Abstract
Advancements in diagnostic tools and curative-intent therapies have improved cancer-specific survival. With prolonged survival, patients are now subject to increased aging and development of cardiovascular risk factors such that further improvements in cancer-specific mortality are at risk of being offset by increased cardiovascular mortality. Moreover, established and novel adjuvant therapies used in cancer treatment are associated with unique and varying degrees of direct as well as indirect myocardial and cardiovascular injury (i.e., cardiotoxicity). Current approaches for evaluating anticancer therapy-induced injury have limitations, particularly lack of sensitivity for early detection of subclinical cardiac and cardiovascular dysfunction. With emerging evidence suggesting early prevention and treatment can mitigate the degree of cardiotoxicity and limit interruption of life-saving cancer therapy, the importance of early detection is increasingly paramount. Newer imaging modalities, functional capacity testing and blood biomarkers have the potential to improve early detection of cardiotoxicity and reduce cardiovascular morbidity and mortality.
KEYWORDS : anthracyclines, biomarkers, cardiotoxicity, chemotherapy, echocardiography, imaging, stress testing, trastuzumab
Mortality rates from cancer have declined over the past 30 years as more effective methods of early detection, pharmacologic treatments and surgical approaches have resulted in significant cancer-related survival gains [1–3]. Long-term survivors with cancer are expected to increase by approximately 30% in the next decade to an estimated 18 million by 2022 in the USA alone [4]. With prolonged survival, cancer patients are now subject to the effects of aging and to the development of other risk factors that determine the long-term risk of cardiovascular disease (CVD) [5–7]. In this setting, CVD may limit the survival gains of oncology and is already the predominant cause of mortality in breast cancer patients over 50 years of age [5,6,8] and a more common contributor than cancer to mortality among older survivors [9,10].
The cardiovascular effects of a prolonged survival are also compounded by those of standard modern cancer therapy. Current anticancer therapies are associated with unique and varying degrees of direct (e.g., myocardial toxicity, ischemia, hypertension, arrhythmias) [11–14] as well as indirect (e.g., unfavorable lifestyle changes) sequential and progressive cardiovascular insults [11]. The most well known direct cardiotoxic effects of cancer therapy historically occur with anthracycline-containing regimens (i.e., doxorubicin, epirubicin). Anthracyclines are still widely used in solid tumors (i.e., breast cancer, osteosarcoma etc.) and hematologic malignancies (Hodgkin's/non-Hodgkin's lymphoma, acute lymphoblastic leukemia etc.) and are well recognized to trigger dose-dependent, cumulative, progressive cardiac dysfunction [15,16] manifest as decreased left ventricular ejection fraction (LVEF) [17–19], and ultimately, symptomatic heart failure (HF) in up to 5% of patients [20]. Newer, targeted agents have dramatically improved the antitumor efficacy of therapies for various cancers but are not without adverse cardiovascular effects [13,21–23]. In fact, many targeted therapies, particularly monoclonal antibodies and tyrosine kinase inhibitors targeting HER-2 (i.e., trastuzumab, pertuzumab etc.), VEGF and VEGF receptors (i.e., bevacizumab, sunitinib, sorafenib etc.), and Abl kinase activity (i.e., imatinib, nilotinib, dasatinib etc.) have been demonstrated to interfere with molecular pathways crucial to cardiovascular health [23,24]. Moreover, cardiac damage inflicted by the newer agents may be additive; specifically, trastuzumab (Herceptin®), a humanized monoclonal antibody used in HER-2 positive early-stage breast cancer has demonstrated synergistic cardiotoxicity when added to anthracyclines [25,26].
The magnitude of cardiovascular morbidity is likely to increase with continual improvements in cancer-specific survival in the setting of sequential use of targeted therapies [23] and the approval of newer antineoplastic agents for which the long-term cardiovascular safety profile is not yet known [27,28]. Going forward, cardiovascular detection and surveillance needs to sufficiently identify patients susceptible to therapy-related cardiotoxicity early to prevent morbidity and mortality, as well as to avoid unnecessary discontinuation of essential anticancer therapy or overtreatment of individuals not at risk.
Current state of cardio-oncology
Oncology and cardiology organizations have attempted to classify cardiotoxicity in terms of overt clinical events and subclinical injury. The National Cancer Institute has developed a comprehensive system for cancer adverse events reporting which includes cardiotoxicity, the common terminology criteria for adverse events. This recognizes a broad array of important cardiac and cardiovascular events as well as subclinical laboratory and ejection fraction changes during cytotoxic therapy. Notably, the National Cancer Institute has kept the traditional criteria that define myocardial toxicity (e.g., left ventricular systolic dysfunction, HF and LVEF decline) separate, rather than unifying these parameters under a comprehensive definition of cardiotoxicity [29]. The Cardiac Review and Evaluation Committee criteria were specifically developed in 2002 during an initial extensive safety review of patients treated in trastuzumab trials and defined cardiotoxicity based on physical signs, symptoms and LVEF [30]. The American College of Cardiology/American Heart Association have developed a staging system (stages A–D) to identify patients during the course of HF development. Asymptomatic patients who receive potential cardiotoxins (stage A) or have asymptomatic left ventricular (LV) dysfunction (stage B) are at risk to develop symptomatic HF (stages C and D) [31]. The transition from American College of Cardiology/American Heart Association stage B asymptomatic to stage C symptomatic HF has been associated with a significant decrease in 5-year survival (96–75%) equivalent to a fivefold increase in mortality risk in a community population [32].
Early detection of cardiotoxicity provides an opportunity to prevent or reverse progression to a more advanced stage (Figure 1); LVEF recovery and cardiac event reduction were more likely with early detection of LV dysfunction and prompt initiation of usual HF therapies in cancer patients with anthracycline-induced cardiomyopathy [33]. In this regard, the evolution of the ‘cardio-oncology’ field, which aims to keep pace with the rapid evolution of cancer therapies and the incidence, magnitude and consequences of their cardiac and cardiovascular side effects, has contributed to improved recognition of the prevalence of treatment-related cardiotoxicity and the importance of early detection [34]. A common pathway for identifying patients at risk for cardiotoxicity, however, remains complicated by the nonspecific nature of treatment-related cardiac and cardiovascular damage, the unpredictability of clinical consequences (e.g., severity, timing etc.), and consequently, uncertainty regarding the optimal strategy for detection.
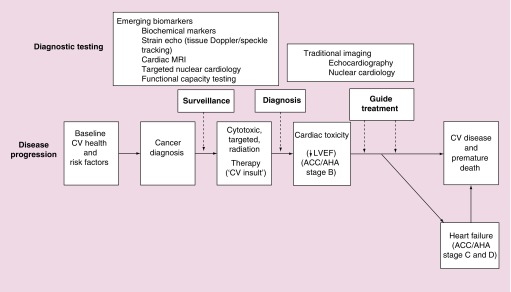
Figure 1. . Cardiovascular disease and detection of cardiotoxicity in cancer.
A schematic representation describing the continuum of cancer treatment, subclinical cardiotoxicity and eventual development of overt cardiovascular disease, in particular, heart failure. Cancer occurs in the setting of the patient's baseline health and risk factors profile. With the administration of potentially cardiotoxic therapies, various surveillance diagnostic strategies may be adopted. Traditional and novel imaging, functional capacity testing and blood-based biomarker strategies have been assessed to detect cardiotoxicity. Once there is evidence of overt cardiac dysfunction (i.e., ACC/AHA stage B heart failure), imaging and blood-based biomarkers may also help to guide treatment.
↓: Decline; ACC/AHA: American College of Cardiology/American Heart Association; CV: Cardiovascular; LVEF: Left ventricular ejection fraction.
Adapted with permission from [36].
Current state of detection of cardiotoxicity
In current oncology practice, the cardiotoxic impact of anticancer therapies is primarily evaluated by resting LVEF. Accurate assessment of the frequency and magnitude of cardiotoxicity is challenging, however, given differences in LVEF cut points, monitoring frequency and measurement modalities used in clinical trials that limit direct comparisons. There are currently no consensus criteria for cardiotoxicity [35–37]; trial-based cardiac safety end points have been heterogeneously defined as either an absolute reduction of LVEF >10% or >15%, an LVEF reduction >10% or >15% to a threshold of <55% or <50%, or any LVEF decline to <50% [38]. Echocardiography, multi-gated acquisition (MUGA) or both have been used most frequently for cardiac assessments in clinical trials and practice, although LVEF measurements by these modalities are not interchangeable [39]. The validity of assumptions common to the spectrum of cardiotoxicity criteria, specifically whether an LVEF decline is always attributable to treatment-related toxicity and whether the absence of an LVEF decline can be interpreted as absence of cardiotoxicity, is also debatable [40]. Moreover, there are currently no adult oncology guidelines in the USA addressing prospective cardiac monitoring in patients treated with potentially cardiotoxic treatment regimens, although clinical practice guidelines have been released in Europe [37]. For certain cardiotoxic agents, such as trastuzumab, serial LVEF measurements are US FDA mandated in the USA [41] and stopping/holding rules (i.e., LVEF decrease of ≥16% or 10–15% below institutional lower limits of normal) that were used in the trastuzumab clinical trials [42–44] during treatment have translated to clinical monitoring practices. However, institutional and provider-based differences remain in approach to long-term cardiac risk surveillance and detection in patients treated with potentially cardiotoxic treatment regimens [45], inconsistency which limits accurate assessment of the incidence of late cardiotoxicity.
There are additional limitations with the current paradigm that uses resting LVEF for surveillance of changes in cardiac and cardiovascular function during and after potentially cardiotoxic anticancer therapy. First, resting LVEF provides a snapshot of cardiac performance under optimal circumstances and may not demonstrate subclinical loss of cardiac reserve from early myocyte damage [46]. Second, resting LVEF principally assesses load-dependent changes in LV cavity size. Changes in loading conditions during chemotherapy are common and, as a result, LVEF may not reflect actual myocardial systolic function [47]. In fact, LVEF may overestimate actual LV health when intrinsic mechanisms are initially compensatory to maintain cardiac output in the face of acute myocardial injury [48]. When such compensation ultimately falters, a drop in LVEF is finally observed that may occur too late to avert irreversible cardiomyopathy [49]. Third, cancer therapy-induced damage often extends beyond the heart and may occur in conjunction with (mal) adaptation in other organ components, which are not evaluated by resting LVEF. Many anticancer therapies cause unique and varying degrees of injury to the cardiovascular system (i.e., pulmonary–vascular/blood–skeletal muscle axis) [50]. For example, radiation and certain forms of systemic therapy (e.g., chemotherapy, molecularly targeted therapies) can cause pulmonary dysfunction, anemia, endothelial dysfunction and pulmonary/systemic arterial stiffness as well as skeletal muscle dysfunction [11,12,51–53]. These direct insults occur in conjunction with ‘indirect’ lifestyle perturbations (e.g., physical inactivity) that synergistically cause marked impairments in cardiovascular reserve capacity [54].
As current detection strategies in the oncology setting focus almost exclusively on LVEF at rest, alternative assessments that provide better measures of cardiac (i.e., myocardial) as well as global cardiovascular function are required to identify those cancer patients at high risk of CVD but who do not experience overt HF or a decline in LVEF. The purpose of this review is to summarize other potential strategies for the early detection of cardiotoxicity, a term used herein to describe cancer therapy-related clinical as well as subclinical cardiac and cardiovascular injury.
Resting left ventricular ejection fraction
In current practice, 2D echocardiography (2DE) and MUGA scanning are most frequently used to evaluate resting LVEF. These techniques have advantages, including portability (for 2DE), ease of use and availability. However, there are also well-described limitations, including limited reproducibility and accuracy for assessment of LV volumes and LVEF by 2DE that limits sensitivity for detection of small changes in LV function and radiation exposure for MUGA [35].
3D echocardiography (3DE) preserves many of the advantages of echocardiography while mitigating some of the limitations that exist with 2DE. In adjuvant breast cancer, 3DE was superior to 2DE, and had similar accuracy to MUGA (with cardiac magnetic resonance [CMR] imaging as the gold standard) for LVEF in early breast cancer [55]. Similarly, 3DE may provide more reliable detection of LVEF changes, which is clinically important given the threshold magnitude of LVEF decline (10%) used to adjudicate subclinical cancer therapy-related cardiac dysfunction by existing criteria [36]. In a prospective comparison of 2DE and 3DE in 56 breast cancer patients, Thavendiranathan et al. [56] found that the temporal variability of LVEF by 3DE was 5–6% compared with 10–13% by 2DE. In the same study, noncontrast 3DE measurement of LVEF had the best intra and interobserver as well as test-retest variability. Owing to its excellent spatial resolution and the ability to image the entirety of the heart, CMR imaging is the accepted gold standard in cardiac volumetric analysis and LVEF assessment [57–59] and may be particularly useful in cases where data regarding cardiac function and volume with echocardiography and MUGA are conflicting or unclear. Despite their superiority for LVEF assessments, both 3DE and CMR are less available than 2DE (Table 1).
Table 1. . Comparison of modalities of detection for cancer therapy-induced cardiotoxicity†.
| |
Echocardiography |
Radionuclide angiogram |
Cardiac magnetic resonance |
Blood biomarkers |
|||
|---|---|---|---|---|---|---|---|
| 2D | 3D | Strain | Troponin | Natriuretic peptides | |||
| Measure(s) |
LVEF; volumes |
LVEF; volumes |
Myocardial deformation |
LVEF; volumes |
LVEF; volumes; myocardial damage/deformation |
Myocardial injury |
Myocardial stretch/stress |
| Availability |
++++ |
+++ |
+++ |
+++ |
+ |
++++ |
++++ |
| Cost |
Low |
Medium |
Medium |
Low |
|||
| Reproducibility |
++ |
+++ |
+++ |
++++ |
++++ |
++++ |
++++ |
| Cardiac structure† |
|
|
|
|
|
|
|
| Temporal resolution |
++++ |
+ |
+++ |
– |
– |
||
| Spatial resolution |
++ |
+ |
+++ |
– |
– |
||
| Myocardial function‡ |
|
|
|
|
|
|
|
| Systolic |
+++ |
+++(+) |
++++ |
++++ |
++++ |
– |
– |
| Diastolic |
++++ |
+ |
++++ |
– |
++ |
– |
– |
| Tissue characterization |
+ |
– |
– |
– |
++++ |
– |
– |
| Potential to detect subclinical cardiac toxicity | Low | Low | Moderate-high | Low | Moderate | High | Unknown |
Increasing ‘+’ corresponds to increasing efficacy. – corresponds to not available.
†Cardiac structure includes visualization and quantification of cardiac chambers (dimension), myocardium (thickness) and valves (mobility, regurgitation, stenosis).
‡Myocardial function includes visualization and quantification of systolic function (e.g., LVEF, strain, strain rate, stroke volume etc.) and diastolic function (e.g., diastolic grade/pattern).
LVEF: Left ventricular ejection fraction.
Adapted with permission from [36].
Among noncancer populations, LVEF has been repeatedly shown to be an important and independent predictor of prognosis and is used frequently in clinical decision making [31,60,61]. In a cancer population, the prognosis of developing LV systolic dysfunction is presumably worse compared with the general population, given the continuous nature of the myocardial insult from cytotoxic therapy. In a clinical trial involving 944 early breast cancer patients with LVEF ≥ 50% by MUGA prior to treatment with doxorubicin-cyclophosphamide-paclitaxel-trastuzumab, LVEF independently predicted cardiac events (HF [36 patients], cardiac death [one patient]) over 7 years follow-up when prechemotherapy LVEF was <55% (i.e., 50–54%) [42]. The predictive utility of LVEF was less apparent among pediatric cancer patients in a retrospective study of 356 children followed over 5 years for possible anthracycline-induced cardiac damage which found that LVEF assessments by 2DE before and during chemotherapy detected only one patient with symptomatic HF and did not impact treatment decisions [62]. Data addressing the utility of systematic follow-up LVEF assessments in adult cancer survivors with a history of cytotoxic therapy are lacking [63]. Recent evidence, however, suggests that by the time an LVEF decline is detected after anthracyclines, recovery of LV function and attenuation of cardiac events in response to treatment may be limited [33], reinforcing the need for more sensitive measures of early myocardial damage and LV dysfunction.
Myocardial deformation imaging
There are several indices available to assess myocardial deformation, including strain, strain rate and torsion. Current echocardiographic assessments of myocardial deformation are based on tissue Doppler imaging (TDI) or speckle-tracking echocardiography (STE), the 2D tracking of unique speckle patterns created by the constructive and destructive interference of ultrasound beams within tissue, which has technical advantages compared with TDI. These speckles are tracked on a frame-by-frame basis and the accuracy of speckle tracking has been validated against sonomicrometry and tagged CMR imaging [64]. Strain reflects the global deformation of ventricular myocardium during the cardiac cycle, is typically measured at peak systole (i.e., at aortic valve closure) and can be determined in the longitudinal (GLS), radial (GRS) and circumferential (GCS) planes [64]. Beyond measuring linear deformations, peak systolic LV torsion by STE can also measure myocardial rotational deformation due to helical orientation of the myocardial fibers, by calculating the maximum instantaneous difference between peak systolic apical rotation relative to basal rotation [65].
These parameters have emerged as more sensitive measures of subclinical LV dysfunction and may be useful approaches for the early detection of cancer therapy-induced cardiotoxicity and for following patients longitudinally [66]. In clinical studies, reduced TDI strain and strain rate revealed impaired myocardial function prior to LVEF decline [67–69] and HF symptoms [70] in patients treated with anthracycline-containing therapy although the predictive value of these parameters was not evaluated. In separate small studies using STE, Sawaya et al. [71,72] found that impaired absolute longitudinal strain (>-19%) at 3 months and Negishi et al. [73] found that relative GLS decline (≥11%) from baseline to 6 months predicted LVEF decline in breast cancer patients treated with trastuzumab with or without anthracyclines. GLS also appears to provide superior prognostic information to LVEF in noncancer populations; a recent meta-analysis showed that GLS independently predicted mortality better than LVEF in 5721 patients with underlying HF, acute myocardial infarction, valvular heart disease and cardiac amyloidosis [61]. Impairments in torsion and torsion velocities in the absence of LVEF changes have been observed 1 month after anthracycline therapy in 25 leukemia/lymphoma patients [74] and after 7 years in 35 anthracycline-treated childhood cancer survivors [75]. Finally, 3D STE is a novel modality that can comprehensively assess LV myocardial mechanics, tracking linear myocardial deformation in multiple dimensions simultaneously as well as torsion and mechanical dyssynchrony, and has demonstrated early promise in this context, with sensitive detection of altered LV mechanics in childhood cancer survivors treated with anthracyclines [76].
Despite their potential, echocardiographic myocardial deformation indices have important considerations currently limiting their widespread use in cardio-oncology. The optimal timing for assessments in relation to chemotherapy completion and cut-off values for positive tests remain undetermined. Strain echocardiography is dependent on high quality images, has variability related to different vendor acquisition and analysis platforms, and uncertainty regarding the optimal parameter of myocardial deformation and inter-institutional reproducibility [35]. However, longitudinal strain has been demonstrated to be more reproducible [77] and the emerging evidence suggests myocardial deformation indices have a potential role for early detection of therapy-related cardiotoxicity that merits further investigation and validation in large cohort studies.
Cardiac MRI
Cardiac MRI has an emerging role in the detection and prognosis of chemotherapy-related cardiotoxicity largely due to its unique ability to directly assess tissue characteristics of the myocardium, such as inflammation/edema, fibrosis and extracellular volume. Hyperemia, as assessed by increased myocardial signal early after contrast administration, on T1-weighted imaging may detect acute myocardial inflammation; among 22 patients receiving anthracyclines, an increase in T1-weighted myocardial signal early after gadolinium administration of ≥5 relative to skeletal muscle from baseline to day 3 of chemotherapy predicted LVEF decline from 67.5 to 51.4% at 28 days [78]. Delayed enhancement imaging has been extensively validated for the detection of myocardial fibrosis, a recognized finding from necropsy studies of anthracycline-induced cardiomyopathy [79]. However, findings using this technique have been mixed; a series of studies from one group reported a strong association of late gadolinium enhancement in the subepicardial lateral wall and cardiotoxicity among anthracycline-trastuzumab-treated HER2+ breast cancer patients [80–82] whereas other studies have found minimal or no late gadolinium enhancement in anthracycline-treated patients [83,84]. T1 mapping is a relatively novel technique with the potential to quantify myocardial extracellular volume which is increased in patients after anthracycline-based chemotherapy, compatible with histopathologic findings [79] and associated with LV dysfunction [85] and reduced peak exercise oxygen consumption (i.e., aerobic capacity) (VO2peak) on functional capacity testing [86] in recent small studies.
Imaging with CMR also provides novel insights on cardiovascular structure and function that are incremental to information provided by other detection modalities. Decreases in LV mass, likely due to anthracycline-induced myocyte loss, correlated with cardiovascular death and decompensated HF over more than 7-year median follow-up among 91 patients with reduced LVEF post-anthracycline therapy [83]. A significant increase in aortic stiffness detected by an increase in pulse wave velocity in patients up to 6 months after receipt of anthracyclines may be associated with vascular injury and a higher risk of future cardiovascular events [52,84]. In summary, CMR may be useful to assess cardiovascular function after chemotherapy and offers a variety of techniques potentially well suited to the study of chemotherapy-induced cardiotoxicity; however, data regarding its diagnostic and prognostic utility in this setting remain limited to small studies and consideration of cost, ease of use and availability issues is warranted.
Nuclear cardiology
New radiopharmaceuticals and technical advances in scintigraphy have contributed to an evolution in nuclear cardiac imaging for cancer patients starting from MUGA, used to quantitate LVEF, to higher order functional techniques capable of visualizing pathophysiologic and neurophysiologic processes at the cardiac tissue level. Molecular imaging techniques including 111In-antimyosin (marker specific for myocardial cell necrosis) and 123I-metaiodobenzylguanidine (imaging the efferent sympathetic nervous innervation) have demonstrated potential as early predictors of chemotherapy-induced cardiotoxicity in anthracycline-treated patients. In one study, 111In-antimyosin uptake was more intense in patients with low LVEF and correlated with LVEF values [87] while increased uptake at intermediate cumulative doses identified patients at risk for cardiotoxicity before LVEF deterioration [88]. The same group showed that patients with more intense 111In-antimyosin uptake at intermediate doses tended to have more severe cardiac functional impairment at maximal cumulative doses [89]. Another small prospective study found that at low doxorubicin and epirubicin dose levels, 111In-antimyosin uptake occurred in the absence of LV systolic and diastolic dysfunction suggesting that 111In-antimyosin is very sensitive in detecting myocyte damage that precedes LV dysfunction [90]. Declines in 123I-metaiodobenzylguanidine uptake have also demonstrated correlation with higher cumulative anthracycline doses and preceded changes in LVEF [89,91]. Nevertheless, despite promising results, these techniques, which were investigated more than a decade ago, have still not been integrated into standard clinical practice.
Stress-related functional testing
Cancer therapy is associated with reduced cardiovascular reserve attributed to either the direct effects of therapy or the indirect effects of therapy-associated lifestyle changes [11,92]. Application of ‘system stress’ (via pharmacologic or exercise) is an established method to detect subclinical impairments in myocardial function and determination of contractile reserve is an independent predictor of prognosis beyond LVEF in patients with coronary artery disease, although these methods have received limited attention in cancer therapy-related cardiotoxicity [54]. Using exercise stress echocardiography in 57 asymptomatic early-stage breast cancer survivors with resting LVEF ≥50%, we found that change in LV stroke volume and cardiac index (from rest) were significantly reduced by 12 and 24%, respectively, compared with controls, suggesting that patients have impaired LV contractile reserve (LVCR) [93]. McKillop et al. [94] examined radionuclide-determined LVEF at rest and during graded exercise testing in 37 patients receiving doxorubicin; exercise LVEF improved the sensitivity for detection of cardiotoxicity from 58 to 100%. Civelli et al. [95] measured LVCR (defined as the difference between peak and resting LVEF) with low-dose dobutamine during and after high-dose chemotherapy in women with advanced breast cancer; an asymptomatic decline in LVCR of ≥5 units from baseline predicted LVEF decline to <50%. By comparison, multiple exercise and pharmacologic stress studies have been performed in anthracycline-treated adult survivors of pediatric malignancies with mixed results regarding the incremental sensitivity of exercise [96–99] or pharmacologic [100,101] stress echocardiography to detect subclinical therapy-induced cardiotoxicity over resting echocardiography alone.
Cancer therapy-related cardiac damage also occurs in conjunction with (mal) adaptation in other organ components [11,54,84,86]. Thus, tools with the ability to evaluate integrated crosstalk between cardiovascular organ components, like VO2peak, may provide a more comprehensive measure of therapy-related global cardiovascular effects in the setting of cancer [54]. As a measure of global cardiovascular function and reserve capacity, VO2peak is also inversely correlated with cardiovascular and all-cause mortality in a broad range of adult populations, including lung cancer [102–105]. In breast cancer, Jones et al. [106] found that despite preserved resting LVEF ≥50%, 130 patients, on average 3 years following the completion of adjuvant therapy, had VO2peak that was 22% below that of sedentary age-matched women without a history of breast cancer. Studies investigating the prognostic value of VO2peak to predict acute and late-occurring cardiac dysfunction and other cardiovascular events in cancer patients are warranted.
Blood-based biomarkers
Blood-based cardiac biomarkers, specifically troponins, have emerged as potentially useful noninvasive markers of cardiotoxicity in cancer based on the findings of numerous small studies. For a comprehensive overview of the utility of blood-based biomarkers for early detection of cardiotoxicity, the reader is referred to prior reviews [107,108]. In brief, a transient rise in cardiac troponin I has been demonstrated to predict the occurrence [109,110] as well as the magnitude of LVEF decline [110–112] in patients with hematologic and solid malignancies receiving high-dose anthracyclines (Table 2). In women receiving anthracycline-trastuzumab-containing therapy, detectable troponin I levels (>0.08 ng/ml) were associated with a 23-fold increased risk of ‘cardiotoxicity’ (LVEF decline >10 to <50%) and an approximately threefold increased risk of LVEF irreversibility following drug discontinuation [113]. Early changes in high- and ultra-sensitive troponin I have shown incremental prognostic power, particularly in combination with STE, to predict HF in early breast cancer patients treated with anthracycline-trastuzumab therapy [71,72]. However, as reported by Sawaya et al. [72], the sensitivity and specificity of ultra-sensitive troponin I for predicting anthracycline-trastuzumab cardiotoxicity are not high at 48% (95% CI: 27–69) and 73% (95% CI: 59–84), respectively, and the clinical utility of troponin measurements is not as well established for other chemotherapeutic regimens [114]. The family of natriuretic peptides (e.g., brain natriuretic peptide, N-terminal pro-brain natriuretic peptide and N-terminal pro-atrial natriuretic peptide) appears to be less reliable than troponins in predicting LVEF decline in the oncology setting [107,115].
Table 2. . Troponin for early detection and prediction of cancer therapy-related cardiotoxicity.
|
Study (year) |
Patient population |
Treatment |
n |
Detection |
Cut-off |
%BM+ |
LVEF modality |
Mean ↓LVEF |
Incidence of ↓LVEF >10% or LVEF < 50% |
Incidence of HF symptoms |
BM predicted (P sig) |
Ref. |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| BM+ | BM- | BM+ | BM- | BM+ | BM- | ↓LVEF | HF | |||||||||
| Sawaya et al. (2011) |
Breast |
ANT, H |
43 |
hsTnI |
0.015 µg/l |
28 |
Echo |
– |
– |
50% |
10% |
– |
– |
Yes |
– |
[71] |
| Sawaya et al. (2012) |
Breast |
ANT, H |
81 |
usTnI |
30 pg/ml |
14 |
Echo |
– |
– |
42% |
27% |
8% |
5% |
Yes |
– |
[72] |
| Fallah-Rad et al. (2011) |
Breast |
FEC, H AC, H |
42 |
cTnT |
0.01 ng/ml |
0 |
Echo |
– |
˜4% |
– |
24% |
– |
24% |
No |
No |
[81] |
| Cardinale et al. (2000) |
Multiple diagnoses |
HDC |
204 |
cTnI |
0.4 ng/ml |
32 |
Echo |
>10% |
˜0% |
29% |
0% |
5% |
0% |
Yes |
Yes† |
[109] |
| Cardinale et al. (2004) |
Multiple diagnoses |
HDC |
703 |
cTnI |
0.08 ng/ml |
30 |
Echo |
– |
– |
71% |
2% |
22% |
0.2% |
Yes |
Yes‡ |
[110] |
| Cardinale et al. (2002) |
Breast |
HDC |
211 |
cTnI |
0.5 ng/ml |
33 |
Echo |
>15% |
˜0% |
– |
– |
14% |
0% |
Yes |
– |
[111] |
| Sandri et al. (2003) |
Multiple diagnoses |
HDC |
179 |
cTnI |
0.08 µg/ml |
32 |
Echo |
18% |
3% |
– |
– |
– |
– |
Yes |
– |
[112] |
| Cardinale et al. (2010) |
Breast |
H |
251 |
cTnI |
0.08 ng/ml |
14 |
Echo |
– |
72% |
7% |
19% |
0% |
Yes |
Yes‡ |
|
[113] |
| Morris et al. (2011) | Breast | ddAC→THL | 95 | cTnI | 0.04–0.06 ng/ml | 67 | RNA | – | – | 5% | 19% | 2% | 7% | No | No | [114] |
†All patients (n = 3) developing HF during study were cTnI+.
‡Elevated cTnI predicted composite cardiac events, including heart failure.
↓: Decline or impairment; Δ: Change; AC: Doxorubicin and cyclophosphamide; ANT: Anthracyclines; BM: Biomarker; cTnI: Cardiac troponin I; cTnT: Cardiac troponin T; dd: Dose dense; FEC: Fluorouracil, epirubicin, cyclophosphamide; H: Trastuzumab; HDC: High-dose chemotherapy; HF: Heart failure; hsTnI: High-sensitivity troponin I; L: Lapatinib; LVEF: Left ventricular ejection fraction; RNA: Radionuclide angiography; T: Paclitaxel; usTnI: Ultra-sensitive troponin I.
Overall, the studies of troponins and natriuretic peptides do have notable limitations. The predictive role of these biomarkers has been investigated in small studies with heterogeneous cancer populations receiving a variety of cytotoxic and targeted therapies [107]. Moreover, standardization of timing of assessments, measurement assays and cut-off points remain undetermined, currently limiting translation into clinical practice.
Conclusion
The current state of the art for detection in cardio-oncology with resting LVEF surveillance is insensitive for identification of cardiotoxicity. Many alternative techniques have been proposed including advanced cardiac imaging modalities, functional capacity testing and blood-based biomarkers but no best approach or combination of approaches has yet to clearly emerge. Research prospectively evaluating the optimal timing and frequency of alternative detection techniques and their prognostic utility is lacking, limiting translation into routine clinical use. Large prospective, multi-institutional studies are needed to determine whether these techniques can be used practically to improve not only detection of cardiotoxicity but also prediction of cardiovascular and overall survival, thereby, informing oncologic and cardiac treatment decisions and facilitating early interventions that may reduce risk of downstream cardiovascular morbidity without compromising the efficacy of anticancer therapy.
Future perspective
Novel imaging and blood-based biomarkers, likely in combination, will become increasingly prevalent in clinical practice for the monitoring and surveillance of cancer patients on treatment and cancer survivors. Research efforts will evolve from the current evidence base dominated by small, single-center studies to more prospective, multi-institutional studies aimed at: delineating the short- and long-term natural history of positive blood and imaging biomarkers and their relationship with clinical events, determining the optimal strategy (type of test, timing, frequency and cut-off points) for detection of early subclinical cardiac and cardiovascular dysfunction in patients receiving conventional and/or novel anticancer therapies, and performing adequately-powered multicenter randomized control trials comparing the efficacy of different strategies to individualize anticancer drug therapy and prevent the development of cardiovascular disease. Ultimately, with an evolving understanding and effective use of these modalities, there is the potential to mitigate cardiac and cardiovascular morbidity and mortality, and improve overall survival in cancer.
EXECUTIVE SUMMARY.
Background
Mortality rates from cancer have declined over the past 30 years and the number of cancer survivors is projected to increase significantly.
With increasing survival, patients are increasingly developing cardiovascular risk factors and cardiovascular disease has emerged to rival cancer as the predominant cause of mortality, particularly among older survivors.
Highly effective conventional and novel anticancer therapeutic agents also have the potential to cause myocardial and cardiovascular injury (i.e., cardiotoxicity) during treatment and may contribute to short- and long-term cardiovascular morbidity and mortality.
Current state of cardio-oncology
The growing importance of anticancer therapy-related cardiotoxicity and its early detection is recognized by cardiology and oncology societies.
The cardio-oncology field has emerged to keep pace with the rapid evolution of cancer therapies and their cardiovascular side effects.
Current state of detection of cardiotoxicity
Surveillance resting left ventricular ejection fraction (LVEF) is the current state of the art for detection of cardiotoxicity.
LVEF, which may be affected by changes in loading conditions and does not account for therapy-induced cardiovascular damage beyond the heart, has limited sensitivity for early detection of cardiotoxicity.
Resting left ventricular ejection fraction
2D echocardiography and multiple-gated acquisition scanning are the predominant modalities currently used for resting evaluation of ejection fraction.
3D echocardiography and cardiac MRI are newer modalities with improved accuracy and reproducibility for LVEF and the potential for detecting small changes in contractility, although availability primarily affects widespread application of these modalities in clinical practice
Myocardial deformation imaging
Myocardial deformation indices (strain, strain rate, torsion) can be measured (most commonly by speckle-tracking echocardiography) and their utility as more sensitive measures of subclinical left ventricular dysfunction has been assessed in small studies.
Global longitudinal strain has been studied most extensively but the optimal deformation parameter for early detection and prediction of cardiotoxicity remains uncertain.
Widespread use of deformation imaging in cardio-oncology has also been limited due to lack of standardization of optimal timing of assessments, cut-off values and variability related to multiple vendor acquisition-analysis platforms.
Cardiac MRI
Cardiac MRI has an emerging role in the detection of cancer therapy-induced cardiotoxicity due to its accuracy for ventricular volumes and ejection fraction and its unique ability to directly assess tissue characteristics of the myocardium.
Nuclear cardiology
Multiple-gated acquisition scanning is well established for its reproducibility quantitating LVEF but provides limited further assessment of cardiac structure and function.
Molecular imaging techniques have demonstrated promising results for early prediction of cardiotoxicity in anthracycline-treated patients but remain outside standard clinical practice.
Exercise-related testing
Cancer therapy has direct and indirect adverse effects on the cardiovascular system and is associated with (mal) adaptation in other organ components within the cardiovascular–pulmonary–hematologic–musculoskeletal system axis.
Functional capacity testing aimed at determination of left ventricular contractile reserve capacity and/or cardiorespiratory respiratory fitness (VO2peak) have demonstrated impairments in cancer patients despite normal resting ejection fraction and may provide a more comprehensive assessment of the global effects of cancer therapy.
Blood biomarkers
Blood-based cardiac biomarkers, specifically troponins, have emerged as potentially useful noninvasive markers of cardiotoxicity in cancer in small studies, although standardization of timing of assessments, measurement assays and cut-off points remain undetermined, limiting translation into clinical practice thus far.
Conclusion
Current standard detection modalities in cardio-oncology (i.e., resting ejection fraction) are insensitive for cardiotoxicity.
Emerging imaging modalities that assess myocardial deformation, functional capacity testing and blood-based biomarkers have potential to improve early detection.
However, evidence with these modalities remains based on small, single-center studies and future investigation with large, prospective studies is required to validate their diagnostic and prognostic utility, determine optimal timing of assessments and standardize cut-off points.
An ultimate goal for cardio-oncology will be improved detection of cardiotoxicity that informs and facilitates how best to balance cancer and cardiovascular treatments and outcomes.
Footnotes
Financial & competing interests disclosure
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as: • of interest
- 1.Jemal A, Ward E, Hao Y, Thun M. Trends in the leading causes of death in the United States, 1970–2002. JAMA. 2005;294(10):1255–1259. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward E, Thun M. Declining death rates reflect progress against cancer. PLoS ONE. 2010;5(3):e9584. doi: 10.1371/journal.pone.0009584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Howlader N, Ries LA, Mariotto AB, Reichman ME, Ruhl J, Cronin KA. Improved estimates of cancer-specific survival rates from population-based data. J. Natl Cancer Inst. 2010;102(20):1584–1598. doi: 10.1093/jnci/djq366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Siegel R, Desantis C, Virgo K, et al. Cancer treatment and survivorship statistics, 2012. CA Cancer J. Clin. 2012;62(4):220–241. doi: 10.3322/caac.21149. [DOI] [PubMed] [Google Scholar]
- 5.Chapman JA, Meng D, Shepherd L, et al. Competing causes of death from a randomized trial of extended adjuvant endocrine therapy for breast cancer. J. Natl Cancer Inst. 2008;100(4):252–260. doi: 10.1093/jnci/djn014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, et al. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. J. Clin. Oncol. 2007;25(31):4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 7.Lloyd-Jones DM, Leip EP, Larson MG, et al. Prediction of lifetime risk for cardiovascular disease by risk factor burden at 50 years of age. Circulation. 2006;113(6):791–798. doi: 10.1161/CIRCULATIONAHA.105.548206. [DOI] [PubMed] [Google Scholar]
- 8.Schairer C, Mink PJ, Carroll L, Devesa SS. Probabilities of death from breast cancer and other causes among female breast cancer patients. J. Natl Cancer Inst. 2004;96(17):1311–1321. doi: 10.1093/jnci/djh253. [DOI] [PubMed] [Google Scholar]
- 9.Patnaik JL, Byers T, Diguiseppi C, Dabelea D, Denberg TD. Cardiovascular disease competes with breast cancer as the leading cause of death for older females diagnosed with breast cancer: a retrospective cohort study. Breast Cancer Res. 2011;13(3):R64. doi: 10.1186/bcr2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Colzani E, Liljegren A, Johansson AL, et al. Prognosis of patients with breast cancer: causes of death and effects of time since diagnosis, age, and tumor characteristics. J. Clin. Oncol. 2011;29(30):4014–4021. doi: 10.1200/JCO.2010.32.6462. [DOI] [PubMed] [Google Scholar]
- 11.Jones LW, Haykowsky MJ, Swartz JJ, Douglas PS, Mackey JR. Early breast cancer therapy and cardiovascular injury. J. Am. Coll. Cardiol. 2007;50(15):1435–1441. doi: 10.1016/j.jacc.2007.06.037. [DOI] [PubMed] [Google Scholar]
- 12.Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J. Am. Coll. Cardiol. 2009;53(24):2231–2247. doi: 10.1016/j.jacc.2009.02.050. [DOI] [PubMed] [Google Scholar]
- 13.Chu TF, Rupnick MA, Kerkela R, et al. Cardiotoxicity associated with tyrosine kinase inhibitor sunitinib. Lancet. 2007;370(9604):2011–2019. doi: 10.1016/S0140-6736(07)61865-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hall PS, Harshman LC, Srinivas S, Witteles RM. The frequency and severity of cardiovascular toxicity from targeted therapy in advanced renal cell carcinoma patients. JACC Heart Fail. 2013;1(1):72–78. doi: 10.1016/j.jchf.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 15.Von Hoff DD, Layard MW, Basa P, et al. Risk factors for doxorubicin-induced congestive heart failure. Ann. Intern. Med. 1979;91(5):710–717. doi: 10.7326/0003-4819-91-5-710. [DOI] [PubMed] [Google Scholar]
- 16.Swain SM, Whaley FS, Ewer MS. Congestive heart failure in patients treated with doxorubicin: a retrospective analysis of three trials. Cancer. 2003;97(11):2869–2879. doi: 10.1002/cncr.11407. [DOI] [PubMed] [Google Scholar]
- 17.Perez EA, Suman VJ, Davidson NE, et al. Effect of doxorubicin plus cyclophosphamide on left ventricular ejection fraction in patients with breast cancer in the North Central Cancer Treatment Group N9831 Intergroup Adjuvant Trial. J. Clin. Oncol. 2004;22(18):3700–3704. doi: 10.1200/JCO.2004.03.516. [DOI] [PubMed] [Google Scholar]
- 18.Meinardi MT, Van Veldhuisen DJ, Gietema JA, et al. Prospective evaluation of early cardiac damage induced by epirubicin-containing adjuvant chemotherapy and locoregional radiotherapy in breast cancer patients. J. Clin. Oncol. 2001;19(10):2746–2753. doi: 10.1200/JCO.2001.19.10.2746. [DOI] [PubMed] [Google Scholar]
- 19.Mackey JR, Martin M, Pienkowski T, et al. Adjuvant docetaxel, doxorubicin, and cyclophosphamide in node-positive breast cancer: 10 year follow-up of the phase 3 randomised BCIRG 001 trial. Lancet Oncol. 2013;14(1):72–80. doi: 10.1016/S1470-2045(12)70525-9. [DOI] [PubMed] [Google Scholar]
- 20.Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br. J. Haematol. 2005;131(5):561–578. doi: 10.1111/j.1365-2141.2005.05759.x. [DOI] [PubMed] [Google Scholar]
- 21.Schmidinger M, Zielinski CC, Vogl UM, et al. Cardiac toxicity of sunitinib and sorafenib in patients with metastatic renal cell carcinoma. J. Clin. Oncol. 2008;26(32):5204–5212. doi: 10.1200/JCO.2007.15.6331. [DOI] [PubMed] [Google Scholar]
- 22.Tocchetti CG, Ragone G, Coppola C, et al. Detection, monitoring, and management of trastuzumab-induced left ventricular dysfunction: an actual challenge. Eur. J. Heart Fail. 2012;14(2):130–137. doi: 10.1093/eurjhf/hfr165. [DOI] [PubMed] [Google Scholar]
- 23.Force T, Kolaja KL. Cardiotoxicity of kinase inhibitors: the prediction and translation of preclinical models to clinical outcomes. Nat. Rev. Drug Discov. 2011;10(2):111–126. doi: 10.1038/nrd3252. [DOI] [PubMed] [Google Scholar]
- 24.Ky B, Vejpongsa P, Yeh ET, Force T, Moslehi JJ. Emerging paradigms in cardiomyopathies associated with cancer therapies. Circ. Res. 2013;113(6):754–764. doi: 10.1161/CIRCRESAHA.113.300218. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Outlines the proposed mechanisms of cardiotoxicity of common and newer anticancer agents.
- 25.Tan-Chiu E, Yothers G, Romond E, et al. Assessment of cardiac dysfunction in a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel, with or without trastuzumab as adjuvant therapy in node-positive, human epidermal growth factor receptor 2-overexpressing breast cancer: NSABP B-31. J. Clin. Oncol. 2005;23(31):7811–7819. doi: 10.1200/JCO.2005.02.4091. [DOI] [PubMed] [Google Scholar]
- 26.Perez EA, Rodeheffer R. Clinical cardiac tolerability of trastuzumab. J. Clin. Oncol. 2004;22(2):322–329. doi: 10.1200/JCO.2004.01.120. [DOI] [PubMed] [Google Scholar]
- 27.Cheng H, Force T. Molecular mechanisms of cardiovascular toxicity of targeted cancer therapeutics. Circ. Res. 2010;106(1):21–34. doi: 10.1161/CIRCRESAHA.109.206920. [DOI] [PubMed] [Google Scholar]
- 28.Telli ML, Hunt SA, Carlson RW, Guardino AE. Trastuzumab-related cardiotoxicity: calling into question the concept of reversibility. J. Clin. Oncol. 2007;25(23):3525–3533. doi: 10.1200/JCO.2007.11.0106. [DOI] [PubMed] [Google Scholar]
- 29.Common Terminology Criteria for Adverse Events V4.03 (CTCAE) http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
- 30.Seidman A, Hudis C, Pierri MK, et al. Cardiac dysfunction in the trastuzumab clinical trials experience. J. Clin. Oncol. 2002;20(5):1215–1221. doi: 10.1200/JCO.2002.20.5.1215. [DOI] [PubMed] [Google Scholar]
- 31.Yancy CW, Jessup M, Bozkurt B, et al. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 2013;62(16):e147–E239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 32.Ammar KA, Jacobsen SJ, Mahoney DW, et al. Prevalence and prognostic significance of heart failure stages: application of the American College of Cardiology/American Heart Association heart failure staging criteria in the community. Circulation. 2007;115(12):1563–1570. doi: 10.1161/CIRCULATIONAHA.106.666818. [DOI] [PubMed] [Google Scholar]
- 33.Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J. Am. Coll. Cardiol. 2010;55(3):213–220. doi: 10.1016/j.jacc.2009.03.095. [DOI] [PubMed] [Google Scholar]; • Demonstrates the importance of early detection of cardiotoxicity as an opportunity for intervention and reversibility of cardiotoxicity.
- 34.Lenihan DJ, Sawyer DB. Heart disease in cancer patients: a burgeoning field where optimizing patient care is requiring interdisciplinary collaborations. Heart Fail. Clin. 2011;7(3):xxi–xxiii. doi: 10.1016/j.hfc.2011.04.001. [DOI] [PubMed] [Google Scholar]
- 35.Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Standardizecardiography and the European Association of Cardiovascular Imaging. J. Am. Soc. Standardizecardiogr. 2014;27(9):911–939. doi: 10.1016/j.echo.2014.07.012. [DOI] [PubMed] [Google Scholar]
- 36.Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126(23):2749–2763. doi: 10.1161/CIRCULATIONAHA.112.100560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Curigliano G, Cardinale D, Suter T, et al. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann. Oncol. 2012;23(Suppl. 7):vii155–vii166. doi: 10.1093/annonc/mds293. [DOI] [PubMed] [Google Scholar]
- 38.Verma S, Ewer MS. Is cardiotoxicity being adequately assessed in current trials of cytotoxic and targeted agents in breast cancer. Ann. Oncol. 2011;22(5):1011–1018. doi: 10.1093/annonc/mdq607. [DOI] [PubMed] [Google Scholar]
- 39.Bellenger NG, Burgess MI, Ray SG, et al. Comparison of left ventricular ejection fraction and volumes in heart failure by standardizecardiography, radionuclide ventriculography and cardiovascular magnetic resonance; are they interchangeable. Eur. Heart J. 2000;21(16):1387–1396. doi: 10.1053/euhj.2000.2011. [DOI] [PubMed] [Google Scholar]
- 40.Ewer MS, Lenihan DJ. Left ventricular ejection fraction and cardiotoxicity: is our ear really to the ground. J. Clin. Oncol. 2008;26(8):1201–1203. doi: 10.1200/JCO.2007.14.8742. [DOI] [PubMed] [Google Scholar]
- 41.Trastuzumab (Herceptin®) Genentech, Inc.; CA, USA: package insert. [Google Scholar]
- 42.Romond EH, Jeong JH, Rastogi P, et al. Seven-year follow-up assessment of cardiac function in NSABP B-31, a randomized trial comparing doxorubicin and cyclophosphamide followed by paclitaxel (ACP) with ACP plus trastuzumab as adjuvant therapy for patients with node-positive, human epidermal growth factor receptor 2-positive breast cancer. J. Clin. Oncol. 2012;30(31):3792–3799. doi: 10.1200/JCO.2011.40.0010. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates the prognostic value of baseline left ventricular ejection fraction (LVEF) in predicting longer-term cardiotoxicity.
- 43.Romond EH, Perez EA, Bryant J, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N. Engl. J. Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 44.Slamon D, Eiermann W, Robert N, et al. Adjuvant trastuzumab in HER2-positive breast cancer. N. Engl. J. Med. 2011;365(14):1273–1283. doi: 10.1056/NEJMoa0910383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmitz KH, Prosnitz RG, Schwartz AL, Carver JR. Prospective surveillance and management of cardiac toxicity and health in breast cancer survivors. Cancer. 2012;118(8 Suppl.):2270–2276. doi: 10.1002/cncr.27462. [DOI] [PubMed] [Google Scholar]
- 46.Ewer MS, Ali MK, Mackay B, et al. A comparison of cardiac biopsy grades and ejection fraction estimations in patients receiving Adriamycin. J. Clin. Oncol. 1984;2(2):112–117. doi: 10.1200/JCO.1984.2.2.112. [DOI] [PubMed] [Google Scholar]
- 47.Delgado V, Mollema SA, Ypenburg C, et al. Relation between global left ventricular longitudinal strain assessed with novel automated function imaging and biplane left ventricular ejection fraction in patients with coronary artery disease. J. Am. Soc. Echocardiogr. 2008;21(11):1244–1250. doi: 10.1016/j.echo.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 48.Mann DL, Bristow MR. Mechanisms and models in heart failure: the biomechanical model and beyond. Circulation. 2005;111(21):2837–2849. doi: 10.1161/CIRCULATIONAHA.104.500546. [DOI] [PubMed] [Google Scholar]
- 49.Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J. Clin. Oncol. 2005;23(34):8597–8605. doi: 10.1200/JCO.2005.02.5841. [DOI] [PubMed] [Google Scholar]
- 50.Jones LW, Eves ND, Haykowsky M, Freedland SJ, Mackey JR. Exercise intolerance in cancer and the role of exercise therapy to reverse dysfunction. Lancet Oncol. 2009;10(6):598–605. doi: 10.1016/S1470-2045(09)70031-2. [DOI] [PubMed] [Google Scholar]
- 51.Lakoski SG, Eves ND, Douglas PS, Jones LW. Exercise rehabilitation in patients with cancer. Nat. Rev. Clin. Oncol. 2012;9(5):288–296. doi: 10.1038/nrclinonc.2012.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chaosuwannakit N, D'agostino R, Jr, Hamilton CA, et al. Aortic stiffness increases upon receipt of anthracycline chemotherapy. J. Clin. Oncol. 2010;28(1):166–172. doi: 10.1200/JCO.2009.23.8527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Beckman JA, Thakore A, Kalinowski BH, Harris JR, Creager MA. Radiation therapy impairs endothelium-dependent vasodilation in humans. J. Am. Coll. Cardiol. 2001;37(3):761–765. doi: 10.1016/s0735-1097(00)01190-6. [DOI] [PubMed] [Google Scholar]
- 54.Koelwyn GJ, Khouri M, Mackey JR, Douglas PS, Jones LW. Running on empty: cardiovascular reserve capacity and late effects of therapy in cancer survivorship. J. Clin. Oncol. 2012;30(36):4458–4461. doi: 10.1200/JCO.2012.44.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional standardizecardiography in breast cancer: comparison with two-dimensional standardizecardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J. Clin. Oncol. 2010;28(21):3429–3436. doi: 10.1200/JCO.2009.26.7294. [DOI] [PubMed] [Google Scholar]
- 56.Thavendiranathan P, Grant AD, Negishi T, Plana JC, Popovic ZB, Marwick TH. Reproducibility of standardizecardiographic techniques for sequential assessment of left ventricular ejection fraction and volumes: application to patients undergoing cancer chemotherapy. J. Am. Coll. Cardiol. 2013;61(1):77–84. doi: 10.1016/j.jacc.2012.09.035. [DOI] [PubMed] [Google Scholar]; • Reports the temporal variability of LVEF assessments by 2D echocardiography in cancer patients being serially monitored during therapy.
- 57.Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82(1):154–163. doi: 10.1161/01.cir.82.1.154. [DOI] [PubMed] [Google Scholar]
- 58.Grothues F, Smith GC, Moon JC, et al. Comparison of interstudy reproducibility of cardiovascular magnetic resonance with two-dimensional standardizecardiography in normal subjects and in patients with heart failure or left ventricular hypertrophy. Am. J. Cardiol. 2002;90(1):29–34. doi: 10.1016/s0002-9149(02)02381-0. [DOI] [PubMed] [Google Scholar]
- 59.Sandner TA, Houck P, Runge VM, et al. Accuracy of accelerated cine MR imaging at 3 Tesla in longitudinal follow-up of cardiac function. Eur. Radiol. 2008;18(10):2095–2101. doi: 10.1007/s00330-008-0993-y. [DOI] [PubMed] [Google Scholar]
- 60.Mcmurray JJ, Adamopoulos S, Anker SD, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2012;33(14):1787–1847. doi: 10.1093/eurheartj/ehs104. [DOI] [PubMed] [Google Scholar]
- 61.Kalam K, Otahal P, Marwick TH. Prognostic implications of global LV dysfunction: a systematic review and meta-analysis of global longitudinal strain and ejection fraction. Heart. 2014;100(21):1673–1680. doi: 10.1136/heartjnl-2014-305538. [DOI] [PubMed] [Google Scholar]
- 62.Watts RG, George M, Johnson WH., Jr Pretreatment and routine standardizecardiogram monitoring during chemotherapy for anthracycline-induced cardiotoxicity rarely identifies significant cardiac dysfunction or alters treatment decisions: a 5-year review at a single pediatric oncology center. Cancer. 2012;118(7):1919–1924. doi: 10.1002/cncr.26481. [DOI] [PubMed] [Google Scholar]
- 63.Carver JR, Shapiro CL, Ng A, et al. American Society of Clinical Oncology clinical evidence review on the ongoing care of adult cancer survivors: cardiac and pulmonary late effects. J. Clin. Oncol. 2007;25(25):3991–4008. doi: 10.1200/JCO.2007.10.9777. [DOI] [PubMed] [Google Scholar]
- 64.Gorcsan J, 3rd, Tanaka H. Standardizecardiographic assessment of myocardial strain. J. Am. Coll. Cardiol. 2011;58(14):1401–1413. doi: 10.1016/j.jacc.2011.06.038. [DOI] [PubMed] [Google Scholar]
- 65.Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc. Imaging. 2008;1(3):366–376. doi: 10.1016/j.jcmg.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 66.Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by standardizecardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J. Am. Coll. Cardiol. 2014;63(25 Pt A):2751–2768. doi: 10.1016/j.jacc.2014.01.073. [DOI] [PubMed] [Google Scholar]
- 67.Ganame J, Claus P, Uyttebroeck A, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J. Am. Soc. Echocardiogr. 2007;20(12):1351–1358. doi: 10.1016/j.echo.2007.04.007. [DOI] [PubMed] [Google Scholar]
- 68.Jurcut R, Wildiers H, Ganame J, et al. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J. Am. Soc. Echocardiogr. 2008;21(12):1283–1289. doi: 10.1016/j.echo.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 69.Hare JL, Brown JK, Leano R, Jenkins C, Woodward N, Marwick TH. Use of myocardial deformation imaging to detect preclinical myocardial dysfunction before conventional measures in patients undergoing breast cancer treatment with trastuzumab. Am. Heart J. 2009;158(2):294–301. doi: 10.1016/j.ahj.2009.05.031. [DOI] [PubMed] [Google Scholar]
- 70.Mercuro G, Cadeddu C, Piras A, et al. Early epirubicin-induced myocardial dysfunction revealed by serial tissue Doppler standardizecardiography: correlation with inflammatory and oxidative stress markers. Oncologist. 2007;12(9):1124–1133. doi: 10.1634/theoncologist.12-9-1124. [DOI] [PubMed] [Google Scholar]
- 71.Sawaya H, Sebag IA, Plana JC, et al. Early detection and prediction of cardiotoxicity in chemotherapy-treated patients. Am. J. Cardiol. 2011;107(9):1375–1380. doi: 10.1016/j.amjcard.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sawaya H, Sebag IA, Plana JC, et al. Assessment of standardizecardiography and biomarkers for the extended prediction of cardiotoxicity in patients treated with anthracyclines, taxanes, and trastuzumab. Circ. Cardiovasc. Imaging. 2012;5(5):596–603. doi: 10.1161/CIRCIMAGING.112.973321. [DOI] [PMC free article] [PubMed] [Google Scholar]; • The largest study thus far to assess the prognostic value of strain and blood-based biomarkers for predicting cardiotoxicity.
- 73.Negishi K, Negishi T, Hare JL, Haluska BA, Plana JC, Marwick TH. Independent and incremental value of deformation indices for prediction of trastuzumab-induced cardiotoxicity. J. Am. Soc. Echocardiogr. 2013;26(5):493–498. doi: 10.1016/j.echo.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 74.Motoki H, Koyama J, Nakazawa H, et al. Torsion analysis in the early detection of anthracycline-mediated cardiomyopathy. Eur. Heart J. Cardiovasc. Imaging. 2012;13(1):95–103. doi: 10.1093/ejechocard/jer172. [DOI] [PubMed] [Google Scholar]
- 75.Cheung YF, Li SN, Chan GC, Wong SJ, Ha SY. Left ventricular twisting and untwisting motion in childhood cancer survivors. Echocardiography. 2011;28(7):738–745. doi: 10.1111/j.1540-8175.2011.01429.x. [DOI] [PubMed] [Google Scholar]
- 76.Yu HK, Yu W, Cheuk DK, Wong SJ, Chan GC, Cheung YF. New three-dimensional speckle-tracking echocardiography identifies global impairment of left ventricular mechanics with a high sensitivity in childhood cancer survivors. J. Am. Soc. Echocardiogr. 2013;26(8):846–852. doi: 10.1016/j.echo.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 77.Risum N, Ali S, Olsen NT, et al. Variability of global left ventricular deformation analysis using vendor dependent and independent two-dimensional speckle-tracking software in adults. J. Am. Soc. Echocardiogr. 2012;25(11):1195–1203. doi: 10.1016/j.echo.2012.08.007. [DOI] [PubMed] [Google Scholar]
- 78.Wassmuth R, Lentzsch S, Erdbruegger U, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging-a pilot study. Am. Heart J. 2001;141(6):1007–1013. doi: 10.1067/mhj.2001.115436. [DOI] [PubMed] [Google Scholar]
- 79.Isner JM, Ferrans VJ, Cohen SR, et al. Clinical and morphologic cardiac findings after anthracycline chemotherapy. Analysis of 64 patients studied at necropsy. Am. J. Cardiol. 1983;51(7):1167–1174. doi: 10.1016/0002-9149(83)90364-8. [DOI] [PubMed] [Google Scholar]
- 80.Wadhwa D, Fallah-Rad N, Grenier D, et al. Trastuzumab mediated cardiotoxicity in the setting of adjuvant chemotherapy for breast cancer: a retrospective study. Breast Cancer Res. Treat. 2009;117(2):357–364. doi: 10.1007/s10549-008-0260-6. [DOI] [PubMed] [Google Scholar]
- 81.Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J. Am. Coll. Cardiol. 2011;57(22):2263–2270. doi: 10.1016/j.jacc.2010.11.063. [DOI] [PubMed] [Google Scholar]
- 82.Fallah-Rad N, Lytwyn M, Fang T, Kirkpatrick I, Jassal DS. Delayed contrast enhancement cardiac magnetic resonance imaging in trastuzumab induced cardiomyopathy. J. Cardiovasc. Magn. Reson. 2008;10:5. doi: 10.1186/1532-429X-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Neilan TG, Coelho-Filho OR, Pena-Herrera D, et al. Left ventricular mass in patients with a cardiomyopathy after treatment with anthracyclines. Am. J. Cardiol. 2012;110(11):1679–1686. doi: 10.1016/j.amjcard.2012.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Drafts BC, Twomley KM, D'agostino R, Jr, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc. Imaging. 2013;6(8):877–885. doi: 10.1016/j.jcmg.2012.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Neilan TG, Coelho-Filho OR, Shah RV, et al. Myocardial extracellular volume by cardiac magnetic resonance imaging in patients treated with anthracycline-based chemotherapy. Am. J. Cardiol. 2013;111(5):717–722. doi: 10.1016/j.amjcard.2012.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Tham EB, Haykowsky MJ, Chow K, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J. Cardiovasc. Magn. Reson. 2013;15:48. doi: 10.1186/1532-429X-15-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Estorch M, Carrio I, Berna L, et al. Indium-111-antimyosin scintigraphy after doxorubicin therapy in patients with advanced breast cancer. J. Nucl. Med. 1990;31(12):1965–1969. [PubMed] [Google Scholar]
- 88.Carrio I, Lopez-Pousa A, Estorch M, et al. Detection of doxorubicin cardiotoxicity in patients with sarcomas by indium-111-antimyosin monoclonal antibody studies. J. Nucl. Med. 1993;34(9):1503–1507. [PubMed] [Google Scholar]
- 89.Carrio I, Estorch M, Berna L, Lopez-Pousa J, Tabernero J, Torres G. Indium-111-antimyosin and iodine-123-MIBG studies in early assessment of doxorubicin cardiotoxicity. J. Nucl. Med. 1995;36(11):2044–2049. [PubMed] [Google Scholar]
- 90.Valdes Olmos RA, Carrio I, Hoefnagel CA, et al. High sensitivity of radiolabelled antimyosin scintigraphy in assessing anthracycline related early myocyte damage preceding cardiac dysfunction. Nucl. Med. Commun. 2002;23(9):871–877. doi: 10.1097/00006231-200209000-00011. [DOI] [PubMed] [Google Scholar]
- 91.Olmos RA, Ten Bokkel Huinink WW, Ten Hoeve RF, et al. Assessment of anthracycline-related myocardial adrenergic derangement by [123I]metaiodobenzylguanidine scintigraphy. Eur. J. Cancer. 1995;31A(1):26–31. doi: 10.1016/0959-8049(94)00357-b. [DOI] [PubMed] [Google Scholar]
- 92.Lakoski SG, Barlow CE, Koelwyn GJ, et al. The influence of adjuvant therapy on cardiorespiratory fitness in early-stage breast cancer seven years after diagnosis: the Cooper Center Longitudinal Study. Breast Cancer Res. Treat. 2013;138(3):909–916. doi: 10.1007/s10549-013-2478-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Khouri MG, Hornsby WE, Risum N, et al. Utility of 3-dimensional standardizecardiography, global longitudinal strain, and exercise stress standardizecardiography to detect cardiac dysfunction in breast cancer patients treated with doxorubicin-containing adjuvant therapy. Breast Cancer Res. Treat. 2014;143(3):531–539. doi: 10.1007/s10549-013-2818-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Mckillop JH, Bristow MR, Goris ML, Billingham ME, Bockemuehl K. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am. Heart J. 1983;106(5 Pt 1):1048–1056. doi: 10.1016/0002-8703(83)90651-8. [DOI] [PubMed] [Google Scholar]
- 95.Civelli M, Cardinale D, Martinoni A, et al. Early reduction in left ventricular contractile reserve detected by dobutamine stress standardize predicts high-dose chemotherapy-induced cardiac toxicity. Int. J. Cardiol. 2006;111(1):120–126. doi: 10.1016/j.ijcard.2005.07.029. [DOI] [PubMed] [Google Scholar]
- 96.Smibert E, Carlin JB, Vidmar S, Wilkinson LC, Newton M, Weintraub RG. Exercise standardizecardiography reflects cumulative anthracycline exposure during childhood. Pediatr. Blood Cancer. 2004;42(7):556–562. doi: 10.1002/pbc.20016. [DOI] [PubMed] [Google Scholar]
- 97.De Souza AM, Potts JE, Potts MT, et al. A stress standardizecardiography study of cardiac function during progressive exercise in pediatric oncology patients treated with anthracyclines. Pediatr. Blood Cancer. 2007;49(1):56–64. doi: 10.1002/pbc.21122. [DOI] [PubMed] [Google Scholar]
- 98.Guimaraes-Filho FV, Tan DM, Braga JC, Rodrigues A, Waib PH, Matsubara BB. Ventricular systolic reserve in asymptomatic children previously treated with low doses of anthracyclines: a longitudinal, prospective exercise standardizecardiography study. Pediatr. Blood Cancer. 2012;59(3):548–552. doi: 10.1002/pbc.24000. [DOI] [PubMed] [Google Scholar]
- 99.Sieswerda E, Kremer LC, Vidmar S, et al. Exercise standardizecardiography in asymptomatic survivors of childhood cancer treated with anthracyclines: a prospective follow-up study. Pediatr. Blood Cancer. 2010;54(4):579–584. doi: 10.1002/pbc.22371. [DOI] [PubMed] [Google Scholar]
- 100.Klewer SE, Goldberg SJ, Donnerstein RL, Berg RA, Hutter JJ., Jr Dobutamine stress standardizecardiography: a sensitive indicator of diminished myocardial function in asymptomatic doxorubicin-treated long-term survivors of childhood cancer. J. Am. Coll. Cardiol. 1992;19(2):394–401. doi: 10.1016/0735-1097(92)90497-b. [DOI] [PubMed] [Google Scholar]
- 101.Lanzarini L, Bossi G, Laudisa ML, Klersy C, Arico M. Lack of clinically significant cardiac dysfunction during intermediate dobutamine doses in long-term childhood cancer survivors exposed to anthracyclines. Am. Heart J. 2000;140(2):315–323. doi: 10.1067/mhj.2000.108237. [DOI] [PubMed] [Google Scholar]
- 102.Gupta S, Rohatgi A, Ayers CR, et al. Cardiorespiratory fitness and classification of risk of cardiovascular disease mortality. Circulation. 2011;123(13):1377–1383. doi: 10.1161/CIRCULATIONAHA.110.003236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108(13):1554–1559. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 104.Jones LW, Watson D, Herndon JE, 2nd, et al. Peak oxygen consumption and long-term all-cause mortality in nonsmall cell lung cancer. Cancer. 2010;116(20):4825–4832. doi: 10.1002/cncr.25396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Jones LW, Hornsby WE, Goetzinger A, et al. Prognostic significance of functional capacity and exercise behavior in patients with metastatic non-small cell lung cancer. Lung Cancer. 2012;76(2):248–252. doi: 10.1016/j.lungcan.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Jones LW, Courneya KS, Mackey JR, et al. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. J. Clin. Oncol. 2012;30(20):2530–2537. doi: 10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]; • Demonstrates that breast cancer survivors have significantly impaired aerobic capacity despite normal resting LVEF.
- 107.Cardinale D, Sandri MT. Role of biomarkers in chemotherapy-induced cardiotoxicity. Prog. Cardiovasc. Dis. 2010;53(2):121–129. doi: 10.1016/j.pcad.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 108.Thigpen SC, Geraci SA. Prediction of anthracycline-induced left ventricular dysfunction by cardiac troponins. South. Med. J. 2012;105(12):659–664. doi: 10.1097/SMJ.0b013e3182749006. [DOI] [PubMed] [Google Scholar]
- 109.Cardinale D, Sandri MT, Martinoni A, et al. Left ventricular dysfunction predicted by early troponin I release after high-dose chemotherapy. J. Am. Coll. Cardiol. 2000;36(2):517–522. doi: 10.1016/s0735-1097(00)00748-8. [DOI] [PubMed] [Google Scholar]
- 110.Cardinale D, Sandri MT, Colombo A, et al. Prognostic value of troponin I in cardiac risk stratification of cancer patients undergoing high-dose chemotherapy. Circulation. 2004;109(22):2749–2754. doi: 10.1161/01.CIR.0000130926.51766.CC. [DOI] [PubMed] [Google Scholar]
- 111.Cardinale D, Sandri MT, Martinoni A, et al. Myocardial injury revealed by plasma troponin I in breast cancer treated with high-dose chemotherapy. Ann. Oncol. 2002;13(5):710–715. doi: 10.1093/annonc/mdf170. [DOI] [PubMed] [Google Scholar]
- 112.Sandri MT, Cardinale D, Zorzino L, et al. Minor increases in plasma troponin I predict decreased left ventricular ejection fraction after high-dose chemotherapy. Clin. Chem. 2003;49(2):248–252. doi: 10.1373/49.2.248. [DOI] [PubMed] [Google Scholar]
- 113.Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J. Clin. Oncol. 2010;28(25):3910–3916. doi: 10.1200/JCO.2009.27.3615. [DOI] [PubMed] [Google Scholar]
- 114.Morris PG, Chen C, Steingart R, et al. Troponin I and C-reactive protein are commonly detected in patients with breast cancer treated with dose-dense chemotherapy incorporating trastuzumab and lapatinib. Clin. Cancer Res. 2011;17(10):3490–3499. doi: 10.1158/1078-0432.CCR-10-1359. [DOI] [PubMed] [Google Scholar]
- 115.Ky B, Putt M, Sawaya H, et al. Early increases in multiple biomarkers predict subsequent cardiotoxicity in patients with breast cancer treated with doxorubicin, taxanes, and trastuzumab. J. Am. Coll. Cardiol. 2014;63(8):809–816. doi: 10.1016/j.jacc.2013.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]