Keywords: nerve regeneration, spinal cord ischemia/reperfusion injury, aldehyde dehydrogenase 2, alcohol, apoptosis, oxidative stress, terminal deoxynucleotidyl transferase dUTP nick-end labeling, neural regeneration
Abstract
Aldehyde dehydrogenase 2 (ALDH2) is an important factor in inhibiting oxidative stress and has been shown to protect against renal ischemia/reperfusion injury. Therefore, we hypothesized that ALDH2 could reduce spinal cord ischemia/reperfusion injury. Spinal cord ischemia/reperfusion injury was induced in rats using the modified Zivin's method of clamping the abdominal aorta. After successful model establishment, the agonist group was administered a daily consumption of 2.5% alcohol. At 7 days post-surgery, the Basso, Beattie, and Bresnahan score significantly increased in the agonist group compared with the spinal cord ischemia/reperfusion injury group. ALDH2 expression also significantly increased and the number of apoptotic cells significantly decreased in the agonist group than in the spinal cord ischemia/reperfusion injury group. Correlation analysis revealed that ALDH2 expression negatively correlated with the percentage of TUNEL-positive cells (r = −0.485, P < 0.01). In summary, increased ALDH2 expression protected the rat spinal cord against ischemia/reperfusion injury by inhibiting apoptosis.
Introduction
Decompression of a previously severely compressed region of the spinal cord could induce spinal cord ischemia/reperfusion injury (SCII), which could lead to further irreversible postoperative complications, such as paraplegia or paraparesis (Taher et al., 2013; Zhu et al., 2013; Yang et al., 2015). SCII includes two phases: 1) immediate spinal cord injury related to acute ischemia; and 2) delayed spinal cord injury involving ischemic cellular death and reperfusion injury.
Although the molecular mechanisms of ischemia/reperfusion injury (IRI) pathogenesis remain poorly understood, evidence suggests that oxidative stress caused by excessive production of reactive oxygen species during the IRI process is a critical factor in direct and subsequent cellular damage (Noiri et al., 2001; Walker et al., 2001; Colón and Miranda, 2016). Lipid peroxidation, which is an important source of oxidative stress, is an autocatalytic mechanism resulting in oxidative destruction of cellular membranes associated with production of toxic, reactive, aldehydic metabolites and cell death (Niki et al., 2005). Reactive aldehydes, such as 4-hydroxy-2-nonenal and malondialdehyde, the major end products of lipid peroxidation, are highly toxic and react with proteins to form various adducts, leading to dysfunctional proteins and subsequent cellular injury (Renner et al., 2005; Conklin et al., 2006; Marchitti et al., 2007). Previous studies of cardiac and renal IRI have suggested that preconditions with the effective activator aldehyde dehydrogenase 2 (ALDH2) or physiological levels of ethanol provide protection for corresponding organs, which consistently correlates with the phosphorylation status of ALDH2, a key metabolic enzyme in the oxidation and detoxification of reactive aldehydes in a range of organs and cell types, to inhibit oxidative stress (Chen et al., 2008; Yuan et al., 2011). ALDH2 is a mitochondrial enzyme and belongs to the ALDH gene family. ALDH2 not only catalyzes oxidation of acetaldehyde to acetic acid in ethanol metabolism, but also acts as a key metabolic enzyme in the detoxification of other reactive aldehydes such as hydroxynonenal (Vasiliou et al., 2005). However, to the best of our knowledge, it is unknown whether ALDH2 has neuroprotective effects by reducing apoptosis under conditions of SCII. The objective of our study was to investigate the early effect of ALDH2 on apoptosis through alcohol administration in rats subjected to SCII.
Materials and Methods
Animals
Thirty adult, male, Sprague-Dawley rats, aged 8–12 weeks and weighing 250–270 g, were obtained from the Animal Laboratory of Second Military Medical University of China (license number: SCXK (Hu) 2013-0006). The study protocol was approved by the Animal Ethics Committee of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, China. The experimental procedure followed the the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE).
The rats were randomly divided into sham operation group (sham group, n = 10), SCII group (I/R group, n = 10), and SCII with alcohol group (agonist group, n = 10). Rats in the sham group underwent surgery without injury. Rats in the I/R and agonist groups suffered from SCII using Zivin's method (Zivin et al., 1980). After successful model establishment, the agonist group received daily consumption of 2.5% alcohol (Sinopharm Chemical Reagent Co., Ltd., Shanghai, China); the other groups were fed water (30 mL per day) for 7 days post-surgery.
SCII modeling and alcohol intervention
The rat model of SCII was performed employing the previously described Zivin's method (Zivin et al., 1980). All rats were prohibited from drinking during the morning of the surgery. Rats were deeply anesthetized with 3% sodium pentobarbital (1 mL/kg, intraperitoneal injection) and placed in a supine position. After making a 4–5-cm medial incision, the abdominal aorta was exposed at the level of the left renal artery. A total of 400 U heparin was injected 4 minutes before aortic occlusion, and spinal cord ischemia was induced by clamping the aorta with a bulldog clamp just below the left renal artery. After occlusion, femoral artery pulsation ceased, and blood flow was obstructed for 40 minutes. The bulldog clamp was removed, and the abdominal wall was closed. Successful rat model of spinal cord ischemia criteria: cessation of abdominal aorta and double hindlimb skin cyanosis. Signs of recanalization blood flow: once the arterial clamp was loosened, abdominal aorta beating recurred and color in the skin of both hindlimbs was bright red.
Motor function assessment
Hind limb motor function assessment was performed at 7 days post-surgery using the Basso, Beattie, and Bresnahan (BBB) motor rating scale (Basso et al., 1995). This BBB scale was based on motor ability following SCII in a rat model. Briefly, the BBB scale was a 22-point scale from 0 to 21. The 0-point indicated no observable hind limb movement, and 21 points indicated consistent and coordinated gait with parallel paw placement of the limbs and consistent trunk stability. Locomotion was scored by two independent observers blind to experimental design.
Sacrifice time
All rats were sacrificed at 7 days post-surgery, and the spinal cords (L2–5) were immediately removed to identify medullospinal pathologic changes, examine ALDH2 protein expression, and observe cell apoptosis, respectively, by hematoxylin-eosin staining, western blot assay, and terminal deoxynucleotidyl transferase dUTP nick-end labeling (TUNEL) assay.
Hematoxylin-eosin staining
The first segment of the spinal cord (L2–5) was dissected and post-fixed in 4% paraformaldehyde overnight. After dehydration, the spinal cords were embedded in paraffin and 5-μm-thick serial coronal sections were collected. To obverse histopathological changes, the sections were further subjected to hematoxylin-eosin staining. Sections (5-μm-thick) were stained with hematoxylin solution for 5 minutes followed by 5 dips in 1% acid ethanol (1% HCl in 70% ethanol) and then rinsed in distilled water. Afterwards, the sections were stained with eosin solution for 3 minutes, followed by dehydration in an alcohol gradient and cleared in xylene. An independent observer, blinded to the experimental design, collected images using an inverted fluorescence microscope (Nikon Eclipse T3-S, Tokyo, Japan).
Western blot assay
The second of spinal cord (L2–5) sample was centrifuged at 1,500 r/min for 30 minutes, after which the supernatant was collected. Determination of protein concentration was performed using the bicinchoninic acid protein assay kit. After transferring 0, 15, 30, 60, 120, 180, 240, and 300 μL of bovine serum albumin (500 μg/mL) into each 2-mL centrifuge tube, 1× PBS was added to each tube to reach a volume of 300 μL. The final concentrations of bovine serum albumin in each centrifuge tube were 0, 25, 50, 100, 200, 300, 400, and 500 μg/mL. Working reagent was prepared by mixing 50 parts bicinchoninic acid reagent A with 1 part bicinchoninic acid reagent B (50:1, reagent A:B). The protein extract (20 μL) was placed in an appropriately labeled test tube, and diluted with PBS 30 times. An additional 200 μL of working reagent was added to each tube and mixed well. The tubes were covered and incubated at 37°C for 30 minutes. The spectrophotometer measured at 550 nm and was set to zero using a cuvette filled only with 1 × PBS. Sample absorbance was then measured within 10 minutes. The sample was electrophoresed in a 5% stacking gel with 80 V constant voltage for 20 minutes, followed by 110 V constant voltage for 3.5 hours. A total of 20 μL was resolved on an electrophoresis gel and then transferred onto polyvinylidene difluoride membranes. Primary antibody specific for ALDH2 (goat monoclonal antibody) (1:500; Santa Cruz Biotechnology, Santa Cruz, CA, USA) was diluted in Tris-buffered saline and Tween 20 (TBST), added to the membrane, and incubated for 60 minutes at room temperature. The membrane was then washed in TBST and incubated in secondary antibody (rabbit anti-goat horseradish peroxidase-labeled polyclonal antibody, 1:10,000; Santa Cruz Biotechnology) for 2 hours at room temperature, followed by TBST washing (3 times) and TBS washing (10 minutes). Protein bands were visualized using enhanced chemiluminescence reagent (Beyotime, Haimen, China), followed by exposure and were photographed to save the experimental results. The ratio of optical density values between ALDH2 and GAPDH bands, which was an indicator of ALDH2 protein expression, was measured using the Gel-Pro analyzer 4.5 (Media Cybernetics, Sarasota, FL, USA).
TUNEL assay
The third spinal cord (L2–5) segment was subjected to antigen retrieval proteinase K (Roche) for 30 minutes, and then washed three times with phosphate-buffered saline (PBS). TUNEL solution preparation and staining were performed according to the assay kit instruction manual (Roche, Basel, Switzerland). Cell quantification was obtained by two observers using an inverted fluorescence microscope at 400× magnification. Cell apoptosis (%) was equal to the number of positive cells/(positive cells + negative cells) × 100%.
Statistical analysis
Statistical analysis was performed using SPSS 19.0 software (IBM, Armonk, IL, USA) and all data are expressed as the mean ± SD. Intergroup differences were analyzed by one-way analysis of variance and Student-Newman-Keuls test. Correlation analysis was examined by Pearson correlation coefficient. Significance was set to P < 0.05.
Results
ALDH2 overexpression affected behavior in SCII rats
Rats in the sham group did not show obvious symptoms of neurological impairments. BBB scores in the I/R (7.20 ± 0.40) and agonist groups (14.10 ± 0.80) significantly decreased compared with the sham group (20.00 ± 0.50) (P < 0.05), and BBB scores significantly improved in the agonist group compared with the I/R group (P < 0.05; Figure 1).
Figure 1.
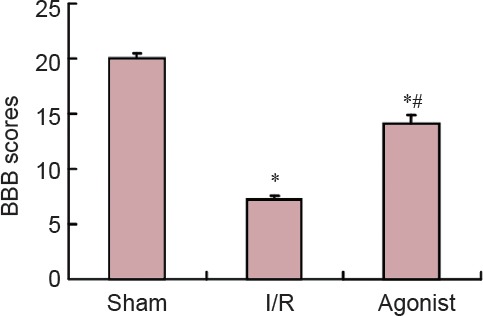
BBB scores in rats at 7 days after surgery.
*P < 0.05, vs. sham group, #P < 0.05, vs. I/R group (mean ± SD, n = 10, one-way analysis of variance and Student-Newman-Keuls test). BBB: Basso, Beattie, and Bresnahan; I/R: ischemia/reperfusion.
ALDH2 overexpression affected histological changes in SCII rats
Hematoxylin-eosin staining results demonstrated well-maintained spinal cord tissues in the sham group, and neurons were morphologically normal with clear karyosomes, uniformly stained cytoplasm, and few vacuolar changes. In the I/R group, the number of normal neurons significantly decreased. Neurons were swollen and pyknotic, and the cytoplasm was stained dark with a great deal of vacuolization. In the agonist group, these pathological changes were markedly reduced compared with the I/R group, and the number of normal neurons was significantly greater than in the I/R group. Most neurons were multipolar with clear karyosomes, with few vacuolar changes (Figure 2).
Figure 2.
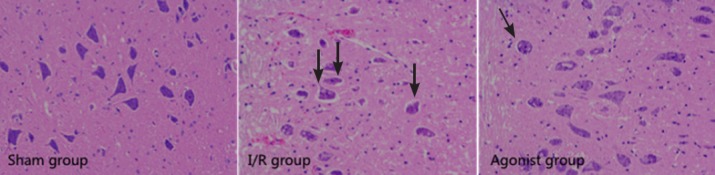
Aldehyde dehydrogenase 2 overexpression affected histomorphology in rats with spinal cord ischemia/reperfusion injury (hematoxylin-eosin staining, ×200).
Sham group: Normal spinal cord tissues; I/R group: serious damage; agonist group: mild damage. Arrows refer to injured cells. I/R: Ischemia/reperfusion.
ALDH2 protein expression
ALDH2 protein expression was significantly improved in the agonist group (0.74 ± 0.04) compared with the I/R group (0.66 ± 0.05) (P < 0.05; Figure 3).
Figure 3.
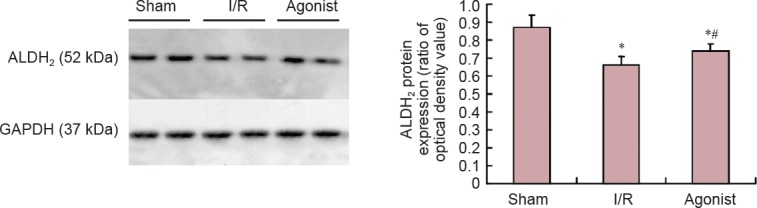
Protein expression in the spinal cord of ALDH2 in the sham, I/R, and agonist groups (western blot assay).
Protein expression of ALDH2 in the agonist group significantly increased compared with the I/R group. *P < 0.05, vs. sham group, #P < 0.05, vs. I/R group (mean ± SD, n = 10, one-way analysis of variance and Student-Newman-Keuls test). ALDH2: Aldehyde dehydrogenase 2; I/R: ischemia/reperfusion; GAPDH: glyceraldehyde 3-phosphate dehydrogenase.
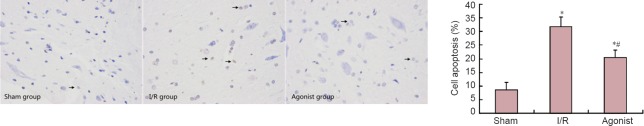
ALDH2 overexpression affected apoptosis in SCII rats
TUNEL staining results demonstrated few TUNEL-positive cells in the sham group (8.47 ± 2.80). The percentage of TUNEL-positive cells was significantly higher in the I/R (31.69 ± 3.66) and agonist groups (20.45 ± 2.72) than in the sham group (P < 0.05). However, the percentage of TUNEL-positive cells was markedly decreased in the agonist group compared with the I/R group (P < 0.05; Figure 4).
Figure 4.
ALDH2 overexpression affected apoptosis in rats with spinal cord ischemia/reperfusion injury (TUNEL assay, × 400).
Sham group: Few TUNEL-positive cells; I/R group: many TUNEL-positive cells (arrows) compared with the agonist group. *P < 0.05, vs. sham group; #P < 0.05, vs. I/R group (mean ± SD, n = 10, one-way analysis of variance and Student-Newman-Keuls test). ALDH2: Aldehyde dehydrogenase 2; I/R: ischemia/reperfusion; TUNEL: terminal deoxynucleotidyl transferase dUTP nick-end labeling.
Correlation between ALDH2 expression and apoptotic rate
The Pearson correlation coefficient between ALDH2 expression (X) and apoptotic rate (Y) was −0.485 in the three groups. Thus, ALDH2 expression and apoptosis rate were negatively correlated (P < 0.01).
Discussion
Although adequate surgical decompression is the preferred treatment for chronic severe compression of the spinal cord and results in favorable surgical outcomes, SCII patients experience unpredictable and disastrous postoperative complications (Taher et al., 2013; Zhu et al., 2013; Yang et al., 2015). Because of the serious consequences of SCII, groups around the world have focused on how to effectively alleviate SCII. The present results demonstrated that moderate alcohol administration following SCII induction provides early protection. After establishment of a rat model of SCII, alcohol-treated rats displayed significantly preserved neurological function characterized by high BBB scores and less histological damage and apoptosis. Results further demonstrated that the protective effect was associated with ALDH2. ALDH2 is a crucial enzyme involved in protecting the IRI by inhibiting oxidative stress. ALDH2 is one of 19 members of the ALDH gene family and is localized within the mitochondria, a major site for reactive oxygen species and reactive aldehyde generation (Vasiliou et al., 2005). ALDH2 not only catalyzes oxidation of acetaldehyde to acetic acid in ethanol metabolism, but also acts as a key metabolic enzyme involved in the detoxification of other reactive aldehydes such as hydroxynonenal (Vasiliou et al., 2005; Chen et al., 2008; Ma et al., 2011; Yuan et al., 2011; Sun et al., 2014). Increasing evidence indicates that ALDH2 plays a protective role in IRI of the heart, kidney, and brain. He et al. (2012) confirmed that the cardioprotective effects of alpha lipoic acid on IRI took place through a mechanism involving ALDH2 activation. Ji et al. (2016) reported that ALDH2 inhibited excessive mitophagy and increased cardiomyocyte following IRI by reducing 4-hydroxy-2-nonenal and reactive oxygen species levels. Yuan et al. (2011) and Zhong et al. (2016) reported that ALDH2 provides protection for kidneys against IRI by enhancing antioxidant capacity and preventing lipid peroxidation. Fu et al. (2014) believed ALDH2 activation could reduce cerebral IRI by decreasing accumulation of reactive aldehydes concomitantly with improvements in brain injury and neurological function. Results from the study clearly showed that increased ALDH2 expression alleviated SCII in rats, which was consistent with the above analysis in other organs. In our study, the agonist group rats, which expressed increased levels of ALDH2, also exhibited significantly improved neurological functions as assessed by BBB scores compared with the I/R group at 7 days post-surgery. Additionally, the pathological changes were markedly reduced, and the number of normal neurons was significantly greater in the agonist group compared with the I/R group. Most neurons were multipolar with clear karyosomes, and there were few vacuolar changes.
Although the underlying mechanisms involved in ALDH2 protection against IRI, previous studies have shown detoxication of reactive aldehydes, reduced reactive oxygen species generation, and protection of mitochondrial function terminally inhibiting endoplasmic reticulum stress, apoptosis, and autophagy (Ma et al., 2011; Pang et al., 2015). The present study focused on the relationship between ALDH2 and apoptosis during SCII.
Inhibition of apoptosis could reduce the extent of injury and preserve neurological function (Lee et al., 2003; Li et al., 2016), which is closely associated with reactive oxygen species accumulation during IRI (Ebert et al., 2014). The anti-apoptotic enzyme ALDH2 has been shown to be involved in cell apoptosis induced by oxidative stress (Zhang et al., 2011; Fan et al., 2013). Ebert et al. (2014) reported that defective regulation of ALDH2-dependent signaling events induces cardiomyocyte apoptosis resulting from increased 4-hydroxy-2-nonenal and reactive oxygen species. When ALDH2 expression is stimulated, levels of 4-hydroxy-2-nonenal, reactive oxygen species, and apoptosis significantly decrease (Pang et al., 2015). Other studies also confirmed that increased ALDH2 expression induced by alcohol or the effective activator Alda-1 reduces cardiomyocyte apoptosis (Chen et al., 2008; Ge et al., 2012; Gao et al., 2015). Zhong et al. (2016) reported that increased ALDH2 expression reduces renal cell apoptosis during IRI. Following liver injury, decreased ALDH2 expression also leads to apoptosis (Zhong et al., 2016). However, it remained to be shown whether ALDH2 exhibits neuroprotective effects by reducing apoptosis in SCII. Results from the present study clearly showed that increased ALDH2 expression stimulated by alcohol resulted in decreased SCII-induced apoptosis.
Although the molecular mechanisms involved in ALDH2 and apoptosis remain poorly understood, increased ALDH2 expression influences apoptosis, which might be associated with subsequent activation of heat shock protein 70, phosphorylation of c-Jun N-terminal kinase, and inhibition of p53 (Li et al., 2004; Harada, et al., 2005; Sun et al., 2014; Zhong et al., 2016). Further studies are needed to determine the relationship between these molecules.
Our study has several limitations that deserve special attention. First, it is necessary to improve accuracy in this experiment by increasing the number of animal models. Second, to determine long-term neurological protection by ALDH2 through alcohol administration, future studies should increase the observation interval time after establishing the rat model of SCII. Third, this study preliminarily discussed the relationship between ALDH2 and SCII, although further studies are needed to determine the involved mechanisms and contributions of ALDH2 to SCII.
In summary, the neurological effects of alcohol on SCII took place through a mechanism involving, at least in part, ALDH2 activation, which effectively inhibited apoptosis. Results from this study could provide a novel therapeutic target for treating spinal cord injury induced by ischemia/reperfusion.
Footnotes
Funding: This research was supported by the Natural Science Research Fund Project of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine of China, No. syz2014-014.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Animal Ethics Committee of Shanghai Ninth People's Hospital, Shanghai Jiao Tong University School of Medicine, China. The experimental procedure followed the United States National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 85-23, revised 1986), and “Consensus Author Guidelines on Animal Ethics and Welfare” produced by the International Association for Veterinary Editors (IAVE). The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Wang J, Li CH, Qiu Y, Song LP, Zhao M
References
- 1.Basso DM, Beattie MS, Bresnahan JC. A sensitive and reliable locomotor rating scale for open field testing in rats. J Neurotrauma. 1995;12:1–21. doi: 10.1089/neu.1995.12.1. [DOI] [PubMed] [Google Scholar]
- 2.Chen CH, Budas GR, Churchill EN, Disatnik MH, Hurley TD, Mochly-Rosen D. Activation of aldehyde dehydrogenase-2 reduces ischemic damage to the heart. Science. 2008;321:1493–1495. doi: 10.1126/science.1158554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Colón JM, Miranda JD. Tamoxifen: an FDA approved drug with neuroprotective effects for spinal cord injury recovery. Neural Regen Res. 2016;11:1208–1211. doi: 10.4103/1673-5374.189164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Conklin D, Prough R, Bhatanagar A. Aldehyde metabolism in the cardiovascular system. Mol Biosyst. 2006;3:136–150. doi: 10.1039/b612702a. [DOI] [PubMed] [Google Scholar]
- 5.Ebert AD, Kodo K, Liang P, Wu H, Huber BC, Riegler J, Churko J, Lee J, de Almeida P, Lan F, Diecke S, Burridge PW, Gold JD, Mochly-Rosen D, Wu JC. Characterization of the molecular mechanisms underlying increased ischemic damage in the aldehyde dehydrogenase 2 genetic polymorphism using a human induced pluripotent stem cell model system. Sci Transl Med. 2014;6:255ra130. doi: 10.1126/scitranslmed.3009027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fan F, Sun A, Zhao H, Liu X, Zhang W, Jin X, Wang C, Ma X, Shen C, Zou Y, Hu K, Ge J. MicroRNA-34a promotes cardiomyocyte apoptosis post myocardial infarction through down-regulating aldehyde dehydrogenase 2. Curr Pharm Des. 2013;19:4865–4873. doi: 10.2174/13816128113199990325. [DOI] [PubMed] [Google Scholar]
- 7.Fu SH, Zhang HF, Yang ZB, Li TB, Liu B, Lou Z, Ma QL, Luo XJ, Peng J. Alda-1 reduces cerebral ischemia/reperfusion injury in rat through clearance of reactive aldehydes. Naunyn Schmiedebergs Arch Pharmacol. 2014;387:87–94. doi: 10.1007/s00210-013-0922-8. [DOI] [PubMed] [Google Scholar]
- 8.Gao Y, Xu Y, Hua S, Zhou S, Wang K. ALDH2 attenuates Dox-induced cardiotoxicity by inhibiting cardiac apoptosis and oxidative stress. Int J Clin Exp Med. 2015;8:6794–6803. [PMC free article] [PubMed] [Google Scholar]
- 9.Ge W, Ren J. mTOR-STAT3-notch signalling contributes to ALDH2-induced protection against cardiac contractile dysfunction and autophagy under alcoholism. J Cell Mol Med. 2012;16:616–626. doi: 10.1111/j.1582-4934.2011.01347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harada M, Qin Y, Takano H, Minamino T, Zou Y, Toko H, Ohtsuka M, Matsuura K, Sano M, Nishi J, Iwanaga K, Akazawa H, Kunieda T, Zhu W, Hasegawa H, Kunisada K, Nagai T, Nakaya H, Yamauchi-Takihara K, Komuro I. G-CSF prevents cardiac remodeling after myocardial infarction by activating the Jak-Stat pathway in cardiomyocytes. Nat Med. 2005;11:305–311. doi: 10.1038/nm1199. [DOI] [PubMed] [Google Scholar]
- 11.He L, Liu B, Dai Z, Zhang HF, Zhang YS, Luo XJ, Ma QL, Peng J. Alpha lipoic acid protects heart against myocardial ischemia-reperfusion injury through a mechanism involving aldehyde dehydrogenase 2 activation. Eur J Pharmacol. 2012;678:32–38. doi: 10.1016/j.ejphar.2011.12.042. [DOI] [PubMed] [Google Scholar]
- 12.Ji W, Wei S, Hao P, Xing J, Yuan Q, Wang J, Xu F, Chen Y. Aldehyde dehydrogenase 2 has cardioprotective effects on myocardial ischaemia/reperfusion injury via suppressing mitophagy. Front Pharmacol. 2016;7:101. doi: 10.3389/fphar.2016.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee P, Sata M, Lefer DJ, Factor SM, Walsh K, Kitsis RN. Fas pathway is a critical mediator of cardiac myocyte death and MI during ischemia-reperfusion in vivo. Am J Physiol Heart Circ Physiol. 2003;284:H456–463. doi: 10.1152/ajpheart.00777.2002. [DOI] [PubMed] [Google Scholar]
- 14.Li SY, Gomelsky M, Duan JH, Zhang ZJ, Gomelsky L, Zhang XC, Epstein PN, Ren J. Overexpression of aldehyde dehydrogenase-2 (ALDH2) transgene prevents acetaldehyde-induced cell injury in human umbilical vein endothelial cells: role of ERK and p38 mitogen-activated protein kinase. J Biol Chem. 2004;279:11244–11252. doi: 10.1074/jbc.M308011200. [DOI] [PubMed] [Google Scholar]
- 15.Li XG, Lin XJ, Du JH, Xu SZ, Lou XF, Chen Z. Combination of methylprednisolone and rosiglitazone promotes recovery of neurological function after spinal cord injury. Neural Regen Res. 2016;11:1678–1684. doi: 10.4103/1673-5374.193250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma H, Guo R, Yu L, Zhang Y, Ren J. Aldehyde dehydrogenase 2 (ALDH2) rescues myocardial ischaemia/reperfusion injury: role of autophagy paradox and toxic aldehyde. Eur Heart J. 2011;32:1025–1038. doi: 10.1093/eurheartj/ehq253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Marchitti SA, Deitrich RA, Vasiliou V. Neurotoxicity and metabolism of the catecholam-inederived 3, 4-dihydroxyphenylacetaldehyde and 3, 4-dihydroxyphenylglycolaldehyde: the role of aldehyde dehydrogenase. Pharmacol Rev. 2007;59:125–150. doi: 10.1124/pr.59.2.1. [DOI] [PubMed] [Google Scholar]
- 18.Niki E, Yoshida Y, Saito Y, Noguchi N. Lipid peroxidation: mechanisms, inhibition, and biological effects. Biochem Biophys Res Commun. 2005;338:668–676. doi: 10.1016/j.bbrc.2005.08.072. [DOI] [PubMed] [Google Scholar]
- 19.Noiri E, Nakao A, Uchida K, Tsukahara H, Ohno M, Fujita T, Brodsky S, Goligorsky MS. Oxidative and nitrosative stress in acute renal ischemia. Am J Physiol Renal Physiol. 2001;281:F948–957. doi: 10.1152/ajprenal.2001.281.5.F948. [DOI] [PubMed] [Google Scholar]
- 20.Pang JJ, Barton LA, Chen YG, Ren J. Mitochondrial aldehyde dehydrogenase in myocardial ischemia-reperfusion injury: from bench to bedside. Sheng Li Xue Bao. 2015;67:535–544. [PubMed] [Google Scholar]
- 21.Renner A, Sagstetter MR, Harms H, Lange V, Götz ME, Elert O. Formation of 4-hydroxy-2-nonenal protein adducts in the ischemic rat heart after transplantation. J Heart Lung Transplant. 2005;24:430–736. doi: 10.1016/j.healun.2004.02.021. [DOI] [PubMed] [Google Scholar]
- 22.Sun A, Cheng Y, Zhang Y, Zhang Q, Wang S, Tian S, Zou Y, Hu K, Ren J, Ge J. Aldehyde dehydrogenase 2 ameliorates doxorubicin-induced myocardial dysfunction through detoxification of 4-HNE and suppression of autophagy. J Mol Cell Cardiol. 2014;71:92–104. doi: 10.1016/j.yjmcc.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 23.Sun A, Zou Y, Wang P, Xu D, Gong H, Wang S, Qin Y, Zhang P, Chen Y, Harada M, Isse T, Kawamoto T, Fan H, Yang P, Akazawa H, Nagai T, Takano H, Ping P, Komuro I, Ge J. Mitochondrial aldehyde dehydrogenase 2 plays protective roles in heart failure after myocardial infarction via suppression of the cytosolic JNK/p53 pathway in mice. J Am Heart Assoc. 2014;3:e000779. doi: 10.1161/JAHA.113.000779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Taher F, Lebl DR, Cammisa FP, Pinter DW, Sun DY, Girardi FP. Transient neurological deficit following midthoracic decompression for severe stenosis: a series of three cases. Eur Spine J. 2013;22:2057–2061. doi: 10.1007/s00586-013-2829-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vasiliou V, Nebert DW. Analysis and update of the human aldehyde dehydrogenase (ALDH) gene family. Hum Genomics. 2005;2:138–143. doi: 10.1186/1479-7364-2-2-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Walker LM, York JL, Imam SZ, Ali SF, Muldrew KL, Mayeux PR. Oxidative stress and reactive nitrogen species generation during renal ischemia. Toxicol Sci. 2001;63:143–148. doi: 10.1093/toxsci/63.1.143. [DOI] [PubMed] [Google Scholar]
- 27.Yang T, Wu L, Wang H, Fang J, Yao N, Xu Y. Inflammation level after decompression surgery for a rat model of chronic severe spinal cord compression and effects on ischemia-reperfusion injury. Neurol Med Chir (Tokyo) 2015;55:578–586. doi: 10.2176/nmc.oa.2015-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yuan Q, Hong S, Han S, Zeng L, Liu F, Ding G, Kang Y, Mao J, Cai M, Zhu Y, Wang QX. Preconditioning with physiological levels of ethanol protect kidney against ischemia/reperfusion injury by modulating oxidative stress. PLoS One. 2011;6:e25811. doi: 10.1371/journal.pone.0025811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang P, Xu D, Wang S, Fu H, Wang K, Zou Y, Sun A, Ge J. Inhibition of aldehyde dehydrogenase 2 activity enhances antimycin-induced rat cardiomyocytes apoptosis through activation of MAPK signaling pathway. Biomed Pharmacother. 2011;65:590–593. doi: 10.1016/j.biopha.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 30.Zhong Z, Hu Q, Fu Z, Wang R, Xiong Y, Zhang Y, Liu Z, Wang Y, Ye Q. Increased expression of aldehyde dehydrogenase 2 reduces renal cell apoptosis during ischemia/reperfusion injury after hypothermic machine perfusion. Artif Organs. 2016;40:596–603. doi: 10.1111/aor.12607. [DOI] [PubMed] [Google Scholar]
- 31.Zhong Z, Ye S, Xiong Y, Wu L, Zhang M, Fan X, Li L, Fu Z, Wang H, Chen M, Yan X, Huang W, Ko DS, Wang Y, Ye Q. Decreased expression of mitochondrial aldehyde dehydrogenase-2 induces liver injury via activation of the mitogen-activated protein kinase pathway. Transpl Int. 2016;29:98–107. doi: 10.1111/tri.12675. [DOI] [PubMed] [Google Scholar]
- 32.Zhu P, Li JX, Fujino M, Zhuang J, Li XK. Development and treatments of inflammatory cells and cytokines in spinal cord ischemia-reperfusion injury. Mediators Inflamm Article. 2013;2013:701970. doi: 10.1155/2013/701970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zivin JA, DeGirolami U. Spinal cord infarction: a highly reproducible stroke model. Stroke. 1980;11:200–202. doi: 10.1161/01.str.11.2.200. [DOI] [PubMed] [Google Scholar]