Abstract
The recent re-emergence of Zika virus (ZIKV)1, a member of the Flaviviridae family, has become a global emergency. Currently, there are no effective methods of preventing or treating ZIKV infection, which causes severe neuroimmunopathology and is particularly harmful to the developing fetuses of infected pregnant women. However, the pathology induced by ZIKV is unique among flaviviruses, and knowledge of the biology of other family members cannot easily be extrapolated to ZIKV. Thus, structure-function studies of ZIKV proteins are urgently needed to facilitate the development of effective preventative and therapeutic agents. Like other flaviviruses, ZIKV expresses an NS2B-NS3 protease, which consists of the NS2B cofactor and the NS3 protease domain and is essential for cleavage of the ZIKV polyprotein precursor and generation of fully functional viral proteins. Here, we report the enzymatic characterization of ZIKV protease, and we identify structural scaffolds for allosteric small-molecule inhibitors of this protease. Molecular modeling of the protease-inhibitor complexes suggests that these compounds bind to the druggable cavity in the NS2B-NS3 protease interface and affect productive interactions of the protease domain with its cofactor. The most potent compound demonstrated efficient inhibition of ZIKV propagation in vitro in human fetal neural progenitor cells and in vivo in SJL mice. The inhibitory scaffolds could be further developed into valuable research reagents and, ultimately, provide a roadmap for the selection of efficient inhibitors of ZIKV infection.
Keywords: Zika virus, Protease, NS3, NS2B, Inhibitors, Flavivirus
1. Introduction
Zika virus (ZIKV) has recently re-merged as a global public health threat (Campos et al., 2015). ZIKV is one of hundreds of known flaviviruses, which include Dengue (DENV), West Nile (WNV), Japanese encephalitis, and tick-borne encephalitis viruses. Flaviviruses are transmitted to humans by mosquitoes and ticks, and are responsible for millions of infections annually. The risk of ZIKV infection has historically been restricted to tropical/subtropical regions, but the virus has now spread into the Southern US. Recent studies have demonstrated that ZIKV infects and is toxic to human neural precursors; however, the molecular mechanism is not precisely known (Azevedo et al., 2016; Cugola et al., 2016; Li et al., 2016; Mlakar et al., 2016).
ZIKV infection is usually accompanied by mild self-limiting symptoms which normally wane in 1–2 weeks. More alarming is the rapidly growing evidence linking ZIKV infection in pregnant women with severe microcephaly in the fetus. In addition, ZIKV infection of adults can cause neuronal pathology such as Guillain-Barré syndrome, a rare rapid-onset acute polyneuropathy caused by an autoimmune response to the peripheral nervous system that may result in life-threatening respiratory failure.
Currently, there are no effective treatments for ZIKV or any other flaviviral infections (Barrows et al., 2016). The pre-existing knowledge of other flaviviral proteins cannot easily be extrapolated because ZIKV causes uniquely severe neurological defects in the fetus. Clearly, a more detailed understanding of ZIKV at the biochemical levels is urgently required.
ZIKV, like other flaviviruses, has a single-stranded, positive-sense ~11 kb RNA genome that encodes a single polyprotein precursor of ~3400 amino acids. Following virus entry into the host cell, the viral RNA serves as an mRNA template for direct translation of the genome into a polyprotein precursor. The precursor comprises three structural (C, prM and E) and seven non-structural (NS1, NS2A, NS2B, NS3, NS4A, NS4B and NS5) proteins arranged in the order C-prM-E-NS1-NS2A-NS2B-NS3-NS4ANS4B-NS5. During translation, the polyprotein precursor is inserted into the endoplasmic reticulum membrane and is processed by a combination of the host and viral proteases to generate the active viral proteins (Fig. 1A). The structural C, prM, and E proteins assemble to build the mature viral particles, while the non-structural proteins are responsible for viral replication and propagation (Sironi et al., 2016).
Fig. 1. Constructs and purification of soluble ZIKV NS2B-NS3pro.
(A) ZIKV polyprotein processing by NS2B-NS3pro and host cell proteases. NS2B-NS3pro cleavage sites are shown by white arrows. Host cell proteases and furin cleavage sites are shown by black arrows. (B) Coomassie staining of purified ZIKV wild-type (WT) and S135A NS2B-NS3pro. M, molecular weight markers. (C) Sequence alignment of the flaviviral NS3 protease domain. Dark blue, regular blue, and light blue highlight the residues identical in 5, 4, and 3 flaviviruses, respectively. An arrow indicates the S135A mutant residue (this mutant residue is numbered according to the ZIKV protease sequence rather than the consensus numbering). (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
The N-terminal region of ZIKV NS3 protein encodes a serine protease (NS3pro; ~180 amino acids) while the C-terminal region encodes a 430–440 residue helicase. The latter is essential for RNA unwinding, capping, and replication. NS2B, which precedes NS3 in the precursor polypeptide, anchors NS2B-NS3pro to the endoplasmic reticulum membrane, functions as a cofactor and promotes the productive folding and activity of NS3pro. In the absence of NS2B, the NS3pro domain is catalytically inert (Aleshin et al., 2007; Erbel et al., 2006).
The 48-amino acid soluble central portion of NS2B mimics the cofactor activity of the entire NS2B sequence. Structural studies have determined that NS2B wraps around NS3pro, completing, in a well-defined fashion, the structure of the active site. These unique cofactor-protease domain interactions are common in flaviviruses. NS2B-NS3pro is responsible for the cleavage of the capsid protein C and for cleavage at the NS2A/NS2B, NS2B/NS3, NS3/NS4A, and NS4B/NS5 boundaries (Table 1) (Luo et al., 2015). Inactivating mutations of the NS3pro cleavage sites in the polyprotein abolish replication of the virus.
Table 1. NS2B-NS3pro cleavage sites in the flaviviral polyprotein.
The cleavage sites in the capsid protein C and at the NS2A/NS2B, NS2B/NS3, NS3/NS4A, and NS4B/NS5 boundaries are shown. ZIKV, GenBank AMB37295; DENV1-4, GenBank P33478, P29990, P27915 and P09866, respectively; WNV, GenBank P06935; JEV, GenBank P19110; YFV, GenBank P19901. Residues homologous to ZIKV are in bold.
| Virus | Capsid C | NS2A/NS2B | NS2B-NS3 | NS3/NS4A | NS4B/NS5 |
|---|---|---|---|---|---|
| ZIKV | V125TRR↓GSAY132 | S1369GKR↓SWPP1376 | T1499GKR↓SGAL1506 | A2116GKR↓GAAF2123 | V2517KRR↓GGGT2524 |
| DENV1 | R97RKR↓SVTM104 | W1341GRK↓SWPL1348 | K1471KQR↓SGVL1478 | A2090GRR↓SVSG2097 | G2489GRR↓GTGA2496 |
| DENV2 | R97RRR↓TAGV104 | S1342KKR↓SWPL1349 | K1472KQR↓AGVL1479 | A2090GRK↓SLTL2097 | N2488TRR↓GTGN2495 |
| DENV3 | K97RKK↓TSLC104 | L1340KRR↓SWPL1347 | Q1470TQR↓SGVL1477 | A2089GRK↓SIAL2096 | T2487GKR↓GTGS2494 |
| DENV4 | G96RKR↓STIT103 | A1341SRR↓SWPL1348 | K1471TQR↓SGAL1478 | S2089GRK↓SITL2096 | T2484PRR↓GTGT2491 |
| WNV | Q101KKR↓GGTA108 | N1367RKR↓GWPA1374 | Y1498TKR↓GGVL1505 | S2117GKR↓SQIG2124 | G2522LKR↓GGAK2529 |
| JEV | Q102NKR↓GGNE109 | N1370KKR↓GWPA1377 | T1501TKR↓GGVF1508 | A2120GKR↓SAVS2127 | S2564LKR↓GRPG2571 |
| YFV | R98KRR↓SHDV105 | F1351GRR↓SIPV1358 | G1481ARR↓SGDV1488 | E2104GRR↓GAAE2111 | T2503GRR↓GSAN2510 |
Taken together, these observations suggest that NS2B-NS3pro is a promising anti-flaviviral drug target. However, the active site of NS3pro is conserved in numerous human serine proteinases. and lacks key structural features that could be exploited to ensure the specificity and potency of flaviviral inhibitors. Therefore, we considered that interference with the productive conformation of NS2B in the NS2B-NS3pro complex might be a superior drug discovery strategy compared with targeting of the protease active site.
Here, we briefly report the enzymatic characterization of the ZIKV NS2B-NS3 protease and the structure-assisted identification of allosteric small-molecule antagonists of its activity. Several compounds were identified that display promising inhibitory properties in vitro and in models of ZIKV infection in vivo. These hit compounds could be further developed to provide valuable research reagents and, ultimately, to generate anti-ZIKV antagonists.
2. Materials and methods
2.1. Reagents
Standard laboratory reagents were purchased from Sigma-Aldrich (St. Louis, MO), unless indicated. Horseradish peroxidase (HRP)-conjugated donkey anti-mouse IgGs and TMB/M substrate were from Jackson ImmunoResearch Laboratories and SurModics IVD, respectively. Myelin basic protein (MBP) was obtained from Meridian Life Science. Furin and both DENV and WNV NS2B-NS3pro were purified as described previously (Gawlik et al., 2009; Shiryaev et al., 2006). Small-molecule compounds were provided by NCI/DTP Open Chemical Repository (https://dtp.cancer.gov), dissolved in 100% DMSO, and stored at −20 °C until use. Purified recombinant ZIKV NS2B-NS3 proteases (wild-type [WT] and S135A inactive mutant) were kindly provided by Dr. Robert C. Liddington (SBP Medical Discovery Institute). In order to obtain E. coli-derived active, autoproteolysis-resistant NS2B-NS3pro, the sequence of the most appropriate construct was design in a way that was highly similar to our previously reported WNV NS2B-NS3pro constructs (Shiryaev et al., 2007) and is as follows: MSVDMYIERAGDITWEKDAEVTGNSPRLDVALDESGDFSLVEDDGPMAGGGGSGGGGSGALWDVPAPKEVKKGETTDGVYRVMTRRLLGSTQVGVGVMQEGVFHTMW(H)VTKGSALRSGEGRLDPYWGDVKQDLVSYCGPWKLDAAWDGHSEVQLLAVPPGERARNIQTLPGIFKTKDGDIGAVALDYPAGTSGSPILDKCGRVIGLYGNGVVIKNGSYVSAITQGRREEETPVECFEPSMLAHHHHHHHH, where the sequence of the flexible linker is in black (underlined), the NS2B co-factor is in blue, NS3pro is in green, and the active site His53 is in parenthesis. To facilitate purification, the construct was C-terminally His-tagged.
2.2. Fluorescent peptide cleavage assay
Peptide cleavage assays of purified ZIKV, WNV, and DENV NS2B-NS3pro and furin were performed in 0.2 ml 20 mM Tris-HCl buffer, pH 8.0, containing 20% glycerol and 0.005% Brij 35. The final concentrations of Pyr-Arg-Thr-Lys-Arg-7-amino-4-methylcoumarin (Pyr-RTKR-AMC) peptide and the enzymes were 20 μM and 20 nM, respectively. The reaction velocity was monitored continuously at λex = 360 nm and λem = 465 nm in a Tecan fluorescence spectrophotometer (Tecan Group Ltd.). All assays were performed in triplicate in 96-well plates.
2.3. Determination of IC50 values of the inhibitory compounds
ZIKV, WNV, and DENV NS2B-NS3pro constructs (20 nM) were pre-incubated for 30 min at 20 °C with individual compounds at the indicated concentrations in 0.1 ml 20 mM Tris-HCl buffer, pH 8.0, containing 20% glycerol and 0.005% Brij 35. Pyr-RTKR-AMC substrate (20 μM) was added in 0.1 ml of the same buffer, and the remainder of the assay procedure was as described above. IC50 values were calculated as the compound concentrations giving half-maximal inhibition of NS2B-NS3pro activity. GraphPad Prism was used as the curve fitting software.
2.4. Cell toxicity assays
Assays were performed in triplicate in 96-well flat-bottomed, white-walled plates. Fetal human NPCs (1 × 104 cells/well) were pre-incubated for 24 h at 37 °C in a 5% CO2 incubator, and compounds were then added for an additional 24 h. Viable cells were counted using an ATP-Lite assay kit (PerkinElmer). TC50 values (compound concentrations giving half-maximal toxicity) were calculated using GraphPad Prism software.
2.5. Cloning, expression, and purification of human Sox2 protein
The DNA sequence of human Sox2 was amplified by PCR using the plasmid pSIN18-hPJK-hSox2-IRES-GFP as a template and 5′-CACCATGTACAACATGATGGAGACGGAG-3′ as the forward primer and 5′-TCAATGGTGGTGATGGTGGTGTCCCTTGTCATCGTCATCTTTGTAGTCTCCTCGCGGTACTAGCATGTGTGAGAGGGGCAGTGTGCC-3′ as the reverse primer (the inserted C-terminal 6 × His and Flag tags and thrombin cleavage site are underlined). The construct was then cloned into the pET101 expression vector (Invitrogen), and the recombinant pET101 plasmid was used to transform competent E. coli BL21 (DE3) Codon Plus cells (Stratagene). Transformed cells were grown at 37 °C in LB broth containing ampicillin (0.1 mg/ml). Cultures were induced with 1 mM isopropyl β-D-thiogalactoside for 16 h at 18 °C. Cells were collected by centrifugation at 5000 g at 4 °C, resuspended in Tris-HCl buffer, pH 8.0, containing 1 M NaCl, and disrupted by sonication. The sonicate was then centrifuged at 40,000 g for 30 min. The construct was purified from the supernatant fraction using a Co2+-chelating Sepharose Fast Flow column (GE Healthcare) equilibrated with 20 mM Tris-HCl buffer, pH 8.0, containing 1 M NaCl. After washing the column with the same buffer containing 35 mM imidazole, bound material was eluted using a 35–500 mM gradient of imidazole. Sox2-containing fractions were combined and further purified by gel filtration using an S200 26/60 column (GE Healthcare) equilibrated with 20 mM Tris-HCl buffer, pH 8.0, containing 150 mM NaCl and 1 mM tris(2-carboxyethyl)phosphine. Purified Sox2 was concentrated to ~1 mg/ml using a 10 kDa-cutoff concentrator (Millipore), flash frozen in small aliquots, and stored at −80 °C. Protein purity was assessed by SDS-PAGE (12% NuPAGE-MOPS, Invitrogen) followed by Coomassie staining or Western blotting with anti-Sox2 antibody (MAB2018, R&D Systems).
2.6. Infection of cultured cells with ZIKV
Human fetal neural progenitor cells (hfNPCs; Thermo Fisher Scientific) were cultured in Matrigel-coated plates in 1:1 DMEM/F12:Neurobasal medium supplemented with B27 (1:1000; Thermo Fisher Scientific), insulin (5 μg/ml), β-fibroblast growth factor (FGF; 25 ng/ml), and epidermal growth factor (EGF; 20 ng/ml). The medium was changed every other day and cells were allowed to reach ~90% confluence prior to passaging. The latest passage used for experiments was P8. For infection, cells were harvested using Accutase (Innovative Cell Technologies), counted, plated in Greiner 96-well imaging plates (1 × 104 cells/well), and allowed to adhere for 16–18 h. Adherent cells were infected with ZIKV (Asian strain, FSS13025, 2010 Cambodian isolate) at a multiplicity of infection (MOI) of 0.1 (103 virus particles/well) and incubated for 72–96 h at 37 °C. Cells were then fixed with 4% paraformaldehyde and analyzed. When present, the inhibitors were added to the cells at the same time as the virus. All assays were performed in triplicate.
2.7. Immunofluorescence staining and EdU labeling
To visualize proliferating cells, cells were labeled with EdU using a Click-iT Plus kit (Invitrogen). EdU was diluted in culture medium and added to the cells for 2 h at 37 °C. Cells were then fixed using 4% paraformaldehyde (10 min at room temperature), blocked with 10% donkey serum in 0.25% Triton X-100 (20 min at room temperature), incubated for 16–18 h at 4 °C with an antibody to mouse flavivirus group antigen (Millipore, MAB10216, clone D1-4G2-4-15) diluted 1:500 in 10% donkey serum/0.25% Triton X-100, and then incubated with an Alexa-conjugated donkey anti-mouse IgG (1:500 dilution). Apoptotic cells were identified by staining for activated Caspase 3 (AC3). Cells were incubated for 16–18 h at 4 °C with a polyclonal anti-AC3 antibody (Promega) diluted 1:250 in 10% donkey serum/0.25% Triton X-100 and then with Alexa-conjugated donkey antirabbit IgG (1:500 dilution). Nuclei were stained for 15 min with Hoechst dye (Thermo Fisher Scientific).
2.8. Image acquisition and analysis
Eight images/well were acquired automatically using an IC200-KIC microscope with a × 20 objective (Vala Sciences). Images were analyzed using Acapella 2.6 software (Perkin Elmer). The nuclei and cell soma were segmented using the Hoechst channel. EdU and flavivirus group antigen staining intensities were quantified in the nuclei and soma, respectively. Individual cells were considered positive based on empirically determined thresholds. The percentage of cells positive for each stain was calculated per well, with at least three wells per condition. Statistical differences were analyzed using Student’s t-test.
2.9. Cleavage of protein targets
Human MBP (4 μg, 10 μM) was co-incubated for 60 min at 37 °C with purified WNV or ZIKV NS2B-NS3pro (0.1, 0.01, and 0.001 μM; enzyme:substrate ratio of 1:100,1:1,000, and 1:10,000, respectively) in 20 μl of 20 mM Tris-HCl buffer, pH 8.0, containing 20% glycerol. Reactions were stopped by addition of 5 × SDS sample buffer and the digests were analyzed by SDS-PAGE using a 4–20% gel.
Purified human Sox2 (2 μg, 3 μM) was co-incubated for 60 min at 37 °C with ZKV NS2B-NS3pro (3.0, 0.3, 0.03 and 0.003 μM; enzyme:substrate ratio of 1:1, 1:10, 1:100 and 1:1,000, respectively) in 20 μl of 50 mM Tris-HCl buffer, pH 8.0, containing 20% glycerol. Reactions were stopped by addition of 5 × SDS sample buffer, and the digests were analyzed by SDS-PAGE followed by western blotting using an anti-Sox2 antibody.
Human fNPCs cells (1 × 105) were seeded for 24 h in Matrigel-coated wells of a 6-well plate in 1:1 DMEM/F12:Neurobasal medium supplemented with B27 (1:1000), insulin (5 μg/ml), β-FGF (25 ng/ml) and EGF (20 ng/ml). Attached cells were infected with ZIKV (Asian strain, FSS13025, 2010 Cambodian isolate) at a MOI of 1.0 (1 × 105 virus particles/well) and then incubated for an additional 48 h at 37 °C. Cells were washed with PBS and lysed in TBS containing 50 mM N-octyl-β-D-glucopyranoside, 1 mM phenylmethylsulphonyl fluoride, 10 mM EDTA, and protease inhibitor cocktail set III. Insoluble material was removed by centrifugation (14,000 g for 20 min). Aliquots of supernatant (20 μg total protein) were separated by SDS-PAGE using a 12% NuPAGE-MOPS gel (Life Technologies) and analyzed by western blotting with anti-Sox2 antibody (MAB2018, R&D Systems) followed by the secondary HRP-conjugated antibody (Jackson ImmunoResearch). Blots were developed with a SuperSignal West Dura Extended Duration Substrate kit (Thermo Fisher Scientific).
2.10. In vivo infection
Three-month-old female SJL mice (Jackson Laboratories) were infected by retro-orbital injection using 2 × 103 PFU of ZIKV (Panama strain, PA259459). On day 1 post-infection, mice were provided with drinking water containing NSC157058 (30 mg/kg) for 5 days. Animals were sacrificed on day 6 and tissue and blood samples were collected. All animal procedures were performed in agreement with the PHS Policy on Humane Care and Use of Laboratory Animals and the protocol approved by the Institutional Animal Care and Use Committee at the SBP Medical Discovery Institute.
2.11. Real-time PCR quantification of ZIKV in mouse plasma
Total RNA was extracted from blood samples (0.1 ml each) using a NucleoSpin RNA Kit (Macherey-Nagel GmbH). RNA concentrations were measured using a NanoDrop spectrophotometer (NanoDrop Technologies) and samples were stored at −80 °C until use. ZIKV-specific primers (ZIKV-835 5′-TTGGTCATGATACTGCTGATTGC-3′, ZIKV-911c 5′-CCTTCCACAAAGTCCCTATTGC-3′) were described previously (Cugola et al., 2016; Lanciotti et al., 2008). Real-time PCR was performed using QuantiTect Reverse Transcription Kit (QIAGEN), SYBR Green I Master Mix, and a LightCycler 480 II instrument (Roche) using the following conditions: initiation at 95 °C for 10 min followed by 50 cycles of 95 °C for 15 s, 60 °C for 30 s, and 72 °C for 30 s. Data were analyzed using LightCycler 480 Software 1.5.0 (Roche). Assay sensitivity was determined using samples of known ZIKV concentration. GraphPad Prism was used as fitting software.
2.12. In silico modeling of ZIKV NS2B-NS3pro complexed with inhibitors
The 3-dimensional structures of inhibitors were downloaded from NCI/DTP chemical data depository (https://wiki.nci.nih.gov/display/NCIDTPdata/Chemical+Data). Docking of NSC157058, NSC86414, and NSC716903 to ZIKV NS2B-NS3pro was performed using SWISSDOCK (www.swissdock.ch) (Grosdidier et al., 2011). ZIKV NS2B-NS3pro (PDB ID: 5t1v) was used as the target structure. To compare a protinin binding modes, the structure of WNV NS2B-NS3pro (PDB ID: 2ijo) was superimposed on that of ZIKV NS2B-NS3pro (PDB ID: 5lc0) (Aleshin et al., 2007; Lei et al., 2016) using the rigid body structural alignment in PyMOL. NS2B-NS3pro images were rendered using PyMOL (version 0.95, DeLano Scientific) and UCSF-Chimera software (Pettersen et al., 2004).
3. Results
3.1. Generation and characterization of ZIKV NS2B-NS3pro constructs
Protein structural analysis and drug design efforts required substantial amounts of proteolytically active recombinant NS2B-NS3pro. Self-proteolysis at the NS2B-NS3 junction typically generates the individual, non-covalently associated NS2B cofactor and NS3pro domains, together with residual intact NS2B-NS3pro in the isolated active protease samples. To prevent self-proteolysis and to obtain the covalently linked, single-chain NS2B-NS3pro construct, we inserted a K48A mutation at the NS2B-NS3 junctional cleavage site, which efficiently inactivated self-cleavage (Shiryaev et al., 2007). This active construct that included the K48A mutation at the C-terminus of the NS2B cofactor (designated wild-type [WT]) was stable, functional, and well suited for our structural and inhibitory studies (Fig. 1B).
Studies by us and others have shown that the central, cytoplasmic portion of the NS2B cofactor is sufficient for productive folding of the NS3pro domain, whereas NS3pro alone is misfolded and catalytically inactive (Falgout et al., 1993; Li et al., 2005; Shiryaev et al., 2006; Yusof et al., 2000). We used a similar approach to express active WT and inactive S135A ZIKV NS2B-NS3pro constructs. The 48-residue central portion of NS2B was linked with NS3pro via a GGGGSGGGGSG linker, and the soluble recombinant constructs (Fig. 1B) were cloned, expressed, and purified (kindly provided by Dr. Robert C. Liddington’s laboratory).
The ZIKV NS2B-NS3pro sequence, particularly the active site region, is homologous to proteases from multiple flaviviruses, including DENV and WNV (Fig. 1C). Because DENV and WNV proteases efficiently cleave the fluorescent peptide Pyr-RTKR-AMC (Shiryaev et al., 2006), which is a common substrate for protease activity assay, we tested whether this peptide was also cleaved by ZIKV NS2B-NS3pro. Indeed, the specific activity of purified ZIKVWT NS2B-NS3pro for Pyr-RTKR-AMC was similar to that of the WNV enzyme (0.75 U/mg versus 1.0 U/mg; Fig. 2A). In contrast, the catalytically inactive NS2B-NS3pro S135A mutant exhibited <5% of the activity of the WT construct. Our results correlate well with the data by Chen et al. (2016) who also reported the cleavage of the Arg/Lys-rich substrate by ZIKV protease.
Fig. 2. Enzymatic characteristics of ZIKV NS2B-NS3pro.
(A) Cleavage of Pyr-RTKR-MCA by purified ZIKV WT and S135A NS2B-NS3pro. WNV NS2B-NS3pro cleavage is shown for reference. Data are the mean ± S.D of n = 5. (B–E) Effect of ions on ZIKV and WNV NS2B-NS3pro activity: (B) Ca2+, (C) Mg2+, (D) Na+, and (E) K+. Data are the mean ± S.D of n = 3. Stars above the bars represent statistical significance determined by Student t-test: *p < 0.05, **p < 0.01, *** – p < 0.001 and ns – not statistically significant.
Similar to other flaviviral proteases, the enzymatic activity of ZIKV NS2B-NS3pro was highly sensitive to metal ions (Shiryaev et al., 2007), and even a low concentration (10 mM) of Na+, K+, Mg+, or Ca2+ inhibited the protease cleavage activity (Fig. 2, B–E). It seems likely that the anionic environment affects the delicate productive interactions of the NS2B cofactor with the NS3pro domain, resulting in reversible inactivation of the protease activity. These findings suggest that unproductively folded structures predominate at high ionic strength and, thus, low ionic strength buffers would be preferred for crystallization of the ZIKV protease.
3.2. MBP is not a substrate for ZIKV NS2B-NS3pro
Surprisingly, despite its high homology to WNV NS2B-NS3pro and its ability to cleave Pyr-RTKR-AMC, ZIKV NS2B-NS3pro did not efficiently cleave myelin basic protein (MBP) in potential cleavage sites (A52PKR↓G56 and R132GK↓G135), while the WNV enzyme readily proteolyzed MBP. To highlight this difference, both the ZIKV- and WNV-treated samples are shown in Fig. 3A. In this regard, ZIKV NS2B-NS3pro is similar to DENV NS2B-NS3 protease, which is also unable to cleave MBP (data not shown). In this respect the link between ZIKV and the incidence of Guillain-Barré syndrome (22), an autoimmune demyelinating disease characterized by extensive degradation of myelin sheath, will need additional mechanistic insights.
Fig. 3. Cleavage of protein targets by ZIKV NS2B-NS3pro.
(A) Myelin basic protein (MBP, 18.5 kDa) is not susceptible to ZIKV NS2B-NS3pro (left panel), but could be readily cleaved by WNV NS2B-NS3pro (enzyme:substrate ratio of 1:10–10,000) (right panel). Please, note that only the pre-existing degraded MBP species rather than newly accumulated cleavage products are present in the left panel. Coomassie Blue staining is shown. (B) Human Sox2 could be partially cleaved by an excess of ZIKV NS2B-NS3pro in vitro. The degradation products the enhanced levels which discriminate the WT samples from the inactive S135A samples are marked by a star symbol. Multiple pre-existing degraded Sox2 species are also visible because of the limited stability of recombinant Sox2. Stars indicate Sox2 cleavage products. Western blot using the Sox2 antibody is shown (C) Sox2 is not cleaved in ZIKV-infected human fetal neural progenitors. Western blot using the Sox2 antibody is shown.
3.3. Assessing neural transcription factor Sox2 as a putative substrate for ZIKV NS2B-NS3pro
ZIKV is known to infect human fetal neural precursor cells (hfNPCs), providing a possible mechanism for the microcephaly observed in the fetuses of infected pregnant women. To determine whether ZIKV NS2B-NS3pro cleaves proteins important for neural precursors, we examined its effect on Sox2 protein, which exhibits likely cleavage sites for NS2B-NS3pro (K95RLR↓A99 and R110PRRK↓T115). Sox2 plays a critical role in neural stem cells by maintaining the activity of multiple genes involved in self-renewal and by priming the epigenetic landscape for the onset of neuronal differentiation (Amador-Arjona et al., 2015; Cimadamore et al., 2011). Notably, Sox2 insufficiency results in a plethora of developmental neuronal malformations in the brain (Cavallaro et al., 2008; Ferri et al., 2004; Hagstrom et al., 2005). To test our suggestion, we, first, incubated purified recombinant Sox2 with purified ZIKV NS2B-NS3pro and examined the digestion products by Western blotting. We found that Sox2 was cleaved by ZIKV NS2B-NS3pro only at a very high enzyme/substrate ratio (Fig. 3B). In contrast, there was no detectable cleavage of cellular Sox2 in hfNPCs cells infected with ZIKV at a MOI of 1 (Fig. 3C). Taken together, these data imply that Sox2 is not a proteolytic target of ZIKV protease in the infected neural cells.
3.4. Aprotinin is an efficient inhibitor of ZIKV NS2B-NS3pro
We previously identified aprotinin (a 60-amino acid bovine pancreatic trypsin inhibitor) as a potent (IC50 20 nM) inhibitor of WNV NS2B-NS3pro (Shiryaev et al., 2006) and successfully solved the X-ray structure of aprotinin-WNV protease co-crystals (Aleshin et al., 2007). Therefore, we asked whether aprotinin might also be an antagonist of ZIKV protease. Indeed, aprotinin efficiently inhibited ZIKV cleavage activity (IC50 ~70 nM; Fig. 4A). Modeling of the ZIKV NS2B-NS3 protease structure (PDB ID: 5t1v) complexed with aprotinin suggested that the aprotinin fold should be similar to that in the co-complex of aprotinin and WNV NS2B-NS3 protease (Fig. 4B). The different residues at positions 85 and 132 in the ZIKV and WNV enzymes (Ser85 vs Gln85 and Ala132 vs Thr132, respectively) likely explains the lower efficiency with which aprotinin inhibits ZIKV NS2B-NS3pro compared with WNV NS2BNS3pro (Fig. 4).
Fig. 4. Inhibitors of ZIKV NS2B-NS3pro.
(A) NSC157058, NSC716897, NSC86314, and aprotinin inhibit the catalytic activity of ZIKV NS2B-NS3pro. Representative dose-response curves are shown. Data are the mean ± S.D of n = 3. (B) Modeling of aprotinin in ZIKV NS2B-NS3pro (PDB 5lc0; right). The X-ray structure of aprotinin in its complex with WNV NS2B-NS3pro (PDB 2ijo) is shown on the left. NS3pro is shown as a cartoon (light brown); the catalytic triad is in red; and NS2B and aprotinin are shown in dark and light blue, respectively. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.5. Small molecule inhibitors of ZIKV NS2B-NS3pro
Small molecule inhibitors targeted to the active site of ZIKV NS2B-NS3pro are likely to cross-react with multiple human serine proteases, leading to off-target toxicity. Therefore, we focused our search for NS2B-NS3pro inhibitors on compounds that bind to viral protease exosites (Johnston et al., 2007; Shiryaev et al., 2011; Shiryaev and Strongin, 2010; Sidique et al., 2009). We tested a focused sub-library of allosteric exosite-targeting inhibitors previously shown to inhibit the structurally homologous WNV NS2B-NS3pro. In addition, we screened >700 structurally similar compounds available from the Conrad Prebys Center for Chemical Genomics (La Jolla, CA). For the screen, ZIKV NS2B-NS3pro was incubated with Pyr-RTKR-AMC substrate in the presence of up to 100 μM compound. The most efficient inhibitors are presented in Table S1. Among the compounds tested, NSC157058, NSC86314, and NSC716903 showed particularly good inhibition of ZIKV NS2B-NS3pro in vitro, with NSC157058 being the most potent (IC50 0.82 μM; Fig. 4, Table 2, Table S1). Importantly, the inhibitors had no effect on furin (IC50 > 100 μM, Table 2), a human serine protease with cleavage sequence preferences common for the flaviviral proteases (Thomas, 2002).
Table 2. Small molecule inhibitors of ZIKV, WNV, and DENV2 NS2B-NS3pro.
TC50, the concentration of the inhibitor which causes death of 50% cells.
| Compound | Structure |
In vitro IC50, μM
|
Cytotoxicity (TC50, μM) | |||
|---|---|---|---|---|---|---|
| ZIKV NS2B-NS3pro | WNV NS2B-NS3pro | DENV2 NS2B-NS3pro | Furin | |||
| NSC157058 |

|
0.82 | 0.74 | >10 | >100 | 257.4 |
| NSC716897 |

|
0.97 | 3.02 | >10 | >100 | 178.4 |
| NSC86314 |
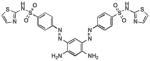
|
1.12 | 0.26 | 2.75 | >100 | 212.5 |
| NSC719147 |

|
2.17 | 3.46 | >10 | >100 | 160.3 |
| NSC716903 |
|
3.01 | 1.26 | >10 | >100 | 127.8 |
| NSC716898 |
|
>10 | 0.44 | >10 | >100 | 235.8 |
| NSC134189 |

|
>10 | 6.25 | >10 | >100 | 132.3 |
3.6. Protein-ligand docking
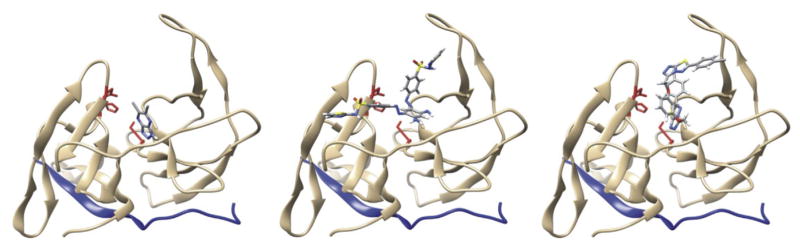
NSC157058, NSC86314, and NSC716903 have been shown to bind to the WNV NS3pro domain exosite, which is distant from the active site cavity (Shiryaev et al., 2011). However, we considered that exosite binding might still interfere with the positioning of the NS2B cofactor relative to the NS3pro active site, thereby inactivating the protease activity. Indeed, modeling of NSC157058, NSC86314, and NSC716903 bound to ZIKV NS2B-NS3pro suggested that these compounds would be capable of binding to the same druggable pocket in ZIKV as they do in WNV and, as a result, were likely to interfere with the productive fold of the NS2B cofactor (Fig. 5).
Fig. 5. Predicted binding mode of NSC157058 (left), NSC86314 (middle), and NSC716903 (right) to ZIKV NS2B-NS3pro.
NS2B-NS3pro is shown as a cartoon (light brown), NS2B is in blue, and the catalytic triad is in red. The ligand structures are multicolored per their atomic composition. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.7. Protease inhibitors protect hfNPCs from ZIKV infection
Because ZIKV NS2B-NS3pro cleavage activity is essential for viral infectivity in vivo, we asked whether incubation of hfNPCs, a primary target of ZIKV, with NSC157058, NSC86314, or NSC716903 could protect them against ZIKV infection. Primary hfNPCs were infected with ZIKV (Asian strain, FSS13025, 2010 Cambodian isolate) at various MOIs. Intracellular ZIKV levels were then assessed by immunofluorescence staining for flavivirus-specific envelope protein, assessed, hfNPC apoptosis was assessed by staining for the presence of active caspase 3 (AC3), and hfNPC proliferation was quantified by incorporation of the DNA-binding dye 5-ethynyl-2′-deoxyuridine (EdU). The cells were monitored continuously using an in vivo imaging platform for up to 96 h postinfection.
The number of infected cells increased progressively up to 72 h in cultures at all MOIs (Fig. 6A and B and C). Although we detected very few AC3-positive apoptotic cells at any time point (~2% AC + cells at MOI = 10 at 96 h), ZIKV infection caused a dramatic decrease in hfNPC proliferation, even at the lowest MOI (Fig. 6D) suggesting a bystander effect that needs to be further investigated. Of note, EdU incorporation also decreased with time in control cultures because the cells approach confluency (Fig. 6D). Collectively, these data demonstrate that ZIKV efficiently infects hfNPCs in vitro.
Fig. 6. Assay of ZIKV infection (Asian strain FSS13025) of primary human fetal neural progenitors.
(A) Micrograph of infected cells (44 h post-infection). Active caspase-3, red; nuclei, blue; ZIKV Envelope protein, green; EdU, white. (B) Infection time course. (C) Number of nuclei in infected cells. (D) Percentage of EdU-positive proliferating cells in the subset of ZIKV-positive cells. At MOI = 0, the percentage of EdU-positive cells is shown relative to the total cell number. Error bars are ± SD. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
To examine the effects of the NS2B-NS3pro inhibitors, hfNPCs were co-incubated with NSC157058, NSC86314, or NSC716903 (up to 100 μM) and then infected with ZIKV at a MOI of 0.1. After 4 days, cell infection was assessed by staining for ZIKV envelope protein. We found that NSC157058 inhibited ZIKV infection with an IC50 ~50 μM without demonstrating significant cytotoxicity (Fig. 7A; Table 2). In contrast, NSC716903 and NSC86314 showed little or no inhibition, and were also more cytotoxic (Fig. 7A). These results provide the first proof of principle that inhibition of NS2B-NS3pro is sufficient to protect hfNPCs from ZIKV infection. Providing further optimization, such allosteric small-molecule viral protease inhibitors could to generate leads for the development of a novel ZIKV therapy.
Fig. 7. NSC157058 inhibits ZIKV infection in human fetal neural progenitors and in SJL mice.
(A) Cells were infected with ZIKV at a MOI of 0.1 and co-incubated with NSC157058, NSC86314, or NSC716903 (0–100 μM) for an additional 4 days. Data are the mean ± S.D of n = 3. Stars above the bars represent statistical significance determined by Student t-test: *p < 0.05. (B) NSC157058 (10 mg/kg) was injected intraperitoneally. NSC157058 concentrations were measured by LC-MS/MS mass spectrometry in plasma samples prepared at 15 min, 30 min, 1 h, and 2 h post-injection. The estimated half-life of NSC157058 was ~20 min (C) SJL mice were infected with ZIKV (2 × 103 PFU; Panama strain, PA 259459). Mice were then provided with water containing NSC157058 (30 mg/kg) for 5 days. On day 6, blood samples were taken and ZIKV RNA was quantified by RT-PCR. Horizontal bars show the mean values, n = 3 mice, p < 0.01 Student t-test. Cytotoxicity of the tested compounds, including NSC157058, NSC86314, or NSC716903, is summarized in Table 2.
3.8. NSC157058 decreases ZIKV infection in mice
Finally, we tested whether NSC157058 could reduce or prevent ZIKV infection in a mouse model. To determine the optimal dose and route of administration, we first investigated the pharmacokinetic properties of NSC157058 after intraperitoneal injection. These experiments revealed rapid plasma clearance, with a half-life of only ~20 min (Fig. 7B). In view of these unfavorable pharmacokinetics, we administered NSC157058 to the mice by adding it to their drinking water. Three-month-old female SJL mice were infected with ZIKV (2 × 103 PFU; Panama strain, PA259459), and their drinking water was supplemented NSC157058 (30 mg/kg) for 5 days. Because mice with an average weight of 20 g consume on average 6 ml water daily, in our experiments each mouse received 0,6 mg inhibitor daily or 30 mg/kg daily. Mice were then sacrificed and the level of ZIKV infection was determined by RT-PCR analysis of plasma. We found that the levels of circulating ZIKV were ~10-fold lower in NSC157058-treated mice than in the untreated control mice (Fig. 7C). These data show that orally bioavailable inhibitor of ZIKV protease activity could be a potent suppressor of ZIKV replication in vivo.
4. Discussion
Despite intense research efforts, there are currently no effective drugs or vaccines for the treatment or prevention of flaviviral infections. The recent outbreak in ZIKV has served to highlight this urgent unmet clinical need, particularly in view of the severe neuropathology inflicted by ZIKV on the developing fetus. Proteolytic processing of the viral polyprotein precursor is a crucial step in ZIKV replication, propagation, and disease progression (Chambers et al., 2005). We analyzed the catalytic activity of a recombinant ZIKV NS2B-NS3pro construct and characterized the effects of small-molecule allosteric inhibitors of its enzymatic activity. We identified an inhibitor that blocked ZIKV infection of hfNPCs in vitro and reduced viral load in ZIKV-infected mice. Our data thus confirm that NS2B-NS3 protease is a highly promising target for the development of anti-flaviviral compounds (28,29).
The homologous flaviviral NS2B-NS3 proteases display similar substrate specificities. These enzymes prefer positively charged Arg and Lys residues at the P1 and P2 positions, and P1′ Gly, Ser, and Thr for the efficient cleavage of the scissile bond. Not surprisingly, the fold of flaviviral NS3 proteases is also similar. The recently solved structures of ZIKV NS2B-NS3pro support this conclusion (Lei et al., 2016; Phoo et al., 2016). Moreover, the structural similarities are consistent with the potent activity of aprotinin as an active site inhibitor of both WNV and ZIKV proteases.
We previously described allosteric inhibitory scaffolds capable of repressing the cleavage activity of WNV and DENV NS2B-NS3pro (23). These inhibitors appeared to interfere with the productive fold of the NS2B cofactor relative to the NS3pro domain (Shiryaev et al., 2011). In the present study, we found that several of the WNV inhibitors were potent inhibitors of ZIKV NS2B-NS3pro, suggesting that some of the WNV and DENV inhibitors could be re-purposed to develop ZIKV inhibitors. Importantly, these compounds target the unique flaviviral protease exosite rather than the active site; therefore, they should have little effect on human serine proteases, as shown here for the furin serine protease. This is critical to reducing the potential for adverse effects due to off-target toxicity.
Noting the strong structural similarity of the catalytic region between the ZIKV NS2B/NS3 and the HCV NS3/NS4A proteases, a similar repurposing approach was recently employed by others (Lee et al., 2017) who evaluated the inhibitory efficacy of 71 of the promising HCV NS3/NS4A inhibitors. As a result, Lee et al. (2017) identified 10 compounds with IC50 values less than 50 μM. Among these compounds there was a single compound that exhibited the 9.5 μM value. However, all of these compounds appear to be less potent against ZIKV NS2B-NS3pro compared with the inhibitors identified here.
We found that compound NSC157058 reduced ZIKV infection of hfNPCs (IC50 ~50 μM) without significant cellular toxicity. Moreover, this inhibitor also significantly (10-fold) reduced the level of circulating ZIKV in infected mice. The fact that this inhibitor shows high aqueous solubility and unfavorable pharmacokinetic parameters bodes well for the development of compounds with improved pharmacodynamics and pharmacokinetics.
In the present study, we identified a small molecule anti-ZIKV scaffold that likely inhibits NS2B-NS3pro activity by interfering with the productive fold of the NS2B cofactor in the two-component protease, thereby inhibiting its cleavage activity and repressing ZIKV infection in both cultured hfNPCs and mice. These compounds could be used as a foundation for the design of more potent and effective ZIKV inhibitors for the treatment and prevention of Guillain-Barré syndrome, fetal malformations, and other neuropathologies linked to ZIKV infection.
Supplementary Material
Acknowledgments
We greatly appreciate the help of Buddy Charbono, Hilda Clarke, and Mary O’Rourke-Braxtan at the Animal Facility of the Sanford-Burnham-Prebys Medical Discovery Institute for their invaluable assistance with the animal studies. We thank Alexander Aleshin, Laurie Bankston, and Robert Liddgnton for providing us with NS2BNS3pro preparations and helpful discussions.
Abbreviations
- AC3
active caspase 3
- DENV
Dengue virus
- EdU
5-ethynyl-2′-deoxyuridine
- HRP
horseradish peroxidase
- hfNPCs
human fetal neural progenitors
- JEV
Japanese encephalitis virus
- MBP
myelin basic protein
- NS
nonstructural
- NS2B-NS3pro
NS2B-NS3 protease
- Pyr-RTKR-AMC
pyroglutamic acid Pyr-Arg-Thr-Lys-Arg-7-amino-4-methylcoumarin
- WNV
West Nile virus
- WT
wildtype
- YFV
Yellow fever virus
- ZIKV
Zika virus
Appendix A. Supplementary data
Supplementary data related to this article can be found at http://dx.doi.org/10.1016/j.antiviral.2017.04.015.
Footnotes
The work was supported, in whole or in part, by National Institutes of Health Grants R21NS100477 (to A.Y.S. and A.V.T.) and R01GM107523 (to P.C.).
Conflict of interest
No author has an actual or perceived conflict of interest with the contents of this article.
Authors contribution
SAS, AYS, and AVT conceived and coordinated the study and wrote the paper. CF, AP, C-TH, NS, AEN, PC, designed, performed and analyzed the experiments. SS provided reagents and helped designed the experiments. All authors reviewed the results and approved the final version of the manuscript.
References
- Aleshin AE, Shiryaev SA, Strongin AY, Liddington RC. Structural evidence for regulation and specificity of flaviviral proteases and evolution of the Flaviviridae fold. Protein Sci. 2007;16:795–806. doi: 10.1110/ps.072753207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amador-Arjona A, Cimadamore F, Huang CT, Wright R, Lewis S, Gage FH, Terskikh AV. SOX2 primes the epigenetic landscape in neural precursors enabling proper gene activation during hippocampal neurogenesis. Proc Natl Acad Sci U S A. 2015;112:E1936–E1945. doi: 10.1073/pnas.1421480112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azevedo RS, Araujo MT, Martins Filho AJ, Oliveira CS, Nunes BT, Cruz AC, Nascimento AG, Medeiros RC, Caldas CA, Araujo FC, Quaresma JA, Vasconcelos BC, Queiroz MG, da Rosa ES, Henriques DF, Silva EV, Chiang JO, Martins LC, Medeiros DB, Lima JA, Nunes MR, Cardoso JF, Silva SP, Shi PY, Tesh RB, Rodrigues SG, Vasconcelos PF. Zika virus epidemic in Brazil. I Fatal disease in adults: clinical and laboratorial aspects. J Clin Virol. 2016;85:56–64. doi: 10.1016/j.jcv.2016.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrows NJ, Campos RK, Powell ST, Prasanth KR, Schott-Lerner G, Soto-Acosta R, Galarza-Munoz G, McGrath EL, Urrabaz-Garza R, Gao J, Wu P, Menon R, Saade G, Fernandez-Salas I, Rossi SL, Vasilakis N, Routh A, Bradrick SS, Garcia-Blanco MA. A screen of FDA-approved drugs for inhibitors of Zika virus infection. Cell Host Microbe. 2016;20:259–270. doi: 10.1016/j.chom.2016.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos GS, Bandeira AC, Sardi SI. Zika virus outbreak, Bahia, Brazil. Emerg Infect Dis. 2015;21:1885–1886. doi: 10.3201/eid2110.150847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavallaro M, Mariani J, Lancini C, Latorre E, Caccia R, Gullo F, Valotta M, DeBiasi S, Spinardi L, Ronchi A, Wanke E, Brunelli S, Favaro R, Ottolenghi S, Nicolis SK. Impaired generation of mature neurons by neural stem cells from hypomorphic Sox2 mutants. Development. 2008;135:541–557. doi: 10.1242/dev.010801. [DOI] [PubMed] [Google Scholar]
- Chambers TJ, Droll DA, Tang Y, Liang Y, Ganesh VK, Murthy KH, Nickells M. Yellow fever virus NS2B-NS3 protease: characterization of charged-to-alanine mutant and revertant viruses and analysis of polyprotein-cleavage activities. J Gen Virol. 2005;86:1403–1413. doi: 10.1099/vir.0.80427-0. [DOI] [PubMed] [Google Scholar]
- Chen X, Yang K, Wu C, Chen C, Hu C, Buzovetsky O, Wang Z, Ji X, Xiong Y, Yang H. Mechanisms of activation and inhibition of Zika virus NS2B-NS3 protease. Cell Res. 2016;26:1260–1263. doi: 10.1038/cr.2016.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cimadamore F, Fishwick K, Giusto E, Gnedeva K, Cattarossi G, Miller A, Pluchino S, Brill LM, Bronner-Fraser M, Terskikh AV. Human ESC-derived neural crest model reveals a key role for SOX2 in sensory neurogenesis. Cell Stem Cell. 2011;8:538–551. doi: 10.1016/j.stem.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cugola FR, Fernandes IR, Russo FB, Freitas BC, Dias JL, Guimarães KP, Benazzato C, Almeida N, Pignatari GC, Romero S, Polonio CM, Cunha I, Freitas CL, Brandão WN, Rossato C, Andrade DG, Faria DeP, Garcez AT, Buchpigel CA, Braconi CT, Mendes E, Sall AA, Zanotto PM, Peron JP, Muotri AR, Beltrão-Braga PC. The Brazilian Zika virus strain causes birth defects in experimental models. Nature. 2016;534:267–271. doi: 10.1038/nature18296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erbel P, Schiering N, D’Arcy A, Renatus M, Kroemer M, Lim SP, Yin Z, Keller TH, Vasudevan SG, Hommel U. Structural basis for the activation of flaviviral NS3 proteases from dengue and West Nile virus. Nat Struct Mol Biol. 2006;13:372–373. doi: 10.1038/nsmb1073. [DOI] [PubMed] [Google Scholar]
- Falgout B, Miller RH, Lai CJ. Deletion analysis of dengue virus type 4 nonstructural protein NS2B: identification of a domain required for NS2B-NS3 protease activity. J Virol. 1993;67:2034–2042. doi: 10.1128/jvi.67.4.2034-2042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferri AL, Cavallaro M, Braida D, Di Cristofano A, Canta A, Vezzani A, Ottolenghi S, Pandolfi PP, Sala M, DeBiasi S, Nicolis SK. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development. 2004;131:3805–3819. doi: 10.1242/dev.01204. [DOI] [PubMed] [Google Scholar]
- Gawlik K, Shiryaev SA, Zhu W, Motamedchaboki K, Desjardins R, Day R, Remacle AG, Stec B, Strongin AY. Autocatalytic activation of the furin zymogen requires removal of the emerging enzyme’s N-terminus from the active site. PLoS One. 2009;4:e5031. doi: 10.1371/journal.pone.0005031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosdidier A, Zoete V, Michielin O. Fast docking using the CHARMM force field with EADock DSS. J Comput Chem. 2011;32:2149–2159. doi: 10.1002/jcc.21797. [DOI] [PubMed] [Google Scholar]
- Hagstrom SA, Pauer GJ, Reid J, Simpson E, Crowe S, Maumenee IH, Traboulsi EI. SOX2 mutation causes anophthalmia, hearing loss, and brain anomalies. Am J Med Genet A. 2005;138A:95–98. doi: 10.1002/ajmg.a.30803. [DOI] [PubMed] [Google Scholar]
- Johnston PA, Phillips J, Shun TY, Shinde S, Lazo JS, Huryn DM, Myers MC, Ratnikov BI, Smith JW, Su Y, Dahl R, Cosford ND, Shiryaev SA, Strongin AY. HTS identifies novel and specific uncompetitive inhibitors of the two-component NS2B-NS3 proteinase of West Nile virus. Assay Drug Dev Technol. 2007;5:737–750. doi: 10.1089/adt.2007.101. [DOI] [PubMed] [Google Scholar]
- Lanciotti RS, Kosoy OL, Laven JJ, Velez JO, Lambert AJ, Johnson AJ, Stanfield SM, Duffy MR. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg Infect Dis. 2008;14:1232–1239. doi: 10.3201/eid1408.080287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H, Ren J, Nocadello S, Rice AJ, Ojeda I, Light S, Minasov G, Vargas J, Nagarathnam D, Anderson WF, Johnson ME. Identification of novel small molecule inhibitors against NS2B/NS3 serine protease from Zika virus. Antivir Res. 2017;139:49–58. doi: 10.1016/j.antiviral.2016.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei J, Hansen G, Nitsche C, Klein CD, Zhang L, Hilgenfeld R. Crystal structure of Zika virus NS2B-NS3 protease in complex with a boronate inhibitor. Science. 2016;353:503–505. doi: 10.1126/science.aag2419. [DOI] [PubMed] [Google Scholar]
- Li H, Saucedo-Cuevas L, Regla-Nava JA, Chai G, Sheets N, Tang W, Terskikh AV, Shresta S, Gleeson JG. Zika virus infects neural progenitors in the adult mouse brain and alters proliferation. Cell Stem Cell. 2016 Nov 3;19(5):593–598. doi: 10.1016/j.stem.2016.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Lim SP, Beer D, Patel V, Wen D, Tumanut C, Tully DC, Williams JA, Jiricek J, Priestle JP, Harris JL, Vasudevan SG. Functional profiling of recombinant NS3 proteases from all four serotypes of dengue virus using tetrapeptide and octapeptide substrate libraries. J Biol Chem. 2005;280:28766–28774. doi: 10.1074/jbc.M500588200. [DOI] [PubMed] [Google Scholar]
- Luo D, Vasudevan SG, Lescar J. The flavivirus NS2B-NS3 protease-helicase as a target for antiviral drug development. Antivir Res. 2015;118:148–158. doi: 10.1016/j.antiviral.2015.03.014. [DOI] [PubMed] [Google Scholar]
- Mlakar J, Korva M, Tul N, Popovic M, Poljsak-Prijatelj M, Mraz J, Kolenc M, Resman Rus K, Vesnaver Vipotnik T, Fabjan Vodusek V, Vizjak A, Pizem J, Petrovec M, Avsic Zupanc T. Zika virus associated with microcephaly. N Engl J Med. 2016;374:951–958. doi: 10.1056/NEJMoa1600651. [DOI] [PubMed] [Google Scholar]
- Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, Ferrin TE. UCSF Chimera–a visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–1612. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- Phoo WW, Li Y, Zhang Z, Lee MY, Loh YR, Tan YB, Ng EY, Lescar J, Kang C, Luo D. Structure of the NS2B-NS3 protease from Zika virus after self-cleavage. Nat Commun. 2016;7:13410. doi: 10.1038/ncomms13410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev SA, Aleshin AE, Ratnikov BI, Smith JW, Liddington RC, Strongin AY. Expression and purification of a two-component flaviviral proteinase resistant to autocleavage at the NS2B-NS3 junction region. Protein Expr Purif. 2007;52:334–339. doi: 10.1016/j.pep.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev SA, Cheltsov AV, Gawlik K, Ratnikov BI, Strongin AY. Virtual ligand screening of the National Cancer Institute (NCI) compound library leads to the allosteric inhibitory scaffolds of the West Nile Virus NS3 proteinase. Assay Drug Dev Technol. 2011;9:69–78. doi: 10.1089/adt.2010.0309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev SA, Ratnikov BI, Chekanov AV, Sikora S, Rozanov DV, Godzik A, Wang J, Smith JW, Huang Z, Lindberg I, Samuel MA, Diamond MS, Strongin AY. Cleavage targets and the D-arginine-based inhibitors of the West Nile virus NS3 processing proteinase. Biochem J. 2006;393:503–511. doi: 10.1042/BJ20051374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiryaev SA, Strongin AY. Structural and functional parameters of the flaviviral protease: a promising antiviral drug target. Future Virol. 2010;5:593–606. doi: 10.2217/fvl.10.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidique S, Shiryaev SA, Ratnikov BI, Herath A, Su Y, Strongin AY, Cosford ND. Structure-activity relationship and improved hydrolytic stability of pyrazole derivatives that are allosteric inhibitors of West Nile Virus NS2B-NS3 proteinase. Bioorg Med Chem Lett. 2009;19:5773–5777. doi: 10.1016/j.bmcl.2009.07.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sironi M, Forni D, Clerici M, Cagliani R. Nonstructural proteins are preferential positive selection targets in Zika virus and related flaviviruses. PLoS Negl Trop Dis. 2016;10:e0004978. doi: 10.1371/journal.pntd.0004978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas G. Furin at the cutting edge: from protein traffic to embryogenesis and disease. Nat Rev Mol Cell Biol. 2002;3:753–766. doi: 10.1038/nrm934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yusof R, Clum S, Wetzel M, Murthy HM, Padmanabhan R. Purified NS2B/NS3 serine protease of dengue virus type 2 exhibits cofactor NS2B dependence for cleavage of substrates with dibasic amino acids in vitro. J Biol Chem. 2000;275:9963–9969. doi: 10.1074/jbc.275.14.9963. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.