Abstract Abstract
The Caesalpinia group is a large pantropical clade of ca. 205 species in subfamily Caesalpinioideae (Leguminosae) in which generic delimitation has been in a state of considerable flux. Here we present new phylogenetic analyses based on five plastid and one nuclear ribosomal marker, with dense taxon sampling including 172 (84%) of the species and representatives of all previously described genera in the Caesalpinia group. These analyses show that the current classification of the Caesalpinia group into 21 genera needs to be revised. Several genera (Poincianella, Erythrostemon, Cenostigma and Caesalpinia sensu Lewis, 2005) are non-monophyletic and several previously unclassified Asian species segregate into clades that merit recognition at generic rank. In addition, the near-completeness of our taxon sampling identifies three species that do not belong in any of the main clades and these are recognised as new monospecific genera. A new generic classification of the Caesalpinia group is presented including a key for the identification of genera, full generic descriptions, illustrations (drawings and photo plates of all genera), and (for most genera) the nomenclatural transfer of species to their correct genus. We recognise 26 genera, with reinstatement of two previously described genera (Biancaea Tod., Denisophytum R. Vig.), re-delimitation and expansion of several others (Moullava, Cenostigma, Libidibia and Erythrostemon), contraction of Caesalpinia s.s. and description of four new ones (Gelrebia, Paubrasilia, Hererolandia and Hultholia), and make 75 new nomenclatural combinations in this new generic system.
Keywords: Mimosoideae-Caesalpinieae-Cassieae clade, Caesalpinioideae, Leguminosae, Fabaceae, generic delimitation, phylogeny, taxonomy
Introduction
Resolving generic limits, reconciling genera with monophyletic groups and establishing stable generic classifications remain some of the most active and at times contentious issues in systematics (Humphreys and Linder 2009, Vences et al. 2013, Garnock-Jones 2014). This is very much the case in the large plant family Leguminosae, where delimitation of genera has been in a state of considerable flux, in large part because of the lack of robust and well-sampled species-level phylogenies (LPWG 2013, and LPWG submitted). In the past three decades, phylogenetic analyses of legume groups with adequate and representative species-level sampling have revealed the non-monophyly of numerous genera previously delimited using morphology alone (e.g. Acacia Mill. [e.g., Murphy 2008, Bouchenak-Khelladi et al. 2010; Miller and Seigler 2012], Piptadenia Benth. [Jobson and Luckow 2007], Monopetalanthus Harms [Wieringa 1999], Hymenostegia Harms [Mackinder et al. 2013; Mackinder and Wieringa 2013; Wieringa et al. 2013], Vigna Savi [Delgado-Salinas et al. 2011], Lonchocarpus Kunth [Da Silva et al. 2012], Poecilanthe Benth. [Meireles et al. 2014], Derris Lour. [Sirichamorn et al. 2014], Otholobium C.H. Stirt. [Egan and Crandall 2008; Dludlu et al. 2013], Dioclea Kunth, and Galactia P. Browne [De Queiroz et al. 2015]). In many other legume groups extensive non-monophyly of genera has been reported, but phylogenies with increased molecular and taxonomic sampling are necessary to provide the robust evidence needed to establish new generic systems (e.g. Bauhinia L., Cynometra L., Maniltoa Scheff., Millettia Wight & Arn., Albizia Durazz., Archidendron F. Muell., Leucochloron Barneby & J. W. Grimes, Entada Adans. (see LPWG 2013 and references therein).
The Caesalpinia group epitomises this generic flux, with persistent doubts about the delimitation of genera over the last 35 years (Gagnon et al. 2013; Fig. 1). This has been due to the difficulties of identifying diagnostic morphological synapomorphies and obtaining adequate sampling of taxa and genes in phylogenetic studies for this large pantropically distributed clade. The group is placed in the newly re-circumscribed subfamily Caesalpinioideae (LPWG submitted; equivalent to the Mimosoideae-Cassieae-Caesalpinieae, MCC clade sensu Doyle (2012); see also LPWG 2013), forming one of the informal groups in tribe Caesalpinieae. The Caesalpinia group was defined by Polhill and Vidal (1981) to include the genera with species that have a large variety of glandular trichomes, prickles and spines as a defense mechanism, and possessing zygomorphic flowers with a somewhat modified lower sepal and stamens crowded around the pistil. It is currently classified into 21 genera (Lewis 2005), but recent studies, and notably Gagnon et al. (2013, 2015), have demonstrated the non-monophly of some of these and the need for a new generic classification (Fig. 1). The group comprises ca. 205 species of small trees, woody shrubs and herbaceous subshrubs, with extremely diverse pollination and seed dispersal syndromes (the diversity of plant forms, flowers and fruits is extensively illustrated for all genera in the taxonomic acount), occurring predominantly in seasonally dry tropical forests and shrublands, but extending in a subset of clades into tropical and warm temperate savannas, tropical wet forests and tropical coastal habitats.
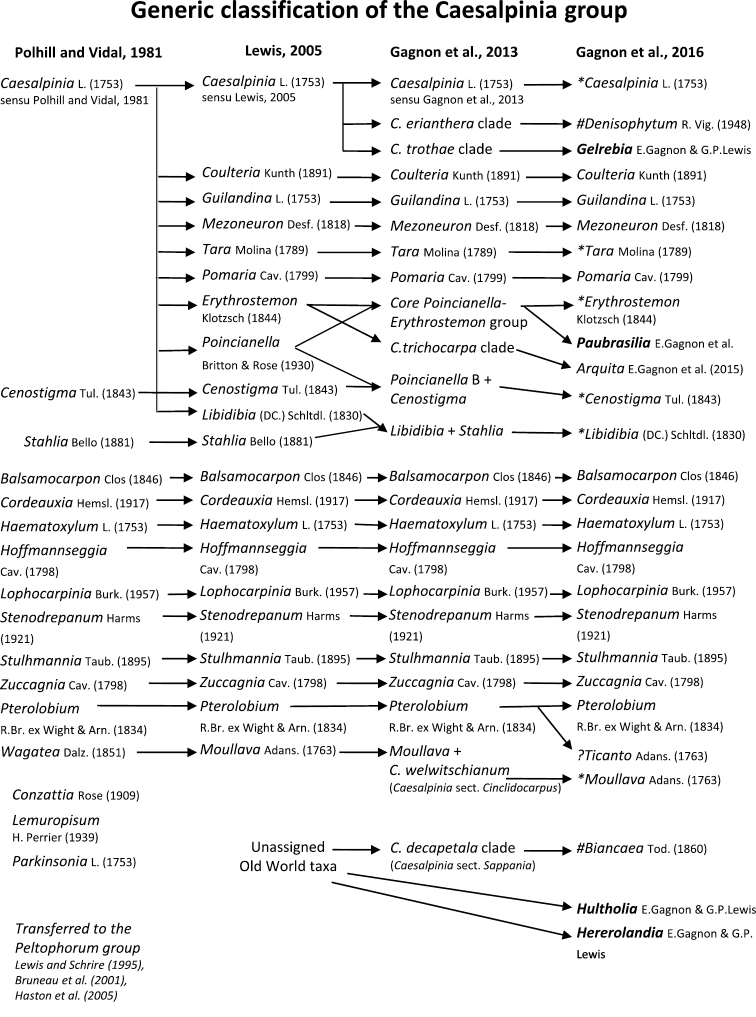
Figure 1.
Comparison of generic classifications for the Caesalpinia group proposed by Polhill and Vidal (1981), Lewis (2005), Gagnon et al. (2013), and this study; names in bold represent new genera described here; prefix * indicates that the description of the genus is emended; prefix # indicates that the genus is being re-instated; prefix ? indicates that the status of the genus is uncertain.
The genus Caesalpinia L. itself has been particularly problematic having been variously circumscribed by different authors. In its broadest sense Caesalpinia comprises ca. 150 species but these have had a tumultuous taxonomic and nomenclatural history, having been placed in up to 30 different genera since the description of the genus in 1753. These changing generic concepts illustrate the difficulties in establishing a stable classification of the group. The proliferation of generic names associated with Caesalpinia s. l. is due in part to the often complex, confusing and highly homoplastic nature of many morphological characters across the group, as well as the occurrence of many narrowly restricted endemics in a group with a pantropical distribution spanning five continents.
Previous molecular and morphologically-based phylogenetic analyses (Lewis and Schrire 1995, Simpson and Miao 1997, Simpson et al. 2003, Nores et al. 2012, Gagnon et al. 2013), including data from floral ontogeny (Kantz and Tucker 1994, Kantz 1996), phytochemistry (Kite and Lewis 1994), wood anatomy (Gasson et al. 2009), and leaf anatomy and secretory structures (Rudall et al. 1994, Lersten and Curtis 1994, 1996, Herendeen et al. 2003), attempted to more clearly delimit monophyletic genera within the Caesalpinia group. However, none of these studies achieved the comprehensive taxon sampling needed to fully understand and synthesize morphological diversity across the group as a whole. Other studies have focused on particular genera or clades, such as Hoffmannseggia Cav. (Simpson et al. 2004, 2005), Pomaria Cav. (Simpson et al. 2006), Mezoneuron Desf. (Clark and Gagnon 2015), and Arquita E. Gagnon, G. P. Lewis & C. E. Hughes (Gagnon et al. 2015). The most recent phylogenetic study (Gagnon et al. 2013), based on a single plastid marker (rps16) and sampling 120 of ca. 205 species (i.e. 58% taxon sampling), suggested that at least 23 genera would need to be recognised due to the non-monophyly of several genera, but lacked sufficient resolution and support as well as critical taxa (notably Lophocarpinia Burkart, Stahlia Bello, Stenodrepanum Harms, Caesalpinia pearsonii L. Bolus and Caesalpinia glandulosa Bertero ex DC.), to confidently propose a comprehensive new generic classification. Here we present a new phylogenetic analysis that samples the full morphological diversity and nearly the entire geographical range of the Caesalpinia group. This analysis is based on five plastid loci and the nuclear ribosomal ITS region, providing improved resolution and support over Gagnon et al. (2013). We use this densely sampled phylogenetic analysis to propose a new generic classification of the Caesalpinia group, in which we recognise 26 genera (with one additional clade tentatively suggested as a 27th genus to be recognised pending additional taxon sampling), provide new or emended generic descriptions, a key to genera and, for genera where no further ambiguity as to species placements exists, the new nomenclatural combinations for species as required.
Material and methods
Taxon sampling
DNA was extracted from herbarium specimens and field-collected silica-dried leaves from wild and, in a few cases, cultivated plants. When possible, multiple individuals per species from different localities were sampled. In addition, previously published sequences (Bruneau et al. 2001, 2008, Simpson et al. 2003, 2005, 2006, Haston et al. 2005, Marazzi et al. 2006, Marazzi and Sanderson 2010, Manzanilla and Bruneau 2012, Nores et al. 2012, Babineau et al. 2013, Gagnon et al. 2013, 2015) were downloaded from GenBank (Appendix 1). All 21 genera belonging to the informal Caesalpinia group (sensu Lewis 2005), including all their type species (except for Mezoneuron Desf.), were sampled.
A total of 429 accessions representing 172 of the ca. 205 species (83.9%) of the Caesalpinia group, and including 131 species previously ascribed to the genus Caesalpinia s. l., were sequenced (Appendix 1). This sampling represents the full geographical range and morphological diversity of the group, with the important exception of seven species from mainland China for which no material was available for study. Several key species, whose phylogenetic and taxonomic affinities were previously unclear, including Caesalpinia digyna Rottler, Caesalpinia tortuosa Roxb., Caesalpinia pellucida Vogel, Caesalpinia glandulosa, and Caesalpinia pearsonii, are analysed here for the first time. Nine outgroup taxa spanning the MCC clade were included: Gymnocladus chinensis Baill., Tetrapterocarpon geayi Humbert (Umtiza grade), Colvillea racemosa Bojer, Conzattia multiflora (B.L. Rob.) Standl. (Peltophorum group) and Cassia javanica L., Pterogyne nitens Tul., Senna alata (L.) Roxb., Senna covesii (A. Gray) H.S. Irwin & Barneby and Senna spectabilis (DC.) H.S. Irwin & Barneby (Cassieae clade).
Molecular methods
Three protocols were used to extract DNA: (1) a modified CTAB protocol (Joly and Bruneau 2006); (2) QIAGEN DNeasy Plant Mini Kit (Mississauga, ON, Canada); or (3) a 4% MATAB protocol (Ky et al. 2000). Six genetic markers were amplified, including the 5.8S subunit and flanking internal transcribed spacers, ITS1 and ITS2, of nuclear ribosomal DNA, and five plastid loci: rps16, the trnD-trnT intergenic spacer, ycf6-psbM, the matK gene and flanking 3’-trnK intron, and the trnL-trnF intron-spacer region. The first four markers were amplified using both standard and nested-PCR protocols, described in Gagnon et al. (2015). The matK-3’-trnK region was amplified using the primers trnK685F (Hu et al. 2000), trnK4La (Wojciechowski et al. 2004), trnK2R* and KC6 (Bruneau et al. 2008), following the protocols described in Bruneau et al. (2008). Because of initially poor amplifications, we designed a new primer, matK-C6-Caesalpinia (5’-GAA TGC TCG GAT AAT TGG TTT-3’), which improved the amplification of the 5’section of this locus. The trnL-trnF intron-spacer region was amplified using the primers trnL-C, -D, -E and -F (Taberlet et al. 1991), using the same protocols as for the rps16 locus (Gagnon et al. 2013), with annealing temperatures varying between 50 and 53 °C. While we attempted to amplify the first four loci for all available material, for the matK-3’trnK and trnL-trnF regions we sequenced a targeted subset of taxa to complement existing data. For problematic samples, including those presenting sequencing problems due to mononucleotide repeats, we used a protocol with Phusion Hot Start II High-Fidelity DNA polymerase (Thermo Scientific, United States), as described by Gagnon et al. (2013), which yields more accurate and longer quality mononucleotide sequence reads (Fazekas et al. 2010).
PCR amplifications were sequenced by Genome Quebec (Montreal, Canada), with Big Dye Terminator 3.1 chemistry on an ABI 3730xl DNA Analyzer (Applied Biosystems, Carlsbad, CA, USA). Geneious (version 5.6-6.1.8, Biomatters, Auckland, New Zealand) was used to assemble chromatograms and inspect and edit contigs. All sequences were submitted to BLAST (Altschul et al. 1990) to verify for non-specific amplification, and eliminated if they did not match Leguminosae sequences in GenBank. GenBank numbers with corresponding locality details and herbarium vouchers are listed in Appendix 1.
Phylogenetic analyses
Sequences were aligned, inspected and manually adjusted using Geneious, and the resulting matrices are available from Dryad Digital Repository (doi: 10.5061/dryad.f4h2h). Regions of ambiguous alignment corresponding mostly to variable mononucleotide and/or tandem repeats were excluded as follows: 42 nucleotides for ITS, 92 for rps16, 146 for trnD-trnT, 157 for ycf6-psbM, 86 for trnL-trnF and 16 for matK-3’trnK. Gaps were coded using simple indel coding (Simmons and Ochoterena 2000) in SeqState 1.4.1 (Müller 2005), retaining only non-autapomorphic indels.
Phylogenetic analyses were carried out on each of the six loci individually and on two concatenated matrices, one with the five plastid loci and a second matrix with all six loci (plastid + ITS). Matrices were concatenated using SequenceMatrix (Vaidya et al. 2011). We used a (ML) approach using RaxML 8.0.0 (Stamatakis 2014) on the CIPRES gateway v.3.3 (Miller et al. 2010). The analyses were conducted using the GTRGAMMA model for the DNA sequences and the BINCAT model for the indel partitions. Bootstrap support was assessed through 1000 non-parametric bootstrap replicates.
Because topological conflicts amongst the six individual gene trees were minimal, and where differences were found these were always only weakly supported (< 60% BS), all subsequent analyses were done on the six-locus concatenated matrix. Initial analyses of this six-locus matrix keeping all accessions of species as separate terminals resulted in a matrix with significant missing data because not all accessions were sequenced for all loci (see Tables 1 and 2). To reduce missing data, multiple accessions of the same species were concatenated if they occurred in the same clade in the preliminary RaxML analyses to maximize the number of loci represented for a species. When more than one sequence per species was available for a given locus, the longest sequence was selected, because we never found any sequence variation in the overlapping sections. This resulted in concatenation of accessions for 16 species (see Appendix 1): Caesalpinia cacalaco Bonpl., Caesalpinia caladenia Standl., Caesalpinia caudata (A. Gray) Fisher, Caesalpinia colimensis F. J. Herm., Caesalpinia epifanioi J. L. Contr., Caesalpinia exilifolia Griseb., Caesalpinia madagascariensis (R. Vig.) Senesse, Caesalpinia melanadenia (Rose) Standl., Caesalpinia mimosoides Lam., Caesalpinia pringlei (Britton & Rose) Standl., Caesalpinia sappan L., Caesalpinia sessilifolia S. Watson, Libidibia sclerocarpa (Standl.) Britton & Rose, Haematoxylum brasiletto H. Karst., Haematoxylum dinteri Harms and Tara spinosa (Molina) Britton & Rose. In addition to concatenating sequences obtained from different accessions of a species, preliminary analyses showed lack of resolution for a few accessions for which only one or two loci were sequenced. To explore the impacts of different levels of missing data, a series of matrices that progressively excluded accessions with five, four, three, two and one missing loci were generated, resulting in six different concatenated matrices (Table 2). Because the matrix containing sequences with no missing data lacked representatives from a number of genera and critical clades or species, a seventh matrix was generated (with 39 taxa) that added an accession from each of these critical taxa to maximise taxonomic representation while minimizing missing data.
Table 1.
Character statistics for the six loci analysed, with the number of accessions for each locus, aligned length (including ambiguous alignment regions), number of indels scored, numbers and % of parsimony informative characters (for both DNA and indel characters), and critical missing genera and taxa.
| Locus | Number of accessions | Aligned length | Number of informative indels | Numbers and % parsimony informative characters | Critical missing genera and taxa |
|---|---|---|---|---|---|
| ITS | 251 | 820 | 113 | 550/891 = 62% | Caesalpinia mimosoidesLophocarpiniaStenodrepanumStahlia |
| rps16 | 298 | 1081 | 45 | 311/1034 = 30% | LophocarpiniaStenodrepanum |
| trnD-trnT | 235 | 1921 | 108 | 513/1883 = 27% | LophocarpiniaStenodrepanum |
| ycf6-psbM | 193 | 1795 | 141 | 540/1779 = 30% | LophocarpiniaStenodrepanum |
| trnL-trnF | 171 | 1347 | 65 | 307/1326 =23% | None |
| matK-3’trnK | 89 | 1839 | 20 | 308/1843 =17% | Caesalpinia mimosoides |
Table 2.
Statistics for the seven combined matrices, with the number of accessions, number of ingroup and outgroup species, % missing data, and missing genera/critical taxa. The results of the parsimony analyses are indicated, with the number of trees retained, the length of the shortest trees (length), (CI), and (RI).
| All sequences | 2 loci + | 3 loci + | 4 loci + | 5 loci + | All 6 loci + | No missing genera | |
|---|---|---|---|---|---|---|---|
| Accessions | 408 | 312 | 223 | 175 | 76 | 30 | 39 |
| Nb. of Caesalpinia group species | 171/~205 | 163/~205 | 128/~205 | 103/~205 | 55/~205 | 26/~205 | 35/~205 |
| Nb. Caesalpinia s.l. species | 130/~155 | 123/~155 | 106/~155 | 84/~155 | 44/~155 | 20/~155 | 23/~155 |
| Outgroup species | 9 | 9 | 9 | 9 | 8 | 4 | 4 |
| % missing data | 61% | 53% | 43% | 38% | 28% | 23% | 30% |
| Missing genera/critical taxa | None | None | 2: Lophocarpinia, Stenodrepanum | 2: Lophocarpinia, Stenodrepanum | 3: Lophocarpinia, Stenodrepanum, Caesalpinia mimosoides | 8: Caesalpinia mimosoides, Cenostigma, Guilandina, Moullava, Lophocarpinia, Pterolobium, Stahlia, Stenodrepanum | None |
| Nb trees found | 50,000 | 50,000 | 50,000 | 50,000 | 7 | 2 | 2 |
| Length | 12,212 | 11,986 | 10,909 | 10,101 | 7,615 | 4,715 | 5405 |
| CI | 0.43 | 0.45 | 0.45 | 0.47 | 0.53 | 0.62 | 0.60 |
| RI | 0.81 | 0.81 | 0.79 | 0.78 | 0.66 | 0.49 | 0.48 |
For these seven concatenanted matrices, phylogenetic analyses were carried out using ML, (MP) and Bayesian methods. For the ML analyses, we used RaxML (Stamatakis 2014) as described above. For MP analyses, PAUP* (Swofford 2003) was used with a two-step approach (Davis et al. 2004) as described in Gagnon et al. (2013), but saving a maximum of 50,000 trees with 5,000 bootstrap replicates, with two trees retained per replicate. Bayesian analyses were conducted in MrBayes 3.2 (Ronquist et al. 2012) using MrModeltest v.2.3 (Nylander 2004) to select the GTR + I + G model for all six loci and the F81-like model for the indel partition. Analyses were run on a high performance computer cluster (Calcul Québec, Université de Montréal, Canada) with two parallel runs of eight (MCMC) chains, four swaps per swapping cycle, and trees sampled every 1000 generations. The stop criterion was set to an average standard deviation of split frequencies that dropped to below the critical value of 0.01. Tracer v.1.6 (Rambaut et al. 2014) was used to ensure effective sample sizes were above 200 and that chains mixed appropriately, with 510,000 and 27 million generations, depending on the size of the matrix. The “burn-in” fraction for all analyses was set to 10%.
Results
Of the six loci, ITS had the highest proportion of parsimony-informative characters (61.7%), followed by ycf6-psbM, rps16, trnD-trnT, trnL-trnC, and matK-3’trnK (Table 1). The concatenated six-locus matrix (aligned length = 8803 bp) included 429 accessions, which was reduced to 408 when accessions were combined for 16 species (see above). Table 2 summarises the number of accessions and species per locus, the percentage of missing data, the number of trees, tree length, CI and RI obtained in the MP analyses for the series of seven concatenated matrices with successively lower numbers of taxa with missing loci.
With the exception of the least informative (trnL-trnF) gene tree, which is poorly resolved (data not shown), the Caesalpinia group is monophyletic in all analyses, generally with high bootstrap and PP support (see Suppl. material 1). The 23 major clades identified from the rps16 phylogeny by Gagnon et al. (2013; Fig. 1) are also generally recovered in each of the individual ML gene trees (Suppl. material 1), as well in the analyses of the matrices combining all six loci, with two notable exceptions. First, in the MP and ML analyses, Lophocarpinia is nested within Haematoxylum, but in the Bayesian analyses Lophocarpinia is sister to Haematoxylum. Second, the genus Pterolobium is also sometimes recovered as non-monophyletic, with Caesalpinia crista nested within it in some of the MP, ML and Bayesian analyses, while in other analyses it is recovered as monophyletic, but with poor to moderate support in the ML and Bayesian analyses of all six loci, with a minimum of 2 to 3 loci per accession (Suppl. material 1).
In addition to these 23 clades (Fig. 1; see Gagnon et al. 2013), four other clades or monospecific lineages were consistently recovered in the MP, ML, and Bayesian analyses of the matrices with all six loci (Suppl. material 1): the three monospecific Caesalpinia echinata, Caesalpinia mimosoides and Caesalpinia pearsonii lineages, and the Caesalpinia crista clade, corresponding to Caesalpinia sect. Nugaria, represented by Caesalpinia crista and Caesalpinia vernalis in the rps16 gene tree of Gagnon et al. (2013), although it is important to note that Caesalpinia vernalis was excluded from later analyses of the concatenated matrices due to missing data and does not appear in Fig. 2 or Fig. 3. In total, this resulted in 27 possible genera in the Caesalpinia group, 26 of which are recognised here (see below). In addition, the MP, ML and Bayesian phylogenies based on the various concatenated datasets were generally congruent as to the relationships amongst these 27 lineages, regardless of the proportion of missing data, or number of missing genera/critical species. Minor differences observed between the topologies lacked support.
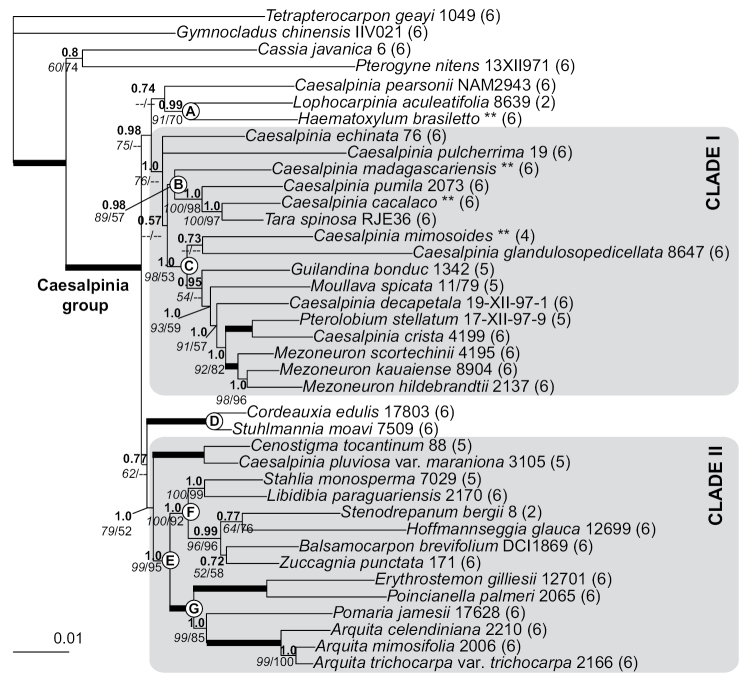
Figure 2.
Phylogeny of the Caesalpinia group. Bayesian phylogram based on 39 accessions, minimizing missing data while maximizing the taxonomic representation of each of the 27 putative genera within the Caesalpinia group. Branch support values are indicated as follows: branches in bold indicate that maximum support has been attained in the parsimony, Maximum Likelihood and Bayesian analyses; otherwise, posterior probabilities are indicated above in bold, with bootstrap support from ML analyses (italicised) and parsimony analyses separated by a slash below the branches.
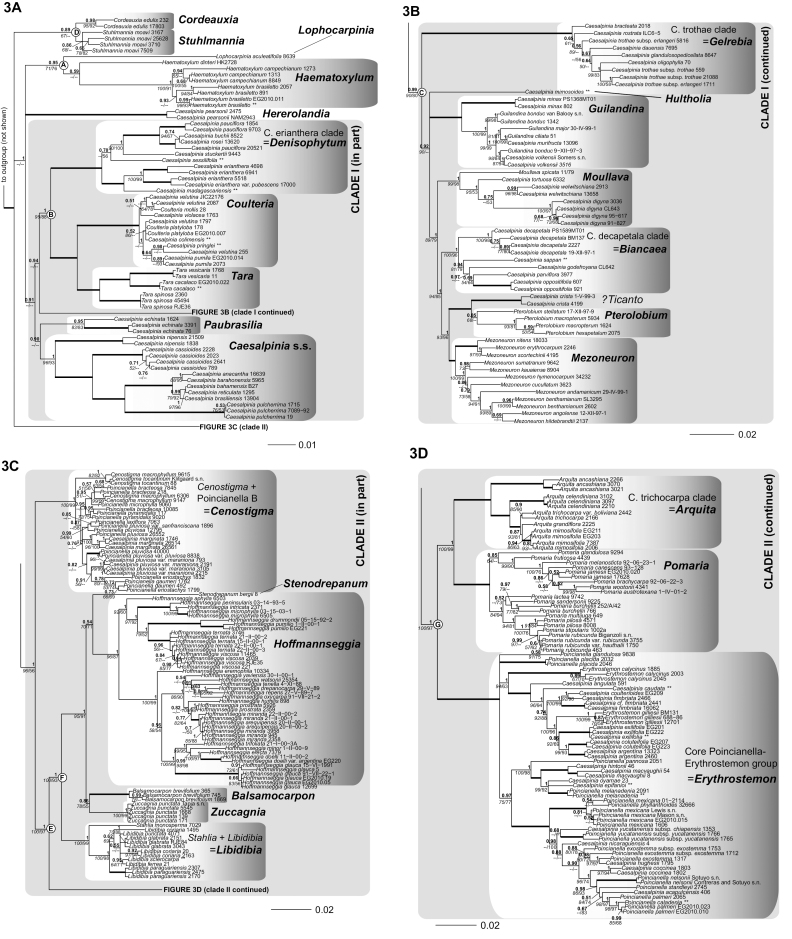
Figure 3.
A–D Phylogeny of the Caesalpinia group. Bayesian phylogram based on 312 accessions, including only accessions with two or more loci. Branch support values are indicated as follows: branches in bold indicate that maximum support has been attained in the MP, ML and Bayesian phylogenetic analyses; otherwise, posterior probabilities are indicated above in bold, with bootstrap support from ML analyses (italicised) and parsimony analyses separated by a slash below the branches; for each terminal, the species name is followed by the collector number of the corresponding voucher (see Appendix 1 for full voucher details); the suffix ** indicates that several sequences from different accessions of the same species were concatenated for analysis (see Appendix 1 for details); for major clades and genera, the names used by Gagnon et al. (2013) are indicated, as well as the corresponding new genera.
Given this congruence among the ML, MP and Bayesian analyses, only the Bayesian topology is presented (Figs 2 and 3A–D) and forms the basis for all subsequent discussion. The first diverging lineages of the Caesalpinia group comprise the species Caesalpinia pearsonii, the Lophocarpinia + Haematoxylum clade (Figs 2 and 3A, clade A), and the Cordeauxia + Stuhlmannia clade (Figs 2 and 3A, clade D). All other genera were placed in two large and robustly supported clades here designated clades I and II (Figs 2 and 3). Clade I (Figs 2 and 3A–B) includes Caesalpinia echinata, Caesalpinia s. s., a clade comprising Tara + Coulteria + the Caesalpinia erianthera clade (Figs 2 and 3A, clade B), as well as a group corresponding to the Caesalpinia trothae clade and all lineages consisting predominantly of Asian liana species (Caesalpinia mimosoides + Guilandina + Moullava + the Caesalpinia decapetala clade + the Caesalpinia crista clade + Pterolobium + Mezoneuron) (Figs 2 and 3B, clade C). Clade II (Figs 2 and 3C–D) includes the Cenostigma-Poincianella B clade as sister to a clade (Figs 2 and 3C, clade E) containing two main lineages: the first comprising Stahlia + Libidibia, Balsamocarpon + Zuccagnia + Stenodrepanum + Hoffmannseggia (Figs 2 and 3C, clade F), and the second made up of the core Poincianella-Erythrostemon group + Pomaria + Arquita (Figs 2 and 3D, clade G).
Although all 27 lineages and all 26 genera are robustly supported, the precise relationships amongst a few genera remain unresolved or are not supported. For example, the position of Caesalpinia echinata lacks support in both the MP and ML analyses (bootstrap support below 50%), while in the Bayesian analyses it is sometimes resolved as sister to Caesalpinia s. s. (PP between 64 and 97), emphasising that this species is phylogenetically isolated and justifying its recognition as a new genus (see below). Similarly, the relationships between Caesalpinia mimosoides, the Caesalpinia trothae clade, and Guilandina are sometimes resolved, but generally with low support, again pointing to the phylogenetic distinctiveness of Caesalpinia mimosoides. Within the core Poincianella-Erythrostemon clade, the relationships of Caesalpinia placida and Caesalpinia glandulosa are unstable, being placed either as sister to a Central American lineage or to a South American lineage. Finally, the position of Stenodrepanum as sister to Hoffmannseggia is consistent across all analyses, but always with low branch support (Fig. 3C).
Discussion
In his generic classification of Caesalpinia s. l., Lewis (2005) suggested that molecular phylogenies with increased taxon sampling were needed to rigourously test the monophyly of the genera he was reinstating and to resolve the relationships of a group of 12 to15 Asian species that could not be placed in any of the proposed segregates. Whilst several recent studies based on single DNA sequence loci or morphology have partially addressed this problem (Simpson et al. 2003, Nores et al. 2012, Gagnon et al. 2013), the results presented here, based on combined analyses of six DNA sequence loci totaling 8.8 kb of DNA sequence data, and sampling 84% of species, provide the most comprehensively sampled and robust phylogeny of the group to date. As seen in many other species-level phylogenetic studies of legume taxa (e.g. Moura et al. 2016, Rando et al. 2016, Simon et al. 2016), the most informative DNA sequence locus is ITS, which has at least twice as many informative characters as the plastid loci included in this study. Near-complete sampling of species across the Caesalpinia group, provides a much more stringent and comprehensive assessment of the monophyly of the subclades, as well as of the homology and interpretation of morphological character evolution within the group. Furthermore, as found in both empirical and simulation studies of other taxa (Wiens 2003, 2006, Phillipe et al. 2004, Pyron et al. 2011, Johnson et al. 2012, Hinchliff and Roalson 2013), the concatenated supermatrix approach used here is shown to be robust to missing data. Of the 21 genera proposed by Lewis (2005; Fig. 1), it is clear that some of these groups, such as the Poincianella-Erythrostemon group (Lewis, 1998), Caesalpinia sensu Lewis (2005) and Cenostigma are non-monophyletic. Our analyses also reveal additional clades of Asian species that do not correspond to any of the genera in the Lewis (2005) classification system. In addition, three species (Caesalpinia echinata, Caesalpinia mimosoides and Caesalpinia pearsonii) are placed outside the clades corresponding to the genera proposed by Lewis (2005) or Gagnon et al. (2013) and comprise phylogenetically isolated monospecific lineages. Based on this new and much more comprehensively sampled phylogeny, thorough review of the literature and detailed survey of the morphological diversity of the group, we propose a new classification recognizing 26 genera corresponding to robustly supported clades found across analyses regardless of the amount of missing data. We also discuss the possibility of recognizing a 27th genus, but more molecular and field sampling, especially of freshly collected field specimens, are needed before naming this clade at generic rank.
Phylogenetic relationships and generic delimitation
In their description of the Caesalpinia group, Polhill and Vidal (1981) remarked that this was one of the most distinctive of the nine informal generic groups in tribe Caesalpinieae, based on several morphological characters, and notably the presence of a lower cucullate sepal on the calyx. Although they included the genera Conzattia, Lemuropisum and Parkinsonia in the Caesalpinia group, these were subsequently shown to belong to the Peltophorum group (Haston et al. 2005). The Caesalpinia group, as circumscribed by Lewis (2005), is here shown to form a robustly supported clade (Figs 2 and 3). All of the 13 genera outside Caesalpinia s. l. form robustly supported monophyletic groups, except Moullava and Cenostigma, which are both recircumscribed and expanded to include extra species that were previously placed in Caesalpinia s.l. Of the original eight genera re-instated by Lewis (2005), five (Tara, Coulteria, Guilandina, Mezoneuron, and Libidibia) also form robust clades in our analyses. These five genera are clearly defined by diagnostic morphological synapomorphies, as discussed in Gagnon et al. (2013).
Libidibia shares many similarities with the monotypic Stahlia from the Caribbean, the two together forming a robustly supported clade (Figs 2 and 3C), prompting re-evaluation of their status as distinct genera. Stahlia has been distinguished by its somewhat fleshy red fruits (Fig. 32A) and singly pinnate leaves. However, the pods of Stahlia are similar to those of some species of Libidibia (especially Libidibia sclerocarpa and some South American species) in terms of shape and lack of dehiscence (Fig. 32A–C and F). All other closely related genera have dehiscent pods. Stahlia has also been differentiated from Libidibia by the presence of pinnate rather than bipinnate leaves as in Libidibia, but the dark punctate gland dots on the undersurface of the leaflets, which are distinctively aligned parallel to the midvein, are also observed in certain species of Libidibia, including Libidibia coriaria and Libidibia ferrea (Simpson et al. 2003, Nores et al. 2012, Gagnon et al. 2013). Elsewhere in the Caesalpinia group, leaf pinnation and the occurrence of pinnate vs. bipinnate leaves can be extremely labile within genera (e.g. Haematoxylum and Cenostigma), within species (e.g. Stuhlmannia moavi), and even within individuals (e.g. Haematoxylum sousanum Cruz Durán & J. Jiménez Ramirez (Durán and Ramirez 2008)). Given these morphological similarities and the apparent lability of leaf division, we conclude that there is no justification for retaining Stahlia and Libidibia as separate genera.
Figure 32.
Libidibia monosperma (Tul.) E. Gagnon & G. P. Lewis. A fruits and foliage (M. F. Gardner, Dominican Republic, Gardner & Knees 7027 (E)) D inflorescence (Carlos Pacheco, Wikicommons (https://commons.wikimedia.org/wiki/File:Stahlia_monosperma_flower_(5840542648).jpg), Puerto Rico, USA, unvouchered). Libidibia paraguariensis (D. Parodi) G. P. Lewis B unripe fruits (C. E. Hughes, Santa Cruz, Bolivia, Hughes 2475 (FHO)). Libidibia glabrata (Kunth) C. Cast. & G. P. Lewis C fruits K inflorescence (C. E. Hughes, La Libertad, Peru, Eastwood et al. RJE85 (FHO)). Libidibia coriaria (Jacq.) Schltdl. E flowers (C. E. Hughes, Estelí, Nicaragua, MacQueen 8 (FHO)) F branch with fruits (C. E. Hughes, Metapan, El Salvador, Lewis 1745 (K)) I bark (C. E. Hughes, Oaxaca, Mexico, Hughes 1933 (FHO)). Libidibia sclerocarpa (Standl.) Britton & Rose, G inflorescence (C. E. Hughes, Oaxaca, Mexico, Lewis 1800 (K)) H bark (C. E. Hughes, Oaxaca, Mexico, Hughes et al. 1494 (FHO)). Libidibia ferrea var. parvifolia (Benth.) L. P. de Queiroz J inflorescence (G. P. Lewis, Bahia, Brazil, unvouchered).
As found previously by Gagnon et al. (2013, 2015), the other three genera recognised by Lewis (2005), Poincianella, Erythrostemon and Caesalpinia s. s., are not supported as monophyletic (Fig. 3A, D). Although Lewis (1998) considered that Poincianella and Erythrostemon together formed a clade, Gagnon et al. (2013, 2015) plus the more densely sampled phylogeny presented here (Fig. 3), show that their species fall into unrelated clades, providing the basis for recognition of three genera. First, a subset of Poincianella species corresponding to the Poincianella B group of Lewis and Schrire (1995) group with Cenostigma (Fig. 3C), as found in the morphological cladistic analysis of Lewis and Schrire (1995). These Poincianella B species differ from the remaining Poincianella and Erythrostemon species in wood anatomy (Gasson et al. 2009) and in their alternate to subopposite leaflets (De Queiroz 2009). While Cenostigma was originally considered as a distinct genus, in part based on its pinnate leaves, two species of the Poincianella B clade (Caesalpinia marginata and Caesalpinia pinnata) also have pinnate leaves. More importantly, several species of Poincianella B have internal secretory cavities in the leaflet lamina and inflorescences (Lersten and Curtis 1994; Rudall et al. 1994), as well as a stellate indumentum on the stems, leaves and/or inflorescences, both of which are considered as diagnostic characters of Cenostigma. These leaf traits are completely lacking in the core Poincianella-Erythrostemon group. In addition, Poincianella B and Cenostigma share robust pods with conspicuously thickened margins (Fig. 30B–E and G), which are absent in the other species of the Poincianella-Erythrostemon group and provide a diagnostic synapomorphy for an expanded Cenostigma including the Poincianella B species. It thus appears that in this group morphological homoplasy (pinnation of leaves, alternate to subopposite leaflets, the presence/absence of stipitate glands, stellate indumentum) has obscured relationships resulting in non-monophyletic genera. Here we expand Cenostigma to include the subset of Poincianella-Erythrostemon group species formerly assigned to Poincianella B by Lewis and Schrire (1995; Fig. 3C).
Figure 30.
Cenostigma macrophyllum Tul. A flower (G. P. Lewis, Piauí, Brazil, Lewis 1342 (K)) B fruit (G. P. Lewis, Brazil, unvouchered). Cenostigma eriostachys (Benth.) E. Gagnon & G. P. Lewis C fruits (C. E. Hughes, Oaxaca, Mexico, Hughes 1935 (FHO)) I flowers (G. P. Lewis, Mexico, MacQueen et al. 408 (K)). Cenostigma pluviosum (DC.) E. Gagnon & G. P. Lewis cf. var. intermedium (G.P. Lewis) E. Gagnon & G. P. Lewis D fruit F inflorescence H a new flush of leaves (E. Gagnon, Bahia, Brazil, H.C. Lima et al. 7901 (RB)). Cenostigma pluviosum var. cabralianum (G. P. Lewis) E. Gagnon & G. P. Lewis E fruits (G. P. Lewis, Brazil, Lewis et al. 2019 (K)). Cenostigma marginatum (Tul.) E. Gagnon & G. P. Lewis G leaves and fruits (C. E. Hughes, Bolivia, Wood et al. 26514 (K)). Cenostigma pluviosum (DC.) E. Gagnon & G. P. Lewis var. pluviosum J inflorescences L inflorescences, foliage and dehisced fruits (C. E. Hughes, Santa Cruz, Bolivia, Wood et al. 26552 (K)). Cenostigma gaumeri (Greenm.) E. Gagnon & G. P. Lewis K inflorescence (C. E. Hughes, Quintana Roo, Mexico, Lewis & Hughes 1762 (K)).
The remaining species of the former Poincianella and Erythrostemon are placed either in an Andean clade of five species, which is sister to Pomaria, or are part of another lineage containing the type species of both Poincianella and Erythrostemon (Fig. 3D). The Andean clade has recently been recognised as the new genus Arquita, based on a combination of morphological, ecological and geographical characters (Gagnon et al. 2015, Fig. 39I–O). In the other lineage, two robustly supported subclades are resolved, one including the type species of Erythrostemon (Erythrostemon gilliesii), and the other the type of Poincianella (Poincianella mexicana; Fig. 3D). While these two subclades could potentially be retained as distinct genera, the unresolved relationships of Caesalpinia glandulosa and Caesalpinia placida at the base of this Poincianella-Erythrostemon lineage in the current phylogeny (Fig. 3D) would entail recognizing two additional monospecific genera to account for these species. We prefer to treat this large Poincianella-Erythrostemon clade as a single genus which comprises a morphologically and ecologically coherent group of shrubs and small treelets in Neotropical seasonally dry tropical forests with a bicentric amphitropical distribution (Lewis 1998, Gagnon et al. 2013). Although there are currently more species under the name Poincianella Britton & Rose (1930), the older name Erythrostemon Klotzsch (1844) takes precedence. As such, Erythrostemon is here re-circumscribed to include Poincianella but excludes the subsets of Poincianella species now transferred to either Cenostigma or Arquita.
Figure 39.
Pomaria pilosa (Vogel) B. B. Simpson & G. P. Lewis. A inflorescences (A. A. Schneider, Flora Digital (http://www.ufrgs.br/fitoecologia/florars/), Rio Grande do Sul, Brazil, unvouchered). Pomaria rubicunda (Vogel) B. B. Simpson & G. P. Lewis B flowers C inflorescences (S. Bordignon, Flora Digital (http://www.ufrgs.br/fitoecologia/florars/), Rio Grande do Sul, Brazil, unvouchered). Pomaria jamesii (Torr. & Gray) Walp. D flower E fruit (P. Alexander, SEINet Arizona Chapter (http://swbiodiversity.org/seinet/imagelib/), Arizona, USA, unvouchered); Pomaria burchellii (DC.) B. B. Simpson & G. P. Lewis subsp. burchellii (captions continued on next page) F flower G habit H fruits (O. Bourquin, Flora of Zimbabwe (http://www.zimbabweflora.co.zw/), Ghanzi district, Botswana, unvouchered). Arquita grandiflora E. Gagnon, G. P. Lewis & C. E. Hughes I flower and buds (C. E. Hughes, Ancash, Peru, Särkinen et al. 2225 (FHO)). Arquita celendiniana (G. P. Lewis & C. E. Hughes) E. Gagnon, G. P. Lewis & C. E. Hughes J flower (E. Gagnon, Cajamarca, Peru, Hughes & al. 3097 (MT)). Arquita trichocarpa (Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes K inflorescence M fruit (E. Gagnon, Salta, Argentina, Gagnon & Atchison 218 (MT)) O habit (E. Gagnon, Jujuy, Argentina, Gagnon et al. 204 (MT)). Arquita ancashiana (Ulibarri) E. Gagnon, G. P. Lewis & C. E. Hughes L undersurface of leaflet (E. Gagnon, Cajamarca, Peru, Hughes et al. 3065 (MT)). Arquita mimosifolia (Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes N fruit (E. Gagnon, Salta, Argentina, Gagnon et al. 203 (MT)).
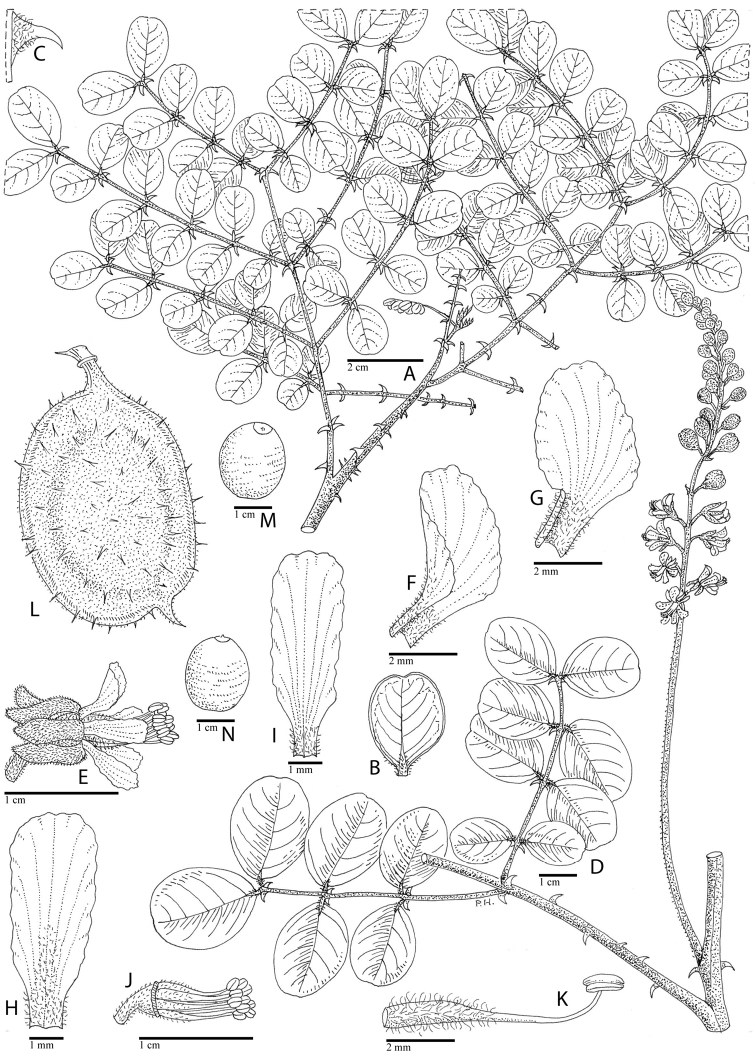
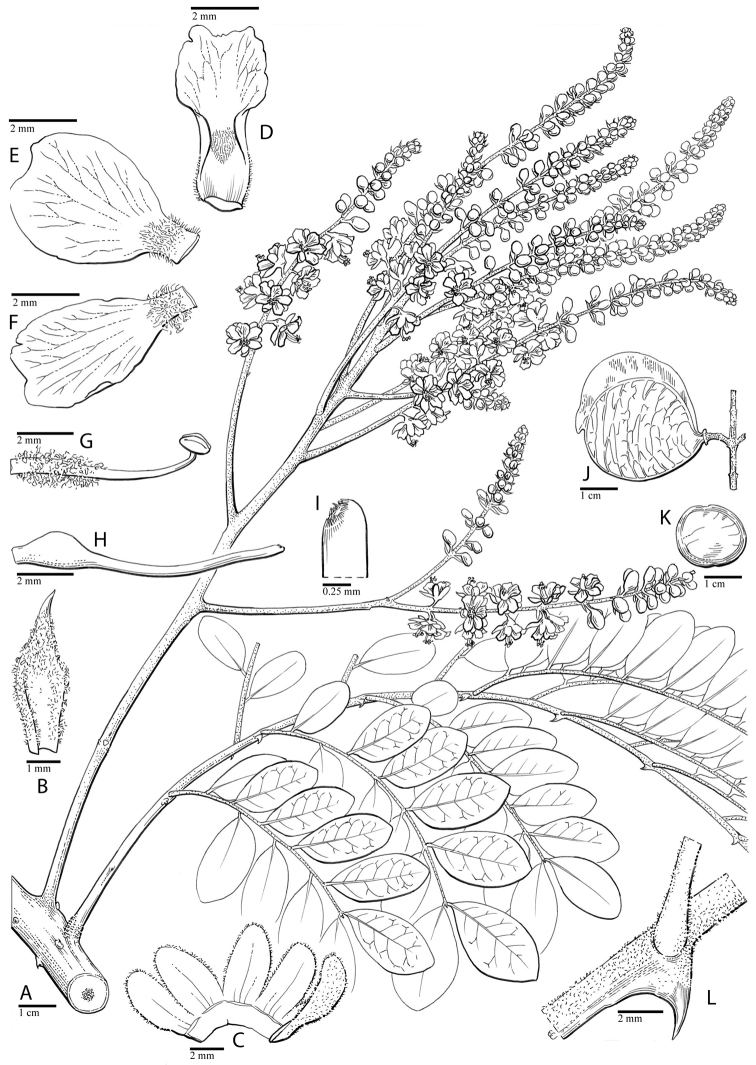
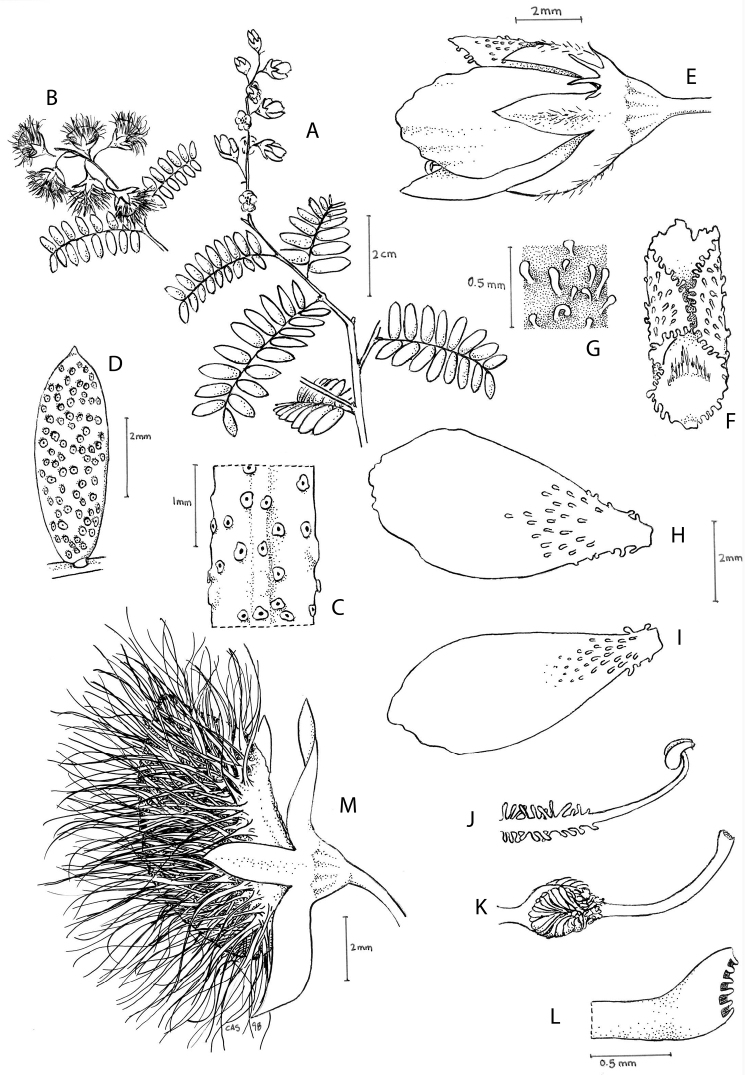
Caesalpinia s.s., as delimited by Lewis (2005), is also non-monophyletic and comprises three independent lineages. The most distinctive of these – the Caesalpinia trothae clade – clearly is not closely related to the remaining Caesalpinia s. s. species (Fig. 3B). This clade consists of African species found in dry forests and thickets from the Horn of Africa, across Tanzania, Botswana, Mozambique, and South Africa to Namibia. Species in this clade share a number of diagnostic morphological synapomorphies: they are all spiny, multi-stemmed shrubs with racemes of reddish-pink to whitish-pink flowers (Fig. 11J); have distinct pyriform pods, with large, rounded, oblique bases and an acute apex; bracts with an aristate tip; and leaflets with translucent dots on the lower surface. However, species delimitation needs to be re-examined. For example, Brenan (1963, 1967) remarked that the rostrate appendage on the calyx, which distinguishes Caesalpinia rostrata, is also found on some specimens of Caesalpinia rubra, bringing into question the distinction of these two species. Despite uncertainty about the number of species, this clade is phylogenetically, morphologically and geographically distinct, clearly meriting recognition as a new genus, here named Gelrebia after the Somali vernacular name for Caesalpinia trothae, which means camel trap and evidently alludes to the highly thorny and impenetrable habit of these plants.
Figure 11.
Caesalpinia bahamensis Lam. A inflorescence D fruits (G. P. Lewis, Cuba, Lewis 1853 (K)). Caesalpinia nipensis Urb. B flowers E fruits (G. P. Lewis, Cuba, Lewis 1838 (K)). Caesalpinia cassioides Willd. C inflorescence (C. E. Hughes, Ancash, Peru, Hughes et al. 2228 (K)). Caesalpinia pulcherrima L. (Sw.) F inflorescence (C. E. Hughes, Sonora, Mexico, unvouchered); Denisophytum pauciflorum (Griseb.) E. Gagnon & G. P. Lewis G flower and leaves (G. P. Lewis, Cuba, Lewis 1854 (K)) H branch with spine-tipped woody protuberances (B. Torke, Cuba, Torke et al. 1424 (NY)). Denisophytum madagascariense R. Vig. I flowers and fruits (G. P. Lewis, Madagascar, Lewis et al. 2158 (K)). Gelrebia trothae (Harms) E. Gagnon & G. P. Lewis J inflorescence (P.J. Cribb, Tanzania, unvouchered).
The other two clades containing members of the former Caesalpinia s. s. lack obvious diagnostic morphological synapomorphies. Both clades include species of shrubs or small treelets that are eglandular and generally spiny (except for one species in each clade), and have explosively dehiscent pods with twisting valves. The type species of Caesalpinia s. s., Caesalpinia brasiliensis, is placed within a clade that includes a set of Caribbean species, most probably pollinated by bats (Koch et al. 2004), the Central American / Mexican Caesalpinia pulcherrima, pollinated by butterflies (Fig. 11F), the northern Andean Caesalpinia cassioides with red, laterally-compressed, tubular corollas, likely pollinated by birds (Fig. 11C), and Caesalpinia nipensis, endemic to the Sierra de Nipe in Cuba, which has a flower morphology and a yellow corolla suggestive of bee pollination (Fig. 11B). As recircumscribed here, a reduced Caesalpinia s. s. is now restricted to the Neotropics with no species now ascribed to this genus in Africa or Asia. The other group, the Caesalpinia erianthera clade (Fig. 3A), contains only yellow-flowered species, but these occur across a strikingly disjunct geographic range in Madasgascar (Caesalpinia madagascariensis, Fig. 11I), Ethiopia, Somalia and the Arabian Peninsula (Caesalpinia erianthera), South America (Caesalpinia stuckertii), Mexico (Caesalpinia sessilifolia), and the Caribbean (Caesalpinia buchii, Caesalpinia pauciflora (Fig. 11G, H) and Caesalpinia rosei). The Caesalpinia erianthera clade is morphologically distinct from its sister clade, the combined Tara + Coulteria clade. This latter clade includes species that are characterised by flowers having a distinctive lower sepal with a cucullate-pectinate margin (although the pectinate margin is absent in Caesalpinia vesicaria, and in Caesalpinia cacalaco the margin is only obscurely pectinate), and pods which are thick and indehiscent (Tara), or thin, chartaceous and indehiscent to tardily and passively dehiscent (Coulteria). Species from the Caesalpinia erianthera clade lack the cucullate-pectinate lower sepal margin and have pods that are explosively dehiscent, with twisting valves. Given the distant phylogenetic placement of the Caesalpinia erianthera clade from both Gelrebia and the recircumscribed Caesalpinia s. s., and its morphological distinctiveness from its sister group, it is clear that the Caesalpinia erianthera clade should also be recognised as a distinct genus. Within this clade, Caesalpinia madagascariensis, endemic to Madagascar, was formerly placed in the monospecific genus Denisophytum, here reinstated with an emended circumscription that includes all species of the Caesalpinia erianthera clade.
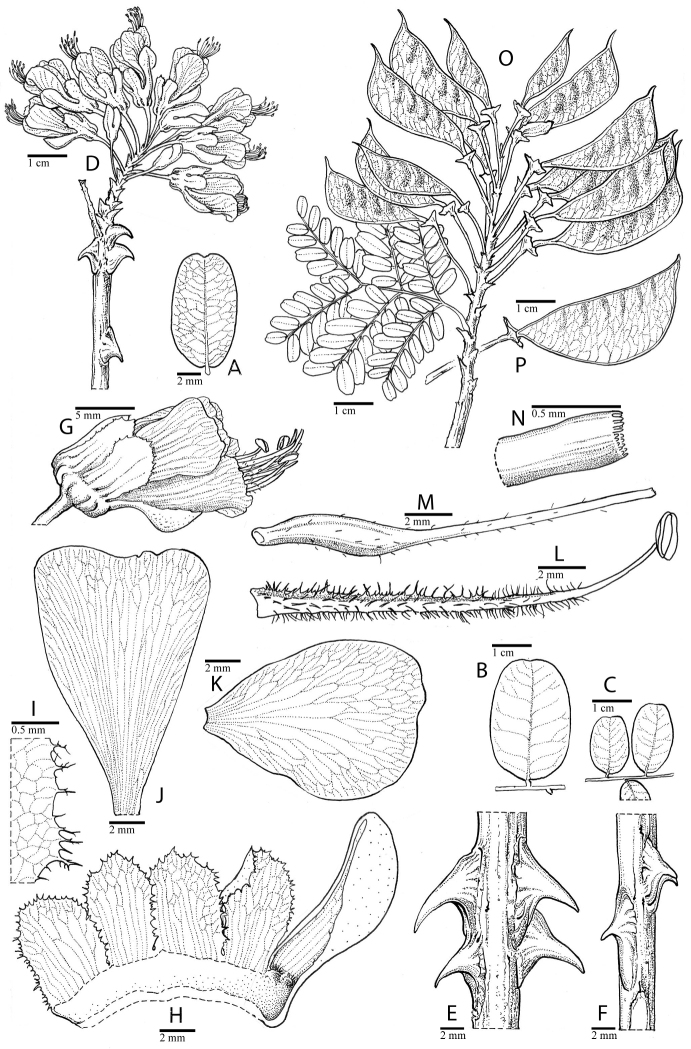
The majority of the rest of the currently unclassified Old World species fall into two main clades, the Caesalpinia decapetala clade and a clade that groups the monospecific genus Moullava, Caesalpinia welwitschiana and two species of Caesalpinia section Cinclidocarpus, which Gagnon et al. (2013) suggested to be closely related to Moullava. The species in these two Old World clades consist of lianas and scrambling shrubs, but are distinguished from the other liana taxa in the Caesalpinia group (which are concentrated in clade C, see Figs 2 and 3B) by their distinctive pods. In the Caesalpinia decapetala clade, the pods are oblong and somewhat laterally compressed, dehiscent along the dorsal suture, and slightly enlarged and truncate towards the apex. In the second clade, all four species have similar rounded, sub-torulose indehiscent pods, with thickened margins, and an exocarp and endocarp that are strongly adnate when dried. It is apparent that both clades merit recognition at the generic level. Based on the preliminary results of Gagnon et al. (2013), Molinari et al. (2016) reinstated the genus Biancaea Todaro (1860) for the Caesalpinia decapetala clade and provided new combinations for three species within the genus. Here we transfer an additional species of Caesalpinia to Biancaea and emend the description of the genus, which was not included in the treatment of Molinari et al. (2016). We also emend the description of Moullava to include three additional species in that genus (Fig. 3B) (see Taxonomic treatment for details).
Monospecific genera
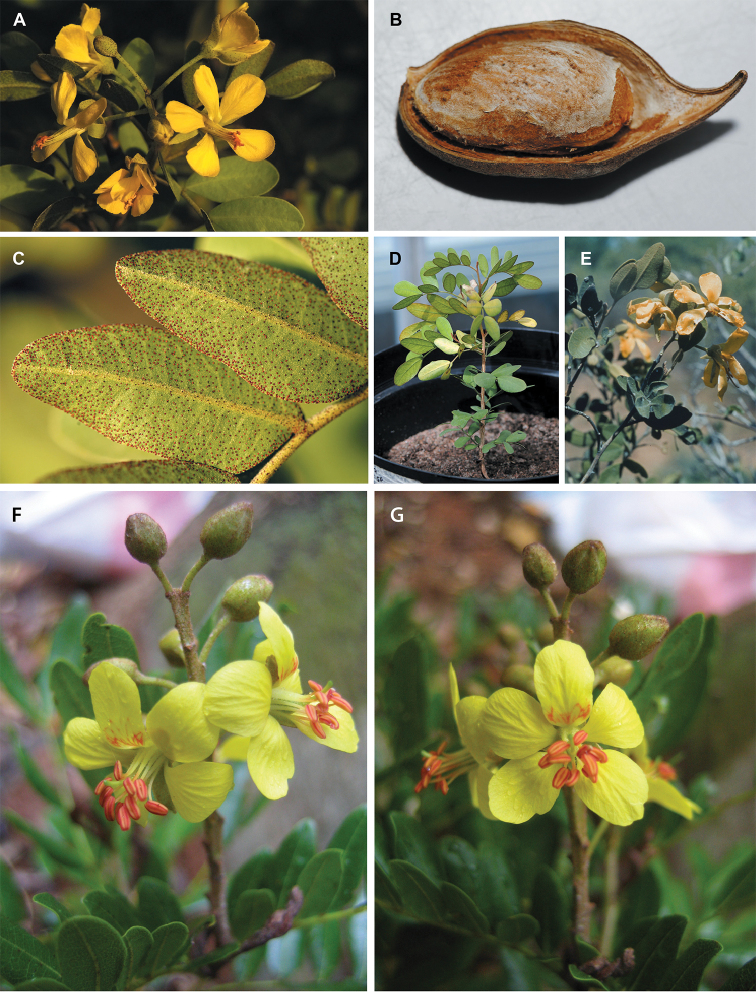
With near-complete taxon sampling and robust support across the phylogeny, it is now clear that the three species, Caesalpinia mimosoides, Caesalpinia pearsonii and Caesalpinia echinata, do not nest within any of the well resolved clades of the Caesalpinia group even though all six loci were sequenced for these species (except for ITS in Caesalpinia mimosoides). The taxonomic placements of these taxa have been problematic in the past, and each species is morphologically unique within the Caesalpinia group, especially with respect to pod morphology. To incorporate these unusual taxa in our generic classification, we propose three new monospecific genera, Hultholia, Hererolandia and Paubrasilia, respectively.
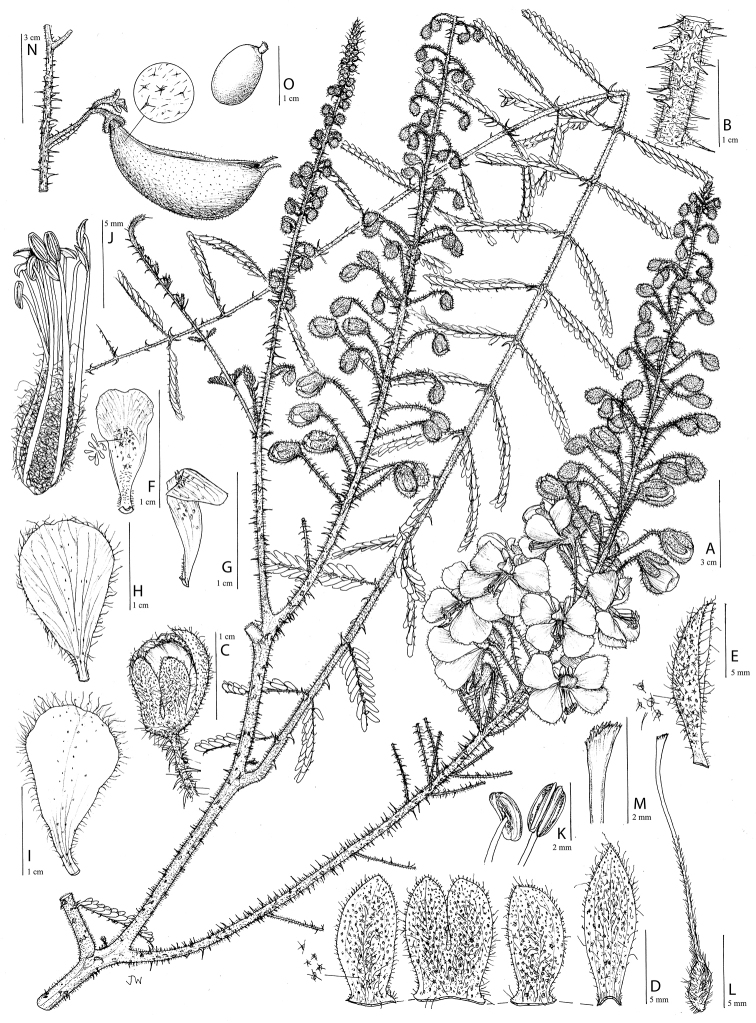
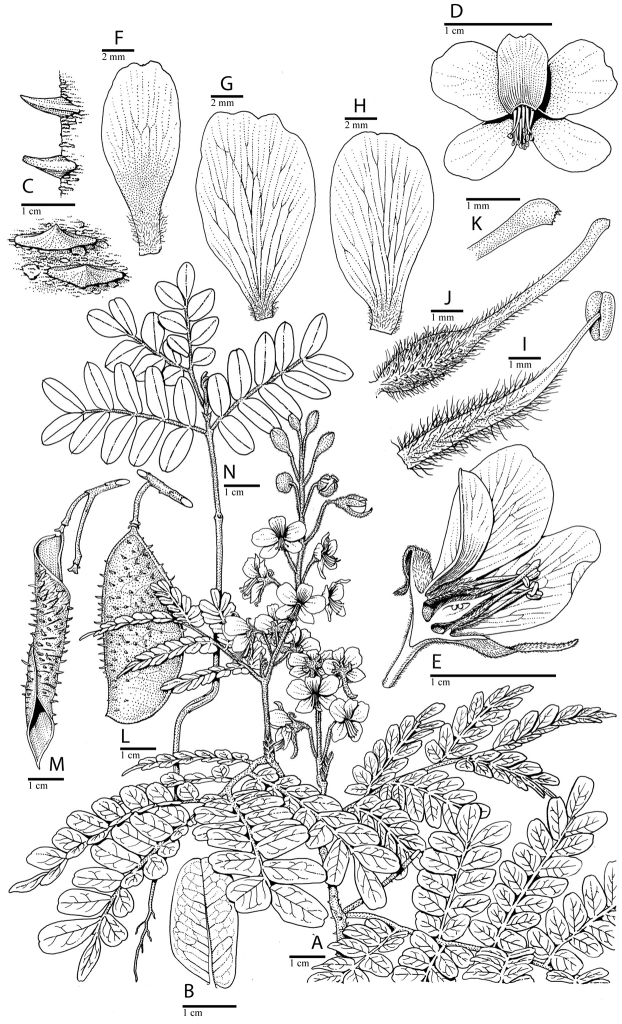
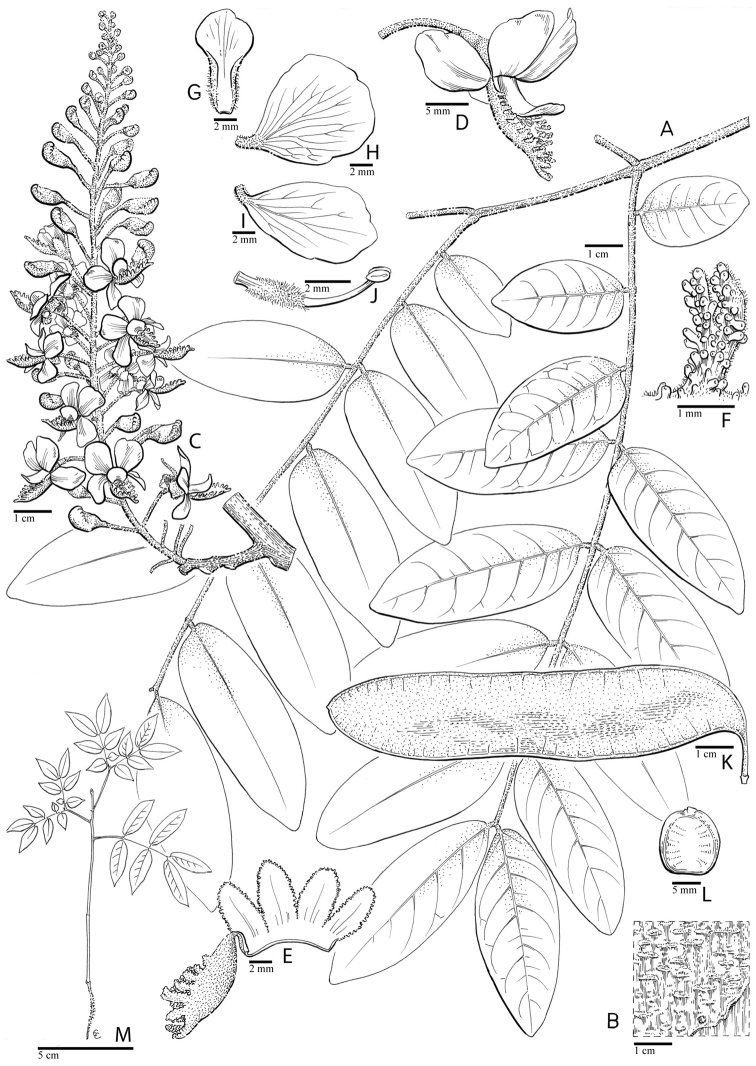
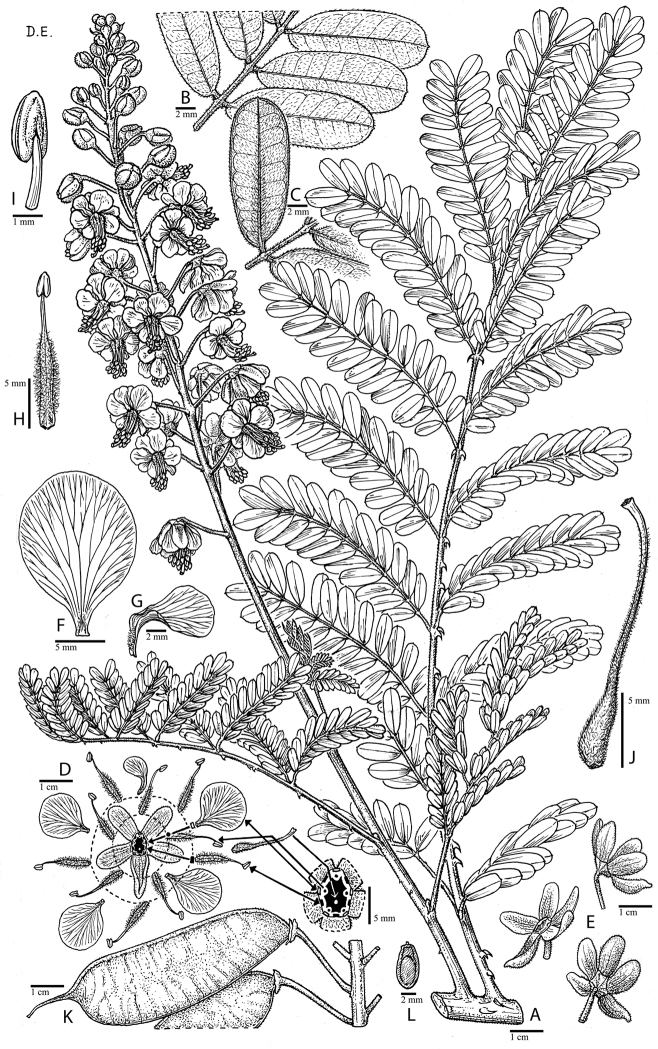
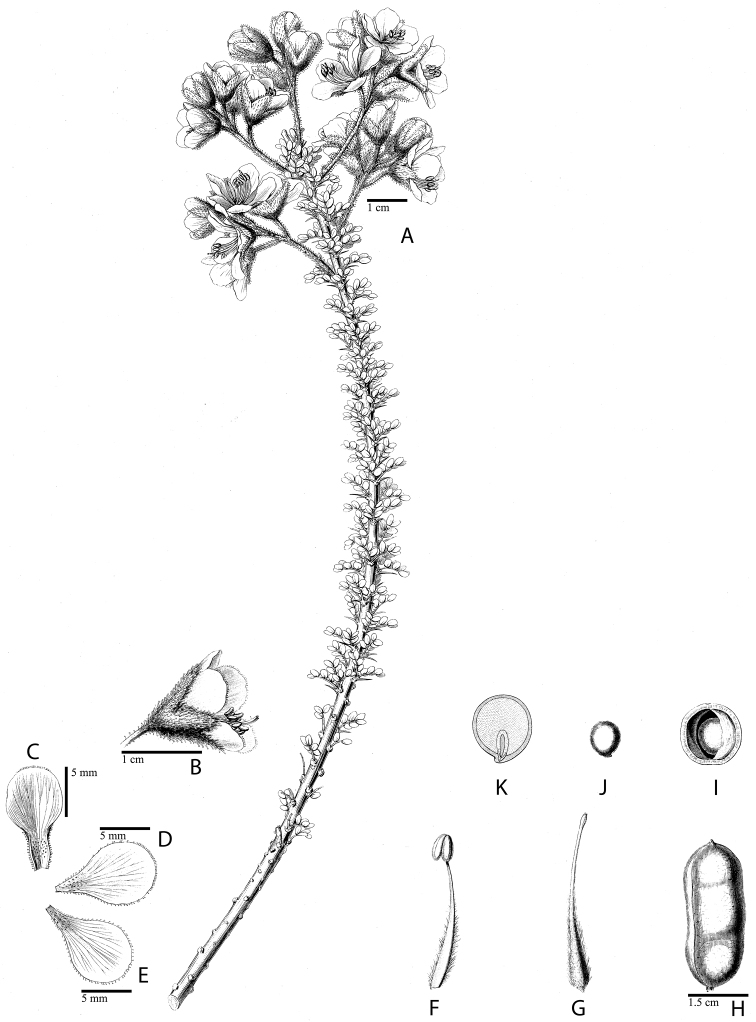
Caesalpinia mimosoides (Figs 17, 18) is a liana found in India, Bangladesh, Thailand, Vietnam, Laos, Myanmar and South-West China. It is morphologically distinct from all other liana species in the Caesalpinia group, because the stem, calyx and fruits are covered in glandular dots, and the pods are falcate, chartaceous and inflated. The robust, needle-like trichomes in Caesalpinia mimosoides, which are present on the stem, inflorescence rachis and pedicels, are also distinctive, and quite different from the more robust and strongly recurved prickles found on stems (and sometimes sparsely at the base of the inflorescences) of other Asian species of the Caesalpinia group. We propose the new generic name Hultholia, to honour the Cambodian taxonomist Dr. Salvamony Hul Thol (see Taxonomic treatment).
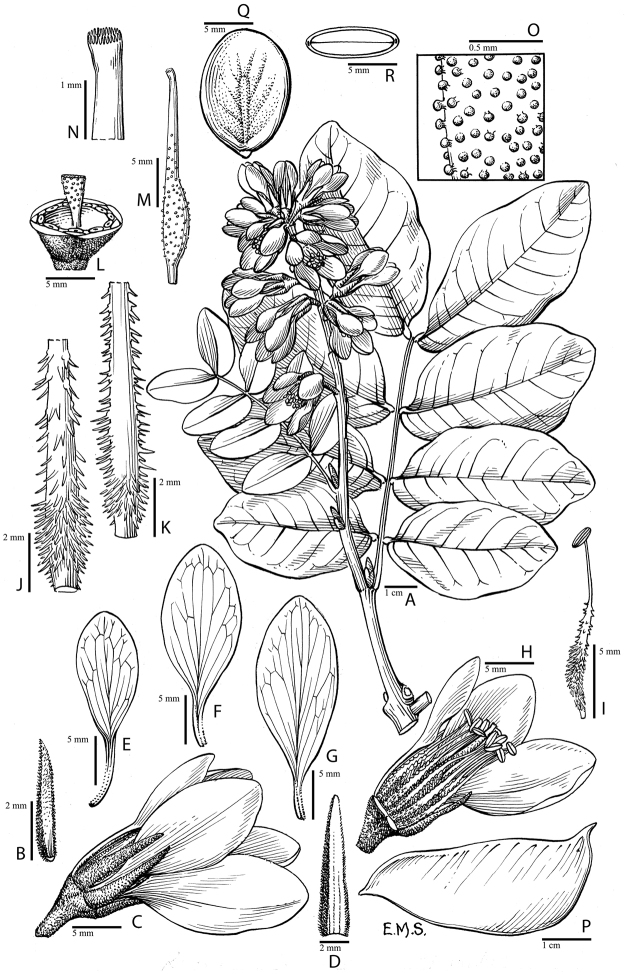
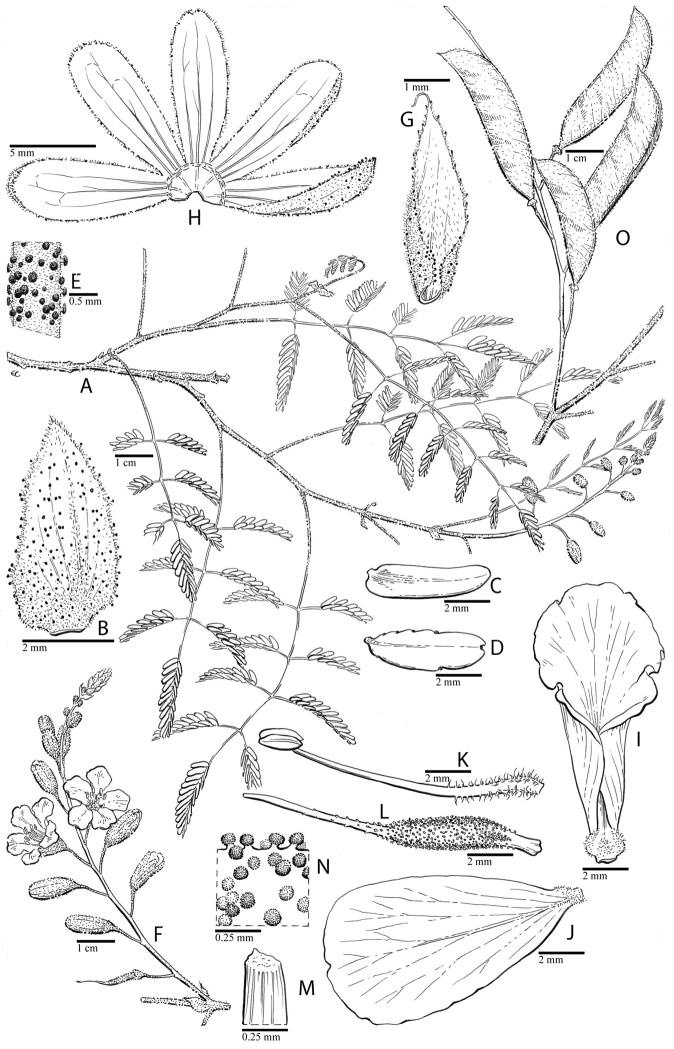
Figure 17.
Hultholia mimosoides (Lam.) E. Gagnon & G. P. Lewis. A habit, including foliage and inflorescences B stem armature detail C bud showing cucullate lower lobe of calyx D calyx lobes outer surface E calyx cucullate lower lobe side view, F median petal inner surface G median petal side view H upper lateral petal inner surface I lower lateral petal inner surface J stamens K anthers dorsal and ventral views L gynoecium M stigma detail N fruit O seed. A–K from Clark 237 L, M from Beusekom & Geesink 4706 N, O from Bunchuai 1342. Drawn by Juliet Williamson.
Figure 18.
Hultholia mimosoides (Lam.) E. Gagnon & G. P. Lewis. A young leaves and inflorescence in bud (J. Jose, Wikicommons (https://commons.wikimedia.org/wiki/File:Caesalpinia_mimosoides_2_at_Kudayathoor.jpg), Kerala, India, unvouchered) B flower (R. Clark, Thailand, Clark et al. 237 (K)) C flowers D immature fruits E mature fruit F habit G open fruit with seeds (V. R. Vinayaraj, Wikicommons (https://commons.wikimedia.org/wiki/Category:Caesalpinia_mimosoides, the basionym of Hultholia mimosoides), India, unvouchered).
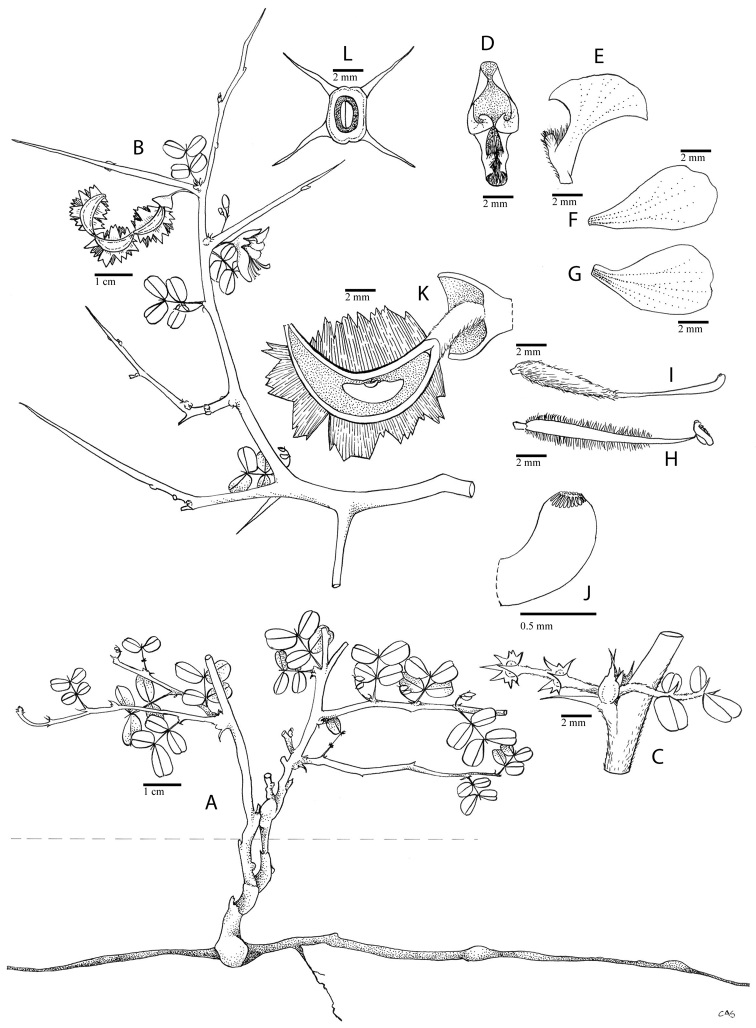
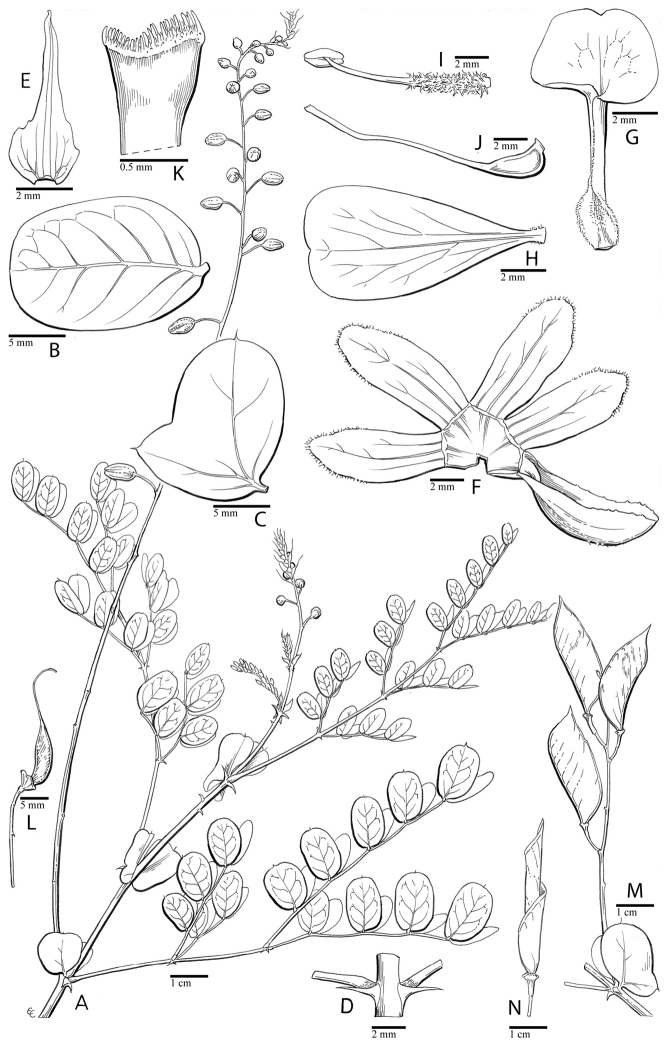
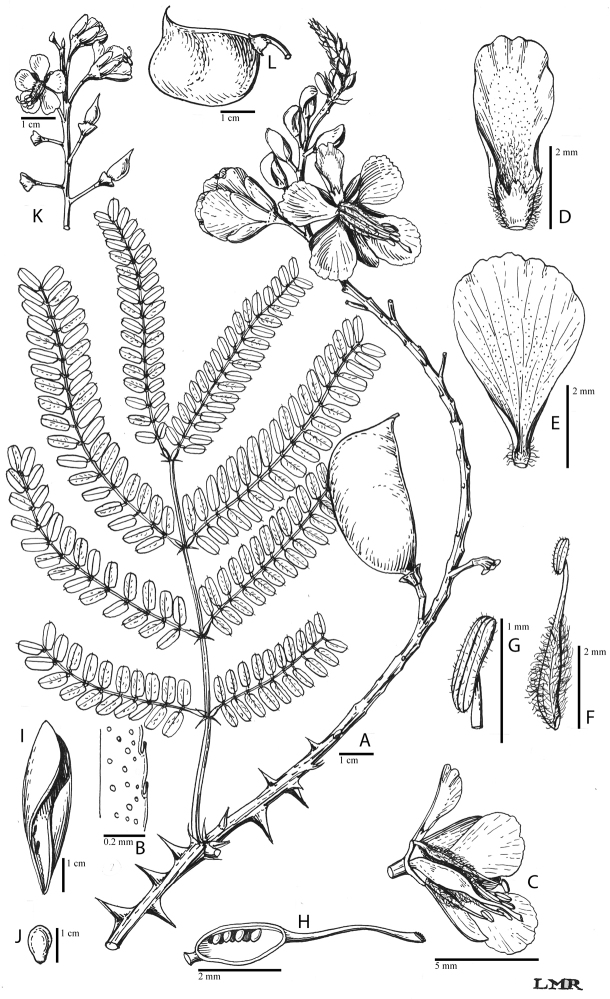
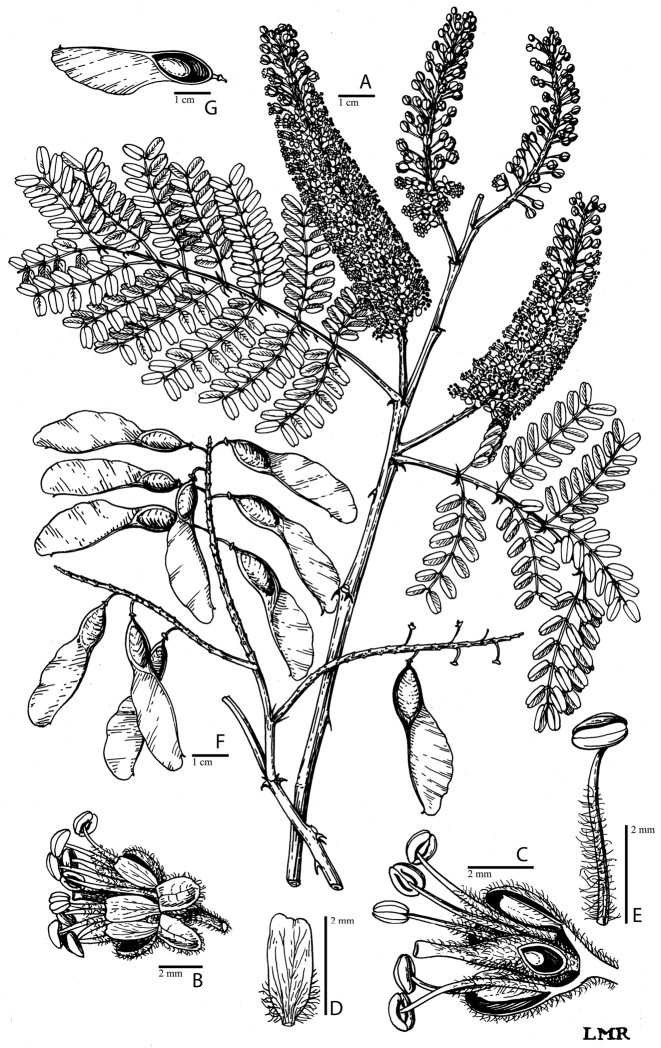
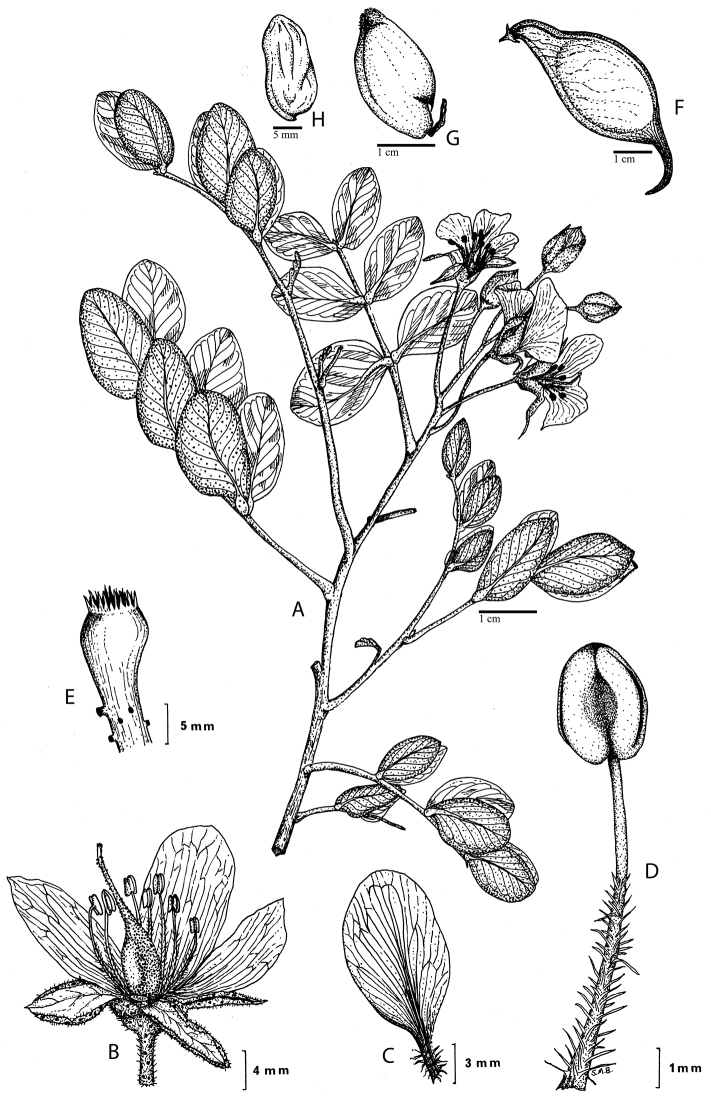
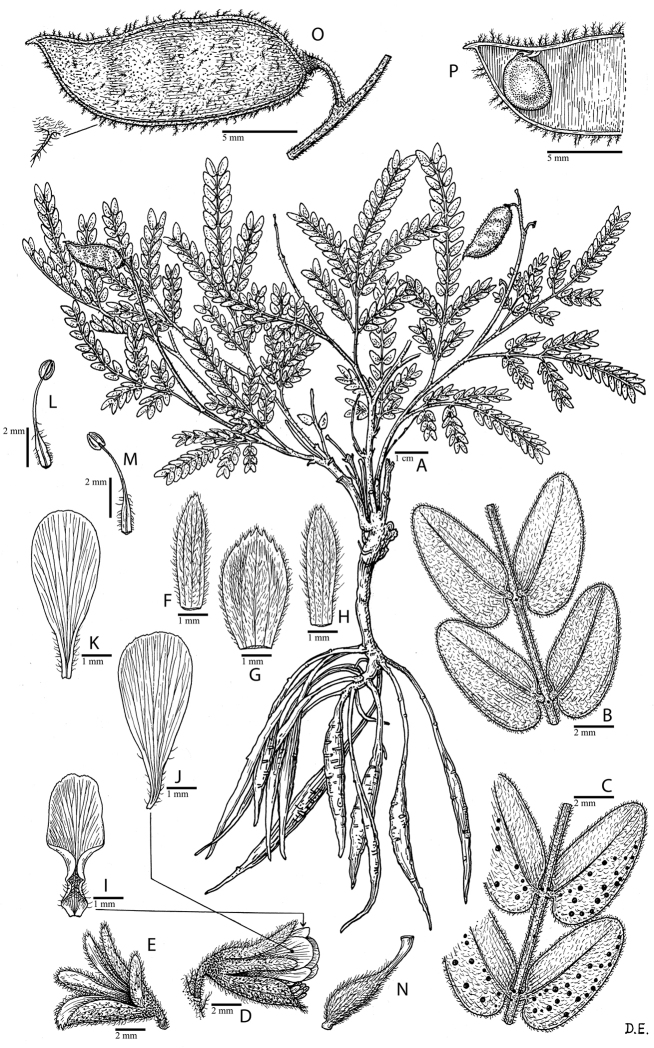
The second unplaced taxon, Caesalpinia pearsonii, differs from the rest of Caesalpinia s. l. primarily by its unusual flattened, circular or semi-circular one-seeded pods, covered in patent red trichomes up to 6 mm long (Fig. 5D). The precise relationships of this rarely collected species, endemic to Namibia, remain uncertain and weakly supported. Our analyses provide only weak support for a sister group relationship to the Lophocarpinia + Haematoxylum clade (Fig. 2), and in most analyses Caesalpinia pearsonii remains unresolved (Fig. 3A). Caesalpinia pearsonii differs from Lophocarpinia and Haematoxylum in having pinnate leaves arranged in fascicles on short brachyblasts, as opposed to the alternate pinnate or bipinnate leaves typical of these latter two genera. In addition, the secondary leaflet venation in Caesalpinia pearsonii is not visible, whereas in Haematoxylum the secondary veins are ascending, and form a sharp angle with the primary vein. Furthermore, armature among these genera differs, with curved and deflexed prickles on the stems and inflorescence rachis in Caesalpinia pearsonii, straight spinescent shoots in Haematoxylum, and straight, conical spines scattered along the branches in Lophocarpinia, which also has distinctively modified lateral, short, spine-tipped branchlets (Fig. 5H). Given the apparently isolated phylogenetic position of this taxon and its morphological distinctiveness, we recognise this species as a new genus, Hererolandia, a name referring to the type locality of Hererolandia pearsonii, which Bolus originally described as coming from “Hereroland” in Namibia, and also chosen to honour the Herero people of that country.
Figure 5.
Hererolandia pearsonii (L. Bolus) E. Gagnon & G. P. Lewis. A shrubby habit B inflorescence C branch showing prickles and leaves D fruits (A. A. Dreyer, Sesriem Canyon, Namibia, unvouchered). Haematoxylum brasiletto H. Karst. E mature fruit dehiscing along the mid-valve (C. E. Hughes, Mexico, unvouchered) F inflorescences and leaves (G. P. Lewis, Mexico, Lewis 2057 (K)) G distinctively fluted trunks (C. E. Hughes, Oaxaca, Mexico, Hughes 1947 (FHO)) Lophocarpinia aculeatifolia (Burkart) Burkart H shrub with flowers, armed with straight conical spines I fruits (R. H. Fortunato, Paraguay, Fortunato 8650 (BAB)).
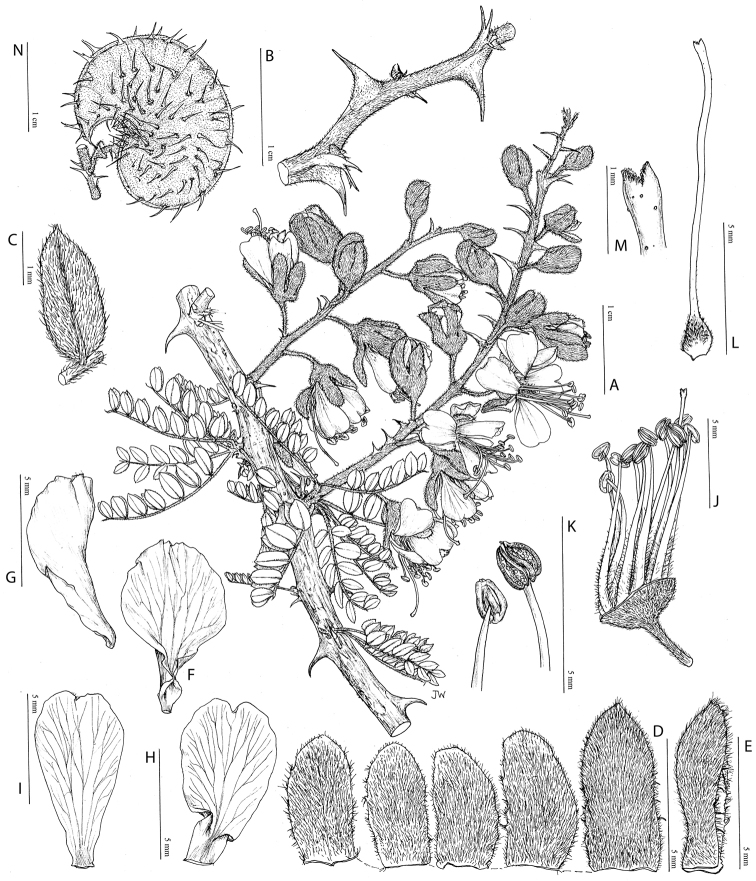
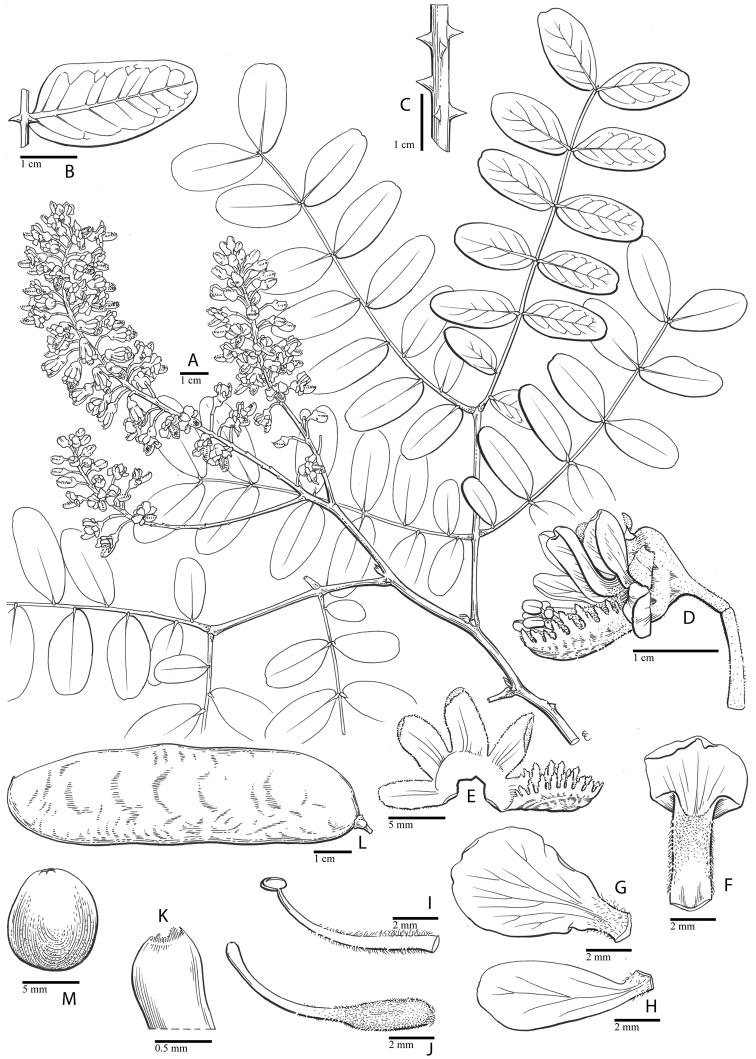
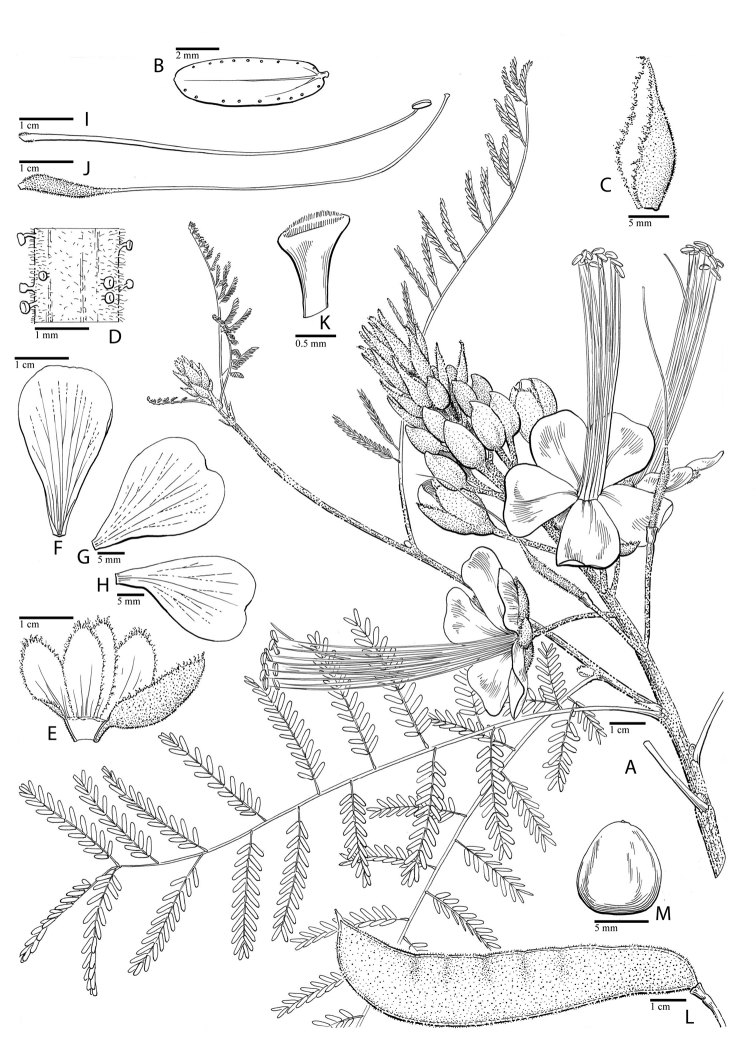
The third unplaced taxon, Caesalpinia echinata, also has several unusual morphological features. The pods of Caesalpinia echinata combine characteristics of Guilandina and Caesalpinia s. s. The patent, sub-woody bristles on the pod valves (Fig. 9B) are reminiscent of Guilandina pods (Fig. 20D and E), but the fruit is laterally compressed with lunate-falcate valves that twist after dehiscence and the seeds are flattened, as in many species of Caesalpinia s. s. In contrast to Caesalpinia s. s. and Guilandina, Caesalpinia echinata has reddish heartwood (Fig. 9F) which is a source of red dye (also found in Caesalpinia sappan in the Caesalpinia decapetala clade and in Haematoxylum). Caesalpinia echinata forms a medium-sized to large tree (Fig. 9E) with unusual upcurved prickles arising from woody protuberances on the trunk and branches (Fig. 9C). In our analyses, multiple accessions of Caesalpinia echinata form a clade in the ITS and ycf6-psbM gene trees and in the combined analysis (Fig. 3A), but in the other plastid gene trees there is no resolution amongst these accessions, suggesting lack of time for coalescence sensu Pennington and Lavin (2016) (Suppl. material 1). Caesalpinia echinata populations along the Atlantic coast of Brazil have been shown to be strongly differentiated genetically (Cardoso et al. 1998, 2005, Lira et al. 2003) and morphologically variable (Lewis 1998, De Lima et al. 2002). Denser sampling and detailed phylogeographical analyses are needed to assess whether these morphotypes represent a continuum or a set of discrete entities worthy of taxonomic recognition. Regardless, we consider that Caesalpinia echinata should be recognised as a distinct genus based on the available morphological and phylogenetic evidence. We propose the genus name Paubrasilia, based on the common name pau-brasil and in reference to the fact that Paubrasilia is the national tree of Brazil with a long and important association with the country.
Figure 9.
Paubrasilia echinata (Lam.) E. Gagnon, H. C. Lima & G. P. Lewis. A flowers (H.C. Lima, Brazil, Lima et al. 2705 (RB)) B fruits (G. P. Lewis, Brazil, unvouchered) C prickles on woody protuberances on a young trunk (E. Gagnon, Bahia, Brazil, Lima et al. 7909 (RB)) D habit (L. P. de Queiroz, Bahia, Brazil, unvouchered) E fluted trunk of a mature individual (E. Gagnon, Bahia, Brazil, Lima et al. 7894 (RB) F cross section of the trunk, showing dark red heartwood (E. Gagnon, Espirito Santo, Brazil, unvouchered), G inflorescences (L. P. de Queiroz, Bahia, Brazil, unvouchered).
Figure 20.
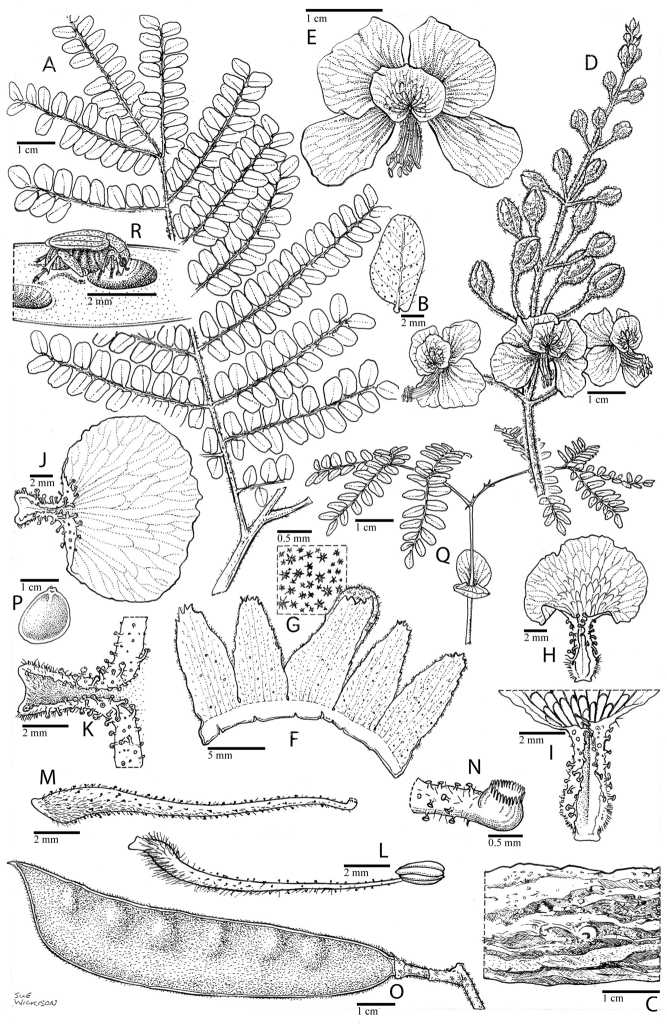
Moullava spicata (Dalzell) Nicolson. A inflorescences B fruit (P. Awale, Flowers of India (http://www.flowersofindia.net/), Maharashtra, India, unvouchered) C flowers (M. Sanjappa, India, unvouchered). Guilandina bonduc L. D young fruits (F. Starr and K. Starr, Starr Environmental (http://www.starrenvironmental.com/images/species/?q=Caesalpinia+bonduc), Florida, USA, unvouchered) E fruits with mature seeds (G. P. Lewis, Madagascar, Du Puy et al. M665 (K)) F inflorescence (M. Sanjappa, India, unvouchered). Biancaea decapetala (Roth) O. Deg. G fruits with seeds H fruit with thickened suture (C. E. Hughes, Ancash, Peru, Hughes et al. 2227 (FHO)) I inflorescence (E. Gagnon, Ancash, Peru, Hughes et al. 3055 (MT)). Biancaea godefroyana (Kuntze) Molinari, Mayta & Sánchez Och. J inflorescences and fruits (F. Xaver, Wikicommons (https://commons.wikimedia.org/wiki/File:Caesalpinia_godefroyana_1.jpg), Cambodia, unvouchered).
Unresolved generic relationships
Three areas of the phylogeny remain unclear and warrant greater sampling before making further adjustments to the generic classification. We hypothesise, based on morphology and preliminary phylogenetic results, that nine species from mainland Asia will form a well-supported clade with Caesalpinia crista (previously referred to as the Caesalpinia nuga clade; Gagnon et al. 2013), which is sister to Pterolobium and which also remains sparsely sampled (Fig. 3B). However, only two of these nine species, Caesalpinia crista and Caesalpinia vernalis (the latter not included in the combined analysis due to missing data, but placed in this clade in the rps16 gene tree in Gagnon et al. (2013)), have been sampled so far. If this putative Caesalpinia crista clade is indeed supported as monophyletic with greater taxon sampling, the oldest available generic name for the clade would be Ticanto Adans. It is notable that two of the species from mainland China (Caesalpinia caesia and Caesalpinia sinense) sometimes have a small wing on the fruit suggesting a fruit intermediate between the typical samara of Pterolobium and the wingless pods of species of the Ticanto clade. This morphological variation highlights the need for thorough sampling and detailed study to arrive at a better understanding of generic delimitation of this group (for more details see Clark 2016).
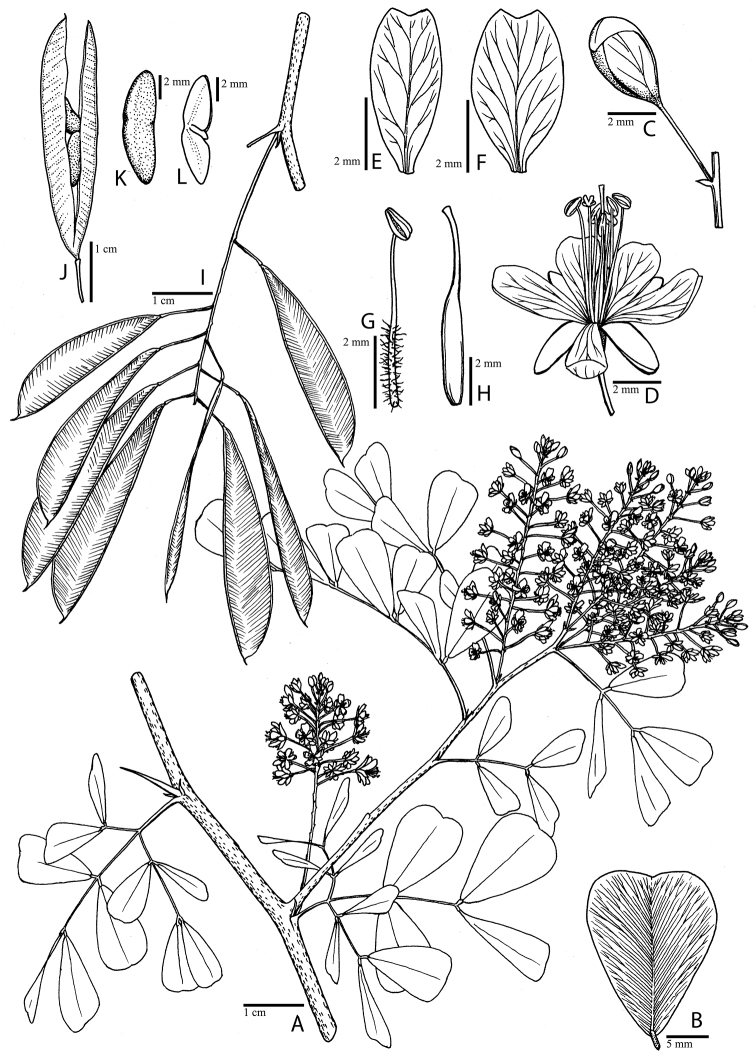
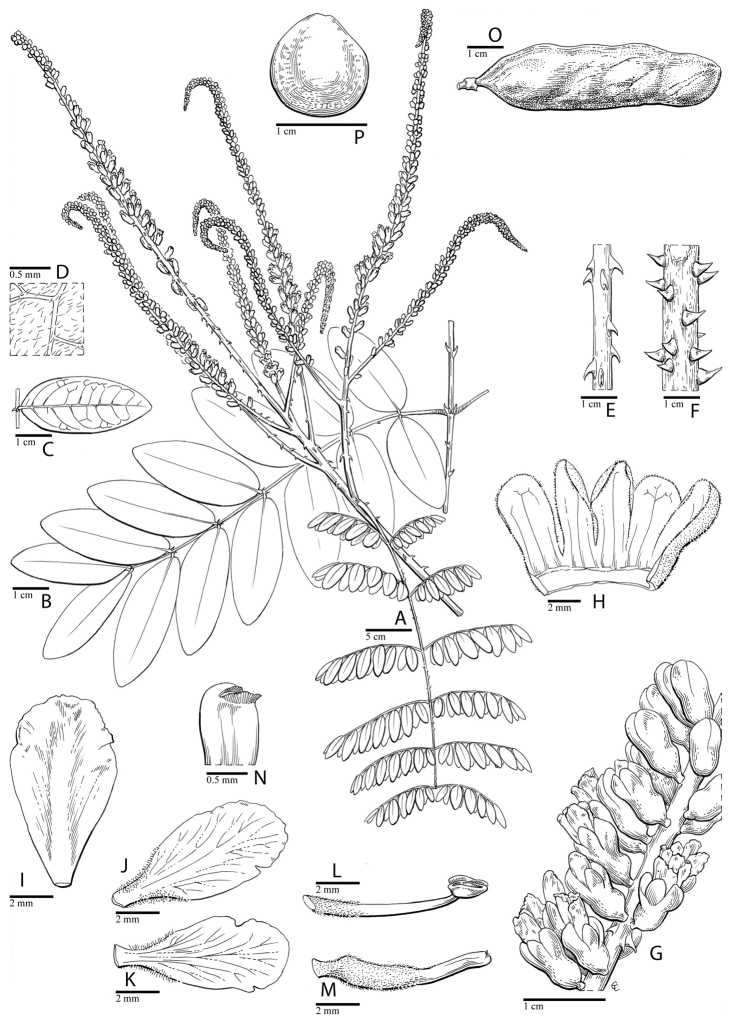
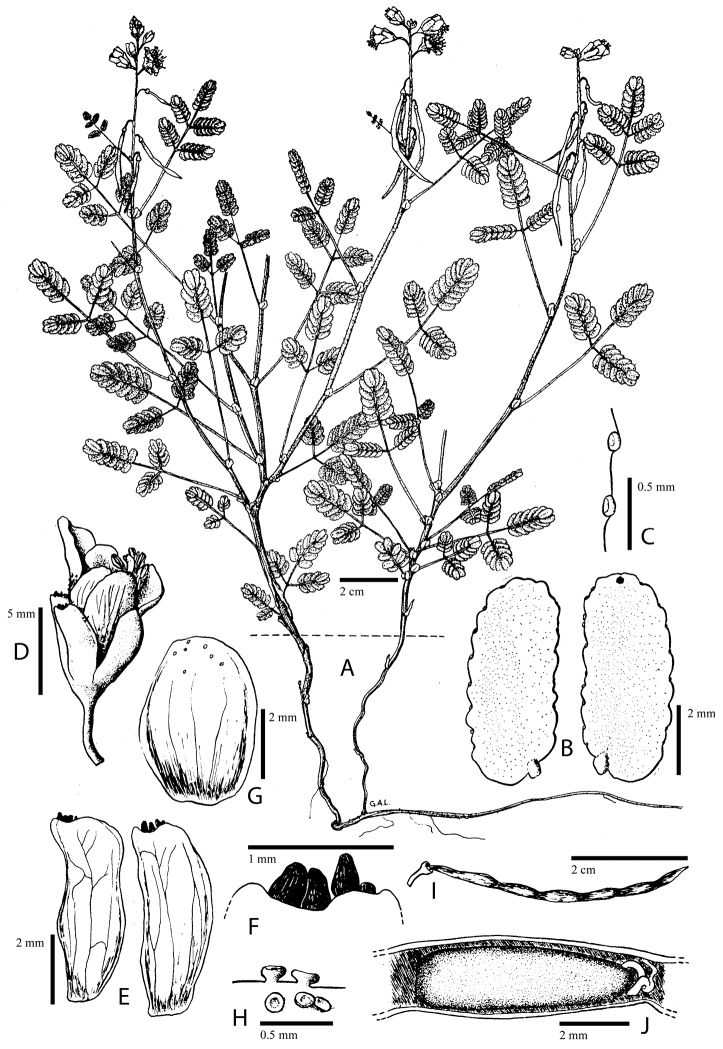
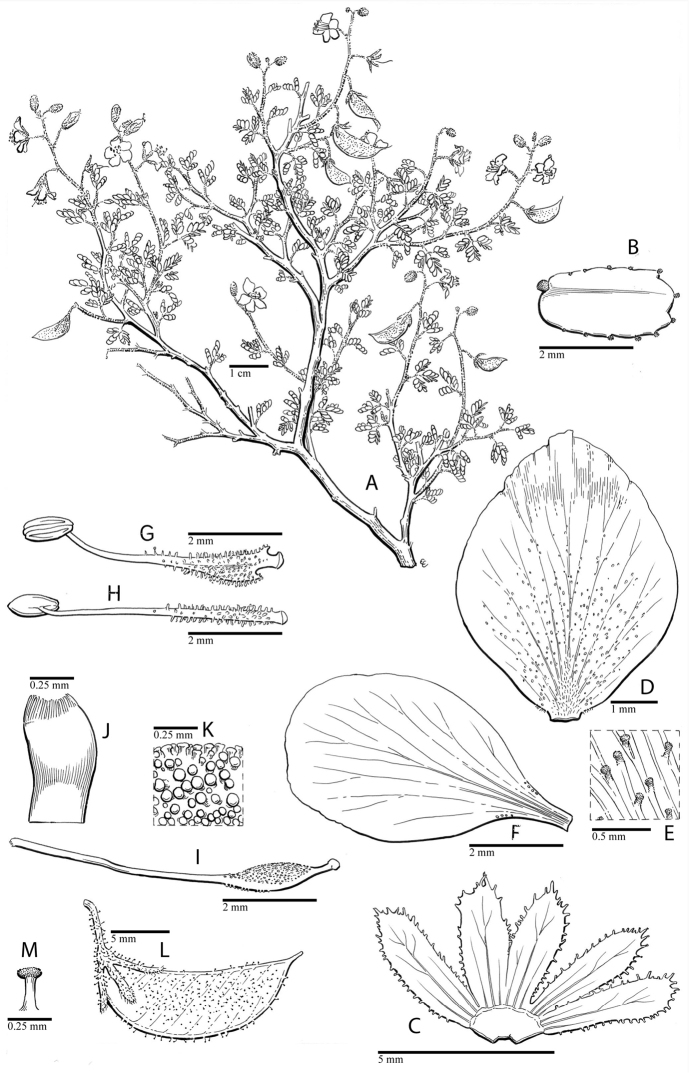
The other questionable taxa are the monospecific genera Lophocarpinia and Stenodrepanum, both of which could potentially be sunk into other genera. However, because only trnL-trnF and matK-3’trnK, the two least informative markers in our study, were sequenced for these two genera, their phylogenetic placements remain weakly or moderately supported. As found by Nores et al. (2012), Lophocarpinia is moderately supported as sister to Haematoxylum (Figs 2 and 3A, clade A). Burkart (1944, 1952) proposed that Lophocarpinia could be synonymised under Haematoxylum due to the strikingly similar vegetative morphology of the two genera, and despite the very distinctive lomentaceous and coarsely serrate-margined winged fruits of Lophocarpinia (Figs 5I and 6). Similarly, Stenodrepanum and Hoffmannseggia are weakly supported as sister taxa, and are distinguished morphologically only by their fruits which are cylindrical and torulose in Stenodrepanum and flattened in Hoffmannseggia (Fig. 34 F, H and K). Although these two generic pairs are differentiated on fruit characters alone, we refrain from proposing any taxonomic changes until additional sequence data can be obtained.
Figure 6.
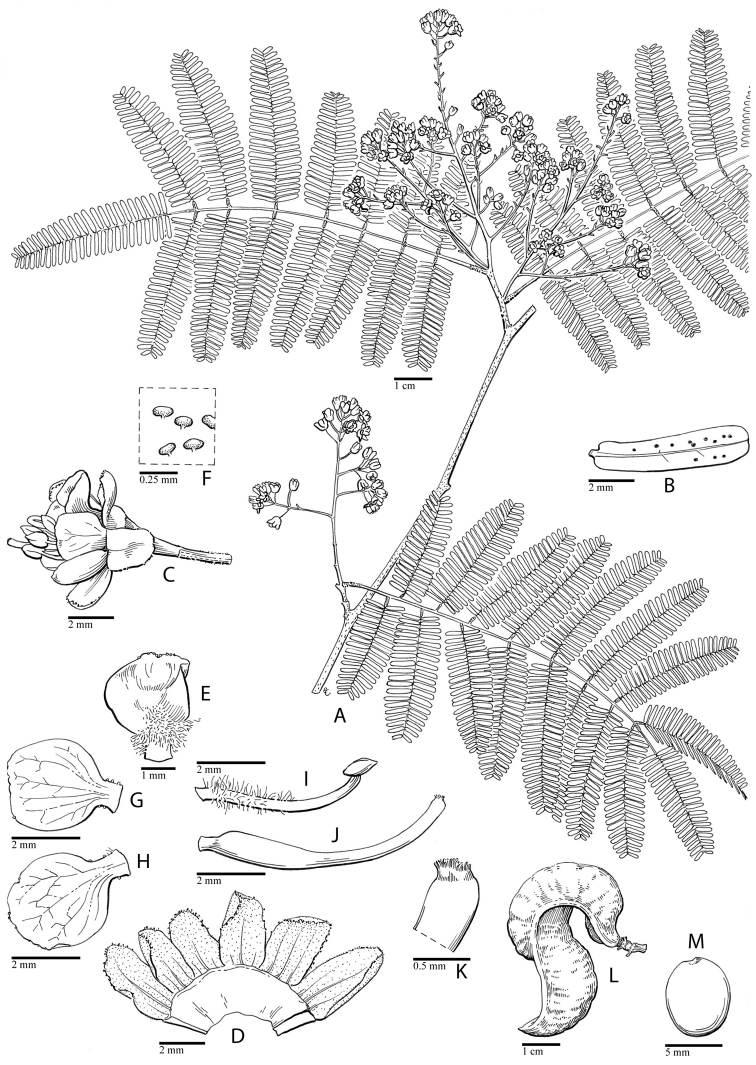
Lophocarpinia aculeatifolia (Burkart) Burkart. A habit B flowering and fruiting branch C detail of leaf attachment D, E median petal front and side views F upper lateral petal G lower lateral petal H stamen I gynoecium J stigma K fruit longitudinal section L fruit cross section. A, B from Burkart 20216 C, K, L after illustration by Burkart D–J from Burkart 20218. Drawn by Christi A. Sobel.
Figure 34.
Balsamocarpon brevifolium Clos. A branch with inflorescence and fruit (M.F. Gardner, Chile, Gardner & Knees 5825 (E)) B fruits with persistent calyx, C habit (P. Baxter, Chile, Baxter et al. DCI 1859 (E)). Zuccagnia punctata Cav. D flowers E fruits (I. Specogna, Flora mendocina (http://www.floramendocina.com.ar/), Mendoza, Argentina, unvouchered). Hoffmannseggia arequipensis Ulibarri F fruits with persistent calyx, and inflorescence (C. E. Hughes, Arequipa, Peru, Hughes et al. 2342 (FHO)). Hoffmannseggia minor (Phil.) Ulibarri, G habit and inflorescence (G. P. Lewis, Bolivia, unvouchered). Hoffmannseggia humilis (Mart. & Galeotti) Hemsl. H fruit with persistent sepals (J. Neff, Puebla, Mexico, unvouchered). Stenodrepanum bergii Harms I habit J inflorescence K fruit (R. H. Fortunato, Argentina, Fortunato 9144 (BAB)).
Morphological variation in the Caesalpinia group
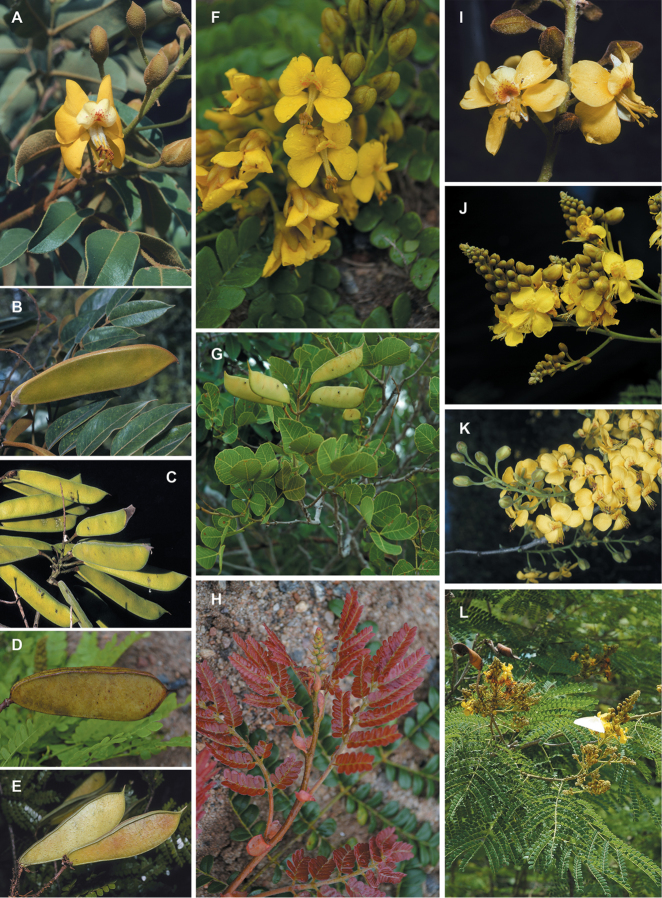
The Caesalpinia group has long been considered a morphologically heterogeneous group, in which morphological homoplasy and convergence have plagued previous attempts to provide a satisfactory generic system (see Lewis and Schrire 1995, Lewis 1998, Gagnon et al. 2013). As circumscribed here, the Caesalpinia group includes 27 robustly supported major lineages (26 of which are formally recognised here as genera). Although there are no unique diagnostic morphological synapomorphies for the clade as a whole, the Caesalpinia group can be recognised by a combination of features, including the presence of glandular trichomes, prickles and spines, bilaterally symmetrical flowers with a somewhat modified lower sepal, and free stamens crowded around the pistil; flowers vary greatly and can be strongly modified depending on pollination system, and fruits across the clade are extremely diverse reflecting a striking variation in seed dispersal strategies. Our new molecular phylogenies (Figs 2, 3) suggest that a number of leaf, armature and fruit characteristics can be used to distinguish genera and delimit the major clades, being exclusive, with minor exceptions, to particular clades. For example, bipinnate leaves with a terminal pinna occur almost exclusively in species of clade II, whereas almost all the species having bipinnate leaves without a terminal pinna are members of clade I. Similarly, clade II contains only species that lack thorns, spines or prickles, and almost all species that lack idioblasts in their leaflets (the latter are also absent in Caesalpinia mimosoides in clade I (Lersten and Curtis 1996) and in Haematoxylum), and almost all species in clade II are characterised by the presence of multi-cellular glandular structures on the stems, leaves and inflorescences (although Haematoxylum dinteri, Caesalpinia mimosoides, and members of Coulteria in clade I also have glandular structures on the margin of the pectinate lower cucullate sepal). In contrast, clade I contains all the species that are armed with spines and prickles along the branches (although Coulteria, Caesalpinia madagascariensis and Caesalpinia nipensis lack thorns, spines or prickles), and which have idioblasts in the lamina of their leaflets. The nearly mutually exclusive distribution of external glands vs. spines+idioblasts gives some support to the idea that these structures constitute alternative plant defense strategies against herbivory (Lersten and Curtis 1994, 1996), even though the role and function of idioblasts and secretory glands in the Caesalpinia group have never been studied in detail.
At the generic level, fruits are highly variable and taxonomically more useful than flowers. Several of the genera we recognise here can be differentiated based on fruit characteristics. For example, the pods of Balsamocarpon, Cenostigma, Guilandina, Haematoxylum, Hererolandia, Hultholia, Libidibia, Lophocarpinia, Moullava, Mezoneuron, Paubrasilia, Pterolobium and Zuccagnia are all distinctive and provide useful diagnostic synapomorphies for these genera (Figs 5, 9, 14, 18, 20, 24, 30, 34). In contrast, only a few floral synapomorphies are diagnostic at the generic level: Guilandina species have sepals that are valvate in bud; in the Balsamocarpon, Zuccagnia, and Hoffmannseggia clade, sepals are persistent until fruiting (Fig. 34), except in Stenodrepanum (Fig. 34); and in Pomaria species, the androecium and gynoecium are cupped in the lower cucullate sepal (Fig. 39A–C, F). In general, however, floral morphology within clades is highly variable reflecting differences in pollination syndromes, including examples of melittophily, chiropterophily, psychophily, phalaenophily and ornithophily, sometimes occurring among closely related congeneric species (e.g. Caesalpinia s. s., as emended here, and Erythrostemon – see above and Figs 11 and 42). These repeated floral morphologies across disparate members of the Caesalpinia group suggest convergent evolution of similar pollination modes in multiple clades across the group.
Figure 14.
Caesalpinia (Coulteria) velutina Britton & Rose. A inflorescence (G. P. Lewis, Guatemala, Lewis et al. 1713 (K)) B fruits (C. E. Hughes, Guatemala, Lewis et al. 1714 (K)). Tara vesicaria (L.) Molinari, Sánchez Och. & Mayta C habit (C. E. Hughes, Tecolostote, Nicaragua, Hughes 1376 (FHO)) H flower (C. E. Hughes, Rivas, Nicaragua, J. A. Hawkins 11 (FHO). Tara spinosa (Molina) Britton & Rose D inflorescence (E. Gagnon, Ancash, Peru, Hughes et al. 3043 (MT)) I unripe fruits (C. E. Hughes, Cajamarca, Peru, Hughes 1996 (FHO)). Tara cacalaco (Humb. & Bonpl.) Molinari & Sánchez Och. E flowers (C. E. Hughes, Puebla, Mexico, Hughes et al. 2169 (FHO)) F unripe fruits (G. P. Lewis, Mexico, MacQueen 488 (K)) G bark (C. E. Hughes, Puebla, Mexico, Hughes et al. 2073 (FHO)).
Figure 24.
Pterolobium stellatum (Forssk.) Brenan. A inflorescences (P. van Wyk, Africa, unvouchered) B fruits (J. Anton-Smith, Africa, unvouchered) C close up of fruits (B. T. Wursten, Flora of Zimbabwe (http://www.zimbabweflora.co.zw/speciesdata/image-display.php?species_id=127190&image_id=1), Zimbabwe, unvouchered). Mezoneuron hildebrandtii Vatke D inflorescences (D. Du Puy, Majunga, Madagascar, Du Puy M286 (P)) E fruits (D. Du Puy, Antsiranana, Madagascar, Du Puy M273 (P)). Mezoneuron kauaiense (H. Mann) Hillebr. F flower and buds I fruit (D. Eickhoff, Wikicommons (https://commons.wikimedia.org/wiki/Category:Mezonevron_kavaiense) cultivated, Hawaii, U.S.A., unvouchered). Caesalpinia crista L. emend. Dandy & Exell (?Ticanto) G flowers H young fruits (P. Grard: Institut Français de Pondichéry, Andhra Pradesh, India, unvouchered).
Figure 42.
Erythrostemon placidus (Brandegee) E. Gagnon & G. P. Lewis. A flowers (C. E. Hughes, Baja California, Mexico, Lewis 2031 (K)). Erythrostemon mexicanus (A. Gray) E. Gagnon & G. P. Lewis B inflorescence (C. E. Hughes, San Luís Potosí, Mexico, Hughes et al. 1606 (FHO)). Erythrostemon coccineus (G. P. Lewis & J. L. Contr.) E. Gagnon & G. P. Lewis C flowers (C. E. Hughes, Oaxaca, Mexico, Lewis et al. 1802 (K)). Erythrostemon pannosus (Brandegee) E. Gagnon & G. P. Lewis (captions continued on next page) D (G. P. Lewis, cultivated in University of Texas from seeds collected in Mexico, B. L. Turner 88 (TEX)). Erythrostemon exostemma (DC.) E. Gagnon & G. P. Lewis E flowers (G. P. Lewis, Comayagua, Honduras, Lewis & Hughes 1709 (K)). Erythrostemon gilliesii (Hook.) Klotzsch F Inflorescences (Stan Shebs, Wikicommons (https://commons.wikimedia.org/wiki/File:Caesalpinia_gilliesii_2.jpg), Nevada, U.S.A., unvouchered). Erythrostemon melanadenius (Rose) E. Gagnon & G. P. Lewis G inflorescence I fruit (C. E. Hughes, Oaxaca, Mexico, Hughes et al. 2091 (FHO)). Erythrostemon hintonii (Sandwith) E. Gagnon & G. P. Lewis H inflorescence J fruit (G. P. Lewis, Mexico, MacQueen et al. 428 (K)). Erythrostemon hughesii (G. P. Lewis) E. Gagnon & G. P. Lewis K unripe, ripe and dehisced fruits and seeds (C.E. Hughes, Oaxaca, Mexico, Lewis et al. 1795 (K)). Erythrostemon nicaraguensis (G. P. Lewis) E. Gagnon & G. P. Lewis L fruits (C. E. Hughes, Esteli, Nicaragua, Hawkins et al. 4 (FHO)). Erythrostemon exilifolius (Griseb.) E. Gagnon & G. P. Lewis M fruits (E. Gagnon, Argentina, Gagnon et al. 203 (MT)) Q flower and buds (E. Gagnon, Catamarca, Argentina, Gagnon & Atchison 222 (MT)). Eythrostemon fimbriatus (Tul.) E. Gagnon & G. P. Lewis N fruits (C. E. Hughes, La Paz Bolivia, Hughes et al. 2441 (FHO)). Erythrostemon cf. fimbriatus (Tul.) E. Gagnon & G. P. Lewis R flowers (C. E. Hughes, Santa Cruz, Bolivia, Hughes et al. 2466 (FHO)). Erythrostemon calycinus (Benth) L.P. Queiroz O flower (G. P. Lewis, Bahia, Brazil, unvouchered). Erythrostemon coulterioides (Griseb. emend. Burkart) E. Gagnon & G. P. Lewis P leaves, inflorescence with flowers and developing fruits (E. Gagnon, Jujuy, Argentina, Gagnon & Atchison 209 (MT).
Taxonomy
Here we present a comprehensive phylogenetically-based and significantly revised generic classification of the Caesalpinia group recognizing 26 genera, including re-instatement of two previously described genera, re-circumscription of eight genera and description of four new genera. A 27th genus (Ticanto) is provisionally indicated, but not formally reinstated. A key to the identification of genera, full generic descriptions, and illustrations of all genera are presented. In addition, we provide new combinations where necessary and where we are confident about species affinities and taxonomy (Biancaea, Cenostigma, Erythrostemon, Hererolandia, Hultholia, Libidibia, Moullava, Paubrasilia) and/or lists of accepted species names (in bold) associated with each genus, as well as references to recently published species-level taxonomic accounts. For the genera Guilandina, Coulteria and Ticanto, only a preliminary list of species names (not bold) is indicated, with no nomenclatural combinations provided. These genera remain poorly understood taxonomically and work is currently ongoing in Coulteria to clarify and delimit species (Sotuyo et al., submitted).
Key to the genera of the Caesalpinia group
Genus 27 Ticanto is provisionally indicated, pending further studies to establish the status of the genus
| 1 | Leaves pinnate | 2 |
| – | Leaves bipinnate | 10 |
| 2 | Armed shrubs or trees, with prickles scattered along the branches, or in pairs below the stipules, or plant with short branches modified into persistent thorns | 3 |
| – | Unarmed shrubs or trees | 6 |
| 3 | Sepals persistent in fruit; fruit a cylindrical pod covered with resinous hairs; pairs of needle-like prickles inserted below the stipules and leaf petiole; endemic to northern Chile, from the Coquibo and La Serena valleys | 20. Balsamocarpon |
| – | Sepals caducous; fruit a flattened and non-resinous pod; widely distributed across Central America, Mexico, the Caribbean, South America and Namibia | 4 |
| 4 | Fruit a lomentum, with 4 coarsely serrate wings, breaking up into one-seeded units (articles | 2. Lophocarpinia |
| – | Fruit unsegmented, without wings | 5 |
| 5 | Fruit sub-circular to sickle-shaped, tardily dehiscent along the sutures, finely pubescent and with robust patent trichomes | 1. Hererolandia |
| – | Fruit oblong to fusiform, dehiscent along the middle of the fruit valves or close to the fruit margin, but never along the sutures, lacking patent trichomes | 3. Haematoxylum |
| 6 | Sepals persistent; fruit a gall-like pod, covered with long bristles | 21. Zuccagnia |
| – | Sepals caducous; fruits ovoid to elliptic pods, not gall-like, glabrous or covered in a different type of indumentum | 7 |
| 7 | Fruit an elastically dehiscent pod, with valves twisting upon dehiscence, laterally-compressed and subligneous to woody, oblanceolate to oblong-elliptic | 8 |
| – | Fruit an indehiscent pod, thickened and fleshy, ovoid or elliptic | 9 |
| 8 | Fruit subligneous, lacking a crest; sepals valvate; restricted to Africa and Madagascar; stellate indumentum lacking | 17. Stuhlmannia |
| – | Fruit woody, with conspicuously thickened sutures, sometimes with a crest proximally on the adaxial side; sepals imbricate; restricted to the Neotopics; stellate indumentum often present | 18. Cenostigma |
| 9 | Fruit elliptic, somewhat thick and fleshy, bright red at maturity, rounded at apex and base, 1–2-seeded; leaflets with black, sessile glands on the under-surface; seeds compressed-turgid; sepals imbricate; endemic to Hispaniola and Puerto Rico | 19. Libidibia monosperma |
| – | Fruit ovoid, apex beaked; 1–4-seeded; leaflets with red glands on the lower surface; seeds ovoid; sepals valvate; endemic to NE Africa | 16. Cordeauxia |
| 10 | Leaves terminating in a pair of pinnae plus a single terminal pinna | 11 |
| – | Leaves terminating in a pair of pinnae | 18 |
| 11 | Plant armed; fruits oblong to fusiform, glabrous, dehiscing along the middle of the valves, or parallel to the margin | 3. Haematoxylum |
| – | Plant unarmed; fruits not dehiscing along the middle of the valves | 12 |
| 12 | Sepals persistent in fruit | 23. Hoffmannseggia |
| – | Sepals caducous in fruit | 13 |
| 13 | Pods cylindrical-torulose; central and western Argentina, in subtropical wooded grassland and scrub, especially on salt pans | 22. Stenodrepanum |
| – | Pods never cylindrical torulose | 14 |
| 14 | Stipules linear, persistent; androecium and gynoecium cupped in the lower cucullate sepal, lower lateral sepals forming a platform at right angles to the abaxial cucullate sepal; pods with simple trichomes, glandular-punctate trichomes, and plumose, dendritic and/or stellate trichomes | 25. Pomaria |
| – | Stipules caducous; androecium and gynoecium not cupped in the lower sepal, deflexed; lateral sepals not forming a platform; fruits glabrous or with simple and/or gland-tipped trichomes, the latter sometimes also dendritic or plumose | 15 |
| 15 | Fruits indehiscent; inflorescence a raceme or panicle, often corymbose; leaflets glabrescent and eglandular, or with glandular dots parallel to the midvein | 19. Libidibia |
| – | Fruits dehiscent, often with twisting valves; inflorescence a raceme or panicle, sometimes pyramidal in shape; leaflets glabrescent to densely pubescent, or with a stellate indumentum; leaflets eglandular, or with dark subepidermal glands, and/or with glandular dots sunken in the margins of the leaflets or parallel to the margin on the abaxial side | 16 |
| 16 | Leaflets alternate, or occasionally nearly opposite (rarely opposite), with dark subepidermal glands (best seen with a x10 hand lens); stellate indumentum sometimes present on foliage and inflorescence rachis; fruit subligneous to woody, with thickened sutures | 18. Cenostigma |
| – | Leaflets always opposite, without dark subepidermal glands; stellate indumentum never present on foliage or rachis; fruit coriaceous to subligneous, sutures not thickened | 17 |
| 17 | Shrubs or small to medium-sized trees varying from (0.5–) 1–12 (–20) meters tall, occasionally functionally herbaceous subshrubs, woody at the base; widespread across low-elevation seasonally dry tropical forests in Mexico, Central America, the Caribbean, and in Caatinga vegetation in Brazil, and in patches of dry forest, deserts, yungas-puna transition zones, and chaco-transition forests in Argentina, Bolivia, Chile and Paraguay; flowers yellow, red, pink or orange, sometimes laterally compressed; ovary eglandular or covered in gland-tipped trichomes, the hairs never dendritic | 26. Erythrostemon |
| – | Small to medium-sized, often decumbent, shrubs, 0.3–2.5 m tall; occurring at mid elevations in dry inter-Andean valleys, in Ecuador, Peru, Bolivia and Argentina; flowers yellow, sometimes all five petals streaked with red markings, never laterally compressed; ovary covered in gland-tipped trichomes, which are sometimes dendritic | 24. Arquita |
| 18 | Plants unarmed | 19 |
| – | Plants armed | 22 |
| 19 | Fruit thin, flat, oblong-elliptic to elliptic, membranaceous to papyraceous, indehiscent; margin of the lower cucullate sepal pectinate-glandular; flowers unisexual; leaflets eglandular | 8. Coulteria |
| – | Fruit an oblong-elliptic pod, elastically dehiscent with twisting valves; margin of the lower cucullate sepal entire; flowers bisexual; leaflets eglandular or with red glands | 20 |
| 20 | Flowers nearly actinomorphic; trees, up to 25 m tall; leaflets eglandular or with red glands; E Africa (Kenya and Tanzania), and N and NW Madagascar | 17. Stuhlmannia |
| – | Flowers clearly zygomorphic; shrubs or small trees, up to 5m tall; leaflets eglandular; Cuba or northern Madagascar (close to Antsiranana) | 21 |
| 21 | Fruits laterally compressed; anthers glabrous; endemic to Cuba (near Moa, in the Sierra de Nipe) | 5. Caesalpinia nipensis |
| – | Fruits inflated and hollow; anthers pubescent; endemic to the northern tip of Madagascar (Orangea peninsula, near Antsiranana) | 6. Denisophytum madagascariense |
| 22 | Trees or erect shrubs | 23 |
| – | Lianas or climbing or trailing shrubs | 27 |
| 23 | Fruits indehiscent, somewhat fleshy, turgid and coriaceous; lower cucullate sepal with a pectinate/fimbriate or entire margin | 7. Tara |
| – | Fruits dehiscent, with valves twisting upon dehiscence, laterally-compressed and subligneous to woody; lower cucullate sepal with an entire margin | 24 |
| 24 | Fruits armed with woody spines, stems with upturned thorns arising from woody protuberances; flowers yellow, the median petal with a conspicuous red blotch on the inner face | 4. Paubrasilia |
| – | Fruits unarmed, stems with straight to deflexed prickles; flowers yellow, white, pink, red or orange | 25 |
| 25 | Flowers pink-purple to whitish pink; bracts broadly ovate to suborbicular with an aristate apex; pyriform pods with rounded, oblique bases; sometimes translucent dots on leaflet lower surface | 9. Gelrebia |
| – | Flowers yellow, red, orange , green or white (horticultural variety sometimes pink); bracts lanceolate to linear with an acute to acuminate apex; pods oblong-elliptic, short-stipitate, with a cuneate base; leaflets eglandular | 26 |
| 26 | Flowers orange, red, green, white, rarely yellow or pink; Central America, Mexico, the Caribbean and the northern Andes (Peru to Colombia) | 5. Caesalpinia |
| – | Flowers yellow, sometimes with red markings on the standard (median petal); Somalia, Ethiopia, Argentina, Paraguay, Mexico, Florida and the Caribbean | 6. Denisophytum |
| 27 | Fruits with a wing, although this sometimes very narrow | 28 |
| – | Fruits without a wing | 31 |
| 28 | Fruit a samara (with a basal 1-seeded chamber and a prolonged upper suture that is broadly winged) | 14. Pterolobium |
| – | Fruit 1 or more seeded, with a longitudinal (often narrow) wing along the upper suture | 29 |
| 29 | Fruit with a wing 2 mm or more wide, chartaceous, coriaceous or ligneous; Africa, Madagascar and SE Asia across the Malay Peninsula and Archipelago to New Guinea, New Caledonia and Australia, one species endemic to Hawaii | 15. Mezoneuron |
| – | Fruit with a wing 2 mm wide or less; coriaceous or ligneous; southern (principally mainland) China, Myanmar (Burma), N Laos and N Vietnam | 30 |
| 30 | Fruit oblong-elliptic, terminating in a sharp beak; 4–9-seeded | 13. Biancaea decapetala |
| – | Fruit rhomboid-circular to sub-elliptic; 1 (rarely 2)–seeded | 27. ? Ticanto (Caesalpinia caesia) |
| 31 | Glands on stems, leaf rachis, inflorescence, and fruits; needle-like trichomes on inflorescence rachis and pedicels | 10. Hultholia |
| – | Plants eglandular; stems with recurved prickles; pedicels and inflorescence peduncle with a few prickles near their bases | 32 |
| 32 | Fruit oblong to oblong-elliptic | 33 |
| – | Fruit broadly elliptic to circular | 34 |
| 33 | Fruit oblong, indehiscent, somewhat fleshy, sub-torulose, with thickened sutures, terminating in an acute apex, exocarp and endocarp strongly adnate; seeds sub-globular | 12. Moullava |
| – | Fruit oblong to oblong-elliptic, laterally compressed, dehiscent, coriaceous to subligneous, with a smooth, regular outer surface, base often much narrower than the truncate apex which terminates in a sharp beak, exocarp and endocarp separate easily; seeds flattened to ellipsoidal | 13. Biancaea |
| 34 | Flowers unisexual, segregated into female and male racemes; fruits usually covered in spinescent bristles; seeds globose, with parallel fracture lines concentric with the small apical hilum | 11. Guilandina |
| – | Flowers bisexual, in racemes; fruits always glabrous; seeds laterally compressed, smooth, without fracture lines | 27. ? Ticanto |
Taxonomic treatment of the genera of the Caesalpinia group
List of accepted genera
1. Hererolandia E. Gagnon & G. P. Lewis, gen. nov.
2. Lophocarpinia Burkart
3. Haematoxylum L.
4. Paubrasilia E. Gagnon, H. C. Lima & G. P. Lewis, gen. nov.
5. Caesalpinia L., descr. emended E. Gagnon & G. P. Lewis
6. Denisophytum R. Vig., descr. emended E. Gagnon & G. P. Lewis
7. Tara Molina, descr. emended E. Gagnon & G. P. Lewis
8. Coulteria Kunth, descr. emended E. Gagnon, Sotuyo, & G. P. Lewis
9. Gelrebia E. Gagnon & G. P. Lewis, gen. nov.
10. Hultholia E. Gagnon & G. P. Lewis, gen. nov.
11. Guilandina L.
12. Moullava Adans., descr. emended E. Gagnon & G. P. Lewis
13. Biancaea Tod., descr. emended E. Gagnon & G. P. Lewis
14. Pterolobium R. Br. ex Wight & Arn.
15. Mezoneuron Desf.
16. Cordeauxia Hemsl.
17. Stuhlmannia Taub.
18. Cenostigma Tul., descr. emended E. Gagnon & G. P. Lewis
19. Libidibia (DC.) Schltdl., descr. emended E. Gagnon & G. P. Lewis
20. Balsamocarpon Clos
21. Zuccagnia Cav.
22. Stenodrepanum Harms
23. Hoffmannseggia Cav.
24. Arquita E. Gagnon, G. P. Lewis & C. E. Hughes
25. Pomaria Cav.
26. Erythrostemon Klotzsch, descr. emended E. Gagnon & G. P. Lewis
?27. Ticanto Adans.
1. Hererolandia
E. Gagnon & G. P. Lewis gen. nov.
urn:lsid:ipni.org:names:77158009-1
Figure 4.
Hererolandia pearsonii (L. Bolus) E. Gagnon & G. P. Lewis. A foliage and inflorescences B stem armature detail C leaflet lower surface D calyx lobes outer surface E lower cucullate calyx lobe side view F median petal inner surface G median petal side view H upper lateral petal inner surface I lower lateral petal inner surface J stamens and part of gynoecium, with calyx lobes removed K anthers dorsal and ventral views L gynoecium M stigma detail, N fruit. A, C–M from Müller 1006, B, N from Geiss et al. 5156. Drawn by Juliet Williamson.
Diagnosis.
Hererolandia most closely resembles Lophocarpinia, but differs in having scattered curved, deflexed prickles on shoots (vs. scattered straight, conical spines, as well as modified, short, lateral, spinescent branchlets), pinnate leaves with (4–) 5–7 (–9) pairs of leaflets, arranged in fascicles (vs. alternate, pinnate leaves with 2–3 pairs of leaflets), and leaflets elliptic to oblong-elliptic (vs. leaflets obovate or elliptic-orbicular). The most distinctive feature of Hererolandia is the thinly woody, laterally compressed, almost circular to strongly sickle-shaped, usually 1-seeded fruit, covered in robust trichomes up to 6 mm long (vs. a segmented, falcate, lomentaceous fruit, with 4 coarsely serrate wings, breaking up into 1-seeded units).
Type.
Hererolandia pearsonii (L. Bolus) E. Gagnon & G. P. Lewis ≡ Caesalpinia pearsonii L. Bolus
Description.
A multi-stemmed shrub to 2 m, but usually less than 1 m tall, armed with curved, deflexed, 7 mm long prickles scattered along the branches; bark white or brown; stems terete and slightly sinuous, with a fine silvery indumentum on the young twigs, older stems glabrescent. Stipules not seen. Leaves pinnate, 7–17 mm long, subsessile, borne in fascicles on short woody brachyblasts that are usually subtended by a pair of tiny (sometimes obscure) prickles; leaflets opposite, (4–) 5–7 (–9) pairs per pinna, eglandular, covered in a fine silvery pubescence, 5–6.5 × 2.5–3 mm, elliptic to oblong-elliptic, apex obtuse, with an acuminate tip, main vein prominent, secondary venation not visible. Inflorescence a short raceme of bisexual flowers, about 5 cm long, usually borne on brachyblasts, covered in a fine silvery pubescence, with prickles along the inflorescence rachis; bracts about 2–3 × 1.5 mm, ovate, apex acute, caducous. Flowers zygomorphic; calyx with a short hypanthium, and 5 free sepals, c. 3–5 mm long, finely white pubescent, with the lower sepal cucullate and covering the other 4 sepals in bud, all sepals caducous, but hypanthium persistent as a ring around the stipe of the fruit; petals 5, yellow, free, c. 6–9 mm long, obovate; stamens 10, free, up to 10 mm long, eglandular, pubescent on the lower half; ovary pubescent, stigma a fringed and slightly indented chamber. Fruit a thinly woody, laterally compressed, almost circular to strongly sickle-shaped pod, c. 2–2.3 × 1–1.5 cm, dehiscing along the sutures, finely pubescent and covered in robust trichomes up to 6 mm long, usually 1-seeded. Seeds laterally compressed, about 6–8 mm long.
Geographic distribution.
A monospecific genus endemic to Namibia, on the Great Escarpment.
Habitat.
Semi-desert and desert areas, on stony, sandy soils.
Etymology.
Semiarid Hereroland, a region of eastern Namibia, is the type locality of Hererolandia pearsonii. The Herero people who inhabit this region are nomadic cattle herders and it is they and their region that are honoured in the name proposed for this monospecific genus, endemic to this restricted area of Namibia.
References.
Bolus (1920); Roux (2003); Curtis and Mannheimer (2005: 227).
1.1. Hererolandia pearsonii
(L. Bolus) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158011-1
Basionym.
Caesalpinia pearsonii L. Bolus, Annals of the Bolus Herbarium 3: 4. 1920.
Type.
NAMIBIA, Ababes, breccia banks of Tsondab River below farm, 29 Dec 1915, Pearson 9162 (holotype: BOL; isotypes: K!, GRA, NBG, PRE).
2. Lophocarpinia
Burkart, Darwiniana 11: 256. 1957
Type.
Lophocarpinia aculeatifolia (Burkart) Burkart ≡ Cenostigma aculeatifolium Burkart.
Description.
Shrub 0.5 (– 3) m tall, armed with scattered straight, conical, 2–5 mm long spines on shoots; leaves and inflorescences crowded on brachyblasts; shoots glabrous, reddish, the lateral ones sometimes, spinescent. Stipules acuminate, caducous. Leaves alternate, paripinnate, 5–10 mm long; leaflets in 2 (– 3) pairs, obovate or elliptic-orbicular, 4–7 × 2–2.4 mm, finely pubescent, eglandular, with a pair of small prickles at the insertions of the leaflets. Inflorescences short, corymbiform, pubescent racemes, each with 3–6 bisexual flowers; bracts small, caducous. Flowers zygomorphic, 1–1.5 cm long; calyx with a turbinate, fleshy hypanthium, and 5 oblong, pubescent, caducous sepals, lower sepal cucullate and covering the other 4 sepals in bud, embracing the androecium and gynoecium at anthesis; petals 5, yellow to yellow-orange, free, the median petal differentiated from the rest by a fleshy claw and wavy blade margins, pubescent; stamens 10, free, filaments pubescent; ovary glabrous; stigma apical, concave. Fruit a lomentum, with 1–5 segments, falcate, with 4 coarsely serrate wings. Seeds ellipsoid to reniform, smooth.
Geographic distribution.
A monospecific genus restricted to Argentina and Paraguay (possibly also occurring in Mato Grosso do Sul, Brazil, pers. comm. H. C. de Lima).
Habitat.
Chaco woodland and seasonally dry tropical to subtropical forest.
Etymology.
From lopho- (Greek: combed or crested) and carpos (Greek: fruit), the fruit has 4 crested wings, the ending -inia signifies a close relationship with Caesalpinia.
References.
2.1. Lophocarpinia aculeatifolia
(Burkart) Burkart
3. Haematoxylum
L., Sp. Pl. 1: 384. 1753
Figure 7.
Haematoxylum campechianum L. A flowering branch B leaflet C flower bud D flower E median petal F lateral petal G stamen H gynoecium I infructescence J dehiscing fruit K seed L embryo. A, B, D–H from Lorence 2746 C from Balfour s.n. I–L from Johnston s.n. Drawn by Eleanor Catherine, originally published in Flore des Mascareignes 80. Légumineuses, page 6, plate 1.
Haematoxylon L., 1764, orthographic variant.
Cymbosepalum Baker, 1895.
Type.
Haematoxylum campechianum L.
Description.
Multi-stemmed shrubs to 3 m, to medium-sized trees, 3–15 m in height, armed with scattered straight conical spines, 0.5–1.5 cm long on shoots, and the short, lateral shoots spinescent; mature trees with conspicuously fluted trunks, shrubs often with ribbed branches; young stems reddish brown to grey, glabrous to pubescent, eglandular (or with stalked glands in Haematoxylum dinteri). Leaves alternate, pinnate or bipinnate (both can be present on the same individual in some species), glabrous to pubescent, eglandular, 1–10 cm long; pinnate leaves with 2–6 pairs of leaflets, 2.5–35 × 3–30 mm, glabrous to slightly pubescent, eglandular; bipinnate leaves with 1–3 pairs of pinnae plus a terminal pinna, each pinna with 2–5 (–6) pairs of leaflets, 5–11 × 2–4.5 mm; leaflets in opposite pairs, obcordate to obovate, apex emarginate to obtuse, base cuneate to attenuate (occasionally obtuse), short-petiolulate; primary vein centric, secondary veins ascending, and forming a sharp angle with the primary vein. Inflorescences terminal or axillary racemes or panicles of pedicellate flowers; rachis and pedicels unarmed, glabrous to pubescent, eglandular or glandular. Flowers bisexual, actinomorphic to zygomorphic; calyx comprising a hypanthium and 5 free sepals that are c. 6–7 mm long, glabrous to pubescent, eglandular or glandular, the lower sepal cucullate and slightly covering the other 4 in bud, sepals caducous, hypanthium persisting in fruit, forming a calyx ring; petals 5, yellow to pale yellow or white, free, imbricate, obovate to oblanceolate, 4–10 mm long; stamens 10, free, filaments pubescent, particularly on the lower half; ovary glabrous to pubescent. Fruit flattened, membranaceous to chartaceous, oblong to fusiform (occasionally falcate), apex rounded to obtuse, base acute, dehiscing along the middle of the valves, or near the margin of the fruit, but never along the sutures, 10–50 × 4–15 mm, 1–3-seeded. Seeds oblong to reniform, flattened, 6–12 × 3.8–5 mm.
Geographic distribution.
Haematoxylum comprises five species: two in Central America (Salvador to Costa Rica), Mexico, South America (Colombia and Venezuela) and the Caribbean (perhaps introduced), two endemic to Mexico and one in Southern Africa (Namibia).
Habitat.
Deserts, seasonally dry tropical semi-deciduous scrub and thorn scrub, sandy river beds and dry rocky hillsides. One species (Haematoxylum campechianum) is known to grow in frequently inundated marshy areas by rivers.
Etymology.
From haemato- (Greek: bloody) and xylon (Greek: wood), alluding to the blood-red heartwood of Haematoxylum campechianum L. which produces a brilliant red dye.
Notes.
There is a key to species by Durán and Sousa, in Novon 23(1): 31–36 (2014).
References.
Standley and Steyermark (1946); Ross (1977: 122–114); Roux (2003); Curtis and Mannheimer (2005: 215); Durán and Ramírez (2008); Barreto Valdés (2013); Durán and Sousa (2014).
3.1. Haematoxylum brasiletto
H. Karst.
3.2. Haematoxylum calakmulense
Cruz Durán & M. Sousa
3.3. Haematoxylum campechianum
L.
3.4. Haematoxylum dinteri
Harms
3.5. Haematoxylum sousanum
Cruz Durán & J. Jiménez Ram.
4. Paubrasilia
E. Gagnon, H. C. Lima & G. P. Lewis gen. nov.
urn:lsid:ipni.org:names:77158010-1
Figure 8.
Paubrasilia echinata (Lam.) E. Gagnon, H. C. Lima & G. P. Lewis. A inflorescences and foliage B leaflet undersurface C bark armature (front and side views) D flower E flower l.s. F median petal G upper lateral petal H lower lateral petal I stamen J gynoecium K stigma L fruit M single valve of dehisced fruit N seedling. A from Glaziou 6839 B, K from Angeli 201 C, M from Lewis et al. 1634 D from Lima et al. 2705 E–J from Ducke 20623 L from Mell s.n., N from Lewis et al. 1624. Drawn by Tim Galloway.
Diagnosis.
Paubrasilia is closely related to Caesalpinia, but differs in habit, forming medium-sized to large trees, 5–15+ m tall, armed with small to large upturned prickles, these usually arising from woody protuberances (vs. shrubs or small to medium sized trees, usually 1–6 m tall, unarmed or armed with curved deflexed prickles, either occurring in pairs at the base of leaves, or scattered on shoots, or both, and sometimes present at the base of trunk). Paubrasilia also differs from Caesalpinia by having alternate pinnae with consistently alternate leaflets (vs. opposite pinnae with opposite to alternate leaflets), the median petal with a blood red central blotch (vs. the median petal lacking a red central blotch) and a spiny, woody, finely pubescent, sub-lunate, 1–2-seeded pod (vs. an unarmed, glabrous, oblong-elliptic, generally 3–7-seeded pod, with a marcescent style forming an acute apex).
Type.
Paubrasilia echinata (Lam.) E. Gagnon, H.C. Lima & G. P. Lewis ≡ Caesalpinia echinata Lam.
Description.
Medium sized to large trees, 5–15+ m tall, armed with small to large upturned prickles, these usually arising from woody protuberances, 1–20 mm long (the prickles often sparse or lacking on more mature specimens and larger, older branches); bark chestnut brown to almost black with greyish pustular lenticels, flaking in large woody plates; heartwood red, with the trunk exuding a red sap when injured. Stipules lanceloate, acute to acuminate, caducous. Leaves bipinnate, ending with a pair of pinnae; petiole and rachis finely tomentose; pinnae alternate, the terminal pair opposite to subopposite, with (2–) 3–20 pairs of pinnae per leaf; leaflets alternate, with (2–) 3–19 (–21) leaflets per pinna (generally the number of leaflets is inversely proportional to their size), 0.9–5 × 0.5–3.6 cm (although some specimens have leaflets up to 12 cm long), leaflet blades coriaceous, broadly oblong to subrhombic, apex rounded, obtuse or emarginate, base asymmetric, eglandular, glabrous, midvein excentric, secondary veins brochidodromous. Inflorescence a terminal, or occasionally axillary, finely tomentose raceme or panicle, with c. 15–40 flowers; bracts broadly ovate-triangular, apex acute to acuminate, less than 1 mm long, pubescent, caducous. Flowers bisexual, zygomorphic; calyx a tomentose hypanthium with 5 sepals, that are c. 5–9 mm long, the lowest sepal cucullate, covering the other 4 in bud, all sepals caducous but the hypanthium persisting as a free ring around the pedicel as the pod matures; petals 5, free, bright yellow, the median petal with a blood-red blotch on the inner face, c. 11–15 × 4–10 mm, all petals eglandular, broadly-obovate to slightly spathulate, the petal claws pubescent; stamens 10, free, 7–9 mm long, eglandular, densely pubescent on lower half; ovary pubescent with small spines intermixed, stigma a subterminal fringed-chamber. Fruit a spiny, finely pubescent, sub-lunate, woody, 5.5–7.3 × 1.9–2.6 cm, elastically dehiscent pod with twisting valves, 1–2-seeded. Seeds laterally compressed, ovate-obovate.
Geographic distribution.
A monospecific genus endemic to Eastern Brazil, in the states of Pernambuco, Bahia, Espirito Santo and Rio de Janeiro. Widely cultivated in Brazil as an ornamental street or park tree, and sometimes in plantations.
Habitat.
Dry coastal cactus scrub often on rocky outcrops, inland in Mata Atlântica, and in tall restinga on well-drained sandy soil.
Etymology.
“Pau-brasil” is the national tree of Brazil, and has long been associated with the country. Its red sap was once used for dying cotton and cloth and its wood is much prized for the manufacture of high quality violin bows. Originally described as Caesalpinia echinata by Lamarck in 1785, it is appropriate that this phylogenetically isolated taxon should be placed in its own monospecific genus and a Latinization of its well-known and much used common name recognises the importance of the species to Brazil. For a detailed account of this iconic species refer to Pau-brasil by E. Bueno [et al.], São Paulo, Axis Mundi (2002).
References.
Lewis (1998: 152–158); Bueno (2002); Cardoso et al. (2005).
4.1. Paubrasilia echinata
(Lam.) E. Gagnon, H. C. Lima & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158012-1
Basionym.
Caesalpinia echinata Lam., Encycl. 1: 461. 1785. Guilandina echinata (Lam.) Spreng., Syst. Veg. 2: 327. 1825.
Type.
[BRAZIL] “In locis mari vicinis non apparet, sed tantum in mediterraneis silvis, unde magno labore ad littoralia vehitur” (Lectotype: [icon] “Ibirapitanga, sive Lignvm Rvbrvm” in Piso, De Indiae utriusque re naturali et medica: 164. 1658, designated here).
Epitype.
An epitype is to be selected in a subsequent paper focussing on the morphotypes of Paubrasilia echinata (De Lima et al., in prep.).
Caesalpinia vesicaria Vell., Fl. Flumin.: 172. 1829, Fl. Flumin. Icon. 4. t. 89. 1831. (“vessicaria”), non L. 1753. .
Type. [BRAZIL], “Habitat silvis maritimis usque ad Molendinum Sacchariferum dictum Itacurussá” (Lectotype: [icon] “Cæsalpinia vessicaria” in Velloso, Fl. Flumin. Icon. 4: t. 89. 1831).
Caesalpinia obliqua Vogel in Linnaea 11: 407. 1837.
Type: BRAZIL, Sellow s.n. (holotype ? B †; isotype P02142646!).
5. Caesalpinia
L. Sp. Pl. 1: 380 1753, descr. emended E. Gagnon & G. P. Lewis
Figure 10.
Caesalpinia cassioides Willd. A median leaflet B, C median leaflets (to show variation) D inflorescence E, F stem armature G flower H calyx opened out I calyx margin J median petal K upper lateral petal, L stamen M gynoecium N stigma O leaf and immature fruits P single immature fruit. A, D, E, Q from Mayolo 325 B, C, R from Silverstone-Sopkin 2004 F from Sandeman 4613 G–P from Silverstone-Sopkin 5139. Drawn by Sue Wickison.
Poinciana L., in part (1753).
Brasilettia (DC.) Kuntze (1891), non sensu Britton & Rose (1930).
Diagnosis.
Caesalpinia resembles Guilandina, but differs in habit, comprising armed shrubs and small trees (vs. armed lianas and scrambling/trailing shrubs). It also differs in having racemes of bisexual flowers (vs. racemes of unisexual flowers), sepals imbricate in bud, with a pronounced lower cucullate sepal (vs. sepals valvate in bud), zygomorphic corollas variable in colour (yellow, white, red, orange, pink or green), with petals extending well beyond the sepals (vs. zygomorphic to sub-actinomorphic, yellow corollas, with petals barely extending beyond the sepals), coriaceous, oblong-elliptic to linear, laterally compressed, glabrous pods (vs. oblong-elliptic inflated pods, usually armed with 5–10 mm long spinescent bristles), and obovoid, laterally compressed seeds (vs. obovoid globular seeds).
Type.
Caesalpinia brasiliensis L.
Emended description.
Shrubs or small trees, usually 1–6 m tall, armed with curved deflexed prickles (except Caesalpinia nipensis which is unarmed), these either in pairs at the base of leaves, or scattered along the shoots (or both), or sometimes on woody protuberances at the base of trunks and stems; young shoots terete, glabrous and eglandular. Stipules not seen. Leaves alternate, bipinnate, c. 4–30 cm long, ending with a pair of pinnae, unarmed, or sometimes with a pair of prickles at the insertion of the pinnae on the leaf rachis, sometimes also at the insertions of the leaflets on the pinna rachis; pinnae opposite, in (1–) 2–6 pairs per leaf; leaflets alternate to opposite, in 3–13 pairs per pinna, short-petiolulate, blades suborbicular, obovate or elliptic, apex mucronate, rounded or emarginate, base cuneiform, rounded or oblique; main vein centric, secondary veins reticulate. Inflorescence a terminal or axillary raceme or panicle of pedicellate, bisexual flowers, c. 5–37 cm long, unarmed; bracts lanceolate or ovate, apex acute to acuminate, caducous. Flowers zygomorphic, c. 13–25 mm long; calyx comprising a hypanthium with 5 sepals, that are each c. 7–17 mm long, glabrous to occasionally finely puberulous, always eglandular, the lower sepal strongly cucullate and covering the other 4 sepals in bud, all sepals caducous, but hypanthium persistent as a free ring around the pedicel as the fruit matures; petals 5, variable in colour (yellow, white, red, orange, or green; certain horticultural varieties are also pink), the corolla also variable in shape (related to different pollination systems: bees, butterflies, birds and bats); stamens 10, free, c. 10–65 mm long, the filaments pubescent, eglandular; ovary glabrous and eglandular. Fruit a wingless, unarmed, coriaceous, glabrous, eglandular, oblong-elliptic, or linear pod, with a marcescent style forming an acute apex, c. 34–120 × 7–26 mm, explosively dehiscent, with twisting valves, 3–7-seeded. Seeds laterally compressed, obovate, up to 10 mm in diameter.
Geographic distribution.
Caesalpinia, as re-circumscribed here, is reduced to around nine species (a detailed taxonomic revision is needed to properly delimit species), and is now restricted to the Neotropics (apart from the pantropically cultivated Caesalpinia pulcherrima). All the Old World species previously included in Caesalpinia s.s. sensu Lewis (2005) are here transferred to other genera. One species (Caesalpinia cassioides) occurs in the northern Andes from Peru to Colombia, one (Caesalpinia pulcherrima) is likely native in Guatemala and the state of Sonora in Mexico), two occur in the Caribbean (one, Caesalpinia nipensis, is endemic to Cuba, the other widely distributed and possibly divisible into six separate species, all of which are listed below). Caesalpinia pulcherrima is a widely cultivated ornamental throughout the tropics. It includes red, orange, pink, and pure yellow-flowered forms and cultivated specimens are usually unarmed and lack bristles (unlike wild specimens which are armed and bristly).
Habitat.
Seasonally dry tropical forests, coastal thicket, bushland and thorn scrub, dry plains and riparian woodland, on soils derived from limestone or sandstone.
Etymology.
Named by Linnaeus for Andrea Cesalpino (1519–1603), Italian naturalist, botanical collector, systematist and philosopher, physician to Pope Clement VIII, professor of medicine and botany in Pisa and Rome.
References.
Britton and Rose (1930); Macbride (1943: 191, 194–195); Ulibarri (1996); Barreto Valdés (2013).
5.1. Caesalpinia anacantha
Urb.
5.2. Caesalpinia bahamensis
Lam.
5.3. Caesalpinia barahonensis
Urb.
5.4. Caesalpinia brasiliensis
L.
5.5. Caesalpinia cassioides
Willd.
5.6. Caesalpinia monensis
Britton
5.7. Caesalpinia nipensis
Urb.
5.8. Caesalpinia pulcherrima
(L.) Sw.
5.9. Caesalpinia secundiflora
Urb.
6. Denisophytum
R. Vig., Notul. Syst. (Paris) 13(4): 349. 1948, descr. emended E. Gagnon & G. P. Lewis
Figure 12.
Denisophytum stuckertii (Hassl.) E. Gagnon & G. P. Lewis. A foliage and inflorescences B median leaflet undersurface C stipule D leaf rachis spines E bract F calyx opened out G median petal H lateral petal I stamen J gynoecium K stigma L developing ovary M infructescence, N single fruit valve after dehiscence. A, B, D–K from Renvoize et al. 3538 C, M from Venturi 7697 L from Ruiz et al. 10488c N from Aguilar 241. Drawn by Eleanor Catherine.
Diagnosis.
Denisophytum is closely related to Tara (Fig. 3), but differs in having flowers with a lower cucullate sepal with an entire margin (vs. a lower cucullate sepal with a pectinate margin), and dehiscent, coriaceous, laterally compressed pods (except for Denisophytum madagascariense which has inflated fruits) (vs. indehiscent, somewhat fleshy, coriaceous pods that are slightly turgid). Morphologically, species of Denisophytum are most likely to be confused with those of Caesalpinia s.s., but no reliable diagnostic characters have been found to differentiate these two genera. The corolla of Denisophytum species is consistently yellow and the flowers are bee pollinated, whereas Caesalpinia s.s. species display a wide range of flower colour (yellow, orange, red, green and white) and pollination syndromes (chiropterophily, ornitophily, psychophily and mellitophily).
Type.
Denisophytum madagascariense R. Vig.
Emended description.
Shrubs to small trees, 0.5–2 (–5) m tall, armed with straight or curved, deflexed prickles, scattered along shoots and also in pairs at the petiole base (except Denisophytum madagascariense which is unarmed); young twigs glabrous to pubescent, eglandular. Stipules either minute or foliaceous and conspicuous, caducous (persistent in Denisophytum stuckertii). Leaves alternate, bipinnate, ending with a pair of pinnae; petiole and rachis glabrous and eglandular, with membranous or spinulose stipels at the insertions of pinnae on the leaf rachis, occasionally also at the insertion of the leaflets on the pinnae; pinnae opposite, in 1–6 pairs per leaf; leaflets opposite, in 2–10 (–11) pairs per pinna, elliptic, obovate to orbicular, with a rounded, acuminate or emarginate apex, c. 2–25 × 3–12 mm, leaflet blades glabrous to pubescent, eglandular. Inflorescence a terminal or axillary raceme; bracts caducous (acuminate and filiform in Denisophytum stuckertii). Flowers bisexual, zygomorphic; calyx a short hypanthium with 5 sepals, c. 4–10 mm long, eglandular, glabrous to finely pubescent, lower sepal cucullate and covering the other 4 sepals in bud, all sepals caducous, leaving a persistent free hypanthium ring on the pedicel as the fruit develops; petals 5, free, yellow, the median petal sometimes with red markings on the inner face of the blade, c. 5–10 mm long, obovate, petal claw almost absent (present in Denisophytum madagascariense); stamens 10, free, filaments pubescent and eglandular (8–11 mm long in Denisophytum madagascariense), anthers dorsifixed, glabrous to pubescent; ovary glabrous. Fruits coriaceous, oblong-elliptic, laterally compressed (but inflated in Denisophytum madagascariense), glabrous, eglandular pods with a tapering, sharp beak, 18–49 × 5–15 mm, elastically dehiscent, with twisting valves. Seeds ovoid, laterally compressed.
Geographic distribution.
Denisophytum comprises nine taxa in eight species, found across North America, South America and Africa, including Madagascar, a classical highly disjunct trans-continental distribution typical of lineages occupying the succulent biome sensu Schrire et al. (2005). Three species are distributed in Mexico, Florida, and the Caribbean, one species is endemic to Paraguay and Argentina, one is endemic to northern Madagascar, and the other three occur in northern Kenya, Somalia and Arabia. An evaluation of species limits is needed in this group.
Habitat.
Low deciduous seasonally dry tropical woodland or scrubland, also in open pineland or coastal plains and foothills. Species in Madagascar and Africa grow in limestone soils.
Etymology.
There is no indication of the etymology of Denisophytum in the posthumous publication of the generic name. Nevertheless, it is quite likely that the author, René Viguier, had intended to honour his friend and collaborator, Marcel Denis, a botanist with expertise in the genus Euphorbia in Madagascar. Sadly, M. Denis passed away prematurely at the age of 33 in 1929 (Allorge and Allorge 1930).
References.
Britton and Rose (1930); Burkart (1936: 84–86); Viguier (1949); Roti-Michelozzi (1957); Brenan (1967); Capuron (1967); Thulin (1983: 16–18; 1993: 344–347); Ulibarri (1996); Du Puy and Rabevohitra (2002); Barreto Valdés (2013).
6.1. Denisophytum bessac
(Chiov.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158013-1
Basionym.
Caesalpinia bessac Chiov., Flora Somala 1: 156. 1929.
Type.
SOMALIA, Uebi, Aug 1891, Robecchi-Bricchetti 622 (FI).
Denisophytum bessac is based on depauperate material and is of dubious status (Thulin, 1993).
6.2. Denisophytum buchii
(Urb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158016-1
Basionym.
Caesalpinia buchii Urb., Symb. Antill. 7(4): 510. 1913.
Type.
HAITI, “inter Gonaïves et Grosmorne ad Perou”, Buch 322 (holotype presumed at B†).
6.3. Denisophytum eriantherum
(Chiov.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158014-1
Basionym.
Caesalpinia erianthera Chiov., Fl. Somala 1: 155. 1929.
Type.
SOMALIA, from Obbia to Wuarandi, Aug 1891, Robecchi-Bricchetti 534 (syntype FI, fragments K!); and Boscaglia between Attod and Doldobscio, Apr 1924, Puccioni & Stefanini 450 (syntype FI).
6.3.1. Denisophytum eriantherum var. eriantherum
6.3.2. Denisophytum eriantherum var. pubescens
(Brenan) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158021-1
Basionym.
Caesalpinia erianthera var. pubescens Brenan, Kew Bull. 17(2): 203. 1963.
Type.
KENYA, Northern Frontier Province, Banessa-Ramu, 23 May 1952, Gillett 13274 (holotype K!; isotype EA).
6.4. Denisophytum madagascariense
R. Vig, Notul. Syst. (Paris) 13(4): 349. 1949
Caesalpinia madagascariensis (R. Vig.) Senesse, Bull. Mus. Nat. Hist. Nat., B, Adansonia. 10(1): 79. 1988.
Type.
MADAGASCAR, Loky R. basin, Perrier de la Bâthie 4147 (holotype P).
Caesalpinia antsiranensis Capuron, Adansonia, sér. 2, 7: 203. 1967.
Type. MADAGASCAR, NE of Diego Suarez [Antsiranana], Orangea, Capuron 22990-SF (holotype P).
6.5. Denisophytum pauciflorum
(Griseb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158017-1
Basionym.
Libidibia pauciflora Griseb., Cat. Pl. Cub.: 78. 1866, (as “Lebidibia”).
Poinciana pauciflora (Griseb.) Small, Fl. SE United States: 59. 1903.
Caesalpinia pauciflora (Griseb.) C. Wright ex Sauvalle, Anal. Acad. Cienc. Med. Habana 5: 404. 1868 [1869].
Type. CUBA or. et occ., Wright 2361 (holotype ?GOET, n.v., isotype K!).
6.6. Denisophytum rosei
(Urb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158018-1
Basionym.
Caesalpinia rosei Urb., Repert. Sp. Nov. Regni Veg. 15: 314. 1918.
Type.
DOMINICAN REPUBLIC (Santo Domingo) prope Azua, Rose, Fitch & Russell 3861 (holotype US, photo K!).
6.7. Denisophytum sessilifolium
(S. Watson) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158015-1
Basionym.
Caesalpinia sessilifolia S. Watson, Proc. Amer. Acad. Arts and Sci. 21: 450 (1886).
Poinciana sessilifolia (S. Watson) Rose, in Contrib. U. S. Nat. Herb. 13(9): 303 (1911).
Type.
MEXICO, Bolson de Mapimi, 10 May 1847, Gregg s.n. (syntype NY); Mexico, Coahuila, on hills and mesas about Jumulco, May 1885, Pringle 202 (syntypes BR, CAS, CORD!, E, F, GH, GOET, JE, K!, MO, PH, SI!, US).
6.8. Denisophytum stuckertii
(Hassl.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158019-1
Basionym.
Caesalpinia stuckertii Hassl., in Repert. Sp. Nov. Reg. Veg. 12: 201 (1913).
Type.
ARGENTINA, Prov. Tucuman, Dept. Bunyacu: prope Cañada Alegre, 5 Jan 1900, Stuckert 21276 (? holotype SI).
Caesalpinia herzogii Harms, in Meded. Rijks-Herb. 27: 38 (1915).
Type. ARGENTINA, Gran Chaco: near Camoteras, Nov 1910, Herzog 1077 (? holotype L).
Caesalpinia stuckertii var. robusta Hassl., in Repert. Sp. Nov. Reg. Veg.12: 202. 1913.
Type. ARGENTINA, Prov. Tucuman, Depto. Bunyacu: Cañada Alegre, 31 Dec 1908, Stuckert 19726 (? holotype SI).
7. Tara
Molina, Saggio Chili 283. 1789, descr. emended E. Gagnon & G. P. Lewis
Figure 13.
Tara spinosa (Molina) Britton & Rose. A habit B leaflet undersurface, C section of young stem D flower E calyx opened out F median petal G upper lateral petal H lower lateral petal I stamen J gynoecium K stigma L fruit M seed. A–K from Lewis 1416 L, M from Filskov et al. 37341. Drawn by Eleanor Catherine.
Coulteria Kunth. 1824, in large part (excluding Coulteria mollis Kunth).
Nicarago Britton & Rose. 1930.
Russellodendron Britton & Rose. 1930.
Diagnosis.
Tara differs from the closely related Coulteria in having racemose or paniculate inflorescences of bisexual flowers (vs. racemose inflorescences of unisexual flowers), indehiscent, laterally compressed, oblong, straight, slightly turgid and somewhat fleshy, coriaceous, sessile pods (vs. chartaceous to papyraceous, laterally-compressed, oblong to elliptic, occasionally suborbicular, pods, with a stipe ca. 4–13 mm long), and ellipsoid (vs. ovate-orbicular to sub-quadrate, compressed) seeds.
Type.
Tara tinctoria Molina ≡ Tara spinosa (Molina) Britton & Rose
Emended description.
Shrubs or trees, 3–5 (– 8) m tall, armed with deflexed prickles on the shoots; twigs glabrous to puberulent. Stipules not seen. Leaves alternate, bipinnate, ending with a pair of pinnae, sometimes armed with prickles at the base of the pinnae and leaflets; pinnae in 2–5 opposite pairs; leaflets opposite, in 1–8 pairs per pinna, obovate, broadly elliptic to oblong-elliptic, apex rounded, obtuse, to slightly emarginate, base equal or asymmetrical, rounded to cuneate, 10–46 × 7–35 mm, eglandular, glabrous or pubescent on lower surface; primary vein centric, secondary venation reticulate. Inflorescences in terminal or axillary racemes or panicles, rachis c. 5–30 cm long, glabrous or puberulous, eglandular, unarmed; bracts minute, usually under 3 mm long, with a long acuminate tip, caducous. Flowers bisexual, zygomorphic; calyx a hypanthium with five sepals that are 6–9 mm long, eglandular, glabrous to puberulous, lower sepal cucullate covering the other 4 sepals in bud, with a pectinate, fimbriate or entire margin, sepals caducous, but the hypanthium persisting as a calyx ring around the pedicel as the pod matures; petals 5, free, yellow, the median petal with red markings, c. 10 mm long; stamens 10, free, the filaments pubescent, eglandular. Fruit an indehiscent, straight, oblong, laterally compressed, slightly turgid and somewhat fleshy, coriaceous pod, 4–15 × 1.2–4 cm, eglandular, often puberulent when young, glabrescent. Seeds ellipsoid, c. 8–10 mm diameter, brown, shiny.
Geographic distribution.
A genus of three species, one in South America (Tara spinosa thought to be native to Peru and Ecuador), one in Mexico (Tara cacalaco) and one in Mexico, Guatemala, Nicaragua and extending into the Caribbean (Tara vesicaria). Tara spinosa is also widely cultivated across the tropics and subtropics (including in the Canary Islands) as a source of tannins and occasionally as an ornamental.
Habitat.
Seasonally dry tropical forest to semi-arid thorn scrub.
Etymology.
Derived from the vernacular name ‘tara’ in Peru, Bolivia and Chile.
Notes.
Based on Gagnon et al. (2013), Molinari-Novoa and Sánchez Ocharan (2016) transfered Caesalpinia cacalaco and Caesalpinia vesicaria to the genus Tara, but did not emend the description of the genus, which we provide above.
References.
Britton and Rose (1930); Sprague (1931); Macbride (1943, as Caesalpinia spinosa, 195-196); Ulibarri (1996); Barreto Valdés (2013); Molinari-Novoa and Sánchez Ocharan (2016).
7.1. Tara cacalaco
(Humb. & Bonpl.) Molinari & Sánchez Och.
7.2. Tara spinosa
(Feuillé ex Molina) Britton & Rose
7.3. Tara vesicaria
(L.) Molinari, Sánchez Och. & Mayta
8. Coulteria
Kunth, Nov. Gen. Sp. 6 ed. fol. 258 (1824), 6 ed. qu. 328. 1824 (excluding t. 568 et 569 which ≡ Tara spinosa (Molina) Britton & Rose. 1824), descr. emended E. Gagnon, Sotuyo & G. P. Lewis
Figure 15.
Caesalpinia (Coulteria) velutina Britton & Rose. A portion of leaf B detail of bark C inflorescence D flower E calyx opened out F detail of calyx lobe G median petal H upper lateral petal I lower lateral petal J stamen K fruit L seed M seedling. A, K from Lewis and Hughes 1714 B–J, M from Lewis and Hughes 1713. Drawn by Eleanor Catherine.
Brasilettia sensu Britton & Rose (1930), non (DC.) Kuntze (1891).
Guaymasia Britton & Rose (1930).
Diagnosis.
Coulteria differs from Tara by its racemose inflorescences of unisexual flowers (vs. inflorescences of racemes and panicles with bisexual flowers), chartaceous to papyraceous, laterally-compressed, oblong to elliptic (occasionally suborbicular) stipitate pods, subtended by a 4–13 mm long stipe (vs. indehiscent, laterally compressed but slightly turgid and somewhat fleshy, coriaceous, straight, oblong, sessile pods), and compressed, ovate-orbicular to sub-quadrate, compressed (vs. ellipsoid) seeds.
Type.
No type designated in the original publication, nor since. Type designated here: Coulteria mollis Kunth.
Emended description.
Trees or shrubs, 3–20 m tall, unarmed; young twigs with a dense velvety-bronze pubescence, glabrescent. Stipules not seen. Leaves alternate, bipinnate, ending in a pair of pinnae; petiole and rachis glabrous or densely velutinous; pinnae in 2–6 pairs; leaflets in (2–) 4–12 (– 14) pairs per pinna, 0.6–8 cm long, elliptic, oblong to ovate, apex obtuse to acute, base narrow, rounded or obtuse, eglandular, glabrous to velvety pubescent; main vein centric, secondary veins brochidodromous. Inflorescence racemose, axillary or terminal, 5–16 (– 25) cm long; bracts minute, with an acute tip, pubescent, caducous. Flowers unisexual, male and female flowers on separate trees, zygomorphic; calyx comprising a hypanthium with 5 sepals, 8–10 mm long, velvety-pubescent, lower sepal cucullate, glandular-pectinate, covering the other 4 sepals in bud; petals 5, yellow, free; male flowers with 10 free stamens, filaments pubescent, eglandular. Fruit chartaceous to papyraceous, laterally-compressed, oblong to elliptic (occasionally suborbicular), indehiscent (or sometimes opening along one suture), wingless, 3–15 × 2–4 cm, with a 4–13 mm long stipe, pendulous, often persisting to next flowering season, eglandular, glabrous to densely velutinous, 1–6-seeded. Seeds ovate orbicular or sub-quadrate, compressed.
Geographic distribution.
A genus of approximately seven species in Mexico and Central America, one species extending to Cuba, Jamaica and Curaçao, one to Venezuela (including Isla Margarita) and Colombia.
Habitat.
Seasonally dry tropical forest, deciduous woodland and dry thorn scrub, some species occurring on limestone.
Etymology.
Named by Kunth for the Irish botanist Thomas Coulter (1793–1846) who collected in central Mexico (1825–1834) and was curator of the herbarium at Trinity College, Dublin, Ireland.
Notes.
A revision of the genus has been submitted by S. Sotuyo, J. L. Contreras, E. Gagnon, and G. P. Lewis. The list of species names presented here simply includes all names associated with the genus Coulteria and will be reduced in the forthcoming taxonomic account.
References.
Britton and Rose (1930: 320–322); Ulibarri (1996); Zamora Villalobos (2010); Sotuyo et al. (submitted)
8.1. Brasilettia glabra
Britton & Rose
8.2. Brasilettia pilosa
Britton
8.3. Brasilettia pubescens
Britton
8.4. Brasilettia pringlei
Britton & Rose
8.5. Brasilettia velutina
Britton & Rose
8.6. Caesalpinia acutifolia
J. R. Johnst.
8.7. Caesalpinia blasiana
M. E. Jones
8.8. Caesalpinia colimensis
J. F. Herm.
8.9. Caesalpinia cubensis
Greenm. ex Combs
8.10. Caesalpinia violacea
(Mill.) Standl.
8.11. Coulteria mollis
Kunth
8.12. Coulteria platyloba
(S. Watson) N. Zamora
8.13. Guaymasia pumila
Britton & Rose
8.14. Peltophorum linnaei
Benth.
8.15. Caesalpinia gracilis
Benth. ex Hemsl.
9. Gelrebia
E. Gagnon & G. P. Lewis gen. nov.
urn:lsid:ipni.org:names:60473338-2
Figure 16.
Gelrebia trothae E. Gagnon & G. P. Lewis subsp. trothae. A part of branch showing inflorescence with flowers and fruits B portion of leaflet margin, lower surface C longitudinal section of flower D median petal inner surface E lateral petal inner surface F stamen G anther H ovary with part of wall removed to expose ovules I fruit valve after dehiscence J seed. Gelrebia trothae subsp. erlangeri (Harms) E. Gagnon & G. P. Lewis K part of inflorescence L fruit. A–H from Milne-Redhead & Taylor 11177 I, J from Ward U27 K from Gillett 13223 L from Hemming 478. Drawn by L. M. Ripley, originally published in F.T.E.A., Leguminosae subfamily Caesalpinioideae, page 34, fig. 5 (1967).
Diagnosis.
Gelrebia is morphologically similar to Caesalpinia s. s. but the two genera differ somewhat in habit, with Gelrebia species being erect to scrambling shrubs (vs. erect shrubs or small trees), in having dark pinkish mauve to light pinkish-white flowers (vs. flowers that are variable in colour, from yellow, white, red and orange to green), and coriaceous, broadly oblong-ovoid to obliquely pyriform pods, with a large, oblique, rounded base (vs. coriaceous, oblong-elliptic to linear pods, with an oblique cuneate base).
Type.
Gelrebia rubra (Engl.) E. Gagnon & G. P. Lewis ≡ Hoffmannseggia rubra Engl.: Caesalpinia rubra (Engl.) Brenan
Description.
Erect to scambling shrubs, 0.3–5 m tall, armed with scattered, straight or curved, deflexed prickles (these 7–20 mm long); stems puberulous to pubescent when young, glabrescent. Stipules not seen. Leaves alternate, bipinnate, ending in a pair of pinnae; pinnae opposite, in 1–17 pairs; leaflets opposite (except in Gelrebia glandulosopedicellata), in 1–33 pairs per pinna, narrowly oblong or oblong-elliptic, 3–11 × 2–5 mm, apex rounded to emarginate, sometimes mucronate, glabrous or sparsely pubescent, lower surface of the blades with numerous subepidermal glands or translucent dots (best seen with a × 10 hand lens or microscope). Inflorescence a terminal or axillary raceme, c. (1–) 2–19 (– 25) cm long, unarmed; bracts broadly ovate to suborbicular, apex aristate, 3–10 mm long, caducous. Flowers bisexual, zygomorphic; calyx comprising a short hypanthium with 5 sepals, c. 5–13 mm long, eglandular, glabrous to finely pubescent, lower sepal strongly cucullate (occasionally with a beaked apex), covering the other 4 sepals in bud before anthesis, all sepals caducous, but hypanthium persisting as a free ring around the pedicel as the pod matures; petals 5, free, dark pinkish mauve to light pinkish-white, c. 7–24 × 5–15 mm, eglandular; stamens 10, free, filaments 8–20 mm long, pubescent and eglandular; ovary glabrous. Fruit a coriaceous, broadly oblong-ovoid to obliquely pyriform pod, apex acute, with a large, oblique, rounded base, c. 15–40 × 12–23 mm, dehiscent along both sutures, glabrous to minutely pubescent, eglandular. Seeds obovoid, laterally compressed.
Geographic distribution.
A genus of nine taxa in eight species, restricted to Africa, in Namibia, Botswana, South Africa, Northern Kenya, Ethiopia, and Somalia. One species also found in the Democratic Republic of the Congo (Zaire, Katanga).
Habitat.
Deciduous bushland, dry woodlands, on rocky ridges, often along dry river beds, or on sandy valley floors. One species also found in degraded savanna, close to termite mounds.
Etymology.
Gelreb or gelrib is the Somali name for Gelrebia trothae subsp. erlangeri (field labels of Dale K724 (“gelrib”) and of Gillett 13223 (“gelreb”) from Kenya), meaning ‘camel trap’ and clearly alluding to the robust deflexed prickles characteristic of the species, and indeed the genus as a whole, which can hinder the passage of camels.
References.
Wilczek (1951); Roti-Michelozzi (1957); Brenan (1963, 1967); Ross (1977: 122–130); Thulin (1980, 1983: 16–18; 1993: 344–347); Germishuizen (1991); Roux (2003); Curtis and Mannheimer (2005: 226–228); Brummitt et al. (2007).
9.1. Gelrebia bracteata
(Germish.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473339-2
Basionym.
Caesalpinia bracteata Germish., Bothalia 21 (2): 153. 1991.
Type.
[South Africa, Cape Province]: “2819 (Ariamsvlei): Kenhardt District, on farm Skroef, near hot spring (Warmbad Noord) on Orange River (-DA)”, 29 Sep 1987, Van Hoepen 1941 (holotype PRE).
9.2. Gelrebia dauensis
(Thulin) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473340-2
Basionym.
Caesalpinia dauensis Thulin, Kew Bull. 34(4): 819. 1980.
Type.
KENYA, 30 km on the Ramu-Malka road, c. 4°04'N, 40°59'E, 8 May 1978, Gilbert & Thulin 1583 (holotype UPS; isotypes BR, EA, K!).
9.3. Gelrebia glandulosopedicellata
(R. Wilczek) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473341-2
Basionym.
Caesalpinia glandulosopedicellata R. Wilczek, Bull. Jard. Bot. Brux. 21: 83. 1951.
Type.
“Congo Belge”, district du Haut-Katanga: environs de Niemba, Schmitz 1595.
9.4. Gelrebia merxmuellerana
(A. Schreib.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473342-2
Basionym.
Caesalpinia merxmuellerana A. Schreib., Mitt. Bot. St. Munchen 16, Beih., Die Gattung Caesalpinia in Südwestafrica, 64. 1980.
Type.
SOUTH WEST AFRICA, Dist. Lüderitz-Süd, Farm Uitsig, Wendt in herb. W. Giess 14713 (holotype M; isotypes K!, PRE, WIND).
9.5. Gelrebia oligophylla
(Harms) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473343-2
Basionym.
Caesalpinia oligophylla Harms, Engl., Bot. Jahrb. Syst. 33: 160. 1902.
Type.
ETHIOPIA, “Arussi Galla”, Apr 1901, Ellenbeck 2038 (holotype B †); SOMALIA, rive dello Scebelia Bulo Burti, 25 Feb 1924, Puccioni & Stefanini 134 (neotype FI, designated by G. Roti-Michelozzi in Webbia 13: 207. 1957).
9.6. Gelrebia rostrata
(N.E.Br.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473344-2
Basionym.
Caesalpinia rostrata N. E. Br., Hooker's Icon. Pl., 28: t. 2702. 1901.
Type.
SOUTH AFRICA, from cultivation in Durban Botanic Garden, raised from seed obtained from “Delagoa Bay”, Maputo (Lourenço Marques), Wood 7943 (holotype K!; isotypes BOL, NH, PRE).
9.7. Gelrebia rubra
(Engl.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158022-1
Basionym.
Hoffmannseggia rubra Engl., Bot. Jahrb. Syst. 10: 25. 1889. Caesalpinia rubra (Engl.) Brenan, Kew Bull. 17(2): 202. 1963.
Type.
NAMIBIA, Karibib Dist., Usakos, Marloth 1432 (holotype ?B; isotypes BOL, PRE).
9.8. Gelrebia trothae
(Harms) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473345-2
Basionym.
Caesalpinia trothae Harms, Engl., Bot. Jahrb. Syst., 26: 277. 1899, as “trothaei”.
Type.
TANZANIA, ?Dodoma District, Ugogo, Chumo Pass, Jan. 1897, von Trotha 186 (holotype B †).
9.8.1. Gelrebia trothae subsp. trothae
9.8.2. Gelrebia trothae subsp. erlangeri
(Harms) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473346-2
Basionym.
Caesalpinia erlangeri Harms, Engl., Bot. Jahrb. Syst. 33: 160. 1902.
Caesalpinia trothae subsp. erlangeri (Harms) Brenan, Kew Bull. 17(2): 20. 1963.
Type.
ETHIOPIA, Galla Sidama, Borana, Tarro Gumbi, Ellenbeck 2071 (holotype B †). Somalia, Dolo, sul Daua, 6 May 1893, Riva 1104 (neotype FI, designated by G. Roti-Michelozzi in Webbia 13: 209, 1957).
10. Hultholia
E. Gagnon & G. P. Lewis gen. nov.
urn:lsid:ipni.org:names:77158067-1
Diagnosis.
Hultholia is closely related and morphologically similar to Guilandina. While both genera form armed lianas, Hultholia differs in having stems with dome-shaped glands intermixed with dense slender, patent, needle-like prickles (vs. stems eglandular and with strongly recurved, robust prickles in Guilandina); both genera have sharp recurved prickles on the leaf and pinnae rachises. Hultholia has bisexual flowers (vs. unisexual flowers on separate female and male racemes in Guilandina), a zygomorphic corolla, with petals extending beyond the sepals, and the median (standard) petal smaller than the other four (vs. a sub-actinomorphic to zygomorphic corolla, with petals only slightly extending beyond the sepals in Guilandina), unarmed, obovoid, falcate, pubescent, vesicular pods (vs. oblong-elliptic, coriaceous, eglandular, inflated pods, usually armed with 5–10 mm long, slender spinescent bristles), and sub-globose, oblong, grey, ca. 10 × 7 mm, smooth seeds (vs. obovoid to globular c. 20 mm in diameter, grey, pale to dark brown or orange seeds, with parallel fracture lines concentric with the small apical hilum).
Type.
Hultholia mimosoides (Lam.) E. Gagnon & G. P. Lewis ≡ Caesalpinia mimosoides Lam.
Description.
Climbing woody shrub; branches densely armed with short, robust, needle-like trichomes; young stems pubescent, with rust-coloured, hyaline hairs and dome-shaped glands, topped with a few hairs. Stipules subulate, 7–15 mm long, pubescent, caducous. Leaves alternate, bipinnate, without a single terminal pinna, 22–40 cm long; pinnae opposite, in 10–30 pairs per leaf, about 3–5 cm long, pubescent, with a pair of deflexed prickles at the insertion of the pinnae on the leaf rachis, and at the insertion of leaflets on the pinnae rachises; leaflets opposite, in 7–20 pairs per pinna, oblong, asymmetric at base, c. 9 × 4 mm, glabrous, eglandular. Inflorescences terminal or leaf-opposed, lax racemes, with 50 or more flowers, 20–40 cm long; rachis and pedicels armed with needle-like, robust trichomes, pubescent and covered with domed, hair-tipped glands. Flowers bisexual, zygomorphic; calyx comprising a hypanthium with 5 sepals 13–16 × 6 mm; hypanthium and sepals pubescent and glandular, the sepal margins sometimes with small stipitate glands, < 1 mm long; petals 5, free, bright yellow, dark glands present on the blade, median (standard) petal c. 8 mm wide and smaller than the 4 lateral petals, that are c. 1.7 × 1.3 cm; stamens 10, free, filaments 1.8 cm long, pubescent at least on the lower ½; ovary densely pubescent, and with glandular dots (often obscured by the dense pubescence). Fruit an obovoid, falcate, vesicular, unarmed, dehiscent pod, sparsely pubescent, particularly along the margin, and with a few obscure stellate hairs, and covered in gland dots, 5–6 × 2.5–3 cm, 1–3-seeded. Seeds sub-globose, oblong, 10 × 7 mm, grey.
Geographic distribution.
The single species is distributed across Asia, in China (Yunnan), Bangladesh, India, Laos, Myanmar (Burma), Thailand and Vietnam.
Habitat.
In secondary thickets and clearings, often on roadsides, up to 1500 m elevation. More information on the ecology of this genus is needed.
Etymology.
The name Hultholia honours the Cambodian botanist Dr. Sovanmoly Hul Thol (born 1946), whose doctoral thesis, “Contribution à la révision de quelques genres de Caesalpiniaceae, representés en Asie” (1976), is an important revision of the Asian species and genera of the Caesalpinia group, and particularly the genus Pterolobium. Dr. Hul Thol retired from the Museum National d’Histoire Naturelle, Paris in 2014, but continues as an honorary researcher. She is a specialist on the flora of Cambodia and South East Asia, directed the publication of multiple volumes of the Flora of Cambodia, Laos and Vietnam from 1995, and is one of the co-founders of the National Herbarium of Cambodia, Royal University of Phnom Penh.
Notes.
Although Hultholia mimosoides is not known to be cultivated, the young, pungent, flowering shoots are sold as a vegetable in markets in Vientiane (Laos) (Vidal and Hul Thol 1976).
References.
Vidal and Hul Thol (1976); Chen et al. (2010a: 42–43).
10.1. Hultholia mimosoides
(Lam.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158068-1
Basionym.
Caesalpinia mimosoides Lam., Encycl. Méth., Bot. 1(2): 462 (1785).
Biancaea mimosoides (Lam.) Tod., Hort. Bot. Panorm. 1(1): 3 (1875).
Type.
Specimen originally from Malabar, sent to Lamarck by Sonnerat (P: Herb. Lamarck, fide Vidal and Hul Thol. 1976).
11. Guilandina
L., Sp. Pl.: 381. 1753
Figure 19.
Guilandina ciliata Bergius ex Wikstrom. A foliage B leaflet undersurface C prickle enlarged to show indumentum D inflorescence and portion of leaf; E flower F, G median petal H upper lateral petal (outer surface) I lower lateral petal (inner surface) J stamens K stamen L fruit M, N seeds. A–C from Ekman 5413 D–K from Curtiss 143 L–N from Pannell 179. Drawn by Pat Halliday.
Bonduc Mill. (1754).
Caesalpinia subgenus Guilandina (L.) Gillis & Proctor (1974).
Type.
Guilandina bonduc L.
Description.
Lianas, woody climbers, scrambling or trailing shrubs, often forming dense tangled clumps, densely armed with recurved prickles on branches and shoots, as well as in pairs at leaf bases (except Caesalpinia murifructa and closely related species in the Caribbean which are unarmed). Stipules foliaceous to subulate, sub-persistent or caducous. Leaves bipinnate, ending with a pair of pinnae, prickles present in pairs at the insertion of pinnae and scattered on the leaf rachis, and at the insertion of leaflets on the pinnae rachises; leaflets oblong, apex obtuse and mucronulate to acuminate, base rounded. Inflorescences supra-axillary or terminal racemes, 30–60 cm long; bracts narrow, lanceolate, aristulate, 1 mm long, to conspicuous and exceeding floral buds, caducous. Flowers unisexual, segregated on separate male and female racemes, the female flowers cryptically bisexual with 10 fully formed stamens, but these produce no pollen; male flowers with a highly reduced, non-functional pistil, zygomorphic to sub-actinomorphic; calyx with a hypanthium and 5 almost equal sepals, these valvate in bud, the lower sepal slightly cucullate, the hypanthium and sepals caducous, leaving no persistent calyx ring, eglandular, without spines (except Madagascan Caesalpinia delphinensis in which the calyx is armed with slender prickles); petals 5, free, yellow, barely exceeding the sepals; stamens 10, free, pubescent near the filament base; ovary usually covered in bristly trichomes, except in a few species, including Caesalpinia solomonensis and Caesalpinia murifructa. Fruits oblong-elliptic, inflated pods, usually armed with 5–10 mm long spinescent bristles, apex terminating in a beak, base acute, 1–4-seeded. Seeds obovoid to globular, c. 2 cm in diameter, smooth, grey, pale to dark brown, or orange, with parallel fracture lines concentric with the small apical hilum.
Geographic distribution.
This pantropical genus lacks a recent global taxonomic account and there are doubts about the number of species, with previous estimates ranging from seven to as many as 19. Species occur from as far north as Japan, south to South Africa, with three species in the Caribbean, one in China, India, Myanmar (Burma), Indo China, Hong Kong and Taiwan, one endemic to Madagascar, one in Australia, and two widespread across the Old and New World tropics.
Habitat.
Coastal thickets on sand, in secondary forest, and lowland rain forest, occasionally on limestone.
Etymology.
Named by Linnaeus for Melchior Wieland (1515–1589), Prussian naturalist, traveller and scholar from Königsberg, who settled in Italy and italianised his name to ‘Guilandini’, or Guilandinus in Latin; he was sent to the Levant, Asia and Africa (1559–1560), was captured by pirates and finally ransomed by Gabriele Falloppio.
Notes.
Pending a complete taxonomic revision, the list of 19 names presented below provides a guide to potential species content in Guilandina, but includes no synonymy and no information on types, nor any new nomenclatural combinations for the five species of Caesalpinia that as yet have no published name in Guilandina.
References.
Britton and Rose (1930: 336–341); Wilczek (1951); Brenan (1967); Gillis and Proctor (1974); Hattink (1974); Vidal and Hul Thol (1976); Du Puy and Rabevohitra (2002: 46–48); Chen et al. (2010a).
11.1. Guilandina barkeriana
(Urb. & Ekman) Britton
11.2. Guilandina bonduc
L.
11.3. Guilandina caymanensis
(Millsp.) Britton & Rose
11.4. Guilandina ciliata
Bergius ex Wikstrom
11.5. Guilandina culebrae
Britton & Wilson ex Britton & Rose
11.6. Caesalpinia delphinensis
Du Puy & Rabev.
11.7. Guilandina glaucophylla
(Urb.) Britton & Rose
11.8. Caesalpinia homblei
R. Wilczek
11.9. Guilandina intermedia
(Urb.) Britton & Rose
11.10. Guilandina major
(DC.) Small
11.11. Caesalpinia minax
Hance
11.12. Caesalpinia murifructa
Gillis & Proctor
11.13. Guilandina portoricensis
Britton & Wilson
11.14. Guilandina socorroensis
Britton & Rose
11.15. Caesalpinia solomonensis
Hattink
11.16. Guilandina sphaerosperma
(Urb. & Ekman) Britton
11.17. Guilandina urophylla
(Donn. Sm.) Britton & Rose
11.18. Caesalpinia volkensii
Harms
11.19. Guilandina wrightiana
(Urb.) Britton & Rose
12. Moullava
Adans., Fam. Pl. 2: 318. 1763, descr. emended E. Gagnon & G. P. Lewis
Figure 21.
Moullava spicata (Dalzell) Nicolson. A flowering branch B single pinna of bipinnate leaf C leaflet undersurface D leaflet undersurface detail E young stem F older stem G part inflorescence H calyx opened out I median petal J upper lateral petal K lower lateral petal L stamen M gynoecium N stigma O fruit P seed. A, G from photo by P. S. Green B–D, H–N from Cult. Foster Bot. Gard. F1901, specimen Hutchinson 2784 E from Critchett 11/79 F from Nana 5620 O, P from Meebold 8605. Drawn by Eleanor Catherine.
Wagatea Dalzell (1851).
Cinclidocarpus Zoll. & Moritzi (1846).
Caesalpinia sect. Cinclidocarpus (Zoll. & Moritzi) Benth. & Hook. (1865).
Diagnosis.
Moullava is related to Mezoneuron, but differs by its fleshy, oblong-elliptic, indehiscent, sub-torulose, wingless pods, with thickened sutures (vs. laterally compressed, chartaceous, coriaceous or ligneous, indehiscent pods, with a longitudinal wing along the upper suture), and by its subglobular (vs. compressed) seeds.
Type.
“H.M. 6 t. 6” (= Rheede`s Hortus Malabaricus 6, plate 6, 1686) = Moullava spicata.
Emended description.
Lianas and scrambling shrubs, armed with deflexed prickles on shoots. Stipules not seen. Leaves alternate, bipinnate, ending with a pair of pinnae, 12–40 cm long, glabrous to pubescent-tomentose, with a pair of prickles at the insertion of each pinna; pinnae opposite, in 7–20 pairs; leaflets in 5–40 opposite pairs per pinna, sessile, narrowly oblong to ovate-oblong, apex rounded to emarginate, sometimes mucronate, base asymmetrical to rounded, blades eglandular, glabrous to pubescent, 4–20 × 2–6 mm. Inflorescence an elongated terminal or axillary raceme, the flowers subsessile, pedicels, when present, 10–25 mm long, the racemes sometimes aggregated into panicles, 8–60 cm long, unarmed or with a few prickles at the base. Flowers bisexual, sub-actinormophic or zygomorphic; calyx comprising a hypanthium with 5 sepals, 6–12 × 2–4 mm, the lower sepal strongly cucullate, covering the other 4 sepals in bud, all sepals eglandular and glabrous; petals 5, free, yellow, the median and lateral petals sometimes streaked red, eglandular; stamens 10, free, barely exserted beyond the corolla, densely pubescent on lower half of filaments, 8–15 mm long; ovary glabrous or pubescent. Fruit fleshy, oblong-elliptic, unarmed, indehiscent, sub-torulose, with thickened sutures, the apex apiculate, 35–50 (–80) × 15–30 mm, drying black (immature fruits of Moullava spicata red-tomentose), exocarp and endocarp strongly adnate, glabrous, 1–4-seeded. Seeds sub-globular, 12–20 mm in diameter, olive-brown to black.
Geographic distribution.
A genus of four species, three in south Asia: India, Nepal, Myanmar (Burma), Thailand, Laos, Cambodia, Sri Lanka, southern China (Yunnan and Hainan), and the Malay Peninsula and Archipelago, and one in Africa: Cameroun, Gabon, the Democratic Republic of Congo, Angola, Zambia (Kabompo Dist.), Uganda and Tanzania (Kigoma Dist.).
Habitat.
The Asian species are found in seasonally dry tropical semi-evergreen forest margins, secondary thickets, and on mountain slopes, up to 1200 m elevation. The African species occurs mostly in riverine habitats in lowland rainforests.
Etymology.
Derived from the vernacular name of Moullava spicata, “mulu” (Malayalam: spiny), a spiny climber.
References.
Brenan (1963, 1967); Hattink (1974); Vidal and Hul Thol (1976); Nicolson (1980); Ansari (1990); Sanjappa (1992: 33); Brummitt et al. (2007, see both Moullava and Mezoneuron welwitschianum); Chen et al. (2010a).
12.1. Moullava digyna
(Rottl.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:namess:77158131-1
Basionym.
Caesalpinia digyna Rottl., Ges. Naturf. Freude Berlin Neue Schriften 4:198–200, pl. 3. 1803.
Type.
[S. INDIA] Marmelon (near Madras), 9 Oct 1799, Rottler s.n. (? B: Herb. Willdenow, K!).
Caesalpinia gracilis Miq., Fl. Ned. Ind. 1:110. 1855.
Type. INDIA, Roxburgh (n.v.).
Caesalpinia oleosperma Roxb., Hort. Bengal. 32. 1814.
Type. JAVA, Horsfield 138 (holotype K!; isotype BM).
Caesalpinia flavicans Grah., Cat.: 5825. 1832, nom. nud.
12.2. Moullava spicata
(Dalzell) Nicolson, Bot. Hist. Hort. Malabaricus [K.S.Manilal]: 184. 1980
Basionym.
Caesalpinia spicata Dalzell, in Hooker’s J. Bot. Kew Gard. Misc. 3: 89 (1851).
Wagatea spicata Dalzell, in Hooker’s J. Bot. Kew Gard. Misc. 3: 89 (1851).
Type.
WESTERN INDIA, Bombay presidency.
Caesalpinia ferox Hohen., Pl. Ind. Or. Exs. No. 414, non Hassk.
Type. Not traced.
Caesalpinia digyna Graham, Cat. 60. 1839, non Rottl. 1803, nom. illeg.
Caesalpinia mimosoides Heyne & Wall, Numer. List n. 5837. 1831, nom. illeg., non Lam.1785.
12.3. Moullava tortuosa
(Roxb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158069-1
Basionym.
Caesalpinia tortuosa Roxb., Fl. Ind. (ed. 1832) 2: 365. 1832.
Type.
Specimen originating from SUMATRA, cultivated in the Botanic Garden of Calcutta, “Hort. Calc. E. Sumatra”, Roxburgh s.n. (holotype: K!).
Caesalpinia acanthobotrya Miq., Fl. Ned. Ind. 1(Suppl.): 108 (1860) & 293 (1861).
Type. W. SUMATRA, prov. Priaman, 1855–60, Diepenhorst HB2240 (holotype U; isotype BO).
Caesalpinia microphylla Buch.-Ham ex Prain, in J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 66: 471. 1897, non Mart. ex G. Don, 1832.
Type. INDIA, Goyalpara, 6 Aug 1908, Wallich 5826 (K!).
Caesalpinia tortuosa var. grandifolia Craib, Fedde Repert. Spec. Nov. Reg. Veg. 12: 392. 1913.
Type. MYANMAR [Burma], Kowpok, Jan 1912, Meebold 17208 (K!).
Caesalpinia cinclidocarpa Miq., in Fl. Ned. Ind 1: 110 (1855).
Type. JAVA, as for Cinclidocarpus nitidus, non Caesalpinia nitida Hassk. (1844).
Cinclidocarpus nitidus Zoll. & Moritzi, in Naturr-Geneesk. Arch. Ned.-Indie 3: 82 (1846).
Type. JAVA, Zollinger 3462 (holotype L; isotypes A, BM, P).
Caesalpinia tortuosa Wall., Numer. List n. 5827 D. 1831, nom. nud.
12.4. Moullava welwitschiana
(Oliv.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158070-1
Basionym.
Mezoneuron welwitschianum Oliv., Fl. Trop. Afr. 2: 261. 1871.
Caesalpinia welwitschiana (Oliv.) Brenan, Kew Bull. 17(2): 203. 1963.
Type.
ANGOLA, Cuanza Norte, Golungo Alto, Welwitsch 608 (holotype LISU; isotypes BM, K!).
13. Biancaea
Tod., Nuovi Gen. Sp. Orto Palermo: 21. 1860, descr. emended E. Gagnon & G. P. Lewis
Figure 22.
Biancaea decapetala (Roth) O. Deg. A flowering branchlet and foliage B, C leaflets viewed from above and below, respectively D flower with parts separated, and centre of flower enlarged E calyx three views F lateral petal G median petal H stamen I anther J gynoecium K fruits L seed. A from Rutherford-Smith 11062 B, C from White 2478 D–J from Chase 4564 K, L from Myre 2528. Drawn by D. Erasmus, originally published in Flora Zambesiaca, vol. 3 part 2, page 182, figure 3.2.39 (2007).
Campecia Adans. 1763; no type species designated, and no species names ever published in this genus. It is thus not possible to apply this name which is rejected against Biancaea.
Caesalpinia sect. Sappania DC. 1825.
Diagnosis.
Biancaea is closely related to Mezoneuron, differing principally in its fruit, a coriaceous, laterally compressed, wingless, dehiscent pod (except Biancaea decapetala, which has somewhat inflated, boat-shaped pods, often with a narrow wing or ridge along the upper suture). In contrast, Mezoneuron has chartaceous, coriaceous or ligneous pods, which are also laterally compressed, but indehiscent, and with a wing along the upper suture. In addition, the ovary of Biancaea species always has a velvety indumentum (vs. glabrous to pubescent in Mezoneuron).
Type.
Biancaea scandens Tod. ≡ Biancaea decapetala (Roth) Deg.
Emended description.
Lianas, climbing or trailing shrubs (1–3 m), or small trees (2.5–10 m), armed with short, slightly recurved prickles, scattered along the branches; young shoots pubescent or glabrescent. Stipules lanceolate-oblong to broadly-ovate, sometimes amplexicaul at base, 3–4 mm to 4.5 cm long, caducous or sub-persistent to persistent. Leaves alternate (except in Biancaea oppositifolia), bipinnate, ending with a pair of pinnae, rachis pubescent (glabrous in Biancaea oppositifolia), armed with pairs of prickles at the base of each pinna, sometimes also scattered on the rachis; pinnae in 4–19 opposite to alternate pairs; leaflets opposite to alternate, in 5–20 pairs per pinna, blade membranous, eglandular, glabrous to pubescent, 10–35 × 4–15 mm (4–10 × 1.5–4.5 cm in Biancaea oppositifolia), oblong-elliptic, apex acute, obtuse, rounded to emarginate, base asymmetric. Inflorescences erect, showy, terminal or axillary racemes or panicles; rachis eglandular, pubescent, unarmed or with a few scattered prickles, mainly near the base; bracts ovate-lanceolate, acuminate, 2–8 mm long, caducous. Flowers bisexual, zygomorphic; calyx with a short hypanthium and 5 sepals, the lower sepal cucullate and covering the other 4 in bud, sepals pubescent (except in Biancaea sappan), caducous, but the hypanthium persisting as a calyx ring around the pedicel as fruits mature; petals 5, free, yellow to white, eglandular, the claws pubescent; the median petal smaller than the other 4, and inrolled towards the centre, lateral petals oblong, obovate to spathulate, 4–10 × 2–8 mm; stamens 10, filaments densely pubescent (most evident at the base), eglandular, 10–15 mm long; ovary densely velutinous. Fruit a coriaceous, glabrous, eglandular, oblong-elliptic to obovate, dehiscent, wingless, laterally compressed (but somewhat inflated and often with a narrow wing along the upper suture in Biancaea decaptala), 4.5–10 × 2–4 cm, 2–8-seeded pod, usually much broader at the rounded to truncate apex, which terminates in a sharp beak. Seeds flat, elliptic, ovoid to orbicular, c. 2 cm in diameter, black or brown.
Geographic distribution.
A genus of six species widespread across southern Asia, from India, to Myanmar (Burma), Thailand, Cambodia, Vietnam, south China, Japan, the Philippines, and the Malay Peninsula and Archipelago, one species endemic to Sabah (near Sandakan). Biancaea decapetala, native to Asia, has been widely introduced across the tropics as a hedge plant or ornamental and is considered to be invasive in South Africa and Hawaii.
Habitat.
Primary forest and forest margins, grasslands, scrub vegetation, riverine habitats, secondary thickets and clearings. From the coast to mountain slopes.
Etymology.
Unknown.
Notes.
Based on the study of Gagnon et al. (2013), Molinari-Novoa et al. (2016) provided some, but not all, of the required nomenclatural transfers to the genus Biancaea. Furthermore, they did not emend the description of the genus, as provided here.
References.
Hattink (1974); Vidal and Hul Thol (1976); Jansen (2005); Brummitt et al. (2007); Chen et al. (2010a); Molinari-Novoa et al. (2016).
13.1. Biancaea decapetala
(Roth) O. Deg., Fl. Hawaiiensis K7. 1936
Basionym.
Reichardia decapetala Roth, Nov. Pl. Sp. 212. 1821.
Caesalpinia decapetala (Roth) Alston, Handb. Fl. Ceylon 6: 89. 1931.
Type. INDIA, (fl.), Heyne s.n. (isotype K!).
Biancaea scandens Tod., in Nuov. Gen. Sp. Pl.: 22. 1860.
Type. “Cortivasi da lungo tempo nel Real Orto Botanico [di Palermo] in piena terra, col nome di Caesalpinia sepiaria”.
Caesalpinia benguetensis Elmer, in Leafl. Philipp. Bot. 1: 226 (1907).
Mezoneuron benguetense (Elmer) Elmer, in Leafl. Philipp Bot 1: 362 (1908).
Type. PHILIPPINES, Luzon, Benguet prov. Baguio, (fl. fr.), Mar 1907, Elmer 8720 (BO, K!, L, PHN).
Caesalpinia japonica Sieb. & Zucc., in Abh. Math.-Phys. Cl. Königl. Bayer Akad. Wiss. 4(2): 117. 1845.
Caesalpinia sepiaria var. japonica (Siebold & Zucc.) Gagnep., in Fl. Indo-Chine 2: 180. 1913.
Caesalpinia sepiaria var. japonica (Siebold & Zucc.) Makino, Ill. Fl. Nippon: 431. 1940.
Caesalpinia decapetala var. japonica (Siebold & Zucc.) H. Ohashi, Fl. E. Himalaya 3: 58. 1975.
Caesalpinia decapetala var. japonica (Siebold & Zucc.) Isely, Mem. New York Bot. Gard. 24(2): 193. 1975.
Type. JAPAN, Siebold & Zuccanini.
Caesalpinia ferox Hassk., Ind. Sem. Hort. Amst. 1841.
Biancaea ferox (Hassk.) Tod., Hort. Bot. Panorm. 1(1): 3. 1875.
Type. probably a living plant in Hort. Bog., fide Hattink (1974).
Caesalpinia sepiaria Roxb., Fl. Ind. 2: 360. 1832. Biancaea sepiaria (Roxb.) Tod., Hort. Bot. Panorm. 1(1): 3. 1875.
Type. INDIA, Roxburgh without number (isotypes: BM, K!, in Hb. Wallich 5834A).
Caesalpinia sepiaria Roxb. var. pubescens T. Tang. & F.T. Wang, Illust. Treat. Prin. Pl. China (Leguminosae): 96. 1955, without Latin description.
Caesalpinia sepiaria Roxb. var. pubescens T. Tang & F. T. Wang ex C. W. Chang, Flora Tsinlingensis 1(3): 444. 1981.
Caesalpinia decapetala (Roth) Alston var. pubescens P. C. Huang, Sylva Sinica 2: 1187. 1985, nom. illeg., without Latin description or type.
Caesalpinia decapetala var. pubescens (T. Tang & F. T. Wang ex C. W. Chang) X. Y. Zhu, in Legumes of China: 5. 2007.
Type. CHINA.
13.2. Biancaea godefroyana
(Kuntze) Molinari, Mayta & Sánchez Och., Weberbauerella 1(11): 3. 2016
Basionym.
Caesalpinia godefroyana Kuntze, Rev. Gen. Pl. 1: 166. 1891.
Type.
VIETNAM (South), Cap St-Jacques (Vung Tau), 18 Mar 1875, Godefroy s.n. (lectotype K!, designated by Vidal and Hul Thol, 1976).
Caesalpinia thorelii Gagnep., Notul. Syst. (Paris). 2: 207. 1912.
Types. VIETNAM, 1er pont de l’avalanche près Saïgon, 14 Jan 1865, Lefèvre, Thorel et Godefroy no. 145 (syntype P02940578!); Cochinchine, Bien-hoa, Nov 1866, Thorel 848 (syntype P02940348!); ad Bienhoa, Pierre 130 (syntype P02940353); Cochinchine, Baria, Baudoin and Talmy 104 (syntype);
13.3. Biancaea millettii
(Hook. & Arn.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158071-1
Basionym.
Caesalpinia millettii Hook. & Arn., Bot. Beechey Voy. 182 (1841[1833]).
Type.
CHINA, Millett s.n. (K!).
Pterolobium subvestitum Hance, J. Bot. 22(12): 365. 1884.
Cantuffa subvestita (Hance) Kuntze, Rev. Gen. Pl. 1: 168. 1891.
Type. CHINA, Kwangtung, Lo Fau Sahn, Faber in herb. Hance 22291 (BM).
13.4. Biancaea oppositifolia
(Hattink) Molinari & Mayta, Weberbauerella 1(11): 3. 2016
Basionym.
Caesalpinia oppositifolia Hattink, Reinwardtia 9(1): 43. 1974.
Type.
MALESIA, Sabah [North Borneo], Ranau Distr. Hot Spring track, 15 Feb 1961, J. Singh 24026 (holotype SAN; isotypes K!, L).
13.5. Biancaea parviflora
(Prain ex King) Mayta & Molinari, Weberbauerella 1(11): 3. 2016
Basionym.
Caesalpinia parviflora Prain ex King, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 66: 230. 1897.
Type.
MALAY PENINSULA, Perak, Relau Tugor, May 1888, Wray 1909 (lectotype CAL, designated by Hattink 1974; isolectotypes K!, SING).
Caesalpinia parviflora var. stipularis Prain, in J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 66: 230. 1897.
Types. MALAY PENINSULA, Perak, Larut, Wray 3983, 3991, 4261 (syntypes).
Caesalpinia stipularis Ridl., in Fl. Malay Penin. 1: 651 (1922), nom. illeg., non Caesalpinia stipularis (Vogel) Benth. (1870) (= Pomaria stipularis (Vogel) B.B. Simpson & G. P. Lewis).
Caesalpinia parviflora var. typica (Prain ex King) Prain, J. Asiat. Soc. Bengal, Pt. 2, Nat. Hist. 60: 230. 1897, nom. illeg.
Caesalpinia borneensis Merr., Univ. Calif. Publ. Bot. 15: 104. 1929.
Type. BORNEO, Tawao, Elphinstone Prov., Oct 1922– Mar 1923, Elmer 21449 (holotype MO; isotypes A, BM, BO, K!, L, NY, P, SING, U, UC).
Caesalpinia macra Craib, Bull. Misc. Inform. Kew 2: 386. 1927.
Type. THAILAND, Saraburi, Muak Lek, 10 Nov 1924, Marcan 1866 (syntype K), Pak Chong, 30 Dec 1923, Marcan 1532 (syntype K).
Caesalpinia minutiflora Elmer, Leafl. Philipp. Bot. 5: 1803. 1913.
Type. PHILIPPINES, Palawan, Puerto Princesa, Mt. Pulgar, Apr 1911, Elmer 12969 (BM, K!, L, P, PNH, U).
13.6. Biancaea sappan
(L.) Tod., Hort. Bot. Panorm. 1(1): 3. 1875
Basionym.
Caesalpinia sappan L., Sp. Pl. 1: 381. 1753.
Type.
SRI LANKA (CEYLON), Hb. Hermann, vol. 4, fol. 31 (holotype BM).
Caesalpinia angustifolia Salisb., Prod.: 326. 1796, nom. illeg.
14. Pterolobium
R. Br. ex Wight & Arn., Prodr: 283. 1834
Figure 23.
Pterolobium stellatum (Forssk.) Brenan. A part of flowering branch B flower C longitudinal section of flower D petal E stamen F infructescence with mature fruits G samara with part cut away to reveal seed. A–E from Richards 11275 F from Eggeling 3400 G from Sandwith 25. Drawn by L. M. Ripley, originally published in Flora of Tropical East Africa, Leguminosae subfamily Caesalpinioideae, page 41, fig. 7 (1967).
Cantuffa J.F. Gmel. (1791).
Reichardia Roth (1821), nom. illeg., non Roth (1787), nec Roth (1800).
Type.
Pterolobium lacerans R. Br. ex Wight & Arn., nom. illeg. (Cantuffa exosa J.F. Gmel. = Pterolobium exosum (J.F. Gmel.) E.G. Baker; this now considered a synonym of Pterolobium stellatum (Forssk.) Brenan).
Description.
Lianas or scrambling / trailing shrubs, armed with prickles on shoots, as well as in pairs at the base of leaves. Stipules small, inconspicuous, subulate or triangular-subulate, caducous. Leaves alternate, bipinnate, ending in a pair of pinnae, 6–30 cm long; petiole and rachis pubescent to sparsely pubescent or glabrous; pinnae opposite, in 5–20 pairs; leaflets opposite, in 6–25 pairs per pinna, linear-oblong to elliptic-oblong, apex rounded to emarginate, sometimes mucronate, eglandular or punctate-glandular, 6–15 × 1.5–10 mm. Inflorescences terminal or axillary racemes, often aggregated into panicles, pubescent to glabrous, 4–25 cm long; bracts small, caducous. Flowers bisexual, sub-actinomorphic to zygormophic; calyx comprising a short hypanthium and 5 sepals, glabrous to pubescent, the lower sepal cucullate, covering the other 4 sepals in bud; petals 5, free, yellow to white, equal to slightly differentiated, claws pubescent, the median petal sometimes inrolled; stamens 10, free, filaments pubescent (occasionally glabrous); ovary pubescent, stigma chambered. Fruit a red to brown samara, the basal seed-containing portion 12–20 × 8–15 mm, reticulate or smooth, glabrous to pubescent, the upper suture much prolonged and broadly winged, the wing 20–45 mm long and usually wider distally, 1 (–2)-seeded.
Geographic distribution.
A genus of 10 species; one in southern tropical Africa, East Africa and Arabia, nine in SE Asia (one endemic to India, two in China, four in Indo-China [one endemic to Thailand, two extending to Malesia], three restricted to the Malay Peninsula and Archipelago [one endemic to the Philippines]).
Habitat.
Seasonally dry tropical upland evergreen forest, riverine and humid forest, woodland and wooded grassland.
Etymology.
From ptero- (Greek: wing) and lobion (Greek: pod, fruit), in reference to the fruit which is a samara.
Notes.
Vidal and Hul Thol (1974) published a revision of Pterolobium, with a key to species. We provide below a list of species currently accepted in the genus, taking into account the treatment of Pterolobium sinense as a synonym of Pterolobium macropterum (Chen et al. 2010b).
References.
Roti-Michelozzi (1957); Brenan (1967: 40–42); Vidal and Hul Thol (1974, 1976); Hul Thol and Hideux (1977); Hou et al. (1996: 654–700); Chen et al. (2010b).
14.1. Pterolobium borneense
Merrill
14.2. Pterolobium densiflorum
Prain
14.3. Pterolobium hexapetalum
(Roth) Santapau & Wagh
14.4. Pterolobium integrum
Craib
14.5. Pterolobium macropterum
Kurz
14.6. Pterolobium membranulaceum
(Blanco) Merrill
14.7. Pterolobium micranthum
Gagnep., emend. Craib
14.8. Pterolobium microphyllum
Miq.
14.9. Pterolobium punctatum
Hemsl.
14.10. Pterolobium stellatum
(Forssk.) Brenan
15. Mezoneuron
Desf., Mém. Mus. Hist. Nat. 4: 245. 1818
Figure 25.
Mezoneuron scortechinii F. Muell. A flowering branch B bract C calyx opened out D median petal E upper lateral petal F lower lateral petal G stamen H gynoecium I stigma J fruit K seed L detail of prickle from leaf. A–I, L from Hoogland 11665 J from Thurtill & Coveny 3880 K from White s.n. 6/1926. Drawn by Eleanor Catherine.
Mezonevron Desf. and Mezoneurum DC. (1825), (orth. vars.).
Caesalpinia subg. Mezoneuron (Desf.) Vidal ex Herend. & Zarucchi (1990).
Type.
Mezoneuron glabrum Desf. ≡ Mezoneuron pubescens Desf.
Description.
Scrambling shrubs or lianas, occasionally medium -sized trees (Mezoneuron kauaiense) to 12 m, usually armed with recurved prickles on stem and leaves, rarely unarmed. Stipules very small, often caducous. Leaves alternate or occasionally opposite, bipinnate, ending in a pair of pinnae; pinnae opposite to sub-opposite, in (1–)2–18 pairs; leaflets opposite to alternate, in 1–15 pairs per pinna, elliptic, oblong, suborbicular to occasionally subrhombic, the base oblique, the apex obtuse to acute. Inflorescences terminal or axillary racemes (often aggregated into panicles); bracteoles small. Flowers bisexual, zygomorphic; calyx comprising a hypanthium and 5 imbricate sepals, the lower sepal cucullate, and overlapping the other 4 in bud; petals 5, free, usually yellow with red markings on the median petal, or occasionally red, pink or cream, the median petal somewhat modified (either with a fleshy ligule or a patch of hairs on the inner surface between the blade and claw, or the petal bilobed); stamens 10, free, filaments alternately longer and shorter, usually all 10 pubescent or villous on lower half, or one or all glabrous; ovary glabrous to hairy, 1-many ovuled, stigma cupular, funnel-shaped, terminal or laterally placed, glabrous, or the rim fimbriate with papillate hairs, not peltate. Fruit laterally compressed, indehiscent, chartaceous, coriaceous or woody, venose, longitudinally and often broadly winged along the upper suture, the wing 1–18 mm wide. Seeds 1–13 per pod, ± transversely arranged in seed chamber, compressed, endosperm lacking.
Geographic distribution.
A genus of 24 extant species, mainly in Asia, extending to Australia, Polynesia, Madagascar and Africa; two species on mainland Africa (one widespread in West Africa, the other in both West, East and Southeast Africa); one endemic to Madagascar; five endemic to New Caledonia; one endemic in Hawaii; one in Vietnam; four endemic to Australia (Queensland and New South Wales); one endemic in the Philippines; one in Australia and Papua New Guinea; nine species more widespread across Asia.
Habitat.
Tropical and subtropical riverine forest, lowland rain forest, swamp forest, seasonally dry forest, thicket, vine forest and wooded grassland, especially along forest and river margins.
Etymology.
From meso- (Greek: middle) or meizon (Greek: greater) and neuron (Greek: nerve), the upper suture of the fruit is bordered by a usually broad longitudinal wing so that the suture appears as a prominent sub-central nerve or vein.
Notes.
The genus has recently been revised by Clark (2016), who provides full synonymy, a key to species, and a list of fossil taxa associated with this genus.
References.
Brenan (1967: 38–40); Hattink (1974); Vidal and Hul Thol (1976); Verdcourt (1979: 18–20); Lock (1989: 25); Herendeen and Zarucchi (1990); Pedley (1997); George (1998: 59–67); Wagner et al. (1999); Du Puy and Rabevohitra (2002: 48–49); Brummitt et al. (2007); Clark and Gagnon (2015); Clark (2016).
15.1. Mezoneuron andamanicum
Prain
15.2. Mezoneuron angolense
Welw. ex Oliv.
15.3. Mezoneuron baudouinii
Guillaumin
15.4. Mezoneuron benthamianum
Baill.
15.5. Mezoneuron brachycarpum
Benth.
15.6. Mezoneuron cucullatum
(Roxb.) Wight & Arn.
15.7. Mezoneuron enneaphyllum
(Roxb.) Wight & Arn. ex Voigt
15.8. Mezoneuron erythrocarpum
(Pedley) R. Clark & E. Gagnon
15.9. Mezoneuron furfuraceum
Prain
15.10. Mezoneuron hildebrandtii
Vatke
15.11. Mezoneuron hymenocarpum
Wight & Arn. ex Prain
15.12. Mezoneuron kauaiense
(H. Mann) Hillebr.
15.13. Mezoneuron latisiliquum
(Cav.) Merr.
15.14. Mezoneuron mindorense
Merr.
15.15. Mezoneuron montrouzieri
Guillaumin
15.16. Mezoneuron nhatrangense
Gagnep.
15.17. Mezoneuron nitens
(F. Muell. ex Benth.) R. Clark & E. Gagnon
15.18. Mezoneuron ouenensis
(Guillaumin) R. Clark
15.19. Mezoneuron pubescens
Desf.
15.20. Mezoneuron rubiginosum
(Guillaumin) R. Clark
15.21. Mezoneuron sinense
Hemsl.
15.22. Mezoneuron schlechteri
(Harms) R. Clark
15.23. Mezoneuron scortechinii
F. Muell.
15.24. Mezoneuron sumatranum
(Roxb.) Wight & Arn.
Fossil taxa
15.25. Mezoneuron claibornensis
(Herendeen & Dilcher) R. Clark & E. Gagnon
15.26. Mezoneruon flumen-viridensis
(Herendeen & Dilcher) R. Clark & E. Gagnon
15.27. Mezoneuron spokanensis
(Knowlton) R. Clark & E. Gagnon
16. Cordeauxia
Hemsl., Bull. Misc. Inform. Kew 1907: 361. 1907
Figure 26.
Cordeauxia edulis Hemsl. A branch with foliage and flowers B flower C petal D stamen E stigma F fruit G seed H seed with testa removed. A, C–E from Thulin & Warfa 4610 B from Hemming 375 F from Wood 2184 G, H from Cordeaux s.n. (type). Drawn by unknown artist.
Figure 27.
Cordeauxia edulis Hemsl. A inflorescence B open fruit with seed C undersurface of leaflets showing glands D young seedling (Jarmo Holopainen, cultivated plants in Sweden and Finland, unvouchered) E branch with flowers (M. Thulin, Somalia, unvouchered). Stuhlmannia moavi Taub. F, G inflorescence (R. Randrianaivo, Madagascar, Radrianaivo 1486 (MO, TAN)).
Type.
Cordeauxia edulis Hemsl.
Description.
Evergreen shrubs, multi-stemmed, to 4 m tall, unarmed, red gland dots on stems. Leaves alternate, pinnate; leaflets in (1–) 2–4 (– 6) pairs per leaf, ovate-oblong, coriaceous, with conspicuous red glands on the lower surface, elliptic-oblong, up to 3 (– 5) × 1.5 (– 2.5) cm. Inflorescence a terminal, few-flowered raceme. Flowers bisexual, sub-actinomorphic; sepals c. 1 cm long, with red gland dots; petals 5, free, yellow, c. 1.5 cm long, clawed; stamens 10, free, filaments pubescent; ovary with red gland dots. Fruit a compressed-ovoid, ligneous, dehiscent pod, 4–6 × 2 cm, with very hard, thick valves, and a cornute beak, 1–4-seeded. Seeds ovoid, 20–45 mm long.
Geographic distribution.
A monospecific genus from NE Africa (Somalia and Ethiopia). Introduced in Israel, Kenya, Sudan, Tanzania, and Yemen (Orwa et al. 2009).
Habitat.
Seasonally dry tropical (semi-desert) bushland and thicket on sand.
Etymology.
Named by Hemsley for Captain H. E. S. Cordeaux (1870–1943), one time H. M. Commissioner in Somalia.
References.
Roti-Michelozzi (1957); Thulin (1983: 20–21; 1993: 348); Brink (2006).
16.1. Cordeauxia edulis
Hemsl.
17. Stuhlmannia
Taub., Engler, Pflanzenw. Ost.-Afr. C: 201. 1895
Figure 28.
Stuhlmannia moavi Taub. A inflorescence and pinnate leaf B flower bract C flower D sepal E median petal F upper lateral petal G lower lateral petal H flower with sepals and petals removed from one side to show arrangement of stamens I stamen J lower portion of stamen filament, seen from inside the flower K lower portion of stamen filament seen from outside the flower L hypathium after fall of sepals, petals and stamens M gynoecium, N stigma and apical portion of style O detail of outer surface of ovary showing sessile glands P fruit Q seed R transverse section of seed. A from Tanner 3167 B, P–R from Tanner 3724 C–O from Tanner 2467. Drawn by E. M. Stones, originally published in Hooker`s Icones Plantarum, Tab. 3626 (1967).
Type.
Stuhlmannia moavi Taub.
Description.
Unarmed trees, to 25 m tall; bark brown, fissured and fibrous; young shoots eglandular or with small red glands. Stipules not seen. Leaves alternate, pinnate or bipinnate and then ending in a pair of pinnae, (1.5–) 5–11 (– 20 cm) long, pinnae in (1–) 2–10 pairs per leaf, with reddish glands; leaflets in 3–12 pairs per pinna, opposite to sub-opposite, elliptic, 7–75 (– 120) × 3–30 (– 60) mm, obtuse at the base and apex, glabrous, eglandular or with red glands on the lower surface. Inflorescence a 2–11 cm long, terminal or axillary raceme; pedicels 3–13 mm long. Flowers bisexual, sub-actinomorphic; calyx comprising a hypanthium and 5 sepals, these 5–6.5 mm long, valvate in bud, caducous; petals 5, free, yellow, the median petal with red markings, obovate, 9–12 × 3–6 mm, apex rounded, median petal slightly smaller than the others; stamens 10, free, 5.5–8 mm long, filaments pubescent; ovary stipitate, with red sessile glands, glabrous to pubescent. Fruit a flattened, oblong, woody, elliptic pod with an acuminate apex, 4.5–6 × 1.5–2 cm, dehiscing along both sutures, valves twisting, glabrous to thinly puberulous. Seeds flattened, sub-circular to ovate, c. 10–13 × 8–9 mm, brown.
Geographic distribution.
A monospecific genus in E Africa (Kenya and Tanzania) and N Madagascar.
Habitat.
Seasonally dry tropical forest, woodland on limestone and in riverine forest.
Etymology.
Named by Taubert for the German naturalist Franz Ludwig Stuhlmann (1863–1928).
References.
Brenan (1967: 45–47); Capuron (1967, under Caesalpinia insolita); Lewis (1996); Du Puy and Rabevohitra (2002: 48, 50, under Caesalpinia insolita); Lemmens (2010).
17.1. Stuhlmannia moavi
Taub.
Caesalpinia insolita (Harms) Brenan & Gillett
Caesalpinia dalei Brenan & Gillett
18. Cenostigma
Tul., Ann. Sci. Nat., Bot., sér. 2. 20: 140. 1843, descr. emended E. Gagnon & G. P. Lewis
Figure 29.
Cenostigma eriostachys (Benth.) E. Gagnon & G. P. Lewis. A part of bipinnate leaf B median leaflet undersurface, C section of branchlet bark D inflorescence E flower F calyx opened out G detail of stellate hairs on calyx H median petal I median petal claw J upper lateral petal K detail of lateral petal claw L stamen M gynoecium N stigma O fruit P seed Q seedling R bruchid emerged from seed. A, B, P–R from Lewis & Hughes 1799 C from Lewis et al. 1719 D–N from Lewis et al. 1718 O from Lewis & Hughes 1775. Drawn by Sue Wickison.
Poincianella Britton & Rose. 1930, pro parte, excluding the type.
Diagnosis.
Cenostigma is morphologically most similar to the genus Erythrostemon. It differs from the latter by its leaves with alternate to subopposite (occasionally opposite) leaflets (vs. leaflets consistently opposite in Erythrostemon). A number of other characters can help to distinguish between the two genera, but these are not constant across species of Cenostigma. For example, a stellate indumentum on the leaflets, inflorescences, and/or sepals is found on some, but not all Cenostigma species, but is always lacking in Erythrostemon. Black subepidermal glands (visible with a × 20 lens) can be found scattered in the undersurface of leaflets and/or on sepals in Cenostigma (vs. these always lacking in Erythrostemon). Cenostigma pods are generally woody with thickened margins or an adaxial, proximal woody ridge or crest (vs. less robust pods lacking any woody ridge or crest in Erythrostemon).
Type.
Cenostigma macrophyllum Tul.
Emended description.
Unarmed multi-stemmed shrubs, small compact trees, (0.3–) 0.5–6 m, or large trees to 35 m tall, the larger trees with fluted trunks at maturity (Cenostigma bracteosum, Cenostigma pluviosum, Cenostigma eriostachys, Cenostigma tocantinum and Cenostigma macrophyllum); bark smooth, or occasionally rough and flaking (some infraspecific taxa of Cenostigma pluviosum), brown, grey, or mottled silver or grey; young shoots terete, glabrous to pubescent, glandular to eglandular. Stipules red, with ciliate margins, broadly ovate with a rounded apex, and caducous in Cenostigma pyramidale, not seen in other species. Leaves alternate, pinnate or bipinnate and then ending in a pair of pinnae plus a single terminal pinna, glabrous to densely pubescent, sometimes with stellate hairs or various types of sessile or stalked glands; petioles (0.1–) 0.6–4.8 (–6) cm, rachis 0.5–17 (– 26.5) cm; species with pinnate leaves (Cenostigma tocantinum, Cenostigma marginatum, Cenostigma pinnatum, and Cenostigma macrophyllum) either with three leaflets or 2–9 pairs of opposite leaflets; species with bipinnate leaves with 1–11 pairs of opposite to alternate pinnae, plus a terminal pinna, each pinna with 3–29 alternate to subopposite (occasionally opposite) individual leaflets; leaflets vary greatly in size, 0.5–15 × 0.1–7 cm, glossy on the upper surface, usually more or less coriaceous (chartaceous in Cenostigma tocantinum), ovate-elliptic, lanceolate with an acute to acuminate apex (some specimens of Cenostigma tocantinum), obovate, oblong-elliptic or suborbicular, apex rounded or emarginate, mucronate, base cuneate, cordate or truncate, the blade often inequilateral at the base, eglandular, or with black subepidermal glands (visible with a × 20 lens) scattered on the undersurface, and/or with conspicuous, sessile or punctate glands on the undersurface or along the margins, in addition to stipitate glands; veins usually prominent, main vein often excentric, secondary venation brochidodromous. Inflorescences either axillary or terminal racemes, these sometimes pyramidal in shape, sometimes aggregated into large showy panicles, inflorescence rachis and pedicels densely tomentose to glabrescent, sometimes covered in stellate hairs, these occasionally intermixed with stipitate glands; pedicels 5–22 mm long, articulated; bracts 2.5–6 mm long, caducous. Flowers bisexual, zygomorphic; calyx a short hypanthium with 5 sepals, 4.5–9 (– 11) mm long, the lower cucullate sepal generally slightly longer than the other four, apices entire or with a fimbriate-glandular margin, puberulous or tomentose, sometimes with a dense stellate indumentum (Cenostigma eriostachys, Cenostigma tocantinum and Cenostigma macrophyllum), the sepal lobes eglandular or with scattered dark, subepidermal glands, caducous, but the hypanthium persisting as a calyx ring in fruit; all 5 petals free and clawed, bright yellow, the median petal (7.5–) 9–15 (– 19) × 5–13 (– 17) mm, with red or orange markings on the inner surface of the blade, suborbicular to elliptic or spathulate, with a thickened, pubescent claw, the outer surface of which has short-stalked glands, these sometimes also on the dorsal surface of the blade, lateral petals 0.9–2.7 × 0.4–2 cm, broadly elliptic, sub-rectangular, obovate or suborbicular, petal claws pubescent and with stalked-glands, these sometimes also on the dorsal surface of the blade; stamens 10, free, filaments (7–) 8–14 (–21) mm long, pubescent on lower ⅔ to ½, with short-stipitate glands along entire length (except in Cenostigma macrophyllum); ovary pubescent with glands intermixed, these sometimes obscured by the indumentum, stigma a terminal fringed-chamber. Fruits laterally compressed, coriaceous to woody pods, (3.8–) 5–14 (– 16) × 1.2–3.3 (– 3.7) cm, with conspicuously thickened margins (an adaxial, proximal woody ridge or crest in Cenostigma macrophyllum), elastically dehiscent (sometimes tardily), the valves twisting at maturity, either glabrous or pubescent, smooth or prominently reticulately veined (on herbarium specimens), usually eglandular or with a few scattered stipitate or sessile glands (densely glandular in Cenostigma microphyllum). Seeds 2–6 (– 8) per pod, ovate-elliptic to ovate-orbicular, 9–19 × (6–) 8–12 × 1–3 mm, ochre, brown, or mottled, shiny.
Geographic distribution.
We recognise 20 taxa in 14 species, all of them neotropical; only two of these taxa do not require new names, while the rest are species of Caesalpinia here transferred to Cenostigma. The majority of species are found in central and NE Brazil, including parts of the Amazon. Two species extend around the circum-Amazonian arc of dry forests and adjacent cerrado, including in Paraguay, Argentina and Bolivia, and one taxon is also found in the seasonally dry inter-Andean valleys of Peru. Species are also found throughout Central America, from Panama northwards and in Mexico, extending to the Caribbean, with endemics in Cuba and Hispaniola.
Habitat.
Seasonally dry tropical forest, bushland and thicket (restinga, caatinga, semi-arid thorn scrub), wooded grassland (cerrado and cerradão) and terra firme forest.
Etymology.
From ceno- (Greek: empty) and stigma, presumably alluding to the chambered stigma (a character of many species of the Caesalpinia Group, and not restricted to Cenostigma).
References.
Lewis (1987: 34–35, 1998); Freire (1994); Ulibarri (1996); De Queiroz (2009: 129–130, see also under Poincianella, 121–128); Warwick and Lewis (2009); Lewis et al. (2010).
18.1. Cenostigma bracteosum
(Tul.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158073-1
Basionym.
Caesalpinia bracteosa Tul., Arch. Mus. Hist. Nat., Paris 4: 141. 1844. Poincianella bracteosa (Tul.) L. P. Queiroz, Leguminosas da Caatinga: 122. 2009.
Type.
BRAZIL, Piauí, Gardner 2144 (holotype P!; isotypes BM!, K!).
18.2. Cenostigma eriostachys
(Benth.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158074-1
Basionym.
Caesalpinia eriostachys Benth., Bot. Voy. Sulphur: 88. 1844.
Poincianella eriostachys (Benth.) Britton & Rose, N. Amer. Fl. 23(5): 332. 1930.
Type.
COSTA RICA, Cocos Island, Barclay s.n. (lectotype K!, designated by Lewis, 1998).
Schizolobium covilleanum Pittier, Contr. U.S. Natl. Herb. 18: 231. 1917, pro parte (flowering material only).
Type. PANAMA, Prov. Coclé, between Aguadulce and Chico River, Pittier 5105.
18.3. Cenostigma gaumeri
(Greenm.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158075-1
Basionym.
Caesalpinia gaumeri Greenm., Publ. Field Mus. Nat. Hist., Bot. Ser. 2: 330. 1912.
Poincianella gaumeri (Greenm.) Britton & Rose, N. Amer. Fl. 23(5): 333. 1930.
Type.
MEXICO, Yucatán, Progresso, 5 Mar 1899, Millspaugh 1675 (holotype F).
Poincianella guanensis Britton, N. Amer. Fl. 23(5): 333. 1930.
Caesalpinia guanensis (Britton) León, Contr. Ocas. Mus. Hist. Nat. Colegio “De La Salle” 9: 12. 1950.
Type. CUBA, Remates de Guane, Pinar del Rio, Apr 1926, Fors 3965 (holotype NY!).
18.4. Cenostigma laxiflorum
(Tul.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158076-1
Basionym.
Caesalpinia laxiflora Tul., Arch. Mus. Hist. Nat., Paris 4: 143. 1844.
Poincianella laxiflora (Tul.) L. P. Queiroz, Leguminosas da Caatinga: 123. 2009.
Type.
BRAZIL, Bahia, near Villa da Barra, Blanchet 3146 (isotypes BM!, BR!, F!, GH!, K!, MG!, P! [P02142655, P02142656, P02142657]).
Caesalpinia laxiflora Tul. var. pubescens Benth., Mart., Fl. Bras. 15(2): 70. 1870.
Type. BRAZIL, Bahia, near Maracás, Martius s.n. (holotype M!; isotypes M!).
18.5. Cenostigma macrophyllum
Tul., Ann. Sc. Nat. 2 Sér. 20: 141, pl. 3. 1843
Type.
BRAZIL, Mato Grosso, 1883, C. Gaudichaud, Herb. Imp. Bras. No. 213 (P03014131!).
Cenostigma gardnerianum Tul., Ann. Sc. Nat. 2 Sér. 20: 141, pl. 3. 1843.
Type. BRAZIL, Piauí, Gardner 2523 (isotype K!).
Cenostigma angustifolium Tul. , Ann. Sc. Nat. 2 Sér. 20: 141, pl. 3. 1843.
Types. BRAZIL, Bahia, Gentio do Ouro: Serra do Açuruá, Blanchet 2798 (syntypes K!, MO!, P 03104099!); Marais de St-Antoine, Blanchet 3144 (syntype P03104095!)
18.6. Cenostigma marginatum
(Tul.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158077-1
Basionym.
Caesalpinia marginata Tul., Arch. Mus. Hist. Nat., Paris 4: 147. 1844.
Type.
BOLIVIA, Chiquitos, near San-Juan (Bois de la Tapira), without date, d’Orbingy 831 (holotype P0242658!).
Cenostigma sclerophyllum Malme, Bih. Kongl. Svenska Vetensk.-Akad. Handl. 25 (11): 24. 1900.
Type. PARAGUAY, Colonia Risso, near Rio Apa, 20 Oct 1893, Malme 1084 (lectotype S!, designated by Lewis (1998); isolectotype S!).
18.7. Cenostigma microphyllum
(Mart. ex G. Don) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158078-1
Basionym.
Caesalpinia microphylla Mart. ex G. Don, Gen. Syst. 2: 431. 1832.
Poincianella microphylla (Mart. ex. G. Don) L. P. Queiroz, Leguminosas da Caatinga: 124. 2009.
Type.
BRAZIL, Bahia, in sylvis catingas, Martius Obsv. 2274 (lectotype M!, designated by Lewis (1998); isolectotypes K!, M!).
18.8. Cenostigma myabense
(Britton) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158079-1
Basionym.
Caesalpinia myabensis Britton, Mem. Torrey Bot. Club 16: 66. 1920.
Poincianella myabensis (Britton) Britton & Rose, N. Amer. Fl. 23(5): 334. 1930.
Type.
CUBA, Oriente, between Holguin and Myabe, Apr 1909, Shafer 1403 (holotype NY!; isotype A!).
Libidibia pauciflora Griseb. var. ? puberula Griseb., Cat. Pl. Cub.: 79. 1866.
Type. CUBA, Wright 2362 (incorrectly given as “1362”).
Caesalpinia hornei Britton, Mem. Torrey Bot. Club 16: 67. 1920.
Poincianella hornei (Britton) Britton & Rose, N. Amer. Fl. 23(5): 333 (1930).
Caesalpinia myabensis var. hornei (Britton) Barreto, Acta Bot. Cub. 89: 5 1992.
Type. CUBA, Ciego de Avila, Camaguey, 3 Sep 1905, Horne 95 (holotype NY!).
Caesalpinia subglauca Britton in Mem. Torrey Bot. Club 16: 66 (1920).
Poincianella subglauca (Britton) Britton & Rose, N. Amer. Fl. 23(5): 333 (1930).
Caesalpinia myabensis var. subglauca (Britton) Barreto, Acta Bot. Cub. 89: 6 (1992).
Type. CUBA, Oriente, near Santiago, Britton et al. 12596 (holotype NY!).
Poincianella clementis Britton, N. Amer. Fl. 23(5): 333. 1930.
Caesalpinia clementis (Britton) León, Contr. Ocas. Mus. Hist. Nat. Colegio “De La Salle” 9: 12. 1950.
Caesalpinia myabensis var. clementis (Britton) Barreto, Acta Bot. Cub. 89: 6. 1992.
Type. CUBA, Oriente, Renté, Santiago, Jul 1919, Clement 135 (holotype NY!; isotype HAC!).
Caesalpinia hermeliae León, Contr. Ocas. Mus. Hist. Nat. Colegio “De La Salle” 9: 12. 1950.
Caesalpinia myabensis var. hermeliae (León) Barreto, Acta Bot. Cub. 89: 5. 1992.
Type. CUBA, Oriente, SW of Holguin, orillas del monte de Caguairanal, 18 Mar 1932, León & Garcia 15501 (holotype LS (transferred to HAC)!; isotypes HAC!, NY!).
18.9. Cenostigma nordestinum
E. Gagnon & G. P. Lewis nom. nov.
urn:lsid:ipni.org:names:77158101-1
Caesalpinia gardneriana Benth., in Mart., Fl. Bras. 15 (2): 68. 1870.
Poincianella gardneriana (Benth.) L. P. Queiroz, Leguminosas da Caatinga: 123. 2009, non Cenostigma gardnerianum Tul. (1843), a synonym of Cenostigma macrophyllum Tul. (1843).
Type.
BRAZIL, Piauí, between Praya Grande and Boa Esperança, Feb 1839, Gardner 2148 (holotype K!; isotype BM!).
18.10. Cenostigma pellucidum
(Vogel) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158080-1
Basionym.
Caesalpinia pellucida Vogel, Linnaea 10: 601. 1836.
Poincianella pellucida (Vogel) Britton & Rose, N. Amer. Flora 23(5): 334. 1930.
Type.
DOMINICAN REPUBLIC, Ehrenberg s.n. (isotype NY!).
18.11. Cenostigma pinnatum
(Griseb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158094-1
Basionym.
Libidibia pinnata Griseb. Cat. Pl. Cub.: 79. 1866 (As “Lebidibia pinnata”).
Caesalpinia pinnata (Griseb.) C. Wright, in Suav., Anales Acad. Ci. Med. Habana 5: 404. 1869.
Poincianella pinnata (Griseb.) Britton & Rose, N. Amer. Fl. 23(5): 335. 1930.
Type.
CUBA, Wright 2360 (holotype GOET!; isotypes GH!, K!, NY!).
Caesalpinia oblongifolia Urban, Symb. Ant. 2: 281 (1900).
Poincianella oblongifolia (Urban) Britton & Rose, N. Amer. Fl. 23(5): 335 (1930).
Type. As for Caesalpinia pinnata.
Poincianella savannarum Britton & Wilson, N. Amer. Fl. 23(5): 335 1930.
Caesalpinia savannarum (Britton & Wilson) León, Contr. Ocas. Mus. Hist. Nat. Colegio “De La Salle” 10 (Fl. Cub. 2): 283. 1951.
Caesalpinia oblongifolia var. savannarum (Britton & Wilson) A. Borhidi & O. Muniz, Bot. Közlem. 62 (1): 25. 1975.
Type. CUBA, Sancti Spiritus, 20 Jul 1915, León & Roca 7835 (holotype NY!).
18.12. Cenostigma pluviosum
(DC.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158081-1
Basionym.
Caesalpinia pluviosa DC., Prodr. 2: 483. 1825.
Poincianella pluviosa (DC.) L. P. Queiroz, Leguminosas da Caatinga: 126. 2009.
Type.
BRAZIL, 1819, Leandro di Sacramento 5 (P02142667!).
18.12.1. Cenostigma pluviosum var. pluviosum
Caesalpinia floribunda Tul., Arch. Mus. Hist. Nat., Paris 4: 140. 1844. Type. BOLIVIA, Prov. de Chiquitos, camino de San Rafel a Santa Ana, [without date], Orbigny 1039 (holotype P02142650!; isotypes G, P02142651!).
Caesalpinia taubertiana S. Moore, Trans. Linn. Soc. London, Bot. 4: 345. 1895. Type. BRAZIL, near Corumbá, Jan 1891–1892, Moore 1037 (holotype BM!; isotype BM!).
18.12.2. Cenostigma pluviosum var. cabralianum
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158096-1
Basionym.
Caesalpinia pluviosa var. cabraliana G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostmeon group: 148. 1998.
Poincianella pluviosa var. cabraliana (G. P. Lewis) L. P. Queiroz, Neodiversity 5(1): 11. 2010.
Type.
BRAZIL, Bahia, Mun. Santa Cruz de Cabrália, c. 12 km NW of Porto Seguro, 27 Nov 1979, Mori et al. 13029 (holotype CEPEC!; isotypes K!, NY).
18.12.3. Cenostigma pluviosum var. intermedium
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158097-1
Basionym.
Caesalpinia pluviosa var. intermedia G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 141. 1998.
Poincianella pluviosa var. intermedia (G. P. Lewis) L. P. Queiroz, Leguminosas da Caatinga: 127. 2009.
Type.
BRAZIL, Bahia, Abaíra, road to Jussiape, 15 Feb 1987, Harley et al. 24326 (holotype SPF; isotype K!).
18.12.4. Cenostigma pluviosum var. maraniona
(G. P. Lewis & C. E. Hughes) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158087-1
Basionym.
Caesalpinia pluviosa var. maraniona G. P. Lewis & C. E. Hughes, Kew Bull. 65(2): 213-217. 2010.
Type.
PERU, Cajamarca, Celendín, Marañón Valley, km 50 rd from Celendín to Leimebamba, 23 Apr 2002, fl. & fr., Hughes, Daza & Forrest 2215 (holotype FHO!; isotypes K!, MOL!).
18.12.5. Cenostigma pluviosum var. paraense
(Ducke) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158088-1
Basionym.
Caesalpinia paraensis Ducke, Archiv. Jard. Bot. Rio de Janeiro 4: 59. 1925.
Caesalpinia pluviosa var. paraensis (Ducke) G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 150. 1998.
Poincianella pluviosa var. paraensis (Ducke) L. P. Queiroz, Neodiversity 5(1): 11. 2010.
Type.
BRAZIL, Pará, near Monte Alegre, Ducke s.n. (BM!, K!, MG, RB).
18.12.6. Cenostigma pluviosum var. peltophoroides
(Benth.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158098-1
Basionym.
Caesalpinia peltophoroides Benth., Mart., Fl. Bras. 15(2): 72. 1870.
Caesalpinia pluviosa var. peltophoroides (Benth.) G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 146. 1998.
Poincianella pluviosa var. peltophoroides (Benth.) L. P. Queiroz, in Neodiversity 5(1): 11. 2010.
Type.
BRAZIL, Rio de Janeiro, Glaziou 1032 (syntypes BM!, BR!, F!, P02142662!); Glaziou 6 (syntype BR!).
18.12.7. Cenostigma pluviosum var. sanfranciscanum
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158089-1
Basionym.
Caesalpinia pluviosa var. sanfranciscana G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 151. 1998.
Poincianella pluviosa var. sanfranciscana (G. P. Lewis) L. P. Queiroz, Leguminosas da Caatinga: 127. 2009.
Type.
BRAZIL, Bahia, 35 km S of Livramento do Brumado, 1 Apr 1991, Lewis & Andrade 1932 (holotype CEPEC!; isotype K!).
18.13. Cenostigma pyramidale
(Tul.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158099-1
Basionym.
Caesalpinia pyramidalis Tul., Arch. Mus. Hist. Nat., Paris 4: 139. 1844.
Poincianella pyramidalis (Tul.) L. P. Queiroz, Leguminosas da Caatinga: 128. 2009.
Type.
BRAZIL, Serra Jacobina, 1841, J. S. Blanchet 3425 (holotype P003790235!; isotypes BM!, BR!, F!, MG!).
18.13.1. Cenostigma pyramidale var. pyramidale
Caesalpinia pyramidalis var. alagoensis Tul., Arch. Mus. Hist. Nat., Paris 4: 140. 1844. Type. BRAZIL, Alagoas, banks of the Rio St. Francisco at Propiá, Feb 1838, Gardner 1278 (holotpye BM!; isotypes F!, GH!, K!, US!).
18.13.2. Cenostigma pyramidale var. diversifolium
(Benth.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158100-1
Basionym.
Caesalpinia pyramidalis var. diversifolia Benth., Mart., Fl. Bras. 15(2): 69. 1870.
Type.
BRAZIL, Maranhão, Jun 1841, Gardner 6006 (lectotype K!, designated by Lewis, 1998; isolectotype BM!).
18.14. Cenostigma tocantinum
Ducke, Arch. Jard. Bot. Rio de Janeiro 29, pl. 10 (1915)
Type.
BRAZIL, Pará, Alcobaça, Rio Tocantins, Ducke s.n., H.A.M.P. no. 15643 (holotype MG).
19. Libidibia
(DC.) Schltdl., in Linnaea 5: 192. 1830, descr. emended E. Gagnon & G. P. Lewis
Figure 31.
Libidibia coriaria. A inflorescences and foliage B leaflet undersurface showing glands C flower D calyx opened out E median petal F detail of glands on back of median petal G upper lateral petal H lower lateral petal I stamen J gynoecium K stigma L fruit M seed. A–C from Hughes 1495 D–M from Macqueen 8. Drawn by Eleanor Catherine.
Caesalpinia section Libidibia DC. (1825).
Stahlia Bello (1881), syn. nov.
Diagnosis.
Libidibia is related to Hoffmannseggia, Stenodrepanum, Balsamocarpon and Zuccagnia but differs in being a genus of medium to tall trees, 6–20 m in height (versus woody based perennial herbs to shrubs, 10 cm to 5 m tall), most species have a distinctive, smooth patchwork bark in shades of white, grey and green (“snake skin bark”) a characteristic not found in the other four genera. Libidibia (except Libidibia monosperma) has bipinnate leaves (Balsamocarpon and Zuccagnia are pinnate) and coriaceous or woody, glabrous, eglandular, indehiscent fruits which dry black (red in Libidibia monosperma) versus thick, turgid, glandular, resinous, indehiscent fruits (Balsamocarpon), or laterally compressed, gall-like, ?indehiscent fruits covered in trichomes (Zuccagnia). Stenodrepanum and Hoffmannseggia are bipinnate but the fruits of most species of Hoffmannseggia are dehiscent with twisting pod valves and persistent sepals (in Libidibia sepals are caducous in fruit); the fruits of Stenodrepanum are narrow, cylindrical and torulose.
Type.
Libidibia coriaria (Jacq.) Schltdl. ≡ Poinciana coriaria Jacq.
Emended description.
Small to medium-sized or large unarmed trees, 6–20+ meters in height; bark hard, smooth, with a patchwork of shades of grey, white and pale green, often referred to as snake skin bark, (except in Libidibia coriaria and Libidibia monosperma, where it is rough and fissured). Stipules not seen. Leaves alternate, bipinnate and ending in a pair of pinnae plus a single terminal pinna, rarely pinnate (Libidibia monosperma); pinnae (in bipinnate species) in 2–10 opposite pairs, plus a single terminal pinna; leaflets opposite, in 3–31 pairs per pinna, ovate, elliptic to oblong, apex rounded, mucronate or acute, base often oblique, subcordate, rounded or obtuse, eglandular or with subsessile gland dots on the undersurface of the blades, on either side of the midvein, glabrous to occasionally puberulous; in bipinnate leaves the leaflets (3–) 4–31 × 2.5–14 mm; in pinnate leaves, leaflets are much larger, c. 40–90 × 15–35 mm. Inflorescences terminal or axillary racemes or panicles, sometimes corymbose, with pedicellate flowers. Flowers bisexual, zygomorphic; calyx comprising a hypanthium and 5 sepals, the lower sepal slightly longer and cucullate in bud, caducous, but hypanthium persisting as a calyx ring around the pedicel as pods mature; petals 5, free, yellow or white, the median petal sometimes flecked or blotched orange or red; stamens 10, free, pubescent on the lower half of the filaments, eglandular (except for Libidibia ferrea, which has stipitate glands); ovary eglandular, glabrous or pubescent. Fruit coriaceous to woody, oblong-elliptic to suborbicular, straight (contorted in Libidibia coriaria), indehiscent, eglandular, glabrous, black (red and somewhat fleshy in Libidibia monosperma), 15–80 × 10–30 mm. Seeds oblong to elliptic, somewhat laterally compressed, smooth.
Geographic distribution.
A genus of ten taxa in seven species in the Neotropics. One species in Mexico, one widespread in Brazil, one in Colombia, Venezuela and the Antilles, one in Colombia, Ecuador and Peru, one in Paraguay, Bolivia, Argentina and SW Brazil, one (Libidibia monosperma, previously in the monospecific genus Stahlia) endemic to Puerto Rico and the Dominican Republic, and Libidibia coriaria widespread throughout Mexico, Central America, the Caribbean and NW South America. Other species perhaps waiting to be discovered and described, both in the field and in herbaria; the genus needs revising.
Habitat.
Seasonally dry tropical forest and thorn scrub (including Brazilian caatinga) and savanna woodland. Libidibia monosperma occurs along the margins of mangrove swamps and in marshy deltas, in drier edaphic conditions.
Etymology.
The name Libidibia is derived from the vernacular name ‘libi-dibi’ or ‘divi-divi’ used for some species.
References.
Britton (1927); Britton and Rose (1930: 221, 318–319); Burkart (1936, Caesalpinia melanocarpa: 78–82); Macbride (1943, Caesalpinia paipai: 193–194); Little and Wadsworth (1964); U.S. Fish and Wildlife Service (1995); Ulibarri (1996); De Queiroz (2009: 130–133); Borges et al. (2012); Barreto Valdés (2013).
19.1. Libidibia coriaria
(Jacq.) Schltdl., Linnaea 5: 193. 1830
Basionym.
Poinciana coriaria Jacq., Select. Stirp. Amer. Hist. 123, pl. 175, f. 36 (flower, fruit and seed). 1763.
Caesalpinia coriaria (Jacq.) Willd., Sp. Pl. 2: 532. 1799.
Type.
Curação, “Habitat in Curação & Carthagenae frequens; in limosis praesertim inudatisque maritimis; ad salinas”, [no date], Jacquin s.n. (holotype probably in W; photo Field Museum 1794 of probable isotype “Hb. Willdenow” (fl.); by micro. Reprod. of the same Hb. Willdenow 8023: SI).
Caesalpinia thomaea Spreng., Syst. Veg. 2: 343. 1825.
Type. “Ins. S. Thomae, Bertero”.
19.2. Libidibia ferrea
(Mart. ex Tul.) L. P. Queiroz, Leguminosas da Caatinga: 130. 2009
Basionym.
Caesalpinia ferrea Mart. ex Tul., Arch. Mus. Hist. Nat. Paris 4: 137. 1844.
Type.
BRAZIL, “Province of Alagoas, Tropical Brazil, Gardner 1277 (holotype P02736428!; isotypes BM!, K!).
19.2.1. Libidibia ferrea var. ferrea
Caesalpinia ferrea var. petiolulata Tul., Arch. Mus. Hist. Nat. Paris 4: 138. 1844. Type. BRAZIL, Piaui (“Piauhy”), 1839, Gardner 2147 (syntypes K!, P02736427!); Bahia, Blanchet 3264 (syntype P02142648!).
Caesalpinia ferrea var. megaphylla Tul., in Arch. Mus. Hist. Nat. Paris 4: 139. 1844. Type. BRAZIL, Piaui (“Piauhy”), dry woods near Villa do Crato, Jan 1839, Gardner 1934 (holotype P02736441!; isotype K!).
19.2.2. Libidibia ferrea var. glabrescens
(Benth.) L. P. Queiroz, Leguminosas da Caatinga: 131. 2009
Basionym.
Caesalpinia ferrea var. glabrescens Benth., Mart., Fl. Brasil 15(2): 70. 1870.
Type.
BRAZIL, Sergipe-Alagoas, “banks of the Rio St. Francisco”, Feb 1838, Gardner 1276 (holotype K).
19.2.3. Libidibia ferrea var. leiostachya
(Benth.) L. P. Queiroz, Neodiversity 5(1): 11. 2010
Basionym.
Caesalpinia ferrea Mart. ex Tul. var. leiostachya Benth., Mart., Fl. Bras. 15(2): 70. 1870. Caesalpinia leiostachya (Benth.) Ducke, Mem. Inst. Oswaldo Cruz 51: 458. 1953.
Type.
BRAZIL “prope Rio de Janeiro juxta viam ad Jacarépaguá ducentem”, 13 Mar 1868, Glaziou 2555 (P02736434!).
19.2.4. Libidibia ferrea var. parvifolia
(Benth.) L. P. Queiroz, Leguminosas da Caatinga: 133. 2009
Basionym.
Caesalpinia ferrea var. parvifolia Benth., Mart., Fl. Brasil 15(2): 70. 1870.
Type.
BRAZIL, “in sylvis catingas de interioribus prov. Bahia”, Martius s.n.
19.3. Libidibia glabrata
(Kunth) C. Castellanos & G. P. Lewis, Revista Acad. Colomb. Ci. Exact. 36(139): 183. 2012
Basionym.
Caesalpinia glabrata Kunth, Nov. Gen. Sp. 6: 326. 1823.
Type.
PERU, “Crescit inter urbem Caxamarcae et pagum Madgalenae, Peruvia”, M. A. Bonpland 3712 (holotype P00679209!; isotype P02142659!, photo K!, photo and fragment F 937253).
Libidibia corymbosa (Benth.) Britton & Killip, Ann. N. Y.Acad. Sci. 35(3): 189 (1936).
Caesalpinia corymbosa Benth., Pl. Hartw.: 117. 1832.
Type. ECUADOR, Guayaquil, [without date], Hartweg 651 (holotype K!; isotypes K!, P! (two sheets: P02737048!, P02737051!), photo at F, no. 1774).
Caesalpinia paipai Ruíz & Pav., Fl. Peruv. 4, Ic. 375. 1830.
Type. PERU, “Limae & Chancay” (lectotype based on Ic. 375, fragment of the material probably used for the illustration “Hb. Ruíz & Pavon, Peru, Chacau” MA: F842538).
Caesalpinia paipai var. pubens J.F. Macbr., Field Mus. Nat. Hist. Bot. Ser. (Fl. Peru) 13, 3, 1: 193. 1943.
Type. PERU, Dpto. Piura: Salitral y Serrán, Mar 1912, Weberbauer 5994 (holotype F).
19.4. Libidibia monosperma
(Tul.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158082-1
Basionym.
Caesalpinia monosperma Tul., Arch. Mus. Hist. Nat. Paris 4: 148. 1844. Stahlia monosperma (Tul.) Urb., Symb. Antill. 2(2): 285. 1900.
Type.
PUERTO RICO, without exact locality or date, A. Plée 713 (lectotype P03090076, designated by Santiago-Valentín, Sánchez-Pinto & Francisco-Ortega, 2015).
Stahlia monosperma var. domingensis Standl, Trop. Woods 40: 16. 1934.
Type. DOMINICAN REPUBLIC, delta of Soco River, J.C. Scarff s.n. (“type” Hb. Field Mus. No. 7147180; Yale No. 27244).
Stahlia maritima Bello, Anales Soc. Esp. Hist. Nat. 10: 255. 1881.
Type. PUERTO RICO, Guánica, in sylvis inter Barina et la Boca, 2 Mar 1886, P. E. E. Sintensis 3876 (neotype NY, designated by Santiago-Valentín, Sánchez-Pinto & Francisco- Ortega, 2015; isoneotypes BM, G, GH, NY, P, W).
19.5. Libidibia paraguariensis
(D. Parodi) G. P. Lewis, in Mabberley, Pl. Book (ed. 3): 1021. 2008
Basionym.
Acacia paraguariensis D. Parodi, Revista Farm. 3: 7. 1862.
Caesalpinia paraguariensis (D. Parodi) Burkart, Darwiniana 10(1): 26. 1952.
Type.
PARAGUAY, “Arbor sylvestris in ripa fluminis Paraguay” (holotype probably at BAF, not found).
Caesalpinia melanocarpa Griseb., Abh. Königl. Ges. Wis. Göttingen (Pl. Lorentz) 19: 80. 1874.
Type. ARGENTINA, Tucumán, infrecuens in sylvis subtropicis et in campis, pr. La Cruz, 20–24 Apr 1872, Lorentz 196. (holotype GOET; isotypes CORD, SI).
Caesalpinia coriaria Micheli, Mem. Soc. Phys. Genève 29(7): 42. 1883, non (Jacq.) Willd. (1799).
Type. PARAGUAY, Assomption in hortis culta, Balansa 1397 and 1397a (syntypes BAF, G, K!).
19.6. Libidibia punctata
(Willd.) Britton, Sci. Surv. Porto Rico & Virgin Islands 5: 378. 1924
Basionym.
Caesalpinia punctata Willd., Enum. Pl. 455. 1809.
Type.
Herb. Willd. 822, plant cult. Source erroneously attributed to Brazil.
Caesalpinia granadillo Pittier, Bol. Cien. Técn. Mus. Com. Venez. 1:56. 1926.
Libidibia granadillo (Pittier) Pittier, Man. Pl. Usual. Venez. (Suppl.): 37. 1939.
Type. VENEZUELA, Zulia: selva montañosa de San Martín, Río Palmar, 15 Oct 1922, Pittier 10515 (holotype VEN, isotypes GH, P02736828!, US!).
Caesalpinia ebano H. Karst., Fl. Columb. 2: 57, pl. 129. 1862.
Libidibia ebano (H. Karst.) Britton & Killip, Ann. New York Acad. Sci. 35(4): 189. 1936.
Type. COLOMBIA, “regiones septentrionales calidus, siccas”.
19.7. Libidibia sclerocarpa
(Standl.) Britton & Rose, N. Amer. Fl. 23 (5): 319. 1930
Basionym.
Caesalpinia sclerocarpa Standl., Contrib. U. S. Nat. Herb. 20(6): 214–215. 1919.
Type.
MEXICO, Oaxaca, between San Geronimo and La Venta, alt. 50 m, 13 Jul 1895, E. W. Nelson 2784 (holotype US 229315).
20. Balsamocarpon
Clos, Fl. Chile. 2(2): 226; Atlas Botanico t. 20. 1846
Figure 33.
Balsamocarpon brevifolium Clos. A flowering stem B flower C median petal D upper lateral petal E lower lateral petal F stamen G gynoecium H fruit I dissected seed J longitudinal section of seed K embryo. Drawn by A. Riocreux, first published in Historia fysica y polica de Chile, Botanica, Atlas, col. 1: t. 20 (1854). Scale bars were estimated for this plate based on descriptions and comparison with herbarium specimens; we were unable to estimate these for F, G, K, J, I.
Type.
Balsamocarpon brevifolium Clos
Description.
Shrub 1–2 m tall, with long terete branches with thin, straight, 3–5 mm long, often caducous spines. Stipules deltoid, hairy, glandular. Leaves in fascicles on short brachyblasts, pinnate, 3–8 mm long; leaflets in 3–4 pairs, elliptic-obovate to orbicular, 1.5–4.5 × 1–2 mm, glabrous, fleshy. Inflorescences composed of short racemes; pedicels and rachis hairy and glandular; bracts deltoid, hairy and glandular. Flowers bisexual, sub-zygomorphic; calyx comprising a hypanthium and 5 sepals, c. 5–6 × 4.2 mm, fimbriate, hairy and with glandular trichomes, sepals persistent in fruit; petals 5, free, yellow, obovate, subequal, short-clawed, 10 × 3–4.5 mm, with glandular trichomes on the dorsal surface; stamens 10, free, filaments pubescent, eglandular; ovary glandular, finely pubescent, stigma a fringed chamber. Fruit a thick, turgid, resinous, glandular, indehiscent pod, 2.5–4 × 1.5 cm, 3–4-seeded.
Geographic distribution.
A monospecific genus endemic to northern Chile, from the Coquibo and La Serena valleys.
Habitat.
Desert scrub, rocky hillsides.
Etymology.
From balsamo- (Gk.: balsam) and carpos (Gk.: fruit), the pods yield a sticky resin traditionally used for tanning.
References.
Burkart (1940: 162); Ulibarri (1996, 2008); Nores et al. (2012).
20.1. Balsamocarpon brevifolium
Clos
21. Zuccagnia
Cav., Icon. 5: 2. 1799
Figure 35.
Zuccagnia punctata Cav.. A flowering branchlet B infructescence C stem section D leaflet E flower (unopened) F median petal G detail of petal glands H upper lateral petal I lower lateral petal J stamen K gynoecium L stigma M fruit. A, D from Tinto 2017 B, M from Wingenroth et al. 354 C, E–L from Cabrera 30149. Drawn by Christi A. Sobel.
Type.
Zuccagnia punctata Cav.
Description.
Shrubs, 1–5 m. Stipules caducous. Leaves alternate, pinnate, (2–) 3–5 (– 6) cm long; leaflets in 5–13 subopposite pairs, elliptic-linear, rarely obovate, 4–14 × 1–3 mm, with glandular dots on both surfaces of the leaflet blades. Inflorescences terminal, erect racemes; bracts deltoid, glabrous, glandular, caducous. Flowers bisexual, zygomorphic; calyx comprising a hypanthium and 5 glabrous sepals, persistent after fruit develops, the lower sepal cucullate and covering the other four in bud; petals 5, free, yellow, obovate to broadly obovate, short-clawed, glandular trichomes on the dorsal surface of the petal blades; stamens 10, free, pubescent; ovary pilose. Fruit an ovoid-acute, oblique, laterally compressed, indehiscent (?), gall-like pod, on a short stipe and covered with long reddish brown bristles, c. 1 × 0.6 cm, 1-seeded.
Geographic distribution.
A monospecific genus restricted to Chile, NW and central-W Argentina.
Habitat.
Dry temperate upland and montane bushland and thickets on sandy plains.
Etymology.
Named by Cavanilles for the Italian physician, traveller and plant collector, Attilio Zuccagni (1754–1807).
References.
Burkart (1952: 184–185); Kiesling et al. (1994: 286); Ulibarri (2005, 2008); Nores et al. (2012).
21.1. Zuccagnia punctata
Cav.
22. Stenodrepanum
Harms, Notizbl. Bot. Gart. Berlin-Dahlem 7: 500. 1921
Figure 36.
Stenodrepanum bergii Harms. A habit B leaflets C glands on the margin of the leaflets D flower E sepals with glands F apical glands of the sepals G lower cucullate sepal H glands on the petals I fruit J position of a seed in the fruit. A–H from Piccini-Leguizamon 1970 I, J from Soriano 787. A drawn by G. A. Larsen, B–J drawn by Emilio A. Ulibarri, originally published in Darwiniana, vol. 21 (nos. 2–4), page 402 (1978).
Type.
Stenodrepanum bergii Harms.
Description.
Suffrutescent shrub, (10–) 20–40 cm tall, with bud-bearing and occasionally tuber-forming roots; glabrous, with globose sessile glands scattered along the branches. Stipules ovate, membranous, 2.5–4 × 2–2.5 mm. Leaves alternate, bipinnate, pinnae in 1–3 pairs plus a single terminal pinna, 4–10 cm long; leaflets in 5–9 pairs per pinna, obtuse, 5–12 × 2–5.5 mm, with a crenulate, glandular margin, and embedded glands on the lower surface. Inflorescence a lax, terminal raceme, 4–14 cm long. Flowers bisexual, zygomorphic; calyx comprising a hypanthium and 5 sepals (these not persisting in fruit), glabrous, glandular, the lower cucullate sepal covering the other four in bud; petals 5, free, yellow, the median petal with red markings, obovate, with stipitate glands on the dorsal surface; stamens 10, free, filaments pubescent and glandular; ovary glandular. Fruit a linear to slightly falcate, cylindrical, torulose pod, 30–60 × 2–2.5 mm, 1–5-seeded. Seeds ovoid.
Geographic distribution.
A monospecific genus endemic to central and western Argentina.
Habitat.
Subtropical wooded grassland and scrub, especially close to salt pans.
Etymology.
From steno- (Greek: narrow) and drepano- (Greek: sickle), in allusion to the narrow sickle-shaped fruit.
References.
Ulibarri (1979, 2008); Kiesling et al. (1994: 285); Caponio et al. (2012); Nores et al. (2012).
22.1. Stenodrepanum bergii
Harms
23. Hoffmannseggia
Cav., Icon. 4: 63. 1798
Figure 37.
Hoffmannseggia pumilio (Griseb.) B. B. Simpson. A habit B median leaflet undersurface C calyx opened out D median petal E detail of glands on dorsal surface of median petal F lateral petal G, H stamens I gynoecium J stigma K detail of glands on ovary L fruit M gland on fruit. A, L–M from Cabrera 30150 B–K from Venturi 8309. Drawn by Eleanor Catherine.
Larrea Ortega (1797), nom. rejec. against Larrea Cav. (1800) in the Zygophyllaceae.
Moparia Britton & Rose (1930).
Type.
Hoffmannseggia falcaria Cav., nom. illeg. = Hoffmannseggia glauca (Ortega) Eifert.
Description.
Perennial woody herbs, most species forming a basal rosette, or subshrubs to 3 m, unarmed, often arising from bud-bearing and tuberous roots, shoots pubescent and with gland-tipped trichomes. Stipules not seen. Leaves alternate, bipinnate, ending in a pair of pinnae plus a single terminal pinna (except for Hoffmannseggia aphylla); pinnae opposite, in 1-13 pairs; leaflets small and numerous, in 2–15 (– 18) pairs per pinna, glabrous to pubescent, and glandular. Inflorescences terminal or axillary racemes; bracts often caducous. Flowers bisexual, zygomorphic; calyx comprising a hypanthium and 5 sepals, these weakly imbricate, persistent as pods mature (except in Hoffmannseggia microphylla and Hoffmannseggia peninsularis, where they are not always persistent); petals 5, free, yellow to orange, the median petal often with red markings; stamens 10, free, filaments pubescent; ovary glabrous to pubescent, eglandular to glandular, stigma apical, concave. Fruit a laterally compressed, straight or sometimes falcate pod, the sutures almost parallel, papery to leathery, glabrous to pubescent, eglandular or with glandular trichomes, indehiscent or dehiscent, with twisting valves. Seeds compressed, ovoid.
Geographic distribution.
Hoffmannseggia comprises 25 taxa in 23 species and occupies a classical amphitropical distribution in the New World with 10 species restricted to North America (southern USA and Mexico), 12 in South America (Peru, Bolivia to south-central Argentina and Chile, mainly Andean), and one species (Hoffmannseggia glauca (Ortega) Eifert) widespread throughout the range of the genus.
Habitat.
Subtropical desert and semi-desert grassland, often in open areas and on disturbed sites, on sandy, rocky or calcareous soils.
Etymology.
Named by Cavanilles for the German botanist, entomologist and ornithologist, Johann Centurius Graf von Hoffmannsegg (1766–1849).
References.
Britton and Rose (1930, under Larrea and Moparia); Burkart (1936); Macbride (1943, under Caesalpinia); Ulibarri (1979, 1996); Simpson (1999); Simpson et al. (2004, 2005); Lewis (1998, see Caesalpinia pumilio: 171–173); Simpson and Ulibarri (2006); Lewis and Sotuyo (2010).
Notes.
A complete synopsis and key to species (except Hoffmannseggia aphylla) is available in Simpson and Ulibarri (2006). A list of accepted species is given below excluding types and synonymy, for which the reader should refer to Simpson and Ulibarri (2006).
23.1. Hoffmannseggia aphylla
(Phil.) G.P. Lewis & Sotuyo
23.2. Hoffmannseggia arequipensis
Ulibarri
23.3. Hoffmannseggia doelli
Phil.
23.2.1. Hoffmannseggia doelli Phil. subsp. doellii
23.2.2. Hoffmannseggia doelli Phil. subsp. argentina
Ulibarri
23.4. Hoffmannseggia drepanocarpa
A. Gray
23.5. Hoffmannseggia drummondii
Torr. & A. Gray
23.6. Hoffmannseggia erecta
Phil.
23.7. Hoffmannseggia eremophila
(Phil.) Burkart ex Ulibarri
23.8. Hoffmannseggia glauca
(Ortega) Eifert
23.9. Hoffmannseggia humilis
(Mart. & Galeotti) Hemsl.
23.10. Hoffmannseggia intricata
Brandegee
23.11. Hoffmannseggia microphylla
Torr.
23.12. Hoffmannseggia minor
(Phil.) Ulibarri
23.13. Hoffmannseggia miranda
Sandwith
23.14. Hoffmannseggia oxycarpa
Benth.
23.14.1. Hoffmannseggia oxycarpa Benth. subsp. oxycarpa
23.14.2. Hoffmannseggia oxycarpa Benth. subsp. arida
(Rose) B. B. Simpson
23.15. Hoffmannseggia peninsularis
(Britton) Wiggins
23.16. Hoffmannseggia prostrata
Lag. ex DC.
23.17. Hoffmannseggia pumilio
(Griseb.) B. B. Simpson
23.18. Hoffmannseggia repens
(Eastw.) Cockerell
23.19. Hoffmannseggia tenella
Tharp & L. P. Williams
23.20. Hoffmannseggia trifoliata
Cav.
23.21. Hoffmannseggia viscosa
(Ruiz & Pav.) Hook.
23.22. Hoffmannseggia watsonii
(Fisher) Rose
23.23. Hoffmannseggia yaviensis
Ulibarri
24. Arquita
E. Gagnon, G. P. Lewis & C. E. Hughes, Taxon 64(3): 479. 2015
Figure 38.
Arquita mimosifolia (Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes. A flowering branchlet B stipule C eglandular leaflet undersurface D glandular leaflet undersurface E detail of glands on stem F inflorescence G bract H calyx opened out I median petal J lateral petal K stamen L gynoecium M stigma N detail of glands on ovary O fruits. A–E, G–N from Kiesling et al. 4990 F from Lorentz s.n. O from Schreiter 68526. Drawn by Eleanor Catherine.
Type.
Arquita mimosifolia (Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes.
Description.
Small to medium-sized, often decumbent shrubs, 0.3–2.5 m in height, slender in stature, usually with glandular trichomes on various parts of the plant; young stems and inflorescence rachises red-orange to maroon. Stipules ovate-obovate to deltoid, chartaceous, 2.5–5.5 mm long, usually with a fimbriate-glandular margin and short-stalked glands (except in some specimens of Arquita ancashiana), caducous. Leaves bipinnate, with 1–5 pairs of pinnae, usually with a single terminal pinna; petiole (0.3–) 0.5–6 cm long; rachis 0.5–6 cm long (but sometimes absent); leaflets in 4–12 opposite pairs per pinna, oblong-obovate, 2.5–10 (– 14) × 1–3.5 (– 6) mm, often with maroon/black glands in depressions on crenulated leaflet margins, and sometimes with occasional sessile black glands on the undersurface of leaflet blades (in Arquita ancashiana the glands are submarginal on the lower half of the basal leaflets of the pinnae). Inflorescences leaf-opposed, determinate racemes (with only 1 to 2 flowers open at a given time), (5–) 7–21 (– 41.5) cm long; bracts lanceolate, acuminate, either eglandular or covered in gland-tipped trichomes, 2.75–7 mm long, caducous. Flowers bisexual, zygomorphic; calyx comprising a hypanthium, and 5 sepals, 6–11 mm long, caducous, the lower sepal cucullate, and sepals either have an entire or glandular-fimbriate margin; petals 5, free, yellow to orange, median petal, sometimes streaked red, 6–17 × 4–12 mm, claw pubescent at the base, either flat or inrolled, sometimes with stipitate-glandular trichomes on the dorsal surface of the whole petal, upper and lower lateral petals 6–17 × 3–12 mm; stamens 10, free, 5–13 mm long, anthers 0.75–2.3 mm long, the stamens deflexed and loosely grouped around the gynoecium; ovary usually covered with gland-tipped trichomes. Fruits laterally compressed, lunate-falcate pods with a marcescent style, covered sparsely to densely with gland-tipped trichomes, these sometimes dendritic, 2–4.7 × (0.7–) 0.9–1 cm. Seeds laterally compressed, ovate-orbicular, 4.5–6 × 3.5–4.5 × 1 mm, the testa shiny olive-grey, sometimes mottled or streaked black.
Geographic distribution.
The genus Arquita comprises six taxa in five species restricted to the Andes in South America, in disjunct inter-Andean valleys, in Ecuador, Peru, Bolivia and Argentina.
Habitat.
Seasonally dry, montane, rupestral habitats in inter-Andean valleys.
Etymology.
The name Arquita derives from the vernacular name of Arquita trichocarpa in Argentina (Ulibarri 1996).
Notes.
A revision of Arquita with a complete key to species is available in Gagnon et al. (Taxon 64(3): 468–490, 2015).
References.
Burkart (1936); Ulibarri (1996); Lewis (1998: 167–171, 174–179); Lewis et al. (2010); Gagnon et al. (2015: 468–490).
24.1. Arquita ancashiana
(Ulibarri) E. Gagnon, G. P. Lewis & C. E. Hughes
24.2. Arquita celendiniana
(G. P. Lewis & C. E. Hughes) E. Gagnon, G. P. Lewis & C. E. Hughes
24.3. Arquita grandiflora
E. Gagnon, G. P. Lewis & C. E. Hughes
24.4. Arquita mimosifolia
(Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes
24.5. Arquita trichocarpa
(Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes
24.5.1. Arquita trichocarpa (Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes var. trichocarpa
24.5.2. Arquita trichocarpa (Griseb.) E. Gagnon, G. P. Lewis & C. E. Hughes var. boliviana
E. Gagnon, G. P. Lewis & C. E. Hughes
25. Pomaria
Cav., Icon. 5: 1. 1799
Figure 40.
Pomaria burchellii (DC.) B. B. Simpson & G. P. Lewis subsp. burchellii. A habit B, C leaflets from above and beneath, respectively D flower E calyx F–H calyx lobes I median petal J upper lateral petal K lower lateral petal L, M stamens N gynoecium O fruit, with enlargement of single trichome P part of single fruit valve showing seed. A–C, O, P from Wild & Drummond 6913 D–N from Galala 72. Drawn by D. Erasmus, originally published in Flora Zambesiaca, vol. 3 part 2, page 185 (2007).
Melanosticta DC. (1825).
Cladotrichium Vogel (1837).
Type.
Pomaria glandulosa Cav.
Description.
Small shrubs, subshrubs or perennial herbs, with a moderate to dense indumentum of simple curled hairs, sometimes also scattered plumose trichomes, intermixed with sessile, oblate glands (drying black) on stems. Stipules laciniate, pubescent, glandular, persistent. Leaves alternate, bipinnate, pinnae in 1–8 (– 11) pairs plus a terminal pinna; leaflets small, in 2–16 (– 27) pairs per pinna, always with multiple sessile glands on their lower surface (these orange in the field, drying black). Inflorescence a terminal or axillary raceme; bracts caducous. Flowers bisexual, zygomorphic; calyx comprising a hypanthium and 5 lanceolate sepals, the lower sepal cucullate, covering the other 4 in bud, and closely embracing the androecium and gynoecium at anthesis, sepals not persistent in fruit; petals 5, free, yellow, white, red or pink; stamens 10, filaments pubescent; ovary sparsely to densely hairy and glandular, stigma lateral. Fruit a linear or sickle-shaped, laterally-compressed pod, apex acute, with a sparse to dense covering of plumose/dendritic or stellate trichomes (these sometimes obscure and restricted to the fruit margin) intermixed with sessile oblate glands (drying black), elastically dehiscent, with twisting valves. Seeds laterally compressed.
Geographic distribution.
A genus of 17 taxa in 16 species: nine in North America (south-eastern USA, central and northern Mexico), four in South America (south-eastern Brazil, Paraguay, and Argentina), and three in southern Africa (Namibia, Botswana and South Africa).
Habitat.
Mainly in subtropical dry grassland and in degraded sites, many on limestone.
Etymology.
Named by Cavanilles for Dominic Pomar, botanist from Valencia, and doctor to Philip III (1598–1621), King of Spain.
Notes.
Revisions of the species of Pomaria are available for North America (Simpson, 1998), South America and Africa (Simpson and Lewis 2003), and southern Africa (under the name Hoffmannseggia, Brummit and Ross 1974). A list of accepted species is given below, but excludes types and synonymy which are available in the aforementioned revisions.
References.
Burkart (1936: 86–90); Brummitt and Ross (1974, as Hoffmannseggia); Ulibarri (1996, 2008); Simpson (1998); Simpson and Lewis (2003); Simpson et al. (2006).
25.1. Pomaria austrotexana
B. B. Simpson
25.2. Pomaria brachycarpa
(A. Gray) B. B. Simpson
25.3. Pomaria burchellii
(DC.) B. B. Simpson & G. P. Lewis
25.4. Pomaria canescens
(Fisher) B. B. Simpson
25.5. Pomaria fruticosa
(S. Watson) B. B. Simpson
25.6. Pomaria glandulosa
Cav.
25.7. Pomaria jamesii
(Torr. & A. Gray) Walp.
25.8. Pomaria lactea
(Schinz) B. B. Simpson & G. P. Lewis
25.9. Pomaria melanosticta
S. Schauer
25.10. Pomaria multijuga
(S. Watson) B. B. Simpson
25.11. Pomaria parviflora
(Micheli) B. B. Simpson & G. P. Lewis
25.12. Pomaria pilosa
(Vogel) B. B. Simpson & G. P. Lewis
25.13. Pomaria rubicunda
(Vogel) B. B. Simpson & G. P. Lewis
25.13.1. Pomaria rubicunda (Vogel) B. B. Simpson & G. P. Lewis var. rubicunda
25.13.2. Pomaria rubicunda (Vogel) B. B. Simpson & G. P. Lewis var. hauthalii
(Harms) B. B. Simpson & G. P. Lewis
25.14. Pomaria sandersonii
(Harv.) B. B. Simpson & G. P. Lewis
25.15. Pomaria stipularis
(Vogel) B. B. Simpson & G. P. Lewis
25.16. Pomaria wootonii
(Britton) B. B. Simpson
26. Erythrostemon
Klotzsch, in Link, Klotzsch & Otto, Icon. Pl. Rar. Horti. Berol. 2: 97, t. 39. 1844, descr. emended E. Gagnon & G. P. Lewis
Figure 41.
Erythrostemon gilliesii (Hook.) Klotzsch. A inflorescence and foliage B leaflet undersurface with submarginal glands C bract D detail of glandular pedicel E calyx opened out F median petal G upper lateral petal H lower lateral petal I stamen J gynoecium K stigma L fruit M seed. A from Venturi 5365 B, L from Kiesling et al. 4891 C–K from Cult. Kew 213-69 01878 M from Lewis 1417. Drawn by Eleanor Catherine.
Poincianella Britton & Rose (1930), pro parte, including the type species Caesalpinia mexicana A. Gray = Poincianella mexicana (A. Gray) Britton & Rose.
Schrammia Britton & Rose (1930).
Diagnosis.
Erythrostemon is closely related to Pomaria, but differs in habit, consisting of large shrubs and small to medium sized trees, or occasionally suffrutices (vs. shrubs, suffrutices, or perennial herbs in Pomaria). It also differs by its ovate-lanceolate to orbicular sepals (vs. linear, laciniate sepals in Pomaria), leaflets that are either eglandular or with conspicuous black sessile glands along the margin, these sometimes sunken in the sinuses of the crenulated margin (vs. leaflets with multiple glandular dots on the lower leaflet surfaces, that are orange in the field, drying black), the androecium and gynoecium free from the calyx (vs. the androecium and gynoecium cupped in the lower cucullate sepal), deflexed petals (vs. the two lower petals forming a horizontal platform above the lower cucullate sepal), and oblong-elliptic pods, the valves chartaceous to slightly woody, glabrous to pubescent, eglandular or with stipitate glands (vs. linear to sickle-shaped pods, the valves glabrous or with plumose trichomes and stipitate glands).
Type.
Erythrostemon gilliesii (Hook.) Klotzsch.
Emended description.
Shrubs or small to medium-sized trees varying from (0.5–) 1–12 (– 20) meters tall, occasionally suffrutices (Erythrostemon nelsonii and Erythrostemon caudatus), unarmed (except Erythrostemon glandulosus); bark variable, smooth or rough, sometimes exfoliating, grey, greyish white, pale brown or reddish brown, often with white or black pustular lenticels; young stems terete (angular in Erythrostemon angulatus), glabrous to densely pubescent, eglandular to densely covered in stipitate-glands. Stipules ovate-lanceolate, ovate to orbicular, apex acute to acuminate, caducous (persistent in Erythrostemon argentinus and Erythrostemon caudatus). Leaves alternate, bipinnate, usually ending in a pair of pinnae plus a single terminal pinna; petioles (0.2–) 0.5–8 (– 10) cm long; rachis (0.5–) 1.2–14.5 (– 21.5) cm long, or lacking; petiole and rachis glabrous to densely pubescent, eglandular or covered in stipitate glands; pinnae in 1–6 (– 15) pairs, plus a terminal pinna (this occasionally lacking); leaflets in 2–13 (– 20) opposite pairs per pinna, size varying from a few mm in length and width (1.4–3 × 0.75–2 mm in Erythrostemon exilifolius), to 5.3 × 2.5 cm, elliptic, oblong-elliptic, obovate, ovate or sub-orbicular, leaflet blades eglandular or with conspicuous black sessile glands along the margin, these sometimes sunken in the sinuses of the crenulated margin. Inflorescence an axillary or terminal raceme. Flowers bisexual, zygomorphic; calyx a short hypanthium with 5 sepals, 4.5–25 mm long, glabrous to pubescent, eglandular or with stipitate-glands, lower sepal cucullate in bud, all sepals caducous, the hypanthium persistent and abscising to form a free ring around the pedicel as the fruit matures; petals 5, free, imbricate, bright golden yellow, to creamish yellow, salmon pink or pink-scarlet, the median petal often with red-orange markings, the corolla diverse in form, the median petal 6–32 × 3.2–20 mm, the lateral petals 6–32 × 3.5–18.5 mm, petal blades eglandular or the dorsal surface covered with stipitate glands, claw margins glabrous to pubescent, eglandular or with gland-tipped trichomes; stamens 10, free, 0.6–3.5 cm long (up to 10 cm in Erythrostemon gilliesii), filaments pubescent, eglandular or with stipitate glands; ovary pubescent, eglandular or with sessile or stipitate glands, stigma a terminal fringed chamber. Fruit a chartaceous to coriaceous or slightly woody, laterally compressed pod, with a marcescent style persisting as a small beak, elastically dehiscent with twisting valves, 2.4–12.5 × 1–2.8 cm, glabrous to pubescent, eglandular or with stipitate glands, (1–) 2–7 (– 8)-seeded. Seeds yellow to ochre-brown, or mottled with grey and black.
Geographic distribution.
The genus comprises 34 taxa in 31 species. Its circumscription is emended here to include many species previously placed in Central American and Mexican Poincianella. 22 species are found across the southern USA, Mexico and Central America, one occurs in the Caribbean (Cuba and Hispaniola), eight occur in South America, with one endemic in the caatinga vegetation of Brazil, and the other seven in Argentina, Bolivia, Chile, and Paraguay.
Habitat.
Low-elevation seasonally dry tropical forests across Mexico, Central America, the Caribbean and in caatinga vegetation in Brazil; also in patches of dry forest, deserts, yungas-puna transition zones, and chaco-transition forests in Argentina, Bolivia, Chile and Paraguay.
Etymology.
From erythro- (Greek: red) and stemon (Greek: stamen), the type species Erythrostemon gilliesii (Wall. ex Hook.) Klotzsch has long red exserted stamens, but this is unusual in the genus as circumscribed here.
Notes.
Species descriptions (under Caesalpinia binomials) are available in Lewis (1998). A key is also available in that revision, but it includes species now considered to belong in Cenostigma, Arquita, and Hoffmannseggia.
References.
Britton and Rose (1930); Burkart (1936: 82–84, 97–108); Ulibarri (1996); Lewis (1998); De Queiroz (2009: 120–121).
26.1. Erythrostemon acapulcensis
(Standl.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158083-1
Basionym.
Caesalpinia acapulcensis Standl., Contr. U.S. Natl. Herb. 20: 213. 1919.
Poincianella acapulcensis (Standl.) Britton & Rose, N. Amer. Fl. 23(5): 329. 1930.
Type.
MEXICO, Guerrero, vicinity of Acapulco, Oct 1894– Mar 1895, Palmer 505 (holotype US!; isotypes F!, GH!, K!, MEXU!, NY!).
26.2. Erythrostemon angulatus
(Hook. & Arn.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158084-1
Basionym.
Zuccagnia ? angulata Hook. & Arn., Bot. Beechy’s Voyage: 22. 1830.
Caesalpinia angulata (Hook. & Arn.) Baill., Adansonia 9: 227. 1870.
Type.
CHILE, Coquimbo (holotype ?E, n.v.).
Caesalpinia angulicaulis Clos, Fl. Chile: 223. 1846.
Type. CHILE, Coquimbo, Andacollo, near the Rio Hurtado, 1837, C. Gay 525 (holotype ?TL, n.v.; isotype SGO).
26.3. Erythrostemon argentinus
(Burkart) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158085-1
Basionym.
Caesalpinia argentina Burkart, Revista Argent. Agron. 3: 105. 1936.
Type.
ARGENTINA, Jujuy, Santa Cornelia, Sierra de Santa Bárbara, Nov 1911, Spegazzini 2159 (holotype LP, isotype SI).
Caesalpinia coulterioides Griseb. Symb. Fl. Argent.: 113. 1879, pro parte.
26.4. Erythrostemon caladenia
(Standl.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158086-1
Basionym.
Caesalpinia caladenia Standl., Contr. U.S. Natl. Herb. 20: 214. 1919.
Poincianella caladenia (Standl.) Britton & Rose, N. Amer. Fl. 23(5): 329. 1930.
Type.
MEXICO, Sonora, c. 5 miles below Minas Nuevas, 12 Mar 1910, Rose et al. 12660 (holotype US!; isotype NY!).
26.5. Erythrostemon calycinus
(Benth.) L. P. Queiroz, in Leguminosas da Caatinga: 121. 2009, as "calycina"
Basionym.
Caesalpinia calycina Benth., Mart., Fl. Brasil. 15(2): 71. 1870.
Type.
BRAZIL, Bahia, near Rio de Contas, Mar 1817, Prinz zu Wied-Neuwied (Princeps Maximilianus Neovidensis) s.n. (holotype BR!).
26.6. Erythrostemon caudatus
(A. Gray) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158090-1
Basionym.
Hoffmannseggia caudata A. Gray, Boston J. Nat. Hist. 6: 179. 1850.
Caesalpinia caudata (A. Gray) E. M. Fisher, Bot. Gaz. 18: 123. 1893.
Schrammia caudata (A. Gray) Britton & Rose, N. Amer. Flora 23(5): 317. 1930.
Type.
U. S. A., Texas, between the Nueces and the Rio Grande, Wright 146 (holotype GH; isotype K!).
26.7. Erythrostemon coccineus
(G. P. Lewis & J. L. Contr.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158120-1
Basionym.
Caesalpinia coccinea G. P. Lewis & J. L. Contr., Kew Bull. 49: 103. 1994.
Type.
MEXICO, Oaxaca State, 27 Mar 1989, Lewis et al. 1802 (holotype MEXU!; isotypes FCME!, FHO!, K!, M!, NY!, SI!).
26.8. Erythrostemon coluteifolius
(Griseb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158091-1
Basionym.
Caesalpinia coluteifolia Griseb., Symb. Fl. Argent.: 111. 1879.
Type.
Argentina, Tucumán, near El Alduralde on the route to Salta, Feb 1873, Lorentz & Hieronymus 1004 (holotype GOET!; isotype CORD).
26.9. Erythrostemon coulterioides
(Griseb. emend. Burkart) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158119-1
Basionym.
Caesalpinia coulterioides Griseb., Symb. Fl. Argent: 113. 1879, (as “coulteriodes”), pro parte quoad material from El Volcan.
Type.
ARGENTINA, Jujuy, Depto. Tumbaya, El Volcán, 12–13 May 1873, Lorentz & Hieronymus 760 (holotype GOET; isotype CORD).
Caesalpinia coulterioides Griseb., emend. Burkart, Revista Argent. Agron. 3: 97. 1936.
26.10. Erythrostemon epifanioi
(J. L. Contr.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158121-1
Basionym.
Caesalpinia epifanioi J. L. Contr., Anales Inst. Biol. Univ. Nac. Auton. Mexico, Bot. 58: 55. 1989.
Type.
MEXICO, Guerrero, Mpio. Mártires de Cuéllar, 18 Feb. 1986, Contreras 1825 (holotype FCME; isotype MEXU).
26.11. Erythrostemon exilifolius
(Griseb.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158092-1
Basionym.
Caesalpinia exilifolia Griseb., Plant. Lorentz: 80. 1874.
Type.
ARGENTINA, Catamarca, near San José, 4 Jan 1872, Lorentz 352 (holotype GOET!).
26.12. Erythrostemon exostemma
(DC.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158093-1
Basionym.
Caesalpinia exostemma DC., Prodr. 2: 483. 1825.
Poincianella exostemma (DC.) Britton & Rose, N. Amer. Fl. 23(5): 328. 1930.
Type.
MEXICO, a painting, one of the copies of Ic. Fl. Mex. 80, represented at G-DC by de Candolle plate 218.
26.12.1. Erythrostemon exostemma subsp. exostemma
? Poinciana compressa Sessé & Mociño ex. G. Don, Gen. Hist. 2: 433 (1832).
? Caesalpinia compressa (G. Don) D. Dietr. Syn. Pl. 2:1494. 1840. Type. MEXICO, Sessé & Mociño, formerly in herb. Lambert– not located in recent times, but a specimen in the Sessé & Mociño herbarium (MA), no. 1097, labelled Poinciana compressa, represents Caesalpinia exostemma according to P. Standley (fide McVaugh, 1987).
Caesalpinia affinis Hemsl., Diag. Pl. Nov. Mexic. 8. 1878.
Poincianella affinis (Hemsl.) Britton & Rose, N. Amer. Fl. 23(5): 328. 1930. Type. GUATEMALA, Skinner s.n. (holotype K!; isotype K!).
Poinciana conzattii Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Poincianella conzattii (Rose) Britton & Rose, N. Amer. Fl. 23(5): 328. 1930.
Caesalpinia conzattii (Rose) Standl., Trop. Woods 37: 34. 1934. Type. MEXICO, Tehuantepec, 1909, Hugo & Conzatti 2444 (holotype US!, national herbarium number 841055).
26.12.2. Erythrostemon exostemma subsp. tampicoanus
(Britton & Rose) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158117-1
Basionym.
Poincianella tampicoana Britton & Rose, N. Amer. Fl. 23(5): 330. 1930.
Caesalpinia tampicoana (Britton & Rose) Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 11(5): 159. 1936.
Caesalpinia exostemma subsp. tampicoana (Britton & Rose) G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 72. 1998.
Type.
MEXICO, Veracruz, vicinity of Pueblo Viejo, 2 km S of Tampico, 1 and 2 Jun 1910. Palmer 556 (holotype US!).
26.13. Erythrostemon fimbriatus
(Tul.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473354-2
Basionym.
Caesalpinia fimbriata Tul., Arch. Mus. Hist. Nat. Paris 4: 145. 1844.
Type.
BOLIVIA, “Chivesivi, Vallé S de La Paz, alt. 8500–12000 ped. angl.”, Pentland 39 (holotype P!; isotype F!).
Caesalpinia bangii Rusby, Mem. Torrey Bot. Club 3(3): 22. 1893.
Type. BOLIVIA, 1891, Bang 757 (holotype NY!; isotypes E!, F!, GH!, K!).
Caesalpinia cromantha Burkart, Revista Argent. Agron. 3(2): 100. 1936.
Type. ARGENTINA, Prov. Salta, Depto. Guachipas, Pampa Grande, Jan 1897, Spegazzini 2198 (holotype SI!; isotype LP).
26.14. Erythrostemon gilliesii
(Hook.) Klotzsch, Ic. Pl. Rar. Horti. Berol. 2 (3): 97, t. 39. 1844
Basionym.
Poinciana gilliesii Wall. ex Hook., Bot. Misc. 1: 129. 1829 [1830].
Caesalpinia gilliesii (Hook.) D. Dietr., Synop. Pl. 2: 1495. 1840.
Type.
ARGENTINA, near Rio Quatro and Rio Quinto, and in La Punta de San Luis, Gillies s.n. (holotype K!).
26.15. Erythrostemon glandulosus
(Bertero ex DC.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158102-1
Basionym.
Caesalpinia glandulosa Bertero ex DC., Prodr. 2: 482. 1825.
Poincianella glandulosa (Bertero ex DC.) Britton & Rose, N. Amer. Fl. 23(5): 336. 1930.
Type.
HISPANIOLA, Bertero 84 (holotype G-DC).
26.16. Erythrostemon hintonii
(Sandwith) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158103-1
Basionym.
Caesalpinia hintonii Sandwith. Kew Bull. 1937: 303. 1937.
Type.
MEXICO, Guerrero, District of Coyuca, Cuajilote, 9 May 1935, Hinton 7746 (holotype K!; isotypes A!, F!, GH!, MEXU).
26.17. Erythrostemon hughesii
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473355-2
Basionym.
Caesalpinia hughesii G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 73. 1998.
Type.
MEXICO, Oaxaca, 5 km W of Rio Grande, 25 Mar 1989, Lewis et al. 1795 (holotype K!; isotypes FCME!, FHO!, K!, MEXU!).
26.18. Erythrostemon laxus
(Benth.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158104-1
Basionym.
Caesalpinia laxa Benth., Pl. Hartw.: 60. 1840.
Poincianella laxa (Benth.) Britton & Rose, N. Amer. Flora 23(5): 329. 1930.
Type.
MEXICO, Oaxaca, Teojomulco, Hartweg 455 (holotype BM!; isotypes E!, K!, MEXU!, photos F!).
26.19. Erythrostemon macvaughii
(J. L. Contr. & G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158111-1
Basionym.
Caesalpinia macvaughii J. L. Contr. & G. P. Lewis, Kew Bull. 47: 309. 1992.
Type.
MEXICO, Guerrero, Mpio. Zirándaro de Chávez, 8 Mar 1988, Contreras 2343 (holotype FCME; isotypes K!, MEXU).
Caesalpinia laxa sensu McVaugh, pro parte quoad McVaugh 22517, non Benth.
26.20. Erythrostemon melanadenius
(Rose) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473356-2
Basionym.
Poinciana melanadenia Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Caesalpinia melanadenia (Rose) Standl., Contr. U.S. Natl. Herb. 23: 425. 1922.
Poincianella melanadenia (Rose) Britton & Rose, N. Amer. Flora 23(5): 334. 1930.
Type.
MEXICO, Puebla, near Tehuacán, 1 Sep 1906, Rose & Rose 11249 (holotype US!).
26.21. Erythrostemon mexicanus
(A. Gray) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473357-2
Basionym.
Caesalpinia mexicana A. Gray, Proc. Amer. Acad. Arts 5: 157. 1861.
Poinciana mexicana (A. Gray) Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Poincianella mexicana (A. Gray) Britton & Rose, N. Amer. Fl. 23(5): 330. 1930.
Type.
MEXICO, Nuevo León, near Monterrey, 11 Feb 1847, Gregg s.n. (lectotype GH!, fide McVaugh, 1987).
26.22. Erythrostemon nelsonii
(Britton & Rose) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158112-1
Basionym.
Poincianella nelsonii Britton & Rose in N. Amer. Fl. 23(5): 331. 1930.
Caesalpinia nelsonii (Britton & Rose) J. L. Contr., Thesis, UNAM, Mexico D.F.: 91. 1991.
Type.
MEXICO, Guerrero, between Copala and Juchitango [Juchitan], 9 Feb 1895, Nelson 2303 (holotype US!; isotypes GH!, NY!, photo MEXU).
26.23. Erythrostemon nicaraguensis
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473358-2
Basionym.
Caesalpinia nicaraguensis G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 86. 1998.
Type.
NICARAGUA, Department of Esteli, Hughes 1406 (holotype MEXU!; isotypes EAP, FHO, K!, NY!).
26.24. Erythrostemon oyamae
(Sotuyo & G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:60473359-2
Basionym.
Caesalpinia oyamae Sotuyo & G. P. Lewis, Brittonia 59: 34. 2007.
Type.
MEXICO, Puebla, Mpio. Acatlán de Osorio, 20 km to the W of Acatlán on the road from Oaxaca City to Izúcar de Matamoros (Hwy. 190), 18°17'N, 98°5'W, 19 Feb 1993, J. A. Hawkins & C. E. Hughes 23 (holotype MEXU; isotypes FHO!, K!, MEXU).
26.25. Erythrostemon palmeri
(S. Watson) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158105-1
Basionym.
Caesalpinia palmeri S. Watson, Proc. Am. Acad. Arts 24: 47. 1889.
Poinciana palmeri (S. Wats.) Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Poincianella palmeri (S. Watson) Britton & Rose, N. Amer. Flora 23(5): 332. 1930.
Type.
MEXICO, Sonora, Guaymas, Jun 1887, Palmer 70 (holotype US!; isotypes GH!, K!, NY!).
Poincianella arida Britton & Rose, N. Amer. Flora 23 (5): 332. 1930.
Caesalpinia arida (Britton & Rose) Wiggins, Contr. Dudley Herb. 3(3): 69. 1940.
Type. MEXICO, Sonora, near Hermosillo, 7 Mar 1910, Rose et al. 12508 (holotype NY!).
26.26. Erythrostemon pannosus
(Brandegee) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158113-1
Basionym.
Caesalpinia pannosa Brandegee, Proc. Calif. Acad. Sci., Ser. 2: 150. 1889. (See also Proc. Calif. Acad. Sci., Ser. 3: 130. 1891).
Poinciana pannosa (Brandegee) Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Poincianella pannosa (Brandegee) Britton & Rose, N. Amer. Flora 23(5): 331. 1930.
Type.
MEXICO, Baja California, San Gregoria, 1 Feb 1889, Brandegee s.n. (lectotype UC!, designated by Lewis 1998).
Caesalpinia mexicana A. Gray var. californica A. Gray, Proc. Amer. Acad. Arts 5: 157. 1861.
Poinciana californica (A. Gray) Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Caesalpinia californica (A. Gray) Standl., Contr. U.S. Natl. Herb. 23: 426. 1922.
Poincianella californica (A. Gray) Britton & Rose, N. Amer. Flora 23(5): 331. 1930.
Type. MEXICO, Baja California, Cape St. Lucas, Aug 1859– Jan 1860, Xantus 29 (lectotype GH!, designated by Lewis 1998; isolectotype NY!).
Caesalpinia arenosa Wiggins, Contr. Dudley Herb. 3(3): 68. 1940.
Type. MEXICO, Baja California, 4 miles S of Guadalupe, 21 Mar 1935, Whitehead 839 (holotype DS).
26.27. Erythrostemon phyllanthoides
(Standl.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158106-1
Basionym.
Caesalpinia phyllanthoides Standl., Contr. U.S. Natl. Herb. 23: 425. 1922. Poincianella phyllanthoides (Standl.) Britton & Rose, N. Amer. Fl. 23(5): 332. 1930.
Type.
MEXICO, Tamaulipas, Hacienda Buena Vista, 18 Jun 1919, Wooton s.n. (holotype US!; isotype NY!).
26.28. Erythrostemon placidus
(Brandegee) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158114-1
Basionym.
Caesalpinia placida Brandegee, Proc. Calif. Acad. Sci., Ser. 2, 3: 131. 1891.
Poinciana placida (Brandegee) Rose, Contr. U.S. Natl. Herb. 13: 303. 1911.
Poincianella placida (Brandegee) Britton & Rose, N. Amer. Fl. 23(5): 331. 1930.
Type.
MEXICO, Baja California, La Paz, 4 Feb 1890, Brandegee s.n. (lectotype UC!, designated by Lewis 1998; isolectotype GH!).
26.29. Erythrostemon robinsonianus
(Britton & Rose) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158116-1
Basionym.
Poincianella robinsoniana Britton & Rose, N. Amer. Fl. 23(5): 330. 1930.
Caesalpinia robinsoniana (Britton & Rose) G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 42. 1998.
Type.
MEXICO, Jalisco, Zapotlán, 25 May 1893, Pringle 5467 (holotype GH!; isotype MEXU!).
Caesalpinia mexicana A. Gray var. pubescens B.L. Rob. & Greenm., Proc. Amer. Acad. Arts 29: 386. 1894.
Type. As above.
26.30. Erythrostemon standleyi
(Britton & Rose) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158115-1
Basionym.
Poincianella standleyi Britton & Rose, N. Amer. Fl. 23(5): 330. 1930.
Caesalpinia standleyi (Britton & Rose) Standl., Publ. Field Mus. Nat. Hist., Bot. Ser. 11(5): 159. 1936.
Type.
MEXICO, Nayarit, Acaponeta, 9 Apr 1910, Rose et al. 14190 (holotype NY!).
26.31. Erythrostemon yucatanensis
(Greenm.) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158107-1
Basionym.
Caesalpinia yucatanensis Greenm., Publ. Field Mus. Nat. Hist., Bot. Ser. 2: 252. 1907.
Poincianella yucatanensis (Greenm.) Britton & Rose, N. Amer. Fl. 23(5): 330. 1930.
Type.
MEXICO, Yucatán, near Izamal, 1895, Gaumer 371 (holotype F!; isotypes F!, K!, NY!).
26.31.1. Erythrostemon yucatanensis subsp. yucatanensis
Caesalpinia recordii Britton & Rose, Trop. Woods 7: 6. 1926.
Poincianella recordii (Britton & Rose) Britton & Rose, N. Amer. Fl. 23(5): 329. 1930. Type. BELIZE, Feb 1926, Record s.n. (holotype US; isotypes F!, GH!, NY!).
26.31.2. Erythrostemon yucatanensis subsp. chiapensis
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158108-1
Basionym.
Caesalpinia yucatanensis subsp. chiapensis G. P. Lewis, Caesalpinia: Revis. Poincianella-Erythrostemon group: 85. 1998.
Type.
MEXICO, Chiapas, c. 4 km from Comalapa on road to La Trinitaria, 27 Feb 1992, Hughes et al. 1684 (holotype K (sheet 2)!, isotypes E!, FHO!, K!, MEXU!, MO!, NY!).
26.31.3. Erythrostemon yucatanensis subsp. hondurensis
(G. P. Lewis) E. Gagnon & G. P. Lewis comb. nov.
urn:lsid:ipni.org:names:77158109-1
Basionym.
Caesalpinia yucatanensis subsp. hondurensis G. P. Lewis, in Caesalpinia: Revis. Poincianella-Erythrostemon group: 86. 1998.
Type.
HONDURAS, Dept. Yoro, lower Aguán Valley, c. 31 km W of Olanchito, 25 Mar 1991, Hughes 1448 (holotype K!; isotype FHO!).
27. ? Ticanto
Adans., Fam. Pl. 2: 319. 1763.
?Ticanto Adans., Fam. Pl. 2: 319. 1763.
Caesalpinia sect. Nugaria DC. 1825.
Type.
“H.M. 6: t. 19” (= Rheede`s Hortus Malabaricus 6, plate 19, 1686).
Notes.
More work is needed to determine whether the species listed below form a clade and merit reinstatement as a distinct genus, or alternatively if the name Ticanto should be synonymised under another genus in the Caesalpinia group. The list of species presented below includes names that most probably belong in Ticanto, but revisionary and phylogenetic work are needed to accurately delimit species, and determine types and synonyms.
References.
Hattink (1974); Vidal and Hul Thol (1976); Chen et al. (2010a).
27.1. Caesalpinia caesia
Handel-Mazzetti
27.2. Caesalpinia chinensis
Roxb.
27.3. Caesalpinia crista
L. emend. Dandy & Exell
27.4. Caesalpinia elliptifolia
S. J. Li, Z. Y. Chen & D. X. Zhang
27.5. Caesalpinia hypoglauca
Chun & How
27.6. Caesalpinia kwangtungensis
Merr.
27.7. Caesalpinia laevigata
Perr.
27.8. Caesalpinia magnifoliolata
Metcalf
27.9. Caesalpinia nuga
(L.) Ait.
27.10. Caesalpinia paniculata
(Lam.) Roxb.
27.11. Caesalpinia rhombifolia
J. E. Vidal
27.12. Caesalpinia scandens
Heyne ex Roth
27.13. Caesalpinia szechuanensis
Craib
27.14. Caesalpinia vernalis
Champion
27.15. Caesalpinia yunnanensis
S. J. Li, D. X. Zhang & Z. Y. Chen
Authors’ contributions
EG, AB, CEH and GPL were involved in study conception and design; EG, AB, CEH, GPL and LPdQ collected and provided herbarium and field samples for analysis; EG generated and assembled all the data, which she was also responsible for analysing and interpreting; EG drafted the manuscript, and critical revisions were provided by AB, CEH, GPL and LPdQ; EG also wrote the key, generic descriptions and provided the list of species belonging to each genus. These were all critically revised by GPL, who completed this work by verifying the nomenclature and identifying types for all species names and synonyms. GPL was also the main instigator behind the new generic names (Paubrasilia, Hultholia, Hererolandia and Gelrebia).
Acknowledgements
This study was supported by a grant from the Natural Sciences and Engineering Research Council of Canada to AB, a Fonds de Recherche Québécois sur la Nature et les Technologies (FRQNT) fellowship to EG and funding from the Claraz Schenkung Foundation, Switzerland for fieldwork. We thank Ruth Clark at Kew, who provided the description of Mezoneuron and helped to revise the key, as well as Nicholas Turland who helped us with the typification of Paubrasilia echinata. We also thank Heather Lindon, for verifying that all our nomenclatural combinations respected the latin and greek rules of grammar. We thank the following herbaria for loans of material and permission to sample leaflets for DNA: A, CAS, CORD, E, FHO, HUEFS, IBSC, INIREB, INPA, K, MEXU, MO, NTBG, NY, P, TEX, US, and WAG. We thank the Arizona-Sonora Desert Museum, D. Aplin, S-J Li, D. Lorence, I. Telford, J. Bruhl, and B.B. Simpson for leaf samples, B. Marazzi, A. Daza, R. Vanni, G. Lopez, and G. Atchison for the help with field work, and F. El Ayadi for technical assistance in the lab. We thank the many artists who illustrated genera in the Caesalpinia group, notably Eleanor Catherine, Margaret Tebbs, Juliet Williamson, Tim Galloway, Pat Halliday, Christi A. Sobel, and Sue Wickison. Thanks also goes to Manuel Belgrano, who helped us secure permission to reproduce a Stenodrepanum illustration, as well as Frances Crawford, who provided material that allowed the Hererolandia illustration to be completed. Finally, we acknowledge the many photographers and websites that gave us permission to reproduce their images in this publication: P. Alexander, J. Anton-Smith, P. Awale, P. Baxter, P.J. Cribb, A.A. Dreyer, R.H. Fortunato, M.F. Gardner, P. Grard (Institut français de Pondichéry), J. Holopainen, J. Neff, J.M. Norres, D. Du Puy, R. Ripley, M. Sanjappa, B.B. Simpson, I. Specogna, F. Starr and K. Starr, B. Torke, M. Thulin, P. van Wyk, V.R. Vinayaraj, and B.T. Wursten; and Flowers of India (http://www.flowersofindia.net), Flora digital (http://www.ufrgs.br/fitoecologia/florars/), Flora Mendocina (http://www.floramendocina.com.ar/), Flora of Zimbabwe (http://www.zimbabweflora.co.zw/), SEINet (http://swbiodiversity.org/seinet/imagelib/), and Wikicommons (https://commons.wikimedia.org/).
Appendix 1
Accessions included in this study. Species of the Caesalpinia group are classified sensu Lewis (2005), and the number of species sampled over the total number of species recognized in the genus is given in parentheses. Type species for genera in the Caesalpinia group are preceded by an asterisk (*). Collector names, numbers (and herbarium acronym) of voucher specimens are listed for all material that was taken from herbarium specimens and for the voucher specimens of seed collections and silica-dried leaf samples, if known. Collection locality indicates the country where the specimen originated, and indicates which accessions were from cultivated specimens; N/A indicates that locality data was not available. Accession numbers are provided for published sequences downloaded directly from GenBank; with the exception of 22 sequences for the species Caesalpinia crista, Caesalpinia decapetala, Caesalpinia sappan, Cenostigma gardnerianum, Coulteria platyloba, Guilandina bonduc, Libidibia coriaria, Poincianella exostemma, Poincianella bracteosa, Poincianella pyramidalis and Pterolobium stellatum, the majority of the sequences come from the following published studies: Bruneau et al. (2001), Simpson et al. (2003), Haston et al. (2005), Simpson et al. (2005), Marazzi et al. (2006), Simpson et al. (2006), Marazzi and Sanderson (2010), Babineau et al. (2013), Gagnon et al. (2013), and Gagnon et al. (2015). Furthermore, certain accessions were combined together in phylogenetic analyses: these accessions are in bold. If there were redundant sequences between these combined accessions, the longest sequence for each available marker were selected (GenBank numbers in bold).
| Genus (no. of species sampled/total no. species) Species |
Voucher specimen (Herbarium) | Collection locality | rps16 | trnD-trnT | ycf6-psbM | ITS | trnL-trnF | matK-3’trnK |
|---|---|---|---|---|---|---|---|---|
| OUTGROUP | ||||||||
| Cassia javanica L. | Fougère-Danezan 6 (MT) | Singapore, cultivated | KF522255 | KX379272 | KX372932 | KX372778 | EU361782 | EU361910 |
| Colvillea racemosa Bojer ex Hook. | Haston V200303 (RNG) | Madagascar | AY899794 | - | - | - | AY899739 | - |
| Colvillea racemosa Bojer ex Hook. | Bruneau 1397 (MT) | Madagascar | - | KX379275 | - | KX372928 | - | KX176814 |
| Conzattia multiflora (B.L. Rob.) Standl. | Du 600 (K), Haston V200303 (RNG) | Mexico | AY899785 | - | - | - | - | - |
| Conzattia multiflora (B.L. Rob.) Standl. | Hughes 1815 (NY) | Mexico | - | KX379270 | - | KX372927 | - | - |
| Conzattia multiflora (B.L. Rob.) Standl. | Simpson 17-XI-97 (TEX) | Mexico | - | - | - | - | AF430770 | - |
| Conzattia multiflora (B.L. Rob.) Standl. | Werling 399 (ASU) | Mexico | - | - | - | - | - | AY386918 |
| Gymnocladus chinensis Baill. | Herendeen II-V-02-1 (US) | USA, cultivated | KF522308 | KX379269 | KX372931 | KX372777 | AY232786 | - |
| Gymnocladus chinensis Baill. | Herendeen 8-V-2003-1 (US) | USA, cultivated | - | - | - | - | - | AY386928 |
| Pterogyne nitens Tul. | Pennington 244 (FHO) | Brazil | AY899747 | - | - | - | AY899689 | - |
| Pterogyne nitens Tul. | Herendeen 13-XII-97-1 (US) | Tanzania | - | KX379276 | KX372936 | KX372782 | - | EU362031 |
| Senna covesii (A. Gray) H.S. Irwin & Barneby | Marazzi BM297 (ARIZ) | USA, cultivated | HM236885 | - | - | - | - | - |
| Senna covesii (A. Gray) H.S. Irwin & Barneby | Wojciechowski 876 (ASU) | USA | - | - | - | KX372779 | EU361835 | AY386850 |
| Senna spectabilis (DC.) H.S. Irwin & Barneby | Marazzi et al. BM029 (PY, CTES, Z) | Paraguay | AM086983 | - | - | - | - | AM086900 |
| Senna spectabilis (DC.) H.S. Irwin & Barneby | Herendeen & Pooma 24-IV-99-6 (US) | Thailand | - | KX379274 | KX372934 | KX372781 | - | - |
| Senna alata (L.) Roxb. | Bruneau 1076 (MT) | Cameroun, cultivated | - | KX379273 | KX372933 | KX372780 | AF365091 | EU362042 |
| Tetrapterocarpon geayi Humbert | Noyes 1049 (K) | Madagascar | AY899742 | - | - | - | AY899684 | - |
| Tetrapterocarpon geayi Humbert | Bruneau & Ranaivojaona 1395 (WAG) | Madagascar | - | KX379271 | KX372935 | KX372783 | - | GU321972 |
| CAESALPINIA GROUP | ||||||||
| Arquita Gagnon et al. (5/5 species) | ||||||||
| *Arquita mimosifolia (Griseb.) E. Gagnon et al. | Gagnon et al. EG203 (MT) | Argentina | KF522160 | KP003760 | KP003707 | KP003654 | - | KX176810 |
| *Arquita mimosifolia (Griseb.) E. Gagnon et al. | Gagnon & Atchison EG211 (MT) | Argentina | KF522159 | KP003759 | KP003706 | KP003653 | - | KX176809 |
| *Arquita mimosifolia (Griseb.) E. Gagnon et al. | Särkinen et al. 2006 (FHO) | Argentina | KF522161 | KP003761 | KP003708 | KP003655 | KX373124 | KX176837 |
| *Arquita mimosifolia (Griseb.) E. Gagnon et al. | Chumley 7387 (TEX) | Argentina | - | - | - | AY549893 | AY535818-AY535805 | - |
| Arquita ancashiana (Ulibarri) E. Gagnon et al. | Hughes et al. 3021 (MT, Z) | Peru | KF522164 | KP003747 | KP003696 | KP003643 | - | KX176804 |
| Arquita ancashiana (Ulibarri) E. Gagnon et al. | Hughes et al. 3070 (MT, Z) | Peru | KF522167 | KP003749 | KP003698 | KP003644 | - | - |
| Arquita ancashiana (Ulibarri) E. Gagnon et al. | Lewis & Klitgaard 2266 (K) | Ecuador | KF522170 | KP003753 | KP003792 | KP003647 |
KX373114
|
- |
| Arquita celendiniana (G.P. Lewis & C.E. Hughes) E. Gagnon et al. | Hughes et al. 2210 (FHO) | Peru | KF522148 | KP003756 | KP003703 | KP003650 | KX373092 | KX176805 |
| Arquita celendiniana (G. P. Lewis & C.E. Hughes) E. Gagnon et al. | Hughes et al. 3097 (MT, Z) | Peru | KF522149 | KP003757 | KP003704 | KP003651 | - | KX176823 |
| Arquita celendiniana (G. P. Lewis & C.E. Hughes) E. Gagnon et al. | Hughes et al. 3102 (MT, Z) | Peru | KF522147 | KO003758 | KP003705 | KP003652 | - | KX176824 |
| Arquita celendiniana (G.P. Lewis & C.E. Hughes) | Pennington 17567 (E) | Peru | - | - | - | KX372914 | - | - |
| Arquita trichocarpa (Griseb.) E. Gagnon et al. var. trichocarpa | Lewis & Klitgaard 2166 (K) | Argentina | KF522163 | KP003762 | KP003709 | KP003659 | AF430740 | KX176828 |
| Arquita trichocarpa var. boliviana E. Gagnon et al. | Hughes et al. 2442 (FHO) | Bolivia | KF522162 | KP003764 | KP003711 | KP003657 | - | KX176833 |
| Arquita grandiflora E. Gagnon et al. | Särkinen et al. 2225 (FHO) | Peru | KF522151 | KP003763 | KP003710 | KP003656 | - | KX176811 |
| Balsamocarpon Clos (1/1 species) | ||||||||
| *Balsamocarpon brevifolium Clos | Baxter et al. DCI 1869 (E) | Chile | KF522135 | KP003801 | KP003743 | KP003689 | EU361739. | KX176815 |
| *Balsamocarpon brevifolium Clos | Taylor 745 (K) | Chile | KF522136 | KX379415 | KX373043 | KX372915 | - | - |
| *Balsamocarpon brevifolium Clos | Coccuci & Sérsic 365 (CORD) | Chile | - | - | - |
AY308548
|
JX219457 | AF430761 |
| Caesalpinia L. sensu Lewis (2005; 21/~25 species) | ||||||||
| *Caesalpinia brasiliensis L. | Léonard & Léonard 13904 (US, K) | Haiti | KF522092 | KX379366 | KX373030 | KX372861 | - | - |
| Caesalpinia anacantha Urb. | Liogier 16639 (P) | Dominican Republic | KX373127 | KX379263 | - | KX372859 | - | - |
| Caesalpinia bahamensis Lam. | Baker B27 (K) | Bahamas | KF522091 | KX379367 | - | KX372862 | - | - |
| Caesalpinia bahamensis Lam. | Michael 8975 (MEXU) | Bahamas | KF522093 | - | - | - | - | - |
| Caesalpinia barahonensis Urb. | Ekman 5965 (K) | Haiti | KF522094 | KX379365 | - | KX372860 | - | - |
| Caesalpinia bracteata Germish. | van Hoepen 2018 (K) | South Africa | KF522258 | KX379345 | KX372952 | KX372784 | - | - |
| Caesalpinia buchii Urb. | Acevedo-Rodriguez et al. 8522 (K, US) | Dominican Republic | KF522115 | KX379341 | KX373021 | KX372870 | - | - |
| Caesalpinia buchii Urb. | Ekman 8491 (K) | Haiti | - | KX379258 | - | - | - | - |
| Caesalpinia cassioides Willd. | Hughes et al. 2023 (FHO) | Peru | KF522097 | KX379358 | KX373036 | KX372855 | - | - |
| Caesalpinia cassioides Willd. | Hughes et al. 2228 (FHO) | Peru | KF522098 | KX379359 | KX373035 | KX372856 | - | - |
| Caesalpinia cassioides Willd. | Hughes et al. 2641 (FHO) | Peru | KF522095 | KX379360 | KX373033 | KX372857 | - | - |
| Caesalpinia cassioides Willd. | Pennington et al. 789 (E) | Peru | KF522096 | KX379361 | KX373034 | KX372858 | KX373102 | - |
| Caesalpinia cassioides Willd. | Lewis et al. 3281 (K) | Ecuador | - | - | - | - | AF430711 | - |
| Caesalpinia dauensis Thulin | Gilbert et al. 7695 (K) | Ethiopia | KF522266 | KX379346 | KX372950 | KX372788 | - | - |
| Caesalpinia erianthera Chiov. | Friis et al. 4698 (K) | Somalia | KF522123 | KX379333 | KX373023 | KX372878 | - | - |
| Caesalpinia erianthera Chiov. | Radcliffe-Smith 5518 (K) | Oman | KF522122 | KX379335 | KX373026 | KX372877 | - | - |
| Caesalpinia erianthera Chiov. | Thulin & Mohamed 6941 (K) | Somalia | KF522125 | KX379332 | KX373024 | KX372879 | - | - |
| Caesalpinia erianthera Chiov. var. erianthera | Thulin 5557 (K) | Somalia | KF522118 | - | - | - | - | - |
| Caesalpinia erianthera var. pubescens Brenan | Boulos et al. 17000 (K) | Yemen | KF522117 | KX379334 | KX373025 | KX372876 | - | - |
| Caesalpinia glandulosopedicellata R. Wilczek | Bamps & Malaisse 8647 (K) | Zaire | KF522261 | KX379343 | KX372953 | KX372787 | KX373118 | KX176838 |
| Caesalpinia madagascariensis (R.Vig) Senesse | Bruneau 1348 (MT) | Madagascar | KF522119 | KX379330 | KX373027 | KX372874 | - | KX176834 |
| Caesalpinia madagascariensis (R.Vig) Senesse | Lewis et al. 2158 (K) | Madagascar | KF522120 | KX379331 | KX373028 | KX372875 | KX373096 | - |
| Caesalpinia nipensis Urb. | Marie-Victorin et al. 21500 (MT) | Cuba | - | KX379414 | - | - | - | - |
| Caesalpinia nipensis Urb. | Marie-Victorin et al. 21509 (MT) | Cuba | KX373129 | KX379267 | - | KX372865 | - | - |
| Caesalpinia nipensis Urb. | Lewis 1838 (K) | Cuba | KX373128 | KX379413 | KX372980 | KX372864 | - | KX176835 |
| Caesalpinia oligophylla Harms. | Hassan 70 (FHO, K) | Somalia | KF522262 | KX379348 | - | KX372786 | - | - |
| Caesalpinia pauciflora (Griseb.) C. Wright | Ekman 9703 (K) | Cuba | KF522124 | KX379338 | KX373020 | KX372872 | - | - |
| Caesalpinia pauciflora (Griseb.) C. Wright | Liogier & Liogier 20521 (NY) | Hispaniola | KF522116 | KX379340 | KX373022 | - | KX373097 | - |
| Caesalpinia pauciflora (Griseb.) C. Wright | Lewis 1854 (K) | Cuba, cultivated | - | KX379339 | - | KX372873 | - | - |
| Caesalpinia pulcherrima (L.) Sw. | Cox 1, RBG Liv.Coll. 1975-3028 (K) | United Kingdom, cultivated | KF522174 | - | - | - | - | - |
| Caesalpinia pulcherrima (L.) Sw. | Fougère-Danezan 19 (MT) | Singapore, cultivated | KF522172 | KX379363 | KX373031 | KF379227 | KX373109 | KX176820 |
| Caesalpinia pulcherrima (L.) Sw. | Lewis & Hughes 1715 (K) | Guatemala | KF522171 | KX379362 | - | KX372853 | AF430733 | - |
| Caesalpinia pulcherrima (L.) Sw. | Montreal Botanical Garden 7089-92 (MT) | Canada, cultivated | KF522173 | KX379364 | KX373032 | KX372854 | - | - |
| Caesalpinia reticulata Britton | Pollard et al. 1295 (K) | Turks & Caicos Islands | KX373130 | KX379368 | - | KX372863 | - | - |
| Caesalpinia rosei Urb. | Ekman H13620 (K, TEX) | Dominican Republic | KX373131 | KX379342 | - | KX372871 | AF430735 | - |
| Caesalpinia rostrata N.E. Br. | ILC6-5 (PRE) | South Africa, cultivated | KX373132 | KX379259 | KX372951 | KX372785 | KX373116 | - |
| Caesalpinia rubra (Engl.) Brenan | de Winter 3164 (K) | South Africa | KF522260 | - | - | - | - | - |
| Caesalpinia rubra (Engl.) Brenan | Oshikoto 1917BD (K) | Namibia | KF522259 | - | - | - | - | - |
| Caesalpinia sessilifolia S. Watson | Palmer 533 (K, MO) | Mexico | KX373133 | KX379336 | KX373018 | KX372868 | - | - |
| Caesalpinia sessilifolia S. Watson | Neff 8-24-91-4 (TEX) | Mexico | - | - | - | - | AF430737 | - |
| Caesalpinia sessilifolia S. Watson | Hinton 24737 (MEXU) | Mexico | KF522121 | - | - | - | - | - |
| Caesalpinia stuckertii Hassl. | Beck 9443 (NY) | Bolivia | KF522126 | KX379337 | KX373019 | KX372869 | KX373095 | - |
| Caesalpinia stuckertii Hassl. | Krapovickas 4626 (K) | Argentina | KF522127 | - | - | - | - | - |
| Caesalpinia trothae subsp. erlangeri (Harms) Brenan | Beckett & White 1711 (K) | Somalia | KF522263 | KX379349 | KX372948 | KX372789 | - | KX176812 |
| Caesalpinia trothae subsp. erlangeri (Harms) Brenan | Thulin & Warfa 5816 (K) | Somalia | KF522267 | KX379344 | - | - | - | - |
| Caesalpinia trothae subsp. erlangeri (Harms) Brenan | Vollesen & Hassan 4873 (K) | Somalia | KF522264 | - | - | - | - | - |
| Caesalpinia trothae Harms subsp. trothae | Bidgood et al. 559 (K) | Tanzania | KF522265 | KX379350 | KX372949 | - | KX373117 | KX176829 |
| Caesalpinia trothae Harms subsp. trothae | Gillett 21088 (K) | Kenya | - | KX379347 | - | KX372790 | - | - |
| Cenostigma Tul. (2/2 species) | ||||||||
| *Cenostigma macrophyllum Tul. | Coradin et al. 6306 (K) | Brazil, Bahia | KF522053 | KX379446 | KX372981 | - | - | - |
| *Cenostigma macrophyllum Tul. | Thomas 9615 (K) | Brazil, Piaui | KF522069 | - | KX372991 | - | - | - |
| *Cenostigma macrophyllum Tul. | de Queiroz 9147 (HUEFS) | Brazil, Bahia | KF522037 | KX379447 | KX372982 | - | - | - |
| *Cenostigma macrophyllum Tul. | Ferreira et al. 6371 (MBG) | From GenBank | - | - | - | - | JX073262 | - |
| Cenostigma tocantinum Ducke | Klitgaard & de Lima 88 (K) | Brazil, cultivated | KF522071 | KP003803 | KP003740 | KP003694 | - | KX176806 |
| Cenostigma tocantinum Ducke | Klitgaard s.n. (INPA) | Brazil | KF522070 | KX379453 | KX372992 | KX372835 | - | - |
| Cenostigma gardnerianum Tul. (synonym of Cenostigma macrophyllum) | (retrieved from GenBank) | Brazil | - | - | - | DQ787400 | - | - |
| Cordeauxia Hemsl. (1/1 species) | ||||||||
| *Cordeauxia edulis Hemsl. | Gillett & Beckett 23305 (K) | Somalia | KF522083 | - | - | - | - | - |
| *Cordeauxia edulis Hemsl. | Hassan 232 (FHO, K) | Somalia | AY899748 | - | - | - | AY899690 | - |
| *Cordeauxia edulis Hemsl. | Kuchar 17803 (K) | Somalia | KF522084 | KX379430 | KX372998 | KX372826 | EU361787 | EU361920 |
| *Cordeauxia edulis Hemsl. | Annable & Collins 3541 (NY) | Hawaii, cultivated | - | - | - | - | AF430771 | - |
| Coulteria Kunth (7/9-10 species) | ||||||||
| *Coulteria mollis Kunth | Way NMLW 28 (K) | Venezuela | KF522187 | KX379403 | KX373051 |
KX372887
|
- | - |
| Coulteria platyloba (S. Watson) N. Zamora | Gagnon & Marazzi, EG2010.007 (MT) | USA, cultivated | KF522175 | KX379407 | KX373057 | KX372894 | - | - |
| Coulteria platyloba (S. Watson) N. Zamora | Espinoza, BioBot01994 BOLD rec.: MHPAF1646-11 |
Costa Rica | - | - | - | - | - | JQ587526 |
| Coulteria platyloba (S. Watson) N. Zamora | Lorea Lozada 685 (MEXU) | Mexico | KF522183 | - | - | - | - | - |
| Coulteria platyloba (S. Watson) N. Zamora | MacQueen 178 (K) | Mexico | KF522178 | KX379410 | KX373059 | KX372892 | - | - |
| Coulteria platyloba (S. Watson) N. Zamora | Steinmann 3199 (INIREB, K) | Mexico | KF522184 | - | - | - | - | - |
| Coulteria platyloba (S. Watson) N. Zamora | Espinoza, BioBot01995 BOLD rec.: MHPAF1647-11 |
Costa Rica | - | - | - | - | - |
JQ587527 |
| Coulteria platyloba (S. Watson) N. Zamora | Espinoza, BioBot01996 BOLD rec.: MHPAF1648-11 |
Costa Rica | - |
- |
- |
- |
- |
JQ587528 |
| Caesalpinia colimensis F. J. Herm. | Sousa 6163 (K) | Mexico | KF522176 | KX379409 | KX373058 | KX372893 | - | - |
| Caesalpinia colimensis F.J. Herm. | Sousa 7659 (TEX) | Mexico | - | - | - | - | AF430713 | - |
| Caesalpinia pringlei (Britton & Rose) Standl. | Cruz Duran 926 (MEXU) | Mexico | KF522180 | - | - | - | - | - |
| Caesalpinia pringlei (Britton & Rose) Standl. | Panero 4037 (TEX) | Mexico | - | - | - | - | AF430732 | - |
| Caesalpinia pumila (Britton & Rose) F.J.Herm. | Gagnon & Marazzi EG 2010.014 (MT) | USA, cultivated | KF522182 | KX379405 | KX373054 | KX372889 | - | - |
| Caesalpinia pumila (Britton & Rose) F.J.Herm. | Lewis et al. 2067 (K) | Mexico | KF522177 | KX379406 | KX373055 | KF379234 | KF379385 | KX176832 |
| Caesalpinia pumila (Britton & Rose) F.J.Herm. | Nabhan et al. 1988 (MEXU) | Mexico | KF522185 | - | - | - | - | - |
| Caesalpinia pumila (Britton & Rose) F.J.Herm. | Cavan 5535 (TEX) | Mexico | - | - | - | - | AF430720 | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Hughes et al. 2087 (FHO) | Mexico | KF522189 | KX379404 | KX373053 | KX372888 | - | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Lewis 1797 (NY) | Mexico | KF522179 | KX379408 | KX373056 | KX372891 | - | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Tenorio 296 (MEXU) | Mexico | KF522191 | - | - | - | - | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Torres 1590 (MEXU) | Mexico | KF522186 | - | - | - | - | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Way et al. JIC 22176 (K) | Mexico | KF522190 | KX379402 | KX373052 | KX372890 | - | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Hughes 255 (FHO) | Guatemala | AY899752 | - | - | - | AY899694 | - |
| Caesalpinia velutina (Britton & Rose) Standl. | Torres 10741 (K) | Mexico | - | - | - | - | AF430741 | - |
| Caesalpinia violacea (Mill.) Standl. | Lewis et al. 1763 (NY) | Mexico | KF522188 | KX379401 | KX373050 | - | KX373100 | JX099334 |
| Caesalpinia violacea (Mill.) Standl. | Tenorio 4442 (MEXU) | Mexico | KF522181 | - | - | - | - | - |
| Erythrostemon (Hook.) Klotzsch (9/9 species) | ||||||||
| *Erythrostemon gilliesii Klotzsch | Marazzi et al. BM131 (CTES, Z) | Argentina | AM086914 | - | - | - | - | AM086829 |
| *Erythrostemon gilliesii Klotzsch | Spellenberg 12701 (MT) | USA, cultivated | KF522296 | KP003786 | KP003729 | KP003681 | JX073265 | JX099328 |
| *Erythrostemon gilliesii Klotzsch | Jodrell 688-86 (K) | Chile | - | - | - | AY549891 | AF430721 | - |
| *Erythrostemon gilliesii Klotzsch | Wojciechowski 882 (ASU) | USA | - | - | - | - | - | AY386845 |
| *Erythrostemon gilliesii Klotzsch | Hick & Bertone 34 (CORD) | Argentina | - | - | - | - | JX219458 | - |
| Erythrostemon calycinus (Benth) L.P. Queiroz | Giulietti 2045 (HUEFS) | Brazil | KF522304 | KX379278 | KX373075 | - | - | - |
| Erythrostemon calycinus (Benth) L.P. Queiroz | Lewis & Andrade 2003 (K) | Brazil | AY899749 | - | - | - | KX373110 | - |
| Erythrostemon calycinus (Benth) L.P. Queiroz | Lewis & Andrade 1885 (K) | Brazil | KF522303 | - | KX373074 | KX372895 | AF430710 | EU361899 |
| Caesalpinia angulata (Hook & Arn.) Baill. | Brownless et al. 591 (E) | Chile | KF522288 | KX379303 | KX373080 | KX372902 | - | - |
| Caesalpinia angulata (Hook & Arn.) Baill. | Nee 37585 (K) | Chile | KF522287 | - | - | - | - | - |
| Caesalpinia argentina Burkart | Hughes et al. 2460 (FHO) | Bolivia | KF522289 | KX379309 | KX373088 | KX372908 | - | - |
| Caesalpinia argentina Burkart | Pennington et al. 13323 (K) | Bolivia | KF522290 | KX379310 | KX373089 | KX372909 | - | - |
| Caesalpinia caudata (A. Gray) Fisher | Simpson I-IV-01-3 (TEX) | USA | KF522298 | KX379277 | KX373084 | - | - | - |
| Caesalpinia caudata (A. Gray) Fisher | Neff 99-3-16-1(TEX) | USA | - | - | - | - | AF430712 | - |
| Caesalpinia coluteifolia Griseb. | Gagnon et al. EG207 (MT) | Argentina | KF522291 | KX379311 | KX373090 | KX372903 | - | - |
| Caesalpinia coluteifolia Griseb. | Gagnon & Atchison EG223 (MT) | Argentina | KF522292 | KX379312 | KX373091 | KX372904 | - | - |
| Caesalpinia coulterioides Griseb. emend Burkart | Gagnon & Atchison EG209 (MT) | Argentina | KF522285 | KX379308 | KX373081 | KX372910 | - | - |
| Caesalpinia exilifolia Griseb. | Gagnon et al. EG201 (MT) | Argentina | KF522295 | KX379307 | KX373085 | KX372907 | - | - |
| Caesalpinia exilifolia Griseb. | Gagnon & Atchison EG219 (MT) | Argentina | KF522293 | KX379306 | KX373086 | KX372906 | - | - |
| Caesalpinia exilifolia Griseb. | Gagnon & Atchison EG222 (MT) | Argentina | KX373134 | KX379257 | KX373087 | KX372905 | - | - |
| Caesalpinia exilifolia Griseb. | Galleto 167 (CORD) | Argentina | - | - | - | - | AF430716 | - |
| Caesalpinia fimbriata Tul. | Hughes et al. 2441 (FHO) | Bolivia | KF522284 | KP003785 | KP003728 | KP003680 | - | - |
| Caesalpinia cf. fimbriata Tul. | Hughes et al. 2466 (FHO) | Bolivia | KF522286 | KX379304 | KX373082 | KX372911 | - | - |
| Caesalpinia fimbriata Tul. | Wood 10627 (K) | Bolivia | KF522211 | - | - | - | - | - |
| Caesalpinia fimbriata Tul. | Solomon & Nee 16062 (NY) | Bolivia | KF522297 | KX379305 | KX373083 | KX372912 | KX373111 | - |
| Guilandina L. (6/7-18 species) | ||||||||
| *Guilandina bonduc L. | Bruneau 1342 (MT) | Madagascar | KF522062 | KX379370 | KX372967 | KX372797 | - | KX176816 |
| *Guilandina bonduc L. | van Balooy s.n., Krukoff coll. (K) | Malaysia | KF522063 | - | - | - | AF430708 | - |
| *Guilandina bonduc L. | Herendeen 9-XII-97-3 (US) | Tanzania | - | - | - | KF379229 | KX373103 | - |
| *Guilandina bonduc L. | Espinoza, BioBot02010 BOLD rec.: MHPAF1662-11 |
Costa Rica | - | - | - | - | - | JQ587518 |
| *Guilandina bonduc L. | Espinoza, BioBot02011 BOLD rec.: MHPAF1663-11 |
Costa Rica | - | - | - | - | - | JQ587519 |
| *Guilandina bonduc L. | Espinoza, BioBot02012 BOLD rec.: MHPAF1664-11 |
Costa Rica | - | - | - | - | - | JQ587520 |
| Guilandina ciliata Wikstr. | Ekman 5413 (K) | Haiti | KX373125 | - | - | - | - | - |
| Guilandina ciliata Wikstr. | Walker 51 (K) | British Virgin Islands | KX373126 | KX379372 | - | KX372798 | - | - |
| Guilandina major L. | Herendeen & Pooma 30-IV-1999-1 (US) | USA, cultivated | KF522253 | KX379374 | KX372965 | - | KX373104 | - |
| Caesalpinia minax Hance | Li Shi Jin 802 (CAS, IBSC) | China | KF522131 | KX379369 | - | KX372926 | - | - |
| Caesalpinia minax Hance | Living collection National Botanic Garden of Belgium 19645275(BR) | China, cultivated | KF522132 | - | - | - | - | - |
| Caesalpinia minax Hance | PS1368MT01, GenBank | N/A | - | - | - | GU217664 | - | HM049550 |
| Caesalpinia murifructa Gillis & Proctor | Gillis 13096 (K) | Bahamas | KF522064 | KX379373 | KX372966 | KX372799 | - | - |
| Caesalpinia volkensii Harms | Archbold 2861 (K) | Tanzania | KF522065 | - | - | - | - | - |
| Caesalpinia volkensii Harms | Friis et al. 3516 (K) | Ethiopia | KF522066 | KX379375 | KX372968 | KX372800 | - | - |
| Caesalpinia volkensii Harms | Somers s.n., RBG Liv.Coll. 1978-891 (K) | Kenya | KF522067 | KX379371 | KX372969 | KX372801 | - | - |
| Haematoxylum L. (3/5 species) | ||||||||
| *Haematoxylum campechianum L. | Bruneau 1313 (MT) | Mexico | KF522200 | KX379329 | KX373039 | - | - | - |
| *Haematoxylum campechianum L. | du Puy et al. M356 (K) | Madagascar | KF522208 | - | - | - | - | - |
| *Haematoxylum campechianum L. | Hughes 1273 (FHO) | Guatemala | AY899754 | - | - | - | AY899697 | - |
| *Haematoxylum campechianum L. | Miller & Morello 8849 (MO) | Dominica | KF522201 | KX379328 | KX373038 | KX372832 | - | - |
| Haematoxylum brasiletto H. Karst. | Bernandes et al. 891 (MO) | Colombia | KF522209 | KX379325 | KX373042 | KX372831 | - | - |
| Haematoxylum brasiletto H. Karst. | Gagnon & Marazzi EG2010.011 (MT) | USA, cultivated | KF522207 | KX379327 | KX373041 | KX372834 | - | - |
| Haematoxylum brasiletto H. Karst. | Gagnon & Marazzi EG2010.013 (MT) | USA, cultivated | KF522206 | KX379326 | KX373040 | KX372833 | - | - |
| Haematoxylum brasiletto H. Karst. | Haston V200307 (RNG), OFI 14/83 (OFI) | Mexico | - | - | - | - | AY899696 | - |
| Haematoxylum brasiletto H. Karst. | Wojciechowski 953 (ASU) | USA | - | - | - | - | - | AY386905 |
| Haematoxylum brasiletto H. Karst. | Lewis et al. 2057 (FHO) | Mexico | AY899753 | - | - | - | AY899695 | - |
| Haematoxylum brasiletto H. Karst. | Simpson 17-XI-97 (TEX) | USA | - | - | - | - | AF430777 | - |
| Haematoxylum dinteri Harms | Sucheach s.n. (OFI), Haston V200308 (RNG) | Namibia | AY899755 | - | - | - | AY899698 | - |
| Haematoxylum dinteri Harms | Millennium seed bank project, HK2728 (K) | Namibia | KX373135 | KX379324 | KX373037 | KX372830 | - | - |
| Hoffmannseggia Cav. (24/24 species) | ||||||||
| *Hoffmannseggia glauca (Ortega) Eifert | Gagnon & Marazzi EG2010.05 (MT) | USA | KF522214 | KX379318 | KX372941 | KX372792 | - | - |
| *Hoffmannseggia glauca (Ortega) Eifert | Gagnon & Marazzi EG2010.19 (MT) | USA | KF522212 | KX379319 | KX372940 | KX372793 | - | - |
| *Hoffmannseggia glauca (Ortega) Eifert | Wojciechowski 1501 (ASU) | USA | - | - | - | - | - | JQ619977 |
| *Hoffmannseggia glauca (Ortega) Eifert | Spellenberg 12699 (MT) | USA | KF522213 | KP003796 | KP003744 | KP003690 | AF365069 | EU361969 |
| *Hoffmannseggia glauca (Ortega) Eifert | Hick & Bertone 5 (CORD) | Argentina | - | - | - | - | JX219459 | JX219465 |
| *Hoffmannseggia glauca (Ortega) Eifert | Cocucci 15-VI-1991 (CORD) | Argentina | - | - | - | X | AF430747 | - |
| *Hoffmannseggia glauca (Ortega) Eifert | Simpson 91-VII-22-1 (TEX) | Mexico | - | - | - | X | AY308488 | - |
| Hoffmannseggia aphylla (Phil.) G.P. Lewis & Sotuyo | Gardner & Knees 6503 (E) | Chile | KF522146 | KX379314 | KX372937 | KX372923 | - | - |
| Hoffmannseggia aphylla (Phil.) G.P. Lewis & Sotuyo | Gardner & Knees 6557 (E) | Chile | KF522144 | - | - | - | - | - |
| Hoffmannseggia arequipensis Ulibarri | Simpson 20-II-00-1 (TEX) | Peru | - | - | - | AY308550 | AY308483 | - |
| Hoffmannseggia arequipensis Ulibarri | Simpson 20-II-00-2 (TEX) | Peru | - | - | - | AY308551 | AY308484 | - |
| Hoffmannseggia doelli Philippi | Simpson 11-II-00-2 (TEX) | Chile | - | - | - | AY308552 | AY308485 | - |
| Hoffmannseggia doelli subsp. argentina Ulibarri | Gagnon & Atchison EG220 (MT, K) | Argentina | KX373136 | KX379320 | KX372943 | KX372791 | - | - |
| Hoffmannseggia drepanocarpa A. Gray | Simpson 29-V-89 (TEX) | Mexico | - | - | - | AY308553 | AF430745 | - |
| Hoffmannseggia drummondii Torr. & A. Gray | Simpson 05-15-92-2 (TEX) | Mexico | - | - | - | AY308554 | AF430747 | - |
| Hoffmannseggia erecta Philippi | Chumley 7379 (TEX) | Argentina | - | - | - | AY308555 | AY308486 | - |
| Hoffmannseggia eremophila (Phil.) Ulibarri | Aranoi & Sequeo 10334 (CORD) | Chile | - | - | - | AY308556 | AY308487 | - |
| Hoffmannseggia humilis (M. Martens & Galeotti) Hemsl. | Mayfield et al. 898 (TEX) | Mexico | - | - | - | AY308559 | AF430748 | - |
| Hoffmannseggia intricata Brandegee | Irwin 2371 (TEX) | Mexico | - | - | - | AY308560 | AY308489 | - |
| Hoffmannseggia microphylla Torr. | Holmgren 6505 (NY) | USA | KF522145 | KX379315 | KX372938 | KX372920 | - | - |
| Hoffmannseggia microphylla Torr. | Simpson 03-15-03-1 (TEX) | Mexico | - | - | - | AY308561 | AF430749 | - |
| Hoffmannseggia minor (Phil.) Ulibarri | Simpson 1-II-00-9 (TEX) | Argentina | - | - | - | AY308562 | AY308534 | - |
| Hoffmannseggia miranda Sandwith | FLSP 945 (NY) | Peru | KF522239 | KX379321 | KX372946 | KX372796 | - | - |
| Hoffmannseggia miranda Sandwith | Hughes & Daza 2358 (FHO) | Peru | KF522240 | KX379322 | KX372945 | KX372795 | - | - |
| Hoffmannseggia miranda Sandwith | Simpson 22-II-00-2 (TEX) | Peru | - | - | - | AY308565 | AY308492 | - |
| Hoffmannseggia miranda Sandwith | Simpson 21-II-00-1 (TEX) | Peru | - | - | - | AY308564 | AY308491 | - |
| Hoffmannseggia miranda Sandwith | Dillon & Dillon 3958 (F) | Peru | - | - | - | AY308563 | AF430750 | - |
| Hoffmannseggia oxycarpa Benth. subsp. arida (Rose) B. B. Simpson | Simpson 91-VII-21-2 (TEX) | Mexico | - | - | - | AY308566 | AF430751 | - |
| Hoffmannseggia peninsularis (Britton) Wiggins | Simpson 03-15-93-5 (TEX) | Mexico | - | - | - | AY308567 | AF430752 | - |
| Hoffmannseggia prostrata DC. | Hughes & Daza 2359 (FHO) | Peru | KF522241 | KX379323 | KX372944 | KX372794 | - | - |
| Hoffmannseggia prostrata DC. | Dillon & Dillon 5926 (F) | Chile | - | - | - | AY308568 | AF430753 | - |
| Hoffmannseggia pumilio (Griseb.) B.B. Simpson | Gagnon & Atchison EG221 (MT, K) | Argentina | KX373137 | KX379268 | - | KX372919 | - | - |
| Hoffmannseggia pumilio (Griseb.) B.B. Simpson | Simpson 1-II-00-1 (TEX) | Argentina | - | - | - | AY308549 | AF430791 | - |
| Hoffmannseggia repens (Eastw.) Cockerell | Simpson 27-V-89-7 (TEX) | USA | - | - | - | AY308569 | AF430755 | - |
| Hoffmannseggia tenella Tharp & L.O. Williams | Neff 4-XI-88 (TEX) | USA | - | - | - | AY308570 | AF430755 | - |
| Hoffmannseggia ternata DC. | Dillon & Dillon 3746 (F) | Peru | - | - | - | AY308571 | AF430756 | - |
| Hoffmannseggia ternata DC. | Simpson 22-II-00-1 (TEX) | Peru | - | - | - | AY308574 | AY308495 | - |
| Hoffmannseggia ternata DC. | Simpson 15-II-00-1 (TEX) | Chile | - | - | - | AY308572 | AY308493 | - |
| Hoffmannseggia ternata DC. | Simpson 21-II-00-2 (TEX) | Peru | - | - | - | AY308573 | AY308494 | - |
| Hoffmannseggia ternata DC. | Simpson 22-II-00-3 (TEX) | Peru | KF522139 | - | - | AY308575 | AY308496 | - |
| Hoffmannseggia trifoliata Cav. | Simpson 21-I-00-3A (TEX) | Argentina | - | - | - | AY308576 | AY308497 | - |
| Hoffmannseggia viscosa Hook.& Arn. | Eastwood et al. RJE35 (FHO) | Peru | KF522138 | KX379317 | KX372942 | KX372924 | - | - |
| Hoffmannseggia viscosa Hook.& Arn. | Hughes et al. 2221 (FHO) | Peru | KF522137 | KX379316 | KX372939 | KX372925 | - | - |
| Hoffmannseggia viscosa Hook.& Arn | Sagastegui 11465 (MO) | Peru | - | - | - | AY308577 | AY308498 | - |
| Hoffmannseggia viscosa Hook.& Arn | Richardson 2039 | Peru | - | - | - | AY308578 | AY308499 | - |
| Hoffmannseggia watsonii (Fisher) Rose | Hunter 25354 (TEX) | Mexico | - | - | - | AY308579 | AY308500 | - |
| Hoffmannseggia yaviensis Ulibarri | Simpson 30-I-00-1 (TEX) | Argentina | - | - | - | AY308580 | AY308501 | - |
| Libidibia (DC.) Schltdl. (6/6-8 species) | ||||||||
| *Libidibia coriaria (Jacq.) Schltdl. | Fougère-Danezan 20 (MT) | Singapore, cultivated | KF522109 | KX379423 | KX373008 | - | - | - |
| *Libidibia coriaria (Jacq.) Schltdl. | Hughes 1495 (K) | Mexico | AY899750 | - | - | - | AY899692 | - |
| *Libidibia coriaria (Jacq.) Schltdl. | Hughes et al. 2163 (FHO) | Mexico | KF522107 | KP003797 | KP003745 | KP003691 | - | |
| *Libidibia coriaria (Jacq.) Schltdl. |
Espinoza, BioBot00788 BOLD rec.: MHPAD924-09 |
Costa Rica | - | - | - | - | - | JQ587521 |
| *Libidibia coriaria (Jacq.) Schltdl. | Espinoza, BioBot00789 BOLD rec.: MHPAD925-09 |
Costa Rica | - | - | - | - | - | JQ587522 |
| *Libidibia coriaria (Jacq.) Schltdl. | Espinoza, BioBot00790 BOLD rec.: MHPAD926-09 |
Costa Rica | - | - | - | - | - | JQ587523 |
| Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz | Fougère-Danezan 21 (MT) | Singapore, cultivated | KF522105 | KX379425 | KX373014 | - | JX073260 | EU361901 |
| Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz | Lewis et al. 1623 (K) | Brazil | KF522114 | - | - | - | - | - |
| Libidibia ferrea (Mart. ex Tul.) L.P. Queiroz | Kew Living coll. 1973-21715 (K) | Brazil | - | - | - | - | AF430718 | - |
| Libidibia glabrata (Kunth) C.Cast. & G.P. Lewis | Delgado 2097 (MEXU) | Peru | KF522103 | - | - | - | - | - |
| Libidibia glabrata (Kunth) C.Cast. & G.P. Lewis | Eastwood et al. RJE84 (FHO) | Peru | KF522102 | KX379427 | KX373010 | KX372913 | - | - |
| Libidibia glabrata (Kunth) C.Cast. & G.P. Lewis | Lewis & Lozano 3043 (K) | Ecuador | KF522101 | KX379428 | KX373012 | - | KX373101 | - |
| Libidibia glabrata (Kunth) C.Cast. & G.P. Lewis | Särkinen et al. 2151 (FHO) | Peru | KF522104 | KX379429 | KX373011 | - | - | - |
| Libidibia glabrata (Kunth) C.Cast. & G.P. Lewis | Lewis & Klitgaard 3337 (K) | Ecuador | - | - | - | - | AF430722 | - |
| Libidibia paraguariensis (Parodi) G.P. Lewis | Hughes et al. 2307 (FHO) | Bolivia | KF522110 | KX379420 | KX373006 | - | - | - |
| Libidibia paraguariensis (Parodi) G.P. Lewis | Hughes et al. 2475 (FHO) | Bolivia | KF522111 | KX379421 | KX373007 | - | - | - |
| Libidibia paraguariensis (Parodi) G.P. Lewis | Lewis & Klitgaard 2170 (K) | Argentina | KF522112 | KX379419 | KX373005 | KF379233 | KX373119 | EU361905 |
| Libidibia paraguariensis (Parodi) G.P. Lewis | Zardini & Velazquez 19907 (K) | Paraguay | KF522113 | - | - | - | - | - |
| Libidibia punctata (Willd.) Britton | Cardenas 4071 (K) | Venezuela | KF522106 | KX379424 | KX373015 | - | - | - |
| Libidibia sclerocarpa (Standl.) Britton & Rose | Lewis & Hughes 1778 (K) | Mexico | KF522108 | KX379426 | KX373013 | - | - | - |
| Libidibia sclerocarpa (Standl.) Britton & Rose | Kew seed collection s.n. | Mexico | - | - | - | - | AF430736 | - |
| Lophocarpinia Burkart (1/1 species) | ||||||||
| *Lophocarpinia aculeatifolia (Burkart) Burkart | Fortunato 8639 (BAB) | Argentina | - | - | - | - | JX219460 | JX219466 |
| Mezoneuron Desf. (11/26 species) | ||||||||
| Mezoneuron andamanicum Prain | Herendeen 29-IV-1999-1 (US) | Thailand | KF522305 | - | KX372957 | KX372815 | - | AY386931 |
| Mezoneuron angolense Welw. ex Oliv. | Herendeen 12-XII-97-1 (US) | Tanzania | - | - | - | - | AF365068 | EU361897 |
| Mezoneuron benthamianum Baill. | Ern 2602 (K) | Togo | KF522196 | KX379388 | KX372960 | KX372818 | - | - |
| Mezoneuron benthamianum Baill. | Morton & Jarr SL3295 (K) | Sierra Leone | KF522195 | KX379387 | KX372959 | KX372817 | - | - |
| Mezoneuron benthamianum Baill. | Vigne 3487 (FHO) | Ghana | KF522197 | - | - | - | - | - |
| Mezoneuron cucullatum (Roxb.) Wight & Arn. | Grierson & Long 3623 (K) | Bhutan | KF522194 | KX379266 | - | KX372819 | - | - |
| Mezoneuron deverdiana Guillaumin | McPherson 6211 (K) | New Caledonia | KF522078 | - | - | - | - | - |
| Mezoneuron hildebrandtii Vatke | Lewis et al. 2137 (K) | Madagascar | KF522198 | KX379386 | KX372958 | KX372816 | KX373107 | KX176807 |
| Mezoneuron hildebrandtii Vatke | Simpson 17-XI-97 (TEX) | Madagascar | - | - | - | - | AF430780 | - |
| Mezoneuron hymenocarpum Prain | Larsen & Larsen 34232 (K) | Thailand | - | KX379390 | KX372956 | KX372820 | - | - |
| Mezoneuron kauaiensis (H. Mann) Hillbr. | Lorence & Wagner 8904 (NTBG) | Hawaii, USA | KF522192 | KX379391 | KX372961 | KX372823 | EU361770 | EU361903 |
| Mezoneuron kauaiensis (H. Mann) Hillbr. | Melville 71/1033 (K) | Hawaii, USA | - | - | - | KX372822 | - | - |
| Mezoneuron scortechinii F. Muell. | Wieringa et al. 4195 (WAG) | Australia | KF522077 | KX379394 | KX372964 | KF379231 | KX373106 | KX176821 |
| Mezoneuron sumatranum (Roxb.) Wight & Arn. | Beaman 9642 (NY, MO) | Malaysia | KF522199 | - | - | KX372821 | - | - |
| Mezoneuron montrouzieri Guillaumin | Pullen 7619 (K) | Papua New Guinea | KF522193 | - | - | - | - | - |
| Caesalpinia erythrocarpa Pedley | Schodde 2246 (K) | Papua New Guinea | KF522257 | KX379393 | KX372963 | KX372813 | - | - |
| Caesalpinia nitens (F. Muell ex Benth.) Pedley | Bean 18033 (MO) | Australia | KF522076 | KX379392 | KX372962 | KX372814 | - | - |
| Moullava Adans. (1/1 species) | ||||||||
| *Moullava spicata (Dalzell) Nicolson | Critchett 11/79 (K) | Zambia, cultivated | KF522252 | KX379378 | - | KX372805 | JX073267 | KX176818 |
| *Moullava spicata (Dalzell) Nicolson | Hutchison 2784 (TEX) | Sri Lanka | - | - | - | - | AF430782 | - |
| Poincianella Britton & Rose (32/~35 species) | ||||||||
| *Poincianella mexicana (A. Gray) Britton & Rose | Hughes et al. 1606 (NY, FHO) | Mexico | KF522218 | KX379296 | KX373061 | - | EU361772 | EU361904 |
| *Poincianella mexicana (A. Gray) Britton & Rose | Delgado 01-2114 (MEXU) | Mexico | KF522219 | - | - | - | EF177387 | - |
| *Poincianella mexicana (A. Gray) Britton & Rose | Lewis s.n., Kew Living Coll. 1973-21714 (K) | Mexico | KF522215 | KP003788 | KP003730 | KP003683 | - | - |
| *Poincianella mexicana (A. Gray) Britton & Rose | Gagnon & Marazzi EG2010.015 (MT) | USA, cultivated | KF522217 | KX379295 | KX373062 | - | - | - |
| *Poincianella mexicana (A. Gray) Britton & Rose | Mason s.n. (K) | USA | - | - | - | AY549892 | AF430727 | - |
| Poincianella aff. mexicana | Contreras s.n. (MEXU) | Mexico | KF522227 | - | - | - | - | - |
| Poincianella acapulcensis (Standl.) Britton & Rose | Lott 3205 (K) | Mexico | KF522233 | - | - | - | - | - |
| Poincianella acapulcensis (Standl.) Britton & Rose | MacQueen et al. 406 (K) | Mexico | KF522235 | KX379280 | KX373065 | - | - | - |
| Poincianella bracteosa (Tul.) L.P. Queiroz | Carvalho-Sobrinho 218 (HUEFS) | Brazil | KF522035 | KX379449 | KX372983 | - | - | - |
| Poincianella bracteosa (Tul.) L.P. Queiroz | de Queiroz 10085 (HUEFS) | Brazil | KF522079 | KX458251 | - | - | - | - |
| Poincianella bracteosa (Tul.) L.P. Queiroz | de Queiroz7845 (HUEFS) | Brazil | KF522036 | KX379448 | - | - | - | - |
| Poincianella bracteosa (Tul.) L.P. Queiroz | (retrieved from GenBank) | Brazil | - | - | - | DQ787395 | - | - |
| Poincianella caladenia (Standl.) Britton & Rose | Contreras 2868 (MEXU) | Mexico | KF522234 | - | - | - | - | - |
| Poincianella caladenia (Standl.) Britton & Rose | Contreras 2818 (MEXU) | Mexico | - | - | - | - | EF177383 | - |
| Poincianella caladenia (Standl.) Britton & Rose | Lewis et al. 2072 (K) | Mexico | KF522228 | KX379285 | KX373066 | - | - | - |
| Poincianella eriostachys (Benth.) Britton & Rose | Hughes 1832 (K) | Mexico | AY899751 | - | - | - | AY899693 | - |
| Poincianella eriostachys (Benth.) Britton & Rose | Lewis et al. 1799 (K) | Mexico | KF522029 | KX379444 | KX372993 | KX372836 | AF430715 | - |
| Poincianella eriostachys (Benth.) Britton & Rose | MacQueen 449 (MEXU) | Mexico | - | - | - | - | EF177389 | - |
| Poincianella exostemma (DC.) Britton & Rose | Contreras s.n. Febrero 2000 (MEXU) | Mexico | KF522237 | - | - | - | - | - |
| Poincianella exostemma (DC.) Britton & Rose subsp. exostemma | Bruneau 1317 (MT) | Mexico | KF522221 | KX379292 | KX373072 | - | - | - |
| Poincianella exostemma (DC.) Britton & Rose subsp. exostemma | Lewis & Hughes 1712, RBG Liv.Coll. 1989-3073 (K) | Guatemala | KF522224 | KX379290 | - | - | AF430717 | - |
| Poincianella exostemma (DC.) Britton & Rose subsp. exostemma | Lewis & Hughes 1753 (K) | Guatemala | KF522222 | KX379291 | KX373071 | - | - | - |
| Poincianella exostemma (DC.) Britton & Rose | Espinoza, BioBot00766 BOLD rec.: MHPAD902-09 |
Costa Rica | - | - | - | - | - | JQ587524 |
| Poincianella exostemma (DC.) Britton & Rose | Espinoza, BioBot00767 BOLD rec.: MHPAD903-09 |
Costa Rica | - | - | - | - | - | JQ587525 |
| Poincianella gaumeri (Greenm.) Britton & Rose | Calzada 19333 (K, MEXU) | Mexico | KF522030 | - | - | - | - | - |
| Poincianella gaumeri (Greenm.) Britton & Rose | Hughes 492 (K) | Mexico | KF522034 | KX379445 | - | - | - | - |
| Poincianella gaumeri (Greenm.) Britton & Rose | Lewis & Hughes 1762 (K) | Mexico | KF522044 | KP003799 | KP003739 | KP003692 | - | - |
| Poincianella glandulosa (DC.) Britton & Rose | Ekman 9838 (K) | Haiti | KX373138 | KX379279 | - | - | - | - |
| Poincianella laxa (Benth.) Britton & Rose | Delgado 2337 (MEXU) | Mexico | KF522274 | - | - | - | - | - |
| Poincianella laxiflora (Tul.) L.P. Queiroz | de Queiroz 7063 (HUEFS) | Brazil | KF522051 | KX379440 | - | KX372849 | - | - |
| Poincianella laxiflora (Tul.) L.P. Queiroz | Lewis & Andrade 2012 (MO) | Brazil | - | - | - | KX372929 | - | - |
| Poincianella melanadenia (Rose) Britton & Rose | Hughes et al. 2091 (FHO) | Mexico | KF522275 | KX379301 | KX373078 | - | - | - |
| Poincianella melanadenia (Rose) Britton & Rose | Contreras 7369 (MEXU) | Mexico | KF522277 | - | - | - | - | - |
| Poincianella melanadenia (Rose) Britton & Rose | Hughes et al. 2074 (FHO) | Mexico | KF522276 | KX379302 | - | KX372896 | - | - |
| Poincianella melanadenia (Rose) Britton & Rose | Sotuyo and Gonzalez s.n. (MEXU) | Mexico | - | - | - | - | DQ208904 | - |
| Poincianella microphylla (Mart. ex. G. Don) L.P. Queiroz | Coradin et al. 5941 (K) | Brazil | KF522040 | - | - | - | - | - |
| Poincianella microphylla (Mart. ex. G. Don) L.P. Queiroz | de Queiroz 9060 (HUEFS) | Brazil | KF522039 | KX379450 | KX372984 | KX372847 | - | - |
| Poincianella nelsonii Britton & Rose | Contreras & Sotuyo s.n. (MEXU) | Mexico | KF522300 | KX379281 | KX373070 | - | - | - |
| Poincianella nelsonii Britton & Rose | Lewis et al. 1794 (K) | Mexico | - | - | - | - | AF430728 | - |
| Poincianella nelsonii Britton & Rose | Sotuyo s.n., RBG Liv.Coll. 2002-3577 (K) | Mexico | KF522301 | KP003789 | KP003731 | KP003684 | EF177385 | - |
| Poincianella palmeri (S. Watson) Britton & Rose | Gagnon et al. EG2010.010 (MT) | USA, cultivated | KF522230 | KX379286 | KX373067 | - | - | - |
| Poincianella palmeri (S. Watson) Britton & Rose | Gagnon et al. EG2010.023 (MT) | USA, cultivated | KF522229 | KX379284 | KX373068 | - | - | - |
| Poincianella palmeri (S. Watson) Britton & Rose | Lewis 2064 (K) | Mexico | KF522232 | - | - | - | - | - |
| Poincianella palmeri (S. Watson) Britton & Rose | Lewis et al. 2065 (K) | Mexico | KF522231 | KP003790 | KP003732 | KP003685 | KX373113 | KF379243 |
| Poincianella pannosa (Standl.) Britton & Rose | Gentry 4365 (MEXU) | Mexico | KF522283 | - | - | - | - | - |
| Poincianella pannosa (Standl.) Britton & Rose | Lewis 2051 (K) | Mexico | KF522282 | KP003791 | KP003734 | KP003686 | KX373112 | - |
| Poincianella pannosa (Standl.) Britton & Rose | Turner s.n. (TEX) | Mexico | - | - | - | AY549890 | AY535804-AY535817 | - |
| Poincianella pellucida (Vogel) Britton & Rose | Ekman 4999 (K) | Haiti | - | KX379452 | - | - | - | - |
| Poincianella phyllanthoides (Standl.) Britton & Rose | Nee 32666 (K) | Mexico | KF522220 | KX379294 | KX373060 | - | - | - |
| Poincianella phyllanthoides (Standl.) Britton & Rose | Steinmann 3718 (INIREB, MEXU) | México | KF522216 | - | - | - | - | - |
| Poincianella placida (Brandegee) Britton & Rose | Lewis et al. 2032 (K) | Mexico | KF522273 | KP003792 | KP003735 | KP003687 | - | - |
| Poincianella placida (Brandegee) Britton & Rose | Lewis 2046 (K) | Mexico | KF522272 | KP003792 | X | KP003687 | KX373122 | |
| Poincianella pluviosa (DC.) L.P. Queiroz | de Queiroz 12795 (HUEFS) | Brazil | KF522049 | KP003800 | KP003735 | KP003687 | - | - |
| Poincianella pluviosa (DC.) L.P. Queiroz | Wood et al. 26552 (K) | Bolivia | KF522047 | X | KX372987 | KX372840 | - | - |
| Poincianella pluviosa (DC.) L.P. Queiroz var. pluviosa | Wood 8838 (K) | Bolivia | KF522052 | KX379442 | - | KX372842 | - | - |
| Poincianella pluviosa (DC.) L.P. Queiroz var. pluviosa | Nee 40000 (K) | Bolivia | KF522054 | - | - | KX372841 | - | - |
| Poincianella pluviosa (DC.) L.P. Queiroz | Nee 38223 (TEX) | Bolivia | - | - | - | - | AF430731 | |
| Poincianella pluviosa var. peltophoroides (DC.) L.P. Queiroz | Lewis et al. 1632 (K, NY) | Brazil | - | - | - | KX372848 | - | - |
| Poincianella pluviosa var. sanfranciscana (G.P. Lewis) L.P. Queiroz | Lewis & Andrade 1896 (K) | Brazil | KF522050 | KX379443 | KX372990 | KX372850 | - | - |
| Poincianella pyramidalis (Tul.) L.P. Queiroz | Dorea 117 (HUEFS) | Brazil | KF522041 | KX379441 | KX372985 | KX372851 | - | - |
| Poincianella pyramidalis (Tul.) L.P. Queiroz | de Queiroz 9020 (HUEFS) | Brazil | KF522042 | KX379451 | KX372986 | KX372852 | - | - |
| Poincianella pyramidalis (Tul.) L.P. Queiroz | Taylor et al. 1361 (MO, NY) | Brazil | - | - | - | KX372930 | - | - |
| Poincianella pyramidalis (Tul.) L.P. Queiroz | Mori & Boom 14207 (K) | Brazil | KF522038 | - | - | - | - | - |
| Poincianella pyramidalis (Tul.) L.P. Queiroz | Sampaio s.n. (retrieved from GenBank) | Brazil | - | - | - | - | - | JX850053 |
| Poincianella standleyii Britton & Rose | Contreras 2745 (K) | Mexico | KF522236 | KX379282 | KX373069 | - | - | - |
| Poincianella yucatanensis (Greenm.) Britton & Rose subsp. yucatanensis | Lewis 1765 (K) | Mexico | KF522280 | KX379288 | KX373063 | - | AF430743 | |
| Poincianella yucatanensis (Greenm.) Britton & Rose subsp. yucatanensis | Lewis & Hughes 1766 (K, NY) | Mexico | KF522281 | KX379289 | - | - | - | - |
| Caesalpinia coccinea G.P. Lewis & J.L. Contr. | Lewis 1802 (K) | Mexico | KF522225 | KX379283 | - | KX372901 | - | - |
| Caesalpinia coccinea G.P. Lewis & J.L. Contr. | Lewis 1803 (K) | Mexico | KF522226 | - | - | - | EF177386 | - |
| Caesalpinia echinata Lam. | Filgueiras 3391 (NY) | Brazil, cultivated | KF522099 | KP003802 | KP003746 | KP003695 | KX373105 | - |
| Caesalpinia echinata Lam. | Lewis et al. 1624 (K) | Brazil | KF522072 | KX379412 | KX373029 | KX372866 | - | KX176825 |
| Caesalpinia echinata Lam. | Miranda 76 (HUEFS) | Brazil | KF522100 | KX379411 | KX373044 | KX372867 | KX373121 | KX176822 |
| Caesalpinia epifanioi J.L. Contr. | Contreras 2039 (K) | Mexico | KF522278 | KP003787 | KP003733 | KP003682 | - | |
| Caesalpinia epifanioi J.L. Contr. | Sotuyo et al. 63 (MEXU) | Mexico | - | - | - | - | DQ208901 | - |
| Caesalpinia epifanioi J.L. Contr. | Sotuyo & Sotuyo 20 (MEXU) | Mexico | KF522279 | - | - | - | - | - |
| Caesalpinia hintonii Sandwith. | Sotuyo 46 (MEXU) | Mexico | KF522270 | KX379299 | KX373076 | KX372897 | DQ208882 | - |
| Caesalpinia hughesii G.P. Lewis | Lewis et al. 1795 (K) | Mexico | KF522223 | KX379293 | KX373073 | - | AF430725 | - |
| Caesalpinia macvaughii J.L. Contr. & G.P. Lewis | Sotuyo et al. 8 (MEXU) | Mexico | KF522299 | KX379297 | KX373077 | KX372898 | KX373108 | - |
| Caesalpinia macvaughii J.L. Contr. & G.P. Lewis | Sotuyo et al. 54 (MEXU) | Mexico | KF522269 | - | - | - | - | - |
| Caesalpinia macvaughii J.L. Contr. & G.P. Lewis | Steinmann 3175 (INIREB, K, MEXU) | Mexico | KF522268 | KX379298 | - | - | DQ208916 | - |
| Caesalpinia marginata Tul. | Dubs 1746 (K) | Brazil | KF522045 | - | - | KX372839 | - | - |
| Caesalpinia marginata Tul. | Wood et al. 26514 (K) | Bolivia | KF522048 | KX379438 | KX372989 | KX372838 | - | - |
| Caesalpinia marginata Tul. | Wood et al. 26561 (K) | Bolivia | KF522046 | KX379439 | KX372988 | KX372837 | - | KX176808 |
| Caesalpinia nicaraguensis G.P. Lewis | Hawkins & Hughes 4 (K) | Nicaragua | KF522302 | - | - | KX372899 | - | - |
| Caesalpinia oyamae Sotuyo & G.P. Lewis | Hawkins & Hughes 23 (FHO, MEXU, TEX) | Mexico | KF522210 | KX379300 | KX373079 | - | AF430724 | - |
| Caesalpinia pluviosa var. maraniona G.P. Lewis & C.E. Hughes | Hughes et al. 2215 (FHO) | Peru | KF522033 | KX379436 | KX372996 | KX372844 | KX373120 | - |
| Caesalpinia pluviosa var. maraniona G.P. Lewis & C.E. Hughes | Hughes et al. 3105 (MT) | Peru | KF522032 | KX379437 | KX372997 | KX372843 | - | KX176836 |
| Caesalpinia pluviosa var. maraniona G.P. Lewis & C.E. Hughes | Pennington et al. 793 (E, K) | Peru | KF522031 | KX379434 | KX372995 | KX372845 | - | - |
| Caesalpinia pluviosa var. maraniona G.P. Lewis & C.E. Hughes | Särkinen et al. 2191 (FHO) | Peru | KF522043 | KX379435 | KX372994 | KX372846 | - | - |
| Caesalpinia yucatanensis subsp. chiapensis G.P. Lewis | Hughes 1353 (FHO) | Mexico | KF522271 | KX379287 | KX373064 | KX372900 | - | - |
| Pomaria Cav. (15/16 species) | ||||||||
| *Pomaria glandulosa Cav. | Ventura & López 9294 (TEX) | Mexico | KF522088 | - | - | AY549901 | AY535823-AY535810 | - |
| Pomaria austrotexana B.B. Simpson | Simpson 1-IV-01-2 (TEX) | USA | - | - | - | AY549895 | AF430757 | - |
| Pomaria brachycarpa (A. Gray) B.B. Simpson | Simpson 92-06-22-3 (TEX) | USA | - | - | - | AY549896 | AF430758 | - |
| Pomaria burchellii (DC.) B.B. Simpson & G.P. Lewis | Mott 766 (MO) | South Africa | - | - | - | AY549897 | AY535819-AY535806 | - |
| Pomaria burchellii (DC.) B.B. Simpson & G.P. Lewis | Klepper 252/A/42 (PRU) | South Africa | - | - | - | AY549898 | AF430744 | - |
| Pomaria canescens (Fisher) B.B. Simpson | Turner et al. 93-128 (TEX) | Mexico | - | - | - | AY549899 | AY535820-AY535807 | - |
| Pomaria fruticosa (S. Watson) B.B. Simpson | Villareal 4439 (TEX) | Mexico | - | - | - | AY549901 | AY535822-AY535809 | - |
| Pomaria jamesii (Torr. & A. Gray) Walp. | Gagnon & Marazzi EG2010.020 (MT) | USA | KF522089 | KX379313 | KX372947 | - | - | KX176830 |
| Pomaria jamesii (Torr. & A. Gray) Walp. | Higgins 17628 (NY) | USA | KF522090 | KP003793 | KP003736 | KP003677 | EU361830 | EU362029 |
| Pomaria lactea (Schinz) B.B. Simpson & G.P. Lewis | Pearson 9742 (MO) | South Africa | - | - | - | AY549904 | AY535824-AY535811 | - |
| Pomaria melanosticta S. Schauer | Simpson 92-06-23-1 (TEX) | USA | - | - | - | AY549905 | AF430760 | - |
| Pomaria multijuga (S. Watson) B.B. Simpson | Engard 649 (TEX) | Mexico | - | - | - | AY549906 |
AY535825- AY535812 |
- |
| Pomaria pilosa (Vogel) B.B. Simpson & G.P. Lewis | Wasum et al. 4571 (NY) | Brazil | - | - | - | AY549900 | AY535821-AY535808 | - |
| Pomaria pilosa (Vogel) B.B. Simpson & G.P. Lewis | Wasum & Bastos 8008 (NY) | Brazil | - | - | - | AY549907 | AY535824-AY535813 | - |
| Pomaria rubicunda (Vogel) B.B. Simpson & G.P. Lewis | Biganzoli et al. s.n. (NY) | Argentina | KF522085 | KP003795 | KP003738 | KP003679 | EU361775 | - |
| Pomaria rubicunda (Vogel) B.B. Simpson & G.P. Lewis | Lima 463 (HUEFS) | Brazil | KP003642 | KP003794 | KP003737 | KP003678 | - | - |
| Pomaria rubicunda (Vogel) B.B. Simpson & G.P. Lewis var. rubicunda | Vanni & Marunak 3755 (NY) | Argentina | - | - | - | AY549909 | AY535827-AY535814 | - |
| Pomaria rubicunda var. hauthalii (Harms) B.B. Simpson & G.P. Lewis | Ibarrola 1750 (US) | Argentina | KF522087 | - | - | AY549908 | AF430723 | - |
| Pomaria sandersonii (Harv.) B.B. Simpson & G.P. Lewis | Hilliard & Burtt 9225 (MO) | South Africa | - | - | - | AY549910 | AY535828-AY535815 | - |
| Pomaria stipularis (Vogel) B.B. Simpson & G.P. Lewis | Jönsson 1002a (A) | Brazil | KF522086 | - | - | AY549911 | AF430739 | - |
| Pomaria wootonii (Britton) B.B. Simpson | Johnston 4341 (TEX) | Mexico | - | - | - | AY549912 | AY535829-AY535816 | - |
| Pterolobium R. Br. ex Wight & Arn. (4/10 species) | ||||||||
| *Pterolobium stellatum (Forssk.) Brenan | Herendeen 17-XII-97-9 (US) | Tanzania | KF522238 | KX379457 | - | KX372812 | KX373115 | EU362032 |
| *Pterolobium stellatum (Forssk.) Brenan | Briden & al. RNB219 (JRAU) BOLD rec. KNPA1387-09 |
South Africa | - | - | - | - | - | JF270908 |
| *Pterolobium stellatum (Forssk.) Brenan | Albers 63080 (TEX) | Ethiopia | - | - | - | - | AF430783 | - |
| Pterolobium hexapetalum (Roth) Santapau & Wagh | Grierson & Long 2075 (P) | Bhutan | KX373139 | - | KX372973 | KX372806 | - | - |
| Pterolobium integrum Craib | van Beusekom 4021 (P) | Thailand | - | KX379456 | - | - | - | - |
| Pterolobium macropterum Kurz | Grierson & Long 1624 (P) | Bhutan | KX373141 | KX379454 | KX372974 | - | - | - |
| Pterolobium macropterum Kurz | Geesink & al. 5934 (P) | Thailand | KX373140 | KX379455 | - | - | - | - |
| Stahlia Bello (1/1 species) | ||||||||
| *Stahlia monosperma (Tul.) Urb. | Gardner 7029 (E) | Dominican Republic | KX373142 | KX379422 | KX373009 | - | EU361838 | EU362050 |
| *Stahlia monosperma (Tul.) Urb. | Proctor 48543 (MO) | Puerto Rico | - | - | - | - | AF430787 | - |
| Stenodrepanum Harms (1/1 species) | ||||||||
| *Stenodrepanum bergii Harms | Hick & Bertone 8 (CORD) | Argentina | - | - | - | - | JX219461 | JX219467 |
| *Stenodrepanum bergii Harms | Hick & Bertone 16 (CORD) | Argentina | - | - | - | - | JX219462 | - |
| Stuhlmannia Taub. (1/1 species) | ||||||||
| *Stuhlmannia moavi Taub. | Luke 3710 (MO, K) | Tanzania | KF522061 | KX379431 | KX373001 | KX372829 | - | - |
| *Stuhlmannia moavi Taub. | Keraudren-Aymonin & Aymonin 25628 (MO) | Madagascar | KF522060 | KX379433 | KX373000 | KX372828 | - | - |
| *Stuhlmannia moavi Taub. | Tanner 2404 (NY) | Tanzania | - | - | - | - | AF430789 | - |
| *Stuhlmannia moavi Taub. | Luke & Robertson 2336 (K) | Kenya | KF522058 | - | - | - | - | - |
| *Stuhlmannia moavi Taub. | Robertson 7509 (K) | Kenya | KF522059 | KX379432 | KX372999 | KX372827 | EU361839 | KX176819 |
| *Stuhlmannia moavi Taub. | Tanner 3167 (K) | Tanzania | AY899765 | - | - | - | AY899707 | - |
| Tara Molina (3/3 species) | ||||||||
| *Tara spinosa (Molina) Britton & Rose | Eastwood et al. RJE36 (FHO) | Peru | KF522128 | KX379398 | KX373046 | - | - | KF379250 |
| *Tara spinosa (Molina) Britton & Rose | Hughes 2360 (FHO) | Peru | KF522129 | KX379399 | KX373045 | KX372881 | - | - |
| *Tara spinosa (Molina) Britton & Rose | Nee 45494 (MO) | Australia, cultivated | KF522130 | KX379400 | KX373047 | KX372880 | - | - |
| *Tara spinosa (Molina) Britton & Rose | Lewis 2200 (K) | Ecuador | - | - | - | KF379235 | KX373099 | - |
| *Tara spinosa (Molina) Britton & Rose | Aronson 7756 (TEX) | Chile | - | - | - | - | AF430738 | - |
| Tara cacalaco (Humb. & Bonpl.) Molinari & Sánchez Och. | Gagnon & Marazzi EG2010.022 (MT) | USA, cultivated | KF522202 | KX379396 | - | KX372885 | - | - |
| Tara cacalaco (Humb. & Bonpl.) Molinari & Sánchez Och. | Soto Nuñez 13682 (MEXU) | Mexico | KF522312 | - | - | - | - | - |
| Tara cacalaco (Humb. & Bonpl.) Molinari & Sánchez Och. | Walker s.n., RBG Liv.Coll. 1986-6481 (K) | Mexico | KF522203 | KX379397 | KX373048 | KX372886 | - | - |
| Tara cacalaco (Humb. & Bonpl.) Molinari & Sánchez Och. | Lewis 1789 (K) | Mexico | - | - | - | - | AF430709 | EU361898 |
| Tara cacalaco (Humb. & Bonpl.) Molinari & Sánchez Och. | Lewis 1788 (K) | Mexico | - | - | KX372884 | - | - | |
| Tara vesicaria (L.) Molinari, Sánchez Och. & Mayta | Hawkins & Hughes 11 (FHO) | Nicaragua | KF522204 | KX379395 | KX373049 | KX372882 | - | - |
| Tara vesicaria (L.) Molinari, Sánchez Och. & Mayta | Lewis & Hughes 1768 (K) | Mexico | KF522205 | - | - | KX372883 | AF430742 | - |
| Zuccagnia Cav. (1/1 species) | ||||||||
| *Zuccagnia punctata Cav. | Fortunato 5545 (MO) | Argentina | KF522142 | KX379417 | KX373002 | KX372917 | - | - |
| *Zuccagnia punctata Cav. | Galleto et al. 171 (CORD) | Argentina | KF522141 | KP003798 | KP003742 | KP003688 | AF430791 | KX176813 |
| *Zuccagnia punctata Cav. | Guglianone et al. 1668 (K, SI) | Argentina | KF522143 | KX379418 | KX373003 | KX372916 | - | - |
| *Zuccagnia punctata Cav. | Lutz 136 (NY) | Argentina | KF522140 | KX379416 | KX373004 | KX372918 | EU361842 | - |
| *Zuccagnia punctata Cav. | Tapia & al. s.n. (CORD) | Argentina | - | - | - | - | JX219463 | JX219468 |
| Unassigned Old World taxa (13/~20 species) | ||||||||
| Caesalpinia crista L. | Herendeen 1-V-99-3 (US) | Thailand | KF522073 | KX379384 | KX372971 | KX372807 | KX373094 | EU361900 |
| Caesalpinia crista L. | Wieringa et al. 4199 (WAG) | Australia, cultivated | KF522074 | KX379385 | KX372972 | KX372808 | - | - |
| Caesalpinia crista L. | PS1367MT01 (retrieved from GenBank) | N/A | - | - | - | - | - | HM049549 |
| Caesalpinia decapetala (Roth) Alston | Marazzi BM137 (Z) | Switzerland, cultivated | AM086910 | - | - | - | - | AM086828 |
| Caesalpinia decapetala (Roth) Alston | Hughes et al. 2227 (FHO) | Peru, cultivated | KF522081 | KX379353 | KX372978 | KX372922 | - | - |
| Caesalpinia decapetala (Roth) Alston | Hooper & Gandhi 2429 (US) | India, cultivated | KF522080 | - | - | - | - | - |
| Caesalpinia decapetala (Roth) Alston | Herendeen & Mbago 19-XII-97-1 (US) | Tanzania | KF522082 | KX379354 | KX372979 | KX372921 | KX373098 | KX176817 |
| Caesalpinia decapetala (Roth) Alston | PS1589MT01 (retrieved from GenBank) | N/A | - | - | - | GU217669 | - | HM049555 |
| Caesalpinia decapetala (Roth) Alston | Corby 2173; Krukoff coll. (K) | N/A | - | - | - | - | AF430714 | - |
| Caesalpinia decapetala (Roth) Alston | (retrieved from GenBank) | N/A | - | - | - | JF708207 | - | - |
| Caesalpinia digyna Rottler | van Beusekom & Phengklai 3036 (P) | Thailand | KX373144 | KX379381 | - | - | - | - |
| Caesalpinia digyna Rottler | Maxwell 91-827 (P) | Thailand | KX373146 | KX379383 | - | KX372803 | - | - |
| Caesalpinia digyna Rottler | Parnell et al. 95-617 (K) | Thailand | KX373145 | KX379265 | - | - | - | |
| Caesalpinia digyna Rottler | Cheng et al. CL 643 (P) | Cambodia | KX373143 | KX379382 | - | KX372802 | - | - |
| Caesalpinia godefroyana Kuntze | Cheng et al. CL 642 (P) | Cambodia | KX373147 | KX379351 | KX372976 | KX372809 | - | - |
| Caesalpinia mimosoides Lam. | Larsen et al. 44653 (MO) | Thailand | KF522251 | KX379357 | KX372955 | - | - | - |
| Caesalpinia mimosoides Lam. | Clark RPC237 (K) | Thailand | KX373148 | KX379262 | KX372954 | - | KX373093 | - |
| Caesalpinia oppositifolia Hattink | Lugas 607 (K) | Malaysia | KF522056 | KX379355 | - | - | - | - |
| Caesalpinia oppositifolia Hattink | Lugas 921 (K) | Malaysia | KF522055 | KX379356 | KX372970 | KX372810 | - | - |
| Caesalpinia parviflora Prain | van Beusekom et al. 3977 (K) | Thailand | KF522057 | KX379389 | KX372977 | KX372811 | - | - |
| Caesalpinia pearsonii Bolus | Strey 2475 (K) | Namibia | KX373150 | KX379377 | KX373017 | KX372825 | - | KX176826 |
| Caesalpinia pearsonii Bolus | Kolberg & Loots, NAM2943-HK1399 (K) | Namibia | KX373149 | KX379376 | KX373016 |
KX372824 |
KX373123 | KX176827 |
| Caesalpinia milettii Hook. & Arn. | Ying 1639 (K) | China | - | KX379260 | - | - | - | - |
| aCaesalpinia sappan L. | Jinawn 76 (K) | Sabah | - | KX379261 | - | - | - | - |
| aCaesalpinia sappan L. | PS1370MT04, (retrieved from GenBank) | N/A | - | - | - | GQ434751 | - | - |
| aCaesalpinia sappan L. | PS1370MT05, (retrieved from GenBank) | N/A |
- |
- | - | - | - | HM049952 |
| bCaesalpinia sappan L. | Gillis 9548 (P) | India | - | KX379352 | - | - | - | - |
| bCaesalpinia sappan L. | (retrieved from GenBank) | N/A | - | - | - | EU243573 | - | - |
| bCaesalpinia sappan L. | PS1370MT01, (retrieved from GenBank) | N/A | - | - | - | - | - | HM049551 |
| Caesalpinia tortuosa Roxb. | Lace 6332 (K) | Burma | - | KX379264 | - | KX372804 | - | - |
| Caesalpinia vernalis Benth. | Li Shi Jin 787 (CAS, IBSC) | China | KF522075 | - | - | - | - | |
| Caesalpinia welwitschiana (Oliv.) Brenan | Bidgood et al. 2913 (K) | Tanzania | KF522133 | KX379379 | - | - | - | - |
| Caesalpinia welwitschiana (Oliv.) Brenan | Malaisse 13658 (K) | Zaire | KF522134 | KX379380 | KX372975 | - | - | KX176831 |
Accessions with the same prefix were combined together.
Accessions with the same prefix were combined together.
Citation
Gagnon E, Bruneau A, Hughes CE, De Queiroz LP, Lewis GP (2016) A new generic system for the pantropical Caesalpinia group (Leguminosae). PhytoKeys 71: 1–160. doi: 10.3897/phytokeys.71.9203
Supplementary materials
Table 3. Summary of the branch support results from phylogenetic analyses of the Caesalpinia group.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Edeline Gagnon, Anne Bruneau, Colin E. Hughes, Luciano Paganucci De Queiroz, Gwilym P. Lewis
Data type: results from phylogenetic analyses
Explanation note: Bootstrap support from the ML analyses of the six individual loci and the combined datasets, as well as Bootstrap support and Posterior probabilities from the parsimony and Bayesian analyses of the combined datasets, for various proposed genera from Gagnon et al. (2013).
References
- Allorge V, Allorge P. (1930) Marcel Denis (1897–1929). Bulletin de la Société Botanique de France 77(4): 693–696. doi: 10.1080/00378941.1930.10837216 [Google Scholar]
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. (1990) Basic local alignment search tool. Journal of Molecular Biology 215: 403–410. doi: 10.1016/S0022-2836(05)80360-2 [DOI] [PubMed] [Google Scholar]
- Ansari AA. (1990) Extended distribution of an endemic monotypic genus Wagatea Dalz. Journal of Economic and Taxonomic Botany 14(3): 746–747. [Google Scholar]
- Babineau M, Gagnon E, Bruneau A. (2013) Phylogenetic utility of 19 low-copy nuclear genes in closely related genera and species of caesalpinioid legumes. Journal of South African Botany 89: 94–105. doi: 10.1016/j.sajb.2013.06.018 [Google Scholar]
- Barreto Valdés A. (2013) Flora de la Republica de Cuba. Serie A, Plantas vasculares. Fasciculo 18. Caesalpiniaceae. Koeltz Scientific Books, Königstein, 1–210. [Google Scholar]
- Bolus L. (1920) Caesalpinia pearsonii. Annals of the Bolus Herbarium 3: 4. [Google Scholar]
- Borges L, Souza L, Guerra M, Machado I, Lewis GP, Lopes A. (2012) Reproductive isolation between diploid and tetraploid cytotypes of Libidibia ferrea (= Caesalpinia ferrea) (Leguminosae): ecological and taxonomic implications. Plant Systematics and Evolution 298(7): 1371–1381. doi: 10.1007/s00606-012-0643-3 [Google Scholar]
- Bouchenak-Khelladi Y, Maurin O, Hurter J, van der Bank M. (2010) The evolutionary history and biogeography of Mimosoideae (Leguminosae): an emphasis on African acacias. Molecular Phylogenetics and Evolution 57(2): 495–508. doi: 10.1016/j.ympev.2010.07.019 [DOI] [PubMed] [Google Scholar]
- Brenan JPM. (1963) Notes on African Caesalpinioideae. Kew Bulletin 17: 197–218. doi: 10.2307/4118939 [Google Scholar]
- Brenan JPM. (1967) Leguminosae, part 2: subfamily Caesalpinioideae. In: Milne-Redhead E, Polhill RM. (Eds) Flora of Tropical East Africa. Crown Agents for Oversea Goverments and Administration, London. [Google Scholar]
- Brink M. (2006) Cordeauxia edulis Hemsl. In: Brink M, Belay G. (Eds) PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale), Wageningen, Netherlands: http://www.prota4u.org/protav8.asp?en=1&p=Cordeauxia+edulis+Hemsl [accessed 15.05.2015] [Google Scholar]
- Britton NL. (1927) Stahlia monosperma, Cobana negra, native of Puerto Rico, Family Caesalpiniaceae. Adansonia 12: 33. [Google Scholar]
- Britton NL, Rose JN. (1930) Caesalpiniaceae, Krameriaceae, (Rosales). North American Flora 23: 301–342. [Google Scholar]
- Brummitt RK, Ross J. (1974) The African species of Hoffmannseggia (Leguminosae-Caesalpinioideae). Kew Bulletin 29(2): 415–424. doi: 10.2307/4108550 [Google Scholar]
- Brummitt RK, Chikuni AC, Lock JM, Polhill RM. (2007) Leguminosae. In: Timberlake JR, Pope GV, Polhill RM, Martins ES. (Eds) Flora Zambesiaca, vol. 3(2). Kew Royal Botanic Gardens, 1–218. [Google Scholar]
- Bruneau A, Herendeen PS, Klitgaard BB, Lewis GP. (2001) Phylogenetic relationships in the Caesalpinioideae (Leguminosae) as inferred from chloroplast trnL intron sequences. Systematic Botany 26: 487–514. doi: 10.1043/0363-6445-26.3.487 [Google Scholar]
- Bruneau A, Mercure M, Lewis GP, Herendeen PS. (2008) Phylogenetic patterns and diversification in the Caesalpinioid legumes. Botany 86: 697–718. doi: 10.1139/B08-058 [Google Scholar]
- Bueno E. (2002) Pau-brasil. Axis Mundi, São Paulo, Brazil, 1–278. [Google Scholar]
- Burkart A. (1936) Las especias argentinas y uruguayas del género Caesalpinia. Revista Argentina de Agronomía 3(3): 67–112. [Google Scholar]
- Burkart A. (1940) Nota sobre algunas Leguminosas indigenas o introducidas en Chile. Revista Chilena Historia Natural 43: 156–164. [Google Scholar]
- Burkart A. (1944) Tres nuevas Leguminosas del Paraguay: coleccionadas por el Señor Teodoro Rojas. Darwiniana 6(3): 477–493. [Google Scholar]
- Burkart A. (1952) Las Leguminosas argentinas silvestres y cultivadas. Buenos Aires, Acme Agency, Buenos Aires, Argentina, 1–569. [Google Scholar]
- Burkart A. (1957) Leguminosas nuevas o críticas, V. Darwiniana 11(2): 256–271. [Google Scholar]
- Caponio I, Anton AM, Fortunato RH, Norrmann GA. (2012) Ploidy dimorphism and reproductive biology in Stenodrepanum bergii (Leguminosae), a rare South American endemism. Genome 55(1): 1–7. doi: 10.1139/G11-067 [DOI] [PubMed] [Google Scholar]
- Capuron R. (1967) Deux Caesalpinia nouveaux pour Madagascar. Adansonia (série 2) 7(2): 199–205. [Google Scholar]
- Cardoso MA, Provan J, Powell W, Ferreira PCG, De Oliveira DE. (1998) High genetic differentiation among remnant populations of the endangered Caesalpinia echinata Lam. (Leguminosae-Caesalpinioideae). Molecular Ecology 7: 601–608. doi: 10.1046/j.1365-294x.1998.00363.x [Google Scholar]
- Cardoso SRS, Provan J, Lira CDF, Pereira LDOR, Ferreira PCG, Cardoso MA. (2005) High levels of genetic structuring as a result of population fragmentation in the tropical tree species Caesalpinia echinata Lam. Biodiversity and Conservation 14(5): 1047–1057. doi: 10.1007/s10531-004-8409-z [Google Scholar]
- Chen D, Zhang D, Hou D. (2010a) Caesalpinia. In: Wu CY, Raven P. (Eds) Flora of China, vol. 10 Missouri Botanical Garden Press, St-Louis, USA, 41–47. [Google Scholar]
- Chen D, Zhang D, Hou D. (2010b) Pterolobium. In: Wu CY, Raven P. (Eds) Flora of China, vol. 10 Missouri Botanical Garden Press, St-Louis, USA, 47–48. [Google Scholar]
- Clark R. (2016) A taxonomic revision of Mezoneuron (Leguminosae: Caesalpinioideae: Caesalpinieae). Phytotaxa 274(1): 1–72. doi: 10.11646/phytotaxa.274.1.1 [Google Scholar]
- Clark R, Gagnon E. (2015) A revision of Mezoneuron (Leguminosae: Caesalpinioideae) in New Caledonia, with perspectives on vegetation, geology, and conservation. Phytotaxa 207(1): 68–92. doi: 10.11646/phytotaxa.207.1.3 [Google Scholar]
- Curtis B, Mannheimer C. (2005) Tree Atlas of Namibia. National Botanical Research Institute, Windhoek, Namibia, 1–674. [Google Scholar]
- Da Silva MJ, De Queiroz LP, Tozzi AMGdeA, Lewis GP, De Sousa AP. (2012) Phylogeny and biogeography of Lonchocarpus sensu lato and its allies in the tribe Millettieae (Leguminosae, Papilionoideae). Taxon 61(1): 93–108. [Google Scholar]
- Davis JI, Stevenson DW, Petersen G, Seberg O, Campbell LM, Freudenstein JV, Goldman DH, Hardy CR, Michelangeli FA, Simmons MP, Specht CD, Vergara-Silva F, Gandolfo M. (2004) A phylogeny of the monocots, as inferred from rbcL and atpA sequence variation, and a comparison of methods for calculating jackknife and bootstrap values. Systematic Botany 29: 467–510. doi: 10.1600/0363644041744365 [Google Scholar]
- De Lima HC, Lewis GP, Bueno E. (2002) Pau-brasil: uma biografia. In: Bueno E. (Ed.) Pau-brasil. Axis Mundi, São Paulo, Brazil, 39–76. [Google Scholar]
- De Queiroz LP. (2009) Leguminosas da Caatinga. Universidade Estadual de Feira de Santana and Associacao Plantas do Nordeste, Brazil, and Kew Royal Botanic Gardens, Richmond, 1–443. [Google Scholar]
- De Queiroz LP, Pastore JFP, Cardoso D, Snak C, Lima ALC, Gagnon E, Vatanparast M, Holland AE, Egan AN. (2015) A multilocus phylogenetic analysis reveals the monophyly of a recircumscribed papilionoid legume tribe Diocleae with well-supported generic relationships. Molecular Phylogenetics and Evolution 90: 1–19. doi: 10.1016/j.ympev.2015.04.016 [DOI] [PubMed] [Google Scholar]
- Delgado-Salinas A, Thulin M, Pasquet R, Weeden N, Lavin M. (2011) Vigna (Leguminosae) sensu lato: the names and identities of the American segregate genera. American Journal of Botany 98(10): 1694–1715. doi: 10.3732/ajb.1100069 [DOI] [PubMed] [Google Scholar]
- Dludlu MN, Stirton CH, Chimphango SBM, Bello A, Muasya AM. (2013) Phylogenetic position of the southern African members of the tribe Psoraleeae based on molecular and morphological data. South African Journal of Botany 89: 150–155. doi: 10.1016/j.sajb.2013.06.019 [Google Scholar]
- Doyle JJ. (2012) Polyploidy in Legumes. In: Soltis PS, Soltis DE. (Eds) Polyploidy and Genome Evolution. Springer-Verlag, Berlin, 47–180. doi: 10.1007/978-3-642-31442-1_9 [Google Scholar]
- Du Puy DJ, Rabevohitra R. (2002) Tribe Caesalpinieae. In: Du Puy DJ, Labat JN, Rabevohitra R, Villiers J, Bosser J, Moat J. (Eds) The Leguminosae of Madagascar. Kew Royal Botanic Gardens, Richmond, 20–59. [Google Scholar]
- Durán RC, Ramírez JJ. (2008) Haematoxylum sousanum (Leguminosae, Caesalpinioideae), una especie nueva del Sur de México. Novon: A Journal for Botanical Nomenclature 18(1): 25–28. doi: 10.3417/2005126 [Google Scholar]
- Durán RC, Sousa SM. (2014) Haematoxylum calakmulense (Leguminosae, Caesalpinioideae), una nueva especie mesoamericana. Novon: A Journal for Botanical Nomenclature 23(1): 31–36. doi: 10.3417/2011106 [Google Scholar]
- Egan AN, Crandall KA. (2008) Incorporating gaps as phylogenetic characters across eight DNA regions: ramifications for North American Psoraleeae (Leguminosae). Molecular Phylogenetics and Evolution 46(2): 532–546. doi: 10.1016/j.ympev.2007.10.006 [DOI] [PubMed] [Google Scholar]
- Fazekas AJ, Steeves R, Newmaster SG. (2010) Improving sequencing quality from PCR products containing long mononucleotide repeats. BioTechniques 48: 277–285. doi: 10.2144/000113369 [DOI] [PubMed] [Google Scholar]
- Freire FT. (1994) Cenostigma tocantinum Ducke (Leg. Caes.): uma redescrição da especie. Bradea 6(36): 297–303. [Google Scholar]
- Gagnon E, Hughes CE, Lewis GP, Bruneau A. (2015) A new cryptic species in a new cryptic genus in the Caesalpinia group (Leguminosae) from the seasonally dry inter-Andean valleys of South America. Taxon 64(3): 468–490. doi: 10.12705/643.6 [Google Scholar]
- Gagnon E, Lewis GP, Sotuyo JS, Hughes CE, Bruneau A. (2013) A molecular phylogeny of Caesalpinia sensu lato: Increased sampling reveals new insights and more genera than expected. South African Journal of Botany 89: 111–127. doi: 10.1016/j.sajb.2013.07.027 [Google Scholar]
- Garnock-Jones PJ. (2014) Evidence-based review of the taxonomic status of New Zealand’s endemic seed plant genera. New Zealand Journal of Botany 52(2): 163–212. doi: 10.1080/0028825X.2014.902854 [Google Scholar]
- Gasson P, Warner K, Lewis GP. (2009) Wood anatomy of Caesalpinia s. s., Coulteria, Erythrostemon, Guilandina, Libidibia, Mezoneuron, Poincianella, Pomaria and Tara (Leguminosae, Caesalpinioideae, Caesalpinieae). IAWA Journal 30(3): 247–276. doi: 10.1163/22941932-90000218 [Google Scholar]
- Germishuizen G. (1991) Caesalpinia bracteata, a new species from the Onseepkans area of the Northern Cape Province. Bothalia 21(2): 152–154. doi: 10.4102/abc.v21i2.876 [Google Scholar]
- George AS. (1998) Caesalpinia. In: McCarthy PM. (Ed.) Flora of Australia, vol. 12 Australian Biological Resources Study; /CSIRO, Melbourne, 59–67. [Google Scholar]
- Gillis WT, Proctor GR. (1974) Caesalpinia subg. Guilandina in the Bahamas. Journal of the Arnold Arboretum 55(3): 425–430. [Google Scholar]
- Haston EM, Lewis GP, Hawkins JA. (2005) A phylogenetic reappraisal of the Peltophorum group (Caesalpinieae: Leguminosae) based on the chloroplast trnL-F, rbcL and rps16 sequence data. American Journal of Botany 92(8): 1359–1371. doi: 10.3732/ajb.92.8.1359 [DOI] [PubMed] [Google Scholar]
- Hattink TT. (1974) A revision of Malesian Caesalpinia, including Mezoneuron (Leguminosae–Caesalpiniaceae). Reinwardtia 9(1): 1–69. [Google Scholar]
- Herendeen PS, Bruneau A, Lewis GP. (2003) Phylogenetic relationships in caesalpinioid legumes: a preliminary analysis based on morphological and molecular data. In: Klitgaard BB, Bruneau A. (Eds) Advances in Legume Systematics, Part 10: Higher Level Systematics. Kew Royal Botanic Gardens, Richmond, 37–62. [Google Scholar]
- Herendeen PS, Zarucchi JL. (1990) Validation of Caesalpinia subgenus Mezoneuron (Desf.) Vidal and new combinations in Caesalpinia for two species of Mezoneuron from Africa. Annals of the Missouri Botanical Garden 77(4): 854–855. doi: 10.2307/2399679 [Google Scholar]
- Hinchliff CE, Roalson EH. (2013) Using supermatrices for phylogenetic inquiry: an example using the sedges. Systematic Biology 62(2): 205–219 doi: 10.1093/sysbio/sys088 [DOI] [PubMed] [Google Scholar]
- Hou D, Larsen K, Larsen SS. (1996) Caesalpiniaceae. In: Hou D, Larsen K, Larsen SS, Laferrier JF, Duyfjes BEE. (Eds) Flora Malesiana, vol. 12(2). Rijksherbarium; /Hortus Botanicus, Leiden University, Leiden, Netherlands, 409–784. [Google Scholar]
- Hu J-M, Lavin M, Wojciechowski MF, Sanderson MJ. (2000) Phylogenetic systematics of the tribe Millettieae (Leguminosae) based on chloroplast trnK/matK sequences and its implications for evolutionary patterns in Papilionoideae. American Journal of Botany 87(3): 418–430. doi: 10.2307/2656638 [PubMed] [Google Scholar]
- Hul Thol S. (1976) Contribution à la révision de quelques genres de Caesalpiniaceae, représentés en Asie. PhD thesis, Université Pierre et Marie Curie; (Paris: VI), France. [Google Scholar]
- Hul Thol S, Hideux M. (1977) Taxonomie du genre Pterolobium (Caesalpiniaceae) avec traitement numérique des caractères macromorphologiques et palynologiques. Bulletin du Muséum National d’Histoire Naturelle (Paris), 3e série (502): 1–165.
- Humphreys AM, Linder HP. (2009) Concept versus data in delimitation of plant genera. Taxon 58(4): 1054–74. http://www.jstor.org/stable/27757002 [Google Scholar]
- Jansen PCM. (2005) Caesalpinia sappan L. In: Jansen PCM, Cardon D. (Eds) PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale). Wageningen, Netherlands: http://www.prota4u.org/protav8.asp?en=1&p=Caesalpinia+sappan+L [accessed 15.05.2015] [Google Scholar]
- Jobson RW, Luckow M. (2007) Phylogenetic study of the genus Piptadenia (Mimosoideae: Leguminosae) using plastid trnl-F and trnK/matK sequence data. Systematic Botany 32(3): 569–575. doi: 10.1600/036364407782250544 [Google Scholar]
- Joly S, Bruneau A. (2006) Incorporating allelic variation for reconstructing the evolutionary history of organisms from multiple genes: an example from Rosa in North America. Systematic Biology 55: 623–636. doi: 10.1080/10635150600863109 [DOI] [PubMed] [Google Scholar]
- Johnson KA, Holland BR, Heslewood MM, Crayn DM. (2012) Supermatrices, supertrees and serendipitous scaffolding: inferring a well-resolved, genus-level phylogeny of Styphelioideae (Ericaceae) despite missing data. Molecular Phylogenetics and Evolution 62(1): 146–158. doi: 10.1016/j.ympev.2011.09.011 [DOI] [PubMed] [Google Scholar]
- Kantz KE. (1996) Floral development in the Caesalpinioid tribe Caesalpinieae (Fabaceae). PhD thesis, Louisiana State University, USA. [Google Scholar]
- Kantz KE, Tucker SC. (1994) Developmental basis of floral characters in the Caesalpinieae. In: Ferguson IK, Tucker SC. (Eds) Advances in Legume Systematics, Part 6: Structural Botany. Kew Royal Botanic Gardens, Richmond, 33–40. [Google Scholar]
- Kiesling R, Mulgura ME, Ulibarri EA. (1994) Flora de San Juan, Republica Argentina, vol. 1 Vazquez Mazzini Editores, Buenos Aires, Argentina, 1–348. [Google Scholar]
- Kite G, Lewis GP. (1994) Chemotaxonomy of seed non-protein amino acids in Caesalpinia s.l. In: Sprent JI, McKey D. (Eds) Advances in Legume Systematics, Part 5: the Nitrogen Factor. Kew Royal Botanic Gardens, Richmond, 101–105. [Google Scholar]
- Koch CU, Guitiérrez JE, Rodriguez A, von Helversen O. (2004) Bat pollination in a Cuban ultramafic endemic Caesalpinia bahamensis Lam. subsp. orientensis Borhidi. In: Boyd RS, Barker AJM, Protor J. (Eds) Ultramafic Rocks: their Soils, Vegetation and Fauna. Science Reviews 2000 Ltd, 243–253. [Google Scholar]
- Ky CL, Barre P, Lorieux M, Trouslot P, Akaffou S, Louarn J, Charrier A, Hamon S, Noirot M. (2000) Interspecific genetic linkagemap, segregation distortion and genetic conversion in coffee (Coffea sp. ) Theoretical and Applied Genetics 101: 669–676. doi: 10.1007/s001220051529 [Google Scholar]
- Lemmens RHMJ. (2010) Stuhlmannia moavi Taub. In: Lemmens RHMJ, Louppe D, Oteng-Amoako AA. (Eds) PROTA (Plant Resources of Tropical Africa / Ressources végétales de l’Afrique tropicale). Wageningen, Netherlands: http://www.prota4u.org/protav8.asp?en=1&p=Stuhlmannia+moavi [accessed 15.05.2015] [Google Scholar]
- Lersten NR, Curtis JD. (1994) Leaf anatomy in Caesalpinia and Hoffmannseggia (Leguminosae, Caesalpinioidae) with emphasis on secretory structures. Plant Systematics and Evolution 192: 231–255. doi: 10.1007/BF00986254 [Google Scholar]
- Lersten NR, Curtis JD. (1996) Survey of leaf anatomy, especially secretory structures, of tribe Caesalpinieae (Leguminosae, Caesalpinioideae). Plant Systematics and Evolution 200: 21–39. doi: 10.1007/BF00984746 [Google Scholar]
- Lewis GP. (1987) Legumes of Bahia. Kew Royal Botanic Gardens, Richmond, 1–369. [Google Scholar]
- Lewis GP. (1996) Notes on Stuhlmannia Taub. and the correct placement of Caesalpinia insolita (Harms) Brenan & J.B. Gillett (Leguminosae: Caesalpinioideae: Caesalpinieae). Kew Bulletin 51(2): 377–379. doi: 10.2307/4119334 [Google Scholar]
- Lewis GP. (1998) Caesalpinia: a revision of the Poincianella-Erythrostemon group. Kew Royal Botanic Gardens, Richmond, 1–233. [Google Scholar]
- Lewis GP. (2005) Tribe Caesalpinieae. In: Lewis G, Schrire B, Mackinder B, Lock M. (Eds) Legumes of the World. Kew Royal Botanic Gardens, Richmond, 127–159. [Google Scholar]
- Lewis GP, Schrire BD. (1995) A reappraisal of the Caesalpinia group (Caesalpinioideae: Caesalpinieae) using phylogenetic analysis. In: Crisp MD, Doyle JJ. (Eds) Advances in Legume Systematics: Part 7, Phylogeny. Kew Royal Botanic Gardens, Richmond, 41–52. [Google Scholar]
- Lewis GP, Sotuyo JS. (2010) Hoffmannseggia aphylla (Leguminosae: Caesalpinieae), a new name for a Chilean endemic. Kew Bulletin 65(2): 221–224. doi: 10.1007/s12225-010-9201-8 [Google Scholar]
- Lewis GP, Hughes CE, Daza Yomona A, Solange Sotuyo J, Simon MF. (2010) Three new legumes endemic to the Marañón Valley, Perú. Kew Bulletin 65(2): 209–220. doi: 10.1007/s12225-010-9203-6 [Google Scholar]
- Lira CF, Cardoso SRS, Ferreira PCG, Cardoso MA, Provan J. (2003) Long-term population isolation in the endangered tropical tree species Caesalpinia echinata Lam. revealed by chloroplast microsatellites. Molecular Ecology 12(12): 3219–3225. doi: 10.1046/j.1365-294X.2003.01991.x [DOI] [PubMed] [Google Scholar]
- Little EL, Wadsworth FH. (1964) Common trees of Puerto Rico and the Virgin islands. U.S. Department of Agriculture, Forest Service, Washington D.C., U.S.A. [Google Scholar]
- Lock JM. (1989) Legumes of Africa: a check-list. Kew Royal Botanic Gardens, Richmond, 1–626. [Google Scholar]
- LPWG [Legume Phylogeny Working Group] (2013) Legume phylogeny and classification in the 21st century: progress, prospects and lessons for other species-rich clades. Taxon 62(2): 217–248. doi: 10.12705/622.8 [Google Scholar]
- LPWG [Legume Phylogeny Working Group] (submitted) A new subfamily classification of the Leguminosae based on a taxonomically comprehensive phylogeny. Taxon.
- Macbride JF. (1943) Caesalpinia L. In: Macbride JF. (Ed.) Flora of Peru, vol. 8, part 3, no. 1: Leguminosae. Field Museum of Natural History, Chicago, USA, 188–197. [Google Scholar]
- Mackinder BA, Saslis-Lagoudakis H, Wieringa JJ, Devey D, Forest F, Bruneau A. (2013) The tropical African legume Scorodophloeus clade includes two undescribed Hymenostegia segregate genera and Micklethwaitia, a rare, monospecific genus from Mozambique. South African Journal of Botany 89: 156–163. doi: 10.1016/j.sajb.2013.07.002 [Google Scholar]
- Mackinder BA, Wieringa JJ. (2013) Annea gen. nov. (Detarieae, Caesalpinioideae, Leguminosae): a home for two species long misplaced in Hymenostegia sensu lato. Phytotaxa 142(1): 1–14. doi: 10.11646/phytotaxa.142.1.1 [Google Scholar]
- Manzanilla V, Bruneau A. (2012) Phylogeny reconstruction in the Caesalpinieae grade (Leguminosae) based on duplicated copies of the sucrose synthase gene and plastid markers. Molecular Phylogenetics and Evolution 65: 149–162. doi: 10.1016/j.ympev.2012.05.035 [DOI] [PubMed] [Google Scholar]
- Marazzi B, Endress PK, De Queiroz LP, Conti E. (2006) Phylogenetic relationships within Senna (Leguminosae, Cassiinae) based on three chloroplast DNA regions: patterns in the evolution of floral symmetry and extrafloral nectaries. American Journal of Botany 93: 288–303. doi: 10.3732/ajb.93.2.288 [DOI] [PubMed] [Google Scholar]
- Marazzi B, Sanderson MJ. (2010) Large-scale patterns of diversification in the widespread legume genus Senna and the evolutionary role of extrafloral nectaries. Evolution 64: 3570–3592. doi: 10.1111/j.1558-5646.2010.01086.x [DOI] [PubMed] [Google Scholar]
- Meireles JE, Tozzi AMGdeA, Lavin M. (2014) A phylogenetic analysis of molecular and morphological data reveals a paraphyletic Poecilanthe (Leguminosae, Papilionoideae). Systematic Botany 39(4): 1142–1149. doi: 10.1600/036364414X683912 [Google Scholar]
- Miller MA, Pfeiffer W, Schwartz T. (2010) Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE) (New Orleans, Louisiana, USA, 14 Nov 2010), 45–52. doi: 10.1109/GCE.2010.5676129 [Google Scholar]
- Miller JT, Seigler D. (2012) Evolutionary and taxonomic relationships of Acacia s.l. (Leguminosae: Mimosoideae). Australian Systematic Botany 25(3): 217–224. doi: 10.1071/SB11042 [Google Scholar]
- Molinari-Novoa EA, Sanchez Ocharan C. (2016) Notulae Nomenclaturales I. Transfers to Tara. Weberbauerella 1(8): 1–6. [Google Scholar]
- Molinari-Novoa EA, Mayta Anco LF, Sanchez Ocharan C. (2016) Notulae Nomenclaturales IV. Transfers to Biancaea. Weberbauerella 1(11): 1–3. [Google Scholar]
- Moura TM, Vatanparast M, Tozzi AMGdeA, Forest F, Wilmot-Dear CM, Simon MF, Mansano VF, Kajita T, Lewis GP. (2016) A molecular phylogeny and new infrageneric classification of Mucuna Adans. (Leguminosae-Papilionoideae) including insights from morphology and hypotheses about biogeography. International Journal of Plant Sciences 177(1): 76–89. doi: 10.1086/684131 [Google Scholar]
- Müller K. (2005) SeqState — primer design and sequence statistics for phylogenetic DNA data sets. Applied Bioinformatics 4: 65–69. doi: 10.2165/00822942-200504010-00008 [DOI] [PubMed] [Google Scholar]
- Murphy D. (2008) A review of the classification of Acacia (Leguminosae, Mimosoideae). Muelleria 26(1): 10–26. [Google Scholar]
- Nicolson DH. (1980) Moullava Adanson, recently Wagatea Dalzell (Fabaceae-Caesalpinioideae). In: Manilal KS. (Ed.) Botany and History of Hortus Malabaricus. Oxford: & IBH Publi. Co., New Delhi, 181–185. [Google Scholar]
- Nores MJ, Simpson BB, Hick P, Anton AM, Fortunato RH. (2012) The phylogenetic relationships of four monospecific caesalpinioids (Leguminosae) endemic to southern South America. Taxon 61(4): 790–802. [Google Scholar]
- Nylander JAA. (2004) MrModeltest 2.3. Evolutionary Biology Centre, Uppsala University, Sweden: https://github.com/nylander/MrModeltest2 [accessed 29.04.2016] [Google Scholar]
- Orwa C, Mutua A, Kindt R, Jamnadass R, Anthony S. (2009) Agroforestree Database: a tree reference and selection guide version 4.0. http://www.worldagroforestry.org/sites/treedbs/treedatabase.asp [accessed 15.05.2015]
- Pedley L. (1997) Notes on Caesalpinia subg. Mezoneuron (Leguminosae: Caesalpinioideae) in Australia. Austrobaileya 5(1): 97–102. [Google Scholar]
- Pennington RT, Lavin M. (2016) , The contrasting nature of woody plant species in different neotropical forest biomes reflects differences in ecological stability. New Phytologist 210: 25–37. doi: 10.1111/nph.13724 [DOI] [PubMed] [Google Scholar]
- Philippe H, Snell EA, Bapteste E, Lopez P, Holland PWH, Casane D. (2004) Phylogenomics of Eukaryotes: impact of missing data on large alignments. Molecular Biology and Evolution 21(9): 1740–1752. doi: 10.1093/molbev/msh182 [DOI] [PubMed] [Google Scholar]
- Polhill RM, Vidal JE. (1981) Caesalpinieae. In: Polhill RM, Raven PH. (Eds) Advances in Legume Systematics, Part 1 Kew Royal Botanic Gardens, Richmond, 81–95. [Google Scholar]
- Pyron RA, Burbrink FT, Colli GR, Oca ANMd, Vitt LJ, Kuczynski CA, Wiens JJ. (2011) The phylogeny of advanced snakes (Colubroidea), with discovery of a new subfamily and comparison of support methods for likelihood trees. Molecular Phylogenetics and Evolution 58(2): 329–342. doi: 10.1016/j.ympev.2010.11.006 [DOI] [PubMed] [Google Scholar]
- Rambaut A, Suchard MA, Xie D, Drummond AJ. (2014) Tracer v.1.6. http://tree.bio.ed.ac.uk/software/tracer/ [accessed 29.04.2016]
- Rando JG, Zuntini AR, Conceição AS, van den Berg C, Pirani JR, De Queiroz LP. (2016) Phylogeny of Chamaecrista ser. Coriaceae (Leguminosae) unveils a lineage recently diversified in Brazilian Campo Rupestre vegetation. International Journal of Plant Sciences 177(1): 3–17. doi: 10.1086/683846 [Google Scholar]
- Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP. (2012) MrBayes 3.2: efficient bayesian phylogenetic inference and model choice across a large model space. Systematic Biology 61: 539–542. doi: 10.1093/sysbio/sys029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross JH. (1977) Fabaceae, subfamily Caesalpinioideae. In: Flora of Southern Africa, vol. 16, no. 2. Botanical Research Institute, Department of Agricultural Technical Services, Pretoria, 1–142.
- Roti-Michelozzi G. (1957) Adumbratio Florae Aethiopicae: 6. Caesalpiniaceae (excl. gen. Cassia). Webbia 13(1): 133–228. [Google Scholar]
- Roux JP. (2003) Caesalpinia. In: Germishuizen G, Meyer L. (Eds) Plants of Southern Africa: An Annotated Checklist, Strelitzia 14 National Botanical Institute, Pretoria. [Google Scholar]
- Rudall PJ, Myers G, Lewis GP. (1994) Floral secretory structures in Caesalpinia sensu lato and related genera. In: Ferguson IK, Tucker S. (Eds) Advances in Legume Systematics, Part 6: Structural Botany. Kew Royal Botanic Gardens, Richmond, 41–52 [Google Scholar]
- Sanjappa M. (1992) Legumes of India. Bishen Singh Mahendra Pal Singh, Dehra Dun, India, 1–338. [Google Scholar]
- Schrire BD, Lewis GP, Lavin M. (2005) Biogeography of the Leguminosae. In: Lewis G, Schrire B, Mackinder B, Lock M. (Eds) Legumes of the World, Kew Royal Botanic Gardens, Richmond, 21–54. [Google Scholar]
- Simmons MP, Ochoterena H. (2000) Gaps as characters in sequence-based phylogenetic analyses. Systematic Biology 49: 369–381. doi: 10.1093/sysbio/49.2.369 [PubMed] [Google Scholar]
- Simon MF, Pastore JFB, Souza AF, Borges LM, Scalon VR, Ribeiro PG, Santos-Silva J, Souza VC, De Queiroz LP. (2016) Molecular phylogeny of Stryphnodendron (Mimosoideae, Leguminosae) and generic delimitations in the Piptadenia group. International Journal of Plant Sciences 177(1): 44–59. doi: 10.1086/684077 [Google Scholar]
- Simpson BB. (1998) A revision of Pomaria (Fabaceae) in North America. Lundellia 1: 46–71. http://w3.biosci.utexas.edu/prc/pdfs/Simpson_Lundellia01.pdf [accessed 29.04.2016] [Google Scholar]
- Simpson BB. (1999) A revision of Hoffmannseggia (Fabaceae) in North America. Lundellia 2: 14–54. http://w3.biosci.utexas.edu/prc/pdfs/Simpson_Lundellia02.pdf [accessed 29.04.2016] [Google Scholar]
- Simpson BB, Lewis GP. (2003) New combinations in Pomaria (Caesalpinioideae: Leguminosae). Kew Bulletin 58(1): 175–184. doi: 10.2307/4119360 [Google Scholar]
- Simpson BB, Miao BM. (1997) The circumscription of Hoffmannseggia (Fabaceae, Caesalpinioideae, Caesalpinieae) and its allies using morphological and cpDNA restriction site data. Plant Systematics and Evolution 205: 157–178. doi: 10.1007/BF01464402 [Google Scholar]
- Simpson BB, Ulibarri EA. (2006) A synopsis of the genus Hoffmannseggia (Leguminosae). Lundellia 9: 7–33. [Google Scholar]
- Simpson BB, Larkin L, Weeks A. (2003) Progress towards resolving the relationships of the Caesalpinia group (Caesalpinieae: Caesalpinioideae: Leguminosae). In: Klitgaard BB, Bruneau A. (Eds) Advances in Legume Systematics, Part 10: Higher Level Systematics. Kew Royal Botanic Gardens, Richmond, 123–148. [Google Scholar]
- Simpson BB, Tate JA, Weeks A. (2004) Phylogeny and character evolution of Hoffmannseggia (Caesalpinieae: Caesalpinioideae: Leguminosae). Systematic Botany 29(4): 933–946. doi: 10.1600/0363644042451044 [Google Scholar]
- Simpson BB, Tate JA, Weeks A. (2005) The biogeography of Hoffmannseggia (Leguminosae, Caesalpinioideae, Caesalpinieae): a tale of many travels. Journal of Biogeography 32(1): 15–27. doi: 10.1111/j.1365-2699.2004.01161.x [Google Scholar]
- Simpson BB, Larkin L, Weeks A, McDill J. (2006) Phylogeny and biogeography of Pomaria (Caesalpinioideae: Leguminosae). Systematic Botany 31(4): 792–804. doi: 10.1600/036364406779695915 [Google Scholar]
- Sirichamorn Y, Adema FACB, Roos MC, van Welzen PC. (2014) Molecular and morphological phylogenetic reconstruction reveals a new generic delimitation of Asian Derris (Fabaceae): reinstatement of Solori and synonymisation of Paraderris with Derris. Taxon 63(3): 522–538. doi: 10.12705/633.13 [Google Scholar]
- Sprague T. (1931) The botanical name of “Tara”. Bulletin of Miscellaneous Information, Royal Botanic Gardens, Kew: (2): 91–96. doi: 10.2307/4102518 [Google Scholar]
- Stamatakis A. (2014) RAxML version 8: a tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 30(9): 1312–1313. doi: 10.1093/bioinformatics/btu033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Standley PC, Steyermark JA. (1946) Haematoxylon L., Flora of Guatemala. Fieldiana: Botanical series 24(5): 137–141. [Google Scholar]
- Swofford DL. (2003) PAUP* Phylogenetic analysis using parsimony (*and other methods). v. 4. Sinauer Associates, Sunderland. [Google Scholar]
- Taberlet P, Gielly L, Pautou G, Bouvet J. (1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology 17(5): 1105–1109. doi: 10.1007/BF00037152 [DOI] [PubMed] [Google Scholar]
- Thulin M. (1980) A new Caesalpinia (Leguminosae) from northeast Kenya. Kew Bulletin 34(4): 819–820. doi: 10.2307/4119075 [Google Scholar]
- Thulin M. (1983) Leguminosae of Ethiopia. Opera Botanica 68: 1–223. [Google Scholar]
- Thulin M. (1993) Flora of Somalia, vol.1, Pteridophyta, Gymnospermae, Angiospermae (Annonaceae-Fabaceae). Kew Royal Botanic Gardens, Richmond, 1–1493. [Google Scholar]
- Ulibarri EA. (1979) Las especies argentinas del genero Hoffmannseggia Cav. (Legum.-Caesalp.). Darwiniana 22(1–3): 135–158. [Google Scholar]
- Ulibarri EA. (1996) Sinopsis de Caesalpinia y Hoffmannseggia (Leguminosae-Caesalpinioideae) de Sud América. Darwiniana 34(1–4): 299–348. [Google Scholar]
- Ulibarri EA. (2005) Zuccagnia punctata (Leguminosae): ¿nuevo o viejo endemismo argentino? Darwiniana 43: 212–215. [Google Scholar]
- Ulibarri EA. (2008) The genera of Caesalpinioideae (Leguminosae) from South America. Darwiniana 46(1): 69–163. [Google Scholar]
- United States Fish and Wildlife Service (1995) Stahlia monosperma (cobana negra) Recovery Plan. U.S. Fish and Wildlife Service, Atlanta, Georgia, 1–15. http://www.fws.gov/caribbean/ES/PDF/Recovery%20Plans/Stahlia%20monosperma.pdf [accessed 01.06.2015] [Google Scholar]
- Vaidya G, Lohman DJ, Meier R. (2011) SequenceMatrix: concatenation software for the fast assembly of multi-gene datasets with character set and codon information. Cladistics 27: 171–180. doi: 10.1111/j.1096-0031.2010.00329.x [DOI] [PubMed] [Google Scholar]
- Vences M, Guayasamin JM, Miralles A, Riva IDL. (2013) To name or not to name: criteria to promote economy of change in Linnaean classification schemes. Zootaxa 3636(2): 201–244. doi: 10.11646/zootaxa.3636.2.1 [DOI] [PubMed] [Google Scholar]
- Verdcourt B. (1979) A manual of New Guinea Legumes, vol. 11 Office of Forests, Division of Botany, Lae, Papua New Guinea, 1–646. [Google Scholar]
- Vidal JE, Hul Thol S. (1974) Révision du genre Pterolobium (Caesalpiniaceae). Bulletin du Muséum National d’histoire naturelle, 3e série 227(15): 1–29. [Google Scholar]
- Vidal JE, Hul Thol S. (1976) Révision des Caesalpinia asiatiques. Bulletin du Muséum National d’histoire naturelle, 3e série 395: 27–81. [Google Scholar]
- Viguier R. (1949) Leguminosae madagascarienses novae. Notulae Systematicae, Herbier du Muséum de Paris 13: 333–369. [Google Scholar]
- Wagner WL, Herbst DR, Sohmer SH. (1999) Manual of the Flowering Plants of Hawai’i, Vols. 1 and 2 University of Hawai’i and Bishop Museum Press, Hawaii, USA. [Google Scholar]
- Warwick MC, Lewis GP. (2009) A revision of Cenostigma (Leguminosae– Caesalpinioideae– Caesalpinieae), a genus endemic to Brazil. Kew Bulletin 64(1): 135–146. doi: 10.1007/s12225-008-9091-1 [Google Scholar]
- Wiens JJ. (2003) Missing data, incomplete taxa, and phylogenetic accuracy. Systematic Biology 52(4): 528–538. doi: 10.1080/10635150390218330 [DOI] [PubMed] [Google Scholar]
- Wiens JJ. (2006) Missing data and the design of phylogenetic analyses. Journal of Biomedical Informatics 39(1): 34–42. doi: 10.1016/j.jbi.2005.04.001 [DOI] [PubMed] [Google Scholar]
- Wieringa JJ. (1999) Monopetalanthus exit: a systematic study of Aphanocalyx, Bikinia, Icuria, Michelsonia and Tetraberlinia (Leguminosae, Caesalpinioideae). PhD thesis, Wageningen Agricultral University, Netherlands. [Google Scholar]
- Wieringa JJ, Mackinder BA, van Proosdij ASJ. (2013) Gabonius gen. nov. (Leguminosae, Caesalpinioideae, Detarieae), a distant cousin of Hymenostegia endemic to Gabon. Phytotaxa 142(1): 15–24. doi: 10.11646/phytotaxa.142.1.2 [Google Scholar]
- Wilczek R. (1951) Deux nouvelles espèces de Caesalpinia du Congo Belge. Bulletin du Jardin botanique de l’État à Bruxelles 21: 83–86. doi: 10.2307/3666811 [Google Scholar]
- Wojciechowski MF, Lavin M, Sanderson MJ. (2004) A phylogeny of legumes (Leguminosae) based on analysis of the plastid matK gene resolves many well-supported subclades within the family. American Journal of Botany 91(11): 1846–1862. doi: 10.3732/ajb.91.11.1846 [DOI] [PubMed] [Google Scholar]
- Zamora Villalobos. (2010) Fabaceae. In: Hammel BE, Grayum MH, Herrera Mora C, Zamora Villalobos N. (Eds) Manual de Plantas de Costa Rica. Missouri Botanical Garden, St-Louis. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table 3. Summary of the branch support results from phylogenetic analyses of the Caesalpinia group.
This dataset is made available under the Open Database License (http://opendatacommons.org/licenses/odbl/1.0/). The Open Database License (ODbL) is a license agreement intended to allow users to freely share, modify, and use this Dataset while maintaining this same freedom for others, provided that the original source and author(s) are credited.
Edeline Gagnon, Anne Bruneau, Colin E. Hughes, Luciano Paganucci De Queiroz, Gwilym P. Lewis
Data type: results from phylogenetic analyses
Explanation note: Bootstrap support from the ML analyses of the six individual loci and the combined datasets, as well as Bootstrap support and Posterior probabilities from the parsimony and Bayesian analyses of the combined datasets, for various proposed genera from Gagnon et al. (2013).