Summary
Sickle cell disease (SCD) is the single most important genetic cause of childhood mortality globally. Tanzania has one of the highest annual births of SCD individuals in the world, estimated to reach 11 000 births a year. Without intervention, 50–90% of children will die in childhood. However, cost-effective interventions have the potential to reduce childhood mortality by up to 70%. The effects of SCD are multi-dimensional, ranging from causing high morbidity and mortality, and reducing the quality of life, to imposing a high socio-economic burden on individuals, families and health systems. In the past 12 years, the SCD programme in Tanzania has developed, with local and global partnerships, a systematic framework for comprehensive research that is integrated into providing healthcare, training and advocacy in SCD. This report outlines the approach and achievements of collective initiatives for management and control of SCD in Tanzania.
Keywords: sickle cell, Tanzania, health, research
Sickle cell disease (SCD) is an inherited disease caused by a single-gene mutation affecting the β-globin gene (HBB) on chromosome 11. It results in abnormality of red blood cells (RBC), which affects their shape and function, and subsequently influencing nearly all organ-systems of the body. The impact of SCD is multi-dimensional, from causing high morbidity and mortality and reducing the quality of life, resulting in a high economic burden for individuals, families and health system. As a result of the significant health, social and economic impact, the United Nations General Assembly recognized SCD as a disease of public health concern in 2009 (United Nations General Assembly Resolution 2009).
The burden of SCD is high based on the annual birth prevalence of SCD. Modelling using demographic information, mortality rates and available interventions estimate that 14 242 000 newborns affected by SCD will be born between 2010 and 2050 (Piel et al, 2013), with 82% being in Sub Saharan Africa. In Tanzania, it is estimated that there are between 8000 and 11 000 children born with SCD annually, making this one of the countries with the highest SCD annual births worldwide (Piel et al, 2013). The prevalence of the sickle heterozygous carrier state (HbAS) in Tanzania is 13% (Makani et al, 2011), with the geographical regions that are most affected being the Eastern coast and the North West region around Lake Victoria. Like many low- and middle-income countries (LMIC), Tanzania is undergoing a demographic transition, with an increase in child survival and life expectancy. The reduction in childhood mortality is thought to be predominantly the result of improvements in hygiene, nutrition, and public health interventions, which has led to a decrease in mortality caused by infectious diseases. Therefore, it is anticipated that there will be an increase in the number of infants with non-communicable diseases, such as SCD, who are surviving and who present for diagnosis and treatment. This demographic transition coupled with increased population growth, will lead to a major increase in the number of SCD births over the next half-century (Weatherall, 2010).
Approach to management of SCD
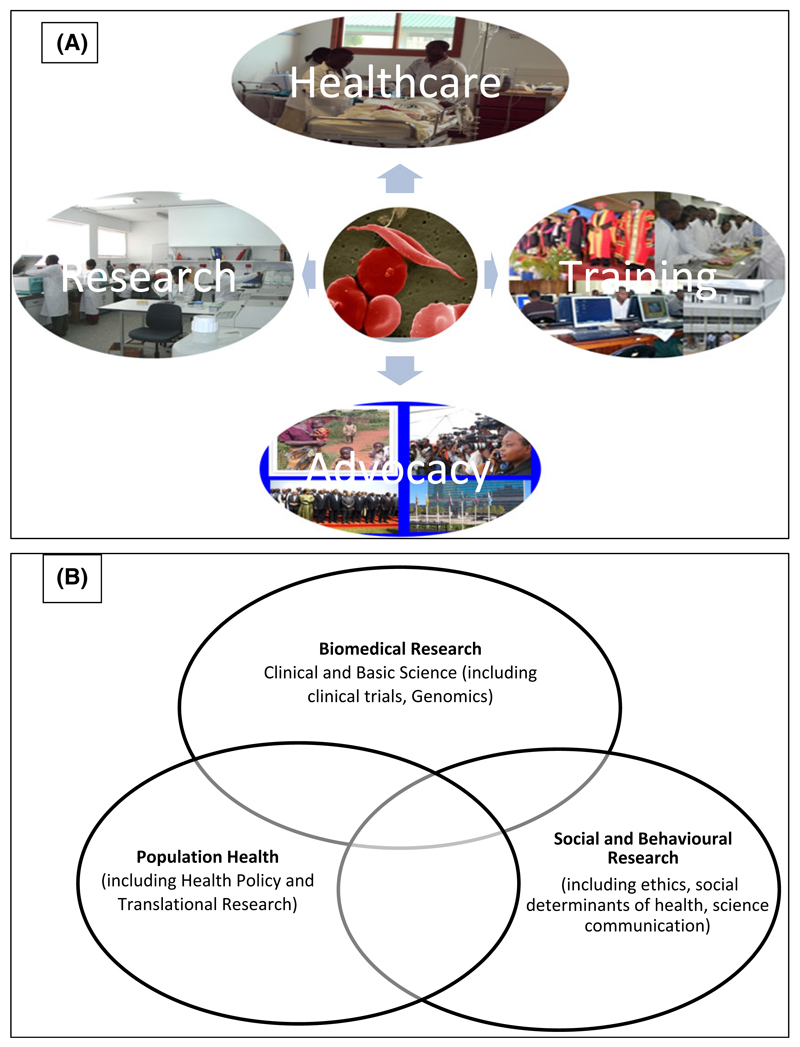
The World Health Organization (WHO) has recommended a set of public health interventions to reduce the burden of SCD. The interventions include improving awareness, early diagnosis and improving the quality of healthcare for affected individuals (WHO 2006). The latter involves providing effective clinical, laboratory, diagnostic and imaging facilities that are appropriate for different levels of the health system. In addition, where appropriate, measures to prevent disease should be taken through the counselling of individuals with a high risk of having a child with SCD (WHO 2010). With this in mind, Muhimbili University of Health and Allied Sciences (MUHAS) (http://www.muhas.ac.tz) worked with Muhimbili National Hospital (MNH) (http://www.mnh.or.tz/) to develop local and global partnerships to establish a systematic framework for comprehensive research that is integrated into providing healthcare, education and advocacy in SCD (Fig 1A).
Fig 1.
(A) Sickle Cell activities that integrate Healthcare, Research, Training and Advocacy. (B) Research themes in the sickle cell programme at Muhimbili.
Healthcare
Background
Muhimbili National Hospital (MNH) (http://www.mnh.or.tz/) is the National Referral Hospital for Tanzania, situated in the commercial capital, Dar es Salaam. It is a tertiary level health facility with a 1500-bed capacity, seeing up to 1200 outpatients per day and admitting 1000–1200 inpatients per week. The hospital has 3000 employees, of which 300 are doctors and 900 nurses. Healthcare for SCD is provided to adults within the Department of Internal Medicine (Clinical Haematology Unit) and to children within the Department of Paediatrics and Child Health. The departments of laboratory services (specifically the haematology unit) and radiology are responsible for providing laboratory tests and imaging facilities for diagnosis and monitoring of SCD-related complications. MNH, a teaching hospital, is affiliated to MUHAS, with university and hospital staff working together to provide healthcare services, conduct research and offer education and training. It is within this framework that the Muhimbili Sickle Cohort (MSC) was developed.
Approach
Muhimbili National Hospital had an existing health service with dedicated SCD clinics for children and adults. In 2004, this framework was used to develop a platform for the integrated programme. Between March 2004 and March 2016, a total of 8484 individuals were seen and given unique demographic identity numbers (demographic ID). There were 5466 (64%) with SCD (HbSS), 2121 (25%) with sickle cell trait (HbAS) and 897 (11%) had normal haemoglobin pattern (HbAA). It is important to note that, apart from a few patients with S/β-thalassaemia, more than 99% of patients have homozygous (SS) SCD. Individuals who were confirmed to have SCD were enrolled into the MSC programme and were assigned a unique SCD identity number (SCD ID). Approximately 150 patients with SCD were seen every week at outpatient clinics for diagnostic testing and follow-up visits, whilst 15 patients were hospitalized every week. Every 2 years, a report was generated from the database of individuals who had not attended the clinic for more than 9 months. These individuals were actively contacted by telephone calls or where possible, home visits. The proportion of SCD patients who were lost to follow-up was 12%. For those families who reported that the SCD individual had died, a verbal autopsy was undertaken by a medical doctor, where feasible.
Diagnostic services
Screening for SCD was done by the sickling test, which uses sodium metabisulphite. The programme was the only public facility to offer 3 types of SCD confirmatory tests, including Haemoglobin Electrophoresis (HBE), Isoelectric focusing (IEF) and High Performance Liquid Chromatography (HPLC). Additional laboratory services that were available included haematology tests (automated haematology analysers, reticulocyte count) as well as rapid diagnostic tests for haemoglobin level and malaria. The programme has access to the following tests through laboratories in MUHAS and MNH: microbiology (automated blood cultures, malaria microscopy, human immunodeficiency virus); biochemistry (haemolytic markers); bone marrow and blood film morphology; iron studies (serum ferritin, soluble transferrin receptors, and serum iron), levels of vitamin B12, serum and red cell folate.
Therapeutic services
Sickle cell disease patients received daily folic acid due to the high prevalence of folic acid deficiency in SCD. Although individuals with SCD have low prevalence of malaria infection, there is a high risk of malaria-related morbidity and mortality, particularly from severe anaemia and increased haemolysis. Therefore, malaria prevention is recommended in SCD with use of insecticide-treated nets, anti-malarial chemoprophylaxis as well as prompt diagnosis and treatment of malaria. In addition to malaria, individuals with SCD are at an increased risk of infection and death from invasive bacterial infections. The proportion of deaths from invasive bacterial infections in SCD has been reported to be as high as 50% in the absence of interventions (Thomas et al, 1982; Leikin et al, 1989; Gill et al, 1995). Septicaemia and meningitis caused by Streptococcus pneumoniae are the leading causes of morbidity and mortality (Aken’ova et al, 1998; Booth et al, 2001; Kizito et al, 2007; Williams et al, 2009; Obaro, 2010), with children below the age of 6 years being reported to have the highest risk. Interventions with daily oral penicillin and pneumococcal conjugate vaccine (PCV) have significantly reduced mortality due to infections in SCD (Gaston et al, 1986; Knight-Madden & Serjeant, 2001; Halasa et al, 2007). With this evidence in mind, daily penicillin prophylaxis was given to children under 6 years of age. In 2012, the Ministry of Health of Tanzania, through its Expanded Programme on Immunization (EPI) schedule, introduced PCV as part of routine immunization of infants, beginning at 6 weeks of age. The immunization coverage in Tanzania ranges between 80% and 90% (http://www.gavi.org/country/tanzania).
Blood transfusion is available for the management of severe anaemia, acute stroke and acute chest syndrome (ACS), but there is no chronic transfusion programme. Surgical services for splenectomy, cholelithiasis and other complications are provided following clinical indications. Although there is overwhelming evidence to support the use of hydroxycarbamide (also termed hydroxyurea) to reduce morbidity and mortality in SCD (Charache et al, 1992; Stegenga et al, 2004) as well as its cost effectiveness (Ojwang et al, 1987), the use of hydroxycarbamide in Tanzania is limited. Within the MSC programme only 1% of SCD patients were on hydroxycarbamide. Although hydroxycarbamide has been approved as a treatment option for SCD in Tanzania, with inclusion in the national essential drug list, it is not consistently available in public and private pharmacies and, when available, it is not affordable for most patients. Together with other initiatives, the programme has partnered with the School of Pharmacy at MUHAS to start formulating hydroxycarbamide locally. This initiative is in its infancy but will go a long way towards increasing access to hydroxycarbamide.
Newborn screening for SCD
The newborn screening (NBS) for SCD project was a 2-year project (January 2015–December 2016) that aimed to introduce newborn screening for SCD as a health programme in Tanzania. The project was funded by UK Agency for International Development (UKAID), from the Department for International Development (DFID). The project worked with the reproductive and child health (RCH) unit in the Ministry of Health and involved 2 hospitals in Dar es Salaam. During the pilot phase, the programme trained 160 nurses on NBS for SCD and 16 laboratory technicians in using isoelectric focusing (IEF) for SCD testing. In addition, the NBS programme developed a national guideline for NBS. During this period, 4002 newborns were screened and 31 SCD babies were diagnosed, giving a birth prevalence of 8 per 1000. The newborn babies who were identified to have SCD were referred to SCD clinics for comprehensive care.
Health education
Health education has been provided to all individuals during hospital visits and was complemented with educational material translated into Kiswahili, which is the local language. Newly diagnosed patients, relatives and guardians were counselled during the enrolment visit. The MSC programme worked with stakeholders to develop a SCD treatment guideline, which was officially adopted at the national level by the Ministry of Health in 2014. Working with the Non-Communicable Diseases (NCD) unit, training on management of SCD was conducted for healthcare workers in different health facilities.
Health policy
Cost-effective interventions (NBS and comprehensive care) can reduce SCD deaths in childhood by up to 70%. The MSC has been working to influence health policy change and Tanzania has recognized SCD as a disease of public health importance. To this end, SCD is included in the strategic and action plan for the prevention and control of NCD (2016–2020). The main objective of the strategy (http://www.moh.go.tz/en/) is to improve the quality of lives of all Tanzanians by reducing the suffering, disease and death caused by NCD with focus on access to quality, sustainable and equitable services. Specifically, the strategy aims to reduce the burden (morbidity, disability and premature mortality) related to NCD by 20% by 2020; to increase the proportion of newborns at health facilities screened for SCD by 50%; to increase the proportion of SCD patients receiving standardized care and treatment by 70% and to increase survival rates of SCD patients by 50% [In Africa, childhood mortality due to SCD is estimated to range between 50% and 90% (Grosse et al, 2011)]. These targets will bring a renewed energy towards reducing the burden of SCD in Tanzania. Tanzania has also worked with other member countries of WHO Regional Office for Africa (AFRO) as part of the Brazzaville declaration for NCD, to include SCD as a priority NCD condition in Africa (WHO, 2011).
Research
Background
In Tanzania, the introduction of targeted, cost-effective interventions in SCD has been hampered by lack of convincing, locally appropriate evidence. To address this gap, MNH started a research study on SCD (MSC) to provide descriptions of the clinical spectrum of SCD, defining the major causes of morbidity and mortality and developing strategies for appropriate interventions.
Approach
In 2004, prospective surveillance was started of SCD individuals who visited MNH during this period and consented to participate in the MSC study. All SCD patients who were identified and enrolled into the cohort received comprehensive care. Clinic visits were scheduled every 3–6 months and active follow-up was done via telephone calls for those who did not attend the clinic for more than 9 months. In addition to their regular appointments, patients were encouraged to self-report events and visit the clinic or any health facility when they had an acute event. There was daily active surveillance of hospitalization events at MNH. There was a standardized system of collection of phenotype data (clinical, laboratory, imaging), which follows principles of patient confidentiality. The MSC has grown to become one of the largest single-centre SCD cohorts in the world, with well-characterized, clinical description over the 12-year period.
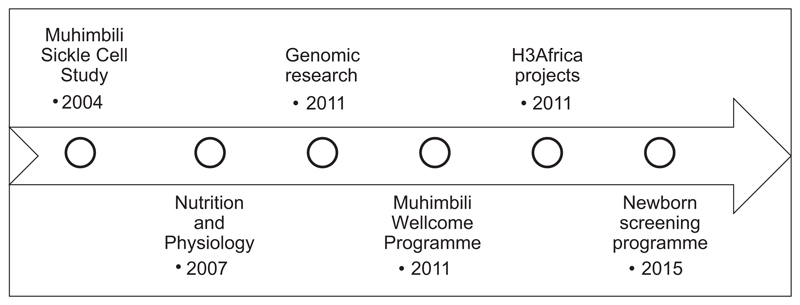
The MSC started as a 4-year Wellcome Trust training fellowship awarded to Julie Makani to conduct research in the clinical-epidemiology of SCD in Tanzania (Fig 2). In 2007, through a Wellcome Trust project grant awarded to Andrew Prentice and Sharon Cox, research on nutrition and physiology in SCD was started. The Muhimbili Wellcome Programme (MWP) was formed in 2011, with core funding from the Wellcome Trust in the form of a programme grant to Charles Newton in collaboration with Ephata Kaaya, Julie Makani and Sharon Cox. In 2008, through collaboration with the Wellcome Trust Sanger Institute and Kings College London, the programme developed a project that aimed to conduct research to determine the genomic determinants of SCD. In 2008, a partnership between the Wellcome Trust and the National Institutes of Health (NIH) funded the Human Hereditary and Health in Africa Initiative, to develop genomic research in Africa (H3Africa Consortium 2014). Through this initiative, the programme in Tanzania collaborated with the University of CapeTown to strengthen bioinformatics capacity for genomic research (Nicola Mulder) and research into the ethical and social determinants of SCD (Ambroise Wonkam). Therefore, the scientific themes include: clinical and basic science research (haematology, neurology, nutrition & physiology and, more recently, genomics); population health research (which includes translational research and development of health policy for SCD) and social and behavioural research (including ethics and social determinants of health and science communication) (Fig 1B). During the 12-year period, the programme has received support and funding from various sources, including the Wellcome Trust and NIH (Table I).
Fig 2.
Timelines of selected research programmes in Sickle cell disease in Muhimbili.
Table I.
Funding for Sickle Cell Disease for research, health and training (Selected funding).
| Project | Funding Source | Period |
|---|---|---|
| Anaemia in SCD | The Wellcome Trust, UK | 2012–2016 |
| H3ABioNET – Bioinformatics Award | NIH – University of Cape Town | 2012–2017 |
| H3Africa Ethics in SCD | NIH – University of Cape Town | 2013–2017 |
| mDOT | University of Pittsburgh | 2015–2017 |
| Muhimbili Wellcome programme | The Wellcome Trust, UK | 2011–2016 |
| Genomic research in SCD | The Wellcome Trust – Sanger | 2011–2015 |
| Vascular Function in SCD | The Wellcome Trust – LSHTM | 2007–2014 |
| Newborn screening for SCD | HDIF, DFID | 2015–2016 |
| Sickle cell project | University of Oxford, UK | 2008–2010 |
| Sickle cell study | The Wellcome Trust, UK | 2004–2007 |
DFID, Department for International Development; HDIF, Human Development Innovation Fund; LSHTM, London School of Hygiene and Tropical Medicine; mDOT, mobile-directly observed therapy for hydroxycarbamide use; NIH, National Institutes of Health; SCD, sickle cell disease.
Achievements
Clinical research
The main objective of the programme was to describe the clinical spectrum of SCD. First, the programme set out to determine the rates and cause of mortality. Data, collected between 2004 and 2009, of 1725 individuals with sickle cell anaemia (12% lost to follow-up) were analysed (Makani et al, 2011). The mortality rates were significantly high, 1·9 per 100 person-years of observation (PYO), when compared with 0·13 in UK and 0·81 in USA. The rates were highest in those under 5 years old: 7·3 in Tanzania compared with 3 per 100 PYO in USA) (Makani et al, 2011). Furthermore, the study found that, despite the high mortality in childhood, SCD individuals were surviving and challenged the dogma that there were few SCD individuals reaching adulthood (Makani et al, 2011), highlighting the need for a health programme for SCD that spans the life span.
Second, the programmes described the role of malaria in SCD, which is considered to be one of the top causes of hospitalization and death in SCD. The programme reported the prevalence, risk factors and outcome of malaria, with low prevalence of malaria in SCD compared to non-SCD populations, confirming the protective effects of sickle haemoglobin (Makani et al, 2010a). However, malaria caused severe morbidity and mortality, findings that were also reported in collaborative work in Kenya (Komba et al, 2009; McAuley et al, 2010). The programme reiterated the importance of management of malaria in SCD: prevention (chemoprophylaxis) as well as prompt diagnosis and treatment. It also highlighted the need to understand the pathogenesis of severe malaria, which is pertinent not only in SCD but also in the non-SCD population.
With regards to understanding mechanisms of protection from sickle haemoglobin (HbS), the programme reported on negative epistasis with fetal haemoglobin (HbF) (Mmbando et al, 2015). This work proposed that high HbF, which is the aim of hydroxycarbamide treatment for SCD, may result in loss of HbS-related malaria protection, resulting in higher malaria-related mortality in SCD. Finally, the precise mechanism of protection conferred by HbF against malaria is unknown. The programme aims to conduct basic science research, using in vitro malaria culture experiments, to explore how HbF confers protection against malaria, providing knowledge on immune mechanisms/targets/pathways that may help in the development of vaccines or medicines.
Finally, as bacterial infection is one of the top three causes of hospitalization and death in SCD (together with anaemia and malaria), the MSC programme conducted studies to determine the prevalence and risk factors associated with invasive bacterial infections (Makani et al, 2015).
Genomic research
SCD has been quoted as a ‘perfect model’ to test the paradigm of translation of research in genomic medicine into improvement in health due to its clinical, environmental, genetic and population diversity. Tanzania conducted one of the first genomic studies in SCD in Africa using a genome-wide approach. 1700 individuals were genotyped using the Human Omnichip 2·5 (Illumina Inc., San Diego, CA, USA), providing assays on 2·4 million single nucleotide polymorphisms (SNPs). The expertise was developed in Tanzania through partnership with Kings College London and the Wellcome Trust Sanger Institute (WTSI) for DNA extraction and quantification well as genotyping using the candidate gene approach, targeted and genome wide sequencing. Siana Nkya Mtatiro conducted this work as part of her PhD under supervision of Stephan Menzel, Sharon Cox and Julie Makani.
The focus of this work was HbF, a major ameliorating factor of clinical illness in SCD. Of the three known genetic factors influencing HbF (chromosomes 2, 6 and 11), only one is prevalent in Tanzania, while the other two are rare in this population (Makani et al, 2010b). Known genetic factors have been reported to account for under 50% of HbF variability in healthy European Caucasians (Thein & Menzel, 2009). The genetic contribution to HbF in Africans is not clear; but is likely to be lower, with figures estimated at 2–20%. The programme conducted the first genome-wide association study to identify new variants (Mtatiro et al, 2014) and also participated in multi-centre studies to further elucidate these genetic loci (Menzel et al, 2014; Mtatiro et al, 2015a).
In addition, the programme reported the pleiotropic effect of HbF-modifier genetic loci (Mtatiro et al, 2015b). Tanzania also participated in development of the white paper for human genomic research in Africa (H3Africa Consortium 2014), with MUHAS participating in 2 research studies funded by the NIH, one which aims to build capacity in bioinformatics (Mulder et al, 2016) and the second exploring ethical issues around genomic research in SCD. Muhimbili is also a site for MalariaGEN, a network for genomic research in malaria (Jallow et al, 2009).
Data management
The MSC study has 12 years of longitudinal data, with accompanying genetic data, making it one of the world’s most valuable biomedical resources in SCD. A database has been developed specifically for the SCD study. Data was then entered into a web-based database system known as MySQL (Sun Microsystems Inc, Santa Clara, California, USA), with individual repeated measures. The data team was responsible for data entry after validation and integrity checks. Note that the study screened both individuals suspected to have SCD and enrolled those with SCD: this consisted of 77 569 recorded visits from 10 748 individuals, both SCD and non-SCD individuals (Size: 220 MB). Genetic data on 1700 SCD individuals with 2·5 million SNPs (Size: 12 GB) was also available. Formats allow sharing and long-term validity, as well as meta-analysis with data from other cohorts. The programme has also developed standardized case report forms, which are specific for screening, enrolment, follow-up and inpatient visits and for central nervous system events.
Bioinformatics
The SCD programme under MUHAS has invested in developing capacity in informatics and analysis for genetic research. For the past 4 years, it has been a member of H3AbioNet – a sustainable African Bioinformatics Network that aims to build bioinformatics infrastructure to support H3Africa and genomics projects in Africa (Mulder et al, 2016). This network is led by Professor Nicky Mulder, University of Cape Town. Bruno Mmbando (PhD) and Raphael Sangeda (PhD) are supported by H3ABioNet to provide expertise in statistics and bioinformatics respectively. This includes maintenance of the database for genotypic and phenotypic data.
Biorepositories
The MSC has 12 years’ worth of biological samples, phenotypic and genotypic data. To build capacity in biological sample archiving, the programme collaborated with Kings College London and WTSI. Archived samples include DNA, red cells, plasma and serum from over 5000 SCD individuals. One of the challenges of cohort studies is the cost of maintaining the biorepository. MUHAS has committed institutional resources to maintain the biorepository and has started discussions with stakeholders to establish an institutional and national repository, to ensure that its optimal use and sustainability.
Education and training
Background
The MSC programme supports education, training and development of capacity in Tanzania in healthcare and biomedical research. The aim is to increase the number and quality of staff in healthcare and academia whilst building a critical mass of highly skilled professionals in science and technology.
Approach
The strategy has been to strengthen existing rather than creating parallel systems of education, training and career development. The programme has contributed to strengthening formal educational programmes at MUHAS as well as scientific programmes leading to Masters in Science (MSc) and Doctor of Philosophy (PhD) for a minimum of 2 and 3 years respectively. The scientific training requires development and conducting a research project with publication in peer-reviewed journals (2 publications for MSc and 4 Publications for PhD).
Achievements
Medical education
Training of medical specialists in haematology and blood transfusion at postgraduate level began in 2007.
Continuing medical education and professional development
The programme has supported in-service training of health personnel at MUHAS, MNH and 3 other hospitals in Dar-es-Salaam. Through the directorate of continuing education and professional development at MUHAS, the programme is working to develop training programmes to strengthen capacity in clinical and laboratory services in haematology and blood transfusion.
Research training
Muhimbili University of Health and Allied Sciences trains 70% of the health professionals in Tanzania in medical and allied health sciences. The MSC programme has supported research training of 46 individuals (21 undergraduate, 17 Masters and PhD, 5 post-doctoral fellows and 2 research projects) between 2004 and 2016 (Table II).
Table II.
Research training.
|
HPLC, high performance liquid chromatography; MNH, Muhimbili National Hospital; MUHAS, Muhimbili University of Health and Allied Sciences; SCD, sickle cell disease.
Advocacy
Background
There is limited awareness regarding the burden of SCD in Tanzania. The MSC recognised that there was a need to address this gap by actively disseminating information and education about SCD and the various activities in health, education and research. The target audiences were patients and affected families, health personnel, academic staff as well as the public. In addition, it was important to increase awareness about the burden of SCD to policy makers and health planners so that appropriate resources could be allocated to improve the lives of individuals with SCD.
Approach
Over the past decade, there have been efforts by patient and advocacy groups to be involved in health and research. Unfortunately, it has been observed that healthcare workers, media professionals and researchers have not adequately interacted with each other, with patients and with the public about SCD. Worldwide, this is not unique to SCD, as other diseases face similar challenges including mechanisms to sustain advocacy with active efforts to encourage patients to be involved in care and research (Balasegaram et al, 2008). In addition, journalists are faced with challenges in reporting technical aspects of science and health (Appiah et al, 2015). Scientists may also need training in communication to help them engage the public effectively. Engaging the media in communicating research has been found to enhance the quality and quantity of media coverage of research (Oronje et al, 2011). The advocacy groups have actively participated in activities on World Sickle Cell Day and during SCD Awareness Month, as well as other public and social media events that aim to raise awareness about SCD in Tanzania.
Achievements
Advocacy activities have been led by the Sickle Cell Foundation of Tanzania (SCFT), a non-governmental organisation. The SCFT was launched in June 2010 with the mission to raise awareness of SCD and contribute to its effective management and treatment of SCD through biomedical science. Over the past 10 years there has been an increase in the number of patient and family advocacy groups, which provide peer education and participate in public advocacy events. These include the Sickle Cell Disease Patients Community (Arafa Said Salim), Sickle Cell Society Tanzania (Yasmin Razak), Sickle Cell Youth Foundation (Yusuph N Gesase) and Tanzania SCD Alliance (Dr Deo Soka). The MSC programme in Tanzania has been actively involved in dissemination of information on health and research activities in SCD using different platforms, such as print (newspapers, magazines) and social media (Facebook, Twitter), television and short documentaries.
World sickle cell day
The United Nations (UN) recognized 19 June as World Sickle Cell Day in 2008. The programme has marked this day with events and activities that aim to raise awareness about SCD. The activities have ranged from simple press conferences to dedicated campaigns, such as the Cycle for Sickle Campaign.
Science communication
In attempts to address gaps in the dissemination of scientific findings, the programme has embarked on an 18-month programme to develop a strategy for public engagement. This project is funded by the Wellcome Trust and aims to bring different stakeholders together, with the, of understanding the challenges of, and opportunities for media reporting on health, specifically SCD. The formative research has concluded. Information obtained will guide formation of a model to effectively engage media and scientist in order to inform the public. The project is coordinated by a public engagement fellow- Promise Mwakale.
The future of SCD in Tanzania
Tanzania will strengthen SCD services in health facilities, with the intention of establishing sickle cell centres at referral and regional hospitals, in regions where there is a high number of SCD patients. These include; Dar -es-Salaam – (MNH, Temeke, Amana and Mwananyamala), Dodoma (Benjamin Mkapa Hospital), Mwanza (Bugando Medical Centre) and Zanzibar (Mnazi Mmjoa Hospital). In areas where there is a low prevalence of disease, e.g. Kilimanjaro and Mbeya, services will be integrated into the departments of paediatrics or internal medicine. These sickle cell centres will provide high quality clinical and laboratory services as well as strengthening health information management systems, with establishment of a sickle cell registry.
To ensure sustainability of SCD activities nationwide, the aim is to strengthen the existing facilities in terms of diagnostic services and clinical care. Tanzania plans to establish a National Sickle Cell Programme that will be responsible for developing a national policy for the control, management and prevention of SCD at the national level. This will be developed within the framework of the national policy of NCD, consolidating all national SCD activities under one umbrella.
Conclusion
The SCD programme has made significant strides in highlighting the burden of SCD individuals in Tanzania. There is now a foundation for future initiatives and the integration of healthcare, research training and advocacy has set an example for a comprehensive approach to disease prevention and control.
Acknowledgements
We acknowledge the contribution of all funding agencies for their unwavering support, the sickle cell programme staff for a decade of dedication and sickle cell patients and their families for their unparalleled resilience.
Sources of funding
Wellcome Trust (UK), Julie Makani Fellowship grant number WT093727MA.
Footnotes
Disclosure and competing interest statement
The authors have no competing interests.
Author contributions
FT identified the reviews sections, reviewed literature and wrote the manuscript; JM critically reviewed the manuscript and designed tables.
Furahini Tluway
References
- Aken’ova YA, Bakare RA, Okunade MA. Septicaemia in sickle cell anaemia patients: the Ibadan experience. The Central African Journal of Medicine. 1998;44:102–104. [PubMed] [Google Scholar]
- Appiah B, Gastel B, Burdine JN, Russell LH. Science reporting in Accra, Ghana: sources, barriers and motivational factors. Public Understanding of Science. 2015;24:23–37. doi: 10.1177/0963662514547478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balasegaram M, Balasegaram S, Malvy D, Millet P. Neglected diseases in the news: a content analysis of recent international media coverage focussing on leishmaniasis and trypanosomiasis. PLoS Neglected Tropical Diseases. 2008;2:e234. doi: 10.1371/journal.pntd.0000234. 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases. 2001;14:e2–e12. doi: 10.1016/j.ijid.2009.03.010. [DOI] [PubMed] [Google Scholar]
- Charache S, Dover GJ, Moore RD, Eckert S, Ballas SK, Koshy M, Milner PF, Orringer EP, Phillips G, Jr, Platt OS. Hydroxyurea: effects on haemoglobin F production in patients with sickle cell anaemia. Blood. 1992;79:2555–2565. [PubMed] [Google Scholar]
- Gaston MH, Verter JI, Woods G, Pegelow C, Kelleher J, Presbury G, Zarkowsky H, Vichinsky E, Iyer R, Lobel JS, Diamond S, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomizedtrial. The New England Journal of Medicine. 1986;314:1593–1599. doi: 10.1056/NEJM198606193142501. [DOI] [PubMed] [Google Scholar]
- Gill FM, Sleeper LA, Weiner SJ, Brown AK, Bellevue R, Grover R, Pegelow CH, Vichinsky E. Clinical events in the first decade in a cohort of infants with sickle cell disease.Cooperative Study of Sickle Cell Disease. Blood. 1995;86:776–783. [PubMed] [Google Scholar]
- Grosse SD, Odame I, Atrash HK, Amendah DD, Piel FB, Williams TN. Sickle cell disease in africa: a neglected cause of early childhood mortality. American Journal of Preventive Medicine. 2011;41:S398–S405. doi: 10.1016/j.amepre.2011.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- H3Africa Consortium. Enabling the genomic revolution in Africa. Science. 2014;344:1346–1348. doi: 10.1126/science.1251546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halasa NB, Shankar SM, Talbot TR, Arbogast PG, Mitchel EF, Wang WC, Schaffner W, Craig AS, Griffin MR. Incidence of invasive pneumococcal disease among individuals with sickle cell disease before and after the introduction of the pneumococcal conjugate vaccine. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America. 2007;44:1428–1433. doi: 10.1086/516781. [DOI] [PubMed] [Google Scholar]
- Jallow M, Teo YY, Small KS, Rockett KA, Deloukas P, Clark TG, Kivinen K, Bojang KA, Conway DJ, Pinder M, Sirugo G, et al. Genome-wide and fine-resolution association analysis of malaria in West Africa. Nature Genetics. 2009;41:657–665. doi: 10.1038/ng.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kizito ME, Mworozi E, Ndugwa C, Serjeant GR. Bacteraemia in homozygous sickle cell disease in Africa: is pneumococcal prophylaxis justified? Archives of Disease in Childhood. 2007;92:21–23. doi: 10.1136/adc.2005.088807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight-Madden J, Serjeant GR. Invasive pneumococcal disease in homozygous sickle cell disease, Jamaican experience 1973–1997. The Journal of Pediatrics. 2001;138:65–70. doi: 10.1067/mpd.2001.109709. [DOI] [PubMed] [Google Scholar]
- Komba AN, Makani J, Sadarangani M, Ajala-Agbo T, Berkley JA, Newton CR, Marsh K, Williams TN. Malaria as a cause of morbidity and mortality in children with homozygous sickle cell disease on the coast of Kenya. Clinical Infectious Diseases. 2009;49:216–222. doi: 10.1086/599834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leikin SL, Gallagher D, Kinney TR, Sloane D, Klug P, Rida W. Mortality in children and adolescents with sickle cell disease. Cooperative Study of Sickle Cell Disease. Pediatrics. 1989;84:500–508. [PubMed] [Google Scholar]
- Makani J, Komba AN, Cox SE, Oruo J, Mwamtemi K, Kitundu J, Magesa P, Rwezaula S, Meda E, Mgaya J, Pallangyo K, et al. Malaria in patients with sickle cell anaemia: burden, risk factors, and outcome at the outpatient clinic and during hospitalization. Blood. 2010a;115:215–220. doi: 10.1182/blood-2009-07-233528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J, Menzel S, Nkya S, Cox SE, Drasar E, Soka D, Komba AN, Mgaya J, Rooks H, Vasavda N, Fegan G, et al. Genetics of foetal hemoglobin in Tanzanian and British patients with sickle cell anaemia. Blood. 2010b;117(4):1390–1392. doi: 10.1182/blood-2010-08-302703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J, Cox SE, Soka D, Komba AN, Oruo J, Mwamtemi H, Magesa P, Rwezaula S, Meda E, Mgaya J, Lowe B, et al. Mortality in sickle cell anemia in africa: a Prospective Cohort Study in Tanzania. PLoS ONE. 2011;6:e14699. doi: 10.1371/journal.pone.0014699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makani J, Mgaya J, Balandya E, Msami K, Soka D, Cox S, Komba N, Rwezaula S, Meda E, Muturi D, Kitundu J, et al. Bacteraemia in sickle cell anaemia is associated with low haemoglobin: a report of 890 admissions to a tertiary hospital in Tanzania. British Journal of Haematology. 2015;171:273–276. doi: 10.1111/bjh.13553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAuley CF, Webb C, Makani J, Macharia A, Uyoga S, Opi DH, Ndila C, Ngatia A, Scott JA, Marsh K, Williams TN. High mortality from Plasmodium falciparum malaria in children living with sickle cell anaemia on the coast of Kenya. Blood. 2010;116:1663–1668. doi: 10.1182/blood-2010-01-265249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel S, Rooks H, Zelenika D, Mtatiro SN, Gnanakulasekaran A, Drasar E, Cox S, Liu L, Masood M, Silver N, Garner C, et al. Global genetic architecture of an erythroid quantitative trait locus, HMIP-2. Annalsof Human Genetics. 2014;78:434–451. doi: 10.1111/ahg.12077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mmbando BP, Mgaya J, Cox SE, Mtatiro SN, Soka D, Rwezaula R, Meda E, Msaki E, Snow RW, Jeffries N, Geller NL, et al. Negative epistasis between sickle and foetal haemoglobin suggests a reduction in protection against malaria. PLoS ONE. 2015;10:e0125929. doi: 10.1371/journal.pone.0125929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtatiro SN, Singh T, Rooks H, Mgaya J, Mariki H, Soka D, Mmbando B, Msaki E, Kolder I, Thein SL, Menzel S, et al. Genome wide association study of foetal hemoglobin in sickle cell anaemia in Tanzania. PLoS ONE. 2014;9:e111464. doi: 10.1371/journal.pone.0111464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtatiro SN, Mgaya J, Singh T, Mariki H, Rooks H, Soka D, Mmbando B, Thein SL, Barrett JC, Makani J, Cox SE, et al. Genetic association of foetal-hemoglobin levels in individuals with sickle cell disease in Tanzania maps to conserved regulatory elements within the MYB core enhancer. BMC Medical Genetics. 2015a;16:4. doi: 10.1186/s12881-015-0148-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mtatiro SN, Makani J, Mmbando B, Thein SL, Menzel S, Cox SE. Genetic variants at HbF-modifier loci moderate anaemia and leukocytosis in sickle cell disease in Tanzania. American Journal of Hematology. 2015b;90:E1–E4. doi: 10.1002/ajh.23859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mulder NJ, Adebiyi E, Alami R, Benkahla A, Brandful J, Doumbia S, Everett D, Fadlelmola FM, Gaboun F, Gaseitsiwe S, Ghazal H, et al. H3ABioNet, a sustainable Pan-African Bioinformatics Network for human heredity and health in Africa. Genome Research. 2016;26:271–277. doi: 10.1101/gr.196295.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Obaro S. Preventable deaths in sickle-cell anaemia in African children. Lancet. 2010;375:460. doi: 10.1016/S0140-6736(10)60193-6. [DOI] [PubMed] [Google Scholar]
- Ojwang PJ, Ogada T, Beris P, Hattori Y, Lanclos KD, Kultar A, Kultar F, Huisman THJ. Haplotypes and alpha globin gene analyses in sickle cell anaemia patients from Kenya. British Journal of Haematology. 1987;65:211–215. doi: 10.1111/j.1365-2141.1987.tb02267.x. [DOI] [PubMed] [Google Scholar]
- Oronje RN, Undie CC, Zulu EM, Crichton J. Engaging media in communicating research on sexual and reproductive health and rights in sub-Saharan Africa: experiences and lessons learned. Health Research Policy and Systems. 2011;9:S7. doi: 10.1186/1478-4505-9-S1-S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piel FB, Hay SI, Gupta S, Weatherall DJ, Williams TN. Global burden of sickle cell anaemia in children under five, 2010–2050: modelling based on demographics, excess mortality, and interventions. PLoS Medicine. 2013;10:e1001484. doi: 10.1371/journal.pmed.1001484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stegenga KA, Ward-Smith P, Hinds PS, Routhieaux JA, Woods GM. Quality of life among children with sickle cell disease receiving chronic transfusion therapy. Journal of Paediatric Oncology Nursing. 2004;21:207–213. doi: 10.1177/1043454204265841. [DOI] [PubMed] [Google Scholar]
- Thein SL, Menzel S. Discovering the genetics underlying foetal haemoglobin production in adults. British Journal of Haematology. 2009;145:455–467. doi: 10.1111/j.1365-2141.2009.07650.x. [DOI] [PubMed] [Google Scholar]
- Thomas AN, Pattison C, Serjeant GR. Causes of death in sickle-cell disease in Jamaica. British Medical Journal (Clin Res Ed) 1982;285:633–635. doi: 10.1136/bmj.285.6342.633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations General Assembly Resolution. Sickle Cell Anaemia as a public health problem. 2009 Resolution 63/237. Available at: https://www.un.org/sg/en/content/sg/statement/2009-06-19/secretary-generals-message-sickle-cell-anaemia.
- Weatherall DJ. The inherited diseases of haemoglobin are an emerging global health burden. Blood. 2010;115:4331–4336. doi: 10.1182/blood-2010-01-251348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. Management of Birth Defects and Haemoglobin Disorders: Report of a Joint WHO-March of Dimes Meeting. World Health Organisation; Geneva: 2006. [Google Scholar]
- WHO. Sickle Cell Disease: A Strategy For The WHO Africa Region. Report of the Regional Director. Document number AFR/RC60/8. Geneva, Switzerland: World Health Organization, Regional Office for Africa; 2010. [Google Scholar]
- WHO. Uniting against NCDs: the time to act is now. The Brazzaville declaration on Noncommunicable diseases prevention and control in the WHO African region. Geneva, Switzerland: World Health Organization, Regional Office for Africa; 2011. [Google Scholar]
- Williams TN, Uyoga S, Macharia A, Ndila C, McAuley CF, Opi DH, Mwarumba S, Makani J, Komba A, Mdiritu MN, Sharif SK, et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case–control study. Lancet. 2009;374:1364–1370. doi: 10.1016/S0140-6736(09)61374-X. [DOI] [PMC free article] [PubMed] [Google Scholar]