Abstract
BACKGROUND
Although married cancer patients have more favorable survival than unmarried patients, reasons underlying this association are not fully understood. The authors evaluated the role of economic resources, including health insurance status and neighborhood socioeconomic status (nSES), in a large California cohort.
METHODS
From the California Cancer Registry, we identified 783,167 cancer patients (386,607 deaths) who were diagnosed during 2000 through 2009 with a first primary, invasive cancer of the 10 most common sites of cancer-related death for each sex and were followed through 2012. Age-stratified and stage-stratified Cox proportional hazard models were used to estimate hazard ratios (HRs) and 95% confidence intervals (95% CIs) for all-cause mortality associated with marital status, adjusted for cancer site, race/ethnicity, and treatment.
RESULTS
Compared with married patients, unmarried patients had an elevated risk of mortality that was higher among males (HR, 1.27; 95% CI, 1.26–1.29) than among females (HR, 1.19; 95% CI, 1.18–1.20; Pinteraction < .001). Adjustment for insurance status and nSES reduced the marital status HRs to 1.22 for males and 1.15 for females. There was some evidence of synergistic effects of marital status, insurance, and nSES, with relatively higher risks observed for unmarried status among those who were under-insured and living in high nSES areas compared with those who were under-insured and living in low nSES areas (Pinteraction = 6.8 × 10−9 among males and 8.2 × 10−8 among females).
CONCLUSIONS
The worse survival of unmarried than married cancer patients appears to be minimally explained by differences in economic resources.
Keywords: health insurance, marriage, mortality, neighborhood socioeconomic status, race/ethnicity
INTRODUCTION
The association of marital status with cancer survival is well established: mortality is lower among married than unmarried patients with cancer irrespective of sex, site, and stage of disease.1–3 In a recent analysis of Surveillance, Epidemiology, and End Results (SEER) data, Aizer et al demonstrated significantly lower cancer-specific mortality among married patients diagnosed with 1 of the 10 most common cancer types4 after adjustment for stage, treatment, age, sex, and county-level socioeconomic status (SES). Increased social support has been suggested as 1 of the primary drivers for the inverse association between being married and cancer mortality, particularly because married cancer patients were more likely than unmarried patients to be diagnosed at an earlier disease stage and to receive definitive treatment.4,5 The protective effect of marriage on survival is greater among males than females and diminishes with increasing age.6,7
Married patients differ from unmarried patients in many ways; they are more likely to engage in healthy behaviors, such as having better diets, engaging in more physical activity, participating in health-prevention measures like cancer screening, and receiving more aggressive treatment.6–8 Less well studied, however, is the impact of economic resources as a contributing factor for marriage-associated survival differences, despite the finding that married individuals generally have greater combined income and access to health insurance than unmarried individuals.9 Moreover, economic disadvantage may interact with marital status to produce heightened disparities in cancer outcomes.10
Therefore, we assessed the extent to which mortality differences between married and unmarried cancer patients are explained by health insurance status and neighborhood SES (nSES). We addressed this question using population-based data from the California Cancer Registry (CCR) for the 10 most common sites of cancer-related deaths, with attention to differences by sex and including data on patient-level health insurance status and neighborhood-level (ie, block-group) SES as indicators of economic resources.
MATERIALS AND METHODS
Case Selection
We obtained data for first primary invasive cancers among individuals aged ≥18 years diagnosed from 2000 through 2009. For males, the cancer sites were prostate, lung and bronchus (“lung”), colon, non-Hodgkin lymphoma (“NHL”), bladder, liver and intrahepatic bile duct (“liver and IBD”), leukemia, pancreas, stomach, and esophagus. For females, the sites were breast; lung; colon; corpus and uterus, not otherwise specified (“uterus”); NHL; ovary; pancreas; leukemia; brain and other nervous system (“brain”); and liver and IBD. The data were obtained from the CCR, which registers cancers from throughout the large and diverse state of California and comprises 3 National Cancer Institute (NCI) SEER program regions and half of the cases in all 18 SEER regions. Of 844,824 cases, we excluded cases diagnosed at autopsy or from death certificate only (n = 9286) and those with unknown follow-up time (n = 4347), unknown marital status (n = 36,937), or unknown treatment (n = 11,087).
Patient characteristics from the CCR (from reporting facilities, with demographic variables based on self-report) included: marital status, age, address and stage at diagnosis, year of diagnosis, race/ethnicity, sex, histology, first course of treatment (surgery, radiation, and/or systemic hormone agents), and primary and secondary sources of payment. By using the payment variables, health insurance status was coded hierarchically as no insurance; any public, military, or any Medicaid/Medi-Cal (including Medicare/Medicaid) insurance; private insurance only; Medicare only or Medicare and private insurance; and unknown. We conducted our primary analyses with marital status coded as married and unmarried (consisting of never married, separated, divorced, and widowed), because mortality risks generally do not vary greatly across subcategories of unmarried status.7
Patient address at diagnosis was geocoded and assigned to a census block group, then linked to an nSES index that was developed previously from principal components analysis, incorporating information on education, occupation, employment, household income, poverty, rent and house values from the Census 2000 Summary File (for cases diagnosed 2000–2005), and American Community Survey data from 2007 to 2011 (for cases diagnosed during 2006–2009).11,12 The hospital that initially reported each case was classified by NCI-designated cancer center status (yes, no).
Determination of Follow-Up and Vital Status
The CCR follows all patients for vital status, collecting information by follow-up from the diagnosing hospital, state and national vital statistics databases, and other sources. Follow-up time for all-cause mortality was computed as the number of days between diagnosis and either death, the date of last known contact, or the end date of follow-up, whichever occurred first. For analyses of cancer-specific death, the underlying cause of death was obtained from death certificates; follow-up was censored at the date of death for those who died from a cause other than the primary cancer.
Statistical Analysis
Chi-square tests were used to compare case characteristics by marital status and sex. We estimated hazard ratios (HR) and 95% confidence intervals (CI) associated with all-cause mortality and cancer-specific mortality using multivariable Cox proportional hazards regression models. Because the HRs for marital status differed significantly by sex, we conducted analyses separately for males and females. We also conducted analyses by age, using age 70 years as a cutoff point, because prior studies have noted that the strength of the correlation between marital status and mortality appears to diminish at this age.2,7
We tested the proportional hazards assumption for each covariate using correlation tests of time versus scaled Schoenfeld residuals. The assumption of proportional hazards was violated for stage and age. Thus, we computed stage-stratified and age-stratified Cox regression models, allowing the baseline hazards to vary by these variables. These models were adjusted for cancer site, race/ethnicity (non-Hispanic white [“white”], non-Hispanic black [“black”], Hispanic, Asian or Pacific Islander [“API”], and other or unknown), treatment (surgery, radiation, hormone agents), nSES (statewide quintiles), and health insurance. Models that included all of the cancer sites combined excluded cases with leukemia, because stage and surgery were not applicable. All statistical tests were 2-sided with an a value of .05. Likelihood ratio tests of interaction were computed based on cross-product terms. Analyses were performed using SAS version 9.3 (SAS Institute, Inc, Cary, NC). We did not obtain informed consent from the patients, because we analyzed de-identified cancer registry data.
RESULTS
The cohort included 393,470 males and 389,697 females. With follow-up through December 31, 2012, there were 1,801,907 and 1,903,874 person-years of follow-up for males and females, respectively, and 204,007 and 182,600 deaths, respectively. Compared with females, males were more likely to be married and were less likely to be widowed (for males: 70% married, 14% never married, 1% separated, 8% divorced, and 7% widowed; for females: 51% married, 15% never married, 1% separated, 11% divorced, and 22% widowed) (Table 1). Nearly all patients (98%) had some type of health insurance, with private insurance being the predominant type. Unmarried patients were more likely than married patients to comprise the very youngest and the very oldest age categories, to live in lower SES neighborhoods, to be uninsured or have public insurance, to be diagnosed at a later stage of disease, not to receive any surgery or radiation, and were more likely to be black but less likely to be API. The proportion of patients who used an NCI-designated cancer center was similar for married and unmarried patients at 9.7% and 9% among unmarried and married males, respectively, and 5.9% and 6.2% among unmarried and married females, respectively (data not shown).
TABLE 1.
Demographic and Clinical Characteristics of Cancer Patients by Sex and Marital Status, California, Diagnoses Years 2000 Through 2009 (N = 783,167)
| Characteristic | Males, n = 393,470, %
|
Females, n = 389,697, %
|
||
|---|---|---|---|---|
| Unmarried, n = 118,126 | Married, n = 275,344 | Unmarried, n = 191,059 | Married, n = 198,638 | |
| Age at diagnosis, y | ||||
| 18–29 | 1.6 | 0.2 | 1.3 | 0.7 |
| 30–39 | 1.9 | 1 | 3 | 4.9 |
| 40–49 | 6.9 | 4.7 | 9.5 | 16.6 |
| 50–59 | 20.6 | 18.7 | 16.5 | 25.6 |
| 60–69 | 27.6 | 32.5 | 20.1 | 25.1 |
| 70–79 | 24.9 | 29.7 | 24.6 | 19.4 |
| 80–89 | 14 | 12.1 | 20.6 | 7.4 |
| >90 | 2.6 | 1 | 4.5 | 0.5 |
| Race/ethnicity | ||||
| Non-Hispanic white | 65 | 65.9 | 67.3 | 66.4 |
| Non-Hispanic black | 12.1 | 6.1 | .8.8 | 4 |
| Hispanic | 15 | 15.2 | 14.9 | 16 |
| Asian/Pacific Islander | 6.2 | 11.2 | 8.2 | 12.9 |
| Other/unknown | 1.7 | 1.6 | 0.9 | 0.7 |
| Neighborhood socioeconomic status, statewide quintiles | ||||
| Quintile 1 (low) | 19.6 | 12.1 | 16 | 10.9 |
| Quintile 2 | 21.1 | 17.2 | 20 | 16.4 |
| Quintile 3 | 21.1 | 20.6 | 21.8 | 20.4 |
| Quintile 4 | 20.2 | 22.8 | 22.2 | 23.6 |
| Quintile 5 (high) | 18.1 | 27.3 | 20 | 28.8 |
| Health insurance | ||||
| None | 3.6 | 1.4 | 1.9 | 1.4 |
| Private only | 36.3 | 49.5 | 41.4 | 61.2 |
| Medicare only or Medicare and private | 14 | 18.7 | 17.4 | 13.8 |
| Any public/Medicaid/military | 41.3 | 27.2 | 34.9 | 20.5 |
| Unknown | 4.8 | 3.2 | 4.4 | 3.1 |
| Cancer site | ||||
| Lung | 21.4 | 15.6 | 19.1 | 12.5 |
| Prostate | 37.2 | 48.9 | NA | NA |
| Breast | NA | NA | 39.1 | 50.6 |
| Colon | 10.4 | 9.8 | 12 | 9 |
| Non-Hodgkin lymphoma | 7.4 | 6.1 | 5.6 | 5.3 |
| Liver and intrahepatic bile duct | 5.1 | 3.5 | 1.9 | 1.4 |
| Pancreas | 3.7 | 3.5 | 4.3 | 3.1 |
| Esophagus | 2.4 | 1.7 | NA | NA |
| Stomach | 3.1 | 3.2 | NA | NA |
| Bladder | 4.6 | 4 | NA | NA |
| Uterus | NA | NA | 8.2 | 8.9 |
| Ovary | NA | NA | 5 | 4.8 |
| Leukemia | 4.5 | 3.7 | 3.1 | 2.7 |
| Brain | NA | NA | 1.7 | 1.8 |
| Stage | ||||
| Local | 42.7 | 52.4 | 40 | 47.7 |
| Regional | 17.8 | 18.8 | 26.3 | 28.1 |
| Distant | 28.4 | 21 | 24.8 | 19 |
| Unknown/NA | 11.1 | 7.9 | 9 | 5.3 |
| Surgery | ||||
| Yes | 35.1 | 43.5 | 64.1 | 76.9 |
| No | 60.4 | 52.8 | 32.8 | 20.4 |
| Unknown/NA | 4.5 | 3.7 | 3.1 | 2.7 |
| Radiation | ||||
| Yes | 24.1 | 27 | 27.1 | 35.2 |
| No | 75.9 | 73 | 72.9 | 64.8 |
| Deaths | 61.9 | 47.6 | 56.3 | 37.8 |
| Follow-up, total person-years | 446,640 | 1,355,267 | 811,423 | 1,092,451 |
Abbreviation: NA, not applicable.
Uninsured males and females had an approximately 25% increased risk of death compared with privately insured patients (Table 2). Patients who had any public insurance also had increased mortality compared with those who had private insurance. Mortality declined with increasing levels of nSES.
TABLE 2.
All-Cause Mortality Associated With Health Insurance Status and Neighborhood Socioeconomic Status Among Cancer Patients Diagnosed From 2000 Through 2009, by Sex, California
| Variable | Males, n = 377,932a
|
Females, n = 378,447a
|
||
|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI)b | Unadjusted HR (95% CI) | Adjusted HR (95% CI)b | |
| Health insurance | ||||
| Private only | 1.0 | 1.0 | 1.0 | 1.0 |
| None | 1.96 (1.90–2.02) | 1.23 (1.20–1.27) | 1.76 (1.70–1.83) | 1.24 (1.19–1.28) |
| Any Medicare | 1.48 (1.47–1.50) | 1.02 (1.01–1.03) | 2.05 (2.02–2.08) | 1.01 (0.99–1.02) |
| Any public/Medicaid/military | 1.80 (1.78–1.82) | 1.11 (1.10–1.12) | 2.08 (2.06–2.11) | 1.13 (1.12–1.14) |
| Unknown | 2.49 (2.43–2.54) | 1.29 (1.26–1.32) | 2.49 (2.44–2.55) | 1.23 (1.20–1.26) |
| Neighborhood SES | ||||
| Quintile 1 (low) | 1.0 | 1.0 | 1.0 | 1.0 |
| Quintile 2 | 0.89 (0.88–0.90) | 0.94 (0.92–0.95) | 0.92 (0.90–0.93) | 0.95 (0.94–0.97) |
| Quintile 3 | 0.78 (0.77–0.80) | 0.88 (0.87–0.90) | 0.83 (0.82–0.84) | 0.90 (0.88–0.91) |
| Quintile 4 | 0.68 (0.67–0.69) | 0.82 (0.80–0.83) | 0.73 (0.72–0.75) | 0.84 (0.82–0.85) |
| Quintile 5 (high) | 0.53 (0.53–0.54) | 0.72 (0.71–0.74) | 0.60 (0.59–0.61) | 0.75 (0.74–0.76) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
The analysis excluded 15,538 males with leukemia and 11,250 females with leukemia.
Models were stratified by stage and age and were adjusted for cancer site, race/ethnicity, treatment, marital status, insurance status, and neighborhood socioeconomic status.
Overall, both unmarried men and unmarried women were more likely to die than their married counterparts, and HRs were significantly higher in men than in women (Table 3). Although HRs for unmarried patients appeared to be slightly higher for those aged <70 years than those aged ≥70 years, elevated risks persisted and were stronger for men than for women in both age groups. Similar associations for marital status were observed across cancer sites (Fig. 1), although the size of the associations varied somewhat, being slightly more pronounced for NHL and prostate cancer in males and for uterine cancer, NHL, and breast cancer in females. Results for cancer-specific mortality were similar to those for all-cause mortality (data not shown).
TABLE 3.
All-Cause Mortality Associated With Being Unmarried (vs Married), by Sex and Age, Among Cancer Patients Diagnosed From 2000 Through 2009, California
| Age at Diagnosis | Males, n = 377,932a
|
Females, n = 378,447a
|
PInteraction for Adjusted HRsc | ||
|---|---|---|---|---|---|
| Unadjusted HR (95% CI) | Adjusted HR (95% CI)b | Unadjusted HR (95% CI) | Adjusted HR (95% CI)b | ||
| All ages | 1.57 (1.56–1.59) | 1.22 (1.21–1.24) | 1.80 (1.78–1.82) | 1.15 (1.14–1.16) | 3.3 × 10−16 |
| <70 y | 1.75 (1.73–1.78) | 1.24 (1.22–1.26) | 1.50 (1.48–1.52) | 1.16 (1.14–1.17) | 1.1 × 10−7 |
| ≥70 y | 1.47 (1.45–1.48) | 1.19 (1.18–1.21) | 1.46 (1.44–1.48) | 1.13 (1.11–1.14) | 5.8 × 10−9 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
The analysis excluded 15,538 males with leukemia and 11,250 females with leukemia.
Models were stratified by stage and age and were adjusted for cancer site, race/ethnicity, treatment, insurance status, and neighborhood socioeconomic status.
P values for the interaction between sex and marital status are shown.
Figure 1.
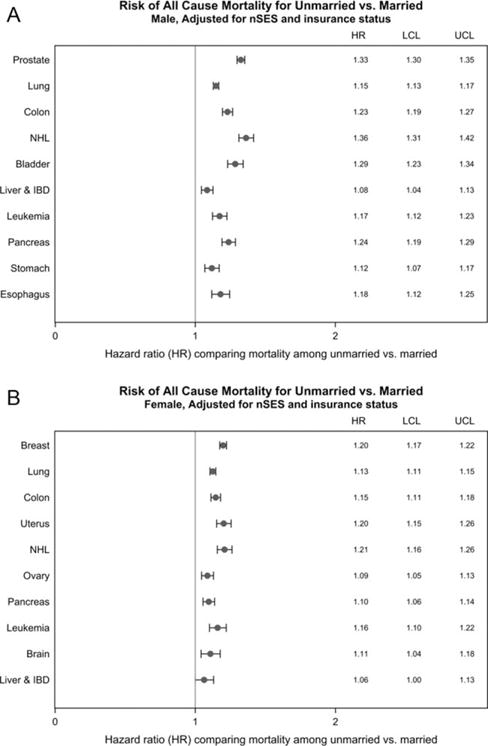
Each site-specific model was stratified by stage and age, and adjusted for race/ethnicity, treatment, marital status, insurance status, and neighborhood SES. IBD indicates intrahepatic bile duct; LCL, lower confidence limit; NHL, non-Hodgkin lymphoma; UCL, upper confidence limit.
HRs associated with specific types of unmarried status for women were similar across groups (compared with married women: HR, 1.15 [95% CI, 1.14–1.17] for never married; HR, 1.16 [95% CI, 1.11–1.22] for separated; HR, 1.16 [95% CI, 1.14–1.17] for divorced; HR, 1.15 [95% CI, 1.13–1.16] for widowed). Among men, however, the HR was highest in those who had never been married (compared with married: HR, 1.26 [95% CI, 1.24–1.28] for never married; HR, 1.17 [95% CI, 1.12–1.22] for separated; HR, 1.23 [95% CI, 1.21–1.25] for divorced; HR, 1.18 [95% CI, 1.16–1.20] for widowed).
Adjustment for both nSES and insurance status only marginally reduced the higher risks associated with being unmarried (Table 4). When stratified on nSES and insurance status, the HRs for marital status did not substantially vary across strata (Table 5). However, there was evidence of an interaction, such that those patients with lower levels of insurance coverage and living in higher SES neighborhoods had the highest risks (Table 5). Stratifying on nSES and adjusting for insurance status did not change associations of marital status and survival for each cancer site, with the exception of NHL among men, for which considerably higher HRs were observed among those living in higher than lower SES neighborhoods (Supporting Figs. 1 and 2; see online supporting information).
TABLE 4.
Risk of All-Cause Mortality Associated With Being Unmarried (vs Married) Among Cancer Patients Diagnosed From 2000 Through 2009, by Sex, California: Effect of Adjusting for Socioeconomic Status and Health Insurance Status
| Model | Covariates in Modelb | All-Cause Mortality Risk Associated With Unmarried Status: HR (95% CI)a
|
|
|---|---|---|---|
| Males, n = 377,932a | Females, n = 378,447a | ||
| 1 | None | 1.37 (1.35–1.38) | 1.26 (1.25–1.27) |
| 2 | Cancer site, race/ethnicity, and treatment | 1.27 (1.26–1.29) | 1.19 (1.18–1.20) |
| 3 | Model 2 + neighborhood SES | 1.24 (1.23–1.26) | 1.17 (1.15–1.18) |
| 4 | Model 2 + health insurance | 1.25 (1.23–1.26) | 1.17 (1.16–1.18) |
| 5 | Model 2 + neighborhood SES + health insurance | 1.22 (1.21–1.24) | 1.15 (1.14–1.16) |
Abbreviations: CI, confidence interval; HR, hazard ratio.
The analysis excluded 15,538 males with leukemia and 11,250 females with leukemia.
Models were stratified by stage and age at diagnosis.
TABLE 5.
Risk of All-Cause Mortality Associated With Being Unmarried (vs Married) Among Cancer Patients Diagnosed From 2000 Through 2009 Stratified by Health Insurance, Neighborhood Socioeconomic Status (SES), and Both Health Insurance and Neighborhood SES, California
| Variable | All-Cause Mortality Risk Associated With Unmarried Status
|
|||
|---|---|---|---|---|
| Males, n = 377,932a
|
Females, n = 378,447a
|
|||
| Unadjusted HR (95% CI) | Adjusted HR (95% CI)b | Unadjusted HR (95% CI) | Adjusted HR (95% CI)b | |
| Health insurancec | ||||
| Low | 1.49 (1.47–1.51) | 1.25 (1.23–1.27) | 1.60 (1.58–1.63) | 1.18 (1.16–1.19) |
| High | 1.44 (1.42–1.46) | 1.21 (1.20–1.23) | 1.73 (1.70–1.75) | 1.15 (1.13–1.16) |
| Neighborhood SESd | ||||
| Low (quintiles 1–3) | 1.48 (1.46–1.49) | 1.25 (1.23–1.26) | 1.67 (1.65–1.69) | 1.17 (1.15–1.18) |
| High (quintiles 4–5) | 1.58 (1.56–1.61) | 1.26 (1.24–1.28) | 1.87 (1.84–1.90) | 1.18 (1.16–1.19) |
| Neighborhood SES/health insurance | ||||
| Low SES/low insurance | 1.40 (1.38–1.42) | 1.22 (1.20–1.24) | 1.54 (1.51–1.57) | 1.15 (1.12–1.17) |
| Low SES/high insurance | 1.36 (1.34–1.39) | 1.19 (1.17–1.21) | 1.61 (1.59–1.64) | 1.13 (1.11–1.15) |
| High SES/low insurance | 1.59 (1.55–1.63) | 1.26 (1.23–1.29) | 1.69 (1.65–1.74) | 1.19 (1.16–1.22) |
| High SES/high insurance | 1.45 (1.42–1.48) | 1.21 (1.19–1.24) | 1.78 (1.75–1.81) | 1.14 (1.12–1.16) |
| Pinteraction comparing adjusted HRs by SES/insurancee | – | 6.8 × 10−9 | – | 8.2 × 10−8 |
Abbreviations: CI, confidence interval; HR, hazard ratio.
The analysis excluded 15,538 males with leukemia and 11,250 females with leukemia.
Models were stratified by stage and age and were adjusted for cancer site, race/ethnicity, and treatment.
Low health insurance indicates none, any public/Medicaid/military, or unknown; high health insurance, private only, Medicare only/Medicare, or private. This variable also was adjusted for neighborhood SES.
Neighborhood SES also was adjusted for health insurance.
P values were determined using the log-likelihood ratio test.
DISCUSSION
In the large, sociodemographically and geographically diverse California population, we observed that health insurance status and nSES did not substantively explain the marital status association for male or female cancer patients, despite having independent associations with allcause mortality. Similar results were observed across cancer sites, although there was some suggestion of a stronger association for sites with more favorable survival (breast, prostate, and NHL). Our results also confirmed previous observations of more pronounced survival benefits associated with marriage for males than for females and some attenuation of the association in older patients, although the protective effects persisted even among elderly patients.
Although the association of marriage and various outcomes, including longevity and cancer, is supported by a large body of research,7 the mechanisms driving this correlation are not fully understood.6,7 Two underlying pathways have been postulated: 1) a “social” pathway, in which greater social support, social integration, social role attainment, and social control are available to married individuals; and 2) a “material” pathway, in which better economic resources, such as greater income, employment, and better health insurance, are available to married individuals.7 Although we were not directly able to test the social pathway and we lacked individual-level SES information on income and employment, our results based on insurance status and nSES provide at least indirect evidence that the survival benefits associated with marriage may not be because of better material resources. Further research should examine how this association is mediated by specific factors within social pathways. Others have demonstrated that specific social advantages of marriage include spousal or children support/encouragement for health-seeking behaviors, such as adherence to recommended health screening and treatment, and health-promoting behaviors, such as more exercise and better diet.6,7 Furthermore, evidence suggests that higher levels of social support are directly correlated with biologic processes that may mitigate the harmful physiologic effects of stress by directly inhibiting tumor progression through immunologic or neuroendocrine pathways.6,7,13 Increased social support has been associated with maintenance of normal diurnal patterns of cortisol14–16 and lower levels of depression,17,18 both of which, in turn, have been associated with lower mortality. A meta-analysis indicated that providing social support to patients with metastatic breast cancer increases 1-year survival.
In this study, we observed evidence of an interaction among marital status, health insurance status, and nSES, with slightly stronger associations for unmarried status and mortality observed among patients with public or no insurance living in higher SES neighborhoods. Our findings may be related to the higher out-of-pocket costs for uninsured individuals,20 with those financial challenges compounded by the higher costs of living in higher SES neighborhoods. In contrast, however, a recent study of women with late-stage colon cancer in California reported that the poorest survival was observed among unmarried women who lived in high poverty areas and were uninsured or had public health insurance.10 The discrepancy in study findings may reflect differences across cancer sites or in the type or geographic level of nSES measure and stages of disease. Our small area-level (block group) nSES measure comprising 7 indicators provides a more comprehensive view of community-level SES than the single measure of poverty at an unspecified geographic level that was used in the colon cancer study. Other studies have reported higher mortality among individuals of low individual-level SES living in the context of high SES communities,21,22 which may reflect increased stress and economic strain among these individuals. The lack of individual-level SES information in population-based cancer registry data limited our ability to further explore this finding.
The stronger survival benefit of marriage among men confirms prior research1,2,23,24 and may provide important clues into underlying mechanisms. Men and women appear to benefit differentially from marriage,25–28 with women benefitting more financially and men benefitting more socially.6,7,25 Furthermore, the adoption of “healthier” lifestyle habits that often accompanies marriage may be greater among men than women.26,27,29 Thus, the greater survival benefits observed in men may reflect sex-specific differences in the relative contributions of the underlying mechanisms. In this context, our finding of larger survival advantages for men than for women, even after controlling for SES and insurance status, underscores the likely importance of socially mediated factors.
Our findings benefit from the strengths of a large and representative data source. However, this study could not account for changes in marital status since diagnosis or specific psychosocial factors. All studies addressing the association of marriage and health outcomes must consider the likelihood of self-selection, ie, individuals who marry are physically, emotionally, or psychologically healthier and/or of higher individual SES than those who do not,6,7,30 which may be especially relevant for the stronger effects observed in men, because unhealthy men may have greater difficulty marrying than unhealthy women.29 Although California is a large and diverse region and the CCR database represents half of the cases reported to the 18-registry SEER program, our results nonetheless may not be generalizable to other US regions. We also lacked granular data on treatments, adherence to practice guidelines, and follow-up care as well as institutional and provider characteristics.
In summary, in a large and representative cohort of men and women diagnosed with 1 of the 10 most common sites of cancer-related death, we observed that the well established survival benefit of marriage operates independently of the economic resources we were able to assess. We also observed evidence of greater survival deficits for unmarried cancer patients with no or public insurance living in higher SES neighborhoods, a new finding that warrants further follow-up. Together, our results suggest that, because economic resources likely play a minimal role in explaining the detrimental survival experienced among unmarried cancer patients, future research that focus on social support and other socially mediated factors associated with marriage may provide an important avenue to inform interventions that improve cancer survival.
Supplementary Material
Acknowledgments
FUNDING SUPPORT
This work was supported by the Stanford Cancer Institute (Scarlett Lin Gomez) and the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California (Principal Investigator, Sally L. Glaser). Support was also provided by the Specialized Cancer Center Support Grant to the University of California, San Diego Moores Cancer Center (CA023100-29). The collection of cancer incidence data used in this study was supported by the California Department of Public Health as part of the statewide cancer reporting program mandated by California Health and Safety Code Section 103885; the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program under contract HHSN261201000140C awarded to the Cancer Prevention Institute of California, contract HHSN261201000035C awarded to the University of Southern California, and contract HHSN261201000034C awarded to the Public Health Institute; and the Centers for Disease Control and Prevention’s National Program of Cancer Registries, under agreement U58DP003862-01 awarded to the California Department of Public Health.
Footnotes
See related article on pages 1570-8, this issue.
The ideas and opinions expressed herein are those of the authors, and endorsement by the State of California, Department of Public Health, the National Cancer Institute, and the Centers for Disease Control and Prevention or their contractors and subcontractors is not intended nor should be concluded.
Additional supporting information may be found in the online version of this article.
CONFLICT OF INTEREST DISCLOSURES
Scarlett Lin Gomez and Christina A. Clarke report grants from Genentech outside the submitted work.
References
- 1.Goodwin JS, Hunt WC, Key CR, Samet JM. The effect of marital status on stage, treatment, and survival of cancer patients. JAMA. 1987;258:3125–3130. [PubMed] [Google Scholar]
- 2.Kravdal O. The impact of marital status on cancer survival. Soc Sci Med. 2001;52:357–368. doi: 10.1016/s0277-9536(00)00139-8. [DOI] [PubMed] [Google Scholar]
- 3.Lai H, Lai S, Krongrad A, Trapido E, Page JB, McCoy CB. The effect of marital status on survival in late-stage cancer patients: an analysis based on Surveillance, Epidemiology, and End Results (SEER) data, in the United States. Int J Behav Med. 1999;6:150–176. doi: 10.1207/s15327558ijbm0602_4. [DOI] [PubMed] [Google Scholar]
- 4.Aizer AA, Chen MH, McCarthy EP, et al. Marital status and survival in patients with cancer. J Clin Oncol. 2013;31:3869–3876. doi: 10.1200/JCO.2013.49.6489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kissane DW. Marriage is as protective as chemotherapy in cancer care. J Clin Oncol. 2013;31:3852–3853. doi: 10.1200/JCO.2013.51.5080. [DOI] [PubMed] [Google Scholar]
- 6.Manzoli L, Villari P, MP G, Boccia A. Marital status and mortality in the elderly: a systematic review and meta-analysis. Soc Sci Med. 2007;64:77–94. doi: 10.1016/j.socscimed.2006.08.031. [DOI] [PubMed] [Google Scholar]
- 7.Rendall MS, Weden MM, Favreault MM, Waldron H. The protective effect of marriage for survival: a review and update. Demography. 2011;48:481–506. doi: 10.1007/s13524-011-0032-5. [DOI] [PubMed] [Google Scholar]
- 8.Seeman TE. Health promoting effects of friends and family on health outcomes in older adults. Am J Health Promot. 2000;14:362–370. doi: 10.4278/0890-1171-14.6.362. [DOI] [PubMed] [Google Scholar]
- 9.Bernstein AB, Cohen RA, Brett KM, Bush M. Marital status is associated with health insurance coverage for working-age women at all income levels, 2007. NCHS Data Brief. 2008;11:1–8. [PubMed] [Google Scholar]
- 10.Levitz NR, Haji-Jama S, Munro T, et al. Multiplicative disadvantage of being an unmarried and inadequately insured woman living in poverty with colon cancer: historical cohort exploration in California [serial online] BMC Womens Health. 2015;15:166. doi: 10.1186/s12905-015-0166-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang J, Schupp CW, Harrati A, Clarke C, Keegan THM, Gomez SL. Developing an area-based socioeconomic measure from American Community Survey data. Fremont, CA: Cancer Prevention Institute of California; 2014. [Google Scholar]
- 12.Yost K, Perkins C, Cohen R, Morris C, Wright W. Socioeconomic status and breast cancer incidence in California for different race/ethnic groups. Cancer Causes Control. 2001;12:703–711. doi: 10.1023/a:1011240019516. [DOI] [PubMed] [Google Scholar]
- 13.Spiegel D, Sephton SE, Terr AI, Stites DP. Effects of psychosocial treatment in prolonging cancer survival may be mediated by neuroimmune pathways. Ann N Y Acad Sci. 1998;840:674–683. doi: 10.1111/j.1749-6632.1998.tb09606.x. [DOI] [PubMed] [Google Scholar]
- 14.Sephton SE, Lush E, Dedert EA, et al. Diurnal cortisol rhythm as a predictor of lung cancer survival. Brain Behav Immun. 2013;30(suppl):S163–S170. doi: 10.1016/j.bbi.2012.07.019. [DOI] [PubMed] [Google Scholar]
- 15.Sephton SE, Sapolsky RM, Kraemer HC, Spiegel D. Diurnal cortisol rhythm as a predictor of breast cancer survival. J Natl Cancer Inst. 2000;92:994–1000. doi: 10.1093/jnci/92.12.994. [DOI] [PubMed] [Google Scholar]
- 16.Turner-Cobb JM, Sephton SE, Koopman C, Blake-Mortimer J, Spiegel D. Social support and salivary cortisol in women with metastatic breast cancer. Psychosom Med. 2000;62:337–345. doi: 10.1097/00006842-200005000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Giese-Davis J, Collie K, Rancourt KM, Neri E, Kraemer HC, Spiegel D. Decrease in depression symptoms is associated with longer survival in patients with metastatic breast cancer: a secondary analysis. J Clin Oncol. 2011;29:413–420. doi: 10.1200/JCO.2010.28.4455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sephton SE, Dhabhar FS, Keuroghlian AS, et al. Depression, cortisol, and suppressed cell-mediated immunity in metastatic breast cancer. Brain Behav Immun. 2009;23:1148–1155. doi: 10.1016/j.bbi.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 19.Mustafa M, Carson-Stevens A, Gillespie D, Edwards AG. Psychological interventions for women with metastatic breast cancer [serial online] Cochrane Database Syst Rev. 2013;6:CD004253. doi: 10.1002/14651858.CD004253.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Machlin SR, Carper K. Statistical brief 441: out-of-pocket health care expenses by age and insurance coverage, 2011. Medical Expenditure Panel Survey. 2014 Jun; Available at: http://meps.ahrq.gov/mepsweb/data_files/publications/st441/stat441.shtml. Accessed June 9, 2015. [PubMed]
- 21.Shariff-Marco S, Yang J, John EM, et al. Impact of neighborhood and individual socioeconomic status on survival after breast cancer varies by race/ethnicity: the neighborhood and breast cancer study. Cancer Epidemiol Biomarkers Prev. 2014;23:793–811. doi: 10.1158/1055-9965.EPI-13-0924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Winkleby M, Cubbin C, Ahn D. Effect of cross-level interaction between individual and neighborhood socioeconomic status on adult mortality rates. Am J Public Health. 2006;96:2145–2153. doi: 10.2105/AJPH.2004.060970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sammon JD, Morgan M, Djahangirian O, et al. Marital status: a gender-independent risk factor for poorer survival after radical cystectomy. BJU Int. 2012;110:1301–1309. doi: 10.1111/j.1464-410X.2012.10993.x. [DOI] [PubMed] [Google Scholar]
- 24.Wang L, Wilson SE, Stewart DB, Hollenbeak CS. Marital status and colon cancer outcomes in US Surveillance, Epidemiology and End Results registries: does marriage affect cancer survival by gender and stage? Cancer Epidemiol. 2011;35:417–422. doi: 10.1016/j.canep.2011.02.004. [DOI] [PubMed] [Google Scholar]
- 25.Lillard LA, Waite LJ. ’Til death do us part: marital disruption and mortality. Am J Sociol. 1995;100:1131–1156. [Google Scholar]
- 26.Kiecolt-Glaser JK, Newton TL. Marriage and health: his and hers. Psychol Bull. 2001;127:472–503. doi: 10.1037/0033-2909.127.4.472. [DOI] [PubMed] [Google Scholar]
- 27.Umberson D. Gender, marital status and the social control of health behavior. Soc Sci Med. 1992;34:907–917. doi: 10.1016/0277-9536(92)90259-s. [DOI] [PubMed] [Google Scholar]
- 28.Jaffe DH, Manor O, Eisenbach Z, Neumark YD. The protective effect of marriage on mortality in a dynamic society. Ann Epidemiol. 2007;17:540–547. doi: 10.1016/j.annepidem.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Wingard DL. The sex differential in morbidity, mortality, and lifestyle. Annu Rev Public Health. 1984;5:433–458. doi: 10.1146/annurev.pu.05.050184.002245. [DOI] [PubMed] [Google Scholar]
- 30.Kravdal O. The poorer cancer survival among the unmarried in Norway: is much explained by comorbidities? Soc Sci Med. 2013;81:42–52. doi: 10.1016/j.socscimed.2013.01.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


