Abstract
Background
Some reported that organizing pneumonia (OP) may occur after influenza A infections including swine-origin influenza A (H1N1). However, OP associated with influenza B infection has never been reported. We report the first case of secondary OP associated with viral pneumonia caused by influenza B.
Case presentation
A 23-year old woman was diagnosed as viral pneumonia caused by type B influenza. Despite of antiviral therapy, abnormal chest shadows were not improved. Bronchoscopy and transbronchial lung biopsy showed organizing pneumonia due to viral pneumonia caused by influenza B. Corticosteroid therapy was started at 30 mg daily (0.5 mg/kg), and the dose was reduced to 25, 20, 15 or 10 mg per day every month with symptomatic and radiological resolution. Even after corticosteroid therapy was discontinued, we did not confirm disease recurrence.
Conclusions
Physicians should be aware of the possibility for SOP and severe viral pneumonia even in case of type B as well as type A influenza infections.
Keywords: Influenza pneumonia, Type B influenza virus, Organizing pneumonia
Background
Organizing pneumonia (OP) is a pulmonary inflammatory condition that occurs in certain clinical settings [1, 2]. In the appropriate clinical-radiologic context, an OP diagnosis can be confirmed with bronchoalveolar lavage fluid (BALF) and transbronchial lung biopsy (TBLB). Some reported that OP may occur after influenza A infections including swine-origin influenza A (H1N1) [3–5]. However, OP associated with influenza B infection has never been reported. We report the first case of secondary OP associated with viral pneumonia caused by influenza B.
Case presentation
A 23-year old woman without relevant medical history visited our outpatient clinic complaining fever, sorethorat and cough lasting for 3 days. She was diagnosed as influenza B infection by nasophayngeal antigen test (Influenza B strain was not confirmed because reverse transcription-polymerase chain reaction was not performed.). She was not obese (body mass index 22.3 kg/m2) and had not received any influenza vaccination in the past. She had no smoking history. She had an allergy history for macrolides antibiotics. Although she had received an antiviral therapy of oseltamivir 150 mg per day, flu symptoms such as fever and cough persisted and she once again came to the clinic on day 4 after starting the therapy. Also, she had an acute respiratory failure (SpO2 93% on 5 L/min O2 mask) with elevation of inflammatory reactions (WBC 18,900 /μL, CRP 11.69 mg/dL). Chest radiography and chest computed tomography (CT) revealed peripheral subpleural opacities (Fig.1) and consolidations. Broad spectrum antimicrobial agent, meropenem, was empirically administered but additional antiviral therapy was not started. A rapid influenza diagnostic test showed that influenza A and B were negative and positive, respectively. Urinary streptococcal antigen and legionella antigen were both negative. No virulent pathogens were detected in the sputum. Therefore, she was diagnosed as having influenza B virus pneumonia and was referred to us. While her respiratory condition was improved, inflammatory reaction and abnormal chest radiographic shadows recurred. Transbronchial lung biopsy (TBLB) revealed lymphocytic alveolitis and organizing pneumonia (Fig 2). No pathogens were found in the BALF. She was diagnosed as secondary OP associated with influenza B pneumonia. Corticosteroid therapy was started and inflammatory reaction and chest radiography were gradually improved. The initial dose of corticosteroid was 30 mg (0.5 mg/kg) daily and tapered with improvement of inflammatory reaction and chest radiography. She was discharged on day 30. The dose was reduced to 25, 20, 15 and 10 mg per day every month with symptomatic and radiological resolution. After corticosteroid therapy was discontinued, disease recurrence was not observed.
Fig. 1.
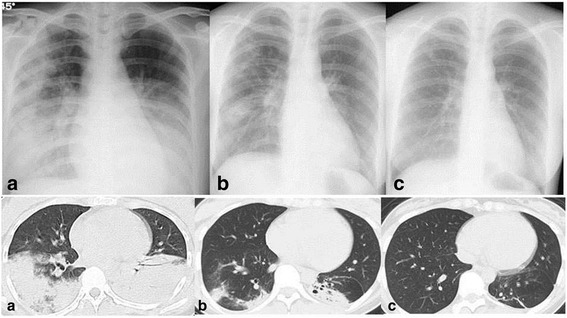
Chest X-ray showed bilateral infliltrates on admission (a upper). After starting PSL therapy on day 25, infiltrates were improved (b upper). Abnormal shadows on Chest X-ray disappeared 6 months after starting corticosteroid therapy (c upper). Chest CT showed consolidations on both lungs on admission (a lower) and the shadows were improved on day 25 after starting corticosteroid therapy (b lower). Six months after starting corticosteroid therapy, the consolidations disappeared (c lower)
Fig. 2.
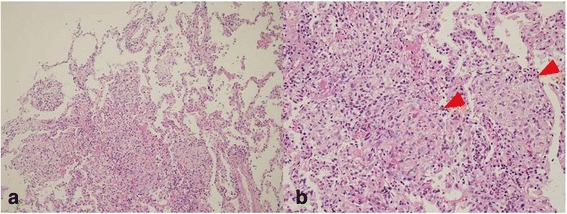
TBLB specimen from the right middle lobe showed intraalveolar granulation tissue with myofibroblasts consistent with organizing pneumonia (Hematoxin-Eosin (HE) × 100) (a). Masson body (red arrow) was seen on the TBLB specimen (HE ×400) (b)
Discussion
In general, influenza B complicating bacterial infection cause a mild disease, but severe pneumonia cases due to influenza B and Streptococcal co-infection have been reported [6–8].
Several mechanisms of such severe co-infection were reported in animal models. The influenza virus could damage respiratory tract epithelium and facilitate bacterial infection. The viruses also alter and/or impair ciliary function of the epithelium. It can change host immunity and inflammatory responses by impairing bacterial clearance or via the inflammatory cascade [9–11]. To the best of our knowledge, this is the first case of SOP due to influenza B virus pneumonia and the second case of severe respiratory failure due to influenza B virus infection not co-infection, following the case reported by Kato [12]. Gutierrez-Pizarraya, et al. reported that the proportion of patients with pneumonia and the rate of admission to the intensive care unit did not differ between cases of influenza A (H1N1) pdm09 and influenza B infection. Notably, the mortality rates were almost similar between patients with influenza A (16.3%) and influenza B (10%) infection [13]. Paddock, et al. documented the pathological findings of the lung autopsy cases of patients who died of influenza B virus infection and demonstrated that diffuse alveolar damage pattern were found in 17.8% of the cases [14]. These suggest that influenza B virus infection could contribute to severe respiratory failure, resulting in poor outcome that is similar to influenza A virus infection.
The standard treatment of influenza virus pneumonia is not established yet. Tanaka reported that the median survival time of post-influenza pneumococcal pneumonia in mice was longer by double dose of peramivir than by single dose of peramivir or single dose of oseltamivir group. Also, the production of inflammatory cytokines/chemokines was also significantly suppressed by double dose of peramivir compared with other two groups. These suggests that double dose of peramivir could contribute to reducing a cytokine storm by influenza virus, resulting in favorable outcomes for influenza virus pneumonia and post-influenza pneumonia patients [15]. In contrast, some demonstrated that double dose of neuraminidase inhibitors are not useful in the treatment for flu compared with the standard dose of oseltamivir [16]. In the study, they concluded that the duration of antiviral therapy was associated with the outcome. Other studies also indicate that early antiviral therapy in patients with severe influenza is associated with both clinical benefits and more rapid viral clearance from the upper respiratory tract samples [17, 18].
Some previously reported cases of SOP caused by influenza virus (Table 1) [3–5, 7, 8]. It is well known that H1N1 and avian flu could result in a cytokine storm. Thus, it is reasonable that SOP could occur in patients infected with these viruses. In terms of radiological findings of influenza A pneumonia, peripheral ground-grass opacities and consolidations has been commonly reported as in our case. Some documented a peripheral distribution of lung opacities in patients with influenza A infection mimicking OP [19–21]. SOP could be seen 2 to 3 weeks after the onset of initial influenza symptoms, whereas influenza viral pneumonia could generally occur in day 4 to 5 after the onset of illness [22, 23]. SOP should be considered if influenza patients have fever, and a dry cough after initial influenza like illness improved, and TBLB should be performed since proper OP treatment requires corticosteroid therapy. All SOP cases following severe influenza virus pneumonia were cured by corticosteroid therapy, except for the case of acute fibrosis organizing pneumonia (AFOP) associated with influenza A/H1N1 pneumonia after lung transplantation [8] as shown in Table 1. These favorable outcomes of SOP could correlate with the clinicopathological features of OP. Such features of OP might be different from that of a fatal interstitial lung disease such as diffuse alveolar damage. Ebina and colleagues reported the disappearance of subpleural and interlobular lymphatics in idiopathic pulmonary fibrosis (IPF) lungs along with a poor lymphogenesis and a significant decrease of alveolar lymphangiogenesis in comparison with cellular non-specific interstitial pneumonia and OP. These changes may exert a detrimental effect on IPF lungs by impairing alveolar clearance [24].
Table 1.
Cases of secondary organizing pneumonia due to influenza virus infection
| Author (Year) | Sex | Age | Type of influenza | Initial treatment | Outcome |
|---|---|---|---|---|---|
| Cornejo R [3] (2010) |
Female | 52 | A | Steroidpulse therapy | Cure |
| Cornejo R [3] (2010) |
Male | 36 | A | Steroidpulse therapy | Cure |
| Gómez-Gómez A [4] (2011) |
Female | 44 | A | Corticosteoroid 0.5 mg/kg |
Cure |
| Gómez-Gómez A [4] (2011) |
Male | 60 | A | Corticosteoroid 0.5 mg/kg |
Cure |
| Torrego A [5] (2010) |
Female | 55 | A | Corticosteoroid 0.75 mg/kg |
Cure |
| Kwok WC [7] (2016) |
Female | 45 | B | Corticosteroid 30 mg daily |
Curea |
| Otto C [8] (2013) |
Female | 66 | B | High dose corticosteroid | Deathb |
| Asai N (2016) |
Female | 22 | B | Corticosteroid 0.5 mg/kg |
Cure |
All cases were diagnosed as organizing pneumonia by transbronchial lung biopsy
a This is a case of secondary organizing pneumonia associated with influenza B and Streptococcal co-infection
b This is a case of acute fibrinous and organizing pneumonia associated with influenza A/H1N1 pneumonia after lung transplantation
AFOP is a distinct reaction pattern in acute respiratory failure with high mortality rate of up to 90%. Current literature reported that cause of AFOP are idiopathic, or collagen vascular diseases, or bacterial infection, drug exposure (e.g. amiodarone, abacavir or statins) [8, 25]. Physicians should know that influenza infection could induce AFOP, resulting in a poor outcome.
Conclusions
Physicians should be aware of the possibility of severe respiratory failure due to influenza B virus infection and secondary OP following influenza B infection, even though once influenza infection was cured.
Acknowledgments
We are grateful for the diligent and thorough critical reading of our manuscript by Dr. Yoshihiro Ohkuni, Chief Physician, Taiyo and Mr. John Wocher, Executive Vice President and Director, International Affairs/International Patient Services, Kameda Medical Center (Japan).
Funding
None declared.
Availability of data and materials
The datasets supporting the conclusions of this article are included within the article.
Abbreviations
- BALF
Bronchoalveolar lavage fluid
- CT
Computed tomography
- IPF
Idiopathic pulmonary fibrosis
- OP
Organizing pneumonia
- TBLB
Transbronchial lung biopsy
Authors’ contributions
NA, NN, YK, YY, HM carried out the clinical follow up. NA draft the manuscript. DS and HS performed microbial testing and NA, NN, YK, YY, HM performed laboratory analysis. HK and MH supervised the antibiotic and antiviral therapy. All authors read and approved the final manuscript.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Written informed consent was obtained from the patient for publication of this report.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nobuhiro Asai, Email: nobuhiro0204@gmail.com.
Toyoharu Yokoi, Email: tyokoi@ekisaikai.or.jp.
Naoya Nishiyama, Email: n.naoyaso@gmail.com.
Yusuke Koizumi, Email: ykoizumi@aichi-med-u.ac.jp.
Daisuke Sakanashi, Email: saka74d@aichi-med-u.ac.jp.
Hideo Kato, Email: katou.hideo.233@mail.aichi-med-u.ac.jp.
Mao Hagihara, Email: hagimao@aichi-med-u.ac.jp.
Hiroyuki Suematsu, Email: hsuemat@aichi-med-u.ac.jp.
Yuka Yamagishi, Email: y.yamagishi@mac.com.
Hiroshige Mikamo, Email: mikamo@aichi-med-u.ac.jp.
References
- 1.Cordier JF. Organizing pneumonia. Thorax. 2000;55:318–328. doi: 10.1136/thorax.55.4.318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vasu TS, Cavallazzi R, Hirani A, Sharma D, Weibel SB, Kane GC. Clinical and radiologic distinctions between secondary bronchiolitis obliterans organizing pneumonia and cryptogenic organizing pneumonia. Respir Care. 2009;54:1028–1032. [PubMed] [Google Scholar]
- 3.Cornejo R, Llanos O, Fernández C, Carlos Díaz J, Cardemil G, Salguero J, et al. Organizing pneumonia in patients with severe respiratory failure due to novel a (H1N1) influenza. BMJ Case Rep. 2010;21:2010. doi: 10.1136/bcr.02.2010.2708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gómez-Gómez A, Martínez-Martínez R, Gotway MB. Organizing pneumonia associated with swine-origin influenza a H1N1 2009 viral infection. AJR Am J Roentgenol. 2011;196:W103–W104. doi: 10.2214/AJR.10.4689. [DOI] [PubMed] [Google Scholar]
- 5.Torrego A, Pajares V, Mola A, Lerma E, Franquet T. Influenza A (H1N1) organising pneumonia. BMJ Case Rep. 2010;27:2010. doi: 10.1136/bcr.12.2009.2531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lam KW, Sin KC, Au SY, Yung SK. Uncommon cause of severe pneumonia: co-infection of influenza B and streptococcus. Hong Kong Med J. 2013;19:545–548. doi: 10.12809/hkmj133835. [DOI] [PubMed] [Google Scholar]
- 7.Kwok WC, Lam SH, Wong MP, Ip MS, Lam DC. Influenza B/streptococcal co-infection complicated by organizing pneumonia. Respirol Case Rep. 2016;4(5):e00170. doi: 10.1002/rcr2.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Otto C, Huzly D, Kemna L, Hüttel A, Benk C, Rieg S, et al. Acute fibrinous and organizing pneumonia associated with influenza a/H1N1 pneumonia after lung transplantation. BMC Pulm Med. 2013;13:30. doi: 10.1186/1471-2466-13-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park K, Bakaletz LO, Coticchia JM, Lim DJ. Effect of influenza a virus on ciliary activity and dye transport function in the chinchilla eustachian tube. Ann Otol Rhinol Laryngol. 1993;102:551–558. doi: 10.1177/000348949310200711. [DOI] [PubMed] [Google Scholar]
- 10.Echchannaoui H, Frei K, Schnell C, Leib SL, Zimmerli W, Landmann R. Toll-like receptor 2-deficient mice are highly susceptible to Streptococcus Pneumoniae meningitis because of reduced bacterial clearing and enhanced inflammation. J Infect Dis. 2002;186:798–806. doi: 10.1086/342845. [DOI] [PubMed] [Google Scholar]
- 11.Dallaire F, Ouellet N, Bergeron Y, Turmel V, Gauthier MC, Simard M, et al. Microbiological and inflammatory factors associated with the development of pneumococcal pneumonia. J Infect Dis. 2001;184:292–300. doi: 10.1086/322021. [DOI] [PubMed] [Google Scholar]
- 12.Kato S, Fujisawa T, Enomoto N, Inui N, Nakamura Y, Suda T. Severe respiratory failure associated with influenza B virus infection. Respirology case report. 2015;3:61–63. doi: 10.1002/rcr2.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gutiérrez-Pizarraya A, Pérez-Romero P, Alvarez R, Aydillo TA, Osorio-Gómez G, Milara-Ibáñez C, et al. Unexpected severity of cases of influenza B infection in patients that required hospitalization during the first postpandemic wave. J Inf Secur. 2012;65:423–430. doi: 10.1016/j.jinf.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Paddock CD, Liu L, Denison AM, Bartlett JH, Holman RC, Deleon-Carnes M, et al. Myocardial injury and bacterial pneumonia contribute to the pathogenesis of fatal influenza B virus infection. J Infect Dis. 2012;205:895–905. doi: 10.1093/infdis/jir861. [DOI] [PubMed] [Google Scholar]
- 15.Tanaka A, Nakamura S, Seki M, Iwanaga N, Kajihara T, Kitano M, et al. The effect of intravenous peramivir, compared with oral oseltamivir, on the outcome of post-influenza pneumococcal pneumonia in mice. Antivir Ther. 2015;20:11–19. doi: 10.3851/IMP2744. [DOI] [PubMed] [Google Scholar]
- 16.South East Asia Infectious Disease Clinical Research Network Effect of double dose oseltamivir on clinical and virological outcomes in children and adults admitted to hospital with severe influenza: double blind randomised controlled trial. BMJ. 2013;30:346. doi: 10.1136/bmj.f3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Neuraminidase Inhibitor Flu Treatment Investigator Group Efficacy and safety of oseltamivir in treatment of acute influenza: a randomised controlled trial. Lancet. 2000;355:1845–1850. doi: 10.1016/S0140-6736(00)02288-1. [DOI] [PubMed] [Google Scholar]
- 18.McGeer A, Green KA, Plevneshi A, Siddiqi N, Raboud J, Low DE, et al. Antiviral therapy and outcomes of influenza requiring hospitalization in Ontario, Canada. Clin Infect Dis. 2007;45:1568–1575. doi: 10.1086/523584. [DOI] [PubMed] [Google Scholar]
- 19.Valente T, Lassandro F, Marino M, Squillante F, Aliperta M, Muto R. H1N1 pneumonia: our experience in 50 patients with a severe clinical course of novel swine-origin influenza a (H1N1) virus (S-OIV) Radiol Med. 2012;117:165–184. doi: 10.1007/s11547-011-0734-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ajlan AM, Quiney B, Nicolaou S, Müller NL. Swine-origin influenza a (H1N1) viral infection: radiographic and CT findings. AJR Am J Roentgenol. 2009;193:1494–1499. doi: 10.2214/AJR.09.3625. [DOI] [PubMed] [Google Scholar]
- 21.Marchiori E, Zanetti G, Hochhegger B, Rodrigues RS, Fontes CA, Nobre LF, et al. High-resolution computed tomography findings from adult patients with influenza a (H1N1) virus-associated pneumonia. Eur J Radiol. 2010;74:93–98. doi: 10.1016/j.ejrad.2009.11.005. [DOI] [PubMed] [Google Scholar]
- 22.Influenza Investigators ANZIC, Webb SA, Pettilä V, Seppelt I, Bellomo R, Bailey M, et al. Critical care services and 2009 H1N1 influenza in Australia and New Zealand. N Engl J Med. 2009;361:1925–1934. doi: 10.1056/NEJMoa0908481. [DOI] [PubMed] [Google Scholar]
- 23.Kumar A, Zarychanski R, Pinto R, Cook DJ, Marshall J, Lacroix J, et al. Critically ill patients with 2009 influenza a(H1N1) infection in Canada. JAMA. 2009;302:1872–1879. doi: 10.1001/jama.2009.1496. [DOI] [PubMed] [Google Scholar]
- 24.Ebina M, Shibata N, Ohta H, Hisata S, Tamada T, Ono M, et al. The disappearance of subpleural and interlobular lymphatics in idiopathic pulmonary fibrosis. Lymphat Res Biol. 2010;8:199–207. doi: 10.1089/lrb.2010.0008. [DOI] [PubMed] [Google Scholar]
- 25.Beasley MB, Franks TJ, Galvin JR, Gochuico B, Travis WD. Acute fibrinous and organizing pneumonia: a histological pattern of lung injury and possible variant of diffuse alveolar damage. Arch Pathol Lab Med. 2002;126:1064–1070. doi: 10.5858/2002-126-1064-AFAOP. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting the conclusions of this article are included within the article.


