Abstract
Background
Diets rich in animal protein, such as the typical American diet, are thought to create a high acid load. An association between acid load and bone loss has led to the idea that providing positive alkaline salt therapy could have beneficial effects on bone metabolism.
Objective
To investigate the effects of potassium citrate (K-citrate), 40 mEq daily, over one year on bone resorption and formation.
Design
A randomized, double-blind, placebo-controlled trial of 83 women with postmenopausal osteopenia. Levels of bone turnover markers, specifically urinary N-telopeptide of collagen type I (u-NTX), amino-terminal propeptide of type I procollagen (P1NP) bone specific alkaline phosphatase (BSAP) and osteocalcin (OC) were compared. Changes in bone mineral density (BMD) were also examined.
Results
K-Citrate decreased both u-NTX (p=0.005) and serum P1NP (p = <0.001) starting at month 1 and continuing through month 12. No significant change was seen in BSAP or OC. No significant change was seen in lumbar or hip BMD between the two groups.
Conclusions
In women with postmenopausal osteopenia, treatment with K- citrate for one year resulted in a significant decrease in markers of turnover. The effect on markers of bone formation was not consistent. K-citrate may serve as a potential treatment for bone loss that is well tolerated without any significant known long term consequences.
INTRODUCTION
In addition to its structural functions, the skeletal system acts as a metabolic buffer to stabilize blood pH and protect against acidosis. However, the consequence of using alkali in the bones as a buffer is increased calcium excretion and decreased bone mass (1). What role subtle, chronic acid retention plays in causing osteoporosis is an important but only partially answered question.
Normal bone remodeling is a complicated process involving many factors. Through direct hormonal control and normal bone turnover, large amounts of minerals are released through bone resorption and subsequently reincorporated into bone both passively and through new bone formation. Metabolic acidosis accelerates this dissolution process by leaching bicarbonate from skeletal tissue to neutralize blood pH. Over time, acute and chronic states of acid excess may lead to persistently increased bone resorption, with the untoward consequence of increased urinary calcium excretion and decreased bone mass (2).
Past studies have pointed to the contribution of acidogenic diets that are rich in sulfur containing amino acids found in diets high in animal protein, being a cause of accelerated bone loss (3). Either a defect in renal acid handling, perhaps age related, or an increase in the acid load presented to the kidney could contribute to this bone loss. Diets high in animal protein lead to an increase in net acid excretion (NAE) because the sulfur containing amino acids are metabolized to sulfuric acid. Such states of protein excess are associated with increased bone resorption, high u-NTX values and increased urinary calcium excretion, all direct consequences of accelerated bone breakdown (4). Increased bone resorption and bone loss predisposes to the loss of structural integrity and increased risk of fracture. Furthermore, increased urinary calcium excretion can predispose to nephrolithiasis. (5).
It has been known that alkali supplementation can improve calcium and phosphorus balance as early as 6 days after treatment. (6). Recent publications report the administration of exogenous alkaline salts may be used to counteract the effects of metabolic acidosis and acidogenic diets. These studies had heterogenous populations and limited controls and were of short duration. The current randomized, placebo controlled trial investigates the effects of 40 mEq K-citrate supplementation in postmenopausal women with osteopenia over a one year period. The present study population is known to be at higher risk for age-related bone loss, hence an appropriate “target” population. K-citrate rather than sodium bicarbonate was chosen to avoid the sodium load. Short term studies have shown that markers of bone formation and calcium balance improve with 90 mEq/day of K-citrate. (6) However sustained supplementation with this dose often leads to gastrointestinal intolerance. K-citrate 40meq is a dose often used to prevent kidney stone recurrence and is well tolerated. Finding effective means of bone protection without significant long term risk could provide added value and benefit in the prevention and treatment of bone loss in postmenopausal women.
SUBJECTS and METHODS
Participants
Subjects were eligible for inclusion if they had osteopenia (T-score at the lumbar spine or hip between −1.0 and −2.5) with no history of fragility fracture and were more than two years post menopause. There is no clear current consensus regarding treatment in this group, therefore randomizing them to an experimental regimen or placebo was not denying them standard of care. Women with T-scores less than −2.5 were eligible if they were unable or unwilling to take any other medications for their bone loss. Exclusion criteria included renal insufficiency (GFR < 30 mL/min or creatinine > 2.0 ng/dL); use of potassium sparing diuretics or potassium supplements; baseline hyperkalemia (K > 5.0 mmol/L); secondary causes of osteoporosis or metabolic bone disease (primary hyperparathyroidism, Paget’s disease of the bone, thyroid disease, or malabsorption); conditions that could affect treatment (untreated Addison’s disease, severe myocardial damage, acute dehydration, delayed gastric emptying, esophageal compression, or intestinal obstruction or stricture); were taking drugs for bone metabolism, thiazide diuretics, anticholinergic medication, or aromatase inhibitors; or active urinary tract infections. Current bisphosphonate users were excluded as were those with a history of bisphosphonate use less than 2 years prior to the start of the study.
Participants were instructed to maintain eating habits throughout the study and were advised to refrain from beginning new medications that may interfere with bone metabolism; if either dietary or medication change occurred, study protocol was followed, but the change was noted in the participant’s chart.
Ethics
Participants were recruited from a single academic center. Subjects underwent screening at the Clinical Translational Science Center (CTSC) at Weill Cornell Medical College (WCMC). Study visits occurred at the CTSC where investigators administered and monitored questionnaires, compliance, adverse events, and endpoint measurements. Subjects were assigned an anonymous study number at the beginning of the trial, which was used to track the participant’s data throughout the study. The protocol was approved by the Institutional Review Board (IRB) and the procedures followed were in accordance with the ethical standards of the IRB and the CTSC. All patients provided informed consent.
Treatment Groups
Participants were assigned to either the treatment or placebo group using a randomization schema generated by the statistician. The randomization method was blocked randomization with a blocking factor of 4. The blocked randomization was not stratified by any other factors. The study was conducted in a double blind manner. The study medication, K-citrate, or placebo, was dispensed through the New York Presbyterian Hospital (NYPH) pharmacy. Bottles in the pharmacy were sequentially numbered and the number was linked to the blocked randomization scheme. Only the statistician and the pharmacist knew the meaning of the numbered codes and only the statistician knew the blocking assignment. Blocked randomization with balanced randomization of each block and blocks of the same size was performed by the RANDOM procedure within the WinPepi Version 11.1.
Investigators who administered questionnaires and assessed compliance, adverse effects or endpoint information were blind to group assignment. Only study investigators were able to enroll participants in the study and assign them to treatment arms. Those assigned to the treatment group received the study drug (40 mEq daily K Citrate: two 10 mEq tablets twice daily); those assigned to the control group received inert tablets of the same quantity. All participants received daily supplementation with Citracal (630 mg calcium citrate and 400 IU vitamin D3 per two caplets). All supplements and medications were provided by Mission Pharmacal/Bayer Pharmaceuticals in Boerne, Texas. Subjects discontinued their prior supplements at the time of entry to the study and were advised to adhere to the standardized supplementation regimen outlined by the protocol.
Measurements and Outcomes
Subjects were evaluated at baseline, 1, 3, 6, and 12 months. The following outcomes were measured: change in bone turnover markers including u-NTX, BSAP, OC and P1NP; changes in 24 hour urinary concentrations of citrate, sulfate, and calcium; and changes in BMD measured from baseline to 12 months. Adverse events and compliance were measured at each visit over the study duration. Adverse events pertained to medication side effects, including, but not limited to, gastrointestinal complaints, nausea, diarrhea, and stomach pain, as well as the development of hyperkalemia or metabolic acidosis. If any of the following occurred, potassium exceeded 5.2 mmol/L; bicarbonate level exceeded 32 mmol/L; creatinine increased by more than 30% or rose above 2.0 ng/dL; or GFR was < 60, study medications were stopped until the parameter normalized, at which point the medication was resumed at half dose: Compliance was assessed by remaining pill count; good compliance was defined at ≤ 20% of pills remaining, or ≤ 18 pills remaining for each 3-month dose allocation.
Baseline measurements included dietary assessment (block food frequency questionnaire) and blood pressure. Laboratory evaluation was performed at the General Core Laboratory at WCMC and included a basic metabolic panel, calcium, albumin and thyroid stimulating hormone (TSH). 25-OH and 1,25(OH)2 Vitamin D were measured by radioimmunoassay (Immunodiagnostic Systems, Scottsdale, Arizona). The interassay coefficient of variation (CV) was <8.2% and <13%, respectively. Intact parathyroid hormone (i-PTH) was measured by immunoradiometric assay (Scantibodies Laboratories, Santee, California; CV <6.4%). Markers of bone turnover included osteocalcin (OC: quantitative immunoradiometric assay, DiaSorin, Stillwater, Minnesota; CV <9.5%), bone specific alkaline phosphatase (BSAP: solid phase monoclonal antibody immunoenzymetric assay, Immunodiagnostics Systems, Scottsdale, Arizona; CV<6.4%), procollagen type 1 amino-terminal propeptide (P1NP: quantitative radioimmunoassay, Orion Diagnostica, Espoo, Finland; CV<9.8%), urinary N-telopeptide (U-NTX: quantitative enzyme-linked immunosorbent assay kit, Wampole Laboratories INC Princeton, New Jersey; CV<5.0%). All specimens were collected as fasting morning samples. The urinary-NTX was a second morning void. The specimens were frozen and batch analyzed. 24 hour urinary collections for calcium, creatinine, sulfate, citrate and sodium were analyzed at Quest Diagnostics. BMD was performed at lumbar spine, total hip and femoral neck using dual-energy X-ray Absorptiometry (DXA) Hologic; Bedford, Massachusetts. The least significant change (LSC) for the DXA was 0.025 at the lumbar spine, 0.025 at the femoral neck and 0.015 at the radius. Two technologists, both certified by the International Society for Clinical Densitometry, performed all DXA testing on the participants.
STATISTICAL CONSIDERATIONS
The analysis of primary and secondary endpoints was performed following intention to treat prinicples using SPSS software. The primary endpoint was percent change from baseline in bone markers at 1,3,6 and 12 months. The secondary endpoints included BMD and urinary levels of citrate, sulfate and calcium measured as the change from baseline to 12 months. The sample size calculation was based on the comparison of mean change in urinary N-telopeptide between the treatment group and the placebo group. The trial was powered at 80% and a 0.05 two-sided significance level was 29 patients per group. After allowing for dropouts, the sample size per group was estimated to be 34 for a total sample size of 68 patients.
Within-group and between group comparisons of all measures were performed by the paired t-test or Wilcoxon signed-rank test, as appropriate. The Pearson correlation coefficient (r) was used to evaluate the correlation between dietary protein and urinary sulfate at baseline. All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. Level of acidosis as determined by abnormal urine tests (low citrate and/or high sulfate and low urinary pH) and dietary intake of acidogenic compounds at baseline, was used a moderator variable. All analyses were performed in SAS Version 9.3 (SAS Institute, Inc., Cary, NC) and SPSS Version 21.0 (SPSS Inc., Chicago, IL).
RESULTS
Baseline Characteristics
A total of 83 subjects were enrolled in this study, with 42 subjects randomized to treatment with 40 meq daily of K-citrate and 41 subjects randomized to placebo. The recruitment period was from August 2006 to April 2011. There was a higher number of subjects with a history of smoking in the placebo group compared to the treatment group (p=0.04). There was no difference in current tobacco use between the groups (p=0.64), however, a greater number of subjects in the placebo group were former smokers (p=0.01). There were also a higher number of subjects in the placebo group who had been taking calcium supplementation at the time of entry into the study (p=0.06). There were no other statistically significant differences between the placebo and K-citrate treated subjects at baseline. (Table 1).
Table 1.
Baseline characteristics in K-citrate versus placebo group.
| Parameter | K-citrate n=42 |
Placebo n=41 |
P-value |
|---|---|---|---|
| Age (years) | 65.1±5.9 | 66.1±7.1 | 0.6 |
| BMI (kg/m2) | 24.0±3.3 | 23.0±2.5 | 0.19 |
| Bisphosphonate Use (number of subjects) | 5 | 9 | 0.18 |
| Fragilty Fracture (number of subjects) | 11 | 9 | 0.71 |
| Smoking History (number of subjects) | 5 | 11 | 0.04 |
| Active Use | 2 | 1 | 0.64 |
| Former Use | 3 | 10 | 0.01 |
| Calcium supplementation (number of subjects) | 16 | 24 | 0.06 |
| Baseline BMD-LS | 0.856±.092 | 0.882±.123 | 0.32 |
| Baseline BMD-TH | 0.791±.084 | 0.811±.085 | 0.32 |
| Baseline BMD-FN | 0.658±.073 | 0.657±.078 | 0.98 |
| iPTH (pg/mL) | 56.9±33.4 | 58.8±25.0 | 0.79 |
| BSAP (ug/L) | 12.2±3.0 | 11.9±3.5 | 0.75 |
| NTX (nmol BCE/nmol creatinine) | 51.6±25.8 | 47.2±19.6 | 0.41 |
| P1NP (microgram/L) | 55.5±23.5 | 50.6±20.1 | 0.32 |
| Osteocalcin (ng/mL) | 6.0±1.6 | 5.7±1.8 | 0.41 |
| 25OHD (ng/mL) | 31.3±13.3 | 35.3±12.7 | 0.19 |
| Dietary Calcium* (mg/d) | 825.4±440.0 | 772.3±367.4 | 0.57 |
| Dietary Vit D* (mg/d) | 155.9±186.9 | 127.1±76.4 | 0.38 |
Calculated by Block Food Frequency Questionnaire
Changes in Bone Turnover with Treatment
Treatment with K-citrate was associated with a significant decrease in the bone resorption marker, u-NTX, at all time points starting at month 1 and continuing through month 12. Urinary NTX in the placebo group decreased significantly at one time point in the study, month 6 (p=0.004). The 12 month time point was not significant (p=0.08) (Figure 1).
Figure 1. Mean urine NTX values.
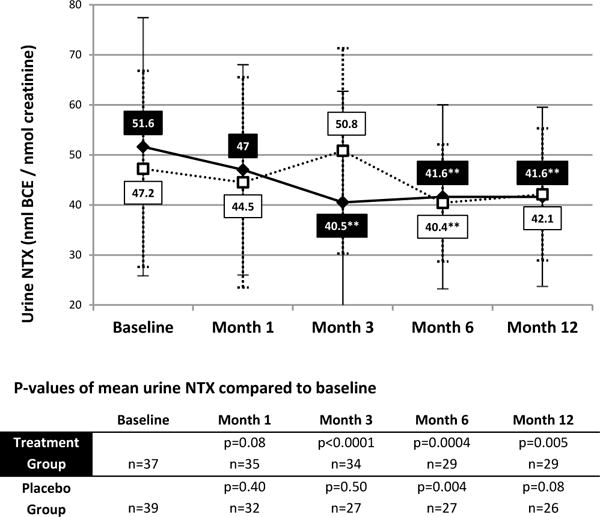

*P-value <0.05 compared to baseline
K-citrate was also associated with a significant decrease in the bone formation measured by P1NP, starting at month 1 and continuing for the duration of the trial. There was no significant change from baseline in the placebo group at month 1 or 3. However, by month 6 and 12 there was a significant decrease in P1NP albeit to a lesser degree. (Figure 2).
Figure 2. Mean P1NP values.


**P-value <0.005 compared to baseline
There were no significant changes in either OC or BSAP in either the treatment or placebo group for the duration of the trial.
Urinary Analysis
Treatment with K- Citrate resulted in a significant increase in 24 hour urine citrate excretion from baseline to month 12 (P=0.006). There was no significant change urinary citrate in the placebo group (p=0.92). The urinary calcium:creatinine (Ca/Cr) ratio was compared in both the placebo and treatment group. In the placebo group, there was no change in the Ca/Cr ratio between baseline and month 12 (P=0.48). Similarly in the treatment group, there was no change in the Ca/Cr ratio between baseline and month 12 (p=0.80). There were no significant changes in either group in urinary sulfate or sodium.
Dietary Analysis
Dietary analysis was conducted to measure the effect of protein intake on level of acidosis. Dietary protein was positively correlated with urinary sulfate at baseline (Pearson correlation coefficient = 0.35, P=0.004.)
Bone Mineral Density
Mean BMD remained stable over the 12 month study period in subjects treated with K-citrate and placebo at all sites.
Compliance and Adverse Events
Compliance was self reported by subjects at each visit and any remaining study drug or calcium supplements were counted when subjects returned their drug bottles. Compliance assessed by pill count was >80% for K-citrate and calcium in both study groups.
Adverse events in relation to the study included fractures, hyperkalemia (K>5.0), and moderate-severe gastrointestinal symptoms. Only one fracture occurred during the study interval in the placebo group (traumatic ankle fracture). There was a higher prevalence of hyperkalemia in the placebo group vs. potassium citrate group (14.6% vs. 4.8%) but was not significant (p=0.16). Moderate to severe gastrointestinal symptoms were more frequently reported in the treatment group (19.0% vs 9.8%, respectively) but was not significant (p=0.23).
DISCUSSION
The present study examines the effect of treatment with K-Citrate 40 meq daily on bone metabolism. K-Citrate significantly decreased u-NTX starting at month 1 and continuing through the duration of the 12 month study period. This decrease in a marker of bone resorption and osteoclast function was clearly associated with K-Citrate treatment and is consistent with the hypothesis that metabolic acidosis increases osteoclast activity (7). Previous studies have confirmed this association. A randomized controlled trial in young women compared the neutralizing effects of calcium carbonate, calcium citrate, and potassium citrate on diet-induced acid load. Results indicated a significant positive effect for potassium citrate in reducing bone resorption (8). Studies in women over the age of 55 corroborate this effect. A randomized control trial in postmenopausal women found sustained systemic alkalization and a significant increase in bone mass as a result of daily 30 mEq oral potassium citrate supplementation (9). A placebo controlled 6-week crossover study reported a decrease in bone resorption markers in response to combined potassium citrate and calcium citrate supplementation (10). However, these trials were either limited by control or duration. The most extensive randomized, placebo controlled trial to date investigated the effects of potassium citrate on bone metabolism, over a 2 year period, in healthy older men and women without osteoporosis. This prospective study reported increased bone mineral density, improved trabecular volume and number, and diminished fracture prediction score with potassium citrate supplementation, compared to placebo (11).
The decrease in bone formation markers was not consistent across markers. PINP decreased in the treatment group and there was no significant change in either osteocalcin or BSAP. These discrepancies in bone formation markers have been previously reported and are poorly understood (11). One confounding factor is the diversity in populations previously evaluated in alkalinization studies. Our population consisted of post-menopausal woman with active bone loss, based on the elevated bone resorptive marker levels when compared to pre-menopausal historical controls (12). The osteopenic post-menopausal group is known to manifest low bone formation making evaluation with less sensitive markers more challenging (8).
The higher urinary citrate seen in the treatment group was indicative of a sustained absorption of base. It was associated with lower urinary sodium and sulfate levels as compared to placebo although these changes did not reach significance. The dosage of 40 meq of K-citrate may not have provided adequate suppression to see a measurable effect on calcium excretion. Higher doses have been associated with significant decreases in calcium excretion (10). Calcium supplementation is often administered clinically twice daily to postmenopausal women with osteopenia and osteoporosis, and therefore using twice daily K-citrate many not be that difficult to implement. However, once daily treatment should be investigated as a future direction.
No significant changes were seen in BMD with DXA analysis between the two groups. The duration of our study was 12 months and may not have been long enough to observe increases in bone density. The dose of 40 meq of K-Citrate may not have been sufficient to produce a change in BMD. Jehle et al.(11) reported significant improvements with a higher dose of 60 meq of K-Citrate in BMD, volumetric density, microarchitecture parameters, and fracture risk assessment using a 24 month model. It is possible that microarchitectural assessments such as high resolution quantitative computed tomography or trabecular bone score measurements may be able to discern differences not able to be seen with routine bone densitometry. These new techniques are promising for closer analysis of changes in bone formation and resorption markers and the correlation with changes in bone microarchitecture before changes in BMD are seen. (13,14)
The current study had several limitations. First, there may have been a dual effect of calcium citrate, which mitigated the net effect of the alkalinizing treatment. Sakhaee et al. investigated the effects of potassium citrate, calcium citrate, and combined treatment, compared to placebo (10). Potassium citrate independently contributed a hypocalciuric effect and increased urinary pH and citrate. Calcium citrate independently reduced PTH and increased calcium absorption. Combined treatment yielded the same independent effects and exhibited significantly decreased bone resorption markers as compared to placebo. However, the change in bone markers was not significant when compared to calcium citrate alone. This is relevant to the present findings in that our placebo group took a calcium supplement in the form of calcium citrate. Since calcium citrate, given to both groups, is also an alkaline salt, the effect of K citrate versus placebo may have been partially masked. We believe that the effect of alkali supplementation on decreasing bone resorption is a base effect rather than the specific base. Dawson-Hughes et al demonstrated a similar effect of decreased bone resorption in subjects treated with sodium and potassium bicarbonate, but not potassium chloride (15). Potassium may be preferable because it avoids the risk of sodium loading and has a history of tolerability in the kidney stone population.
Second, the dose of K-citrate may not have been sufficient to produce a change in BMD. With a higher dose a greater effect on BMD may have been observed. However, this dose was chosen to avoid significant GI side effects. Third, the duration of treatment may have been too short to see changes in BMD. More sensitive indices that might reveal early, favorable changes in bone morphometry, such as histological analyses, high resolution quantitative computed tomography or trabecular bone score may have been more appropriate.
There was a difference in the two groups with respect to history of tobacco use and calcium supplementation with more subjects in the placebo group with a history of smoking and greater use of calcium supplements which may have impacted our results. However, there was no difference in current tobacco use between the groups and given the negative impact of tobacco on bone appears to decrease after smoking cessation, the effect of former tobacco was not likely to have had a substantial impact on our results (16,17). Despite robust calcium supplementation provided to participants, urinary calcium levels did not change during the study among subjects. This was true for total urinary calcium when assessed as the calcium/creatinine ratio. This finding likely reflects the high intakes of subjects at baseline, particularly those in the placebo group. As all subjects were recruited throughout the year and followed for one year, seasonal variations in vitamin D and subsequently calcium were unlikely to have played a major role. The subjects in both groups were equally compliant with study medication and calcium supplements.
In conclusion, supplementation with potassium citrate, in combination with calcium citrate, significantly reduces bone resorption in postmenopausal osteopenic women. Additional long-term studies are needed to confirm this effect. There are presently many questions regarding how and whether to treat postmenopausal women with osteopenia, in whom side effects of treatment may outweigh the benefits. While our results suggest that potassium citrate may have beneficial skeletal effects, additional long-term studies are needed to further investigate the role of this agent for the treatment of postmenopausal women with osteopenia.
Acknowledgments
NSG and EMS provided study oversight. EMS, RSB and JSR designed the research. NSG, RK, and EMS conducted research. PC, NSG and RK analyzed data and performed statistical analysis. NSG wrote the paper and had primary responsibility for final content. RK, RSB, EMS and JSR helped in the writing of the paper. NSG had primary responsibility for final content.
Sources of Support:
1. Private Grant to John S. Rodman, MD
2. Clinical Translational Science Center of Weill Cornell Medical College:Research reported in this publication was supported by the National Center for Advancing Translational Science of the National Institute of Health under Award Number UL1-TR000457.
3. Bayer Mission Pharmaceuticals provided study medications but had no involvement in design, analysis or interpretation of results.
4. Paul Christos was partially supported by the following grant: Clinical Translational Science Center UL1-TR000457-06).
Abbreviations
- BMD
bone mineral density
- BSAP
bone specific alkaline phosphatase
- K-Citrate
potassium citrate
- NAE
net acid excretion
- OC
osteocalcin
- PINP
amino-terminal propeptide of type I procollagen
- u-NTX
urinary N-telopeptide of collagen type
References
- 1.Green J, Kleeman CR. The role of bone in the regulation of systemic acid-base balance. Contrib Nephrol. 1991;91:61–76. doi: 10.1159/000420160. Review. [DOI] [PubMed] [Google Scholar]
- 2.Assapun J, Charoenphandhu N, Krishnamra N. Early acceleration phase and late stationary phase of remodeling imbalance in long bones of male rats exposed to long-standing acidemia: a 10-month longitudinal study using bone histomorphometry. Calcif Tissue Int. 2009 Jul;85(1):1–9. doi: 10.1007/s00223-009-9254-6. [DOI] [PubMed] [Google Scholar]
- 3.Lemann J, Bushinksy D, Hamm L. Bone buffering of acid and base in humans. Am J Physiol. 2003;285:F811–F32. doi: 10.1152/ajprenal.00115.2003. [DOI] [PubMed] [Google Scholar]
- 4.Jajoo R, Song L, Rasmussen H, Harris SS, Dawson-Hughes B. Dietary acid-base balance, bone resorption and calcium excretion. J Am Coll Nutr. 2006 Jun;25(3):224–30. doi: 10.1080/07315724.2006.10719536. [DOI] [PubMed] [Google Scholar]
- 5.Marangella M, Di Stefano M, Casalis S, Berutti S, D’Amelio P, Isaia GC. Effects of potassium citrate supplementation on bone metabolism. Calcif Tissue Int. 2004 Apr;74(4):330–5. doi: 10.1007/s00223-003-0091-8. [DOI] [PubMed] [Google Scholar]
- 6.Sebastian Anthony, Harris Steven T, Ottaway Joan H, Todd Karen M, Morris R Curtis., Jr Improved Mineral Balance and Skeletal Metabolism in Postmenopausal Women Treated with Potassium Bicarbonate. N Engl J Med. 1994;330:1776–1781. doi: 10.1056/NEJM199406233302502. [DOI] [PubMed] [Google Scholar]
- 7.Bushinsky D. Acid-base imbalance and the skeleton. Eur J Nutr. 2001;40:238–244. doi: 10.1007/s394-001-8351-5. [DOI] [PubMed] [Google Scholar]
- 8.Karp HJ, Ketola ME, Lamberg-Allardt CJ. Acute effects of calcium carbonate, calcium citrate and potassium citrate on markers of calcium and bone metabolism in young women. Br J Nutr. 2009 Nov;102(9):1341–7. doi: 10.1017/S0007114509990195. [DOI] [PubMed] [Google Scholar]
- 9.Jehle S, Zanetti A, Muser J, Hulter HN, Krapf R. Partial neutralization of the acidogenic Western diet with potassium citrate increases bone mass in postmenopausal women with osteopenia. J Am Soc Nephrol. 2006 Nov;17(11):3213–22. doi: 10.1681/ASN.2006030233. [DOI] [PubMed] [Google Scholar]
- 10.Sakhaee K, Maalouf NM, Abrams SA, Pak CY. Effects of potassium alkali and calcium supplementation on bone turnover in postmenopausal women. J Clin Endocrinol Metab. 2005 Jun;90(6):3528–33. doi: 10.1210/jc.2004-2451. [DOI] [PubMed] [Google Scholar]
- 11.Jehle S, Hulter HN, Krapf R. Effect of potassium citrate on bone density, microarchitecture, and fracture risk in healthy older adults without osteoporosis: a randomized controlled trial. J Clin Endocrinol Metab. 2013 Jan;98(1):207–17. doi: 10.1210/jc.2012-3099. [DOI] [PubMed] [Google Scholar]
- 12.Garnero P, Shih WJ, Gineyts E, Karpf DB, Delmas PD. Comparison of new biochemical markers of bone turnover in late postmenopausal osteoporotic women in response to alendronate treatment. J Clin Endocrinol Metab. 1994 Dec;79(6):1693–700. doi: 10.1210/jcem.79.6.7989477. [DOI] [PubMed] [Google Scholar]
- 13.Boutroy S, Bouxsein ML, Munoz F, Delmas PD. In vivo assessment of trabecular bone microarchitecture by high-resolution peripheral quantitative computed tomography. J Clin Endocrinol Metab. 2005 Dec;90(12):6508–15. doi: 10.1210/jc.2005-1258. Epub 2005 Sep 27. [DOI] [PubMed] [Google Scholar]
- 14.Liu XS, Stein EM, Zhou B, Zhang CA, Nickolas TL, Cohen A, Thomas V, McMahon DJ, Cosman F, Nieves J, Shane E, Guo XE. Individual trabecula segmentation (ITS) based morphological analyses and microfinite element analysis of HRpQCT images discriminate postmenopausal fragility fractures independent of DXA measurements. J Bone Miner Res. 2012 Feb;27(2):263–72. doi: 10.1002/jbmr.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dawson-Hughes B, Harris SS, Palermo NJ, Castaneda-Sceppa C, Rasmussen HM, Dallal GE. Treatment with potassium bicarbonate lowers calcium excretion and bone resorption in older men and women. J Clin Endocrinol Metab. 2009 Jan;94(1):96–102. doi: 10.1210/jc.2008-1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Cornuz J, Feskanich D, Willett W, Colditz G. Smoking, smoking cessation, and risk of hip fracture in women. Am J Med. 1999 Mar;106:311–314. doi: 10.1016/s0002-9343(99)00022-4. [DOI] [PubMed] [Google Scholar]
- 17.Olofsson H, Byberg L, Mohsen R, Melhus H, Lithell H, Michaelsson K. Smoking and the risk of fracture in men. J Bone Miner Res. 2005 Feb;20:1208–1215. doi: 10.1359/JBMR.050208. [DOI] [PubMed] [Google Scholar]


