Abstract
Background:
Urothelial bladder cancer (UBC) is characterised by a high risk of recurrence. Patient monitoring is currently based on iterative cystoscopy and on urine cytology with low sensitivity in non-muscle-invasive bladder cancer (NMIBC). Telomerase reverse transcriptase (TERT) is frequently reactivated in UBC by promoter mutations.
Methods:
We studied whether detection of TERT mutation in urine could be a predictor of UBC recurrence and compared this to cytology/cystoscopy for patient follow-up. A total of 348 patients treated by transurethral bladder resection for UBC were included together with 167 control patients.
Results:
Overall sensitivity was 80.5% and specificity 89.8%, and was not greatly impacted by inflammation or infection. TERT remaining positive after initial surgery was associated with residual carcinoma in situ. TERT in urine was a reliable and dynamic predictor of recurrence in NMIBC (P<0.0001). In univariate analysis, TERT positive-status after initial surgery increased risk of recurrence by 5.34-fold (P=0.0004). TERT positive-status was still associated with recurrence in the subset of patients with negative cystoscopy (P=0.034).
Conclusions:
TERT mutations in urine might be helpful for early detection of recurrence in UBC, especially in NMIBC.
Keywords: cystoscopy, cytology, non-invasive prognostic marker, recurrence, TERT, transurothelial bladder resection, urine, urothelial bladder cancer
Diagnosis and treatment of patients presenting an urothelial bladder cancer (UBC) is a major challenge for clinicians. Prognosis for UBC is tightly correlated to stage and grade; the two main entities depend on infiltration of the muscle layer and are non-muscle-invasive bladder cancer (NMIBC) and muscle-invasive bladder cancer (MIBC). The association of urine cytology and cystoscopy is the current gold standard to detect recurrence and monitor UBC. The main advantage of urine cytology is that it is non-invasive, cheap, and easy to perform. Its high specificity (90–98%) makes cytology an interesting test to monitor high-grade tumours, with sensitivity up to 90% in pTis (Grossman et al, 2007; Geavlete et al, 2012). Unfortunately, overall sensitivity to detect tumour cells ranges from 22 to 62% (Koss et al, 1985; Piaton et al, 2004; Bassi et al, 2005) making it unsuitable for low-grade lesions (Fontaniere et al, 2001). Although the current gold standard is cystoscopy and cytology, it is subjective and may vary with the experience of the observers (Miremami and Kyprianou, 2014). This is particularly a problem in some conditions (elderly patients or those with neurogenic bladder with a chronic urinary infection, inflammatory bladder etc.). Therefore objective biological markers are required. To date, several urinary biomarkers have been described, some of them being more sensitive than urine cytology. However, they are lacking specificity, especially in the conditions cited above (Raitanen et al, 2001; Raitanen and FinnBladder Group, 2008; Rosser et al, 2014; Chou et al, 2015).
TERT (telomerase reverse transcriptase) is an essential safeguard of genomic integrity, responsible for telomere maintenance. In aging or damaged cells, TERT activity is physiologically shut down leading to shortened telomeres and induction of a form of cell death called senescence. Escaping senescence is a hallmark of cancer (Hanahan and Weinberg, 2011) and nearly all tumours develop genetic or epigenetic strategies to avoid elimination by increasing TERT activity (Schmidt and Cech, 2015). Two recurrent somatic mutations (C228T and C250T) have been identified in the TERT promoter in melanoma (Horn et al, 2013; Huang et al, 2013) as well as in various other tumours including bladder cancers (Killela et al, 2013; Wu et al, 2014). TERT promoter mutations have previously been described at high frequencies across stages in malignant bladder tumours, but the prognostic value in urine is unclear (Allory et al, 2014; Ward et al, 2016).
In this context, we evaluated, in a large prospective cohort, the use of non-invasive detection of TERT promoter mutations in urine as a predictive marker of recurrences and compared this to cytology and cystoscopy.
Materials and methods
Patients
Eighty-three patients from the Urology department of Lyon Sud university hospital (Lyon, France) presenting UBC irrespective of histological stage, were initially enrolled in a previous study (PHRC2006 - no. 27–46) between 2000 and 2008 (Descotes et al, 2014). The cohort was then prospectively increased up to 348 patients in 2014 (Table 1). Informed consent was obtained from patients to use their specimens for research purposes, as required by the French Committee for the Protection of Human Subjects. This study complies with the latest version of the Declaration of Helsinki and general guidelines for good clinical practice. Treatments (intravesical BCG or mitomycin) and follow-up were based on European Association of Urology (EAU) guidelines. For each patient an initial urine sample was obtained at the time of tumour resection by cystoscopy (transurethral bladder resection, (TUBR)). Subsequent urine samples were collected during follow-up consultations. No extra samples of urines were collected for this study, because analyses were performed on residual materials obtained for cytology. During follow-up some patients presented a recurrence based on cystoscopy. For 50 of them, recurrence was not confirmed by pathologists on surgical material obtained by TUBR. We further referred to these cases as ‘non-tumour’ pathology post-TUBR.
Table 1. Characteristics of tumours and patients with TUBR.
|
TERT promoter mutation |
||||
|---|---|---|---|---|
| Variables | n | Mutated (n=280) | Wild type (n=68) | P-valuea |
|
Age at diagnosis (years) | ||||
| Median (range) | 348 | 74 (34–97) | 68 (27–95) | 0.0017 |
|
Sex | ||||
| Female | 52 | 39 (75.0%) | 13 (25.0%) | 0.2816 |
| Male | 296 | 241 (81.4%) | 55 (18.6%) | |
|
Tumour stage | ||||
| pTa | 199 | 158 (79.4%) | 41 (21.6%) | 0.4979 |
| pT1 | 76 | 59 (77.6%) | 17 (22.4%) | |
| >pT1 | 61 | 52 (85.2%) | 9 (14.8%) | |
| pTis concomitant | 12 | 11 (91.7%) | 1 (8.3%) | |
|
Histological grade | ||||
| Low-grade | 144 | 107 (74.3%) | 37 (25.7%) | 0.0150 |
| High-grade | 204 | 173 (84.8%) | 31 (15.2%) | |
|
Cytology | ||||
| Negative | 115 | 84 (73.0%) | 31 (27.0%) | 0.1103 |
| AUC-USb | 19 | 17 (89.5%) | 2 (10.5%) | |
| Low-grade | 97 | 79 (81.4%) | 18 (18.6%) | |
| AUC-Hb | 8 | 6 (75.0%) | 2 (25.0%) | |
| High-grade | 109 | 94 (86.2%) | 15 (13.8%) | |
Abbreviations: AUC-H=Atypical urothelial cells of high grade; AUC-US=atypical urothelial cells of undetermined significance; TERT=telomerase reverse transcriptase.
P-values correspond to χ2-test or Mann-Whitney test (age).
Atypical urothelial cells of undetermined significance (AUC-US) or cannot exclude high grade (AUC-H).
To assess specificity, 167 patients without UBC were also included as controls (Table 2): 89 from healthy individuals or from patients consulting for low urinary tract symptoms or urinary incontinence (excluding patients reporting macroscopic and microscopic haematuria), 17 neurogenic bladder, 10 infectious urines, and 42 patients diagnosed with any other type of cancer (prostate, kidney, intestine, etc.). Of note, patients presenting with macroscopic and microscopic haematuria were excluded from this control cohort because it might be linked to an undiagnosed UBC.
Table 2. Pathological data and TERT status in 167 urines without bladder cancer.
| Variable | Category | n | Mutated TERT |
|---|---|---|---|
| Benign bladder lesion or healthy individuals | Total | 125 | 10 |
| Neurological bladder | 17 | 1 | |
| Infectious | 10 | 0 | |
| Healthy urines | 89 | 9 | |
| Other cancers | Total | 42 | 7 |
| Prostate | 33 | 4 | |
| Kidney | 5 | 2 | |
| Other | 4 | 1 |
Abbreviation: TERT=telomerase reverse transcriptase.
Cytology
Urine samples were fixed with a Carbowax solution of 20% polyethylene glycol 1500 (Merck, Darmstadt, Germany) in 50% ethanol and treated as previously described (Collin-Chavagnac et al, 2010). Urothelial cells were considered high-grade when they displayed an increased nucleus/cytoplasm (N/C) ratio, hyperchromatism, and markedly irregular nuclear borders or prominent nucleoli, and low-grade when they formed papillary fronds, had an increased N/C ratio, and a slightly irregular nuclear shape, or showed numerous elongated cells with slight nuclear abnormalities, as previously described (Layfield et al, 2004). We categorised cytological results as positive or negative for high-grade urothelial tumour cells. Urine classified as high-grade or AUC-H were considered positive (Piaton et al, 2014), whereas normal, inflammatory, reactive, and degenerative urothelial findings were considered negative.
Histopathology
Tumour stage and histological grade were assessed according to the International Union Against Cancer–tumour, node, metastases system and the 2004 World Health Organization classification (Sauter, 2004). Histopathology served as the gold standard for cancer diagnosis.
Molecular testing
A 25-ml sample of fresh urine was centrifuged at 800 g for 10 min and the cell pellet (urine sediment) was rinsed in PBS and frozen at −80 °C until use. DNA was extracted from urine sediment using Circulating Nucleic Acid Kit QIAamp according to the manufacturer’s recommendations (Qiagen, Hilden, Germany). Mutations of TERT promoter were analysed by nested PCR and Sanger sequencing. The first PCR (forward 5′-CACCCGTCCTGCCCCTTCACCTT-3′ and reverse 5′-GGCTTCCCACGTGCGCAGCAGGA-3′) amplifies a 275- bp fragment that is used as matrix for a second PCR (forward 5′-CCCCTTCACCTTCCAGCTC-3′ and reverse 5′-GCCGCGGAAAGGAAGG-3′) amplifying a fragment of 118 bp carrying the points −124 mutation (C228T) and −146 (C250T). PCR products were then sequenced according to the Sanger method.
Statistical analysis
Quantitative variables were compared between groups using Mann-Whitney test and categorical variables using the χ2-test or Fisher's exact test. Survival analyses were performed in the population of patients with positive TERT mutation at the time of TUBR. Analysis was restricted to superficial UBC stages, excluding pTis stage. Absence of recurrence was defined as no event with a minimum follow-up of 6 months. Median follow-up was 11.3 months (range: (1.3–117)). The Kaplan-Meier method was used to estimate recurrence-free survival (RFS), and curves were compared using the Log-rank test. Univariate and multivariate analysis were performed using Cox proportional hazard model with 95% confidence intervals (CI). All tests were set at the significance level of P<0.05. Statistical analyses were performed using the IBM SPSS Statistics software (release 19).
Results
Recurrent somatic TERT promoter mutations in urine from UBC patients
Urine from 348 patients was collected at the time of initial TURB and tested for TERT promoter mutations. This cohort included 275 NMIBC (pTa or pT1), 61 MIBC (>pT1), and 12 carcinoma in situ (CIS). The overall TERT mutation rate was 80.5% (280 out of 348). TERT positivity in urine was stage-independent; sensitivity was 79.4 in pTa and 77.6% in pT1 NMIBC, increasing to 85.2 in MIBC (>pT1) and 91.7% in pTis. There was a higher frequency of TERT mutations among high-grade tumours than among low-grade ones (P=0.0193); sensitivity in low-grade lesions was 74.3% (Table 1). Among TERT promoter hotspot mutations, C228T was the most prevalent (235 out of 280, 83.9%), followed by C250T (35 out of 280). There were also rarer substitutions in some patients (C228A and CC242TT), and in two patients concomitant mutations of both C228T and C250T.
Comparison of TERT mutations to cytology for the detection of UBC
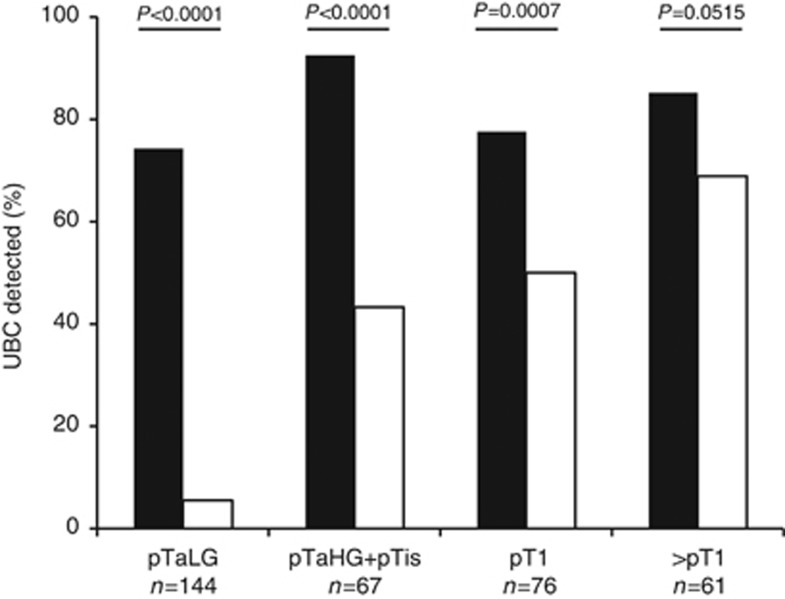
TERT positivity was not significantly correlated with urine cytology classification (Table 1). Regardless of tumour stage, sensitivity of TERT mutations (280 out of 348, 80.5%) was significantly higher than urine cytology (117 out of 348, 33.6%) (χ2-test P<0.0001). The overall sensitivity to detect UBC was for stratification of NMIBC in three groups (low-grade pTa, high-grade pTa/pTis and pT1) showed a sensitivity of cytology of 5.5%, 43.3%, and 50%, respectively whereas sensitivity of TERT remained high whatever the group (74.3%, 92.5%, and 77.6%, respectively; Figure 1). Of note, sensitivity of TERT in MIBC (>pT1) was not significantly different from cytology (P=0.0515).
Figure 1.
Distribution of somatic TERT promoter mutation and positive urine cytology among tumour stage in the 348 UBC cohort. Black bars, TERT mutated; HG, high-grade; LG, low-grade; White bars, positive cytology.
Specificity of TERT mutation detection in urine
To assess the specificity of this test we included 167 ‘non-UBC’ patients including 125 with benign bladder lesion or healthy individuals, and 42 with other cancers (Table 2). Specificity was 92.0% (115 out of 125) in those with benign bladder lesion or healthy individuals, and 83.3% in cancer patients (35 out of 42). Among those with infectious or inflammatory urines (neurogenic bladder), only one presented a TERT mutation (specificity 96.3%, 26 out of 27). As prostate cancer could have been associated with TERT mutation, we also studied a group of patients with prostate cancer and without known UBC. In this group, specificity of TERT mutations was 87.9% (29 out of 33).
Detection of residual CIS after TUBR
Analysis of follow-up urines from the 348 patients found that in some cases TERT remained positive after TUBR whereas histopathology on the surgical specimen was negative for UBC, which could point to the presence of residual CIS. To test this hypothesis we analysed the association between TERT status and presence of pTis lesions detected during follow-up in patients with ‘non-tumour’ pathology post-TUBR (n=50; Table 3). Among the 13 patients with recurrence of a confirmed CIS within 6 months after initial TUBR, 10 (76.9%) had follow-up urine (1 month post-surgery) that remained positive for TERT mutation, whereas among the 35 who did not have recurrence, 35.1% remained positive. TERT mutation was significantly associated with 6-month recurrence of pTis (P=0.0214).
Table 3. Correlation of somatic TERT promoter mutation with the appearance of pTis stage in the evolution of the disease in a subset of 50 patients with non-tumour stage at the resection.
| pTis stage | n | Mutated TERT | P-valuea |
|---|---|---|---|
| Yes | 13 | 10 (76.9%) | |
| No | 37 | 13 (35.1%) | 0.0214 |
Abbreviation: TERT=telomerase reverse transcriptase.
P-value corresponds to Fisher's exact test.
Prediction of recurrence in NMIBC by TERT in urine
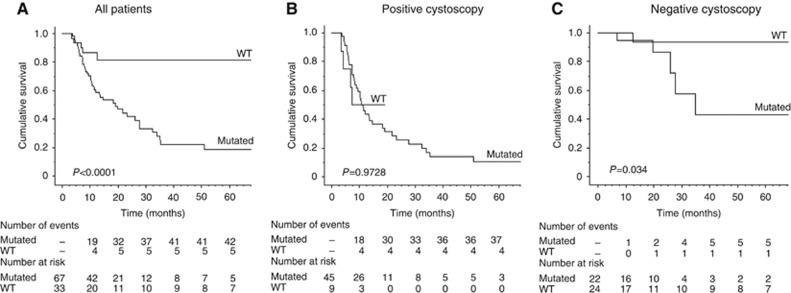
Patients presenting with NMIBC are known to be at risk of recurrence with more invasive tumours. We therefore evaluated whether TERT in urine could detect such recurrence, by analysing the association of TERT status with RFS in 100 patients with a minimum follow-up of 6 months and initially presenting a NMIBC without pTis (because it is known to increase risk of recurrence). Presence of TERT promoter mutation in urine was strongly associated with recurrence in these NMIBC patients (Figure 2A, P<0.0001). Results of univariate RFS analysis showed that TERT mutation was associated with a risk of recurrence that increased 5.34-fold in the NMIBC subset (95% CI 2.11–13.55; P=0.0004). This association did not persist in multivariate analysis when associated with cystoscopy in the Cox regression model (HR 1.72, 95% CI 0.61–4.81 P=0.3015). However when patients were stratified on cystoscopy status, TERT mutation positivity was still significantly associated with recurrence (Figure 2C, P=0.034) in the negative cystoscopy subset (46 out of 100 patients), despite only a few numbers of events (5 out of 6 were TERT mutated). TERT mutation did not provide additional information in terms of RFS in the positive cystoscopy subset (n=54; Figure 2B, P=0.9728).
Figure 2.
Recurrence-free survival and TERT status. Kaplan-Meier curves for RFS probabilities according to somatic TERT promoter mutation alone (A) or associated with positive cystoscopy (B) and negative cystoscopy (C) in the NMIBC subset (n=100).
Dynamic follow-up of NMIBC patients using TERT
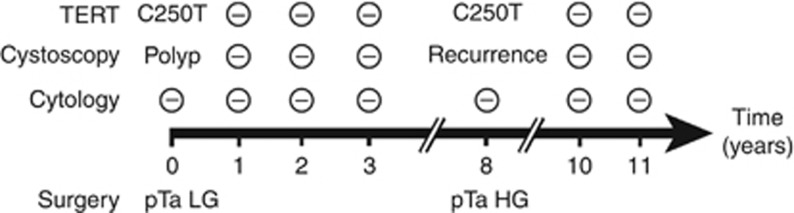
We also observed that presence of TERT mutations in urine was a dynamic marker of recurrence. A frequent scenario is exemplified by the case presented in Figure 3. This patient was initially treated by TUBR for a NMIBC classified as low-grade pTa. He presented a C250T mutation that became negative in the follow-up urines. Seven years later, TERT became positive again, associated with cystoscopic signs of recurrence. A tumour classified as high-grade pTa was resected. Since this second surgery (three years follow-up) urines have remained negative for TERT mutation and the patient has not experienced recurrence. Of note, urine cytology remained negative throughout progression of the disease, including initial tumour and recurrence.
Figure 3.
Example of somatic TERT promoter mutation status and patient outcome. HG, high-grade; LG, low-grade; −, negative result.
Discussion
Results of this large cohort study demonstrate that detecting TERT promoter mutations in urine is a non-invasive and sensitive way to detect UBC lesions, even of low-grade, where cytology is not sensitive enough. TERT may help to detect recurrence earlier and to better adapt follow-up frequency and treatment. We further showed that TERT remained positive after TUBR was significantly associated with residual CIS, which is difficult to detect by standard cystoscopy. This could explain why TERT remained a predictor of recurrence even in the negative cystoscopy group. Therefore TERT testing could help to better identify the group of patients for whom to consider hexaminolevulinate fluorescence cystoscopy.
However, around 20% of patients did not show positive TERT in urine after initial TUBR and remained negative during follow-up. Because TERT is known to be reactivated by mechanisms other than mutations of its promoter, it may be of interest to study TERT expression and activity in these non-mutated patients (Hurst et al, 2014). Importantly we also found that detection of TERT mutation remained highly specific in inflammatory or infectious urines where previously described urinary biomarkers are known to give false-positive results (Raitanen et al, 2001; Chou et al, 2015). This is an important issue since this type of urine is frequent in non-UBC patients. Furthermore, TERT could also help clinicians to distinguish recurrence from inflammatory scar in case of suspicious cystoscopy.
The study does have some limitations. The main one being the single-centre design that could introduce some positive bias in analysing the performance of this marker.
A prospective study, investigating combination of TERT with FGFR3 and OTX1 as diagnostic urinary markers during follow-up of patients with primary NMIBC, recently confirmed the interest of this panel in patients with negative cystoscopy (Beukers et al, 2017). It would be important to define the negative predictive value of TERT mutation as a single marker in case of suspicious cystoscopy (inflammatory lesions, scar post BCG therapy, etc.), and its positive predictive value in case of negative cystoscopy and cytology.
Conclusions
Detection of TERT promoter mutations in urine is a reliable non-invasive prognostic marker for recurrence in UBC, especially in NMIBC where cytology does have some limitations.
Acknowledgments
This study was supported by the French Ministry of Health (PHRC National 2006). We would to thank Florence Morin for her technical support, Dr Pierre Sujobert for his constructive comments on this manuscript and Philip Robinson (Hospices Civils de Lyon) for editorial help.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
All named authors declare that they have no competing interest. They have agreed to the submission and have participated in the study to a sufficient extent to be named as authors.
References
- Allory Y, Beukers W, Sagrera A, Flández M, Marqués M, Márquez M, van der Keur KA, Dyrskjot L, Lurkin I, Vermeij M, Carrato A, Lloreta J, Lorente JA, Carrillo-de Santa Pau E, Masius RG, Kogevinas M, Steyerberg EW, van Tilborg AAG, Abas C, Orntoft TF, Zuiverloon TCM, Malats N, Zwarthoff EC, Real FX (2014) Telomerase reverse transcriptase promoter mutations in bladder cancer: high frequency across stages, detection in urine, and lack of association with outcome. Eur Urol 65: 360–366. [DOI] [PubMed] [Google Scholar]
- Bassi P, De Marco V, De Lisa A, Mancini M, Pinto F, Bertoloni R, Longo F (2005) Non-invasive diagnostic tests for bladder cancer: a review of the literature. Urol Int 75: 193–200. [DOI] [PubMed] [Google Scholar]
- Beukers W, van der Keur KA, Kandimalla R, Vergouwe Y, Steyerberg EW, Boormans JL, Jensen JB, Lorente JA, Real FX, Segersten U, Orntoft TF, Malats N, Malmström P-U, Dyrskjot L, Zwarthoff EC (2017) FGFR3, TERT and OTX1 as a urinary biomarker combination for surveillance of patients with bladder cancer in a large prospective multicenter study. J Urol 197: 1410–1418. [DOI] [PubMed] [Google Scholar]
- Chou R, Gore JL, Buckley D, Fu R, Gustafson K, Griffin JC, Grusing S, Selph S (2015) Urinary biomarkers for diagnosis of bladder cancer: a systematic review and meta-analysis. Ann Intern Med 163: 922–931. [DOI] [PubMed] [Google Scholar]
- Collin-Chavagnac D, Marçais C, Billon S, Descotes F, Piaton E, Decaussin M, Rodriguez-Lafrasse C, Ruffion A (2010) Quantitative loss of heterozygosity analysis for urothelial carcinoma detection and prognosis. Urology 76: 515.e1–e7. [DOI] [PubMed] [Google Scholar]
- Descotes F, Dessen P, Bringuier PP, Decaussin M, Martin PM, Adams M, Villers A, Lechevallier E, Rebillard X, Rodriguez-Lafrasse C, Devonec M, Paparel P, Perrin P, Lazar V, Ruffion A (2014) Microarray gene expression profiling and analysis of bladder cancer supports the sub-classification of T1 tumours into T1a and T1b stages. BJU Int 113: 333–342. [DOI] [PubMed] [Google Scholar]
- Fontaniere B, Ranchere-Vince D, Landry JL, Colombel M, Chopin D, Gattegno B (2001) [Quality criteria in urinary cytology for tumor diagnosis]. Prog Urol 11: 867–875. [PubMed] [Google Scholar]
- Geavlete B, Jecu M, Multescu R, Geavlete P (2012) Narrow-band imaging cystoscopy in non-muscle-invasive bladder cancer: a prospective comparison to the standard approach. Ther Adv Urol 4: 211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman HB, Gomella L, Fradet Y, Morales A, Presti J, Ritenour C, Nseyo U, Droller MJ PC B302/01 Study Group (2007) A phase III, multicenter comparison of hexaminolevulinate fluorescence cystoscopy and white light cystoscopy for the detection of superficial papillary lesions in patients with bladder cancer. J Urol 178: 62–67. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA (2011) Hallmarks of cancer: the next generation. Cell 144: 646–674. [DOI] [PubMed] [Google Scholar]
- Horn S, Figl A, Rachakonda PS, Fischer C, Sucker A, Gast A, Kadel S, Moll I, Nagore E, Hemminki K, Schadendorf D, Kumar R (2013) TERT promoter mutations in familial and sporadic melanoma. Science 339: 959–961. [DOI] [PubMed] [Google Scholar]
- Huang FW, Hodis E, Xu MJ, Kryukov GV, Chin L, Garraway LA (2013) Highly recurrent TERT promoter mutations in human melanoma. Science 339: 957–959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst CD, Platt FM, Knowles MA (2014) Comprehensive mutation analysis of the TERT promoter in bladder cancer and detection of mutations in voided urine. Eur Urol 65: 367–369. [DOI] [PubMed] [Google Scholar]
- Killela PJ, Reitman ZJ, Jiao Y, Bettegowda C, Agrawal N, Diaz LA, Friedman AH, Friedman H, Gallia GL, Giovanella BC, Grollman AP, He T-C, He Y, Hruban RH, Jallo GI, Mandahl N, Meeker AK, Mertens F, Netto GJ, Rasheed BA, Riggins GJ, Rosenquist TA, Schiffman M, Shih I-M, Theodorescu D, Torbenson MS, Velculescu VE, Wang T-L, Wentzensen N, Wood LD, Zhang M, McLendon RE, Bigner DD, Kinzler KW, Vogelstein B, Papadopoulos N, Yan H (2013) TERT promoter mutations occur frequently in gliomas and a subset of tumors derived from cells with low rates of self-renewal. Proc Natl Acad Sci USA 110: 6021–6026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koss LG, Deitch D, Ramanathan R, Sherman AB (1985) Diagnostic value of cytology of voided urine. Acta Cytol 29: 810–816. [PubMed] [Google Scholar]
- Layfield LJ, Elsheikh TM, Fili A, Nayar R, Shidham V Papanicolaou Society of Cytopathology (2004) Review of the state of the art and recommendations of the Papanicolaou Society of Cytopathology for urinary cytology procedures and reporting: the Papanicolaou Society of Cytopathology Practice Guidelines Task Force. Diagn Cytopathol 30: 24–30. [DOI] [PubMed] [Google Scholar]
- Miremami J, Kyprianou N (2014) The promise of novel molecular markers in bladder cancer. Int J Mol Sci 15: 23897–23908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piaton E, Decaussin-Petrucci M, Mege-Lechevallier F, Advenier A-S, Devonec M, Ruffion A (2014) Diagnostic terminology for urinary cytology reports including the new subcategories ‘atypical urothelial cells of undetermined significance’ (AUC-US) and ‘cannot exclude high grade’ (AUC-H). Cytopathology 25: 27–38. [DOI] [PubMed] [Google Scholar]
- Piaton E, Hutin K, Faÿnel J, Ranchin M-C, Cottier M (2004) Cost efficiency analysis of modern cytocentrifugation methods versus liquid based (Cytyc Thinprep) processing of urinary samples. J Clin Pathol 57: 1208–1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raitanen M-P FinnBladder Group (2008) The role of BTA stat Test in follow-up of patients with bladder cancer: results from FinnBladder studies. World J Urol 26: 45–50. [DOI] [PubMed] [Google Scholar]
- Raitanen MP, Kaasinen E, Lukkarinen O, Kauppinen R, Viitanen J, Liukkonen T, Tammela TL Finnbladder Group (2001) Analysis of false-positive BTA STAT test results in patients followed up for bladder cancer. Urology 57: 680–684. [DOI] [PubMed] [Google Scholar]
- Rosser CJ, Chang M, Dai Y, Ross S, Mengual L, Alcaraz A, Goodison S (2014) Urinary protein biomarker panel for the detection of recurrent bladder cancer. Cancer Epidemiol Biomarkers Prev 23: 1340–1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauter G (2004) In: Pathology and Genetics of Tumours of the Urinary System and Male Genital Organs. Eble JN, Sauter G, Epstein JI, Sesterhenn IA (eds). IARC Press: Lyon, France.
- Schmidt JC, Cech TR (2015) Human telomerase: biogenesis, trafficking, recruitment, and activation. Genes Dev 29: 1095–1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward DG, Baxter L, Gordon NS, Ott S, Savage RS, Beggs AD, James JD, Lickiss J, Green S, Wallis Y, Wei W, James ND, Zeegers MP, Cheng KK, Mathews GM, Patel P, Griffiths M, Bryan RT (2016) Multiplex PCR and next generation sequencing for the non-invasive detection of bladder cancer. PloS One 11: e0149756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S, Huang P, Li C, Huang Y, Li X, Wang Y, Chen C, Lv Z, Tang A, Sun X, Lu J, Li W, Zhou J, Gui Y, Zhou F, Wang D, Cai Z (2014) Telomerase reverse transcriptase gene promoter mutations help discern the origin of urogenital tumors: a genomic and molecular study. Eur Urol 65: 274–277. [DOI] [PubMed] [Google Scholar]