Abstract
Praziquantel (PZQ) is an effective chemotherapy for schistosomiasis mansoni and a mainstay for its control and potential elimination. However, it does not prevent against reinfection, which can occur rapidly in areas with active transmission. A guide to ranking the risk factors for Schistosoma mansoni reinfection would greatly contribute to prioritizing resources and focusing prevention and control measures to prevent rapid reinfection. The objective of the current study was to explore the relationship among the socioeconomic, demographic, and epidemiological factors that can influence reinfection by S. mansoni one year after successful treatment with PZQ in school-aged children in Northeastern Minas Gerais state Brazil. Parasitological, socioeconomic, demographic, and water contact information were surveyed in 506 S. mansoni-infected individuals, aged 6 to 15 years, resident in these endemic areas. Eligible individuals were treated with PZQ until they were determined to be negative by the absence of S. mansoni eggs in the feces on two consecutive days of Kato-Katz fecal thick smear. These individuals were surveyed again 12 months from the date of successful treatment with PZQ. A classification and regression tree modeling (CART) was then used to explore the relationship between socioeconomic, demographic, and epidemiological variables and their reinfection status. The most important risk factor identified for S. mansoni reinfection was their “heavy” infection at baseline. Additional analyses, excluding heavy infection status, showed that lower socioeconomic status and a lower level of education of the household head were also most important risk factors for S. mansoni reinfection. Our results provide an important contribution toward the control and possible elimination of schistosomiasis by identifying three major risk factors that can be used for targeted treatment and monitoring of reinfection. We suggest that control measures that target heavily infected children in the most economically disadvantaged households would be most beneficial to maintain the success of mass chemotherapy campaigns.
Introduction
Despite recent progress in controlling Schistosoma mansoni infection in Latin America and the Caribbean (LAC) countries, this parasitic nematode remains a major public health problem in Brazil and in other low and middle income countries in the region [1]. Brazil has the largest number of S. mansoni cases among the LAC countries with approximately 6 million people currently infected, and 25 million at a risk of infection [2]. Although S. mansoni infection occurs when individuals enter fecally contaminated water inhabited by infected intermediate hosts (Biomphalaria glabrata), the transmission of this neglected tropical disease also has a complex social, biological, and environmental basis that must be considered for successful control and elimination programs. While the importance of these factors in the occurrence of schistosomiasis has been intensively studied [3–5], the complexity of socioeconomic and behavioral factors can vary considerably on a local level as well as on a global scale. For example, a study conducted on the shores and islands of Lake Victoria in Nyanza Province, Kenya showed that human population density was a major factor driving Schistosoma transmission, with individual socioeconomic factors appearing not to significantly affect transmission [6]. On the other hand, in a study conducted in Porto de Galinhas in Northeastern Brazil, the education level of heads of families and low household income were the major risk factors influencing infection [7]. Thus, mapping the risk factors for S. mansoni transmission depends on identifying the local context in which transmission occurs, which facilitates the implementation and optimization of schistosomiasis control programs. The planning and implementation of effective schistosomiasis control programs demands consideration of the major risk factors of particular communities, including their unique ecological, social, and behavioral determinants and the interaction of these determinants within the context of the control programs [8,9]. An approach that can identify a hierarchy of risk factors and their interaction at the local level could form the basis for an effective community-based control program, as it would help prioritize both the foci and the methods for the prevention or control of transmission [10]. Many previous epidemiological studies of schistosomiasis used multinomial models, such as logistic regression, which uses linear combinations as the primary method of expressing associations between variables and outcomes such as infection or reinfection [11,12]. These models do not estimate the interaction of the explanatory variables nor do they rank the variables according to their relative importance in light of multiple interactions among the variables. Hierarchical ranking has become an increasingly useful tool for such analyses as it enables exploration of the relationship among different risk factors, giving a better understanding of their actual importance and the interplay of these factors on infection or reinfection. More specifically, the classification and regression tree (CART) technique is an especially useful decision support tool in this context, with its advantage over the multivariate analysis described in several studies [10,13,14, 15]. CART has been used in ranking determinants of Taenia solium infection [10], malaria infection [13], as well as for other diseases [16,17], but not for schistosomiasis infection or reinfection. The objective of the current study was to apply CART analysis to determine the relative importance and relationships among different factors that can predict risk of reinfection with S. mansoni in school-aged children in Northeastearn Brazil, who had been successfully treated with Praziquantel (PZQ) and then followed for 12 months post-treatment to determine their level of reinfection.
Methods
Study area and population
This is a longitudinal study of school-aged children conducted in five municipalities (Itaobim, Jequitinhonha, Joaima, Monte Formoso and Ponto dos Volantes) in the Jequitinhonha Valley in northern Minas Gerais State (MG). This region is known as one of the poorest regions in Brazil, with low social economic indexes. These municipalities together have a population of 76,074 with approximately 36% living in rural areas. The area is endemic for S. mansoni and is drained by streams and rivers. The main sources of income are subsistence agriculture, animal husbandry, and small-scale commerce.
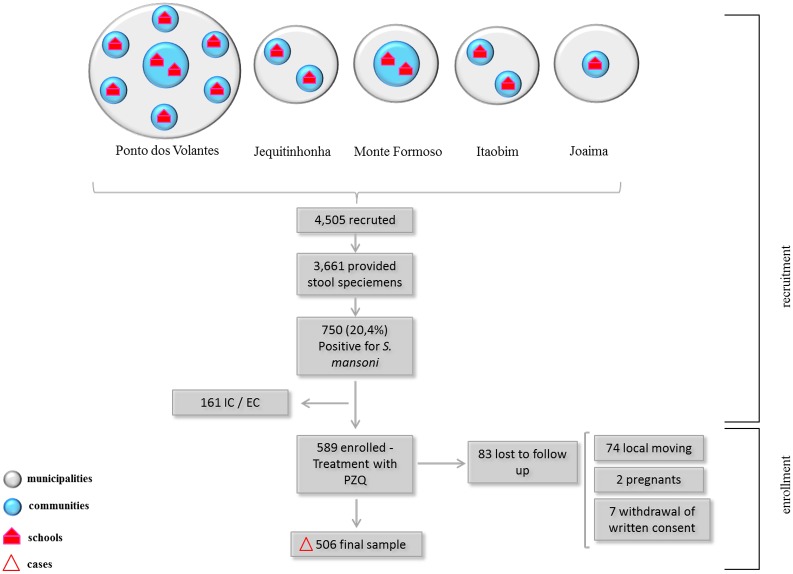
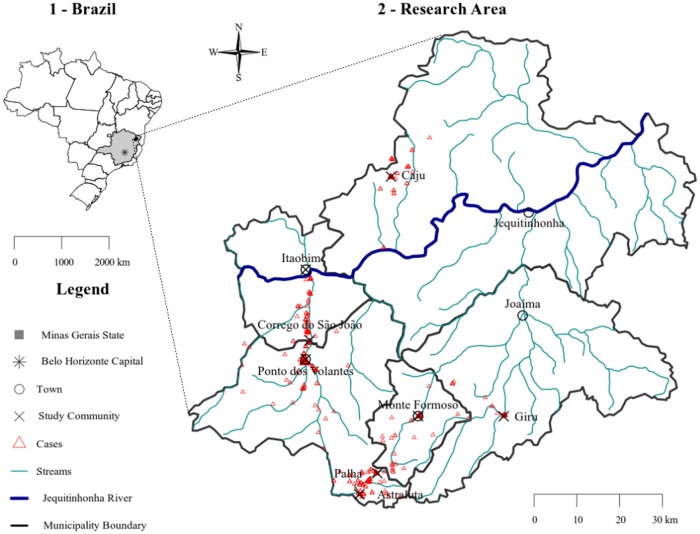
Thirteen communities were indicated by the schistosomiasis control programs of the municipalities as having a higher probability of finding S. mansoni positive cases. Students from 15 local public schools, eight in Ponto dos Volantes, two in Jequitinhonha, Monte Formoso and Itaobim and a single in Joaíma were chosen for the current study. According to the list of all students from those schools, 4,505 elementary and high school students of both sexes were eligible. These students and their parents or legal guardians were invited by members of the study team to participate in the parasitological screening. After obtaining a written consent, 81.2% (n = 3,661) of the students provided stool specimens, with 20.4% (n = 750) identified as positive for S. mansoni by conventional microscopy (see Methods below). Parents and legal guardians of the infected children were then visited and informed about the study and their children invited to participate. Only children who were positive for S. mansoni and between the ages of 6 to 15 years (inclusive) and not pregnant or breastfeeding were enrolled in the study (Fig 1). The final study area was comprised of eight communities as shown in Fig 2.
Fig 1. Flow chart representing the recruitment and enrollment phases of the study.
Municipalities are represented in grey, communities in blue, schools in red and the final sample represented by triangle. IC / EC: inclusion and exclusion criteria; PZQ: Praziquantel.
Fig 2. Map showing the distribution of communities in Northeastern, Minas Gerais State Brazil in which participants were enrolled into the longitudinal component of the study.
Map created in R statistical software (https://www.r-project.org/) from public data of the Brazilian Institute of Geography and Statistics—IBGE, available at: http://mapas.ibge.gov.br/bases-e-referenciais/bases-cartograficas/malhas-digitais.html and the National Water Agency—ANA (2010) Hydrography 1: 1,000,000 (georeferenced digital base), available at: http://hidroweb.ana.gov.br/HidroWeb.asp?TocItem=4100.
Parasitological methods
All study participants received two name-coded, 80-ml plastic containers for the collection of fecal samples. They were instructed to deposit one fecal sample per day in the container and return each sample immediately to collection points, where they were stored at 4°C. Two slides for each stool sample (four slides per individual) were prepared within 24h of collection, using the Kato-Katz fecal thick smear method [18]. For quality control, 10% of the slides were randomly selected and examined by a senior microscopist at the Centro de Pesquisas René Rachou, FIOCRUZ, in Belo Horizonte, MG. The intensity of S. mansoni infection was estimated from the mean egg count per gram of feces (epg) and categorized into classes of egg counts as recommended by World Health Organization (WHO) [19]: low (1–99 epg), moderate (100–399 epg) and high (≥400 epg). The moderate and high infected groups were combined into a single group for analyses and as such our analyses were based on two groups: low burden (1–99 epg) and moderate to high burden (≥ 100 epg).
Stool samples were collected one week before and one year after successful chemotherapy by PZQ as determined by the absence of eggs in feces by the Kato Katz method. At baseline (D-0), between March and December 2014 in the different study communities, treatment was performed with the standard single oral dose of 60 mg/kg of PZQ administered with direct observation and under medical supervision as recommended by the Brazilian Ministry of Health [20]. Individuals infected with geohelminths were also treated with a single oral dose of 400 mg of albendazole under the same conditions.
Four weeks after PZQ treatment, two stool specimens were collected from each individual on two consecutive days to determine the effectiveness of the treatment. Individuals who remained infected received a single dose of PZQ at 60 mg/kg orally until there was an absence of S. mansoni eggs four weeks after treatment (successful treatment). One year after the successful treatment (Day 360), two stool specimens were again collected from each individual and examined for S. mansoni infection, with reinfection defined as the presence of a single S. mansoni egg by microscopy. Positive cases were retreated with PZQ at 60 mg/kg orally until successful treatment was reached as described above. Reinfection was considered the presence of a single S. mansoni eggs in the feces by microscopy 360 days post successful treatment with PZQ.
Socioeconomic and water contact surveys
Questionnaires as previously described [21, 22] were applied to obtain demographic (age, gender, place of residence, education of household head), socioeconomic (income, wealth, occupation, quality of housing, presence and type of latrine, number of persons per room and water supply) and water contact behavior information for all participants at baseline. Data was collected by graduate students, specifically trained and supervised by the team leader. Parents answered the questions on socioeconomic status and water contact for children less than 10 years of age.
The socioeconomic information was scored to determine the socioeconomic status (SES) of families using the method of Gwatkin et al. [23]. This instrument considers household appliances, car ownership, type of water used, etc. as a gauge of a household socioeconomic status rather than monthly income alone. The asset questionnaire is binary (“yes” or “no”) and is summarized and scored. The sum of scores allows the separation of families into wealth quintiles with cut-off points as follows: low (less than -0.66129); second (-0.66129 to 0.02018); third (0.02018 to 0.51776); fourth (0.51776 to 1.00965) and high (above 1.00965). The lowest quintile was designated as “extremely poor”, the second quintile as “very poor”, the third quintile as “poor”, the fourth quintile as “medium”, and the fifth as “high” socioeconomic status. In this study, all families were ranked in the three lowest quintiles.
All participants were asked about the type and frequency of their weekly contact with potentially S. mansoni infected water sources for domestic, recreational, hygienic, and occupational purposes. Contact with the surface of water bodies, including streams, canals, springs, and ponds, was also included because of the widespread distribution of the intermediate host Biomphalaria glabrata in the study area. The weekly frequency of water contact data was used to calculate the exposure index or Total Body Minutes (TBM) developed by Kloos et al. [24]. All predictor variables used in the CART analysis are described in Table 1.
Table 1. Predictor variables in the CART analysis for risk of S. mansoni reinfection.
| Variable classes | Predictor variables |
|---|---|
| Host characteristics | Age |
| Gender | |
| Education of household head | |
| Intensity of infection | |
| Socioeconomic level | |
| Household characteristics | Locality (rural or urban) |
| Presence of latrine | |
| Type of latrine | |
| Type of walls | |
| Type of floor | |
| Treated water supply | |
| Water contact | Total TBM |
| Washing clothes | |
| Washing utensils | |
| Washing animals | |
| Washing parts of the body | |
| Bathing | |
| Swimming | |
| Playing | |
| Fishing | |
| Watering plants | |
| Irrigation | |
| Collecting sand at stream | |
| Fetching stream water | |
| Crossing streams | |
| Cleaning streams |
Statistical analysis
All data were double entered by two individuals into Excel spreadsheets (Microsoft Office Excel 2007, Microsoft Corp., Redmond, WA). Then the two data sets were crosschecked by a third individual and merged into a single data set for analysis. If a discrepancy was identified, data were again checked and if necessary return to subject. Chi-square test was used to test for difference in proportions with a statistical significance at a p<0.05. The variables with p-values <0.20 in the univariate analysis were selected. Subsequently multivariate adjusted analysis and a Poisson regression with robust variances were used. The magnitude of associations was estimated by relative risk (RR), assuming a 95% confidence interval (CI) as precision measurement. Variables remaining statistically significant below 5% were retained in the final model.
Quality evaluation of the model was carried out by calculating its coefficient of R2 determination and by goodness-of-fit test. Co-linearity and interaction between the variables in the final model were also tested. The software Statistical Package for Social Sciences (SPSS Inc., Chicago, USA) version 19.0 and the Statistical Software for Professionals (STATA) Version 12 were used for data analysis.
The Classification and Regression Tree (CART) model was used to show the relationship among risk factors that lead to a higher risk of reinfection and their hierarchical classification. The classification model was developed using the method first introduced by Breiman [25], known as Decision Trees (S1 Box). CART is a non-linear and non-parametric model that is fitted by binary recursive partitioning of multidimensional covariate space [25]. Classification trees are used to analyze categorical health outcomes, while regression trees serve to assess continuous health outcomes [15]. See S1 Box for the details of CART analysis.
Ethical approval
Ethical approval was obtained by the Brazilian National Ethics Committee (CONEP-Number 531.282). Prior to the commencement of the study, a written consent was obtained from all parents and legal guardians of participants and informed assent obtained from students aged ≥ 7 years.
Results
Study sample
A total of 589 students aged 6 to 15 years old (inclusive) from 10 schools in eight communities were included in the study sample. Eighty-three students were lost to follow-up after one year: 74 (89.1%) moved to other areas, 2 (2.4%) got pregnant and 7 (8.4%) withdrawal of written consent. The final sample for the study consisted of 506 individuals infected with S. mansoni. The population of the study are mostly from poor families, with 82.6% of them receiving government assistance through the “Bolsa Família Program”, a social welfare initiative of federal assistance. They typically live in houses with no sewage system, with only 57.1% of the households having treated water.
Risk factor analysis
A total of 506 children and adolescents infected with S. mansoni participated in the study, and 111 of the 506 were reinfected one year after treatment. The intensity of infection with S. mansoni before treatment (baseline) was determined by geometric mean eggs per gram of feces (epg) to be 79.26 (95% CI, 23.90, 134.61). Treatment with PZQ resulted in substantial reductions both on the number of individuals reinfected with S. mansoni and a reduction in their egg counts (epg) 12 months after successful treatment (Study Day 360). The reinfection rate one year after treatment was 21.9% (n = 111) and the intensity of infection was 20.49 epg (95% CI, 2.85, 38.13). Table 2 shows that reinfected individuals were mostly males (n = 65, 58.6%), aged from 11 to 15 years (50.5%) (n = 56), living in rural areas (n = 72, 64.9%), with a moderate to high intensity of infection of (n = 71, 64%),and a household head with 1 to 4 years of schooling (n = 55, 49.5%) and classified as very poor household (n = 61, 55%) (Table 2). When comparing “reinfected” individuals with those who were not reinfected, we observed a significant difference (p <0.001) in intensity of infection since most of the reinfected individuals had moderate/high intensity of infection.
Table 2. Baseline and reinfection demographic and socioeconomic factors and intensity of infection of reinfected and non-reinfected students.
| Study Sample (n = 506) | Reinfected (n = 111) | Not reinfected (n = 395) | p | ||||
|---|---|---|---|---|---|---|---|
| Variables | n | % | n | % | n | % | |
| Gender | |||||||
| Male | 297 | 58.7 | 65 | 58.6 | 232 | 58.7 | 0.974 |
| Female | 209 | 41.3 | 46 | 41.4 | 163 | 41.3 | |
| Age (years) | |||||||
| 5–10 | 225 | 44.5 | 56 | 50.5 | 170 | 43 | 0.223 |
| 11–15 | 281 | 55.5 | 55 | 49.5 | 225 | 57 | |
| Local | |||||||
| Rural | 318 | 62.8 | 72 | 64.9 | 246 | 62.3 | 0.618 |
| Urban | 188 | 37.2 | 39 | 35.1 | 149 | 37.7 | |
| Intensity of infection | |||||||
| Moderate-high | 209 | 41.3 | 71 | 64.0 | 138 | 34.9 | <0.001 |
| Low | 297 | 58.7 | 40 | 36.0 | 257 | 65.1 | |
| Education (Household head) | |||||||
| None | 136 | 26.9 | 30 | 27.0 | 106 | 26.8 | |
| Grades 1–4 | 216 | 42.7 | 55 | 49.5 | 161 | 40.8 | 0.145 |
| Grades > 4 | 154 | 30.4 | 26 | 23.4 | 128 | 32.4 | |
| Economic classification* | |||||||
| Extremely poor | 184 | 36.4 | 39 | 35.1 | 145 | 36.7 | |
| Very poor | 240 | 47.4 | 61 | 55.0 | 179 | 45.3 | 0.073 |
| Poor | 82 | 16.2 | 11 | 9.9 | 71 | 18 | |
*Note that no household reached the “medium” or “high” economic strata.
A Poisson regression model adjusted for demographic, socioeconomic, water contact and intensity of infection before treatment was performed. The results showed that only the variable “intensity of infection” at baseline remained independently associated with reinfection one year after treatment. Individuals who had moderate/heavy intensity of infection before treatment had a higher risk of reinfection for S. mansoni (RR = 2.52; 95% CI, 1.78, 3.56.
Classification and regression trees (CART)
According to the overall discriminatory power in the analysis (Table 3), the intensity of infection at baseline was the most important factor (100.0) in predicting the risk of reinfection with S. mansoni. This was followed by Total Body Minutes in potentially S. mansoni contaminated water (94.9) and by washing parts of the body in potentially S. mansoni contaminated water (75.3). Other factors are shown in Table 3.
Table 3. Ranking of reinfection risk factors by overall discriminatory power.
| Variable | Power |
|---|---|
| Baseline intensity of infection | 100.0 |
| Total TBM | 94.9 |
| Washing parts of the body | 75.3 |
| Type of latrine | 36.0 |
| Type of floor | 26.3 |
| Socioeconomic level | 25.6 |
| Playing | 25.5 |
| Presence of latrine | 25.0 |
| Age | 23.9 |
| Washing animals | 22.2 |
| Bathing | 21.5 |
| Swimming | 16.8 |
| Education of household head | 14.1 |
| Fetching water | 13.4 |
| Gender | 12.7 |
| Crossing streams | 10.6 |
| Fishing | 10.1 |
| Chemically treated water | 7.5 |
| Locality | 7.3 |
| Collecting sand at streams | 6.7 |
| Type of walls | 3.1 |
| Washing clothes | 0.0 |
| Washing utensils | 0.0 |
| Watering plants | 0.0 |
| Irrigation | 0.0 |
| Cleaning stream | 0.0 |
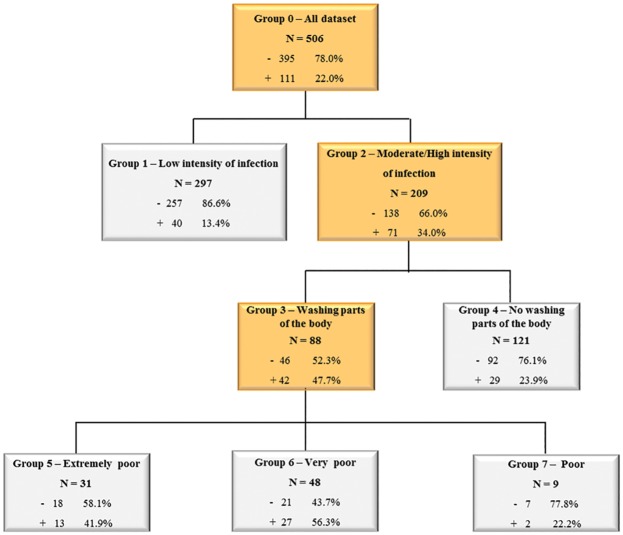
The classification tree (Fig 3) showed that at the root, 111 (22%) of the 506 individuals were reinfected with S. mansoni. The intensity of infection (expressed as an EPG) at baseline was the first “splitter”, with individuals having a moderate to heavy intensity of infection (n = 209) at baseline having a greater risk of becoming reinfected (34.0%) than individuals with a low intensity of infection at baseline (13.4%). Moreover, in the former group (second splitter), reinfection was higher among moderate to heavily infected individuals who had a higher mean TBM for washing parts of the body (47.7%) than for those with lower mean TBM (23.9%). For those with higher mean TBM for washing parts of the body, socioeconomic classification was the best discriminator of their placement in this group. Reinfection was higher in the very poor socioeconomic strata (56.3%) and in the extremely poor strata (41.9%) if compared with reinfection in the “poor” socioeconomic strata (22.2%) (Fig 3).
Fig 3. Classification tree of the risk factors for reinfection.
The internal nodes are highlighted in orange. The plus sign (+) represents positivity for reinfection and the minus sign (-) represents lack of reinfection.
CART analysis showed that individuals who had a low pre-treatment intensity of infection had substantially lower risk of reinfection than the rest of the sample (13.4% versus 22.0%). Among those with moderate-high intensity of infection, CART analysis split them further into homogeneous reinfection risk groups. Groups 4 and 7 are nearly equal with the rest of the sample and are composed of participants who either do not wash parts of their bodies in potentially S. mansoni contaminated water nor wash parts of their bodies in potentially S. mansoni contaminated water, but are in the “poor” socioeconomic strata. The two remaining groups, composed by those who wash parts of their body in potentially S. mansoni contaminated water and in the “extremely poor” socioeconomic strata have a higher risk of reinfection than the overall sample (56.3% and 41.9, respectively).
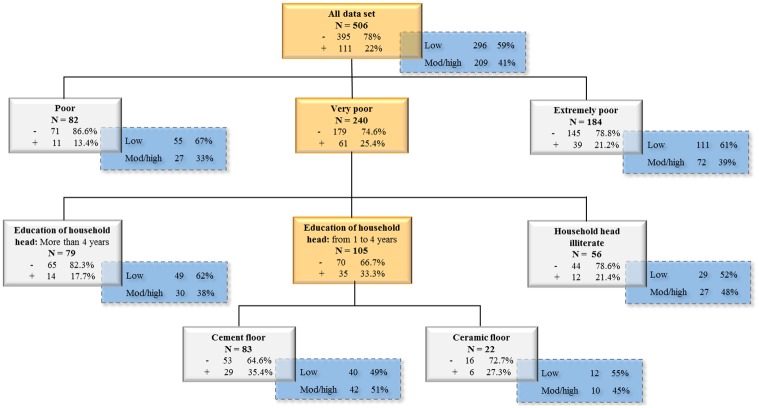
It is possible that the variable “intensity of infection” was affected by the other variables in CART, making it the causal factor that best describes reinfection, since it is the first splitter of the tree (Fig 3) and with the greatest discriminatory power (Table 3). Therefore, the variable intensity of infection at baseline was removed, and CART analysis runned on weka, generating a different classification tree. With the resulting tree, the rate of low and moderate/high intensity of infection in each final node was manually calculated and put together to be able to visualize the behavior of both variables: reinfection and intensity of infection. The resulting analysis showed other variables not present in the first tree (Fig 3), but important risk factors for reinfection (Fig 4). The tree showed that in the first splitter the very poor and the extremely poor individuals had higher reinfection rates (25.4% and 21.2% respectively) than the poor individuals (13.4%). In the former group, reinfection was higher among children whose head of household had 1–4 years of education (33.3%) or were illiterate (21.4%) in comparison with literate heads of households (17.7%). For those with head of household with a low level of education, the type of floor was the best discriminator of reinfection. Over one third of the 83 (35.4%) reinfected individuals lived in houses with cement floor, but only 27.3% of the 22 individuals living in houses with ceramic tile floors were reinfected.
Fig 4. Classification tree of the risk factors for reinfection excluding the variable intensity of infection at baseline.
The internal nodes are highlighted in orange. The plus sign (+) represents positivity for reinfection and the minus sign (-) represents no reinfection. The crosshatched boxes (blue) show the percentages of those with low and moderate/high intensity of infection in each final node.
Other factors also significantly influenced reinfection. Using the resulting tree structure of re-run CART without intensity of infection, the percentages of low and moderate/high intensity of infection category were manually calculated for each leaf of the tree and put together (Fig 4). The effects of two variables (education of household head and type of floor) were hidden by the presence of the initial intensity of infection as a possible explanatory variable. Indeed, these variables may also explain the initial intensity of infection. The percentages of those with low and moderate/high intensity of infection were calculated and results are shown in a crosshatched box attached to each final node in Fig 4. We observed that, by considering the final groups, the percentage of reinfection is positively correlated with the percentage of moderate/high intensity of infection. For example, the individuals classified as poor had a lower risk of reinfection (13.4% in the group versus 22% in the entire sample) and they too have a lower risk of moderate/high infection intensity in the crosshatched box (33% in the group versus 41% in the entire sample). In this study the two trees analysis indicate that intensity of infection, water contact, poverty and education are important risk factors for reinfection in the endemic areas studied.
Discussion
Schistosomiasis is a disease with complex transmission dynamics that require sophisticated statistical analyses at the local level to capture the relationships among various risk factors and the hierarchical importance of each risk factor on infection and reinfection. Indeed, surveys in S. mansoni endemic areas often generate large and complex data sets, with a broad range of variables, requiring the application of appropriate statistical analyses. CART is a statistical method that allows the hierarchical analyses of such complex data sets and generates a classification tree, that uses all possible combinations to show relationships between variables and rank the potential factors related [15], enabling the application of these results to control and possibly eliminate S. mansoni in endemic areas. In CART analysis, the discriminatory power of the model provides a ranking based on the overall contribution of each variable in the construction of the tree. This hierarchical ranking indicates the importance of each independent variable as a predictor, while the tree allows the exploration of relationships between variables and their relative importance [15]. In this study, some risk factors appear in the first tree from the root (Fig 3) in a different ranking than their hierarchical importance shown in the discriminatory power in Table 3. This is due to the tree being able to show the splitter variables that increase the differentiation between groups (reinfected and non-reinfected), conditioned on the values of previous variables. This creates a set of hierarchically nested groups showing a highly homogeneous reinfection behavior. In general, the high risk factors determined by CART and the most important variables presented in the discriminatory power table (Table 3) are quite similar. The arrangement and sequence of risk factors in the tree shows the interaction and relationship among these variables that result in reinfection.
In the current study, the final regression model showed that the moderate/high intensity of infection at baseline was the only risk factor for reinfection. The CART analysis confirms the final Poisson regression model, in addition, identified other important risk factors in predicting reinfection according to their importance, showing the advantages of this method. CART has the advantage of dealing with multi-collinearity, multi-level interactions and can identify risk factors according to their importance, different from multinomial models such as logistic regression (15).
As mentioned, a critical risk factor for reinfection was intensity of infection at baseline. This may be related to the fact that in our studies, we focused on a population of young individuals that are constantly exposed to contaminated water. In this context, it is possible that an increase on reinfection levels may be a consequence of cumulative infections due to the lack of an effective immune response that allows for the killing of all the incoming parasites leaving some to develop to the adult stage. Therefore, it is possible that the observed infection levels, after treatment, may be due to the, yet, ineffective immune response of the young individuals to new incoming infection in these endemic areas [26, 27].
Heavily infected individuals have most of the clinical consequences of schistosomiasis and are the major source of infection for the rest of the community [19]. Anemia is more common in children with moderate to heavy S. mansoni infection and the prevalence of hepatomegaly increases in heavily infected children [28]. High and moderate intensity of infection are also associated with increased risk of morbidity in schistosomiasis such as liver fibrosis, and genitourinary and gastrointestinal abnormalities [28–33].
However, there is increasing evidence demonstrating that light infections are epidemiologically important since they contribute significantly to maintain the transmission cycle [34].
The CART analysis also showed that besides moderate to heavy pre-treatment infection intensities, other risk factors were important in predicting reinfection. Several water contact activities, specifically washing parts of the body, were identified as important risk factors for reinfection as shown by the ranking of its discriminatory power and its appearance as a main “splitter” in the first tree (Fig 3). These activities are often associated with a short duration and medium degree of body exposure compared to other water contact activities such as bathing, swimming, playing and washing clothes and utensils.
Studies in Brazil evaluating the risks of infection by Matoso et al. [5] showed an increased risk in children and young adults who fished and crossed streams. Similar observations with schoolchildren have been shown by Massara et al. [35], who observed that activities with both high and low water contact exposure (e.g., bathing, fishing, crossing streams, working in agriculture, watering plants and working in sand extraction) were significantly associated with infection. Studies with school age children in Sub Saharan Africa were done mostly with S. hematobium and showed controversial results. Satayathum et al. [36] found that the frequency of water contact was not a predictor of risk for S. hematobium infection or reinfection. On the other hand, Rudge et al. [37] observed that particular activities associated with a long duration and a high degree of body surface exposure (e.g., swimming, bathing and fishing) were strongly associated with S. hematobium infection. These results suggest that the frequency, duration, and the percentage of body exposure in water do not need to be exceptionally high to result in high infection rates. In our CART analysis, washing parts of the body was observed to be an important risk factor for reinfection in children with.moderate/high intensity of infection.
Another important risk factor observed was SES classification. This factor was detected both on the first and second tree of the CART analysis. In the second tree, two other socioeconomic indicators (education of household head of family and type of floor) also showed that lower SES classification was an important determinant for reinfection, regardless of infection intensity at baseline. One possible explanation is that lower SES classification reduces access to safe water supplies and sanitation as well as to health care, thus increasing the risk of reinfection and morbidity [31]. It is important to note that the great majority of schistosomiasis infected people live in marginalized, low-income, resource-limited regions with inadequate sanitation [38, 39]. This situation reaffirms what is known about the relationship between social resources, economic status, and schistosomiasis infection at the community and the household level, particularly among school children in low-income localities as shown in a meta analysis by Houweling et al. [40]. Brazil has numerous low-income places, where schistosomiasis has long be in transmission, further supporting our findings [41, 42].
In summary, the most important risk factors for reinfection among school age children as determined by our CART analysis were intensity of infection at baseline, lower SES classification, and the head of the households level of education, all of which are useful and easily obtained indicators of risk of reinfection. As such, efforts to control schistosomiasis reinfection can be stratified to target the treatment and monitoring of such at risk children. For example, when baseline classification has been determined, school aged children with moderate to high intensity of infection may be a priority for treatment, as this group has a greater likelihood of becoming reinfected than other children in the community. In the absence of classification, surrogate indicators of reinfection such as low household economy or the low level of education of the head of the household may enabled targeted treatment or monitoring. In this case, the data generated by census (such as by the Instituto Brasileiro de Geografia e Estatística-IBGE) could contribute to screening the regions where income and education are low and through socioeconomics questionnaires would be identified target families for treatment. This analytic approach may help to guide the Brazilian government's schistosomiasis control program action proposal of which calls for treating communities in high-risk areas to reduce disease transmission and morbidity. The ranking of independent factors may help prioritize prevention and control efforts focusing on specific factors and thus facilitate planning and organizing efficient and cost-effective strategies in the prevention and control of schistosomiasis.
Supporting information
(DOC)
(SAV)
Data Availability
All relevant data are in the paper and its Supporting Information files.
Funding Statement
This study was funded by National Institutes of Health - Tropical Medicine Research Centers (TMRC, grant number AI098507), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Instituto Nacional de Ciência e Tecnologia em Doenças Tropicais (INCT-DT, grant number 465229/2014-0), Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). The TMRC program supports tropical medicine research in endemic international areas and in populations most affected. CNPq and FAPEMIG support research in all different scientific areas and support studies on tropical diseases. CAPES supported fellowships for: Rodrigo Correa-Oliveira (Universidade Federal de Ouro Preto – UFOP)-Senior Fellowship and Roberta de Oliveira Prado-Senior Postdoctoral fellowship (PNPD2256/2011). Leonardo Ferreira Matoso received Junior Postdoctoral fellowships from CNPq (151240/2013-2) and CAPES (PNPD/CAPES) and Gisele Nepomuceno Andrade received support from Ciências sem Fronteiras-CNPq (234399/2014-7). Only the authors Andréa Gazzinelli and Rodrigo Corrêa-Oliveira were awardees by TMRC grant for the development of this study. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Taylor M. Global trends in schistosomiasis control. Bull World Health Organ. 2008;86(10):738 doi: 10.2471/BLT.08.058669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO—World Health Organization. Elimination of schistosomiasis from low transmission areas. 2009;1–31.
- 3.Mbanefo EC, Huy NT, Wadagni AA, Eneanya CI, Nwaorgu O, Hirayama K. Host Determinants of Reinfection with Schistosomes in Humans: A Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2014;8(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grimes JET, Croll D, Harrison WE, Utzinger J, Freeman MC, Templeton MR. The Relationship between Water, Sanitation and Schistosomiasis: A Systematic Review and Meta-analysis. PLoS Negl Trop Dis. 2014;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matoso LF, Oliveira-Prado R, Abreu MNS, Fujiwara RT, Loverde PT, Kloos H, et al. Longitudinal analysis of antigen specific response in individuals with schistosoma mansoni infection in an endemic area of Minas Gerais, Brazil. Trans R Soc Trop Med Hyg. 2013;107(12):797–805. doi: 10.1093/trstmh/trt091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagi S, Chadeka EA, Sunahara T, Mutungi F, Justin YK, Kaneko S, et al. Risk factors and spatial distribution of Schistosoma mansoni infection among primary school children in Mbita District, Western Kenya. PLoS Negl Trop Dis. 2014;8 (7):e2991 doi: 10.1371/journal.pntd.0002991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.de Souza Gomes E, Leal-Neto O, de Oliveira FJ, Campos J, Souza-Santos R, Barbosa C. Risk analysis for occurrences of schistosomiasis in the coastal area of Porto de Galinhas, Pernambuco, Brazil. BMC Infect Dis. 2014;14(1):101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gazzinelli A, Correa-Oliveira R, Yang GJ, Boatin BA, Kloos H. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6(4):e1603 doi: 10.1371/journal.pntd.0001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Inobaya MT, Olveda RM, Chau TN, Olveda DU, Ross AG. Prevention and control of schistosomiasis: a current perspective. Res Rep Trop Med. 2014;2014(5):65–75. doi: 10.2147/RRTM.S44274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mwape KE, Phiri IK, Praet N, Dorny P, Muma JB, Zulu G, et al. Study and ranking of determinants of taenia solium infections by classification tree models. Am J Trop Med Hyg. 2015;92(1):56–63. doi: 10.4269/ajtmh.13-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dawaki S, Al-Mekhlafi HM, Ithoi I, Ibrahim J, Abdulsalam AM, Ahmed A, et al. The menace of schistosomiasis in Nigeria: Knowledge, attitude, and practices regarding schistosomiasis among rural communities in Kano State. PLoS One. 2015;10(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li HM, Zhou CH, Li ZS, Deng ZH, Ruan CW, Zhang QM, Zhu TJ, Xu LQ CY. Enterobius vermicularis infection in children in Gaozhou, Guangdong, China. Infect Dis Poverty. 2015;2(4):28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Protopopoff N, Van Bortel W, Speybroeck N, Van Geertruyden JP, Baza D, D’Alessandro U, et al. Ranking malaria risk factors to guide malaria control efforts in African highlands. PLoS One. 2009;4(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yewhalaw D, Legesse W, Van Bortel W, Gebre-Selassie S, Kloos H, Duchateau L, et al. Malaria and water resource development: the case of Gilgel-Gibe hydroelectric dam in Ethiopia. Malar J. 2009;8:21 doi: 10.1186/1475-2875-8-21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tang ND, Erhart A, Speybroeck N, Hung LX, Thuan LK, Hung CT, et al. Malaria in Central Vietnam: analysis of risk factors by multivariate analysis of risk factors by multivariate analysis and classification tree models. Malar J. 2008;7:28 doi: 10.1186/1475-2875-7-28 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Besga A, Gonzalez I, Echeburua E, Savio A, Ayerdi B, Chyzhyk D, et al. Discrimination between Alzheimer’s Disease and Late Onset Bipolar Disorder Using Multivariate Analysis. Front Aging Neurosci. 2015;7:231 doi: 10.3389/fnagi.2015.00231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Esteban C, Arostegui I, Garcia-Gutierrez S, Gonzalez N, Lafuente I, Bare M, et al. A decision tree to assess short-term mortality after an emergency department visit for an exacerbation of COPD: a cohort study. Respir Res. 2015;16:151 doi: 10.1186/s12931-015-0313-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Katz N, Chaves A, Pellegrino J. A simple device for quantitative stool thick-smear technique in Schistosomiasis mansoni. Rev Inst Med Trop Sao Paulo. 1972;14(6):397–400. [PubMed] [Google Scholar]
- 19.WHO Expert Committee on the Control of Schistosomiasis (2001, Geneva Switzerland), World Health Organization. Prevention and control of schistosomiasis and soil-transmitted helminthiasis: report of a WHO expert committee. WHO Tech Rep Ser. 2002;(912):57 p. [PubMed]
- 20.Brasil, Ministério da Saúde, 2008. Esquistossomose. In Vigilância em Saúde: Dengue, Esquistossomose, Hanseníase, Malária, Tracoma e Tuberculose. Série A. Normas e Manuais Técnicos. Cadernos de Atenção Básica n° 21, 2ª Edição (MS/SAB/DAB), Brasília, pp. 48–65.
- 21.Gazzinelli A, Velasquez-Melendez G, Crawford SB, LoVerde PT, Correa-Oliveira R, Kloos H. Socioeconomic determinants of schistosomiasis in a poor rural area in Brazil. Acta Trop. 2006;99(2–3):260–71. doi: 10.1016/j.actatropica.2006.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gazzinelli A, Bethony J, Fraga LA, LoVerde PT, Correa-Oliveira R, Kloos H. Exposure to Schistosoma mansoni infection in a rural area of Brazil. I: water contact. Trop Med Int Health. 2001. February;6(2):126–35. [DOI] [PubMed] [Google Scholar]
- 23.Gwatkin, D R., Rutstein S, Johnson K, Suliman E, Wagstaff, Amouzou A. Socio Economic Differences in Health, Nutrition, and Population –BRAZIL, 2007. [PubMed]
- 24.Kloos H, Rodrigues JCAP, Pereira WR, Velásquez-Meléndez G, LoVerde P, Oliveira RC, et al. Combined methods for the study of water contact behavior in a rural schistosomiasis-endemic area in Brazil. Acta Trop. 2006;97(1):31–41. doi: 10.1016/j.actatropica.2005.08.006 [DOI] [PubMed] [Google Scholar]
- 25.Brieman L, Friedmen J, Olshen R, Stone C. Classification and regression trees. Pacific Grove, CA:Wadsworth,1984. [Google Scholar]
- 26.Black CL, Steinauer ML, Mwinzi PNM, Evan Secor W, Karanja DM, Colley DG. Impact of intense, longitudinal retreatment with praziquantel on cure rates of schistosomiasis mansoni in a cohort of occupationally exposed adults in western Kenya. Trop Med Int Health. 2009;14(4):450–7. doi: 10.1111/j.1365-3156.2009.02234.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Black CL, Mwinzi PNM, Muok EMO, Abudho B, Fitzsimmons CM, Dunne DW, et al. Influence of exposure history on the immunology and development of resistance to human Schistosomiasis mansoni. PLoS Negl Trop Dis. 2010;4(3):e637 doi: 10.1371/journal.pntd.0000637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Samuels AM, Matey E, Mwinzi PNM, Wiegand RE, Muchiri G, Ireri E, et al. Schistosoma mansoni morbidity among school-aged children: A SCORE Project in Kenya. Am J Trop Med Hyg. 2012;87(5):874–82. doi: 10.4269/ajtmh.2012.12-0397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brouwer KC, Ndhlovu PD, Wagatsuma Y, Munatsi A, Shiff CJ. Epidemiological assessment of Schistosoma haematobium-induced kidney and bladder pathology in rural Zimbabwe. Acta Trop. 2003;85(3):339–47. [DOI] [PubMed] [Google Scholar]
- 30.Vennervald BJ, Kenty L, Butterworth AE, Kariuki CH, Kadzo H, Ireri E, et al. Detailed clinical and ultrasound examination of children and adolescents in a Schistosoma mansoni endemic area in Kenya: Hepatosplenic disease in the absence of portal fibrosis. Trop Med Int Heal. 2004;9(4):461–70. [DOI] [PubMed] [Google Scholar]
- 31.King CH, Dickman K, Tisch DJ. Reassessment of the cost of chronic helmintic infection: A meta-analysis of disability-related outcomes in endemic schistosomiasis. Lancet. 2005;365(9470):1561–9. [DOI] [PubMed] [Google Scholar]
- 32.Leenstra T, Acosta LP, Langdon GC, Manalo DL, Su L, Olveda RM, et al. Schistosomiasis japonica, anemia, and iron status in children, adolescents, and young adults in Leyte, Philippines. Am J Clin Nutr. 2006;83(2):371–9. [DOI] [PubMed] [Google Scholar]
- 33.King CH, Sturrock RF, Kariuki HC, Hamburger J. Transmission control for schistosomiasis—why it matters now. Trends Parasitol. 2006;22:575–82. doi: 10.1016/j.pt.2006.09.006 [DOI] [PubMed] [Google Scholar]
- 34.Grenfell RFQ, Silva-Moraes V, Taboada D, de Mattos ACA, de Castro AKS, Coelho PMZ. Immunodiagnostic methods: what is their role in areas of low endemicity? ScientificWorldJournal. 2012;2012:593947 doi: 10.1100/2012/593947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Massara CL, Peixoto SV, Barros Hda S, Enk MJ, Carvalho Odos S, Schall V. Factors associated with schistosomiasis mansoni in a population from the municipality of Jaboticatubas, State of Minas Gerais, Brazil. Mem Inst Oswaldo Cruz. 2004;99(5 Suppl 1):127–34. [DOI] [PubMed] [Google Scholar]
- 36.Satayathum SA, Muchiri EM, Ouma JH, Whalen CC, King CH. Factors affecting infection or reinfection with Schistosoma haematobium in Coastal Kenya: survival analysis during a nine-year, school-based treatment program. Am J Trop Med Hyg. July 2006; 75(1): 83–92. [PMC free article] [PubMed] [Google Scholar]
- 37.Rudge JW, Stothard JR, Basáñez MG, Mgeni AF, Khamis IS, Khamis AN, et al. Micro-epidemiology of urinary schistosomiasis in Zanzibar: Local risk factors associated with distribution of infections among schoolchildren and relevance for control. Acta Trop. 2008;105(1):45–54. doi: 10.1016/j.actatropica.2007.09.006 [DOI] [PubMed] [Google Scholar]
- 38.Lustigman S, Prichard RK, Gazzinelli A, Grant WN, Boatin BA, McCarthy JS, Basáñez MG. A research agenda for helminth diseases of humans: the problem of helminthiases. PLoS Negl Trop Dis. 2012;6(4):e1582 doi: 10.1371/journal.pntd.0001582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gazzinelli A, Correa-Oliveira R, Yang GJ, Boatin BA, Kloos H. A research agenda for helminth diseases of humans: social ecology, environmental determinants, and health systems. PLoS Negl Trop Dis. 2012;6(4):e1603 doi: 10.1371/journal.pntd.0001603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Houweling TAJ, Karim-Kos HE, Kulik MC, Stolk WA, Haagsma JA, Lenk EJ, et al. (2016) Socioeconomic Inequalities in Neglected Tropical Diseases: A Systematic Review. PLoS Negl Trop Dis. 2016;12;10(5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kloos H, Correa-Oliveira R, Oliveira Quites HF, Caetano Souza MC, Gazzinelli A. Socioeconomic studies of schistosomiasis in Brazil: a review. Acta Trop. 2008. Nov-Dec;108(2–3):194–201. doi: 10.1016/j.actatropica.2008.07.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rollemberg CV, Silva MM, Rollemberg KC, Amorim FR, Lessa NM, Santos MD, et al. Predicting frequency distribution and influence of sociodemographic and behavioral risk factors of Schistosoma mansoni infection and analysis of co-infection with intestinal parasites. Geospat Health. 2015. May 18;10(1):303 doi: 10.4081/gh.2015.303 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOC)
(SAV)
Data Availability Statement
All relevant data are in the paper and its Supporting Information files.