Significance
The accelerating loss of coastal foundation species impairs the delivery of vital ecosystem services on which nearly half the human population depends. Recognizing how loss of habitat-forming species such as seagrasses and oysters can be offset is therefore essential. This paper demonstrates that in areas where native foundation species are absent, nonnative habitat formers can amplify the production of diverse ecosystem functions that underpin provisioning of services to humans, such as food production. Our findings suggest that in areas where native foundation species have been lost, invasive habitat formers may be considered as a tool to enhance multiple ecosystem functions.
Keywords: exotic plant, ecosystem engineer, novel facilitation, conservation, biodiversity
Abstract
While invasive species often threaten biodiversity and human well-being, their potential to enhance functioning by offsetting the loss of native habitat has rarely been considered. We manipulated the abundance of the nonnative, habitat-forming seaweed Gracilaria vermiculophylla in large plots (25 m2) on southeastern US intertidal landscapes to assess impacts on multiple ecosystem functions underlying coastal ecosystem services. We document that in the absence of native habitat formers, this invasion has an overall positive, density-dependent impact across a diverse set of ecosystem processes (e.g., abundance and richness of nursery taxa, flow attenuation). Manipulation of invader abundance revealed both thresholds and saturations in the provisioning of ecosystem functions. Taken together, these findings call into question the focus of traditional invasion research and management that assumes negative effects of nonnatives, and emphasize the need to consider context-dependence and integrative measurements when assessing the impact of an invader, including density dependence, multifunctionality, and the status of native habitat formers. This work supports discussion of the idea that where native foundation species have been lost, invasive habitat formers may be considered as sources of valuable ecosystem functions.
The large-scale degradation of marine ecosystems is unequivocal (1–4). Recent assessments suggest that ∼20% of coral reefs (5), 30% of seagrasses (6), 45% of salt marshes (7), and 90% of oyster reefs (8) have been lost worldwide, implying that many coastal ecosystems around the world have been converted from complex biogenic habitats to barren sedimentary systems. Because a significant proportion of society depends on these coastal ecosystems to generate >1014 USD in services each year through storm protection, food production, and tourism (1, 9), conservation resources of >109 USD are spent globally each year in an attempt to reverse the decline of these coastal foundation species (10, 11). Yet many efforts to restore coastal habitats have achieved only limited success despite high investment per hectare (12–14). It is therefore pertinent that research examine different means by which the loss of marine foundation species can be offset in order to provide society with the goods and services needed to prosper.
Nonnative species often threaten biodiversity (15) and incur substantial costs (16), but recent reviews have also pointed out their potential value for conservation (17, 18). Related analyses also suggest biases that favor finding negative effects, and, as a consequence, the potential benefits of invasive species may have been overlooked (19, 20). For example, most studies (i) examine impacts when invasive species are displacing functionally similar native species (21–24), (ii) consider only one or few related response variables when assessing the impacts of invaders (19, 20), (iii) are conducted on small spatial scales (e.g., in aquatic systems typically ≤1 m2; refs. 25 and 26), and (iv) rarely incorporate multiple abundance levels, despite the fact that impacts depend fundamentally on the abundance of the invader (26, 27).
Because managers often need to conserve ecosystem functions where native foundation species have been lost (e.g., kelp forest retreat due to warming waters, coral reef loss due to multiple stressors, oyster reef decline due to overharvesting and diseases, seagrass loss to eutrophication and warming; refs. 5, 8, 14, 28, 29), recognizing that nonnative species may enhance ecosystem functions could influence management decisions (17, 18). For example, in coastal areas that have experienced degradation of native foundation species, such as on coral reefs (5, 30), seagrass beds (6), and oyster reefs (8, 12), invasive foundation species could have significant positive effects, particularly if they replace and substitute ecological functions that have otherwise been lost (18, 21, 22, 31). With the exception of highly managed systems, such as agricultural fields, society values ecosystems that simultaneously provide many different and complementary functions and services (1, 9). In order to facilitate informed management decisions, impacts of invaders should therefore, whenever possible, be assessed on multiple ecosystem functions (32), at realistic spatial scales (e.g., landscape; refs. 26 and 33), and across a wide range of realistic invader abundances (26, 27, 34). Only then will managers have critical information needed to better predict when and where invasion impacts will be positive vs. negative.
To address this research gap, we focused on the nonnative seaweed Gracilaria vermiculophylla (hereinafter Gracilaria) that has invaded shallow soft-bottom lagoons and estuaries throughout coastlines of the North Atlantic (35). Gracilaria is expected to have negative ecological effects where native foundation species are present (24), but where native habitat formers are absent or have declined, Gracilaria can generate mosaics of vegetated habitats that could constitute hotspots of ecosystem functions (23). An initial survey of two sites along the North Carolina coast revealed high abundance and variability in Gracilaria cover (mean ± SD, 14 ± 23%, but also often with 100% cover; Fig. 1 A–C). In North Carolina alone, estimates suggest that 97% of seagrasses (36), 90% of oyster reefs (4, 37), and 12% of salt marshes (7) have been lost relative to their historical extent, a pattern similar to losses of native coastal foundation species throughout the biogeographical region that Gracilaria has invaded (e.g., refs. 4, 12, 14, 36, 37) and, more generally, to global losses (6–8). Gracilaria’s invasion of North Carolina mudflats provides a unique system for testing how a nonnative foundation species affects ecosystem functioning where native analogs are absent or have declined significantly in recent times (6, 38). We hypothesized that invasion by a novel foundation species, in the absence of native foundation species, would have positive density-dependent effects on multiple ecosystem functions, including nursery habitat production, sediment stabilization, and wave attenuation, for which coastal habitats are highly valued (9).
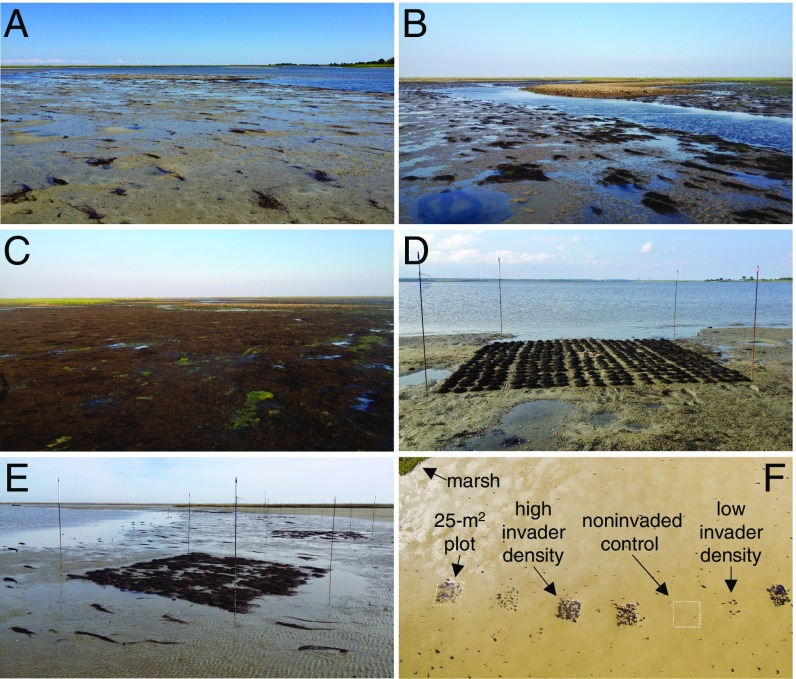
Fig. 1.
Foundation species invasion and experimental manipulation. (A–C) Gracilaria vermiculophylla at typical low cover around Zeke’s Island (A) and at intermediate and high cover around Masonboro Island (B and C) in North Carolina. (D–F) Initial manipulation of Gracilaria cover (D) and ground-level (E) and bird’s-eye (F) views of the experimental plots at various invader densities on intertidal mudflats. (F) Image courtesy of Devon Eulie (photographer).
To test this hypothesis, we manipulated six densities of Gracilaria in 48 large (25 m2) plots over a 10-mo period (Fig. 1 D–F). On a monthly basis, we measured seven ecosystem functions (abundance and taxonomic richness of epifauna and nursery species, flow attenuation, sediment stabilization, and decomposition) that underpin services for which coastal ecosystems are highly valued (Table S1). To simplify our analyses and remove temporal autocorrelation, we calculated the time-averaged, density-dependent response of each ecosystem function (in each plot) over the course of the experiment. Given that several previous studies have documented nonlinear relationships between the structure and functions of coastal habitats (39–41), we analyzed response functions using a common set of linear, saturating, and exponential models with Gracilaria cover as the explanatory variable. Model selection was based on the Akaike information criterion, corrected for small sample size (AICc).
Table S1.
Ecosystem services, and underlying processes and functions, for Gracilaria supported by this study
| Ecosystem services | Ecosystem processes and functions | Proxy measured herein | Effect |
| Raw materials and food | Generates biological productivity and diversity | Epifauna abundance | + |
| Epifauna richness | + | ||
| Coastal protection | Attenuates and/or dissipates tidal currents and waves | Dissolution of gypsum blocks | + |
| Erosion control | Provides sediment stabilization and soil retention | Sediment stabilization | 0 |
| Water purification | Provides nutrient and pollution uptake, as well as retention and particle deposition | Not measured here | NA |
| Maintenance of fisheries | Provides suitable reproductive habitat and nursery grounds, sheltered living space | Nursery abundance | + |
| Nursery richness | + | ||
| Carbon sequestration | Generates biogeochemical activity, sedimentation, and biological productivity | Decomposition of marsh plants | 0 |
| Tourism, recreation, education, and research | Provides unique and aesthetic submerged vegetated landscape, suitable habitat for diverse flora and fauna | This study, taken as a whole | + |
NA, not available. Table adapted from that presented in ref. 9 for seagrasses. Ecosystem services reflect benefits (of monetary value) provided to humanity and are underpinned by ecosystem processes and functions. The proxy measured herein outlines the actual variant(s) of those processes and functions that we quantified, and the effect indicates the sign of Gracilaria’s impact on each service, supported by the proxies measured in our study.
Results
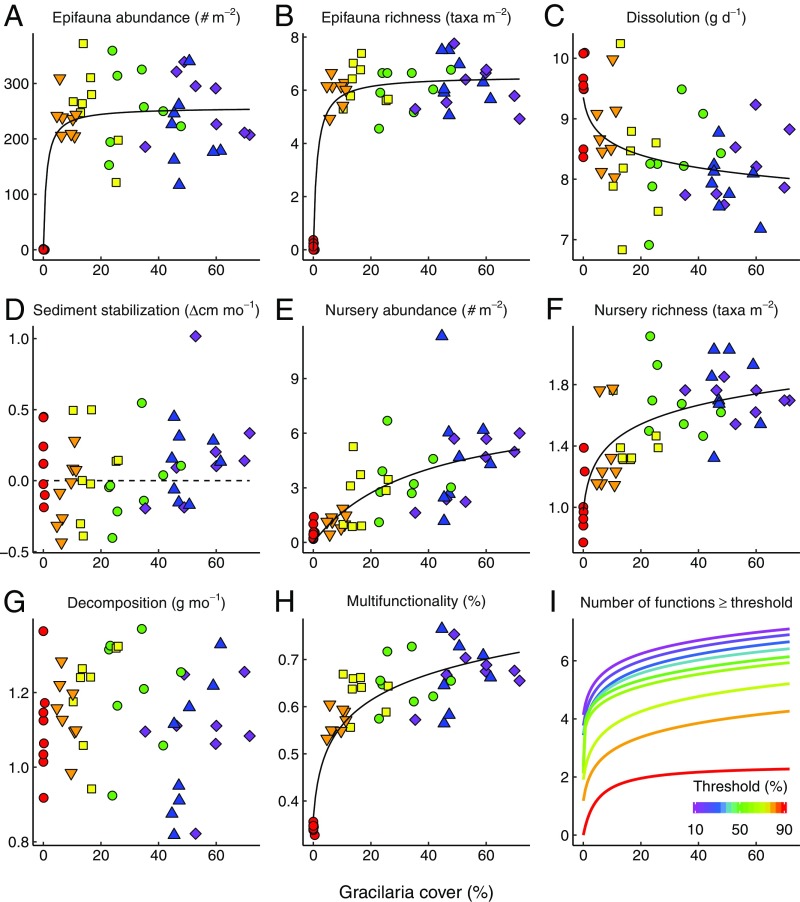
We first examined the relationships between Gracilaria cover and individual ecosystem functions. To assess whether Gracilaria cover affects biological productivity and diversity (underpinning the service of raw materials and food), we sampled the abundance and richness of epifauna in a 0.25-m2 quadrat in each plot. We found positive, hyperbolic relationships between Gracilaria cover and both epifauna abundance (Fig. 2A) and richness (Fig. 2B). In both cases, saturation was reached rapidly; on average, epifauna abundance and richness were more than three orders of magnitude greater in the presence than in the absence of Gracilaria. The epifauna community was dominated by small herbivorous crustaceans and gastropods. The effect of increasing Gracilaria cover on flow attenuation (measured as the dissolution of gypsum blocks) underlying coastal protection was logarithmic and positive (Fig. 2C). We did not find a significant relationship between Gracilaria cover and sediment stabilization, a process that underpins erosion control (Fig. 2D), although there was a nonsignificant tendency for plots with higher Gracilaria cover to accumulate sediments (plots with >55% cover appeared to accumulate sediments; Fig. 2D). The effect of Gracilaria cover on abundance (Fig. 2E) and taxonomic richness (Fig. 2F) of nursery species, functions underlying the maintenance of fisheries, were strongly positive, with hyperbolic and logarithmic response functions, respectively. Plots containing Gracilaria had >400% higher abundances of nursery species than those without Gracilaria, dominated by commercially important species, including croaker (fish), blue crabs, and penaeid shrimp (Fig. S1). In contrast to the epifauna data, nursery function increased more slowly with the abundance of the invader, demonstrating markedly different levels of ecosystem functioning across our density treatments. We did not observe any significant relationship between Gracilaria cover and decomposition (Fig. 2G), a function underlying carbon sequestration. Overall, the effect of increasing Gracilaria cover on the abundance and richness of epifauna and nursery species and flow attenuation was highly significant (P < 0.001), positive, and saturating (Fig. 2 A–C, E, and F).
Fig. 2.
Density-dependent impacts of an invasive foundation species on coastal ecosystem functions. (A) Epifauna abundance. (B) Number of epifauna taxa. (C) Gypsum dissolution (a proxy for flow attenuation; data were reflected before calculating multifunctionality, so that reduced dissolution corresponded to increased flow attenuation). (D) Sediment stabilization, the change in substrate elevation per month. Values above the dashed line represent accretion, whereas those below represent erosion. (E) Abundance of nursery species, i.e., mobile macrofauna and juvenile species of commercial importance. (F) Taxonomic richness of nursery species. (G) Decomposition (biomass of Spartina stems lost). (H) Multifunctionality index; the average of the seven standardized functions in percent. (I) Raw number of functions exceeding threshold levels in each plot against Gracilaria cover, for thresholds ranging from 10% (magenta) to 90% (red) of the maximum indicated on the color scale below. Points are time-averaged responses of each function vs. the average Gracilaria cover of each plot (n = 48). Colors and symbols correspond to the six density treatments (number of stakes arranged in squared grids: red circles, 0; orange triangles, 9; yellow squares, 36; green circles, 100; blue triangles, 225; purple diamonds, 400). The best-fitting models determined by AICc are shown where significant (F1,46 ≥ 17.61; P < 0.001). In I, the form of the relationship was logarithmic up to the 40% threshold (blue-green), then changed to a power function at 50% (green), back to logarithmic at 70% (yellow), and then to a hyperbolic function at 90% (red).
Fig. S1.
(A) Seining a plot to obtain nursery abundance and richness functions. (B–D) Commercially important species commonly retained by the seine include juvenile croaker, blue crabs, and adult penaeid shrimp.
We next examined the density-dependent effects of Gracilaria on the simultaneous performance of multiple functions (multifunctionality) as described previously (32). Our analysis revealed a strong positive, logarithmic relationship between Gracilaria cover and ecosystem multifunctionality, measured as the mean standardized index of all seven functions in percent (Fig. 2H). To further quantify whether Gracilaria enhances the simultaneous maintenance of multiple ecosystem functions, we assessed the effect of Gracilaria cover on the ability to achieve single ecosystem multifunctionality thresholds by counting the number of functions in each plot maintained above a given threshold (defined between 10% and 90%). Although the form of this response varied slightly depending on the threshold examined, the relationship between Gracilaria cover and the number of functions maintained above a given threshold was significantly positive across all thresholds up to 90% (Fig. 2I).
Discussion
Our results provide large-scale experimental evidence for a positive relationship between the abundance of an invasive habitat-forming seaweed and ecosystem multifunctionality in the context of absent native foundation species. The ecological functions measured in our experiment were not chosen at random, but rather were selected as proxies for processes that underpin essential services provided by seagrass ecosystems (9), which are among the most important coastal systems worldwide (1). Given that native foundation species have been severely reduced in this region (4, 7, 36, 37), and considering that our sampling encompassed measurements of disparate ecological functions in large plots, included temporal variation, and was conducted more than 5 y after the initial invasion (35), we argue that our findings are representative of how this foundation species affects shallow, soft-bottom ecosystems in which native foundation species have been lost or are absent (23, 31, 42).
Whereas many invasive species threaten biodiversity, ecosystem functioning, and human well-being (1, 15, 16, 43), our field experiment revealed that Gracilaria can provide multiple ecosystem functions by creating novel habitat in an otherwise barren sedimentary landscape, increasing epifaunal biodiversity and the biomass of important fishery species (nursery functions) and decreasing hydrodynamic forces. The increase in epifaunal biodiversity is supported by the results of previous investigations (23, 31, 42, 44, 45), although those studies quantified impacts from only a few experimental densities and on smaller spatial scales. Our results are also consistent with a growing body of work demonstrating the importance of positive interactions in ecological communities, especially under conditions of elevated physical stress that dominate degraded coastal habitats targeted for restoration (31, 38, 46). Furthermore, we note that Gracilaria represents a typical foundation species (sensu ref. 47) because it increases biodiversity through habitat formation and delivers multiple ecosystem functions, analogous to trees in forests (48), salt marshes (38), oyster reefs (8, 12), seagrasses (6), mussel beds (46), and many other sessile taxa (1, 9). Our results therefore support the need for an experimental and comprehensive examination of invasive species' effects before ascribing a sign (+ or −) to their overall impact (18, 19).
Although we did not identify the underpinning mechanisms by which Gracilaria increases ecosystem multifunctionality, our results suggest that, by forming a complex biogenic habitat, Gracilaria (i) occupies the fundamental niche of a foundation species, (ii) directly increases biodiversity, (iii) indirectly increases ecosystem functioning (via its effect on biodiversity), and (iv) thereby provides density-dependent functions comparable to those of functionally similar natives (20–22, 31, 44, 45).
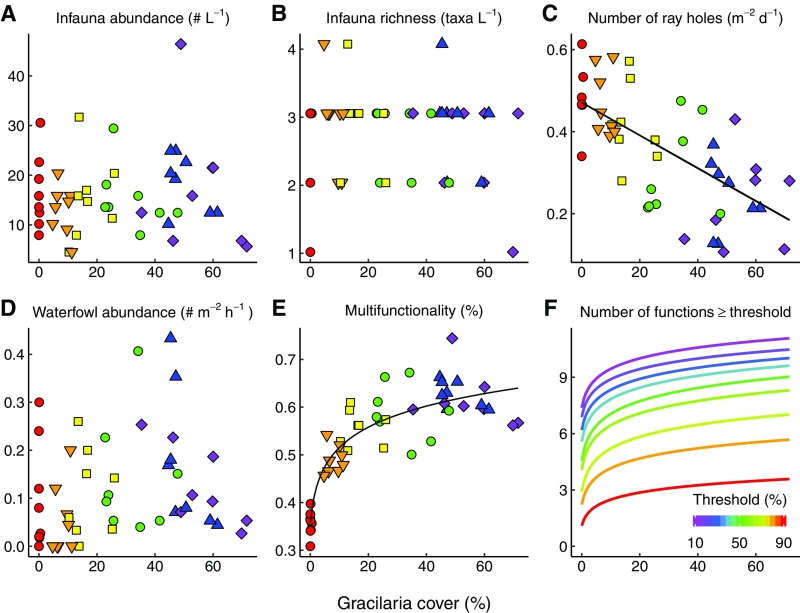
It is possible that Gracilaria has negative effects on ecosystem functions that were not measured here (24) or that could emerge if Gracilaria were to accumulate in very large densities in stagnant waters (49). However, we found no negative effect of Gracilaria on either infauna or waterfowl activity, and only a weak negative effect on ray foraging activity even at the highest densities (SI Text and Fig. S2; these additional functions were measured only at the end of the experiment and thus were excluded from the formal analysis). It is likely that decreased ray foraging could facilitate commercially and recreationally important bivalves like clams, mussels, and oysters, similar to how Gracilaria modifies other predator–prey interactions in this system (50).
Fig. S2.
Effects of Gracilaria cover on supporting variables characteristic of soft-sediment communities and multifunction indices when including all 11 response variables in the analysis (i.e., the original 7 plus 4 supporting variables). These supporting variables were, in contrast to the functions analyzed in the main text, measured only near the end of our experiment (months 8–10). (A) Infauna abundance. (B) Infauna richness, the number of infauna taxa. (C) Bivalve predation pressure measured by ray foraging activity, evident from their holes. (D) Waterfowl abundance, measured as the average number of birds present in each plot during a 15-min period. (E) Multifunctionality index, the average of the 11 standardized functions in percent. (F) The raw number of functions exceeding threshold levels in each plot against Gracilaria cover, for thresholds ranging from 10% (magenta) to 90% (red) of the maximum indicated on the color scale. Points are time-averaged responses of each function vs. the average Gracilaria cover of each plot (n = 48). Colors and symbols correspond to the six density treatments (number of stakes arranged in squared grids: red circles, 0; orange triangles, 9; yellow squares, 36; green circles, 100; blue triangles, 225; purple diamonds, 400). The model of best fit determined by AICc is shown where significant at the 0.05 level. The form of the relationship between Gracilaria cover and multifunction indices is logarithmic in E and across all thresholds in F.
Ecologists are becoming increasingly aware that native foundation species provide ecosystem functions in nonlinear ways (39–41). Our experimental findings demonstrate similar nonlinearity in ecosystem functions provided by a nonnative foundation species that has invaded thousands of kilometers of coastline throughout the Northern Hemisphere. We also found that the slopes and saturation points in these nonlinear functions vary widely for different ecosystem functions. Scale dependency is likely an important factor contributing to this variation. For example, functions such as wave attenuation and sediment stabilization may manifest on larger scales and at higher densities (39), whereas functions associated with biodiversity, such as productivity and nursery habitats, appear to operate on smaller scales and at lower densities (i.e., with early thresholds as shown here and in ref. 41). Although curve shapes of multifunctionality are likely to depend on the functions included in the analysis, our supplementary analysis (adding four processes measured at the end of our experiment) showed similarly curved functional responses, suggesting that our results are robust (Fig. S2). Nevertheless, to better understand the impacts of nonnative coastal foundation species, future studies should manipulate more densities (including both high and low extremes) and measure more ecosystem functions across different environmental gradients (e.g., salinity, depth, latitude).
Management Implications.
Human activities are accelerating the losses of species that provide the foundation for coastal habitats, including seagrasses, oysters, and salt marshes (3, 4, 6–8, 12), and, consequently, the services that these species provide (1, 2, 9). Restoration is an important tool for mitigating coastal degradation, but despite recent advances (e.g., ref. 38), current practices have yet to succeed at scales sufficiently large to match the scale of degradation. For example, a recent synthesis (13) suggests relatively low survival of restored seagrasses (41%), salt marshes (65%), and oyster reefs (56%) in the short-term (≤2 y) despite mean restoration costs of ∼$700,000, $1,040,000, and $860,000 (2010 USD) per hectare for these three coastal habitats, respectively (Table S2). Moreover, these appraisals do not take into account that (i) the cost and success of simultaneously eradicating nonnative analogs is uncertain (51); (ii) coastal management is becoming increasingly complicated as global anthropogenic stressors, such as climate change, eutrophication, and overharvesting, alter the context for future native and invasive species interactions (52–54); and (iii) managers and researchers often do not know whether invasive foundation species are drivers, passengers, or back-seat drivers of ecological change, even though each of these scenarios requires a unique management and restoration approach (55, 56). Currently, it appears that replacement of many native by nonnative foundation species, such as Wadden Sea mussels by invasive oysters and Mediterranean seagrasses by the nonnative seaweed Caulerpa, are driven more by external factors such as warming and eutrophication than by competitive interactions among foundation species (57–59).
Table S2.
Overall and total cost per unit area and success of restoration projects for seagrass, salt marsh, and oyster reef ecosystems in developed countries
| Ecosystem | Restoration cost (2010 US$) per ha | Total restoration cost (2010 US$) per ha | Restoration success | |||||
| No. | Median | Mean | No. | Median | Mean | No. | % survival | |
| Seagrass | 64 | 106 782 | 399 532 | 22 | 383 672 | 699 525 | 114 | 41.3 |
| Salt marsh | 73 | 67 128 | 1 804 779 | 40 | 151 129 | 1 042 116 | 28 | 64.8 |
| Oyster reef | 23 | 66 821 | 386 783 | 5 | 189 665 | 859 080 | 64 | 56.2 |
Adapted from Bayraktarov et al. (13). Total restoration cost includes only studies that reported both capital and operating costs. The success of restoration projects is measured as a percent survival of restored organisms.
Based on our case study, we recognize several advantages and disadvantages associated with passive “do nothing, laissez faire” vs. active “remove and replace” approaches to the management of invasive foundation species. For this Gracilaria invasion in an area where native foundation species have severely declined, our results suggest that a passive approach could help sustain fishery outputs, invertebrate biodiversity, and erosion control, without causing reductions in extant native foundation species. Akin to other invasions, however, Gracilaria may still increase “global homogenization” (60) and could have unknown long-term evolutionary consequences (61). In addition, Gracilaria could potentially (i) have negative impacts on cryptic and rare endemic species (which would require a massive sampling effort to detect), (ii) decrease the likelihood of native habitat restoration success, and (iii) accumulate in extreme densities, where it may enhance the risk of local anoxia that can cause mortality of fish and benthic invertebrates (49, 62). For an active remove and replace strategy, managers, in contrast, would seek to remove Gracilaria and restore economically and culturally important native foundation species to regain their associated benefits that support high biodiversity, sustainable fisheries, and regulation of erosional forces caused by wind and waves. Native oysters, seagrasses, and marshes typically have more stable biomass across seasons than Gracilaria, thereby providing more stable ecosystem functions. These native foundation species also have stronger legacy effects, as relic bivalves and macrophyte rhizomes can accumulate over time and thereby enhance ecological functions over longer time scales (63, 64). Finally, in contrast to Gracilaria and other invasive seaweeds, native marshes, oysters, and seagrasses have not been shown to cause large-scale anoxia events and associated faunal die-offs.
Nevertheless, despite the benefits of a remove and replace strategy, this approach has clear associated risks and costs. First, the initial economic cost is fantastically high, and both the removal of invasive species and subsequent restoration of native species carry a high risk of failure. The remove and replace approach may also result in reintroduction, and will necessitate long-term monitoring and management of restored natives (13, 51). Clearly, more research is needed to provide better data for evaluating these management approaches. For example, it will be important to study (i) facilitative vs. antagonistic impacts of Gracilaria on seagrasses, oysters, and marshes, and whether these native foundation species can recolonize invaded areas; (ii) long-term multigenerational impacts of Gracilaria on invertebrates, fish, and birds to, for example, document whether this invasion leads to evolutionary traps or whether there are cryptic native species that are lost or decimated following the invasion (61); and (iii) economic, environmental, and cultural costs and benefits associated with the contrasting management schemes (16). However, until such research has been carried out, managers should pragmatically acknowledge and incorporate the possibility of positive ecosystem functions delivered by nonnative foundation species into their decision making processes for conservation strategies (17, 18), especially when native foundation species are absent and eradication and restoration is infeasible.
Conclusions.
Our large-scale field experiment documented positive density-dependent relationships between Gracilaria abundance and many individual and integrative ecosystem functions. Consequently, if native foundation species are absent and restoration is infeasible, then actively incorporating established nonnative foundation species into conservation and management strategies may have stronger than expected benefits for the provisioning of coastal ecosystem services. We hope that this work will stimulate new thinking and innovative research on the impacts of nonnative foundation species, which are likely to become increasingly common community members in our rapidly changing world.
Materials and Methods
Study System.
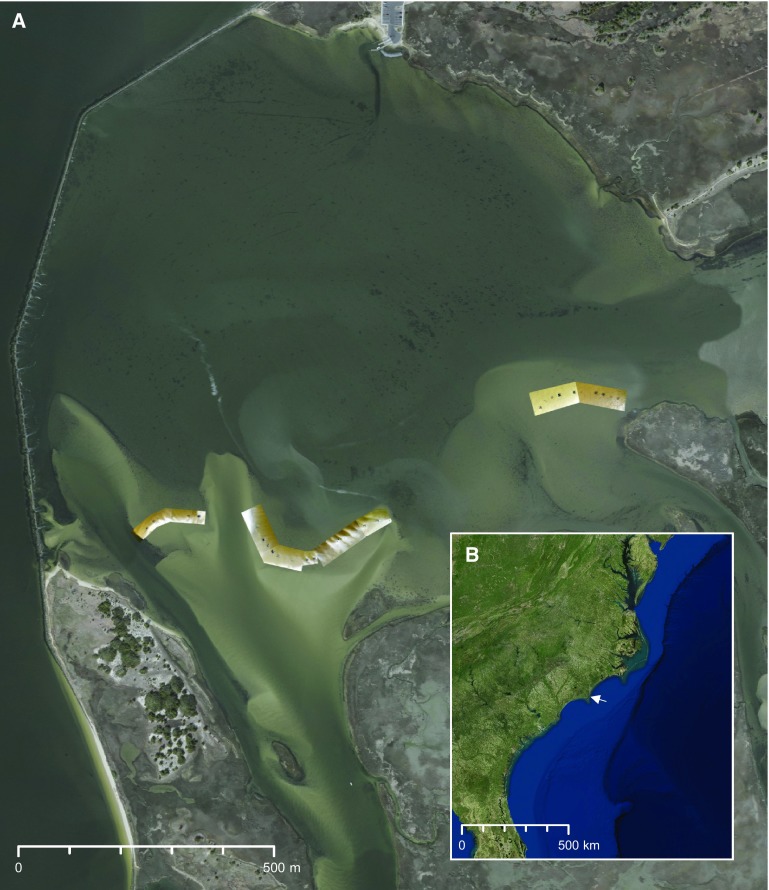
The experiment was carried out on intertidal mud and sandflats located within the Zeke’s Island National Estuarine Research Reserve (NERR), a shallow lagoon-like estuarine complex in the lower Cape Fear River, NC (Fig. S3). Tides are semidiurnal and range from 1.1 to 1.7 m above mean lower low water (MLLW). Salinity ranges from 20 to 35 ppt depending on rainfall and tidal currents. Like much of the Atlantic coast of the southeastern US, this estuary was once dominated by such species as salt marsh cordgrass (Spartina alterniflora) in the high-intertidal zone, oyster reefs (Crassostrea virginica) in the mid-intertidal zone, and mudflats in the low-intertidal zone, in which Gracilaria is becoming increasingly common (23). The infaunal polychaete Diopatra cuprea is relatively common in some locations and can facilitate Gracilaria by incorporating fronds into its tube caps (31, 65). At the study site, Gracilaria forms extensive meadows and is typically the only macrophyte on the intertidal flats (Fig. 1 A–C); the ephemeral green seaweed Ulva spp. is sometimes found in low abundance in spring.
Fig. S3.
(A) Aerial imagery of the study site in the basin of Zeke’s Island NERR. Georeferenced photos are overlaid to show the experimental plots on the three intertidal flats. The Federal Point revetment separates the shallow basin from the lower Cape Fear River estuary to the west. Note the distribution of Gracilaria (black patterns) throughout the basin. (B) Satellite imagery of the eastern seaboard of the US. The white arrow denotes the location of the study site slightly North of Cape Fear, NC. Georeferenced photos in A courtesy of Devon Eulie (photographer).
To investigate the potential extent of Gracilaria impacts, we quantified Gracilaria cover along 14 intertidal line transects in Zeke’s Island NERR (33.95 N, 77.94 W) and eight transects in Masonboro Island NERR (34.14 N, 77.85 W) during 2013. Cover was quantified during low tide in 10–15 0.25-m2 quadrats positioned haphazardly (>1 m apart) along each transect running from high to low intertidal (∼0 m MLLW) perpendicular to the water line. Replicate transects were separated by at least 5 m. Gracilaria cover was scored within each quadrat using a modified Braun–Blanquet method, in which the quadrat was divided into a 5 × 5 grid and each square was scored as 0 for <50% cover or 1 for >50% cover. In instances where the cumulative score was 0 despite the presence of a small frond of algae in the quadrat, it was scored as 0.5. The cumulative scores of each quadrat were divided by the total possible score (i.e., 25) and multiplied by 100 to obtain Gracilaria cover in percent. The mean cover across all transects and sites was 13.8% (SD = 23.3%, n = 304), suggesting high variability of Gracilaria, consistent with previous reports (35).
Experiment.
We manipulated Gracilaria cover across six density treatments (n = 8 per treatment) in a large-scale field experiment using 25-m2 plots (Fig. 1 D–F). We selected three low-intertidal flats spanning >1 km in the reserve that differed in terms of area, flow regimes, Gracilaria cover, grain size, and proximity to the Spartina salt marsh. The three flats represented the continuum of estuarine habitats where Gracilaria naturally occurs in this area. We established the 48 plots along the mean low water line at 5-m intervals by adding 3-m steel rebar 1.2 m into the substrate at each plot corner. Treatments were randomly assigned to the plots to avoid potentially confounding small-scale effects of site (and all plots had only a few Diopatra tubes). Gracilaria was fixed in a plot with metal “U-pegs” (constructed from clothes hangers; ref. 66) by physically staking handful-sized “clumps” of loose thalli to the sediment surface. Pegs were flushed with the sediment surface to avoid above-surface experimental artifacts. Thus, our six treatments were based on the total number of pegs per 25 m2 (arranged in squared grids) as follows: 0 (0 × 0), 9 (3 × 3), 36 (6 × 6), 100 (10 × 10), 225 (15 × 15), and 400 (20 × 20). We acknowledge that Gracilaria density thereby covaried with peg density; however, all pegs were flushed with or slightly below the sediment surface and thus did not affect any ecosystem functions above the sediment surface. More importantly, all peg densities were relatively low, corresponding to a maximum of 16 pegs per m2 (a maximum of 4 pegs per epifauna quadrat and no pegs in any infaunal cores). Specific tests of peg artifacts comparing 1-m2 plots with 0 vs. 16 pegs (without any Gracilaria) demonstrated that even at the highest densities used in our experiment, pegs had no effect on the abundance and taxonomic richness of epifauna or infauna, decomposition, dissolution, bird or ray feeding activities, or average multifunctionality (one-way ANOVA, P ≥ 0.38 for all responses; Table S3).
Table S3.
Results of the artifact control experiment testing the effects of peg density on each response variable in Masonboro Island NERR
| Response variable | No. of pegs per m−2 | df | F | P value | Adjusted R2 | |
| 0 | 16 | |||||
| Epifauna abundance, no. per m−2 | 46.50 ± 34.60 | 41.00 ± 24.63 | 14 | 0.13 | 0.72 | −0.06 |
| Epifauna richness, taxa per m−2 | 4.62 ± 1.09 | 4.00 ± 1.58 | 14 | 0.82 | 0.38 | −0.01 |
| Dissolution, mass lost in g d−1 | 8.27 ± 1.70 | 7.66 ± 1.72 | 14 | 0.51 | 0.49 | −0.03 |
| Decomposition, mass lost in g mo−1 | 0.98 ± 0.11 | 0.97 ± 0.16 | 13 | 0.01 | 0.92 | −0.08 |
| Infauna abundance, no. per L−1 | 66.21 ± 24.68 | 65.36 ± 28.33 | 14 | 0.00 | 0.95 | −0.07 |
| Infauna richness, taxa per L−1 | 4.51 ± 1.07 | 4.05 ± 1.10 | 14 | 0.69 | 0.42 | −0.02 |
| Number of ray holes, m−2 d−1 | 0.03 ± 0.02 | 0.03 ± 0.03 | 14 | 0.08 | 0.78 | −0.07 |
| Multifunctionality, % | 0.55 ± 0.08 | 0.55 ± 0.15 | 13 | 0.01 | 0.91 | −0.08 |
Pairs (n = 8) of 1-m2 plots were established 1 m apart on an intertidal flat with low Gracilaria density. The distance between pairs was at least 5 m. Treatments of either 0 or 16 pegs m−2 (corresponding to the highest peg density used in our experiment) were assigned at random within each pair of plots by flipping a coin. The experiment was maintained for 30 d, during which the responses were measured in each plot as described in the text. Data are presented as mean ± SD for each treatment, corresponding to the lowest (0 m−2) vs. highest (16 m−2) peg density used in our experiment. Results of one-way ANOVA are given on the right. Note that all P values were >0.35 and absolute values of R2 were <0.1, documenting that the pegs used in our experiment to control the abundance of Gracilaria did not by themselves affect any response variables.
Gracilaria was collected from nearby locations and added to plots in the U-peg grids in August 2013 (Fig. 1 D and E). Treatments were maintained and response variables were quantified approximately monthly from September 2013 to June 2014 (treatments were maintained and measured at total of 10 times). At each plot visit, we quantified the cover of Gracilaria (in 10 randomly placed 0.25-m2 quadrats per plot) and seven ecosystem functions (see the next section for details) before maintaining Gracilaria densities by replenishing U-pegs devoid of Gracilaria and manually removing Gracilaria from control plots.
To examine the effect of Gracilaria on epifauna, we positioned a 0.25-m2 quadrat in the center of each plot and collected all Gracilaria and associated epifauna into a zip-top bag. Thus, here epifauna refers to organisms associated with both Gracilaria and the sediment surface (23). In the laboratory, Gracilaria was rinsed in freshwater and shaken for ∼1 min to remove epifauna, which were captured in a 500-μm sieve. The wet biomass of Gracilaria was quantified after centrifugation to remove excess water. Epifauna were identified and enumerated to broad taxonomic groupings (typically family level) under a stereomicroscope (∼18×; Nikon SMZ800). For simplicity, all faunal data were standardized to unit area. Taxonomic richness was rescaled to unit area using the species–area relationship and assuming a conservative z value of 0.15 (67).
To quantify whether Gracilaria attenuates hydrodynamic forces, we used gypsum dissolution blocks (68). Gypsum dissolves at a rate proportional to water velocity and thus represents an integrated proxy for tidal currents and wave exposure (69). We created gypsum blocks as hemispheres (⌀ = 6.5 cm) from dental plaster (Die Keen; Heraeus Kalzer), covered on the bottom with two layers of polyurethane to ensure that an equal surface area would be subject to dissolution (70). Gypsum blocks were dried at 60 °C for a minimum of 24 h, after which the initial mass was recorded and one block was deployed flush with the substrate surface in the center of each plot for 4 d. Following retrieval, the gypsum blocks were dried and reweighed, and the dissolution rate was calculated as grams of gypsum dissolved per day. Because lower dissolution rates indicate greater flow reduction, dissolution rate was calculated using the equation –fi + max(fi), so that greater flow reduction corresponds with a positive contribution to ecosystem functioning (32).
To examine the effect of Gracilaria on sediment stabilization, we marked all corner poles at 20 cm above the substrate surface in August 2013, then measured the distance between the marking and the substrate surface with a ruler to the nearest 0.5 cm at the end of each month. We calculated the monthly (30 d) change in height in cm by subtracting the final distance from the initial distance to the substrate (using the average of the four corners per plot) and correcting for the time interval between measurements. Accretion and erosion are represented as positive and negative values, respectively.
To assess the effectiveness of Gracilaria as a nursery habitat for commercially and recreationally important species, we sampled the entire plot using a 1.2-m-high × 6.7-m-wide nylon seine net (The Fish Net Company; mesh size 3.175 mm) during a falling tide (Fig. S1). On completion of a pass, we swiftly pulled the net taught, tilted it into a horizontal position, and lifted it from the water into an adjacent boat (R\V Adelaide) in a single motion. Organisms (>1 cm) retained on the boat were identified to the family level and enumerated before being returned to the water. Abundances were reported per unit area (dividing by 25 m2), and richness data were rescaled to unit area using the species–area relationship and assuming a conservative z value of 0.15 (67).
We quantified the effect of Gracilaria on decomposition processes as described previously (71). Standing dead Spartina stems were collected from adjacent salt marshes, washed, and dried at 60 °C for a minimum of 72 h (until no further weight loss occurred). We pooled multiple stems to achieve an initial mass of 7.0 ± 0.5 g and placed them inside a mesh litter bag, which was closed and deployed on the sediment surface in the center of each plot. Bags were retrieved just before the next treatment maintenance. Remaining stem material was washed, dried, and weighed, and the decomposition rate was recorded as the mass lost in grams per month.
Data Analysis.
We calculated the average response of each function in each plot using the full 10-mo dataset (48 plots sampled each month; Dataset S1), such that each plot is represented by a single value in each plot of Fig. 2. We analyzed the abundance and taxonomic richness (of epifauna and nursery species) as separate functions because abundance more directly affects services like carbon sequestration and the maintenance of fisheries, whereas richness reflects taxonomic complementarity (i.e., different taxa support different functions; e.g., ref. 71) and redundancy (i.e., different taxa support similar functions), which can affect the resilience of ecosystem processes. To assess whether Gracilaria cover enhances the simultaneous performance of all seven measured functions, we used the multifunc package (version 0.7.0; https://github.com/jebyrnes/multifunc) and the averaging and single threshold approaches outlined previously (32). The averaging approach determines the average level of a suite of functions by standardizing each function to a common scale and taking their mean. After reflecting dissolution (presented in its raw form in Fig. 2C) to represent a positive contribution to functioning, we calculated an average multifunctionality index (in %) for each plot (Fig. 2H). Here we assume that high values of each of our functions correspond to high ecosystem functioning (i.e., higher values of sediment stabilization denote a higher level of performance for this function). The average multifunctionality index can be interpreted as the average level of all seven functions; however, this index cannot be used to interpret whether all functions are being performed simultaneously at a high level, given that functions performed at low levels can be averaged out by those performed at high levels. Thus, we also tallied the number of functions, at their standardized function value, in each plot that surpassed each of nine threshold levels (10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, and 90% of maximum functioning). Threshold index scores, which range from none to all seven functions, can be interpreted simply as the number of functions performed above a given threshold level in a plot (Fig. 2I).
Because recent experiments and ecological theory suggest that relationships between the structure and function of coastal habitats are characterized by thresholds and nonlinear limiting functions (39–41), we analyzed the relationship between Gracilaria cover and each response variable individually by comparing five models (41, 72). Using nonlinear least squares (73), we fit null, linear, log, hyperbolic, and power relationships for each response using the average Gracilaria cover (in %) of each plot as the explanatory variable. Model selection was based on AICc (74, 75). For each response variable, we compared the null model (i.e., just the intercept, a straight horizontal line representing the mean response) with the model of best fit using one-way ANOVA. We report the significance of the Gracilaria cover treatment as the probability (P) of obtaining the model, given that the null hypothesis is true. Model fits, AICc values, AICc weights, and parameter estimates for each ecosystem function and multifunction response variable are reported in Dataset S2. All analyses were conducted in R version 3.3.1 (76).
SI Text
At the end of the experiment, we measured four additional functions (months 8–10). Because we did not have seasonal data for these responses, we excluded them from the main analysis of multifunction effects; however, here we analyze them using the same statistical analysis described in the main text.
To sample benthic infauna, triplicate core samples (5-cm diameter, 15-cm depth, 294.5-cm3 volume) were obtained equidistant along a diagonal transect of each plot on June 25, 2014. The three sediment core samples from each plot were pooled into a zip-top bag. On return to the laboratory, the contents of each bag were drained and rinsed over a 1-mm mesh sieve to remove fine sediments. Infauna retained on the sieve were preserved in 75% ethanol. The 1-mm mesh size was chosen to concentrate sampling efforts on juvenile and early life stages of crustaceans, mollusks, and larger polychaete taxa. Infauna were identified and enumerated under a stereomicroscope (∼18×; Nikon SMZ800) to families and, in some cases, phyla. Infaunal data were standardized and rescaled to unit volume (using the reciprocal of 0.8836 L and a conservative z value of 0.15) following the same methods described previously for epifauna and nursery functions.
To evaluate the effect of Gracilaria on ray foraging activity, we counted the number of ray holes in each plot on three or four different days in a given month. Here we report the average number of ray holes standardized to unit area (by dividing by 25 m2) during a given low tide on a single day (Fig. S2C).
To investigate the association of waterfowl with Gracilaria, we delimited the 48 plots into four sites based on spatial proximity (plots 1–12, 13–24, 25–36, and 37–48) and surveyed all waterfowl activity occurring within a site (containing 12 plots) over a 15-min period during low tide. Bird counts were made through binoculars from our research vessel from a distance of ∼100 m, to avoid disturbances arising from our presence. We tallied the number of birds initially present and that became present within the boundaries of each plot during the observation period. After completing the 15-min observation of a site, we moved to a new vantage point to observe the next 12 plots. By repeating this procedure at all sites, we sampled all 48 plots with equivalent effort in a ∼1-h period. Because measurements were made on one to three different days in a given month, we present the average number of birds tallied per unit area (by dividing by 25 m2) per unit time (by multiplying by 4; 15 min × 4 = 60 min = 1 h) of low tide (Fig. S2D).
We did not observe any significant relationships between Gracilaria cover and infauna abundance (Fig. S2A), the number of infauna taxa (Fig. S2B), or waterfowl activities (although there was a tendency for waterfowl to be more abundant in plots with intermediate to high Gracilaria cover; Fig. S2D). However, we found an inverse linear relationship between Gracilaria cover and ray foraging activity (Fig. S2C), suggesting that Gracilaria may decrease predation on common infauna species (50), including bivalves, such as economically important quahog clams. Therefore, because increased survival of these commercially important bivalves is considered desirable, the ray foraging data were considered (similar to dissolution in Fig. 2C) before calculating multifunctionality to represent a positive contribution to functioning. Analogous to our main analysis, our supporting analysis revealed a strong positive, logarithmic relationship between Gracilaria cover and ecosystem multifunctionality, measured as the mean standardized index of all 11 functions in percent (Fig. S2E). Similarly, the relationship between Gracilaria cover and the number of functions maintained above a given threshold was significantly positive and logarithmic across all thresholds from 10% to 90% (Fig. S2F).
In summary, these results corroborate our main analysis and suggest that Gracilaria did not have a substantial negative impact on any of the components of the intertidal community that we measured at the end of the experiment (77).
Supplementary Material
Acknowledgments
We thank A. Brown, K. Johnson, M. Michel, J. Mejaski, I. Depa, S. Wall, S. Delaney, M. Henry, J. Walsh, M. Aiello, T. Miller, R. Accomazzo, G. Rowan, N. Crealese, M. Liberti, M. Varner, L. Smith, D. Chase, D. Blackstock, J. Coates, W. Vallecillo, B. Williams, D. Hines, and D. Eulie for assistance with research; the North Carolina National Estuarine Research Reserve for site access; and M. Durako, W. Freshwater, L. Dodd, J. Fodrie, J. Hench, L. Cahoon, M. Posey, Q. He, E. Schrack, S. Zhang, J. Morton, J. Byrnes, J. Lefcheck, and two referees for helpful comments that improved this work. This work was supported in part by the National Science Foundation (CAREER Award 1056980, to B.R.S.), Duke University, the Stolarz Foundation, and the University of North Carolina Wilmington. M.S.T. was funded by the Marsden Fund of the Royal Society of New Zealand (13‐UOC‐106). A.P.R. was supported in part by National Science Foundation Grant 1459384.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The plot-level data used to generate all analyses and figures of this paper are tabulated in Dataset S1 and have been made available digitally via GitHub (https://github.com/apramus/invFSxfunc).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700353114/-/DCSupplemental.
References
- 1.Millennium Ecosystem Assessment . Ecosystems and Human Well-Being: Current State and Trends. Island Press; Washington, DC: 2005. [Google Scholar]
- 2.Worm B, et al. Impacts of biodiversity loss on ocean ecosystem services. Science. 2006;314:787–790. doi: 10.1126/science.1132294. [DOI] [PubMed] [Google Scholar]
- 3.Lotze HK, et al. Depletion, degradation, and recovery potential of estuaries and coastal seas. Science. 2006;312:1806–1809. doi: 10.1126/science.1128035. [DOI] [PubMed] [Google Scholar]
- 4.Jackson JBC, et al. Historical overfishing and the recent collapse of coastal ecosystems. Science. 2001;293:629–637. doi: 10.1126/science.1059199. [DOI] [PubMed] [Google Scholar]
- 5.Wilkinson C. Status of Coral Reefs of the World. Global Coral Reef Monitoring Network and Reef and Rainforest Research Center; Townsville, Australia: 2008. [Google Scholar]
- 6.Waycott M, et al. Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci USA. 2009;106:12377–12381. doi: 10.1073/pnas.0905620106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gedan KB, Silliman BR. Patterns of salt marsh loss within coastal regions of North America: Presettlement to present. In: Silliman BR, Grosholz T, Bertness MD, editors. Human Impacts on Salt Marshes: A Global Perspective. Univ of California Press; Berkeley: 2009. pp. 253–266. [Google Scholar]
- 8.Beck MW, et al. Oyster reefs at risk and recommendations for conservation, restoration, and management. Bioscience. 2011;61:107–116. [Google Scholar]
- 9.Barbier EB, et al. The value of estuarine and coastal ecosystem services. Ecol Monogr. 2011;81:169–193. [Google Scholar]
- 10.Pendleton L. Measuring and Monitoring the Economic Effects of Habitat Restoration: A Summary of a NOAA Blue Ribbon Panel. Nicholas Institute for Environmental Policy Solutions, Duke University; Durham, NC: 2010. [Google Scholar]
- 11.Schrack E, Beck M, Brumbaugh R, Crisley K. Restoration Works: Highlights from a Decade of Partnership Between The Nature Conservancy and the National Oceanic and Atmospheric Administration’s Restoration Center. Nature Conservancy; Arlington, VA: 2012. [Google Scholar]
- 12.Coen LD, et al. Ecosystem services related to oyster restoration. Mar Ecol Prog Ser. 2007;341:303–307. [Google Scholar]
- 13.Bayraktarov E, et al. The cost and feasibility of marine coastal restoration. Ecol Appl. 2016;26:1055–1074. doi: 10.1890/15-1077. [DOI] [PubMed] [Google Scholar]
- 14.Lefcheck JS, Wilcox DJ, Murphy RR, Marion SR, Orth RJ. Multiple stressors threaten the imperiled coastal foundation species eelgrass (Zostera marina) in Chesapeake Bay, USA. Glob Change Biol. 2017;23:1–17. doi: 10.1111/gcb.13623. [DOI] [PubMed] [Google Scholar]
- 15.Vilà M, et al. Ecological impacts of invasive alien plants: A meta-analysis of their effects on species, communities and ecosystems. Ecol Lett. 2011;14:702–708. doi: 10.1111/j.1461-0248.2011.01628.x. [DOI] [PubMed] [Google Scholar]
- 16.Pimentel D, Zuniga R, Morrison D. Update on the environmental and economic costs associated with alien-invasive species in the United States. Ecol Econ. 2005;52:273–288. [Google Scholar]
- 17.D’Antonio C, Meyerson LA. Exotic plant species as problems and solutions in ecological restoration: A synthesis. Restor Ecol. 2002;10:703–713. [Google Scholar]
- 18.Schlaepfer MA, Sax DF, Olden JD. The potential conservation value of non-native species. Conserv Biol. 2011;25:428–437. doi: 10.1111/j.1523-1739.2010.01646.x. [DOI] [PubMed] [Google Scholar]
- 19.Hulme PE, et al. Bias and error in understanding plant invasion impacts. Trends Ecol Evol. 2013;28:212–218. doi: 10.1016/j.tree.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Thomsen MS, et al. Impacts of marine invaders on biodiversity depend on trophic position and functional similarity. Mar Ecol Prog Ser. 2014;495:39–47. [Google Scholar]
- 21.Rodriguez LF. Can invasive species facilitate native species? Evidence of how, when, and why these impacts occur. Biol Invasions. 2006;8:927–939. [Google Scholar]
- 22.Wallentinus I, Nyberg CD. Introduced marine organisms as habitat modifiers. Mar Pollut Bull. 2007;55:323–332. doi: 10.1016/j.marpolbul.2006.11.010. [DOI] [PubMed] [Google Scholar]
- 23.Byers JE, Gribben PE, Yeager C, Sotka EE. Impacts of an abundant introduced ecosystem engineer within mudflats of the southeastern US coast. Biol Invasions. 2012;14:2587–2600. [Google Scholar]
- 24.Thomsen MS, et al. A meta-analysis of seaweed impacts on seagrasses: Generalities and knowledge gaps. PLoS One. 2012;7:e28595. doi: 10.1371/journal.pone.0028595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powell KI, Chase JM, Knight TM. A synthesis of plant invasion effects on biodiversity across spatial scales. Am J Bot. 2011;98:539–548. doi: 10.3732/ajb.1000402. [DOI] [PubMed] [Google Scholar]
- 26.Thomsen MS, Wernberg T, Olden JD, Griffin JN, Silliman BR. A framework to study the context-dependent impacts of marine invasions. J Exp Mar Biol Ecol. 2011;400:322–327. [Google Scholar]
- 27.Parker IM, et al. Impact: Toward a framework for understanding the ecological effects of invaders. Biol Invasions. 1999;1:3–19. [Google Scholar]
- 28.Steneck RS, et al. Kelp forest ecosystems: Biodiversity, stability, resilience and future. Environ Conserv. 2002;29:436–459. [Google Scholar]
- 29.Mumby PJ, Hastings A, Edwards HJ. Thresholds and the resilience of Caribbean coral reefs. Nature. 2007;450:98–101. doi: 10.1038/nature06252. [DOI] [PubMed] [Google Scholar]
- 30.Gardner TA, Côté IM, Gill JA, Grant A, Watkinson AR. Long-term region-wide declines in Caribbean corals. Science. 2003;301:958–960. doi: 10.1126/science.1086050. [DOI] [PubMed] [Google Scholar]
- 31.Thomsen MS, et al. Habitat cascades: The conceptual context and global relevance of facilitation cascades via habitat formation and modification. Integr Comp Biol. 2010;50:158–175. doi: 10.1093/icb/icq042. [DOI] [PubMed] [Google Scholar]
- 32.Byrnes JEK, et al. Investigating the relationship between biodiversity and ecosystem multifunctionality: Challenges and solutions. Methods Ecol Evol. 2014;5:111–124. [Google Scholar]
- 33.Schmidt AL, Scheibling RE. Effects of native and invasive macroalgal canopies on composition and abundance of mobile benthic macrofauna and turf-forming algae. J Exp Mar Biol Ecol. 2007;341:110–130. [Google Scholar]
- 34.Thiele J, Kollmann J, Markussen B, Otte A. Impact assessment revisited: Improving the theoretical basis for management of invasive alien species. Biol Invasions. 2010;12:2025–2035. [Google Scholar]
- 35.Freshwater DW, et al. Distribution and identification of an invasive Gracilaria species that is hampering commercial fishing operations in southeastern North Carolina, USA. Biol Invasions. 2006;8:631–637. [Google Scholar]
- 36.Lotze HK. Historical reconstruction of human-induced changes in US estuaries. Oceanogr Mar Biol Annu Rev. 2010;48:267–338. [Google Scholar]
- 37.Zu Ermgassen PSE, et al. Historical ecology with real numbers: Past and present extent and biomass of an imperiled estuarine habitat. Proc R Soc B. 2012;279:3393–3400. doi: 10.1098/rspb.2012.0313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Silliman BR, et al. Facilitation shifts paradigms and can amplify coastal restoration efforts. Proc Natl Acad Sci USA. 2015;112:14295–14300. doi: 10.1073/pnas.1515297112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Barbier EB, et al. Coastal ecosystem-based management with nonlinear ecological functions and values. Science. 2008;319:321–323. doi: 10.1126/science.1150349. [DOI] [PubMed] [Google Scholar]
- 40.Koch EW, et al. Non-linearity in ecosystem services: Temporal and spatial variability in coastal protection. Front Ecol Environ. 2009;7:29–37. [Google Scholar]
- 41.Angelini C, et al. Foundation species’ overlap enhances biodiversity and multifunctionality from the patch to landscape scale in southeastern United States salt marshes. Proc R Soc B. 2015;282:20150421. doi: 10.1098/rspb.2015.0421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nyberg CD, Thomsen MS, Wallentinus I. Flora and fauna associated with the introduced red alga Gracilaria vermiculophylla. Eur J Phycol. 2009;44:395–403. [Google Scholar]
- 43.Wilcove DS, Rothstein D, Dubow J, Phillips A, Losos E. Quantifying threats to imperiled species in the United States. Bioscience. 1998;48:607–615. [Google Scholar]
- 44.Johnston CA, Lipcius RN. Exotic macroalga Gracilaria vermiculophylla provides superior nursery habitat for native blue crab in Chesapeake Bay. Mar Ecol Prog Ser. 2012;467:137–146. [Google Scholar]
- 45.Wright JT, Byers JE, DeVore JL, Sotka EE. Engineering or food? Mechanisms of facilitation by a habitat forming invasive seaweed. Ecology. 2014;95:2699–2706. [Google Scholar]
- 46.Silliman BR, et al. Whole-community facilitation regulates biodiversity on Patagonian rocky shores. PLoS One. 2011;6:e24502. doi: 10.1371/journal.pone.0024502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Dayton PK. Toward an understanding of community resilience and the potential effects of enrichments to the benthos at McMurdo Sound. In: Parker BC, editor. Antarctica. Proceedings of the Colloquium on Conservation Problems in Antarctica. Allen Press; Lawrence, KS: 1972. pp. 81–95. [Google Scholar]
- 48.Ellison AM, et al. Loss of foundation species: Consequences for the structure and dynamics of forested ecosystems. Front Ecol Environ. 2005;3:479–486. [Google Scholar]
- 49.Lyons DA, et al. Macroalgal blooms alter community structure and primary productivity in marine ecosystems. Glob Change Biol. 2014;20:2712–2724. doi: 10.1111/gcb.12644. [DOI] [PubMed] [Google Scholar]
- 50.Cordero ALH, Seitz RD. Structured habitat provides a refuge from blue crab, Callinectes sapidus, predation for the bay scallop, Argopecten irradians concentricus (Say 1822) J Exp Mar Biol Ecol. 2014;460:100–108. [Google Scholar]
- 51.Myers JH, Simberloff D, Kuris AM, Carey JR. Eradication revisited: Dealing with exotic species. Trends Ecol Evol. 2000;15:316–320. doi: 10.1016/s0169-5347(00)01914-5. [DOI] [PubMed] [Google Scholar]
- 52.Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS. Five potential consequences of climate change for invasive species. Conserv Biol. 2008;22:534–543. doi: 10.1111/j.1523-1739.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 53.Lawler JJ, Olden JD. Reframing the debate over assisted colonization. Front Ecol Environ. 2011;9:569–574. [Google Scholar]
- 54.Pyke CR, et al. Current practices and future opportunities for policy on climate change and invasive species. Conserv Biol. 2008;22:585–592. doi: 10.1111/j.1523-1739.2008.00956.x. [DOI] [PubMed] [Google Scholar]
- 55.Didham RK, Tylianakis JM, Hutchison MA, Ewers RM, Gemmell NJ. Are invasive species the drivers of ecological change? Trends Ecol Evol. 2005;20:470–474. doi: 10.1016/j.tree.2005.07.006. [DOI] [PubMed] [Google Scholar]
- 56.Bauer JT. Invasive species: “Back-seat drivers” of ecosystem change? Biol Invasions. 2012;14:1295–1304. [Google Scholar]
- 57.Jaubert JM, et al. Re-evaluation of the extent of Caulerpa taxifolia development in the northern Mediterranean using airborne spectrographic sensing. Mar Ecol Prog Ser. 2003;263:75–82. [Google Scholar]
- 58.Marbà N, Duarte CM. Mediterranean warming triggers seagrass (Posidonia oceanica) shoot mortality. Glob Change Biol. 2010;16:2366–2375. [Google Scholar]
- 59.Nehls G, Diederich S, Thieltges DW, Strasser M. Wadden Sea mussel beds invaded by oysters and slipper limpets: Competition or climate control? Helgol Mar Res. 2006;60:135–143. [Google Scholar]
- 60.Olden JD. Biotic homogenization: A new research agenda for conservation biogeography. J Biogeogr. 2006;33:2027–2039. [Google Scholar]
- 61.Strayer DL, Eviner VT, Jeschke JM, Pace ML. Understanding the long-term effects of species invasions. Trends Ecol Evol. 2006;21:645–651. doi: 10.1016/j.tree.2006.07.007. [DOI] [PubMed] [Google Scholar]
- 62.Thomsen MS, Wernberg T. The devil in the detail: Harmful seaweeds are not harmful to everyone. Glob Change Biol. 2015;21:1381–1382. doi: 10.1111/gcb.12772. [DOI] [PubMed] [Google Scholar]
- 63.Gutièrrez JL, Jones CG, Strayer DL, Iribarne OO. Mollusks as ecosystem engineers: The role of shell production in aquatic habitats. Oikos. 2003;101:79–90. [Google Scholar]
- 64.Mazarrasa I, et al. Seagrass meadows as a globally significant carbonate reservoir. Biogeosciences. 2015;12:4993–5003. [Google Scholar]
- 65.Thomsen MS, McGlathery K. Facilitation of macroalgae by the sedimentary tube forming polychaete Diopatra cuprea. Estuar Coast Shelf Sci. 2005;62:63–73. [Google Scholar]
- 66.Fonseca MS, Kenworthy WJ, Thayer GW. Guidelines for the Conservation and Restoration of Seagrasses in the United States and Adjacent Waters. NOAA Coastal Ocean Office; Silver Spring, MD: 1998. [Google Scholar]
- 67.Powell KI, Chase JM, Knight TM. Invasive plants have scale-dependent effects on diversity by altering species-area relationships. Science. 2013;339:316–318. doi: 10.1126/science.1226817. [DOI] [PubMed] [Google Scholar]
- 68.Brisson CP, Coverdale TC, Bertness MD. Salt marsh die-off and recovery reveal disparity between the recovery of ecosystem structure and service provision. Biol Conserv. 2014;179:1–5. [Google Scholar]
- 69.Bruno JF. Facilitation of cobble beach plant communities through habitat modification by Spartina alterniflora. Ecology. 2000;81:1179–1192. [Google Scholar]
- 70.Bertness MD, Trussell GC, Ewanchuk PJ, Silliman BR. Do alternate stable community states exist in the Gulf of Maine rocky intertidal zone? Ecology. 2002;83:3434–3448. [Google Scholar]
- 71.Hensel MJS, Silliman BR. Consumer diversity across kingdoms supports multiple functions in a coastal ecosystem. Proc Natl Acad Sci USA. 2013;110:20621–20626. doi: 10.1073/pnas.1312317110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Scherber C, et al. Bottom-up effects of plant diversity on multitrophic interactions in a biodiversity experiment. Nature. 2010;468:553–556. doi: 10.1038/nature09492. [DOI] [PubMed] [Google Scholar]
- 73.Grothendieck G. 2013 nls2: Non-linear regression with brute force. R package version 0.2. https://cran.r-project.org/web/packages/nls2/index.html.
- 74.Burnham KP, Anderson DR. Model Selection and Multi-Model Inference: A Practical Information-Theoretic Approach. Springer; New York: 2002. [Google Scholar]
- 75.Mazerolle MJ. 2016 AICcmodavg: Model selection and multimodel inference based on (Q)AIC(c). R package version 2.0-4. https://cran.r-project.org/web/packages/AICcmodavg/index.html.
- 76.R Core Team . R: A Language and Environment for Statistical Computing. The R Foundation for Statistical Computing; Vienna, Austria: 2016. [Google Scholar]
- 77.Peterson CH, Peterson NM. The Ecology of Intertidal Flats of North Carolina: A Community Profile. US Fish and Wildlife Service, Office of Biological Services; Slidell, LA: 1979. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.