Abstract
Migraine is a debilitating condition that affects hundreds of millions of people worldwide. A subset of these patients experience chronic migraine, resulting in long-term disability and a severely lowered quality of life. The development of novel migraine therapies has been slow, partially due to the small number of predictive animal models. We have recently developed a novel model of chronic migraine-associated pain, using the known human migraine trigger, nitroglycerin. Injection of nitroglycerin evokes an acute mechanical hyperalgesia, which is sensitive to the acute migraine therapy sumatriptan. In addition, chronic administration of nitroglycerin produces a progressive and sustained decrease in basal mechanical responses; and this hypersensitivity is blocked by migraine preventatives such as topiramate. This mouse model of chronic migraine can be used to study the mechanisms underlying progression of migraine from an episodic to a chronic disorder, and for identifying and screening novel acute and preventive migraine therapies.
Keywords: headache, pain, hyperalgesia, rodent, nitric oxide, nitroglycerin
Introduction
Migraine is one of the most common disorders affecting the general population, resulting in severe and substantial episodic disability and lost productivity worldwide. In the United States there are over 30 million migraine sufferers, of which 3 million experience chronic migraine, defined as over 15 headache days/month (Bigal, Serrano, Reed, & Lipton, 2008; ICHD, 2013; Stewart, Wood, Reed, Roy, & Lipton, 2008; Stovner et al., 2007; Victor, Hu, Campbell, Buse, & Lipton, 2010). Despite the very high prevalence of migraine, therapeutic strategies are limited, and less than 50% of chronic migraineurs are satisfied with their treatment (M.E. Bigal et al., 2008). A significant obstacle to the identification of new headache therapies has been the difficulty in modeling these disorders in animals. Our lab has dedicated significant effort to developing a novel model of chronic migraine (Pradhan, Smith, McGuire, et al., 2014; Pradhan, Smith, Zyuzin, & Charles, 2014; Tipton, Tarash, McGuire, Charles, & Pradhan, 2015). Our model specifically examines increased pain sensitivity that is associated with migraine. Allodynia, the perception of normally innocuous sensory stimuli as uncomfortable, can be used as a quantifiable measure of the sensory dysregulation that accompanies migraine. A significant percentage of migraine patients experience tactile allodynia, and this phenomenon is associated with increased migraine frequency and severity (M. E. Bigal et al., 2008; Burstein, Collins, & Jakubowski, 2004; Burstein et al., 2010; Charles & Brennan, 2008).
One approach to modeling acute migraine is the quantification of increased sensory sensitivity in response to known migraine triggers. Nitroglycerin (NTG) reliably triggers headache in normal subjects, and migraine without aura in migraine susceptible patients (Afridi et al., 2005; Christiansen, Thomsen, Daugaard, Ulrich, & Olesen, 1999; Iversen, Olesen, & Tfelt-Hansen, 1989; Olesen, 2008); NTG-evoked migraine is a commonly used experimental model in humans (Olesen, 2008, 2010). NTG-evoked hyperalgesia in rodents has been developed as a model for sensory hypersensitivity associated with migraine (E.A. Bates et al., 2010; Markovics et al., 2012). Acute nitroglycerin was previously shown to produce thermal and mechanical allodynia in mice that was reversed by the migraine therapies sumatriptan (E.A. Bates et al., 2010) and a CGRP receptor antagonist (Capuano, Greco, Navarra, & Tringali, 2014). In addition, in a transgenic mouse model of familial migraine, animals expressing a human migraine gene (casein kinase 1 delta) showed a greater sensitivity to NTG-evoked hyperalgesia compared to wild-type controls (Brennan et al., 2013). Further, NTG has also been shown to produce light-aversive behavior (Markovics et al., 2012), and increased meningeal blood flow in mice (Greco et al., 2011; Markovics et al., 2012). Taken together, these results indicate that NTG may effectively model migraine-like symptoms in rodents (Erdener & Dalkara, 2014).
Migraine begins as an episodic disorder, but as with other types of pain, it can develop into a chronic condition. NTG has primarily been used to study acute migraine-associated symptoms, and we adapted this model to study chronic migraine. Using chronic intermittent injection of NTG, we have developed a test that models the progression of migraine from an acute to chronic state (Pradhan, Smith, McGuire, et al., 2014). In this model, each NTG treatment evokes a severe acute hyperalgesia which peaks at 2 hours and lasts for several hours after each injection; and is inhibited by the migraine abortive therapy sumatriptan (Pradhan, Smith, McGuire, et al., 2014; Pradhan, Smith, Zyuzin, et al., 2014). In addition, chronic treatment with NTG also results in the development of a progressive and sustained basal hypersensitivity, in which animals are sensitive to mechanical stimulation days after NTG administration (Pradhan, Smith, McGuire, et al., 2014; Tipton et al., 2015). These results are consistent with clinical observations of patients with chronic migraine in whom allodynia may occur both between and during migraine attacks. Furthermore, we have found that migraine preventatives, such as topiramate and propranolol, can block this basal hypersensitivity (Pradhan, Smith, McGuire, et al., 2014; Tipton et al., 2015). We propose that the chronic intermittent injection of NTG can be used as a model of chronic migraine-associated pain.
Basic Protocol 1
Basic Protocol Title: Assessment of chronic migraine-associated pain in mice
In order to model chronic migraine-associated pain, NTG is administered systemically every second day for 9 days (5 injections total). Animals are first habituated to the testing racks for 2 days prior to testing. On test days, basal responses are assessed before NTG administration, and post-treatment responses are determined 2 hours after NTG injection. Briefly, mechanical sensitivity is determined using manual von Frey hair stimulation. In this method, a series of monofilaments are applied to the plantar surface of the hindpaw. The 50% threshold for mechanical response is determined using the up-and-down method (Chaplan, Bach, Pogrel, Chung, & Yaksh, 1994). In this case, the plantar surface of the animal hindpaw will be stimulated with a series of eight von Frey monofilaments (bending force ranging from 0.008 to 2 g); and depending on the response to the tested filament, the next filament is either heavier (up) or lighter (down). At the end of the post-treatment test animals are returned to their home cages until the next test day (48 hours later). All experiments must be performed in accordance with the institution's animal care committee guidelines.
Materials
C57BL/6 mice, 8-24 weeks old, group housed (we usually use mice from Jackson Labs). We have also tested C57BL/6 mice from Charles River, and mice on a 50:50 C57BL6:Sv129 background, with similar results. We use both male and female mice, usually an equal number in the same experiment.
Saline (0.9% Sodium Chloride Injection, USP, 100 mL VIAFLEX Plastic Container Multi Pack; Ref No. 2B1307)
Nitroglycerin (5 mg/mL) (From American Regent, NDC 0517-4810-25), stored at room temperature in low-light (eg. in a drawer or cabinet).
5-15 ml tubes (for diluted NTG)
Quiet testing room with low light conditions (∼30-50 lux)
Mesh stand for testing (eg. IITC Inc mesh stand part 410)
Mouse plexiglass testing boxes (eg. IITC Inc animal enclosure part 433)
von Frey filaments – (weight in grams/size): 0.008/1.65, 0.04/2.44, 0.07/2.83, 0.16/3.22, 0.4/3.61, 0.6/3.84, 1/4.08, 2/4.31 (eg. BioSeb, Model: Bio-VF-M, or Stoelting Touch Test Sensory Probes)
1 mL syringes with a tuberculin slip tip, sterile (from BD, Ref No. 309659)
26 ½ G needles, sterile (From BD, Ref No. 305111)
Sharps box
Diaper/Absorbent pads
Digital Scale to weigh animals
Permanent Marker
Notebook
Pen
Timer
Up-and-down calculator http://www.u.arizona.edu/∼michaelo/jflashdixon.html
Protocol steps—Step annotations
Allow mice to habituate to your animal colony for at least 1 week. We recommend ordering mice at 8-10 weeks of age, and group housing them 2-5 mice/cage under standard housing conditions. We have tested mice up to 6 months old with similar results. Our standard experiments run 24 mice at a time.
-
After acclimation to the vivarium/animal colony, animals are habituated to the behavioral testing room. This is a separate room with low light conditions (∼30-50 lux), which houses the mesh stand and testing boxes. Place diaper pads down on the table below the mesh rack for easy clean-up. Before placing mice on the rack, label their tails for identification with permanent marker (stripes, numbers, letters, etc). Place each mouse in a testing box on the mesh rack in a randomized fashion. Write down the order in which mice are placed on the rack, and be sure to consistently place the same mouse in the same position. Once all mice have been placed onto the mesh rack, allow them to habituate to the rack for 20-30 minutes. Gently remove each animal from the rack and place it back in its home cage, and return the cages to the vivarium. Repeat this habituation process for 2-3 days before beginning the chronic NTG experiment, ideally at the same time every day.
Ideally, the behavioral testing room should be separate from the vivarium, and should only be used for pain testing during the days of habituation and testing. This room should be quiet, with low-light conditions (∼30-50 lux), and minimal disruptions during testing to minimize animal stress and variability.
-
On day 1 of testing, prepare NTG in a tube prior to the start of the experiment. The dose of NTG used is 10 mg/kg, and it is injected in a volume of 10 ml/kg; therefore a 1 mg/ml solution is required. NTG should be prepared fresh on each test day. Note that half of your animals will receive control (saline) injections, and half will receive NTG. Thus, only prepare the drug necessary for a given test day (See example 1).
Example 1:- If you have 24 mice, 12 will receive vehicle and 12 will receive NTG. The dose of NTG is 10 mg/kg and it is injected in a 10 ml/kg volume, so you must prepare a 1 mg/ml solution (Concentration2). Therefore if your mice are 25 g, you will need to prepare 3.5ml of NTG (0.25 ml/mouse × 12 mice = 3 ml + 0.5 ml extra; Volume2).
- The NTG stock solution is 5 mg/ml (Concentration1), therefore:
- Concentration1 × Volume1= Concentration2 × Volume2
- (5 mg/mL) V1= (1mg/mL)*(3.5mL)
- V1=0.7mL
To prepare the 1mg/mL solution, 0.7mL of stock NTG (5 mg/mL) is diluted in 2.8mL of saline. Be aware that NTG is light-sensitive, and binds to soft plastics (Yuen, Denman, Sokoloski, & Burkman, 1979). Keep 5 mg/mL NTG in the glass, light-proof container until ready to dilute on each test day.
Once NTG has been prepared, bring all of the materials to the behavioral testing room. Bring the mice to the testing room, and place each mouse on the rack to habituate for 15-20 minutes. Be sure to place each mouse in the same box every time.
-
After the habituation period, begin testing. The von Frey filaments are applied by approaching the plantar surface of the paw from the underside of the mesh stand. Always start by testing the 0.4g (3.61) von Frey filament first. In all cases, the tip of the filament is pressed against the plantar surface of one hindpaw. Always test the same hindpaw in all animals (e.g. our lab always tests the left hindpaw). Before testing a filament make sure that the animal is standing on all four paws. The tip of the filament is placed at the center of the plantar surface, and avoid the fat pads associated with each digit (Figure 1). Gently push the filament against the surface of the hindpaw until it bends, and maintain for 1-3 seconds. A response is defined as withdrawal, shaking, or licking of the paw. If the animal does not give a response, repeat this procedure with the next heaviest filament (0.6g/3.84). If the animal does respond, repeat this procedure with the next lightest filament (0.16g/3.22). Responses to each filament are recorded. Denote a lack of response to a filament with “O” in your lab notebook, and a response with the letter “X”. After the animal responds for the first time, continue testing 4 more filaments with the up-down method. Write down the pattern of responses, and the final filament tested. Each mouse should be tested to completion before moving to the next mouse. It should take approximately 30-60s to test each mouse. Repeat this procedure for every mouse. These are the basal thresholds. See example 2.
Example 2:-
The first mouse has a basal threshold of OOXOXXO, and the last filament tested was 3.61. This means that there was no response at 3.61 (O), no response at 3.84 (O), response at 4.08 (X), no response at 3.84 (O), response at 4.08 (X), response at 3.84 (X), and no response at 3.61 (O). This pattern and ending filament will be used to determine the mechanical threshold. We refer experimenters to the following papers for the formulas to calculate the thresholds (Chaplan et al., 1994; Dixon, 1965). The mean interval in this example is 0.354, which is the average interval between filaments. There is also an online calculator at the below site: http://www.u.arizona.edu/∼michaelo/jflashdixon.htmlThe average basal threshold for a naïve mouse is between 0.8-1.4 g using this method. To decrease variability, always test the animals around the same time of day, avoiding the times closest to the change in light cycle. For example, we test primarily between 8-15:00h.
-
-
Once the basal thresholds have been assessed for all mice, counterbalance the mice into separate NTG and control groups. There should be an equal number of animals in each group, and if using both sexes, they should be evenly distributed between groups.
The experimenter should be blinded to the treatment group when testing these animals. If possible, the NTG and saline tubes can be blinded beforehand. Alternatively, the animals' weights and groups can be recorded on one piece of paper that is hidden during basal and post-treatment testing.
-
Remove each animal from the testing box and weigh it on the digital scale. Record the weight of the animal, and administer an intraperitoneal injection of saline or 10 mg/kg NTG. See Example 3.
Example 3:- After assessing the basal threshold, the mouse weighs 27g. This mouse will receive 0.27mL by intraperitoneal injection.
-
After the first mouse has been injected, place it back in its home cage. Set the timer for 2 hours. Repeat this procedure for all the animals, and note the weight of each mouse.
Although you will be testing 2 hours after the i.p. injection, the animals need to habituate to the rack prior to testing. Therefore, you will return to the behavioral testing room within 1 hour and 40 minutes post-injection.
-
After 1 hour and 40 minutes, return to the behavioral room and place each mouse back in the same position on the rack. Allow the mice to habituate for 20 minutes. At the 2 hour time point, begin assessing the post-treatment responses. Post-treatment thresholds are assessed in the same manner as the basal thresholds (Step 5).
To decrease variability, the same experimenter should test both the basal and post-treatment responses.
Once all post-treatment responses have been determined, place each mouse back in its home cage. Return them to the animal colony. This testing procedure will be repeated every other day for 9 days (i.e. days 1,3,5,7, and 9).
Day 9 is the final test day. At the end of this experiment, you can continue analyzing the animals for recovery, in which case basal responses will be assessed for up to 2 weeks following the final treatment.
Figure 1.
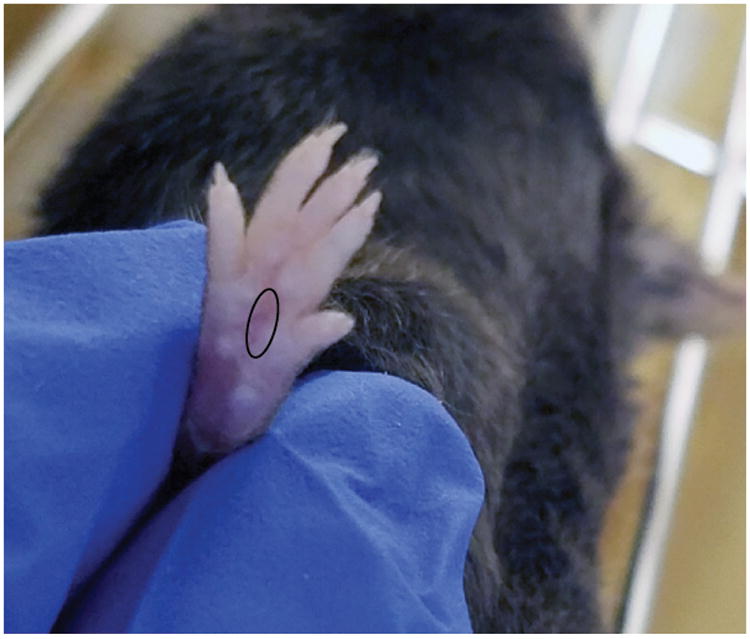
Representative image of where to test on the mouse hindpaw.
Reagents and Solutions
Nitroglycerin (kept in a light-proof glass bottle at room temperature). Note the expiration date on the bottle.
Make a fresh batch of diluted NTG on each test day in a separate drug tube.
Saline (kept at room temperature)
Commentary
Background Information
NTG is a known migraine trigger, and is used as a human experimental model of migraine (Iversen et al., 1989). Acute administration of NTG has been used to model acute migraine in mice (E.A. Bates et al., 2010). In this acute study, similar doses of NTG produced mechanical and thermal hyperalgesia which was blocked by the migraine medication sumatriptan. This paper also showed that NTG administration increased expression of the immediate early gene cFos in the upper cervical spinal cord and the trigeminal nucleus caudalis, two regions involved with migraine pathogenesis (E. A. Bates et al., 2010). For the chronic migraine model, we modified this acute protocol by giving NTG every second day for 9 days, and assessing basal and post-treatment responses. The chronic intermittent administration of NTG results in the development of a basal hypersensitivity to mechanical stimulation. In addition, each injection of NTG also produces an acute mechanical hyperalgesia, which is inhibited by the migraine abortive therapy sumatriptan (Pradhan, Smith, McGuire, et al., 2014; Pradhan, Smith, Zyuzin, et al., 2014). Both the chronic hypersensitivity and acute hyperalgesia are blocked by the migraine preventatives topiramate (Pradhan, Smith, McGuire, et al., 2014) and propranolol (Tipton et al., 2015), supporting the notion that this model reflects a chronic migraine-like state. This model can also be adapted to test for thermal responses. We have tested the effects of chronic NTG in a warm water tail immersion assay (46-48°C) (Pradhan, Smith, Zyuzin, et al., 2014), and we see similar changes in pain sensitivity. In addition, others have reported that acute and chronic NTG can produce light aversion, which models the photophobia associated with migraine (Farajdokht, Babri, Karimi, & Mohaddes, 2016; Markovics et al., 2012; Sufka et al., 2016). Taken together, multiple lines of evidence indicate that NTG administration in rodents can be used to effectively model migraine-associated behaviors.
Critical Parameters
Female mice
Female C57BL/6 mice have been tested extensively in this model. Their responses are slightly more variable, and they are more sensitive compared to males (Pradhan, Smith, McGuire, et al., 2014).
Vehicle Preparation
The vehicle control used in this protocol is 0.9% saline. However, the 5 mg/ml NTG from American Regent is in 30% propylene glycol, 30% ethanol, and water. After dilution to a 1 mg/ml solution, the NTG injected into the mice is in a 6% propylene glycol, 6% ethanol, 0.9% saline solution. We have tested this vehicle previously, and found that it did not produce any mechanical sensitivity, and effects were comparable to saline injection (Pradhan, Smith, McGuire, et al., 2014).
Associative learning
Animals are tested repeatedly in this paradigm, which can result in associative learning. Specifically, mice can associate the mechanical stimulus with a paw withdrawal, and therefore show reduced thresholds which may be interpreted as increased nociception. We have explored this issue by treating animals with NTG or vehicle as described, but only testing them on the first and last days (days 1 and 9), thus avoiding repeated testing. Under these conditions, chronic intermittent NTG administration still produced a highly significant and severe basal hypersensitivity (Pradhan, Smith, McGuire, et al., 2014). These results indicate that the learning associated with repeated testing provides a minor contribution to the observed mechanical hyperalgesia.
Troubleshooting
Habituation to the mesh rack will help to reduce variability. Without habituation, the mice explore the novel environment, which can produce false positives when testing von Frey filaments. In addition, the behavior room should be quiet, and low-light conditions (∼30-50 lux) are preferable.
Statistical Analyses
Data is typically expressed as mean ± s.e.m. A n of at least 8 animals is recommended for statistical power, and we encourage investigators to perform a data analysis based on their variability. For acute pain experiments or dose response curves, one-way ANOVAs are performed. For chronic pain experiments, two-way repeated measures ANOVA is performed, with the factors of treatment (vehicle/NTG) and time (days 1, 3, 5, 7 and 9); with the basal and post-treatment responses analyzed separately. Holm-Sidak, Tukey's or Dunnett's can be used for post-hoc analysis. A significance level of p<0.05 is used.
Understanding Results
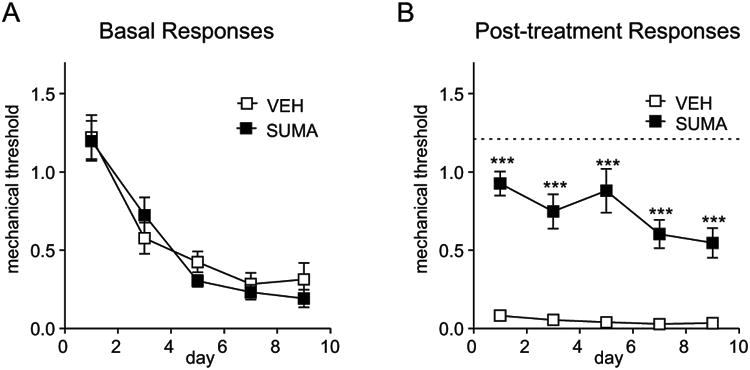
In C57BL/6 mice, each injection of NTG injection should result in a significantly reduced threshold within 2 hours of administration (post-treatment responses, Figure 2b). In addition, repeated injection of NTG will also produce a progressive decrease in basal mechanical responses (basal responses, Figure 2a). By day 9, the basal response for NTG mice will be almost as low as after NTG administration. The interpretation of these results is that the acute NTG-evoked hyperalgesia (post-treatment responses) models an acute migraine attack, whereas the basal hypersensitivity models the progression of migraine from an episodic to chronic state. The chronic intermittent administration of saline will not significantly change from basal responses observed on day 1. There will be a certain amount of variability, and likely a small decrease due to habituation over the treatment paradigm, but these responses will not vary significantly from the initial naïve response.
Figure 2.

Chronic intermittent nitroglycerin produces a sustained basal hypersensitivity, and evokes acute hyperalgesia. Male C57BL/6J mice were treated every other day for 9 days with nitroglycerin (10 mg/kg, i.p.) or 0.9% saline control. A) Basal mechanical responses, assessed prior to vehicle or nitroglycerin administration, significantly decreased in the nitroglycerin group during the treatment period (n=12/group, 2-way RM ANOVA and Holm-Sidak post-hoc analysis. p<0.001 for drug, time and interaction; ***p<0.001 as compared to vehicle. B) Post-treatment mechanical responses, assessed 2 hours after vehicle or nitroglycerin injection, were significantly decreased in the nitroglycerin group on each test day. 2-way RM ANOVA, p<0.001 for time.
We have validated this assay using a number of migraine medications, including sumatriptan (Pradhan, Smith, McGuire, et al., 2014; Pradhan, Smith, Zyuzin, et al., 2014). Triptans are acute migraine treatments, and are not used as preventatives. We have previously tested sumatriptan within this model, and given it 1h15min after NTG. We found that sumatriptan treatment did not affect the basal hypersensitivity induced by chronic NTG (Figure 2A). However, sumatriptan does inhibit the acute hyperalgesia evoked by NTG, which is measured 2h after NTG/VEH injection (Figure 2B). Once the experimenter is able to proficiently assess the basal and post-treatment thresholds following chronic NTG/control treatment, this assay can be adapted to test different hypotheses. For example, this model can be used to screen novel migraine therapies (Pradhan, Smith, Zyuzin, et al., 2014; Tipton et al., 2015).
Time Considerations
The two habituation days take approximately 30-40 minutes. The experiment is conducted every second day over 9 days. On each test day, one can expect to spend about 3-4 hours from start to finish. If an individual is new to testing with the von Frey hair filaments, we recommend that s/he practice assessing baseline thresholds before performing the experiment. Once the thresholds range from 0.8-1.2, then the individual can confidently begin testing NTG-treated animals.
Figure 3.
Acute, but not chronic, hyperalgesia induced by nitroglycerin is blocked by sumatriptan. Male and female C57BL/6J mice were treated every other day for 9 days with nitroglycerin (10 mg/kg, i.p.), and 1h15min later with vehicle (0.9% saline) or sumatriptan (0.6 mg/kg i.p.). A) Basal mechanical responses, assessed prior to nitroglycerin administration, significantly decreased in both vehicle and sumatriptan groups during the treatment period. B) Post-treatment mechanical responses, assessed 45 min after sumatriptan/vehicle injection (2h post-nitroglycerin), indicate that nitroglycerin produces an acute hyperalgesia (vehicle group) which is significantly inhibited by sumatriptan. (n=11/group, 2-way RM ANOVA and Holm-Sidak post-hoc analysis. p<0.001 for drug, time and interaction; ***p<0.001 as compared to vehicle. This figure was previously published by Pradhan et al., Characterization of a novel model of chronic migraine, Pain, 155 (2):269-274; http://journals.lww.com/pain/pages/articleviewer.aspx?year=2014&issue=02000&article=00010&type=abstract.
Significance Statement.
Chronic migraine is a highly debilitating disorder that affects approximately 3 million people in the United States. Current therapies are poorly tolerated or ineffective, and a large proportion of chronic migraine patients are dissatisfied with their treatments. Considering the magnitude of this disorder, there are relatively few animal models of acute migraine, and even fewer of chronic migraine. In this unit, we describe a novel model of chronic migraine using repeated administration of the known human migraine trigger, nitroglycerin. This mouse model can be used to screen novel pharmacological therapies for migraine. Furthermore, this model can also be used to understand the mechanisms that promote the movement of migraine from an acute to chronic condition.
Acknowledgments
This work was supported by NIH grants DA031243 and DA040688, and DOD grant PR141746.
Literature Cited
- Afridi SK, Matharu MS, Lee L, Kaube H, Friston KJ, Frackowiak RS, Goadsby PJ. A PET study exploring the laterality of brainstem activation in migraine using glyceryl trinitrate. Brain. 2005;128(Pt 4):932–939. doi: 10.1093/brain/awh416. [DOI] [PubMed] [Google Scholar]
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, et al. Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30(2):170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates EA, Nikai T, Brennan KC, Fu YH, Charles AC, Basbaum AI, et al. Ahn AH. Sumatriptan alleviates nitroglycerin-induced mechanical and thermal allodynia in mice. Cephalalgia. 2010;30(2):170–178. doi: 10.1111/j.1468-2982.2009.01864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Ashina S, Burstein R, Reed ML, Buse D, Serrano D, Lipton RB. Prevalence and characteristics of allodynia in headache sufferers: a population study. Neurology. 2008;70(17):1525–1533. doi: 10.1212/01.wnl.0000310645.31020.b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bigal ME, Serrano D, Reed M, Lipton RB. Chronic migraine in the population: burden, diagnosis, and satisfaction with treatment. Neurology. 2008;71(8):559–566. doi: 10.1212/01.wnl.0000323925.29520.e7. [DOI] [PubMed] [Google Scholar]
- Brennan KC, Bates EA, Shapiro RE, Zyuzin J, Hallows WC, Huang Y, et al. Ptacek LJ. Casein kinase idelta mutations in familial migraine and advanced sleep phase. Sci Transl Med. 2013;5(183):183ra156. doi: 10.1126/scitranslmed.3005784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burstein R, Collins B, Jakubowski M. Defeating migraine pain with triptans: a race against the development of cutaneous allodynia. Ann Neurol. 2004;55(1):19–26. doi: 10.1002/ana.10786. [DOI] [PubMed] [Google Scholar]
- Burstein R, Jakubowski M, Garcia-Nicas E, Kainz V, Bajwa Z, Hargreaves R, et al. Borsook D. Thalamic sensitization transforms localized pain into widespread allodynia. Ann Neurol. 2010;68(1):81–91. doi: 10.1002/ana.21994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capuano A, Greco MC, Navarra P, Tringali G. Correlation between algogenic effects of calcitonin-gene-related peptide (CGRP) and activation of trigeminal vascular system, in an in vivo experimental model of nitroglycerin-induced sensitization. Eur J Pharmacol. 2014;740:97–102. doi: 10.1016/j.ejphar.2014.06.046. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53(1):55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Charles A, Brennan KC. A touch of increased pain: cutaneous allodynia in migraine. Ann Neurol. 2008;63(2):130–132. doi: 10.1002/ana.21323. [DOI] [PubMed] [Google Scholar]
- Christiansen I, Thomsen LL, Daugaard D, Ulrich V, Olesen J. Glyceryl trinitrate induces attacks of migraine without aura in sufferers of migraine with aura. Cephalalgia. 1999;19(7):660–667. doi: 10.1046/j.1468-2982.1999.019007660.x. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. The Up-and-Down Method for Small Samples. Journal of the American Statistical Association. 1965;60(312):967–978. doi: 10.2307/2283398. [DOI] [Google Scholar]
- Erdener SE, Dalkara T. Modelling headache and migraine and its pharmacological manipulation. Br J Pharmacol. 2014;171(20):4575–4594. doi: 10.1111/bph.12651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farajdokht F, Babri S, Karimi P, Mohaddes G. Ghrelin attenuates hyperalgesia and light aversion-induced by nitroglycerin in male rats. Neurosci Lett. 2016;630:30–37. doi: 10.1016/j.neulet.2016.07.026. [DOI] [PubMed] [Google Scholar]
- Greco R, Meazza C, Mangione AS, Allena M, Bolla M, Amantea D, et al. Tassorelli C. Temporal profile of vascular changes induced by systemic nitroglycerin in the meningeal and cortical districts. Cephalalgia. 2011;31(2):190–198. doi: 10.1177/0333102410379887. [DOI] [PubMed] [Google Scholar]
- ICHD. The International Classification of Headache Disorders, 3rd edition (beta version) Cephalalgia. 2013;33(9):629–808. doi: 10.1177/0333102413485658. [DOI] [PubMed] [Google Scholar]
- Iversen HK, Olesen J, Tfelt-Hansen P. Intravenous nitroglycerin as an experimental model of vascular headache. Basic characteristics. Pain. 1989;38(1):17–24. doi: 10.1016/0304-3959(89)90067-5. [DOI] [PubMed] [Google Scholar]
- Markovics A, Kormos V, Gaszner B, Lashgarara A, Szoke E, Sandor K, et al. Helyes Z. Pituitary adenylate cyclase-activating polypeptide plays a key role in nitroglycerol-induced trigeminovascular activation in mice. Neurobiol Dis. 2012;45(1):633–644. doi: 10.1016/j.nbd.2011.10.010. [DOI] [PubMed] [Google Scholar]
- Olesen J. The role of nitric oxide (NO) in migraine, tension-type headache and cluster headache. Pharmacol Ther. 2008;120(2):157–171. doi: 10.1016/j.pharmthera.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Olesen J. Nitric oxide-related drug targets in headache. Neurotherapeutics. 2010;7(2):183–190. doi: 10.1016/j.nurt.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, McGuire B, Tarash I, Evans CJ, Charles A. Characterization of a novel model of chronic migraine. Pain. 2014;155(2):269–274. doi: 10.1016/j.pain.2013.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan AA, Smith ML, Zyuzin J, Charles A. delta-Opioid receptor agonists inhibit migraine-related hyperalgesia, aversive state and cortical spreading depression in mice. Br J Pharmacol. 2014;171(9):2375–2384. doi: 10.1111/bph.12591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart WF, Wood C, Reed ML, Roy J, Lipton RB. Cumulative lifetime migraine incidence in women and men. Cephalalgia. 2008;28(11):1170–1178. doi: 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- Stovner L, Hagen K, Jensen R, Katsarava Z, Lipton R, Scher A, et al. Zwart JA. The global burden of headache: a documentation of headache prevalence and disability worldwide. Cephalalgia. 2007;27(3):193–210. doi: 10.1111/j.1468-2982.2007.01288.x. [DOI] [PubMed] [Google Scholar]
- Sufka KJ, Staszko SM, Johnson AP, Davis ME, Davis RE, Smitherman TA. Clinically relevant behavioral endpoints in a recurrent nitroglycerin migraine model in rats. J Headache Pain. 2016;17:40. doi: 10.1186/s10194-016-0624-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipton AF, Tarash I, McGuire B, Charles A, Pradhan AA. The effects of acute and preventive migraine therapies in a mouse model of chronic migraine. Cephalalgia. 2015 doi: 10.1177/0333102415623070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: a life-span study. Cephalalgia. 2010;30(9):1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]
- Yuen PH, Denman SL, Sokoloski TD, Burkman AM. Loss of nitroglycerin from aqueous solution into plastic intravenous delivery systems. J Pharm Sci. 1979;68(9):1163–1166. doi: 10.1002/jps.2600680928. [DOI] [PubMed] [Google Scholar]


