Abstract
Background
Fractalkine/CX3CR1 signalling has been implicated in many neurodegenerative and neurological diseases of the central nervous system (CNS). This signalling pathway plays an important role in regulating reactive oxygen species (ROS), as well as itself being altered in conditions of oxidative stress. Here, we investigated the effects of recombinant fractalkine (rCX3CL1) in models of hydrogen peroxide (H2O2)-induced demyelination and astrocyte toxicity, within organotypic cerebellar slice cultures.
Methods
Organotypic cerebellar slice cultures were generated from postnatal day 10 C57BL/6J mice to assess myelination. Immunohistochemistry was used to measure the degree of myelination. Fluorescent images were obtained using a leica SP8 confocal microscope and data analysed using ImageJ software.
Results
We show here, for the first time, that rCX3CL1 significantly attenuated bolus H2O2-induced demyelination as measured by expression of myelin basic protein (MBP) and attenuated reduced vimentin expression. Using the GOX-CAT system to continuously generate low levels of H2O2 and induce demyelination, we observed similar protective effects of rCX3CL1 on MBP and MOG fluorescence, although in this model, the decrease in vimentin expression was not altered.
Conclusions
This data indicates possible protective effects of fractalkine signalling in oxidative stress-induced demyelination in the central nervous system. This opens up the possibility of fractalkine receptor (CX3CR1) modulation as a potential new target for protecting against oxidative stress-induced demyelination in both inflammatory and non-inflammatory nervous system disorders.
Keywords: Fractalkine, CX3CL1, GOX-CAT, Hydrogen peroxide, demyelination
Background
Fractalkine (CX3CL1) and its receptor, fractalkine receptor (CX3CR1), are constitutively expressed in the central nervous system. This is in contrast to most other chemokines, whose expression in the brain is only detected during inflammation [1–3]. One of the unique features of fractalkine is its ability to exist as both a membrane-tethered adhesion molecule and as a soluble chemotactic ligand [4]. In naive rodent brains, CX3CR1 has been localised to microglia [1, 5] and shown to be expressed intracellularly by neurons [5]. Fractalkine is shown to be localised to neurons [1, 5] and astrocytes [5, 6]. It is thought that fractalkine expression in the brain is an important mediator of neuronal-glial communication. It is also thought to play a homeostatic role in normal physiological functions with the ability to regulate immune responses during times of injury [7, 8].
Several studies provide evidence of neurotoxic insults increasing the expression of fractalkine on neurons and astrocytes as well as increased microglial migration [9–11]. In the rodent experimental autoimmune encephalomyelitis (EAE) model for multiple sclerosis (MS), an increase in astrocytic levels of fractalkine was observed at sites of inflammation, while neuronal fractalkine remained unchanged. Microglial CX3CR1 expression has also been reported to be increased around active demyelinating lesions [12]. Further experiments suggest that fractalkine plays a direct neuroprotective role in the regulation of inflammation under oxidative and ischemic conditions [13, 14]. Fractalkine has also been shown to promote human monocyte survival through a reduction in intracellular reactive oxygen species (ROS) [15]. However, studies also show that ROS such as hydrogen peroxide (H2O2) can enhance the expression of fractalkine and other adhesion molecules on endothelial cells, which may contribute to vascular injury [16]. Thus, evidence suggests that fractalkine can be neuroprotective or neurotoxic depending on the cellular environment [17–19].
Ischemic brain injury has been associated with the generation of excess ROS, which can lead to oxidative stress and, in turn, neuronal damage [20]. Increases in ROS production have also been shown to affect myelin-producing oligodendrocytes. High concentrations of iron and polyunsaturated fatty acids combined with low levels of glutathione make oligodendrocytes an ideal target for oxidative stress [21]. Decreasing the levels of ROS surrounding these oligodendrocytes has been shown to increase myelin production with a concurrent reduction in the anti-oxidative response elements Nrf2 and hemeoxygenase-1 (HO-1) [21, 22].
Earlier work in our lab has investigated fractalkine signalling as well as the effects of glucose oxidase-catalase (GOX-CAT) and bolus H2O2 on cell viability of isolated astrocyte cultures [6, 23]. We have also established previously a model of GOX-CAT-induced demyelination [23] as well as bolus H2O2 to compare low-continuous versus high-acute levels of oxidative stress in organotypic cerebellar slice cultures. In these studies, we have shown that GOX-CAT and bolus H2O2 can cause demyelination, which is attenuated by the MS drug, FTY720/Gilenya [23]. Due to documented protective effects of fractalkine in oxidative stress conditions [13–15] and the particular susceptibility of oligodendrocytes to oxidative stress [21], here, we build on these previous works and investigated the protective effects of recombinant fractalkine in an organotypic slice culture system investigating myelination under oxidative stress conditions.
Methods
Compounds and treatments
Recombinant mouse CX3CL1/fractalkine chemokine domain (R&D systems; 458-MF) was used at various concentrations as indicated in the figure legends. Hydrogen peroxide (H2O2; Sigma, 216763) was prepared fresh for every experiment by diluting appropriately in serum-free media. Glucose oxidase solution (GOX; G0543) and catalase solution (CAT; C3155) were both sourced from Sigma. GOX was supplied at a concentration of 200 U/mg, where 1 unit of enzyme activity oxidises 1 μM of d-glucose per minute to H2O2. GOX was used at a fixed concentration of 0.1 units/ml (i.e. 1:100,000 dilution) throughout all experiments. CAT was supplied at a concentration of 30,000 U/mg, where 1 unit of enzyme activity decomposes 1 μM of H2O2 per minute to H2O. CAT was used at varying concentrations from 0.03 units/ml (i.e. 1:1,000,000 dilution) to 300 units/ml (i.e. 1:100 dilution) as indicated in the figure legends. All GOX-CAT treatments were allowed to equilibrate for approximately 15 min prior to addition to slice cultures.
Mouse organotypic cerebellar slice culture
Cerebellar slice cultures were prepared as described previously [23–27] using brain tissue isolated from both male and female postnatal day 10 C57BL/6J mice, according to the protocol reported earlier [26]. Mice were decapitated; cerebellar tissue was removed from the skull and separated from the hindbrain with spatulas on ice. The cerebellum was cut into 400-μm parasagittal slices using a McIlwain tissue chopper. Slices were separated into individual slices under a dissection microscope. Four slices were grown on each cell culture insert (Millicell, PICMORG50). Slices were cultured in media containing 50% Opti-MEM (Invitrogen, 11058021), 25% Hanks’ buffered salt solution (HBSS, Invitrogen, 14025-050), 25% heat inactivated horse serum (Biosera, HO-290) supplemented with 2 mM Glutamax (Invitrogen, 35050-038), 28 mM d-glucose (Sigma, G8769), 1% pen/strep, and 10 mM HEPES (Sigma, H3784) for 12–14 days in vitro. Slice cultures were grown at 35.5 °C and 5% CO2 in a humidified incubator. Slices were starved in serum-free media for 4 h prior to all treatments.
Immunohistochemistry
Immunohistochemistry for organotypic slices was performed by washing the slices twice in PBS. The slices were then fixed by incubation in 4% formaldehyde solution (Sigma, F1635) for 10 min. The slices were washed twice in PBS and incubated overnight in blocking buffer (PBS supplemented with 10% BSA and 0.5% Triton X-100). For all antibody dilutions, PBS supplemented with 2% BSA and 0.1% Triton-X 100 was used. Slices were incubated for 24 h at 4 °C in primary antibody, washed three times in PBS supplemented with 0.01% Triton X-100 and incubated overnight at 4 °C in secondary antibody. The slices were washed again three times and mounted on glass cover slides in antifade reagent (life technologies, S36936) and the edges sealed with varnish. Samples were stored at 4 °C in the dark until imaged. Slices were visualised with a Leica SP8 confocal microscope at × 10 magnifications. The primary antibodies used were myelin basic protein (MBP) (Abcam, ab40390), myelin oligodendrocyte glycoprotein (MOG, Millipore; MAB5680), neurofilament heavy (NFH, Millipore; ab5539) and vimentin (Santa Cruz, sc373717). Secondary fluorescent antibodies used were goat anti-rabbit Alexa 488 (Invitrogen, A11008), goat anti-chicken Alexa 633 (Invitrogen A21103) and anti-mouse Dylight 549 (Jackson Immunoresearch, 115-506-068). Four slices per condition were grown and six to eight images per slice were taken to cover the whole slice, thereby limiting bias and variation between conditions. Values in graphs represent the mean fluorescent intensity of approximately 130 measurements with standard error of the mean. Confocal images were captured as 12-bit.lif files of 1024 × 1024 pixel resolution. Image acquisition settings were kept constant across treatments. Image analysis was conducted using ImageJ software (https://imagej.nih.gov/ij/).
Statistical analysis
Using ImageJ, confocal images were split into single channels and a minimum threshold was adjusted to remove background staining/noise. Arbitrary fluorescence values were averaged across the four slices per condition to give a single value for the total fluorescent intensity of that condition. All statistical analysis was performed using GraphPad Prism 5. In experiments where three or more groups were compared, an ordinary one-way analysis of variance (ANOVA) was performed and was followed by post hoc tests. Tukey’s post hoc test was used for experiments where all columns were compared to each other. Student’s t test was used to compare the means between two groups. Detailed data analysis methods are provided in the figure legends and ‘Results’ sections.
Results
Fractalkine (CX3CL1) prevents bolus H2O2-induced demyelination in cerebellar slices
Oxidative stress is thought to play a role in ageing as well as many neurodegenerative diseases [28]. Fractalkine and CX3CR1 expression has been shown to be altered in demyelinating lesions associated with EAE [12, 29]. To determine whether the soluble fractalkine ligand (sCX3CL1) is protective of myelin, in an environment of oxidative stress, we pre-treated cerebellar slices for 1 h with recombinant fractalkine (rCX3CL1; R&D systems; cat#458-MF) prior to addition of 0.5 mM bolus H2O2. Our results show that, after 18 h of bolus H2O2 treatment, there is a significant decrease in MBP fluorescence (59.6 ± 2.5%), which was significantly attenuated in groups pre-treated with rCX3CL1 (94.5 ± 10.3%) (Student’s t test, ### p < 0.001, and one-way ANOVA and Tukey’s post hoc test, *p < 0.05) (Fig. 1B(i)). We also note a small decrease in NFH fluorescence in comparison to the control; however, this was not found to be significant (81.7 ± 14.6%) (Fig. 1B(ii)). This data shows that rCX3CL1 may have protective effects on myelin state in the cerebellum, in an environment of oxidative stress.
Fig. 1.
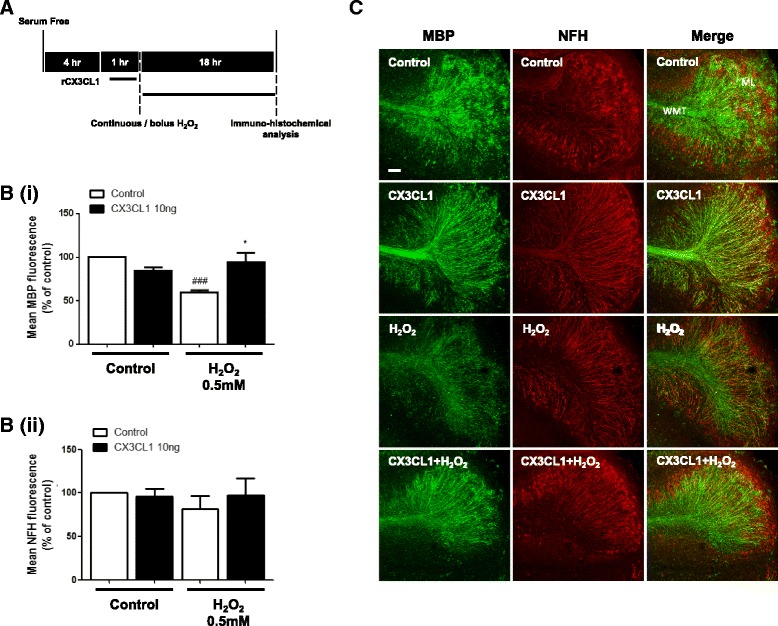
Fractalkine prevents bolus H2O2-induced demyelination in cerebellar slice cultures. a Experimental timeline shown. b(i) Organotypic cerebellar slices were pre-treated with fractalkine (CX3CL1; 10 ng) for 1 h prior to addition of bolus H2O2 (0.5 mM) for 18 h. Graph shows a significant decrease in MBP fluorescence after H2O2 treatment. This effect is rescued with CX3CL1 treatment. b(ii) NFH shows no significant decrease with bolus H2O2 treatment compared to control. c Images are representative of three separate experiments. Confocal images taken at × 10 magnification. Scale bar, 100 μm. Molecular layer (ML) and white matter tract (WMT) labelled. Data expressed as mean ± SEM; Student’s t test, ### p < 0.001; and one-way ANOVA and Tukey’s post hoc test, *p < 0.05
Fractalkine (CX3CL1) prevents bolus H2O2-induced astrocyte cell death in cerebellar slice cultures
CX3CL1 has been shown to have anti-apoptotic effects on human monocytes through a reduction in intracellular oxidative stress [15]. In certain diseases, the upregulation of CX3CL1 has been shown to help prevent microgliosis, through the activation of the NRF2 transcription factor and upregulation of heme oxygenase 1 proteins [30]. Therefore, in addition to the positive effects of rCX3CL1 on myelination in an organotypic slice model of oxidative stress, we next examined the effect of 0.5 mM bolus H2O2 on astrocytes. Vimentin fluorescence was significantly decreased through the addition of bolus H2O2 (74.2 ± 4.0%) (Fig. 2a, b). Importantly, pre-treatment with rCX3CL1 (10 ng/ml) significantly prevented this loss in vimentin fluorescence (127.7 ± 12.6%) (Student’s t test, ## p < 0.01, and one-way ANOVA and Tukey’s post hoc test, **p < 0.01) (Fig. 2b). These results show that rCX3CL1 displays a significant protective effect on astrocytes in cerebellar tissue when exposed to large concentrations of H2O2.
Fig. 2.
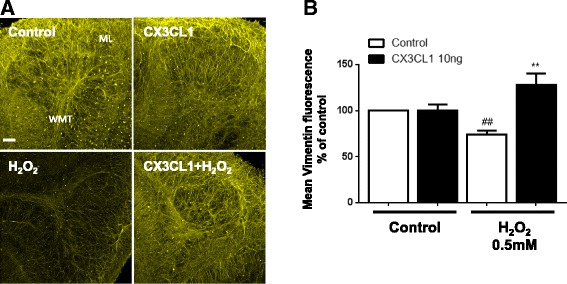
Fractalkine prevents bolus H2O2-induced astrocyte cell death in cerebellar slice cultures. Organotypic cerebellar slices were pre-treated with fractalkine (CX3CL1; 10 ng/ml) for 1 h prior to addition of bolus H2O2 (500 μM) for 18 h. a Images are representative of three separate experiments. Confocal images taken at × 10 magnification. Scale bar, 100 μm. Molecular layer (ML) and white matter tract (WMT) labelled. b Graph shows a significant decrease in vimentin fluorescence when treated with bolus H2O2. This effect is rescued when pre-treated with fractalkine. Data expressed as mean ± SEM; Student’s t test, ## p < 0.01; and one-way ANOVA and Tukey’s post hoc test, **p < 0.01
GOX-CAT-induced demyelination is prevented with fractalkine (CX3CL1) treatment
Administration of large bolus doses of H2O2 is now being challenged as an inaccurate model of oxidative stress, largely because it involves non-physiological H2O2 concentrations [31]. Bolus H2O2 is also said to be metabolised within minutes [32]. Alternatively, the glucose oxidase-catalase (GOX-CAT) system is thought to produce physiologically appropriate concentrations of H2O2 over a physiologically appropriate time [23, 33]. Therefore, differing biological outcomes may result from either large bolus or low continuous H2O2 delivery. Given we find that rCX3CL1 protects myelin and astrocytes from the effects of a large bolus dose of H2O2 (Figs. 1 and 2), we next investigated if rCX3CL1 would have similar protective effects when using the GOX-CAT model. Cerebellar slices were treated with 100 ng/ml, 10 ng/ml or 1 ng/ml of rCX3CL1 for 1 h prior to treatment with GOX-CAT (GOX dilution 1:100,000, CAT dilution 1:500,000) for 18 h. This GOX-CAT treatment caused significant demyelination (70.8 ± 4.6%, Student’s t test, ### p < 0.001; n = 4) (Fig. 3b–d), which was attenuated by pre-treatment for 1 h with rCX3CL1 (100 ng/ml) (105.4 ± 9.0%, one-way ANOVA and Tukey’s post hoc test, **p < 0.01; n = 4) (Fig. 3b). These effects of rCX3CL1 were concentration-dependent, where pre-treatment with 10 ng/ml (94.9 ± 4.7%) and 1 ng/ml of rCX3CL1 (95.8 ± 7.8%) did not have a significant protective effect (one-way ANOVA and Tukey’s post hoc test) (Fig. 3c, d). In the conditions tested, we observed no significant difference in NFH fluorescence (Fig. 3e–g). There were also no significant effects of rCX3CL1 treatment alone on MBP fluorescence, at any of the three concentrations tested (100 ng/ml, 105.8 ± 9.2%; 10 ng/ml, 127.3 ± 27.9%; 1 ng/ml, 112.0 ± 18.3%) and NFH fluorescence (100 ng/ml, 107.1 ± 12.7%; 10 ng/ml, 112.2 ± 11.2%; 1 ng/ml, 98.9 ± 13.9%) (Fig. 3e–g).
Fig. 3.
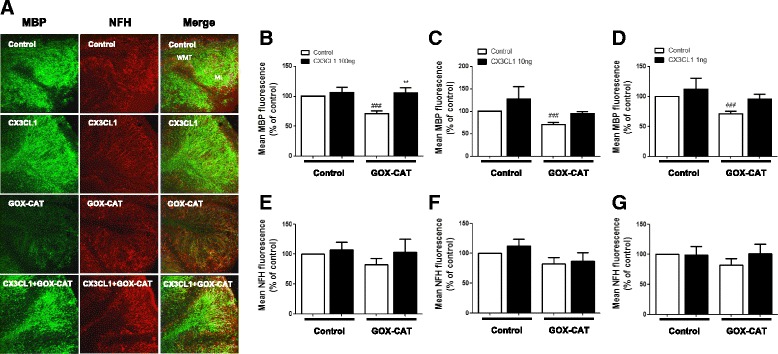
Fractalkine prevents oxidative stress-induced demyelination in cerebellar slice cultures. Organotypic cerebellar slices were pre-treated with fractalkine (CX3CL1; 100,10 or 1 ng/ml) for 1 h prior to treatment with glucose oxidase (GOX) and catalase (CAT); concentrations were maintained constant at 1:100,000 and 1:500,000, respectively, to generate low levels of H2O2 continuously. a Images are representative of fractalkine (CX3CL1) at 100 ng/ml treatment group. Confocal images taken at × 10 magnification. Scale bar, 100 μm. Molecular layer (ML) and white matter tract (WMT) labelled. b Graph shows a significant decrease in MBP fluorescence with GOX-CAT treatment. This effect is rescued with fractalkine treatment at 100 ng/ml but not at c 10 ng/ml or d 1 ng/ml. NFH shows no significant decrease in fluorescence with GOX-CAT treatment (e–g). Graphs expressed as mean ± SEM; Student’s t test, ### p < 0.001; and one-way ANOVA, **p < 0.01; n = 5
GOX-CAT-induced reduction in MOG is attenuated by fractalkine
In vitro studies on oxidative stress have led to mixed reports as to the protective [15] or toxic effects [16, 34] of fractalkine. Like many other cytokines, fractalkine has been shown to be either anti-inflammatory [35] or neurotoxic [36] depending on the circumstances. It has been suggested that the timing and concentration of fractalkine administration is of importance in determining the response of neurons and glia to potential neurodegeneration [37]. Given we find that soluble fractalkine attenuates GOX-CAT-induced decrease in MBP (Fig. 3), we further investigated the effects of GOX-CAT and recombinant fractalkine (rCX3CL1) on levels of MOG. We report that GOX-CAT treatment caused a significant decrease in MOG fluorescence compared to control (100 vs 62.7 ± 7.4%). Pre-treatment with rCX3CL1 (100 ng/ml) for 1 h produced significant protective effects on MOG levels (110.7 ± 11.9%, Student’s t test, ### p < 0.001, and one-way ANOVA, *p < 0.05; n = 5) (Fig. 4B(i)). As before, we show that neither rCX3CL1 nor GOX-CAT significantly reduce NFH fluorescence (Fig. 4B(ii)). These finding suggest fractalkine can significantly attenuate the toxic effects of low continuous H2O2 on myelin proteins.
Fig. 4.
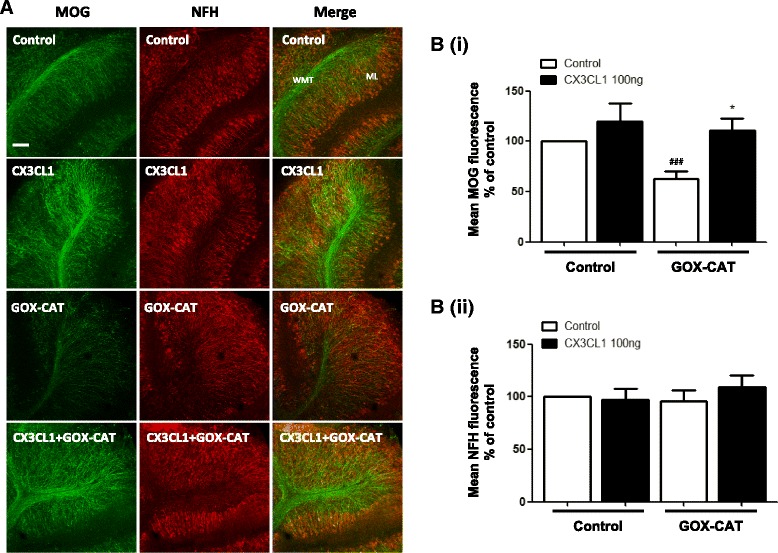
GOX-CAT-induced reduction in MOG is attenuated by fractalkine. Organotypic cerebellar slices were pre-treated with fractalkine (CX3CL1; 100 ng/ml) for 1 h prior to addition of glucose oxidase (GOX; 1:100,000) and catalase (CAT; 1:500,000). a Images are representative of five separate experiments. Scale bar, 100 μm. Molecular layer (ML) and white matter tract (WMT) labelled. Confocal images taken at × 10 magnification. b(i) Graph shows a significant decrease in MOG fluorescence with GOX-CAT treatment. This effect is rescued with fractalkine treatment at 100 ng/ml. b(ii) NFH shows no significant decrease in fluorescence with GOX-CAT treatment. Graphs expressed as mean ± SEM; Student’s t test, ### p < 0.001; and one-way ANOVA, *p < 0.05; n = 5
Fractalkine does not protect astrocytes from low continuous H2O2 exposure
Many in vitro studies on the effects of oxidative stress on astrocytes have used large bolus H2O2 concentrations in order to measure biological effects [38–40]. In this study, we have used the GOX-CAT system to generate low continuous concentrations of H2O2 in order to induce demyelination in cerebellar slice cultures. In this model, fluorescence levels of the astrocyte marker vimentin decreased significantly when exposed to GOX-CAT (GOX dilution 1:100,000, CAT dilution 1:500,000) for 18 h (66.9 ± 5.9%) (Fig. 5b, c). Pre-treatment with rCX3CL1 for 1 h at 100 ng/ml (70.4 ± 11.8%) (Fig. 5b) and 10 ng/ml (69.6% +/− 7.4%) (Fig. 5c) (one-way ANOVA and Tukey’s post hoc test, n = 4) were unable to significantly attenuate this effect of GOX-CAT treatment on astrocytes. This data shows that even though rCX3CL1 is able to significantly attenuate the bolus H2O2-induced decrease in vimentin fluorescence (see Fig. 2), rCX3CL1 is unable to counteract the toxic effects of low continuous H2O2. These results may be explained by astrocytes displaying a higher vulnerability to continuous low-level concentrations of H2O2, in comparison to transient large bolus concentrations.
Fig. 5.
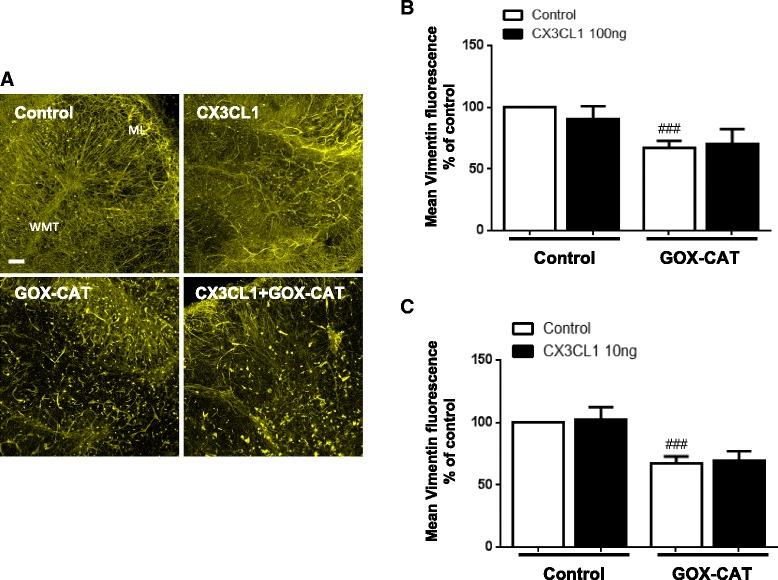
Fractalkine does not protect astrocytes from low continuous H2O2 exposure. Cerebellar slices were pre-treated with fractalkine (CX3CL1; 100 or 10 ng/ml) for 1 h prior to addition of glucose oxidase (GOX; 1:100,000) and catalase (CAT; 1:500,000) in order to generate low levels of H2O2 continuously. a Images are representative of five separate experiments. Confocal images taken at × 10 magnification. Scale bar, 100 μm. Molecular layer (ML) and white matter tract (WMT) labelled. b, c Graph shows a significant decrease in vimentin fluorescence when treated with GOX-CAT. Fractalkine treatment does not prevent this astrocyte cell death. Data expressed as mean ± SEM; Student’s t test, ### p < 0.001; and one-way ANOVA with Tukey’s post hoc test
Discussion
High concentrations of H2O2 can cause oxidation of proteins and lipids resulting in cellular damage [41]. To date, most studies have focused on bolus H2O2 treatments for studying the toxic effects of H2O2 on isolated cells. We and others have previously shown that addition of GOX-CAT enzymes allows for the generation of low-sustained levels of H2O2 in vitro, which may better mimic the sustained rise in oxidative stress levels seen in neurological disorders [23, 42, 43]. Given that a large amount of oxidative damage is associated with the initiation and progression of many neurological diseases, it is important to identify new cellular targets for preventing oxidative injury. Fractalkine is a chemokine which can regulate neuronal and glial cell responses to oxidative stress [14, 30]. Here, we investigated the effects of recombinant fractalkine (rCX3CL1) in cerebellar slices treated with bolus H2O2 or GOX-CAT-mediated H2O2. Addition of fractalkine to cerebellar slices, prior to H2O2-induced oxidative stress, significantly attenuates the loss of myelin associated with toxic levels of H2O2. Turning our attention to astrocytes, fractalkine significantly attenuates bolus H2O2-induced decrease in vimentin fluorescence but has little to no protective effect over GOX-CAT treatment. Overall, these studies suggest that fractalkine may protect astrocytes against highly concentrated but short lived H2O2 insults but may be limited in its protective effects against low-sustained levels of H2O2.
Protective effects of fractalkine on myelination
The involvement of fractalkine in regulating the oxidative stress response has been documented. Administration of fractalkine prior to ischemic insult in wild-type rodents has been shown to produce neuroprotective effects, with smaller infarct sizes and a reduced mortality rate reported [19]. In contrast, studies with CX3CR1−/− and CX3CL1−/− rodents following ischemic injury had a better outcome in comparison to their wild-type counterparts which suggests that fractalkine contributes to neurotoxicity [18, 19]. This apparent contradiction highlights the complex signalling pathways evoked by fractalkine suggesting it can be protective or toxic depending on the microenvironment. In our studies, we show that H2O2-induced demyelination, as expressed by a decrease in the levels of MBP and MOG fluorescence, can be attenuated through the addition of fractalkine, prior to H2O2 insult. Of note, the fractalkine chemokine domain and not full-length fractalkine, which includes the mucin stalk, was used in this study. Addition of recombinant fractalkine (rCX3CL1) prior to H2O2 insult may be an important factor for the protective effects observed.
Contrasting responses of astrocytes to bolus- and GOX-CAT-generated H2O2
Studies have suggested that different sub-populations of astrocytes in the brain can vary considerably in sensitivity to bolus H2O2. For example, hippocampal astrocytes are sensitive to concentrations as low as 50 μM, whereas cortical and cerebellar astrocytes are resistant to 500 μM H2O2 [44–46]. Astrocytes may also vary in their sensitivity towards the method of H2O2 delivery as it has been suggested that different or opposing biological outcomes may be elicited by cells in response to bolus or GOX-CAT H2O2 [31]. Using organotypic cerebellar slice cultures, decreased fluorescent intensity of vimentin, an intermediate filament protein in astrocytes, is caused by both bolus- and GOX-CAT-generated H2O2. In contrast to GOX-CAT-generated H2O2, rCX3CL1 significantly attenuated bolus H2O2-induced decrease in vimentin fluorescence. The protective effects of rCX3CL1 on bolus H2O2 insult may be due to the short half-life of H2O2 in cell culture [33, 40]. Similarly, the lack of protective effects observed in GOX-CAT-treated slices may be due to the half-life of rCX3CL1, which for chemokines is generally thought to be short [28, 47, 48]. In addition, persistent H2O2 signalling due to GOX-CAT activity, which is likely beyond the duration of rCX3CL1 signalling, outlasts any protective effects of rCX3CL1. Thus, rCX3CL1 may be protective of astrocytes in conditions of short-lasting bolus H2O2 treatments, but not in long-lasting continuous H2O2.
Conclusions
In conclusion, we demonstrate that fractalkine signalling pathways may not be protective of vimentin within cerebellar slices from continuous, low concentrations of H2O2 whereas it has significant protective effects on vimentin when exposed to a once off, large bolus concentration of H2O2 as observed through an increase in vimentin fluorescence. We also show in our study that rCX3CL1 may protect myelin from pathological levels of H2O2 during oxidative stress conditions.
Acknowledgements
Not applicable
Funding
This work was supported, in part, by Trinity College Dublin, Ireland, and the Health Research Board, Ireland.
Availability of data and materials
We agree to share out data obtained in this study.
Abbreviations
- CNS
Central nervous system
- CX3CL1
Fractalkine
- CX3CR1
Fractalkine receptor
- EAE
Experimental autoimmune encephalomyelitis
- GOX-CAT
Glucose oxidase-catalase
- H2O2
Hydrogen peroxide
- HO-1
Hemeoxygenase-1
- MBP
Myelin basic protein
- ML
Molecular layer
- MOG
Myelin oligodendrocyte glycoprotein
- MS
Multiple sclerosis
- NFH
Neurofilament heavy
- rCX3CL1
Recombinant fractalkine
- ROS
Reactive oxygen species
- WMT
White matter tract
Authors’ contributions
KKD conceived the study. SOS and KKD designed the experiments. SOS performed and analysed the experiments. SOS and KKD wrote the paper. Both authors read and approved the final manuscript.
Ethics approval
All animal experiments were carried out in accordance with protocols approved by the ethics committee of Trinity College Dublin and according to EU legislation for the protection of animals used for scientific purposes.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Sinead A. O’Sullivan, Email: sosulli1@tcd.ie
Kumlesh K. Dev, Phone: +353 1 896 4180, Email: devk@tcd.ie
References
- 1.Harrison JK, Jiang Y, Chen S, Xia Y, Maciejewski D, McNamara RK, Streit WJ, Salafranca MN, Adhikari S, Thompson DA, et al. Role for neuronally derived fractalkine in mediating interactions between neurons and CX3CR1-expressing microglia. Proc Natl Acad Sci U S A. 1998;95:10896–10901. doi: 10.1073/pnas.95.18.10896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nishiyori A, Minami M, Ohtani Y, Takami S, Yamamoto J, Kawaguchi N, Kume T, Akaike A, Satoh M. Localization of fractalkine and CX3CR1 mRNAs in rat brain: does fractalkine play a role in signaling from neuron to microglia? FEBS Lett. 1998;429:167–172. doi: 10.1016/S0014-5793(98)00583-3. [DOI] [PubMed] [Google Scholar]
- 3.Pan Y, Lloyd C, Zhou H, Dolich S, Deeds J, Gonzalo JA, Vath J, Gosselin M, Ma J, Dussault B, et al. Neurotactin, a membrane-anchored chemokine upregulated in brain inflammation. Nature. 1997;387:611–617. doi: 10.1038/42491. [DOI] [PubMed] [Google Scholar]
- 4.Harrison JK, Fong AM, Swain PA, Chen S, Yu YR, Salafranca MN, Greenleaf WB, Imai T, Patel DD. Mutational analysis of the fractalkine chemokine domain. Basic amino acid residues differentially contribute to CX3CR1 binding, signaling, and cell adhesion. J Biol Chem. 2001;276:21632–21641. doi: 10.1074/jbc.M010261200. [DOI] [PubMed] [Google Scholar]
- 5.Hughes PM, Botham MS, Frentzel S, Mir A, Perry VH. Expression of fractalkine (CX3CL1) and its receptor, CX3CR1, during acute and chronic inflammation in the rodent CNS. Glia. 2002;37:314–327. doi: 10.1002/glia.10037. [DOI] [PubMed] [Google Scholar]
- 6.O'Sullivan SA, Gasparini F, Mir AK, Dev KK. Fractalkine shedding is mediated by p38 and the ADAM10 protease under pro-inflammatory conditions in human astrocytes. J Neuroinflammation. 2016;13:189. doi: 10.1186/s12974-016-0659-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tsou CL, Haskell CA, Charo IF. Tumor necrosis factor-alpha-converting enzyme mediates the inducible cleavage of fractalkine. J Biol Chem. 2001;276:44622–44626. doi: 10.1074/jbc.M107327200. [DOI] [PubMed] [Google Scholar]
- 8.Boddeke EW, Meigel I, Frentzel S, Biber K, Renn LQ, Gebicke-Harter P. Functional expression of the fractalkine (CX3C) receptor and its regulation by lipopolysaccharide in rat microglia. Eur J Pharmacol. 1999;374:309–313. doi: 10.1016/S0014-2999(99)00307-6. [DOI] [PubMed] [Google Scholar]
- 9.Cardona AE, Pioro EP, Sasse ME, Kostenko V, Cardona SM, Dijkstra IM, Huang D, Kidd G, Dombrowski S, Dutta R, et al. Control of microglial neurotoxicity by the fractalkine receptor. Nat Neurosci. 2006;9:917–924. doi: 10.1038/nn1715. [DOI] [PubMed] [Google Scholar]
- 10.Cho SH, Sun B, Zhou Y, Kauppinen TM, Halabisky B, Wes P, Ransohoff RM, Gan L. CX3CR1 protein signaling modulates microglial activation and protects against plaque-independent cognitive deficits in a mouse model of Alzheimer disease. J Biol Chem. 2011;286:32713–32722. doi: 10.1074/jbc.M111.254268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garcia JA, Pino PA, Mizutani M, Cardona SM, Charo IF, Ransohoff RM, Forsthuber TG, Cardona AE. Regulation of adaptive immunity by the fractalkine receptor during autoimmune inflammation. J Immunol. 2013;191:1063–1072. doi: 10.4049/jimmunol.1300040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sunnemark D, Eltayeb S, Nilsson M, Wallstrom E, Lassmann H, Olsson T, Berg AL, Ericsson-Dahlstrand A. CX3CL1 (fractalkine) and CX3CR1 expression in myelin oligodendrocyte glycoprotein-induced experimental autoimmune encephalomyelitis: kinetics and cellular origin. J Neuroinflammation. 2005;2:17. doi: 10.1186/1742-2094-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen M, Luo C, Penalva R, Xu H. Paraquat-induced retinal degeneration is exaggerated in CX3CR1-deficient mice and is associated with increased retinal inflammation. Invest Ophthalmol Vis Sci. 2013;54:682–690. doi: 10.1167/iovs.12-10888. [DOI] [PubMed] [Google Scholar]
- 14.Zujovic V, Schussler N, Jourdain D, Duverger D, Taupin V. In vivo neutralization of endogenous brain fractalkine increases hippocampal TNFalpha and 8-isoprostane production induced by intracerebroventricular injection of LPS. J Neuroimmunol. 2001;115:135–143. doi: 10.1016/S0165-5728(01)00259-4. [DOI] [PubMed] [Google Scholar]
- 15.White GE, McNeill E, Channon KM, Greaves DR. Fractalkine promotes human monocyte survival via a reduction in oxidative stress. Arterioscler Thromb Vasc Biol. 2014;34:2554–2562. doi: 10.1161/ATVBAHA.114.304717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen DL, Chen TW, Chien CT, Li PC. Intravenous low redox potential saline attenuates FeCl3-induced vascular dysfunction via downregulation of endothelial H2O2, CX3CL1, intercellular adhesion molecule-1, and p53 expression. Transl Res. 2011;157:306–319. doi: 10.1016/j.trsl.2010.12.012. [DOI] [PubMed] [Google Scholar]
- 17.Wu XM, Liu Y, Qian ZM, Luo QQ, Ke Y. CX3CL1/CX3CR1 axis plays a key role in ischemia-induced oligodendrocyte injury via p38MAPK signaling pathway. Mol Neurobiol. 2015;53:4010-4018. [DOI] [PubMed]
- 18.Soriano SG, Amaravadi LS, Wang YF, Zhou H, Yu GX, Tonra JR, Fairchild-Huntress V, Fang Q, Dunmore JH, Huszar D, Pan Y. Mice deficient in fractalkine are less susceptible to cerebral ischemia-reperfusion injury. J Neuroimmunol. 2002;125:59–65. doi: 10.1016/S0165-5728(02)00033-4. [DOI] [PubMed] [Google Scholar]
- 19.Cipriani R, Villa P, Chece G, Lauro C, Paladini A, Micotti E, Perego C, De Simoni MG, Fredholm BB, Eusebi F, Limatola C. CX3CL1 is neuroprotective in permanent focal cerebral ischemia in rodents. J Neurosci. 2011;31:16327–16335. doi: 10.1523/JNEUROSCI.3611-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Radak D, Resanovic I, Isenovic ER. Link between oxidative stress and acute brain ischemia. Angiology. 2014;65:667–676. doi: 10.1177/0003319713506516. [DOI] [PubMed] [Google Scholar]
- 21.Gruber RC, LaRocca D, Minchenberg SB, Christophi GP, Hudson CA, Ray AK, Shafit-Zagardo B, Massa PT. The control of reactive oxygen species production by SHP-1 in oligodendrocytes. Glia. 2015;63:1753–1771. doi: 10.1002/glia.22842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fernandez-Gamba A, Leal MC, Maarouf CL, Richter-Landsberg C, Wu T, Morelli L, Roher AE, Castano EM. Collapsin response mediator protein-2 phosphorylation promotes the reversible retraction of oligodendrocyte processes in response to non-lethal oxidative stress. J Neurochem. 2012;121:985–995. doi: 10.1111/j.1471-4159.2012.07742.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Sullivan SA, Velasco-Estevez M, Dev KK. Demyelination induced by oxidative stress is regulated by sphingosine 1-phosphate receptors. Glia. 2017;65:1119–1136. [DOI] [PubMed]
- 24.O'Sullivan C, Dev KK. Galactosylsphingosine (psychosine)-induced demyelination is attenuated by sphingosine 1-phosphate signalling. J Cell Sci. 2015;128:3878–3887. doi: 10.1242/jcs.169342. [DOI] [PubMed] [Google Scholar]
- 25.Pritchard AJ, Mir AK, Dev KK. Fingolimod attenuates splenocyte-induced demyelination in cerebellar slice cultures. PLoS One. 2014;9:e99444. doi: 10.1371/journal.pone.0099444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sheridan GK, Dev KK. S1P1 receptor subtype inhibits demyelination and regulates chemokine release in cerebellar slice cultures. Glia. 2012;60:382–392. doi: 10.1002/glia.22272. [DOI] [PubMed] [Google Scholar]
- 27.O'Sullivan C, Schubart A, Mir AK, Dev KK. The dual S1PR1/S1PR5 drug BAF312 (Siponimod) attenuates demyelination in organotypic slice cultures. J Neuroinflammation. 2016;13:31. doi: 10.1186/s12974-016-0494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Beal MF. Mitochondria take center stage in aging and neurodegeneration. Ann Neurol. 2005;58:495–505. doi: 10.1002/ana.20624. [DOI] [PubMed] [Google Scholar]
- 29.Zhu W, Acosta C, MacNeil B, Cortes C, Intrater H, Gong Y, Namaka M. Elevated expression of fractalkine (CX3CL1) and fractalkine receptor (CX3CR1) in the dorsal root ganglia and spinal cord in experimental autoimmune encephalomyelitis: implications in multiple sclerosis-induced neuropathic pain. Biomed Res Int. 2013;2013:480702. doi: 10.1155/2013/480702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lastres-Becker I, Innamorato NG, Jaworski T, Rabano A, Kugler S, Van Leuven F, Cuadrado A. Fractalkine activates NRF2/NFE2L2 and heme oxygenase 1 to restrain tauopathy-induced microgliosis. Brain. 2014;137:78–91. doi: 10.1093/brain/awt323. [DOI] [PubMed] [Google Scholar]
- 31.Sobotta MC, Barata AG, Schmidt U, Mueller S, Millonig G, Dick TP. Exposing cells to H2O2: a quantitative comparison between continuous low-dose and one-time high-dose treatments. Free Radic Biol Med. 2013;60:325–335. doi: 10.1016/j.freeradbiomed.2013.02.017. [DOI] [PubMed] [Google Scholar]
- 32.Mueller S. Sensitive and nonenzymatic measurement of hydrogen peroxide in biological systems. Free Radic Biol Med. 2000;29:410–415. doi: 10.1016/S0891-5849(00)00261-6. [DOI] [PubMed] [Google Scholar]
- 33.Mueller S, Millonig G, Waite GN. The GOX/CAT system: a novel enzymatic method to independently control hydrogen peroxide and hypoxia in cell culture. Adv Med Sci. 2009;54:121–135. doi: 10.2478/v10039-009-0042-3. [DOI] [PubMed] [Google Scholar]
- 34.Xuan W, Wu B, Chen C, Chen B, Zhang W, Xu D, Bin J, Liao Y. Resveratrol improves myocardial ischemia and ischemic heart failure in mice by antagonizing the detrimental effects of fractalkine*. Crit Care Med. 2012;40:3026–3033. doi: 10.1097/CCM.0b013e31825fd7da. [DOI] [PubMed] [Google Scholar]
- 35.Zujovic V, Benavides J, Vige X, Carter C, Taupin V. Fractalkine modulates TNF-alpha secretion and neurotoxicity induced by microglial activation. Glia. 2000;29:305–315. doi: 10.1002/(SICI)1098-1136(20000215)29:4<305::AID-GLIA2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 36.Mattison HA, Nie H, Gao H, Zhou H, Hong JS, Zhang J. Suppressed pro-inflammatory response of microglia in CX3CR1 knockout mice. J Neuroimmunol. 2013;257:110–115. doi: 10.1016/j.jneuroim.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sheridan GK, Murphy KJ. Neuron-glia crosstalk in health and disease: fractalkine and CX3CR1 take centre stage. Open Biol. 2013;3:130181. doi: 10.1098/rsob.130181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ito JI, Nagayasu Y, Ogawa T, Okihara H, Michikawa M. Biochemical properties in membrane of rat astrocytes under oxidative stress. Brain Res. 2015;30(1615):1–11. [DOI] [PubMed]
- 39.Kim S, Kwon J. [6]-shogaol attenuates neuronal apoptosis in hydrogen peroxide-treated astrocytes through the up-regulation of neurotrophic factors. Phytother Res. 2013;27:1795–1799. doi: 10.1002/ptr.4946. [DOI] [PubMed] [Google Scholar]
- 40.Dringen R, Hamprecht B. Involvement of glutathione peroxidase and catalase in the disposal of exogenous hydrogen peroxide by cultured astroglial cells. Brain Res. 1997;759:67–75. doi: 10.1016/S0006-8993(97)00233-3. [DOI] [PubMed] [Google Scholar]
- 41.Barbouti A, Doulias PT, Nousis L, Tenopoulou M, Galaris D. DNA damage and apoptosis in hydrogen peroxide-exposed Jurkat cells: bolus addition versus continuous generation of H(2)O(2) Free Radic Biol Med. 2002;33:691–702. doi: 10.1016/S0891-5849(02)00967-X. [DOI] [PubMed] [Google Scholar]
- 42.Denes A, Ferenczi S, Halasz J, Kornyei Z, Kovacs KJ. Role of CX3CR1 (fractalkine receptor) in brain damage and inflammation induced by focal cerebral ischemia in mouse. J Cereb Blood Flow Metab. 2008;28:1707–1721. doi: 10.1038/jcbfm.2008.64. [DOI] [PubMed] [Google Scholar]
- 43.Wang P, Xie K, Wang C, Bi J. Oxidative stress induced by lipid peroxidation is related with inflammation of demyelination and neurodegeneration in multiple sclerosis. Eur Neurol. 2014;72:249–254. doi: 10.1159/000363515. [DOI] [PubMed] [Google Scholar]
- 44.Desagher S, Glowinski J, Premont J. Astrocytes protect neurons from hydrogen peroxide toxicity. J Neurosci. 1996;16:2553–2562. doi: 10.1523/JNEUROSCI.16-08-02553.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Feeney CJ, Frantseva MV, Carlen PL, Pennefather PS, Shulyakova N, Shniffer C, Mills LR. Vulnerability of glial cells to hydrogen peroxide in cultured hippocampal slices. Brain Res. 2008;1198:1–15. doi: 10.1016/j.brainres.2007.12.049. [DOI] [PubMed] [Google Scholar]
- 46.Ferrero-Gutierrez A, Perez-Gomez A, Novelli A, Fernandez-Sanchez MT. Inhibition of protein phosphatases impairs the ability of astrocytes to detoxify hydrogen peroxide. Free Radic Biol Med. 2008;44:1806–1816. doi: 10.1016/j.freeradbiomed.2008.01.029. [DOI] [PubMed] [Google Scholar]
- 47.Lambeir AM, Proost P, Durinx C, Bal G, Senten K, Augustyns K, Scharpe S, Van Damme J, De Meester I. Kinetic investigation of chemokine truncation by CD26/dipeptidyl peptidase IV reveals a striking selectivity within the chemokine family. J Biol Chem. 2001;276:29839–29845. doi: 10.1074/jbc.M103106200. [DOI] [PubMed] [Google Scholar]
- 48.Greer IA, Lyall F, Perera T, Boswell F, Macara LM. Increased concentrations of cytokines interleukin-6 and interleukin-1 receptor antagonist in plasma of women with preeclampsia: a mechanism for endothelial dysfunction? Obstet Gynecol. 1994;84:937–940. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
We agree to share out data obtained in this study.


