Abstract
Aberrant Ca2+ release-activated Ca2+ (CRAC) channel activity has been implicated in a number of human disorders, including immunodeficiency, autoimmunity, occlusive vascular diseases and cancer, thus placing CRAC channels among the important targets for the treatment of these disorders. We briefly summarize herein the molecular basis and activation mechanism of CRAC channel and focus on discussing several pharmacological inhibitors of CRAC channels with respect to their biological activity, mechanisms of action and selectivity over other types of Ca2+ channel in different types of cells.
Keywords: : Ca2+ release-activated Ca2+ channel, CRAC channel inhibitor, ORAI1, STIM1, store-operated Ca2+ entry
As a universal and remarkably versatile second messenger, cytoplasmic Ca2+ is important in mediating fundamental biological processes including gene expression, cell proliferation, differentiation and apoptosis [1,2]. There are two major sources contributing to the increase of cytoplasmic Ca2+ concentration, in other words, the release of stored Ca2+ within the endoplasmic reticulum (ER) or sarcoplasmic reticulum (SR) and the influx of extracellular Ca2+ across the plasma membrane. Store-operated Ca2+ entry (SOCE) is a unique mechanism to generate cytoplasmic Ca2+ signals that combines these two processes, which is triggered by depletion of intracellular Ca2+ stores (mainly ER), and subsequently followed by Ca2+ influx across the plasma membrane by the opening of Ca2+ channels [3–5].
The prototypical store-operated Ca2+ channel is the Ca2+ release-activated Ca2+ (CRAC) channel [6–9], which is widely distributed and involved in the regulation of a myriad of cellular activities in different cell types, including various subsets of T cells [10], B cells [11], mast cells [12], endothelial cells [13], platelets [14], vascular smooth muscle cells [15] and skeletal muscle cells [16]. It has an extremely low conductance (in the range of fS compared with pS of most Ca2+ channels) but is a highly Ca2+-selective channel (PCa/PNa: >1000) that opens in response to Ca2+ depletion in intracellular Ca2+ stores [17]. The opening of CRAC channel leads to the activation of diverse downstream signaling pathways that regulate cytokine production, gene expression, cell growth, proliferation, differentiation and even cell death.
In recent years, aberrant CRAC channel activity has been noted in several human diseases, including severe combined immunodeficiency (SCID) disorders [18], allergy [19], thrombosis [20], acute pancreatitis [21], inflammatory bowel disease [22] and cancer [23–25], which leads to an increasing interest in developing small molecule compounds that suppress aberrant CRAC channel function.
Molecular basis & activation of CRAC channels
CRAC channel is composed of two key components, STIM (STIM1 and STIM2) [26,27] and ORAI (ORAI1, ORAI2 and ORAI3) [18,28–29], with the combination of STIM1/ORAI1 prevails in most cells and thus best characterized.
STIM1, originally identified as a tumor suppressor protein [30], is one of the two elementary components of SOCE, and functions as an ER Ca2+ sensor by detecting the fluctuation of Ca2+ concentration in the ER stores [26,27]. STIM1 is a single-pass transmembrane protein located in the ER membrane. The amino-terminal portion of STIM1 is located within the ER lumen [17], composed of an ER retention sequence, a canonical Ca2+-binding EF-hand domain, a ‘hidden’ EF-hand domain [31] that does not bind Ca2+ and a sterile α-motif (SAM) domain that mediates STIM1 dimerization/oligomerization [32]. The cytosolic domain of STIM1 includes three putative coiled-coiled domains (CC1-3), the CRAC activation domain (SOAR/CAD) that is essential for the gating of ORAI1 [33,34], a serine/proline-rich domain, a TxIP motif associated with microtubule plus-end-tracking protein EB1, and a polybasic C-tail that facilitates efficient targeting of STIM1 toward the plasma membrane through physical association with phosphoinositides embedded in the inner leaflet of the plasma membrane [7,35]. As a homolog of STIM1, STIM2 has also been found to act as ER Ca2+ sensor but has a lower affinity to luminal Ca2+ and also associates with ORAI proteins to regulate Ca2+ influx [36]. However, its physiological functions are less well understood [37].
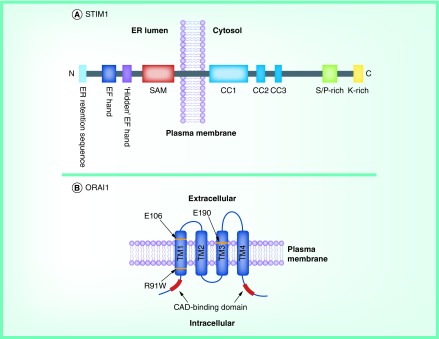
ORAI1 protein has been recognized as the ion pore-forming subunit of CRAC channels [7,29]. ORAI2 and ORAI3, human homologs of ORAI1, also form Ca2+-selective store-operated channels when co-expressed with STIM1 [38]. All three ORAI proteins have four putative transmembrane-spanning domains (TM1–4), one intracellular and two extracellular loop regions, and they are localized to the plasma membrane with their N- and C-termini facing the cytoplasm (Figure 1) [39].
Figure 1. . Domain architecture of STIM1 and ORAI1.
(A) STIM1 is a single-pass transmembrane protein located in the ER membrane. The N terminus is located within the ER lumen and contains an ER-retention sequence, a canonical Ca2+-binding EF-hand domain, a ‘hidden’ EF-hand domain and a sterile α-motif domain. The C terminus contains three putative coiled-coiled domains (CC1–3), a serine/proline-rich domain, and a polybasic C-tail. (B) ORAI1 bears four putative transmembrane-spanning domains (TM1-4), one intracellular and two extracellular loop regions. Residues at position E106 and E190 determine the channel selectivity and the dominant-negative mutant R91W has been found to be related to severe combined immunodeficiency.
ER: Endoplasmic reticulum; SAM: Sterile α-motif.
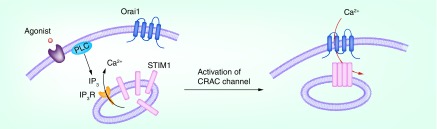
Upon binding of their cognate ligands or antigens, the cell-surface receptors such as receptor tyrosine kinases (RTKs) or G-protein-coupled receptors (GPCRs) activate phospholipase C (PLC) to hydrolyze the membrane phospholipid phosphatidyl-4,5-bisphosphate to generate inositol-1,4,5-trisphosphate (IP3) [40], followed by release of Ca2+ from the Ca2+ stores. So far, the IP3-sensitive ER is the major store that is coupled to CRAC channel activation. The loss of Ca2+ from the ER results in Ca2+ dissociation from the luminal Ca2+-binding EF hand of STIM1 [26,27], leading to the unfolding of EF-SAM domain, followed by STIM1 oligomerization through a mechanism that involves both the luminal and cytosolic domains [32,41–42], which is the essential step responsible for STIM1 conformational switch [43–45], subsequent accumulation at endoplasmic reticulum–plasma membrane (ER–PM) junctions and ultimate activation of ORAI channels [46]. The STIM1 oligomers then migrate from the bulk ER to specialized ER–PM junctions [47,48], during which STIM1 captures diffusing ORAI1 channels, and interaction between the amino and carboxyl termini of ORAI1 with SOAR/CAD on STIM1 leads to the opening of CRAC channel (Figure 2) [49–54].
Figure 2. . Activation of release-activated Ca2+ channel.
The increase of IP3 concentration induced by activation of PLC activates endoplasmic reticulum (ER)-resident IP3 receptors (IP3R) and causes the release of Ca2+ from ER, which leads to oligomerization and conformational switch of STIM1. The activated STIM1 oligomers then move toward the ER–plasma membrane junctions and trigger Ca2+ influx through direct interaction with an opening of ORAI1 Ca2+ channels in the plasma membrane.
CRAC: Release-activated Ca2+; PLC: Phospholipase C.
Pharmacological inhibitors of CRAC channels
The identification of the molecular identities of CRAC channel kindled an intense interest in the search of small molecule modulators of CRAC channel. CRAC channel modulators may work by targeting either ORAI or STIM to regulate the overall level of CRAC channel activity. The compounds may either modulate channel activity by targeting STIM1 or acting directly at the pore of the ORAI channel by blocking the pore or interfering with the STIM–ORAI interaction.
Although both STIM1 and ORAI1 are widely expressed in a variety of tissues, the major clinical manifestations of patient with CRAC channelopathies are surprisingly limited to the immune system, skeletal muscle and ectodermally derived tissues [7], which agrees well with phenotypes observed in mice with targeted disruptions of the murine Orai1, Stim1 and/or Stim2 [55,56]. These findings indicated that therapies specifically targeting CRAC channels may serve as improved immunomodulators with high selectivity and low toxicity compared with currently US FDA approved immunosuppressive agents, such as cyclosporin A and FK506 that often cause undesired off-target toxicity in patients [57].
Although a number of agents that inhibit CRAC channels have been developed [58–61], most of them by far have not reached clinical trials, primarily owing to their poor selectivity and high toxicity. Nonetheless, a member of the CalciMedica series has reached Phase I clinical trials and it is highly anticipated to reach the milestone of FDA approval in drug development [62]. Apart from this, some CRAC modulators may provide promising lead structures for developing CRAC channel inhibitors with improved specificity and higher potency in the near future. Here we discuss a number of pharmacological agents that are most commonly used to inhibit CRAC channel activity, which are also helpful for understanding the physiological roles and dissecting the structure–function relation of the CRAC channel.
Lanthanides
Similar to other Ca2+ entry pathways, store-operated Ca2+ channels could also be inhibited by divalent and trivalent cations. Particularly, CRAC channels show high sensitivity to complete blockade by the trivalent ion La3+ (lanthanum) and Gd3+ (gadolinium) at submicromolar concentration range [63]. This unique feature has been often used to distinguish CRAC channels from other types of less Ca2+ selective channels (e.g., TRP channels) [64–66]. The concentrations of Gd3+ used to effectively block the endogenous CRAC channel exert no significant inhibitory effect on TRP channels.
Mutation of several key acidic residues in the TM1–TM2 loop of ORAI1 (D110, D112 and D114) reduced the CRAC channel's selectivity for Ca2+ and decreased the inhibitory potency of the lanthanides, implying that the binding site of the trivalent ion La3+ and Gd3+ is located at or nearby that region of ORAI1 [67,68]. However, in the recent determined x-ray crystal structure of Drosophila Orai, Gd3+ situates at the same site (E106 in human ORAI1), rather than the acidic region in the first extracellular loop that is proposed to coordinate Ca2+ [69].
Lanthanides also showed inhibitory activity against other cationic ion channels, for example, voltage-gated calcium channels and TRP channels [70,71], which limited their potential use in developing CRAC channel inhibitors. Moreover, because the lanthanide salts of other multivalent anions and proteins are insoluble, their utility is also limited in many other applications.
Imidazole compounds
Imidazole antimycotic SKF-96365 (1) was one of the first identified CRAC channel inhibitors for experimental use [58,72], and the structurally related imidazole compounds econazole (2) and miconazole (3), which are primarily used as antimycotics [58], also suppress CRAC channel activity (Figure 3).
Figure 3. . Chemical structures of typical imidazole release-activated Ca2+ channel inhibitors.
SKF-96365 (1); econazole (2); miconazole (3).
SKF-96365 inhibited thapsigargin-induced SOCE in Jurkat T cells with an IC50 value (measured by I CRAC, the current generated by the opening of CRAC channels) of 12 μM in a dose-dependent manner (different groups reported varying IC50 values, which might be mainly due to the methods used to measure CRAC channel activity and the potencies are also cell-type dependent) [72]. Although this compound inhibits agonist-mediated Ca2+ influx in many cell types, it also suppresses the activity of other ion channels, such as voltage-gated calcium channels, nonselective cation channels and cyclic AMP-gated Cl− channels with comparable potencies [72,73]. Econazole and miconazole also exhibit a lack of specificity to CRAC channel, thereby limiting its further clinical use as specific CRAC channel modulators.
Diphenylboronate compounds
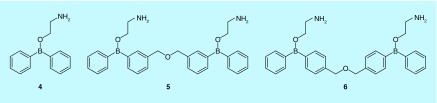
2-Aminoethyldiphenyl borate (2-APB, 4) has been widely used to characterize the activity of CRAC channel [74]. Its pharmacology is complex with an intriguing biphasic effect on CRAC channel activation. At low concentrations (1–10 μM), 2-APB potentiates CRAC channel activity; while at higher concentrations (20–100 μM), it often causes a transient activation of CRAC channel followed by strong inhibition [75,76]. In recent years, two 2-APB analogs DPB162-AE (5) and DPB163-AE (6), which were identified by Mikoshiba's group, have drawn attention for their higher potencies and greater specificity than 2-APB in terms of suppressing SOCE (Figure 4) [77].
Figure 4. . Chemical structures of 2-APB and its analogs.
2-APB (4); DPB162-AE (5); DPB163-AE (6).
2-APB was initially speculated to inhibit SOCE through its inhibitory effect for IP3 receptors [78–81]. However, it has been clarified that the inhibition of SOCs has no direct relation with IP3 [75,82–83].
In recent years, it has been found that formation of STIM1 puncta (clusters of STIM1 at ER–PM regions just below the PM) could be prevented by high concentrations (50 μM) of 2-APB, suggesting that 2-APB could inhibit SOCE through affecting movement of STIM1 [84,85]. However, the inhibition of STIM1-puncta formation by 2-APB could be overcome by coexpression with ORAI1, even though the inhibition of SOCE is still effective [85,86]. These findings imply that the inhibitory effect of 2-APB on SOCE might be due to its effects on one or more steps in the following molecular events: STIM1 multimerization, STIM1 conformational switch, STIM1–ORAI1 interaction or the ORAI channel itself.
Interestingly, the effects of 2-APB on ORAI vary between different ORAI isoforms [85,87]. For example, at high concentrations (50 μM) of 2-APB, ORAI1-mediated Ca2+ entry is initially activated, which is quickly followed by a complete inhibition. 2-APB only partially inhibits ORAI2-mediated SOCE. Surprisingly, 2-APB at high concentrations activate, rather than inhibit, ORAI3 channel [88,89]. The different properties of these three ORAI proteins give hope to develop small molecules which could selectively target one specific ORAI protein.
Although 2-APB is widely used to modulate CRAC channel activity, it could also affect the activities of potassium channels [90], SERCA pumps [91] and mitochondrial Ca2+ efflux [75]. Notably, 2-APB has been reported to activate the heat-gated recombinant TRPV1, TRPV2 and TRPV3 channels [92].
2-APB derivatives DPB162-AE and DPB163-AE are isomers in chemical structure, which only differ in the linker chain between their two diphenyl groups. In STIM1–ORAI1 overexpressing cells, DPB163-AE had a biphasic effect on SOCE, which is similar to 2-APB but exhibits a higher potency with an IC50 of about 600 nM. DPB162-AE, on the other hand, solely inhibited SCOE with an IC50 of approximately 200 nM, which is two orders of magnitude more potent than 2-APB [76,93]. Similar to 2-APB, both DPB162-AE and DPB163-AE suppressed ORAI1 currents and partially inhibited ORAI2 currents. However, unlike 2-APB, they failed to activate ORAI3 channels at higher concentrations in the absence of STIM1, an observation that could be attributed to their larger size, relative to 2-APB, that likely prohibits access to the ORAI3 pore [77].
The interaction between the STIM1–ORAI1 activating region (SOAR) of STIM1 and a combination of the C- and N-termini of ORAI1 leads to the coupling of STIM1 and ORAI1. The single binding pocket formed by C- and N-termini of ORAI1 plays an important role in both SOAR-binding and gating of the channel [94]. Recent researches have shown that DPB162-AE, not acting as an ORAI1 channel pore blocker, does not prevent the STIM1–ORAI1 interaction but potently inhibits the activation of STIM1-mediated ORAI1 channel [93,94]. Using a unique point mutation in the SOAR of STIM1 (F394H), which prevents both physical binding between SOAR and ORAI1 as well as functional coupling to activate the ORAI1 channel [94], DPB162-AE was found to restore SOAR–ORAI1 binding rapidly but restore ORAI1-mediated Ca2+ entry slowly. These findings reveal that DPB162-AE seems to be a potent and relatively specific STIM1–ORAI1 functional uncoupler, and probably acts directly on the coupling interface between SOAR and ORAI1.
In contrast to 2-APB, DPB162-AE has little effect on TRPC channels, L-type Ca2+ channels or Ca2+ pumps at 2 μM, the maximal CRAC channel-mediated SOCE inhibitory level of DPB162-AE [93,95–97].
Pyrazole compounds: the BTPs
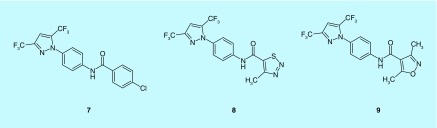
A series of bis(trifluoromethyl)pyrazoles compounds, known as BTP1 (7), BTP2 (8) and BTP3 (9), were initially identified as inhibitors of NFAT activation and T-cell cytokine production by Abbott Laboratories [98–100]. Interestingly, unlike other well-known NFAT inhibitors such as FK506 and cyclosporin A, the BTPs inhibited NFAT nuclear translocation without direct effect on the phosphatase activity of calcineurin, implying a probable effect on the upstream Ca2+ signal [98,99]. Indeed, the BTPs were later found to be capable of inhibiting SOCE in many cells at low micromolar to nanomolar concentrations with considerable selectivity over voltage-gated Ca2+ entry [61]. In particular, BTP2 (also known as YM-58483) inhibited thapsigargin-induced Ca2+ influx in Jurkat T cells with an IC50 value of 100 nM and did not inhibit phosphorylation of PLCγ1 and TCR-mediated Ca2+ release from the stores (Figure 5) [101].
Figure 5. . Chemical structures of the BTPs.
BTP1 (7); BTP2 (8); BTP3 (9).
BTP2 has been reported to inhibit CRAC channels in human T cells with an IC50 of about 10 nM [102], which is one order of magnitude higher than the IC50 value described above. The most probable reason for the discrepancy in IC50 values is that the former experiment was performed via preincubation of cells with BTP2 for 18–24 h to reach full inhibition [102], while cytoplasmic Ca2+ concentrations in the later study were measured shortly (only a few minutes) after BTP2 treatment [101]. Moreover, it was also found that CRAC channel inhibition mediated by BTP2 was affected by the external Ca2+ concentration: higher external Ca2+ concentrations are correlated with reduced inhibitory effect on the CRAC channel [102].
A recent study has shown that drebrin, an actin reorganizing protein, is identified as a potential binding site for BTP2 [103]. Knockdown of drebrin by siRNA inhibited SOCE to the same extent as inhibition by BTP2. Thus, the authors of the report concluded that drebrin may play a role in regulating SOCs by affecting the actin cytoskeleton, and that BTP2 may act by inhibiting drebrin. However, previous studies have shown that the actin cytoskeleton is not considered to play a major role in SOCE [104].
Although BTP2 does not inhibit voltage-gated Ca2+ channels or K+ channels [101,102], it activates TRPM4 channels and inhibits the activities of TRPC3 and TRPC5 channels [105,106].
Pyrazole compounds: the Pyrs
As discussed above, BTP2 (also known as Pyr2, 8) could also inhibit TRPC3 channel activity while it acts on CRAC channels. Recently, the ability of three pyrazole derivative compounds Pyr3 (10), Pyr6 (11) and Pyr10 (12) in inhibiting Ca2+ entry have been examined in terms of TRPC/CRAC selectivity in HEK293 cells overexpressing TRPC3 channels and RBL-2H3 cells expressing CRAC channels (Figure 6) [107].
Figure 6. . Chemical structures of the Pyrs.
Pyr2 (8); Pyr3 (10); Pyr6 (11); Pyr10 (12).
Structurally, Pyr6 and Pyr10 have similar structures to the BTPs bearing two trifluoromethyl groups in the C3 and C5 position of the pyrazole ring, which are important in CRAC channel inhibitory activity of BTP2 [108]. Interestingly, Pyr3 shares a carboxylate group in the C4 position of the pyrazole ring instead of the trifluoromethyl group in the C3 position, which appears to contribute to maintaining its inhibitory activity, and the trichloroacryl group of the side chain also seems to be necessary for its inhibitory activity toward both CRAC and TRPC3 channel [107].
Interestingly, Pyr6 displays higher potency to inhibit Ca2+ entry mediated by CRAC channel than TRPC3, while Pyr10 exhibits significant selectivity for TRPC3-mediated Ca2+ entry. By comparison, Pyr2 and Pyr3 do not show any appreciable selectivity for them [107].
Pyrazole compounds: the GSKs
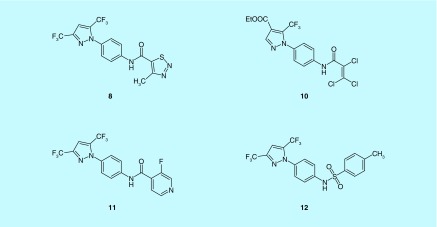
Recently, several novel pyrazole compounds, GSK-5498A (13), GSK-5503A (14) and GSK-7975A (15), which were developed by GlaxoSmithKline, have been identified as selective CRAC channel inhibitors (Figure 7) [109–111].
Figure 7. . Chemical structures of the GSKs.
GSK-5498A (13); GSK-5503A (14); GSK-7975A (15).
Electrophysiological experiment showed that GSK-5498A inhibits I CRAC with an IC50 value of about 1 μM in human embryonic kidney cells stably expressing STIM1 and ORAI1 [110]. GSK-5503A and GSK-7975A inhibited STIM1 mediated ORAI1 and ORAI3 currents with an IC50 value of about 4 μM in HEK293 cells [111].
FRET experiments implied that the GSK compounds did not affect STIM1 oligomerization or STIM1–ORAI1 interaction. Compared with wild-type ORAI1, the less Ca2+-selective mutant E106D ORAI1 pore requires at least tenfold higher concentrations of GSKs for inhibition, thus pointing to the possibility that these compounds may act by altering the ORAI pore geometry [111].
A recent study has found that blockade of CRAC channels by GSK-7975A effectively precludes palmitoleic acid ethyl ester (POAEE), an important mediator of alcohol-related pancreatitis, from evoking sustained elevation of the Ca2+ concentration in the pancreatic acinar cells, activation of protease and necrosis of pancreatic acinar cell [21]. Given these findings, the authors indicated that pharmacological CRAC channel blockade could be applied as a potentially rational therapy against severe acute pancreatitis, which is life-threatening but lacks effective treatment thus far [21,112].
Interestingly, although these GSK compounds were found to have little or no effect on many other ion channels, they potently block TRPV6 channels [111,113].
Synta 66
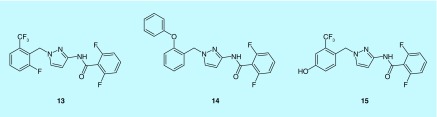
Synta 66 (16), a selective CRAC channel inhibitor developed by Synta pharmaceuticals, has drawn extensive attentions in recent years [114]. The structure of Synta 66 is similar to Pyr6 (11), whose 3,5-bistrifluoromethyl pyrazole ring is replaced with 2,5-dimethoxy benzene ring (Figure 8).
Figure 8. . Comparison of the chemical structures of Synta 66 and Pyr6.
Pyr6 (11); Synta 66 (16).
This compound inhibits I CRAC with an IC50 value of 1.4 μM in RBL cells and has no effect on plasma membrane Ca2+ ATPase pump and inwardly rectifying K+ channels [114,115]. It has also been found that the compound inhibits expression of T-bet and production of IL-2, IL-17 and IFN-γ in biopsy specimens isolated from inflamed areas of IBD patients [115]. The results suggested that Synta 66 could be applied to further investigation of the CRAC channels functions in T-cell signaling and IBD.
A scrutiny of the selectivity of Synta 66 assessed by a panel of 50 specific radioligand-binding assays suggests that, at a concentration of 10 μM, it exerts no significant effect on a series of receptors, enzymes and ion channel targets [115]. Although there are more and more studies employing Synta 66 for probing the physiological role of CRAC channels, the mechanism of action for this compound has not yet been fully clarified.
ML-9
ML-9 (17), an inhibitor of myosin light chain kinase (MLCK), has been found to reversibly inhibit SOCE with an IC50 of approximately 10 μM [116,117]. In HEK293 cells, ML-9 was found to similarly inhibit SOCE and I CRAC [117].
ML-9 inhibits SOCE at least partially by reversing the formation of STIM1 puncta and blocking its movement to ER–PM junctions. Interestingly, the inhibitory effect of ML-9 does not seem to be related to its well-known inhibition of MLCK [117]. Although STIM1 appears to be the molecular target of ML-9-mediated inhibition on SOCE, it is still unclear on the specific site in STIM1 that ML-9 may act on.
Diethylstilbestrol
Diethylstilbestrol (DES; 18), a synthetic estrogen agonist, inhibits SOCE in a range of cell types including mast cells, vascular smooth muscle cells and rat microglia [118,119]. DES inhibits I CRAC in RBL cells with an IC50 of approximately 0.6 μM and does not affect TRPM7 channels at a similar concentration range. Interestingly, if it is applied intracellularly, its inhibitory effect on I CRAC disappears, thus raising the speculation that it might act on the extracellular regions on CRAC channel. Although it might be applied to investigate the function of CRAC channels in vitro, it could not be used in clinical setting due to its activation on estrogen receptors.
Carboxyamidotriazole
Carboxyamidotriazole (CAI; 19) is a potential anticancer drug which has been tested in Phase I and Phase II clinical trials for its activity of inhibiting angiogenesis, tumor growth, invasion and metastasis [120,121]. CAI was initially identified as an inhibitor of SOCE in nonexcitable cells [122]. CAI inhibits I CRAC with an IC50 value of approximately 0.5 μM in HEK293 cells [120,121]. Carboxyamidotriazole suppresses I CRAC by reducing the production of IP3 and depolarizing mitochondria, which induces Ca2+-dependent inactivation of the CRAC channels [123–125]. Therefore, although CAI does inhibit I CRAC, it seems to act in an indirect manner.
RO2959
RO2959 (20), synthesized by Synta Pharmaceutical Corp., has been identified as a novel, potent and selective I CRAC inhibitor by Roche [126]. RO2959 inhibits I CRAC with an IC50 value of about 400 nM in RBL-2H3 cells, which has been preincubated with RO2959 [126]. Moreover, due to its ability of inhibiting I CRAC, RO2959 could potently inhibit human TCR-mediated SOCE, T-cell proliferation, cytokine production and gene expression [126]. In T-REx-CHO cells which could stably express STIM1/ORAI1, STIM1/ORAI2 or STIM1/ORAI3, RO2959 inhibits ORAI1 to a greater extent than ORAI2 and ORAI3 [126], which implies that the compound could be applied as a selective ORAI1 inhibitor. RO2959 had no significant inhibitory effect on a variety of cellular receptors, transporters and ion channels, such as GABA receptors, dopamine transporter, 5-HT transporter, Kv channels, Cl- channels, TRPC1, TRPM2, TRPM4 and Cav1.2 channels, which showed the high action selectivity of the compound [126]. Notably, TRPC1 channel has some similarities with CRAC channel, a number of drug candidates developed as CRAC channel inhibitors also act on TRPC1 channel. Although TRPC1 can contribute to SOCE along with ORAI1 and STIM1, it also participates in other signaling events that are independent on store depletion. If the CRAC channel inhibitors could potently act on TRPC1, one would therefore expect undesired off-target side effects. Thus, for CRAC channel inhibitors, it is necessary to examine the effect on TRPC1 channels.
Although RO2959 has been shown to be a potent and selective CRAC channel inhibitor, the in vivo efficacy and the exact mechanism of action warrants further investigation.
Linoleic acid
More recently, linoleic acid (21), an 18-C polyunsaturated fatty acid (PUFA), has been reported to effectively inhibit antigen- or thapsigargin-mediated SOCE in mast cells by acute addition at micromolar concentrations [127]. Interestingly, stearic acid, the 18-C saturated fatty acid, does not inhibit SOCE. The authors found that linoleic acid inhibited SOCE by affecting STIM1 oligomerization and subsequent STIM1/ORAI1 coupling. The authors further argue that linoleic acid inhibited STIM1/ORAI1 coupling by disrupting potential electrostatic interactions between STIM1 and ORAI1 [127]. Further studies are needed to delineate its mechanism of action and examine its selectivity over other types of ion channels (Figure 9).
Figure 9. . Chemical structures of several pharmacological inhibitors of release-activated Ca2+ channels.
ML-9 (17); Diethylstilbestrol (18); Carboxyamidotriazole (19); RO2959 (20); linoleic acid (21).
1-Phenyl-3-(1-phenylethyl)urea derivatives
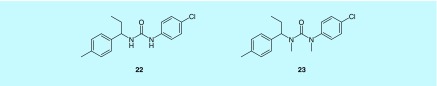
A series of 1-phenyl-3-(1-phenylethyl)urea derivatives has been recently identified as CRAC channel inhibitors. As the lead compound, compound 22 could inhibit Ca2+ influx with IC50 of 3.25 ± 0.17 μM in HEK293 cells stably co-expressing ORAI1 and STIM1 [128]. The Ca2+ influx assay and electrophysiological experiments showed that compound 22 could partially inhibit Ca2+ entry in constitutively opened CRAC channels which were formed by ORAI1-SS (monomer ORAI1 covalently linked with two S336–485 domains) and completely inhibit the Ca2+ entry and the current mediated by the opened STIM1-free V102A channel (a mutant of ORAI1), which is a constitutively opened CRAC channel, even in the absence of STIM1. Furthermore, this compound could specifically reduce ORAI1/STIM1-mediated Ca2+ entry, while exhibited no inhibitory effect on other ORAI channels. These results indicated that compound 22 inhibits CRAC channel by specifically targeting ORAI1 [128].
A total of 40 derivatives have been synthesized, and their primary structure–activity relationships (SARs) study showed that the alkyl substituent on the α-position of the N-phenylethyl group is vital for their inhibition on Ca2+ influx [128]. Notably, among these derivatives, compound 23 exhibited low cytotoxicity and relatively improved inhibition of IL-2 production in the Jurkat cell line [128].
It is encouraging that compound 22 could inhibit CRAC channel by specifically targeting ORAI1, however, its mechanism of action and selectivity over other types of ion channels still warrants further studies (Figure 10).
Figure 10. . Chemical structures of two 1-phenyl-3-(1-phenylethyl)urea derivatives.
Compound 22 and compound 23.
The CalciMedica series
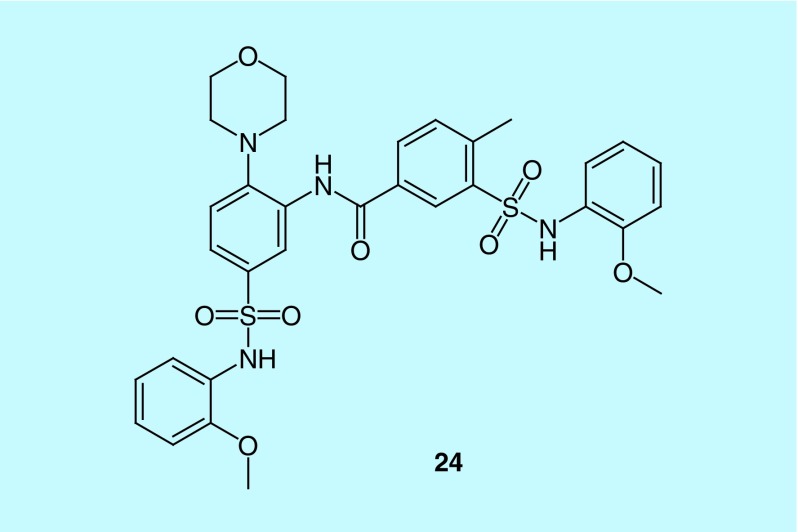
CalciMedica has been actively developing novel, potent and specific CRAC channel inhibitors in the past decade. It is worth noting that CM2489, developed by this biotech company, is the only CRAC channel inhibitor tested in human and has completed Phase I clinical trials for treating moderate-to-severe plaque psoriasis [129]. Although there is no chemical structure information disclosed for this compound, through the patent published by CalciMedica, we could find that most compounds bear phenyl or heterocyclic groups linked by carboxyamide, sulfoxamide or alkyl chain (take compound 24 for example) [62], which may help us to estimate the skeleton structure of CM2489 for designing novel CRAC channel inhibitors (Figure 11).
Figure 11. . An example of release-activated Ca2+ channel modulators developed by CalciMedica.
Compound 24.
Conclusion
CRAC channels, fundamental to human immune cell function, have shown the physiological importance in many cell types. With the discoveries of STIM1 and ORAI1 proteins, the molecular components of CRAC channel have been identified, which facilitates the functional studies of CRAC channels in a wide range of cellular systems. In the last three decades, several classes of CRAC channel inhibitors have been developed. Although most of them have not reached clinical trials due to their poor selectivity and high toxicity, there are some selective CRAC channel inhibitors that might hold promise for further drug development. Most notably, CM2489 [129], developed by CalciMedica, has reached clinical trials, which is the first CRAC channel inhibitor that has completed the Phase I clinical trials. Unfortunately, there is no sufficient public information available for us to have a thorough study of this compound.
In addition to CM2489, RO2959, recently developed by Synta, has also been shown to act as a selective CRAC channel inhibitor by targeting ORAI1. Thus, developing drugs that target particular component(s) of CRAC channel might be the most efficient and effective way to improve the selectivity and specificity.
Future perspective
Given that CRAC channels have emerged as an attractive target for developing new therapies for autoimmune disorders, allergy, thrombosis and cancer, more and more pharmaceutical companies including Hoffmann-La Roche and GSK are joining the efforts to develop CRAC channel inhibitors. As summarized by Pevarello and colleagues [62], the publication of related patents has been kept growing. Although no CRAC channel inhibitors have reached the milestone of FDA approval and clinical use, the increasing attention paid by pharmaceutical companies, together with our deeper understanding of the activation and regulatory mechanisms of CRAC channel and the advent of novel optogenetic tools to manipulate CRAC channel activity [130–132], would certainly expedite the quest for new drugs that specifically target CRAC channels to treat human disorders associated with dysregulated Ca2+ influx (Table 1).
Table 1. . The potencies of leads from each series and their proposed mechanism of action.
| Species | CRAC channel inhibitory activity | Selectivity | Proposed mechanism of action |
|---|---|---|---|
| Lanthanides |
Complete blockade at submicromolar concentration range [63] |
Block other cationic ion channels, such as voltage-gated calcium channels and TRP channels [70,71] |
Directly block ORAI1 [69] |
| SKF-96365 |
IC50: 12 μM (ICRAC) [72] |
Suppresses voltage-gated calcium channels, nonselective cation channels and cyclic AMP-gated Cl− channels [72,73] |
Has not yet been fully clarified |
| 2-APB |
Activates at low micromolar and inhibits at high micromolar [75,76] |
Affect the activities of potassium channels, SERCA pumps, heat-gated recombinant TRPV1, TRPV2 and TRPV3 channels [90–92] |
Might act on STIM1 multimerization, STIM1–ORAI1 interaction or the ORAI channel itself [85,86] |
| DPB162-AE |
IC50: 200 nM [93] |
Relatively selective [93,95–97] |
Probably acts directly on the coupling interface between SOAR and ORAI1 [93,94] |
| BTP2 |
Inhibited thapsigargin-induced Ca2+ influx in Jurkat T cells with an IC50 of 100 nM [101] |
Activates TRPM4 channels and inhibits the activities of TRPC3 and TRPC5 channels [105,106] |
Has not yet been fully clarified |
| GSK-7975A |
IC50: 4 μM (ICRAC in HEK293 cells) [111] |
Potently blocks TRPV6 channels [111,113] |
May act by altering the ORAI pore geometry [111] |
| Synta 66 |
IC50: 1.4 μM (ICRAC in RBL cells) [114,115] |
Relatively selective [115] |
Has not yet been fully clarified |
| ML-9 |
Reversibly inhibit SOCE with an IC50 of approximately 10 μM [116,117] |
Inhibits MLCK [116] |
Might target STIM1 [117] |
| DES |
IC50: 0.6 μM (ICRAC in RBL cells) [118,119] |
Activates estrogen receptors [118] |
Might act on the extracellular regions on CRAC channel [118,119] |
| CAI |
IC50: ˜0.5 μM (ICRAC in HEK293 cells) [120,121] |
Not very selective |
Reduces the production of IP3 and depolarizes mitochondria [123–125] |
| RO2959 |
IC50: 400 nM (ICRAC in RBL-2H3 cells) [126] |
Relatively selective [126] |
Has not yet been fully clarified |
| Linoleic acid |
Inhibit antigen- or thapsigargin-mediated SOCE in mast cells by acute addition at micromolar concentrations [127] |
Has not yet been examined |
Inhibits SOCE by affecting STIM1 oligomerization and subsequent STIM1/ORAI1 coupling [127] |
| 1-phenyl-3-(1-phenylethyl)urea | Inhibits Ca2+ influx with IC50 of ˜3 μM in HEK293 cells [128] | Has not yet been examined | Targets ORAI1 [128] |
Executive summary.
Store-operated Ca2+ entry (SOCE) constitutes one of the major Ca2+ entry routes in nonexcitable cells and is implicated in a variety of fundamental biological processes.
Ca2+ release-activated Ca2+ (CRAC) channel, which is widely distributed and involved in the regulation of many cellular functions in different cell types, is one of the most well-studied prototypical form of store-operated Ca2+ channels.
Aberrant CRAC channel activity is associated with human disorders involving the immune system, as well as tumor growth and cancer metastasis.
CRAC channel is composed of stromal interaction molecule (STIM) and ORAI, with the combination of STIM1/ORAI1 most well characterized.
STIM1 functions as an ER Ca2+ sensor and ORAI1 is the ion pore-forming subunit of CRAC channel. The dynamic coupling between STIM1 and ORAI1 is the key step for the opening of CRAC channel.
Major clinical manifestations of CRAC channelopathies in human are limited to the immune system, skeletal muscle and ectodermally derived tissues. CRAC channels can thus serve as an ideal target for developing novel immunomodulators with improved biosafety profiles.
Although most agents developed as CRAC channel inhibitors have not reached clinical trials owing to their poor selectivity and high toxicity, the clarification of molecular basis of CRAC channel is anticipated to expedite the development of drug candidates that specially target STIM and/or ORAI. Such compounds might hold great promise to serve as selective CRAC channel inhibitors to treat human disorders arising from imbalanced Ca2+ homeostasis.
Footnotes
Financial & competing interests disclosure
This work and the authors of this manuscript have been supported by the Major Project of Science and Technology of Shandong Province (2015ZDJS04001), the Fundamental Research Funds of Shandong University (2014JC008) and the NIH (R01GM112003). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest; •• of considerable interest
- 1.Clapham DE. Calcium signaling. Cell. 2007;131(6):1047–1058. doi: 10.1016/j.cell.2007.11.028. [DOI] [PubMed] [Google Scholar]
- 2.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat. Rev. Mol. Cell Biol. 2003;4(7):517–529. doi: 10.1038/nrm1155. [DOI] [PubMed] [Google Scholar]
- 3.Putney JW. A model for receptor-regulated calcium entry. Cell Calcium. 1986;7(1):1–12. doi: 10.1016/0143-4160(86)90026-6. [DOI] [PubMed] [Google Scholar]
- 4.Putney JW. Capacitative calcium entry: from concept to molecules. Immunol. Rev. 2009;231(1):10–22. doi: 10.1111/j.1600-065X.2009.00810.x. [DOI] [PubMed] [Google Scholar]
- 5.Prakriya M, Lewis RS. Store-operated calcium channels. Physiol. Rev. 2015;95(4):1383–1436. doi: 10.1152/physrev.00020.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cahalan MD, Chandy KG. The functional network of ion channels in T lymphocytes. Immunol. Rev. 2009;231(1):59–87. doi: 10.1111/j.1600-065X.2009.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hogan PG, Lewis RS, Rao A. Molecular basis of calcium signaling in lymphocytes: STIM and ORAI. Annu. Rev. Immunol. 2010;28:491–533. doi: 10.1146/annurev.immunol.021908.132550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lewis RS. Store-operated calcium channels: new perspectives on mechanism and function. Cold Spring Harb. Perspect. Biol. 2011;3(12):a003970. doi: 10.1101/cshperspect.a003970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Parekh AB. Store-operated CRAC channels: function in health and disease. Nat. Rev. Drug Discov. 2010;9(5):399–410. doi: 10.1038/nrd3136. [DOI] [PubMed] [Google Scholar]; •• Summarized the progress in the study of the gating and function of Ca2+ release-activated Ca2+ (CRAC) channels with their relation with human disease prior to 2010.
- 10.Shaw PJ, Feske S. Regulation of lymphocyte function by ORAI and STIM proteins in infection and autoimmunity. J. Physiol. 2012;590(Pt 17):4157–4167. doi: 10.1113/jphysiol.2012.233221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maus M, Medgyesi D, Kiss E, et al. B cell receptor-induced Ca2+ mobilization mediates F-actin rearrangements and is indispensable for adhesion and spreading of B lymphocytes. J. Leukoc. Biol. 2013;93(4):537–547. doi: 10.1189/jlb.0312169. [DOI] [PubMed] [Google Scholar]
- 12.Vig M, Dehaven WI, Bird GS, et al. Defective mast cell effector functions in mice lacking the CRACM1 pore subunit of store-operated calcium release-activated calcium channels. Nat. Immunol. 2008;9(1):89–96. doi: 10.1038/ni1550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trebak M. STIM1/Orai1, I(CRAC) and endothelial SOC. Circ. Res. 2009;104(9):e56–e57. doi: 10.1161/CIRCRESAHA.109.196105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tolhurst G, Carter RN, Amisten S, Holdich JP, Erlinge D, Mahaut-Smith MP. Expression profiling and electrophysiological studies suggest a major role for Orai1 in the store-operated Ca(2+) influx pathway of platelets and megakaryocytes. Platelets. 2008;19(4):308–313. doi: 10.1080/09537100801935710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gandhirajan RK, Meng S, Chandramoorthy HC, et al. Blockade of NOX2 and STIM1 signaling limits lipopolysaccharide-induced vascular inflammation. J. Clin. Invest. 2013;123(2):887–902. doi: 10.1172/JCI65647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lyfenko AD, Dirksen RT. Differential dependence of store-operated and excitation-coupled Ca(2+) entry in skeletal muscle on STIM1 and Orai1. J. Physiol. 2008;586(Pt 20):4815–4824. doi: 10.1113/jphysiol.2008.160481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cahalan MD. STIMulating store-operated Ca(2+) entry. Nat. Cell Biol. 2009;11(6):669–677. doi: 10.1038/ncb0609-669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Feske S, Gwack Y, Prakriya M, et al. A mutation in Orai1 causes immune deficiency by abrogating CRAC channel function. Nature. 2006;441(7090):179–185. doi: 10.1038/nature04702. [DOI] [PubMed] [Google Scholar]
- 19.Peters-Golden M, Gleason MM, Togias A. Cysteinyl leukotrienes: multi-functional mediators in allergic rhinitis. Clin. Exp. Allergy. 2006;36(6):689–703. doi: 10.1111/j.1365-2222.2006.02498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Braun A, Varga-Szabo D, Kleinschnitz C, et al. Orai1 (CRACM1) is the platelet SOC channel and essential for pathological thrombus formation. Blood. 2008;113(9):2056–2063. doi: 10.1182/blood-2008-07-171611. [DOI] [PubMed] [Google Scholar]
- 21.Gerasimenko JV, Gryshchenko O, Ferdek PE, et al. Ca(2+) release-activated Ca(2+) channel blockade as a potential tool in antipancreatitis therapy. Proc. Natl Acad. Sci. USA. 2013;110(32):13186–13191. doi: 10.1073/pnas.1300910110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feske S. Calcium signalling in lymphocyte activation and disease. Nat. Rev. Immunol. 2007;7(9):690–702. doi: 10.1038/nri2152. [DOI] [PubMed] [Google Scholar]
- 23.Yang S, Zhang JJ, Huang X-Y. Orai1 and STIM1 are critical for breast tumor cell migration and metastasis. Cancer Cell. 2009;15(2):124–134. doi: 10.1016/j.ccr.2008.12.019. [DOI] [PubMed] [Google Scholar]
- 24.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 25.Xie J, Pan H, Yao J, Zhou Y, Han W. SOCE and cancer: recent progress and new perspectives. Int. J. Cancer. 2016;138(9):2067–2077. doi: 10.1002/ijc.29840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liou J, Kim ML, Heo WD, et al. STIM is a Ca(2+) sensor essential for Ca(2+)-store-depletion-triggered Ca(2+) influx. Curr. Biol. 2005;15(13):1235–1241. doi: 10.1016/j.cub.2005.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang SL, Yu Y, Roos J, et al. STIM1 is a Ca(2+) sensor that activates CRAC channels and migrates from the Ca(2+) store to the plasma membrane. Nature. 2005;437(7060):902–905. doi: 10.1038/nature04147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vig M, Peinelt C, Beck A, et al. CRACM1 is a plasma membrane protein essential for store-operated Ca2+ entry. Science. 2006;312(5777):1220–1223. doi: 10.1126/science.1127883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang SL, Yeromin AV, Zhang XHF, et al. Genome-wide RNAi screen of Ca(2+) influx identifies genes that regulate Ca(2+) release-activated Ca(2+) channel activity. Proc. Natl Acad. Sci. USA. 2006;103(24):9357–9362. doi: 10.1073/pnas.0603161103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sabbioni S, Veronese A, Trubia M, et al. Exon structure and promoter identification of STIM1 (alias GOK), a human gene causing growth arrest of the human tumor cell lines G401 and RD. Cytogenet. Cell Genet. 1999;86(3–4):214–218. doi: 10.1159/000015341. [DOI] [PubMed] [Google Scholar]
- 31.Lewis RS. The molecular choreography of a store-operated calcium channel. Nature. 2007;446(7133):284–287. doi: 10.1038/nature05637. [DOI] [PubMed] [Google Scholar]
- 32.Stathopulos PB, Zheng L, Li G-Y, Plevin MJ, Ikura M. Structural and mechanistic insights into STIM1-mediated initiation of store-operated calcium entry. Cell. 2008;135(1):110–122. doi: 10.1016/j.cell.2008.08.006. [DOI] [PubMed] [Google Scholar]
- 33.Baba Y, Hayashi K, Fujii Y, et al. Coupling of STIM1 to store-operated Ca(2+) entry through its constitutive and inducible movement in the endoplasmic reticulum. Proc. Natl Acad. Sci. USA. 2006;103(45):16704–16709. doi: 10.1073/pnas.0608358103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yuan JP, Zeng W, Dorwart MR, Choi Y-J, Worley PF, Muallem S. SOAR and the polybasic STIM1 domains gate and regulate the Orai channels. Nat. Cell Biol. 2009;11(3):337–343. doi: 10.1038/ncb1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhou Y, Srinivasan P, Razavi S, et al. Initial activation of STIM1, the regulator of store-operated calcium entry. Nat. Struct. Mol. Biol. 2013;20(8):973–981. doi: 10.1038/nsmb.2625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brandman O, Liou J, Park WS, Meyer T. STIM2 is a feedback regulator that stabilizes basal cytosolic and endoplasmic reticulum Ca(2+) levels. Cell. 2007;131(7):1327–1339. doi: 10.1016/j.cell.2007.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hoth M, Niemeyer BA. Chapter ten – the neglected CRAC proteins: Orai2, Orai3, and STIM2. Curr. Top. Membr. 2013;71:237–271. doi: 10.1016/B978-0-12-407870-3.00010-X. [DOI] [PubMed] [Google Scholar]
- 38.Gwack Y, Srikanth S, Feske S, et al. Biochemical and functional characterization of Orai proteins. J. Biol. Chem. 2007;282(22):16232–16243. doi: 10.1074/jbc.M609630200. [DOI] [PubMed] [Google Scholar]
- 39.Prakriya M. The molecular physiology of CRAC channels. Immunol. Rev. 2009;231(1):88–98. doi: 10.1111/j.1600-065X.2009.00820.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berridge MJ. Inositol trisphosphate and calcium signalling. Nature. 1993;361(6410):315–325. doi: 10.1038/361315a0. [DOI] [PubMed] [Google Scholar]
- 41.Stathopulos PB, Li G-Y, Plevin MJ, Ames JB, Ikura M. Stored Ca2+ depletion-induced oligomerization of stromal interaction molecule 1 (STIM1) via the EF-SAM region: an initiation mechanism for capacitive Ca2+ entry. J. Biol. Chem. 2006;281(47):35855–35862. doi: 10.1074/jbc.M608247200. [DOI] [PubMed] [Google Scholar]
- 42.Covington ED, Wu MM, Lewis RS. Essential role for the CRAC activation domain in store-dependent oligomerization of STIM1. Mol. Biol. Cell. 2010;21(11):1897–1907. doi: 10.1091/mbc.E10-02-0145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Soboloff J, Rothberg BS, Madesh M, Gill DL. STIM proteins: dynamic calcium signal transducers. Nat. Rev. Mol. Cell Biol. 2012;13(9):549–565. doi: 10.1038/nrm3414. [DOI] [PMC free article] [PubMed] [Google Scholar]; • A comprehensive review on STIM1 and regulatory mechanisms of SOCE.
- 44.Ma G, Wei M, He L, et al. Inside-out Ca(2+) signalling prompted by STIM1 conformational switch. Nat. Commun. 2015;6:7826. doi: 10.1038/ncomms8826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fahrner M, Muik M, Schindl R, et al. A coiled-coil clamp controls both conformation and clustering of stromal interaction molecule 1 (STIM1) J. Biol. Chem. 2014;289(48):33231–33244. doi: 10.1074/jbc.M114.610022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luik RM, Wang B, Prakriya M, Wu MM, Lewis RS. Oligomerization of STIM1 couples ER calcium depletion to CRAC channel activation. Nature. 2008;454(7203):538–542. doi: 10.1038/nature07065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liou J, Fivaz M, Inoue T, Meyer T. Live-cell imaging reveals sequential oligomerization and local plasma membrane targeting of stromal interaction molecule 1 after Ca(2+) store depletion. Proc. Natl Acad. Sci. USA. 2007;104(22):9301–9306. doi: 10.1073/pnas.0702866104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Várnai P, Tóth B, Tóth DJ, Hunyady L, Balla T. Visualization and manipulation of plasma membrane-endoplasmic reticulum contact sites indicates the presence of additional molecular components within the STIM1–Orai1 complex. J. Biol. Chem. 2007;282(40):29678–29690. doi: 10.1074/jbc.M704339200. [DOI] [PubMed] [Google Scholar]
- 49.Gudlur A, Zhou Y, Hogan PG. Chapter two – STIM–ORAI interactions that control the CRAC channel. Curr. Top. Membr. 2013;71:33–58. doi: 10.1016/B978-0-12-407870-3.00002-0. [DOI] [PubMed] [Google Scholar]; • An authoritative summary on protein–protein interactions between STIM and ORAI proteins that control CRAC channel activation.
- 50.Zhou Y, Meraner P, Kwon HT, et al. STIM1 gates the store-operated calcium channel ORAI1 in vitro . Nat. Struct. Mol. Biol. 2010;17(1):112–116. doi: 10.1038/nsmb.1724. [DOI] [PMC free article] [PubMed] [Google Scholar]; • First demonstration of direct gating and opening of ORAI1 Ca2+ channel by recombinant STIM1.
- 51.Yang X, Jin H, Cai X, Li S, Shen Y. Structural and mechanistic insights into the activation of stromal interaction molecule 1 (STIM1) Proc. Natl Acad. Sci. USA. 2012;109(15):5657–5662. doi: 10.1073/pnas.1118947109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kawasaki T, Lange I, Feske S. A minimal regulatory domain in the C terminus of STIM1 binds to and activates ORAI1 CRAC channels. Biochem. Biophys. Res. Commun. 2009;385(1):49. doi: 10.1016/j.bbrc.2009.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Muik M, Fahrner M, Derler I, et al. A cytosolic homomerization and a modulatory domain within STIM1 C terminus determine coupling to ORAI1 channels. J. Biol. Chem. 2009;284(13):8421–8426. doi: 10.1074/jbc.C800229200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Park CY, Hoover PJ, Mullins FM, et al. STIM1 clusters and activates CRAC channels via direct binding of a cytosolic domain to Orai1. Cell. 2009;136(5):876–890. doi: 10.1016/j.cell.2009.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Oh-Hora M, Yamashita M, Hogan PG, et al. Dual functions for the endoplasmic reticulum calcium sensors STIM1 and STIM2 in T cell activation and tolerance. Nat. Immunol. 2008;9(4):432–443. doi: 10.1038/ni1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gwack Y, Srikanth S, Oh-Hora M, et al. Hair loss and defective T- and B-cell function in mice lacking ORAI1. Mol. Cell. Biol. 2008;28(17):5209–5222. doi: 10.1128/MCB.00360-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kiani A, Rao A, Aramburu J. Manipulating immune responses with immunosuppressive agents that target NFAT . Immunity. 2000;12(4):359–372. doi: 10.1016/s1074-7613(00)80188-0. [DOI] [PubMed] [Google Scholar]
- 58.Franzius D, Hoth M, Penner R. Non-specific effects of calcium entry antagonists in mast cells. Pflügers Arch. 1994;428(5):433–438. doi: 10.1007/BF00374562. [DOI] [PubMed] [Google Scholar]
- 59.Clementi E, Meldolesi J. Pharmacological and functional properties of voltagemi independent Ca2+ channels. Cell Calcium. 1996;19(4):269–279. doi: 10.1016/s0143-4160(96)90068-8. [DOI] [PubMed] [Google Scholar]
- 60.Putney JW. Pharmacology of capacitative calcium entry. Mol. Interv. 2001;1(2):84–94. [PubMed] [Google Scholar]
- 61.Sweeney ZK, Minatti A, Button DC, Patrick S. Small-molecule inhibitors of store-operated calcium entry. ChemMedChem. 2009;4(5):706–718. doi: 10.1002/cmdc.200800452. [DOI] [PubMed] [Google Scholar]; • An excellent review on SOCE inhibitors developed before 2010.
- 62.Pevarello P, Cainarca S, Liberati C, Tarroni P, Piscitelli F, Severi E. Ca2+ release-activated Ca2+ channel inhibitors. Pharm. Pat. Anal. 2014;3(2):171–182. doi: 10.4155/ppa.14.7. [DOI] [PubMed] [Google Scholar]; •• An excellent patent review on CRAC channel modulators developed by pharmaceutical companies within the recent 5 years.
- 63.Hoth M, Penner R. Calcium release-activated calcium current in rat mast cells. J. Physiol. 1993;465:359–386. doi: 10.1113/jphysiol.1993.sp019681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yeromin AV, Zhang SL, Jiang W, Yu Y, Safrina O, Cahalan MD. Molecular identification of the CRAC channel by altered ion selectivity in a mutant of Orai. Nature. 2006;443(7108):226–229. doi: 10.1038/nature05108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Mcnally BA, Prakriya M. Permeation, selectivity and gating in store-operated CRAC channels. J. Physiol. 2012;590(Pt 17):4179–4191. doi: 10.1113/jphysiol.2012.233098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mason MJ, Mahaut-Smith MP, Grinstein S. The role of intracellular Ca2+ in the regulation of the plasma membrane Ca2+ permeability of unstimulated rat lymphocytes. J. Biol. Chem. 1991;266(17):10872–10879. [PubMed] [Google Scholar]
- 67.Mcnally BA, Yamashita M, Engh A, Prakriya M. Structural determinants of ion permeation in CRAC channels. Proc. Natl Acad. Sci. USA. 2009;106(52):22516–22521. doi: 10.1073/pnas.0909574106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Vig M, Beck A, Billingsley JM, et al. CRACM1 multimers form the ion-selective pore of the CRAC channel. Curr. Biol. 2006;16(20):2073–2079. doi: 10.1016/j.cub.2006.08.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hou X, Pedi L, Diver MM, Long SB. Crystal structure of the calcium release-activated calcium channel Orai. Science (New York) 2012;338(6112):1308–1313. doi: 10.1126/science.1228757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Reichling DB, Macdermott AB. Lanthanum actions on excitatory amino acid-gated currents and voltage-gated calcium currents in rat dorsal horn neurons. J. Physiol. 1991;441:199–218. doi: 10.1113/jphysiol.1991.sp018746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Clapham DE, Runnels LW, Strubing C. The trp ion channel family. Nat. Rev. Neurosci. 2001;2(6):387–396. doi: 10.1038/35077544. [DOI] [PubMed] [Google Scholar]
- 72.Chung SC, Mcdonald TV, Gardner P. Inhibition by SK&F 96365 of Ca2+ current, IL-2 production and activation in T lymphocytes. Br. J. Pharmacol. 1994;113(3):861–868. doi: 10.1111/j.1476-5381.1994.tb17072.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Singh A, Hildebrand ME, Garcia E, Snutch TP. The transient receptor potential channel antagonist SKF96365 is a potent blocker of low-voltage-activated T-type calcium channels. Br. J. Pharmacol. 2010;160(6):1464–1475. doi: 10.1111/j.1476-5381.2010.00786.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ma H-T, Patterson RL, Van DB, et al. Requirement of the inositol trisphosphate receptor for activation of store-operated Ca2+ channels. Science. 2000;287(5458):1647–1651. doi: 10.1126/science.287.5458.1647. [DOI] [PubMed] [Google Scholar]
- 75.Prakriya M, Lewis RS. Potentiation and inhibition of Ca(2+) release-activated Ca(2+) channels by 2-aminoethyldiphenyl borate (2-APB) occurs independently of IP(3) receptors. J. Physiol. 2001;536(Pt 1):3–19. doi: 10.1111/j.1469-7793.2001.t01-1-00003.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma H-T, Venkatachalam K, Parys JB, Gill DL. Modification of store-operated channel coupling and inositol trisphosphate receptor function by 2-aminoethoxydiphenyl borate in DT40 lymphocytes. J. Biol. Chem. 2002;277(9):6915–6922. doi: 10.1074/jbc.M107755200. [DOI] [PubMed] [Google Scholar]
- 77.Goto J-I, Suzuki AZ, Ozaki S, et al. Two novel 2-aminoethyl diphenylborinate (2-APB) analogs differentially activate and inhibit store-operated Ca(2+) entry via STIM proteins. Cell Calcium. 2010;47(1):1–10. doi: 10.1016/j.ceca.2009.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Maruyama T, Kanaji T, Nakade S, Kanno T, Mikoshiba K. 2APB, 2-aminoethoxydiphenyl borate, a membrane-penetrable modulator of Ins(1,4,5)P3-induced Ca2+ release. J. Biochem. 1997;122(3):498–505. doi: 10.1093/oxfordjournals.jbchem.a021780. [DOI] [PubMed] [Google Scholar]
- 79.Sugawara H, Kurosaki M, Takata M, Kurosaki T. Genetic evidence for involvement of type 1, type 2 and type 3 inositol 1,4,5-trisphosphate receptors in signal transduction through the B-cell antigen receptor. EMBO J. 1997;16(11):3078–3088. doi: 10.1093/emboj/16.11.3078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Van Rossum DB, Patterson RL, Ma H-T, Gill DL. Ca2+ entry mediated by store depletion, S-nitrosylation, and TRP3 channels: comparison of coupling and function. J. Biol. Chem. 2000;275(37):28562–28568. doi: 10.1074/jbc.M003147200. [DOI] [PubMed] [Google Scholar]
- 81.Bootman MD, Collins TJ, Mackenzie L, Roderick HL, Berridge MJ, Peppiatt CM. 2-aminoethoxydiphenyl borate (2-APB) is a reliable blocker of store-operated Ca2+ entry but an inconsistent inhibitor of InsP3-induced Ca2+ release. FASEB J. 2002;16(10):1145–1150. doi: 10.1096/fj.02-0037rev. [DOI] [PubMed] [Google Scholar]
- 82.Gregory RB, Rychkov G, Barritt GJ. Evidence that 2-aminoethyl diphenylborate is a novel inhibitor of store-operated Ca2+ channels in liver cells, and acts through a mechanism which does not involve inositol trisphosphate receptors. Biochem. J. 2001;354(Pt 2):285–290. doi: 10.1042/0264-6021:3540285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Iwasaki H, Mori Y, Hara Y, Uchida K, Zhou H, Mikoshiba K. 2-aminoethoxydiphenyl borate (2-APB) inhibits capacitative calcium entry independently of the function of inositol 1,4,5-trisphosphate receptors. Receptors Channels. 2001;7(6):429–439. [PubMed] [Google Scholar]
- 84.Peinelt C, Lis A, Beck A, Fleig A, Penner R. 2-aminoethoxydiphenyl borate directly facilitates and indirectly inhibits STIM1-dependent gating of CRAC channels. J. Physiol. 2008;586(Pt 13):3061–3073. doi: 10.1113/jphysiol.2008.151365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Dehaven WI, Smyth JT, Boyles RR, Bird GS, Putney JW. Complex actions of 2-Aminoethyldiphenyl borate on store-operated calcium entry. J. Biol. Chem. 2008;283(28):19265–19273. doi: 10.1074/jbc.M801535200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Navarro-Borelly L, Somasundaram A, Yamashita M, Ren D, Miller RJ, Prakriya M. STIM1–Orai1 interactions and Orai1 conformational changes revealed by live-cell FRET microscopy. J. Physiol. 2008;586(Pt 22):5383–5401. doi: 10.1113/jphysiol.2008.162503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lis A, Peinelt C, Beck A, et al. CRACM1, CRACM2, and CRACM3 are store-operated Ca2+ channels with distinct functional properties. Curr. Biol. 2007;17(9):794–800. doi: 10.1016/j.cub.2007.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhang SL, Kozak JA, Jiang W, et al. Store-dependent and -independent modes regulating Ca(2+) release-activated Ca(2+) channel activity of human Orai1 and Orai3. J. Biol. Chem. 2008;283(25):17662–17671. doi: 10.1074/jbc.M801536200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schindl R, Bergsmann J, Frischauf I, et al. 2-aminoethoxydiphenyl borate alters selectivity of Orai3 channels by increasing their pore size. J. Biol. Chem. 2008;283(29):20261–20267. doi: 10.1074/jbc.M803101200. [DOI] [PubMed] [Google Scholar]
- 90.Wang Y, Deshpande M, Payne R. 2-aminoethoxydiphenyl borate inhibits phototransduction and blocks voltage-gated potassium channels in Limulus ventral photoreceptors. Cell Calcium. 2002;32(4):209–216. doi: 10.1016/s0143416002001562. [DOI] [PubMed] [Google Scholar]
- 91.Missiaen L, Callewaert G, De Smedt H, Parys JB. 2-aminoethoxydiphenyl borate affects the inositol 1,4,5-trisphosphate receptor, the intracellular Ca2+pump and the non-specific Ca2+leak from the non-mitochondrial Ca2+stores in permeabilized A7r5 cells. Cell Calcium. 2001;29(2):111–116. doi: 10.1054/ceca.2000.0163. [DOI] [PubMed] [Google Scholar]
- 92.Hu H-Z, Gu Q, Wang C, et al. 2-aminoethoxydiphenyl borate is a common activator of TRPV1, TRPV2, and TRPV3. J. Biol. Chem. 2004;279(34):35741–35748. doi: 10.1074/jbc.M404164200. [DOI] [PubMed] [Google Scholar]
- 93.Hendron E, Wang X, Zhou Y, et al. Potent functional uncoupling between STIM1 and Orai1 by dimeric 2-aminodiphenyl borinate analogs. Cell Calcium. 2014;56(6):482–492. doi: 10.1016/j.ceca.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang X, Wang Y, Zhou Y, et al. Distinct Orai-coupling domains in STIM1 and STIM2 define the Orai-activating site. Nat. Commun. 2014;5:3183–3183. doi: 10.1038/ncomms4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nazıroğlu M, Özgül C, Çelik Ö, Çiğ B, Sözbir E. Aminoethoxydiphenyl borate and flufenamic acid inhibit Ca2+ influx through TRPM2 channels in Rat dorsal root ganglion neurons activated by ADP-ribose and rotenone. J. Membr. Biol. 2011;241(2):69–75. doi: 10.1007/s00232-011-9363-9. [DOI] [PubMed] [Google Scholar]
- 96.Chokshi R, Fruasaha P, Kozak JA. 2-Aminoethyl diphenyl borinate (2-APB) inhibits TRPM7 channels through an intracellular acidification mechanism. Channels. 2012;6(5):362–369. doi: 10.4161/chan.21628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kovacs G, Montalbetti N, Simonin A, et al. Inhibition of the human epithelial calcium channel TRPV6 by 2-aminoethoxydiphenyl borate (2-APB) Cell Calcium. 2012;52(6):468–480. doi: 10.1016/j.ceca.2012.08.005. [DOI] [PubMed] [Google Scholar]
- 98.Djuric SW, Bamaung NY, Basha A, et al. 3,5-bis(trifluoromethyl)pyrazoles: a novel class of NFAT transcription factor regulator. J. Med. Chem. 2000;43(16):2975–2981. doi: 10.1021/jm990615a. [DOI] [PubMed] [Google Scholar]
- 99.Trevillyan JM, Chiou XG, Chen Y-W, et al. Potent inhibition of NFAT activation and T cell cytokine production by novel low molecular weight pyrazole compounds. J. Biol. Chem. 2001;276(51):48118–48126. doi: 10.1074/jbc.M107919200. [DOI] [PubMed] [Google Scholar]
- 100.Chen Y-W, Smith ML, Chiou GX, et al. TH1 and TH2 cytokine inhibition by 3,5-bis(trifluoromethyl)pyrazoles, a novel class of immunomodulators. Cell. Immunol. 2002;220(2):134–142. doi: 10.1016/s0008-8749(03)00005-4. [DOI] [PubMed] [Google Scholar]
- 101.Ishikawa J, Ohga K, Yoshino T, et al. A pyrazole derivative, YM-58483, potently inhibits store-operated sustained Ca2+ influx and IL-2 production in T lymphocytes. J. Immunol. 2003;170(9):4441–4449. doi: 10.4049/jimmunol.170.9.4441. [DOI] [PubMed] [Google Scholar]
- 102.Zitt C, Strauss B, Schwarz EC, et al. Potent inhibition of Ca2+ release-activated Ca2+ channels and T-lymphocyte activation by the pyrazole derivative BTP2. J. Biol. Chem. 2004;279(13):12427–12437. doi: 10.1074/jbc.M309297200. [DOI] [PubMed] [Google Scholar]
- 103.Mercer JC, Qi Q, Mottram LF, et al. Chemico-genetic identification of drebrin as a regulator of calcium responses. Int. J. Biochem. Cell Biol. 2010;42(2):337–345. doi: 10.1016/j.biocel.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ribeiro CMP, Reece J, Putney JW. Role of the cytoskeleton in calcium signaling in NIH 3T3 cells: an intact cytoskeleton is required for agonist-induced [Ca2+] i signaling, but not for capacitative calcium entry. J. Biol. Chem. 1997;272(42):26555–26561. doi: 10.1074/jbc.272.42.26555. [DOI] [PubMed] [Google Scholar]
- 105.Takezawa R, Cheng H, Beck A, et al. A pyrazole derivative potently inhibits lymphocyte Ca2+ influx and cytokine production by facilitating transient receptor potential melastatin 4 channel activity. Mol. Pharmacol. 2006;69(4):1413–1420. doi: 10.1124/mol.105.021154. [DOI] [PubMed] [Google Scholar]
- 106.He L-P, Hewavitharana T, Soboloff J, Spassova MA, Gill DL. A functional link between store-operated and TRPC channels revealed by the 3,5-bis(trifluoromethyl)pyrazole derivative, BTP2. J. Biol. Chem. 2005;280(12):10997–11006. doi: 10.1074/jbc.M411797200. [DOI] [PubMed] [Google Scholar]
- 107.Schleifer H, Doleschal B, Lichtenegger M, et al. Novel pyrazole compounds for pharmacological discrimination between receptor-operated and store-operated Ca(2+) entry pathways. Br. J. Pharmacol. 2012;167(8):1712–1722. doi: 10.1111/j.1476-5381.2012.02126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Law M, Morales JL, Mottram LF, Iyer A, Peterson BR, August A. Structural requirements for the inhibition of calcium mobilization and mast cell activation by the pyrazole derivative BTP2. Int. J. Biochem. Cell Biol. 2011;43(8):1228–1239. doi: 10.1016/j.biocel.2011.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Ashmole I, Duffy SM, Leyland ML, Morrison VS, Begg M, Bradding P. CRACM/Orai ion channel expression and function in human lung mast cells. J. Allergy Clin. Immunol. 2012;129(6–3) doi: 10.1016/j.jaci.2012.01.070. 1628–1635.e1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Rice LV, Bax HJ, Russell LJ, et al. Characterization of selective calcium-release activated calcium channel blockers in mast cells and T-cells from human, rat, mouse and guinea-pig preparations. Eur. J. Pharmacol. 2013;704(1–3):49–57. doi: 10.1016/j.ejphar.2013.02.022. [DOI] [PubMed] [Google Scholar]
- 111.Derler I, Schindl R, Fritsch R, et al. The action of selective CRAC channel blockers is affected by the Orai pore geometry. Cell Calcium. 2013;53(2):139–151. doi: 10.1016/j.ceca.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pandol SJ, Saluja AK, Imrie CW, Banks PA. Acute pancreatitis: bench to the bedside. Gastroenterology. 2007;132(3):1127–1151. doi: 10.1053/j.gastro.2007.01.055. [DOI] [PubMed] [Google Scholar]
- 113.Owsianik G, D'hoedt D, Voets T, Nilius B. Structure–function relationship of the TRP channel superfamily. Rev. Physiol. Biochem. Pharmacol. 2006;156:61–90. [PubMed] [Google Scholar]
- 114.Ng SW, Di Capite J, Singaravelu K, Parekh AB. Sustained activation of the tyrosine kinase Syk by antigen in mast cells requires local Ca2+ influx through Ca2+ release-activated Ca2+ channels. J. Biol. Chem. 2008;283(46):31348–31355. doi: 10.1074/jbc.M804942200. [DOI] [PubMed] [Google Scholar]
- 115.Di Sabatino A, Rovedatti L, Kaur R, et al. Targeting gut T cell Ca2+ release-activated Ca2+ channels inhibits T cell cytokine production and T-Box transcription factor T-Bet in inflammatory bowel disease. J. Immunol. 2009;183(5):3454–3462. doi: 10.4049/jimmunol.0802887. [DOI] [PubMed] [Google Scholar]
- 116.Watanabe H, Takahashi R, Zhang X-X, Kakizawa H, Hayashi H, Ohno R. Inhibition of agonist-induced Ca2+ entry in endothelial cells by myosin light-chain kinase inhibitor. Biochem. Biophys. Res. Commun. 1996;225(3):777–784. doi: 10.1006/bbrc.1996.1250. [DOI] [PubMed] [Google Scholar]
- 117.Smyth JT, Dehaven WI, Bird GS, Putney JW. Calcium store-dependent and independent reversal of stim1 localization and function. J. Cell Sci. 2008;121(Pt 6):762–772. doi: 10.1242/jcs.023903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zakharov SI, Smani T, Dobrydneva Y, et al. Diethylstilbestrol is a potent inhibitor of store-operated channels and capacitative Ca2+ influx. Mol. Pharmacol. 2004;66(3):702–707. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- 119.Ohana L, Newell EW, Stanley EF, Schlichter LC. The Ca2+ release-activated Ca2+ current (ICRAC) mediates store-operated Ca2+ entry in rat microglia. Channels. 2009;3(2):129–139. doi: 10.4161/chan.3.2.8609. [DOI] [PubMed] [Google Scholar]
- 120.Kohn EC, Reed E, Sarosy GA, et al. A phase I trial of carboxyamido-triazole and paclitaxel for relapsed solid tumors: potential efficacy of the combination and demonstration of pharmacokinetic interaction. Clin. Cancer Res. 2001;7(6):1600–1609. [PubMed] [Google Scholar]
- 121.Hussain MM, Kotz H, Minasian L, et al. Phase II trial of carboxyamidotriazole in patients with relapsed epithelial ovarian cancer. J. Clin. Oncol. 2003;21(23):4356–4363. doi: 10.1200/JCO.2003.04.136. [DOI] [PubMed] [Google Scholar]
- 122.Rodland KD, Wersto RP, Hobson S, Kohn EC. Thapsigargin-induced gene expression in nonexcitable cells is dependent on calcium influx. Mol. Endocrinol. 1997;11(3):281–291. doi: 10.1210/mend.11.3.9894. [DOI] [PubMed] [Google Scholar]
- 123.Hoth M, Button DC, Lewis RS. Mitochondrial control of calcium-channel gating: a mechanism for sustained signaling and transcriptional activation in T lymphocytes. Proc. Natl Acad. Sci. USA. 2000;97(19):10607–10612. doi: 10.1073/pnas.180143997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Glitsch MD, Bakowski D, Parekh AB. Store-operated Ca(2+) entry depends on mitochondrial Ca(2+) uptake. EMBO J. 2002;21(24):6744–6754. doi: 10.1093/emboj/cdf675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Mignen O, Brink C, Enfissi A, et al. Carboxyamidotriazole-induced inhibition of mitochondrial calcium import blocks capacitative calcium entry and cell proliferation in HEK-293 cells. J. Cell Sci. 2005;118(23):5615–5623. doi: 10.1242/jcs.02663. [DOI] [PubMed] [Google Scholar]
- 126.Chen G, Panicker S, Lau K-Y, et al. Characterization of a novel CRAC inhibitor that potently blocks human T cell activation and effector functions. Mol. Immunol. 2013;54(3–4):355–367. doi: 10.1016/j.molimm.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 127.Holowka D, Korzeniowski MK, Bryant KL, Baird B. Polyunsaturated fatty acids inhibit stimulated coupling between the ER Ca(2+) sensor STIM1 and the Ca(2+) channel protein Orai1 in a process that correlates with inhibition of stimulated STIM1 oligomerization. Biochim. Biophys. Acta. 2014;1841(8):1210–1216. doi: 10.1016/j.bbalip.2014.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Zhang H-Z, Xu X-L, Chen H-Y, et al. Discovery and structural optimization of 1-phenyl-3-(1-phenylethyl)urea derivatives as novel inhibitors of CRAC channel. Acta. Pharmacol. Sin. 2015;36(9):1137–1144. doi: 10.1038/aps.2015.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Jairaman A, Prakriya M. Molecular pharmacology of store-operated CRAC channels. Channels. 2013;7(5):402–414. doi: 10.4161/chan.25292. [DOI] [PMC free article] [PubMed] [Google Scholar]; •• Has introduced the molecular basis of CRAC channels in detail and summarized some traditional CRAC channel inhibitors.
- 130.He L, Zhang Y, Ma G, et al. Near-infrared photoactivatable control of Ca2+ signaling and optogenetic immunomodulation. eLife. 2015;4 doi: 10.7554/eLife.10024. http://www.ncbi.nlm.nih.gov/pmc/articles/PMC4737651/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kyung T, Lee S, Kim JE, et al. Optogenetic control of endogenous Ca2+ channels in vivo . Nat. Biotech. 2015;33(10):1092–1096. doi: 10.1038/nbt.3350. [DOI] [PubMed] [Google Scholar]
- 132.Ishii T, Sato K, Kakumoto T, et al. Light generation of intracellular Ca(2+) signals by a genetically encoded protein BACCS. Nat. Commun. 2015;6:8021. doi: 10.1038/ncomms9021. [DOI] [PMC free article] [PubMed] [Google Scholar]