Abstract
Inherited genetic variants contribute to risk factors for developing an alcohol use disorder, and polymorphisms may inform precision medicine strategies for treating alcohol addiction. Targeting genetic mutations linked to alcohol phenotypes has provided promising initial evidence for reducing relapse rates in alcoholics. Although successful in some studies, there are conflicting findings and the reports of adverse effects may ultimately limit their clinical utility, suggesting that novel pharmacogenetic targets are necessary to advance precision medicine approaches. Here, we describe promising novel genetic variants derived from preclinical models of alcohol consumption and dependence that may uncover disease mechanisms that drive uncontrolled drinking and identify novel pharmacogenetic targets that facilitate therapeutic intervention for the treatment of alcohol use disorder.
Keywords: : alcohol use disorder, K+ channels, neuroimmune genes, pharmacogenetics, preclinical models, RAS signaling, tachykinins
Alcohol use disorder (AUD) is a chronic neurological disorder characterized by an inability to regulate alcohol intake despite a host of serious and harmful health, societal and personal consequences [1]. The current pharmacotherapies available for AUD treatment have been met with variable outcomes that are modest at best and thus have not been widely embraced by the medical community. This gap in treatment options provides an opportunity to explore new avenues toward identifying novel pharmacotherapeutic targets. As with other addictive disorders, AUD is influenced, to a considerable degree, by genetics, with heritability estimates upward of 60% [2,3]. Despite the comparatively large influence of genetics, the exact etiology of AUD is by no means simple or straightforward, which underscores the difficulty in effective treatment of this disorder. In this review, we provide an evaluation of our understanding of the genetics of AUD in relation to current precision medicine approaches. Demonstrating the considerable heterogeneity that exists within the AUD population and understanding the contribution of genetic variation to treatment response provides a framework for promoting a pharmacogenetic approach. Moreover, understanding the genetic variation among individuals with AUD in treatment response will provide valuable information that will allow for personalized treatment plans.
A diagnosis of AUD is based strictly on a set of clinical diagnostic criteria, because as of yet, there are no reliable biomarkers to identify AUD or subpopulations that might be more or less responsive to certain medications. This means that, at best, we are treating AUD by trial and error on a, presumably, largely heterogeneous population. There are currently only three US FDA approved pharmacotherapies for treating AUD, naltrexone, acamprosate and disulfiram, all of which, however, have been met with limited success. This limited success is, in large part, attributable to the heterogeneity of the AUD patient population [4]. The interest in understanding the variable treatment responses in AUD patients has led to over a decade worth studies examining the pharmacogenetics of AUD (e.g., [5]). During this time, a number of SNPs have been identified and associate with various aspects of alcohol drinking and dependence. Because a number of recent reviews have detailed these SNPs [4,6–8], we will briefly highlight a few previously identified SNPs in the human AUD population with druggable targets and discuss how this has informed pharmacotherapeutic interventions. The remainder of the review will focus on newly identified pharmacogenetic targets from preclinical models that may provide novel treatment strategies for AUD. Finally, we incorporate transcriptome changes reported for these targets in postmortem brain tissue from alcoholics and provide initial supportive evidence for SNPs in these same genes that associate with alcohol consumption and dependence in humans.
Current precision medicine approaches for treating AUD
One of the earlier reported genetic variations associated with AUD was the GABAA receptor. Specifically, a decrease in the prevalence and frequency of the major allele (G1) of the GABRB3 was associated with increased severity of alcoholism [9]. Subsequent to this, SNPs in genes coding for a number of additional GABAA receptor subunits were also found to be associated with alcohol dependence and subjective effects of alcohol, including: GABRA1 [10], GABRA2 [11–18], GABRG1 [19,20], GABRG3 [21]. There is a long history of GABAergic pharmacotherapies in treating AUD; unfortunately, drugs that target GABAA receptor subtypes have largely been abandoned due to the significant cross-tolerance with alcohol, the additive depressant effects on the CNS and the significant abuse potential. Thus, benzodiazepines have been relegated for use during the acute detoxification period [22].
In addition to the GABAergic receptor system, SNPs have been reported in numerous other neurotransmitter systems, producing a number of potential druggable targets, some with more promise than others. Polymorphisms in a number of cholinergic receptors have been implicated in the risk for AUD, associated with frequency of binge drinking, and subjective response to alcohol, including CHRM2 [23,24], CHRNA4 [25–27], CHRNA5 [28], CHRNB2 [26] and the CHRNA5–CHRNA3–CHRNB4 cluster [29]. Recent advances in the treatment of nicotine dependence have given way to a number of potential pharmacotherapeutics to treat AUD. Mecamylamine, a nonselective nicotinic receptor antagonist, and varenicline, a partial agonist of the α4β2* nicotinic receptors (*indicates the possible presence of other nicotinic receptor subunits in the pentameric complex), have both shown potential for reducing alcohol consumption in individuals who smoke and have AUD [30–33], and recent evidence suggests that varenicline may be effective independent of smoking status [34]. Unfortunately, to our knowledge, none of the studies presently published have assessed efficacy by genotype interactions, information that could provide insight for enhanced treatment effectiveness in AUD subpopulations.
There have been several SNPs that show some potential in predicting pharmacotherapy treatment response. The most well known of these, perhaps, is the polymorphism in OPRM1 gene. Several studies suggest that the adenine to guanine substitution at position 118 (A118G), which results in a likely loss of function and expression of the μ-opioid receptor in the G allele carriers [35,36], is predictive of naltrexone response in individuals with AUD [37–40]. Additionally, this predictive relationship is further supported by a similar relationship between the analogous SNP (C77G in the OPRM1 gene) in rhesus macaques and alcohol consumption and naltrexone response [41]. As well, a reverse translational approach examining the A118G SNP in a humanized mouse model show similar enhancements in alcohol consumption and response to naltrexone in the mice homozygous for the G allele [42,43]. While encouraging, these studies are tempered by a number of conflicting reports showing inconsistencies or no predictive relationship between the A118G polymorphism and naltrexone response [44–47]. The relationship between the OPRM1 gene and naltrexone response in individuals with AUD is further complicated by the possible influence of other concomitantly expressed SNPs, for example, DAT1 [48–50].
A number of SNPs in the serotonergic system have also been associated with AUD, including HTR1B [51–53], HTR2A [54–57], and more recently, HTR3A and HTR3B [58] as well as SLC6A4 [58,59]. In an initial study, genotypic combinations of polymorphisms in the HTR3A, HTR3B and SLC6A4 genes predicted treatment outcome with the 5-HT3 antagonist, ondansetron, among alcohol-dependent individuals [60]. These results, while promising, should be taken with caution, as more data are needed to validate the predictive abilities of these genotypic variations.
And while genotypic variations can be useful in predicting responsiveness to pharmacotherapeutics interventions, they can also be used to predict likelihood of adverse effects of the pharmacological intervention which may limit its utility, as is the case with topiramate. Topiramate is an anticonvulsant that works through a number of different mechanisms, including antagonism at AMPA and Kainate receptors, allosteric modulation of GABAA receptors, inhibition of voltage-gated Na+ and Ca2+ channels, and enhancement of K+ conductance. Topiramate has shown great promise as a potential pharmacotherapeutic in the treatment of AUD, reducing alcohol consumption and increasing abstinent periods [61,62] and reducing alcohol craving [63]. However, it is commonly associated adverse drug effects including, fatigue, sleepiness and nervousness, among others [64], thus as with many AUD treatments, overall response to treatment with topiramate varied. Thus, using a reverse pharmacogenetics approach, interrogation of SNPs in the genes encoding the Kainate receptor subunits (GRIK1 and GRIK2), which topiramate most potently and selectively inhibits, was initiated to determine potential variants that might predict better treatment outcomes. An SNP in the GRIK1 gene was found to be significantly associated with adverse effects of topiramate [65], which, in turn, moderates the therapeutic response in individuals with AUD [64,66].
These examples highlight the importance of examining the genotypic variation among individuals with AUD, but even with all this potential, we are still met with limited treatment efficacy and only minor progress over the course of decades of study. While the ultimate goal is to determine pharmacogenetic targets for use in treating the clinical population, identifying and understanding the influence of individual SNPs, alone and in combination, poses a number of difficulties and current studies vary in power, size, subject demographics, SNP examined and outcome. Additionally, these issues can be somewhat mitigated by utilizing preclinical models to facilitate a higher throughput strategy of identifying SNPs and testing potential druggable targets for the treatment of AUD prior to testing in clinical populations, rather than concurrently or post hoc to garner clarity. The remainder of this review will focus on several more recently identified genetic variations identified in preclinical models of AUD. Specifically, we will discuss promising preclinical evidence linking alcohol drinking with SNPs in genes encoding K+ channels, neuroimmune signaling proteins, neurokinin receptors and the RAS superfamily of proteins (Table 1). In a number of the preclinical studies, there is evidence showing reductions in alcohol consumption when these targets are pharmacologically manipulated, providing initial validation that these genes are promising pharmacogenetic targets for treating AUD.
Table 1. . Genes with polymorphisms related to alcohol consumption, dependence and other alcohol-related behaviors.
| Gene | Protein | Function | SNP | PMID |
|---|---|---|---|---|
|
RAS signaling family | ||||
|
RASGRF2 |
Ras-specific guanine nucleotide-releasing factor 2 |
Ras and RAC1 activator |
rs26907 |
21471458 |
|
NF1 |
Neurofibromin |
Negative regulator of Ras GAP |
Numerous |
25483400 |
|
KALRN |
Kalirin |
Rho GTPase activator |
rs6438839, rs4634050 |
27092175 |
|
RSU1 |
Ras suppressor protein 1 |
Rac1 suppressor |
Numerous |
26170296 |
|
Rab42 |
Ras-related protein Rab-42 |
Putative Ras activator |
NR |
25833023 |
|
Rap1gap |
Rap1 GTPase-activating protein 1 |
Rap1 suppressor |
NR |
25833023 |
|
PPAR family | ||||
|
PPARA |
PPAR-α |
Nuclear receptor that binds peroxisome proliferators |
NR |
25516156 |
|
PPARG |
PPAR-γ |
Nuclear receptor that binds peroxisome proliferators |
NR |
25516156 |
|
PPARGC1A |
PPAR-γ coactivator 1-α |
Transcriptional coactivator |
NR |
25516156 |
|
K+ channel family | ||||
|
KCNB2 |
KV2.2 |
Voltage-gated delayed-rectifier |
rs2128158, rs2929567 & cis-eQTL |
23953852 |
|
KCND2 |
KV4.2 |
Voltage-gated A-type |
rs728115, rs17142876 |
21956439, 22488850, 25603899 |
|
KCNMA1 |
KCa1.1 |
Calcium- and voltage-activated, Large conductance |
rs717207, rs12219105 |
21314694, 20201924 |
|
KCNQ1 |
KV7.1 |
Voltage-gated, M-current delayed rectifier |
rs12574151 |
20201924 |
|
KCNQ5 |
KV7.5 |
Voltage-gated, M-current delayed rectifier |
rs3799285 |
21314694 |
|
Kcnq2 |
KV7.2 |
Voltage-gated, M-current delayed rectifier |
rs27642425, rs2971971 & cis-QTL |
26104325, 25581648 |
|
Kcnj10 |
KIR4.1 |
Inwardly rectifying |
rs46006714 |
19053975 |
|
Neurokinin family | ||||
|
TACR1 |
NK1R |
Gq-GPCR activated by substance P |
rs6715729–rs735668–rs6741029 |
19553914 |
| Tacr1 | NK1R | Gq-GPCR activated by substance P | NR | 23419547 |
NR: Not reported; PMID: PubMed identification number.
Preclinical pharamcogenetic targets for treating alcohol addiction
K+ channels
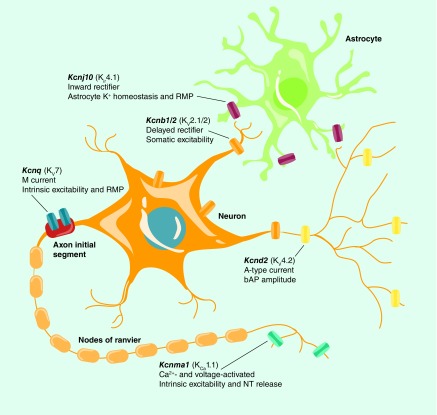
Although topiramate appears quite effective at reducing alcohol craving and consumption in dependent individuals, its utility as a pharmacotherapeutic for AUD is tempered by a host of adverse side effects. Recent interest has been directed toward other well-tolerated anticonvulsants that target potassium (K+) channels to potentially fill this gap in treatment options. Potassium channels represent one of the most diverse groups of ion channels with 79 genes encoding K+ channel subunits (KCN*), and functional channels formed in a monomeric or heteromeric fashion giving rise to many more functional channels than genes [67,68]. Potassium channels generally fall into one of four major categories based on their physiological, pharmacological and structural characteristics: voltage-gated, calcium-activated, inwardly rectifying and two-pore domain [68]. All K+ channels are responsible, in some respect, for stabilizing the membrane potential, stimulating repolarization and regulating neuronal excitability (see Figure 1) [67,68]. While better known for their role in epileptic disorders [68,69], a number of clinical and preclinical studies have identified a potential role for genetic variants in K+ channel genes as mediators of alcohol consumption and dependence [70–78].
Figure 1. . Schematic identifying prominent subcellular localization of K+ channels in neurons and astroglia with SNPs and correlations related to alcohol consumption.
bAP: Back-propagating action potential; NT: Neurotransmitter; RMP: Resting membrane potential.
Images were acquired with permission from [79] and subsequently modified.
Mutations in human KCNQ2 and KCNQ3, which encode the KV7.2 and KV7.3 subunits of the voltage-gated K+ channels responsible for M-current, result in reduced function of KV7 channels and seizures associated with epilepsy suggesting that these channels could be key targets in regulating neuronal hyperexcitability associated with a host of disorders, including alcohol dependence [80]. Recent preclinical evidence showed that acute alcohol directly inhibits KV7 channel activity resulting in reduced M-current and a corresponding increase in the firing rate of hippocampal pyramidal neurons and dopaminergic VTA neurons [81,82]. In addition, a recent study reported that 10 mM alcohol blocked Drosophila KCNQ currents and flies with KCNQ loss-of-function in dopaminergic neurons showed increased tolerance and sensitivity to the sedating effects of alcohol exposure [83]. Interestingly, several studies have implicated the Kcnq family of genes in preclinical rodent alcohol drinking models. Kcnq2 expression was increased in the ventral striatum of mice selectively bred for high alcohol consumption/low withdrawal severity compared with those bred for low alcohol consumption/high withdrawal severity (Table 2) [84]. Additionally, these authors found that Kcnq2 was a positional candidate within the cis-eQTL for alcohol consumption and withdrawal. Kcnq2 and Kcnq3 were reported to lie within multiple alcohol-related QTLs and two candidate SNPs in Kcnq2 were identified that differentiated between high drinking and low drinking mouse strains [74]. Another study examining alcohol consumption in the BXD recombinant inbred strains of mice showed that differential expression of Kcnq5 across strains negatively correlated with drinking, further implicating this family of K+ channels in regulating alcohol drinking [76]. Using whole genome sequencing, a recent study reported that Kcnq5 was differentially expressed between low and high alcohol drinking rat lines [85], and multiple reports identify transcript changes in the KCNQ family of genes in alcoholic brain and preclinical models (Table 2). It should be noted that transcriptome changes in expression of KCNQ and other alcohol-sensitive K+ channel genes are not reported consistently in preclinical and human postmortem studies [86–88]. However, in support of the evidence linking KCNQ and alcoholism, SNPs associated with human alcohol consumption have also been reported in KCNQ1 and KCNQ5 [89,90]. To test the viability of the Kcnq family of genes as a pharmacogenetics target, several preclinical studies have demonstrated that systemic administration of the KV7 channel opener, retigabine, significantly reduces alcohol consumption in both rats and mice [74,76,91], and is most efficacious in those that display a heavy drinking phenotype [74,76]. What is particularly encouraging about these studies is that retigabine is already US FDA approved to treat epileptic disorders and is well-tolerated in the clinical population, and while there is some evidence that alcohol alters pharmacokinetics of retigabine in moderate drinkers, it did not alter the pharmacodynamics measures or the adverse effects of retigabine [92]. Additionally, retigabine shows minimal off target effects in rodents at doses that alter alcohol consumption, giving KV7 channels substantial potential as a pharmacogenetic target for treating AUD.
Table 2. . K+ channel genes that are altered by chronic alcohol drinking or alcohol dependence in brain from human alcoholics or animal models.
| Gene | Species | Diagnosis or preclinical model | Region | Direction of change | PubMed ID |
|---|---|---|---|---|---|
|
KCNB1 |
Human |
Alcohol abusers |
Hippocampus |
↑ |
26041984 |
|
Kcnb1 |
Mouse |
Chronic intermittent alcohol in BXD recombinant inbred strains |
Prefrontal cortex, nucleus accumbens |
NR |
27838001, 27432260 |
|
KCNB1/2 |
Human |
DSM-IV for alcohol abuse |
Superior frontal gyrus |
NR |
25450227 |
|
KCND2 |
Human |
DSM-IV for alcohol abuse |
Amygdala, frontal cortex |
↓ |
22302827 |
|
Kcnd2 |
Mouse |
Chronic intermittent alcohol in BXD recombinant inbred strains |
Prefrontal cortex, nucleus accumbens |
NR |
27838001, 27432260 |
|
KCNJ10 |
Human |
Alcoholics (>80 g alcohol/day) |
Superior frontal cortex |
↓ |
11141048 |
|
KCNJ10 |
Human |
Alcoholics (>0.50 g alcohol/day) |
Hippocampus |
↓ |
23981442 |
|
KCNMA1 |
Human |
DSM-IV for alcohol abuse |
Frontal cortex |
↓ |
22302827 |
|
Kcnma1 |
Mouse |
Chronic intermittent alcohol in BXD recombinant inbred strains |
Prefrontal cortex, nucleus accumbens |
NR |
27838001, 27432260 |
|
KCNQ2 |
Human |
Alcoholics (>100 g alcohol/day) |
Amygdala |
↑ |
20153402 |
|
Kcnq2 |
Mouse |
Continuous alcohol access |
Amygdala (synaptoneurosomes) |
↓ |
25135349 |
|
Kcnq2 |
Mouse |
Chronic intermittent alcohol |
Prefrontal cortex |
↑ |
25803291 |
|
Kcnq2 |
Mouse |
High drinking/low withdrawal and low drinking/high withdrawal mouse lines |
Ventral striatum |
NR |
25581648 |
|
Kcnq2 |
Mouse |
High and low alcohol drinking mouse strains |
Whole brain |
↑ |
16618939 |
|
KCNQ2/3 |
Human |
DSM-IV for alcohol abuse |
Frontal cortex |
↑ |
22302827 |
|
Kcnq3 |
Rat |
Continuous alcohol access & alcohol self-administration |
Nucleus accumbens core |
↓ |
19666046, 18405950 |
|
Kcnq5 |
Mouse |
Chronic intermittent alcohol in BXD recombinant inbred strains |
Prefrontal cortex |
NR |
27838001, 27432260 |
|
Kcnq5 |
Rat |
Continuous alcohol access |
Dorsal hippocampus |
↑ |
12462420 |
| Kcnq5 | Rat | High and low alcohol drinking rat lines | Whole genome | NR | 27490364 |
NR: Not reported; PMID: PubMed identification number.
In addition to Kcnq genes, a number of other K+ channel SNPs (Table 1) and transcriptome changes (Table 2) in human alcoholics and preclinical models have been reported that present unique opportunities for the development of small molecules to target these channels. One such target is the inwardly rectifying K+ channel 4.1 protein (KIR4.1), encoded by the Kcnj10 gene. KIR4.1 is largely expressed on glial cells and is involved in K+ buffering action of astrocytes [93], and mutations in Kcnj10 result in increased seizure susceptibility [94,95]. The genomic location of Kcnj10 also maps onto QTLs for both alcohol preference [96] and withdrawal [97,98], making Kcnj10 an attractive candidate gene. Interestingly, C57BL/6J mice show a downregulation in brain levels of Kcnj10 transcript in comparison with DBA/2J mice [99], and DBA/2J mice express a SNP in Kcnj10 (C → G), which generates a missense mutation in KIR4.1, resulting in greater susceptibility to seizures. In an elegant demonstration, Zou and colleagues showed that when C allele carriers (C57BL/6J allele) were crossed with G allele carriers (DBA/2J allele), the F2 offspring showed a genotype-dependent preference for alcohol, with animals carrying the C allele having the highest preference [99]. In support of these findings, analogous SNPs in KCNJ10 have been reported to alter seizure activity in humans [100], and KCNJ10 transcripts are reduced in postmortem brain tissue from the superior frontal cortex of human alcoholics [101]. Progress is limited by the lack of pharmacological tools to manipulate KIR4.1, but these results are encouraging and represents a promising opportunity for drug development.
A number of other voltage-gated K+ channel genes have been associated with AUD as genome wide association studies (GWAS) become more prevalent. The gene encoding the KV2.2 channel protein, KCNB2, was found in a cis-eQTL for alcohol consumption, and two SNPs in the KCNB2 gene are associated with maximum number of drinks consumed in 24 h [102]. Additionally, transcript levels of the gene encoding the KV2.1 channel protein, KCNB1, was increased in hippocampus from alcoholic postmortem brain [103], and expression of both KCNB1 and KCNB2 in the superior frontal gyrus were strongly associated with lifetime drinking in alcoholics [78]. In a preclinical model, expression of Kcnb1 in the nucleus accumbens correlated negatively with alcohol intake in BXD mice [76], indicating a potential role of the KCNB family in regulating alcohol consumption across species. Another voltage-gated K+ channel identified through GWAS exploration was KCND2 that encodes for the KV4.2 channel that regulate rapidly inactivating A-type K+ current (I A). A SNP in KCND2 was found in the top ten SNPs associated with alcohol and nicotine dependence and alcohol dependence alone [104–106]. And finally, an SNP in the KCNMA1 gene, which encodes the α1 subunit of KCa1.1 was significantly associated with the development of alcohol dependence [89,90] and subjective response to alcohol [107]. These associations are supported by experimental evidence showing a negative correlation between Kcnma1 and alcohol consumption [76] and a role for this gene in the development of tolerance [108–110]. Given that many of these K+ channel genes are altered in postmortem brain tissue from alcoholics (Table 2) [78,103,111], further preclinical research into the potential involvement of KV2.1/2, KV4.1 and KCa1.1 in regulating alcohol consumption is warranted, but will require better pharmacological tools.
Ras signaling
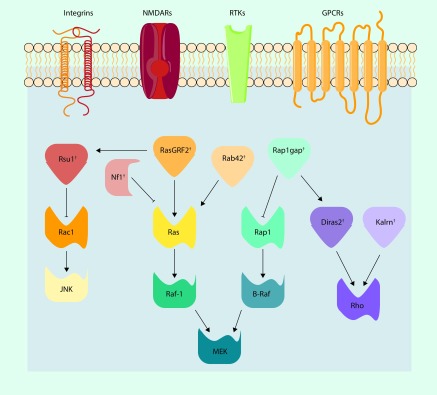
The RAS superfamily of GTPase signaling proteins function as binary molecular switches by cycling between active GTP-bound and inactive GDP-bound states to modulate a diverse range of intracellular processes. In the mature brain, RAS GTPases control synaptic and morphological plasticity that are important for memory formation [112] and, as described below, are implicated in alcohol addiction. Indeed, converging evidence has demonstrated that SNPs in RAS signaling proteins are prominent in preclinical and clinical genetic studies on alcohol drinking and alcohol dependence (Figure 2). An elegant cross-species study linked Rsu1/β-integrin/Rac1 signaling with actin cycling and alcohol consumption in Drosophila [113]. These authors also reported that polymorphisms in RSU1 are associated with alcohol dependence in adults and increased NAc activation during reward processing in adolescents. Kras+/− mice do not escalate their drinking following induction of alcohol dependence [114], and knockout of Nf1, a negative regulator of Ras, does not affect drinking in multiple models of heavy drinking but prevents escalation of intake in alcohol-dependent mice [115]. SNPs in NF1 associate with alcohol dependence and alcoholism severity in African and European–Americans [115], whereas Rab42 (putative Ras activator) and Rap1gap (a negative regulator of Rap-1A) SNPs associate with acute functional tolerance in LXS recombinant inbred strains of mice [116]. Interestingly, a GWAS meta-analysis reported that an SNP in RASGRF2 (rs26907), another Ras activator, was associated with alcohol intake [117]. A comprehensive follow-up functional characterization study targeting Ras–GRF2 demonstrated reduced alcohol consumption and preference and attenuated alcohol-induced dopamine release in ventral striatum in mice with genetic depletion of the Rasgrf2 gene [118]. Knockdown of Rasgrf2 reduced intrinsic excitability of VTA dopamine neurons likely mediated by a change I A. Interestingly, KV4 channels control I A current amplitude and firing of dopamine neurons [119], and, as discussed above, SNPs in genes that encode KV4 channels associate risk for developing alcohol and nicotine co-dependence [105,106].
Figure 2. . SNPs in RAS superfamily of GTP hydrolysis-coupled signal transduction relay proteins related to alcohol intake and alcohol-related behaviors.
Plasma-membrane bound integrins, NMDARs, RTKs, and GPCRs can provide an activation trigger for RAS signal transduction. Functional SNPs in proteins in the Ras, Rac, and Rho pathways may impact cellular proliferation and adhesion, membrane trafficking, and cytoskeletal morphology. †RAS superfamily-related proteins with SNPs or genetic variations correlated with alcohol intake/behaviors.GPCR: G-protein coupled receptor; NMDAR: NMDA-type glutamate receptor; RTK: Receptor tyrosine-kinase.
Images were acquired from [79] and subsequently modified.
Impaired impulsivity and altered brain responsivity in areas responsible for executive functioning and reward processing are risk factors for developing an AUD [120,121]. A recent preclinical study examined expression patterns of cortical genes and impulsive behavior in BXD strains of mice [122]. PFC transcript levels of Kalrn, a gene encoding the Rho activating GTPase kalirin, significantly correlated with premature responding in BXDs tested on the 5-choice serial reaction time task. Of the human homologs of genes that correlated with premature responding in mice, SNPs in KALRN were associated with enhanced activation of the ventral striatum during anticipation of rewards and an increased frequency of binge drinking in adolescents [122]. The basolateral amygdala sends excitatory projections to the PFC, and a recent paper reported reduced KALRN expression in the basolateral amygdala from human alcoholic postmortem tissue [111]. An exploratory bioinformatics study identified Di-Ras2, a Rho activator, as a key synaptic protein that discriminated between low and heavy drinking cynomolgus macaques [123]. In addition, PFC expression levels of Diras2 negatively correlated with drinking in BXDs, and DIRAS2 is found within multiple published QTLs for alcohol drinking and dependence in human and rodent studies [123]. Similar to KALRN, DIRAS2 expression is significantly reduced in the basolateral amygdala of alcoholics [111]. Importantly, data from Ron and colleagues showing that pharmacological inhibition of H- and K-Ras signaling with the farnesyltransferase, FTI-276, in the nucleus accumbens reduces voluntary alcohol drinking and alcohol self-administration in rats [124] validates the RAS superfamily as pharmacogenetic targets for treatment of AUD. The high rate of K-Ras mutations in multiple forms of cancer has led to the development of anticancer drugs that target the RAS signaling system that are now under clinical trials [125]. Progress in anticancer drug development may identify novel pharmacogenetic therapies that could be tested in treatment-seeking alcoholics with SNPs in RAS genes.
Neurokinin receptor 1
The tachykinins are a family of neuromodulatory peptides, including substance P (SP), neurokinin-A and neurokinin-B, which bind with varying affinities to the family of neurokinin receptors, NK1R, NK2R or NK3R [126]. For the purposes of this review, emphasis will be placed on the NK1R and its preferential endogenous ligand, SP, due to its recently discovered role in drug addiction. Activation of NK1R, a Gq-coupled GPCR, by SP results in a host of downstream consequences, including: endocrine secretion, transmission of pain signals, vasodilation and neuroimmune signaling [127], and more recently modulating stress and anxiety [128]. NK1R are located throughout the brain, including regions involved in mediating the reinforcing properties of alcohol, like the ventral tegmental area [129], ventral striatum [130–132] and extended amygdala [133,134]. Preclinical data implicate a role for NK1R signaling in opiate reward and reinforcement [133,135–138]. Given the known overlap in mechanisms between opiates and alcohol [139], it is not surprising that more recently NK1R have also been implicated in modulating alcohol consumption and reward [140–142]. Additionally, the NK1R seems to be particularly involved in the stress-related aspects of alcohol consumption, as NK1R antagonism reduced stress-induced, but not cue-induced, reinstatement of alcohol seeking [143,144] and escalation of alcohol consumption [140].
Interestingly, it has recently been shown that the alcohol-preferring (P) rats, which are selectively bred for high alcohol preference, display increased sensitivity to the effects of NK1R antagonism on alcohol self-administration and motivation to work for alcohol compared with their Wistar parent strain [145]. What is more intriguing about the genetically selected P rats is that they show an upregulated NK1R system, that is, increased transcript levels of Tacr1 and Tac1 [145]. Furthermore, Schank and colleagues also showed the presence of a G–C SNP in the transcriptional start sequence for Tacr1 and an increased transcription factor binding in the presence of the C allele, indicative of increased transcriptional activation of Tacr1. The importance of this finding is underscored by the fact that 100% of the P rats expressed the C allele, whereas only 18% of the Wistar controls did, suggesting that the SNP in Tacr1, and thereby signaling at the NK1R, contributes to a predisposition toward alcohol preference, and predicts the response to drugs that target NK1R. These findings are in line with the human literature that has found several SNPs in the TACR1 gene that are associated with a risk for alcohol dependence [146]. Additionally, there is some evidence that NK1R antagonists, like aprepitant which is US FDA approved for the treatment of nausea and emesis, can be utilized off-label for the treatment of stress, anxiety and depression [147–149] as well as reducing alcohol craving and stress-induced craving [141]. Together with the preclinical evidence suggesting that a SNP in the Tacr1 gene can result in increased alcohol consumption and increased responsiveness to NK1R antagonism, these studies strongly support increased efforts in identifying the functional consequences of polymorphisms in Tacr1 and Tac1 on a molecular and cellular level, as well as clinical studies incorporating prospective genotyping to determine explicitly the relationship between genotypic variation and efficacy of NK1R inhibition.
Neuroimmune signaling
Preclinical studies provide emerging evidence that neuroimmune genes are promising targets for treating AUD [150]. Meta-analyses of gene expression studies in alcohol preferring strains of mice and postmortem tissue from human alcoholics revealed dysregulation of neuroimmune and neuroinflammatory signaling pathways [151]. Null mutant mice for six of these dysregulated immune candidate genes (B2m, Ctsf, Ctss, Il1rn, Cd14 and Il6) showed reduced voluntary alcohol intake and preference [151]. The findings from this study validate the use of genomic analysis for identifying functional groups of genes that regulate alcohol consumption and, in doing so, indicated a novel role for neuroimmune signaling in controlling drinking. PPARs are nuclear proteins that regulate gene expression and control neuroimmune responses and inflammation [152]. Expression of PPARD (PPARδ subunit) and PPARGC1A (PPAR-γ coactivator) are altered in the basolateral and central amygdala of alcoholics [111], and PPAR agonists show promise in preclinical models of alcohol drinking, stress-induced relapse and withdrawal [153–157]. Because of the strong preclinical pharmacology and genetic data linking PPAR signaling with alcohol intake, a clinical trial is currently underway to determine if the PPAR agonist fenofibrate will decrease craving for alcohol following cue-exposure and reduce the number of drinks consumed in alcohol-dependent subjects (ClinicalTrial.gov trial number: NCT02158273). A recent study demonstrated that SNPs in PPARG and PPARGC1A were associated with alcohol dependence in European–American alcoholics [155]. Moreover, these authors also reported that PPARA and PPARG were associated with a DSM-IV criterion for an alcohol withdrawal phenotype. Future studies are necessary to determine if polymorphisms in PPARG and PPARGC1A moderate behavioral responses to treatment with PPAR agonists in alcoholics.
Future perspective
In this review, we have demonstrated that the complex nature of the genetic basis of AUD can influence precision medicine approaches for treating alcohol addiction. The diverse, heterogeneous population of individuals diagnosed with AUD makes determining pharmacogenetic targets from human GWAS difficult in that the inherently qualitative nature of defining the AUD phenotype could lead to false positives within a study or the masking of relevant results due to influence and interaction of, and often times lack of control for, other factors such as comorbid disease states (e.g., impulsivity, anxiety, depression, co-dependence on other drugs of abuse, among others). Additionally, the stringent nature of GWAS in general means that, unless GWAS sample sizes are increased significantly, SNPs that may be related to the disease state could be masked in an effort to avoid false positives. In some studies, pharmacogenetic targets previously identified through clinical studies have failed to reveal viable targets and resulted in inconsistent success in predicting treatment response. In an effort to gain a better understanding and produce higher throughput results, we highlight a number of translational findings from rodent genetic analyses and preclinical pharmacology studies where there is supporting genetic evidence in clinical studies. Although these SNPs do not always achieve genome wide significance for alcohol use disorder [158], compelling preclinical evidence identified potential candidate genes and polymorphisms to pharmacologically target in future clinical investigations.
How can the addiction biology field use these preclinical findings to advance precision medicine approaches for the treatment of AUD? First, additional preclinical studies are necessary to determine what, if any, functional adaptations are produced by the SNPs in genes linked to alcohol drinking in rodents. In doing so, this functional characterization may elucidate the neurobiological impact of these genetic variants on alcohol dependence and heavy alcohol drinking. Targeted studies to validate these SNPs in human alcoholics and identification of suitable drugs with US FDA-approval will be crucial prior to embarking on large-scale clinical trials in treatment-seeking alcoholics. As discussed in this review, there are a number of promising preclinical candidate targets raising the question of how to prioritize future preclinical and clinical studies. While it is clear that preclinical models of alcohol consumption provide us with a means to determine functionally relevant SNPs and genetic targets, an additional challenge for future studies will be for medicinal chemists to develop small molecules that preferentially target proteins with these polymorphisms to reverse any gain or loss of function. Regardless of these practical and theoretical challenges, preclinical genetic variation studies will inform precision medicine approaches that will advance treatment of AUD. Further validation of these candidate SNPs is necessary in preclinical models and clinical studies.
Executive summary.
Precision medicine & alcohol use disorder
While there are promising initial clinical studies demonstrating the utility of precision medicine approaches for treating alcohol use disorder (AUD), there are conflicting findings and reports of adverse side effects that suggest novel pharmacogenetic targets are necessary.
Potassium channels
There are mutations in KCNQ, KCNJ10, KCNB, KCND2 and KCNMA1 that associate with heavy alcohol drinking and alcohol dependence in preclinical and clinical studies.
Preclinical pharmacological evidence demonstrating the ability to reduce alcohol drinking by targeting these K+ channels supports future pharmacogenetic studies for treating alcohol addiction.
RAS signaling
Many genes and SNPs in the RAS superfamily signaling pathways are implicated in alcohol intake and dependence in rodents and humans.
Pharmacologically inhibiting RAS signaling reduces drinking in rodents thus initially validating the RAS superfamily as pharmacogenetic targets for the treatment of AUD.
Tachykinins
Neurokinin 1 receptors are particularly involved in the stress-related aspects of alcohol consumption.
SNPs in the Tacr1 gene contribute to a predisposition toward alcohol preference in rodents and associate with a risk for alcohol dependence in humans.
Neuroimmune signaling
Neuroimmune genes are promising targets for treating AUD.
Expression of peroxisome proliferator-activated receptor genes is altered in preclinical models of alcohol addiction and SNPs in this gene family associate with alcohol dependence and an alcohol withdrawal phenotype.
Conclusion
Preclinical genetic variation studies provide strong support for future precision medicine approaches that will advance treatment of AUD.
Footnotes
Financial & competing interests disclosure
This work was funded by NIH grants AA020930 and AA023288. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
- 1.NIAAA. Alcohol Facts and Statistics. 2016. www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/alcohol-facts-and-statistics
- 2.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat. Rev. Genet. 2005;6(7):521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 3.Enoch MA, Goldman D. The genetics of alcoholism and alcohol abuse. Curr. Psychiatry Rep. 2001;3(2):144–151. doi: 10.1007/s11920-001-0012-3. [DOI] [PubMed] [Google Scholar]
- 4.Heilig M, Goldman D, Berrettini W, O'Brien CP. Pharmacogenetic approaches to the treatment of alcohol addiction. Nat. Rev. Neurosci. 2011;12(11):670–684. doi: 10.1038/nrn3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goldman D, Oroszi G, O'Malley S, Anton R. COMBINE genetics study: the pharmacogenetics of alcoholism treatment response: genes and mechanisms. J. Stud. Alcohol Suppl. 2005;15:56–64. doi: 10.15288/jsas.2005.s15.56. discussion 33. [DOI] [PubMed] [Google Scholar]
- 6.Bell RL, Hauser S, Rodd ZA, et al. A genetic animal model of alcoholism for screening medications to treat addiction. Int. Rev. Neurobiol. 2016;126:179–261. doi: 10.1016/bs.irn.2016.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helton SG, Lohoff FW. Pharmacogenetics of alcohol use disorders and comorbid psychiatric disorders. Psychiatry Res. 2015;230(2):121–129. doi: 10.1016/j.psychres.2015.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Seneviratne C, Johnson BA. Advances in medications and tailoring treatment for alcohol use disorder. Alcohol Res. 2015;37(1):15–28. [PMC free article] [PubMed] [Google Scholar]
- 9.Noble EP, Zhang X, Ritchie T, et al. D2 dopamine receptor and GABA(A) receptor beta3 subunit genes and alcoholism. Psychiatry Res. 1998;81(2):133–147. doi: 10.1016/s0165-1781(98)00084-5. [DOI] [PubMed] [Google Scholar]
- 10.Dick DM, Plunkett J, Wetherill LF, et al. Association between GABRA1 and drinking behaviors in the collaborative study on the genetics of alcoholism sample. Alcohol Clin. Exp. Res. 2006;30(7):1101–1110. doi: 10.1111/j.1530-0277.2006.00136.x. [DOI] [PubMed] [Google Scholar]
- 11.Edenberg HJ, Dick DM, Xuei X, et al. Variations in GABRA2, encoding the alpha 2 subunit of the GABA(A) receptor, are associated with alcohol dependence and with brain oscillations. Am. J. Hum. Genet. 2004;74(4):705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enoch MA, Schwartz L, Albaugh B, Virkkunen M, Goldman D. Dimensional anxiety mediates linkage of GABRA2 haplotypes with alcoholism. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2006;141B(6):599–607. doi: 10.1002/ajmg.b.30336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bierut LJ, Agrawal A, Bucholz KK, et al. A genome-wide association study of alcohol dependence. Proc. Natl Acad. Sci. USA. 2010;107(11):5082–5087. doi: 10.1073/pnas.0911109107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Covault J, Gelernter J, Hesselbrock V, Nellissery M, Kranzler HR. Allelic and haplotypic association of GABRA2 with alcohol dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2004;129B(1):104–109. doi: 10.1002/ajmg.b.30091. [DOI] [PubMed] [Google Scholar]
- 15.Li D, Sulovari A, Cheng C, Zhao H, Kranzler HR, Gelernter J. Association of gamma-aminobutyric acid A receptor alpha2 gene (GABRA2) with alcohol use disorder. Neuropsychopharmacology. 2014;39(4):907–918. doi: 10.1038/npp.2013.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zintzaras E. Gamma-aminobutyric acid A receptor, alpha-2 (GABRA2) variants as individual markers for alcoholism: a meta-analysis. Psychiatr. Genet. 2012;22(4):189–196. doi: 10.1097/YPG.0b013e328353ae53. [DOI] [PubMed] [Google Scholar]
- 17.Uhart M, Weerts EM, Mccaul ME, et al. GABRA2 markers moderate the subjective effects of alcohol. Addict. Biol. 2013;18(2):357–369. doi: 10.1111/j.1369-1600.2012.00457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pierucci-Lagha A, Covault J, Feinn R, et al. GABRA2 alleles moderate the subjective effects of alcohol, which are attenuated by finasteride. Neuropsychopharmacology. 2005;30(6):1193–1203. doi: 10.1038/sj.npp.1300688. [DOI] [PubMed] [Google Scholar]
- 19.Plawecki MH, Wetherill L, Vitvitskiy V, et al. Voluntary intravenous self-administration of alcohol detects an interaction between GABAergic manipulation and GABRG1 polymorphism genotype: a pilot study. Alcohol Clin. Exp. Res. 2013;37(Suppl. 1):E152–E160. doi: 10.1111/j.1530-0277.2012.01885.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ray LA, Hutchison KE. Associations among GABRG1, level of response to alcohol, and drinking behaviors. Alcohol Clin. Exp. Res. 2009;33(8):1382–1390. doi: 10.1111/j.1530-0277.2009.00968.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dick DM, Edenberg HJ, Xuei X, et al. Association of GABRG3 with alcohol dependence. Alcohol Clin. Exp. Res. 2004;28(1):4–9. doi: 10.1097/01.ALC.0000108645.54345.98. [DOI] [PubMed] [Google Scholar]
- 22.Zindel LR, Kranzler HR. Pharmacotherapy of alcohol use disorders: seventy-five years of progress. J. Stud. Alcohol Drugs Suppl. 2014;75(Suppl. 17):79–88. doi: 10.15288/jsads.2014.s17.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Luo X, Kranzler HR, Zuo L, Wang S, Blumberg HP, Gelernter J. CHRM2 gene predisposes to alcohol dependence, drug dependence and affective disorders: results from an extended case–control structured association study. Hum. Mol. Genet. 2005;14(16):2421–2434. doi: 10.1093/hmg/ddi244. [DOI] [PubMed] [Google Scholar]
- 24.Wang JC, Hinrichs AL, Stock H, et al. Evidence of common and specific genetic effects: association of the muscarinic acetylcholine receptor M2 (CHRM2) gene with alcohol dependence and major depressive syndrome. Hum. Mol. Genet. 2004;13(17):1903–1911. doi: 10.1093/hmg/ddh194. [DOI] [PubMed] [Google Scholar]
- 25.Coon H, Piasecki TM, Cook EH, et al. Association of the CHRNA4 neuronal nicotinic receptor subunit gene with frequency of binge drinking in young adults. Alcohol Clin. Exp. Res. 2014;38(4):930–937. doi: 10.1111/acer.12319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ehringer MA, Clegg HV, Collins AC, et al. Association of the neuronal nicotinic receptor beta2 subunit gene (CHRNB2) with subjective responses to alcohol and nicotine. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2007;144B(5):596–604. doi: 10.1002/ajmg.b.30464. [DOI] [PubMed] [Google Scholar]
- 27.Kim SA, Kim JW, Song JY, Park S, Lee HJ, Chung JH. Association of polymorphisms in nicotinic acetylcholine receptor alpha 4 subunit gene (CHRNA4), μ-opioid receptor gene (OPRM1), and ethanol-metabolizing enzyme genes with alcoholism in Korean patients. Alcohol. 2004;34(2–3):115–120. doi: 10.1016/j.alcohol.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Wang JC, Grucza R, Cruchaga C, et al. Genetic variation in the CHRNA5 gene affects mRNA levels and is associated with risk for alcohol dependence. Mol. Psychiatry. 2009;14(5):501–510. doi: 10.1038/mp.2008.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schlaepfer IR, Hoft NR, Collins AC, et al. The CHRNA5/A3/B4 gene cluster variability as an important determinant of early alcohol and tobacco initiation in young adults. Biol. Psychiatry. 2008;63(11):1039–1046. doi: 10.1016/j.biopsych.2007.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mckee SA, Harrison EL, O'Malley SS, et al. Varenicline reduces alcohol self-administration in heavy-drinking smokers. Biol. Psychiatry. 2009;66(2):185–190. doi: 10.1016/j.biopsych.2009.01.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitchell JM, Teague CH, Kayser AS, Bartlett SE, Fields HL. Varenicline decreases alcohol consumption in heavy-drinking smokers. Psychopharmacology (Berl) 2012;223(3):299–306. doi: 10.1007/s00213-012-2717-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Verplaetse TL, Pittman BP, Shi JM, Tetrault JM, Coppola S, McKee SA. Effect of lowering the dose of varenicline on alcohol self-administration in drinkers with alcohol use disorders. J. Addict. Med. 2016;10(3):166–173. doi: 10.1097/ADM.0000000000000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chi H, De Wit H. Mecamylamine attenuates the subjective stimulant-like effects of alcohol in social drinkers. Alcohol Clin. Exp. Res. 2003;27(5):780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- 34.Erwin BL, Slaton RM. Varenicline in the treatment of alcohol use disorders. Ann. Pharmacother. 2014;48(11):1445–1455. doi: 10.1177/1060028014545806. [DOI] [PubMed] [Google Scholar]
- 35.Bond C, Laforge KS, Tian M, et al. Single-nucleotide polymorphism in the human mu opioid receptor gene alters beta-endorphin binding and activity: possible implications for opiate addiction. Proc. Natl Acad. Sci. USA. 1998;95(16):9608–9613. doi: 10.1073/pnas.95.16.9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang Y, Wang D, Johnson AD, Papp AC, Sadee W. Allelic expression imbalance of human mu opioid receptor (OPRM1) caused by variant A118G. J. Biol. Chem. 2005;280(38):32618–32624. doi: 10.1074/jbc.M504942200. [DOI] [PubMed] [Google Scholar]
- 37.Ray LA, Hutchison KE. Effects of naltrexone on alcohol sensitivity and genetic moderators of medication response: a double-blind placebo-controlled study. Arch. Gen. Psychiatry. 2007;64(9):1069–1077. doi: 10.1001/archpsyc.64.9.1069. [DOI] [PubMed] [Google Scholar]
- 38.Oslin DW, Berrettini W, Kranzler HR, et al. A functional polymorphism of the μ-opioid receptor gene is associated with naltrexone response in alcohol-dependent patients. Neuropsychopharmacology. 2003;28(8):1546–1552. doi: 10.1038/sj.npp.1300219. [DOI] [PubMed] [Google Scholar]
- 39.Oroszi G, Anton RF, O'Malley S, et al. OPRM1 Asn40Asp predicts response to naltrexone treatment: a haplotype-based approach. Alcohol Clin. Exp. Res. 2009;33(3):383–393. doi: 10.1111/j.1530-0277.2008.00846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Anton RF, Oroszi G, O'Malley S, et al. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch. Gen. Psychiatry. 2008;65(2):135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Barr CS, Chen SA, Schwandt ML, et al. Suppression of alcohol preference by naltrexone in the rhesus macaque: a critical role of genetic variation at the micro-opioid receptor gene locus. Biol. Psychiatry. 2010;67(1):78–80. doi: 10.1016/j.biopsych.2009.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bilbao A, Robinson JE, Heilig M, et al. A pharmacogenetic determinant of μ-opioid receptor antagonist effects on alcohol reward and consumption: evidence from humanized mice. Biol. Psychiatry. 2015;77(10):850–858. doi: 10.1016/j.biopsych.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 43.Ramchandani VA, Umhau J, Pavon FJ, et al. A genetic determinant of the striatal dopamine response to alcohol in men. Mol. Psychiatry. 2011;16(8):809–817. doi: 10.1038/mp.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gelernter J, Gueorguieva R, Kranzler HR, et al. Opioid receptor gene (OPRM1, OPRK1, and OPRD1) variants and response to naltrexone treatment for alcohol dependence: results from the VA Cooperative Study. Alcohol Clin. Exp. Res. 2007;31(4):555–563. doi: 10.1111/j.1530-0277.2007.00339.x. [DOI] [PubMed] [Google Scholar]
- 45.Ooteman W, Naassila M, Koeter MW, et al. Predicting the effect of naltrexone and acamprosate in alcohol-dependent patients using genetic indicators. Addict Biol. 2009;14(3):328–337. doi: 10.1111/j.1369-1600.2009.00159.x. [DOI] [PubMed] [Google Scholar]
- 46.Tidey JW, Monti PM, Rohsenow DJ, et al. Moderators of naltrexone's effects on drinking, urge, and alcohol effects in non-treatment-seeking heavy drinkers in the natural environment. Alcohol Clin. Exp. Res. 2008;32(1):58–66. doi: 10.1111/j.1530-0277.2007.00545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ziauddeen H, Nestor LJ, Subramaniam N, et al. Opioid antagonists and the A118G polymorphism in the mu-opioid receptor gene: effects of GSK1521498 and naltrexone in healthy drinkers stratified by OPRM1 genotype. Neuropsychopharmacology. 2016;41(11):2647–2657. doi: 10.1038/npp.2016.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schacht JP, Anton RF, Voronin KE, et al. Interacting effects of naltrexone and OPRM1 and DAT1 variation on the neural response to alcohol cues. Neuropsychopharmacology. 2013;38(3):414–422. doi: 10.1038/npp.2012.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anton RF, Voronin KK, Randall PK, Myrick H, Tiffany A. Naltrexone modification of drinking effects in a subacute treatment and bar-lab paradigm: influence of OPRM1 and dopamine transporter (SLC6A3) genes. Alcohol Clin. Exp. Res. 2012;36(11):2000–2007. doi: 10.1111/j.1530-0277.2012.01807.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ray LA, Bujarski S, Squeglia LM, Ashenhurst JR, Anton RF. Interactive effects of OPRM1 and DAT1 genetic variation on subjective responses to alcohol. Alcohol Alcohol. 2014;49(3):261–270. doi: 10.1093/alcalc/agt183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cao J, Larocque E, Li D. Associations of the 5-hydroxytryptamine (serotonin) receptor 1B gene (HTR1B) with alcohol, cocaine, and heroin abuse. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2013;162B(2):169–176. doi: 10.1002/ajmg.b.32128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lee SY, Lin WW, Huang SY, et al. The relationship between serotonin receptor 1B polymorphisms A-161T and alcohol dependence. Alcohol Clin. Exp. Res. 2009;33(9):1589–1595. doi: 10.1111/j.1530-0277.2009.00990.x. [DOI] [PubMed] [Google Scholar]
- 53.Contini V, Bertuzzi GP, Polina ER, et al. A haplotype analysis is consistent with the role of functional HTR1B variants in alcohol dependence. Drug Alcohol Depend. 2012;122(1–2):100–104. doi: 10.1016/j.drugalcdep.2011.09.020. [DOI] [PubMed] [Google Scholar]
- 54.Cao J, Liu X, Han S, Zhang CK, Liu Z, Li D. Association of the HTR2A gene with alcohol and heroin abuse. Hum. Genet. 2014;133(3):357–365. doi: 10.1007/s00439-013-1388-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Jakubczyk A, Klimkiewicz A, Kopera M, et al. The CC genotype in the T102C HTR2A polymorphism predicts relapse in individuals after alcohol treatment. J. Psychiatr. Res. 2013;47(4):527–533. doi: 10.1016/j.jpsychires.2012.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wrzosek M, Jakubczyk A, Wrzosek M, et al. Serotonin 2A receptor gene (HTR2A) polymorphism in alcohol-dependent patients. Pharmacol. Rep. 2012;64(2):449–453. doi: 10.1016/s1734-1140(12)70787-9. [DOI] [PubMed] [Google Scholar]
- 57.Jakubczyk A, Wrzosek M, Lukaszkiewicz J, et al. The CC genotype in HTR2A T102C polymorphism is associated with behavioral impulsivity in alcohol-dependent patients. J. Psychiatr. Res. 2012;46(1):44–49. doi: 10.1016/j.jpsychires.2011.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Enoch MA, Gorodetsky E, Hodgkinson C, Roy A, Goldman D. Functional genetic variants that increase synaptic serotonin and 5-HT3 receptor sensitivity predict alcohol and drug dependence. Mol. Psychiatry. 2011;16(11):1139–1146. doi: 10.1038/mp.2010.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Seneviratne C, Franklin J, Beckett K, et al. Association, interaction, and replication analysis of genes encoding serotonin transporter and 5-HT3 receptor subunits A and B in alcohol dependence. Hum. Genet. 2013;132(10):1165–1176. doi: 10.1007/s00439-013-1319-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Johnson BA, Seneviratne C, Wang XQ, Ait-Daoud N, Li MD. Determination of genotype combinations that can predict the outcome of the treatment of alcohol dependence using the 5-HT(3) antagonist ondansetron. Am. J. Psychiatry. 2013;170(9):1020–1031. doi: 10.1176/appi.ajp.2013.12091163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Johnson BA, Ait-Daoud N, Bowden CL, et al. Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet. 2003;361(9370):1677–1685. doi: 10.1016/S0140-6736(03)13370-3. [DOI] [PubMed] [Google Scholar]
- 62.Johnson BA, Rosenthal N, Capece JA, et al. Topiramate for treating alcohol dependence: a randomized controlled trial. JAMA. 2007;298(14):1641–1651. doi: 10.1001/jama.298.14.1641. [DOI] [PubMed] [Google Scholar]
- 63.Johnson BA, Rosenthal N, Capece JA, et al. Improvement of physical health and quality of life of alcohol-dependent individuals with topiramate treatment: US multisite randomized controlled trial. Arch. Intern. Med. 2008;168(11):1188–1199. doi: 10.1001/archinte.168.11.1188. [DOI] [PubMed] [Google Scholar]
- 64.Feinn R, Curtis B, Kranzler HR. Balancing risk and benefit in heavy drinkers treated with topiramate: implications for personalized care. J. Clin. Psychiatry. 2016;77(3):e278–e282. doi: 10.4088/JCP.15m10053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ray LA, Miranda R, Jr, Mackillop J, et al. A preliminary pharmacogenetic investigation of adverse events from topiramate in heavy drinkers. Exp. Clin. Psychopharmacol. 2009;17(2):122–129. doi: 10.1037/a0015700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kranzler HR, Covault J, Feinn R, et al. Topiramate treatment for heavy drinkers: moderation by a GRIK1 polymorphism. Am. J. Psychiatry. 2014;171(4):445–452. doi: 10.1176/appi.ajp.2013.13081014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Hille B. Ion Channels of Excitable Membranes. Sinauer Associates, Inc.; MA, USA: 2001. Potassium channels and chloride channels. [Google Scholar]
- 68.Maljevic S, Lerche H. Potassium channels: a review of broadening therapeutic possibilities for neurological diseases. J. Neurol. 2013;260(9):2201–2211. doi: 10.1007/s00415-012-6727-8. [DOI] [PubMed] [Google Scholar]
- 69.Maljevic S, Lerche H. Potassium channel genes and benign familial neonatal epilepsy. Prog. Brain Res. 2014;213:17–53. doi: 10.1016/B978-0-444-63326-2.00002-8. [DOI] [PubMed] [Google Scholar]
- 70.Hopf FW, Simms JA, Chang SJ, Seif T, Bartlett SE, Bonci A. Chlorzoxazone, an SK-type potassium channel activator used in humans, reduces excessive alcohol intake in rats. Biol. Psychiatry. 2011;69(7):618–624. doi: 10.1016/j.biopsych.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Mulholland PJ, Hopf FW, Bukiya AN, et al. Sizing up ethanol-induced plasticity: the role of small and large conductance calcium-activated potassium channels. Alcohol Clin. Exp. Res. 2009;33(7):1125–1135. doi: 10.1111/j.1530-0277.2009.00936.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mulholland PJ, Becker HC, Woodward JJ, Chandler LJ. Small conductance calcium-activated potassium type 2 channels regulate alcohol-associated plasticity of glutamatergic synapses. Biol. Psychiatry. 2011;69(7):625–632. doi: 10.1016/j.biopsych.2010.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Padula A, Mcguier N, Griffin W, Lopez M, Becker H, Mulholland P. Novel anticonvulsants for reducing alcohol consumption: a review of evidence from preclinical rodent drinking models. OA Alcohol. 2013;1(1):2. doi: 10.13172/2053-0285-1-1-446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mcguier NS, Griffin WC, 3rd, Gass JT, Padula AE, Chesler EJ, Mulholland PJ. Kv7 channels in the nucleus accumbens are altered by chronic drinking and are targets for reducing alcohol consumption. Addict. Biol. 2016;21(6):1097–1112. doi: 10.1111/adb.12279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Padula AE, Griffin WC, 3rd, Lopez MF, et al. KCNN genes that encode small-conductance Ca2+-activated K+ channels influence alcohol and drug addiction. Neuropsychopharmacology. 2015;40(8):1928–1939. doi: 10.1038/npp.2015.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rinker JA, Fulmer DB, Trantham-Davidson H, et al. Differential potassium channel gene regulation in BXD mice reveals novel targets for pharmacogenetic therapies to reduce heavy alcohol drinking. Alcohol. 2016;58:33–45. doi: 10.1016/j.alcohol.2016.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mayfield J, Blednov YA, Harris RA. Behavioral and genetic evidence for GIRK channels in the CNS: role in physiology, pathophysiology, and drug addiction. Int. Rev. Neurobiol. 2015;123:279–313. doi: 10.1016/bs.irn.2015.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Farris SP, Arasappan D, Hunicke-Smith S, Harris RA, Mayfield RD. Transcriptome organization for chronic alcohol abuse in human brain. Mol. Psychiatry. 2015;20(11):1438–1447. doi: 10.1038/mp.2014.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Servier. www.servier.com
- 80.Maljevic S, Wuttke TV, Lerche H. Nervous system KV7 disorders: breakdown of a subthreshold brake. J. Physiol. 2008;586(7):1791–1801. doi: 10.1113/jphysiol.2008.150656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moore SD, Madamba SG, Siggins GR. Ethanol diminishes a voltage-dependent K+ current, the M-current, in CA1 hippocampal pyramidal neurons in vitro . Brain Res. 1990;516(2):222–228. doi: 10.1016/0006-8993(90)90922-x. [DOI] [PubMed] [Google Scholar]
- 82.Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J. Neurophysiol. 2007;97(3):1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Cavaliere S, Gillespie JM, Hodge JJ. KCNQ channels show conserved ethanol block and function in ethanol behaviour. PLoS ONE. 2012;7(11):e50279. doi: 10.1371/journal.pone.0050279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Metten P, Iancu OD, Spence SE, et al. Dual-trait selection for ethanol consumption and withdrawal: genetic and transcriptional network effects. Alcohol Clin. Exp. Res. 2014;38(12):2915–2924. doi: 10.1111/acer.12574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Lo CL, Lossie AC, Liang T, et al. High resolution genomic scans reveal genetic architecture controlling alcohol preference in bidirectionally selected rat model. PLoS Genet. 2016;12(8):e1006178. doi: 10.1371/journal.pgen.1006178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Melendez RI, Mcginty JF, Kalivas PW, Becker HC. Brain region-specific gene expression changes after chronic intermittent ethanol exposure and early withdrawal in C57BL/6J mice. Addict Biol. 2012;17(2):351–364. doi: 10.1111/j.1369-1600.2011.00357.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Liu J, Lewohl JM, Harris RA, Dodd PR, Mayfield RD. Altered gene expression profiles in the frontal cortex of cirrhotic alcoholics. Alcohol Clin. Exp. Res. 2007;31(9):1460–1466. doi: 10.1111/j.1530-0277.2007.00444.x. [DOI] [PubMed] [Google Scholar]
- 88.Liu J, Lewohl JM, Harris RA, et al. Patterns of gene expression in the frontal cortex discriminate alcoholic from nonalcoholic individuals. Neuropsychopharmacology. 2006;31(7):1574–1582. doi: 10.1038/sj.npp.1300947. [DOI] [PubMed] [Google Scholar]
- 89.Edenberg HJ, Koller DL, Xuei X, et al. Genome-wide association study of alcohol dependence implicates a region on chromosome 11. Alcohol Clin. Exp. Res. 2010;34(5):840–852. doi: 10.1111/j.1530-0277.2010.01156.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kendler KS, Kalsi G, Holmans PA, et al. Genomewide association analysis of symptoms of alcohol dependence in the molecular genetics of schizophrenia (MGS2) control sample. Alcohol. Clin. Exp. Res. 2011;35(5):963–975. doi: 10.1111/j.1530-0277.2010.01427.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Knapp CM, O'Malley M, Datta S, Ciraulo DA. The Kv7 potassium channel activator retigabine decreases alcohol consumption in rats. Am. J. Drug Alcohol Abuse. 2014;40(3):244–250. doi: 10.3109/00952990.2014.892951. [DOI] [PubMed] [Google Scholar]
- 92.Crean CS, Tompson DJ. The effects of ethanol on the pharmacokinetics, pharmacodynamics, safety, and tolerability of ezogabine (retigabine) Clin. Ther. 2013;35(1):87–93. doi: 10.1016/j.clinthera.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 93.Nwaobi SE, Cuddapah VA, Patterson KC, Randolph AC, Olsen ML. The role of glial-specific Kir4.1 in normal and pathological states of the CNS. Acta Neuropathol. 2016;132(1):1–21. doi: 10.1007/s00401-016-1553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ferraro TN, Golden GT, Smith GG, et al. Fine mapping of a seizure susceptibility locus on mouse Chromosome 1: nomination of Kcnj10 as a causative gene. Mamm. Genome. 2004;15(4):239–251. doi: 10.1007/s00335-003-2270-3. [DOI] [PubMed] [Google Scholar]
- 95.Shang L, Lucchese CJ, Haider S, Tucker SJ. Functional characterisation of missense variations in the Kir4.1 potassium channel (KCNJ10) associated with seizure susceptibility. Brain Res. Mol. Brain Res. 2005;139(1):178–183. doi: 10.1016/j.molbrainres.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 96.Tarantino LM, Mcclearn GE, Rodriguez LA, Plomin R. Confirmation of quantitative trait loci for alcohol preference in mice. Alcohol Clin. Exp. Res. 1998;22(5):1099–1105. [PubMed] [Google Scholar]
- 97.Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J. Neurosci. 1997;17(10):3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Buck KJ, Finn DA. Genetic factors in addiction: QTL mapping and candidate gene studies implicate GABAergic genes in alcohol and barbiturate withdrawal in mice. Addiction. 2001;96(1):139–149. doi: 10.1046/j.1360-0443.2001.96113910.x. [DOI] [PubMed] [Google Scholar]
- 99.Zou SB, Weng J, Symons MN, Singh SM. Role of potassium channel gene Kcnj10 in ethanol preference in C57bl/6J and DBA/2J mice. Alcohol. Clin. Exp. Res. 2009;33(3):394–399. doi: 10.1111/j.1530-0277.2008.00848.x. [DOI] [PubMed] [Google Scholar]
- 100.Buono RJ, Lohoff FW, Sander T, et al. Association between variation in the human KCNJ10 potassium ion channel gene and seizure susceptibility. Epilepsy Res. 2004;58(2–3):175–183. doi: 10.1016/j.eplepsyres.2004.02.003. [DOI] [PubMed] [Google Scholar]
- 101.Lewohl JM, Wang L, Miles MF, Zhang L, Dodd PR, Harris RA. Gene expression in human alcoholism: microarray analysis of frontal cortex. Alcohol Clin. Exp. Res. 2000;24(12):1873–1882. [PubMed] [Google Scholar]
- 102.Pan Y, Luo X, Liu X, et al. Genome-wide association studies of maximum number of drinks. J. Psychiatr. Res. 2013;47(11):1717–1724. doi: 10.1016/j.jpsychires.2013.07.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Farris SP, Harris RA, Ponomarev I. Epigenetic modulation of brain gene networks for cocaine and alcohol abuse. Front. Neurosci. 2015;9:176. doi: 10.3389/fnins.2015.00176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zuo L, Gelernter J, Zhang CK, et al. Genome-wide association study of alcohol dependence implicates KIAA0040 on chromosome 1q. Neuropsychopharmacology. 2012;37(2):557–566. doi: 10.1038/npp.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zuo L, Zhang F, Zhang H, et al. Genome-wide search for replicable risk gene regions in alcohol and nicotine co-dependence. Am. J. Med. Genet. B Neuropsychiatr. Genet. 2012;159B(4):437–444. doi: 10.1002/ajmg.b.32047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Buhler KM, Gine E, Echeverry-Alzate V, Calleja-Conde J, De Fonseca FR, Lopez-Moreno JA. Common single nucleotide variants underlying drug addiction: more than a decade of research. Addict. Biol. 2015;20(5):845–871. doi: 10.1111/adb.12204. [DOI] [PubMed] [Google Scholar]
- 107.Schuckit MA, Wilhelmsen K, Smith TL, et al. Autosomal linkage analysis for the level of response to alcohol. Alcohol Clin. Exp. Res. 2005;29(11):1976–1982. doi: 10.1097/01.alc.0000187598.82921.27. [DOI] [PubMed] [Google Scholar]
- 108.Davies AG, Pierce-Shimomura JT, Kim H, et al. A central role of the BK potassium channel in behavioral responses to ethanol in C. elegans . Cell. 2003;115(6):655–666. doi: 10.1016/s0092-8674(03)00979-6. [DOI] [PubMed] [Google Scholar]
- 109.Ghezzi A, Al-Hasan YM, Larios LE, Bohm RA, Atkinson NS. slo K(+) channel gene regulation mediates rapid drug tolerance. Proc. Natl Acad. Sci. USA. 2004;101(49):17276–17281. doi: 10.1073/pnas.0405584101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Pietrzykowski AZ, Martin GE, Puig SI, Knott TK, Lemos JR, Treistman SN. Alcohol tolerance in large-conductance, calcium-activated potassium channels of CNS terminals is intrinsic and includes two components: decreased ethanol potentiation and decreased channel density. J. Neurosci. 2004;24(38):8322–8332. doi: 10.1523/JNEUROSCI.1536-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ponomarev I, Wang S, Zhang L, Harris RA, Mayfield RD. Gene coexpression networks in human brain identify epigenetic modifications in alcohol dependence. J. Neurosci. 2012;32(5):1884–1897. doi: 10.1523/JNEUROSCI.3136-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ye X, Carew TJ. Small G protein signaling in neuronal plasticity and memory formation: the specific role of ras family proteins. Neuron. 2010;68(3):340–361. doi: 10.1016/j.neuron.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ojelade SA, Jia T, Rodan AR, et al. Rsu1 regulates ethanol consumption in Drosophila and humans. Proc. Natl Acad. Sci. USA. 2015;112(30):E4085–E4093. doi: 10.1073/pnas.1417222112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Repunte-Canonigo V, van der Stap LD, Chen J, et al. Genome-wide gene expression analysis identifies K-ras as a regulator of alcohol intake. Brain Res. 2010;1339:1–10. doi: 10.1016/j.brainres.2010.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Repunte-Canonigo V, Herman MA, Kawamura T, et al. Nf1 regulates alcohol dependence-associated excessive drinking and gamma-aminobutyric acid release in the central amygdala in mice and is associated with alcohol dependence in humans. Biol. Psychiatry. 2015;77(10):870–879. doi: 10.1016/j.biopsych.2014.07.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Bennett B, Larson C, Richmond PA, et al. Quantitative trait locus mapping of acute functional tolerance in the LXS recombinant inbred strains. Alcohol Clin. Exp. Res. 2015;39(4):611–620. doi: 10.1111/acer.12678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Schumann G, Coin LJ, Lourdusamy A, et al. Genome-wide association and genetic functional studies identify autism susceptibility candidate 2 gene (AUTS2) in the regulation of alcohol consumption. Proc. Natl Acad. Sci. USA. 2011;108(17):7119–7124. doi: 10.1073/pnas.1017288108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Stacey D, Bilbao A, Maroteaux M, et al. RASGRF2 regulates alcohol-induced reinforcement by influencing mesolimbic dopamine neuron activity and dopamine release. Proc. Natl Acad. Sci. USA. 2012;109(51):21128–21133. doi: 10.1073/pnas.1211844110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Liss B, Franz O, Sewing S, Bruns R, Neuhoff H, Roeper J. Tuning pacemaker frequency of individual dopaminergic neurons by Kv4.3L and KChip3.1 transcription. EMBO J. 2001;20(20):5715–5724. doi: 10.1093/emboj/20.20.5715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Devito EE, Meda SA, Jiantonio R, Potenza MN, Krystal JH, Pearlson GD. Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology. 2013;38(10):1854–1863. doi: 10.1038/npp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Cservenka A. Neurobiological phenotypes associated with a family history of alcoholism. Drug Alcohol Depend. 2016;158:8–21. doi: 10.1016/j.drugalcdep.2015.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Pena-Oliver Y, Carvalho FM, Sanchez-Roige S, et al. Mouse and human genetic analyses associate kalirin with ventral striatal activation during impulsivity and with alcohol misuse. Front. Genet. 2016;7:52. doi: 10.3389/fgene.2016.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nimitvilai S, Uys JD, Woodward JJ, et al. Orbitofrontal neuroadaptations and cross-species synaptic biomarkers in heavy drinking macaques. J. Neurosci. 2017 doi: 10.1523/JNEUROSCI.0133-17.2017. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ben Hamida S, Neasta J, Lasek AW, et al. The small G protein H-Ras in the mesolimbic system is a molecular gateway to alcohol-seeking and excessive drinking behaviors. J. Neurosci. 2012;32(45):15849–15858. doi: 10.1523/JNEUROSCI.2846-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Asati V, Mahapatra DK, Bharti SK. K-Ras and its inhibitors towards personalized cancer treatment: pharmacological and structural perspectives. Eur. J. Med. Chem. 2017;125:299–314. doi: 10.1016/j.ejmech.2016.09.049. [DOI] [PubMed] [Google Scholar]
- 126.Pennefather JN, Lecci A, Candenas ML, Patak E, Pinto FM, Maggi CA. Tachykinins and tachykinin receptors: a growing family. Life Sci. 2004;74(12):1445–1463. doi: 10.1016/j.lfs.2003.09.039. [DOI] [PubMed] [Google Scholar]
- 127.Douglas SD, Leeman SE. Neurokinin-1 receptor: functional significance in the immune system in reference to selected infections and inflammation. Ann. NY Acad. Sci. 2011;1217:83–95. doi: 10.1111/j.1749-6632.2010.05826.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ebner K, Singewald N. The role of substance P in stress and anxiety responses. Amino Acids. 2006;31(3):251–272. doi: 10.1007/s00726-006-0335-9. [DOI] [PubMed] [Google Scholar]
- 129.Lessard A, Pickel VM. Subcellular distribution and plasticity of neurokinin-1 receptors in the rat substantia nigra and ventral tegmental area. Neuroscience. 2005;135(4):1309–1323. doi: 10.1016/j.neuroscience.2005.07.025. [DOI] [PubMed] [Google Scholar]
- 130.Schildein S, Agmo A, Huston JP, Schwarting RK. Intraaccumbens injections of substance P, morphine and amphetamine: effects on conditioned place preference and behavioral activity. Brain Res. 1998;790(1–2):185–194. doi: 10.1016/s0006-8993(98)00062-6. [DOI] [PubMed] [Google Scholar]
- 131.Elliott PJ, Nemeroff CB, Kilts CD. Evidence for a tonic facilitatory influence of substance P on dopamine release in the nucleus accumbens. Brain Res. 1986;385(2):379–382. doi: 10.1016/0006-8993(86)91087-5. [DOI] [PubMed] [Google Scholar]
- 132.Loonam TM, Noailles PA, Yu J, Zhu JP, Angulo JA. Substance P and cholecystokinin regulate neurochemical responses to cocaine and methamphetamine in the striatum. Life Sci. 2003;73(6):727–739. doi: 10.1016/s0024-3205(03)00393-x. [DOI] [PubMed] [Google Scholar]
- 133.Gadd CA, Murtra P, De Felipe C, Hunt SP. Neurokinin-1 receptor-expressing neurons in the amygdala modulate morphine reward and anxiety behaviors in the mouse. J. Neurosci. 2003;23(23):8271–8280. doi: 10.1523/JNEUROSCI.23-23-08271.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ayanwuyi LO, Stopponi S, Ubaldi M, et al. Neurokinin 1 receptor blockade in the medial amygdala attenuates alcohol drinking in rats with innate anxiety but not in Wistar rats. Br. J. Pharmacol. 2015;172(21):5136–5146. doi: 10.1111/bph.13280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Murtra P, Sheasby AM, Hunt SP, De Felipe C. Rewarding effects of opiates are absent in mice lacking the receptor for substance P. Nature. 2000;405(6783):180–183. doi: 10.1038/35012069. [DOI] [PubMed] [Google Scholar]
- 136.Robinson JE, Fish EW, Krouse MC, Thorsell A, Heilig M, Malanga CJ. Potentiation of brain stimulation reward by morphine: effects of neurokinin-1 receptor antagonism. Psychopharmacology (Berl) 2012;220(1):215–224. doi: 10.1007/s00213-011-2469-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Ripley TL, Gadd CA, De Felipe C, Hunt SP, Stephens DN. Lack of self-administration and behavioural sensitisation to morphine, but not cocaine, in mice lacking NK1 receptors. Neuropharmacology. 2002;43(8):1258–1268. doi: 10.1016/s0028-3908(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 138.Barbier E, Vendruscolo LF, Schlosburg JE, et al. The NK1 receptor antagonist L822429 reduces heroin reinforcement. Neuropsychopharmacology. 2013;38(6):976–984. doi: 10.1038/npp.2012.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Mulholland PJ, Chandler LJ, Kalivas PW. Signals from the fourth dimension regulate drug relapse. Trends Neurosci. 2016;39(7):472–485. doi: 10.1016/j.tins.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Thorsell A, Schank JR, Singley E, Hunt SP, Heilig M. Neurokinin-1 receptors (NK1R:s), alcohol consumption, and alcohol reward in mice. Psychopharmacology (Berl.) 2010;209(1):103–111. doi: 10.1007/s00213-010-1775-1. [DOI] [PubMed] [Google Scholar]
- 141.George DT, Gilman J, Hersh J, et al. Neurokinin 1 receptor antagonism as a possible therapy for alcoholism. Science. 2008;319(5869):1536–1539. doi: 10.1126/science.1153813. [DOI] [PubMed] [Google Scholar]
- 142.Baek MN, Jung KH, Halder D, et al. Artificial microRNA-based neurokinin-1 receptor gene silencing reduces alcohol consumption in mice. Neurosci. Lett. 2010;475(3):124–128. doi: 10.1016/j.neulet.2010.03.051. [DOI] [PubMed] [Google Scholar]
- 143.Schank JR, King CE, Sun H, et al. The role of the neurokinin-1 receptor in stress-induced reinstatement of alcohol and cocaine seeking. Neuropsychopharmacology. 2014;39(5):1093–1101. doi: 10.1038/npp.2013.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Schank JR, Pickens CL, Rowe KE, et al. Stress-induced reinstatement of alcohol-seeking in rats is selectively suppressed by the neurokinin 1 (NK1) antagonist L822429. Psychopharmacology (Berl.) 2011;218(1):111–119. doi: 10.1007/s00213-011-2201-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Schank JR, Tapocik JD, Barbier E, et al. Tacr1 gene variation and neurokinin 1 receptor expression is associated with antagonist efficacy in genetically selected alcohol-preferring rats. Biol. Psychiatry. 2013;73(8):774–781. doi: 10.1016/j.biopsych.2012.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Seneviratne C, Ait-Daoud N, Ma JZ, Chen G, Johnson BA, Li MD. Susceptibility locus in neurokinin-1 receptor gene associated with alcohol dependence. Neuropsychopharmacology. 2009;34(11):2442–2449. doi: 10.1038/npp.2009.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Ratti E, Bellew K, Bettica P, et al. Results from 2 randomized, double-blind, placebo-controlled studies of the novel NK1 receptor antagonist casopitant in patients with major depressive disorder. J. Clin. Psychopharmacol. 2011;31(6):727–733. doi: 10.1097/JCP.0b013e31823608ca. [DOI] [PubMed] [Google Scholar]
- 148.Trist DG, Ratti E, Bye A. Why receptor reserve matters for neurokinin1 (NK1) receptor antagonists. J. Recept. Signal Transduct. Res. 2013;33(6):333–337. doi: 10.3109/10799893.2013.843194. [DOI] [PubMed] [Google Scholar]
- 149.Ratti E, Bettica P, Alexander R, et al. Full central neurokinin-1 receptor blockade is required for efficacy in depression: evidence from orvepitant clinical studies. J. Psychopharmacol. 2013;27(5):424–434. doi: 10.1177/0269881113480990. [DOI] [PubMed] [Google Scholar]
- 150.Warden A, Erickson E, Robinson G, Harris RA, Mayfield RD. The neuroimmune transcriptome and alcohol dependence: potential for targeted therapies. Pharmacogenomics. 2016;17(18):2081–2096. doi: 10.2217/pgs-2016-0062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Blednov YA, Ponomarev I, Geil C, Bergeson S, Koob GF, Harris RA. Neuroimmune regulation of alcohol consumption: behavioral validation of genes obtained from genomic studies. Addict. Biol. 2012;17(1):108–120. doi: 10.1111/j.1369-1600.2010.00284.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Straus DS, Glass CK. Anti-inflammatory actions of PPAR ligands: new insights on cellular and molecular mechanisms. Trends Immunol. 2007;28(12):551–558. doi: 10.1016/j.it.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 153.Stopponi S, Somaini L, Cippitelli A, et al. Activation of nuclear PPAR-γ receptors by the antidiabetic agent pioglitazone suppresses alcohol drinking and relapse to alcohol seeking. Biol. Psychiatry. 2011;69(7):642–649. doi: 10.1016/j.biopsych.2010.12.010. [DOI] [PubMed] [Google Scholar]
- 154.Bahi A, Nurulain SM, Ojha S. Ethanol intake and ethanol-conditioned place preference are reduced in mice treated with the bioflavonoid agent naringin. Alcohol. 2014;48(7):677–685. doi: 10.1016/j.alcohol.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 155.Blednov YA, Benavidez JM, Black M, et al. Peroxisome proliferator-activated receptors alpha and gamma are linked with alcohol consumption in mice and withdrawal and dependence in humans. Alcohol Clin. Exp. Res. 2015;39(1):136–145. doi: 10.1111/acer.12610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blednov YA, Black M, Benavidez JM, Stamatakis EE, Harris RA. PPAR agonists: II. Fenofibrate and tesaglitazar alter behaviors related to voluntary alcohol consumption. Alcohol. Clin. Exp. Res. 2016;40(3):563–571. doi: 10.1111/acer.12972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Ferguson LB, Most D, Blednov YA, Harris RA. PPAR agonists regulate brain gene expression: relationship to their effects on ethanol consumption. Neuropharmacology. 2014;86:397–407. doi: 10.1016/j.neuropharm.2014.06.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Xu K, Kranzler HR, Sherva R, et al. Genomewide association study for maximum number of alcoholic drinks in European Americans and African Americans. Alcohol. Clin. Exp. Res. 2015;39(7):1137–1147. doi: 10.1111/acer.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]