Alzheimer's disease (AD) is a neurodegenerative disorder that primarily compromises memory formation and storage. Several hypotheses regarding the pathogenesis of AD have been proposed; however, no cure is available to date. Here we describe the calcium hypothesis of AD, which is gaining popularity. We present data supporting this hypothesis and focus on a recently discovered calcium-signaling pathway that is dysregulated in AD and propose targets for the development of disease-modifying therapies.
Alzheimer's disease (AD) is the most common reason for dementia in the elderly. There is currently no cure for AD. Scientists and physicians worldwide are working diligently to pinpoint the problems in AD brains. There are many hypotheses regarding the pathogenesis of AD; in this article, we will summarize one of these, the calcium hypothesis. The calcium hypothesis states that disruption of Ca2+ signaling/homeostasis via abnormal functioning of calcium handling proteins such as ion channels underlies the pathogenesis of AD. This hypothesis has gained popularity in recent years as other hypotheses concerning AD (e.g., the amyloid hypothesis) have so far failed to yield disease-modifying therapies. Yet, there is evidence for important crosstalk between dysregulated calcium signaling and amyloid pathology in AD. It was proposed that Aβ peptides (the main constituent of amyloid plaques) form Ca2+ permeable pores and bind to and modulate NMDAR, AMPAR, mGluR5 and VGCC, leading to the overfilling of neurons with calcium ions (for review see [1]). It was also proposed that genetically inherited mutations in presenilins influence the production of Aβ42 and increase the Aβ42/Aβ40 ratio [2,3].
Increased cytosolic levels of Ca2+ in neurons have been observed in multiple studies involving cellular and mouse models of AD as well as in cells derived from AD patients. Moreover, Ca2+ accumulates in intracellular stores such as endoplasmic reticulum (ER) and mitochondria. What is the source of increased calcium content? The majority of early-onset familial AD (FAD) cases are caused by missense mutations in PSEN1 and PSEN2 genes. Around 226 AD-causing mutations in PSEN1 have been identified [4]. To explain neuronal Ca2+ overloading, it has been proposed that mutant PS decrease activity of intracellular ER Ca2+ release channels such as RyanRs [5] and IP3Rs [6], modulate the function of the SERC) [7], and/or affect store-operated Ca2+ influx [8,9]. Presenilins, together with nicastrin, anterior pharynx-defective 1 and presenilin enhancer 2, form the γ-secretase complex that cleaves several substrates including APP. Sequential cleavage of APP by β- and γ-secretases constitutes the amyloidogenic pathway that leads to production of toxic Aβ peptides. There was a long-standing debate on whether mutations in PS1 and PS2 proteins cause a loss or gain in γ-secretase function and on whether this loss or gain in function is full or partial [10,11]. This debate was continued recently by Shen, Kelleher and colleagues. They generated two novel presenilin knock-in (KI) models that express the FAD mutations L435F or C410Y [12]. Shen, Kelleher and colleagues report that these mutations cause complete loss of PS1 function in vivo and they propose to extrapolate their finding to the whole field of FAD saying that this may be the mechanism of the disease [12]. However, γ-secretase is not a simple molecule it has many different substrates as well as it exists in four different forms [13,14]. Development of AD preventing therapies based on modulation of γ-secretase function requires exquisite precision [14]. The unfortunate outcome of the recent semagacestat trial in Phase III [15] highlights the potential hazards associated with modulation of γ-secretase function, suggesting the need for further research to comprehensively delineate all γ-secretase functions at a precise, mechanistic level before adequate therapeutics can be developed that rely on targeting this multifaceted protease.
Are there any other functions of presenilins? We recently discovered a new function, namely the formation of ER membrane-delimited ion channels with a passive, low conductance Ca2+ leak. Many, but not all, FAD mutations selectively disrupt this function, causing overfilling of ER with Ca2+ [16,17]. In support of our hypothesis, the archaeal homolog of presenilin 1 (PSH1) was recently crystallized, revealing a water-filled hole within the protein that traverses the lipid bilayer and presumably permits the flow of Ca2+ ions [18]. Notably, the ER leak channel function involves the holoprotein form of PSs, whereas γ-secretase function requires a cleaved form of PSs[16]. Because the FAD-causing M146V mutation disrupts the PS leak function [16,17], we used the PS1-M146V-knockin (KI) mouse model of AD to study the role of dysregulated ER Ca2+ homeostasis in AD pathology. We observed that the ER was overfilled with Ca2+, in line with a diminished ER Ca2+ leak. To compensate and reduce excessive ER Ca2+ loading, KI neurons downregulate STIM2-dependent neuronal store-operated Ca2+ entry (nSOC) [19], which may be related to cognitive decline, because STIM2 knockout mice harbor striking spatial learning deficits [20].
What is the function of nSOC in neurons? There is limited information regarding this question. Our laboratory was among the first to demonstrate that nSOC regulates the stability of dendritic spines [19]. Dendritic spines are protrusions on the dendritic shaft where synapses form. There are three groups of spines that can be classified morphologically. Mushroom spines have a big head and thin neck, thin spines have a small diameter head and thin neck and stubby spines have no distinguishable borders between head and neck. Mushroom spines are stable structures that make functionally stronger synapses and are therefore responsible for memory storage [21]. Thin spines are suggested to be ‘learning spines’ since they scale with increases and decreases in synaptic activity [21]. The function of stubby spines is unclear. It was recently observed that hippocampal spines are less stable [22] in comparison to cortical spines [23]. What underlies the enhanced plasticity of hippocampal spines? How does this influence memory formation and storage? These questions remain unanswered, but we and others have proposed that cognitive decline in AD reflects the preferential elimination of hippocampal mushroom spines during the progression of the disease. We have demonstrated that tonic nSOC is necessary for stability of mushroom spines [19]. Consistent with this, hippocampal STIM2 downregulation is correlated with worse mini-mental state exam scores in sporadic AD patients [19].
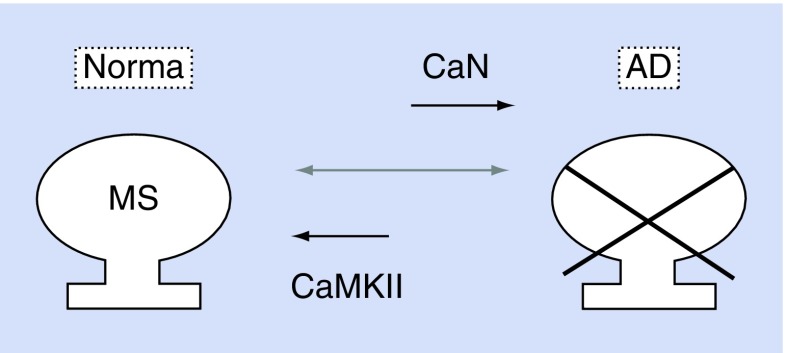
How does nSOC regulate mushroom spine maintenance? We suspect that nSOC regulates the stability of mushroom spines via the activity of Ca2+/CaMKII. CaMKII activity is associated with the formation of long-term potentiation and is highly expressed in synaptic spines. During synaptic loss, decreased CaMKII phosphorylation appears to coincide with increased CaN activity. Indeed, CaN phosphatase activity is enhanced in aging neurons and plays an important role in increased long-term depression [24,25]. We observed that the STIM2-nSOC-CaMKII mushroom spine maintenance pathway is also disrupted in recently developed APP-KI mouse models of AD [26], in conditions of amyloid toxicity [27], in aging neurons and in sporadic AD brains [19]. Intriguingly, pharmacological or genetic rescuing of this pathway in KI mice restores mushroom spines as well as expression of synaptic proteins such as pCaMKII and postsynaptic density protein 95 [19,26,27]. Therefore, we think that the formation and stability of mushroom spines is regulated by the balance of CaMKII and CaN activity. Accordingly, insufficient CaMKII activity and/or disproportionate CaN activity drives mushroom spine elimination, synapse loss and the manifestation of cognitive deficits (Figure 1). On the other hand, if CaMKII activity outweighs CaN activity then the stability of mushroom spines is preserved and cognitive functions are normal. We therefore propose that the STIM2-nSOC-CaMKII pathway may constitute attractive target for the development of AD-preventing therapies.
Figure 1. . Schema representing the CaMKII/CaN balance in dendritic spines.
If the balance is shifted toward favoring CaN activity then mushroom spines are eliminated, synapses are destroyed and cognitive dysfunctions start to appear. If CaMKII activity is maintained and outweighs CaN activity, then stability of mushroom spines is preserved and cognitive functions are normal.
AD: Alzheimer's disease MS: Mushroom spines.
Acknowledgements
We are thankful to Daniel Ryskamp for critical reading and suggestions on how to improve the manuscript. We thank Polina Plotnikova for administrative help.
Footnotes
Financial & competing interests disclosure
I Bezprozvanny is a holder of the Carl J and Hortense M Thomsen Chair in Alzheimer's Disease Research. This work was equally supported by the NIH grant R01NS080152 (IB), Russian Scientific Fund grant 14-25-00024 (IB), by state grant number 17.1360.2014/К (IB), and by the Dynasty Foundation grant DP-B-11/14 (EP). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Bezprozvanny I. Calcium signaling and neurodegenerative diseases. Trends Mol. Med. 2009;15(3):89–100. doi: 10.1016/j.molmed.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Elder GA, Gama Sosa MA, de Gasperi R, Dickstein DL, Hof PR. Presenilin transgenic mice as models of Alzheimer's disease. Brain Struct. Funct. 2010;214(2–3):127–143. doi: 10.1007/s00429-009-0227-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hwang DY, Chae KR, Kang TS, et al. Alterations in behavior, amyloid beta-42, caspase-3, and Cox-2 in mutant PS2 transgenic mouse model of Alzheimer's disease. FASEB J. 2002;16(8):805–813. doi: 10.1096/fj.01-0732com. [DOI] [PubMed] [Google Scholar]
- 4.Alzforum. Http://www.Alzforum.Org/mutations
- 5.Stutzmann GE, Smith I, Caccamo A, Oddo S, Laferla FM, Parker I. Enhanced ryanodine receptor recruitment contributes to CA2+ disruptions in young, adult, and aged Alzheimer's disease mice. J. Neurosci. 2006;26(19):5180–5189. doi: 10.1523/JNEUROSCI.0739-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung KH, Shineman D, Muller M, et al. Mechanism of CA2+ disruption in Alzheimer's disease by presenilin regulation of insp(3) receptor channel gating. Neuron. 2008;58(6):871–883. doi: 10.1016/j.neuron.2008.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Green KN, Demuro A, Akbari Y, et al. Serca pump activity is physiologically regulated by presenilin and regulates amyloid beta production. J. Cell Biol. 2008;181(7):1107–1116. doi: 10.1083/jcb.200706171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leissring MA, Akbari Y, Fanger CM, Cahalan MD, Mattson MP, Laferla FM. Capacitative calcium entry deficits and elevated luminal calcium content in mutant presenilin-1 knockin mice. J. Cell Biol. 2000;149(4):793–798. doi: 10.1083/jcb.149.4.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yoo AS, Cheng I, Chung S, et al. Presenilin-mediated modulation of capacitative calcium entry. Neuron. 2000;27(3):561–572. doi: 10.1016/s0896-6273(00)00066-0. [DOI] [PubMed] [Google Scholar]
- 10.de Strooper B. Loss-of-function presenilin mutations in Alzheimer disease. EMBO Rep. 2007;8(2):141–146. doi: 10.1038/sj.embor.7400897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chavez-Gutierrez L, Bammens L, Benilova I, et al. The mechanism of gamma-secretase dysfunction in familial Alzheimer disease. EMBO J. 2012;31(10):2261–2274. doi: 10.1038/emboj.2012.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xia D, Watanabe H, Wu B, et al. Presenilin-1 knockin mice reveal loss-of-function mechanism for familial Alzheimer's disease. Neuron. 2015;85(5):967–981. doi: 10.1016/j.neuron.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Strooper B. Aph-1, Pen-2, and nicastrin with presenilin generate an active gamma-secretase complex. Neuron. 2003;38(1):9–12. doi: 10.1016/s0896-6273(03)00205-8. [DOI] [PubMed] [Google Scholar]
- 14.de Strooper B. Lessons from a failed gamma-secretase Alzheimer trial. Cell. 2014;159(4):721–726. doi: 10.1016/j.cell.2014.10.016. [DOI] [PubMed] [Google Scholar]
- 15.Doody RS, Raman R, Farlow M, et al. A Phase 3 trial of semagacestat for treatment of Alzheimer's disease. N. Engl. J. Med. 2013;369(4):341–350. doi: 10.1056/NEJMoa1210951. [DOI] [PubMed] [Google Scholar]
- 16.Tu H, Nelson O, Bezprozvanny A, et al. Presenilins former calcium leak channels, a function disrupted by mutations linked to familial Alzheimer's disease. Cell. 2006;126:981–993. doi: 10.1016/j.cell.2006.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nelson O, Supnet C, Tolia A, Horre K, de Strooper B, Bezprozvanny I. Mutagenesis mapping of the presenilin 1 calcium leak conductance pore. J. Biol. Chem. 2011;286(25):22339–22347. doi: 10.1074/jbc.M111.243063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Li X, Dang S, Yan C, Gong X, Wang J, Shi Y. Structure of a presenilin family intramembrane aspartate protease. Nature. 2013;493(7430):56–61. doi: 10.1038/nature11801. [DOI] [PubMed] [Google Scholar]
- 19.Sun S, Zhang H, Liu J, et al. Reduced synaptic STIM2 expression and impaired store-operated calcium entry cause destabilization of mature spines in mutant presenilin mice. Neuron. 2014;82(1):79–93. doi: 10.1016/j.neuron.2014.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berna-Erro A, Braun A, Kraft R, et al. STIM2 regulates capacitive CA2+ entry in neurons and plays a key role in hypoxic neuronal cell death. Sci. Signal. 2009;2(93):ra67. doi: 10.1126/scisignal.2000522. [DOI] [PubMed] [Google Scholar]
- 21.Bourne J, Harris KM. Do thin spines learn to be mushroom spines that remember? Curr. Opin. Neurobiol. 2007;17(3):381–386. doi: 10.1016/j.conb.2007.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Attardo A, Fitzgerald JE, Schnitzer MJ. Impermanence of dendritic spines in live adult ca1 hippocampus. Nature. 2015 doi: 10.1038/nature14467. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zuo Y, Lin A, Chang P, Gan WB. Development of long-term dendritic spine stability in diverse regions of cerebral cortex. Neuron. 2005;46(2):181–189. doi: 10.1016/j.neuron.2005.04.001. [DOI] [PubMed] [Google Scholar]
- 24.Foster TC, Sharrow KM, Masse JR, Norris CM, Kumar A. Calcineurin links CA2+ dysregulation with brain aging. J. Neurosci. 2001;21(11):4066–4073. doi: 10.1523/JNEUROSCI.21-11-04066.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jouvenceau A, Dutar P. A role for the protein phosphatase 2b in altered hippocampal synaptic plasticity in the aged rat. J. Physiol. Paris. 2006;99(2–3):154–161. doi: 10.1016/j.jphysparis.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 26.Zhang H, Wu L, Pchitskaya E, Zaharova O, Saito T, Saido T, Bezprozvanny I. Neuronal store-operated calcium entry and mushroom spine loss in app knock-in mouse model of Alzheimer's disease. J. Neurosci. 2015 doi: 10.1523/JNEUROSCI.1034-15.2015. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Popugaeva E, Pchitskaya E, Speshilova A, et al. STIM2 protects hippocampal mushroom spines fromamyloid synaptotoxicity. Mol. Neurodegener. 2015;10(1):37. doi: 10.1186/s13024-015-0034-7. [DOI] [PMC free article] [PubMed] [Google Scholar]