Abstract
Background and Purpose
Since the SAMMPRIS trial, aggressive medical management (AMM), which includes dual antiplatelet therapy (DAPT) and high-dose statin (HDS) therapy, is recommended for patients with symptomatic ICAD. However, limited data on the “real-world” application of this regimen exist. We hypothesized that recurrent stroke risk among patients treated with AMM is similar to the medical arm of the SAMMPRIS cohort.
Methods
Using a prospective registry, we identified all patients admitted between August 2012 and March 2015 with 1) confirmed ischemic stroke (IS) or transient ischemic attack (TIA); 2) independently adjudicated symptomatic ICAD; and 3) follow-up at 30 days. We analyzed 30-day risk of recurrent IS stratified by treatment: 1) AMM: DAPT plus HDS therapy, 2) HDS alone, and 3) DAPT alone. We also assessed 30-day risk among patients who met prespecified SAMMPRIS eligibility criteria.
Results
Among 99 patients who met study criteria (51.5% male, 54.5% black, mean age 68.2 ± 11.2 years), 49 (48.5%) patients were treated with AMM, 69 (69.7%) with DAPT, and 73 (73.7%) with HDS therapy. At 30 days, 20 (20.2%) patients had recurrent strokes in the territory of stenosis. Compared to the risk in the medical arm of SAMMPRIS (4.4%), the 30-day risk of recurrent stroke was 20.4% in AMM patients, 21.5% in HDS patients, 22.4% in DAPT patients, and 23.2% in SAMMPRIS-eligible patients (all p<0.001).
Conclusions
Recurrent stroke risk within 30 days in patients with symptomatic ICAD was higher than that observed in the medical arm of SAMMPRIS even in the subgroup receiving aggressive medical management. Replication of the SAMMPRIS findings requires further prospective study.
Keywords: acute stroke, stenosis, cerebrovascular accident, therapy
Subject terms: atherosclerosis, stenosis, vascular disease, cerebrovascular disease/stroke
Introduction
Intracranial atherosclerotic disease (ICAD) is a major cause of stroke worldwide and portends a high risk of recurrent stroke.1 The Stenting and Aggressive Medical Management for Preventing Recurrent Stroke in Intracranial Stenosis (SAMMPRIS) trial included aggressive medical management (AMM) characterized by use of dual antiplatelet therapy (DAPT: aspirin 325 mg plus clopidogrel 75 mg daily for 90 days), high-dose statin (HDS) therapy with rosuvastatin, and other risk factor and lifestyle modification.2 The trial was stopped prematurely after finding that AMM alone was superior to stent placement plus AMM. Since the publication and in the subsequent guidelines, AMM with DAPT and HDS therapy is recommended (Class IIb, level of evidence B) in patients with symptomatic ICAD causing 70–99% stenosis.3
No trial, however, has compared AMM to non-AMM approaches and demonstrated its superiority in patients with symptomatic ICAD. Furthermore, there are limited data on the “real world” application of this regimen,4 and its effect on stroke recurrence. In a prospective cohort of patients with symptomatic ICAD admitted to an urban academic medical center, we hypothesized that 30-day recurrent stroke risk among patients treated with AMM would be similar to that observed in the medical arm of the SAMMPRIS trial.
Methods
Standard Protocol Approvals, Registrations, and Patient Consents
The study was approved by the local Institutional Review Board. With informed consent, consecutive patients ≥18 years with a confirmed acute ischemic stroke (AIS) or transient ischemic attack (TIA) between August 1, 2012 through March 13, 2015 were enrolled in the Northwestern University Brain Attack Registry. Written informed consent was obtained from the patient or their legally authorized representative.
Selection of cohort
We defined AIS as sudden onset of neurologic deficits lasting >24 hours without alternative diagnosis and/or confirmation of acute ischemic stroke on diffusion-weighted imaging (DWI).5 In patients in whom DWI was not performed (n=2), the diagnosis was made clinically based on the duration of symptoms and/or computerized tomography (CT) imaging findings. TIA was defined as sudden onset of neurologic deficits lasting <24 hours without alternative diagnosis and no acute lesion on DWI. Patients with confirmed AIS or TIA due to moderate to severe intracranial stenosis involving any of the following arteries were included: intracranial internal carotid artery (ICA), middle cerebral artery (MCA), intracranial vertebral artery (VA), and basilar artery (BA). Stenosis of the vessel was determined using a modified WASID method on CTA and DSA when available, with moderate stenosis defined at 50–69% stenosis and severe stenosis defined as 70–99% stenosis; among those with only MRA performed (n=36), we measured degree of stenosis as >50% by the modified WASID method and further categorized stenosis as 50–69% when flow gap was not present and 70–99% stenosis when flow gap was present.6–7 Symptomatic ICAD required that the infarct on DWI or CT was in the vascular distribution of the stenotic artery without another causative mechanism found on diagnostic testing. Board-certified vascular neurologists prospectively reviewed clinical and radiographic data to determine Trial of Org 10172 in Acute Stroke Treatment (TOAST) subtype8 for each confirmed case; adjudication was made by consensus to avoid inter-rater reliability concerns.9 Consecutive patients with the following criteria were included: 1) AIS or TIA as previously defined and 2) independently adjudicated symptomatic ICAD.
Index evaluation of subjects
Demographics, initial National Institutes of Health Stroke Scale (NIHSS) score during index hospitalization, risk factors and comorbidities, hospital course, and treatments were collected prospectively. Hypertension, diabetes mellitus, dyslipidemia, prior stroke, and cardiac disease (history or angina, myocardial infarction, coronary bypass or intervention, or congestive heart failure) were defined by documented history, active medications, or clinical or laboratory findings at presentation. We also collected baseline low-density lipoprotein (LDL) and glycosylated hemoglobin levels at index hospitalization. Baseline brain and vascular imaging was independently reviewed by two investigators (R.S and S.P) for presence of acute infarcts on DWI or CT along with location, degree of stenosis, and vascular territory, blinded to outcome data.
Aggressive medical management protocol
Eligible patients with symptomatic ICAD were treated according to guidelines during the study period (Note: a change in guidelines supporting DAPT occurred in 2014).3 The use of DAPT along with HDS therapy was determined per attending stroke physician discretion. Patients also received standard inpatient counseling regarding diet and lifestyle modifications during the index hospitalization. Blood pressure medications were initiated during hospitalization whenever possible with titration towards <140/90 mm Hg (<130/80 mm Hg in diabetics) over a period of 2–4 weeks. Patients were instructed and scheduled to follow-up in vascular neurology clinic or with their primary care provider to ensure blood pressure medications were titrated towards this goal. Statin medications were started to target a low-density lipoprotein (LDL) goal of ≤70 mg/dL. For patients at goal of 70 mg/dL at time of hospitalization, pre-stroke statin dosing was continued or low-dose statin medication was started with monitoring of lipid levels by the primary care physician or stroke neurologist in the outpatient setting.
Outcomes
Recurrent AIS in the territory of the symptomatic stenotic artery within 30 days of index event was the primary outcome. We prospectively monitored for post-stroke in-hospital medical complications10 including recurrent ischemic stroke after index AIS or TIA. Recurrent IS after hospitalization was determined via telephonic interview supplemented by utilizing an electronic surveillance system of hospital records at any of 3 health system hospitals with confirmation by manual review of the medical record in all instances of reported recurrent AIS or TIA.
Statistical analysis
Data are expressed as number (percent), mean (standard deviation [SD]), or median (interquartile range [IQR]) as appropriate. Baseline characteristics of our cohort to the SAMMPRIS cohort were compared using Fisher’s exact test with a p-value of <0.05 considered to be significant. We calculated 30-day risk of recurrent AIS in the territory of the stenotic artery and 95% confidence intervals using the Wald method and compared these rates with those reported in the SAMMPRIS trial using chi-square tests. We assessed risk of recurrent AIS in 3 categories of medical management: 1) AMM defined as DAPT plus HDS therapy; 2) HDS therapy without DAPT; and 3) DAPT without HDS therapy; we also assessed the risk in those meeting the following SAMMPRIS criteria: age (30–80 years), severe degree of stenosis, and pre-stroke modified Rankin scale ≤3. In secondary analyses, we compared risks across demographic and risk factor groups, and in 2 subgroups recently identified in SAMMPRIS as having elevated risk: those with prior infarcts and not on statin therapy at baseline.11 We performed univariable and multivariable logistic regression for predictors of the outcome or dependent variable (recurrent stroke within 30 days) including demographic, clinical, imaging, laboratory, and treatment variables. A p-value <0.05 was considered significant in univariate comparisons; however, using the Bonferroni method, we selected a p-value <0.0125 for the comparison of recurrent stroke risks across 4 pre-specified subgroups in our primary analysis. All analyses were conducted using Statistical Package for Social Sciences version 23.0 (IBM, Armonk, NY).
Results
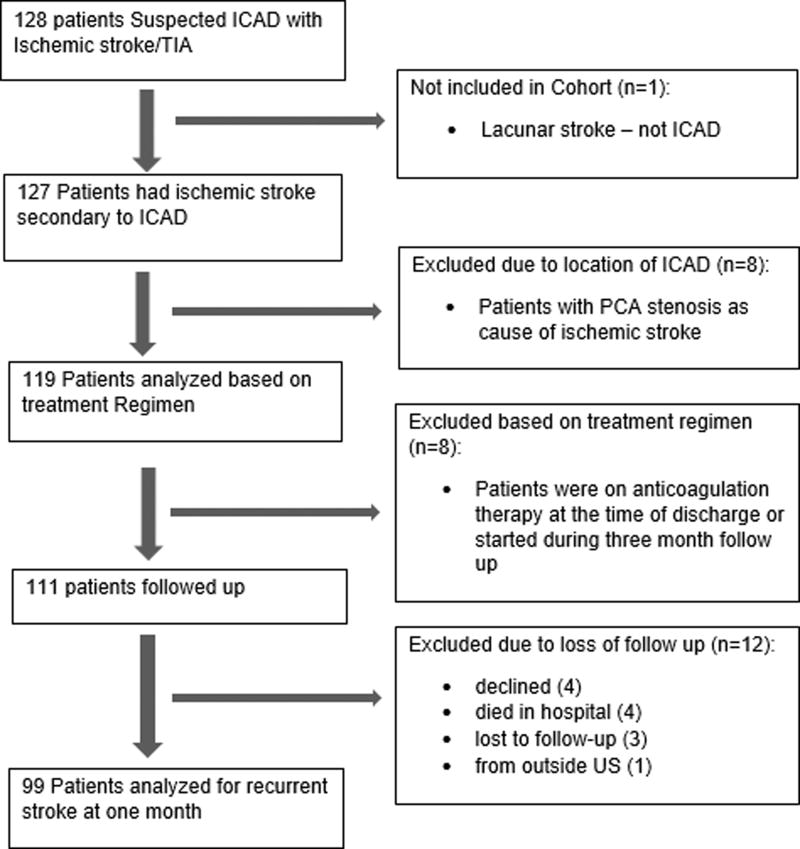
Among 99 consecutive patients (mean age 68.2 ± 11.2 years; 51.5% male; 38.4% white; 54.5% black; 7.1% Hispanic) included for analysis following exclusion and loss to follow-up (Figure 1), the intracranial ICA (37.4%) and MCA (30.3%) were the most commonly involved territories. Severe stenosis was noted in 69.7% with remainder having moderate stenosis. Table 1 provides the clinical and radiographic characteristics of the analyzed cohort compared with medical arm of SAMMPRIS. Our cohort was older, had more severe strokes, and had greater proportions of men, blacks, and diabetes but less dyslipidemia than the SAMMPRIS cohort. Medical management in our cohort included DAPT (67.7%), HDS therapy (65.7%), and both (48.5%). Documented reasons for non-adherence to AMM regimen are provided in Table 2.
Figure one.
Flowchart of study cohort assembly
Table 1.
Baseline characteristics of patients in the cohort compared to the SAMMPRIS cohort
| Variable | Cohort (n=99) | SAMMPRIS (n=227) |
P- value |
|---|---|---|---|
| Mean age (SD) in years | 68.2 (11.2) | 59.5 (11.8) | <0.001 |
| Male, n (%) | 51 (51.5) | 82 (36.1) | 0.013 |
| Race, n (%) | <0.001 | ||
| White | 38 (38.4) | 161 (70.9) | |
| African-American | 53 (53.5) | 50 (22.0) | |
| Other | 8 (8.1) | 16 (7.0) | |
| Hypertension, n (%) | 81 (81.8) | 203 (89.4) | 0.088 |
| Diabetes mellitus, n (%) | 56 (56.6) | 103 (45.4) | 0.082 |
| Hemoglobin A1c, n (%) | |||
| <5.7%, n (%) | 28 (28.3) | - | |
| 5.8 – 7.9%, n (%) | 42 (42.4) | - | |
| >7.9%, n (%) | 29 (29.3) | - | |
| Dyslipidemia, n (%) | 79 (79.8) | 203 (89.4) | 0.031 |
| LDL level, n (%) | |||
| <70 mg/dL, n (%) | 20 (20.2) | - | |
| 70–100 mg/dL, n (%) | 33 (33.3) | - | |
| >100 mg/dL, n (%) | 46 (46.5) | - | |
| Coronary artery disease, n (%) | 22 (22.2) | 59 (26.0) | 0.559 |
| Prior history of ischemic stroke or TIA, n (%) | 45 (45.5) | 58 (25.6) | <0.001 |
| Current smoking, n (%) | 23 (23.2) | 69 (30.4) | 0.235 |
| Statin therapy, n (%) | 53 (53.5) | 196 (86.3) | |
| NIHSS score >1, n (%) | 62 (62.6) | 88 (38.8) | <0.001 |
| Location of ICAD, n (%) | 0.002 | ||
| Middle cerebral artery | 30 (30.3) | 105 (46.3) | |
| Intracranial vertebral artery | 16 (16.2) | 22 (9.7) | |
| Intracranial internal carotid artery | 37 (37.4) | 49 (21.6) | |
| Basilar artery | 16 (16.2) | 51 (22.5) | |
| Degree of stenosis of ICAD, n (%) | <0.001 | ||
| Moderate | 30 (30.3) | N/A | |
| Severe | 69 (69.7) | 227 (100) | |
| Prior infarcts in the territory, n (%) | 30 (40.8) | 75 (33.0) | 0.700 |
| Discharge Medications | <0.001 | ||
| DAPT | 67 (67.5) | 227 (100) | |
| HDS | 65 (65.4) | 227 (100) | |
| DAPT plus HDS therapy | 48 (48.4) | 227 (100) |
Table 2.
Documented reasons for non-adherence to AMM during index hospitalization
| Number of Patients | |
|---|---|
| Reasons for not prescribing DAPT, n (%) | 32 |
| Intracranial hemorrhage | 4 (12.5%) |
| History of gastrointestinal bleeding | 1 (3.1%) |
| Clinical trial enrollment | 1 (3.1%) |
| Large burden of infarct and risk of hemorrhagic conversion | 1 (3.1%) |
| Recent surgery | 3 (9.4%) |
| Prior to 2014 revised guidelines | 22 (68.8%) |
| Reasons for not prescribing HDS, n (%) | 34 |
| At goal LDL ≤70 mg/dL | 25 (73.5%) |
| Prior intolerance to HDS | 3 (8.8%) |
| History of liver disease | 1 (2.9%) |
| History of rhabdomylosis | 1 (2.9%) |
| Prior documented statin intolerance | 1 (2.9%) |
| Interaction with other medications | 1 (2.9%) |
| Unknown | 2 (5.9%) |
The risk of recurrent AIS in the territory of the stenotic artery following the index event was 20.2% (95% CI 13.5–29.2%) at 30 days: 9.1% (95% 4.9–16.4%) during the index hospitalization versus 11.1% (95% CI 6.3–18.8%) after hospital discharge. Those with and without recurrent stroke did not differ based on age, sex, ethnicity, risk factors, stroke severity, baseline LDL and HgA1c levels, prior infarcts, and degree of stenosis in symptomatic artery (Table 3). In multivariable analysis including sex, race (black vs. non-black), diabetes, stenosis location (vertebrobasilar vs. other), stenosis grade (severe vs. moderate), prior infarcts, baseline statin use, and AMM at discharge, no factor was independently associated with 30-day recurrent stroke.
Table 3.
Comparison of 30-day recurrent stroke risk by demographic, clinical, serologic, and imaging factors
| Variable | Recurrent stroke (n=20) |
No recurrent stroke (n=79) |
P-value |
|---|---|---|---|
| Mean age (SD) in years | 65.9 (10.6) | 68.8 (11.3) | 0.303 |
| Male, n (%) | 14 (70.0) | 37 (46.8) | 0.068 |
| Race, n (%) | 0.445 | ||
| White | 7 (35.0) | 31 (39.2) | |
| African-American | 10 (50.0) | 43 (54.4) | |
| Other | 3 (15.0) | 5 (6.3) | |
| Hypertension, n (%) | 16 (80.0) | 65 (82.3) | 0.813 |
| Diabetes mellitus, n (%) | 8 (40.0) | 49 (62.0) | 0.075 |
| Hemoglobin A1c, n (%) | 0.894 | ||
| <5.7%, n (%) | 6 (30.0) | 22 (27.8) | |
| 5.8–7.9%, n (%) | 9 (45.0) | 33 (41.8) | |
| >7.9%, n (%) | 5 (25.0) | 24 (30.4) | |
| Dyslipidemia, n (%) | 19 (95.0) | 68 (86.1) | 0.275 |
| LDL level, n (%) | 0.313 | ||
| <70 mg/dL, n (%) | 4 (20.0) | 16 (20.3) | |
| 70–100 mg/dL, n (%) | 4 (20.0) | 29 (36.7) | |
| >100 mg/dL, n (%) | 12 (60.0) | 34 (43.0) | |
| Coronary artery disease, n (%) | 5 (25.0) | 17 (21.5) | 0.738 |
| Prior history of ischemic stroke or TIA, n (%) | 8 (40.0) | 37 (46.8) | 0.583 |
| Current smoking, n (%) | 3 (15.0) | 20 (25.3) | 0.329 |
| Statin therapy, n (%) | 9 (45.0) | 44 (55.7) | 0.392 |
| NIHSS score >1, n (%) | 13 (65.0) | 49 (62.0) | 0.806 |
| Vertebrobasilar location, n (%) | 5 (25.0) | 27 (34.2) | 0.433 |
| Severe stenosis, n (%) | 16 (80.0) | 53 (67.1) | 0.262 |
| Prior infarcts in the territory, n (%) | 9 (45.0) | 31 (39.7) | 0.670 |
| Aggressive medical management, n (%) | 10 (50.0) | 38 (48.1) | 0.879 |
| Dual anti-platelet therapy, n (%) | 15 (75.0) | 52 (65.8) | 0.433 |
| High-dose statin therapy, n (%) | 14 (70.0) | 51 (64.6) | 0.647 |
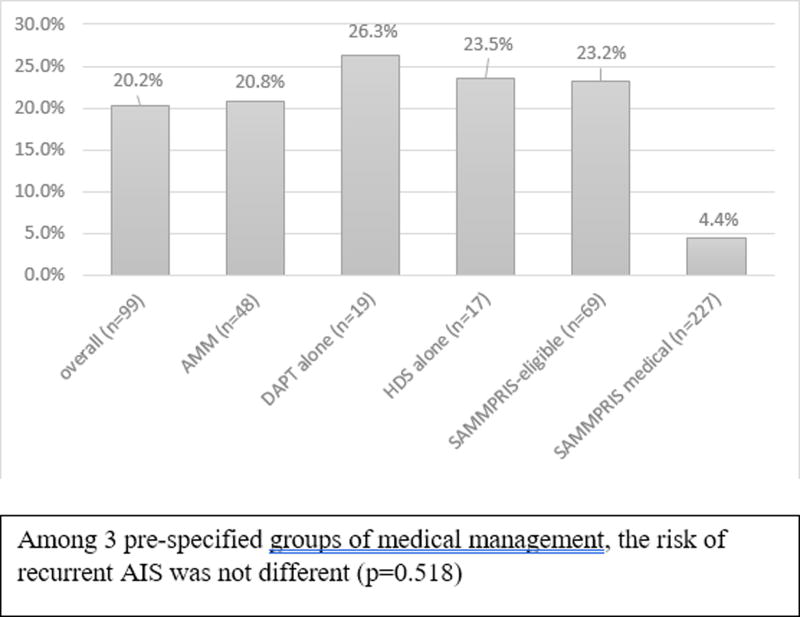
Among 3 pre-specified groups of medical management, the risk of recurrent AIS was not different (p=0.518). Each medical management subgroup had higher 30-day risk of recurrent AIS in the territory of the stenotic artery than observed in the medical arm of SAMMPRIS (Figure 2): AMM (20.8% vs. 4.4%, p<0.001); HDS therapy without DAPT (23.5% vs. 4.4%, p<0.001); and DAPT without HDS therapy (26.3% vs. 4.4%, p<0.001). The SAMMPRIS-eligible patients in our cohort also had higher risk (23.2% vs. 4.4%, p<0.001) compared to that in SAMMPRIS.
Figure two.
30-day risk of recurrent stroke in the territory of the symptomatic stenotic artery overall and by subgroups and compared to the medical arm of SAMMPRIS
Discussion
In a single-center, urban, longitudinal cohort study, we observed higher rates of recurrent AIS within 30 days when compared to the SAMMPRIS trial even in those treated with AMM consisting of DAPT plus HDS therapy. Furthermore, we found no baseline demographic, serologic, clinical, or imaging predictors of recurrent stroke. Our findings suggest that real-world application of AMM may be suboptimal and may not reproduce the results from the SAMMPRIS trial. We attribute this to multiple factors including 1) difficulty in uniformly implementing AMM, especially lifestyle modification, 2) selection bias that may have excluded some patients with high risk of early recurrence in SAMMPRIS, and 3) possible genetic, clinical, and environment/socioeconomic differences between our cohort and the SAMMPRIS medical cohort.
Lifestyle intervention with close monitoring was a key component of aggressive medical management in the SAMMPRIS trial. In addition to DAPT, HDS therapy, and blood pressure management, AMM included lifestyle coaching and modifications. Indeed, in a subgroup analysis of SAMMPRIS, compliance with the lifestyle modification program resulted in improved risk factor control.12 Other studies in patients with stable coronary artery disease also demonstrated intensive risk factor management alone was equivalent to endovascular intervention plus intensive medical management in preventing cardiac ischemic events, suggesting that lifestyle modifications should be a major component in the management of atherosclerotic disease.13
In the SAMMPRIS trial, a lifestyle coach was provided to patients and they underwent close monitoring and frequent follow-up with the study investigators. Absent the clinical trial environment, however, compliance with and affordability of medications may be more difficult to achieve. Others have noted that replicating the results of clinical trials are met with real-world challenges.14 In clinical practice, lack of access to resources and motivation are significant barriers for optimal medical management. Indeed, patients who are more actively engaged in their medical condition and treatment may be more willing to participate in clinical trials and thus more adherent to the medications and treatments in comparison to the general population.15 Similar difficulty in reproducing trial results has been observed in heart failure patients.16 Furthermore, when preventive or treatment regimens are complex and/or require lifestyle modification of existing habits, non-adherence can be as high as 70%.17 Past studies have also shown that counseling on diet and exercise alone achieve little improvement in risk factor profiles.18 Though a small study applying AMM in 22 patients noted high rates of risk factor control and no recurrent events at 1 year,4 our study suggests that AMM including lifestyle modification, but without a program such as the INTERxVENT program used in SAMMPRIS, may be difficult to replicate without the trial infrastructure and improved affordability.
We included a broader range of patients with symptomatic ICAD in our study. Inclusion of patients who may have been unstable due to active plaques or hemodynamic failure could have contributed to higher rates of ischemic stroke especially in the first week in our study compared to the SAMMPRIS trial. The SAMMPRIS trial did not enroll patients with fluctuating symptoms or with deterioration within 24 hours prior to randomization. In the WASID trial, patients whose index event occurred 17 days or before randomization had a significantly higher risk of recurrent ischemic stroke than patients who were randomized after 17 days.19 Although the median time from stroke to enrollment in the SAMMPRIS was 7 days, we speculate that some high-risk patients with early recurrent ischemic strokes may have been excluded or not considered for the trial, leading to an under-estimate of actual recurrent ischemic stroke risk in patients with symptomatic ICAD. While others have identified clinical and imaging factors associated with increased recurrent AIS risk including prior infarcts in the territory of the stenotic artery and no statin use at enrollment,11 we were unable to confirm these findings.
Finally, we postulate that DAPT may be less effective in particular subgroups of patients. Based on the CLAIR20 and CARESS21 trials, short-term DAPT (three months or less) has been posited to reduce the risk of ischemic stroke from artery-artery embolism. Indeed, the strong unexpected results of SAMMPRIS in the medical arm have been attributed in part to DAPT.22 However, no subsequent studies have been able to confirm or refute this finding. A recent subgroup analysis of the CHANCE trial, which utilized a 3-week DAPT regimen after minor stroke and TIA,23 found that DAPT provided no reduction of AIS events in the one-third of patients with symptomatic ICAD. Another factor may be genetic differences that affect clopidogrel metabolism. Patients who are carriers of the CYP2C19 loss-of-function alleles are clopidogrel non-responders.24 Though we might speculate that poor responsiveness to clopidogrel was a potential explanation of our results, it is unlikely that a majority of our patients harbored the mutation.
Our findings must be viewed in the context of several limitations. First, this was a single-center prospective non-randomized cohort study and thus may not generalize to other settings as our population was predominantly black, older, urban, and had a higher prevalence of diabetes than the cohort analyzed in SAMMPRIS. Second, the study did not match the exact eligibility criteria of the SAMMPRIS trial, limiting comparison as patients were included that may not have been eligible for enrollment in the SAMMPRIS trial. Third, we did not assess or enforce compliance to medications at follow-up; assessment of compliance would require a standardized method often only employed in clinical trials. Fourth, some patients were not provided DAPT or HDS therapy for reasons that are outlined in Table 2. A survey of practitioners after SAMMPRIS noted that only 45% routinely used DAPT in patients with symptomatic ICAD, suggesting that adherence to this class IIb recommendation may be far from uniform.25 Fifth, we also did not measure the degree of stenosis via catheter angiography, which could lead to an inaccurate characterization of the degree of stenosis. Lastly, the small sample size could result in type I error and an over-estimation of risk and type II error and inability to identify predictors of 30-day recurrent stroke risk. Given these limitations and observational nature of this cohort, we refrain from drawing definitive conclusions.
In a prospective cohort, we observed that the rate of recurrent ischemic stroke in patients with symptomatic ICAD was higher than in the medical arm of the SAMMPRIS trial. Real-life application and replication of SAMMPRIS trial results are warranted. Alternatively, our data provides rationale for improved risk factor and lifestyle management in clinical practice. Indeed, reimbursement for such activity may be necessary. Further investigation is also needed to determine the pathophysiological processes mediating recurrent ischemic stroke in symptomatic ICAD, especially in the first 30 days. Understanding mechanisms of early recurrence will aid in developing targeted therapies for patients with this condition.
Acknowledgments
None
Sources of Funding:
None
Andrew M. Naidech recives support from NIHS/NINDS for the following grants K18 HS023437 Sameer A. Ansari receives support from NIH/NINDS for the following grants 1R21HL130969, 1U01NS092076 (MyRIAD), 13GRNT17340018, 14GRNT20380798
Shyam Prabhakaran receives support from NIH/NINDS as co-PI for MyRIAD
Footnotes
Disclosures:
Rajbeer Singh Sangha has no relevant disclosures to report.
Carlos Corado has no relevant disclosures to report.
References
- 1.Gorelick PB, Wong KS, Bae H-J, Pandey DK. Large artery intracranial occlusive disease: a large worldwide burden but a relatively neglected frontier. Stroke. 2008;39:2396–2399. doi: 10.1161/STROKEAHA.107.505776. [DOI] [PubMed] [Google Scholar]
- 2.Chimowitz MI, Lynn MJ, Derdeyn CP, Turan TN, Fiorella D, Lane BF, et al. Stenting versus aggressive medical therapy for intracranial arterial stenosis. N Engl J Med. 2011;365:993–1003. doi: 10.1056/NEJMoa1105335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kernan WN, Ovbiagele B, Black HR, Bravata DM, Chimowitz MI, Ezekowitz MD, et al. Guidelines for the Prevention of Stroke in Patients With Stroke and Transient Ischemic Attack. Stroke. 2014;45:2160–236. doi: 10.1161/STR.0000000000000024. [DOI] [PubMed] [Google Scholar]
- 4.Nahab F, Kingston C, Frankel MR, Dion JE, Crawley CM, Mitchell B, et al. Early Aggressive Medical Management for patients with Symptomatic Intracranial Stenosis. Journal of Stroke and Cerebrovascular Diseases. 2013;22:87–92. doi: 10.1016/j.jstrokecerebrovasdis.2011.06.012. [DOI] [PubMed] [Google Scholar]
- 5.Sacco RL, Kasner SE, Broderick JP, Caplan LR, Connors JJ, Culebras A, et al. An updated definition of stroke for the 21st century: a statement for healthcare professionals from the American Heart Association/ American Stroke Association. Stroke. 2013;44:2064–2089. doi: 10.1161/STR.0b013e318296aeca. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Feldmann E, Wilterdink JL, Kosinski A, Lynn M, Chimowitz MI, Sarafin J, et al. The Stroke Outcomes and Neuroimaging of Intracranial Atherosclerosis (SONIA) trial. Neurology. 2007;68:2099–106. doi: 10.1212/01.wnl.0000261488.05906.c1. [DOI] [PubMed] [Google Scholar]
- 7.Samuels OB, Joseph GJ, Lynn MJ, Smith HA, Chimowitz MI. A standardized method for measuring intracranial arterial stenosis. AJNR Am J Neuroradiol. 2000;21:643–646. [PMC free article] [PubMed] [Google Scholar]
- 8.Adams HP, Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute IS: definitions for use in a multicenter clinical trial. Trial of Org 10172 in Acute Stroke Treatment (TOAST) Stroke. 1993;24:35–41. doi: 10.1161/01.str.24.1.35. [DOI] [PubMed] [Google Scholar]
- 9.Goldstein LB, Jones MR, Matchar DB, Edwards LJ, Hoff J, Chilukuri V, et al. Improving the reliability of stroke subgroup classification using the Trial of ORG 10172 in Acute Stroke Treatment (TOAST) criteria. Stroke. 2001;32:1091–1098. doi: 10.1161/01.str.32.5.1091. [DOI] [PubMed] [Google Scholar]
- 10.Shah SV, Corado C, Bergman D, Curran Y, Bernstein RA, Naidech AM, et al. Impact of Poststroke Medical Complications on 30-Day Readmission Rate. J Stroke Cerebrovasc Dis. 2015;24:1969–77. doi: 10.1016/j.jstrokecerebrovasdis.2015.04.037. [DOI] [PubMed] [Google Scholar]
- 11.Waters MF, Hoh BL, Lynn MJ, Kwon HM, Turan TN, Derdeyn CP, et al. Factors Associated with Recurrent Ischemic Stroke in the Medical Group of the SAMMPRIS trial. JAMA Neurol. 2016;73:308–15. doi: 10.1001/jamaneurol.2015.4315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Turan TN, Nizam A, Lynn MJ, Montgomery J, Derdeyn CP, Fiorella D, et al. Relationship Between Compliance With the Lifestyle Modification Program and Risk Factor control in the Stenting and Aggressive Medical Management for Preventing Recurrent Stroke In Intracranial Stenosis (SAMMPRIS) Trial. Stroke. 2014;45 doi: 10.1016/j.jstrokecerebrovasdis.2017.10.017. ATMP105. [DOI] [PubMed] [Google Scholar]
- 13.Boden WE, O’Rouke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, et al. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 14.Nallamothu BK, Hayward RA, Bates ER. Beyond the Randomized Clinical Trial: The Role of Effectiveness Studies in Evaluating Cardiovascular Therapies. Circulation. 2008;118:1294–1303. doi: 10.1161/CIRCULATIONAHA.107.703579. [DOI] [PubMed] [Google Scholar]
- 15.Van Onzenoort HAW, Menger FE, Neef C, Verberk WJ, Kroon AA, Leeuw PW, et al. Participation in a Clinical Trial Enhances Adherence and Persistence to Treatment: A Retrospective Cohort Study. Hypertension. 2011;58:573–578. doi: 10.1161/HYPERTENSIONAHA.111.171074. [DOI] [PubMed] [Google Scholar]
- 16.McAlister FA, Stewart S, Ferrua S, McMurray JJ. Multidisciplinary strategies for the management of heart failure patients at high risk for admission: a systematic review of randomized trials. J Am Coll Cardiol. 2004;44:810–819. doi: 10.1016/j.jacc.2004.05.055. [DOI] [PubMed] [Google Scholar]
- 17.Martin LR, Williams SL, Haskard KB, DiMatteo MR. The challenge of patient adherence. Ther Clin Risk Manag. 2005;1:189–199. [PMC free article] [PubMed] [Google Scholar]
- 18.Joseph LN, Babikian VL, Allen NC, Winter MR. Risk Factor Modification in Stroke Prevention: The Experience of a Stroke Clinic. Stroke. 1999;30:16–20. doi: 10.1161/01.str.30.1.16. [DOI] [PubMed] [Google Scholar]
- 19.Kasner SE, Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, et al. Predictors of ischemic stroke in the territory of a symptomatic intracranial arterial stenosis. Circulation. 2006;113:555–63. doi: 10.1161/CIRCULATIONAHA.105.578229. [DOI] [PubMed] [Google Scholar]
- 20.Wong KS, Chen C, Fu J, Chang HM, Suwanwela NC, Huang YN, et al. Clopidogrel plus aspirin versus aspirin alone for reducing embolization in patients with acute symptomatic cerebral or carotid artery stenosis (CLAIR study): a randomized, open-label, blinded-endpoint trial. Lancet Neurol. 2010;9:489–97. doi: 10.1016/S1474-4422(10)70060-0. [DOI] [PubMed] [Google Scholar]
- 21.Markus HS, Droste DW, Kaps M, Larrue V, Lees KR, Siebler M, et al. Dual antiplatelet therapy with clopidrogrel and aspirin in symptomatic carotid stenosis evaluated using Doppler embolic signal detection: the Clopidogrel and Aspirin for Reduction of Emboli in Symptomatic Carotid Stenosis (CARESS) trial. Circulation. 2005;111:2233–40. doi: 10.1161/01.CIR.0000163561.90680.1C. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi S, Turan TN, Lynn MJ, Derdeyn CP, Fiorella D, Janis LS, et al. Do Patient Characteristics Explain the Differences in Outcome Between Medically Treated Patients in SAMMPRIS and WASID? Stroke. 2015;46:2562–7. doi: 10.1161/STROKEAHA.115.009656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu L, Wong KL, Leng X, Pu Y, Wang Y, Jing J, et al. Dual antiplatelet therapy in stroke and ICAS: Subgroup analysis of CHANCE. Neurology. 2015;85:1154–62. doi: 10.1212/WNL.0000000000001972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Y, Zhao X, Lin J, Li H, Johnston SC, Lin Y, et al. Association Between CYP2C19 Loss-of-Function Allele Status and Efficacy of Clopidogrel for Risk Reduction Among Patients With Minor Stroke or Transient Ischemic Attack. JAMA. 2016;316:70–78. doi: 10.1001/jama.2016.8662. [DOI] [PubMed] [Google Scholar]
- 25.Turan TN, Cotsonis G, Lynn MJ, Wooley RH, Swanson S, Williams JE, et al. Intracranial stenosis: impact of randomized trials on treatment preferences of US neurologists and neurointerventionists. Cerebrovasc Dis. 2014;37:203–211. doi: 10.1159/000358120. [DOI] [PMC free article] [PubMed] [Google Scholar]