Abstract
Study objective
To evaluate effect of parecoxib sodium pretreatment combined with dexmedetomidine on early postoperative cognitive dysfunction in elderly patients after shoulder arthroscopy.
Design
Randomized, double-blind study.
Setting
University-affiliated teaching hospital.
Patients
One hundred and fifty-two elderly patients scheduled for shoulder arthroscopy.
Interventions
At 15 min before the induction of anesthesia, 152 patients received intravenously parecoxib sodium 40 mg and dexmedetomidine at a dose of 0.5 μg/kg over 15 min, followed by a continuous infusion at a rate of 0.5 μg/kg/h until the end of surgery. Then all patients who received postoperative patient-controlled intravenous analgesia were divided 2 groups: sufentanil(0.04μg/kg/h, S group), sufentanil (0.04μg/kg/h) plus dexmedetomidine(0.06μg/kg/h) (SD group).
Measurements
The mini–mental status examination score in SD group was significantly higher than S group at 1, 2 and 7 days after surgery. The incidence of postoperative cognitive dysfunction during 7 days after surgery in S and SD groups was respectively 17.1% and 6.7%. Compared with the S group, the visual analogue scale scores at rest and upon movement were significantly lower at 6, 14, 24, 36 and 48 h after surgery in SD group; analgesia pump liquid amount during 24 h after surgery and number of rescue analgesia during 48 h after surgery were significantly lower in SD group. Jugular venous oxygen partial pressure and jugular venous oxygen saturation values in SD group were significantly higher than S group at postoperative 24 h. The occurrence of nausea and vomiting within 48 h after surgery in SD group were significantly lower than S group. We found no complications including respiratory depression and sinus bradycardia within 48 h after surgery in all patients.
Conclusions
Parecoxib sodium pretreatment combined with dexmedetomidine could reduce the incidence of early postoperative cognitive dysfunction in elderly patients. This might be related to the improvement of postoperative analgesia effect and cerebral oxygen metabolism in patients.
Keywords: Cyclooxygenase-2 inhibitor, Dexmedetomidine, Postoperative cognitive dysfunction, Brain, Pain, Elderly
Highlights
-
•
To explore synergistic effect of Parecoxib sodium and dexmedetomidine combined application.
-
•
Parecoxib sodium pretreatment combined with perioperative dexmedetomidine administration can decrease the incidence of POCD.
-
•
Parecoxib sodium pretreatment combined with using dexmedetomidine in perioperation can provide good analgesia.
-
•
Parecoxib sodium pretreatment combined with using dexmedetomidine in perioperation can improve postoperative SjvO2 and PjvO2.
1. Introduction
Shoulder arthroscopy is one of the effective means of treatment shoulder surgery. But due to the shoulder is a special part, in which tourniquet could not be used. In order to maintain a clear operative field and reduce bleeding, there is a need for intraoperative controlled hypotension and intra-articular pressure washing [1]. However, intraoperative cerebral blood flow reduction and postoperative acute pain are easy to induce postoperative cognitive dysfunction in elderly patients with degenerative changes of brain cells.
Postoperative cognitive dysfunction (POCD) is a common complication of central nervous system in elderly patients, and POCD has been shown to be temporary cognitive decline associated with the surgery [2]. POCD is not only related to the anesthetic and surgery-related factors [3], [4], but also related to acute postoperative pain [5]. Acute postoperative pain management technique is an important risk factor of POCD, and appropriate management techniques that alleviate postoperative pain can improve postoperative cognitive function in patients. Parecoxib sodium is a highly selective cyclooxygenase-2 (COX-2) inhibitor. Studies have shown that parecoxib sodium preventive analgesia could enhance analgesic effects [6], [7]. Dexmedetomidine is a specific a2-adrenergic receptor agonist, which has analgesic, sedative and hypnotic, anxiolytic effects. Studies have found that dexmedetomidine had brain protective effects [8], [9], it could also enhance the analgesic effect of opioids [10]. However, the mechanisms of their action are different, it remains unclear whether the combination has a synergistic effect. This study was designed to investigate the effect of the pre-administration of parecoxib sodium combined with dexmedetomidine on early postoperative cognitive function in elderly patients after controlled hypotension shoulder arthroscopy and to observe the adverse events during 48 h of after surgery.
2. Materials and methods
2.1. Patients and study design
The protocol was approved by the Ethics Committee of Jiaxing the Second Hospital (Jiaxing, China). The study was conducted in accordance with the guidelines of Good Clinical Practice and the principles expressed in the Declaration of Helsinki. The study was registered at chictr.org(ChiCTR-INR-17010347). Each patient signed an informed consent for participation.
From June 2013 to May 2016, patients who were undergoing elective shoulder arthroscopy, according with American Society of Anesthesiologists (ASA) grade II–III, ≥ 60 years old were selected, and received 48 h patient-controlled intravenous analgesia (PCIA) after surgery for this study. The patients were randomly divided into two groups (n = 76 each) by an independent anesthetist. The patients and investigators were not informed of the groupings of the patients. Exclusion criteria: the patients refused to participate, patients with a history of neurological and psychiatric disorders, patients who take psychotropic drugs, patients with a history of alcohol abuse or drug dependence, patients who often use pain medications, patients who receive opioid and dexmedetomidine for allergies, patients with mini–mental status examination (MMSE) scores < 23, patients with chronic obstructive pulmonary disease, patients with a history of heart block or sinus bradycardia.
2.2. Anesthesia management
All patients had no premedication. After arriving at the operating room, all patients were monitored using 5-lead electrocardiography(ECG), noninvasive blood pressure (NIBP), pulse oximetry (SpO2), partial pressure of end-tidal CO2 (PetCO2), and temperature. After local anesthesia, an arterial cannula was placed in the left or right radial artery to monitor invasive arterial blood pressure. The electrodes of the bispectral index (BIS, Aspect, USA) was placed on the side of the patient's forehead to measure the depth of sedation. At fifteen minutes before the induction of anesthesia, parecoxib sodium 40 mg (Batch number: J20130044, Pharmacia and Upjohn Inc., USA) diluted with 5 ml of saline was intravenously injected in patients in every group. At the same time, dexmedetomidine was intravenously infused at a loading dose of 0.5 μg/kg over 15 min, followed by a continuous infusion at a rate of 0.5 μg/kg/h until the end of surgery in two groups, (Batch number: 13071134, Jiangsu HengRui Medicine Co., Ltd). Anesthesia was induced by intravenous administration of midazolam (0.04 mg/kg), propofol (1.5–2 mg/kg), sufentanil (0.4–0.6 μg/kg), and cisatracurium (0.2 mg/kg). Patients underwent endotracheal intubation for mechanical ventilation, with a tidal volume of 8–10 ml/kg, respiratory rate of 12–14 times/min, inspiratory to expiratory ratio of 1:2, and PetCO2 was maintained at 35–45 mm Hg (1 mm Hg = 0.133 kPa). After endotracheal intubation, under the guidance of ultrasound, the intrescalene brachial plexus block was performed, 0.375% ropivacaine 20 ml was used for the block and the block was one shot, and right internal jugular vein catheter was retrogradely placed to the external auditory canal level (internal jugular bulb) to prepare for blood sampling. All patients were in lateral position.
Intravenous infusion of propofol (4–8 mg/kg/h) and cis-atracurium (0.1–0.2 mg/kg/h), and remifentanil (0.05–0.25 μg/kg/min) was adjusted according to MAP in operation. BIS values were maintained at 40–55. Arterial pressure transducers was placed in the external auditory canal level. Mean arterial pressure (MAP) was maintained at 60–65 mm Hg, but for patients with a history of hypertension, MAP was decreased to a baseline of 75–80%. MAP was lower than 55 mm Hg or MAP decreased to < 30% of the baseline values in patients with a history of hypertension, phenylephrine was administered, while 0.5 mg atropine was given if the HR fell to < 50 bpm. The treatments were repeated if necessary. Nasopharyngeal temperature was maintained ≥ 36 °C. At the start of skin closure time, all intravenous infusion anesthetics were stopped; and the analgesia pump was connected. Before connecting analgesia pump, 0.06 μg/kg of sufentanil was intravenously injected. Postoperative analgesics were used for PCIA. Operative time, transfusion volume, urine volume, and recovery time were recorded. The occurrence of hypotension and sinus bradycardia were also recorded.
2.3. Postoperative pain management
The degree of pain at rest and upon movement was measured at 1, 3, 6, 14, 24, 36 and 48 h after surgery using the 11-point numerical the visual analogue scale (VAS) (0 = no pain, 10 = most severe pain). Analgesia pump parameters were set to a background flow of 2 ml/h, PCA 1.5 ml, and a lockout time of 5 min, maximal 6 ml/h. If the patient's rest VAS score is ≥ 4, 2 ml of analgesia pump liquid was given by the investigator.
2.4. Endpoints
The primary outcome measures were mini–mental status examination scores 1 day before surgery and 1, 2 and 7 days after surgery and the occurrence of post-operative cognitive dysfunction during 7 days after surgery in the two groups. The secondary outcome measures were jugular venous oxygen partial pressure and jugular venous oxygen saturation, pain intensity, analgesia pump liquid amount within 24 h after surgery and number of rescue analgesia during 48 h after surgery, level of sedation and concerning adverse effects.
At preoperative 1 day and postoperative 1, 2 and 7 days, MMES score was used to evaluate cognitive function, which was defined according to the MMSE score [11]. MMSE score out of 30 points, postoperative MMSE score was ≤ 2 points compared with preoperative baseline to reflect POCD [12]. POCD incidence was recorded within seven days postoperative.
At immediately controlled hypotension, controlled hypotension 1 h, postoperative 24 h, blood samples was taken from the radial artery and internal jugular vein bulb for blood gas analysis. Arterial blood lactic acid(LAC), base excess(BE), arterial oxygen saturation(PaO2), jugular venous oxygen partial pressure(PjvO2) and jugular venous oxygen saturation(SjvO2) values were measured and recorded.
Ramsay score was used to assess the level of sedation (1 points: anxiety; 2 points: quiet cooperation with directional force; 3 points: sleepiness, but the order reaction; 4: sleeping, quick response to tapping theglabella and loud auditory; 5: sleeping, delayed response to tapping theglabella and loud auditory; 6 points: deep sleep state, it is difficult to wake up). The degree of pain at rest and upon movement was measured at 1, 3, 6, 14, 24, 36 and 48 h after surgery using the 11-point numerical the visual analogue scale (VAS) (0 = no pain, 10 = most severe pain). The 24-h analgesia pump liquid amount was recorded, the occurrence of nausea and vomiting, itching, shiver, respiratory depression and sinus bradycardia were observed within 48 h after surgery.
2.5. Statistical analysis
The sample size was calculated on the basis of our preliminary experiments difference of 20% in the incidence of POCD during 7 days after surgery. For a study power of 90% (α = 0.05, β = 0.1), the required sample size per group was calculated to be 68, a dropout rate of 10%, the final sample size was determined to be 75 patients each group.
Quantitative variables are presented as mean ± standard deviation(SD) or median with interquartile range. Categorical data were reported as frequencies. Enumeration data and categorical variables were analyzed using x2 or Fisher's exact tests. Continuous variables were tested with MannWhitney U test or t-test depending on the distribution of the data. P-value < 0.05 was considered to be statistically significant. Data were analyzed using SPSS 19.0 (Inc., Chicago, IL, USA).
3. Results
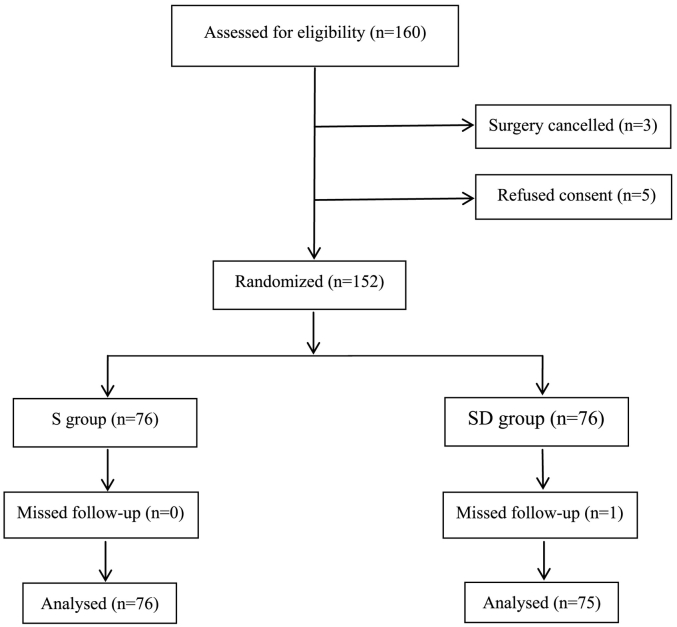
160 elderly patients were recruited in accordance with the inclusion criteria. However, 8 patients were subsequently excluded: 5 patients declined to give consent; surgery was cancelled for 3 patients. The remaining 152 patients were included in randomization and data analysis (Fig. 1). Baseline characteristics of patients in the two groups were comparable (Table 1). There were no significant differences in age, height, body weight, ASA grade (P > 0.05, Table 1). No significant differences could be seen between the two groups in terms of the intraoperative data and recovery time at PACU, sinus bradycardia (P > 0.05, Table 1).
Fig. 1.
Patient flowchart.
Table 1.
Baseline characteristics of patients and procedures.
| Variables | S group | SD group | P values |
|---|---|---|---|
| Age, y | 65.0 ± 5.8 | 65.5 ± 5.3 | 0.568 |
| Body weight, kg | 67.2 ± 6.2 | 66.3 ± 6.4 | 0.377 |
| Height, cm | 166.3 ± 6.1 | 167.6 ± 5.9 | 0.197 |
| ASAII/III | 63/13 | 60/16 | 0.536 |
| Duration of surgery, min | 148.4 ± 19.8 | 150.7 ± 20.2 | 0.492 |
| Compound sodium chloride, ml | 1599.9 ± 188.8 | 1659.4 ± 220.8 | 0.076 |
| Urine output, ml | 375.4 ± 76.2 | 397.1 ± 67.4 | 0.065 |
| Recovery time at PACU, min | 31.1 ± 4.4 | 29.9 ± 4.3 | 0.094 |
| Sinus bradycardia, (n)% | (12)15.8% | (13)17.1% | 0.827 |
Data are expressed as means ± SD or number (%).
ASA, American Society of Anesthesiologists; PACU, postanesthesia care unit.
The MMSE score in SD group was higher than S group at 1, 2 and 7 days after surgery (P < 0.05, Table 7). The incidence of postoperative cognitive dysfunction during 7 days after surgery in S and SD groups was respectively 17.1% and 6.7%.
Table 7.
MMSE scores measured at different time points.
| Variable | Time points | S group | SD group | P values |
|---|---|---|---|---|
| MMES | Before surgery 1d | 28.3 ± 1.3 | 28.5 ± 1.2 | 0.329 |
| After surgery 1d | 24.6 ± 1.3 | 25.2 ± 1.1a | 0.003 | |
| After surgery 2d | 25.0 ± 1.1 | 25.6 ± 1.1a | 0.002 | |
| After surgery 7d | 26.7 ± 1.2 | 27.4 ± 1.3a | 0.001 |
Data are expressed as means ± SD.
MMSE, mini–mental status examination.
P < 0.05 VS S group.
There were no significant differences in LAC, BE, and PaO2 values at immediately controlled hypotension, controlled hypotension 1 h, postoperative 24 h among the two groups(P > 0.05, Table 2). Compared with the S group, SjvO2 and PjvO2 values at postoperative 24 h were significantly higher in SD groups (P < 0.05, Table 3).
Table 2.
LAC, BE and PaO2 values at different time points.
| Variables | Time points | S group | SD group | P values |
|---|---|---|---|---|
| LAC (mmol/L) |
Immediately controlled hypotension | 1.02 ± 0.41 | 1.07 ± 0.38 | 0.425 |
| Controlled hypotension 1 h | 1.14 ± 0.44 | 1.09 ± 0.47 | 0.522 | |
| Postoperative 24 h | 1.08 ± 0.40 | 1.00 ± 0.39 | 0.205 | |
| BE (mmol/L) |
Immediately controlled hypotension | − 0.47 ± 2.00 | − 0.55 ± 1.76 | 0.790 |
| Controlled hypotension 1 h | − 0.92 ± 2.09 | − 1.01 ± 2.26 | 0.785 | |
| Postoperative 24 h | − 0.47 ± 1.51 | − 0.52 ± 1.41 | 0.825 | |
| PaO2 (mm Hg) |
Immediately controlled hypotension | 271.4 ± 75.7 | 284.1 ± 85.3 | 0.334 |
| Controlled hypotension 1 h | 265.3 ± 69.1 | 273.3 ± 66.4 | 0.464 | |
| Postoperative 24 h | 96.8 ± 8.6 | 97.9 ± 8.8 | 0.451 |
Data are expressed as means ± SD.
LAC, Arterial blood lactic acid; BE, base excess; PaO2, arterial oxygen saturation.
Table 3.
SjvO2 and PjvO2 values at different time points.
| Variables | Time points | S group | SD group | P values |
|---|---|---|---|---|
| SjvO2(%) | Immediately controlled hypotension | 70.6 ± 4.7 | 70.3 ± 5.0 | 0.738 |
| Controlled hypotension 1 h | 69.0 ± 4.7 | 68.5 ± 4.9 | 0.480 | |
| Postoperative 24 h | 61.7 ± 4.5 | 63.3 ± 4.9a | 0.039 | |
| PjvO2 (mm Hg) |
Immediately controlled hypotension | 48.3 ± 3.8 | 48.4 ± 3.4 | 0.841 |
| Controlled hypotension 1 h | 47.2 ± 3.3 | 47.5 ± 2.8 | 0.633 | |
| Postoperative 24 h | 40.2 ± 3.9 | 41.8 ± 2.7a | 0.003 |
Data are expressed as means ± SD.
SjvO2, jugular venous oxygen saturation.
PjvO2, jugular venous oxygen partial pressure.
P < 0.05 VS S group.
Compared with the S group, Ramsay score was significantly higher in SD group at 1 h after surgery(P < 0.05, Table 4); the VAS scores at rest and upon movement were significantly lower at 6, 14, 24, 36 and 48 h after surgery in SD group (P < 0.05, Table 4); analgesia pump liquid amount during 24 h after surgery and Number of rescue analgesia during 48 h after surgery were significantly lower in SD group (P < 0.05, Table 5); the occurrence of nausea and vomiting within 48 h after surgery were significantly lower in SD group(P < 0.05, Table 6). We found no complications including respiratory depression and sinus bradycardia within 48 h after surgery (P > 0.05, Table 6).
Table 4.
Ramsay, VAS scores during 48 h after surgery.
| Variables | Time points | S group | SD group | P values |
|---|---|---|---|---|
| Ramsay | 1 h | 2(1–3) | 2.5(2–4)a | 0.027 |
| 3 h | 2(2–2) | 2(2–3) | 0.081 | |
| 6 h | 2(2–2) | 2(2–2) | 1.000 | |
| 14 h | 2(2–2) | 2(2–2) | 1.000 | |
| 24 h | 2(2–2) | 2(2–2) | 1.000 | |
| 36 h | 2(2–2) | 2(2–2) | 1.000 | |
| 48 h | 2(2–2) | 2(2–2) | 1.000 | |
| Rest VAS | 1 h | 1(0–2) | 1(0–2) | 0.828 |
| 3 h | 2(0–3) | 2(0–3) | 0.852 | |
| 6 h | 3(2–4) | 2(2–4)a | 0.006 | |
| 14 h | 3(2–5) | 2(2–3)a | 0.000 | |
| 24 h | 2(1–4) | 2(0–3)a | 0.029 | |
| 36 h | 2(1–4) | 2(0–3)a | 0.032 | |
| 48 h | 1(0–3) | 0(0–3)a | 0.015 | |
| Movement VAS | 1 h | 1(0–2) | 1(0–2) | 0.832 |
| 3 h | 2(0–3) | 2(0–3) | 0.768 | |
| 6 h | 4(2–6) | 4(2–5)a | 0.044 | |
| 14 h | 4(2–6) | 4(2–5)a | 0.033 | |
| 24 h | 4(2–5) | 3(2–5)a | 0.007 | |
| 36 h | 3(2–5) | 3(2–4)a | 0.005 | |
| 48 h | 3(2–5) | 3(2–4)a | 0.001 |
Data are expressed as median with interquartile range.
VAS, the visual analogue scale.
P < 0.05 VS S group.
Table 5.
Analgesia pump liquid amount during 24 h after surgery and number of rescue analgesia during 48 h after surgery.
| Variables | S group | SD group | P values |
|---|---|---|---|
| Analgesia pump liquid amount during 24 h after surgery(ml) | 48(48–60) | 48(48–54)a | 0.021 |
| Number of rescue analgesia during 48 h after surgery(n)% | (22)28.9% | (10)13.2%a | 0.017 |
Data are expressed as median with interquartile range and frequencies.
P < 0.05 VS S group.
Table 6.
Adverse Effects during 48 h after surgery.
| Adverse reactions | S group | SD group | P values |
|---|---|---|---|
| Nausea | 21.1% | 9.2%a | 0.042 |
| Vomiting | 17.1% | 6.6%a | 0.045 |
| Itching | 5.3% | 5.3% | 1.000 |
| Shiver | 6.6% | 6.6% | 1.000 |
| Respiratory depression | 0 | 0 | – |
| Sinus bradycardia | 0 | 0 | – |
Data are expressed as rate.
P < 0.05 VS S group.
4. Discussion
The randomized clinical trial showed that at 15 min before the induction of anesthesia, intravenously infusion of parecoxib sodium 40 mg and dexmedetomidine at a dose of 0.5 μg/kg over 15 min, followed by a continuous infusion at a rate of 0.5 μg/kg/h until the end of surgery, then infusion of a combination of dexmedetomidine (0.06μg/kg/h) and sufentanil (0.04μg/kg/h) as PCA during the initial 48 h after shoulder arthroscopy could reduce POCD incidence during 7 days after surgery, improve postoperative cerebral oxygen metabolism, minimize analgesic consumption, decrease postoperative pain scores, and reduce the incidence of sufentanil-induced nausea and vomiting, without the occurrence of clinically relevant bradycardia, oversedation, and respiratory depression.
In order to maintain a clear operative field and reduce bleeding, there is a need for intraoperative controlled hypotension in shoulder arthroscopy. The decreased self-regulating ability of elderly patients and intraoperative hypotension can easily lead to an imbalance in the environment and cerebral insufficiency, and increase the risk of POCD [13]. Reduced intraoperative cerebral oxygen saturation can lead to the occurrence of POCD [14]. This study showed that for patients in each group, there were no significant changes in LAC, BE and PaO2 at each time point among the two groups, urine volume, SjvO2 and PjvO2 were normal, this prompts that internal environment and tissue perfusion were good. This study showed that in the intraoperative and postoperative period, combined with dexmedetomidine administration could improve SjvO2 and PjvO2 at 24 h after surgery.
POCD is a common complication of central nervous system in elderly patients. POCD is not only related to the self-factors and surgery-related factors, but also related to acute postoperative pain. Study found more severe postoperative pain and more opioid could cause more incidence of postoperative delirium [15]. Studies confirmed that parecoxib sodium pretreatment might improve pain threshold, and reduce postoperative pain [16], [17]. Intraoperative dexmedetomidine infusion could certainly reduce the amount of opioid [18]. The postoperative infusion of dexmedetomidine could certainly improve the analgesics effect of opioids and reduce the amount of opioid [19]. This study also obtained similar results, and found that parecoxib sodium pretreatment combined perioperative application of dexmedetomidine provided a better sparing effect on opioids, and reduced postoperative pain. Animal experimental study showed this cox-2 mediated neurodegeneration is related to impairments of memory parameters [20]. Clinical study confirmed that parecoxib sodium premedication could reduce the incidence of POCD, and the percent of POCD in parecoxib sodium group was 28.6% [21], and intraoperative dexmedetomidine administration could also reduce the incidence of POCD, the percent of POCD in DEX group was 26.6% [22]. Dexmedetomidine may involve the following mechanisms to improve cognitive dysfunction: 1. inhibited inflammation [23]. 2. Improved analgesic effect [19]. 3. Protected the central nervous system [8]. 4. Improved sleep quality [24]. This study showed that the incidence of POCD in SD group (6.7%) was lower than S group (17.1%). Furthermore, the pre-administration of parecoxib sodium combined with perioperative dexmedetomidine administration could certainly reduce the incidence of POCD. This suggests that the scheme for improving POCD in elderly patients may be more reasonable.
A rational regimen is not only related to efficacy, but is also related to the occurrence and severity of adverse reactions. Even with better efficacy, the occurrence of serious adverse events would affect the quality of life of patients. Therefore, patients will be given a lower rating. The highest postoperative incidence of adverse reactions in the two groups of patients was nausea, followed by vomiting; and other types of adverse events were low. The most dangerous complication of opioids was respiratory depression, and the study found that no respiratory depression occurred within 48 h postoperative. The main side effect of dexmedetomidine administration in patients was caused by sinus bradycardia. However, this study did not find such complication within 48 h postoperatively.
In the present study, there were some limitations. First, cognitive function in patients was only observed within seven days. Furthermore, the long-term effect of cognitive function in elderly patients remains to be further explored. Second, due to the study time and other reasons, the sample size was not big enough. Furthermore, this is only a single-center study; and there is a need for a multi-center study with a large sample size. Finally, parecoxib sodium pretreatment combined with dexmedetomidine administration can reduce the incidence of POCD in elderly patients, however, its mechanism of action needs to be further explored in future studies.
In conclusion, parecoxib sodium pretreatment combined with perioperative dexmedetomidine administration could better decrease the incidence of POCD after controlled hypotension shoulder arthroscopy, provide a good effect on postoperative pain and postoperative opioid consumption, and improve postoperative cerebral oxygen metabolism. This scheme may be a way to improve early postoperative cognitive dysfunction in elderly patients.
Funding
This work was supported by Jiaxing science and Technology Bureau (2013, AY21043-4).
Conflicts of interest
The authors report no conflicts of interest.
References
- 1.Gillespie R., Shishani Y., Streit J. The safety of controlled hypotension for shoulder arthroscopy in the beach-chair position. J Bone Joint Surg Am. 2012;94:1284–1290. doi: 10.2106/JBJS.J.01550. [DOI] [PubMed] [Google Scholar]
- 2.Newman S., Stygall J., Hirani S. Postoperative cognitive dysfunction after noncardiac surgery: a systematic review. Anesthesiology. 2007;106:572–590. doi: 10.1097/00000542-200703000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Moller J.T., Cluitmans P., Rasmussen L.S. Long-term postoperative cognitive dysfunction in the elderly: ISPOCD1 study. Lancet. 1998;351:857–861. doi: 10.1016/s0140-6736(97)07382-0. [DOI] [PubMed] [Google Scholar]
- 4.Williams-Russo P., Sharrock N.E., Mattis S. Cognitive effects after epidural VS general anesthesia in older adults. A randomized trial. JAMA. 1995;274:44–50. [PubMed] [Google Scholar]
- 5.Wang Y., Sands L.P., Vaurio L. The effects of postoperative pain and its management on postoperative cognitive dysfunction. Am J Geriatr Psychiatry. 2007;15:50–59. doi: 10.1097/01.JGP.0000229792.31009.da. [DOI] [PubMed] [Google Scholar]
- 6.Malan T.P., Marsh G., Hakki S.I. Parecoxib sodium, a parenteral cyclooxygenase 2 selective inhibitor, improves morphine analgesia and is opioid-sparing following total hip arthroplasty. Anesthesiology. 2003;98:950–956. doi: 10.1097/00000542-200304000-00023. [DOI] [PubMed] [Google Scholar]
- 7.Ng A., Smith G., Davidson A.C. Analgesic effects of parecoxib following total abdominal hysterectomy. Br J Anaesth. 2003;90:746–749. doi: 10.1093/bja/aeg139. [DOI] [PubMed] [Google Scholar]
- 8.Bell M.T., Puskas F., Bennett D.T. Dexmedetomidine, an α-2a adrenergic agonist, promotes ischemic tolerance in a murine model of spinal cord ischemia-reperfusion. J Thorac Cardinvasc Surg. 2014;147:500-6. doi: 10.1016/j.jtcvs.2013.07.043. [DOI] [PubMed] [Google Scholar]
- 9.Sanders R.D., Sun P., Patel S. Dexmedetomidine provides cortical neuroprotection: impact on anaesthetic-induced neuroapoptosis in the rat developing brain. Acta Anaesthesiol Scand. 2010;54:710–716. doi: 10.1111/j.1399-6576.2009.02177.x. [DOI] [PubMed] [Google Scholar]
- 10.Iwakiri H., Oda Y., Asada A. The efficacy of continuous infusion of low dose dexmedetomidine for postoperative patients recovering in general wards. Eur J Anaesthesiol. 2012;29:251–254. doi: 10.1097/EJA.0b013e3283529ba8. [DOI] [PubMed] [Google Scholar]
- 11.Radtke F.M., Franck M., Herbig T.S. Incidence and risk factors for cognitive dysfunction in patients with severe systemic disease. J Int Med Res. 2012;40:612–620. doi: 10.1177/147323001204000223. [DOI] [PubMed] [Google Scholar]
- 12.Mondimore F.M., Damlouji N., Folstein M.F. Post-ECT confusional states associated with elevated serum anticholinergic levels. Am J Psychiatry. 1983;140:930–931. doi: 10.1176/ajp.140.7.930. [DOI] [PubMed] [Google Scholar]
- 13.Chanques G., Jaber S. I'm ready to add a simple test for my anesthesia consultation to screen patients at risk to develop cognitive dysfunction. Ann Fr Anesth Reanim. 2013;32:546–547. doi: 10.1016/j.annfar.2013.07.797. [DOI] [PubMed] [Google Scholar]
- 14.Tang L., Kazan R., Taddei R. Reduced cerebral oxygen saturation during thoracic surgery predicts early postoperative cognitive dysfunction. Br J Anaesth. 2012;108:623–629. doi: 10.1093/bja/aer501. [DOI] [PubMed] [Google Scholar]
- 15.Leung J.M., Sands L.P., Lim E. Does preoperative risk for delirium moderate the effects of postoperative pain and opiate use on postoperative delirium? Am J Geriatr Psychiatry. 2013;21:946–956. doi: 10.1016/j.jagp.2013.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu J., Liu Z., Xia K. Effect of preemptive analgesia with parecoxib sodium in patients undergoing radical resection of lung cancer. Int J Clin Exp Med. 2015;8:14115–14118. [PMC free article] [PubMed] [Google Scholar]
- 17.Bajaj P., Ballary C.C., Dongre N.A. Comparison of the effects of parecoxib and diclofenac in preemptive analgesia: a prospective, randomized, assessor-blind, single-dose, parallel-group study in patients undergoing elective general surgery. Curr Ther Res Clin Exp. 2004;65:383–397. doi: 10.1016/j.curtheres.2004.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.ISchnabel A., Meyer-Frießem C.H., Reichl S.U. Is intraoperative dexmedetomidine a new option for postoperative pain treatment? A meta-analysis of randomized controlled trials. Pain. 2013;154:1140–1149. doi: 10.1016/j.pain.2013.03.029. [DOI] [PubMed] [Google Scholar]
- 19.Nie Y., Liu Y., Luo Q. Effect of dexmedetomidine combined with sufentanil for post-caesarean section intravenous analgesia: a randomised, placebo-controlled study. Eur J Anaesthesiol. 2014;31:197–203. doi: 10.1097/EJA.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 20.Sil S., Ghosh T. Role of cox-2 mediated neuroinflammation on the neurodegeneration and cognitive impairments in colchicine induced rat model of Alzheimer's disease. J Neuroimmunol. 2016;15:115–124. doi: 10.1016/j.jneuroim.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 21.Tian Y., Zhao P., Li L. Pre-emptive parecoxib and post-operative cognitive function in elderly patients. Int Psychogeriatr. 2014;15:1–8. doi: 10.1017/S1041610214001951. [DOI] [PubMed] [Google Scholar]
- 22.Chen J., Yan J., Han X. Dexmedetomidine may benefit cognitive function after laparoscopie cholecystectomy in elderly patients. Exp Ther Med. 2013;5:489–494. doi: 10.3892/etm.2012.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Qiao H., Sanders R.D., Ma D. Sedation improves early outcome in severely septic Sprague Dawley rats. Crit Care. 2009;13(4):R136. doi: 10.1186/cc8012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Su X., Meng Z.T., Wu X.T. Dexmedetomidine for prevention of delirium in elderly patients after non-cardiacsurgery: a randomised, double-blind, placebo-controlled trial. Lancet. 2016;388:1893–1902. doi: 10.1016/S0140-6736(16)30580-3. [DOI] [PubMed] [Google Scholar]