Abstract
The study aimed to investigate the clinical significance of fibrous sheath interacting protein 1 (FSIP1) in bladder cancer, and its potential relevance to the survival of patients with bladder cancer. A total of 225 surgical excised-bladder cancer tissues were collected from the patients with the follow-up data >5 years. The FSIP1 expressions were assayed using immunohistochemistry. The messenger RNA (mRNA) and/or protein levels of FSIP1 in fresh bladder tumor tissues as well as bladder cancer cell lines were measured by quantitative real-time polymerase chain reaction (PCR) and Western blotting analysis. The correlation of FSIP1 expression with clinicopathological parameters was also evaluated. Western blotting analysis revealed that FSIP1 protein was detected in 94.1% (16/17) of bladder tumor specimens and in all three bladder cancer cell lines (5637, BIU-87, and T24 in particular), with significantly higher expression than those of their controls. Quantitative real-time PCR demonstrated an increased FSIP1 mRNA expression level in bladder cancer tissues than in normal adjacent tissues (P=0.012). FSIP1 overexpression showed good correlation with tumor stage and lymph node metastasis (P=0.027 and 0.000, respectively). Positive FSIP1 expression was independently associated with an unfavorable overall and disease-free survival by multivariate Cox regression (P=0.037 and 0.019, respectively). FSIP1 overexpression is associated with unfavorable prognosis in patients with bladder cancer. Thus, FSIP1 represents a potential therapeutic or predictive target for bladder cancer.
Keywords: bladder cancer, fibrous sheath interacting protein 1, prognosis, survival, metastasis
Introduction
Bladder cancer remains one of the most common malignancies with a high rate of recurrence and increased incidence year by year. Urothelial cell carcinoma (UCC) is the most common type of bladder cancer, and it represents the fourth most common malignancy in men with a prevalence three times higher than that among women.1,2 Clinical and pathological data suggest that UCC is characterized by two distinct phenotypes with high- and low-grade differentiation.3,4 Previous studies reported that UCC shows excellent prognosis with no signs of muscle invasion in ~75% of the patients (cancer in situ, Ta, and T1), whereas the rest of the patients present with aggressive form of UCC that invades the intrinsic muscular layers and is associated with a poor prognosis.5,6 There are several known risk factors for bladder cancer, including genetics, diets, smoking, environmental and occupational exposure, gender, and so on.7–9 Despite numerous advances in recent decades, identifying new molecular targets and developing novel therapeutic strategies for achieving long-term survival of such patients are needed. A better understanding of tumor pathogenesis and metastasis will help to discover novel therapeutic targets that are highly specific for bladder cancer.
Fibrous sheath interacting protein 1 (FSIP1) gene or known as HSD10 is a recently discovered gene that expresses in airway epithelial cells, which involves the regulation of amyloid β precursor protein.10 FSIP1 mainly functions through protein–protein binding.11 Most of the research on FSIP1 has mainly focused on breast cancer and lung cancer.12–14 To date, the biological role of FSIP1 in various human cancers, including bladder cancer, remains unknown. We hypothesize that FSIP1 is a potential molecular target for treating bladder cancer or a prognostic indicator of survival because it expressed abnormally in arsenic-related bladder cancer.15 The previous clinical study failed to reveal a correlation between FSIP1 expression and prognosis; however, this could be attributed to the small sample size applied in the study. Little is known about the clinical relevance and biological functions of FSIP1 in bladder cancer. Therefore, the role of FSIP1 in bladder cancer will require further investigation in a study with a larger sample size. Understanding the predictive and diagnostic role of FSIP1 in bladder cancer provides more insights on FSIP1 as a potential therapeutic target for bladder cancer.
This study focused on the clinical significance of FSIP1 in bladder cancer. The expression level of FSIP1 was determined in the bladder tumor tissues (BTs) vs matched adjacent noncancerous tissues (ANTs). In addition, the correlation between FSIP1 expression level and clinicopathological characteristics was analyzed. Moreover, the impact of FSIP1-positive expression on the prognosis of bladder cancer patients was estimated. The purpose of this study was to investigate the expression of FSIP1 protein in bladder cancer and to explore the possible correlations between FSIP1 expression and clinicopathological characteristics as well as prognosis of bladder cancer patients.
Methods and materials
Clinical data and tissue specimens
This study was approved by the Review Board of Shengjing Hospital. Written informed consents were obtained from all of the patients prior to inclusion. Clinicopathological data were collected, including age, gender, number of tumors, clinical stage, tumor size and grade, lymph node metastasis, and so on. A total of 29 pairs of fresh BT and matched ANT specimens were obtained from patients who underwent a cystectomy for bladder cancer at the Department of Urology in Shengjing Hospital of China Medical University from December 2016 to February 2017. None of these patients had experienced a preoperative chemotherapy or radiotherapy. The fresh samples were immediately frozen in liquid nitrogen and stored at −80°C for future determination of FSIP1 expression by western blotting analysis and quantitative real-time polymerase chain reaction (PCR). All BTs were validated as urothelial carcinoma by pathological examination. The histological diagnosis and tumor grade were evaluated independently by two pathologists according to the World Health Organization classification guidelines.16 ANTs were confirmed as normal tissues by pathologic histological examination. Unfortunately, these samples were missing the follow-up data, and only tumor grade and stage were available. In addition, 225 paraffin-embedded bladder cancer tissues of the patients from January 2009 to January 2012 with at least a 5-year follow-up (final follow-up in January 2017) were assayed for the protein expression of FSIP1 using immunohistochemistry and included in prognosis analysis. Patients’ characteristics are shown in Table 1.
Table 1.
The comparison of clinicopathological characteristics between FSIP1-positive and FSIP1-negative bladder cancer patients (n=225)
| Variables | N | FSIP1-negative | FSIP1-positive | P-value |
|---|---|---|---|---|
| Gender | 0.063 | |||
| Female | 34 | 21 | 13 | |
| Male | 191 | 85 | 106 | |
| Age (years) | 0.108 | |||
| <60 | 121 | 63 | 58 | |
| ≥60 | 104 | 43 | 61 | |
| Tumor grade | 0.682 | |||
| G1/2 | 122 | 59 | 63 | |
| G3 | 103 | 47 | 56 | |
| Clinical stage | 0.027 | |||
| Ta–T1 | 91 | 51 | 40 | |
| T2–T4 | 134 | 55 | 79 | |
| Tumor number | 0.203 | |||
| Unifocal | 112 | 48 | 64 | |
| Multifocal | 113 | 58 | 55 | |
| Tumor size | 0.765 | |||
| <3 cm | 125 | 60 | 65 | |
| ≥3 cm | 100 | 46 | 54 | |
| Lymph node metastasis | 0.000 | |||
| No (N0) | 151 | 91 | 60 | |
| Yes (N1–N3) | 74 | 15 | 59 |
Note: The P-value was calculated using chi-square test or Fisher’s exact test.
Abbreviation: FSIP1, fibrous sheath interacting protein 1.
Immunohistochemistry and scoring
Tumor tissues were fixed with 10% neutral formalin, followed by embedding in paraffin and sectioning at 4-μm thickness. Immunohistochemical staining was performed using an S-P staining kit (Ultrasensitive, MaiXin, Fuzhou, China). Sections were boiled in citrate buffer (pH 6.0) for 2 min in an autoclave for antigen retrieval. Then sections were treated with 3% hydrogen peroxide for 15 min for blocking endogenous peroxidase and then incubated with FSIP1 rabbit polyclonal antibody (1:500 dilution, Santa Cruz Biotechnology, Santa Cruz, CA, USA) overnight at 4°C. After rinsing with phosphate-buffered saline (PBS), the sections were incubated with the secondary biotinylated swine anti-rabbit antibody (Dako Inc., Denmark). Next, sections were covered with horseradish peroxidase (HRP)-conjugated streptavidin. After that, the immunological reaction was visualized using a 3,3′-diaminobenzidine kit (MaiXin, China) and counterstained with Gill’s hematoxylin. Staining that replacement of the primary antibody with PBS served as a negative control.
All the slides were inspected independently by two pathologists in a blinded manner, and FSIP1 staining was scored in five randomly selected views per slide. Cytoplasmic or membrane staining was considered as a positive FSIP1 expression. The semiquantitative scale was used, including assessment of both staining intensity and the percentage of positive cells. The intensity of FSIP1 staining was rated as follows: 0 absent, 1 weak, and 2 strong. The area percentage of stained cells was scored as 1, 1%–25%; 2, 26%–50%; 3, 51%–75%; and 4, 76%–100%. The scores of intensity and percentage were multiplied. The final FSIP1 expression was categorized into negative (total score <4) or positive (total score ≥4).
Cell culture
The immortalized, normal human uroepithelium cell line SV-HUC-1 and the human bladder cancer cell lines 5637, BIU-87, and T24 were purchased from the American Type Culture Collection (Manassas, VA, USA). The cells were grown in RPMI 1640 media (Invitrogen, Carlsbad, CA, USA) containing 10% fetal bovine serum and maintained in a sterile incubator at 37°C and 5% CO2. Cells were passaged every 2 days by digestion with trypsin (Invitrogen).
Western blotting analysis
Total proteins were extracted using a protein extraction kit (ProMab Biotechnologies, Richmond, CA, USA). Lysates were centrifugated at 12,000 rpm for 5 min at 4°C. Total protein level in the supernatant was quantified using bicinchoninic acid assay (Santa Cruz Biotechnology, USA). Subsequently, samples were subjected to sodium dodecyl sulfate-polyacrylamide gel electrophoresis and then transferred onto equilibrated polyvinylidene fluoride membrane (Millipore, Bradford, MA, USA). The membrane was blocked with 5% non-fat dried milk in Tris-buffered saline-Tween 20 solution and incubated with rabbit anti-FSIP1 antibody at a dilution of 1:200 (Abcam, Cambridge, UK) at 4°C overnight. After thorough washing, membranes were incubated with HRP-conjugated anti-rabbit secondary antibody at a dilution of 1:1,000 (Cell Signaling Technology, Danvers, MA, USA) at 37°C for 2 h. The bands of targeted proteins were visualized on the membrane using a Pierce enhanced chemiluminescence system kit (Thermo Fisher Scientific, Rockford, IL, USA) and quantitatively analyzed using a DNR BioImaging System (DNR, Jerusalem, Israel). β-actin served as a loading control for normalization.
RNA isolation and quantitative real-time PCR
Total RNA was isolated from tissue specimens or cultured cells using TRIzol reagent (Invitrogen) according to the instructions of the manufacturers. The first strand complementary DNA (cDNA) was synthesized using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems, Foster City, CA, USA). Quantitative real-time PCR was performed using the SYBR Green PCR Master Mix (Applied Biosystems) on the 7500 Real-Time PCR System (Applied Biosystems). The sequences of the primers were as follows: FSIP1, 5′-GCTCAGGGGTAAACACAACC-3′ (forward); 5′-GCTCAACCAGCCTTTTCTTC-3′ (reverse), and β-actin, 5′-CATGTACGTTGCTATCCAGGC-3′ (forward); 5′-CTCCTTAATGTCACGCACGAT-3′ (reverse). The reactions were started at 95°C for 10 min, and then 40 cycles at 95°C for 15 sec; 60°C for 30 sec; and finally, 72°C for 20 sec. The Ct values of the samples were calculated, and the relative quantities of FSIP1 messenger RNA (mRNA) were analyzed using the 2−ΔΔCt method and β-actin served as the endogenous control for normalization. All samples were assayed in triplicate to ensure the accuracy of results.
Statistical analysis
The SPSS statistical software (version 19.0, SPSS Inc., Chicago, IL, USA) was used for statistical data analyses. Chi-square test was used to assess the clinicopathologic data, and t-test was used to compare densitometry data. Survival curves were plotted using the Kaplan–Meier method. The overall survival and disease-free survival of patients were compared using the log-rank test. Univariate and multivariate Cox regression analyses were conducted to identify the prognostic factors for survival in bladder cancer patients. Values are expressed as mean ± SD. P<0.05 was considered statistically significant.
Results
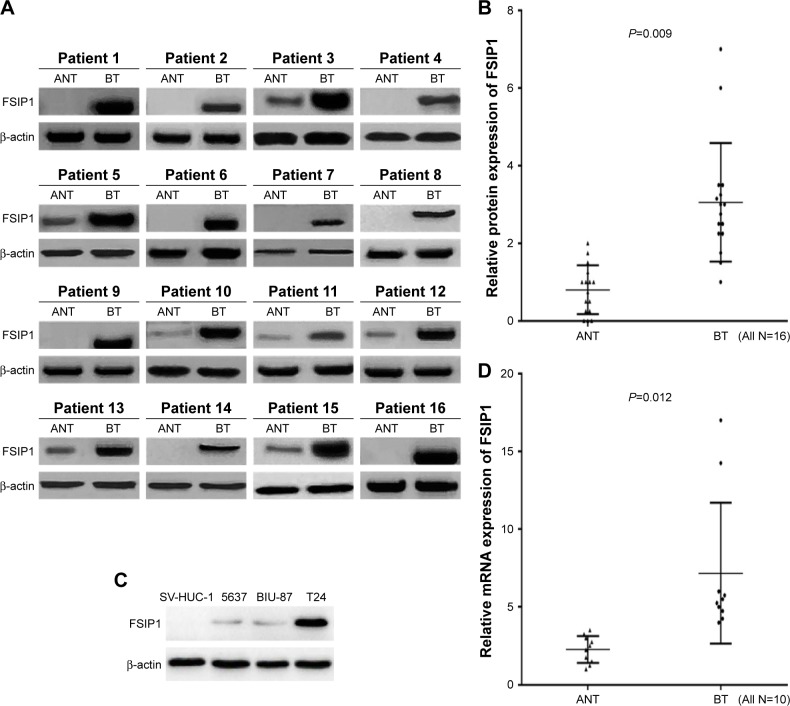
The mRNA and protein levels of FSIP1 were elevated in human bladder cancer. Western blotting and quantitative real-time PCR analysis showed that the mRNA and protein levels of FSIP1 in BTs were significantly higher than that in matched ANTs (Figure 1A, B, and D). Western blotting assay showed that 16 out of 17 (94.1%) BTs from patients exhibited higher FSIP1 protein expression levels compared with their matched ANTs. Similarly, higher FSIP1 mRNA expression levels were observed as compared with matched ANTs by quantitative real-time PCR analysis in 10 of 12 (83.3%) patients. In addition, the comparative analysis showed that the FSIP1 protein expression was significantly elevated in three bladder cancer cell lines (5637, BIU-87, and T24) compared to normal human urothelium cells SV-HUC-1 (Figure 1C). These results suggest that FSIP1 expression is typically elevated in human bladder cancer.
Figure 1.
Expression of FSIP1 in bladder cancer cell lines and human bladder tumor tissues.
Notes: (A, B) Western blotting analysis (16 paired) showed that the expression of FSIP1 in BTs was significantly higher than that in matched ANTs (P=0.009). (C) Western blotting analysis showed that FSIP1 protein expressions were significantly increased in three human bladder cancer cell lines (5637, BIU-87, and T24 in particular), compared with normal human urothelium cells SV-HUC-1. (D) Quantitative real-time PCR analysis showed an increased messenger RNA expression of FSIP1 in BTs as compared with ANTs (n=10, P=0.012).
Abbreviations: ANTs, adjacent noncancerous tissues; BTs, bladder tumor tissues; FSIP1, fibrous sheath interacting protein 1; PCR, polymerase chain reaction.
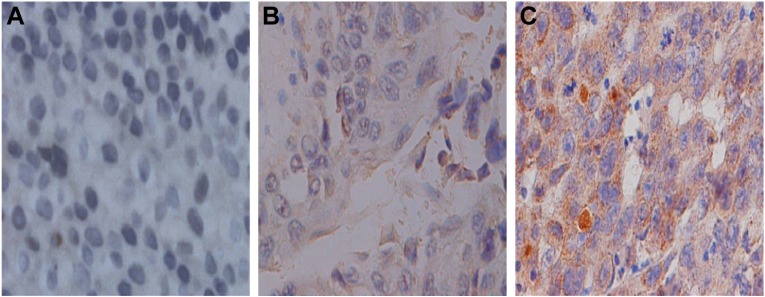
FSIP1 expression is strongly correlated with the clinicopathological characteristics of bladder cancer patients. To investigate the relationship between FSIP1 expression and the clinicopathological parameters of bladder cancer patients, FSIP1 expression was examined in 225 bladder cancer tissues by immunohistochemical analysis (Figure 2). The comparison of the clinicopathological characteristics between FSIP1-positive and FSIP1-negative patients is summarized in Table 1. Overall, FSIP1 was detected in 52.9% (119/225) in BT by immunohistochemical staining. There were no statistically significant differences regarding gender, age, tumor grade, tumor number, and size between FSIP1-positive and FSIP1-negative groups (P>0.05; Table 1). But FSIP1-positive group was associated with advanced clinical stage (T2–T4 vs Ta–T1, P=0.027) and lymph node metastasis (yes vs no, P=0.000). Spearman’s correlation analysis revealed strong correlations between FSIP1 expression and clinical stage and lymph node metastasis (P=0.027 and 0.000, respectively; Table 2).
Figure 2.
Immunohistochemical staining of fibrous sheath interacting protein 1 in bladder cancer tissues (×400 magnification).
Notes: (A) Negative expression (0 score); (B) weak positive expression (2 scores); (C) strong positive expression (6 scores).
Table 2.
Correlation between fibrous sheath interacting protein 1 expression and the clinicopathological characteristics of bladder cancer by Spearman’s correlation analysis
| Variables | Spearman’s R (P-value) |
|---|---|
| Gender | 0.124 (0.064) |
| Age | 0.107 (0.109) |
| Tumor grade | 0.027 (0.684) |
| Clinical stage | 0.147 (0.027) |
| Tumor number | 0.085 (0.205) |
| Tumor size | 0.020 (0.766) |
| Lymph node metastasis | 0.376 (0.000) |
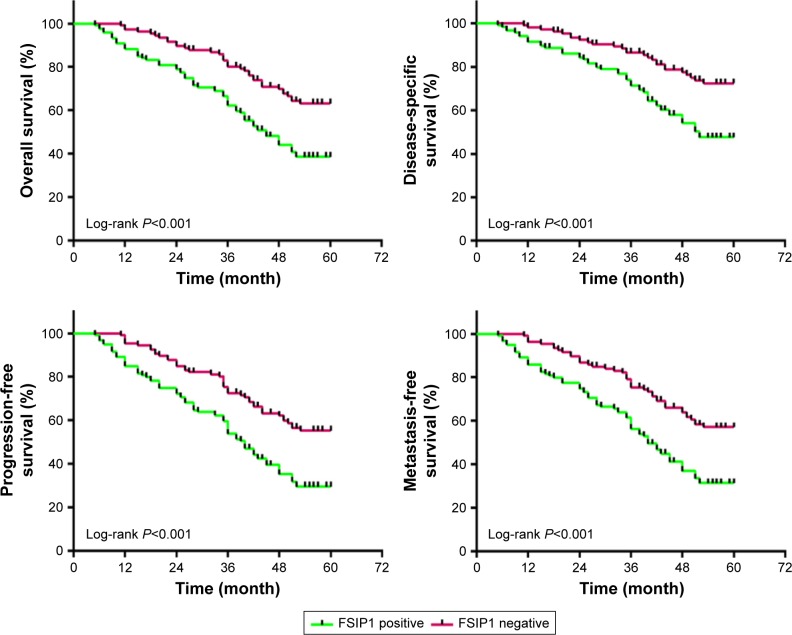
FSIP1-positive expression predicts an unfavorable prognosis in bladder cancer patients. We performed a survival analysis to address whether FSIP1-positive expression affected the prognosis of bladder cancer patients. FSIP1 expressions in 225 cases of paraffin-embedded bladder cancer tissues were examined by immunohistochemical staining, and the follow-up data were analyzed. The Kaplan–Meier curves of survival and log-rank analyses are shown in Figure 3. The 5-year overall survival rate of patients was 38.65% in the FSIP1-positive group, as compared with 63.08% in the FSIP1-negative group. The 5-year cumulative disease-specific survival rate, progression-free survival rate, and metastasis-free survival rate in the FSIP1-positive group were 47.61%, 29.51%, and 31.27%, respectively, which were significantly lower than those in the FSIP1-negative group (all P<0.05).
Figure 3.
Kaplan–Meier survival analysis of overall survival, disease-specific survival, progression-free survival, and metastasis-free survival between FSIP1-positive and FSIP1-negative bladder cancer patients (n=225).
Note: The log-rank test was used to calculate the P-value (all P<0.01).
Abbreviation: FSIP1, fibrous sheath interacting protein 1.
FSIP1-positive expression was an independent prognostic factor for bladder cancer patients. In the present study, univariate and multivariate Cox regression models were performed to investigate the prognostic factors for overall survival and disease-free survival in bladder cancer patients. The FSIP1-positive expression, multifocal tumors, tumor grade G3, clinical stage T2–4, and lymph node metastasis were identified as unfavorable prognostic factors based on the univariate analysis (P<0.05; Table 3). Multivariate Cox regression analysis showed that tumor grade G3 (hazard ratio [HR] 3.015, 95% CI 1.859–4.891, P=0.000), multifocal tumors (HR 1.644, 95% CI 1.063–2.542, P=0.025), clinical stage T2–4 (HR 3.796, 95% CI 2.038–7.072, P=0.000), lymph node metastasis (HR 1.934, 95% CI 1.196–3.128, P=0.007), and FSIP1-positive expression (HR 1.704, 95% CI 1.090–2.664, P=0.019) were independent unfavorable prognostic factors for disease-free survival, whereas tumor grade G3 (HR 4.066, 95% CI 2.263–7.305, P=0.000), clinical stage T2~4 (HR 7.018, 95% CI 2.819–17.472, P=0.000), lymph node metastasis (HR 1.835, 95% CI 1.106–3.044, P=0.019), and FSIP1-positive expression (HR 1.671, 95% CI 1.033–2.704, P=0.037) were risk factors for overall survival (Table 4).
Table 3.
The prognostic factors for overall and disease-free survival in bladder cancer patients by univariate Cox regression analysis (n=225)
| Variables | Disease-free survival
|
Overall survival
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender (male vs female) | 1.596 | 0.930–2.740 | 0.090 | 1.376 | 0.784–2.416 | 0.266 |
| Age (≥60 years vs <60 years) | 1.050 | 0.739–1.492 | 0.785 | 1.040 | 0.711–1.520 | 0.840 |
| Tumor grade (G3 vs G1/2) | 5.717 | 3.825–8.543 | 0.000 | 9.387 | 5.654–15.586 | 0.000 |
| Clinical stage (T2–4 vs Ta–1) | 9.064 | 5.322–15.437 | 0.000 | 19.374 | 8.470–44.314 | 0.000 |
| Tumor number (multifocal vs unifocal) | 2.929 | 2.010–4.269 | 0.000 | 2.736 | 1.822–4.107 | 0.000 |
| Tumor size (≥3 cm vs <3 cm) | 1.139 | 0.801–1.620 | 0.469 | 1.156 | 0.790–1.691 | 0.456 |
| Lymph node metastasis (yes vs no) | 5.759 | 3.980–8.332 | 0.000 | 6.086 | 4.074–9.093 | 0.000 |
| Fibrous sheath interacting protein 1 expression (positive vs negative) | 2.094 | 1.451–3.022 | 0.000 | 2.115 | 1.419–3.154 | 0.000 |
Abbreviation: HR, hazard ratio.
Table 4.
The prognostic factors for overall and disease-free survival in bladder cancer patients by multivariate Cox regression analysis (n=225)
| Variables | Disease-free survival
|
Overall survival
|
||||
|---|---|---|---|---|---|---|
| HR | 95% CI | P-value | HR | 95% CI | P-value | |
| Gender (male vs female) | 1.273 | 0.730–2.221 | 0.395 | 1.152 | 0.642–2.067 | 0.636 |
| Age (≥60 years vs <60 years) | 0.866 | 0.596–1.260 | 0.506 | 0.873 | 0.585–1.303 | 0.506 |
| Tumor grade (G3vs G1/2) | 3.015 | 1.859–4.891 | 0.000 | 4.066 | 2.263–7.305 | 0.000 |
| Clinical stage (T2–4 vs Ta–1) | 3.796 | 2.038–7.072 | 0.000 | 7.018 | 2.819–17.472 | 0.000 |
| Tumor number (multifocal vs unifocal) | 1.644 | 1.063–2.542 | 0.025 | 1.378 | 0.871–2.179 | 0.170 |
| Tumor size (≥3 cm vs <3 cm) | 0.993 | 0.679–1.453 | 0.972 | 1.124 | 0.745–1.696 | 0.576 |
| Lymph node metastasis (yes vs no) | 1.934 | 1.196–3.128 | 0.007 | 1.835 | 1.106–3.044 | 0.019 |
| Fibrous sheath interacting protein 1 expression (positive vs negative) | 1.704 | 1.090–2.664 | 0.019 | 1.671 | 1.033–2.704 | 0.037 |
Abbreviation: HR, hazard ratio.
Discussion
Bladder cancer represents a common and hazardous malignancy and is responsible for significant morbidity worldwide.17–19 At present, pathological stage and grade are still the determinate parameters for predicting prognosis and decision-making in the management of bladder cancers.20 There is still a lack of generally accepted methods, especially tumor biomarkers, to determine the prognosis of bladder cancer. The identification of a certain protein that is related to the prognosis of bladder cancer patients may not only reveal a potential molecular mechanism underlying the initiation and progression of cancer but also offer a potential novel target for the treatment of bladder cancer.
The recently discovered FSIP1 gene is a single-copy gene located on chromosome 15. It is a definite protein-coding gene.13 FSIP1 has been reported to anomalously engage in the tumor cell mitotic network.21 Moreover, there is evidence to support FSIP1 as a potential target for steroid receptor coactivator-3, a gene responsible for cancer progression and poor prognosis. Previous studies demonstrated elevated FSIP1 level in breast cancer, indicating the involvement of FSIP1 in the pathogenesis of such disease.12,22,23 In addition, evidence showed that regulation of FSIP1 is associated with arsenic-related bladder cancer, especially the FSIP1 single-nucleotide polymorphism closely linked to invasion and differentiation of bladder carcinoma.15 However, the FSIP1 expression level in bladder cancer tissues and its clinical significance still lack investigation.
In the current study, a cohort of 225 bladder cancer samples was assayed for FSIP1 expression by immunohistochemical staining. FSIP1 showed positive expression in 52.9% tumor specimens and it is predictive of poor prognosis in patients with bladder cancer. FSIP1 overexpression in bladder tumor is usually associated with more advanced clinical stage and lymph node metastasis, although no association between FSIP1 expression and tumor grade was observed. These findings were also confirmed by the results of both quantitative real-time PCR and Western blotting analysis, which demonstrated the high FSIP1 expression in BTs and the poor expression of FSIP1 expression in ANTs. And also, FSIP1 overexpressed in all the three bladder cancer cell lines tested (5637, BIU-87, and T24 in particular). As FSIP1 overexpression is closely related to advanced clinical stage, especially lymph node metastasis, it appears to exhibit significantly elevated expression levels relative to the higher pT and pN status. The expression level of FSIP1 protein was closely associated with unfavorable clinicopathological characteristics. The positive FSIP1 expression in bladder cancer multivariate Cox proportional hazard model confirmed that a positive FSIP1 expression was an independent prognostic predictor, which was correlated with a worse overall and disease-specific survival for bladder cancer patients. The new insight of this study provides strong evidence that FSIP1 plays a significant role in the progression of bladder cancer, and it can serve as a novel prognostic indicator of unfavorable survival and a target for bladder cancer therapy. A functional study is further needed to explore the molecular mechanisms of FSIP1 on the biological behaviors of bladder cancer.
Conclusion
FSIP1 overexpression showed good correlation with tumor stage and lymph node metastasis; positive FSIP1 expression was independently associated with an unfavorable overall and disease-free survival of bladder cancer. Thus, it potentially serves as a valuable therapeutic or predictive target for bladder cancer. In future research, we will further identify the mechanisms by which FSIP1 contributes to tumorigenesis and metastasis of bladder cancer.
Acknowledgments
The authors thank Dr Xin Su for the evaluation of immunohistochemistry and Prof Zhengwei Yuan for the comments on the manuscript.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Chavan S, Bray F, Lortet-Teulent J, Goodman M, Jemal A. International variations in bladder cancer incidence and mortality. Eur Urol. 2014;66(1):59–73. doi: 10.1016/j.eururo.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 2.Mahdavifar N, Ghoncheh M, Pakzad R, Momenimovahed Z, Salehiniya H. Epidemiology, incidence and mortality of bladder cancer and their relationship with the development index in the world. Asian Pac J Cancer Prev. 2016;17(1):381–386. doi: 10.7314/apjcp.2016.17.1.381. [DOI] [PubMed] [Google Scholar]
- 3.Eifler JB, Barocas DA, Resnick MJ. Predictors of outcome in bladder cancer. J Natl Compr Canc Netw. 2014;12(11):1549–1554. doi: 10.6004/jnccn.2014.0154. [DOI] [PubMed] [Google Scholar]
- 4.Salama RH, Selem TH, El-Gammal M, Elhagagy AE, Bakar SM. Urinary tumor markers could predict survival in bladder carcinoma. Indian J Clin Biochem. 2013;28(3):265–271. doi: 10.1007/s12291-012-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Garg M. Prognostic and therapeutic applications of the molecular events in clinical management of urothelial carcinoma of bladder. J ExpTher Oncol. 2014;10(4):301–316. [PubMed] [Google Scholar]
- 6.Harshman LC, Preston MA, Bellmunt J, Beard C. Diagnosis of bladder carcinoma: a clinician’s perspective. Surg Pathol Clin. 2015;8(4):677–685. doi: 10.1016/j.path.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 7.Schwingshackl L, Hoffmann G. Adherence to mediterranean diet and risk of cancer: an updated systematic review and meta-analysis of observational studies. Cancer Med. 2015;4(12):1933–1947. doi: 10.1002/cam4.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cumberbatch MG, Cox A, Teare D, Catto JW. Contemporary occupational carcinogen exposure and bladder cancer: a systematic review and meta-analysis. JAMA Oncol. 2015;1(9):1282–1290. doi: 10.1001/jamaoncol.2015.3209. [DOI] [PubMed] [Google Scholar]
- 9.Dobruch J, Daneshmand S, Fisch M, et al. Gender and bladder cancer: a collaborative review of etiology, biology, and outcomes. Eur Urol. 2016;69(2):300–310. doi: 10.1016/j.eururo.2015.08.037. [DOI] [PubMed] [Google Scholar]
- 10.Kim JY, Kim JH, Park TJ, et al. Positive association between aspirin-intolerant asthma and genetic polymorphisms of FSIP1: a case-case study. BMC Pulm Med. 2010;10:34. doi: 10.1186/1471-2466-10-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gjerstorff MF, Andersen MH, Ditzel HJ. Oncogenic cancer/testis antigens: prime candidates for immunotherapy. Oncotarget. 2015;6(18):15772–15787. doi: 10.18632/oncotarget.4694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang H, Luo M, Jin Z, et al. Expression and clinicopathological significance of FSIP1 in breast cancer. Oncotarget. 2015;6(12):10658–10666. doi: 10.18632/oncotarget.3381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chapman KB, Prendes MJ, Kidd JL, Sternberg H, West MD, Wagner J. Elevated expression of cancer/testis antigen FSIP1 in ER-positive breast tumors. Biomark Med. 2013;7(4):601–611. doi: 10.2217/bmm.13.58. [DOI] [PubMed] [Google Scholar]
- 14.Mao Y, Xu R, Liu X, Shi W, Han Y. Elevated fibrous sheath interacting protein 1 levels are associated with poor prognosis in non-small cell lung cancer patients. Oncotarget. 2017;8(7):12186–12193. doi: 10.18632/oncotarget.14575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karagas MR, Andrew AS, Nelson HH, et al. SLC39A2 and FSIP1 polymorphisms as potential modifiers of arsenic-related bladder cancer. Hum Genet. 2012;131(3):453–461. doi: 10.1007/s00439-011-1090-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kluth LA, Black PC, Bochner BH, et al. Prognostic and prediction tools in bladder cancer: a comprehensive review of the literature. Eur Urol. 2015;68(2):238–253. doi: 10.1016/j.eururo.2015.01.032. [DOI] [PubMed] [Google Scholar]
- 17.Khan R, Ibrahim H, Tulpule S, Iroka N. Bladder cancer in a young patient: undiscovered risk factors. Oncol Lett. 2016;11(5):3202–3204. doi: 10.3892/ol.2016.4355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Telli O, Sarici H, Ozgur BC, et al. Urothelial cancer of bladder in young versus older adults: clinical and pathological characteristics and outcomes. Kaohsiung J Med Sci. 2014;30(9):466–470. doi: 10.1016/j.kjms.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Borden LS, Jr, Clark PE, Hall MC. Bladder cancer. Curr Opin Oncol. 2003;15(3):227–233. doi: 10.1097/00001622-200305000-00009. [DOI] [PubMed] [Google Scholar]
- 20.Mathieu R, Lucca I, Rouprêt M, Briganti A, Shariat SF. The prognostic role of lymphovascular invasion in urothelial carcinoma of the bladder. Nat Rev Urol. 2016;13(8):471–479. doi: 10.1038/nrurol.2016.126. [DOI] [PubMed] [Google Scholar]
- 21.Brown PR, Miki K, Harper DB, Eddy EM. A-kinase anchoring protein 4 binding proteins in the fibrous sheath of the sperm flagellum. Biol Reprod. 2003;68(6):2241–2248. doi: 10.1095/biolreprod.102.013466. [DOI] [PubMed] [Google Scholar]
- 22.Labhart P, Karmakar S, Salicru EM, et al. Identification of target genes in breast cancer cells directly regulated by the SRC-3/AIB1 coactivator. Proc Natl Acad Sci U S A. 2005;102(5):1339–1344. doi: 10.1073/pnas.0409578102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shou J, Massarweh S, Osborne CK, et al. Mechanisms of tamoxifen resistance: increased estrogen receptor-HER2/neu cross-talk in ER/HER2-positive breast cancer. J Natl Cancer Inst. 2004;96(12):926–935. doi: 10.1093/jnci/djh166. [DOI] [PubMed] [Google Scholar]