprotective agent in Alzheimer's disease and other neurological disorders due to retention of antioxidant and anti-inflammatory activities (Sabogal et al., 2015). QC was reported to act as a pro-antioxidant and thus curb the oxidative stress dependent release of neuroinflammatory mediators like interleukin (IL-6, IL-1β) and tumor necrosis factor (i.e. TNF-α) (Figure 7) (Sun et al., 2015). In addition to this, QC was reported to decrease the expression of inducible cyclooxygenase-2 and thus protect the dopaminergic neurons in SNpc (Pany et al., 2014). Piperine is a natural component reported to have antioxidant and anti-inflammatory activities in in vitro studies (Shrivastava et al., 2013). It has been evaluated that piperine at low dose reduces neuronal loss and doubles the plasma concentration of some herbal antioxidants (Wadhwa et al., 2014).
Figure 7.
Quercetin acts as a potential neuroprotective agent through antioxidant and anti-inflammatory mechanisms.
Intranigral administration of MPTP is readily converted to MPP+ ions by monoamine oxidases. These ions are highly lipophilic, enter the mitochondria of neurons and reduce the adenosine 5′-triphosphate synthesis by interfering with ETC. Further, they increase inflammatory response by activating COX/LOX enzymes and ultimately cause degeneration of dopaminergic neurons. Flavanoids through antioxidant and anti-inflammatory mechanisms protects the dopaminergic neurons against oxidative insult and acts as potential neuroprotective agents. MPTP: 1-Methyl-4-phenyl-1,2,3,6-tetrahydropyridine; MPP: 1-methyl-4-phenylpyridinium; ROS: Reactive oxygen species; COX/LOX: Cyclooxygenase/Lipooxygenase.
In summary, the present findings confirmed the neuroprotective effect of combined administration of QC and piperine against MPTP-induced motor deficits, oxidative stress, neuroinflammatory and neurotransmitter alterations. The present findings suggest that QC prevents MPTP-induced degeneration of dopaminergic neurons through its powerful antioxidant and anti-inflammatory mechanisms supported by its metal ion scavenging properties. Therefore, the use of these antioxidants alone or in combination with available drugs may serve as potential therapeutic targets for the treatment of neurodegenerative disorders like PD.
Acknowledgments
The authors are thankful to Science and Engineering Board (SERB), Department of Science and Technology, Government of India, New Delhi for providing financial assistance under Fast Track Scheme (DST-SERB-FTYS) (SB/FT/LS-139/2012) to Dr. PK.
Footnotes
Conflicts of interest: None declared.
Research ethics: The experimental protocol was reviewed and approved by the Institutional Animal Ethics Committee (ISFCP/IAEC/CPCSCE/M15/P276/2015), and experiments were conducted in compliance with the guidelines of the Indian National Science Academy (INSA) for the Use and Care of Experimental Animals and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association of Veterinary Editors (IAVE). The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Li CH, Song LP, Zhao M
References
- 1.Ahn TB, Jeon BS. The role of quercetin on the survival of neuron-like PC12 cells and the expression of α-synuclein. Neural Regen Res. 2015;10:1113–1119. doi: 10.4103/1673-5374.160106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bhardwaj RK, Glaeser H, Becquemont L, Klotz U, Gupta SK, Fromm MF. Piperine, a major constituent of black pepper, inhibits human P-glycoprotein and CYP3A4. J Pharmacol Exp Ther. 2002;302:645–650. doi: 10.1124/jpet.102.034728. [DOI] [PubMed] [Google Scholar]
- 3.Bournival J, Quessy P, Martinoli MG. Protective effects of resveratrol and quercetin against MPP+-induced oxidative stress act by modulating markers of apoptotic death in dopaminergic neurons. Cell Mol Neurobiol. 2009;29:1169–1180. doi: 10.1007/s10571-009-9411-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Caravaggio F, Nakajima S, Plitman E, Gerretsen P, Chung JK, Iwata Y, Graff-Guerrero A. The effect of striatal dopamine depletion on striatal and cortical glutamate: A mini-review. Prog Neuropsychopharmacol Biol Psychiatry. 2016;65:49–53. doi: 10.1016/j.pnpbp.2015.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chakraborty J, Singh R, Dutta D, Naskar A, Rajamma U, Mohanakumar KP. Quercetin improves behavioral deficiencies, restores astrocytes and microglia, and reduces serotonin metabolism in 3-nitropropionic acid-induced rat model of Huntington's disease. CNS Neurosci Ther. 2014;20:10–19. doi: 10.1111/cns.12189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chao Y, Wong SC, Tan EK. Evidence of inflammatory system involvement in Parkinson's disease. Biomed Res Int. 2014;2014:308654. doi: 10.1155/2014/308654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.di Michele F, Luchetti S, Bernardi G, Romeo E, Longone P. Neurosteroid and neurotransmitter alterations in Parkinson's disease. Front Neuroendocrinol. 2013;34:132–142. doi: 10.1016/j.yfrne.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 8.Ellman GL. Tissue sulfhydryl groups. Arch Biochem Biophys. 1959;82:70–77. doi: 10.1016/0003-9861(59)90090-6. [DOI] [PubMed] [Google Scholar]
- 9.Fu SP, Wang JF, Xue WJ, Liu HM, Liu BR, Zeng YL Liu JX. Anti-inflammatory effects of BHBA in both in vivo and in vitro Parkinson's disease models are mediated by GPR109A-dependent mechanisms. J Neuroinflammation. 2015;12:1–4. doi: 10.1186/s12974-014-0230-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzales GB, Smagghe G, Wittevrongel J, Huynh NT, Van Camp J, Raes K. Metabolism of Quercetin and Naringenin by Food-Grade Fungal Inoculum, Rhizopus azygosporus Yuan et Jong (ATCC 48108) J Agric Food Chem. 2016;64:9263–9267. doi: 10.1021/acs.jafc.6b04124. [DOI] [PubMed] [Google Scholar]
- 11.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126:131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 12.Grosch J, Winkler J, Kohl Z. Early degeneration of both dopaminergic and serotonergic axons–A common mechanism in Parkinson's disease. Front Cell Neurosci. 2016;10:293. doi: 10.3389/fncel.2016.00293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Guo C, Sun L, Chen X, Zhang D. Oxidative stress, mitochondrial damage and neurodegenerative diseases. Neural Regen Res. 2013;8:2003–2014. doi: 10.3969/j.issn.1673-5374.2013.21.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hajj R, Milet A, Toulorge D, Cholet N, Laffaire J, Foucquier J, Robelet S, Mitry R, Guedj M, Nabirotchkin S, Chumakov I, Cohen D. Combination of acamprosate and baclofen as a promising therapeutic approach for Parkinson's disease. Sci Rep. 2015;5:16084. doi: 10.1038/srep16084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hutter-Saunders JA, Gendelman HE, Mosley RL. Murine motor and behavior functional evaluations for acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) intoxication. J Neuroimmune Pharmacol. 2012;7:279–288. doi: 10.1007/s11481-011-9269-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto K, Tsuji A, Kawai Y, Terao J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects in rats. Free Radic Biol Med. 2011;51:1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- 17.Karunanithi K, Annadurai A, Krishnamoorthy M, Elumalai P, Manivasagam T. 1-Methyl 4-phenyl 1, 2, 3, 6-tetrahydropyridine is a potent neurotoxin: Gamma-tocopherol recuperate behavior, dopamine, and oxidative stress on Parkinsonic mice. Int J Nutr Pharmacol Neurol Dise. 2011;1:139. [Google Scholar]
- 18.Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson's disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 19.Kumar P, Kalonia H, Kumar A. Role of LOX/COX pathways in 3-nitropropionic acid-induced Huntington's disease-like symptoms in rats: protective effect of licofelone. Br J Pharmacol. 2011;164(2b):644–654. doi: 10.1111/j.1476-5381.2011.01418.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar P, Kalonia H, Kumar A. Possible GABAergic mechanism in the neuroprotective effect of gabapentin and lamotrigine against 3-nitropropionic acid induced neurotoxicity. Eur J Pharmacol. 2012;674(2-3):265–274. doi: 10.1016/j.ejphar.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 21.Lasley SM, Gilbert ME. Rat hippocampal glutamate and GABA release exhibit biphasic effects as a function of chronic lead exposure level. Toxicol Sci. 2002;66:139–147. doi: 10.1093/toxsci/66.1.139. [DOI] [PubMed] [Google Scholar]
- 22.Lee DW, Andersen JK. Iron elevations in the aging Parkinsonian brain: a consequence of impaired iron homeostasis? J Neurochem. 2010;112:332–339. doi: 10.1111/j.1471-4159.2009.06470.x. [DOI] [PubMed] [Google Scholar]
- 23.Liu J, Huang D, Xu J, Tong J, Wang Z, Huang L, Yang Y, Bai X, Wang P, Suo H, Ma Y, Yu M, Fei J, Huang F. Tiagabine protects dopaminergic neurons against neurotoxins by inhibiting microglial activation. Sci Rep. 2015;5:15720. doi: 10.1038/srep15720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 25.Meredith GE, Rademacher DJ. MPTP mouse models of Parkinson's disease: an update. J Parkinsons Dis. 2011;1:19–33. doi: 10.3233/JPD-2011-11023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pany SU, Pal AB, Sahu PK. Neuroprotective effect of quercetin in neurotoxicity induced rats: role of neuroinflammation in neurodegeneration. Asian J Pharm Clin Res. 2014;7:152–156. [Google Scholar]
- 27.Patel AB, de Graaf RA, Mason GF, Rothman DL, Shulman RG, Behar KL. The contribution of GABA to glutamate/glutamine cycling and energy metabolism in the rat cortex in vivo. Proc Natl Acad Sci U S A. 2005;102:5588–5593. doi: 10.1073/pnas.0501703102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 6th edition. San Diego: Academic Press; 2007. [Google Scholar]
- 29.Reinboth M, Wolffram S, Abraham G, Ungemach FR, Cermak R. Oral bioavailability of quercetin from different quercetin glycosides in dogs. Br J Nutr. 2010;104:198–203. doi: 10.1017/S000711451000053X. [DOI] [PubMed] [Google Scholar]
- 30.Sabogal-Guáqueta AM, Muñoz-Manco JI, Ramírez-Pineda JR, Lamprea RM, Osorio E, Cardona-Gómez GP. The flavonoid quercetin ameliorates Alzheimer's disease pathology and protects cognitive and emotional function in aged triple transgenic Alzheimer's disease model mice. Neuropharmacology. 2015;93:134–145. doi: 10.1016/j.neuropharm.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sandhir R, Mehrotra A. Quercetin supplementation is effective in improving mitochondrial dysfunctions induced by 3-nitropropionic acid: implications in Huntington's disease. Biochim Biophys Acta. 2013;1832:421–430. doi: 10.1016/j.bbadis.2012.11.018. [DOI] [PubMed] [Google Scholar]
- 32.Shrivastava P, Vaibhav K, Tabassum R, Khan A, Ishrat T, Khan MM, Ahmad A, Islam F, Safhi MM, Islam F. Anti-apoptotic and anti-inflammatory effect of Piperine on 6-OHDA induced Parkinson's rat model. J Nutr Biochem. 2013;24:680–687. doi: 10.1016/j.jnutbio.2012.03.018. [DOI] [PubMed] [Google Scholar]
- 33.Singh S, Kumar P. Neuroprotective activity of curcumin in combination with piperine against quinolinic acid induced neurodegeneration in rats. Pharmacology. 2016;97(3-4):151–160. doi: 10.1159/000443896. [DOI] [PubMed] [Google Scholar]
- 34.Sun GY, Chen Z, Jasmer KJ, Chuang DY, Gu Z, Hannink M, Simonyi A. Quercetin attenuates inflammatory responses in BV-2 microglial cells: Role of MAPKs on the Nrf2 pathway and induction of heme oxygenase-1. PLoS One. 2015;10:e0141509. doi: 10.1371/journal.pone.0141509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wadhwa S, Singhal S, Rawal S. Bioavailability enhancement by piperine: a review. Asian J Biomed Pharm Sci. 2014;4:1–8. [Google Scholar]
- 36.Wang Q, Liu Y, Zhou J. Neuroinflammation in Parkinson's disease and its potential as therapeutic target. Transl Neurodegener. 2015;4:19. doi: 10.1186/s40035-015-0042-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamaguchi H, Shen J. Absence of dopaminergic neuronal degeneration and oxidative damage in aged DJ-1-deficient mice. Mol Neurodegener. 2007;2:10. doi: 10.1186/1750-1326-2-10. [DOI] [PMC free article] [PubMed] [Google Scholar]