Significance
The physical dynamics of the body are central to the generation and maintenance of the human gait cycle. The ability to exploit the force of gravity and bodily inertia increases the energetic efficiency of locomotion by minimizing the need for internally generated muscular forces and simplifies control by obviating the need to actively guide each body segment. Here we explore how these principles generalize to situations in which foot placement is constrained, as when walking over a rocky trail. Walkers can exploit external forces to efficiently traverse extended stretches of complex terrain provided that visual information about the upcoming ground surface is available during a particular (critical) phase of the gait cycle between midstance of the preceding step and toe-off.
Keywords: human locomotion, visual control, foot placement, biomechanics, inverted pendulum
Abstract
To walk efficiently over complex terrain, humans must use vision to tailor their gait to the upcoming ground surface without interfering with the exploitation of passive mechanical forces. We propose that walkers use visual information to initialize the mechanical state of the body before the beginning of each step so the resulting ballistic trajectory of the walker’s center-of-mass will facilitate stepping on target footholds. Using a precision stepping task and synchronizing target visibility to the gait cycle, we empirically validated two predictions derived from this strategy: (1) Walkers must have information about upcoming footholds during the second half of the preceding step, and (2) foot placement is guided by information about the position of the target foothold relative to the preceding base of support. We conclude that active and passive modes of control work synergistically to allow walkers to negotiate complex terrain with efficiency, stability, and precision.
Humans and other animals are remarkable in their ability to take advantage of what is freely available in the environment to the benefit of efficiency, stability, and coordination in movement. This opportunism can take on at least two forms, both of which are evident in human locomotion over complex terrain: (i) harnessing external forces to minimize the need for self-generated (i.e., muscular) forces (1), and (ii) taking advantage of passive stability to simplify the control of a complex movement (e.g., ref. 2). In the ensuing section, we explain how walkers exploit external forces and passive stability while walking over flat, obstacle-free terrain.* We then generalize this account to walking over irregular surfaces by explaining how walkers can adapt gait to terrain variations while still reaping the benefits of the available mechanical forces and inherent stability. This account leads to hypotheses about how and when walkers use visual information about the upcoming terrain and where that information is found. We derive several predictions from these hypotheses and then put them to the test in three experiments.
Passive Control in Human Walking
The basic movement pattern of the human gait cycle arises primarily from the phasic activation of flexor and extensor muscle groups by spinal-level central pattern generators, regulated by sensory signals from lower limb proprioceptors and cutaneous feedback from the plantar surface of the foot. This low-level neuromuscular circuitry serves to maintain the rhythmic physical oscillations that define locomotor behavior (see ref. 3 for review). This section will provide an overview of the basic biomechanics of the bipedal gait cycle to show how these inherent physical dynamics contribute to the passive stability and energetic efficiency of human locomotion.
During the single support phase of the bipedal gait cycle, when only one foot is in contact with the ground, a walker shares the physical dynamics of an inverted pendulum. The body’s center of mass (COM) acts as the bob of the pendulum and is supported above a single point of rotation in the planted foot (4, 5). At the onset of the single support phase—that is, at “toe off,” when the nonsupporting leg breaks contact with ground—momentum carries the COM up to a maximum height at midstance and then down again until the swinging foot contacts the ground, which is called “heel strike” (Fig. 1A). As the COM increases its height during the first half of the single support phase, some of the kinetic energy of the COM is transferred into potential energy, which is then transferred back into kinetic energy as the COM drops down to its original height in the latter half of the step. In the ideal case, the exchange between kinetic and potential energy is perfectly lossless and symmetric, and human walking closely approximates an idealized inverted pendulum acting conservatively. As such, humans can harness gravity and inertia and exploit the inverted pendulum dynamics of their bodies to traverse the distance traveled during the single support phase at a minimal cost in terms of work and muscle force (6, 7).
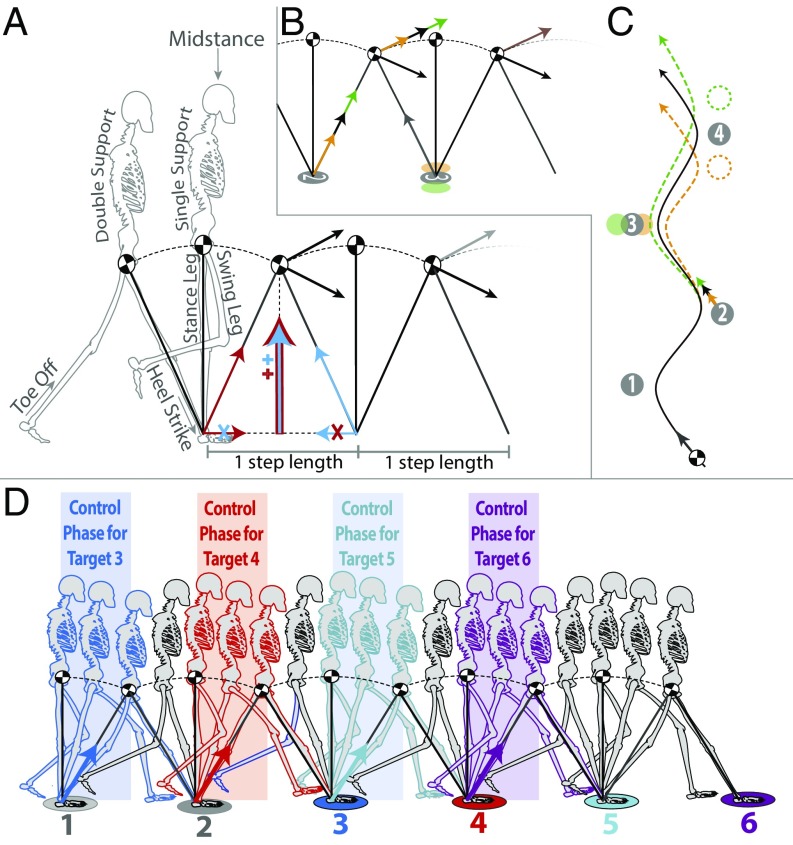
Fig. 1.
(A) A conceptual diagram of the steady-state gait cycle. In the step-to-step transition, positive and negative work from the trailing and leading legs (red and blue arrows, respectively) redirect the COM and establish the conditions leading into the next step. (B) The ballistic trajectory of the COM during a step is defined by two factors—the location of the planted foot and the magnitude of the push-off force from the trailing limb. (C) By adjusting these factors, a walker can tailor the passive trajectory of the COM in an oncoming step to facilitate a step onto a target foothold. (D) The critical control phase for targets 3–6 (see also Movie S1).
Of course, in reality walking does incur costs. A primary determinant of the metabolic cost of walking is restoration of the energy lost when the swinging foot contacts the ground (refs. 8 and 9, but see ref. 10). Ground contact by the swinging foot marks the end of the single-support phase and the beginning of the double-support phase, during which the COM must be redirected from a downward trajectory to the upward trajectory it will need for the coming step (11). Upon ground contact, the force applied by the leading leg has a horizontal component opposite to the direction of motion of the COM; that is, the leading leg performs negative work on the COM. This collision is dissipative, which means that some mechanical energy is lost and that positive work must be performed to maintain forward progress (12). To counteract this loss of energy, the trailing leg applies a push-off force beginning toward the end of the single support phase and continuing through the double-support phase (13, 14). The vertical component of the push-off force from the trailing leg sums with the vertical component of the force from the leading leg to direct the COM into the upward trajectory that it will need for the next step (Fig. 1A). If the forward horizontal component of the push-off force is equal to the backward horizontal component of the force from the leading foot, the two forces cancel out and the mechanical state of the body will be the same at the onset in the upcoming step as it was in the previous one. Both the negative work performed by the leading leg and the positive work performed by the trailing leg involve muscle activation and therefore incur some metabolic cost. However, healthy human walkers adopt strategies that reduce the cost of the step-to-step transition to the degree possible (8, 15). Thus, by exploiting the inverted pendulum dynamics of the single-support phase and using the push-off force to restore the energy lost during the step-to-step transition, humans are able to generate a steady-state gait cycle with minimal energetic cost (9, 12, 16).
The second form of opportunism by the motor system during walking involves reliance on control strategies that take advantage of passive stability. When such strategies are adopted, small perturbations are automatically dissipated over subsequent steps, making it possible for stable walking behavior to emerge with no active, sensory-driven control (17). In human walking, passive stability arises because the loss of energy at collision increases with walking speed (13). As such, a small forward perturbation applied to the COM at midstep increases the speed of the COM, which in turn increases the collision loss so the additional energy that is injected into the system by the perturbation will be dissipated at collision (15). Although the basin of attraction for bipedal gait may be relatively shallow, spinal-level reflex pathways (3) may serve to facilitate gait-restorative responses that take advantage of this passive stability (18–20).†
Active Control of Walking
These insights imply that certain desirable characteristics of movement, such as coordination, efficiency, and stability, can emerge without active (i.e., perceptually guided) control. Nevertheless, humans are not locked into rigid patterns of movement. The spinal and subcortical activity underlying the basic rhythmic pattern of the preferred gait cycle that humans adopt during steady-state walking over flat terrain can be modulated by descending signals from the cortex to accommodate variations in task demands and variations in the environment (3, 21, 22). Such descending signals are essential when walking over complex terrain, where it may not be an option to place the feet in the position that is ideal for the exploitation of the body’s inverted pendulum dynamics. Instead, walkers must identify potential footholds in the upcoming terrain and regulate the movement of their body to ensure that the foot lands on a suitable location.
Recent research on the neural underpinnings of visually guided walking in cats suggests that area 5 of the posterior parietal cortex (PPC) may play an essential role in integrating visual signals into the gait cycle to facilitate the control of foot placement during locomotion over complex terrain (21, 23). This area has been implicated in the control of reaching to remembered locations in primates (24, 25) and may play an analogous role in stepping behavior during locomotion. In vivo recordings from cats walking on a treadmill revealed that neurons from this region increase their firing rates in the 2–3 steps before the step over an obstacle (22), suggesting that this area could be involved in encoding the position of upcoming obstacles relative to the moving observer. This estimate may then be used by the motor cortex to generate the descending signals that modulate the activity of synergistic muscle groups in the limbs to enact the gait adjustment necessary to account for the upcoming obstacle (or target foothold) (23).
When walking over complex terrain, visual information about the upcoming path must be integrated into the ongoing gait cycle to adjust the basic locomotor pattern to fit the available footholds. Although visual information about the world is often noisy, humans appear to use state estimation to derive accurate estimates of target footholds to support precise foot placement (26). This visual information does not need to be continuously available; human walkers are able to walk successfully over constrained terrain with only intermittent visual information about the upcoming path (27–29). The ability to step successfully on a target that is not continuously visible is a hallmark of the feedforward control that underlies visually guided locomotion.
The feedforward plans necessary to traverse an obstacle or other locomotor impediment must be enacted based on visual information gathered during uninterrupted locomotion. When subjects’ vision was occluded several steps before traversing an obstacle, they performed successfully provided that the occlusion occurred during uninterrupted locomotion (30). However, if subjects stopped walking after their vision was occluded, performance degraded and collisions with the obstacle became more frequent. The visual information necessary to cross complex terrain may be sampled by briefly fixating obstacles and targets approximately two step lengths in advance (31–33) or by relying on peripheral vision (34, 35). When terrain variations can be detected far in advance, visually guided adjustments to gait parameters are observed as early as three to four steps ahead and spread out over the remaining steps (36). Occasionally, a more rapid response is needed—when the location of an intended target changes midstep or an obstacle suddenly appears or changes in size, humans are capable of redirecting the trajectory of their feet with short latency (∼120 ms) (37–40). These studies highlight some of the various roles of active, vision-based control in the guidance of walking over complex terrain.
How Are Passive and Active Modes of Control Used During Walking over Complex Terrain?
The imperative to adapt gait to the layout of obstacles and safe footholds entails neuromuscular intervention that interferes with the natural passive trajectory of the body. During locomotion over uneven terrain, steps become more variable in both length and width, mechanical work increases at the hips and knees, and muscle activation increases in several proximal leg muscles, all of which lead to an increase in energy expenditure (41, 42). Does this mean that walkers must give up entirely on exploiting external forces and passive stability when walking over complex terrain, or is it possible to simultaneously use information about the upcoming terrain to modulate gait while still taking advantage of the benefits of passive control?
We propose that the latter could be achieved by using information about upcoming terrain to initialize the mechanical state of the body before the beginning of each step, such that the resulting ballistic trajectory of the body will facilitate stepping on the upcoming target footholds. In this way, a walker can make small adaptations to their energetically optimized preferred gait cycle to accommodate upcoming environmental impediments. To explain how such a strategy would work, we first identify the mechanical determinants of the passive trajectory of the body during the single support phase that could serve as control variables for the walker.
If we assume that the single support phase is adequately modeled by a simple inverted pendulum, the ballistic trajectory of the walker’s COM during the single support phase depends on two factors—the position of the planted foot and the initial conditions (position and velocity) of the COM at toe off (Fig. 1B). During steady-state walking over flat, obstacle-free terrain, the push-off force restores the energy that is lost during step-to-step transition, resulting in a regular, roughly sinusoidal path of the COM between each foothold (Fig. 1C, black line). However, a walker seeking to step onto a particular foothold could also purposefully alter the direction and magnitude of that push off force to cause the COM to follow a different trajectory during the subsequent single support phase. The walker could achieve the same effect by maintaining a normal push-off force and altering the location of the planted foot that defines the base of the pendulum in the coming step (Fig. 1C, green and orange lines).
To summarize, the two determinants of the passive COM trajectory (the position of the planted foot and the push-off force from the trailing foot) are potential control variables that walkers may use to initialize the mechanical state of the body before toe-off to tailor the ballistic trajectory of the COM to the upcoming terrain. By making small adjustments to the placement of a step or by adjusting the restorative push-off force to be either more or less than the magnitude required to exactly recoup the energy loss of heel strike, the walker can alter the parameters of the steady-state, preferred gait cycle to accommodate upcoming terrain. These adjustments to the basic pattern of muscle activation underlying the gait cycle may arise from descending cortical signals that support visually guided locomotion (21).
Once the step is initiated, the body can simply be allowed to follow its natural pendular trajectory to the next target with no need for energetically costly midflight corrections that interfere with that trajectory. In this regard, this strategy relies on active control based on visual information about upcoming terrain but also takes full advantage of the passive dynamics inherent to bipedal walking.
The Critical Control Phase Hypothesis
The idea that the control of foot placement over complex terrain serves to maintain an energetically efficient exploitation of the body’s physical dynamics motivates a hypothesis about when visual information about the upcoming terrain is most critical. This hypothesis has two components and will be referred to as the critical control phase hypothesis.
The first component concerns how far in advance walkers must be able to sample visual information about the upcoming terrain. A visual control strategy based on manipulation of the ballistic trajectory of the COM during the single support phase would require walkers to see the terrain far enough in advance to be able to make the appropriate adjustments to the two determinants of that trajectory. That is, tailoring the mechanical state of the body appropriately to allow a walker to step on a target foothold (say, with the right foot) requires the walker to see that target before the mechanics of the relevant step have been defined. Given the inverted pendulum dynamics of the single support phase, this means the walker must see the right foothold before the left foot, which defines the base of the upcoming inverted pendulum, has contacted the ground and before the push-off force from the supporting leg has been enacted. As such, the walker must see the terrain relevant to a right foothold while the right foot is still on the ground (and vice versa for the left foot). Put another way, to tailor the mechanics of the body to enable stepping to a specific foothold requires a walker to see the terrain relevant to that foothold by around midstance of the previous single-support phase—roughly two step lengths of visual look ahead (Fig. 1D).‡
Indeed, restricting a walker’s ability to see upcoming terrain to less than two step lengths results in decreases in stepping accuracy and walking speed (43). Furthermore, the trajectory of the COM is less like that of a passively moving inverted pendulum compared with when walkers can see two or more step lengths ahead, suggesting that walkers need at least two step lengths of visual look-ahead to exploit their inverted pendulum dynamics when walking over complex terrain (44). Interestingly, these findings converge with studies of gaze behavior that demonstrate that walkers tend to look about two steps ahead when walking over complex terrain (32). In addition, Zaytsev et al. (45) recently showed that a dynamic bipedal walking model with realistic actuators could reach almost any desired target velocity or foothold location by using a two-step-ahead planning window. These results suggest that the tendency of human walkers to look approximately two step lengths ahead during locomotion over complex terrain (31–33) may be rooted in the physical dynamics of the bipedal gait cycle.
The second part of the critical control phase hypothesis concerns when visual information about upcoming terrain is no longer needed. Do walkers require visual information about the location of an upcoming target foothold throughout the entire step, or can they achieve comparable levels of stepping accuracy if they stop sampling information partway through the step or even before the step is initiated? If, in fact, walkers attempt to initialize each step so that the body can follow a ballistic trajectory to the target, then the role of visual information about a target is primarily to allow the walker to tailor the two determinants of the ballistic trajectory. Importantly, this strategy relies on feed-forward control—walkers use information about the upcoming path to establish the mechanical state of the body before the upcoming step so that the natural dynamics of their body will carry them in the desired direction (Fig. 1D). Any active control of the trajectory of the swinging leg during the single-support phase would require the walker to use muscular forces to interfere with the ballistic trajectory of the body. As such, we should expect that visual information about the target should not be necessary once the step to that target is initiated.
Indeed, subjects walking across a path of small target footholds showed a negligible decrease in stepping accuracy when the targets became invisible at toe-off of the step to the target (46). However, stepping accuracy decreased dramatically if the target became invisible at any point before toe-off. In short, it appears that once the foot has left the ground toward a target foothold, no further visual information about that target is required for accurate foot placement. This result entails that any vision-based control of the trajectory of the foot during a step must take place during the preceding step.
Taken together, these results suggest that there is a critical phase within the bipedal gait cycle for the visual control of foot placement when walking over complex terrain. When guiding the placement of a particular step, visual information about the terrain relevant to that foothold becomes maximally important following midstance of the preceding single-support phase but is no longer necessary once the foot leaves the ground on its way to the selected foothold (Fig. 1D and Movie S1).
Results
Testing the Critical Phase Hypothesis (Experiment 1).
Although the findings of Matthis and Fajen (43, 44) and Matthis, Barton, and Fajen (46) are consistent with the critical control phase hypothesis, they are not sufficient to conclude that visual information is primarily used during the latter part of the preceding step because the two components of the critical phase hypothesis were tested in separate experiments. The first aim of this study was to provide a direct test by combining the manipulations used in the previous studies in a single experimental task. Subjects were instructed to walk over a series of small targets that did not appear until they fell within a threshold distance of the subject and then disappeared when they fell within a second threshold distance. This manipulation was applied to each target individually, with the exception of the first two, which were visible throughout the entire trial (stepping accuracy to the first two targets was not included in later analysis).
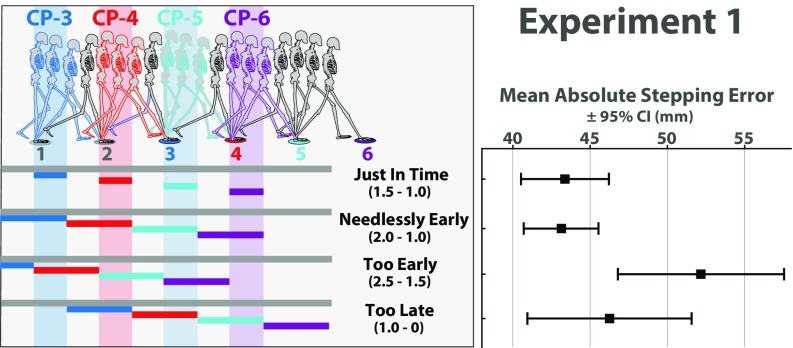
We tested four conditions with different triggers for target visibility and invisibility (Fig. 2; see Materials and Methods for details and Movie S2). In one of the conditions (depicted in the first row of Fig. 2), each target first appeared when the subject was in midstance of the step to the preceding target and disappeared at toe off of the step to the target (that is, it was visible from 1 1/2 to 1 steps before the target, see also Materials and Methods). The colored horizontal bars in Fig. 2 indicate the location of the walker’s COM when the target with the corresponding color was visible (e.g., the third target, which is depicted in red, was visible when the subject was within the range of positions indicated by the red horizontal bar). To help visualize the timing of target visibility relative to the critical control phase for each target, Fig. 2 also includes colored vertical bands showing the critical control phase for each target. Notice that, in this condition, the horizontal bars corresponding to target visibility are aligned with the vertical bands indicating the critical control phase; that is, each target was visible during the critical control phase and only at that time. As such, we call this the “Just in Time” condition. As shown in Fig. 2, Right, the mean absolute stepping error in this condition was 43.4 mm (95% CI [40.5, 46.3]).
Fig. 2.
(Left) Depiction of four target visibility manipulations used in experiment 1 (see Movie S2). Horizontal bars indicate the location of the walker’s COM when the target of the corresponding color was visible. Vertical bands indicate hypothesized critical control phase for each target. (Right) Mean absolute stepping error and 95% CI for each condition in experiment 1.
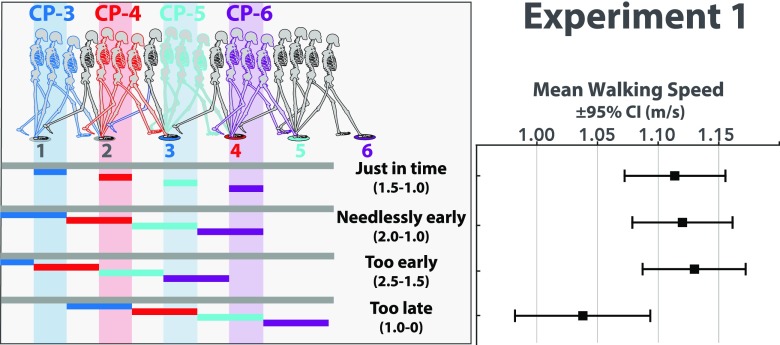
Next, let us compare performance in the Just in Time condition to performance in the three other conditions in which targets were visible for twice as long (one full step) during, before, or after the critical control phase. If the last half of the preceding step is truly the critical phase of the gait cycle during which visual information must be available, then extending the duration of visibility to include the previous 1/2 step amounts to making targets visible needlessly early and should not improve performance. Indeed, stepping accuracy in the “Needlessly Early” condition was not significantly different (t11 = –0.24, P = 0.81, d = 0.07; mean difference relative to Just in Time condition = –0.25, 95% CI [–2.51, 2.01]). When targets were visible for a full step before the critical control phase (“Too Early” condition), stepping error was significantly worse (t11 = 3.42, P < 0.01, d = 0.99; mean difference relative to Just in Time condition = 8.78, 95% CI [3.13, 14.43]). In the “Too Late” condition, each target first appeared at toe-off of the step to that target and remained visible. Stepping error was not significantly worse in this condition (t11 = 0.92, P = 0.38, d = 0.27; mean difference relative to Just in Time condition = 2.92, 95% CI [–4.03, 9.87]). However, subjects walked slower in the Too Late condition than they did in each of the other conditions (Fig. S1). Although the decrease in walking speed is small (∼0.1 m/s), paired-sample t tests comparing each of the three other conditions to the Too Late condition confirmed that these differences were statistically significant (t11 = 5.33, P < 0.01, d = 1.54 for Just in Time vs. Too Late; t11 = 5.20, P < 0.01, d = 1.50 for Needlessly Early vs. Too Late; t11 = 6.04, P < 0.01, d = 1.74 for Too Early vs. Too Late). Thus, subjects were able to maintain stepping accuracy in this condition but only by trading off on walking speed. This trade-off is consistent with behavior observed while attempting to avoid stepping on obstacles under analogous visibility constraints (43, 44).
Fig. S1.
(Left) Depiction of four target visibility manipulations used in experiment 1 (see Movie S2). Horizontal bars indicate the location of the walker’s COM when the target of the corresponding color was visible. Vertical bands indicate hypothesized critical control phase for each target. (Right) Mean walking speed (m/s) and 95% CI for each condition in experiment 1.
Taken together, it is apparent that the best overall performance was found in the two conditions for which targets were visible during the critical control phase. In one of those conditions (i.e., the Just in Time condition), targets were visible for only half a step, supporting the idea that the usefulness of visual information is determined more by the phasic timing of its availability within the gait cycle than by the duration of target visibility.
The Two Targets Hypothesis.
The findings of experiment 1 demonstrate that the objective to exploit one’s biomechanical structure during walking constrains when visual information about the upcoming terrain is needed. Next, we consider whether biomechanical factors similarly constrain where the information that is needed to guide foot placement is found; that is, which regions of the upcoming terrain must be visible to the walker. Again, the proposal that walkers attempt to exploit their biomechanical structure by initializing the upcoming step leads to a testable hypothesis. To avoid having to make midflight adjustments, the push-off force from the trailing foot should be adjusted to the position of the target at the end of the upcoming step relative to the position of the previous target (8). For example, when the distance between the two upcoming targets is short, a weak push-off force would be sufficient; a longer separation between upcoming targets would require a stronger push-off force. The point is that a walker attempting to initialize the upcoming step with an appropriate push-off force needs to know the position (both distance and direction) of target N relative to the preceding foothold (target N–1). That is, target N–1 serves as a visual indication of the origin of the reference frame within which the location of target N must be perceived. This leads to the hypothesis that the relevant visual information is found in the region of the upcoming terrain that includes the two upcoming targets. We refer to this as the two targets hypothesis and derive from it the following prediction—that foot placement should be less accurate when the relative position of the two upcoming targets is perceived less accurately.
Testing the Two Targets Hypothesis (Experiment 2A).
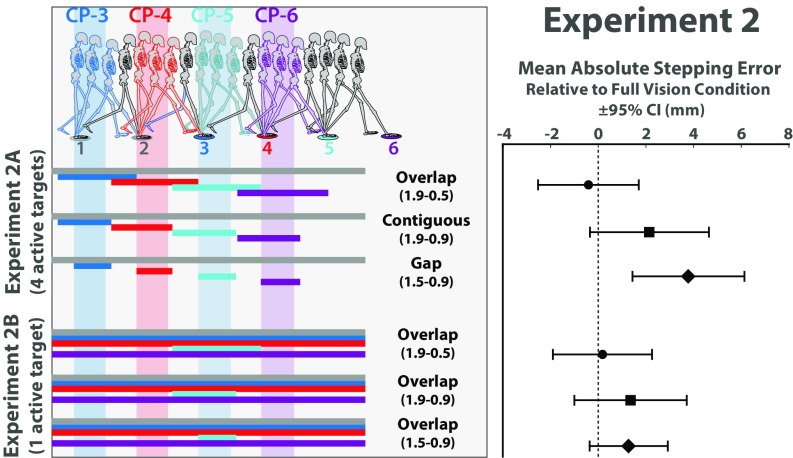
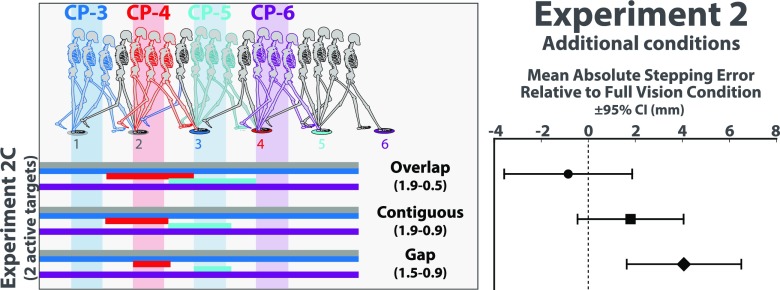
Our approach to testing the two targets hypothesis was to vary whether the two upcoming targets were simultaneously visible (see Movie S3 for animations depicting the conditions used in experiment 2A). If the two targets are visible at different points in time, their relative position should be perceived less accurately, which should translate into less accurate foot placement. In one of the conditions, depicted in the first row of Fig. 3 and the first animation in Movie S3, each of the manipulated targets was visible only when the subject was between roughly two steps and 1/2 a step from that target. The first two targets were always visible as in experiment 1. Recalling that the horizontal bars indicate the location of the walker’s COM when each target was visible, it is apparent that each target appeared before the previous target turned off. As such, there was a brief period, lasting about 1/2 a step, during which the two upcoming targets were simultaneously visible. We refer to this as the Overlap condition, as there was an overlap in time in the visibility of the two upcoming targets. In the Gap condition, depicted in the third row of Fig. 3 and the third animation in Movie S3, each of the manipulated targets was visible when the subject was between roughly 1 1/2 steps and 1 step. The two upcoming targets were not simultaneously visible in this condition; rather, there was a gap in time in the visibility of the two upcoming targets. There was also a condition in which each target turned on around the same time that the previous target turned off; that is, target visibility was “contiguous” in time (second row of Fig. 3, second animation of Movie S3).
Fig. 3.
(Left) Depiction of target visibility manipulations used in experiments 2A (Top) and 2B (Bottom). See Movies S3 and S4. Horizontal bars indicate the location of the walker’s COM when the target of the corresponding color was visible. Vertical bands indicate hypothesized critical control phase for each target. The same three visibility manipulations were used in both experiments but were applied to targets 3–6 in experiment 2A and to targets 4 or 5 in experiment 2B. (Right) Mean absolute stepping error relative to full vision control condition and 95% CI for each condition in experiments 2A and 2B.
The analyses in this experiment focused on stepping error relative to a full-vision control condition in which all six targets remained visible for the entire trial. If the two targets hypothesis is correct, stepping error should be at full-vision level in the Overlap condition because the two upcoming targets are simultaneously visible and higher in the Gap condition in which the targets were not simultaneously visible. The results were consistent with these predictions (Fig. 3, Right). Stepping error was at baseline levels when the two upcoming targets were simultaneously visible (t17 = –0.4, P = 0.69, d = 0.09; mean difference relative to full vision condition = –0.41, 95% CI [–2.55, 1.74]), allowing for their relative position to be accurately perceived. When there was a gap in time in the visibility of upcoming targets (i.e., when relative target position was more difficult to accurately estimate), stepping error was significantly higher (t17 = 3.35, P < 0.01, d = 0.79; mean difference relative to full vision condition = 3.78, 95% CI [1.40, 6.17]). As one would expect, performance was in between in the condition in which target visibility was contiguous in time (t17 = 1.79, P = 0.09, d = 0.42; mean difference relative to full vision condition = 2.15, 95% CI [–0.38, 4.68]).
Ruling out the Influence of Duration of Target Visibility (Experiments 2B).
Although the results are consistent with the two targets hypothesis, there is an alternative explanation for the aforementioned findings that needs to be considered. To make pairs of consecutive targets simultaneously visible, it was necessary to make each target visible for a longer duration. As such, the results of experiment 2A do not rule out the possibility that subjects were more accurate in the Overlap condition simply because targets were visible for more time. To test this possibility, experiment 2 also included another set of conditions (experiment 2B) in which the same visibility manipulations were applied to just one of the targets rather than to the last four (Fig. 3). In other words, five of the six targets were visible throughout the entire trial, and one (either the fourth or fifth target) was initially off, briefly appeared, and then disappeared (see Movie S4). The point of manipulating the visibility of just one target is that the duration of target visibility and the overlap in target visibility do not covary as they do when manipulating the visibility of two or more consecutive targets (i.e., as in experiment 2A). Because the target before the manipulated target is always visible, there is overlap in target visibility in all three conditions, regardless of whether the manipulated target is visible for 1 1/2, 1, or 1/2 a step. This is evident in the bottom of Fig. 3, which illustrates that when target 5 (the manipulated target, blue bars) is visible, target 4 (magenta bars) is also visible. As such, the difference in stepping error across conditions that was found in experiment 2A when four targets were manipulated should be erased.
The results were consistent with this prediction. As shown in Fig. 3, stepping error for the manipulated target did not significantly differ across conditions and was not significantly different from full vision in any condition (t17 = 0.18, P = 0.86, d = 0.04 for the 1.9–0.5 condition; t17 = 1.20, P = 0.25, d = 0.28 for the 1.9–0.9 condition; t17 = 1.62, P = 0.12, d = 0.38 for the 1.5–0.9 condition). This allows us to rule out the possibility that subjects were more accurate in the Overlap condition of experiment 2A simply because targets were visible for more time and bolsters the conclusion that walkers rely on information about the relative position of the two upcoming targets to modulate the push-off force from the trailing foot.
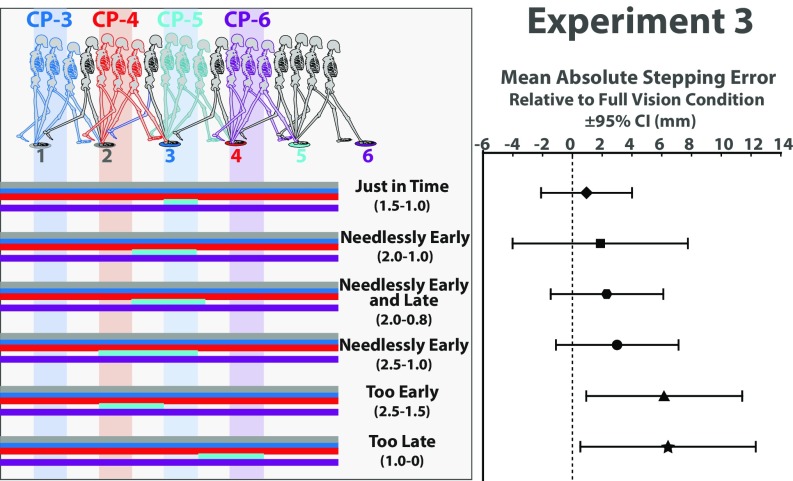
Minimal Visibility Conditions (Experiment 3).
Taken together, the critical control phase hypothesis and the two targets hypothesis delineate the minimal visibility conditions that must be satisfied to attain the same level of foot placement accuracy that is possible when vision is unrestricted: Visual information about the location of a given target relative to the preceding target must be available during the last half of the preceding step. When the necessary and sufficient conditions are not satisfied, foot placement accuracy should degrade. Making targets visible beyond these minimal conditions, including the full-vision condition, should not result in further reductions in stepping error.
These predictions were tested in experiment 3 by manipulating the visibility of a single target (as in experiment 2B) across six different conditions that varied in the timing and duration of target visibility (Fig. 4). In the Just in Time condition, the manipulated target was visible for 1/2 a step during and only during the critical control phase. Because the previous target was always visible, visual information about the location of the manipulated target relative to the previous target was available. In other words, the minimal conditions were satisfied. As predicted, stepping error was not significantly different from the full vision control condition (t11 = 0.67, P = 0.52, d = 0.19). In the next three conditions in Fig. 4, the duration of target visibility was expanded to one full step, 1 1/5 steps, and 1 1/2 steps while keeping the manipulated target visible during the critical control phase. These three conditions represent conditions in which the duration of target visibility is needlessly expanded. Again, stepping error was not significantly different from full vision in any of these conditions (P > 0.05). In the last two conditions, the manipulated target was visible for one full step but before or after the critical control phase. Despite the fact that the target was visible for twice as long as it was in the Just in Time condition, stepping error was significantly greater (t11 = 2.56, P < 0.05, d = 0.74 for the 2.5–1.5 condition; t11 = 2.38, P < 0.05, d = 0.69 for the 1.0–0 condition).
Fig. 4.
(Left) Depiction of target visibility manipulations used in experiment 3. Horizontal bars indicate the location of the walker’s COM when the target of the corresponding color was visible. Vertical bands indicate hypothesized critical control phase for each target. Target visibility manipulation was applied to either target 4 or target 5. (Right) Mean absolute stepping error relative to full vision control condition and 95% CI for each condition in experiment 3.
The results of experiment 3 provide compelling support for the predicted minimal conditions that were derived from the critical control phase and two targets hypotheses. Even a brief glimpse of the two upcoming targets is sufficient, as long as that information is available at just the right time—that is, during the critical phase for the visual control of walking.
Discussion
Summary of Findings.
When humans walk over complex terrain, it is necessary to make gait adjustments to accommodate obstacles and irregularities in the locations of safe footholds. However, that does not mean that each and every detail of the movement needs to be controlled. Active control may be needed only to occasionally tweak the mechanical state of the body so that the movement can be largely under passive control. The findings of the present study suggest that these adjustments happen during the latter part of the stance phase on the leg that is intended for the target foothold and are based on visual information about the position of the intended target relative to the location where the foot will be planted during the step to that target. Importantly, this is not merely a descriptive account of when visual information is needed and where that information is found. Both of these statements derive from the hypothesis that walkers attempt to exploit their biomechanical structure when walking over complex terrain, just as they do in flat, obstacle-free environments.
This strategy for visual control offers some distinct advantages. Once the upcoming step is initialized, the walker can land on the upcoming target by simply allowing the body to follow its natural, pendular trajectory. Because there is no need to actively steer during this phase, the walker can begin to use information about the terrain near the next step, so that that step can also be completed under passive control. In this sense, the strategy can be envisioned as an ongoing cycle of passive movement interspersed with brief intervals of active control, allowing walkers to navigate extended stretches of complex terrain with the efficiency and stability that best approximates that which is achieved when walking over simple terrain.
Further studies are needed to determine whether this conclusion generalizes to uneven terrain with raised obstacles and to other locomotor speeds and gaits. Interestingly, Warren (47) reported that runners must have visual information about the two upcoming targets during the flight phase before heel strike on the first target. This is strikingly similar to the results of the present study and suggests that the critical phase hypothesis and two targets hypothesis may generalize to running.
The findings also converge with recommendations found in manuals describing the proper use of the long cane by blind walkers, which suggest tapping the region of the ground “in front of the foot which is about to be brought forward” (ref. 48, p. 251; see also refs. 49 and 50). This method provides the walker with haptic information about the terrain relevant to the upcoming step precisely during the critical control phase of the human gait cycle.
Lastly, the findings have implications for gaze behavior and peripheral vision. Although walkers visually control their movements with respect to one target at a time, the two targets hypothesis and the results of experiments 2 and 3 indicate that the relevant information for each step is found in the position of each target relative to the previous target. Because walkers can only look at one target at a time, peripheral vision may be necessary to detect this information. If the walker had a condition that impaired peripheral vision, the relative positions of consecutive targets could not be accurately estimated and stepping accuracy would be expected to degrade as it did in the Gap condition of experiment 2A. Findings from previous studies in which the lower visual field is occluded (e.g., by wearing goggles) are consistent with a role for peripheral vision (e.g., see ref. 35 for a review).
The Critical Control Phase and the Role of Spatial Memory.
On the assumption that walkers attempt to move as ballistically as possible during the single support phase of the gait cycle, the end of the critical phase can be demarcated by a discrete change in the mechanical state of the walker (i.e., toe off). In contrast, the beginning of this phase is not so clearly defined. Any adjustment to the two determinants of the ballistic trajectory of the COM during the single support phase (discussed above) must occur before the onset of the single support phase, and as such, visual information about upcoming targets will become increasingly important leading up to that point. However, in principle there is no reason why a walker could not begin making gait adjustments far in advance. Nevertheless, the empirical result that subjects showed degraded performance in the Too Early condition but not the Just In Time condition shows that subjects have difficulty stepping accurately to targets that are not visible after midstance of the preceding step. The difference in behavior in those two conditions is especially notable because the difference in timing between those two conditions was extremely small (half a step, or roughly 200 ms). Although there are clear mechanical reasons for the difference in performance between the Too Late and Just in Time conditions, what could account for the decrease in stepping accuracy in the Too Early condition?
First, it is important to note that planning multiple steps ahead will yield diminishing returns for each additional step planned. Mechanical simulation studies of bipedal walking based on a linear inverted pendulum model show that planning more than two or three steps ahead yields only negligible increases in control authority (51, 52). In addition, due to the inescapable variability of motor behavior, any multistep plan will be subject to accumulating error on each successive step. In short, even if walkers were able to plan many steps into the future, doing so might not yield significant advantages over the shorter planning window entailed by the control phase strategy.
With this consideration in mind, it is possible that the control phase strategy of visually guided walking represents an attempt to balance between the conflicting needs to plan for future steps while simultaneously executing the current step. The interpretation is supported by investigations of gaze behavior in an object manipulation task, which have found that subjects appear to minimize the use of short-term/working memory during the execution of a hand–eye coordination task. Rather than storing the task-relevant properties of objects in memory, subjects appeared to use a “just in time” strategy§ whereby they only acquired necessary information just at the point that it was required for the task (53, 54). When walking over terrain where each step requires precise placement, a walker must visually identify and prepare for upcoming steps while simultaneously stepping toward the remembered location of the target identified in the preceding step. The execution of the guided steps may exact its own cost, which may interfere with subjects’ ability to maintain a precise spatial memory of the location of upcoming targets if they are not visible during the time that the necessary gait modifications need to be made (i.e., during the critical control phase). Thus, because the advantages to planning multiple steps ahead may be minimal anyway, walkers may adopt a strategy based on planning for the upcoming step only during the critical phase for visual control of the upcoming step.
In short, there are clear mechanical reasons why the end of the critical control phase would occur at toe off—any control enacted after that point would interfere with the natural, inverted pendulum-like trajectory of the body during the single support phase. The reason for the empirically identified onset of the critical phase (i.e., midstance) is not as obvious. We argue that this timing may arise from the interaction between visual working memory and the dual-task nature of visually guided walking over terrain with consecutive constrained footholds, but this explanation is largely speculative. Further research may be needed to identify the factors that contribute to the phase-specific use of visual information to guide foot placement in complex terrain.
Blending Active and Passive Modes of Control.
The results of the present study also have implications for the more general question of how actors blend active and passive modes of control. This issue has been extensively investigated in the context of a ball bouncing task in which the subject repeatedly bounces a ball on a racket while attempting to maintain a regular rhythm and bounce height. Ball bouncing is analogous to bipedal walking insofar as it involves the periodic regulation of a rhythmic process that is largely defined by its physical dynamics. Schaal et al. (2) identified a simple, passively stable solution to this problem: If the racket hits the ball during the deceleration phase of the upswing, small variations in the velocity of the ball at impact will die out on their own over repeated cycles, and peak ball height will return to a constant value. As such, the actor does not need to rely on visual information about the motion of the ball to adjust the motion of the racket on each and every cycle. In principle, the ball-bouncing task can be performed with eyes closed. In practice, active, perceptually guided adjustments do play a role (55–57), but it is also clear that actors take advantage of the inherent passive stability (2, 56–58). Taken together, the findings point to a blending of these two modes, which Siegler et al. (55) called mixed control, in which actors actively regulate racket movements based on perceptual information but also attempt to keep the system near the region of passive stability. Relying on mixed control has the advantage of shortening the time to return to steady-state bouncing after a perturbation and eliminates the need for a switching mechanism to turn active control on or off depending on whether the system is outside of or within the region of passive stability. Likewise, remaining near the maximally stable region has the advantage of minimizing the adjustments needed to compensate for variability and keeps the behavior more stable.
Whereas in ball bouncing actors attempt to maintain steady-state behavior from cycle to cycle and only use active control to respond to perturbations, walking over complex terrain requires the adjustment of every step to accommodate variations in the environment. As such, one might expect active control to be the dominant mode during the walking task, with passive control used only on steps for which foot placement is unconstrained. The results of the present study suggest otherwise. In the locomotor task, the role that active control plays and the information upon which it relies is dictated by the imperative to let passive control dominate the movement. Thus, even for a task that cannot be successfully performed without active control, active control appears to function in the service of passive control. Information is necessary for adaptive flexibility, but—as in ball bouncing—behavior is ultimately organized around solutions that exploit passive forces and passive stability (59).
Scope, Limitations, and Future Directions.
The purpose of this study (along with refs. 43, 44, and 46) was to identify the minimum requirements for the availability of visual information to support normal levels of stepping accuracy when walking over complex terrain. Within the precision of our methods, those minimum requirements were satisfied in the Just in Time condition of experiment 3, in which subjects displayed nominal stepping performance when visual information about the position of a target foothold relative to the previous base of support was available during the critical control phase. However, our methodology of precisely coupling the visibility of targets to subjects’ gait cycle afforded experimental control at the cost of ecological validity. Although our results are consistent with many studies of gaze behavior during locomotion, these behaviors may be affected by subtle changes in the task when visual information is unconstrained. For instance, it has been shown that subjects’ ability to step accurately on targets in an otherwise unconstrained walkway was correlated with the duration of the time that they fixated the target (60–62). This finding would appear to conflict with our finding that the availability of visual information about target location is not necessary after toe off, except that the targets in those experiments were always separated by at least two steps’ worth of empty walkway. This separation mitigated the dual-task nature of simultaneous step planning and execution that was present in our studies, which may explain the differences in behavior. Similarly, subjects were able to successfully step over an obstacle that was occluded during the last five steps of the approach (30), but this may not have been possible if there had been other impediments in the space before the obstacle.
Human behavior is nothing if not adaptable, and we should expect that subjects’ use of visual information will vary with the specific constraints of the task that is being performed (53). Nevertheless, the results of this study reveal the way that the minimum requirements for successful locomotion over complex terrain are shaped by the biomechanics and physical dynamics of bipedal gait. Future research will examine walkers’ gaze behavior as they traverse rough terrain to understand how the constraints described by the control phase hypothesis extrapolate to more natural locomotor environments. In addition, although much of our interpretation of the results of these studies focus on the regulation of the push-off force, we do not directly measure this aspect of subjects’ locomotor behavior. Future work may record muscle activity and ground reaction force during walking over visually specified targets to test the specific biomechanical predictions afforded by the control phase hypothesis about the visual control of foot placement walking over complex terrain.
Concluding Remarks
A central component of the characterization of walking introduced in this study is that humans attempt to move ballistically during the single support phase, allowing the trajectory of their body to be largely determined by their physical structure, momentum, and the force of gravity. Of course, the muscles do play a role, even during the single support phase when muscle cocontraction is required to support body weight and maintain stiffness of the knee (63). Although the details of muscle work and energetic costs throughout the gait cycle is an area of active discussion (e.g., refs. 64 and 65), it is undeniable that bipedal gait is accompanied by significant physical forces, and as such these forces may be harnessed for the purposes of minimizing energetic costs.
Human motor control is often quite miserly with respect to energetic costs. Walkers readily adjust their preferred locomotor patterns to achieve increases in energetic efficiency, even when such increases are small and brought about by artificial manipulations, such as by applying springs to the lower limbs (66, 67) or by moving the belts on a split belt treadmill at different speeds (68). From this perspective, it should come as no surprise that the visual control of walking over complex terrain is similarly organized around the goal of minimizing the energetic cost of locomotion. Rather than using visual feedback to enact continuous, feedback-driven muscular control of the COM and lower limbs, walkers use visual information in a feedforward manner to tailor the initial conditions of each step to the upcoming path. From a neural perspective, visual signals mediated through the posterior parietal and motor cortices may generate the descending signals needed to modulate the ongoing rhythmic oscillations of the subcortical and spinal networks to generate the appropriate muscular impulses for the coming step (21, 23). In this way, visual control allows walkers to traverse complex terrain while exploiting the same dynamics that underlie the energetic efficiency of human locomotion in flat, obstacle-free environments.
Materials and Methods
Participants.
Fourteen subjects [5 females, 9 males; mean age = 18.7 (18–21) years] participated in experiment 1, 18 subjects [7 females, 11 males; mean age = 19.1 (18–21) years] participated in experiment 2, and 12 subjects [5 females, 7 males; mean age = 20.0 (18–22) years] participated in experiment 3. Data from two subjects in experiment 1 were excluded because their average stepping accuracy across all conditions was more than 2 SDs from the mean. Mean height, weight, and leg length for participants in each experiment are given in Table 1. Participants were recruited from psychology courses and received extra credit for participating. All participants reported normal or corrected-to-normal vision, and none reported any motor impairments. The protocol was approved by the Institutional Review Board at Rensselaer Polytechnic Institute, and all subjects gave informed consent before performing the experiment.
Table 1.
Participant height, weight, and leg length
| Average height, m (range) | Average weight, kg (range) | Average leg length, m (range) | |
| Experiment 1 | 1.73 (1.55–1.85) | 76 (50–118) | 0.91 (0.81–0.98) |
| Experiment 2A and B | 1.73 (1.58–1.85) | 66 (51–85) | 0.94 (0.85–1.04) |
| Experiment 3 | 1.67 (1.58–1.83) | 63 (45–79) | 0.89 (0.83–0.99) |
Experimental Apparatus and Setup.
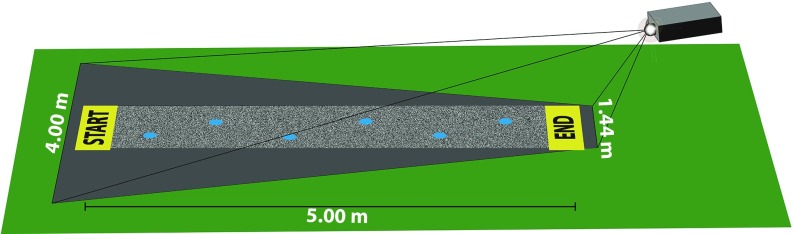
All three experiments made use of a custom-built experimental apparatus that synchronizes a full body motion capture system to an LCD projector that displays virtual target footholds on the floor (Fig. 5). The system detects when a subject’s foot lands on one of the virtual targets in real time and can render the terrain visible or invisible based on the subject’s location in the room. The setup used a 14-camera Vicon Motion Capture system running Vicon Nexus 1.7 software. The motion capture system tracked the positions of a set of retroreflective markers that were attached to black spandex suits worn by subjects in accordance with the Vicon Plug-In Gait Full Body (SACR) marker set included in the Nexus software package. A Sanyo PLC-XP45 projector was used to display virtual obstacles onto the floor at a resolution of 1024 × 768 with a brightness of 3500 ANSI lumens. The projector was located 1.75 m behind the end position at a height of 1.15 m pointed at the ground at a low angle of incidence (∼20 degrees; Fig. 5). The motion capture and projector systems were integrated using custom software. The projector position was calibrated using a method inspired by the Tsai calibration algorithm (69). For this method, calibration points defined in pixel coordinates (i.e., xy coordinates on a computer monitor) were displayed on the laboratory floor, and a marker tracked by the motion capture system was placed on each projected point. The position of the marker in world coordinates (i.e., as defined by the motion capture system) and the position of the corresponding calibration point in pixel coordinates were used to calculate the transformation between screen coordinates (pixels) to world coordinates (millimeters).
Fig. 5.
Experimental set-up (shown with all targets visible). Reprinted with permission from ref. 46 (Matthis et al.), copyright Association for Research in Vision and Ophthalmology.
Task and Procedure.
The basic task required subjects to walk across a path of six small circular target footholds (radius, 50 mm) that covered the space between the start and end locations. The path between the start and end boxes was overlaid by a scintillating white noise texture designed to prevent subjects from using the carpet texture as a landmark to keep track of targets after they were made invisible. To define the target configurations used in this study, the experimenter recorded their normal footfall pattern when walking from the start to end location without targets. The target configurations were then obtained by pseudorandomly placing the targets within a 300 mm × 300 mm box centered on each step in this pattern of foot falls. To scale the task to each subject’s body size, the distances between the targets were multiplied by the ratio of the subject’s leg length to the leg length of the experimenter who defined the original footfall pattern.
Subjects completed the experiment barefoot to avoid any irregularities caused by differences in footwear. They were asked to walk at a brisk pace from a start position (marked with tape on a carpeted floor) to an end position 5.0 m away. Subjects initiated each trial by clicking a button on a wireless mouse. They were instructed to walk so as to place the ball of their foot (specifically, the mocap marker attached to the second metatarsal head of the foot) as close to the center of the target as possible. At each step, if the marker was within the target radius, the computer speakers played a high-pitched tone to indicate that the subject had successfully hit the target. Otherwise, a low-pitched tone was played to indicate that the target had been missed. If the subject failed to reach the end position in 5 s, the trial was terminated and rerun. This constraint ensured that subjects had to walk at an average speed of at least 1.0 m/s to complete the trial in time. Trials in which the subject missed one or more targets were not rerun.
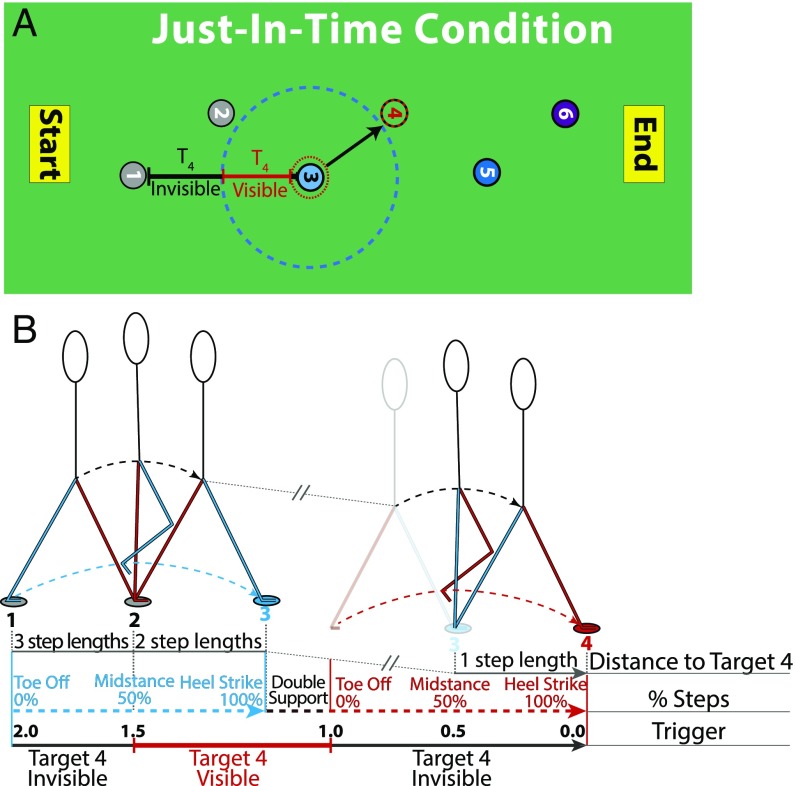
The visibility of target footholds was controlled using a combination of an “invisibility trigger” (similar to the one used in ref. 46) and a “visibility trigger.” These triggers were circular regions centered on each target that trigger a change in the target’s visibility when the subject’s foot entered into its radius. Visibility triggers caused targets to go from invisible to visible, whereas invisibility triggers did the opposite. By combining these two types of triggers, we could ensure that targets were only visible to the subject at a specified phase of their gait cycle as they traversed the path of target footholds (Fig. 6A).
Fig. 6.
Schematic depiction of the target visibility manipulation used in the Just in Time condition applied to target 4. (A) Target 4 becomes visible when the subject’s foot crosses the visibility trigger surrounding target 3 (blue dashed circle) and remains so until the foot crosses the invisibility trigger (red dotted circle). (B) The steps to target 3 (blue arrows) and target 4 (red arrows) shown with time progressing from left to right. The visibility of target 4 is shown on the black and red arrow, along with the trigger number associated the changing visibility.
Fig. 6B shows the steps to target 3 and target 4 unfolding continuously in time. The right leg (colored blue in Fig. 6B) leaves target 1 and crosses the visibility trigger centered on target 3 (blue dotted line in Fig. 6A) ∼50% of the way through that step (during midstance, when the walker is directly above the supporting limb and roughly two step lengths from target 4). At this point, target 4 becomes visible and remains so through the double-support phase. It then becomes invisible when the left foot (colored red in Fig. 6B) leaves target 2 at toe off and remains so for the entire step toward Target 4 (red dotted arrow). The other visibility manipulations used in this study can also be understood using this representation.
Fig. 6B is also useful for understanding the visual look-ahead distance that was available to subjects in the different visibility conditions. When the right (blue-colored) foot crosses the visibility trigger that causes target 4 to become visible, the vertical projection of the subject’s head is approximately two step lengths from the location of target 4. Thus, the 1.5 trigger used in the Just in Time condition provided subjects with two step lengths of visual look ahead. Put another way, the 1.5 trigger in this study is equivalent to the two step-length visibility windows used in Matthis and Fajen (43, 44).
As a result of processing time and the network communication between the computer running the motion capture system and the computer controlling the projector, there was an 80–90-ms lag between the instant that a subject reached a visibility or invisibility trigger (in the real world) and the instant that the display was updated. Thus, a visibility trigger that was set to be 0.5 steps would actually make the target visible at ∼0.35 steps. To compensate for this lag, triggers were programmed to be 15% larger than the desired visibility manipulation. Thus, to achieve an actual 0.5 step visibility trigger, the trigger would be programmed to be 0.65 steps. To make targets appear or disappear at the beginning of a step, we placed the trigger around the preceding target and set the radius to 0.1 steps. We then delayed the visibility manipulation by 130 ms so that the target appeared or disappeared near toe off. All adjusted triggers were tested before the experiment and verified for each subject following data collection. By comparing motion capture data to logs from the computer controlling the projector, we were able to verify that the experienced trigger corresponded to the intended visibility windows described here.
Design.
Each experimental session comprised two blocks of trials with a short break between blocks. In each block, trials were presented in subblocks of 12 trials of the same condition (i.e., with the same visibility and invisibility triggers). The order of conditions within each block was randomized across subjects and the position of each target varied across repetitions within a subblock as described in Task and Procedure. The second block was identical to the first, except that the order of conditions (i.e., subblocks) was reversed. In total, each subject completed 24 repetitions of each of the experimental conditions. In experiments 1 and 3, subjects also completed 12 repetitions of target-free walking before the first subblock.
Experiment 1 also included three conditions that were not reported in the main text or Fig. 2, and experiment 2 also included a set of conditions in which the same manipulations shown in Fig. 3 were applied to two consecutive targets. The data from these conditions were not reported in the main text or figures in the interest of brevity but can be seen in Figs. S2 and S3). The findings from these conditions are consistent with the interpretation given in the main text.
Fig. S2.
(Left) Depiction of three additional target visibility manipulations used in experiment 1 that were not reported in the original manuscript. Horizontal bars indicate the phase of the gait cycle when the target of the corresponding color was visible. Vertical bands indicate hypothesized critical control phase for each target. (Right) Mean absolute stepping error and 95% CI for each condition. Note that stepping error is less than it is in the Just in Time and Needlessly Early conditions (see Fig. 2). This is consistent with the two targets hypothesis, as there is an overlap in the visibility of the two upcoming targets in all three conditions shown in this figure.
Fig. S3.
(Left) Depiction of target visibility manipulations used in experiment 2C with two active targets. Horizontal bars indicate the phase of the gait cycle when the target of the corresponding color was visible. Vertical bands indicate hypothesized critical control phase for each target. (Right) Mean absolute stepping error relative to full vision control condition and 95% CI for each condition in experiment 2C. Note that results are nearly identical to those in experiment 2A with four active targets but different from those in experiment 2B with one active target (see Fig. 3). This is consistent with the two targets hypothesis, as the degree of overlap in the visibility of consecutive targets varies across conditions as it does in experiment 2A.
Analyses.
In all experiments, stepping accuracy was calculated by taking the root mean square of the Euclidean distance between the marker on the second metatarsal head of the foot and the center of the target. Stepping accuracy was analyzed for targets 3–6 in experiment 1, as the visibility manipulations did not apply to targets 1 and 2. Because young, healthy adults reach steady-state walking speed within the first two steps (70), this also helped to ensure that the analyses were based on the portion of each trial during which subjects were moving at a constant speed.
In experiment 2A, stepping accuracy was only analyzed for targets 4 and 5 to allow for comparison between the results of experiment 2A and 2C. In experiment 2b and experiment 3, stepping accuracy was only analyzed from the target that was affected by the visibility manipulation.
Supplementary Material
Acknowledgments
We thank Gaby Ciavardoni and Kevin Sullivan for their programming contributions and Evelyn Hinojosa and Dylan Brion for their assistance with data collection. This research was supported by National Science Foundation Grant 1431078 and National Institutes of Health Grant 1R01EY019317.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
*The forthcoming description of the human gait cycle is an adaptation of the “simplest walking model,” which is an attempt to develop the most parsimonious description of bipedal walking that still captures key characteristics of human locomotion (13, 71, 72). Although more complete musculoskeletal models can be invaluable for determining the contributions of individual muscles to locomotor behavior (63), such models are computationally expensive and conceptually difficult to analyze. By abstracting the highly complex action of human locomotion down to the simplest possible biomechanical model, it is possible to explore how the control of human locomotion is organized around the underlying physical dynamics of bipedal gait (16, 73–75).
†Passive stability has its limits. Larger perturbations will result in shorter steps, which dissipate less energy and require active compensation to avoid falling (15). Furthermore, walkers are passively stable along the axis of forward progress but not along the medial-lateral axis (76). Active control is needed to stabilize the body in response to lateral perturbations. Such responses rely on sensory (e.g., visual, vestibular) information, involve adjustments to foot placement that affect step width, and exact some metabolic cost (77, 78).
‡A step length is defined as the distance between two subsequent footfalls (Fig. 1A) and is distinct from a step, which refers to the action of lifting the foot and moving it to a new location. The term visual look ahead refers to a linear distance from the vertical projection of the walker’s head onto the ground plane. As shown in Fig. 1A, a visual look ahead of two step lengths would allow a walker to see a target foothold when he or she is halfway through the step to the preceding target (i.e., 1 1/2 steps in advance). See the Materials and Methods for additional discussion.
§The similar naming of the Just in Time condition in our experiments and the Just in Time gaze allocation strategy in ref. 54 was coincidental, though it is an interesting example of convergent research.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1611699114/-/DCSupplemental.
References
- 1.Bernstein N. Pergamon Press; New York: 1967. The coordination and regulation of movements. [Google Scholar]
- 2.Schaal S, Atkeson CG, Sternad D. One-handed juggling: A dynamical approach to a rhythmic movement task. J Mot Behav. 1996;28:165–183. doi: 10.1080/00222895.1996.9941743. [DOI] [PubMed] [Google Scholar]
- 3.Rossignol S, Dubuc R, Gossard J-P. Dynamic sensorimotor interactions in locomotion. Physiol Rev. 2006;86:89–154. doi: 10.1152/physrev.00028.2005. [DOI] [PubMed] [Google Scholar]
- 4.Cavagna GA, Margaria R. Mechanics of walking. J Appl Physiol. 1966;21:271–278. doi: 10.1152/jappl.1966.21.1.271. [DOI] [PubMed] [Google Scholar]
- 5.Cavagna GA, Kaneko M. Mechanical work and efficiency in level walking and running. J Physiol. 1977;268:467–481. doi: 10.1113/jphysiol.1977.sp011866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mochon S, McMahon TA. Ballistic walking. J Biomech. 1980;13:49–57. doi: 10.1016/0021-9290(80)90007-x. [DOI] [PubMed] [Google Scholar]
- 7.Kuo AD. The six determinants of gait and the inverted pendulum analogy: A dynamic walking perspective. Hum Mov Sci. 2007;26:617–656. doi: 10.1016/j.humov.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 8.Kuo AD, Donelan JM, Ruina A. Energetic consequences of walking like an inverted pendulum: Step-to-step transitions. Exerc Sport Sci Rev. 2005;33:88–97. doi: 10.1097/00003677-200504000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Donelan JM, Kram R, Kuo AD. Mechanical work for step-to-step transitions is a major determinant of the metabolic cost of human walking. J Exp Biol. 2002;205:3717–3727. doi: 10.1242/jeb.205.23.3717. [DOI] [PubMed] [Google Scholar]
- 10.Neptune RR, Zajac FE, Kautz SA. Muscle mechanical work requirements during normal walking: The energetic cost of raising the body’s center-of-mass is significant. J Biomech. 2004;37:817–825. doi: 10.1016/j.jbiomech.2003.11.001. [DOI] [PubMed] [Google Scholar]
- 11.Adamczyk PG, Kuo AD. Redirection of center-of-mass velocity during the step-to-step transition of human walking. J Exp Biol. 2009;212:2668–2678. doi: 10.1242/jeb.027581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donelan JM, Kram R, Kuo AD. Simultaneous positive and negative external mechanical work in human walking. J Biomech. 2002;35:117–124. doi: 10.1016/s0021-9290(01)00169-5. [DOI] [PubMed] [Google Scholar]
- 13.Kuo AD. Energetics of actively powered locomotion using the simplest walking model. J Biomech Eng. 2002;124:113–120. doi: 10.1115/1.1427703. [DOI] [PubMed] [Google Scholar]
- 14.Zelik KE, Takahashi KZ, Sawicki GS. Six degree-of-freedom analysis of hip, knee, ankle and foot provides updated understanding of biomechanical work during human walking. J Exp Biol. 2015;218:876–886. doi: 10.1242/jeb.115451. [DOI] [PubMed] [Google Scholar]
- 15.Kuo AD, Donelan JM. Dynamic principles of gait and their clinical implications. Phys Ther. 2010;90:157–174. doi: 10.2522/ptj.20090125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donelan JM, Kram R, Kuo AD. Mechanical and metabolic determinants of the preferred step width in human walking. Proc Biol Sci. 2001;268:1985–1992. doi: 10.1098/rspb.2001.1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeer T. Passive dynamic walking. Int J Robot Res. 1990;9:62–82. [Google Scholar]
- 18.Ahn J, Hogan N. Walking is not like reaching: Evidence from periodic mechanical perturbations. PLoS One. 2012;7:e31767. doi: 10.1371/journal.pone.0031767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ahn J, Hogan N. A simple state-determined model reproduces entrainment and phase-locking of human walking. PLoS One. 2012;7:e47963. doi: 10.1371/journal.pone.0047963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ahn J, Hogan N. Long-range correlations in stride intervals may emerge from non-chaotic walking dynamics. PLoS One. 2013;8:e73239. doi: 10.1371/journal.pone.0073239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McVea DA, Pearson KG. Object avoidance during locomotion. In: Sternad D, editor. Advances in Experimental Medicine and Biology. Springer; Boston, MA: 2009. pp. 293–315. [DOI] [PubMed] [Google Scholar]
- 22.Andujar JE, Lajoie K, Drew T. A contribution of area 5 of the posterior parietal cortex to the planning of visually guided locomotion: Limb-specific and limb-independent effects. J Neurophysiol. 2010;103:986–1006. doi: 10.1152/jn.00912.2009. [DOI] [PubMed] [Google Scholar]
- 23.Drew T, Marigold DS. Taking the next step: Cortical contributions to the control of locomotion. Curr Opin Neurobiol. 2015;33:25–33. doi: 10.1016/j.conb.2015.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Kalaska JF. Parietal cortex area 5 and visuomotor behavior. Can J Physiol Pharmacol. 1996;74:483–498. [PubMed] [Google Scholar]
- 25.Lacquaniti F, Guigon E, Bianchi L, Ferraina S, Caminiti R. Representing spatial information for limb movement: Role of area 5 in the monkey. Cereb Cortex. 1995;5:391–409. doi: 10.1093/cercor/5.5.391. [DOI] [PubMed] [Google Scholar]
- 26.Maeda RS, O’Connor SM, Donelan JM, Marigold DS. Foot placement relies on state estimation during visually guided walking. J Neurophysiol. 2016;117:480–491. doi: 10.1152/jn.00015.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Patla AE, Adkin A, Martin C, Holden R, Prentice S. Characteristics of voluntary visual sampling of the environment for safe locomotion over different terrains. Exp Brain Res. 1996;112:513–522. doi: 10.1007/BF00227957. [DOI] [PubMed] [Google Scholar]
- 28.Patla AE. Understanding the roles of vision in the control of human locomotion. Gait Posture. 1997;5:54–69. [Google Scholar]
- 29.Barton SL, Matthis JS, Fajen BR. Visual regulation of gait: Zeroing in on a solution to the complex terrain problem. J Exp Psychol Hum Percept Perform. 2017 doi: 10.1037/xhp0000435. [DOI] [PubMed] [Google Scholar]
- 30.Patla AE, Greig M. Any way you look at it, successful obstacle negotiation needs visually guided on-line foot placement regulation during the approach phase. Neurosci Lett. 2006;397:110–114. doi: 10.1016/j.neulet.2005.12.016. [DOI] [PubMed] [Google Scholar]
- 31.Marigold DS, Patla AE. Gaze fixation patterns for negotiating complex ground terrain. Neuroscience. 2007;144:302–313. doi: 10.1016/j.neuroscience.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 32.Patla AE, Vickers JN. How far ahead do we look when required to step on specific locations in the travel path during locomotion? Exp Brain Res. 2003;148:133–138. doi: 10.1007/s00221-002-1246-y. [DOI] [PubMed] [Google Scholar]
- 33.Patla AE, Vickers JN. Where and when do we look as we approach and step over an obstacle in the travel path? Neuroreport. 1997;8:3661–3665. doi: 10.1097/00001756-199712010-00002. [DOI] [PubMed] [Google Scholar]
- 34.Franchak JM, Adolph KE. Visually guided navigation: Head-mounted eye-tracking of natural locomotion in children and adults. Vision Res. 2010;50:2766–2774. doi: 10.1016/j.visres.2010.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Marigold DS. Role of peripheral visual cues in online visual guidance of locomotion. Exerc Sport Sci Rev. 2008;36:145–151. doi: 10.1097/JES.0b013e31817bff72. [DOI] [PubMed] [Google Scholar]
- 36.Laurent M, Thomson JA. The role of visual information in control of a constrained locomotor task. J Mot Behav. 1988;20:17–37. doi: 10.1080/00222895.1988.10735430. [DOI] [PubMed] [Google Scholar]
- 37.Marigold DS, Weerdesteyn V, Patla AE, Duysens J. Keep looking ahead? Re-direction of visual fixation does not always occur during an unpredictable obstacle avoidance task. Exp Brain Res. 2007;176:32–42. doi: 10.1007/s00221-006-0598-0. [DOI] [PubMed] [Google Scholar]
- 38.Patla AE, Beuter A, Prentice S. A two stage correction of limb trajectory to avoid obstacles during stepping. Neurosci Res Commun. 1991;8:153–159. [Google Scholar]
- 39.Day BL, Reynolds RF. Vestibular reafference shapes voluntary movement. Curr Biol. 2005;15:1390–1394. doi: 10.1016/j.cub.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 40.Weerdesteyn V, Nienhuis B, Hampsink B, Duysens J. Gait adjustments in response to an obstacle are faster than voluntary reactions. Hum Mov Sci. 2004;23:351–363. doi: 10.1016/j.humov.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 41.Voloshina AS, Kuo AD, Daley MA, Ferris DP. Biomechanics and energetics of walking on uneven terrain. J Exp Biol. 2013;216:3963–3970. doi: 10.1242/jeb.081711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Voloshina AS, Ferris DP. Biomechanics and energetics of running on uneven terrain. J Exp Biol. 2015;218:711–719. doi: 10.1242/jeb.106518. [DOI] [PubMed] [Google Scholar]
- 43.Matthis JS, Fajen BR. Visual control of foot placement when walking over complex terrain. J Exp Psychol Hum Percept Perform. 2014;40:106–115. doi: 10.1037/a0033101. [DOI] [PubMed] [Google Scholar]
- 44.Matthis JS, Fajen BR. Humans exploit the biomechanics of bipedal gait during visually guided walking over complex terrain. Proc R Soc B Biol Sci. 2013;280:20130700. doi: 10.1098/rspb.2013.0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zaytsev P, Hasaneini SJ, Ruina A. 2015 IEEE International Conference on Robotics and Automation (ICRA) IEEE; New York: 2015. Two steps is enough: No need to plan far ahead for walking balance; pp. 6295–6300. [Google Scholar]
- 46.Matthis JS, Barton SL, Fajen BR. The biomechanics of walking shape the use of visual information during locomotion over complex terrain. J Vis. 2015;15:10. doi: 10.1167/15.3.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Warren WH. Action modes and laws of control for the visual guidance of action. Adv Psychol. 1988;50:339–379. [Google Scholar]
- 48.Miller ME, Hoover RE. Foot travel without sight. Outlook Blind. 1946;40:244–251. [Google Scholar]
- 49.Miller J. Vision, a component of locomotion. Physiotherapy. 1967;53:326–332. [PubMed] [Google Scholar]
- 50.Rodgers MD, Emerson RW. Human factor analysis of long cane design: Weight and length. J Vis Impair Blind. 2005;99:622–632. [Google Scholar]
- 51.Koolen T, De Boer T, Rebula J, Goswami A, Pratt J. Capturability based analysis and control of legged locomotion, part 2: Application to three simple gait models. Control. 2010;(January):1–15. [Google Scholar]
- 52.Rebula J, Koolen IT, De Boer T. Capturability Based Analysis and Control of Legged Locomotion, Part 1 : Concepts and Definitions. Control. 2010;(January):1–11. [Google Scholar]
- 53.Hayhoe M, Ballard D. Eye movements in natural behavior. Trends Cogn Sci. 2005;9:188–194. doi: 10.1016/j.tics.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 54.Ballard DH, Hayhoe MM, Pelz JB. Memory representations in natural tasks. J Cogn Neurosci. 1995;7:66–80. doi: 10.1162/jocn.1995.7.1.66. [DOI] [PubMed] [Google Scholar]
- 55.Siegler IA, Bardy BG, Warren WH. Passive vs. active control of rhythmic ball bouncing: the role of visual information. J Exp Psychol Hum Percept Perform. 2010;36:729–750. doi: 10.1037/a0016462. [DOI] [PubMed] [Google Scholar]
- 56.Sternad D, Duarte M, Katsumata H, Schaal S. Bouncing a ball: tuning into dynamic stability. J Exp Psychol Hum Percept Perform. 2001;27:1163–1184. doi: 10.1037//0096-1523.27.5.1163. [DOI] [PubMed] [Google Scholar]
- 57.Wei K, Dijkstra TMH, Sternad D. Passive stability and active control in a rhythmic task. J Neurophysiol. 2007;98:2633–2646. doi: 10.1152/jn.00742.2007. [DOI] [PubMed] [Google Scholar]
- 58.de Rugy A, Wei K, Müller H, Sternad D. Actively tracking ‘passive’ stability in a ball bouncing task. Brain Res. 2003;982:64–78. doi: 10.1016/s0006-8993(03)02976-7. [DOI] [PubMed] [Google Scholar]
- 59.Warren WH. The dynamics of perception and action. Psychol Rev. 2006;113:358–389. doi: 10.1037/0033-295X.113.2.358. [DOI] [PubMed] [Google Scholar]
- 60.Chapman GJ, Hollands MA. Evidence that older adult fallers prioritise the planning of future stepping actions over the accurate execution of ongoing steps during complex locomotor tasks. Gait Posture. 2007;26:59–67. doi: 10.1016/j.gaitpost.2006.07.010. [DOI] [PubMed] [Google Scholar]
- 61.Chapman GJ, Hollands MA. Evidence for a link between changes to gaze behaviour and risk of falling in older adults during adaptive locomotion. Gait Posture. 2006;24:288–294. doi: 10.1016/j.gaitpost.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 62.Young WR, Hollands MA. Can telling older adults where to look reduce falls? Evidence for a causal link between inappropriate visual sampling and suboptimal stepping performance. Exp Brain Res. 2010;204:103–113. doi: 10.1007/s00221-010-2300-9. [DOI] [PubMed] [Google Scholar]
- 63.McGowan CP, Neptune RR, Clark DJ, Kautz SA. Modular control of human walking: Adaptations to altered mechanical demands. J Biomech. 2010;43:412–419. doi: 10.1016/j.jbiomech.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuo AD, Donelan JM. Comment on “Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking” (Neptune et al., 2001) and “Muscle mechanical work requirements during normal walking: The energetic cost of raising the body’s center-of-mass is significant” (Neptune et al., 2004) J Biomech. 2009;42:1783–1785, author reply 1786–1789. doi: 10.1016/j.jbiomech.2009.03.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Neptune RR, Zajac FE, Kautz SA. Author’s response to comment on “Contributions of the individual ankle plantar flexors to support, forward progression and swing initiation during walking” (Neptune et al., 2001) and “Muscle mechanical work requirements during normal walking: The energetic cost of raising the body’s center-of-mass is significant.”. J Biomech. 2009;42:1786–1789. doi: 10.1016/j.jbiomech.2009.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Snaterse M, Ton R, Kuo AD, Donelan JM. Distinct fast and slow processes contribute to the selection of preferred step frequency during human walking. J Appl Physiol (1985) 2011;110:1682–1690. doi: 10.1152/japplphysiol.00536.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Selinger JC, O’Connor SM, Wong JD, Donelan JM. Humans can continuously optimize energetic cost during walking. Curr Biol. 2015;25:2452–2456. doi: 10.1016/j.cub.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 68.Finley JM, Bastian AJ, Gottschall JS. Learning to be economical: The energy cost of walking tracks motor adaptation. J Physiol. 2013;591:1081–1095. doi: 10.1113/jphysiol.2012.245506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Tsai B. A versatile camera calibration technique for high-accuracy 3D machine vision meteorology using off-the-shelf TV camera and lenses. IEEE J Robot Autom. 1987;RA-3:323–344. [Google Scholar]
- 70.Muir BC, Rietdyk S, Haddad JM. Gait initiation: The first four steps in adults aged 20-25 years, 65-79 years, and 80-91 years. Gait Posture. 2014;39:490–494. doi: 10.1016/j.gaitpost.2013.08.037. [DOI] [PubMed] [Google Scholar]
- 71.Garcia M, Chatterjee A, Ruina A, Coleman M. The simplest walking model: Stability, complexity, and scaling. J Biomech Eng. 1998;120:281–288. doi: 10.1115/1.2798313. [DOI] [PubMed] [Google Scholar]
- 72.Collins S, Ruina A, Tedrake R, Wisse M. Efficient bipedal robots based on passive-dynamic walkers. Science. 2005;307:1082–1085. doi: 10.1126/science.1107799. [DOI] [PubMed] [Google Scholar]
- 73.Holt KJ, Jeng SF, Rr RR, Hamill J. Energetic cost and stability during human walking at the preferred stride velocity. J Mot Behav. 1995;27:164–178. doi: 10.1080/00222895.1995.9941708. [DOI] [PubMed] [Google Scholar]
- 74.Holt KG, Hamill J, Andres RO. The force-driven harmonic oscillator as a model for human locomotion. Hum Mov Sci. 1990;9:55–68. [Google Scholar]
- 75.Kuo AD. A simple model of bipedal walking predicts the preferred speed-step length relationship. J Biomech Eng. 2001;123:264–269. doi: 10.1115/1.1372322. [DOI] [PubMed] [Google Scholar]
- 76.Kuo AD. Stabilization of lateral motion in passive dynamic walking. Int J Robot Res. 1999;18:917–930. [Google Scholar]
- 77.Bauby CE, Kuo AD. Active control of lateral balance in human walking. J Biomech. 2000;33:1433–1440. doi: 10.1016/s0021-9290(00)00101-9. [DOI] [PubMed] [Google Scholar]
- 78.Donelan JM, Shipman DW, Kram R, Kuo AD. Mechanical and metabolic requirements for active lateral stabilization in human walking. J Biomech. 2004;37:827–835. doi: 10.1016/j.jbiomech.2003.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.