Abstract
Objectives
To demonstrate a new clinically translatable ultrasound molecular imaging approach, modulated acoustic radiation force-based imaging, which is capable of rapid and reliable detection of inflammation as validated in mouse abdominal aorta.
Materials and Methods
Animal studies were approved by the Institutional Animal Care and Use Committee at the University of Virginia. C57BL/6 mice stimulated with tumor necrosis factor-α (TNF-α), or fed with a high-fat diet, were used as inflammation (MInflammation) and diet-induced obesity (DIO) (MDIO) models, respectively. C57BL/6 mice, not exposed to TNF-α or DIO, were used as controls (MNormal). P-selectin-targeted (MBP-selectin), vascular cell adhesion molecule (VCAM)-1-targeted (MBVCAM-1), and isotype control (MBControl) microbubbles were synthesized by conjugating anti-P-selectin, anti-VCAM-1, and isotype control antibodies to microbubbles, respectively. The abdominal aortas were imaged for 180 s during a constant infusion of microbubbles. A parameter, residual-to-saturation ratio (RSR), was used to assess P-selectin and VCAM-1. Statistical analysis was performed with the Student's-t test.
Results
For the inflammation model, RSR of the MInflammation + MBP-selectin group was significantly higher (40.9%, p < 0.0005) than other groups. For the DIO model, RSR of the MDIO + MBVCAM-1 group was significantly higher (60.0%, p < 0.0005) than other groups. Immunohistochemistry staining of the abdominal aorta confirmed the expression of P-selectin and VCAM-1.
Conclusion
A statistically significant assessment of P-selectin and VCAM-1 in mouse abdominal aorta was achieved. This technique yields progress toward rapid targeted molecular imaging in large blood vessels, and thus has the potential for early diagnosis, treatment selection, and risk stratification of atherosclerosis.
Keywords: Ultrasound molecular imaging, microbubble, acoustic radiation force, abdominal aorta, inflammation, atherosclerosis
1. Introduction
Stroke is the fourth leading cause of death in the United States and the direct and indirect cost of stroke was $33.6 billion in 2011 (1). Approximately 15% of stroke incidents are the result of emboli derived from carotid atherosclerosis (2). The current standard of care for carotid atherosclerosis addresses the disease only after the plaque has developed. Detection is often achieved using anatomically-based imaging modalities to measure the luminal stenosis (3–5). However, luminal stenosis has been shown to be an inaccurate predictor of stroke risk (6–8). Conversely, inflammatory responses of atherosclerosis progress silently for decades prior to plaque formation (9,10). Consequently, a method capable of imaging vascular inflammation would enable early diagnosis and risk assessment of atherosclerosis (10). Molecular imaging could achieve this goal by directly visualizing the molecular markers, which are expressed on endothelial cells and associated with vascular inflammation. For example, P-selectin and vascular cell adhesion molecule-1 (VCAM-1) are markers associated with vascular inflammation (10–12). Due to its favorable sensitivity, specificity, safety, cost and availability, ultrasound molecular imaging has been implemented for the detection of vascular inflammation in pre-clinical studies (13–15). Unfortunately, existing ultrasound molecular imaging, which is mainly based on either waiting period method (12) or the pre-burst minus post-burst method (13,16), faces substantial challenges when applied in large blood vessels (17,18). The imaging procedure times are lengthy (30 – 40 min) due to multiple microbubble injections and long waiting periods (Table 1). The length and complexity of these imaging protocols could delay clinical adoption and increase procedure costs. Recently, a new ultrasound molecular imaging strategy, referred to as modulated Acoustic Radiation Force (ARF)-based imaging, has demonstrated rapid (180 s) and quantitative measurements of molecular marker concentration in large vessels (19–21).
Table 1. Pre-clinical applications of ultrasound molecular imaging in large blood vessels or heart.
| Application | Animal model | Biomarker | Mode | Method | ARF* | Procedure time† (min) | p value‡ | Reference |
|---|---|---|---|---|---|---|---|---|
| Inflammation | Rat, heart | ICAM-1 | Contrast | Pre-burst minus post-burst | No | 3 + 20§ + 3 = 26 | < 0.005 | (29) |
| Inflammation | Rat, heart | Selectins | CPS | Pre-burst minus post-burst | No | 4 + 60 + 4 = 68 | < 0.002 | (30) |
| Inflammation | Mouse, heart | P-selectin | CPS | Pre-burst minus post-burst | No | 10 + 20§ + 10 = 40 | < 0.01 | (31) |
| Inflammation | Swine, carotid artery | VCAM-1 | Contrast | Pre-burst minus post-burst | No | 9 + 20 + 9 = 38 | < 0.05 | (32) |
| Atherosclerosis | Mouse, thoracic aorta | VCAM-1 | CPS | Pre-burst minus post-burst | No | 10 + 20§ + 10 = 40 | < 0.001 | (10) |
| Atherosclerosis | Mouse, thoracic aorta | P-selectin, VCAM-1 | CPS | Pre-burst minus post-burst | No | 10 + 20§ + 10 = 40 | < 0.01 | (11) |
| Inflammation | Mouse, abdominal aorta | P-selectin, VCAM-1 | B-mode | Modulated ARF | Yes | 0 + 0 + 3 = 3ǁ | < 0.0005 | This study |
Acoustic radiation force. Destruction pulses were not considered as ARF pulses in the table.
Time for a complete measurement in format: Time for control microbubble injection + Waiting period for clearance + Time for targeted microbubble injection = Procedure time.
The p value between targeted group and various controls.
Waiting period for clearance of freely circulating microbubbles was not specified; estimated waiting period is 20 min.
Single injection of targeted microbubbles required; no waiting period.
In this study, for the first time, we validated this technique for rapid assessment of P-selectin and VCAM-1 in the abdominal aorta of a murine model in just 180 s. The results demonstrate rapid and reliable targeted molecular imaging in a pre-clinical setting. The approach has potential, after considerable further development and validation, as a candidate for clinical usage. It has the potential for early and more accurate diagnosis of atherosclerosis. Although the mouse model involves vessels which are smaller in diameter with lower mean velocities than in human major arterial vessels, it should be borne in mind that the wall shear rate in a mouse is approximately twenty-fold higher (22).
2. Materials and Methods
Mice Preparation
All animal studies were performed under a protocol approved by the Institutional Animal Care and Use Committee at the University of Virginia. The markers of P-selectin and VCAM-1 were selected for microbubble targeting within the abdominal aorta in C57BL/6 female mice (The Jackson Laboratory, Bar Harbor, ME, USA). For the inflammation model (10,12), up-regulation of P-selectin in mice (MInflammation) was achieved by intraperitoneal injection of tumor necrosis factor-α (T7539, Sigma-Aldrich, St Louis, MO, USA) approximately 2.5 h before the start of imaging (0.5 μg/mouse). C57BL/6 mice (MNormal), without any injections, were used as controls. For the DIO model (23), 30 week-old mice (MDIO) were fed with a lard-based high-fat diet (HFD) (60% kcal fat, D12492, Research Diets, New Brunswick, NJ, USA) from 6 weeks of age. Age-matched control mice (MNormal) were fed with a regular low-fat chow diet continuously.
Microbubble Preparation
Biotinylated microbubbles (average diameter = 2.2 μm, standard deviation of diameter = 1.5 μm, and resonance frequency ≈ 4.4 MHz) were synthesized using previously described methods (24,25). P-selectin-targeted microbubbles (MBP-selectin) were prepared by conjugating biotinylated monoclonal anti-P-selectin antibody (RB40.34, CD62P, BD Biosciences, San Diego, CA, USA) to the microbubble using streptavidin linker (AnaSpec, Fremont, CA, USA) (12,26). Briefly, microbubbles were first subjected to repeated centrifugal wash in degasses normal saline to remove excess biotin-lipid, followed by the addition of streptavidin, another centrifugation, and addition of biotinylated antibody, followed by the final centrifugal wash. For the control microbubbles (MBControl) used in the inflammation model, isotype control antibody (IgG1, BD Biosciences, San Diego, CA, USA) was conjugated to the microbubble shell. For the VCAM-1-targeted microbubbles (MBVCAM-1), biotinylated monoclonal anti-VCAM-1 antibody (MVCAM.A, CD106, BD Biosciences, San Diego, CA, USA) was conjugated to the microbubble shell (26). For the control microbubbles (MBControl) used in the DIO model, isotype control antibody (IgG2a, BD Biosciences, San Diego, CA, USA) was conjugated to the microbubble shell. All the antibodies were added to the microbubbles at a concentration of 1.5 μg per 107 microbubbles to obtain maximal surface coverage (∼ 105 antibody molecules per microbubble) (12).
Modulated Acoustic Radiation Force (ARF)-based Imaging
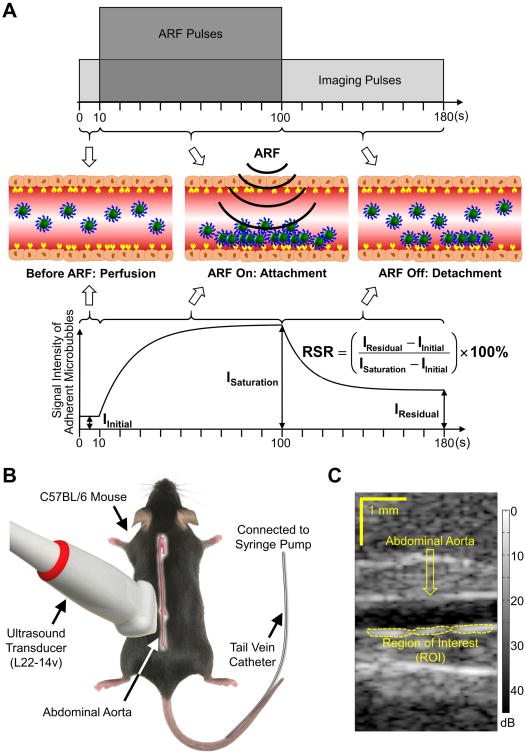
The details of the modulated Acoustic Radiation Force (ARF)-based beam sequence have been described in previous studies (19,20). Briefly, the sequence collected image data continuously for 180 s at a frame rate of approximately 6 Hz (Figure 1, A). The signal intensity along the bottom wall of the abdominal aorta was detected and quantified. Residual-to-saturation ratio (RSR) was extracted from the signal intensity profiles. The microbubble dynamics in response to the modulated ARF-based beam sequence has been optically verified via microscopy (21). The sequence was implemented on a programmable Verasonics ultrasound system (Vantage 256, Verasonics, Kirkland, WA, USA) equipped with an L22-14v linear array transducer. The acoustic properties of both imaging and ARF pulses of the sequence are described in Table 2.
Figure 1. The modulated ARF-based imaging.
A, Schematic diagram illustrating the imaging sequence (top), microbubble dynamic behaviors (middle), and the signal intensity curve of adherent microbubbles and RSR (bottom). B, Mouse was placed in the prone position with the ultrasound transducer imaging the abdominal aorta. Microbubble infusion was administered via a tail vein catheter connected to a syringe pump. C, A sample B-mode image of the mouse abdominal aorta.
Table 2. Acoustic parameters of imaging and ARF pulses.
| Transducer type | L22-14v* | |
|---|---|---|
|
|
||
| Pulse type | Imaging pulse | ARF pulse |
| Frequency (MHz) | 20.8 | 4.5 |
| Pulse length (cycle) | 1 | 10 |
| PRF† (Hz) | 6 | 6×103 |
| PNP‡ (MPa) | 1.13 | 1.33 |
| MI§ | 0.28 | 0.63 |
PNP and MI were measured at the depth of 8 mm.
Average pulse repetition frequency.
Peak-negative pressure.
Mechanical index.
In Vivo Imaging of Mice
The mice were initially anesthetized in the induction chamber using 2.5% isoflurane (Henry Schein, Dublin, OH, USA). They were then transferred to a heated imaging stage (TM150, Indus Instruments, Webster, TX, USA) and laid in the prone position (Figure 1, B). They were kept under anesthesia (2% isoflurane) throughout the course of the experiment. Hair on the left side of the back was removed for imaging. A tail-vein catheter was affixed to administer the microbubbles while imaging. The location and orientation of the ultrasound transducer was carefully adjusted to obtain optimal images of the abdominal aorta (Figure 1, C). The imaging depth (distance between transducer and bottom wall of abdominal aorta) ranged between 6 to 10 mm depending on the mouse body size. Different mice were used in each group to achieve independent measurements. Microbubbles were administered by constant infusion (50×106 microbubbles in 150 μL sterile saline, infusion rate of approximately 2.5×105 microbubbles/s) via the tail vein catheter using a syringe pump (PHD ULTRA, Harvard Apparatus, Holliston, MA, USA). Imaging was performed approximately 20 s after the start of microbubble infusion.
Experimental Framework
The experiments were designed to have the mice and microbubble combinations illustrated in Table 3.
Table 3. Mice and microbubble combinations.
| Inflammation model | DIO model | ||||
|---|---|---|---|---|---|
|
| |||||
| MNormal | MInflammation | MNormal | MDIO | ||
|
|
|
||||
| MBControl | 4 mice | 5 mice | MBControl | 5 mice | 5 mice |
| MBP-selectin | 4 mice | 6 mice | MBVCAM-1 | 5 mice | 6 mice |
Data Analysis
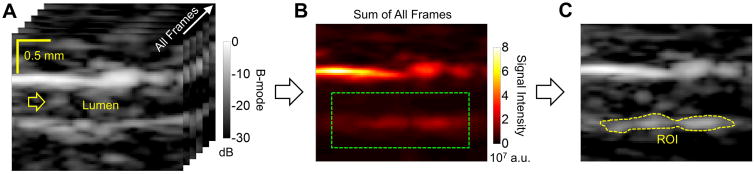
The region of interest (ROI) was located in the middle of bottom wall of the abdominal aorta. The following steps were used to reduce the effects of physiological motion on ROI location (Figure 2). (1) All image frames acquired during 180 s were summed together. (2) A 2 mm × 0.8 mm searching window on the bottom vessel wall was used to establish the ROI. (3) The area within the searching window with signal intensity higher than approximately 20% of the maximum intensity was defined as the ROI. The signal intensity curve of adherent microbubbles was calculated by averaging signal intensities within the ROI for all image frames. All signal intensity curves reported in this paper were normalized, which was achieved by the following steps: During the 180 s imaging period, the minimum signal intensity was normalized to 0, and the maximum signal intensity was normalized to 1. All data analysis was performed in the MATLAB (MathWorks, Natick, MA, USA) environment.
Figure 2. A depiction of data processing in the study.
A, A stack of all B-mode image frames obtained using the modulated ARF pulse sequence showing the mouse abdominal aorta. Scale bars are applicable to all three sub-figures. B, The sum of all image frames. The green window (2 mm × 0.8 mm) shows a zoomed-in region on the bottom vessel wall used to establish the region of interest (ROI). The area within the green window with signal magnitude greater than approximately 20% of the maximum magnitude was defined as ROI (C). The signal intensity curve of bottom vessel wall was calculated by averaging signal intensities within the ROI.
Immunohistochemistry Staining
Immunohistochemistry staining for P-selectin and VCAM-1 was performed on 5 μm-thick paraffin-embedded sections of the abdominal aorta for several mice in each group (10). The antibodies used for immunohistochemistry were as follows: P-selectin (sc-6941, Santa Cruz Biotechnology, Dallas, TX, USA) and VCAM-1 (sc-1504, Santa Cruz Biotechnology, Dallas, TX, USA). The primary antibodies were detected using a Vectastain Elite ABC Kit (Vector Laboratories, Burlingame, CA, USA) after microwave treatment with Antigen Unmasking Solution (Vector Laboratories, Burlingame, CA, USA). Visualization was performed with 3,3′-diaminobenzidine (DAB) chromagen (Acros Organics, Geel, Belgium). Counter staining was performed using Hematoxylin 1 (Richard-Allan Scientific, Kalamazoo, MI, USA). Negative control experiments were performed by omitting the primary antibodies.
Statistics
All data were expressed as mean ± standard deviation. Statistical analysis was performed using the Statistics and Machine Learning Toolbox in MATLAB. An unpaired Student's t-test was used, and differences were considered statistically significant with a p-value less than 0.05.
3. Results
RSR Detected P-selectin and VCAM-1 Markers with Statistical Significance
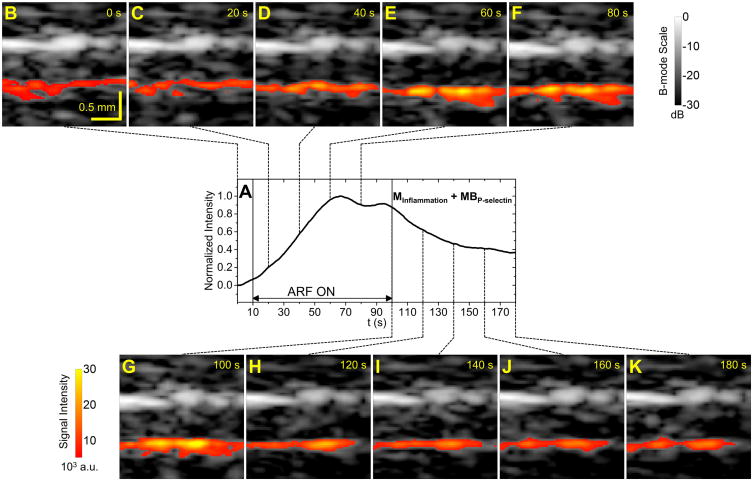
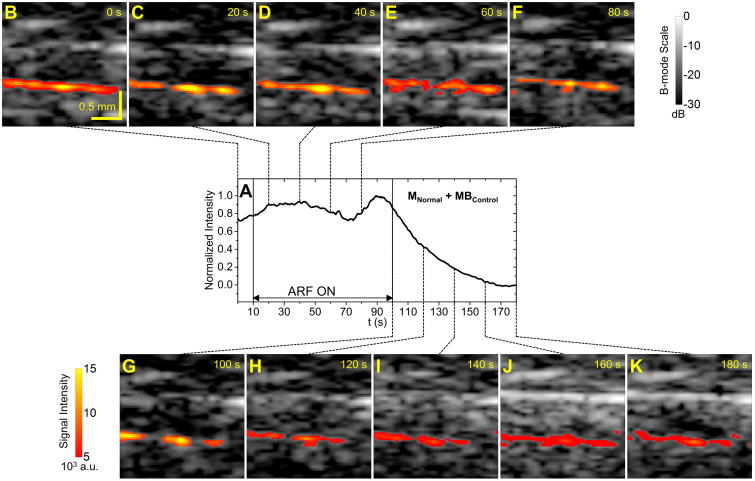
Representative signal intensity curves of the bottom vessel wall of the abdominal aorta from the groups of MInflammation + MBP-selectin and MNormal + MBControl are illustrated in Figure 3 and Figure 4, respectively. With targeted microbubbles, signal intensity increased with application of ARF and decreased to a residual level after cessation of ARF (Figure 3). With control microbubbles, signal intensity decreased to a lower level than the baseline after cessation of ARF (Figure 4).
Figure 3. Example signal intensity curve and corresponding ultrasound images for targeted group.
A, Signal intensity curve for one trial from the group of MInflammation + MBP-selectin. ARF was applied from 10 s to 100 s. B to K, Corresponding B-mode images overlaid with color-coded microbubble signal along the bottom vessel wall at different time points.
Figure 4. Example signal intensity curve and corresponding ultrasound images for control group.
A, Signal intensity curve for one trial from the group of MNormal + MBControl. ARF was applied from 10 s to 100 s. B to K, Corresponding B-mode images overlaid with color-coded microbubble signal along the bottom vessel wall at different time points.
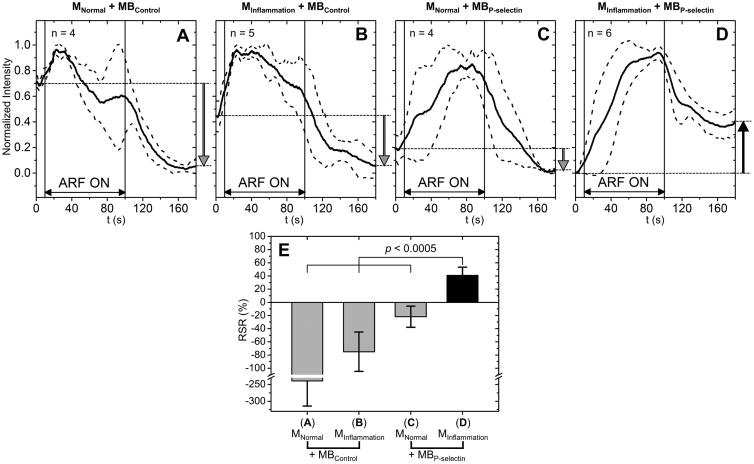
The average signal intensities for the inflammation model are shown in Figure 5. Solid lines indicate the mean and dotted lines indicate the error bars (mean ± standard deviation). Based on the normalization method described before, the values of average signal intensity curves were between 0 and 1. The initial values at 0 s varied depending on the lowest values during the 180 s imaging period. With the application of ARF, signal intensities increased in all groups. After the cessation of ARF, there was a residual intensity above the baseline only in the group of MInflammation + MBP-selectin. For the other three groups, few adherent microbubbles remained attached on the vessel wall after the cessation of ARF. Due to the shadowing effects of freely circulating microbubbles, the residual intensities were lower than the baselines at 0 s. RSR for the group of MInflammation + MBP-selectin was 40.9%, significantly higher (p < 0.0005) than that of any other group (-239% for MNormal + MBControl, -74.9% for MInflammation + MBControl, and -21.9% for MNormal + MBP-selectin).
Figure 5. Signal intensity curves and RSRs for inflammation model.
Signal intensity curves for the groups of: MNormal + MBControl (A), MInflammation + MBControl (B), MNormal + MBP-selectin (C), and MInflammation + MBP-selectin (D). Solid lines indicate the mean and dotted lines indicate the error bars (mean ± standard deviation). E, RSR for all groups. RSR for the group of MInflammation + MBP-selectin was significantly higher than that of other groups (p < 0.0005).
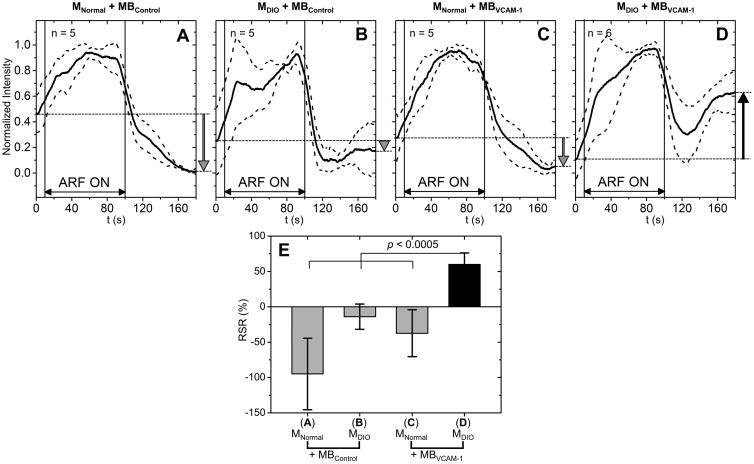
The average signal intensities for the DIO model are illustrated in Figure 6. RSR for the group of MDIO + MBVCAM-1 was 60.0%, significantly higher (p < 0.0005) than that of any other group (-94.9% for MNormal + MBControl, -13.9% for MDIO + MBControl, and -37.4% for MNormal + MBVCAM-1). Consequently, RSR could be used to detect P-selectin and VCAM-1 with statistical significance in inflammation and DIO models, respectively.
Figure 6. Signal intensity curves and RSRs for DIO model.
Signal intensity curves for the groups of: MNormal + MBControl (A), MDIO + MBControl (B), MNormal + MBVCAM-1 (C), and MDIO + MBVCAM-1 (D). Solid lines indicate the mean and dotted lines indicate the error bars (mean ± standard deviation). E, RSR for all groups. RSR for the group of MDIO + MBVCAM-1 was significantly higher than that of other groups (p < 0.0005).
Immunohistochemistry Staining Confirmed Expression of P-selectin and VCAM-1
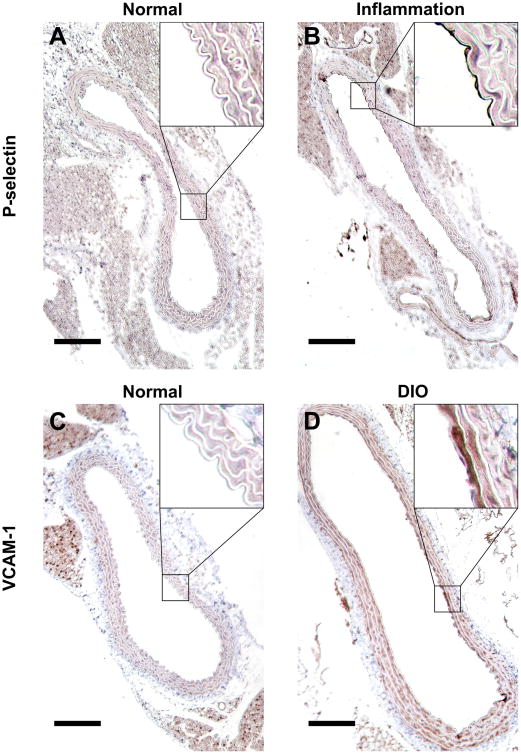
Representative images of immunohistochemistry staining of the abdominal aorta are shown in Figure 7. MNormal demonstrates minimal endothelial P-selectin staining (Figure 7, A) while MInflammation demonstrates P-selectin expression mostly localized to the luminal endothelial surface (Figure 7, B). Additionally, MNormal exhibits minimal endothelial VCAM-1 staining (Figure 7, C) while MDIO exhibits VCAM-1 expression localized to the luminal endothelium (Figure 7, D).
Figure 7. Immunohistochemistry staining of the abdominal aorta.
P-selectin staining of MNormal (A) and MInflammaiton (B). VCAM-1 staining of MNormal (C) and MDIO (D). Scale bar = 150 μm.
4. Discussion
In this study, modulated ARF-based imaging was demonstrated to be effective for rapid assessment of P-selectin and VCAM-1 in the mouse abdominal aorta. Existing pre-clinical studies for detection of inflammation and atherosclerosis using ultrasound molecular imaging have been based mostly on traditional waiting period method (12) or pre-burst minus post-burst method (13) without ARF (Table 1). However, in large blood vessels, relatively high shear forces necessitate the use of ARF to enhance microbubble targeting (27). In order to reliably push microbubbles to the vessel wall in a human, we envision that it will be necessary to operate during the diastolic phase and rely on radiation forces that have been shown, using an extrapolation from an experimentally validated theory, to yield translation velocities up to 17 mm/s using acoustic intensities that are simultaneously FDA intensity compliant and microbubble non-destructive (28). The use of ARF in this study reduces microbubble targeting time from a previous 3 – 10 min down to 1.5 min. Additionally, the traditional method requires a second control microbubble injection and imaging to estimate the “non-specific background”. A waiting period (≥ 20 min) is required for clearance of previously injected microbubbles. These two extra steps can result in a complex imaging protocol and long imaging procedure time (30 – 60 min). In the new method, by monitoring the intensity level after cessation of ARF, RSR allows for quantification of the molecularly bound microbubbles and assessment of the density of molecular markers without any control injections (19,20). Consequently, a single targeted microbubble injection and imaging is sufficient to obtain measurements in only 180 s.
The results demonstrated that RSR can be used to detect molecular markers with statistical significance in both inflammation and DIO models. Compared to other pre-clinical studies (10,11,29–32), our technique exhibited a very low p value (p < 0.0005) between the targeted group and various controls (Table 1). Furthermore, the present study demonstrates that the measurements are insensitive to the imaging path length in vivo (ranged from 6 – 10 mm depending on mouse size). Consequently, RSR is a reliable and accurate indicator to assess P-selectin and VCAM-1 expression levels.
Limitations of the Study
The signal intensity curve in response to the modulated ARF-based imaging sequence was a result of both primary and secondary radiation forces. In this study, we did not elucidate the detailed mechanisms of the microbubble binding dynamics. A previous study (21) demonstrated that secondary radiation forces generate aggregates of adherent microbubbles with similar imaging sequences. More experiments would be required to examine the contributions of primary and secondary radiation forces, respectively.
Although the proposed imaging technique has been demonstrated effective in vivo in this study, it will face challenges when applied in the clinical environments in the future. The algorithm may need to be optimized to account for the lower frequencies and greater depths in human. It may also require refinement to accommodate the presence of ultrasound artifacts (e.g. clutter and reverberation). The effects of physiological motion have been reduced in the study. However, in clinical application settings, more advanced motion-correction methods are required to eliminate motion artifacts. In addition, this modulated ARF-based imaging technique could only detect molecular markers on the distal side of vessel wall relative to the transducer location. Consequently, effective detection of inflammation relies on the assumption that molecular markers for inflammation are approximately symmetrically distributed on the inner surface of blood vessels. In clinical applications, asymmetric distribution of molecular markers may significantly impact detection efficacy. Furthermore, the validation of this imaging technique was completed based on relatively simple flow profiles without complex flow patterns that include turbulence and backflow. As a result, more studies are required to demonstrate the robustness of this technique in realistic physiological conditions.
Conclusion
In conclusion, this ultrasound-based non-invasive technique could reliably and accurately detect molecular markers. It exhibits many desirable characteristics: (1) no separate control injections required; (2) short protocol with a procedure time of 180 s and no extra waiting period; (3) high detection specificity; (4) imaging path length-insensitive measurements; (5) low-cost, radiation-free, clinical translatable, and ready for rapid adoption. This is the first in vivo validation of this technique to assess P-selectin and VCAM-1 expression in the abdominal aorta of a murine model. Significantly, this technique has the potential for clinical applications in large blood vessels for the early diagnosis, treatment selection, and risk stratification of atherosclerosis.
Acknowledgments
Disclosure of Funding:This work was supported by NIH R01 EB001826, NIH R01 HL111077, and the Virginia Center for Innovative Technology (CIT) Commonwealth Research Commercialization Fund Award MF14F-002-LS. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Contributor Information
Shiying Wang, Department of Biomedical Engineering, University of Virginia, Charlottesville, VA 22908, USA
Sunil Unnikrishnan, Department of Biomedical Engineering, University of Virginia, Charlottesville, VA 22908, USA
Elizabeth B. Herbst, Department of Biomedical Engineering, University of Virginia, Charlottesville, VA 22908, USA
Alexander L. Klibanov, Division of Cardiovascular Medicine and Department of Biomedical Engineering, University of Virginia, Charlottesville, VA 22908, USA.
F. William Mauldin, Jr, Department of Biomedical Engineering, University of Virginia, Charlottesville, VA 22908, USA.
John A. Hossack, Department of Biomedical Engineering and Electrical and Computer Engineering, University of Virginia, Charlottesville, VA 22908, USA, 415 Lane Road, MR5 Building, Room 2121, Charlottesville, VA 22908, USA, Phone: (434) 243-5866; Fax: (434) 982-3870.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics - 2015 update: a report from the American Heart Association. Circulation. 2015;131(4):e29–322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Markus HS, Siegel JE, Topakian R, et al. The Asymptomatic Carotid Emboli Study: study design and baseline results. Int J Stroke. 2009;4(5):398–405. doi: 10.1111/j.1747-4949.2009.00339.x. [DOI] [PubMed] [Google Scholar]
- 3.Lovett JK, Gallagher PJ, Hands LJ, Walton J, Rothwell PM. Histological correlates of carotid plaque surface morphology on lumen contrast imaging. Circulation. 2004;110(15):2190–2197. doi: 10.1161/01.CIR.0000144307.82502.32. [DOI] [PubMed] [Google Scholar]
- 4.Miralles M, Merino J, Busto M, Perich X, Barranco C, Vidal-Barraquer F. Quantification and characterization of carotid calcium with multi-detector CT-angiography. Eur J Vasc Endovasc Surg. 2006;32(5):561–567. doi: 10.1016/j.ejvs.2006.02.019. [DOI] [PubMed] [Google Scholar]
- 5.Mathiesen EB, Bønaa KH, Joakimsen O. Echolucent plaques are associated with high risk of ischemic cerebrovascular events in carotid stenosis: the tromsø study. Circulation. 2001;103(17):2171–2175. doi: 10.1161/01.cir.103.17.2171. [DOI] [PubMed] [Google Scholar]
- 6.Golledge J, Siew DA. Identifying the carotid “high risk” plaque: is it still a riddle wrapped up in an enigma? Eur J Vasc Endovasc Surg. 2008;35(1):2–8. doi: 10.1016/j.ejvs.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Trivedi RA, U-King-Im JM, Graves MJ, Gillard J, Kirkpatrick PJ. Non-stenotic ruptured atherosclerotic plaque causing thrombo-embolic stroke. Cerebrovasc Dis. 2005;20(1):53–55. doi: 10.1159/000086424. [DOI] [PubMed] [Google Scholar]
- 8.Halliday A, Mansfield A, Marro J, et al. Prevention of disabling and fatal strokes by successful carotid endarterectomy in patients without recent neurological symptoms: randomised controlled trial. Lancet. 2004;363(9420):1491–1502. doi: 10.1016/S0140-6736(04)16146-1. [DOI] [PubMed] [Google Scholar]
- 9.Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- 10.Kaufmann BA, Sanders JM, Davis C, et al. Molecular imaging of inflammation in atherosclerosis with targeted ultrasound detection of vascular cell adhesion molecule-1. Circulation. 2007;116(3):276–284. doi: 10.1161/CIRCULATIONAHA.106.684738. [DOI] [PubMed] [Google Scholar]
- 11.Kaufmann BA, Carr CL, Belcik JT, et al. Molecular imaging of the initial inflammatory response in atherosclerosis: implications for early detection of disease. Arterioscler Thromb Vasc Biol. 2010;30(1):54–59. doi: 10.1161/ATVBAHA.109.196386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindner JR, Song J, Christiansen J, Klibanov AL, Xu F, Ley K. Ultrasound assessment of inflammation and renal tissue injury with microbubbles targeted to P-selectin. Circulation. 2001;104(17):2107–2112. doi: 10.1161/hc4201.097061. [DOI] [PubMed] [Google Scholar]
- 13.Caskey CF, Hu X, Ferrara KW. Leveraging the power of ultrasound for therapeutic design and optimization. J Control Release. 2011;156(3):297–306. doi: 10.1016/j.jconrel.2011.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Deshpande N, Needles A, Willmann JK. Molecular ultrasound imaging: current status and future directions. Clin Radiol. 2010;65(7):567–581. doi: 10.1016/j.crad.2010.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abou-Elkacem L, Bachawal SV, Willmann JK. Ultrasound molecular imaging: Moving toward clinical translation. Eur J Radiol. 2015;84(9):1685–1693. doi: 10.1016/j.ejrad.2015.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang S, Herbst EB, Mauldin FWJ, Diakova GB, Klibanov AL, Hossack JA. Ultra-low-dose ultrasound molecular imaging for the detection of angiogenesis in a mouse murine tumor model: How little can we see? Invest Radiol. 2016;51(12):758–766. doi: 10.1097/RLI.0000000000000310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mauldin FW, Dhanaliwala AH, Patil AV, Hossack JA. Real-time targeted molecular imaging using singular value spectra properties to isolate the adherent microbubble signal. Phys Med Biol. 2012;57(16):5275–5293. doi: 10.1088/0031-9155/57/16/5275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang S, Mauldin FW, Jr, Klibanov AL, Hossack JA. Shear forces from flow are responsible for a distinct statistical signature of adherent microbubbles in large vessels. Mol Imaging. 2013;12(6):1–13. [PMC free article] [PubMed] [Google Scholar]
- 19.Wang S, Hossack JA, Klibanov AL, Mauldin FW. Binding dynamics of targeted microbubbles in response to modulated acoustic radiation force. Phys Med Biol. 2014;59(2):465–484. doi: 10.1088/0031-9155/59/2/465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang S, Mauldin FW, Klibanov AL, Hossack JA. Ultrasound-based measurement of molecular marker concentration in large blood vessels: a feasibility study. Ultrasound Med Biol. 2015;41(1):222–234. doi: 10.1016/j.ultrasmedbio.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang S, Wang CY, Unnikrishnan S, Klibanov AL, Hossack JA, Mauldin FW. Optical verification of microbubble response to acoustic radiation force in large vessels with in vivo results. Invest Radiol. 2015;50(11):772–784. doi: 10.1097/RLI.0000000000000185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greve JM, Les AS, Tang BT, et al. Allometric scaling of wall shear stress from mice to humans: quantification using cine phase-contrast MRI and computational fluid dynamics. Am J Physiol Heart Circ Physiol. 2006;291(4):H1700–H1708. doi: 10.1152/ajpheart.00274.2006. [DOI] [PubMed] [Google Scholar]
- 23.Xu H, Barnes GT, Yang Q, et al. Chronic inflammation in fat plays a crucial role in the development of obesity-related insulin resistance. J Clin Invest. 2003;112(12):1821–1830. doi: 10.1172/JCI19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil AV, Rychak JJ, Allen JS, Klibanov AL, Hossack JA. Dual frequency method for simultaneous translation and real-time imaging of ultrasound contrast agents within large blood vessels. Ultrasound Med Biol. 2009;35(12):2021–2030. doi: 10.1016/j.ultrasmedbio.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Klibanov AL, Rychak JJ, Yang WC, et al. Targeted ultrasound contrast agent for molecular imaging of inflammation in high-shear flow. Contrast Media Mol Imaging. 2006;1(6):259–266. doi: 10.1002/cmmi.113. [DOI] [PubMed] [Google Scholar]
- 26.Ferrante EA, Pickard JE, Rychak J, Klibanov A, Ley K. Dual targeting improves microbubble contrast agent adhesion to VCAM-1 and P-selectin under flow. J Control Release. 2009;140(2):100–107. doi: 10.1016/j.jconrel.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rychak JJ, Klibanov AL, Ley KF, Hossack JA. Enhanced targeting of ultrasound contrast agents using acoustic radiation force. Ultrasound Med Biol. 2007;33(7):1132–1139. doi: 10.1016/j.ultrasmedbio.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 28.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112(5 Pt 1):2183–2192. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 29.Weller GE, Lu E, Csikari MM, et al. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108(2):218–224. doi: 10.1161/01.CIR.0000080287.74762.60. [DOI] [PubMed] [Google Scholar]
- 30.Villanueva FS, Lu E, Bowry S, et al. Myocardial ischemic memory imaging with molecular echocardiography. Circulation. 2007;115(3):345–352. doi: 10.1161/CIRCULATIONAHA.106.633917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaufmann BA, Lewis C, Xie A, Mirza-Mohd A, Lindner JR. Detection of recent myocardial ischaemia by molecular imaging of P-selectin with targeted contrast echocardiography. Eur Heart J. 2007;28(16):2011–2017. doi: 10.1093/eurheartj/ehm176. [DOI] [PubMed] [Google Scholar]
- 32.Masseau I, Davis MJ, Bowles DK. Carotid inflammation is unaltered by exercise in hypercholesterolemic Swine. Med Sci Sports Exerc. 2012;44(12):2277–2289. doi: 10.1249/MSS.0b013e318266af0a. [DOI] [PMC free article] [PubMed] [Google Scholar]