Abstract
Endocrine therapy has become one of most effective forms of targeted adjuvant therapy for hormone-sensitive breast cancer and may be given after surgery or radiotherapy, and also prior, or subsequent to chemotherapy. Current commonly used drugs for adjuvant endocrine therapy can be divided into following three classes: selective estrogen receptor modulators, aromatase inhibitors and selective estrogen receptor downregulators. Tumor cells can develop resistance to endocrine therapy, a major obstacle limiting the success of breast cancer treatment. The complicated crosstalk, both genomic and nongenomic, between estrogen receptors and growth factors was considered to be a crucial factor contributing to endocrine resistance. However, resistance to this therapy is thought to be a progressive, step-wise process, and the underlying mechanism remains unclear. In this review, we summarize the possible biological and molecular mechanisms that underlie endocrine resistance, and discuss some novel strategies to overcoming these issues.
Keywords: : breast cancer, drug resistance, endrocrine therapeutics, estrogen receptor
Breast cancer is the leading cause of death for women in many countries including the USA and western European countries. Approximately 5% of breast cancer patients are positive for estrogen receptor (ER) expression at diagnosis [1]. This is consistent with the crucial role of estrogen and its receptors in breast cancer etiology and progression, and with the role played by estrogen as tumor promoters. Both clinical observations and experimental studies from our laboratory and other research groups suggested that estrogen and its receptors might affect the therapeutic efficacy of antineoplastic drugs, thus paving a way for the development of therapies that aim to block estrogen stimulation and also its receptors [2,3]. Indeed, since a century ago, Sir George Beaston first reported that oophorectomy could result in tumor remission in women with metastatic breast cancer (MBC), so far endocrine therapy has developed rapidly with various types of antiestrogens including selective ER modulators (SERMs), such as tamoxifen, which block the activity of ER; selective ER downregulators (SERDs), such as fulvestrant, which induce destabilization and degradation of ER; and aromatase inhibitors (AIs), which reduce the production of estrogen in peripheral tissues and within the tumors through inhibition of the enzyme aromatase. These various types of endocrine therapy have been used successfully to cause significant reduction of cancer recurrence and death.
Unfortunately, not all patients with ER positive (ER+) tumors respond to endocrine manipulation (de novo resistance), or substantially, those ER+ patients who initially response would later become refractory to the therapy (acquired resistance). Cumulative data showed that ER status and mutation as well as its complicated crosstalk with the growth factors may contribute to endocrine resistance. These come largely from preclinical models of endocrine resistance as well as a greater understanding of the molecular mechanisms by which estrogen works to stimulate the growth of the tumor. Based on these approaches, several attractive strategies such as manipulation of growth factor signaling networks and the use of tyrosine kinase and multikinase inhibitors emerged, that may delay or even overcome the resistance of breast tumors to antiestrogen therapy. Some clinical trials are underway to test the idea that GFR signaling contributes to de novo or acquired endocrine resistance.
Current status of endocrine therapy
Commonly used antiestrogen agents: SERMs, SERDs & AIs
Selective ER modulators (SERMs) are a family of synthetic molecules. They usually bind to ERs throughout the body and act as tissue-specific estrogen agonists or antagonists. They prevent the growth of breast cancer cells by taking place of estrogen in the receptors to avoid the harmful effects of estrogens. Tamoxifen, the first SERM used in clinics for the treatment of ER-positive MBC, has been demonstrated successfully in suppressing the recurrence of breast cancer and reducing the incidence of contralateral second primary breast tumors by 50%. Coupled to its antagonist activity in the breast, tamoxifen, however, is associated with a two- to four-fold increased risk of endometrial cancer due to its estrogen agonist in the uterus. This limits the wide use of tamoxifen in the postmenopausal population with breast cancer. In 2007, another SERM Evista (raloxifene) was approved by US FDA for reduction in the risk of invasive breast cancer in postmenopausal women with osteoporosis. Raloxifene showed positive outcome in the treatment of invasive, ER-positive breast cancer without increasing the risk of endometrial cancer. In addition, FDA recently approved another SERM Fareston (toremifene) for the treatment of ER+ advanced breast cancer (ABC). Similar to tamoxifen, toremifene binds specifically to ER, thereby interferes with the estrogen-mediated growth stimuli in mammary tumor cells, but toremifene does not increase the risk of endometrial cancer.
Fulvestrant belongs to a class of agents known as selective ER downregulator (SERDs), which competitively binds to the ER with a much greater affinity than that of SERMs. As a pure ER antagonist, fulvestrant completely abrogates estrogen-sensitive gene transcription thus ensuring no cross resistance with other antihormonal agents. Several preclinical studies showed that fulvestrant has the ability in suppressing cellular levels of ER protein and inhibiting ER-induced cell proliferation. Our laboratory previously demonstrated that fulvestrant could reverse ER-mediated paclitaxel drug resistance through establishing a pair of isogenic ER+/ER- breast cell line in vitro [4], and consequently this result was then confirmed in animal models [2]. Moreover, in a Phase I trial involving 30 postmenopausal volunteers, intramuscular injection of 250 mg fulvestrant showed that fulvestrant, unlike tamoxifen, exhibited no observed estrogen-agonistic effects on human endometrium during a 14-day period of administration. As the only clinically available SERDs used in breast cancer treatment, fulvestrant has been licensed for the treatment of postmenopausal women with ER+ ABC that have progressed or recurred on prior endocrine therapy [5].
The third-generation aromatase inhibitors (AIs) anastrozole, letrozole and exemstane have provided novel approaches to the endocrine treatment of breast cancer. There are two types of aromatase inhibitors, steroidal/irreversible (anastrozole and letrozole) and nonsteroidal/reversible (exemestane) inhibitors of estrogen synthesis. By blocking the aromatase enzyme, AIs suppress plasma estrogen levels thus reducing growth-stimulatory effects of estrogens in ER+ breast cancer. These drugs are challenging tamoxifen as gold standard in the treatment of postmenopausal women with metastatic or ABC. A substantial body of clinical evidence demonstrated that AIs are superior to tamoxifen as first-line therapy for MBC or ABC. For example, letrozole was significantly superior to tamoxifen in time to progression, time to treatment failure and overall objective response rate in a Phase III study [6]. Moreover, AIs also showed to be potential agents for preventing ER+ breast cancer in high-risk postmenopausal women.
Drug resistance to endocrine therapy: preclinical & clinical observations
Although current endocrine therapies for women with ER+ breast cancer have led to substantial improvements in outcomes, their success is limited by either intrinsic resistance or acquired resistance. A third of women with early-stage breast cancer treated with tamoxifen may be refractory within 2–5 years or develop resistance to the drug with ongoing treatment [7,8]. Moreover, for postmenopausal women, long-term use of tamoxifen may double the risk of endometrial cancer. As a result, AIs and fulvestrant were usually used to follow or replace tamoxifen as second and now first-line endocrine therapy. Several clinical trials suggested that, in comparison with tamoxifen, AIs exhibited enhanced antitumor efficacy, and more importantly, might be effective to those tamoxifen-resistant patients [9]. Additionally, fulvestrant alone or combined with letrozole also showed a strong possibility of delaying the emergence of acquired resistance in postmenopausal women. Even with an initial response to the treatment, resistance to AIs and fulvestrant eventually occur. An understanding of the underlying mechanisms of resistance to these agents is, therefore, an access for the appropriate delivery of treatment to responsive patients. For example, EGFR/HER2 pathway has been well elucidated and many ongoing clinical trials are carried out to evaluate therapeutic efficacy of combination of endocrine agents and HER2-targeted drugs. Details on this part will be discussed in later sections. Other mechanisms contributing to endocrine therapy including loss of ER expression, ER mutation, cell cycle alternation and growth factor-driven pathways will also be described in the following sections.
Possible mechanisms of endocrine therapy resistance
Loss of ER expression & ER mutation & endocrine resistance
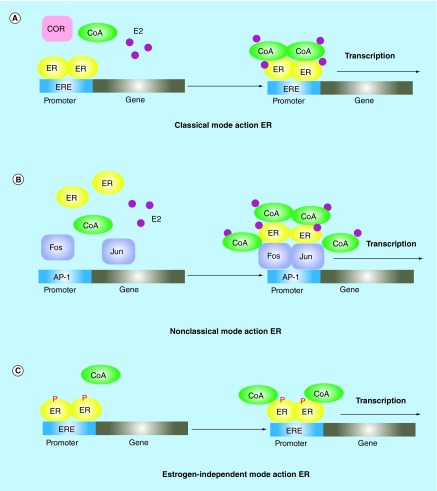
Breast cancer is a classical hormone-dependent tumor which relies on estrogen to stimuli cell growth and proliferation. It is well known that 70% of breast tumors are hormone receptor positive. ERs refer to a family of nuclear transcriptional regulators which play an important role in development and progression of breast cancer. There are two isoforms of ERs, ER-α and ER-β, that are encoded by separate genes located on different chromosomes. As the role of ER-β in endocrine resistance remains inconsistent, herein, we will limit our discussion on ‘ER-α’ which will refer to ‘ER’ in the following sections. ER, including nuclear ER and membrane ER, acts through genomic (nuclear) and nongenomic (membrane) pathways. Estrogens exert most of their regulatory potential on ER-regulated gene expression through nuclear-initiated steroid signaling (NISS) pathways – this is called genomic action of ER. Figure 1A & B shows classical and nonclassical modes of NISS. In addition, estrogen-ER signaling can also be activated by estrogen-independent manner through phosphorylation at specific ER sites targeted by kinases including protein kinase A (PKA) and c-Src (Figure 1C). Estrogens can also bind to membrane ER that cooperates, as a dimmer, with membrane-bound proteins or other co-activators which activate the membrane-initiated steroid signaling (MISS) pathways, which is called nongenomic action of ER (Figure 2).
Figure 1. . Classical and nonclassical and legend-independent mode of nuclear-initiated steroid signaling.
ER, in its classical mode action (A), directly binds to specific DNA response elements called EREs. Estrogen(E2)-bound ER generally recruits CoA complexes to induce gene transcription. In ERs nonclassical mode action (B), ER regulates gene transcription through protein–protein interactions (e.g., with Fos/Jun family members) with other transcription factors, particularly members of the Ap-1 families. In contrast to estrogen-dependent manner of ER action, ER signaling can be activated by phosphorylation at specific ER sites (C). Together, all of these nuclear ER genomic activities are called nuclear-initiated steroid signaling.
CoA: Coactivator; ER: Estrogen receptor; ERE: Estrogen response element.
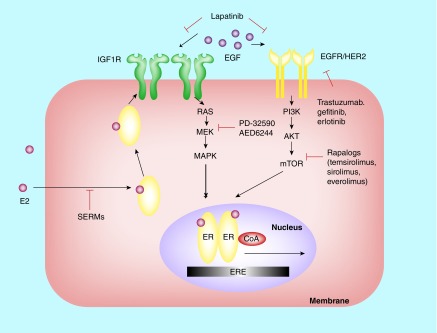
Figure 2. . Integration of genomic and nongenomic ER action and its crosstalk with growth factor receptor pathways in breast cancer: a working model.
In ER-positive breast tumors, genomic ER activity meditated by nuclear-initiated steroid signaling always predominates (see Figure 1), although some nongenomic signaling on acting ER that resides at the membrane and/or cytoplasm also occurs. This situation is usually upon acquisition of tamoxifen resistance or adaptation to hormone deprivation. Therefore, several tyrosine kinase receptors such as HER2 and EGFR as well as IGFR signaling become activated. Tyrosine kinase receptor-induced kinases phosphorylate nuclear ER and its CoA, thus promoting genomic ER activity and enhancing gene expression. As a result, genomic and nongenomic activities of ER and their crosstalk with growth factor tyrosine kinase pathways cooperative in promoting gene transcription, thus leading to endocrine resistance. Targeting the growth factor receptor pathway at different nodal points using antibodies (trastuzumab) and tyrosine kinase inhibitors (gefitinib, erlotinib and lapatinib) or other signal transduction inhibitors (e.g., mTOR inhibitors and MEK inhibitors) can eliminate the molecular crosstalk and overcome endocrine resistance.
CoA: Coactivator; ER: Estrogen receptor; ERE: Estrogen response element.
Adapted with permission from [10].
Theoretically, the molecular features of ER play an important role in determining the outcome of endocrine therapy. Generally speaking, the loss of ER expression and ER mutations are two main aspects of the mechanisms of ER-mediated antiestrogen therapy resistance. ER expression is a critical predictor of response to endocrine therapy, so it is obvious that the lack of ER expression will result in de novo resistance to antiestrogen therapy [11]. Actually, the loss of ER expression occurs only in a minority (15–20%) of resistant breast cancers. The fact is that most of primary ER-positive patients will develop endocrine resistance, implying that ER status and functions may be affected by some altered ways. For example, the loss of ER has been associated with aberrant methylation of CpG islands, located in the 5′ regulatory regions of the ER gene. This abnormal methylation could account for transcriptional inactivation of the ER gene and induce hormone resistance in some human breast cancers. Interestingly, ER gene methylation alone does not always induce the loss of ER expression, for there are still 35% ER/progesterone receptor (PR)-positive tumors also exhibit substantial ER gene methylation. On the other hand, some other studies indicated that histone deacetylation may contribute to ER silencing in some breast tumors as well. Several studies showed that co-treatment with a histone deacetylase (HDAC) inhibitor and a DNMT1 inhibitor to interfere with histone HDAC1or HDAC2 could restore the expression of ER gene in ER-negative breast cancer cells, and more importantly to restore tamoxifen sensitivity in ER-negative breast cancer cells MDA-MB-435 both in vitro and in vivo [12]. These findings suggest that HDAC and DNMT inhibitors may be developed as novel therapeutic strategies in the treatment of ER-negative breast cancers.
Mutation of ER could also impact on the response to endocrine therapy [13]. ER gene mutations such as deletion and point mutation were acquired in tamoxifen-resistant ER-positive cell lines MCF-7 and T47D. Herynk and Fuqua also described the correlation of ER mutations and human disease including the response and resistance to tamoxifen [13]. However, such mutations of ER were rare in clinical samples, estimated to be present in only 1% of breast tumors.
PR & endocrine resistance
The PR, as an estrogen-related gene, is expressed in half of patients with ER+ breast tumors. So it is not strange that ER+/PR+ tumors are more common than ER+/PR- tumors. Moreover, the clinical outcomes of endocrine therapy between these two subsets of patients are not the same. Several clinical studies have shown that ER+/PR+ tumors are more responsive to SERMs therapy than ER+/PR- tumors, while their response to anastrazole is of little difference. Similarly, two neoadjuvant trials regarding the role of PR in response to AIs showed a better response to endocrine therapy in PR+ tumors than PR- tumors. On the other hand, a multivariate analysis showed that the absence of PR expression in metastatic breast tumors was associated with disease progression. These findings indicated that loss of PR in ER+ breast tumors could serve as a predictor of endocrine therapy outcome [14]. Several studies reported that several growth factors of breast cancer could directly downregulate PR levels via PI3K/Akt/mTOR pathway and also reduce ER expression level and activity. Sian Tovey and colleagues showed that PR and HER1–3 status could be used to predict for early relapse in ER+ tamoxifen-treated breast cancer patients [15]. Additionally, HER-1 and HER-2 levels were significantly higher in the ER+/PR- patients than that of in ER+/PR+ patients, and several clinical observations suggested that such high levels of HER-1 and HER-2 were associated with tamoxifen resistance. The understanding why PR-negative tumors respond poorly to endocrine therapy and its correlation with high growth factor activity could be developed as better therapeutic strategies.
Crosstalk between ER & EGFR/HER2 & endocrine resistance
Although hormone receptor status are reliable markers for predicting the response to endocrine therapy, both preclinical and clinical evidence suggested that HER2 overexpression confers resistance to antiestrogen agents, even in the presence of hormone receptors [16]. HER2 is a member of EGF or ErbB family of receptor tyrosine kinase. Overexpression of HER2, which occurs in approximately 30% of metastatic breast tumors, is associated with an increased tendency for metastasis as well as decreased disease-free and overall survival rates. Retrospective analyses of clinical studies have shown a poor outcome in patients with HER2 overexpression breast tumors when treated with tamoxifen. For patients treated with tamoxifen who express high level of HER2. Moreover, the HER2 extracellular domain (ECD) circulating levels also implied the ability to predict worse outcome after hormonal therapy. A clinical trial reported by Lipton et al., elevated serum concentrations of extracellular domain of HER2 resulted in worse outcome [17].
A deeper understanding of the role of HER2 in endocrine resistance focuses on the crosstalk between HER2 and ER signaling pathways. Benz first reported that HER2 transfection into hormone-dependent breast cancer cell MCF-7 could mediate tamoxifen resistance [18]. Another in vitro study showed that long-term exposure of ER-positive breast cancer cell MCF-7 to tamoxifen developed resistant clones, and these clones were detected to have increased levels of phosphorylated and total EGFR and HER2 expression, as well as downstream ERK1/2. Therefore, the growth of these tamoxifen-resistant MCF-7 cells was completely repressed by EGFR-targeted tyrosine kinase inhibitor gefitinib. In vivo work also confirmed that HER2 crosstalk with ER co-activator A1B1 could enhance the estrogen agonist activity of tamoxifen-bound ER. Tamoxifen significantly stimulated growth of MCF-7/HER2–18 tumors, which express high levels of both HER2 and A1B1, but antagonized the parental MCF-7 tumors, which have high A1B1 but low HER2 expression. In HER2 overexpressing tumors, peptide growth factors as well as estrogen and tamoxifen activate EGFR and HER2 signal pathways via ongenomic activities. Some downstream kinase including ERK1, 2, MAPK and AKT can phosphorylate ER and functionally activate A1B1, thus establishing crosstalk nuclear tamoxifen–ER complex and their co-activators (CoA) but not co-suppressors (CoR) to stimulate cell survival and proliferation. This pathway interaction could be completely blocked by gefitinib. Gefitinib ability, which prevented the activation of ER and A1B1, as well as reduced the recruitment of co-activator complexes, could eliminate the crosstalk and restore the tamoxifen's antitumor effects. In addition, cross talk between HER2 and membrane ER also contributes to tamoxifen resistance. In the study conducted by Chung et al. in HER2 overexpressing BT474 cells, there were direct physical associations between HER2 and cell membrane ER [19]. Moreover, HER2 blocked cell membrane ER-initiated apoptosis and indirectly offset the nuclear ER effect on growth inhibition. Collectively, all these data suggested that through suppressing HER2 pathway, cell membrane-ER coupled apoptotic pathway as well as acquired nuclear ER activity could be regulated, thus sensitizing breast cancer cell to tamoxifen and other endocrine therapeutics.
Strategies to overcoming endocrine resistance
Combination of anti-HER family agents with endocrine therapy
As outlined above, abundant preclinical evidences suggested that crosstalk between ER and HER2 signaling pathways significantly contribute to antiestrogen resistance. Indeed, it has been well proved clinically that patients with hormone receptor-positive (HR+)/HER2-positive (HER2+) disease are less likely to response to tamoxifen and other antiestrogen agents than those with HR+/HER2- disease. Therefore, various clinical trials have been designed to examine the potential of combining treatments that target HER2 and ER signaling pathways (Table 1). TAnDEM was the first randomized Phase III study to combine a hormone agent (anastrozole) and anti-HER2 agent trastuzumab but not chemotherapy as a treatment for HER2+/HR+ metastatic breast cancer (MBC) [20]. In this clinical trial, 208 postmenopausal women with HER2+/HR+ breast cancer were enrolled and finally there were 187 withdrawals due to progressive disease (PD). In the anastrozole alone group, 73/104 patients who experienced PD received trastuzumab-containing regimen. The results from this trial showed that compared with patients treated with anastrozole alone, anastrozole plus trastuzumab improved progression-free survival and time to progression but not overall survival (OS) for the patients with HER2+ tumors. Another clinical trial showed that the combining treatment of letrozole and trastuzumab produced durable responses consistently lasting at least 1 year in a quarter of the patients with HER2+/HR+ ABC. However, nearly half of the patients experienced early PD, indicating that the combining treatment was inactive in this subgroup [21].
Table 1. . Complete and ongoing Phase II or III clinical trials of combining treatments that target HER family and ER signaling pathways.
| Study | Patient characteristics | Study phase number | Treatments | Status | Ref. |
|---|---|---|---|---|---|
| TAnDEMH |
ER2+, HR+ MBC |
III 207 |
Anastrozole ± trastuzumabcomplete |
Completed |
[22] |
| Marcom PK |
HER2+, ER+ and/or PR+ ABC |
II 33 |
Letrozole ± trastuzumabcomplete |
Completed |
[23] |
| Johnston S |
HER2+, HR+ MBC |
III 1286 |
Letrozole ± lapatinibcomplete |
Completed |
[24] |
|
NCT01160211 |
HR+, HER2+ MBC |
III 525† |
AI + lapatinib/trastuzumab/lapatinib + trastuzumab |
Open |
[25] |
|
NCT00999804 |
HER2+ MBC |
II 96† |
Herceptin/lapatinib ± letrozoleopen |
Open |
[26] |
|
NCT00759642 |
HR+, HER2- ABC, (failed prior antihormone therapy) |
II 36† |
Lapatinib alone |
Open |
[27] |
| NCT00788194 |
HER2+ ER+ ABC (progressing after AI therapy) |
III 396† |
Fulvestrant ± lapatinib and/or AI |
Open |
[35] |
| NCT1275859 | ER+, HER2+ MBC | II 32† | Lapatinib + letrozole | Open |
†Targeted number.
ABC: Advanced breast cancer; AI: Aromatase inhibitor; ER+: Estrogen receptor-positive; HER2+: Human epidermal factor-positive; HR+: Hormone receptor positive; MBC: Metastatic breast cancer; PR+: Progesterone receptor-positive.
Since trastuzumab mainly depresses the growth of cells overexpressing HER2, lapatinib, another oral tyrosine kinase inhibitor of TK domain of both HER1 and HER2, was tested as monotherapy or combination with hormonal therapies. Several clinical studies suggested that lapatinib plus letrozole showed significant clinical benefit in patients with HER2+/HR+ MBC. The largest clinical trial so far which enrolled 1286 patients compared treatments of letrozole-lapatinib combination and letrozole alone for HR+, HER2+ breast cancer patients. In this study, for patients with MBC that co-expresses HER2 and HR (n = 219), the combined target therapy significantly enhanced progression-free survival and clinical benefit rates [28]. As to the patients with HER2-negative MBC who experienced endocrine therapy resistance, the combination of letrozole and lapatinib may induce clinical benefit, but need more patients to confirm the result. As shown in Table 1, some of the ongoing clinical trials that evaluated therapeutic efficacy of combined lapatinib and endocrine therapy as first- or second-line therapy. It may take a few more years for us to clarify whether this approach will gain significant efficacy in the treatment of breast cancer.
Endocrine therapy combined with mTOR inhibitors
The PI3K–AKT–mTOR pathway has also been implicated to play a crucial role in tumor proliferation and progression. Several studies showed that the upregulation of the PI3K–AKT–mTOR pathway interacts with the ER pathway and confers endocrine resistance. Some in vitro studies suggested that combining specific mTOR inhibitor everolimus (RAD001) with antiestrogen agents is synergistic in breast cancer cell lines such as MCF-7 and T47D. Another preclinical study showed that co-treatment with low concentrations of mTOR inhibitor RAD001 combining with either letrozole or fulvestrant could restore the response of the resistant cells with high Akt activity. In addition, it could also resensitize breast cancer cells that did not respond to letrozole or fulvestrant as a single agent [29]. A recent clinical study enrolled 270 postmenopausal women with operable ER+ breast cancer suggested that compared with patients treated with letrozole alone, the combination of everolimus and letrozole resulted in greater tumor shrinkage and a greater reduction in cell proliferation [30]. Following these results, a multicenter, Israeli Phase II open study evaluating treatment with RAD001 (10 mg daily) combined with letrozole in postmenopausal women after recurrence or progression on tamoxifen, anastrozole or examestane was initiated recently [31]. Additionally, for patients with newly diagnosed ER-positive breast cancer, letrozole combined with everolimus as presurgical therapy was also tested in a recent complete clinical trial. In this study, 255 patients were treated with 2.5 mg letrozole and everolimus or 2.5 mg letrozole alone [32]. The results of this study have not been posted yet. In addition to everolimus, another mTOR inhibitor temsirolimus (CCI-779) also showed synergistic antitumor activity with antiestrogens in hormone-response breast cancer. The combination of temsirolimus and antiestrogen ERA293 could efficiently inhibit the growth of breast cancer MCF-7 cells in vitro, which temsirolimus could inhibit the transcriptional activity of ER via the mTOR pathway. A Phase II study enrolled 109 patients showed that temsirolimus alone exhibited antitumor activity in heavily pretreated patients with locally advanced or MBC [33]. A Phase II randomized open-label study was recently completed. In this clinical trial, postmenopausal women with locally advanced or MBC were treated with letrozole in combination with two dose levels and schedules of oral temsirolimus (10 mg daily and intermittent 30 mg daily for 5 days every 2 weeks) or letrozole alone [34]. However, the results of this study might be not available because the participants who completed the full trial only accounted approximately 3% (4/108). Although some preclinical and clinical studies have shown the possibility of mTOR inhibitor as a neuadjuvant therapy for patients with acquired endocrine resistance, more clinical trials are needed to confirm this issue.
Conclusion & future perspective
This review summarized the current understanding of endocrine resistance mechanisms and some novel strategies to overcome the resistance. Briefly, ER modification, the interaction between ER and its co-regulators and some alternative growth signaling are main aspects of the underlying mechanisms. The above knowledge fueled the development of strategies to prevent and/or overcome endocrine therapies by combining hormonal agents with drugs targeting several escape pathways, with an attempt to block all the tumor survival escapes. Evidence from preclinical and clinical studies suggested that treatment targeting both ER and growth factors pathways may hold a promising future in overcoming endocrine resistance and probably also to anti-HER therapy resistance. Future research efforts should be focused on optimizing individualized therapeutic regimens combining endocrine therapy with one or more pathway-targeted agents for hormone-response breast cancer patients.
Executive summary.
Endocrine therapy drugs can be summarized as three types: selective estrogen receptor modulators, selective ER downregulators and aromatase inhibitors.
Current endocrine therapies are limited by either intrinsic resistance or acquired resistance.
Mutations of ER, as well as crosstalk with bypass pathways, such as the HER2 pathway, are considered a major cause of endocrine resistance.
Combination of HER family inhibitors with endocrine therapy has shown clinical benefit.
Combination of mTOR inhibitors with endocrine therapy has shown clinical benefit.
Footnotes
Financial & competing interests disclosure
This work was supported in part by NNSF Grants (81071880 and 30973456 to W Fan), and NIH CA92880 (to W Fan). The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.Cazzaniga M, Bonanni B. Breast cancer chemoprevention: old and new approaches. J. Biomed. Biotechnol. 2012:985620. doi: 10.1155/2012/985620. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chang J, Sui M, Fan W. Estrogen receptor alpha attenuates therapeutic efficacy of paclitaxel on breast xenograft tumors. Breast Cancer Res. Treat. 2012;134(3):969–980. doi: 10.1007/s10549-012-1994-8. [DOI] [PubMed] [Google Scholar]
- 3.Sui M, Huang Y, Park BH, Davidson NE, Fan W. Estrogen receptor alpha mediates breast cancer cell resistance to paclitaxel through inhibition of apoptotic cell death. Cancer Res. 2007;67(11):5337–5344. doi: 10.1158/0008-5472.CAN-06-4582. [DOI] [PubMed] [Google Scholar]
- 4.Sui M, Jiang D, Hinsch C, Fan W. Fulvestrant (ici 182,780) sensitizes breast cancer cells expressing estrogen receptor alpha to vinblastine and vinorelbine. Breast Cancer Res. Treat. 2010;121(2):335–345. doi: 10.1007/s10549-009-0472-4. [DOI] [PubMed] [Google Scholar]
- 5.Schwartzberg LS, Wang G, Somer BG, et al. Phase ii trial of fulvestrant with metronomic capecitabine for postmenopausal women with hormone receptor-positive, her2-negative metastatic breast cancer. Clin. Breast Cancer. 2014;14(1):13–19. doi: 10.1016/j.clbc.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Mouridsen H, Gershanovich M, Sun Y, et al. Superior efficacy of letrozole versus tamoxifen as first-line therapy for postmenopausal women with advanced breast cancer: results of a Phase III study of the International Letrozole Breast Cancer Group. J. Clin. Oncol. 2001;19(10):2596–2606. doi: 10.1200/JCO.2001.19.10.2596. [DOI] [PubMed] [Google Scholar]
- 7.Osborne CK. Tamoxifen in the treatment of breast cancer. N. Engl. J. Med. 1998;339(22):1609–1618. doi: 10.1056/NEJM199811263392207. [DOI] [PubMed] [Google Scholar]
- 8.Early Breast Cancer Trialists' Collaborative G. Effects of chemotherapy and hormonal therapy for early breast cancer on recurrence and 15 year survival: an overview of the randomised trials. Lancet. 2005;365(9472):1687–1717. doi: 10.1016/S0140-6736(05)66544-0. [DOI] [PubMed] [Google Scholar]
- 9.Strasser-Weippl K, Goss PE. Advances in adjuvant hormonal therapy for postmenopausal women. J. Clin. Oncol. 2005;23(8):1751–1759. doi: 10.1200/JCO.2005.11.038. [DOI] [PubMed] [Google Scholar]
- 10.Ring A, Dowsett M. Mechanisms of tamoxifen resistance. Endocr. Relat. Cancer. 2004;11(4):643–658. doi: 10.1677/erc.1.00776. [DOI] [PubMed] [Google Scholar]
- 11.Khan SA, Rogers MA, Khurana KK, Meguid MM, Numann PJ. Estrogen receptor expression in benign breast epithelium and breast cancer risk. J. Natl Cancer Inst. 1998;90(1):37–42. doi: 10.1093/jnci/90.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Fan J, Yin WJ, Lu JS, et al. Er alpha negative breast cancer cells restore response to endocrine therapy by combination treatment with both HDAC inhibitor and DNMT inhibitor. J. Cancer Res. Clin. Oncol. 2008;134(8):883–890. doi: 10.1007/s00432-008-0354-x. [DOI] [PubMed] [Google Scholar]
- 13.Herynk MH, Fuqua SA. Estrogen receptor mutations in human disease. Endocr. Rev. 2004;25(6):869–898. doi: 10.1210/er.2003-0010. [DOI] [PubMed] [Google Scholar]
- 14.Bardou VJ, Arpino G, Elledge RM, Osborne CK, Clark GM. Progesterone receptor status significantly improves outcome prediction over estrogen receptor status alone for adjuvant endocrine therapy in two large breast cancer databases. J. Clin. Oncol. 2003;21(10):1973–1979. doi: 10.1200/JCO.2003.09.099. [DOI] [PubMed] [Google Scholar]
- 15.Tovey S, Dunne B, Witton CJ, Forsyth A, Cooke TG, Bartlett JM. Can molecular markers predict when to implement treatment with aromatase inhibitors in invasive breast cancer? Clin. Cancer Res. 2005;11(13):4835–4842. doi: 10.1158/1078-0432.CCR-05-0196. [DOI] [PubMed] [Google Scholar]
- 16.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-her2/neu cross-talk in ER/HER2-positive breast cancer. J. Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]
- 17.Lipton A, Ali SM, Leitzel K, et al. Serum her-2/neu and response to the aromatase inhibitor letrozole versus tamoxifen. J. Clin. Oncol. 2003;21(10):1967–1972. doi: 10.1200/JCO.2003.09.098. [DOI] [PubMed] [Google Scholar]
- 18.Benz CC, Scott GK, Sarup JC, et al. Estrogen-dependent, tamoxifen-resistant tumorigenic growth of MCF-7 cells transfected with HER2/neu. Breast Cancer Res. Treat. 1992;24(2):85–95. doi: 10.1007/BF01961241. [DOI] [PubMed] [Google Scholar]
- 19.Chung YL, Sheu ML, Yang SC, Lin CH, Yen SH. Resistance to tamoxifen-induced apoptosis is associated with direct interaction between HER2/neu and cell membrane estrogen receptor in breast cancer. Int. J. Cancer. 2002;97(3):306–312. doi: 10.1002/ijc.1614. [DOI] [PubMed] [Google Scholar]
- 20.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized Phase III tandem study. J. Clin. Oncol. 2009;27(33):5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 21.Marcom PK, Isaacs C, Harris L, et al. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res. Treat. 2007;102(1):43–49. doi: 10.1007/s10549-006-9307-8. [DOI] [PubMed] [Google Scholar]
- 22.Kaufman B, Mackey JR, Clemens MR, et al. Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results from the randomized Phase III TAnDEM study. J. Clin. Oncol. 2009;27(33):5529–5537. doi: 10.1200/JCO.2008.20.6847. [DOI] [PubMed] [Google Scholar]
- 23.Marcom PK, Isaacs C, Harris L. The combination of letrozole and trastuzumab as first or second-line biological therapy produces durable responses in a subset of HER2 positive and ER positive advanced breast cancers. Breast Cancer Res. Treat. 2007;102(1):43–49. doi: 10.1007/s10549-006-9307-8. [DOI] [PubMed] [Google Scholar]
- 24.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 2009;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 25.http://clinicaltrials.gov/ct2/show/NCT01160211 ClinicalTrialsDatabase: NCT01160211.
- 26.https://clinicaltrials.gov/ct2/show/NCT00999804 ClinicalTrials Database: NCT00999804.
- 27.https://clinicaltrials.gov/ct2/show/NCT00759642 ClinicalTrials Database: NCT00759642.
- 28.Johnston S, Pippen J, Jr, Pivot X, et al. Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone receptor-positive metastatic breast cancer. J. Clin. Oncol. 2009;27(33):5538–5546. doi: 10.1200/JCO.2009.23.3734. [DOI] [PubMed] [Google Scholar]
- 29.Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mtor and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin. Cancer Res. 2005;11(14):5319–5328. doi: 10.1158/1078-0432.CCR-04-2402. [DOI] [PubMed] [Google Scholar]
- 30.Baselga J, Semiglazov V, Van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J. Clin. Oncol. 2009;27(16):2630–2637. doi: 10.1200/JCO.2008.18.8391. [DOI] [PubMed] [Google Scholar]
- 31.https://clinicaltrials.gov/ct2/show/NCT01231659 ClinicalTrials Database: NCT01231659.
- 32.https://clinicaltrials.gov/ct2/show/NCT00107016 ClinicalTrials Database: NCT00107016.
- 33.Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (cci-779), a novel inhibitor of mtor, in heavily pretreated patients with locally advanced or metastatic breast cancer. J. Clin. Oncol. 2005;23(23):5314–5322. doi: 10.1200/JCO.2005.66.130. [DOI] [PubMed] [Google Scholar]
- 34.https://clinicaltrials.gov/ct2/show/NCT00062751 ClinicalTrials Database: NCT00062751.
- 35.Harvard University Herbaria & Libraries. http://kiki.huh.harvard.edu/databases/specimen_search.php?mode=details&id=537950