
Keywords: nerve regeneration, nerve remodeling, peripheral nerve, acetylcholinesterase staining, muscle denervation, neural anastomosis, nerve conduit, neural regeneration
Abstract
Motor nerves and sensory nerves conduct signals in different directions and function in different ways. In the surgical treatment of peripheral nerve injuries, the best prognosis is obtained by keeping the motor and sensory nerves separated and repairing the nerves using the suture method. However, the clinical consequences of connections between sensory and motor nerves currently remain unknown. In this study, we analyzed the anatomical structure of the rat femoral nerve, and observed the motor and sensory branches of the femoral nerve in the quadriceps femoris. After ligation of the nerves, the proximal end of the sensory nerve was connected with the distal end of the motor nerve, followed by observation of the changes in the newly-formed regenerated nerve fibers. Acetylcholinesterase staining was used to distinguish between the myelinated and unmyelinated motor and sensory nerves. Denervated muscle and newly formed nerves were compared in terms of morphology, electrophysiology and histochemistry. At 8 weeks after connection, no motor nerve fibers were observed on either side of the nerve conduit and the number of nerve fibers increased at the proximal end. The proportion of newly-formed motor and sensory fibers was different on both sides of the conduit. The area occupied by autonomic nerves in the proximal regenerative nerve was limited, but no distinct myelin sheath was visible in the distal nerve. These results confirm that sensory and motor nerves cannot be effectively connected. Moreover, the change of target organ at the distal end affects the type of nerves at the proximal end.
Introduction
After peripheral nerve injury, the motor endplate of the target organ or organs that are innervated by the injured nerve will gradually undergo atrophy and irreversible fibrosis if the nerve is not repaired as soon as possible. Several approaches to the repair of peripheral nerve injuries are currently the subject of considerable research interest, including promoting the regeneration of proximal fibers; minimizing atrophy of the effector and maintaining function while waiting for ingrowth of the proximal fiber; and creating conditions that promote the accurate crossing and regeneration of different fibers. In some specific types of peripheral nerve injury, the injured nerve cannot be directly sutured. This limitation has led investigators to use another nerve to provide trophic support to the denervated muscle (Karnovsky and Roots, 1964). Surgeons generally aim to repair injured nerves using material from the same type of nerve, such as using motor-nerve tissue to repair injured motor nerves. However, several investigators have demonstrated that neurotrophins derived from sensory nerve fibers are able to promote the development of motor nerves (Karnovsky and Roots, 1964; Lohof et al., 1993; Kucera et al., 1995; Wang et al., 1995). Based on the findings of these previous studies, we hypothesize that nerve regeneration is induced by signaling molecules released from the distal effector (Zhang et al., 2015). Thus, changes to the target organ following injury lead to the formation of newly formed nerves proximal to the location of the injury (Zhang et al., 2015). The purpose of this study is to test this hypothesis by observing the short-term changes taking place in sensory nerves following sensory-motor nerve crossing using a rat model of nerve regeneration involving crossing of the proximal stump of the saphenous nerve to the distal stump of the quadriceps.
Materials and Methods
Animals
Eighteen 8-week-old female Sprague-Dawley rats weighing 200–250 g were purchased from the Beijing Vital River Laboratory Animal Technology Co., Ltd., Beijing, China (license No. SYXK (Jing) 2016-0009). All rats were housed in cages in specific pathogen-free conditions under a 12-hour light/dark cycle with free access to food and water. The use of the animals was approved by the Ethics Committee and Experimental Animal Center of Peking University People's Hospital (2013-59).
Establishment of nerve regeneration
All rats were intraperitoneally anesthetized using a 1% sodium pentobarbital solution and then randomly divided into three groups, with six rats in each group. In animals undergoing sensory-motor nerve crossing the femoral nerve on the right side was exposed and the saphenous and motor nerves were then transected at 5 mm and 3 mm from the neuromuscular junction, respectively. The proximal sensory nerve was then crossed at 2 mm from the distal quadriceps stump using a degradable chitin conduit with 9-0 nylon suture. The control group was used to monitor the status of the uninjured saphenous nerve. The effects of sensory-motor nerve crossing were observed at 4 and 8 weeks postoperatively in different groups of animals.
Biological test
Postsurgical healing of the operative incisions, nutritional status and the extent of locomotor co-ordination of the right lower limb were monitored in all animals after surgery. The function of the lower limbs was measured by holding the tail of each rat while the rat attempted to hold onto a stick using the upper limbs, the ability of the rat to hold onto the stick using its lower limbs was then observed (Eberhardt et al. 2006).
Electrophysiological studies
After anesthetization, the right femoral nerve of each rat was exposed. The tissue was carefully separated from the nerve and examined under a light microscope (AmScope, Irvine, CA, USA). The surroundings were kept moist using a normal saline solution. To test the nerve conduction velocity, a stimulating electrode (MedlecSynergy; Oxford Instrument Inc., Oxford, UK) was placed 5 mm from the femoral nerve branch. The recording electrode and the reference electrode were then positioned at the proximal and distal ends of the quadriceps, respectively. The ground electrode was placed in the gluteus maximus. The surface of the muscle was then dried and a small piece of pledget was placed beneath the nerve, and above the muscle. Nerves were stimulated at a frequency of 5 Hz, with a stimulus duration of 0.1 ms and a current intensity of 0.09 mA. The compound muscle action potential of the quadriceps was recorded.
Restoration ratio of quadriceps wet weight
After isolating the entire quadriceps muscle, the muscles were washed and dried using absorbent paper (Taizhou AoKe, Taizhou, China), and then weighed on an electronic scale (Yuyao JiMing, Zhejiang Province, China). The restoration ratio of muscle wet weight was determined using the formula: wet weightexperimental side/wet weightcontrol side × 100%.
Hematoxylin-eosin staining
After dissection and fixation in a 4% paraformaldehyde solution dissolved in distilled water for 12 hours, the quadriceps pieces were washed with distilled water, dehydrated using an ethanol solution, soaked in wax, and sliced into 3-μm-thick sections using a rotary microtome. These sections were stained using a hematoxylin and eosin solution. This approach enabled the degree of atrophy of the quadriceps to be observed.
Acetylcholinesterase (AchE) staining
Paraformaldehyde-fixed nerves located 5 mm proximal or 5 mm distal to the conduit were cut into 18-μm-thick sections. Nerve sections were stained to investigate the presence of AchE using the method previously described by Karnovsky and Roots (1964). The number of myelinated fibers was counted and the percentage of area of the unmyelinated nerves was determined and compared between sections. All images were evaluated using Image Pro Plus 6.0 (Media Cybernetics, Rockville, MD, USA) and PS CS3 (Adobe, San Jose, CA, USA) image-analysis software. All data obtained from rats undergoing sensory-motor nerve crossing were compared with the equivalent data obtained using similar analyses of samples from control rats.
Statistical analysis
Statistical analyses were performed using SPSS 20.0 statistical analysis software (IBM, Armonk, NY, USA). All results are presented as the mean ± SE or the mean ± SD. Differences between groups were tested for statistical significance using a paired t-test. Differences that exceeded the 95% confidence interval (P < 0.05) were considered statistically significant.
Results
Biological performance
All rats survived the surgical procedure. All the incisions healed, and no obvious differences in co-ordination were observed between the two lower limbs. When injured rats’ grabbed a stick using their upper limbs, the right lower limbs appeared to flex, with a reduced ability to extend the knees. This phenomenon was more apparent among rats in the 8-week postoperative group (Figure 1).
Figure 1.

Weakness in extending the right knee.
Electrophysiological results
The site of injury of each rat was examined under a microscope and the conduit was retained in all animals. None of the connective tissue surrounding the site of injury was stongly adherent, and no neuromas were detected. A newly formed nerve could be observed passing through the conduit from the proximal end to the distal end at 4 weeks postsurgery. No major differences in morphological appearance of the site of injury were observed at 4 or 8 weeks after surgery (Figure 2A). Isolated nerves from rats in the control group had detectable compound action potentials; however, no such activities were detected in any rats in either of the experimental groups (Figure 2B, C).
Figure 2.
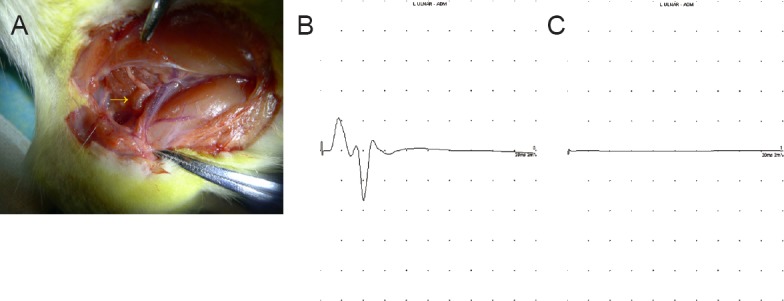
Electrophysiological function in rats with and without sensory-motor nerve crosses.
(A) A newly-formed nerve crossing the conduit. (B, C) Typical compound muscle action potentials of rats in the control (B) and experimental (C) groups. A regular compound action potential was present in rats in the control group, but no impulses were observed among rats in the experimental groups.
Histological and morphometric studies
The extent of atrophy of the quadriceps was significantly worse among rats in the 8-week postoperative group than among those in the 4-week postoperative group (29.7 ± 10.5% vs. 45.1 ± 13.1%, P ≤ 0.05). Examinations of hematoxylin-eosin stained muscle sections from rats in the control group revealed the presence of a normal tissue architecture (Figure 3A), while similar examinations of muscle sections from rats in either of the two experimental groups revealed the presence of considerable nerve-fiber atrophy (Figure 3B, C).
Figure 3.
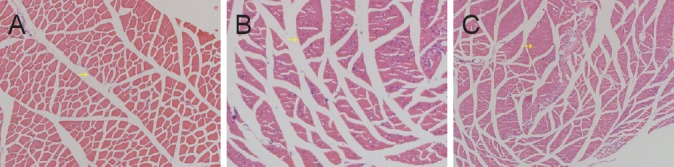
Morphometric changes in the quadriceps of rats that underwent sensory-motor nerve crosses (hematoxylin-eosin staining, × 100).
(A) Quadriceps of a rat in the control group. (B, C) Quadriceps of rats in the sensory-motor nerve cross groups at 4 weeks (B), and 8 weeks (C) after surgery. The diameter of the muscle fibers decreased while the number of muscle fibers per unit area increased, and the muscle-fiber boundary became blurred.
After AchE staining, the mean number of myelinated fibers in the saphenous nerve was 1,260.8 ± 107.5. The mean number of fibers in the same position among rats in the 4-week postoperative group was 1,609.8 ± 50.4, and 1,399.4 ± 102.1 among those in the 8-week postoperative group. The area of the autonomic nerves at the proximal end of the conduit in the general regenerative nerve was reduced compared with that of control mice (strong staining is indicated by the arrow in Figure 4). The percentage of the image area containing autonomic nerves in muscle sections from rats in the 4-week or 8-week postoperative groups was significantly lower than that of muscle sections obtained from rats in the control group (P = 0.005; Table 1). However, at the other end, dark staining was visible in the entire sections of the nerves, with an unclear myelin sheath (Figure 4). No motor fibers were observed on two sides of the conduit in sections from either of the experimental groups.
Figure 4.
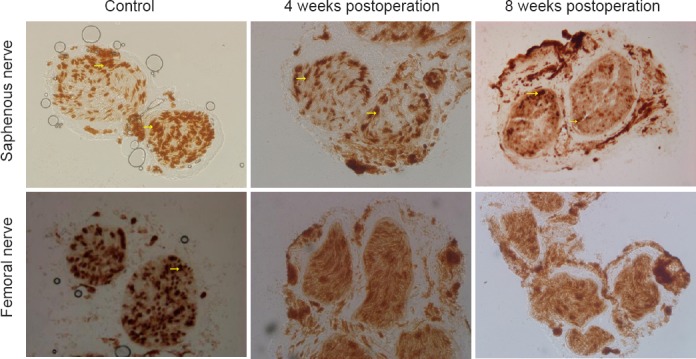
Morphological changes in the saphenous and femoral nerves following crossing of the proximal stump of the saphenous nerve to the distal stump of the quadriceps nerve (acetylcholinesterase staining, × 200).
Upper panel: A strong positive reaction, indicating the presence of abundant acetylcholinesterase enzymes was observed in the unmyelinated zone (arrows) owing to the presence of sympathetic nerves. Lower panel: Sympathetic postganglionic fibers (arrow) in the femoral nerve were similar in appearance to those of the saphenous nerve, although the two nerves can be distinguished by the presence of an obvious circular limit, reflecting the presence of a medullary sheath.
Table 1.
Percentage of area occupied by autonomic nerves in the general saphenous nerve within 5 mm of the proximal end of the conduit in rat models of nerve regeneration, relative to controls

Discussion
The optimal approach to the repair of injured peripheral nerves has been a subject of substantial research interest. In situations where nerve crossing has taken place during the repair process, the repaired fibers of injured peripheral nerves could encounter motor, sensory, and/or autonomic nerves. If inappropriate matching of nerve stumps takes place during re-innervation, the subsequent nerve cannot become functional. Nevertheless, researchers have thus far been unable to explain the consequences of forming such nerves. Weiss (1935, 1945) attempted to sew the sensory nerve to denervated muscle. In the first reported experiment of this kind, the aim was to determine whether or not a functional neuromuscular junction can form following such an approach. Although the results were negative, Weiss confirmed that the muscle fiber, which was connected to the sensory nerve, did become less atrophied. Hynes et al. (1997) also demonstrated that muscle atrophy was ameliorated following regeneration of the sensory nerve, and that the muscle fiber structure was also improved. However, as reported by the author, the conclusion was not convincing, because possible contributions of other regenerative pathways were not ruled out. Karpati et al. (1983), following extensive research, identified the presence of a newly formed sensory nerve and neuromuscular junction in previously denervated skeletal muscle, although these investigators still suggested that the regeneration was mediated by trophic support from motor nerves. Following these findings, Bain et al. (2001) cut the tibial nerve and then sutured the transected distal tibial nerve stump to the common peroneal nerve, thus minimizing the possibility of regeneration owing to ectopic release of neurotrophic factors. The conclusion of these experiments was the same as those of previous investigations: the muscles were connected with sensory nerves, and the fibers underwent great improvements in muscle structure and function, as demonstrated by improvements in muscle M-waves, twitch tension and tetanic tension.
At present, substantial evidence exists that the factors secreted by muscles or sensory nerves can provide trophic support to denervated muscle fibers. For example, denervated muscle fibers can be protected by the application of exogenous ciliary neurotrophic factor (Helgren et al., 1994). Furthermore, neurotrophin 3 and brain-derived neurotrophic factor have also been shown to promote the regeneration of skeletal muscle innervation (Karnovsky and Roots, 1964; Lohof et al., 1993; Kucera et al., 1995; Wang et al., 1995). Following injury, the newly-injured nerve will undergo changes in the neurotrophins it secretes, including increased levels of neurotrophin 4 and brain-derived neurotrophic factor (Funakoshi et al., 1993).
The process of myelin sheath degradation after muscle denervation was originally described by Sunderland and Bradley (1950). Following myelin degradation, a basement membrane is generated by Schwann cells, which provides support for nerve repair and regeneration processes. Sensory nerves might have a role in the formation of the basement membrane (Bunge et al., 1982). The phenomenon of sensory-nerve-mediated trophic support promoting the regeneration of motor nerves has now been confirmed (Bunge et al., 1982); however, the mechanism of this effect remains unknown. The aim of our experiments was to determine whether a change of target organ after injury can affect the newly-formed axon, and this information would then enable the mechanisms underlying sensory-nerve-mediated trophic support to be established through further research. At present, we know the rate of nerve regeneration is 1 mm/day in humans, and that nerve regeneration in rats is faster than in humans (Zhu, 2007). According to this speed of regeneration, the newly formed nerve can pass through the anastomosis at approximately 2 weeks after nerve regeneration. The distance from the femoral nerve branches to the muscle in rats is approximately 1.5 cm; therefore, the results of the electrophysiological tests were expected to be positive, as reported elsewhere (Hynes et al., 1997); however, no nerve function was detected at either 4 or 8 weeks after injury. This finding might indicate that insufficient time was allowed for nerve regeneration to take place.
In this study, the number of newly-formed nerve fibers in the proximal stump increased at 4 weeks after surgery, and then decreased at 8 weeks. This decrease in the number of nerve fibers towards that of rats in the control group can be explained by an increase in the number of nerve fibers sprouting from the proximal stump following injury. Furthermore, at 8 weeks after surgery, the area of the autonomic nerves on the proximal side of the conduit in the regenerating nerve was significantly lower than that of the control group. The area containing the autonomic nerves was smaller in the motor nerve than in the sensory nerve, we suspect that the change of target organ resulted in alterations in the proportion of different nerve fibers present in the newly formed sensory nerves. At the distal end of the conduit, strong staining was visible in whole sections of the nerves with a lack of an obvious myelin sheath. This apparent lack of myelination was very different from the proximal end, probably because after motor-nerve collapse, the autonomic nerves are able to grow into the neural membrane of the original motor nerve having passed through the conduit because both nerves release the same neurotransmitter. Finally, the duration of the postsurgical observation period was too short for a recovery of nerve function to be observed, despite a recovery of nerve function being the expected finding of the electrophysiological experiments.
A notable weakness of this study is the lack of sham surgical procedures conducted in the control group. As a result of this, we cannot be certain whether or not all of the observed effects are caused by the experimental procedures.
In this study, we attempted to change the target organ by crossing the proximal sensory nerve stump with the distal motor nerve stump, but effective connection did not occur. The change of target organ appears to have altered the proportion of different nerve fibers present in the newly-formed sensory nerves. These alterations indicate that changes in the target organ are able to affect sensory nerve regeneration. After the sensory branch is connected with the motor nerve, the proportion of different nerve fibers present in the newly formed nerve is similar to that of the nerve that had the same source, meaning the same neurotransmitter or the same physiological role.
Footnotes
Funding: This research was supported by a grant from the Ministry of Science and Technology 973 Project Planning of China, No. 2014CB542201; a grant from National High-Technology Research and Development Program of China (863 Program), No. SS2015AA020501; the National Natural Science Foundation of China, No. 31571235, 31571236, 31271284, 31171150; a grant from the Ministry of Education Innovation Team of China, No. IRT1201; the Educational Ministry New Century Excellent Talents Support Project of China, No. BMU20110270; a grant from the Ministry of Health of the Public Welfare Industry Special Scientific Research of China, No. 201302007.
Conflicts of interest: None declared.
Research ethics: The study protocol was approved by the Ethics Committee of Ethics Committee and Experimental Animal Center of Peking University People's Hospital, China (approval No. 2013-59). The experiment followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 8023, revised 1978), and “Consensus Author Guidelines on Animal Ethics and Welfare” procluced by the International Association for Veterinary Editors (IAVE). The article was prepared in accordance with the “Animal Research: Reporting of In Vivo Experiments Guidelines” (ARRIVE Guidelines).
Contributor agreement: A statement of “Publishing Agreement” has been signed by an authorized author on behalf of all authors prior to publication.
Plagiarism check: This paper has been checked twice with duplication-checking software iThenticate.
Peer review: A double-blind and stringent peer review process has been performed to ensure the integrity, quality and significance of this paper.
Copyedited by Sidaway P, Stow A, Yu J, Li CH, Qiu Y, Song LP, Zhao M
References
- 1.Bain JR, Veltri KL, Chamberlain D, Fahnestock M. Improved functional recovery of denervated skeletal muscle after temporary sensory nerve innervation. Neuroscience. 2001;103:503–510. doi: 10.1016/s0306-4522(00)00577-7. [DOI] [PubMed] [Google Scholar]
- 2.Bunge MB, Williams AK, Wood PM. Neuron-Schwann cell interaction in basal lamina formation. Dev Biol. 1982;92:449–460. doi: 10.1016/0012-1606(82)90190-7. [DOI] [PubMed] [Google Scholar]
- 3.Eberhardt KA, Irintchev A, Al-Majed AA, Simova O, Brushart TM, Gordon T, Schachner M. BDNF/TrkB signaling regulates HNK-1 carbohydrate expression in regenerating motor nerves and promotes functional recovery after peripheral nerve repair. Exp Neurol. 2006;198:500–510. doi: 10.1016/j.expneurol.2005.12.018. [DOI] [PubMed] [Google Scholar]
- 4.Funakoshi H, Frisen J, Barbany G, Timmusk T, Zachrisson O, Verge VM, Persson H. Differential expression of mRNAs for neurotrophins and their receptors after axotomy of the sciatic nerve. J Cell Biol. 1993;123:455–465. doi: 10.1083/jcb.123.2.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Helgren ME, Squinto SP, Davis HL, Parry DJ, Boulton TG, Heck CS, Zhu Y, Yancopoulos GD, Lindsay RM, DiStefano PS. Trophic effect of ciliary neurotrophic factor on denervated skeletal muscle. Cell. 1994;76:493–504. doi: 10.1016/0092-8674(94)90113-9. [DOI] [PubMed] [Google Scholar]
- 6.Hynes NM, Bain JR, Thoma A, Veltri K, Maguire JA. Preservation of denervated muscle by sensory protection in rats. J Reconstr Microsurg. 1997;13:337–343. doi: 10.1055/s-2007-1006413. [DOI] [PubMed] [Google Scholar]
- 7.Karnovsky MJ, Roots L. A “Direct-Coloring” thiocholine method for cholinesterases. J Histochem Cytochem. 1964;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- 8.Karpati G, Armani M, Carpenter S, Prescott S. Reinnervation is followed by necrosis in previously denervated skeletal muscles of dystrophic hamsters. Exp Neurol. 1983;82:358–365. doi: 10.1016/0014-4886(83)90408-9. [DOI] [PubMed] [Google Scholar]
- 9.Kucera J, Ernfors P, Walro J, Jaenisch R. Reduction in the number of spinal motor neurons in neurotrophin-3-deficient mice. Neuroscience. 1995;69:321–330. doi: 10.1016/0306-4522(95)00221-4. [DOI] [PubMed] [Google Scholar]
- 10.Lohof AM, Ip NY, Poo MM. Potentiation of developing neuromuscular synapses by the neurotrophins NT-3 and BDNF. Nature. 1993;363:350–353. doi: 10.1038/363350a0. [DOI] [PubMed] [Google Scholar]
- 11.Sunderland S, Bradley KC. Denervation atrophy of the distal stump of a severed nerve. J Comp Neurol. 1950;93:401–409. doi: 10.1002/cne.900930304. [DOI] [PubMed] [Google Scholar]
- 12.Wang T, Xie K, Lu B. Neurotrophins promote maturation of developing neuromuscular synapses. J Neurosci. 1995;15:4796–4805. doi: 10.1523/JNEUROSCI.15-07-04796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Weiss P. Experimental innervation of muscles by the central ends of afferent nerves (establishment of a one-neurone connection between receptor and effector organ), with functional tests. J Comp Neurol. 1935;61:135–174. [Google Scholar]
- 14.Weiss P. Sensory-motor nerve crossesin therat. J Neurophysiol. 1945;8:173–193. [Google Scholar]
- 15.Zhang P, Zhang L, Zhu L, Chen F, Zhou S, Tian T, Zhang Y, Jiang X, Li X, Zhang C, Xu L, Huang F. The change tendency of PI3K/Akt pathway after spinal cord injury. Am J Transl Res. 2015;7:2223–2232. [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu J. Effects of immunosuppressant FK506 on nerve regeneration. In: Gu LQ, editor. Modern Peripheral Neurological Surgery. Shanghai: Centry; 2007. pp. 236–243. [Google Scholar]


