Despite a broad understanding of the molecular and genetic complexity of acute myeloid leukemia (AML), the only immune therapy known to provide a significant improvement in outcome over standard chemotherapy is allogeneic hematopoietic stem cell transplant (HSCT). However, many patients, especially the elderly, are not eligible for allogeneic HSCT because of the rigorous conditioning and complications from the treatment including serious and sometimes fatal graft-versus-host disease (GVHD). The five-year survival rates in elderly AML patients (≥65 yrs) are below 10%1. Moreover, certain molecular aberrations associated with AML, such as the FLT3 internal tandem duplication (ITD) and FLT3 point mutations have an especially adverse prognosis and a high probability of relapse 2–4. Therefore, novel approaches for the treatment of AML represent an unmet therapeutic need.
Recently, the genetic modification of T cells with chimeric antigen receptors (CARs) that directly target tumor-associated antigens has shown success in the clinic when targeting CD19 in acute lymphoblastic leukemia (ALL) and chronic lymphocytic leukemia (CLL)5. However, identification of tumor-associated surface antigens that can be targeted by CAR immune cells for the treatment of cancer such as AML has proven to be challenging.
FLT3 is a member of the class III receptor tyrosine kinases, and its variable expression can be found on leukemia blasts from over 90% AML patients6, 7, regardless of CD34 expression (Figure 1a). Approximately 20% of 30 samples of newly diagnosed AML patients, who consented to our protocol approved by the OSU institutional review board, with detailed clinical information that we screened showed nearly uniform high-density surface expression of FLT3, i.e. ~90% blasts are positive for FLT3 expression with high level of median fluorescence intensity (Figure 1a and Supplementary Table 1). FLT3 is expressed on approximately 50% of normal hematopoietic stem cells (HSCs) and partially on dendritic cells but is largely not expressed on lymphocytes in cord blood (Supplementary Figure 1).
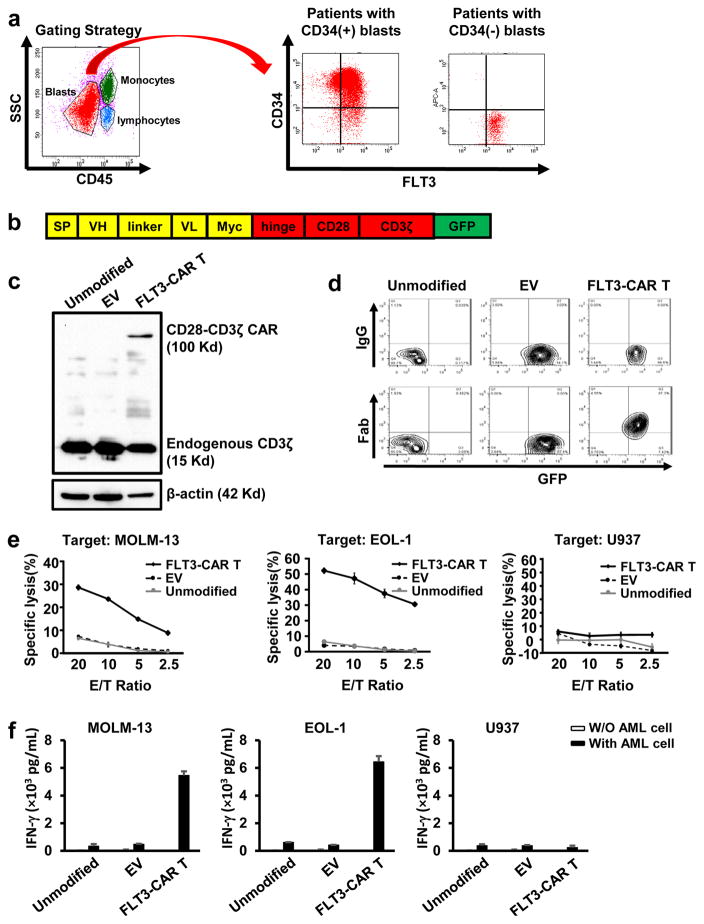
Figure 1. Generation of T cells expressing FLT3-CAR and their cytotoxicity and cytokine production against FLT3(+) and FLT3(−) AML cell lines.
(a) Flow cytometric analysis of FLT3 expression on the surface of AML blasts from patients with CD34(+) or CD34(−) AML blasts. The two patients shown are representative of those 20% with nearly uniform FLT3 expression on the surface of AML blasts among 30 patients that were screened. Note: in some cases, AML cells may be at the place of monocytes in the gating strategy. (b) Schematic representation of the manufactured FLT3-CAR lentiviral construct. SP, signal peptide; VH, variable heavy chain; L, linker; VL, variable light chain. Myc, Myc gene sequence; Hinge, CD8 alpha hinge chain; CD28, a T cell co-stimulatory molecule. (c) Immunoblot analysis of unmodified primary T cells, primary T cells infected with the pCDH empty vector, and primary T cells infected with the vector expressing the FLT3-CAR. Top row: blotted with an anti-CD3ζ antibody to detect ectopic CD28-CD3ζ CAR expression; Middle row: blotted with an anti-CD3ζ antibody to detect endogenous CD3ζ expression; Bottom row: blotted with an anti-β-actin antibody for demonstration of equal loading. (d) Unmodified primary T cells, primary T cells infected with the pCDH empty vector (EV), primary T cells expressing FLT3-CAR were analyzed by flow cytometry after cells were stained with biotin-labeled goat anti-mouse Fab-specific antibody or goat IgG isotype control as well as streptavidin. (e) Cytotoxicity of FLT3-CAR T cells and control cells against MOLM-13 (FLT3+), EOL-1 (FLT3+), or U937 AML (FLT3−) cell lines. (f) ELISA analysis of IFN-γ secretion by FLT3-CAR T cells and their control cells in the presence of indicated AML cell lines. “Unmodified” denotes unmodified T cells, “EV” denotes empty vector-transduced T cells, and “FLT3-CAR T” denotes FLT3-CAR-transduced T cells (c–f). Error bars, standard deviation.
We therefore engineered primary T cells of healthy donors to express a FLT3-specific, second-generation CAR harboring CD28, a co-stimulatory signaling domain, and CD3ζ. Sequences for variable regions of heavy (VH) and light (VL) chains were originally derived from a hybridoma8. The VH-linker-VL fragment was incorporated in frame with the CD28–CD3ζ portion (Figure 1b). The entire anti-FLT3-scFv-CD28-CD3ζ fragment was then subcloned into the lentiviral vector pCDH. Next, we performed lentiviral transduction of primary T cells with the generated FLT3-CAR construct. The generated CAR T cells were lysed in laemmli buffer. Lysates were separated by SDS–PAGE gel and transferred to a PVDF membrane. The membrane was immunoblotted with mouse anti-human CD3ζ mAb and then with a horseradish peroxidase–conjugated goat anti-mouse IgG antibody. Immunoblotting results showed that the CAR was selectively expressed in the T cells (Figure 1c). To detect the expression of FLT3-CAR, especially the scFv portion on the T cell surface, transduced T cells were incubated with biotinlabeled goat anti-mouse (Fab)2 polyclonal antibody or normal polyclonal goat immunoglobulin G (IgG) antibody as an isotype control, followed by staining with allophycocyanin (APC)-conjugated streptavidin. Flow analysis indicated that the FLT3-CAR was successfully expressed on the cell surface of engineered T cells (Figure 1d).
A standard 4-hour 51Cr release assay was performed as previously described9–11 to detect cytotoxicity of FLT3-CAR T cells against six AML cell lines expressing FLT3 (MOLM-13, EOL1, Kasumi, OCI/AML3, MV4-11, THP1), which was substantial, and against one AML cell line lacking expression of FLT3 (U937), which was not significant. Non-infected and empty vector-transduced T cells showed no appreciable cytotoxicity (Figure 1e, Supplementary Figure 2, and data not shown) 12. We also found that FLT3-CAR T cells eradiated FLT3(+) AML cells in a time-dependent fashion (Supplementary Figure 3). The ability of FLT3-CAR T cells to secrete interferon gamma (IFN-γ) upon recognition of AML cells was also assessed. For this purpose, AML cells were co-cultured with an equal number of FLT3-CAR T cells in 96-well V-bottom plates at 37 °C for 24 hours with either AML cell lines expressing FLT3 (MOLM-13 or EOL1) or with an AML cell line lacking expression of FLT3 (U937). Cell-free supernatants were harvested and the levels of IFN-γ were measured by ELISA, as previously described9, 12. Only wells containing both FLT3-CAR T cells and FLT3(+) AML blasts showed substantial IFN-γ production (Figure 1f). The same results were obtained when Kasumi and OCI/AML3 cell lines were used (data not shown). Co-culture of these target cells with FLT3-CAR T cells also led to significantly higher levels of IL-2 secretion compared to control T cells (Supplementary Figure 4 and data not shown). Comparable assays measuring cytotoxicity (Figure 2a) and IFN-γ (Figure 2b) were performed for FLT3-CAR T cells co-cultured with primary AML patient peripheral blood mononuclear cells (PBMCs) containing ~90% FLT3(+) AML blasts, or with primary AML patient PBMC with a comparable percentage of FLT3(−) AML blasts. Enhanced cytotoxicity and IFN-γ secretion were observed in co-cultures of patient samples with FLT3(+) blasts but not in those patients with FLT3(−) blasts (Figures 2a, 2b and Supplementary Figures 5 and 6). Similar data were observed for patient samples with the genetic mutation of FLT3-ITD (Supplementary Figure 7).
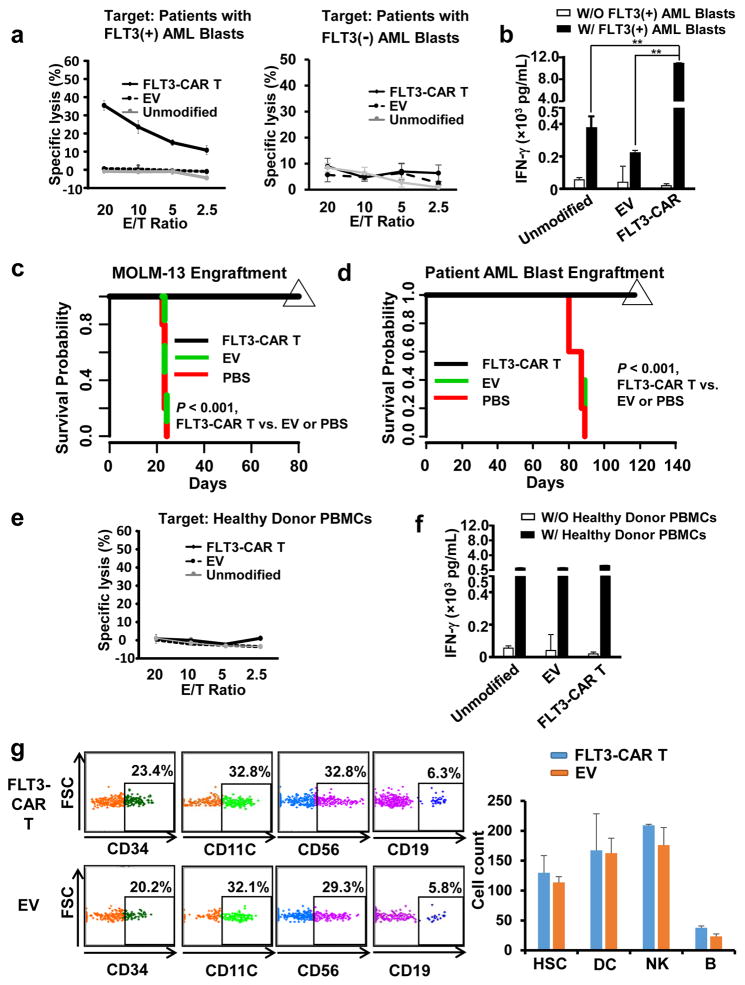
Figure 2. FLT3-CAR T cells show enhanced cytotoxicity and cytokine production against primary AML patient blasts in vitro and against a FLT3(+) AML cell line and patient blasts in vivo, while are not toxic to hematopoiesis.
(a) Cytotoxicity of FLT3-CAR T cells against PBMCs of a representative AML patient containing ~90% FLT3(+) AML blasts, or a representative AML patient containing a comparable percentage of FLT3(−) AML blasts. Similar data of four more patients with ~90% FLT3(+) AML blasts are shown in Supplementary Figure 5. (b) ELISA analysis of IFN-γ secretion by FLT3-CAR T cells against PBMCs of AML patients containing ~90% FLT3(+) AML blasts. Shown here is one patient, representative of all five patients with FLT3 (+) AML blasts. (c) Survival of MOLM-13-bearing mice treated with primary FLT3-CAR T cells, empty vector-transduced T cells, or PBS via tail vein injections. Mice treated with weekly injections of 5 × 106 effector cells for 3 weeks showed 100% survival at 80 days, compared to the two control-treated groups that demonstrated 100% mortality by day 25, as determined by Kaplan-Meier survival curves (n = 5 for each group). (d) 5 × 106 patient PBMC containing ~90% FLT3(+) AML blasts were injected into NSG mice. On day 66 following tumor engraftment, mice were infused with 5 × 106 effect cells weekly for three weeks. Mice treated with weekly injections of 5 × 106 effector cells for three weeks show 100% continued survival at 120 days, compared to the two control-treated groups that demonstrated 100% mortality by day 90, as determined by Kaplan-Meier survival curves (n = 5 for each group). Data presented are representative of three AML patients with similar data. (e). The unmodified T cells, empty-vector transduced T cells, or FLT3-CAR T cells were co-cultured with PBMCs of normal donors for 4 hr, followed by standard 51Cr release assays. Identical effector cells were co-cultured with PBMCs of normal donors for 24 hr after which IFN-γ was measured by ELISA assays. (g). 1 × 106 FLT3-CAR T cells or empty vector-transduced T cells together with 2.5 ×105 human CD34(+) HSCs were simultaneously i.v. injected into NSGS mice that express human IL3, GM-CSF, and SCF. One month and three months later, mice were sacrificed to quantify human CD34(+) HSC and their differentiation as measured by mature lymphocytes and myeloid cells in bone marrow (BM). CD3, CD19, CD56, CD16, and CD14 were used to define lineage cells (Lin). Data shown are for one month engraftment, which are similar to three months’, demonstrating no difference between mice infused with 1 × 106 FLT3-CAR T cells or empty vector-transduced T cells. HSC is defined as CD34(+)Lin(−), DC as CD11c(+)HLDR(+), NK cells as CD56(+)CD3(−)CD19(−), and B cells as CD19(+)CD14(−)CD3(−). n = 4 for EV and n=3 for FLT3-CAR T. “Unmodified” denotes unmodified T cells, “EV” denotes empty vector-transduced T cells, and “FLT3-CAR T” denotes FLT3-CAR-transduced T cells (a–g). ** denotes P < 0.01. Error bars, standard deviation.
Next, we performed an in vivo treatment of AML-bearing mice. For this purpose, MOLM-13 cells, which are FLT3(+) were retrovirally transduced with Pinco-pGL3-luc/GFP virus expressing firefly luciferase, and GFP-positive cells were FACS-sorted, yielding MOLM-13-GL3 cells. NOD scid gamma (NSG) mice were intravenously injected with 8 × 106 MOLM-13-GL3 cells in 400 μL of PBS via tail vein on day 0 to establish an orthotopic xenograft AML model. Mice were intravenously administered with 5 × 106 effector cells, i.e., primary FLT3-CAR T cells or empty vector-transduced primary T cells, in 400 μL of PBS via tail vein injections for 3 times, weekly, starting at day 9. Mice were intraperitoneally infused with D-luciferin (150 mg/kg body weight), anesthetized with isoflurane, and imaged using the In Vivo Imaging System (IVIS) with Living Image® software on day 17 to determine the efficacy of the two doses. We observed that infusion of FLT3-CAR T cells showed significant anti-leukemic activity (Supplementary Figure 8). We repeated this experiment with the MV4-11 AML mouse model, in which 5 × 106 tumor cells were injected and a low and a high dose of FLT3-CAR T cells, 2 × 105 and 3 × 106 per mouse, respectively, were administered. Results showed that both the low and the high dose of FLT3-CAR T cells had higher levels of antitumor activity against MV4-11 AML cells than empty-vector-transduced T cells (Supplementary Figure 9). Also, we observed that three-weekly administrations of FLT3-CAR T cells prolonged the survival of MOLM-13-implanted mice to achieve a survival rate of 100% on day 80, when all mice were sacrificed without evidence of leukemia (Figure 2c). We also engrafted 5 × 106 patient PBMC containing ~90% FLT3(+) primary AML patient blasts in NSG mice and performed weekly treatment for three weeks with 5 × 106 FLT3-CAR T cells per injection, starting at 66 days post tumor cell implantation. Data showed that FLT3-CAR T cell treatment also resulted in a survival rate of 100% at day 120 while 100% of the mice in control-treated groups died by day 90 (Figure 2d). Of note, human FLT3 CAR T cells had no activity on murine Flt3(+) AML cells (Supplementary Figure 10) and in the MV4-11 mouse model, human FLT3 CAR T cells persisted and proliferated up to 7 weeks but could not be detected at 12 weeks post cell fusion, assessed by flow cytometry (Supplementary Figure 11 and data not shown).
To test whether FLT3-CAR T cells are potentially safe, we first co-cultured PBMCs of healthy donors with FLT3-CAR T cells or control T cells. No enhanced cytotoxicity or IFN-γ secretion was observed for FLT3-CAR T cells compared to empty vector–transduced T cells when co-cultured with PBMCs from healthy donors or CD34(+) HSCs isolated from cord blood (Figures 2e and 2f and Supplementary Figure 12). Next, we performed an in vivo assay for toxicity against CD34(+) HSCs by FLT3-CAR T cells. For this purpose, 1 × 106 FLT3-CAR T cells or empty vector-transduced T cells together with 2.5 × 105 human CD34(+) HSCs isolated from cord blood were simultaneously i.v. injected into NSG mice expressing human IL3, GM-CSF and SCF (i.e., NSGS mice). One month and three months later, there was no difference in the quantity and proportion of human CD34(+) and differentiated mature lymphocytes (e.g., NK and B cells) and myeloid cells (e.g., dendritic cells) in mouse bone marrow of mice receiving FLT3-CAR T cells or empty vector-transduced T cells (Figure 2g and Supplementary Figure 13). These data suggest that FLT3-CAR T cells do not affect the capacity for HSC engraftment and differentiation.
In this report, we describe the generation of a new CAR to arm T cells for the treatment of FLT3(+) AML, with our in vivo preclinical data showing 100% survival in orthotopic models of a human FLT3(+) AML cell line and FLT3(+) primary AML blasts from patients. For patients with FLT3 expression on the surface of leukemic blasts, FLT3-CAR T cells could be considered for treatment of refractory disease or up front treatment in the elderly with AML, given their dismal prognosis with standard therapy1. Further, FLT3-CAR T cells could be considered following attainment of complete remission in patients deemed at a high risk of relapse13. AML patients whose blasts contain the FLT3-ITD might be especially suitable for the CAR T cell therapy because of their poor prognosis2, 4 and because the FLT3-CAR T cells generated in this study can target both WT and mutant FLT3.
Our data suggest that FLT3 is an AML-associated antigen that can be targeted by FLT3-CAR T cells. Other CAR T cells used for the treatment of AML recognize CD123 or CD33, which are highly expressed on some AML blasts but also on normal cells such as HSCs and partially differentiated myeloid cells14. Thus, the possibility of toxicity, including myeloablation, could occur14. Although our in vivo studies conducted in mice, which should have had a different cytokine profile as in humans, FLT3 CAR T cells may offer an alternative strategy for application of CAR T cell therapy in AML, because our data indicate that FLT3 CAR T cells do not deplete CD34(+) HSCs and preserve HSC differentiation, at least by the in vivo assays performed in this study. Utilizing multiple CARs to target different AML-associated antigens (e.g., FLT3, CD123, and CD33) may also be necessary in many cases of AML, as some tumor-associated antigens may not be expressed on all leukemia cells or the entire leukemic stem cell population. Further, AML blasts may also lose surface expression of a particular tumor antigen under the pressure of CAR T therapy15, 16.
In summary, our study showed that FLT3 can be targeted by FLT3-CAR T cells for the treatment FLT3(+) AML. FLT3-CAR T cells may provide a new immunotherapeutic approach for AML patients.
Supplementary Material
Acknowledgments
This project was supported in part by a grant from the Gabrielle’s Angel Cancer Research Foundation as well as grants from the National Institutes of Health (CA155521, CA210087, CA068458, CA095426, CA185301, and P30 CA16058). This project was also supported in part by an American Cancer Society Research Scholar Grant RSG-14-243-01-LIB and a grant from the Leukemia & Lymphoma Society. The authors are grateful to David Lucas and Donna Bucci at the Leukemia Tissue Bank Shared Resource of the OSU Comprehensive Cancer Center and James Cancer Hospital for providing AML patient samples.
Footnotes
Conflict of Interest
A patent application partially based on this study has been submitted.
References
- 1.Dores GM, Devesa SS, Curtis RE, Linet MS, Morton LM. Acute leukemia incidence and patient survival among children and adults in the United States, 2001–2007. Blood. 2012 Jan 05;119(1):34–43. doi: 10.1182/blood-2011-04-347872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Whitman SP, Maharry K, Radmacher MD, Becker H, Mrozek K, Margeson D, et al. FLT3 internal tandem duplication associates with adverse outcome and gene- and microRNA-expression signatures in patients 60 years of age or older with primary cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. Blood. 2010 Nov 04;116(18):3622–3626. doi: 10.1182/blood-2010-05-283648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Whitman SP, Ruppert AS, Radmacher MD, Mrozek K, Paschka P, Langer C, et al. FLT3 D835/I836 mutations are associated with poor disease-free survival and a distinct gene-expression signature among younger adults with de novo cytogenetically normal acute myeloid leukemia lacking FLT3 internal tandem duplications. Blood. 2008 Feb 01;111(3):1552–1559. doi: 10.1182/blood-2007-08-107946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whitman SP, Archer KJ, Feng L, Baldus C, Becknell B, Carlson BD, et al. Absence of the wild-type allele predicts poor prognosis in adult de novo acute myeloid leukemia with normal cytogenetics and the internal tandem duplication of FLT3: a cancer and leukemia group B study. Cancer research. 2001 Oct 01;61(19):7233–7239. [PubMed] [Google Scholar]
- 5.Maude SL, Frey N, Shaw PA, Aplenc R, Barrett DM, Bunin NJ, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. The New England journal of medicine. 2014 Oct 16;371(16):1507–1517. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Meshinchi S, Appelbaum FR. Structural and functional alterations of FLT3 in acute myeloid leukemia. Clin Cancer Res. 2009 Jul 1;15(13):4263–4269. doi: 10.1158/1078-0432.CCR-08-1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rosnet O, Buhring HJ, Marchetto S, Rappold I, Lavagna C, Sainty D, et al. Human FLT3/FLK2 receptor tyrosine kinase is expressed at the surface of normal and malignant hematopoietic cells. Leukemia. 1996 Feb;10(2):238–248. [PubMed] [Google Scholar]
- 8.Rappold I, Ziegler BL, Kohler I, Marchetto S, Rosnet O, Birnbaum D, et al. Functional and phenotypic characterization of cord blood and bone marrow subsets expressing FLT3 (CD135) receptor tyrosine kinase. Blood. 1997 Jul 01;90(1):111–125. [PubMed] [Google Scholar]
- 9.Chu J, He S, Deng Y, Zhang J, Peng Y, Hughes T, et al. Genetic modification of T cells redirected toward CS1 enhances eradication of myeloma cells. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014 Aug 01;20(15):3989–4000. doi: 10.1158/1078-0432.CCR-13-2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Han J, Chu J, Keung Chan W, Zhang J, Wang Y, Cohen JB, et al. CAR-Engineered NK Cells Targeting Wild-Type EGFR and EGFRvIII Enhance Killing of Glioblastoma and Patient-Derived Glioblastoma Stem Cells. Scientific reports. 2015 Jul 09;5:11483. doi: 10.1038/srep11483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chu J, Deng Y, Benson DM, He S, Hughes T, Zhang J, et al. CS1-specific chimeric antigen receptor (CAR)-engineered natural killer cells enhance in vitro and in vivo antitumor activity against human multiple myeloma. Leukemia. 2014 Apr;28(4):917–927. doi: 10.1038/leu.2013.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yu J, Mao HC, Wei M, Hughes T, Zhang J, Park IK, et al. CD94 surface density identifies a functional intermediary between the CD56bright and CD56dim human NK-cell subsets. Blood. 2010 Jan 14;115(2):274–281. doi: 10.1182/blood-2009-04-215491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ng SW, Mitchell A, Kennedy JA, Chen WC, McLeod J, Ibrahimova N, et al. A 17-gene stemness score for rapid determination of risk in acute leukaemia. Nature. 2016 Dec 07; doi: 10.1038/nature20598. [DOI] [PubMed] [Google Scholar]
- 14.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Preclinical targeting of human acute myeloid leukemia and myeloablation using chimeric antigen receptor-modified T cells. Blood. 2014 Apr 10;123(15):2343–2354. doi: 10.1182/blood-2013-09-529537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruella M, Barrett DM, Kenderian SS, Shestova O, Hofmann TJ, Perazzelli J, et al. Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. The Journal of clinical investigation. 2016 Oct 03;126(10):3814–3826. doi: 10.1172/JCI87366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013 Apr 18;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.