Abstract
A workshop entitled “Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate” (held in Rockville, MD, September 19, 2016) was organized by the Radiation Research Program and Radiation Oncology Branch of the Center for Cancer Research (CCR) of the National Cancer Institute (NCI), to identify critical research areas and directions that will advance the understanding of radiation-induced fibrosis (RIF) and accelerate the development of strategies to mitigate or treat it. Experts in radiation biology, radiation oncology and related fields met to identify and prioritize the key areas for future research and clinical translation. The consensus was that several known and newly identified targets can prevent or mitigate RIF in pre-clinical models. Further, basic and translational research and focused clinical trials are needed to identify optimal agents and strategies for therapeutic use. It was felt that optimally designed preclinical models are needed to better study biomarkers that predict for development of RIF, as well as to understand when effective therapies need to be initiated in relationship to manifestation of injury. Integrating appropriate endpoints and defining efficacy in clinical trials testing treatment of RIF were felt to be critical to demonstrating efficacy. The objective of this meeting report is to (a) highlight the significance of RIF in a global context, (b) summarize recent advances in our understanding of mechanisms of RIF, (c) discuss opportunities for pharmacological mitigation, intervention and modulation of specific molecular pathways, (d) consider the design of optimal clinical trials for mitigation and treatment and (e) outline key regulatory nonprescriptive frameworks for approval.
INTRODUCTION
A workshop entitled “Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate” was organized by the Radiation Research Program (RRP) and the Radiation Oncology Branch of the Center for Cancer Research (CCR) of the National Cancer Institute (NCI) was held at NCI, Rockville, MD, on September 19, 2016. The workshop was designed to answer several key questions (Table 1). The goals of the workshop and the significance of the problem were framed by Pataje Prasanna and Norman Coleman (RRP, NCI). One of the most common adverse effects of radiotherapy is fibrosis, which can occur in many organ systems, including skin and lung. Understanding the mechanisms of radiation-induced fibrosis (RIF) requires consideration of a variety of molecular and tissue based mechanisms, which may be organ and radiation dose specific, such as immunologic activation/polarization, activation of damage response signaling pathways, wound healing response and inflammation. Similarly, the effects of radiation on endothelial cells, stem cells, and other important components of tissue may vary by tissue type and dose delivered, complicating the application of anti-fibrotic therapy. As normal tissues vary in radiobiologically important ways, understanding the issues surrounding therapy related normal tissue toxicity will allow accelerated discovery, development, and translation of next generation mitigators as well as repurposing pharmaceuticals that are already approved for other indications.
TABLE 1.
Focus Areas and Key Considerations Discussed at the “Radiation-Induced Fibrosis: Mechanisms and Opportunities to Mitigate” Workshop, in September 2016, at National Cancer Institute
| Category | |
|---|---|
| Mechanisms of fibrosis |
|
| Potential targets |
|
| Biomarkers |
|
| Clinical trial design |
|
| Clinical translation of mitigators and treatments for RIF: regulatory issues |
|
Although several targets for prevention, mitigation and treatment of RIF have been explored preclinically, their clinical translation lags resulting in a disparity between research and use in the clinic. To bridge this gap, carefully designed clinical trials for mitigators of normal tissue injury, considering inclusion criteria, measures of efficacy, predictive biomarkers and correlative measures of efficacy are critical for successful clinical translation of preclinical work.
The number of cancer survivors is increasing each year. With radiation used as curative therapy in an increasing number of cases worldwide, with an estimated 7 million cancer patients treated with radiotherapy in 2012, the importance of addressing normal tissue toxicity and to improve post-treatment quality of life (QOL) is of increasing importance (1). The complexity of the interplay of several factors, including treatment and patient related factors, impact of tumor, organ site and other molecular factors are important considerations. The chronology of radiation injury suggests that specific targets may be useful at different points after injury. The workflow of developing clinical mitigators of RIF must consider this complexity and optimally requires collaboration between academic investigators, small businesses and cooperative groups (2).
MECHANISMS OF RADIATION-INDUCED FIBROSIS
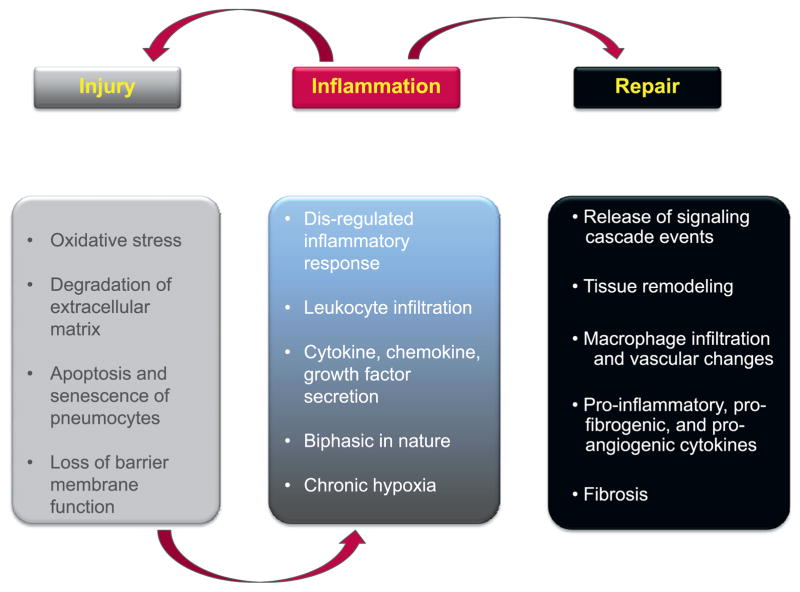
A broad overview of RIF in the context of pulmonary fibrosis is shown in Fig. 1; however, RIF can occur in many organ systems.
FIG. 1.
Mechanisms of pulmonary fibrosis. Injury, inflammation and repair each play contributing roles to RIF. The immediate injury can result in loss of parenchyma, loss of barrier function and an initiation of inflammation. Acute inflammation, although noted to be critical for wound repair, can result in additional injury, oxidative stress and eventual tissue remodeling and chronic inflammation. The chronic inflammatory process further contributes to ongoing injury and stress. Late RIF in the lung is a failed attempt at regeneration of lost tissue.
Three speakers highlighted the current understanding of mechanisms of RIF. Michael Freeman (Vanderbilt University, Nashville, TN) highlighted the basic pathologic findings of radiation-induced pulmonary injury, including the importance of reactive oxygen species (ROS) in the pathophysiology of the process. Radiation pulmonary injury may initially manifest as pneumonitis and then can transition into chronic debilitating fibrosis, characterized by loss of normal alveolar structure, disorganized thickening of septa, collapse of alveolar space, and the replacement of parenchyma with fibrotic tissue (3). Pathophysiological ROS are hypothesized to be central to the etiology of radiation-induced pulmonary fibrosis (RIPF) (3). While the premise is based on clinical and preclinical observations, the underlying molecular mechanisms are currently not well understood.
In 1983, Emerit et al. (4) reported that subcutaneous injection of copper/zinc superoxide dismutase (SOD) into patients suffering from severe radiation-induced necrosis resulted in significant mitigation of the radiation injury. This initial clinical observation preceded a report by Balliet et al. (5) that 3 weeks of intramuscular injection of liposomal encapsulated SOD significantly mitigated radiation therapy-induced fibrosis. Fifty patients originally treated with radiation therapy for various cancers (breast, prostate, melanoma, etc.) developed fibrotic lesions in the field of treatment. Strikingly, SOD treatment mitigated the fibrotic lesions. Delanian et al. (6) reported that 34 patients who developed RIF involving skin and underlying tissues as a result of radiation therapy were treated with liposomal SOD and followed for an average of 5 years. SOD treatment produced complete response in 17% of patients. Studies such as these provided clinical evidence but lacked molecular insight.
Preclinical studies have shown that intra-tracheal administration of plasmid/liposome SOD2 (7) or transgenic expression of extracellular SOD (8) reduced the severity of RIPF. SOD treatment reduced expression of inflammatory cytokines such as IL-1 and TNF-α, as well as the profibrotic cytokine TGF-β (7). Tissue remodeling, breathing abnormality, and collagen deposition were reduced while survival increased (7, 8). Complementing these studies are those of Citrin et al. (9) and Choi et al. (10) who identified NADPH oxidases, a superoxide-generating family of enzymes, as major sources of oxidative stress that contributes to RIPF.
Other than activation of latent TGF-β (11), that in turn increases generation of ROS via activation of NADPH oxidases in a feed-forward response (12, 13), the molecular targets impacted by pathogenic pulmonary oxidant stress have not been well described. Emerging research indicates that isolevuglandins (IsoLGs) can contribute to the pathogenesis of diseases driven by oxidative stress (14–17). IsoLGs, also referred to as isoketals, are a family of eight γ-ketoaldehyde regioisomers formed by nonenzymatic rearrangement of endoperoxide intermediates produced when arachidonic acid or its phospholipid esters undergo free radical mediated cyclooxygenation. Two of the regioisomers can also be formed by enzymatic reaction (18, 19). IsoLGs rapidly adduct the α-amino group of protein lysines. Accumulation of IsoLG adducted protein can evolve into proteotoxic events and is associated with progressive Alzheimer’s disease, ethanol-induced liver damage, asthma, atherosclerosis, and hypertension, all of which are oxidative stress-associated diseases (14–17).
Nrf2, a transcription factor that promotes antioxidant gene expression [e.g., HO1, SOD1, SOD2, GCLC, GCLM and CAT (20, 21)], is essential for maintaining ROS homeostasis (22). IsoLG adduction of protein was significantly increased in the lungs of Nrf2-deficient mice, consistent with Nrf2’s role in antioxidant metabolism. Conversely, IsoLG adduction of protein in alveolar type 1 and 2 pneumocytes, alveolar endothelial cells, and bronchial club cells was diminished in Nox2 null mice (23). These genetic studies linked oxidant challenge to IsoLG adduction. Idiopathic pulmonary fibrosis (IPF) is a disease that is promoted by oxidant stress (24). Mont et al. (23) investigated the question of whether IsoLGs could be found in human IPF. Their investigation found that idiopathic lung fibrotic foci contain significant levels of IsoLG adducted protein.
As 70% of X- and gamma-ray photons traversing a tissue interact with water molecules that rapidly decompose into hydroxyl radicals (•OH), hydrogen radicals (•H), hydrogen peroxide, superoxide and solvated electrons (25), the question of whether irradiation could generate IsoLG-modified protein in cell culture and/or in irradiated murine lung was addressed. Consistent with the chemistry of IsoLG formation, IsoLG adducted protein was found in both irradiated cells and most importantly, adduction of pulmonary parenchyma increased in a progressive manner over a 16-week interval postirradiation (26). This is direct evidence for the presence of chronic oxidative injury in irradiated lung. It was then determined that cells could tolerate basal levels of modification but exceeding these levels triggered apoptosis (26).
IsoLG-modified proteins were immuno-affinity purified from sham-treated and irradiated (5 Gy) endothelial cells and analyzed by LC-MS. Gene ontology analysis revealed that proteins in numerous cellular pathways were susceptible to IsoLG modification. The analysis identified collagen 1α1 among the many proteins adducted. Adduction of collagen 1α1 by IsoLGs in IPF samples was confirmed by confocal microscopy co-localization immunofluorescence studies (26). Biochemical approaches demonstrated that IsoLG modification of collagen 1α1 impaired its degradation by MMP1, suggesting that adduction has the potential to impair resolution of established fibrosis (26).
It is well established that most chronic fibrotic diseases have in common a state of persistent injury (27). Thus, it was of interest to observe that ionizing radiation produced a state of chronic IsoLG protein-modification that correlated with chronic apoptosis and RIF in a murine model (28). IsoLG-modified proteins were also a prominent feature of IPF. Demonstration that IsoLGs adducted collagen 1α1 in irradiated cells and in IPF patients and the knowledge that adducted collagen impaired MMP1 degradation suggests that IsoLGs have the potential to induce a state of chronic injury that can lead to apoptosis and to impair resolution of established fibrosis.
Thomas Wynn [National Institute of Allergy and Infectious Diseases (NIAID), Bethesda, MD)] described the importance of persistent or dysregulated IL-13 responses as key drivers of fibrosis in multiple organ systems. Type 2 immune responses are characterized by presence of T helper 2 (TH2) cells, cytokines such as IL-4, IL-5, IL-9 and IL-13 and IL-4 and/or IL-13-induced macrophages, among other immune and inflammatory cells (29). Type 2 immunity can exhibit host protective and pathogenic functions, as evidenced by its role in resistance to parasitic infections (30) and several autoimmune diseases (31, 32).
Macrophages are a critical regulator of type-2 cytokine driven inflammatory disease (33). Type 2 cytokines such as IL-4 and IL-13, are sufficient to induce nonpolarized tissue resident macrophages to differentiate into pro-fibrotic, alternatively activated macrophages that have been associated with tissue repair and fibrosis. Indeed, alternatively-activated macrophages, induced by IL-13 have been reported to regulate the fibrotic responses to injury, such as those induced by radiation (34). Deficiency of IL-13 or the use of agents that scavenge IL-13 have been successful in ameliorating fibrosis induced by a number of pathogenic stimuli, including irradiation, through suppression of type 2 driven inflammation (34–39).
Although IL-13 deficient mice are resistant to many types of fibrosis, tissues from these mice are characterized by increased IFN-γ production and inflammatory activity. In general, IFN-γ exhibits anti-fibrotic activity (40) and IFN-γ effector function is antagonized by type 2 cytokines, such as IL-4 (41). To investigate the role of IFN-γ, novel IL-13−/−/IFN-γ−/− double cytokine-deficient mice were developed and fibrotic disease progression was examined in models of type 2-driven fibrosis (42). As predicted, fibrosis in the lung and liver were both highly dependent on IL-13, but examination of these tissues in the context of IL-13 deficiency revealed increased IFN-γ production and inflammatory activity. Surprisingly; however, an even greater reduction in fibrosis was observed in IL-13/IFN-γ double deficient mice. The increased protection was associated with marked decreases in Tgfb1, Mmp12 and Timp1 mRNA expression in the tissues; reduced inflammation; and decreased expression of important pro-inflammatory mediators such as TNF-α. Experiments conducted with neutralizing monoclonal antibodies to IL-13 and IFN-γ validated the findings with the genetically deficient mice.
Although IL-13 has emerged as a promising therapeutic target for several diseases that are characterized by persistent or dysregulated type 2 cytokine responses, including radiation toxicities, these studies suggest that in some cases, rebound inflammation driven by the type 1 cytokine IFN-γ may be an unwanted complication resulting from IL-13 blockade. In fact, it is tempting to speculate that rebound inflammation may be a common side effect associated with anti-fibrotic therapy, as wound healing and fibrosis are tightly linked with the suppression of inflammation. Indeed, the pro-fibrotic cytokine TGF-β also serves as a potent anti-inflammatory mediator, with deficiencies in TGF-β1 or TGF-β1 signaling linked with the development of numerous autoimmune and inflammatory diseases. Consequently, therapeutics targeting core pro-fibrotic pathways will need to be carefully evaluated for this potential undesired side effect. These findings suggest that highly effective anti-fibrotic therapies may need to be combined with therapeutics that mitigate this rebound inflammation, as this will likely lead to a more substantial and sustained reduction in fibrosis.
Deborah Citrin (CCR, NCI, Bethesda, MD) discussed recent evidence supporting stem cell senescence as a driver of RIF. Cellular senescence is an irreversible suspension of cell growth that can occur in a variety of cell types as a normal consequence of aging or because of exposure to toxic insults. Replicative senescence occurs because of telomere shortening over the lifespan of a cell. In contrast, stress induced senescence can result from DNA damage from irradiation, genotoxic agents, or other sources of ROS. Replicative and stress induced senescence have been linked to a variety of aging related illnesses in diverse tissues (43).
Recently, senescence has been described as a potential mechanism of late injury after irradiation (9). In a murine model of RIPF, senescence of type II alveolar cells (AECII) was observed in a time and dose dependent fashion after exposure to fibrogenic doses of irradiation. This is of importance given that AECII function as an alveolar stem cell, replenishing both AECII and AECI, after injury. Isolated AECII depletion has been shown to be sufficient to induce pulmonary fibrosis in murine models, suggesting that AECII depletion after irradiation can contribute to RIPF. Consistent with the importance of AECII in repopulating the alveolus after irradiation, AECII depletion occurred in proportion to the initial apoptotic response and the induction of senescence in the lung fibrosis model.
Lending further support to the hypothesis that radiation-induced senescence plays an important role in the development of RIPF, delivery of an agents that inhibits senescence in AECII senescence after irradiation are also capable of mitigating fibrosis. NADPH oxidases (NOX) are membrane bound enzymes that produce superoxide anion, a free radical. Production of superoxide by NOX have been implicated as a source of additional injury in conditions such as acute respiratory distress syndrome and oxygen toxicity. In the RIF mouse model (in which thorax is selectively irradiated), superoxide production is detected several weeks after radiation injury, providing a potential source of chronic oxidative stress and senescence (9). NOX inhibition after irradiation was sufficient to reduce super-oxide production and AECII senescence. Collectively, these data suggest that chronic oxidative stress contributes to AECII senescence after irradiation, and that inhibiting superoxide production may provide a viable option for mitigation of fibrosis.
Aside from the impact senescence has on replicative potential, senescent cells can also alter the local environment in tissues through paracrine mechanisms. Senescent cells secrete a complex mixture of pro-inflammatory and immunomodulatory cytokines commonly termed the “senescence associated secretory phenotype (SASP).” A number of these SASP cytokines have previously been implicated in RIF, such as IL-6, IL-1β, EGF, VEGF, MMPs, TIMPs, ICAM-1 and EGFR (44). Importantly, there is evidence that irradiated senescent stem cells can in turn induce senescence of surrounding stem cells through elaboration of the SASP (9). These findings suggest that inhibition of SASP proteins may be an opportunity to inhibit the pro-inflammatory and pro-fibrotic nature of senescent cells.
Support for the concept that inhibiting the SASP can provide a therapeutic opportunity to mitigate RIF has come from additional work in the murine lung fibrosis model. One member of the SASP that has also been implicated in RIF is plasminogen activator inhibitor-1 (PAI-1). PAI-1 inhibits tissue plasminogen activator and urokinase, the proteolytic activators of plasminogen. Thus, PAI-1 activity inhibits the proteolytic degradation of fibrin by plasmin (45). In the presence of PAI-1, the fibrin matrix is stabilized, providing a scaffold for fibrosis. Independently, PAI-1 acts downstream of p53 to induce senescence (46). Thus, PAI-1 functions in a pro-fibrotic context but also as a member of the SASP that can in turn induce senescence.
Delivery of a recombinant PAI-1 protein capable of inhibiting the anti-proteolytic activity of PAI-1 to mice that received fibrosis evoking thoracic irradiation was shown to reduce collagen accumulation (47). Mice treated with radiation and the truncated protein had increased MMP-3 expression and increased fibrin degradation compared to irradiated mice treated with vehicle. Similarly, delivery of the recombinant protein reduced the accumulation of senescent AECII after irradiation. Importantly, treatment of primary AECII with the truncated PAI-1 protein was capable of inhibiting radiation-induced senescence, further supporting that inhibiting the SASP after radiation can directly impact stem cell senescence.
Signaling through the mammalian target of rapamycin multi protein complex 1 (mTORC1) has also been implicated as a central mediator of cellular senescence (48) and the SASP. Delivery of rapamycin to irradiated mice has similarly been demonstrated to reduce pulmonary fibrosis, AECII senescence, and elaboration of SASP cytokines (49). The usefulness of rapamycin in this context is uncertain given that rapamycin has been linked to pulmonary toxicity when given at high doses, although anti-fibrotic efficacy was seen with chronic dosing at lower dose levels (50). Several alternative dual and single TORC inhibitors are in clinical development may demonstrate utility in this setting.
Although preventing premature senescence has shown promise in preventing RIF, there is increasing interest in agents that can clear prematurely senescent cells from tissues using “senolytic” drugs. Indeed, clearance of prematurely senescent cells with such an agent (e.g. ABT263) has been shown to improve bone marrow clonogenic capacity after total-body irradiation (51). Evidence of the ability of these agents to treat or mitigate RIF is eagerly awaited.
Understanding the mechanisms by which radiation induces fibrosis in a variety of tissues, where these mechanisms could be tissue specific, offers new opportunities to discover targets and develop strategies to mitigate or treat fibrosis.
POTENTIAL TARGETS FOR MITIGATION AND TREATMENT OF FIBROSIS
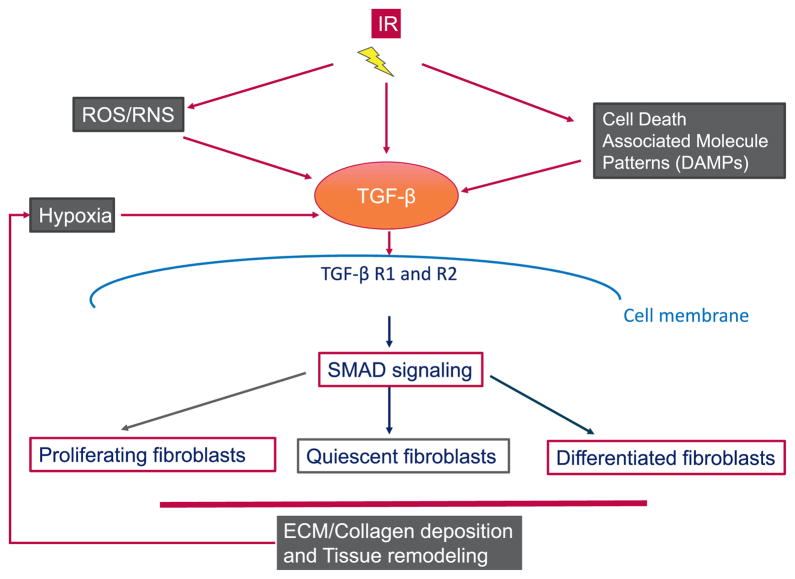
There were four speakers in the session, “Potential targets for the mitigation and treatment of fibrosis”. Mary Helen Barcellos-Hoff (University of California, San Francisco, CA) highlighted the critical role played by TGF-β, a widely-distributed cytokine whose activity is controlled by secretion as a latent complex that is sequestered in the extracellular matrix. Specifically, she described the importance of the rapid and persistent TGF-β activation in tissues, tumors and cells after exposure to ionizing radiation, and described data supporting the role of TGF-β as a driver of fibrosis. Key implications of radiation effect on TGF-β is depicted in Fig. 2. The pleiotropic biological responses to TGF-β were noted to depend in cell type and context, as well as the duration and degree of exposure. As indicated above, there is abundant evidence that TGF-β is a key component of the fibrotic response. Determining whether and when TGF-β inhibition might be clinically viable for the mitigation of fibrosis depends on understanding its activity in irradiated tissues.
FIG. 2.
The central role of TGF-β in radiation fibrosis. TGF-β activation after irradiation can occur via reactive oxygen species or other indirect mechanisms. Once activated, TGF-β signaling results in activation of SMAD signaling pathways, with can stimulate fibroblast proliferation and extracellular matrix (ECM) deposition. These changes can contribute to hypoxia, which further contributes to the progression of fibrosis. Note: DAMPs are Death Associated Molecule Patterns.
Three TGF-β ligands (TGF-β1, TGF-β2 and TGF-β3) act through TGF-β type 1 (TGF-βR1) and type 2 receptors(TGF-βR2)(52). The three TGF-β ligand genes share a high degree of sequence homology in the ligand region and functional overlap; however, TGF-β1 is the best studied isoform. During protein synthesis, the precursor protein is proteolyzed to yield a C-terminal peptide that dimerizes, forming the mature cytokine. The cleaved N-terminal peptide forms a 75 kDa glycosylated homodimer called the latency associated peptide (LAP) (53) that acts as a chaperone to ensure proper folding of TGF-β and provides a secretory signal for the molecule. The latent TGF-β (LTGF-β) complex, also referred to as the small latent complex, is produced by the noncovalent association of LAP with the TGF-β cytokine. In some instances, LAP is covalently bound to the TGF-β cytokine via a disulfide bond, resulting in the “large latent complex,” which is more readily sequestered in the extracellular space.
Thus, latent TGF-β remains abundant in the extracellular space but biologically inactive until the appropriate stimuli result in the dissociation of LAP from the TGF-β cytokine. Activation of TGF-β by stimuli such as proteases, ROS, and radiation liberates TGF-β to bind to cell surface type I and type II receptors, that in turn activate receptor mediated SMAD 2 and 3 proteins. Phosphorylated SMAD 2/3 proteins binding in an enhanced fashion to co-SMAD 4 and the complex is retained in the nucleus, where it mediates transcription of a diverse set of genes modulating differentiation, function, proliferation or apoptosis [reviewed in (54)]. Despite over two decades of study, novel TGF-β actions are still frequently described and the importance of this molecule continues to broaden.
Activation of TGF-β is required for the cytokine to bind to cell surface receptors. Thus, the modes of activation are critical determinants of its biological activity (53, 55). Although all three isoforms bind to the same cell surface receptors, evidence from knockout studies suggests that the specificity of effect of each isoform may result from differential susceptibility to varying modes of activation of the latent complex (56–58). Although activation of LTGF-β can occur in solution after exposure to acidic or basic solutions (59), these methods are relatively inefficient. Most mechanisms suggesting activation are not physiologically relevant. Most LTGF-β activation mechanisms require interaction with one or more additional proteins localized on the cell surface (53).
Rapid activation of LTGF-β is observed in vivo after exposure to ionizing radiation (60, 61). ROS are a product of the interaction of ionizing radiation with water or with cell membranes. Solution sources of ROS generated by Fenton chemistry efficiently release biologically active TGF-β from recombinant LTGF-β in the absence of cells or other proteins (62, 63). Since ROS are efficient at driving LTGF-β activation that does not require additional cellular machinery, and since ROS are widely generated via cellular metabolism, inflammation, and irradiation, LTGF-B may function as a sensitive extracellular sensor of oxidative stress (63). In the Fenton chemistry cell-free model for generating ROS, the hydroxyl radical was identified as the critical ROS required for activation.
The redox sensitive mechanism of activation is restricted to LTGF-β1 (62). The lesser degree of sequence homology in LAP between isoforms (34–38%) compared to the latent TGF-β proteins (75% sequence homology) suggests that the LAP component may provide differential susceptibility to modes of activation. The efficiency of the oxidative activation mechanism and the specificity of the mechanism of LTGF-β1 suggests a major role of the latent complex as a tissue sensor of oxidative stress (64). This sensitive redox switch provides a mechanism for liberation of active TGF-β in response to irradiation.
Given the associations with ROS and inflammation, there is clear basis for predicting these as mechanisms perpetuating TGF-β activity that contributes to RIF. The key questions are to identify who is at risk and to determine if blocking TGF-β can ameliorate RIF once it is clinically evident. Given that radiation-induced TGF-β activity also opposes therapeutic benefit by promoting the DNA damage response and hence cell survival [reviewed in (65)], it is also of interest to evaluate whether a short course of TGF-β inhibition during radiotherapy can both improve response to radiotherapy and reduce risk of fibrosis in sensitive organs.
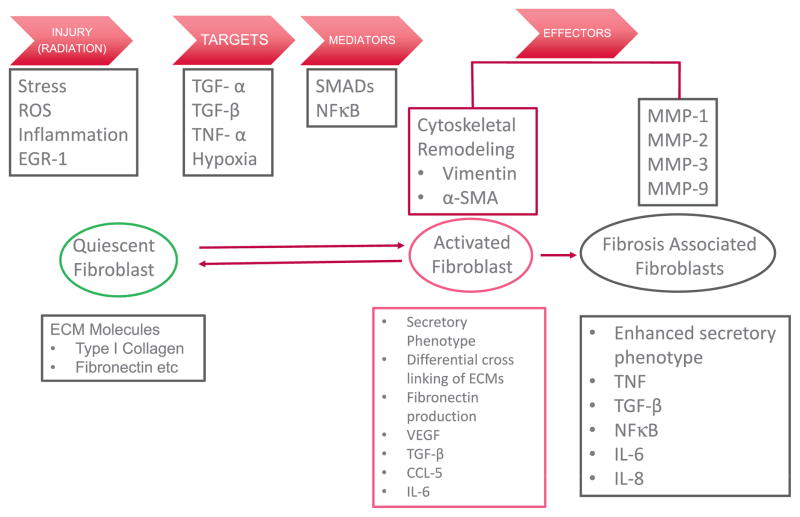
Mansoor M. Ahmed (RRP, NCI) described the TNF-α signaling axis as a major hub that constantly interacts with other signaling pathways to regulate various cellular, tissue, organ and immune function, involving radiation injury, targets, mediators, and effectors (Fig. 3). His talk focused on how the function of TNF-α modulates RIF in pre-clinical and clinical settings and included a critical outlook in terms of how TNF-α function can be regulated at the promoter and protein level. A description of several agents, peptides, antibodies, and small molecules that alter TNF-α function were described as relevant targets in RIF.
FIG. 3.
Onset and manifestation of fibrosis. Quiescent fibroblasts, which are characterized the presence of extracellular matrix (ECM), Type I collagens, and fibronectin are activated by external stress such as ROS, TGF-β, hypoxia or other cytokines. Activated fibroblasts have plasticity and are characterized by a secretory phenotype, leading to differential cross linking of ECM, production of fibronectin, TGF-β, CCL-5 and IL-6. Activated fibroblasts also demonstrate cytoskeletal remodeling. Fibrosis associated fibroblasts are characterized by an enhanced secretory phenotype and production of MMP-1, -2, -3 and -9.
He described that while TGF-α is an important player in regulating RIF as well as from other insults, TNF-α with or without TGF-α can play role in fibrosis, more importantly in certain cell types (66). In a nonradiation insult, induction of cardiac fibrosis in an experimental mouse model was correlated with increased serum level of TGF-α and decreased TNF-α (67). In high-dose spatially fractionated GRID radiation treated patients with bulky tumor a significant elevation of TNF-α with concomitant decrease in TGF-β was observed and this decrease was found to be an indicator of having a low risk of developing post-radiotherapy fibrosis (68). Further, TNF-α promoted acute apoptosis in the lungs of ultra-high dose-rate FLASH (≥40 Gy/s)-irradiated animals and triggered dramatic pulmonary edema, consistent with enhanced vascular permeability. However, TNF-α did not induce lung fibrosis in FLASH-irradiated animals within the time range investigated suggesting that protection against vascular apoptosis is only a part of the non-fibrogenic character of FLASH (69). In both cases of high-dose GRID and high-dose-rate FLASH radiotherapy, when TNF-α is engaged in targeting vascular apoptosis, TGF-β function is down-modulated and hence disengages onset of fibrosis events in the irradiated tissue.
The above observations can be explained in the context of relevance to radiation injury, irrespective of cell type, based on the axis of signal transduction pathways that drive functions of TNF-α and TGF-β. It is well documented that both TGF-β and TNF-α are robustly induced by ionizing radiation (70, 71). Functional regulation of these two cytokines demonstrate a common key transcription factor, EGR-1, that is inducible by ionizing radiation (71). Hence, the signal-transduction axis between TGF-β and TNF-α can become complex depending on the target, cell type and potentially radiation dose (Fig. 3). TNF-α has dual function in regulating response to radiation. Radio-inducible EGR-1 mediated TNF-α elevation can directly activate extrinsic cell death pathway via ceramide/TNFR to induce vascular and epithelial apoptosis with concomitant increase in TNF-α mediated elevation of NFκB activity (71). Interestingly, radiation-induced EGR-1 can directly interact with p65 to block NFκB functions and directly TNF-α functions to engage in apoptosis (71). On the other, radiation-induced activated TGF-β requires downstream receptors and SMADs (70) to initiate a cascade of events leading to fibrosis. However, certain critical TGF-β downstream effector pathway genes that play roles in fibrosis are directly regulated by NFκB that includes fibronectin (72), Collagen I and III (73), MMP-1 and 2 (74) and TIMP-1 (75).
There are several FDA approved inhibitors of TNF-α including small molecules and other drugs that disrupt the signal transduction pathway, starting from immature TNF-α to inhibiting TNF-TNFR interaction, are available on the market (76). Open-label uncontrolled studies with TNF-α inhibitors in systemic sclerosis appear to be promising; however, from the existing literature, there are concerns that treatment with TNF-α antagonists could lead to progression of fibrosis. Therefore, TNF-α antagonists should not be used in daily clinical practice for the treatment of patients with fibrotic diseases until these concerns are cleared (77). Hence, it is not clear on the definitive clinical effects of TNF-α antagonists in mitigating RIF as more placebo-controlled trials are warranted.
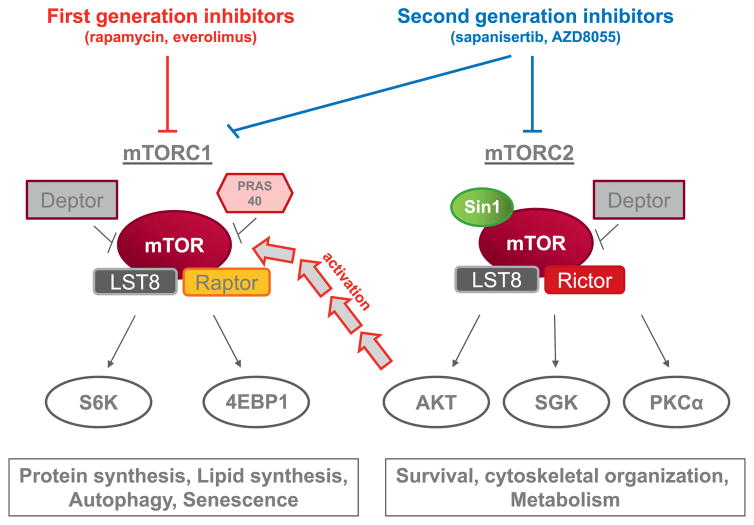
Iris Eke (CCR, NCI) described the role of mTOR targeting agents for mitigation of RIF. The mammalian target of rapamycin (mTOR) is a serine/threonine kinase known for its role in tumor proliferation. mTOR serves as a catalytic subunit of two functionally distinct complexes, mTOR complex 1 (mTORC1) and mTOR complex 2 (mTORC2), both of which phosphorylate several downstream targets (Fig. 4) (78). Hereby, it regulates multiple essential cellular processes such as protein synthesis, survival, metabolism and the cellular stress response. Small molecule inhibitors targeting either mTORC1 (e.g. temsirolimus, everolimus) or both complexes (sapanisertib, AZD8055) are already in clinical trials for different tumor types (79).
FIG. 4.
Targeting mTOR in fibrosis. First generation mTOR inhibitors are effective at targeting the mTORC1 complex, which is implicated in the control of protein and limped synthesis, autophagy, and senescence. mTORC1 inhibition may not suppress the pathway sufficiently in some cases due to compensatory Akt activation. Second generation agents capable of suppressing mTORC1 and mTORC2 more effectively blunt signaling through these pathways.
Recent studies indicate that mTOR signaling is involved in pulmonary fibrosis. Histologic analysis of lung tissue from patients with IPF revealed a correlation of mTOR expression with fibrosis grade and decline of pulmonary function (80). Further, in fibrotic lung tissue of mice treated with bleomycin, phosphorylation of the mTOR downstream target S6 was strongly enhanced in comparison to control mice without drug-induced pulmonary fibrosis (81). When mTORC1 was over-activated in alveolar epithelial cells by down regulation of TSC1, an inhibitor of mTORC1, mice showed increased mortality and lung fibrosis after bleomycin exposure (81). Accordingly, RNAi-mediated knockdown of mTOR or mTOR inhibition using rapamycin results in reduced collagen synthesis of fibroblasts in vitro (82). Not only drug-induced fibrosis, but also lung fibrosis after fractionated thoracic radiotherapy seems to be modulated by mTOR signaling (49). Targeting mTORC1 with a low dose of rapamycin during and after radiotherapy significantly prolonged the median survival compared with the control group, which only received fractionated radiation (49). In parallel, the number of fibrotic foci and the hydroxyproline levels in the fibrotic lung tissue as a marker of collagen content was strongly reduced. Mechanistically, the authors found differential inflammatory cytokine expression, an attenuated radiation-induced accumulation of immune cells including lymphocytes and macrophages, and reduced senescence of pneumocytes after radiation when mTOR was inhibited (49).
The use of mTORC1 inhibitors in clinical trials has shown that these drugs are generally well tolerated with only few serious adverse effects (83, 84). One potentially life-threatening adverse effect is the noninfectious pneumonitis affecting approximately 2–36% of patients receiving everolimus or temsirolimus therapy (83). This pulmonary toxicity is characterized by inflammatory infiltration of lung interstitium leading to clinical symptoms such as cough, dyspnea, hemoptysis, and fever (84). The pathogenesis of this adverse effect is not known to date. Although these findings can put into question the usage of mTOR inhibitors to prevent pulmonary toxicity, it must be considered that most drug-related pneumonitis in tumor patients occurred under much higher drug concentrations compared to the doses, which were effective to reduce drug- or radiation-induced fibrotic processes.
Inhibition of the mTOR pathway to mitigate drug or RIPF achieved promising results in preclinical studies. Many questions still remain regarding the optimal translation of these agents as a therapy for the mitigation of RIF, including whether these strategies can successfully be translated into patient treatment. Defining the appropriate time for beginning treatment and identifying patients that would benefit most from mTOR inhibition would be critical for effective clinical translation. Further, it is not clear if there is any risk for drug-related pneumonitis when the mTOR inhibitors are used at very low doses, as has been used in preclinical models (49, 85). Clarifying these points might be of value to optimize radiation therapy by reducing treatment-related side effects on normal tissue.
Molykutty Aryankalayil (CCR, NCI) presented recent studies focusing on microRNAs (miRNAs) as a therapeutic target in RIF. miRNAs function as endogenous oligonucleotides capable of producing a global effect on the gene expression of major regulatory pathways (86). Each of these master regulators silence/modulate genetic expression patterns of multiple complementary target mRNAs. Particular classes of miRNAs have been identified as strong regulators of fibrotic processes, namely “fibromiRs” and “redoximiRs,” effective genetic regulators of ROS generation (87, 88). Furthermore, miRNAs have also been found to influence various pathways comprising TGF-β, SMADs, and other extracellular matrix (ECM) proteins that are predominantly influential to both universal and organ-specific fibrogenesis (89, 90).
Identification and classification of recognized biomarkers, prior to the observation of fibrotic signs, has the potential to allow for medical intervention to impede irreparable fibrotic scarring resulting from radiotherapy. Beyond the ubiquitous fibro-regulatory physiological ramifications of certain miRNA families, miRNA organ specific expression patterns facilitate their use as biomarkers for sub-clinical or early clinical indicators of fibrotic scaring.
Theoretically, this could be used to alter therapy. Precise ionizing radiation response patterns of miRNA expression and target gene regulation in both an in-vitro tumor model and normal endothelial cells have already been described (91, 92). A recent study by Simone et al. reported radiation-induced dysregulation of key fibrosis related miRNAs, like miR-15a, miR-21 and miR-34a, in a murine skin model (93).
The influence of miRNAs on numerous adverse cardiovascular fibrotic events has been extensively published (94). Tissue specific investigations of profibrotic miRNA in isolated organ fibrogenesis demonstrate that radiotherapy induction of a single aberrantly expressed miRNA can impact numerous fibrotic pathways. Targeting profibrotic miRNAs using small molecule inhibitors or sponges, which can bind to the miRNA-mRNA binding sites, will prevent corresponding mRNA degradation, which in turn, helps to restore anti-fibrotic mRNA levels. In IPF, a disease with currently no cure, miR-21 upregulation in the lungs of mice was associated with pro-fibrogenic activity of TGF-β1, which were rescued with the administration of miR-21 antisense probes (95). Kidney fibrotic nephropathy also manifests significantly upregulated miR-21, and was alleviated by both constitutive knockdowns and anti-miR-21 oligonucleotide (96).
Antifibrogenic microRNAs like miR-9-5p, miR-29, miR-153, possess endogenous protective activity, which can be mimicked and intensified, enabling the down regulation of the pro-fibrotic mRNAs involved in fibrogenic pathways (97, 98). The mRNA and protein targets of components like miR-9 and miR-29 repression represent new objectives for antisense-oligo factors and inhibitory small molecules to disrupt the development of fibrosis. Radiation-induced miRNA miR-210, with recognized roles in DNA damage repair, radioresistance, and homeostatic maintenance are being identified as RIF regulators (97, 99). Hamama et al. reported the overexpression of miR-210 in radiation-induced intestinal enteropathy and transmural fibrosis, and its repression by a combination of anti-fibrotic drugs (100).
Subsequent to the manifestation of fibrosis triggered by injury, chronic disease, or as a complication of surgical/radiological medical interventions, clinicians possess limited therapeutic options for treatment. Antisense inhibitors of profibrotic miRNA targets or anti fibrotic miRNA mimics are becoming one appealing path for pharmaceutical development using miRNA based technology (Table 2) (101–105). In addition to these instruments, the capacity of small molecule inhibitors targeting specific miRNAs has been demonstrated as a potential form of targeting miRNAs in cancer therapy (106). Drug screens for these classes of small molecule inhibitors can identify small molecules capable of targeting a single profibrotic miRNA, yet still regulating multiple mRNAs fundamental to fibrotic development. Advanced delivery methods, such as spherical nucleic acid nanoparticle delivery design, are uncovering startling mechanisms of RNA based oligonucleotide delivery (107).
TABLE 2.
Oligonucleotide-Based Drugs Targeting miRNAs
| Target | Drug name | Mechanism of action | Disease |
|---|---|---|---|
| miR-29b | MRG-201 | miRNA Mimic | Fibrosis |
| miR-195 (miR-15 family) | NA | Antisense inhibitor | Post-myocardial infarction remodeling |
| CTGF | RXI-109 | Antisense inhibitor | Scar prevention, keloid formation |
| miR-34 | MRX-34 | miRNA mimic | Cancer |
| miR-122 | Miravirsen | miRNA inhibitor | Hepatitis C |
| miR-122 | RG-101 | miRNA inhibitor | Hepatitis C |
| miR-103 and miR-107 | RG-125 | miRNA inhibitor | Nonalcoholic steatohepatitis |
| miR-21 | RG-012 | miRNA inhibitor | Alport syndrome, cancer, fibrosis |
miRNA-based therapeutics along with the identification of the players, pathways, and complex interactions of “radiofibromiRs” may add to the arsenal of RIF treatment. These key components of the larger RNA world have revealed an ideal system in which to develop and identify biomarkers, small drug compound targets, antisense miRNA inhibitors, and miRNA mimic design for treatment of fibro proliferative disorders. miRNA based therapeutics represent a new category of pharmaceuticals capable of regulating the expression of key signaling pathways in fibrogenesis, which are not currently attained through the traditional medicines.
PREDICTIVE BIOMARKERS FOR RADIATION FIBROSIS
Individual variations in the response of normal tissue to radiation among patients also present a risk in terms of treatment outcome and post-treatment QOL. Therefore, early predictive biomarkers of adverse response such a RIF will be of immense value. Ruth Wilkins (Health Canada, Ottawa, Canada) reviewed the status of biomarkers for predicting RIF. The risk of damage from radiotherapy is affected not only by the treatment schedule and total dose, but also by individual variations in response to radiation. Prediction of such individual variations in normal tissue characteristics could be useful when tailoring radiotherapy regimens to obtain an optimal therapeutic ratio while minimizing late toxicity.
Clinical evidence of individual variation in late normal tissue response to radiation treatment was first described in the late 1980s and early 1990s in both fibroblasts and lymphocytes (108–110). It was demonstrated that intrinsic in vitro radiosensitivity of normal cells correlates with radiotherapy induced normal tissue damage; however, with mixed results. These studies were based on the hypothesis that, if radiation response was based on a genetic variation, cells from normal tissues would reflect this intrinsic radiosensitivity. Recently, there has been renewed effort into identifying biological markers for both normal tissue and tumor response including both acute and late effects.
There is a large range of biomarkers that have been examined for correlation to RIF including cell death, DNA double-strand break repair, apoptosis, chromosome aberrations and emerging “omic technologies”. These endpoints are typically examined in fibroblasts grown from patient skin biopsies or in lymphocytes or plasma from patient blood samples.
Some of the earliest attempts to identify predictive assays examined correlations between cell killing in fibroblasts derived from skin biopsies and the degree of toxicity after radiotherapy. These studies looked at clonogenic survival after an in vitro exposure of ionizing radiation to determine whether the cellular radiosensitivity was an indicator of normal tissue toxicity. Overall, results were controversial with some studies demonstrating a correlation with surviving fraction after an in vitro exposure to the cells with risk of fibrosis (111, 112) while others found little or no correlation (113). A few studies also attempted to link the risk of RIF with the induction and repair of DNA double strand breaks using cells grown from different individuals. Even though there was a larger variation with the residual damage after a large in vitro exposure, there was still little correlation between double strand breaks and RIF (114–116). Thus, the utility of the assay for predicting RIF was not supported, and has been abandoned due to the invasiveness of the sampling, difficulty of the assay and the length time required to determine the response.
Greater success was achieved in correlating damage in lymphocytes with RIF, specifically lethal aberrations after 6 Gy exposure in vitro (117). This study found more fibrosis in patients with higher levels of in vitro induced lethal aberrations. In addition, using three color whole chromosome FISH, Keller et al. found higher levels of breaks, complex chromosomal rearrangements and translocations, in in vitro irradiated lymphocytes from patients who had developed grade 3 or greater late radiation toxicity including fibrosis (118). This study; however, was based on only 5 sensitive and 11 normally responding patients.
The existence of several genetic mutations in DNA repair pathways (e.g., ATM) that are related to radiosensitivity supports the hypothesis that radiosensitivity has a genetic component. There have been many studies attempting to identify specific gene mutations that confer radiosensitivity; however, many of these are limited by patient number and lack of repeated studies. However, there has been some success in identifying patients with increased RIF when several genomic endpoints have been combined. Alsbeih et al. demonstrated that head and neck cancer patients with a higher grade of fibrosis also had a higher number of risk alleles (119). Similarly, Terranzzino et al. found the number of breast cancer patients with grade 2 or 3 fibrosis increased with the number of concomitant genetic risk factors (120). This strategy has been applied to gene expression analysis to devise a response signature related to radiosensitivity. For example, in one study on fibroblasts from 136 head and neck cancer patients, gene expression after an in vitro exposure allowed the separation of patients into sensitive and resistant responders. Of the resistant population, none of the patients developed RIF while 34% of the sensitive responders developed severe fibrosis (121).
With the deluge of genomic data now available in the literature and the variation in responses, the opportunity has arisen to conduct meta-analysis on these data sets. This allows the results of many studies to be compared to determine whether there are significant associations between specific mutations. One such study conducted a meta-analysis on single nucleotide polymorphisms (SNP) in TGF-β1, which has been highly studied due to its strong association with the fibrogenic response pathway. This analysis included 5,555 patients from 21 different cohorts including breast, cervical and prostate cancer. The results of this analysis did not confirm previously reported associations between a particular TGF-β1 SNP and an increase risk of RIF; however, it did demonstrate the value to pooling data from many studies (122). It also demonstrated the value of large scale collaborations for sharing and developing expertise, harmonizing procedures and addressing many challenges associated with radiogenomics.
This collaborative group has now been developed into a radiogenomics consortium, which allowed the first genome-wide association study (GWAS) on late radiotherapy toxicity. The type of analysis makes no assumption on which genes may be important in a process and is only now possible due to the rapid reduction in the costs of genotyping. The study was performed in 1,850 breast and prostate cancer patients and provided evidence of a true association between common genetic variant and genotoxicity (123). None of the SNPs found to be associated to late radiotoxicity were previously identified with prostate or breast cancer susceptibility or late radiotoxicity; however, there are biologically plausible mechanisms for the associations found.
Several biomarkers implicated in the mechanism for RIF have been investigated as predictors for fibrosis. For example, although increased levels of TGF-β1 after radiotherapy have been correlated to radiation-induced lung injury, there is mixed evidence for pretreatment levels being indicators of late tissue damage (124, 125). Other proinflammatory cytokines have been shown to be involved in radiation-induced toxicity although the predictive value of these proteins has not been clinically validated (126). With recent advances in high-throughput screening, the validation of many candidate targets is now possible.
Although the prevailing assumption is that radiosensitivity should track with RIF, this has not been supported by a wide range of efforts, perhaps due either to the complexity of the response or the lack of powered studies. One exception is with the radiation-induced lymphocyte apoptosis assay, specifically with respect to CD8 T-lymphocytes. Based on methods first developed by Crompton et al. (127), prospective studies have demonstrated a correlation between a decrease in radiation-induced apoptosis after an 8 Gy exposure in vitro and an increase in RIF (128, 129). This method has the advantage of being simple, rapid, reproducible and minimally invasive. Although the predictive value of this assay is high, there is still much discussion on the mechanistic explanation for this correlation (129). At this time, radiation-induced lymphocyte apoptosis appears to be the most promising approach and has been validated in prospective clinical studies.
The research to identify a predictive biomarker has been limited by the number of patients included, the sensitivity, specificity and reproducibility of the biomarkers and confounding effects. To overcome some of these challenges, work needs to continue on method development and harmonization such that large, multi-center studies can be conducted to increase patient numbers. The use of multivariate analysis may be the key through the comparison of multiple endpoints, gene panels or gene wide association studies. Moreover, new technologies continue to be developed, which could be applied to this field including microRNA or Raman spectroscopy. This work is essential for identifying radiosensitive patients prior to treatment so that treatments can be modified to mitigate adverse effects.
CLINICAL TRIAL DESIGN FOR MITIGATORS AND TREATMENT OF RIF
A major focus of the workshop was refining strategies for clinical translation of agents with demonstrated efficacy in preclinical models. Three speakers focused on optimal clinical trial design for the study of agents with demonstrated preclinical efficacy in treating or mitigating RIF with specific examples. Benjamin Movsas (Henry Ford Hospital, Detroit, MI) noted that there are important issues to consider when designing a clinical trial to decrease the adverse effects of radiation. In 2010, the NCI sponsored a meeting on this topic resulting in the following guideline: “Decreasing the Adverse Effects of Cancer Therapy: NCI Guidance for the Clinical Development of Radiation Injury Mitigators” (130). Some key points discussed included utilizing optimal endpoints, such as biological/physical correlates and validated patient reported outcomes (PROs). The funding for such studies may be available, among other sources, from Small Business Innovation Research grants (SBIR), Small Business Technology Transfer Research grants (SBTTR) and/or from other National Institutes of Health (NIH) research project grants (2).
An example of such a clinical trial, funded by the NIH SBIR mechanism, is a study of BIO 300, an orally available nanoparticle formulation of genistein (2). This agent was initially developed by the U.S. Government [Armed Forces Radiobiology Research Institute (AFRRI), Bethesda, MD] for warfighters as a countermeasure for high doses of radiation exposure and later licensed by Humanetics Corporation (Edina, Minnesota) to be developed as both a medical radiation countermeasure and in clinical oncology as a radiomodulator. Genistein is molecularly classified as an isoflavone, a small molecule, which is naturally found in soy. Biochemically, genistein functions as a tyrosine kinase inhibitor, a free-radical scavenger, and a weak phytoestrogen. These properties of BIO 300 are associated with multiple biological effects including: the regulation of cell-cycle, reduction of ROS, and modulation of the inflammatory cytokine response. The benefits of dietary genistein have been the subject of many preclinical studies, but its clinical efficacy as a therapeutic was ultimately limited by its insolubility in water and thus poor bioavailability. These limitations of genistein were overcome by the development of a novel, wet-milled nanosuspension of synthetic genistein (BIO 300), which has significantly improved bioavailability and near-linear dose kinetics when compared to ordinary genistein. BIO 300 is extremely stable, and can be stored at room temperature. Multiple formulations of BIO 300 have been developed, which allow for either oral or parenteral dosing.
Preclinical studies at Henry Ford Hospital demonstrated that the daily oral administration of BIO 300 in a mouse model significantly reduced radiation-induced damage to normal lung tissue. At the same time, BIO 300 did not protect tumors from radiation-induced killing. Rather, BIO 300 + radiation was more effective than radiation alone in slowing the growth of tumors (131).
The clinical trial (NCT02567799) focuses on patients receiving concurrent chemoradiation for non-small cell lung cancer (https://clinicaltrials.gov/ct2/show/NCT02567799?term=NCT02567799&rank=1). Its basic design involves three cohorts with dose escalation of the BIO 300 to look for potential dose limiting toxicities and the adverse event profile. The protocol allows for a fourth cohort to be enrolled at either the maximum tolerated dose or the optimal biological dose, based upon safety and efficacy endpoints. Importantly, other key endpoints have been added in order to gain the greatest amount of knowledge from this clinical study. Indeed, the “failure rate” on clinical trials can be reduced by applying the principles of “Phase zero” trials during clinical development. Phase zero trials are typically first-in-human trials that are designed to evaluate the pharmacokinetic and pharmacodynamic properties of investigational agents in small numbers of patients before initiating larger trials. Similar principles can be employed in more traditional early-phase trials involving patients with cancer, to inform the design of subsequent, larger trials (132).
The primary objective of the BIO 300 clinical trial is to describe any dose limiting toxicities of the combination of BIO300 with chemoradiation and to determine the recommended/optimal dose of this combination. Beyond this, the BIO 300 lung cancer trial does include pharmacokinetics of BIO 300 and importantly, it also examines the effect of BIO 300 on the pharmacokinetics of the two chemotherapeutics to rule out any effects on drug exposure due to the combination. Other secondary objectives include the rate of local progression as defined by Response Evaluation Criteria in Solid Tumors s#x0005B;RECIST (version 1.1)] criteria, as well as overall survival and progression free survival. Importantly, this study also includes biologic endpoints to study the pharmacodynamic effects of BIO 300 in combination with chemoradiation by quantifying levels of serum biomarkers. In particular, cytokines involved in the inflammatory response and/or fibrotic response are being studied (such as TGF-β, IL-1, IL-4, IL-10). This study also includes a key imaging endpoint to study radiation lung fibrosis using 4D-CT ventilation scans. Ventilation images can be acquired by a method based on four-dimensional (4D) computed tomography (CT) and image processing/analysis referred to as CT ventilation imaging (133). CT ventilation imaging has the potential for widespread clinical implementation, as 4D-CT is routinely acquired for radiation treatment planning. Validation studies for CT ventilation imaging have been focused on cross-modality image comparisons. For example, studies with mechanically ventilated sheep have demonstrated strong correlations between CT ventilation and xenon-CT ventilation. Human studies have also reported reasonable correlations with ventilation scintigraphy, single-proton emission CT (SPECT) ventilation and other modalities (134). Pulmonary function testing is also included as an objective measure.
Importantly, this study also includes QOL measures to incorporate the patient perspective. Clinicians often underestimate the frequency and severity of patients’ symptoms and, therefore, the published data underestimate the true toxicity burden (135). In this regard, the Patient-Reported Outcomes Common Terminology Criteria for Adverse Events (PRO-CTCAE) is a novel patient-centered approach to adverse event (AE) reporting. The power of the PRO-CTCAE is that it intertwines the patient perspective directly into the AE reporting using a validated methodology that can facilitate informed decision making. When it comes to optimally understanding and appreciating the patient experience, our patients want us to “PRO”ceed with PROs (136). For example, the critical importance of PROs was found in RTOG 0617, a randomized clinical trial in locally advanced non-small-cell lung cancer of radiation dose escalation in combination with chemotherapy. Overall, the study showed a worse survival on the higher RT dose arm. Nevertheless, based on provider-reported toxicity scores, few differences were evident between the two arms (137). In the study, QOL was assessed prospectively by a validated lung cancer instrument, the Functional Assessment of Cancer Therapy-Trial Outcome Index (FACT-TOI). Data were analyzed based upon clinically meaningful changes (138). At the primary time point (3 months), there was a much greater clinically meaningful decline in QOL in the higher radiation dose arm. Beyond the radiation dose level, the baseline QOL also predicted for survival multivariate analysis (P < 0.02). Indeed, every 10 points higher on the FACT-TOI corresponded to a 14% decreased risk of death. This study also suggested a clinically meaningful difference in QOL at a year from treatment favoring IMRT, over the 3D method.
Thus, beyond assessing for dose limiting toxicities and pharmacokinetics, the BIO300 clinical lung study also includes biological markers, pulmonary function tests, 4D-CT ventilation scans and validated PRO instruments. By including these additional endpoints, this study will enable us to learn as much as possible from the important clinical trial experience and thereby can inform the design of a subsequent larger trial.
Mitchell Anscher (Virginia Commonwealth University, Richmond, VA) noted that in designing clinical trials to combat RIF, one must first decide whether the primary goal is prevention, mitigation or treatment (139). Prevention trials deliver therapies prior to and concurrently with radiation exposure. Examples of such a trial are RTOG 98–01, in which patients with non-small cell lung cancer received 2 cycles of induction chemotherapy followed by concurrent chemoradiotherapy and were randomized to receive the free radical scavenger amifostine or placebo simultaneously with the chemoradiation portion of the treatment (140), and a recently reported trial in which lovastatin was given concurrently and after radiation therapy for prostate cancer in an attempt to prevent the development of radiation-induced late rectal injury after treatment for prostate cancer (141). In mitigation trials, therapies are given after radiation has been completed, but before the onset of clinically evident injury. For example, administration of angiotensin converting enzyme (ACE) inhibitors after total body irradiation to prevented renal injury (142). In contrast to mitigation studies, treatment trials deliver therapy after radiation therapy to patients who have overt evidence of clinical injury. Examples of such a trial would be the randomized phase III placebo controlled study reported by Delanian et al. (143) in which patients were randomized to Vitamin E plus pentoxifylline, Vitamin E plus placebo, pentoxifylline plus placebo or double placebo for superficial RIF, and the randomized double-blind placebo controlled trial of bevacizumab vs placebo for central nervous system radio-necrosis (144).
Each of the three approaches has advantages and disadvantages. Prevention and mitigation studies, if successful, will reduce the incidence and severity of the complication in question, an important public health goal, given the number cancer patients treated with radiation. However, because most significant radiation-induced injuries develop in a minority of treated patients, these studies will require large numbers of patients, most of whom will not benefit from treatment, and consequently only commonly occurring clinically significant problems can be studied. In addition, these trials require long follow-up to detect significant beneficial effect, owing to the delayed onset of many late radiation toxicities. Prevention trials also must address the concern that the agent to be tested does not protect the tumor from the cytotoxic effects of radiation in addition to the normal tissues. Treatment trials have the disadvantage that patients may require long term and possibly lifelong treatment to prevent relapse of their symptoms (145). On the other hand, these trials will require far fewer patients, as every patient under study has the injury in question and each patient can serve as his/her own control, thus potentially avoiding the need for large phase III trials. In addition, short-term and intermediate PRO and QOL endpoints can be used to gather a preliminary estimate of the efficacy of the therapy. Treatment trials also avoid the issue of tumor protection in most cases, as these patients generally will have been cured of their cancer.
In the past, it was thought that longstanding radiation injury was irreversible (146). However, an increasing body of evidence suggests that radiation-induced late toxicity may be, at least in part, reversible (143, 145), and is often manageable with a multidisciplinary team approach (147–149). Since this is a relatively new area of clinical research, carefully defining meaningful endpoints and adopting standardized, reliable and validated instruments to quantify outcomes is critically important. Although a single toxicity may be the driver of study design and ultimately determine the number of patients that need to be enrolled, it is rare that only a single organ is exposed when irradiating tumors to curative doses. Even when that is the case, each organ has multiple functional components, often with different tolerance limits, that when injured manifest damage in different ways. Furthermore, studies designed to prevent, mitigate or treat a complication in one organ may change the pattern of injury expression such that adverse events in one or more adjacent organs dominate the clinical picture (150, 151). Thus, it will be important to collect baseline and sequential data reflecting the impact of radiation exposure on all organs at risk (OARs).
Several different categories of outcomes will need to be considered when designing trials pertaining to late radiation injury. The current standard approach is the Common Toxicity Criteria for Adverse Events (CTCAE), developed by the NIH specifically for measuring the impact of cancer treatments (152, 153), and its use has become standard practice in cooperative group cancer clinical trials. The CTCAE is a comprehensive list of graded adverse events, broken down by system organ class, based on the Medical Dictionary for Regulatory Activities. Some adverse events are defined clinically, e.g., palpitations, whereas others are defined by laboratory and/or imaging features. This instrument is not specific for radiation-related injuries, nor does it take the time course of injury development into account, so it does not specifically distinguish between acute vs. late toxicity. Moreover, as CTCAE is not a PRO instrument scales, it may underestimate complication rates compared to PROs (154).
More recently, the importance PROs and QOL measures have been recognized. These measures are felt to represent a more accurate portrayal of the impact of cancer treatment from the patient’s perspective than do physician reported outcomes (154). Several reliable and validated instruments exist to capture these data, and the most appropriate instrument(s) will vary depending on the organs and adverse events in question. In addition, PRO version of the CTCAE has been developed recently (155). It is recommended that researchers consult with experts in this field when trying to decide on the optimal instruments to incorporate into their trials.
In addition to PROs and QOL measures, most studies will include some objective measures of organ function, and incorporate imaging as an additional objective measure of treatment efficacy. Choosing the optimal battery of tests will require consultation with specialists in other fields to ensure that appropriate endpoints are being addressed. Combinations of studies that address both global organ function as well as document evidence of focal organ damage within the irradiated field may give the most useful objective data, and these measures can be followed serially to track the course of the condition in question. For example, in evaluating patients undergoing radiation to the brain, MRI may provide the necessary anatomic information, but serial neurologic and neuropsychiatric testing will be required to detect evidence of functional impairment (144).
The choice of the therapy to be tested as a potential treatment against radiation injury is a critical one. Because of the long-term nature of these trials, and the probability that chronic therapy will be required, only orally administered agents are truly practical. Also, investigators must decide whether to test agents early in the development pipeline versus choosing drugs already on the market for which evidence exists of potential efficacy against radiation injury (141, 156). The use of established agents has major advantages in terms of study design and costs, but may face more funding hurdles, especially if the patent on the drug in question has expired and the manufacturer sees little opportunity for profit in the future from repurposing its product.
The question of correlative science will also need to be addressed. It is strongly recommended that investigators build correlative studies into clinical trials addressing the treatment of radiation toxicity. At the very least, specimen banks of plasma, serum and white cells should be created and stored, as these will provide a gold mine for current and future studies of biomarkers and predictive assays that may better guide future therapies against radiation injury (157).
The final speaker for the clinical trial session was Eric Cohen (University of Maryland, Baltimore, MD) who recounted his experience with the clinical development of ACE inhibitors as a method to prevent late radiation injury of lung and kidney. The fibrosis of radiation nephropathy occurs several months after a sufficient irradiation dose in rodent models that correspond closely to the classical radiation nephropathy seen in humans (158). It involves all renal compartments: vascular, glomerular, tubular, and interstitial (159). Established renal fibrotic injury can be quantified by scoring Masson trichrome stained tissue sections for the blue staining collagen, and by the occurrence of fibrotic narrowing of the glomerulo-tubular neck (160). The progression of those stenotic necks to atubular glomeruli is stopped by use of the ACE inhibitor captopril (161). The latter is an example of a use of an ACE inhibitor as a treatment. ACE inhibitors are very effective when used preventively, i.e. when started before irradiation and continued thereafter (162). ACE inhibitors are also effective when used as mitigators, i.e. when started after irradiation but before typical manifestations of injury (163). Other agents that are effective in mitigating the fibrosis of experimental radiation nephropathy are selenium compounds and an analogue of epoxyeicosatrienoic acid (164).
Captopril was tested as a mitigator of late radiation injury in patients undergoing hematopoietic stem cell transplantation that was preceded by total body irradiation (142). This masked and placebo controlled trial showed favorable trends for less renal and lung injury in subjects on captopril compared to those on placebo. Tissue histology was not done as part of this trial, so a benefit on fibrosis per se cannot be affirmed.
The fibrosis of radiation-induced lung injury occurs several months after a sufficient irradiation dose in humans or in rodent models. It involves the lung parenchyma, including vasculature, interalveolar septa, and pleura. This can be prevented by use of captopril in rodent studies that use a thoracic irradiation model, i.e. when that ACE inhibitor is started before irradiation (165). Many ACE inhibitors are also effective as mitigators of lung fibrosis in similar rodent models (166). Intriguing data have been reported that an antibody against connective tissue growth factor can reverse RIPF (167). These data require confirmation.
ACE inhibitors are associated with less radiation pneumonitis and fibrosis in subjects undergoing radiotherapy for lung cancer when those subjects were coincidentally taking ACE inhibitors for another medical indication (168–170). These reports show the importance of observational clinical studies. It is possible that coincidental use of other agents may affect the occurrence of pneumonitis and fibrosis. That could be a favorable effect or an unfavorable one.
Captopril was used in RTOG-0123 to test whether it could mitigate radiation-induced lung injury. Recruitment was insufficient to show significance of the effect of captopril (171). A new trial of ACE inhibitors is underway to test whether those drugs can mitigate radiation-induced lung injury [NCT01754909 (https://clinicaltrials.gov/ct2/show/NCT01754909?term=NCT01754909&rank=1)] and is funded by the Department of Veterans Affairs. This trial is testing enalapril vs. placebo to mitigate both pneumonitis and fibrosis, the former as clinical grade 2 pneumonitis and the latter as quantified by CT scanning. To date in this trial, the study drug has been well tolerated. Pneumonitis rates have been higher than expected, perhaps because the study subjects are followed closely. Occurrence of fibrosis has not yet been analyzed in this trial.
In regard to recommendations for clinical translation, trials to mitigate RIF should compare the proposed test article to placebo. The study subjects should be susceptible to fibrosis of mid-range severity. Serologic and noninvasive biomarkers of fibrosis should be studied in these subjects, if possible. Power calculations will define whether one center or more than one center is needed to enroll sufficient subjects to enable robust conclusions. Further, ongoing observational trials that record late fibrotic radiation injuries are of tremendous importance. Observations of late fibrotic radiation injuries remain vitally important. Quantitative clinical reports establish ongoing occurrence rates of those injuries and they enable correlative studies of non-invasive fibrosis markers as is done for liver disease (172).
REGULATORY CONSIDERATIONS
For a drug to be marketed in the United States, a sponsor must demonstrate efficacy with an acceptable safety profile in adequate and well-controlled clinical trials in the patient population for which the drug is indicated. Pharmaceuticals intended to prevent, mitigate or treat RIF are regulated by the Center for Drug Evaluation and Research (CDER). In general, the FDA Division selected to regulate a drug will depend on the organ for which the drug is exerting an effect. For example, a drug intended to treat RIPF would be regulated in the Division of Pulmonary, Allergy and Rheumatology Products in the Office of Drug Evaluation II, and a drug intended to treat radiation-induced skin fibrosis would be regulated in the Division of Dermatology and Dental Products in the Office of Drug Evaluation III.
Efficacy has been defined as clinical benefit to the patient and can be demonstrated through a variety of clinical outcomes, including improved function, symptomatic improvement, or effects on established surrogate endpoints. Clinical trials with radiotherapy protectants usually have two objectives: (i) to assess the amelioration of radiation toxicity, and (ii) to determine whether anticancer activity is compromised by the protectant with endpoints such as time to progression or overall survival (173). The appropriate primary endpoint(s) for a clinical trial will depend on the specific disease site, organ of interest and intent of therapy. Efficacy endpoints may include clinician-reported safety data, improved physical function such as pulmonary function tests, or improved PROs. For a PRO measure to be used as an efficacy endpoint to support approval and/or labeling claims, PRO endpoint(s) must be generated from a well defined and reliable PRO instrument, and appropriate statistical methods must be applied to generate substantial evidence of a meaningful treatment effect (174). In addition to support for efficacy, PRO measures can provide important descriptive patient-centered data to further inform the safety and tolerability of a cancer therapy; an important trial objective across all stages of clinical development.
Given the lack of regulatory precedent for marketed products intended to treat, mitigate, or prevent RIF, the FDA strongly recommends obtaining feedback during the drug development process in the form of meetings (175). For example, a type B meeting, also known as a milestone meeting, is an opportunity for Sponsors to meet with the FDA to discuss the development program at predefined milestones. Examples of type B meetings include pre-IND meetings, end-of-phase 2 meetings, and pre-New Drug Application or Pre-Biologics License Applications meetings. Sponsors may also submit special protocol assessments to confirm the acceptability of endpoints and statistical analysis plans prior to initiating trials anticipated to support drug marketing applications (176). A special protocol assessment agreement indicates concurrence by the FDA with the adequacy and acceptability of specific critical elements of overall protocol design, including entry criteria, trial design, endpoints and planned analyses. These elements are critical to ensuring that the trial has the potential to meet regulatory requirements for approval.
SUMMARY OF MEETING CONCLUSIONS AND AREAS FOR FUTURE FOCUS
RIF is a chronic, progressive complication of radiation with few effective therapies. Identification of patients at highest risk of this toxicity through a combination of biomarkers, dosimetry, and patient risk factors would allow testing of potentially efficacious agents earlier during fibrosis, at a time when the disease may have a higher likelihood of stabilization or reversibility. Early intervention may improve ultimate QOL and preserve function compared to late treatment. Animal models should consider the timing of treatment in relation to radiation (mitigation, treatment) that is intended for clinical translation. The use of animal models in preclinical studies may enhance the success of attempting to identify biomarkers of fibrosis. Assessment of tumor response with agents that mitigate or treat injury, and ideally prioritizing agents that have single agent efficacy or the capacity to radiosensitive, is likely to enhance acceptance of these treatments. A better understanding of the complex interaction between tumor and normal tissue in the context of fibrosis is required to optimally manage this toxicity while enhancing tumor cure.
Clinical translation requires careful consideration of an appropriate objective measure, and the inclusion of PROs are strongly encouraged. Additional biomarkers, tailored to the agent in question, and correlative studies, such as imaging and functional studies, should be included to allow careful description of therapeutic benefit and to enhance the mechanistic understanding of any response.
Acknowledgments
The authors are grateful to the organizers and participants in the workshop, especially to the speakers who assisted in the preparation of the manuscript. This work was primarily supported by the Radiation Research Program and in part by the intramural program of the NCI (Center for Cancer Research), NIH and the NIAID, NIH. DC received support from CCR, NCI (ZIA BC 010850). AJW was supported by Office of Hematology and Oncology Products, CDER, FDA. MF obtained support from NHLBI, NIH under grant RO1HL112286. EPC was supported by a Merit Review Award, IO1 CX000569, from the U.S. Veterans Affairs, Clinical Sciences Research and Development Program. BM received funding from NCI’s SBIR Development Center under contract with Humanetics Corp. [Edina, Minnesota; HHSN261201200078C (Fast Track Phase I and Phase II)] to develop BIO 300 (IND119332) as a radiation modulator for use during radiotherapy of lung cancer and conduct Phase I/II clinical trial, NCT02567799 (www.clinicaltrials.gov). MSM acknowledges support from the Radiation Research Society. Conflicts of interest: BM receives support from Varian Inc. and Philips Inc.
References
- 1.Prasanna PG, Stone HB, Wong RS, Capala J, Bernhard EJ, Vikram B, et al. Normal tissue protection for improving radiotherapy: Where are the gaps? Transl Cancer Res. 2012;1:35–48. [PMC free article] [PubMed] [Google Scholar]
- 2.Prasanna PG, Narayanan D, Hallett K, Bernhard EJ, Ahmed MM, Evans G, et al. Radioprotectors and radiomitigators for improving radiation therapy: The Small Business Innovation Research (SBIR) Gateway for Accelerating Clinical Translation. Radiat Res. 2015;184:235–48. doi: 10.1667/RR14186.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding NH, Li JJ, Sun LQ. Molecular mechanisms and treatment of radiation-induced lung fibrosis. Curr Drug Targets. 2013;14:1347–56. doi: 10.2174/13894501113149990198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Emerit J, Michelson AM, Robert HG, Chomette G, Guerin RA, Blondon J, et al. Superoxide dismutase treatment of 2 cases of radiation-induced sclerosis. Sem Hop. 1983;59:277–81. [PubMed] [Google Scholar]
- 5.Baillet F, Housset M, Michelson AM, Puget K. Treatment of radiofibrosis with liposomal superoxide dismutase. Preliminary results of 50 cases. Free Radic Res Commun. 1986;1:387–94. doi: 10.3109/10715768609051643. [DOI] [PubMed] [Google Scholar]
- 6.Delanian S, Baillet F, Huart J, Lefaix JL, Maulard C, Housset M. Successful treatment of radiation-induced fibrosis using liposomal Cu/Zn superoxide dismutase: clinical trial. Radiother Oncol. 1994;32:12–20. doi: 10.1016/0167-8140(94)90444-8. [DOI] [PubMed] [Google Scholar]
- 7.Epperly M, Bray J, Kraeger S, Zwacka R, Engelhardt J, Travis E, et al. Prevention of late effects of irradiation lung damage by manganese superoxide dismutase gene therapy. Gene Ther. 1998;5:196–208. doi: 10.1038/sj.gt.3300580. [DOI] [PubMed] [Google Scholar]
- 8.Kang SK, Rabbani ZN, Folz RJ, Golson ML, Huang H, Yu D, et al. Overexpression of extracellular superoxide dismutase protects mice from radiation-induced lung injury. Int J Radiat Oncol Biol Phys. 2003;57:1056–66. doi: 10.1016/s0360-3016(03)01369-5. [DOI] [PubMed] [Google Scholar]
- 9.Citrin DE, Shankavaram U, Horton JA, Shield W, 3rd, Zhao S, Asano H, et al. Role of Type II Pneumocyte Senescence in Radiation-Induced Lung Fibrosis. J Natl Cancer Inst. 2013;105:1474–84. doi: 10.1093/jnci/djt212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Choi SH, Kim M, Lee HJ, Kim EH, Kim CH, Lee YJ. Effects of NOX1 on fibroblastic changes of endothelial cells in radiationinduced pulmonary fibrosis. Mol Med Rep. 2016;13:4135–42. doi: 10.3892/mmr.2016.5090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–83. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 12.Herrera B, Murillo MM, Alvarez-Barrientos A, Beltran J, Fernandez M, Fabregat I. Source of early reactive oxygen species in the apoptosis induced by transforming growth factor-beta in fetal rat hepatocytes. Free Radic Biol Med. 2004;36:16–26. doi: 10.1016/j.freeradbiomed.2003.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Hecker L, Vittal R, Jones T, Jagirdar R, Luckhardt TR, Horowitz JC, et al. NADPH oxidase-4 mediates myofibroblast activation and fibrogenic responses to lung injury. Nat Med. 2009;15:1077–81. doi: 10.1038/nm.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Davies SS, Bodine C, Matafonova E, Pantazides BG, Bernoud-Hubac N, Harrison FE, et al. Treatment with a gamma-ketoaldehyde scavenger prevents working memory deficits in hApoE4 mice. J Alzheimers Dis. 2011;27:49–59. doi: 10.3233/JAD-2011-102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kirabo A, Fontana V, de Faria AP, Loperena R, Galindo CL, Wu J, et al. DC isoketal-modified proteins activate T cells and promote hypertension. J Clin Invest. 2014;124:4642–56. doi: 10.1172/JCI74084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roychowdhury S, McMullen MR, Pritchard MT, Li W, Salomon RG, Nagy LE. Formation of gamma-ketoaldehyde-protein adducts during ethanol-induced liver injury in mice. Free Radic Biol Med. 2009;47:1526–38. doi: 10.1016/j.freeradbiomed.2009.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bernoud-Hubac N, Alam DA, Lefils J, Davies SS, Amarnath V, Guichardant M, et al. Low concentrations of reactive gamma-ketoaldehydes prime thromboxane-dependent human platelet aggregation via p38-MAPK activation. Biochim Biophys Acta. 2009;1791:307–13. doi: 10.1016/j.bbalip.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 18.Brame CJ, Salomon RG, Morrow JD, Roberts LJ., 2nd Identification of extremely reactive gamma-ketoaldehydes (isolevuglandins) as products of the isoprostane pathway and characterization of their lysyl protein adducts. J Biol Chem. 1999;274:13139–46. doi: 10.1074/jbc.274.19.13139. [DOI] [PubMed] [Google Scholar]
- 19.Salomon RG, Bi W. Isolevuglandin adducts in disease. Antioxid Redox Signal. 2015;22:1703–18. doi: 10.1089/ars.2014.6154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McMahon M, Itoh K, Yamamoto M, Chanas SA, Henderson CJ, McLellan LI, et al. The Cap’n’Collar basic leucine zipper transcription factor Nrf2 (NF-E2 p45-related factor 2) controls both constitutive and inducible expression of intestinal detoxification and glutathione biosynthetic enzymes. Cancer research. 2001;61:3299–307. [PubMed] [Google Scholar]
- 21.Taylor RC, Acquaah-Mensah G, Singhal M, Malhotra D, Biswal S. Network inference algorithms elucidate Nrf2 regulation of mouse lung oxidative stress. PLoS Comput Biol. 2008;4:e1000166. doi: 10.1371/journal.pcbi.1000166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kovac S, Angelova PR, Holmstrom KM, Zhang Y, Dinkova-Kostova AT, Abramov AY. Nrf2 regulates ROS production by mitochondria and NADPH oxidase. Biochim Biophys Acta. 2015;1850:794–801. doi: 10.1016/j.bbagen.2014.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mont S, Davies SS, Roberts Second LJ, Mernaugh RL, McDonald WH, Segal BH, et al. Accumulation of isolevuglandin-modified protein in normal and fibrotic lung. Sci Rep. 2016;6:24919. doi: 10.1038/srep24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hecker L, Cheng J, Thannickal VJ. Targeting NOX enzymes in pulmonary fibrosis. Cell Mol Life Sci. 2012;69:2365–71. doi: 10.1007/s00018-012-1012-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collinson E, Dainton FS, Kroh J. Effects of linear energy transfer on the radiolysis of water and heavy water. Nature. 1960;187:475–7. doi: 10.1038/187475a0. [DOI] [PubMed] [Google Scholar]
- 26.Mont S, Davies SS, Roberts LJ, Mernaugh RL, McDonald WH, Segal BH, et al. Accumulation of isolevuglandin-modified protein in normal and fibrotic lung. Sci Rep-Uk. 2016:6. doi: 10.1038/srep24919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wynn TA. Cellular and molecular mechanisms of fibrosis. J Pathol. 2008;214:199–210. doi: 10.1002/path.2277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Travis EL, Rachakonda G, Zhou X, Korhonen K, Sekhar KR, Biswas S, et al. NRF2 deficiency reduces life span of mice administered thoracic irradiation. Free Radic Biol Med. 2011;51:1175–83. doi: 10.1016/j.freeradbiomed.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wynn TA. Type 2 cytokines: mechanisms and therapeutic strategies. Nat Rev Immunol. 2015;15:271–82. doi: 10.1038/nri3831. [DOI] [PubMed] [Google Scholar]
- 30.Urban JF, Jr, Noben-Trauth N, Donaldson DD, Madden KB, Morris SC, Collins M, et al. IL-13, IL-4Ralpha, and Stat6 are required for the expulsion of the gastrointestinal nematode parasite Nippostrongylus brasiliensis. Immunity. 1998;8:255–64. doi: 10.1016/s1074-7613(00)80477-x. [DOI] [PubMed] [Google Scholar]
- 31.Anthony RM, Kobayashi T, Wermeling F, Ravetch JV. Intravenous gammaglobulin suppresses inflammation through a novel T(H)2 pathway. Nature. 2011;475:110–3. doi: 10.1038/nature10134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.West GA, Matsuura T, Levine AD, Klein JS, Fiocchi C. Interleukin 4 in inflammatory bowel disease and mucosal immune reactivity. Gastroenterology. 1996;110:1683–95. doi: 10.1053/gast.1996.v110.pm8964392. [DOI] [PubMed] [Google Scholar]
- 33.Borthwick LA, Barron L, Hart KM, Vannella KM, Thompson RW, Oland S, et al. Macrophages are critical to the maintenance of IL-13-dependent lung inflammation and fibrosis. Mucosal Immunol. 2016;9:38–55. doi: 10.1038/mi.2015.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung SI, Horton JA, Ramalingam TR, White AO, Chung EJ, Hudak KE, et al. IL-13 is a therapeutic target in radiation lung injury. Sci Rep. 2016;6:39714. doi: 10.1038/srep39714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mentink-Kane MM, Cheever AW, Thompson RW, Hari DM, Kabatereine NB, Vennervald BJ, et al. IL-13 receptor alpha 2 down-modulates granulomatous inflammation and prolongs host survival in schistosomiasis. Proc Natl Acad Sci U S A. 2004;101:586–90. doi: 10.1073/pnas.0305064101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jakubzick C, Choi ES, Joshi BH, Keane MP, Kunkel SL, Puri RK, et al. Therapeutic attenuation of pulmonary fibrosis via targeting of IL-4- and IL-13-responsive cells. J Immunol. 2003;171:2684–93. doi: 10.4049/jimmunol.171.5.2684. [DOI] [PubMed] [Google Scholar]
- 37.Chiaramonte MG, Cheever AW, Malley JD, Donaldson DD, Wynn TA. Studies of murine schistosomiasis reveal interleukin-13 blockade as a treatment for established and progressive liver fibrosis. Hepatology. 2001;34:273–82. doi: 10.1053/jhep.2001.26376. [DOI] [PubMed] [Google Scholar]
- 38.Chiaramonte MG, Donaldson DD, Cheever AW, Wynn TA. An IL-13 inhibitor blocks the development of hepatic fibrosis during a T-helper type 2-dominated inflammatory response. J Clin Invest. 1999;104:777–85. doi: 10.1172/JCI7325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chiaramonte MG, Schopf LR, Neben TY, Cheever AW, Donaldson DD, Wynn TA. IL-13 is a key regulatory cytokine for Th2 cell-mediated pulmonary granuloma formation and IgE responses induced by Schistosoma mansoni eggs. J Immunol. 1999;162:920–30. [PubMed] [Google Scholar]
- 40.Gurujeyalakshmi G, Giri SN. Molecular mechanisms of antifibrotic effect of interferon gamma in bleomycin-mouse model of lung fibrosis: downregulation of TGF-beta and procollagen I and III gene expression. Exp Lung Res. 1995;21:791–808. doi: 10.3109/01902149509050842. [DOI] [PubMed] [Google Scholar]
- 41.Baumjohann D, Ansel KM. MicroRNA-mediated regulation of T helper cell differentiation and plasticity. Nat Rev Immunol. 2013;13:666–78. doi: 10.1038/nri3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ramalingam TR, Gieseck RL, Acciani TH, KMH, Cheever AW, Mentink-Kane MM, et al. Enhanced protection from fibrosis and inflammation in the combined absence of IL-13 and IFN-gamma. J Pathol. 2016;239:344–54. doi: 10.1002/path.4733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Baker DJ, Childs BG, Durik M, Wijers ME, Sieben CJ, Zhong J, et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016;530:184–9. doi: 10.1038/nature16932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Coppe JP, Desprez PY, Krtolica A, Campisi J. The senescence-associated secretory phenotype: the dark side of tumor suppression. Annu Rev Pathol. 2010;5:99–118. doi: 10.1146/annurev-pathol-121808-102144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Loskutoff DJ, Quigley JP. PAI-1, fibrosis, and the elusive provisional fibrin matrix. J Clin Invest. 2000;106:1441–3. doi: 10.1172/JCI11765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kortlever RM, Higgins PJ, Bernards R. Plasminogen activator inhibitor-1 is a critical downstream target of p53 in the induction of replicative senescence. Nat Cell Biol. 2006;8:877–84. doi: 10.1038/ncb1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chung EJ, McKay-Corkum G, Chung S, White A, Scroggins BT, Mitchell JB, et al. Truncated plasminogen activator inhibitor-1 protein protects from pulmonary fibrosis mediated by irradiation in a murine model. Int J Radiat Oncol Biol Phys. 2016;94:1163–72. doi: 10.1016/j.ijrobp.2015.11.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kolesnichenko M, Hong L, Liao R, Vogt PK, Sun P. Attenuation of TORC1 signaling delays replicative and oncogenic RAS-induced senescence. Cell Cycle. 2012;11:2391–401. doi: 10.4161/cc.20683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chung EJ, Sowers A, Thetford A, McKay-Corkum G, Chung SI, Mitchell JB, et al. mTOR inhibition with rapamycin mitigates radiation-induced pulmonary fibrosis in a murine model. Int J Radiat Oncol Biol Physics. 2016;96:857–866. doi: 10.1016/j.ijrobp.2016.07.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Barber NA, Ganti AK. Pulmonary toxicities from targeted therapies: a review. Target Oncol. 2011;6:235–43. doi: 10.1007/s11523-011-0199-0. [DOI] [PubMed] [Google Scholar]
- 51.Chang J, Wang Y, Shao L, Laberge RM, Demaria M, Campisi J, et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nat Med. 2016;22:78–83. doi: 10.1038/nm.4010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shi Y, Massagué J. Mechanisms of TGF-beta signaling from cell membrane to the nucleus. Cell. 2003;113:685–700. doi: 10.1016/s0092-8674(03)00432-x. [DOI] [PubMed] [Google Scholar]
- 53.Annes JP, Munger JS, Rifkin DB. Making sense of latent TGF{beta} activation. J Cell Sci. 2003;116:217–24. doi: 10.1242/jcs.00229. [DOI] [PubMed] [Google Scholar]
- 54.Derynck R, Akhurst RJ, Balmain A. TGF-b signaling in tumor suppression and cancer progression. Nature Genet. 2001;29:117–29. doi: 10.1038/ng1001-117. [DOI] [PubMed] [Google Scholar]
- 55.Rifkin D. Latent transforming growth factor-beta (TGF-beta) binding proteins: orchestrators of TGF-beta availability. J Biol Chem. 2005;280:7409–12. doi: 10.1074/jbc.R400029200. [DOI] [PubMed] [Google Scholar]
- 56.Kaartinen V, Voncken JW, Shuler C, Warburton D, Bu D, Heisterkamp N, et al. Abnormal lung development and cleft palate in mice lacking TGF-b3 indicates defects of epithelial-mesenchymal interaction. Nat Gen. 1995;11:415–21. doi: 10.1038/ng1295-415. [DOI] [PubMed] [Google Scholar]
- 57.Glick A, Kulkarni A, Tennenbaum T, Hennings HFK, O’Reilly M, Sporn MB, Karlsson S, Yuspa SH. Loss of expression of transforming growth factor beta in skin and skin tumors is associated with hyperproliferation and a high risk for malignant conversion. Proc Natl Acad Sci U S A. 1993;90:6076–80. doi: 10.1073/pnas.90.13.6076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sanford L, Ormsby I, Gittenberger-de GA, Sariola H, Friedman RBG, Cardell EL, Doetschman T. TGFbeta2 knockout mice have multiple developmental defects that are non-overlapping with other TGFbeta knockout phenotypes. Development. 1997;124:2659–70. doi: 10.1242/dev.124.13.2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Brown PD, Wakefield LM, Levinson AD, Sporn MB. Physiochemical activation of recombinant latent transforming growth factor-beta’s 1, 2, and 3. Growth Factors. 1990;3:35–43. doi: 10.3109/08977199009037500. [DOI] [PubMed] [Google Scholar]
- 60.Barcellos-Hoff MH. A novel redox mechanism for TGF-beta activation. Molecular biology of the cell. 1994;5:139a. [Google Scholar]
- 61.Barcellos-Hoff MH. Radiation-induced transforming growth factor b and subsequent extracellular matrix reorganization in murine mammary gland. Cancer research. 1993;53:3880–6. [PubMed] [Google Scholar]
- 62.Jobling MF, Mott JD, Finnegan M, Erickson AC, Taylor SE, Ledbetter S, et al. Isoform specificity of redox-mediated TGF-b activation. Radiation research. 2006;166:839–48. doi: 10.1667/RR0695.1. [DOI] [PubMed] [Google Scholar]
- 63.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-b1. Molec Endocrin. 1996;10:1077–83. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 64.Barcellos-Hoff MH, Derynck R, Tsang ML-S, Weatherbee JA. Transforming growth factor-b activation in irradiated murine mammary gland. J Clin Invest. 1994;93:892–9. doi: 10.1172/JCI117045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Barcellos-Hoff MH, Cucinotta FA. New tricks for an old fox: impact of TGFbeta on the DNA damage response and genomic stability. Sci Signal. 2014;7:re5. doi: 10.1126/scisignal.2005474. [DOI] [PubMed] [Google Scholar]
- 66.Distler JH, Schett G, Gay S, Distler O. The controversial role of tumor necrosis factor alpha in fibrotic diseases. Arthritis Rheum. 2008;58:2228–35. doi: 10.1002/art.23645. [DOI] [PubMed] [Google Scholar]
- 67.Fedulov AV, Ses TP, Gavrisheva NA, Rybakova MG, Vassilyeva JG, Tkachenko SB, et al. Serum TGF-beta 1 and TNF-alpha levels and cardiac fibrosis in experimental chronic renal failure. Immunol Invest. 2005;34:143–52. [PubMed] [Google Scholar]
- 68.Sathishkumar S, Dey S, Meigooni AS, Regine WF, Kudrimoti MS, Ahmed MM, et al. The impact of TNF-alpha induction on therapeutic efficacy following high dose spatially fractionated (GRID) radiation. Technol Cancer Res Treat. 2002;1:141–7. doi: 10.1177/153303460200100207. [DOI] [PubMed] [Google Scholar]
- 69.Favaudon V, Caplier L, Monceau V, Pouzoulet F, Sayarath M, Fouillade C, et al. Ultrahigh dose-rate FLASH irradiation increases the differential response between normal and tumor tissue in mice. Sci Transl Med. 2014;6:245ra93. doi: 10.1126/scitranslmed.3008973. [DOI] [PubMed] [Google Scholar]
- 70.Dancea HC, Shareef MM, Ahmed MM. Role of Radiation-induced TGF-beta Signaling in Cancer Therapy. Mol Cell Pharmacol. 2009;1:44–56. doi: 10.4255/mcpharmacol.09.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ahmed MM. Regulation of radiation-induced apoptosis by early growth response-1 gene in solid tumors. Curr Cancer Drug Targets. 2004;4:43–52. doi: 10.2174/1568009043481704. [DOI] [PubMed] [Google Scholar]
- 72.Kumar M, Allison DF, Baranova NN, Wamsley JJ, Katz AJ, Bekiranov S, et al. NF-kappaB regulates mesenchymal transition for the induction of non-small cell lung cancer initiating cells. PLoS One. 2013;8:e68597. doi: 10.1371/journal.pone.0068597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ferri N, Garton KJ, Raines EW. An NF-kappaB-dependent transcriptional program is required for collagen remodeling by human smooth muscle cells. J Biol Chem. 2003;278:19757–64. doi: 10.1074/jbc.M212714200. [DOI] [PubMed] [Google Scholar]
- 74.Vincenti MP, Brinckerhoff CE. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002;4:157–64. doi: 10.1186/ar401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Wilczynska KM, Gopalan SM, Bugno M, Kasza A, Konik BS, Bryan L, et al. A novel mechanism of tissue inhibitor of metalloproteinases-1 activation by interleukin-1 in primary human astrocytes. J Biol Chem. 2006;281:34955–64. doi: 10.1074/jbc.M604616200. [DOI] [PubMed] [Google Scholar]
- 76.Watson B. TNF inhibitors: a review of the recent patent literature. IDrugs. 2002;5:1151–61. [PubMed] [Google Scholar]
- 77.Jain A, Singh JA. Harms of TNF inhibitors in rheumatic diseases: a focused review of the literature. Immunotherapy. 2013;5:265–99. doi: 10.2217/imt.13.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35. doi: 10.1038/nrm3025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Guertin DA, Sabatini DM. The pharmacology of mTOR inhibition. Sci Signal. 2009;2:pe24. doi: 10.1126/scisignal.267pe24. [DOI] [PubMed] [Google Scholar]
- 80.Park JS, Park HJ, Park YS, Lee SM, Yim JJ, Yoo CG, et al. Clinical significance of mTOR, ZEB1, ROCK1 expression in lung tissues of pulmonary fibrosis patients. BMC Pulm Med. 2014;14:168. doi: 10.1186/1471-2466-14-168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gui YS, Wang L, Tian X, Li X, Ma A, Zhou W, et al. mTOR Overactivation and Compromised Autophagy in the Pathogenesis of Pulmonary Fibrosis. PLoS One. 2015;10:e0138625. doi: 10.1371/journal.pone.0138625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shegogue D, Trojanowska M. Mammalian target of rapamycin positively regulates collagen type I production via a phosphatidylinositol 3-kinase-independent pathway. J Biol Chem. 2004;279:23166–75. doi: 10.1074/jbc.M401238200. [DOI] [PubMed] [Google Scholar]
- 83.Albiges L, Chamming’s F, Duclos B, Stern M, Motzer RJ, Ravaud A, et al. Incidence and management of mTOR inhibitor-associated pneumonitis in patients with metastatic renal cell carcinoma. Ann Oncol. 2012;23:1943–53. doi: 10.1093/annonc/mds115. [DOI] [PubMed] [Google Scholar]
- 84.Atkinson BJ, Cauley DH, Ng C, Millikan RE, Xiao L, Corn P, et al. Mammalian target of rapamycin (mTOR) inhibitor-associated non-infectious pneumonitis in patients with renal cell cancer: predictors, management, and outcomes. BJU Int. 2014;113:376–82. doi: 10.1111/bju.12420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kaplan B, Qazi Y, Wellen JR. Strategies for the management of adverse events associated with mTOR inhibitors. Transplant Rev (Orlando) 2014;28:126–33. doi: 10.1016/j.trre.2014.03.002. [DOI] [PubMed] [Google Scholar]
- 86.Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fierro-Fernandez M, Miguel V, Lamas S. Role of redoximiRs in fibrogenesis. Redox Biol. 2016;7:58–67. doi: 10.1016/j.redox.2015.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pottier N, Cauffiez C, Perrais M, Barbry P, Mari B. FibromiRs: translating molecular discoveries into new anti-fibrotic drugs. Trends Pharmacol Sci. 2014;35:119–26. doi: 10.1016/j.tips.2014.01.003. [DOI] [PubMed] [Google Scholar]
- 89.Yang C, Zheng SD, Wu HJ, Chen SJ. Regulatory Mechanisms of the Molecular Pathways in Fibrosis Induced by MicroRNAs. Chin Med J (Engl) 2016;129:2365–72. doi: 10.4103/0366-6999.190677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang Y, Huang XR, Wei LH, Chung AC, Yu CM, Lan HY. miR-29b as a therapeutic agent for angiotensin II-induced cardiac fibrosis by targeting TGF-beta/Smad3 signaling. Mol Ther. 2014;22:974–85. doi: 10.1038/mt.2014.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.John-Aryankalayil M, Palayoor ST, Makinde AY, Cerna D, Simone CB, 2nd, Falduto MT, et al. Fractionated radiation alters oncomir and tumor suppressor miRNAs in human prostate cancer cells. Radiat Res. 2012;178:105–17. doi: 10.1667/rr2703.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Palayoor ST, John-Aryankalayil M, Makinde AY, Falduto MT, Magnuson SR, Coleman CN. Differential expression of stress and immune response pathway transcripts and miRNAs in normal human endothelial cells subjected to fractionated or single-dose radiation. Mol Cancer Res. 2014;12:1002–15. doi: 10.1158/1541-7786.MCR-13-0623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Simone BA, Ly D, Savage JE, Hewitt SM, Dan TD, Ylaya K, et al. microRNA alterations driving acute and late stages of radiation-induced fibrosis in a murine skin model. Int J Radiat Oncol Biol Phys. 2014;90:44–52. doi: 10.1016/j.ijrobp.2014.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wang J, Liew OW, Richards AM, Chen YT. Overview of MicroRNAs in Cardiac Hypertrophy, Fibrosis, and Apoptosis. Int J Mol Sci. 2016:17. doi: 10.3390/ijms17050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Liu G, Friggeri A, Yang Y, Milosevic J, Ding Q, Thannickal VJ, et al. miR-21 mediates fibrogenic activation of pulmonary fibroblasts and lung fibrosis. J Exp Med. 2010;207:1589–97. doi: 10.1084/jem.20100035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Chau BN, Xin C, Hartner J, Ren S, Castano AP, Linn G, et al. MicroRNA-21 promotes fibrosis of the kidney by silencing metabolic pathways. Sci Transl Med. 2012;4:121ra18. doi: 10.1126/scitranslmed.3003205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Chan YC, Banerjee J, Choi SY, Sen CK. miR-210: the master hypoxamir. Microcirculation. 2012;19:215–23. doi: 10.1111/j.1549-8719.2011.00154.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Liang C, Li X, Zhang L, Cui D, Quan X, Yang W. The anti-fibrotic effects of microRNA-153 by targeting TGFBR-2 in pulmonary fibrosis. Exp Mol Pathol. 2015;99:279–85. doi: 10.1016/j.yexmp.2015.07.011. [DOI] [PubMed] [Google Scholar]
- 99.Grosso S, Doyen J, Parks SK, Bertero T, Paye A, Cardinaud B, et al. MiR-210 promotes a hypoxic phenotype and increases radioresistance in human lung cancer cell lines. Cell Death Dis. 2013;4:e544. doi: 10.1038/cddis.2013.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hamama S, Noman MZ, Gervaz P, Delanian S, Vozenin MC. MiR-210: A potential therapeutic target against radiation-induced enteropathy. Radiother Oncol. 2014;111:219–21. doi: 10.1016/j.radonc.2013.10.030. [DOI] [PubMed] [Google Scholar]
- 101.van Rooij E, Sutherland LB, Thatcher JE, DiMaio JM, Naseem RH, Marshall WS, et al. Dysregulation of microRNAs after myocardial infarction reveals a role of miR-29 in cardiac fibrosis. Proc Natl Acad Sci U S A. 2008;105:13027–32. doi: 10.1073/pnas.0805038105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li Z, Rana TM. Therapeutic targeting of microRNAs: current status and future challenges. Nat Rev Drug Discov. 2014;13:622–38. doi: 10.1038/nrd4359. [DOI] [PubMed] [Google Scholar]
- 103.Agostini M, Knight RA. miR-34: from bench to bedside. Oncotarget. 2014;5:872–81. doi: 10.18632/oncotarget.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, et al. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–94. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 105.Jopling CL, Yi M, Lancaster AM, Lemon SM, Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–81. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 106.del Monroig PC, Chen L, Zhang S, Calin GA. Small molecule compounds targeting miRNAs for cancer therapy. Adv Drug Deliv Rev. 2015;81:104–16. doi: 10.1016/j.addr.2014.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Choi CH, Hao L, Narayan SP, Auyeung E, Mirkin CA. Mechanism for the endocytosis of spherical nucleic acid nanoparticle conjugates. Proc Natl Acad Sci U S A. 2013;110:7625–30. doi: 10.1073/pnas.1305804110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bentzen SM, Overgaard M, Overgaard J. Clinical correlations between late normal tissue endpoints after radiotherapy: implications for predictive assays of radiosensitivity. Eur J Cancer. 1993;29A:1373–6. doi: 10.1016/0959-8049(93)90004-y. [DOI] [PubMed] [Google Scholar]
- 109.Cole J, Arlett CF, Green MH, Harcourt SA, Priestley A, Henderson L, et al. Comparative human cellular radiosensitivity: II. The survival following gamma-irradiation of unstimulated (G0) T-lymphocytes, T-lymphocyte lines, lymphoblastoid cell lines and fibroblasts from normal donors, from ataxia-telangiectasia patients and from ataxia-telangiectasia heterozygotes. Int J Radiat Biol. 1988;54:929–43. doi: 10.1080/09553008814552331. [DOI] [PubMed] [Google Scholar]
- 110.Turesson I. Individual variation and dose dependency in the progression rate of skin telangiectasia. Int J Radiat Oncol Biol Phys. 1990;19:1569–74. doi: 10.1016/0360-3016(90)90374-s. [DOI] [PubMed] [Google Scholar]
- 111.Geara FB, Peters LJ, Ang KK, Wike JL, Brock WA. Prospective comparison of in vitro normal cell radiosensitivity and normal tissue reactions in radiotherapy patients. Int J Radiat Oncol Biol Phys. 1993;27:1173–9. doi: 10.1016/0360-3016(93)90540-c. [DOI] [PubMed] [Google Scholar]
- 112.Johansen J, Bentzen SM, Overgaard J, Overgaard M. Evidence for a positive correlation between in vitro radiosensitivity of normal human skin fibroblasts and the occurrence of subcutaneous fibrosis after radiotherapy. Int J Radiat Biol. 1994;66:407–12. doi: 10.1080/09553009414551361. [DOI] [PubMed] [Google Scholar]
- 113.Russell NS, Grummels A, Hart AA, Smolders IJ, Borger J, Bartelink H, et al. Low predictive value of intrinsic fibroblast radiosensitivity for fibrosis development following radiotherapy for breast cancer. Int J Radiat Biol. 1998;73:661–70. doi: 10.1080/095530098141915. [DOI] [PubMed] [Google Scholar]
- 114.Dickson J, Magee B, Stewart A, West CM. Relationship between residual radiation-induced DNA double-strand breaks in cultured fibroblasts and late radiation reactions: a comparison of training and validation cohorts of breast cancer patients. Radiother Oncol. 2002;62:321–6. doi: 10.1016/s0167-8140(01)00432-7. [DOI] [PubMed] [Google Scholar]
- 115.Dikomey E, Brammer I, Johansen J, Bentzen SM, Overgaard J. Relationship between DNA double-strand breaks, cell killing, and fibrosis studied in confluent skin fibroblasts derived from breast cancer patients. Int J Radiat Oncol Biol Phys. 2000;46:481–90. doi: 10.1016/s0360-3016(99)00335-1. [DOI] [PubMed] [Google Scholar]
- 116.Kiltie AE, Ryan AJ, Swindell R, Barber JB, West CM, Magee B, et al. A correlation between residual radiation-induced DNA double-strand breaks in cultured fibroblasts and late radiotherapy reactions in breast cancer patients. Radiother Oncol. 1999;51:55–65. doi: 10.1016/s0167-8140(99)00030-4. [DOI] [PubMed] [Google Scholar]
- 117.Hoeller U, Borgmann K, Bonacker M, Kuhlmey A, Bajrovic A, Jung H, et al. Individual radiosensitivity measured with lymphocytes may be used to predict the risk of fibrosis after radiotherapy for breast cancer. Radiother Oncol. 2003;69:137–44. doi: 10.1016/j.radonc.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 118.Keller U, Kuechler A, Liehr T, Muller E, Grabenbauer G, Sauer R, et al. Impact of various parameters in detecting chromosomal aberrations by FISH to describe radiosensitivity. Strahlenther Onkol. 2004;180:289–96. doi: 10.1007/s00066-004-1200-y. [DOI] [PubMed] [Google Scholar]
- 119.Alsbeih G, Al-Harbi N, Al-Hadyan K, El-Sebaie M, Al-Rajhi N. Association between normal tissue complications after radiotherapy and polymorphic variations in TGFB1 and XRCC1 genes. Radiat Res. 2010;173:505–11. doi: 10.1667/RR1769.1. [DOI] [PubMed] [Google Scholar]
- 120.Terrazzino S, La Mattina P, Gambaro G, Masini L, Franco P, Canonico PL, et al. Common variants of GSTP1, GSTA1, and TGFbeta1 are associated with the risk of radiation-induced fibrosis in breast cancer patients. Int J Radiat Oncol Biol Phys. 2012;83:504–11. doi: 10.1016/j.ijrobp.2011.06.2012. [DOI] [PubMed] [Google Scholar]
- 121.Andreassen CN, Overgaard J, Alsner J. Independent prospective validation of a predictive test for risk of radiation induced fibrosis based on the gene expression pattern in fibroblasts irradiated in vitro. Radiother Oncol. 2013;108:469–72. doi: 10.1016/j.radonc.2013.08.029. [DOI] [PubMed] [Google Scholar]
- 122.Barnett GC, Elliott RM, Alsner J, Andreassen CN, Abdelhay O, Burnet NG, et al. Individual patient data meta-analysis shows no association between the SNP rs1800469 in TGFB and late radiotherapy toxicity. Radiother Oncol. 2012;105:289–95. doi: 10.1016/j.radonc.2012.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Barnett GC, Thompson D, Fachal L, Kerns S, Talbot C, Elliott RM, et al. A genome wide association study (GWAS) providing evidence of an association between common genetic variants and late radiotherapy toxicity. Radiother Oncol. 2014;111:178–85. doi: 10.1016/j.radonc.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 124.Li C, Wilson PB, Levine E, Barber J, Stewart AL, Kumar S. TGF-beta1 levels in pre-treatment plasma identify breast cancer patients at risk of developing post-radiotherapy fibrosis. Int J Cancer. 1999;84:155–9. doi: 10.1002/(sici)1097-0215(19990420)84:2<155::aid-ijc11>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 125.Novakova-Jiresova A, Van Gameren MM, Coppes RP, Kampinga HH, Groen HJ. Transforming growth factor-beta plasma dynamics and post-irradiation lung injury in lung cancer patients. Radiother Oncol. 2004;71:183–9. doi: 10.1016/j.radonc.2004.01.019. [DOI] [PubMed] [Google Scholar]
- 126.Fleckenstein K, Gauter-Fleckenstein B, Jackson IL, Rabbani Z, Anscher M, Vujaskovic Z. Using biological markers to predict risk of radiation injury. Semin Radiat Oncol. 2007;17:89–98. doi: 10.1016/j.semradonc.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 127.Crompton NE, Ozsahin M. A versatile and rapid assay of radiosensitivity of peripheral blood leukocytes based on DNA and surface-marker assessment of cytotoxicity. Radiat Res. 1997;147:55–60. [PubMed] [Google Scholar]
- 128.Azria D, Riou O, Castan F, Nguyen TD, Peignaux K, Lemanski C, et al. Radiation-induced CD8 T-lymphocyte Apoptosis as a Predictor of Breast Fibrosis After Radiotherapy: Results of the Prospective Multicenter French Trial. EBioMedicine. 2015;2:1965–73. doi: 10.1016/j.ebiom.2015.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ozsahin M, Crompton NE, Gourgou S, Kramar A, Li L, Shi Y, et al. CD4 and CD8 T-lymphocyte apoptosis can predict radiation-induced late toxicity: a prospective study in 399 patients. Clin Cancer Res. 2005;11:7426–33. doi: 10.1158/1078-0432.CCR-04-2634. [DOI] [PubMed] [Google Scholar]
- 130.Movsas B, Vikram B, Hauer-Jensen M, Moulder JE, Basch E, Brown SL, et al. Decreasing the adverse effects of cancer therapy: National Cancer Institute guidance for the clinical development of radiation injury mitigators. Clin Cancer Res. 2011;17:222–8. doi: 10.1158/1078-0432.CCR-10-1402. [DOI] [PubMed] [Google Scholar]
- 131.Brown SL, Kaytor MD, Lapanowski K, McFall D, Zenk JL, Zenk RJ, et al. BIO300 (genistein oral nanosuspension) protects normal lung tissue and radiosensitizes tumor tissue in a mouse xenograft model of non-small cell lung cancer (NSCLC). 60th Annual Meeting of Radiation Research Society; Sept. 21–24, 2014; Las Vegas: Radiation Research Society; 2014. [Google Scholar]
- 132.Doroshow JH, Parchment RE. Oncologic phase 0 trials incorporating clinical pharmacodynamics: from concept to patient. Clin Cancer Res. 2008;14:3658–63. doi: 10.1158/1078-0432.CCR-07-4562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Guerrero T, Sanders K, Noyola-Martinez J, Castillo E, Zhang Y, Tapia R, et al. Quantification of regional ventilation from treatment planning CT. Int J Radiat Oncol Biol Phys. 2005;62:630–4. doi: 10.1016/j.ijrobp.2005.03.023. [DOI] [PubMed] [Google Scholar]
- 134.Reinhardt JM, Ding K, Cao K, Christensen GE, Hoffman EA, Bodas SV. Registration-based estimates of local lung tissue expansion compared to xenon CT measures of specific ventilation. Med Image Anal. 2008;12:752–63. doi: 10.1016/j.media.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Basch E. The missing voice of patients in drug-safety reporting. N Engl J Med. 2010;362:865–9. doi: 10.1056/NEJMp0911494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Movsas B. PROceeding with the patient-reported outcomes (PROs) version of the common terminology criteria for adverse events. JAMA Oncol. 2015;1:1059–60. doi: 10.1001/jamaoncol.2015.2689. [DOI] [PubMed] [Google Scholar]
- 137.Bradley JD, Paulus R, Komaki R, Masters G, Blumenschein G, Schild S, et al. Standard-dose versus high-dose conformal radiotherapy with concurrent and consolidation carboplatin plus paclitaxel with or without cetuximab for patients with stage IIIA or IIIB non-small-cell lung cancer (RTOG 0617): a randomised, two-by-two factorial phase 3 study. Lancet Oncol. 2015;16:187–99. doi: 10.1016/S1470-2045(14)71207-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cella D, Eton DT, Lai JS, Peterman AH, Merkel DE. Combining anchor and distribution-based methods to derive minimal clinically important differences on the Functional Assessment of Cancer Therapy (FACT) anemia and fatigue scales. J Pain Symptom Manage. 2002;24:547–61. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 139.Moulder JE, Cohen EP. Future strategies for mitigation and treatment of chronic radiation-induced normal tissue injury. Semin Radiat Oncol. 2007;17:141–8. doi: 10.1016/j.semradonc.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 140.Movsas B, Scott C, Langer C, Werner-Wasik M, Nicolaou N, Komaki R, et al. Randomized trial of amifostine in locally advanced non-small-cell lung cancer patients receiving chemotherapy and hyperfractionated radiation: radiation therapy oncology group trial 98–01. J Clin Oncol. 2005;23:2145–54. doi: 10.1200/JCO.2005.07.167. [DOI] [PubMed] [Google Scholar]
- 141.Anscher MS, Chang MG, Moghanaki D, Rosu M, Mikkelsen RB, Holdford D, et al. A phase II study to prevent radiation-induced rectal injury with lovastatin. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Cohen EP, Bedi M, Irving AA, Jacobs E, Tomic R, Klein J, et al. Mitigation of late renal and pulmonary injury after hematopoietic stem cell transplantation. Int J Radiat Oncol Biol Phys. 2012;83:292–6. doi: 10.1016/j.ijrobp.2011.05.081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Delanian S, Porcher R, Balla-Mekias S, Lefaix JL. Randomized, placebo-controlled trial of combined pentoxifylline and tocopherol for regression of superficial radiation-induced fibrosis. J Clin Oncol. 2003;21:2545–50. doi: 10.1200/JCO.2003.06.064. [DOI] [PubMed] [Google Scholar]
- 144.Levin VA, Bidaut L, Hou P, Kumar AJ, Wefel JS, Bekele BN, et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79:1487–95. doi: 10.1016/j.ijrobp.2009.12.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Delanian S, Porcher R, Rudant J, Lefaix JL. Kinetics of response to long-term treatment combining pentoxifylline and tocopherol in patients with superficial radiation-induced fibrosis. J Clin Oncol. 2005;23:8570–9. doi: 10.1200/JCO.2005.02.4729. [DOI] [PubMed] [Google Scholar]
- 146.Anscher MS. The irreversibility of radiation-induced fibrosis: fact or folklore? J Clin Oncol. 2005;23:8551–2. doi: 10.1200/JCO.2005.03.6194. [DOI] [PubMed] [Google Scholar]
- 147.Andreyev HJ. Pelvic radiation disease. Colorectal Dis. 2015;17:2–6. doi: 10.1111/codi.12812. [DOI] [PubMed] [Google Scholar]
- 148.Andreyev HJ, Benton BE, Lalji A, Norton C, Mohammed K, Gage H, et al. Algorithm-based management of patients with gastrointestinal symptoms in patients after pelvic radiation treatment (ORBIT): a randomised controlled trial. Lancet. 2013;382:2084–92. doi: 10.1016/S0140-6736(13)61648-7. [DOI] [PubMed] [Google Scholar]
- 149.Andreyev HJ, Muls AC, Norton C, Ralph C, Watson L, Shaw C, et al. Guidance: The practical management of the gastrointestinal symptoms of pelvic radiation disease. Frontline Gastroenterol. 2015;6:53–72. doi: 10.1136/flgastro-2014-100468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, et al. Using plasma transforming growth factor beta-1 during radiotherapy to select patients for dose escalation. J Clin Oncol. 2001;19:3758–65. doi: 10.1200/JCO.2001.19.17.3758. [DOI] [PubMed] [Google Scholar]
- 151.Anscher MS, Marks LB, Shafman TD, Clough R, Huang H, Tisch A, et al. Risk of long-term complications after TFG-beta1-guided very-high-dose thoracic radiotherapy. Int J Radiat Oncol Biol Phys. 2003;56:988–95. doi: 10.1016/s0360-3016(03)00184-6. [DOI] [PubMed] [Google Scholar]
- 152.Trotti A, Byhardt R, Stetz J, Gwede C, Corn B, Fu K, et al. Common Toxicity Criteria: version 2.0. An improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys. 2000;47:13–47. doi: 10.1016/s0360-3016(99)00559-3. [DOI] [PubMed] [Google Scholar]
- 153.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, et al. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–81. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 154.Chen J, Ou L, Hollis SJ. A systematic review of the impact of routine collection of patient reported outcome measures on patients, providers and health organisations in an oncologic setting. BMC Health Serv Res. 2013;13:211. doi: 10.1186/1472-6963-13-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Basch E, Reeve BB, Mitchell SA, Clauser SB, Minasian LM, Dueck AC, et al. Development of the National Cancer Institute’s patient-reported outcomes version of the common terminology criteria for adverse events (PRO-CTCAE) J Natl Cancer Inst. 2014:106. doi: 10.1093/jnci/dju244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Anscher MS, Chang MG, Moghanaki D, Rosu M, Mikkelsen RB, Holdford D, et al. Lovastatin may reduce the risk of erectile dysfunction following radiation therapy for prostate cancer. Acta Oncol. 2016:1–6. doi: 10.1080/0284186X.2016.1223882. [DOI] [PubMed] [Google Scholar]
- 157.Alam A, Mukhopadhyay ND, Ning Y, Reshko LB, Cardnell RJ, Alam O, et al. A Preliminary Study on Racial Differences in HMOX1, NFE2L2, and TGFbeta1 Gene Polymorphisms and Radiation-Induced Late Normal Tissue Toxicity. Int J Radiat Oncol Biol Phys. 2015;93:436–43. doi: 10.1016/j.ijrobp.2015.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Luxton RW. Radiation nephritis. A long-term study of 54 patients. Lancet. 1961;2:1221–4. doi: 10.1016/s0140-6736(61)92590-9. [DOI] [PubMed] [Google Scholar]
- 159.Cohen EP, Molteni A, Hill P, Fish BL, Ward WF, Moulder JE, et al. Captopril preserves function and ultrastructure in experimental radiation nephropathy. Lab Invest. 1996;75:349–60. [PubMed] [Google Scholar]
- 160.Cohen EP, Robbins ME, Whitehouse E, Hopewell JW. Stenosis of the tubular neck: a possible mechanism for progressive renal failure. J Lab Clin Med. 1997;129:567–73. doi: 10.1016/s0022-2143(97)90011-1. [DOI] [PubMed] [Google Scholar]
- 161.Cohen EP, Regner K, Fish BL, Moulder JE. Stenotic glomerulotubular necks in radiation nephropathy. J Pathol. 2000;190:484–8. doi: 10.1002/(SICI)1096-9896(200003)190:4<484::AID-PATH529>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 162.Cohen EP, Moulder JE, Fish BL, Hill P. Prophylaxis of experimental bone marrow transplant nephropathy. J Lab Clin Med. 1994;124:371–80. [PubMed] [Google Scholar]
- 163.Moulder JE, Cohen EP, Fish BL. Captopril and losartan for mitigation of renal injury caused by single-dose total-body irradiation. Radiat Res. 2011;175:29–36. doi: 10.1667/RR2400.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Sieber F, Muir SA, Cohen EP, Fish BL, Mader M, Schock AM, et al. Dietary selenium for the mitigation of radiation injury: effects of selenium dose escalation and timing of supplementation. Radiat Res. 2011;176:366–74. doi: 10.1667/rr2456.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Molteni A, Moulder JE, Cohen EF, Ward WF, Fish BL, Taylor JM, et al. Control of radiation-induced pneumopathy and lung fibrosis by angiotensin-converting enzyme inhibitors and an angiotensin II type 1 receptor blocker. Int J Radiat Biol. 2000;76:523–32. doi: 10.1080/095530000138538. [DOI] [PubMed] [Google Scholar]
- 166.Gao F, Fish BL, Moulder JE, Jacobs ER, Medhora M. Enalapril mitigates radiation-induced pneumonitis and pulmonary fibrosis if started 35 days after whole-thorax irradiation. Radiat Res. 2013;180:546–52. doi: 10.1667/RR13350.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Lipson KE, Wong C, Teng Y, Spong S. CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair. 2012;5:S24. doi: 10.1186/1755-1536-5-S1-S24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Kharofa J, Cohen EP, Tomic R, Xiang Q, Gore E. Decreased risk of radiation pneumonitis with incidental concurrent use of angiotensin-converting enzyme inhibitors and thoracic radiation therapy. Int J Radiat Oncol Biol Phys. 2012;84:238–43. doi: 10.1016/j.ijrobp.2011.11.013. [DOI] [PubMed] [Google Scholar]
- 169.Jenkins P, Welsh A. Computed tomography appearance of early radiation injury to the lung: correlation with clinical and dosimetric factors. Int J Radiat Oncol Biol Phys. 2011;81:97–103. doi: 10.1016/j.ijrobp.2010.05.017. [DOI] [PubMed] [Google Scholar]
- 170.Harder EM, Park HS, Nath SK, Mancini BR, Decker RH. Angiotensin-converting enzyme inhibitors decrease the risk of radiation pneumonitis after stereotactic body radiation therapy. Pract Radiat Oncol. 2015;5:e643–9. doi: 10.1016/j.prro.2015.07.003. [DOI] [PubMed] [Google Scholar]
- 171.Small W, Jr, James JL, Moore TD, Fintel DJ, Lutz ST, Movsas B, et al. Utility of the ACE Inhibitor Captopril in Mitigating Radiation-associated Pulmonary Toxicity in Lung Cancer: Results From NRG Oncology RTOG 0123. Am J Clin Oncol. 2016 doi: 10.1097/COC.0000000000000289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Patel K, Shackel NA. Current status of fibrosis markers. Curr Opin Gastroenterol. 2014;30:253–9. doi: 10.1097/MOG.0000000000000059. [DOI] [PubMed] [Google Scholar]
- 173.FDA. Clinical Trial Endpoints for the Approval of Cancer Drugs and Biologics. Washington, D.C: Food and Drug Administration, U.S. Department of Health and Human Services; 2007. ( http://www.fda.gov/downloads/Drugs/Guidances/ucm071590.pdf) [Google Scholar]
- 174.FDA. Guidance for Industry: Patient-Reported Outcome Measures: Use in Medical Product Development to Support Labeling Claims. Washington D.C: Food and Drug Administration, U.S. Department of Health and Human Services; 2009. ( http://www.fda.gov/downloads/drugs/guidances/ucm193282.pdf) [Google Scholar]
- 175.FDA. Formal Meetings Between the FDA adn Sponsors or Applicants. Washington D.C: Food and Drug Administration, U.S. Department of Health and Human Services; 2009. ( http://www.fda.gov/downloads/drugs/guidances/ucm153222.pdf) [Google Scholar]
- 176.FDA. Special Protocol Assessment. Washington D.C: Food and Drug Administration, U.S. Department of Health and Human Services; 2016. ( http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/UCM498793.pdf) [Google Scholar]