Abstract
Objective
The role of influenza virus in patients presenting at ED during seasonal-epidemic periods has not previously been specified. Our objective was to determine its frequency according to clinical presentation.
Methods
This is a prospective observational study conducted during three-consecutive seasonal Influenza epidemics (2013–2015), including patients presenting i) community-acquired pneumonia (CAP); ii) severe acute symptoms (SAS): respiratory failure (RF), hemodynamic failure (HF), cardiac failure (CF), and miscellaneous symptoms (M); iii) symptoms suggesting influenza (PSSI). Patients were tested for influenza using specific PCR on naso-pharyngeal swabs.
Results
Of 1,239 patients, virological samples were taken from 784 (63.3%), 213 (27.2%) of whom were positive for the influenza virus: CAP 52/177 (29.4%), SAS 115/447 (25.7%) and PSSI 46/160 (28.8%) (p = 0.6). In the SAS group positivity rates were: RF 76/263 (28.9%), HF 5/29 (17.2%), CF 15/68 (22.1%), and M 19/87 (21.8%) (p = 0.3). Among the major diagnostic categories, the influenza virus positivity rates were: asthma 60/231 (26%), acute exacerbation of chronic obstructive pulmonary disease 18/86 (20.9%), HIV 5/21 (23.8%) and cardiac failure 33/131 (25.2%). The positivity of the samples has not been associated (p>0.1) nor the presence of signs of severity or admission rate in medical ward nor intensive care unit.
Conclusions
Our results indicate that during seasonal influenza epidemics, Influenza virus-positivity rate is similar in patients attending ED for influenza-compatible clinical features, patients with acute symptoms including pneumonia, respiratory, hemodynamic and cardiac distress, and patients presenting for acute decompensation of chronic respiratory and cardiac diseases.
Introduction
Seasonal influenza occurs in epidemic peaks during the winter periods. Particular climate conditions, including cold and humidity, school holidays, and the characteristics of the likely circulating viral strain and vaccination coverage, are associated with epidemic peaks that vary in intensity in terms of the number of patients and the severity of the observed cases [1].
There is an increase in the number of acute asthma episodes during the winter period [2], as well as in acute exacerbations of chronic obstructive pulmonary disease (AE-COPD) [3], decompensation of cardiac diseases [4], hospitalizations, particularly among the elderly [5], and in pulmonary, cardiovascular, and neuromuscular complications [6–8]. This means that the reception capacities of the emergency services and intensive care units (ICU) can rapidly become increasingly stretched [9].
Determination of the number of lengthy hospital stays, primarily for respiratory and hemodynamic decompensations related to respiratory viruses, and to influenza viruses in particular, is a strategic priority, in order to promote improvements in prevention, diagnostics, and therapeutics, as well as in care delivery [10]. The influenza virus has been identified as the causative agent of 2.2–18% of lung diseases [11–13], up to 10% of asthma [2] and AE-COPD episodes [14], 4–33% of patient admissions to ICU for community-acquired pneumonia (CAP) [15], and 3.4% of all admissions to ICU [16]. Nevertheless, to our knowledge, no study has evaluated the role of the influenza virus in episodes of respiratory, cardiac, or hemodynamic distress during periods of influenza epidemic in the ED.
The objective of this study was to identify the number of patients infected with the influenza virus among those presenting at the ED with pneumonia or severe acute symptoms or presenting symptoms suggesting Influenza, as well as the impact of the influenza virus on the severity of illness and the fate of the patients.
Material and methods
Study design and setting
This was a prospective observational study conducted as part of a continuous quality improvement program for the diagnosis and treatment of viral infections in the ED of the hospital Bichat—Claude Bernard (BCB), Paris, France. The BCB is an academic, 1,000-bed hospital, and its ED receives 80,000 visits each year.
Selection of participants
Adults attending the BCB ED during three consecutive periods of seasonal influenza epidemic (2013–2015) were included. The National Institute for Public Health Surveillance annually determines the beginning and the end of the seasonal influenza epidemic, according to the number of cases reported by the influenza surveillance networks, [17].
In accordance with the inclusion criteria, three groups were defined on the basis of the initial reason for presentation in the ED. Groups were defined by ED physician during ED stay: (1) Patients presenting with acute respiratory symptoms suggesting CAP (CAP group); (2) Patients presenting with severe acute symptoms (SAS group) [18]: polypnea, cyanosis, oxygen saturation Spa02 <95%, pneumonia, wheeze, tachycardia, hypotension, areas of mottled skin, malaise, change in mental status, and oliguria; and (3) patients with symptoms suggesting Influenza virus infection (fever and cough or myalgias or rhinorrhea) and with underlying conditions for severe influenza (PSSI group): age ≥65 years, asthma, bronchopulmonary dysplasia, cystic fibrosis, chronic respiratory failure, cardiac failure, cardiac valvulopathy, congenital heart disease, cardiovascular disease, renal failure, nephrotic syndrome, sickle-cell anemia, hepatic failure, diabetes, systemic corticosteroid therapy, leukemia or lymphoma, immunosuppression, cancer, HIV infection, CD4 lymphocytes count, obesity, and pregnancy [19]. Patients in the SAS group were classified into four subgroups on the basis of their main reason for visiting the ED: a) respiratory failure (RF); b) hemodynamic failure (HF); c) cardiac failure (CF); and d) miscellaneous (M).
For each patient meeting the inclusion criteria, a standard case report form, which included demographic and clinical data, was completed by emergency physicians as a part of a quality of care program. The data were prospectively recorded and collected from the computerized emergency database system (Urqual®, McKesson International, Paris, France), and the final disposition decision was recorded (Medical or Intensive Care Unit).
The rate of influenza in presentations of asthma, acute exacerbation of chronic obstructive pulmonary disease (AE-COPD), HIV infected patients and acute decompensation of cardiac failure was calculated on the basis of the final diagnosis of emergency (ICD-10).
Interventions
Nasopharyngeal samples were obtained from patients with CAP, severe acute conditions, or underlying clinical conditions for severe influenza at the time of study enrollment. It was also possible to include some patients with an indication of hospitalization, patients residing in elderly care centers, healthcare workers, and people living with a person with underlying clinical conditions for severe influenza.
Swab samples were placed in universal vials in universal transport media (Sigma-Virocult®, MW951S) and stored at + 4°C if not tested immediately. All samples were processed for influenza detection within 36 hours, using the Xpert® Flu PCR test (Cepheid, California, USA.). This test also allowed the classification of influenza viruses.
Bacteriological findings were obtained from electronic clinical records.
Ethics statement
Data collection and storage by the Urqual® Emergency Database was approved by the French National Commission for Data Protection and Liberties. Anonymized data was extracted from the CNR-M database. The Emergency Ethics Committee for Biomedical Research of Assistance Publique-Hôpitaux de Paris approved this study.
Analysis
In order to describe the study population, quantitative variables have been expressed as mean and standard deviation, and qualitative variables as numbers of patients and percentages. A Chi 2 or Fisher’s test and a Student’s t-test or Wilcoxon test were used to compare qualitative and quantitative variables between study groups. The significance threshold was set at p = 0.05. Statistical analyses were performed using Statistica® software (StatSoft).
Results
Characteristics of study subjects
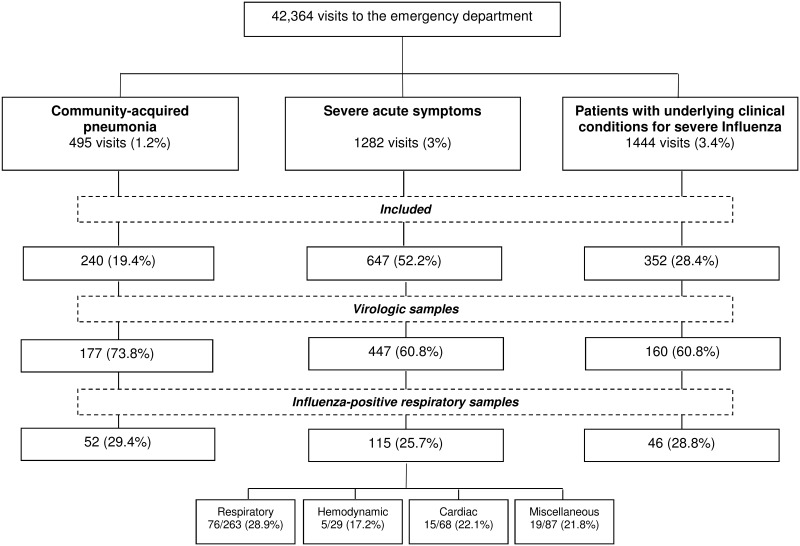
During the three consecutive periods, 42,364 ED visits were recorded. On the basis of the reason for presentation and the ICD-10 coding, 3,221 visits (7.6%) were considered for inclusion in our study. Of these, 1,239 (38.5%) were actually included, as follows: 240 (19.4%) cases of CAP, 647 (52.2%) patients with SAS, and 352 (28.4%) cases of PSSI. Fig 1 shows a flowchart relating to the study population.
Fig 1. Study flowchart.
Table 1 shows the main characteristics of the patients included, according to the reason for ED presentation. It can be observed that the CAP and SAS groups more frequently had underlying clinical conditions, putting them at risk of severe influenza, than did the group of patients with clinical symptoms suggestive of influenza, but without signs of seriousness.
Table 1. The main characteristics of the study groups.
| Community-acquired pneumonia | Severe acute symptoms | Patients with underlying conditions for severe influenza | P | ||||
|---|---|---|---|---|---|---|---|
| n = 240 | n = 647 | n = 352 | |||||
| moy±DS | moy±DS | moy±DS | |||||
| n | % | n | % | n | % | ||
| Risk factors for severe influenza infection | |||||||
| Age | 68.5±18.7 | 70.6±18.5 | 55±21.1 | 0.002 | |||
| Age ≥65 years | 153 | 63.8 | 444 | 68.6 | 127 | 36.1 | 0.00001 |
| Asthma | 44 | 18.7 | 259 | 40.6 | 38 | 11.2 | 0.00001 |
| Bronchopulmonary dysplasia | 5 | 2.1 | 20 | 3.1 | 1 | 0.3 | 0.01 |
| Cystic fibrosis | 23 | 9.8 | 34 | 5.3 | 13 | 3.9 | 0.009 |
| Chronic respiratory failure | 19 | 8.1 | 94 | 14.8 | 10 | 3 | 0.000001 |
| Cardiac failure | 27 | 11.5 | 126 | 19.8 | 20 | 5.9 | 0.000001 |
| Cardiac valvulopathy | 9 | 3.8 | 31 | 4.9 | 4 | 1.2 | 0.01 |
| Congenital heart disease | 3 | 1.3 | 14 | 2.2 | 4 | 1.2 | 0.4 |
| Cardiovascular disease | 14 | 6 | 57 | 9 | 10 | 3 | 0.002 |
| Renal failure | 21 | 8.9 | 68 | 10.7 | 19 | 5.6 | 0.03 |
| Nephrotic syndrome | 1 | 0.4 | 1 | 0.2 | 4 | 1.2 | 0.09% |
| Sickle-cell anemia | 0 | 0 | 1 | 0.2 | 3 | 0,9 | 0.1 |
| Hepatic failure | 1 | 0.4 | 3 | 0.5 | 0 | 0 | 0.5 |
| Diabetes | 37 | 15.7 | 123 | 19.3 | 43 | 12.7 | 0.03 |
| Systemic corticosteroid therapy | 14 | 6 | 43 | 6.8 | 18 | 5.3 | 0.7 |
| Leukemia or Lymphoma | 6 | 2.6 | 5 | 0.8 | 2 | 0.6 | 0.05 |
| Immunosuppression | 14 | 6 | 24 | 3.8 | 18 | 5.3 | 0.3 |
| Cancer | 11 | 4.7 | 31 | 4.9 | 11 | 3.3 | 0.5 |
| HIV infection | 10 | 4.3 | 14 | 2.2 | 11 | 3.3 | 0.2 |
| CD4 count | 196±347 | 145±174 | 15–19 | 0.4 | |||
| Obesity | 11 | 4.70% | 44 | 6.9 | 9 | 2.7 | 0.02 |
| Pregnancy | 1 | 0.4 | 6 | 0,9 | 4 | 1.2 | 0.6 |
| At least one of the above | 177 | 75.3 | 640 | 52.7 | 339 | 27.9 | 0.00001 |
| Signs of severity | |||||||
| Polypnea | 34 | 14.17% | 124 | 12.41% | 0 | 0 | 0.5 |
| Cyanosis | 5 | 2.08% | 28 | 2.80% | 0 | 0 | 0.0002 |
| Oxygen saturation Spa02 < 95% | 108 | 45.00% | 416 | 41.64% | 0 | 0 | 0.00001 |
| Pneumonia | 118 | 49.2 | 167 | 25.8 | 0 | 0 | 0.00001 |
| Wheeze | 52 | 21.67% | 259 | 25,93% | 0 | 0 | 0.0001 |
| Tachycardia | 35 | 14.58% | 120 | 12.01% | 0 | 0 | 0.000001 |
| Hypotension | 17 | 7.08% | 45 | 4.50% | 0 | 0 | 0.000001 |
| Areas of mottled skin | 9 | 3.75% | 42 | 4.20% | 0 | 0 | 0.000001 |
| Discomfort | 10 | 4.17% | 39 | 3.90% | 0 | 0 | 0.00002 |
| Changes in mental status | 12 | 5% | 37 | 3.70% | 0 | 0 | 0.00004 |
| Oliguria | 2 | 0.83% | 5 | 0.50% | 0 | 0 | 0.2 |
| At least one of the above | 177 | 73.8% | 591 | 59.2% | 0 | 0 | 0.00001 |
Virological results
Virological samples were taken from 784/1,239 (63.3%) patients, as follows: CAP 177 (73.8%), SAS 447 (69.1%), and PSSI 160 (45.5%). Of these patients, 213 (27.2%) were positive for the influenza virus. The influenza positivity rate in each of the groups was as follows: CAP 52/177 (29.4%), SAS 115/447 (25.7%), and PSSI 46/160 (28.8%) (p = 0.6).
In the SAS group, the influenza positivity rates were according to the reason of presentation as follows: RF 76/263 (28.9%), HF 5/29 (17.2%), CF 15/68 (22.1%), and M 19/87 (21.8%) (p = 0.3). On the basis of the final diagnosis, the influenza virus positivity rates of the virological samples were as follows: asthma 60/231 (26%), AE-COPD 18/86 (20.9%), HIV 5/21 (23.8%), and acute cardiac failure 33/131 (25.2%).
The overall rate and the groups' positivity rates were not significantly different between the three-seasonal influenza epidemic periods (p = 0.5). The distribution of influenza strains among study groups is presented in Table 2.
Table 2. Influenza strains distribution among main study groups.
| Community-acquired pneumonia | Severe acute symptoms | Patients with underlying conditions for severe influenza | P | ||||
|---|---|---|---|---|---|---|---|
| n = 177 | n = 447 | n = 160 | |||||
| n | % | n | % | n | % | ||
| Positive influenza | 52 | 29.4 | 115 | 25.7 | 46 | 28.8 | 0.6 |
| Influenza A | 25 | 14.1 | 69 | 15.4 | 20 | 12.5 | 0.7 |
| Influenza A H3N2 | 21 | 11.9 | 60 | 13.4 | 17 | 16.6 | 0.6 |
| Influenza A H1N1 | 9 | 5.1 | 28 | 5.6 | 8 | 5 | 0,9 |
| Influenza B | 22 | 12.4 | 30 | 6.7 | 21 | 13.1 | 0.02 |
Bacteriological results
Among the patients with virological samples, 92/784 (11.7%) had positive blood or respiratory bacteriological samples: Community acquired pneumonia: 32/177 (18%); Severe acute symptoms: 58/447 (13%); Patients with underlying conditions for severe influenza: 2/160 (1.3%). Streptococcus pneumonia (36/92 (39.1%) and Staphylococccus aureus 24/92 (26.1%) were the most common pathogens. Haemophilus influenza, Klebsiella pneumonia, Mycoplasma pneumonia, Pseudomonas aeruginosa and Streptococcus pyogenes explain the remaining isolates. Procalcitonin tests according to study groups were as follows (n (% of patients with procalcitonin >0.15μg/L), mean±SD (patients with procalcitonin >0.15μg/L): Community acquired pneumonia: 48/177 (27.1%), 0.1.9±2.2; Severe acute symptoms: 148/447 (33.1%), 2.2±4.1; Patients with underlying conditions for severe influenza: 24/160 (15%), 0.2±0.4. Both differences were significant between study groups (p = 0.000006 and p<0.0001).
Severity criteria as a function of influenza infection
Comparisons of frequency of severity criteria with regard to Influenza virus virological results are presented in Table 3. There was no difference between patients with positive samples and those with negative samples.
Table 3. Frequency of severity criteria as a function of influenza results.
| Negative influenza | Positive influenza | P | |||
|---|---|---|---|---|---|
| n = 571 | n = 213 | ||||
| n | % | n | % | ||
| Polypnea | 82 | 14.4% | 39 | 18.3% | 0.2 |
| Cyanosis | 19 | 3.3% | 6 | 2.8% | 0.7 |
| Oxygen saturation Spa02 < 95% | 280 | 49% | 107 | 50.2 | 0.8 |
| Pneumonia symptoms and signs | 149 | 26.1% | 57 | 26.7% | 0.3 |
| Wheeze | 162 | 28.4% | 68 | 31.9% | 0.3 |
| Tachycardia | 92 | 16.1% | 26 | 12.2 | 0.2 |
| Hypotension | 35 | 6.1% | 14 | 6.6% | 0.8 |
| Areas of mottled skin | 27 | 4.7% | 12 | 5.6% | 0.6 |
| Discomfort | 20 | 3.5 | 9 | 4.2% | 0.6 |
| Changes in mental status | 21 | 3.7% | 13 | 6.1% | 0.1 |
| Oliguria | 2 | 0.35% | 3 | 1.4% | 0.09 |
| At least one of the above | 401 | 70.2% | 151 | 70.9 | 0,9 |
| Positive bacteriological sample | 31 | 5.4% | 61 | 28.6% | 0.000004 |
Final disposition decision
Overall, 538/774 (71.1%) patients were admitted, 48 (6.2%) in ICU and 490 (63.3%) in medical ward. The ICU and medical ward admission rates as a function of study groups and Influenza results are presented in Table 4. There was no difference between the groups with regard to the virological results. Positive bacteriological sample and positive PCT value were not associated with final disposition decision (Table 4). In total, 11/784 (1.4%) patients died within the first 48 hours.
Table 4. Impact of influenza-positive virological samples on final disposition decision.
| Intensive care unit | Medical ward | Discharged | P | |
|---|---|---|---|---|
| n (%) | n (%) | n (%) | ||
| All groups | 0.5 | |||
| Negative influenza | 36 (6.3%) | 361 (63.2%) | 165 (28.9%) | |
| Positive influenza | 12 (5.6%) | 129 (60.6%) | 71 (33.3%) | |
| Community-acquired pneumonia | 0.9 | |||
| Negative influenza | 4 (3.2%) | 91 (72.8%) | 29 (23.2%) | |
| Positive influenza | 2 (3.9%) | 36 (69.2%) | 14 (26.9%) | |
| Severe acute symptoms | 0.5 | |||
| Negative influenza | 30 (9.3%) | 213 (65.7%) | 81 (25%) | |
| Positive influenza | 9 (7.9%) | 78 (68.4%) | 27 (23.7%) | |
| Respiratory | 0.8 | |||
| Negative influenza | 16 (8.8%) | 121 (66.5%) | 45 (24.7%) | |
| Positive influenza | 8 (10.7%) | 51 (68%) | 16 (21.3%) | |
| Hemodynamic | 0.5 | |||
| Negative influenza | 4 (17.4%) | 14 (60.9%) | 5 (21.7%) | |
| Positive influenza | 1 (20%) | 4 (80%) | 0 (0%) | |
| Cardiac | 0.2 | |||
| Negative influenza | 10 (19.2%) | 31 (59.6%) | 11 (21.2%) | |
| Positive influenza | 0 (0%) | 11 (73.3%) | 4 (26.7%) | |
| Miscellaneous | 0.8 | |||
| Negative influenza | 0 (0%) | 47 (70.2%) | 20 (29.9%) | |
| Positive influenza | 0 (0%) | 12 (63.2%) | 7 (36.8%) | |
| Patients with underlying conditions for severe Influenza | 0.1 | |||
| Negative influenza | 2 (1.8%) | 57 (50%) | 55 (48.3%) | |
| Positive influenza | 1 (2.2%) | 15 (32.6%) | 30 (65.2%) | |
| Positive bacteriological sample | 0.4 | |||
| Negative influenza | 22 (71%) | 8 (25.6%) | 1 (3.2%) | |
| Positive influenza | 46 (75.4%) | 14 (23%) | 1 (1.6%) |
Discussion
The results of our study indicate that the frequency of influenza-positive samples taken from symptomatic patients who presented at the ED was between 25.7% and 29.4%. Numerous clinical reasons for presentation at the ED were associated with influenza viruses, including acute decompensation of chronic cardiac and respiratory diseases, as well as acute episodes of community acquired pneumonia and respiratory and hemodynamic distress.
It has been reported that the frequency of the lung disease, the hospitalization rate and the mortality associated with Influenza-related pneumonia is increasing [20]. The frequency of lung damage is higher in patients with underlying cardiac or pulmonary diseases [21,22] and Influenza has been associated with increased mortality [23]. In the present study, 58% of the patients had at least one underlying condition for severe influenza [18], and 59% of patients had at least one severity criteria [19]. Respiratory distress was the main symptom, with up to 45% of patients exhibiting signs of clinically significant respiratory distress. It has previously been reported that 16% of cases of seasonal influenza occur in clinical pulmonary patients [24], 36% may develop acute pneumonia [24,25] and 20% may develop respiratory failure [25–28]. Our data support this finding, in that, among the proven cases of influenza virus, 49.2% had CAP, 25.8% had SAS with acute pneumonia, and up to 50% of patients had signs of respiratory distress. Neurological, hemodynamic, and cardiovascular complications have also previously been described in the context of severe infections that have been complicated by influenza [27,28]; in the present study, 12% of patients with influenza had hemodynamic disorders and 6% had neurological disorders. Some of the sepsis-like events that were observed in the group with hemodynamic failure could be related to frequent bacterial superinfection, which has been described in such patients [29].
Whereas only 5.6% of the patients with negative Influenza test had a bacterial coinfection, this figure reached 28.6% of patients with positive Influenza test. This feature indicates that Influenza may facilitate bacterial infection as previously resported [30]. Otherwise, bacterial coinfection was clearly more frequent in Community acquired pneumonia and Severe acute symptoms groups, both more frequently associated with severity criteria. However, coinfection was not associated with increased ICU and Medical ward admission rates. It has been reported that coinfection was frequently associated with severity criteria and ICU admission [31].
We found that the overall influenza positivity rate was 27.2%, and that, according to the reason for presentation in the ED (CAP 29.4%, SAS 25.7%, and PSSI 28.8%), the rates observed in the different groups were not significantly different. With regard to the final diagnostic categories (asthma (26%), EA-COPD (20.9%), HIV 5/21 (23.8%), and acute cardiac failure (25.2%) a high frequency was observed, but there was no difference between these categories. Our results indicate a higher frequency than that which was found in the few previous studies conducted in the ED, where, during the flu epidemic, the positivity rate of respiratory samples was low [32]. Our study shows that the positivity rates of respiratory specimens are very similar between patients with clinical symptoms suggestive of influenza than in patients with acute decompensation of chronic diseases. Most importantly, some of these clinical features are not usually recognized as being associated, or possibly associated, with the influenza virus.
It has previously been reported that the influenza virus has been isolated in 2.2% to 18% of people with CAP [11–13] and in 5% of people with severe acute respiratory infection admitted to ICU [33]. In the present study, the medical ward and ICU admission rates of people who had influenza-positive virus samples were 71.8% and 3.4%, respectively, while previously reported admission rates to MSW and ICU were 26% [12] and 15% [26,34], respectively. We found that the positivity of the influenza virus samples did not increase the frequency of the severity criteria or the rate of admissions to MSW or ICU, which corresponds to the results of previous studies [32,35]. An increase in ED attendance and hospitalizations due to decompensation of respiratory and cardiac pathologies during the winter period have previously been reported, but no direct link with epidemic influenza episodes was established [20,29]. Viruses can account for 30% of cardiac decompensations, but the influenza virus was isolated in only 3% of patients in an earlier study [14]. In the present study, up to 30% of episodes of acute cardiac failure, asthma, and AE-COPD were associated with the influenza virus. Our results indicate that influenza may account for 17%–29% of patients with severity criteria.
Strengths and limitations
Ours was a monocentric study in an urban environment in a large city in Europe. Thus, our results cannot be generalized and require local assessments. It is accepted that clinical variables are insensitive and non-specific in predicting influenza [26,36–38], with worse results in adults and the elderly [34]. Therefore, among the non-tested patients, a number may have had undocumented influenza, which could alter the reported rates. The isolation of respiratory viruses is possible in asymptomatic individuals but Influenza detection is likely associated with the illness under evaluation [39]. Thus, positive samples can be considered clinically significant. Otherwise, the sample size was sufficient to evaluate the distribution of positive viral samples among the study groups, and the rates of inclusion and withdrawals appeared to be satisfactory.
Conclusion
Our findings indicate that, during seasonal influenza epidemic episodes, 25–30% of emergency cases have positive influenza specimens, and this applies both to people experiencing symptoms that are suggestive of influenza and to people with acute respiratory infectious episodes, or episodes of respiratory, cardiac, or hemodynamic failure. High rates of medical ward and ICU hospitalizations amongst our entire population and the frequency of influenza virus among people whose clinical picture is not usually associated with influenza suggests that the risk of nosocomial influenza transmission must be considered as high during seasonal epidemic periods. As nosocomial influenza is currently recognized as an emerging concern [38], indications of isolation and treatment should be extended to these clinical situations during times of influenza epidemics. Our study thus opens up new prospects for research on indications of treatment and nosocomial transmission of influenza in the ED and hospital stay after through ED admission.
Data Availability
Data are available at https://osf.io/n79hg/.
Funding Statement
The author(s) received no specific funding for this work.
References
- 1.Thomas RE. Do we have enough evidence how seasonal influenza is transmitted and can be prevented in hospitals to implement a comprehensive policy? Vaccine. 2016;34(27):3014–21. doi: 10.1016/j.vaccine.2016.04.096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gerke AK, Yang M, Tang F, Foster ED, Cavanaugh JE, Polgreen PM. Association of hospitalizations for asthma with seasonal and pandemic influenza. Respirology. 2014;19(1):116–21. doi: 10.1111/resp.12165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Donaldson GC, Wedzicha JA. The causes and consequences of seasonal variation in COPD exacerbations. Int J Chron Obstruct Pulmon Dis. 2014;9:1101–10. doi: 10.2147/COPD.S54475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boari B, Mari E, Gallerani M, Fabbian F, Pala M, Tiseo R, et al. Temporal variation of heart failure hospitalization: does it exist? Rev Cardiovasc Med. 2011;12(4):211–8. [DOI] [PubMed] [Google Scholar]
- 5.Fiore AE, Ujeki TM, Broder K. Prevention and control of influenza with vaccines: recommendation of the Advisory Committee on Immunization Practices (ACIP) 2010. MMWR Recommen Rep, 2010;59(35):1147. [PubMed] [Google Scholar]
- 6.Rothberg MB, Haessler SD, Brown RB. Complications of viral influenza. Am J Med. 2008;121(4):258–64. doi: 10.1016/j.amjmed.2007.10.040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Walter ND, Taylor TH, Shay DK, Thompson WW, Brammer L, Dowell SF, et al. Active Bacterial Core Surveillance Team. Influenza circulation and the burden of invasive pneumococcal pneumonia during a non-pandemic period in the United States. Clin Infect Dis. 2010;50(2):175–83. doi: 10.1086/649208 [DOI] [PubMed] [Google Scholar]
- 8.Brundage JF. Interactions between influenza and bacterial respiratory pathogens: implications for pandemic preparedness. Lancet Infect Dis. 2006;6(5):303–12. doi: 10.1016/S1473-3099(06)70466-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schanzer DL, Schwartz B. Impact of seasonal and pandemic influenza on emergency department visits, 2003–2010, Ontario, Canada. Acad Emerg Med. 2013;20(4):388–97. doi: 10.1111/acem.12111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.World Health Organization. Battle against respiratory viruses (BRaVe) initiative 2012. http://www.who.int/influenza/patient_care/clinical/brave/en/.
- 11.Irving SA, Patel DC, Kieke BA, Donahue JG, Vandermause MF, Shay DK, et al. Comparison of clinical features and outcomes of medically attended influenza A and influenza B in a defined population over four seasons: 2004–2005 through 2007–2008. Influenza Other Respir Viruses. 2012;6(1):37–43. doi: 10.1111/j.1750-2659.2011.00263.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Grijalva CG, Zhu Y, Williams DJ, Self WH, Ampofo K, Pavia AT, et al. Association Between Hospitalization With Community-Acquired Laboratory-Confirmed Influenza Pneumonia and Prior Receipt of Influenza Vaccination. JAMA. 2015;314(14):1488–97. doi: 10.1001/jama.2015.12160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Farida H, Gasem MH, Suryanto A, Keuter M, Zulkarnain N, Satoto B, et al. Viruses and Gram-negative bacilli dominate the etiology of community-acquired pneumonia in Indonesia, a cohort study. Int J Infect Dis. 2015;38:101–7. doi: 10.1016/j.ijid.2015.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Clark TW, Medina MJ, Batham S, Curran MD, Parmar S, Nicholson KG. Adults hospitalised with acute respiratory illness rarely have detectable bacteria in the absence of COPD or pneumonia; viral infection predominates in a large prospective UK sample. J Infect. 2014;69(5):507–15. doi: 10.1016/j.jinf.2014.07.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rice TW, Rubinson L, Uyeki TM, Vaughn FL, John BB, Miller RR 3rd, et al. A virus and bacterial coinfection in the United States. Crit Care Med. 2012;40(5):1487–98. doi: 10.1097/CCM.0b013e3182416f23 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ortiz JR, Neuzil KM, Shay DK, Rue TC, Neradilek MB, Zhou H, et al. The burden of influenza-associated critical illness hospitalizations. Crit Care Med. 2014;42(11):2325–32. doi: 10.1097/CCM.0000000000000545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.INVS. Grippe: généralités. 2016. http://invs.santepubliquefrance.fr/Dossiers-thematiques/Maladies-infectieuses/Maladies-a-prevention-vaccinale/Grippe/Grippe-generalites/Donnees-de-surveillance.
- 18.Lam J, Nikhanj N, Ngab T, Tennant R, Shahedi K, Mathisen G, et al. Severe cases of pandemic H1N1 pneumonia and respiratory failure requiring intensive care. J Intensive Care Med. 2011;26(5):318–25. doi: 10.1177/0885066610392684 [DOI] [PubMed] [Google Scholar]
- 19.Shah NS, Greenberg JA, McNulty MC, Gregg KS, Riddell J, Mangino JE,et al. Severe Influenza in 33 US Hospitals, 2013–2014: Complications and Risk Factors for Death in 507 Patients. Infect Control Hosp Epidemiol. 2015;36(1):1251–60. [DOI] [PubMed] [Google Scholar]
- 20.Wuerth BA, Bonnewell JP, Wiemken TL, Arnold FW. Trends in Pneumonia Mortality Rates and Hospitalizations by Organism, United States, 2002-2011(1). Emerg Infect Dis. 2016;22(9):1624–7. doi: 10.3201/eid2209.150680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oliveira EC, Marik PE, Colice G. Influenza pneumonia: a descriptive study. Chest. 2001;119(6):1717–23. [DOI] [PubMed] [Google Scholar]
- 22.Murata Y, Walsh EE, Falsey AR. Pulmonary complications of interpandemic influenza A in hospitalized adults. J Infect Dis. 2007;195(7):1029–37. doi: 10.1086/512160 [DOI] [PubMed] [Google Scholar]
- 23.Ortiz JR, Neuzil KM, Cooke CR, Neradilek MB, Goss CH, Shay. Influenza pneumonia surveillance among hospitalized adults may underestimate the burden of severe influenza disease. PLoS One. 2014. November 25;9(11):e113903 doi: 10.1371/journal.pone.0113903 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maruyama T, Fujisawa T, Suga S, Nakamura H, Nagao M, Taniguchi K, et al. Outcomes and Prognostic Features of Patients With Influenza Requiring Hospitalization and Receiving Early Antiviral Therapy: A Prospective Multicenter Cohort Study. Chest. 2016;149(2):526–34. doi: 10.1378/chest.14-2768 [DOI] [PubMed] [Google Scholar]
- 25.Garg S, Jain S, Dawood FS, Jhung M, Pérez A, D'Mello T, et al. Pneumonia among adults hospitalized with laboratory-confirmed seasonal influenza virus infection-United States, 2005–2008. BMC Infect Dis. 2015;15:369 doi: 10.1186/s12879-015-1004-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Loubet P, Samih-Lenzi N, Galtier F, Vanhems P, Loulergue P, Duval X, et al. Factors associated with poor outcomes among adults hospitalized for influenza in France: A three-year prospective multicenter study. J Clin Virol. 2016;79:68–73. doi: 10.1016/j.jcv.2016.04.005 [DOI] [PubMed] [Google Scholar]
- 27.Leung CH, Tseng HK, Wang WS, Chiang HT, Wu AY, Liu CP. Clinical characteristics of children and adults hospitalized for influenza virus infection. J Microbiol Immunol Infect. 2014;47(6):518–25. doi: 10.1016/j.jmii.2013.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Rendón-Ramirez EJ, Ortiz-Stern A, Martinez-Mejia C, Salinas-Carmona MC, Rendon A, Mata-Tijerina VL, et al. TGF-β Blood Levels Distinguish Between Influenza A (H1N1) pdm09 Virus Sepsis and Sepsis due to Other Forms of Community-Acquired Pneumonia. Viral Immunol. 2015;28(5):248–54. doi: 10.1089/vim.2014.0123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rodríguez AH, Avilés-Jurado FX, Díaz E, Díaz E, Schuetz P, Trefler SI, et al. Procalcitonin (PCT) levels for ruling-out bacterial coinfection in ICU patients with influenza: A CHAID decision-tree analysis. J Infect. 2016;72(2):143–51. doi: 10.1016/j.jinf.2015.11.007 [DOI] [PubMed] [Google Scholar]
- 30.Klein EY, Monteforte B, Gupta A, Jiang W, May L, Hsieh YH, et al. The frequency of influenza and bacterial coinfection: a systematic review and meta-analysis. Influenza Other Respir Viruses. 2016;10(5):394–403. doi: 10.1111/irv.12398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Damasio GA, Pereira LA, Moreira SD, Duarte dos Santos CN, Dalla-Costa LM, Raboni SM. Does virus-bacteria coinfection increase the clinical severity of acute respiratory infection? J Med Virol. 2015;87(9):1456–61. doi: 10.1002/jmv.24210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Monmany J, Rabella N, Margall N, Domingo P, Gich I, Vázquez G. Unmasking influenza virus infection in patients attended to in the emergency department. Infection. 2004;32(2):89–97. doi: 10.1007/s15010-004-3088-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sakr Y, Ferrer R, Reinhart K, Beale R, Rhodes A, Moreno R, et al. The Intensive Care Global Study on Severe Acute Respiratory Infection (IC-GLOSSARI): a multicenter, multinational, 14-day inception cohort study. Intensive Care Med. 2016;42(5):817–28. doi: 10.1007/s00134-015-4206-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Falsey AR, Baran A, Walsh EE. Should clinical case definitions of influenza in hospitalized older adults include fever? Influenza Other Respir Viruses. 2015;9 Suppl 1:23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Self WH, Williams DJ, Zhu Y, Ampofo K, Pavia AT, Chappell JD, et al. Respiratory Viral Detection in Children and Adults: Comparing Asymptomatic Controls and Patients With Community-Acquired Pneumonia. J Infect Dis. 2016;213(4):584–91. doi: 10.1093/infdis/jiv323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yang JH, Huang PY, Shie SS, Yang S, Tsao KC, Wu TL, et al. Predictive Symptoms and Signs of Laboratory-confirmed Influenza: A Prospective Surveillance Study of Two Metropolitan Areas in Taiwan. Medicine (Baltimore). 2015;94(44):e1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shah SC, Rumoro DP, Hallock MM, Trenholme GM, Gibbs GS, Silva JC, et al. Clinical predictors for laboratory-confirmed influenza infections: exploring case definitions for influenza-like illness. Infect Control Hosp Epidemiol. 2015;36(3):241–8. doi: 10.1017/ice.2014.64 [DOI] [PubMed] [Google Scholar]
- 38.Ishiguro T, Takayanagi N, Kanauchi T, Uozumi R, Kawate E, Takaku Y, et al. Clinical and Radiographic Comparison of Influenza Virus-associated Pneumonia among Three Viral Subtypes. Intern Med. 2016;55(7):731–7. doi: 10.2169/internalmedicine.55.5227 [DOI] [PubMed] [Google Scholar]
- 39.Byington CL, Ampofo K, Stockmann C, Adler FR, Herbener A, Miller T, et al. Community Surveillance of Respiratory Viruses Among Families in the Utah Better Identification of Germs-Longitudinal Viral Epidemiology (BIG-LoVE) Study. Clin Infect Dis. 2015;61(8):1217–24 doi: 10.1093/cid/civ486 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data are available at https://osf.io/n79hg/.