Abstract
The aim of this work was to evaluate the effect of feeding management during the first month of life (natural with the mother, NAT, or artificial with milk replacer, ART) on the rumen microbial colonization and the host innate immune response. Thirty pregnant goats carrying two fetuses were used. At birth one kid was taken immediately away from the doe and fed milk replacer (ART) while the other remained with the mother (NAT). Kids from groups received colostrum during first 2 days of life. Groups of four kids (from ART and NAT experimental groups) were slaughtered at 1, 3, 7, 14, 21 and 28 days of life. On the sampling day, after slaughtering, the rumen content was sampled and epithelial rumen tissue was collected. Pyrosequencing analyses of the bacterial community structure on samples collected at 3, 7, 14 and 28 days showed that both systems promoted significantly different colonization patterns (P = 0.001). Diversity indices increased with age and were higher in NAT feeding system. Lower mRNA abundance was detected in TLR2, TLR8 and TLR10 in days 3 and 5 compared to the other days (7, 14, 21 and 28). Only TLR5 showed a significantly different level of expression according to the feeding system, presenting higher mRNA abundances in ART kids. PGLYRP1 showed significantly higher abundance levels in days 3, 5 and 7, and then experienced a decline independently of the feeding system. These observations confirmed a highly diverse microbial colonisation from the first day of life in the undeveloped rumen, and show that the colonization pattern substantially differs between pre-ruminants reared under natural or artificial milk feeding systems. However, the rumen epithelial immune development does not differentially respond to distinct microbial colonization patterns.
Introduction
Ruminants harbor a complex and diverse microbial ecosystem in their rumen that allows them to covert digested plant material into edible high nutritive quality products (meat and milk) [1]. At birth the rumen is not yet developed and functional. The process of bacterial colonization in the developing rumen is key for the achievement of rumen functions, which are a prerequisite for weaning and can thereafter affect efficiency and stability of digestion [2,3]. There is increasing evidence that populations established in early life of the animal may persist into later life, exerting physiological, metabolic and immunological effects, and offering potential of ‘programming’ the microbiome of the adult animal and potentially the animal’s performance [4,5]. However, the window of time in which the ecosystem is most sensitive to alteration is yet unknown and describing the sequential colonization in different feeding systems could help to design efficient intervention strategies to manipulate rumen microbial colonization in early life. Two main systems exist for rearing offspring in ruminant production. In commercial dairy systems, newborns are typically separated from the dam after birth and fed either milk replacer or whole milk; in contrast, in meat and extensive production systems, the offspring remains with the dam until weaning. These two systems therefore imply differences in regards to milk type (whole milk vs. milk replacer) and presence/absence of older companion, which can not be addressed separately.
Ruminant placenta impedes the transfer of Immunoglobulins (Ig) from the dam to the fetus, consequently, the consumption of colostrum by offspring has a fundamental role in the acquisition of passive immunity [6,7]. The Ig are the principal agents that protect the gut epithelium against pathogenic microorganisms, and IgG antibodies express multifunctional activities, including complement activation, bacterial opsonisation and agglutination, and act by binding to specific sites on the surfaces of most infectious agents or products, either inactivating them or reducing infection [8]. However, less clear is how the colonization of commensal microbiota interacts with the host immune system.
The ruminal epithelium is continuously exposed to commensal microbiota, pathogens and dietary antigens and provides a critical barrier between the host and the gut environment. Toll-like receptors (TLRs), peptidoglycan recognition proteins (PGLYRP1), and antimicrobial peptides (β-defensin) have been reported to interact with microbes to maintain gastro-intestinal homeostasis, including the rumen [9]. Toll-like receptors are present on a wide range of cells and can detect conserved molecular products of microorganisms [10]. It is important to understand host-microbiome interactions within the context of individual animal species and specific management practices. Data is now being generated revealing significant associations between the early microbiome, development of the gut immune system and the growth and health of newborns [11]. However, little information is available specific to the rumen ecosystem.
The aim of the present study was to evaluate the effect of feeding management during the first month of life (natural feeding with the dam, NAT, or artificial with milk replacer and isolated from adult animals, ART) on the sequential rumen bacterial colonization and the associated host immune response.
Material and methods
All management and experimental procedures involving animals were performed by trained personnel in strict accordance with the Spanish guidelines (RD 1201/2005 of October 10, 2005) for experimental animal protection at the Estación Experimental del Zaidín (CSIC). Experimental protocols were approved (October 1, 2012) by the Ethics Committee for Animal Research at the Animal Nutrition Unit.
Diets
Twenty eight pregnant Murciano-Granadina goats carrying two fetuses were selected and kept in individual pens (1.7 × 1.2 m) with free access to water. They were fed alfalfa hay ad libitum once per day (in g/kg of DM: OM, 880; CP, 214; ether extract, 13.6; NDF, 419: ADF, 244; and ADL, 61) and a supplement 600 g/d fed twice per day (0900 and 1500 h) based on (g/kg) wheat shorts (350), corn shorts (100), corn grain (50), barley grain (160), soybean hulls (90), soybean meal (90), sunflower meal (120), CaO (22), NaCl (3.5), calcium salts (4.5), and trace minerals and vitamins supplement (10; in g/kg of DM: OM, 893; CP, 170; ether extract, 33.9; NDF, 342; ADF, 142; and ADL, 34.3).
Kids had free access to the same hay and were offered ad libitum a commercial starter concentrate based on (g/kg): wheat shorts (50), corn shorts (50), corn grain (150), oat grain (260), milk powder (190), soybean meal (172), sunflower meal (120), NaCl (3.5), and calcium salts (4.5; in g/kg of DM: OM, 925; CP, 162; ether extract, 35; NDF, 163; and ADF, 78).
Experimental design
The experimental period commenced after parturition. All does gave birth to two kids, one of which remained with the dam (NAT) while the other was taken immediately away and fed colostrum for 48 hours and milk replacer (ART) (Sello azul, Lemansa, León, Spain). Colostrum from each goat mother was fed to both of her twin kids. Groups of four kids (from both ART and NAT experimental groups) were randomly selected and slaughtered at 1, 3, 5, 7, 14, 21 and 28 days of life. On the sampling day blood samples were taken for Ig quantification in plasma. After slaughtering, rumen content was sampled and aliquots immediately stored at -80°C for volatile fatty acids (VFA) [4] and molecular analyses. Samples of the rumen epithelium (approximately 500 mg) were biopsied, from the ventral sac and washed immediately after collection with 0.01 M phosphate-buffered saline (PBS) buffer (pH 6.8). The samples of cleaned tissue were then transferred to RNA-later solution (Qiagen Ltd, West Sussex, UK) and stored at −80°C until further analysis. Rumen digesta samples from day 1 contained very low volume and could only be used for VFA analyses.
Pyrosequencing and sequence analysis
Total DNA was extracted from 0.5 g of each sample collected on days 3, 7, 14 and 28 using QIAGEN QIAamp® DNA stool mini kits (Qiagen Ltd, West Sussex, UK). The yield and purity of the extracted DNA were assessed using NanoDrop® ND-1000 Spectrophotometer (NanoDrop Technologies, Wilmington, USA). Amplification of the bacterial V1-V3 regions of 16SrRNA was performed using the primer pair 27F and 533R (27F 5' GTT TGA TCC TGG CTC AG 3' and 533r 5' TTA CCG CGG CTG CTG GCA C 3'). Primers incorporated 10nt barcode tags allowing samples to be multiplexed and Roche/454 adaptors. PCRs were performed, per triplicate, in a total volume each one of 25 μL containing 10x PCR buffer, 10 mM dNTP mix, 10 pmol/μL of forward and reverse primers, 1U FastStart Polymerase, and 1 μL of DNA template. The amplification conditions were: an initial denaturation step at 95°C for 2 min; 30 cycles of denaturation at 95°C for 30s, annealing at 55°C for 30s, and elongation at 72°C for 2 min; and a final extension step at 72°C for 7 min The size of the PCR products was then checked on a 1% agarose gel electrophoresis. Following this, triplicates were pooled together and products were then purified using the short fragment removal method described by Roche using their GS FLX amplicon DNA preparation guide and AMPure beads. The purified PCR products were quantified using Quant-iT PicoGreen dsDNA quantification kit (Invitrogen) and mixed in equimolar amounts to 107 molecules/ μL sample. The amplicon pooled libraries were pyrosequenced on a Roche 454 FLX Titanium.
Sequence preprocessing and statistical analysis
Short read sequence data generated was analyzed using QIIME: Quantitative Insights Into Microbial Ecology software package [12]. Raw sequences were passed through Acacia for 454 error correcting [13]. Error corrected sequences were then de-multiplexed in QIIME based on their unique barcode, clustering of sequences to OTUs of 97% similarity were performed using closed-reference OTU picking method uclust [14]. Taxonomic assignment of sequences was performed against the Greengenes database [15] using the RDP classifier software [16]. Alpha and beta diversity and significant change of OTU’s were performed in the R packages ade4, Phyloseq, and vegan [17,18,19]. The significances of grouping in the PCoA plots were tested by analysis of dissimilarity (ADONIS) with 999 permutations. The sequences obtained in this paper were deposited in the European Nucleotide Archive (ENA) under the project number PRJEB20457.
RNA extraction and gene expression quantification
Total RNA samples were extracted from 50–100 mg of rumen epithelial samples. They were homogenized with 0.9 mm stainless steel bead and 1 ml of TRIzol Reagent (Invitrogen, CA, USA), using a Bullet Blender® homogenizer (Next Advance, Inc. NY, USA). The homogenate was incubated for 5 min at RT to allow dissociation of nucleoprotein complexes before adding 200 μL chloroform mL–1 of TRIZOL containing tissue homogenate. The solution was mixed briefly, incubated for 3 min at RT, and then centrifuged at 12 000 × g for 10 min at 4°C. The aqueous phase was collected, precipitated with 500 μL isopropanol mL–1 of TRIZOL containing tissue homogenate, incubated for 5 min at RT and then applied to an RNeasy Mini-column (Qiagen, UK) before spinning for 15 s at 8000 × g. The flow through was discarded and RNA bound to the column was DNase treated using RNase-Free DNase Set (Qiagen) and collected in a 50 μL volume. The RNA quantity was measured using ND 1000 spectrophotometer (NanoDrop Technologies, Wilmington) and RNA integrity number was measured using Bioanalyzer 2100 (Agilent Technologies, Santa Clara, CA).
The extracted total RNA was reversed transcribed using QuantiTect Reverse Transcription Kit (Qiagen, UK) according to the protocol. The RNA was used as template for qRT-PCR analysis to evaluate the expression of genes encoding 10 bovine TLR, β-defensin and peptidoglycan recognition protein 1 (PGLYRP1) in the rumen epithelium using the gene specific primer pairs (S1 Table) relative to β-actin expression. For each PCR reaction, 25 ng of cDNA was amplified with each primer set using the following parameters: 55°C for 2 min to eliminate carry-over dUTP, 95°C for 8.5 min, then 45 cycles of 95°C for 15 s; 58°C for 30 s; and 72°C for 30 s on the Bio-Rad iCycler (Bio-Rad Laboratories Ltd., Mississauga, ON). Amplification data are expressed as change in Cycle threshold (ΔCt) and calculated as follows: (ΔCt = Cycle threshold of Gene of Interest—Cycle threshold of β-actin). A smaller ΔCt value equates to more abundant transcript.
Quantification of immunoglobulins
Plasma samples were centrifuged at 3000 × g for 5 min at 4°C just before the analysis. Immunoglobulin A and G were measured from plasma using an ELISA quantification kit from Bethyl Laboratoires (Montgomery, TX), following the manufacturer´s instruction. IgG determination in plasma samples were determined between 1:150000 and 1:50000 dilutions for 3–7 d and 14-28d, respectively. IgA determination in plasma samples was determined between 1:1000 and 1:200 dilutions at 3 and 14 or 28, respectively. Data were expressed as mg or μg per millilitre of sample for IgG or IgA, respectively. Duplicate determinations were performed on each plate.
Statistical analyses
Data were analyzed using the SAS PROC MIXED procedure (SAS Inst. Inc., Cary, NC). The statistical model used included the effects of feeding management to kids, the age and the feeding management × age interaction as fixed effects. Animal effect was considered random. When feeding management × age interaction was significant (P < 0.05), differences between treatment means were evaluated using the pdiff option of the LS means statement in the MIXED procedure of SAS and declared significant at P < 0.05. A tendency (T) was considered when P-values were <0.1.
Results
Bacterial community
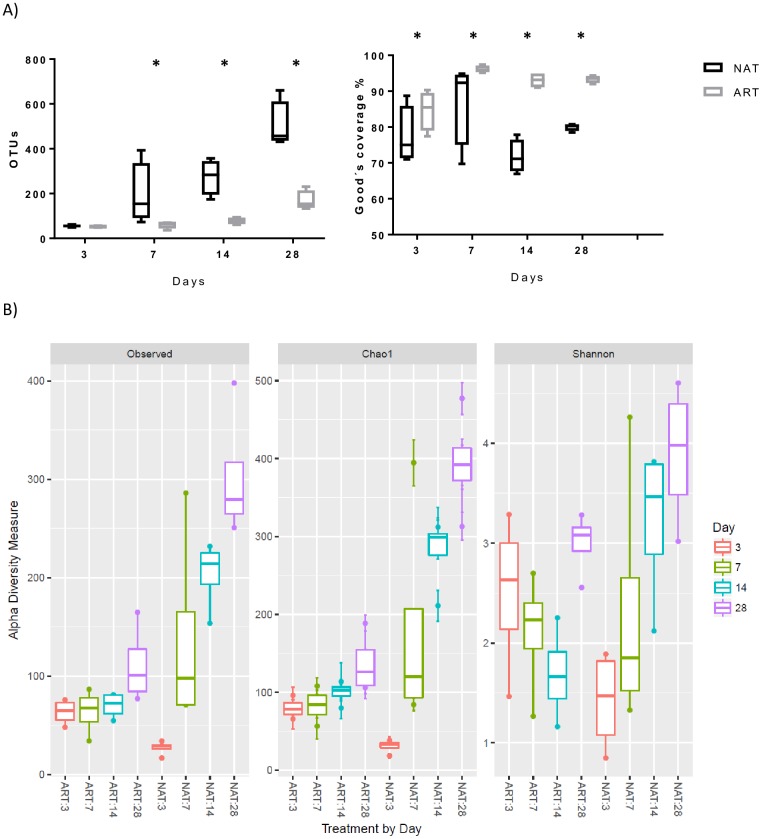
A total number of 141197 reads, with an average of 4412 ± 2464 sequence reads were observed per sample. The average length of sequence reads after primer removal was 470 bp. The overall number of OTUs detected by the analysis reached 1556, based on 97% nucleotide sequence identity between reads and increased with age. Rumen contents from NAT kids presented greater number of OTUs than in ART kids from day 7 (Fig 1A). The bacterial diversity (measured as different indexes, Fig 1B) increased significantly with age and was greater in NAT kids.
Fig 1. Alpha diversity measures for rumen bacteria across different feeding system the first month of life.
Total Observed taxonomic units, Chao1 estimates and Shannon diversity index. Boxplots indicate the first and third quartiles with the median value indicated as a horizontal line the whickers extend to 1.5 times the inter quartile range. NAT, natural; ART, artificial feeding management.
OTU diversity and similarity analysis
When assessing the data by principle coordinate analysis, a clear pattern was observed according to feeding management (Fig 2A) (P = 0.001) and age (Fig 2B). Pairwise comparison between different ages of the animals showed that day 3 presented a significantly different bacterial community (P = 0.001). Clear differences between the community at days 7 and 28 were found (P = 0.004).
Fig 2. Principal coordinate analysis (Bray Curtis distance) comparing changes in rumen bacterial community.
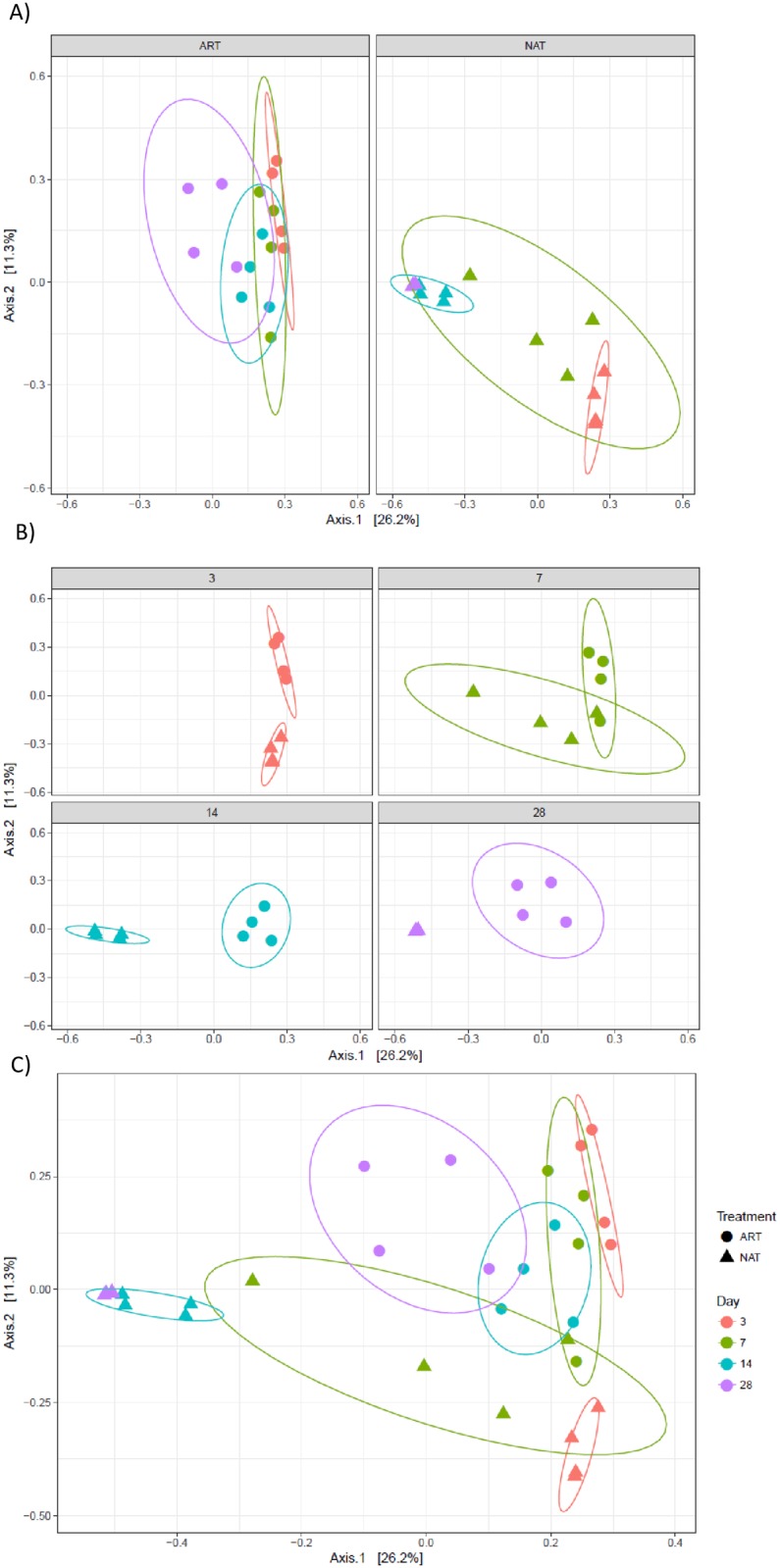
(A) age within feeding system (NAT or ART). (B) feeding system within each age (3, 7, 14 and 28 days). (C) age and feeding system.
Taxonomic composition of ruminal bacterial community
Overall, the abundance of 17 phyla was detected in rumen content samples, these were Actinobacteria, Bacteroidetes, Chloroflexi, Cyanobacteria, Elusimicrobia, Fibrobacteres, Firmicutes Fusobacteria, Lentisphaerae, Proteobacteria, Spirochaetes, SR1, Synergistetes, Tenericutes, TM7, Verrucomicrobia and WPS-2. Among them, Proteobacteria, Firmicutes and Bacteroidetes were identified as the dominant phyla regardless of the age group, but their ratio and composition differed considerably between NAT and ART kids. In both experimental groups Bacteroidetes and Proteobacteria were the two main phyla that colonized the rumen at day 3, reaching proportions as high as 73 and 67% of total sequences, for NAT and ART, respectively. Afterward, the relative abundance of Proteobacteria declined rapidly with age. In contrast, Firmicutes, Fibrobacter and Cyanobacteria increased with age significantly. The Fusobacterium peaked at day 7 (P = 0.03). The phylum Bacteroidetes was the dominant from day 7 onwards in both feeding managements. The relative abundance of Spirochaetes phylum was significantly higher in NAT feeding system (P = 0.014).
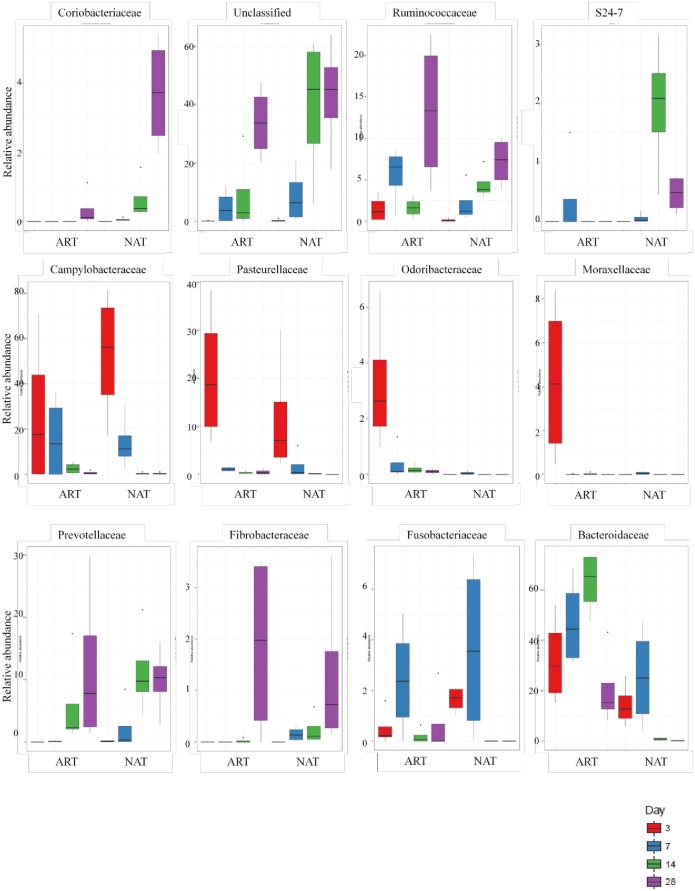
Of the 88 families detected, 15 changed significantly over the experimental period (Fig 3). The Coriobacteriaceae, Ruminococcaceae, S24-7, Prevotellaceae, Fibrobacteraceae and Alcaligenaceae families increased with time. Similarly, unclassified sequences abundance increased with age (P = 0.001). Conversely, Campylobacteraceae, Pasteurellaceae, Odoribacteraceae and Moraxellaceae abundance decreased with time. The abundance of Bacteroidaceae family was affected by feeding system (P = 0.0001). The effect between feeding system and age modified Coriobacteriaceae and S24-7 families, being more abundant in NAT feeding as animals were older.
Fig 3. Family level taxonomic composition of the ruminal bacterial community at different ages of kids (% of sequences).
Of the 132 genera detected, 17 were over 0.1% relative abundance and changed significantly over the experimental study (S1 Fig). The Aggregatibacter, Actinobacillus, Arcobacter, Acinetobacter, Butyricimonas, Haemophilys and Mannheimia relative abundance decreased very rapidly with age. In contrast, Fibrobacter, Moryella, Mogibacterium, Prevotella, Ruminococcus, Succiniclasticum, Sutterella and TG5 genera increased with age. The Bacteroides and Fusobacterium were dominant in their families and followed the same pattern as described for the family, similarly to unclassified sequences. The genus Moryella, Bacteroides and Succiclasticum were affected by feeding system, Bacteroides being higher in ART (P = 0.0002) and Succiniclasticum (P = 0.03) in NAT feeding system. The relative abundance of Moryella and TG5 genus was significantly higher in NAT feeding system with older kids (P = 0.0003 and P = 0.03, respectively). On the contrary, Actinobacillus genus peaked at day 3 in the rumen of ART kids (P = 0.03).
TLRs, β-defensin and PGLYRP1 expression
The expressions level of genes of interest in the rumen epithelia of kids were measured using qRT-PCR relative to β-actin expression (Malmuthuge et al., 2010) using the ΔCt value. Expression of the 10 TLR was detected from day 3 (Table 1). The lowest expression levels corresponded to TLR 2 and 5, while the highest was TLR6. Lower mRNA abundance was detected in TLR2, TLR8 and TLR10 in days 3 and 5 compared to the other days (7, 14, 21 and 28). Only TLR5 showed a significantly different level of expression according to the feeding system, presenting higher mRNA abundances in ART kids. PGLYRP1 showed significantly higher abundance levels in days 3, 5 and 7, and then experienced a decline independently of the feeding system.
Table 1. Expression of 10 TLRs, β-defensin and peptidoglycan recognition protein 1(PGLYRP1) in rumen of kids at 3, 5, 7, 14, 21 and 28 days old fed on two feeding systems (Natural and Artificial).
ΔCt = Ct (TLRs) − Ct (βactin), lower ΔCt represent higher mRNA abundance level and vice versa.
| Feeding | Age | TLR1 | TLR2 | TLR3 | TLR4 | TLR5 | TLR6 | TLR7 | TLR8 | TLR9 | TLR10 | β-defensine | PGLYRP1 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Natural | 3 | 11.52 | 23.92 | 12.01 | 9.64 | 22.34 | 9.95 | 10.81 | 15.04 | 11.77 | 17.00 | 16.78 | 10.81 |
| 5 | 11.51 | 24.19 | 12.79 | 10.53 | 24.03 | 9.58 | 11.18 | 14.88 | 12.21 | 16.60 | 15.58 | 10.74 | |
| 7 | 10.11 | 18.90 | 10.89 | 9.32 | 23.97 | 8.76 | 9.12 | 11.73 | 10.31 | 14.31 | 15.80 | 10.58 | |
| 14 | 11.40 | 20.36 | 10.85 | 10.58 | 25.22 | 8.61 | 12.42 | 12.76 | 13.17 | 15.04 | 17.63 | 15.44 | |
| 21 | 11.31 | ND | 11.95 | 10.71 | 23.69 | 10.40 | 12.64 | 13.05 | 11.33 | 15.21 | 15.11 | 15.48 | |
| 28 | 11.87 | 19.62 | 10.66 | 10.33 | 22.15 | 9.07 | 9.71 | 13.10 | 10.47 | 15.71 | 15.38 | 14.53 | |
| Artificial | 3 | 10.48 | 24.79 | 10.53 | 9.99 | 20.78 | 8.59 | 10.95 | 14.45 | 11.45 | 16.47 | 15.79 | 10.70 |
| 5 | 10.58 | 24.74 | 10.94 | 10.15 | 20.86 | 8.88 | 10.98 | 14.07 | 12.17 | 16.78 | 15.21 | 10.44 | |
| 7 | 11.07 | 19.97 | 11.44 | 10.18 | 23.72 | 8.86 | 10.54 | 13.41 | 11.51 | 15.89 | 15.33 | 11.31 | |
| 14 | 11.62 | 18.99 | 11.86 | 10.68 | 22.63 | 9.01 | 10.31 | 13.50 | 11.18 | 15.82 | 15.87 | 11.81 | |
| 21 | 11.28 | ND | 10.72 | 10.15 | 23.04 | 9.23 | 10.57 | 13.21 | 11.08 | 16.15 | 15.00 | 14.49 | |
| 28 | 11.64 | 20.19 | 10.68 | 10.41 | 23.23 | 9.43 | 10.60 | 13.89 | 11.37 | 15.68 | 15.10 | 14.89 | |
| SED | 0.638 | 1.121 | 0.916 | 1.092 | 1.384 | 0.612 | 1.814 | 1.122 | 1.487 | 0.831 | 1.249 | 1.721 | |
| P-value | Feeding | 0.47 | 0.49 | 0.13 | 0.88 | 0.0235 | 0.078 | 0.61 | 0.41 | 0.87 | 0.10 | 0.13 | 0.28 |
| Age | 0.081 | <0.0001 | 0.39 | 0.51 | 0.06 | 0.15 | 0.54 | 0.0201 | 0.52 | 0.0108 | 0.22 | <0.0001 | |
| Interaction | 0.18 | 0.62 | 0.099 | 0.85 | 0.16 | 0.065 | 0.49 | 0.43 | 0.56 | 0.29 | 0.89 | 0.39 |
SED: standard error of difference.
ND: Not determined
Immunoglobulin concentration
The IgG levels in plasma (Table 2) were affected by feeding system and age, being higher the first 7 days of age in NAT group compared to ART, and then became progressively similar from day 14 onwards. Feeding system did not have any effect on IgA levels in plasma of kids; however the titers were highest on days 1 and 3 and started declining from day 5 onwards.
Table 2. Immunoglobulin A and G were measured from plasma at different ages and feeding systems in kids.
Results were expressed as mg/ml and μg/ml for G and A, respectively.
| Feeding | Age | IgG | IgA |
|---|---|---|---|
| Natural | 1 | 27.4a A | 157.5A |
| 3 | 20.9 A | 181.3A | |
| 5 | 10.8 BC | 122.1B | |
| 7 | 14.5a B | 82.4BC | |
| 14 | 4.5 C | 16.8C | |
| 21 | 3.8 C | 16.7C | |
| 28 | 3.5 C | 3.7C | |
| Artificial | 1 | 15.8b | 192.2 |
| 3 | 17.01 | 187.9 | |
| 5 | 5.9 | 92.0 | |
| 7 | 8.9b | 104.7 | |
| 14 | 5.9 | 95.5 | |
| 21 | 3.8 | 9.7 | |
| 28 | 3.8 | 6.7 | |
| SED | 3.039 | 28.76 | |
| P Value | Feeding | 0.002 | 0.254 |
| Age | <0.0001 | <0.0001 | |
| Feeding x Age | 0.034 | 0.504 |
SED: standard error of difference.
a–cWithin a column, means without a common superscript letter differ according to feeding;
A–CWithin a column, means without a common superscript letter differ according to age, p ≤ 0.05 (LSD test).
Discussion
This experiment used the two main offspring rearing systems in ruminant livestock to assess whether this differential management exerted any effect on rumen bacterial colonisation and innate immune response. The aim was not to compare the effect of two milk types (natural vs. milk replacer) with inherent differences in composition, but the whole management system, which also accounts for presence/absence of the dam. A previous work from the same experimental trial [20] described the effects on bacterial, protozoal and archaeal biomass colonization, rumen development and fermentation. The results confirmed substantial microbial colonisation from the first day of life in the undeveloped rumen. Moreover, the concentration of bacteria was higher in the rumen of NAT kids on days 3, 5, 7 and 14 and of protozoa from day 3 onward. The objective of the present work was to complement the previous observations by describing the sequence of colonization of bacterial groups to confirm a differential pattern between two feeding systems and the link with the host immune response.
Rumen bacterial community composition and diversity indices
In ruminants, the gastrointestinal tract, including the forestomach complex, is sterile at birth but it is rapidly colonized after. Previous work emphasizes the essential role that the dam plays as a ‘microbial inoculator’ [21] from several sources: vaginal canal, fecal material, colostrum, skin and saliva. The primo-colonizing bacteria may play an important role in shaping the biotope for strictly anaerobic microbial populations colonizing rapidly afterward [22]. Our results are consistent with those reported recently [23–26], microbial diversity and species richness increased over time when microbiota becomes more mature and stable as the animal is aging. In the NAT feeding system group, diversity was higher and the community structure different from ART group in which animals were separated from the doe after birth.
Based on the current analysis, the dynamic of the bacterial community establishment in kids could be divided in three steps, including a progressive change in the bacterial community composition. The first 2–3 days of life correspond to the initial phase of colonization corresponding to the pioneer colonizing bacteria [27] and the profile of bacterial community was completely different from all the later ages, as it was described at day 2 by Rey et al. [28]. Bacteria belonging to Proteobacteria, Bacteroidetes, Firmicutes, Actinobacteria and Fusobacteria phyla were present at this stage, and the Proteobacteria phylum represented more than 50% of total bacteria. These phyla comprise both obligate and facultative anaerobic bacteria genera, which is consistent with other authors [28,29,30] who also observed a large proportion of both facultative and strictly anaerobic bacterial communities between 2 and 3 days of age of ruminants. In goat kids, Jiao et al. [20] noted that on the day of birth, measurements of the phylum Proteobacteria far exceeded other phyla (90.13%), with the majority belonging to the genus Escherichia (80.8%) that comprises facultative anaerobic bacteria. This microbiota might be derived from that of the mother’s vagina, skin, colostrum, or nearby environment, and may have a particular importance in scavenging oxygen diffusing from the capillary network, thereby creating ecological conditions suitable for the establishment of anaerobic communities [31]. In our work, Arcobacter was present in high proportion during the first week of life in agreement with Jiao et al. [25], who found this genus in the rumen epithelial bacteria community of goats at the first week of life. Arcobacter is aerotolerant and the majority of these bacteria are oxidase-positive microaerophiles indicating an electron transport chain with molecular oxygen as terminal electron acceptor [32].
In the second stage of colonization kids switched from colostrum to mother´s milk (NAT) or milk replacer (ART). Jiao et al. [25] observed remarkable changes in ruminal epithelial bacterial communities during the first week after birth with a rapid change in the rumen environment as it was observed in our study in rumen content from goats. This change was probably due to the ‘pioneer’ community that allowed the establishment of other bacteria and plays an important function in the early maturation of the microbial community. Thus changes in the rumen environment were probably related to a switch from aerobic or facultative anaerobic to strictly anaerobic bacteria, as previously described by Jami et al. [23] in colostrum fed calves. After day 3, the bacterial colonization presented large differences in the community assemblies among individual kids, supporting the concept of a interaction between the microbiota and the host [28,33,34].
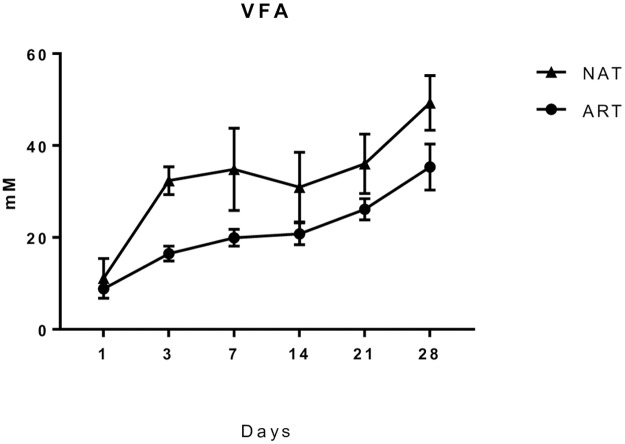
The third stage of colonization covers from day 14 to 28 or weaning. During this stage, starter concentrate intake (and some small pieces of forage) progressively increased their proportion in the diet and bacterial community presented a fluctuation according to two main elements: feeding behavior and probably the presence/absence of adult animals. From day 14, the community no longer exhibited clear time-related changes. As noted earlier, at this stage, the concentrate intake by kids increased and this was reflected in an increase in VFA concentration (Fig 4). It has been suggested that the earlier the animals start consuming solid feed, the quicker their rumen bacterial community becomes more similar to that in adults, probably selecting bacterial microbial taxa that are more specific and adapted to new substrates [35].
Fig 4. Total VFA concentration in the rumen of kids fed on natural or artificial systems.
Jami et al. [23] reported that the matured bacterial community was not detected before month 6 of age, when Bacteroidetes, Firmicutes, and Proteobacteria were among the dominant phyla. However, more recently it has been reported that the colonization by rumen epithelial bacteria is age-related and achieved at 2 months of life [25]. In agreement with previous studies in cattle [23,35], our results suggest that the developing rumen comprises the same dominant phyla as that in the more mature, although the relative abundances vary with stage of development and diet. In our study, Bacteroidetes, Firmicutes, and Proteobacteria accounted for a total of 84.6 and 86.9% in the rumen of NAT and ART feeding group at day 28, respectively, Bacteroidetes being the dominant phylum. This in agreement with previous studies who reported that Bacteroidetes was the most abundant phyla from days 19 to 83 in pre-weaned calves [28] and that the same three phyla were dominant regardless of the age group (0–70 d) in rumen epithelial bacteria [25]. The abundance of Proteobacteria decreased while the abundance of Bacteroidetes and Firmicutes increased with age as it has been also detected in rumen epithelial bacterial diversity in goats [25]. This phylum was dominated by the g. Prevotella despite decreasing in abundance in NAT group. The dominance of g. Prevotella in the mature ruminal community has been reported previously in cattle [36,37,38] and in recent international survey of the rumen and foregut microbial communities in 742 samples collected from 32 ruminant species across 35 countries from different geographical regions [6] reported that the most abundant and prevalent taxa within the rumen microbiota was Prevotella genus. The Bacteroides genus was present in high abundance in the rumen of ART group from day 3 to 28. Our results indicate that Bacteroides is linked to the consumption of high caloric milk replacer in pre-weaned kids, as it was also described in pre-weaned calves by Meale et al. [35]. They linked the carbohydrate composition of the diet during the transition from milk replacer to starter with changing the ratio of Bacteroidetes to Firmicutes in the rumen.
With regards to the bacterial colonization pattern in NAT vs. ART kids, some differences were detected. Fibrobacter (one of the main cellulolytic phyla in the rumen) was present in NAT group at day 7 onward; however it did colonize the rumen of ART group until day 28, similarly to Succiniclasticum which ferment succinate to propionate. However, the abundance of these phylums is highly variable among individual animals and diets [6], this positively correlated with concentrate intake and VFA concentration. The greater VFA concentration observed in the rumen of NAT kids may suggest a relationship between the abundance of Moryella sp. with total VFA production as the major metabolic end product are acetic, butyric and lactic acids. However, it remains unknown whether the distinct bacterial colonization between animals reared naturally or artificially may have effects on the animal digestive performance later in life.
The differences observed in rumen bacterial colonization between NAT and ART kids may be ascribed to different factors: intake pattern of solid feed and the presence (NAT)/absence (ART) of the dam as inoculator of rumen bacteria through saliva, faces, etc… De Paula Vieira et al. [39] observed that the presence of an older companion with pre-weaned calves stimulated feeding behavior and growth before and after weaning from milk. A differential pattern in solid feed intake between NAT and ART kids may have provided a different substrate to ferment and therefore different bacterial groups promoted. The pattern of VFA concentration in the rumen as animals aged (Fig 4) suggests that NAT kids had greater feed intakes than ART ones. This is supported by results from De Paula Vieira et al. [39], which showed that calves reared in the presence of older companions had more frequent and longer visits to the feeder, which could be a result of social learning [40]. Anderson et al. [41] showed that introducing solid feed for early weaning (3 weeks) in calves promoted greater microbial abundance in the rumen as compared to calves weaned conventionally (6 weeks), but no assessment of the composition of the microbiota was performed. Early studies [42,43] reported that giving forage or forage plus concentrate around weaning determined the concentration of some anaerobic bacteria (lactobacilli and lactate-utilizing cocci).
The second factor that may promote a different colonization according to feeding management is the presence of dam and the associated increased in the availability of microorganisms in the environment, allowing earlier (and different) inoculation of microbes in the digestive tract of the NAT kids as compared to ART ones. Previous work using twins emphasized the essential role that the dam plays as a ‘microbial inoculator’ [21]. A direct contact with the mother offers a constant source of microbes through mouth, faeces, skin and milk, which are not available for the artificially reared kids. This would explain the greater number of OTUs and bacterial diversity observed in NAT kids. Another distinctive feature between NAT and ART kids was the almost absence of protozoal biomass in the rumen of artificially reared kids, as protozoa can only be inoculated in the rumen by direct contact with adult animals through saliva [20]. Protozoa are not essential for the normal rumen functioning [44]; however, the presence/absence of protozoa has been associated with the structure of different bacterial and methanogens communities and different rumen fermentation pattern [45,46]. For example, rumen protozoa have been associated with greater butyrate concentration [47], and butyrate is one of the main energy sources of epithelial cells and therefore promotes the development of the rumen papillae absorptive area [48], which could represent a clear advantage to overcome the stress associated with transition from liquid to solid diet at weaning. Indeed, supplementing butyric acid to young animals during pre-weaning has been shown to adapt rumen to postpartum diet and accelerate rumen development [49–50]. Although it has been shown that inoculation of protozoa later in life (either artificially or by grouping young animals with faunated ones) is possible, it remains unknown whether the indirect effect on the microbial community persists later in life [2].
TLRs, β-defensin and PGLYRP1 expression
TLRs comprise a family of pattern-recognition receptors that detect conserved molecular products of microorganism, such as lipolysaccharide (LPS) and lipoteichoic acid (LTA). Viruses, bacteria, fungi and protozoa display several different molecular patterns that are recognized by different TLRs [51]. In the present study, TLRs 1, 2, 5, 8 and 10 displayed an age-dependent expression. The general pattern involved an increased in gene expression between days 5 and 7 and then a subsequent decrease and stabilization. The increase in expression coincides with the sharp raise in VFA concentration and microbial biomass colonization. However, although colonization continued in following days, this did not trigger higher expression levels. This is in agreement with Malmuthuge et al. [9] that reported an age-related down-regulation of the expression level of TLRs in the gastrointestinal tract of calves. Likewise, Teran et al. [52] found that the TLR expression levels in the blood of infants were down-regulated with increasing age, while memory of T cells increased in number. These changes are consistent with a decrease in innate immune responses that is balanced by an increase in adaptive immune response with increasing age. Down-regulation with increasing age has been suggested as one mechanism by which the host avoids unnecessary inflammatory responses to commensal microbiota [11] and our results support such hypothesis. TLR2 play very important roles in the host response to the cell wall components of pathogenic gram-positive bacteria, which can directly affect gut health [53] and also recognizes another component, bacterial lipopeptide, alone or cytoplasmic domain of TLR2 can form functional pairs with TLR1, TLR6 or TLR10 [54]. Interestingly, the two main phyla that colonized the rumen (Bacteroidetes and Proteobacteria) are mainly gram-negative. However with age, proportions of colonizing groups changed: Proteobacteria decreased and Firmicutes increased, most of which are gram-positive. Our results suggest that variations in bacterial population with time may represent variations in bacterial ligands which could play an important role in modulating TLRs expression and innate mucosal immune responses. Although TLRs do not, as a rule, exhibit specificity for a single microbial product, they are individually responsive to a limited group of molecules.
The observed increased in TLR10 expression as rumen developed might be an indication of variation in the gut maturity of animals, suggesting that higher expression of TLR10 might be very important in early epithelial immune system development. As TLR10 is a link between adaptive and innate immune responses [55], further studies on TLR10 and its functions in the gastrointestinal tract may lead to better understanding of the role of TLR10 in gastrointestinal tract health.
The expression of TLR5 was the only one that significantly differed between feeding systems, although mostly in the first week of life. TLR5 recognises bacterial flagellin, a principal component of Gram-positive and Gram-negative bacterial flagella. The interaction between TLR5 and flagellin also leads to the expression of antiapoptotic genes that are correlated with the protective effect of the receptor against normal commensal such as E. coli [56].
In an attempt to identify links between TLR expression and microbial colonization, we assessed potential correlation with the abundance of different bacterial groups and no significant relationships were observed (data not included). Apart from differences in bacterial groups abundance, the main feature that can distinguish NAT and ART groups is the presence/absence of protozoa. Unlike bacteria and viruses, protozoans often differentiate within the host into discrete forms that are morphologically and molecularly distinct. Recognition of protozoan pathogens by the host innate immune system presents further challenges, because, like their hosts, they are eukaryotic organisms. Nevertheless, protozoan-associated molecular patterns exist that are recognized by TLRs. These include dominant surface glycolipids (glycosylphosphatidylinositol anchors that are recognized by TLR2 and TLR4) and genomic DNA that activates TLR9 [57]. However, none of these three TLR showed a significant (P > 0.05) correlation with protozoan biomass (r = -0.12, 0.28 and 0.02, respectively for TLR 2, 4 and 9), suggesting that symbiotic protozoa can occupy ecological niches in the rumen without triggering a specific immune response at epithelium level.
The β-Defensin and PGLYRP1 are secretory antimicrobial defense molecules that are capable of recognizing and killing of pathogens [58]. Peptidoglycan recognition protein 1 mainly kills gram-positive bacteria however bovine PGLYRP1 has an affinity to kill fungi, and neutralize lipoteichoic acid, and lipopolysaccharides as well [59]. Peptidoglycan recognition protein 1 is exclusively expressed in polymorphonuclear leukocytes [59], suggesting the need for microbial products to reach into the epithelial tissue to activate PGLYRP1. Our results showed a marked decreased in PGLYRP1 levels of expression as rumen matured and no effect of feeding system. Malmuthuge et al. [9] observed very low expression of PGLYRP1 prior weaning and higher at 6 months of age in calves rumen. The different temporal sequence between our work and that of Malmuthuge makes comparisons very difficult. It has been noted that the first five days of life there is different gut permeability to allow immunoglobulins ingested from colostrum and allows more bacterial products to reach the mucosal barrier and stimulate the expression of host pattern recognition receptors [11]. This fact could be related with the higher abundance of mRNA the first week of life.
Plasma immunoglobulins concentration
Although colostrum contains several types of immunoglobulins (IgG, IgA, IgM), IgG constitutes approximately 85% of the total, therefore, measurement of kid plasma IgG level is the standard for determining the level of passive transfer of immunity. Failure of passive transfer has been associated with decreased first and second lactation milk production and an increased culling rate during the first lactation [60–61].
Despite ART kid goats being fed the same colostrum as NAT kids during the first 48 hours of life, NAT group presented higher IgG levels due to continuous access to the mother´s udder for 24 hours. Differences due to feeding systems were observed only during the first week of life. IgG levels decreased from day 5 and values were stable from day 14 onwards. This agrees with Guidry et al. [62] who reported that in the transition from colostrum to mature milk, Ig levels decrease sharply during the first five days post-partum. The ruminant´s gastrointestinal tract is designed to temporarily allow the absorption of large molecules, including immunoglobulins, during the first 12 to 24 h of life. After this period, intestinal closure is produced [63]. Contrary to IgG, IgA levels did not differ between experimental groups. IgA in plasma provides the source for secretory IgA via gut epithelia or through saliva, which in ruminants represent the main vehicle of introducing immunoglobulins into the rumen [2]. Subharat et al. [64] recently showed that IgA resist longer in the rumen to degradation than IgG, due to the secretory component of IgA which makes the immunoglobulin more resistant to proteases [65].
In this experiment IgA levels were highest during the first 3 days of life and then declined sharply from day 14. There is ample evidence that primary gut microbial colonization after birth provides the antigenic stimulus for development of IgA responses [66]. Early work by Sharpe et al. [67] highlighted the strong link between rumen microbial colonization and specific antigen production. Unfortunately, after molecular techniques developed to study microbial ecosystem no further work has been conducted in ruminants reared under different conditions in early life. In our work, animals experienced different microbial colonization patterns, mainly, higher bacterial and protozoal biomass and more diverse bacterial community in NAT than in ART kids. However, this different pattern was not reflected in alterations in total IgA concentration in plasma. Further studies could address the response in secretory IgA in saliva, and paying attention on specific IgA in relation to the groups that first colonizing the rumen.
Conclusions
Our results confirmed a diverse bacterial colonisation from the first day of life in the undeveloped rumen, and show that the colonization pattern substantially differs between pre-ruminants reared under natural or artificial feeding systems. The rumen epithelial immune responded quickly to the early colonization but did not develop differential response to distinct colonizing patterns, suggesting that the niches are open to first colonizers. Longer-term trials are suggested to assess the persistence of differences in adult animals and the metabolic implications.
Supporting information
(PDF)
(TIF)
Acknowledgments
This research has been funded by MINECO (grants AGL2011-27218 and BFU2014-57964-R).
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research has been funded by MINECO (www.mineco.gob.es): grants AGL2011-27218 and BFU2014-57964-R.
References
- 1.Henderson G, Cox F, Ganesh S, Jonker A, Young W, Global Rumen Census Collaborators, et al. Rumen microbial community composition varies with diet and host, but a core microbiome is found across a wide geographical range. Sci Rep. 2015; 5: 14567 doi: 10.1038/srep14567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heinrichs J. Rumen development in the dairy calf. Adv Dairy Technol. 2005; 17: 179–187. [Google Scholar]
- 3.Yáñez-Ruiz DR, Abecia L, Newbold CJ. Manipulating rumen microbiome and fermentation through interventions during early life: a review. Front Microbiol. 2015; 14; 6:1133 doi: 10.3389/fmicb.2015.01133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yáñez-Ruiz DR, Macías B, Pinloche E, Newbold CJ. The persistence of bacterial and methanogenic archaeal communities residing in the rumen of young lambs. FEMS Microbiol Ecol. 2010; 72: 272–278. doi: 10.1111/j.1574-6941.2010.00852.x [DOI] [PubMed] [Google Scholar]
- 5.Abecia L, Martín-García AI, Martínez G, Newbold CJ, Yáñez-Ruiz DR. Nutritional intervention in early life to manipulate rumen microbial colonization and methane output by kid goats postweaning. J Anim Sci. 2013; 91: 4832–4840. doi: 10.2527/jas.2012-6142 [DOI] [PubMed] [Google Scholar]
- 6.Lascelles AK. The immune system on the ruminant mammary gland and its role in the control of mastitis. J Dairy Sci. 1979; 62: 154–167. [DOI] [PubMed] [Google Scholar]
- 7.Lilius EM, Marnila P. The role of colostral antibodies in prevention of microbial infections. Curr Opin Infect Dis. 2001; 14: 295–300. [DOI] [PubMed] [Google Scholar]
- 8.Maslowski KM, Mackay C. Diet, gut microbiota and immune responses. Nat Immunol. 2011; 12: 5–9. doi: 10.1038/ni0111-5 [DOI] [PubMed] [Google Scholar]
- 9.Malmuthuge N, Li M, Fries P, Griebel PJ, Guan LL. Regional and age dependent changes in gene expression of toll-like receptors and key antimicrobial defense molecules throughout the gastrointestinal tract of dairy calves. Vet Immunol Immunopathol. 2012; 146: 18–26. doi: 10.1016/j.vetimm.2012.01.010 [DOI] [PubMed] [Google Scholar]
- 10.Van dA, Van dW, Verstraete W, Possemiers S. The host selects mucosal and luminal associations of coevolved gut microorganisms: a novel concept. FEMS Microbiol. Rev. 2011; 35: 681–704. doi: 10.1111/j.1574-6976.2011.00270.x [DOI] [PubMed] [Google Scholar]
- 11.Malmuthuge N, Griebel PG, Guan LL. The gut microbiome and its potential role in the development and function of newborn calf gastrointestinal tract. Front Vet Sci. 2015; 2: 36 doi: 10.3389/fvets.2015.00036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD. QIIME allows analysis of high-throughput community sequencing data. Nat Methods. 2010; 7: 335–336. doi: 10.1038/nmeth.f.303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bragg L, Stone G, Imelfort M, Hugenholtz P, Tyson GW. Fast, accurate error-correction of amplicon pyrosequences using Acacia. Nat Methods. 2012; 9: 425–426. doi: 10.1038/nmeth.1990 [DOI] [PubMed] [Google Scholar]
- 14.Edgar RC. UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat Methods. 2013; 10: 996–998. doi: 10.1038/nmeth.2604 [DOI] [PubMed] [Google Scholar]
- 15.McDonald D, Price MN, Goodrich J, Nawrocki EP, Desantis TZ, Probst A, et al. An improved greengenes taxonomy with explicit ranks for ecological and evolutionary analyses of bacteria and archaea. ISME J. 2012; 6: 610–618. doi: 10.1038/ismej.2011.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Q, Garrity GM, Tiedje JM, Cole JR. Naïve Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol. 2007; 73: 5261–5267. doi: 10.1128/AEM.00062-07 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chessel D, Dufour AB, Thioulouse J. The ade4 package-I-one-table methods. R News 2004; 4 5–10. [Google Scholar]
- 18.McMurdie P J, Holmes S. phyloseq: an R package for reproducible interactive analysis and graphics of microbiome census data. PLoS ONE 2013; 8:e61217 doi: 10.1371/journal.pone.0061217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oksanen J, Guillaume Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, et al. Package ‘vegan’ Community Ecology Package Version 2.4–3 April 12, 2017.
- 20.Abecia L, Ramos-Morales E, Martinez-Fernandez G, Arco A, Martín-García AI, Newbold CJ, et al. Feeding management in early life influences microbial colonisation and fermentation in the rumen of newborn goat kids. Anim Prod Sci. 2014; 54: 1449–1454. [Google Scholar]
- 21.Skillman LC, Evans PN, Naylor GE, Morvan B, Jarvis GN, Joblin KN. 16S ribosomal DNA-directed PCR primers for ruminal methanogens and identification of methanogens colonising young lambs. Anaerobe 2004; 10: 277–285. doi: 10.1016/j.anaerobe.2004.05.003 [DOI] [PubMed] [Google Scholar]
- 22.Fonty G, Chaucheyras-Durand F. Les ecosystemes digestifs In: Les Communautes microbiennes du tube digestif des mammiferes: Diversite et structure. Tec&Doc Lavoisier, Paris, France: 2007. pp. 71–126. [Google Scholar]
- 23.Jami E, Israel A, Kotser A, Mizrahi I. Exploring the bovine rumen bacterial community from birth to adulthood. ISME J. 2013; 7: 1069–1079. doi: 10.1038/ismej.2013.2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Malmuthuge N, Griebel PJ, Guan LL. Taxonomic identification of commensal bacteria associated with the mucosa and digesta throughout the gastrointestinal tracts of preweaned calves. Appl Environ Microbiol. 2014; 80: 2021–2028. doi: 10.1128/AEM.03864-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jiao J, Huang J, Zhou C, Tan Z. Taxonomic identification of ruminal epithelial bacterial diversity during rumen developmentin goats. Appl Environ Microbiol. 2015; 81: 3502–3509. doi: 10.1128/AEM.00203-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang W, Li C, Li F, Wang X, Zhang X, Liu T, et al. Effects of early feeding on the host rumen transcriptome and bacterial diversity in lambs. Sci Rep. 2016: 31; 6:32479 doi: 10.1038/srep32479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Alexander M. Microbial Ecology. New York, USA: John Wiley and Sons Inc; 1971. [Google Scholar]
- 28.Rey M, Enjalbert F, Combes S, Cauquil L, Bouchez O, Monteils V. Establishment of ruminal bacterial community in dairy calves from birth to weaning is sequential. J Appl Microbiol. 2013; 116: 245–257. doi: 10.1111/jam.12405 [DOI] [PubMed] [Google Scholar]
- 29.Fonty G, Gouet P, Jouany JP, Senaud J. Establishment of the microflora and anaerobic fungi in the rumen of lambs. J Gen Microbiol. 1987; 133: 1835–1843. [Google Scholar]
- 30.Minato H, Otsuka M, Shirasaka S, Itabashi H, Mitsumori M. Colonization of microorganisms in the rumen of young calves. J Gen Appl Microbiol. 1992; 38: 447–456. [Google Scholar]
- 31.Steele MA, Penner GB, Chaucheyras-Durand F, Guan LL. Development and physiology of the rumen and the lower gut: Targets for improving gut health. J Dairy Sci. 2016; 99: 4955–4966. doi: 10.3168/jds.2015-10351 [DOI] [PubMed] [Google Scholar]
- 32.Lancaster CR, Simon J. Succinate:quinone oxidoreductases from epsilon-proteobacteria. Biochim Biophys Acta. 2002; 17; 1553(1–2): 84–101. [DOI] [PubMed] [Google Scholar]
- 33.Ley RE, Hamady M, Lozupone C, Turnbaugh PJ, Ramey RR, Bircher JS, et al. Evolution of mammals and their gut microbes. Science. 2008; 20; 320(5883):1647–1651. doi: 10.1126/science.1155725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sekirov I, Finlay BB. The role of the intestinal microbiota in enteric infection. J Physiol. 2009; 1; 587(Pt 17): 4159–4167. doi: 10.1113/jphysiol.2009.172742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meale SJ, Li S, Azevedo P, Derakhshani H, Plaizier JC, Khafipour E, et al. Development of ruminal and fecal microbiomes are affected by weaning but not weaning strategy in dairy calves. Front Microbiol. 2016; 7: 582 doi: 10.3389/fmicb.2016.00582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Stevenson DM, Weimer PJ. Dominance of Prevotella and low abundance of classical ruminal bacterial species in the bovine rumen revealed by relative quantification real-time PCR. Appl Microbiol Biotechnol 2007; 75: 165–174. doi: 10.1007/s00253-006-0802-y [DOI] [PubMed] [Google Scholar]
- 37.Jami E, Mizrahi I. Composition and similarity of bovine rumen microbiota across individual animals. PLoS ONE 2012: 7(3): e33306 doi: 10.1371/journal.pone.0033306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hernandez-Sanabria E, Goonewardene LA, Wang Z, Durunna ON, Moore SS, Guan LL. Impact of feed efficiency and diet on adaptive variations in the bacterial community in the rumen fluid of cattle. Appl Environ Microbiol. 2012; 78: 1203–1214. doi: 10.1128/AEM.05114-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.De Paula Vieira A, de Passillé AM, Weary DM. Effects of the early social environment on behavioral responses of dairy calves to novel events. J Dairy Sci. 2012; 95(9): 5149–5155. doi: 10.3168/jds.2011-5073 [DOI] [PubMed] [Google Scholar]
- 40.Galef BG. Jr., Giraldeau LA. Social influences on foraging in vertebrates: causal mechanisms and adaptive functions. Anim Behav. 2001; 61: 3–15. doi: 10.1006/anbe.2000.1557 [DOI] [PubMed] [Google Scholar]
- 41.Anderson K, Nagaraja T, Morrill J. Ruminal metabolic development in calves weaned conventionally or early. J Dairy Sci. 1987: 70; 1000–1005. doi: 10.3168/jds.S0022-0302(87)80105-4 [DOI] [PubMed] [Google Scholar]
- 42.Eadie JM, Hobson PN, Mann SO. A relationship between some bacteria, protozoa and diet in early weaned calves. Nature. 1959: 183; 624–625. [DOI] [PubMed] [Google Scholar]
- 43.Ziolecki A, Briggs CA. The microflora of the rumen of young calf: II. Source, nature and development. J. Appl. Bacteriol. 1961; 24: 148–163. [Google Scholar]
- 44.Williams A. G., Coleman G. S. The Rumen Protozoa. New York, NY: Springer-Verlag; 1992. [Google Scholar]
- 45.Yáñez-Ruiz DR, Williams S, Newbold CJ. The effect of absence of protozoa on rumen biohydrogenation and the fatty acid composition of lamb muscle. Br J Nutr. 2007; 97: 938–948. doi: 10.1017/S0007114507675187 [DOI] [PubMed] [Google Scholar]
- 46.Belanche A, de la Fuente G, Newbold CJ. Study of methanogen communities associated with different rumen protozoal populations. FEMS Microbiol. Ecol. 2014; 90: 663–677. doi: 10.1111/1574-6941.12423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Brossard L, Martin C, Chaucheyras-Durand F, Michalet-Doreau B. Protozoa involved in butyric rather than lactic fermentative pattern during latent acidosis in sheep. Reprod Nutr Dev. 2004; 44: 195–206. [DOI] [PubMed] [Google Scholar]
- 48.Kato S, Sato K, Chida H, Roh SG, Ohwada S, Sato S, et al. Effects of Na-butyrate supplementation in milk formula on plasma concentrations of GH and insulin, and on rumen papilla development in calves. J Endocrinol. 2011; 211: 241–248. doi: 10.1530/JOE-11-0299 [DOI] [PubMed] [Google Scholar]
- 49.Kowalski ZM, Górka P, Flaga J, Barteczko A, Burakowska K, Oprządek J, et al. Effect of microencapsulated sodium butyrate in the close-up diet on performance of dairy cows in the early lactation period. J Dairy Sci. 2015; 98:3284–3291. doi: 10.3168/jds.2014-8688 [DOI] [PubMed] [Google Scholar]
- 50.Górka P, Kowalski ZM, Pietrzak P, Kotunia A, Jagusiak W, Holst JJ, et al. Effect of method of delivery of sodium butyrate on rumen development in newborn calves. J Dairy Sci. 2011; 94: 5578–5588. doi: 10.3168/jds.2011-4166 [DOI] [PubMed] [Google Scholar]
- 51.Mogensen TH. Pathogen recognition and inflammatory signaling in innate immune defenses. Clin Microbiol Rev. 2009; 22: 240–273. doi: 10.1128/CMR.00046-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Teran R, Mitre E, Vaca M, Erazo S, Oviedo G, Hubner MP, et al. Immune system development during early childhood in tropical Latin America: evidence for the age-dependent down regulation of the innate immune response. Clin Immunol. 2011: 138, 299–310. doi: 10.1016/j.clim.2010.12.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang ZL. Role of Toll-like receptors in regulatory functions of T and B cells. Chin Sci Bull. 2008; 53: 1121–1127. [Google Scholar]
- 54.Akira S, Uematsu S, Takeuchi O. Pathogen recognition and innate immunity. Cell. 2006: 24; 124: 783–801. [DOI] [PubMed] [Google Scholar]
- 55.Guan Y, Ranoa RE, Jiang S, Mutha SK, Li X, Baudry J, Tapping RI. Human TLRs 10 and 1 share common mechanisms of innate immune sensing but not signaling. J Immunol. 2010; 184: 5094–5103. doi: 10.4049/jimmunol.0901888 [DOI] [PubMed] [Google Scholar]
- 56.Hayashi F, Smith KD, Ozinsky A, Hawn TR, Yi EC, Goodlett DR, et al. The innate immune response to bacterial flagellin is mediated by Toll- like receptor 5. Nature. 2001; 410: 1099–1103. doi: 10.1038/35074106 [DOI] [PubMed] [Google Scholar]
- 57.Gazzinelli RT, Denkers EY. Protozoan encounters with Toll-like receptor signalling pathways: implications for host parasitism. Nature Rev Immunol. 2004; 6: 895–906. [DOI] [PubMed] [Google Scholar]
- 58.Osanai A, Sashinami H, Asano K, Li S, Hu D, Nakane A. Mouse peptidoglycan recognition protein PGLYRP-1 plays a role in the host innate immune response against Listeria monocytogenes infection. Infect Immun. 2011; 79: 858–866. doi: 10.1128/IAI.00466-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dziarski R, Gupta D. The peptidoglycan recognition proteins (PGRPs). Genome Biol. 2006; 7: 232 doi: 10.1186/gb-2006-7-8-232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeNise SK, Robison JD, Stott GH, Armstrong DV. Effects of passive immunity on subsequent production in dairy heifers. J Dairy Sci. 1989; 72: 552–554. doi: 10.3168/jds.S0022-0302(89)79140-2 [DOI] [PubMed] [Google Scholar]
- 61.Faber SN, Faber NE, McCauley TC, Ax RL. Case Study: Effects of colostrum ingestion on lactational performance. Prof Anim Scientist 2005; 21: 420–425. [Google Scholar]
- 62.Guidry AJ, Butler JE, Pearson RE, Weiland B. Ig A, IgG1, IgG2, IgM and BSA secretion by the bovine mammary gland throughout lactation. Vet Immunol Immunopathol. 1980; 1: 329–341. [DOI] [PubMed] [Google Scholar]
- 63.Bush LJ, Staley TE. Absorption of colostral immunoglobulins in newborn calves. J Dairy Sci. 1980; 63: 672–680. doi: 10.3168/jds.S0022-0302(80)82989-4 [DOI] [PubMed] [Google Scholar]
- 64.Subharat S, Shu D, Zheng T, Buddle BM, Janssen PH, Luo D, et al. Vaccination of cattle with a methanogen protein produces specific antibodies in the saliva which are stable in the rumen. Vet Immunol Immunopathol. 2015; 164: 201–207. doi: 10.1016/j.vetimm.2015.02.008 [DOI] [PubMed] [Google Scholar]
- 65.Snoeck V, Peters IR, Cox E. The IgA system: a comparison of structure and function in different species. Vet Res. 2006; 37: 455–467. doi: 10.1051/vetres:2006010 [DOI] [PubMed] [Google Scholar]
- 66.Kaetzel CS. Cooperativity among secretory IgA, the polymeric immunoglobulin receptor, and the gut microbiota promotes host-microbial mutualism. Immunol Lett. 2014; 162: 10–21. doi: 10.1016/j.imlet.2014.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Sharpe ME, Latham M J, Reiter B. The immune response of the hosts animal to bacteria in the rumen and caecum In McDonald IW, Warner ACI (Eds). Digestion and Metabolism in the Ruminant. The University of New England, Sydney, NSW, Australia, 1975. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.