Significance
Physiology and behavior are subject to daily cycles. In Drosophila melanogaster, two clusters of clock neurons—morning (M cells) and evening (E cells) oscillators—are largely responsible for activity bursts at dawn and dusk. In contrast, male–female pairs of flies follow a distinct pattern: low activity at dusk, followed by male courtship activity during the night, referred to as “male sex drive rhythm” (MSDR). Here we report that males lacking Salt-inducible kinase 3 (SIK3) expression in M cells exhibit a short period of MSDR but a long period of single-fly locomotor rhythm (SLR) because circadian nucleocytoplasmic shuttling of Histone deacetylase 4 (HDAC4) is disrupted. We conclude that SIK3–HDAC4 signaling in M cells regulates MSDR by regulating the molecular oscillation in DN1 neurons.
Keywords: SIK3, HDAC4, male sex drive rhythm, circadian rhythms, Drosophila
Abstract
The physiology and behavior of many organisms are subject to daily cycles. In Drosophila melanogaster the daily locomotion patterns of single flies are characterized by bursts of activity at dawn and dusk. Two distinct clusters of clock neurons—morning oscillators (M cells) and evening oscillators (E cells)—are largely responsible for these activity bursts. In contrast, male–female pairs of flies follow a distinct pattern, most notably characterized by an activity trough at dusk followed by a high level of male courtship during the night. This male sex drive rhythm (MSDR) is mediated by the M cells along with DN1 neurons, a cluster of clock neurons located in the dorsal posterior region of the brain. Here we report that males lacking Salt-inducible kinase 3 (SIK3) expression in M cells exhibit a short period of MSDR but a long period of single-fly locomotor rhythm (SLR). Moreover, lack of Sik3 in M cells decreases the amplitude of PERIOD (PER) cycling in DN1 neurons, suggesting that SIK3 non–cell-autonomously regulates DN1 neurons’ molecular clock. We also show that Sik3 reduction interferes with circadian nucleocytoplasmic shuttling of Histone deacetylase 4 (HDAC4), a SIK3 phosphorylation target, in clock neurons and that constitutive HDAC4 localization in the nucleus shortens the period of MSDR. Taking these findings together, we conclude that SIK3–HDAC4 signaling in M cells regulates MSDR by regulating the molecular oscillation in DN1 neurons.
The physiology and behavior of most animals undergo daily oscillations, which are controlled by a small set of clock neurons in the brain. In mammals, a heterodimeric complex between CLOCK (CLK) and BMAL1 activates transcription of Period (Per1 and Per2) and Cryptochrome (Cry1 and Cry2) genes, and their protein products in turn inhibit the activity of CLK/BMAL1. Likewise, Drosophila heterodimeric complexes between CLK and CYCLE (CYC) activate the genes period (per) and timeless (tim), and their respective protein products repress CLK/CYC. These conserved negative-feedback loops, which include several kinases, produce rhythmic transcription profiles in numerous genes (1).
The Drosophila brain contains ∼150 clock neurons which are divided into seven clusters based on their anatomical locations and functional characteristics: the small and large ventral lateral neurons (sLNvs and lLNvs), the dorsal lateral neurons (LNds), the lateral posterior neurons (LPNs), and three dorsal neuron clusters (DN1–3) (2). Some of these clock neurons have distinct functions in circadian locomotor behavior. Specifically, four sLNvs, also referred to as “morning” (M) cells, express the neuropeptide pigment-dispersing factor (PDF) and control the timing of morning locomotor activity during light:dark (LD) cycles; these neurons are also the key pacemaker neurons in constant darkness (DD). The fifth, PDF−, sLNv and the LNds, referred to as “evening” (E) cells, are required for the generation of the evening activity peak in LD cycles (3). Communication between various groups of cells within this interconnected neural network enhances the synchrony of molecular oscillation in each neuron (4–7).
Ventral lateral neuron (LNv)-derived PDF plays a critical role in regulating the molecular clock (8). Specifically, PDF participates in synchronization of clock neurons by up-regulating cAMP (6), which activates PKA, which in turn regulates the stability of PER (9) and TIM (10, 11) in PDF receptor (PDFR)-expressing target neurons (4–6). Thus, Pdf+ sLNvs serve as master pacemakers that reset other clock neurons daily, thereby dictating the pace of behavioral rhythms even when flies are kept in DD (12, 13).
Locomotor activity is the best-characterized circadian behavior in Drosophila, but numerous other behaviors, such as courtship and mating, sleep, and feeding, are under strong circadian influence (14–18). We have previously shown that male–female pairs of flies exhibit activity patterns strikingly distinct from those of singly kept males or females (i.e., single-fly locomotor rhythm or SLR) or same-sex pairs of flies (16). The activity pattern of male–female pairs, which we refer to as “male sex-drive rhythm” (MSDR), is characterized by a trough at subjective dusk, followed by a sharp increase in male-driven courtship activity (especially “following” behavior) that peaks during the subjective night (16–18). A functional molecular clock in both Pdf+ LNvs (17) and DN1 neurons (17, 18) is necessary and sufficient for proper MSDR. However, few other cellular and molecular components contributing to MSDR have been identified to date. Specifically, information is lacking about both the molecular and cellular identity of downstream effectors of the main clock components that are important for MSDR.
Here we report the identification of two downstream effectors of the molecular clock that play distinct roles in MSDR and SLR. Using an RNAi screen for kinases, we show that Salt-inducible kinase 3 (SIK3) is a critical component for circadian behavior. Sik3 knockdown in subsets of clock neurons (DN1 neurons or Pdf+ LNvs) causes a short period of MSDR, whereas the period length of SLR is slightly shortened with Sik3 knockdown in DN1 neurons and is slightly elongated with Sik3 knockdown in sLNvs. We also find that transcriptional activity of Histone deacetylase 4 (HDAC4) is regulated by SIK3 in a circadian manner. Finally, Sik3 reduction in Pdf+ LNvs reduces the amplitude of PER oscillation in DN1 neurons and shortens the length of the MSDR period, suggesting that SIK3–HDAC4 signaling plays an important role in the determination of MSDR period by modulating the intercellular communication between clock neurons.
Results
Sik3 Plays a Key Role for MSDR in Clock Neurons.
Because many protein kinases serve as core clock components that regulate SLR (19), we reasoned that some might also regulate MSDR. We therefore performed an RNAi screen targeting about 150 protein kinases (as well as other proteins of interest) within the male courtship circuit. This neural circuit, defined by the expression of FRUITLESSM (FRUM), consists of ∼2,000 neurons that control all aspects of male courtship behavior (20). We analyzed locomotor activity of male–female pairs in DD and found that knockdown of three kinases known to be involved in regulating the circadian clock (sgg, CkIIβ, and dbt), as well as Sik3, affected MSDR (Table S1). Specifically, we observed that loss of Sik3 in fruitless neurons (fru > Dcr2, Sik3RNAi) significantly reduced rhythmicity and shortened the period length of MSDR but did not affect SLR (Fig. 1 A and A′ and Table S2). The FRUM circuit includes many clock neurons such as the LNvs, LNds, and DN1 neurons (17, 21), and we investigated whether expressing Sik3 in clock neurons within the FRUM network of males is sufficient to restore MSDR by using cry-GAL80 to block Sik3RNAi expression in all clock neurons (fru > Dcr2, Sik3RNAi, cry-GAL80). Indeed, these males exhibited both higher rhythmicity and normal period length in MSDR (Fig. 1 A and A′ and Table S2).
Table S1.
MSDR screening of kinase RNAi lines
| Line no. | CG no. | Symbol | Name | MSDR by fru |
| 1 | CG9236 | CG9236 | — | SRR |
| 2 | CG11165 | CG11165 | — | R |
| 3 | CG4204 | Elongin-B | Elongin B | R |
| 4 | CG11105 | CG42683 | — | R |
| 5 | CG17998 | Gprk2 | G protein-coupled receptor kinase 2 | R |
| 6 | CG2621 | Sgg | Shaggy | A |
| 8 | CG34384 | CG34384 | — | R |
| 11 | CG12408 | TpnC4 | Troponin C isoform 4 | R |
| 12 | CG10014 | CG10014 | — | R |
| 13 | CG10260 | PI4KIIIalpha | Phosphatidylinositol 4-kinase III alpha | R |
| 14 | CG32217 | Su(Tpl) | Su(Tpl) | R |
| 15 | CG5874 | Nelf-A | Negative elongation factor A | AAR′A |
| 16 | CG32498 | Dnc | Dunce | R |
| 17 | CG31119 | HdacX | Histone deacetylase X | R |
| 18 | CG7646 | CG7646 | — | R |
| 19 | CG17769 | And | Androcam | R |
| 20 | CG31758 | Pde1c | Phosphodiesterase 1c | ARAR |
| 21 | CG10261 | aPKC | Atypical protein kinase C | R |
| 22 | CG3263 | Pka-R1 | cAMP-dependent protein kinase R1 | ADR′R′ |
| 23 | CG11245 | Pkcdelta | Protein kinase C delta | R |
| 24 | CG12196 | Egg | Eggless | R |
| 25 | CG14944 | Pde1c | Phosphodiesterase 1c | R |
| 26 | CG15177 | CG15177 | — | R |
| 27 | CG5182 | Pk34A | Pk34A | R |
| 28 | CG7641 | Nca | Neurocalcin | R |
| 29 | CG9151 | Acj6 | Abnormal chemosensory jump 6 | SARR |
| 30 | CG1906 | Alph | Alphabet | R |
| 32 | CG14692 | CG14692 | — | R |
| 33 | CG11325 | GRHR | Gonadotropin-releasing hormone receptor | R |
| 36 | CG1210 | Pdk1 | Phosphoinositide-dependent kinase 1 | R |
| 37 | CG12066 | Pka-C2 | cAMP-dependent protein kinase 2 | AR |
| 38 | CG7050 | Nrx-1 | Neurexin 1 | R |
| 39 | CG10033 | For | Foraging | R |
| 40 | CG17493 | CG17493 | — | ARR |
| 41 | CG3857 | CG3857 | — | SAR |
| 42 | CG7393 | p38b | p38b | R |
| 44 | CG10371 | Plip | PTEN-like phosphatase | R |
| 46 | CG33338 | p38c | p38c | R |
| 47 | CG17032 | CG17032 | — | R |
| 48 | CG9554 | Eya | Eyes absent | R |
| 49 | CG5216 | Sir2 | Sir2 | R |
| 50 | CG2984 | Pp2C1 | Protein phosphatase 2C | R |
| 51 | CG31757 | Pde1c | Phosphodiesterase 1c | A |
| 54 | CG12151 | Pdp | Pyruvate dehydrogenase phosphatase | R |
| 55 | CG14080 | Mkp3 | Mitogen-activated protein kinase phosphatase 3 | ARR′ |
| 56 | CG5744 | Frq1 | Frequenin 1 | R |
| 57 | CG6939 | Sbf | SET domain binding factor | LRAD |
| 58 | CG2048 | Dco | Discs overgrown | R |
| 59 | CG4945 | CG4945 | — | R |
| 60 | CG17100 | Cwo | Clockwork orange | R |
| 61 | CG2096 | Flw | Flapwing | R |
| 62 | CG11217 | CanB2 | Calcineurin B2 | R |
| 63 | CG7001 | Pk17E | Protein kinase-like 17E | R |
| 64 | CG8402 | PpD3 | Protein phosphatase D3 | R |
| 65 | CG2256 | CG2256 | — | R |
| 66 | CG6518 | InaC | inactivation no after potential C | R |
| 67 | CG32812 | CG32812 | — | R |
| 68 | CG11221 | CG11221 | — | R |
| 70 | CG4006 | Akt1 | Akt1 | R |
| 71 | CG4965 | Twe | Twine | R |
| 72 | CG5671 | Pten | Pten | R |
| 73 | CG2128 | Hdac3 | Histone deacetylase 3 | R |
| 74 | CG17603 | Taf1 | TBP-associated factor 1 | R |
| 76 | CG9291 | Elongin-C | Elongin C | R |
| 77 | CG1954 | Pkc98E | Protein C kinase 98E | AR′R′ |
| 78 | CG4141 | Pi3K92E | Pi3K92E | R |
| 79 | CG4252 | Mei-41 | Meiotic 41 | R |
| 80 | CG3324 | Pkg21D | cGMP-dependent protein kinase 21D | R |
| 81 | CG4379 | Pka-C1 | cAMP-dependent protein kinase 1 | R |
| 82 | CG8822 | PpD6 | Protein phosphatase D6 | R |
| 83 | CG5974 | Pll | Pelle | R |
| 84 | CG9088 | Lid | Little imaginal discs | ARR |
| 85 | CG2995 | G9a | G9a | R |
| 86 | CG5229 | Chm | Chameau | LLLL |
| 87 | CG5026 | CG5026 | — | R |
| 88 | CG5373 | Pi3K59F | Phosphatidylinositol 3 kinase 59F | LRA |
| 89 | CG8472 | Cam | Calmodulin | ARARR |
| 91 | CG33554 | Nipped-A | Nipped-A | R |
| 92 | CG7109 | Mts | Microtubule star | A |
| 94 | CG5907 | Frq2 | Frequenin 2 | R |
| 95 | CG11621 | Pi3K68D | Phosphatidylinositol 3 kinase 68D | R |
| 96 | CG6622 | Pkc53E | Protein C kinase 53E | R |
| 97 | CG17291 | Pp2A-29B | Protein phosphatase 2A at 29B | A |
| 98 | CG6571 | RdgC | Retinal degeneration C | SRR′ |
| 99 | CG1747 | Sk1 | Sphingosine kinase 1 | A |
| 100 | CG5247 | Irbp | Inverted repeat-binding protein | R |
| 101 | CG12559 | Rl | Rolled | R |
| 102 | CG1455 | CanA1 | Calcineurin A1 | R |
| 103 | CG18801 | Ku80 | Ku80 | A |
| 104 | CG6920 | Mus309 | Mutagen-sensitive 309 | LAR |
| 105 | CG2647 | Per | Period | R |
| 106 | CG15178 | Sowi | Solwind | R |
| 107 | CG12151 | Pdp | Pyruvate dehydrogenase phosphatase | AAR |
| 108 | CG5680 | Bsk | Basket | A |
| 109 | CG16708 | Cerk | Ceramide kinase | R |
| 110 | CG5169 | GckIII | Germinal center kinase III | R |
| 111 | CG4132 | Pkaap | Pkaap | SRR |
| 113 | CG1098 | Madm | MLF1-adaptor molecule | AR |
| 115 | CG17100 | Cwo | Clockwork orange | R |
| 116 | CG5643 | Wdb | Widerborst | DARR |
| 117 | CG15793 | Dsor1 | Downstream of raf1 | R |
| 118 | CG5085 | Sirt2 | Sirt2 | R |
| 119 | CG6593 | Pp1alpha-96A | Protein phosphatase 1alpha at 96A | R |
| 120 | CG6235 | Tws | Twins | R |
| 121 | CG5475 | Mpk2 | Mpk2 | R |
| 122 | CG6238 | Ssh | Slingshot | R |
| 123 | CG15862 | Pka-R2 | cAMP-dependent protein kinase R2 | R |
| 126 | CG1954 | Pkc98E | Protein C kinase 98E | A |
| 128 | CG5974 | Pll | Pelle | L |
| 133 | CG4007 | Nrk | Neurospecific receptor kinase | R |
| 134 | CG4290 | Sik2 | Salt-inducible kinase 2 | ARR |
| 136 | CG17598 | CG17598 | — | R |
| 140 | CG6551 | Fu | Fused | R |
| 142 | CG6772 | Slob | Slowpoke binding protein | R |
| 143 | CG8173 | CG8173 | — | RRR |
| 150 | CG17309 | Csk | C-terminal Src kinase | R |
| 151 | CG4488 | Wee | Wee | R |
| 153 | CG2028 | CkIalpha | Casein kinase I alpha | R |
| 158 | CG7207 | Cert | Ceramide transfer protein | R |
| 159 | CG5650 | Pp1-87B | Protein phosphatase 1 at 87B | R |
| 162 | CG9222 | CG9222 | — | R |
| 163 | CG17698 | CG17698 | — | R |
| 165 | CG2577 | CG2577 | — | R |
| 169 | CG17216 | KP78b | KP78b | R |
| 171 | CG34356 | CG34356 | — | AAAR |
| 181 | CG8789 | Wnd | Wallenda | R |
| 186 | CG14305 | CG14305 | — | R |
| 187 | CG3969 | PR2 | Fak-like tyrosine kinase | R |
| 188 | CG2845 | Phl | Pole hole | R |
| 192 | CG1344 | CG1344 | — | R |
| 193 | CG8057 | Alc | Alicorn | R |
| 194 | CG2252 | Fs(1)h | Female sterile (1) homeotic | AAA |
| 195 | CG9962 | CG9962 | — | A |
| 198 | CG11489 | Srpk79D | Serine-arginine protein kinase at 79D | R |
| 209 | CG11420 | Png | Pan gu | R |
| 211 | CG8874 | Fps85D | Fps oncogene analog | R |
| 221 | CG10673 | CG10673 | — | R |
| 223 | CG17256 | Nek2 | Nek2 | AR′D |
| 224 | CG4523 | Pink1 | PTEN-induced putative kinase 1 | R |
| 230 | CG6775 | Rg | Rugose | A |
| 232 | CG8726 | CG8726 | — | A |
| 234 | CG8637 | Trc | Tricornered | RRR |
| 240 | CG14992 | Ack | Ack | R |
| 242 | CG3172 | Twf | Twinfilin | R |
| 243 | CG12306 | Polo | Polo | R |
| 245 | CG3086 | MAPk-Ak2 | MAP kinase activated protein-kinase-2 | R |
| 246 | CG14217 | Tao | Tao | R |
| 248 | CG33553 | Doa | Darkener of apricot | RRR |
| 249 | CG4551 | Smi35A | Smell impaired 35A | R |
| 251 | CG6620 | Ial | IplI-aurora-like kinase | R |
| 253 | CG1973 | Yata | Yata | RR |
| 255 | CG9774 | Rok | Rho-kinase | RR |
| 256 | CG4353 | Hep | Homopterous | R |
| 257 | CG3068 | Aur | Aurora | R |
| 258 | CG11245 | Pkcdelta | Protein kinase C delta | R |
| 259 | CG7177 | Wnk | — | RL |
| 261 | CG7525 | Tie | Tie-like receptor tyrosine kinase | RR |
| 262 | CG14030 | Bub1 | Bub1 homolog | RRR |
| 263 | CG12147 | CG12147 | — | RRA |
| 264 | CG15072 | Sik3 | Salt-inducible kinase 3 | AAA |
| 265 | CG7616 | CG7616 | — | R |
| 266 | CG8878 | CG8878 | — | RRD |
| 268 | CG17348 | Drl | Derailed | R |
| 269 | CG3105 | Pask | PAS kinase | R |
| 271 | CG6875 | Asp | Abnormal spindle | R |
| 272 | CG3277 | CG3277 | — | R |
| 274 | CG5408 | Trbl | Tribbles | R |
| 275 | CG31711 | CG42367 | — | R |
| 276 | CG18069 | CaMKII | Calcium/calmodulin-dependent protein kinase II | RRRR |
| 277 | CG15224 | CkIIbeta | Casein kinase II beta subunit | AADL |
| 278 | CG8866 | CG8866 | — | R |
| 280 | CG9374 | Ikb1 | Ikb1 | R |
| 284 | CG33519 | Unc-89 | Unc-89 | R |
| 287 | CG8914 | CkIIbeta2 | Casein kinase II beta2 subunit | RRR |
| 288 | CG12559 | Rl | Rolled | R |
| 289 | CG1210 | Pdk1 | Phosphoinositide-dependent kinase 1 | LA |
| 290 | CG1210 | Pdk1 | Phosphoinositide-dependent kinase 1 | A |
| 291 | CG3857 | CG3857 | — | R |
| 292 | CG14692 | CG14692 | — | R |
| 294 | CG14692 | CG14692 | — | DAR |
| 295 | CG34356 | CG34356 | — | DAR |
| 296 | CG7097 | Hppy | Happyhour | RA |
| 298 | CG7111 | Rack1 | Receptor of activated protein kinase C 1 | R |
| 299 | CG14692 | CG14692 | — | R |
| 300 | CG12066 | Pka-C2 | cAMP-dependent protein kinase 2 | R |
| 301 | CG32703 | Erk7 | Extracellularly regulated kinase 7 | R |
| 302 | CG1210 | Pdk1 | Phosphoinositide-dependent kinase 1 | AAA |
| 304 | CG32743 | NonC | No-on-and-no-off transient C | R |
| 306 | CG34356 | CG34356 | — | RRR |
| 307 | CG34356 | CG34356 | — | R |
| 308 | CG34356 | CG34356 | — | R |
| 311 | CG4629 | CG4629 | — | R |
| 313 | CG12559 | Rl | Rolled | R |
| 314 | CG12559 | Rl | Rolled | R |
| 315 | CG5650 | Pp1-87B | Protein phosphatase 1 at 87B | R |
| 316 | CG3835 | CG3835 | — | RRR |
| 317 | CG3835 | CG3835 | — | R |
| 319 | CG7001 | Pk17E | Protein kinase-like 17E | RRR |
| 320 | CG7001 | Pk17E | Protein kinase-like 17E | RRR |
| 321 | CG7001 | Pk17E | Protein kinase-like 17E | R |
| 323 | CG8878 | CG8878 | — | R |
| 324 | CG8878 | CG8878 | — | R |
| 325 | CG17256 | Nek2 | Nek2 | R |
| 326 | CG17256 | Nek2 | Nek2 | R |
| 328 | CG34412 | Tlk | Tousled-like kinase | AARR |
| 330 | CG34412 | Tlk | Tousled-like kinase | R |
| 331 | CG34412 | Tlk | Tousled-like kinase | R |
| 332 | CG34412 | Tlk | Tousled-like kinase | ARDD |
| 333 | CG34412 | Tlk | Tousled-like kinase | R |
| 334 | CG33553 | Doa | Darkener of apricot | R |
| 335 | CG33553 | Doa | Darkener of apricot | R |
| 336 | CG33553 | Doa | Darkener of apricot | LR |
| 337 | CG33553 | Doa | Darkener of apricot | R |
| 338 | CG8866 | CG8866 | — | R |
| 339 | CG8866 | CG8866 | — | R |
| 340 | CG17090 | Hipk | Homeodomain interacting protein kinase | R |
| 341 | CG17090 | Hipk | Homeodomain interacting protein kinase | R |
| 343 | CG8767 | Mos | Mos | R |
| 344 | CG8767 | Mos | Mos | R |
| 345 | CG8767 | Mos | Mos | R |
| 346 | CG12147 | CG12147 | — | R |
| 348 | CG12147 | CG12147 | — | R |
| 349 | CG11221 | CG11221 | — | R |
| 350 | CG11221 | CG11221 | — | R |
| 351 | CG11221 | CG11221 | — | R |
| 354 | CG6498 | CG6498 | — | LLR′R′ |
| 355 | CG6498 | CG6498 | — | R |
| 356 | CG6498 | CG6498 | — | RRR |
| 358 | CG1107 | Aux | Auxillin | DRRR |
| 359 | CG14217 | Tao | Tao | R |
| 361 | CG6027 | Cdi | Center divider | R |
| 365 | CG2048 | Dco | Discs overgrown (dbt) | AARA |
| 366 | CG33553 | Doa | Darkener of apricot | LRLR |
This table is related to Fig. 1. MSDR of fru > Dcr2, geneX-RNAi males for each RNAi line was measured for 5 d under DD, and rhythmicity and tau were determined by Faas software. Each letter in the “MSDR by fru” column represents the MSDR of an individual male. Each RNAi line was tested once (the initial round of measurement), and more males were tested if the result of the initial round was “not R.” For example, the initial male of line no. 1 showed shorter MSDR in the initial round, but other two males showed normal MSDR at the second-round measurement. A, arrhythmic; D, dead during the measurement; L, rhythmic and longer tau compared with fru > Dcr2 male; R, rhythmic and normal tau; R′, rhythmic and normal tau with low power or activity; S, rhythmic and shorter tau compared with fru > Dcr2 male.
Fig. 1.
Sik3 is essential for proper MSDR. (A) Males in which Sik3 expression was knocked down by RNAi in fruM-positive neurons (bar 3), show a low rhythmicity and short period in MSDR but not in SLR. Period length is completely restored and rhythmicity is mostly restored by Sik3 expression in clock neurons (bar 4). (B) Males in which Sik3 expression was knocked down by RNAi in Pdf+ LNvs (bars 3 and 5), in DN1 neurons (bars 7 and 9), and in a majority of clock cells (bar 11) show a short MSDR period. SIk3 knockdown produces a slight extension of the SLR period in LNvs and a shortening of the SLR period in DN1 neurons. Black bars represent experimental genotypes; white bars represent control genotypes. Numbers within the bars denote total number of flies in “Rhythmic flies” and total number of rhythmic flies in “Period.” Error bars represent the SEM. *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 in rhythmic flies (χ2 test) and period (one-way ANOVA followed by Tukey’s multiple comparison test). (A′ and B′) Behavior actograms for MSDR and SLR of the experimental genotypes in A and B, respectively. Averaged activity traces for MSDR and SLR are double plotted. Complex trough phases in MSDR are observed with Sik3 reduction in M cells (B′). All measurements were done under DD. Gray and black bars below the graphs denote subjective day and night, respectively. The numbers below the graphs indicate the averaged period with SEM. RNAi is abbreviated as “i.”
Table S2.
MSDR and SLR of males with Sik3 knockdown in fruitless and in subsets of clock neurons
 |
This table is related to Figs. 1 and 5. Genotypes of experimental flies (E1 to E23) are shown in black, and appropriate control (C) genotypes are shown in blue. Asterisks indicate a significant difference between experimental and control males: *P < 0.05, **P < 0.01, ***P < 0.001, and ****P < 0.0001 in rhythmic flies (χ2 test), period, and power (one-way ANOVA followed by Tukey’s multiple comparison test). Cont, the control genotype(s) to which each experimental genotype is compared for evaluating statistical significance; Rhy flies, rhythmic flies.
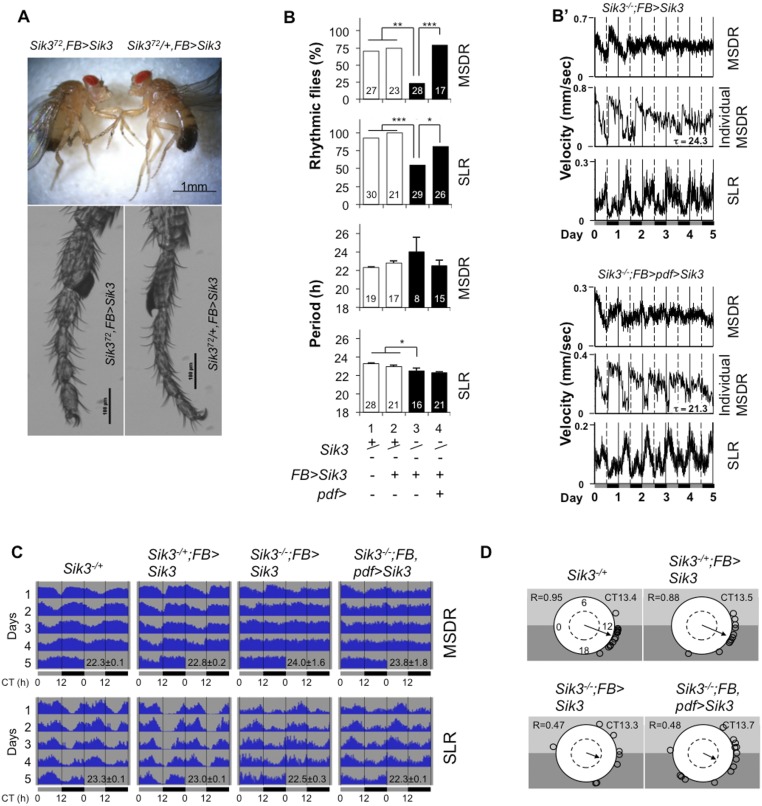
A role of SIK3 in MSDR is also supported by behavioral rhythm phenotypes of Sik3-mutant males. Homozygous loss-of-function Sik372 flies die at an early larval stage, but their lethality can be rescued by restoring Sik3 expression in fat body (FB) cells (22) (Sik3−/−, FB > Sik3) (Fig. S1A). Such males exhibit lower rhythmicity and variable periods in both MSDR and SLR (Figs. S1 B and B′ and S2 and Table S3). Also restoring Sik3 expression in the Pdf+ LNvs of such males (Sik3−/−, FB > Sik3, Pdf > Sik3) rescued both MSDR and SLR rhythmicity, albeit phase coherence among rhythmic individuals in MSDR was still low (Fig. S1 B–D and Table S3) (17, 23). However, Sik3-mutant flies in which Sik3 expression was driven in the majority of clock neurons (Sik3−/−, FB > Sik3, cry > Sik3) or in all clock neurons (Sik3−/−, FB > Sik3, tim > Sik3), in addition to fat body cells were arrhythmic in both SLR and MSDR (Table S3). We note that overexpression of Sik3 in cry neurons (which include some non-clock neurons) of otherwise wild-type flies also causes arrhythmicity (cry > Sik3) (Table S4), suggesting that improper levels of SIK3 in most clock neurons or even in non-clock neurons interfere with proper rhythmicity in both single-fly and courtship settings.
Fig. S1.
Morphological (A) and behavior (B–D) phenotypes of Sik3 mutants in MSDR and SLR. (A) Homozygous Sik372 males (Left) show a morphological phenotype in legs and sometimes in wings. (B) Sik3 mutant flies with Sik3 function rescued in fat body (FB) cells show low rhythmicity in both SLR and MSDR (bar 3), which is rescued by additional Sik3 expression in the Pdf neurons (bar 4). Numbers within the bars denote number of samples. Error bars represent SEM. *P < 0.05, **P < 0.01, and ***P < 0.001 in rhythmic flies (χ2 test) and period (one-way ANOVA followed by Tukey’s multiple comparison test). (B′) Averaged and representative individual activity traces for MSDR and averaged SLR of the experimental genotypes in B. Note that the low phase coherence (shown in D) causes low amplitude in MSDR. All behavioral measurements were done under DD. Gray and black bars below the plots denote subjective day and night, respectively. (C) Averaged activity of rhythmic flies in Table S3 is double plotted. The averaged period with SEM is shown below the graphs. (D) Circular phase analysis of MSDR for individual flies revealed that Sik3 expression in M cells does not rescue the low phase coherence phenotype in MSDR of Sik3-mutant males. Small circles represent the estimated trough phase of individual rhythmic flies (Table S3). Vector length and position indicate phase concentration (R) and averaged phase, respectively. An internal dotted circle represents a value of 0.5 in phase coherence. The trough phase is shown in CT hours. This figure is related to Fig. 1.
Fig. S2.
Distribution of MSDR and SLR period lengths. Periods of rhythmic flies in MSDR (A) and SLR (B) (Table S2) are shown in box plots with whiskers with 0.5 interquartile ranges. Dots indicate outliers. This figure is related to Figs. 1 and 6.
Table S3.
MSDR and SLR of Sik3 mutant males
 |
This table is related to Figs. 1 and 5. Genotypes of experimental flies (E1 to E23) are shown in black, and appropriate control (C) genotypes are shown in blue. Asterisks indicate a significant difference between experimental and control males: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 in rhythmic flies (χ2 test), period, and power (one-way ANOVA followed by Tukey’s multiple comparison test). Cont, the control genotype(s) to which each experimental genotype is compared for evaluating statistical significance; Rhy flies, rhythmic flies.
Table S4.
MSDR and SLR of males overexpressing wild-type Sik3 in subsets of clock neurons
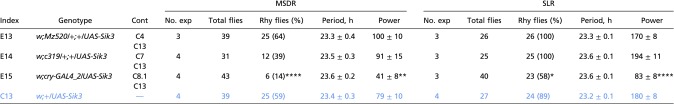 |
This table is related to Figs. 1 and 5. Genotypes of experimental flies (E1 to E23) are shown in black, and appropriate control (C) genotypes are shown in blue. Asterisks indicate a significant difference between experimental and control males: *P < 0.05, **P < 0.01, and ****P < 0.0001 in rhythmic flies (χ2 test), period, and power (one-way ANOVA followed by Tukey’s multiple comparison test). Cont, the control genotype(s) to which each experimental genotype is compared for evaluating statistical significance; Rhy flies, rhythmic flies.
To explore the cellular requirements for SIK3 in MSDR and SLR further, we knocked down its expression in defined subsets of clock neurons by using various GAL4 lines: Pdf-GAL4 and Mz520 express GAL4 in PDF+ M cells; Clk4.1M and c319 express GAL4 in a subset of DN1 neurons; and cry-GAL4 express GAL4 in the majority of clock neurons. Reduction of SIK3 in M cells (Pdf > Dcr2, Sik3RNAi and Mz520 > Dcr2, Sik3RNAi), DN1 neurons (Clk4.1M > Dcr2, Sik3RNAi and c319 > Dcr2, Sik3RNAi) or a majority of clock neurons (cry > Dcr2, Sik3RNAi) shortens the period but does not affect the rhythmicity of MSDR (with the exception Pdf > Dcr2, Sik3RNAi males) (Fig. 1 B and B′, Figs. S3 and S4A, and Table S2). In contrast, the SLR period of such males is extended (Pdf > Dcr2, Sik3RNAi and Mz520 > Dcr2, Sik3RNAi), slightly shortened (Clk4.1M > Dcr2, Sik3RNAi and c319 > Dcr2, Sik3RNAi), or normal (cry > Dcr2, Sik3RNAi), suggesting that Sik3 plays distinct roles in regulating period length in MSDR and SLR. We also noted that males with down-regulated SIK3 in PDF neurons (Pdf > Dcr2, Sik3RNAi and Mz520 > Dcr2, Sik3RNAi), although having an overall short period phenotype, show signs of behavioral complexity in their MSDR, with irregular period and multiple rhythmic components seen in 5 of 24 males (Fig. 1B′).
Fig. S3.
Behavior actograms for MSDR and SLR of various controls. Dashed lines in Mz520 > Dcr2, nmoi indicate a constant short trough phase of MSDR by nmo reduction. RNAi is abbreviated as “i.” This figure is related to Figs. 1 and 6.
Fig. S4.
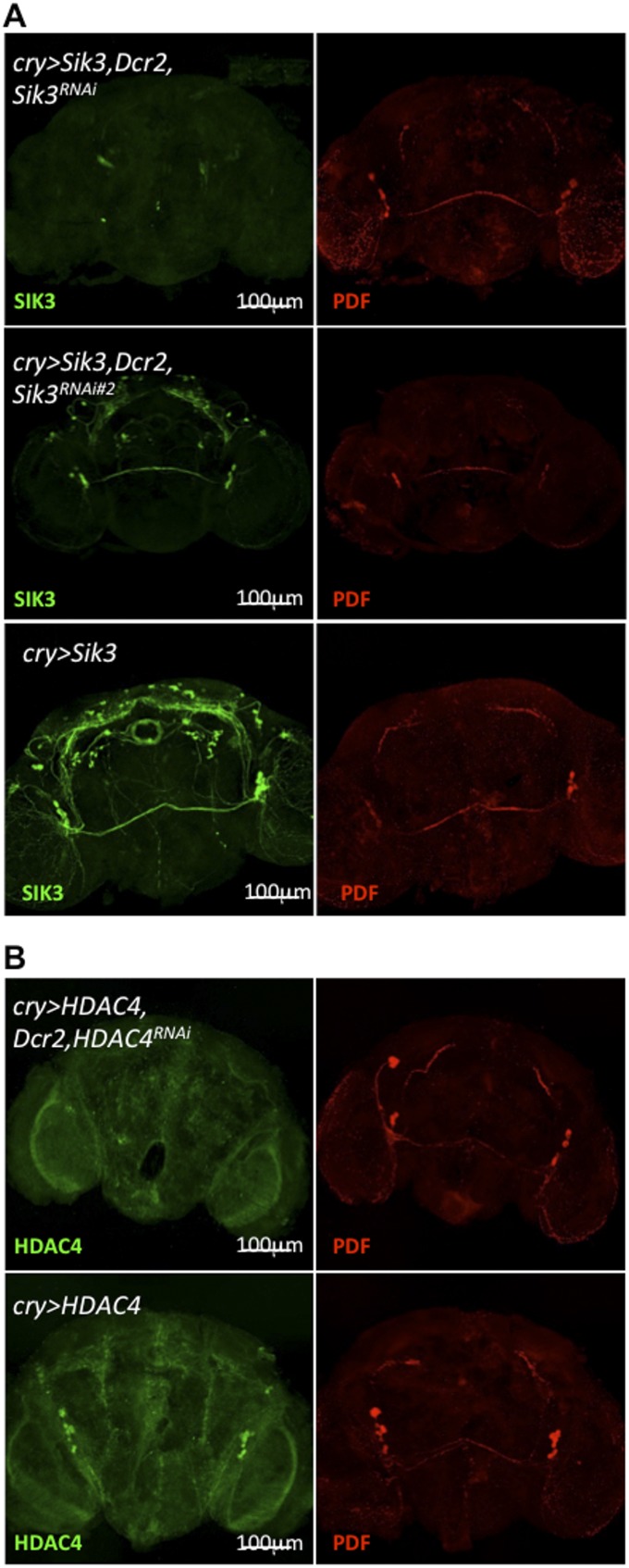
Sik3RNAi and HDAC4RNAi significantly reduce the expression of SIK3 and HDAC4 protein, respectively, in the brain. Epitope-tagged SIK3 (A), HDAC4 (B) and endogenous PDF were detected with anti-HA, anti-FLAG, and anti-PDF antisera, respectively. Reduction of SIK3 protein is much weaker in a second SIK3RNAi line (Sik3RNAi#2), the likely reason that Mz520 > Dcr2, Sik3RNAi#2 flies fail to show an MSDR phenotype (Table S2). Male brains were fixed at ZT5–8. This figure is related to Figs. 1 and 6.
SIK3 Is Necessary for Robustness and Period Determination of Molecular Oscillation in Clock Cells.
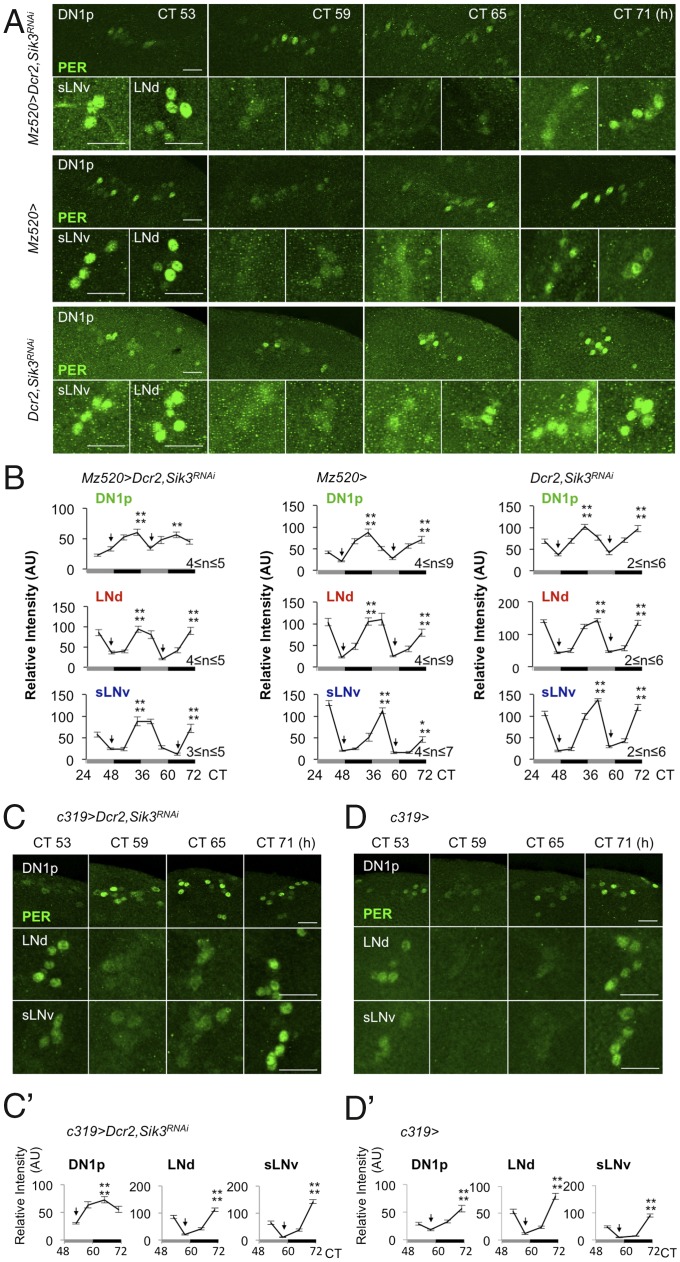
The molecular clock runs at the same speed in all clock neurons. Manipulating the speed of the molecular clock in M cells has shown that these cells regulate the period length of other clock neurons in DD (12, 13) and that cell–cell communication by the Pdf/Pdfr system plays a central role in such oscillator coupling (13). Consistent with this finding, knockdown of nemo (nmo), an essential kinase involved in regulating the molecular clock (24, 25) in M cells (Mz520 > Dcr2, nmoRNAi) shortens period length of both MSDR and SLR by about 2 h (Fig. S3 and Table S5). Thus, our observation that loss of SIK3 in M cells severely shortens the period length of MSDR while having a mild opposite effect on SLR is surprising and suggests that this kinase may play a role in oscillator coupling between specific sets of clock neurons. Importantly, SIK3 reduction in M cells does not interfere with their morphology (Fig. S5), making it unlikely that the observed behavioral phenotypes are caused by developmental defects. We therefore investigated whether cycling of the molecular clock was affected differently in various sets of clock neurons of flies lacking SIK3 only in M cells. We found that the amplitude of PER cycling in DN1 neurons was dampened on the third day of DD, and phases were different from those in sLNvs and LNds (Fig. 2 A and B and Fig. S6 A and B). Although, as expected, the sLNvs and LNds showed robust PER rhythms peaking in the late subjective night and early subjective day, the amplitude of PER oscillation in DN1 neurons of experimental flies was weaker than that of control flies (Fig. 2B and Fig. S6 A and B). Similar observations were made when females were present (Fig. S6C) or when using a different GAL4 driver for Pdf+ neurons (Fig. S6D). PER rhythms appear to lose amplitude and, based on the timing of the weakened peaks and troughs, seem to run fast, which is consistent with a faster MSDR observed in these males. Because DN1 neurons are likely nonhomogeneous and fall into functionally distinct groups (26), it is possible that specific subsets of DN1 neurons are differentially sensitive to the loss of SIK3 in M cells, possibly thereby contributing to the poor overall rhythmicity of DN1 neurons. We also observed that the trough phase of MSDR is normal during day 1 of DD (Fig. 1B′), suggesting that a day or two of constant conditions might be required for the DN1 neurons to loose their robust molecular oscillation driven by signals from the M cells.
Table S5.
MSDR and SLR of males with altered clock-related gene expressions in Pdf-expressing neurons
 |
This table is related to Figs. 1 and 5. Genotypes of experimental flies (E1 to E23) are shown in black, and appropriate control (C) genotypes are shown in blue. Asterisks indicate a significant difference between experimental and control males: *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 in rhythmic flies (χ2 test), period, and power (one-way ANOVA followed by Tukey’s multiple comparison test). Cont, the control genotype(s) to which each experimental genotype is compared for evaluating statistical significance; Rhy flies, rhythmic flies.
Fig. S5.
GFP and Sik3RNAi are driven by two Pdf-specific GAL4 drivers, Mz520 (A) or Pdf-GAL4 (B). The brains of indicated genotypes of flies were collected and stained with anti-GFP and anti-PDF antisera at ZT2 (Left) or ZT14 (Right). Reduction of Sik3 expression does not affect the morphology of Pdf neurons. Constitutive Sik3 reduction in Pdf neurons during development does not cause defects in Pdf neuron morphology. This figure is related to Fig. 1.
Fig. 2.
Sik3 knockdown in M cells or DN1 neurons changes the amplitude or phase of PER cycling in DN1ps. (A) PER expression in clock neurons of brains from flies of the indicated genotypes kept in DD were stained with anti-PER antibody at four time points in the third subjective day and night. (Scale bar: 10 μm.) (B) Relative intensity of PER expression in DN1ps (Top), LNds (Middle), and sLNvs (Bottom). The values at all peaks are significantly higher than the values at the troughs (arrows). n indicates the number of hemispheres measured. A second, independent experiment yielded similar results (Fig. S6A). Error bars represent the SEM. **P < 0.01 and ****P < 0.0001 (one-way ANOVA followed by Tukey’s multiple comparison test). AU, arbitrary units (C and D) The indicated genotypes were stained as in A at four time points in the third subjective day and night. (Scale bar: 10 μm.) (C′ and D′) Relative intensity of PER expression as described in B (4 ≤ n ≤ 6). Error bars represent the SEM. ****P < 0.0001 (one-way ANOVA followed by Tukey’s multiple comparison test).
Fig. S6.
Sik3 knockdown in sLNvs changes the amplitude and phase of PER cycling in DN1ps. Shown is the relative intensity of nuclear PER expression in sLNvs, LNds, and DN1ps at different circadian times of flies kept in DD. (A) Measurements as in Fig. 2B of another biological sample (2 ≤ n ≤ 6). (B–D) Measurements from third subjective night to the fourth subjective day of single (5 ≤ n ≤ 6) (B) or female-paired (n = 6) (C) Sik3RNAi males and control males and singly kept Sik3RNAi males and controls in which SIK3 was knocked down with another driver (Pdf-GAL4; 5 ≤ n ≤ 10) (D). In all cases, PER cycling of experimental flies is out of phase or there is no clear peak in DN1 neurons as compared with LNds or sLNvs. (E and F) Dcr2 expression does not cause low amplitude or a phase shift in PER cycling in DN1 neurons. Measurements were from the third subjective day to the third subjective night (E: 6 ≤ n ≤ 8; F: 3 ≤ n ≤ 6). Error bars represent SEM. **P < 0.01 and ****P < 0.0001 (one-way ANOVA followed by Tukey’s multiple comparison test); ns, not significant. This figure is related to Fig. 2.
In summary, these results, combined with the MSDR observations, indicate that the amplitude of PER cycling is dampened in DN1 neurons when SIK3 is missing in M cells and that the communication between these two groups of circadian neurons is disrupted. How the weak molecular oscillation in DN1 neurons accelerates MSDR but slows down SLR is not yet clear, but the loss in DN1 amplitude might be indicative of DN1 neurons free-running with different periods. Interestingly, manipulation of PDF/PDFR signaling can lead to behavioral splitting in SLR [i.e., single flies expressing locomotor rhythms with two or more simultaneous periods (27)] as well as dissociations of molecular rhythms in the DN1 neurons (28). Thus our results indicate that the loss of SIK3 in M cells disrupts communications between these Pdf+ circadian neurons and the DN1 neurons. Because a reduction of Sik3 in DN1 neurons also shortens the MSDR period (Clk4.1M > Dcr2, Sik3RNAi and c319 > Dcr2, Sik3RNAi) (Fig. 1 B and B′ and Table S2), we measured PER cycling in DN1 neurons of these flies and found it to be uncoupled from the sLNvs, but the synchronicity of PER cycling appeared robust within the DN1 group (Fig. 2 C and D and Fig. S2 C′ and D′). In any case, these observations suggest that Sik3 is involved in the determination of MSDR period length by modulating the molecular oscillation in clock neurons through a cell-autonomous as well as a non–cell-autonomous pathway.
Morning Activity Is Affected by SIK3 Down-Regulation in sLNvs.
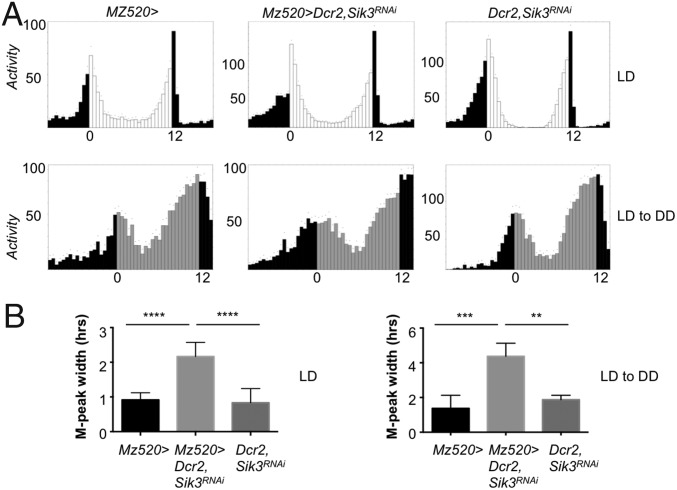
Flies adopt a crepuscular locomotor pattern under LD conditions, with a morning and an evening bout of activity. Morning activity is under the control of the Pdf+ sLNvs (M cells) and their targets, the DN1 neurons, whereas the LNds and the PDF− sLNvs (E cells) control evening activity (29–33). Both control flies and flies in which Sik3 was down-regulated in the sLNvs showed clear morning and evening anticipatory behavior under LD cycles, as expected. However, the morning peak of activity was broader in Sik3 RNAi flies (Fig. 3A, Upper and Fig. 3B, Left). In control flies activity increased steadily until the light-on transition, but Sik3 RNAi flies showed little or no increase in activity in the hours before light-on transition, suggesting that the morning peak phase is advanced. Because flies have a strong startle response when lights are turned on, we also monitored activity at the transition between LD to DD to visualize the morning peak better (Fig. 3A, Lower and Fig. 3B, Right). Under these conditions, we observed that morning activity was also distributed over a longer period in Sik3 RNAi flies. We conclude that reducing Sik3 levels in sLNvs affects the precise timing of morning increase and decrease in activity. The broad morning peak, which was not caused by DCR2 overexpression (Fig. S7), might be the result of weakened coupling between DN1 neurons and sLNvs, because both neuronal groups contribute to the control of morning activity. The presence of what appeared to be two small activity maxima in all M peaks recorded at the LD-to-DD transition might be a further indication that the M peak is splitting in two components as a result of sLNv–DN1 desynchronization. Unfortunately, the amplitude of the M peak on the second day of DD was too low to be informative. We also observed that the E peak in Sik3 RNAi flies was slightly delayed during the first day of DD (Fig. 3A, Lower), as expected from the slightly long-period phenotype in DD.
Fig. 3.
The morning activity peak is broader in Sik3 RNAi flies. (A) Average locomotor activity of 23–32 flies under LD (Upper) or during the transition from LD to DD (Lower). White bars represent day, gray bars represent subjective day, and black bars represent night or subjective night. ZT or CT is indicated on the x axes. The morning activity peak appears to be broader in flies expressing Sik3 dsRNAs in sLNvs. (B) Quantification of the width of the morning activity peak at 80% of peak activity under LD (Left, six independent experiments) and during the transition from LD to DD (Right, four independent experiments) confirms that morning activity is spread over a longer period. **P < 0.01, ***P < 0.001, ****P < 0.001 (one-way ANOVA analysis followed by Tukey’s multiple comparisons test; ANOVA P < 0.0001 for LD and P < 0.001 for LD to DD).
Fig. S7.
The broad morning peak was not caused by Dcr2 overexpression. Average locomotor activity of 62–64 flies under LD (Upper) or during the transition from LD to DD (Lower). White bars represent day, gray bars represent subjective day, and black bars represent night or subjective night. ZT or CT is indicated on the x axis. This figure is related to Fig. 3.
HDAC4, a SIK3 Phosphorylation Target, Exhibits Nucleocytoplasmic Cycling in a Subset of Clock Cells.
HDAC4, a class IIa histone deacetylase, is a well-established SIK3 target in fat body cells (22). There, phosphorylation of HDAC4 by SIK3 leads to association with 14-3-3 proteins, which are involved in various signaling pathways (34), and sequestration of HDAC4 in the cytoplasm (35). Lack of SIK3 renders HDAC4 dephosphorylated at three serine phosphorylation sites, permitting HDAC4 translocation into the nucleus, where it exerts its transcriptional regulatory activity on various gene promoters. Furthermore, HDAC4 has a binding motif for Myocyte enhancer factor 2 (MEF2) (35), a transcription factor implicated in the regulation of circadian behavior (36, 37), and a circadian role for HDAC4 is also supported by a circadian locomotor activity phenotype in flies carrying a hypomorphic HDAC4 allele (HDAC4KG09091) (38). We therefore examined whether HDAC4 is expressed in clock neurons and investigated its potential function in SLR and MSDR. First, we analyzed expression of HDAC4NP1617, a GAL4 insertion into the promoter region of the gene, and found that GAL4 is active in a majority of lateral clock neurons (Fig. 4 A and B), a finding consistent with elevated HDAC4 mRNA expression in clock neurons (see supporting information in ref. 39) and in the brain (FlyAtlas, ref. 40). It is worth mentioning that HDAC4NP1617 activity is detected in only a few posterior DN1s (DN1ps) (Fig. 4C). To determine whether HDAC4 undergoes nucleocytoplasmic shuttling, we took advantage of FLAG-tagged HDAC4 transgenes, UAS-HDAC4 and UAS-HDAC43A [encoding a SIK3 phosphorylation-defective HDAC4 (22)] and evaluated cellular localization at different times of day (Fig. 4 D and E). Antibody staining of the brains of cry > HDAC4 flies revealed that wild-type HDAC4 cycles between the nucleus and cytoplasm in a subset of clock cells, specifically in sLNvs (Fig. 4 D and D′) but not in lLNvs (Fig. S8A). Nucleocytoplasmic cycling was also observed in two of three CRY+ and PDFR+ LNds, whereas three CRY− and PDFR− LNds exhibit constitutive cytoplasmic HDAC4 expression (Fig. S8 B and C′). Consistent with SIK3 having a role in HDAC4 phosphorylation and nucleocytoplasmic cycling in fat cells, we found that HDAC43A is continuously localized to the nucleus of sLNvs and LNds (Fig. 4 E and E′ and Fig. S8 D and D′).
Fig. 4.
HDAC4 cycles between the cytoplasm and nucleus in subsets of clock neurons. (A–C) HDAC4 expression in adult male brains of HDAC4NP1617/Y;UAS-mCD8GFP/+ flies. An overview is shown in A. Lateral brain regions are shown in B (Upper, LNds; Lower, LNvs), and the region containing DN1 neurons is shown in C. HDAC4NP1617 (representing HDAC4)–expressing neurons are shown in green (visualized by anti-GFP antibody staining). Arrowheads indicate CLK+ neurons (visualized by anti-CLK antibody staining). Numbers in A and C indicate GFP+ LNd and LNvs (A) and DN1ps (C). 4 ≤ n ≤ 6. MB, mushroom body; MNSC, median neurosecretory cells. (D and E) Nucleocytoplasmic localization of wild-type HDAC4 (D) and phosphorylation-defective HDAC43A (E). Clock cells (sLNvs) in brains dissected from cry-GAL4/UAS-HDAC4-FLAG flies and cry-GAL4/UAS-HDAC43A-FLAG flies were visualized using anti-FLAG (green) and anti-CLK (red) antibodies at the indicated time points. White asterisks indicate lLNvs. (Scale bars: 5 μm.) (D′ and E′) Graphs display sLNvs with nuclear HDAC4 (D′) or HDAC43A (E′) at different times of day. Error bars represent SEM. Letters in graphs indicate significant differences in values (P < 0.05, ANOVA followed by Tukey’s multiple comparisons).
Fig. S8.
Nucleocytoplasmic cycling of HDAC4 in CRY+ LNds. (A) The graph displays lLNvs with nuclear HDAC4 at different times of day. There is no significant difference in values (ANOVA followed by Tukey’s multiple comparisons, P < 0.05). (B and C) HDAC4 nucleocytoplasmic localization cycles in CRY+ and PDFR+ LNds (cry-GAL4/UAS-HDAC4-FLAG in B and Mai179/Pdf-GAL80/UAS-HDAC4-FLAG in C). Note that Mai179 labels only CRY+ and PDFR+ cells in LNds. Arrows and arrowheads in B indicate LNds with nuclear-localized HDAC4 and putative CRY− and PDFR− LNds, respectively. HDAC4, CLK, and PER were visualized by anti-FLAG, anti-CLK, and anti-PER antibodies, respectively. (Scale bars: 5 μm.) (D–F) HDAC4 localization in LNds of flies overexpressing HDAC43A (cry-GAL4/UAS-HDAC43A-FLAG) (D), overexpressing ΔCYC (cry-GAL4/UAS-ΔCYC/UAS-HDAC4-FLAG) (E), or reducing Sik3 by RNAi (cry-GAL4/UAS-Dcr2/UAS-Sik3RNAi/UAS-HDAC4-FLAG) (F). (Scale bars: 5 μm.) (B′ –F′) Graphs display LNds with nuclear HDAC4 (B′, C′, E,′ and F′) or HDAC43A (D′) in brains shown in B–F at different times of day. Letters in graphs indicate significant differences in values (P < 0.05, ANOVA followed by Tukey’s multiple comparisons). This figure is related to Figs. 4 and 5.
To test whether the molecular clock in general and SIK3 specifically are required for nucleocytoplasmic shuttling of HDAC4 in sLNvs, we first overexpressed a dominant negative form of the core clock protein CYC (41) in clock neurons (cry>ΔCYC, HDAC4); this overexpression leads to misregulation of CLK/CYC downstream targets. Indeed, nucleocytoplasmic shuttling of HDAC4 was diminished in clock cells of such fly brains, leaving HDAC4 localized predominantly in the cytoplasm (Fig. 5 A and A′ and Fig. S8 E and E′). Conversely, when brains of flies with knocked-down SIK3 function were examined, we found that HDAC4 was localized in the nucleus of sLNvs at all times (Fig. 5 B and B′ and Fig. S8 F and F′). In summary, these data indicate that HDAC4 is expressed in a subset of clock cells and shuttles between the cytoplasm and nucleus in most sLNvs and some LNds in a clock/SIK3-dependent fashion, reminiscent of nutrient-dependent nucleocytoplasmic shuttling of HDAC4 in fat cells (22). Direct visualization using a specific antibody may confirm the expression and shuttling of endogenous HDAC4 in the future.
Fig. 5.
HDAC4 cycling is clock and SIK3 dependent (A) Disruption of the molecular clock interferes with nucleocytoplasmic HDAC4 shuttling. Flies overexpressing ΔCYC (cry-GAL4/UAS-ΔCYC/UAS-HDAC4-FLAG) exhibit cytoplasm-centric HDAC4 but constitutive nuclear CLK localization in sLNvs. (The antibodies used were the same as used in Fig. 4D) White asterisks indicate lLNvs. (B) Sik3 knockdown leads to constitutive nuclear localization of HDAC4. cry-GAL4/UAS-Dcr2/UAS-Sik3RNAi/UAS-HDAC4-FLAG flies show constitutive localization of HDAC4 in sLNvs. (A′ and B′) The graphs display sLNvs with nuclear HDAC4 at different times of day. Error bars represent SEM. There is no significant difference in values (P < 0.05, ANOVA followed by Tukey’s multiple comparisons).
Constitutive Nuclear Localization of HDAC4 in sLNvs Shortens the Length of the MSDR but Not of the SLR Period.
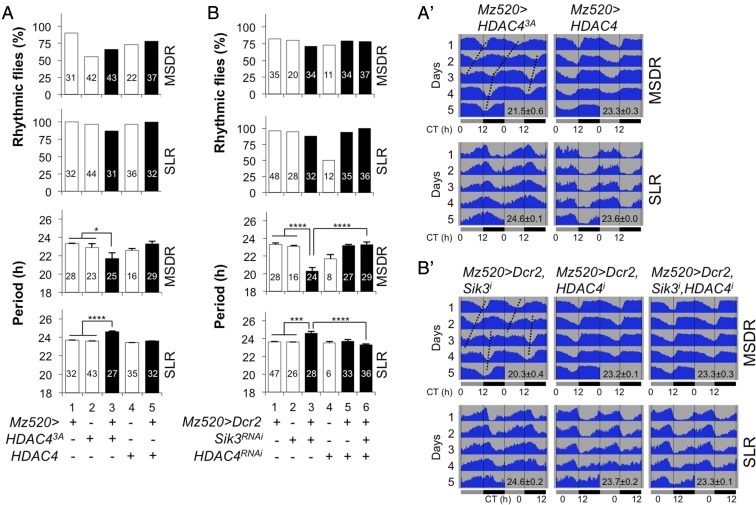
Sik3 reduction leads to constitutive nuclear localization of HDAC4 in clock neurons (Fig. 5 B and B′) and causes a shorter period of MSDR (Fig. 1B). If constitutive nuclear HDAC4 localization were sufficient to do so, we would expect that overexpression of HDAC43A would mimic this phenotype. Indeed, flies overexpressing HDAC43A (Mz520 > HDAC43A), but not wild-type HDAC4 (Mz520 > HDAC4), in M cells show behavioral phenotypes similar to those of flies with Sik3 down-regulation in the same neurons (Mz520 > Dcr2, Sik3RNAi): a shorter period of MSDR and a slightly longer period of SLR (Fig. 6 A and A′, Fig. S3B, and Table S6).
Fig. 6.
Constitutive nuclear localization of HDAC4 in M cells causes a short MSDR period. (A) Overexpression of phosphorylation-defective HDAC43A (bar 3), but not wild-type HDAC4 (bar 5), in M cells shortens the period length of MSDR but not of SLR. Note that SLR is extended in flies expressing HDAC43A. (B) Simultaneous knockdown of HDAC4 and Sik3 in M cells (box 6) rescues MSDR and SLR phenotypes of flies in which Sik3 is knocked down alone (box 3). In A and B experimental genotypes are shown in black, and control genotypes are shown in white. Numbers within the bars denote n. Error bars represent SEM. *P < 0.05, ***P < 0.001, ****P < 0.0001 in rhythmic flies (χ2 test) and period (ANOVA followed by Tukey’s multiple comparisons). (A′ and B′) Behavior actograms for MSDR and SLR of the experimental genotypes in A and B. As in Fig. 1B′, complex trough phases in MSDR are observed with the overexpression of HDAC43A in M cells. Concurrent reduction of Sik3 and HDAC4 expression by RNAi rescues the short and complex period MSDR phenotype caused by the reduction of Sik3 alone.
Table S6.
MSDR and SLR of males overexpressing wild-type and phosphorylation-defective HDAC4 in Pdf-expressing neurons
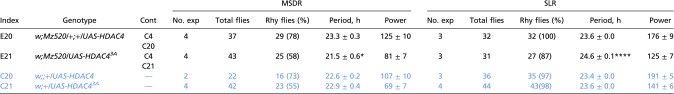 |
This table is related to Figs. 1 and 5. Genotypes of experimental flies (E1 to E23) are shown in black, and appropriate control (C) genotypes are shown in blue. Asterisks indicate a significant difference between experimental and control males: *P < 0.05 and ****P < 0.0001 in rhythmic flies (χ2 test), period, and power (one-way ANOVA followed by Tukey’s multiple comparison test). Cont, the control genotype(s) to which each experimental genotype is compared for evaluating statistical significance; Rhy flies, rhythmic flies.
To investigate whether nucleocytoplasmic HDAC4 oscillation is necessary for proper circadian behavior, we examined MSDR and SLR in flies lacking HDAC4 in M cells (Mz520 > Dcr2, HDAC4RNAi) (Fig. S4B). This manipulation had no effect on rhythmicity or period length in either circadian output behavior (Fig. 6 B and B′, Fig. S3B, and Table S7). This finding and the observation that HDAC4 phosphorylation by SIK3 is necessary to keep HDAC4 in the cytoplasm led us to suspect that double knockdown of HDAC4 and SIK3 would alleviate the period length phenotype of flies lacking only SIK3. This notion indeed proved to be the case (Fig. 6 B and B′, and Table S7). Taking these findings together, we conclude that constitutive nuclear localization of HDAC4 in M cells is the main cause of the different period phenotypes in MSDR and SLR and that HDAC4 nucleocytoplasmic oscillation is dispensable for proper MSDR and SLR.
Table S7.
MSDR and SLR of males with Sik3 and/or HDAC4 knockdown in Pdf-expressing neurons
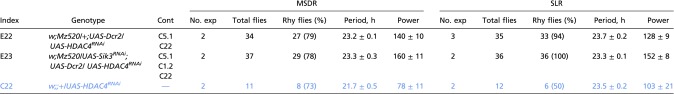 |
Discussion
SIK–HDAC (class IIa) signaling is evolutionarily conserved from worm (42) to mammals (43), operating in a number of tissues, including the nervous system (42), liver (22), and muscle (43). In mice, SIK1-HDAC signaling is important for muscle integrity by regulating the activity of the transcription factor MEF2 (43). In the fly, SIK3–HDAC4 signaling was shown to control the expression of lipolytic and gluconeogenic genes in the fat body (22). Furthermore, both Drosophila HDAC4 and MEF2 have been implicated in circadian rhythm, as has the related HDAC5 gene in mice (36–38). In this paper we have established a critical role for SIK3 in two circadian behaviors, single-fly locomotor activity and male sex drive, respectively.
SIK3 and HDAC4 Differentially Impact MSDR and SLR.
MSDR is mediated through the activity of Pdf+ LNvs and DN1 neurons (17). We therefore specifically targeted SIK3 in either group of circadian neurons using RNAi. Strikingly, SIK3 knockdown in LNvs shortened the period length of MSDR but slightly yet reproducibly lengthened that of SLR (Fig. 1B and Table S2). The loss of PER rhythm amplitude observed specifically in the DN1 neurons and its apparent phase advance on the second or third day of constant conditions would fit with these observations (Fig. 2 A and B and Fig. S6 A–E). The advance would be symptomatic of DN1 neurons free-running with a short period and thus presumably explains the short-period MSDR. The loss of amplitude could indicate that a small subset of DN1 neurons runs at a different pace, perhaps explaining the slightly long period of the SLR. Indeed, both SLR and MSDR depend on sLNvs driving DN1 neurons (17, 18, 31–33). The broader M peak might also be an early sign that DN1 neurons are not as coherent, even under LD, because the DN1 neurons function downstream of the sLNvs to control morning anticipatory activity (31–33). The same short-period DN1 neurons might drive the M peak and MSDR. Unfortunately, the amplitude of the M peak in DD was too low to be able to determine whether it free-runs with a short period.
Knockdown of SIK3 in DN1 neurons shortened the period length of MSDR that is well correlated with shortened PER oscillations in DN1 neurons, and these flies show subtle but significantly shortened period length in SLR (Fig. 1B and Table S2). Together, these findings suggest that SIK3 is a key component in molecular oscillator coupling between sLNvs and DN1 neurons and that its role is especially important for maintaining an appropriate MSDR period length. However, we cannot exclude the possibility that SIK3 also influences the period of the circadian pacemaker in a neuron-specific manner (i.e., in the DN1 neurons), as was proposed for SGG and CKII (44).
We also demonstrated that HDAC4 cycles in a SIK3-dependent fashion between the cytoplasm and the nucleus in the M cells. Because M-cell restricted overexpression of phosphorylation-defective, constitutively nuclear-located HDAC43A, but not wild-type HDAC4, mimics the phenotype of flies lacking SIK3 in these cells, we suggest that HDAC4 is a critical component for the transduction of the circadian intercellular signal from M cells to DN1 neurons. However, the function of SIK3 in oscillator coupling is unlikely to be mediated by HDAC4 in DN1 neurons, because most of these neurons do not express HDAC4 (Fig. 4C). Another potential SIK3 phosphorylation target such as CREB or CRTC, which are implicated in cAMP-mediated signaling and the circadian clock (45–48), may play a role in the regulation of oscillator coupling in DN1 neurons for MSDR.
Circadian Control of SIK3 Activity.
SIK3-dependent circadian shuttling of HDAC4 in sLNvs implies that the activity of SIK3 is under circadian control. How is SIK3 activity regulated in sLNvs? In fat cells (and rat adipocytes) SIK3 activity is dependent on nutrition status and is regulated indirectly through neurosecretory signaling: In well-fed flies, SIK3 is thought to be indirectly activated by insulin-like peptides (ILPs), whereas in starved flies, it is inhibited by adipokinetic hormone (AKH) (22). SIK3 activity itself is regulated via phosphorylation by AKT1 (activated by ILPs) and cAMP-dependent protein kinase A (PKA) (activated by AKH) (22). These kinases target distinct but overlapping sets of serine and threonine residues, and thus it appears that SIK3 activity is dependent on the particular phosphorylation pattern at these sites. Intriguingly, it has been reported that PDF stabilizes PER by increasing cAMP levels and PKA activity in Pdfr+ clock neurons (including M cells) at dawn (9), a time when HDAC4 is activated and translocated into the nucleus (Fig. 4 D and D′). PDF thus could be an indirect circadian regulator of SIK3 activity via PKA. However, the reduction of PDF in M cells did not shorten the MSDR period length (Table S5), suggesting that PDF signaling probably does not regulate SIK3. Moreover, RNAi-mediated knockdown of MEF2, which regulates SIK3–HDAC in the mouse (43), had no effect on MSDR. Future experiments will be needed to investigate how SIK3 activity is regulated and how HDAC4 controls intercellular communications between M cells and DN1 neurons.
SIK3 Is Necessary for the Robust Circadian Molecular Oscillations in Specific Subsets of Clock Cells.
How does the lack of SIK3 in M cells (i.e., sLNvs) alter the robustness of PER cycling in some (DN1 neurons) but not other (sLNvs and LNds) PDFR-expressing clock cells? One possibility might be the manner by which sLNvs communicate with other clock cells. Functional and anatomical studies including GFP Reconstitution Across Synaptic Partners strongly suggest that at least some DN1 neurons are direct downstream targets of sLNvs (31, 33), and hence accurately timed communication between these neurons likely occurs through synapses, which we propose rely on SIK3 function in LNvs. In contrast, autocrine (sLNvs) and paracrine (LNds) communication likely occurs via untargeted release of PDF, a process we suggest is not dependent on SIK3. The projections of sLNvs to DN1 neurons, in addition to PDF-containing dense core vesicles, harbor small clear vesicles that house classical neurotransmitters (49, 50), raising the possibility that communication between sLNvs and DN1 neurons pertinent to the robust amplitude of PER oscillation in DN1 neurons is mediated by an as yet unidentified HDAC4-dependent signal (Fig. 7). Moreover, DN1 neurons are probably heterogeneous in function, and thus it is quite likely that only some of these cells respond to the sLNv-derived and SIK3–HDAC4–dependent signal, whereas another either overlapping or entirely distinct group of DN1 neurons is responsive to the sLNv-derived PDF. In this context, it is worth noting that sLNvs also express the small neuropeptide F (sNPF) (51). Moreover, a discrete requirement for both PDF-mediated and classical neurotransmitter signaling has been proposed for distinct aspects of SLR (52), and glycine in sLNvs was recently proposed to coordinate locomotor behavior and appears either to accelerate or to slow down circadian oscillators in specific neuronal groups (53). Future studies will be necessary to identify the LNv-derived signal that maintains the appropriate amplitude and speed of the clock in DN1 neurons to coordinate MSDR and SLR.
Fig. 7.
A model for SIK3 function in circadian neurons. (A) In the sLNvs, SIK3 controls the phosphorylation and therefore the cellular localization of HDAC4. Because HDAC4 localization is rhythmic, we propose that SIK3 activity is under circadian clock control. Alternatively, an HDAC4 phosphatase could be under circadian control. We also propose that HDAC4 regulates the release of a synchronizing cue secreted by the sLNvs that targets the DN1 neurons (red arrow). In the DN1 neurons, SIK3 mediates their synchronization with the sLNvs via phosphorylation of an unknown target protein (green). (B) When SIK3 is down-regulated in the sLNvs, rhythmic HDAC4 phosphorylation and nucleus/cytosol shuttling is lost. Thus, the synchronizing signal is constantly inhibited, leading to poor synchronization of DN1 neurons and thus to fast MDSR and slow SLR. (C) When SIK3 is down-regulated in the DN1 neurons, they are uncoupled from the sLNv synchronizing signal, and their circadian clock runs too fast. As a result, both SLR and MDSR run fast.
It is surprising that the loss of SIK3 in sLNvs results in a long SLR and a short MSDR, whereas the loss of SIK3 in the DN1 neurons shortens both SLR and MSDR, because in either case it appears that the DN1 neurons are disconnected from the sLNvs (Fig. 7). One explanation could be that SIK3 is differentially modulated in different subpopulations of DN1 neurons by the sLNv synchronizing cue, thus resulting in DN1 desynchronization in flies lacking SIK3 in the sLNvs. However, when SIK3 is missing in DN1 neurons, they all adopt a short period by default.
Conclusion
In summary, our work unexpectedly reveals the existence of a SIK3–HDAC4 regulatory pathway that allows the M cells—the critical circadian pacemaker neurons of the fly brain—to control specific circadian neurons and behaviors. This pathway could prove particularly important in explaining how circadian behaviors can be differentially modulated in response to environmental conditions or internal states. Indeed, the ability to tune and prioritize specific behaviors in a daily manner to minimize energy expenditure and to maximize fitness and reproductive output is critical for animals. Given the strong neural and molecular homologies between the circadian system of fruit flies and mammals, it will be particularly interesting to determine whether the SIK–HDAC pathway is also active in VIP (vasoactive intestinal polypeptide-expressing) neurons of the mammalian suprachiasmatic nucleus and, if so, whether it also controls specific circadian behaviors.
Materials and Methods
Fly Strains.
Fly strains used in this study were obtained from the following resources: p{GawB}HDAC4NP1617, UAS-mCD8GFP, UAS-Dcr2, UAS-HDAC4RNAi-P{TRiP.HM05035}attP2 (Bloomington Stock Center); UAS-Sik3RNAi-kk109965, (referred to simply as “Sik3RNAi”), UAS-Sik3RNAi-kk104229 (Sik3RNAi#2), UAS-nmoRNAi-kk109009, and UAS-Mef2RNAi-GD15549 (Vienna Drosophila Stock Center); Sik372, FB-GAL4, UAS-Sik3-HA, UAS-HDAC4-FLAG, and UAS-HDAC43A-FLAG (22), the kind gift of B. Wang, University of California, San Francisco; UAS-CYCΔ (41); Clk4.1M-GAL4 (31, 32); Pdf-GAL4 (30), the kind gift of P. Hardin, Texas A&M University, College Station, TX; fruGAL4 (20), the kind gift of B. J. Dickson, Janelia Farm, Ashburn, VA; cry-GAL80 (30); UAS-Pdf (8), the kind gift of M. Rosbash, Brandeis University, Waltham, MA; Mz520, the kind gift of F. Rouyer, Université Paris-Saclay, Gif-sur-Yvette, France; c319 (www.fly-trap.org/); and cry-GAL4_2 (17).
Behavioral Assays.
For MSDR and SLR assays, we used 10- to 17-d-old males of the indicated genotypes as test subjects and virgin w1118 females (1–8 d of age) as target objects. Five- to seven-day-old males were entrained under 12:12 LD conditions in vials (about 40 flies per vial) for 7–10 d. Virgin females were kept in vials with agarose containing 500 mM sucrose. A single male and a single female were placed in the 15-mm diameter arena (24-well tissue-culture plates with apple juice agarose medium) before lights-off time [time 0; zeitgeber time (ZT)12]. Flies were videotaped (time lapse, one frame every 3 s) at ∼23 °C for 132 h under constant darkness with IR light. The activity of males was analyzed by EthoVison 3.1 (Noldus). In previous reports (16, 17) we measured the proximity between male and female pairs; in this report we measured the locomotor activity of male flies because of its robustness. Data were analyzed using FaasX software 1.6b (F. Rouyer). Rhythmic flies were defined by χ2 periodgram analysis with the following criteria (filter on): power ≥20, width ≥2 h, with selection on period = 24 ± 8 h. The bin size was 20 min, and data length was 5 d (the initial 12 h were cut off). Only samples in which flies stayed alive throughout the entire recording were included in the analysis. For MSDR, a fraction of “rhythmic” males were assigned as “arrhythmic” by the video observation because they showed lower “average bin activity” calculated by FaasX (less than 20 arbitrary units) caused by noncourtship behavior. Rhythmic flies were used to calculate Period and Power.
Circadian Locomotor Rhythms.
Three- to five-day-old males (12–32 flies per genotype and per experiment) were loaded into glass tubes and recorded using the TriKinetics Drosophila Activity Monitor system as previously described (32). LD activity profiles were generated by averaging locomotor activity measured during three LD cycles, using the eduction function of FAAS-X (29). LD-to-DD transition profiles were similarly generated from data collected between ZT14 in the last night of LD and circadian time (CT)14 in the first day of DD. To quantify the width of the morning peak of activity in LD, an observer blind to genotypes determined the time at which activity first reached 80% of the maximum activity observed before the light-on transition. For example, if 80% of maximum activity was reached 2 h before the light-on transition, the width of the morning peak was 2 h. For the LD-to-DD transition, the width of the M peak was defined as the difference between the time at which 80% of peak activity was first and last reached.
Immunocytochemistry.
Males (∼20 per vial) were entrained, and heads were collected at different times of day. The males reported in Fig. S6C were initially entrained as a group. A male then was paired with a female in each arena and was kept in DD for 2–4 d. Fixed brains were placed in primary antisera solution at 4 °C for 48 h. Rabbit anti-PER, guinea pig anti-CLK, rabbit anti-PDF (54) (the kind gift of P. Hardin, Texas A&M University, College Station, TX), chicken anti-GFP (Molecular Probes), and mouse anti-FLAG and chicken anti-HA (Sigma) were diluted at 1:15,000, 1:3,000, 1:1,000, 1:3,000, 1:500, and 1:500, respectively, in blocking solution (PBS containing 0.1% Triton X-100 and 5% heat-inactivated goat serum, 5% BSA, and 0.3% Na-deoxycholate). Brains were rinsed five times for 1 h and then were placed in secondary anti-sera for 48 h at 4 °C; 1:200 dilutions of goat anti-rabbit Alexa 647, goat anti-guinea pig Cy3, goat anti-chicken Alexa 488, goat anti-mouse Alexa 488, and goat anti-chicken Alexa 555 (Molecular Probes) in blocking solution were used. Confocal images were captured with a Nikon A1R Confocal Microscopy System.
Quantification of PER Intensity.
PER immunoreactivity quantification was restricted to the nuclear area as determined by CLK immunoreactivity. After background subtraction, intensity values of cells in each cluster were calculated. n indicates the number of clock neuron clusters, which contain at most five sLNv, six LNds and 17 DN1p neurons, respectively (Fig. 2 B, C′, and D′ and Fig. S6 A–F).
Cell Count of Nuclear-Localized HDAC4.
The quantification of HDAC4-FLAG immunoreactivity was restricted to the nuclear area as determined by CLK immunoreactivity. The whole-cell area was estimated to be about twice as large as the nucleus. After background subtraction, cells in which the intensity value of the nucleus was more than 20 arbitrary units higher than that of the cytosolic compartment were defined as cells with nuclear-localized HDAC4. The size of the nucleus was used to differentiate sLNvs from lLNvs. n indicates the number of clock neuron clusters (Figs. 4 D′ and E′ and 5 A′ and B′ and Fig. S8 A, B′, C′, D′, E′, and F′).
Statistical Analysis.
All χ2 tests were done with Microsoft Excel. For period length and power of MSDR and SLR, relative PER signal intensity measurements, measurements of M-peak width, and HDAC4 subcellular localization measurements, ANOVAs were performed with the PRISM software (GraphPad Software, Inc.), followed by Tukey’s multiple comparisons tests.
Acknowledgments
We thank B. Wang, P. Hardin, B. J. Dickson, M. Rosbash, and F. Rouyer for various fly strains; P. Hardin for helpful comments on the manuscript; D. Bilodeau-Wentworth and Chunyan Yuan for performing locomotor behavior experiments; and P. Lamba for quantification of M peaks. This work was supported by National Institute on Deafness and Other Communication Disorders Grant R01DC0113967 and National Institute of General Medical Sciences (NIGMS) Grant R01GMDC05606 (to H.A.). P.E. is supported by Maximizing Investigators’ Research Award (R35) GM1180875 from the NIGMS.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1620483114/-/DCSupplemental.
References
- 1.Hardin PE. The circadian timekeeping system of Drosophila. Curr Biol. 2005;15:R714–R722. doi: 10.1016/j.cub.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 2.Nitabach MN, Taghert PH. Organization of the Drosophila circadian control circuit. Curr Biol. 2008;18:R84–R93. doi: 10.1016/j.cub.2007.11.061. [DOI] [PubMed] [Google Scholar]
- 3.Rieger D, Shafer OT, Tomioka K, Helfrich-Förster C. Functional analysis of circadian pacemaker neurons in Drosophila melanogaster. J Neurosci. 2006;26:2531–2543. doi: 10.1523/JNEUROSCI.1234-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hyun S, et al. Drosophila GPCR Han is a receptor for the circadian clock neuropeptide PDF. Neuron. 2005;48:267–278. doi: 10.1016/j.neuron.2005.08.025. [DOI] [PubMed] [Google Scholar]
- 5.Lear BC, et al. A G protein-coupled receptor, groom-of-PDF, is required for PDF neuron action in circadian behavior. Neuron. 2005;48:221–227. doi: 10.1016/j.neuron.2005.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Mertens I, et al. PDF receptor signaling in Drosophila contributes to both circadian and geotactic behaviors. Neuron. 2005;48:213–219. doi: 10.1016/j.neuron.2005.09.009. [DOI] [PubMed] [Google Scholar]
- 7.Collins B, et al. Differentially timed extracellular signals synchronize pacemaker neuron clocks. PLoS Biol. 2014;12:e1001959. doi: 10.1371/journal.pbio.1001959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Renn SC, Park JH, Rosbash M, Hall JC, Taghert PH. A pdf neuropeptide gene mutation and ablation of PDF neurons each cause severe abnormalities of behavioral circadian rhythms in Drosophila. Cell. 1999;99:791–802. doi: 10.1016/s0092-8674(00)81676-1. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Guo F, Shen J, Rosbash M. PDF and cAMP enhance PER stability in Drosophila clock neurons. Proc Natl Acad Sci USA. 2014;111:E1284–E1290. doi: 10.1073/pnas.1402562111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Seluzicki A, et al. Dual PDF signaling pathways reset clocks via TIMELESS and acutely excite target neurons to control circadian behavior. PLoS Biol. 2014;12:e1001810. doi: 10.1371/journal.pbio.1001810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guo F, Cerullo I, Chen X, Rosbash M. PDF neuron firing phase-shifts key circadian activity neurons in Drosophila. Elife. 2014;3:e02780. doi: 10.7554/eLife.02780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stoleru D, Peng Y, Nawathean P, Rosbash M. A resetting signal between Drosophila pacemakers synchronizes morning and evening activity. Nature. 2005;438:238–242. doi: 10.1038/nature04192. [DOI] [PubMed] [Google Scholar]
- 13.Yao Z, Shafer OT. The Drosophila circadian clock is a variably coupled network of multiple peptidergic units. Science. 2014;343:1516–1520. doi: 10.1126/science.1251285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hardeland R. Species differences in the diurnal rhythmicity of courtship behaviour within the Melanogaster group of the genus Drosophila. Anim Behav. 1972;20:170–174. doi: 10.1016/s0003-3472(72)80188-x. [DOI] [PubMed] [Google Scholar]
- 15.Sakai T, Ishida N. Circadian rhythms of female mating activity governed by clock genes in Drosophila. Proc Natl Acad Sci USA. 2001;98:9221–9225. doi: 10.1073/pnas.151443298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fujii S, Krishnan P, Hardin P, Amrein H. Nocturnal male sex drive in Drosophila. Curr Biol. 2007;17:244–251. doi: 10.1016/j.cub.2006.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujii S, Amrein H. Ventral lateral and DN1 clock neurons mediate distinct properties of male sex drive rhythm in Drosophila. Proc Natl Acad Sci USA. 2010;107:10590–10595. doi: 10.1073/pnas.0912457107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hamasaka Y, Suzuki T, Hanai S, Ishida N. Evening circadian oscillator as the primary determinant of rhythmic motivation for Drosophila courtship behavior. Genes Cells. 2010;15:1240–1248. doi: 10.1111/j.1365-2443.2010.01456.x. [DOI] [PubMed] [Google Scholar]
- 19.Zheng X, Sehgal A. Speed control: Cogs and gears that drive the circadian clock. Trends Neurosci. 2012;35:574–585. doi: 10.1016/j.tins.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stockinger P, Kvitsiani D, Rotkopf S, Tirián L, Dickson BJ. Neural circuitry that governs Drosophila male courtship behavior. Cell. 2005;121:795–807. doi: 10.1016/j.cell.2005.04.026. [DOI] [PubMed] [Google Scholar]
- 21.Hanafusa S, Kawaguchi T, Umezaki Y, Tomioka K, Yoshii T. Sexual interactions influence the molecular oscillations in DN1 pacemaker neurons in Drosophila melanogaster. PLoS One. 2013;8:e84495. doi: 10.1371/journal.pone.0084495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang B, et al. A hormone-dependent module regulating energy balance. Cell. 2011;145:596–606. doi: 10.1016/j.cell.2011.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Levine JD, Funes P, Dowse HB, Hall JC. Resetting the circadian clock by social experience in Drosophila melanogaster. Science. 2002;298:2010–2012. doi: 10.1126/science.1076008. [DOI] [PubMed] [Google Scholar]
- 24.Chiu JC, Ko HW, Edery I. NEMO/NLK phosphorylates PERIOD to initiate a time-delay phosphorylation circuit that sets circadian clock speed. Cell. 2011;145:357–370. doi: 10.1016/j.cell.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yu W, Houl JH, Hardin PE. NEMO kinase contributes to core period determination by slowing the pace of the Drosophila circadian oscillator. Curr Biol. 2011;21:756–761. doi: 10.1016/j.cub.2011.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yoshii T, Todo T, Wülbeck C, Stanewsky R, Helfrich-Förster C. Cryptochrome is present in the compound eyes and a subset of Drosophila’s clock neurons. J Comp Neurol. 2008;508:952–966. doi: 10.1002/cne.21702. [DOI] [PubMed] [Google Scholar]
- 27.Choi C, et al. Cellular dissection of circadian peptide signals with genetically encoded membrane-tethered ligands. Curr Biol. 2009;19:1167–1175. doi: 10.1016/j.cub.2009.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshii T, et al. The neuropeptide pigment-dispersing factor adjusts period and phase of Drosophila’s clock. J Neurosci. 2009;29:2597–2610. doi: 10.1523/JNEUROSCI.5439-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Grima B, Chélot E, Xia R, Rouyer F. Morning and evening peaks of activity rely on different clock neurons of the Drosophila brain. Nature. 2004;431:869–873. doi: 10.1038/nature02935. [DOI] [PubMed] [Google Scholar]
- 30.Stoleru D, Peng Y, Agosto J, Rosbash M. Coupled oscillators control morning and evening locomotor behaviour of Drosophila. Nature. 2004;431:862–868. doi: 10.1038/nature02926. [DOI] [PubMed] [Google Scholar]
- 31.Zhang L, et al. DN1(p) circadian neurons coordinate acute light and PDF inputs to produce robust daily behavior in Drosophila. Curr Biol. 2010;20:591–599. doi: 10.1016/j.cub.2010.02.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang Y, Liu Y, Bilodeau-Wentworth D, Hardin PE, Emery P. Light and temperature control the contribution of specific DN1 neurons to Drosophila circadian behavior. Curr Biol. 2010;20:600–605. doi: 10.1016/j.cub.2010.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cavanaugh DJ, et al. Identification of a circadian output circuit for rest:activity rhythms in Drosophila. Cell. 2014;157:689–701. doi: 10.1016/j.cell.2014.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Burbelo PD, Hall A. 14-3-3 proteins. Hot numbers in signal transduction. Curr Biol. 1995;5:95–96. doi: 10.1016/s0960-9822(95)00022-4. [DOI] [PubMed] [Google Scholar]
- 35.Yang X-J, Seto E. The Rpd3/Hda1 family of lysine deacetylases: From bacteria and yeast to mice and men. Nat Rev Mol Cell Biol. 2008;9:206–218. doi: 10.1038/nrm2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanchard FJ, et al. The transcription factor Mef2 is required for normal circadian behavior in Drosophila. J Neurosci. 2010;30:5855–5865. doi: 10.1523/JNEUROSCI.2688-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sivachenko A, Li Y, Abruzzi KC, Rosbash M. The transcription factor Mef2 links the Drosophila core clock to Fas2, neuronal morphology, and circadian behavior. Neuron. 2013;79:281–292. doi: 10.1016/j.neuron.2013.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogg PC, et al. Class IIa histone deacetylases are conserved regulators of circadian function. J Biol Chem. 2014;289:34341–34348. doi: 10.1074/jbc.M114.606392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kula-Eversole E, et al. Surprising gene expression patterns within and between PDF-containing circadian neurons in Drosophila. Proc Natl Acad Sci USA. 2010;107:13497–13502. doi: 10.1073/pnas.1002081107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chintapalli VR, Wang J, Dow JA. Using FlyAtlas to identify better Drosophila melanogaster models of human disease. Nat Genet. 2007;39:715–720. doi: 10.1038/ng2049. [DOI] [PubMed] [Google Scholar]
- 41.Tanoue S, Krishnan P, Krishnan B, Dryer SE, Hardin PE. Circadian clocks in antennal neurons are necessary and sufficient for olfaction rhythms in Drosophila. Curr Biol. 2004;14:638–649. doi: 10.1016/j.cub.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 42.van der Linden AM, Nolan KM, Sengupta P. KIN-29 SIK regulates chemoreceptor gene expression via an MEF2 transcription factor and a class II HDAC. EMBO J. 2007;26:358–370. doi: 10.1038/sj.emboj.7601479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Berdeaux R, et al. SIK1 is a class II HDAC kinase that promotes survival of skeletal myocytes. Nat Med. 2007;13:597–603. doi: 10.1038/nm1573. [DOI] [PubMed] [Google Scholar]
- 44.Top D, Harms E, Syed S, Adams EL, Saez L. GSK-3 and CK2 kinases converge on timeless to regulate the master clock. Cell Rep. 2016;16:357–367. doi: 10.1016/j.celrep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Screaton RA, et al. The CREB coactivator TORC2 functions as a calcium- and cAMP-sensitive coincidence detector. Cell. 2004;119:61–74. doi: 10.1016/j.cell.2004.09.015. [DOI] [PubMed] [Google Scholar]
- 46.Takemori H, Kajimura J, Okamoto M. TORC-SIK cascade regulates CREB activity through the basic leucine zipper domain. FEBS J. 2007;274:3202–3209. doi: 10.1111/j.1742-4658.2007.05889.x. [DOI] [PubMed] [Google Scholar]
- 47.Jagannath A, et al. The CRTC1-SIK1 pathway regulates entrainment of the circadian clock. Cell. 2013;154:1100–1111. doi: 10.1016/j.cell.2013.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakamoto K, et al. Clock and light regulation of the CREB coactivator CRTC1 in the suprachiasmatic circadian clock. J Neurosci. 2013;33:9021–9027. doi: 10.1523/JNEUROSCI.4202-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yasuyama K, Meinertzhagen IA. Synaptic connections of PDF-immunoreactive lateral neurons projecting to the dorsal protocerebrum of Drosophila melanogaster. J Comp Neurol. 2010;518:292–304. doi: 10.1002/cne.22210. [DOI] [PubMed] [Google Scholar]
- 50.Miskiewicz K, Pyza E, Schürmann FW. Ultrastructural characteristics of circadian pacemaker neurones, immunoreactive to an antibody against a pigment-dispersing hormone in the fly’s brain. Neurosci Lett. 2004;363:73–77. doi: 10.1016/j.neulet.2004.03.051. [DOI] [PubMed] [Google Scholar]
- 51.Johard HA, et al. Peptidergic clock neurons in Drosophila: Ion transport peptide and short neuropeptide F in subsets of dorsal and ventral lateral neurons. J Comp Neurol. 2009;516:59–73. doi: 10.1002/cne.22099. [DOI] [PubMed] [Google Scholar]
- 52.Choi C, et al. Autoreceptor control of peptide/neurotransmitter corelease from PDF neurons determines allocation of circadian activity in Drosophila. Cell Rep. 2012;2:332–344. doi: 10.1016/j.celrep.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frenkel L, et al. Organization of circadian behavior relies on glycinergic transmission. Cell Rep. 2017;19:72–85. doi: 10.1016/j.celrep.2017.03.034. [DOI] [PubMed] [Google Scholar]
- 54.Cheng Y, Hardin PE. Drosophila photoreceptors contain an autonomous circadian oscillator that can function without period mRNA cycling. J Neurosci. 1998;18:741–750. doi: 10.1523/JNEUROSCI.18-02-00741.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]