Significance
The immune system is important for host defense against invading pathogens by producing proinflammatory cytokines and IFNs. IL-12 is a vital proinflammatory cytokine that combines innate immunity with adaptive immunity. In our study, we find that CA-VI B preferentially expressed in macrophages can interact with PRMT5, consequently suppressing H3R8me2s modification in Il12 promoters to promote IL-12 production to trigger an antibacterial immune response. Our study adds insight about the function of CA family members in innate immune response by selectively inducing cytokine IL-12 production through regulating histone arginine modification, which is independent of its carbonic anhydrase activity.
Keywords: Car6-b, IL-12, PRMT5, innate immunity, epigenetic modification
Abstract
Interleukin-12 (IL-12) is critical for induction of protective immunity against intracellular bacterial infection. However, the mechanisms for efficient induction of IL-12 in innate response remain poorly understood. Here we report that the B type of carbonic anhydrase 6 (Car6-b, which encoded CA-VI B) is essential for host defense against Listeria monocytogenes (LM) infection by epigenetically promoting IL-12 expression independent of its carbonic anhydrase activity. Deficiency of Car6-b attenuated IL-12 production upon LM infection both in vitro and in vivo. Car6−/− mice were more susceptible to LM infection with less production of IL-12. Mechanistically, the nuclear localized CA-VI B selectively promotes IL-12 expression by interaction with protein arginine N-methyltransferase 5 (PRMT5), which reduces symmetric dimethylation of histone H3 arginine 8 modification (H3R8me2s) at Il12 promoters to facilitate chromatin accessibility, selectively enhancing c-Rel binding to the Il12b promoter. Our findings add insights to the epigenetic regulation of IL-12 induction in innate immunity.
Innate immune response is critical for host defense against invading pathogens. Microbial and dangerous signals induce pattern-recognition receptor (PRR)-dependent activation of inflammatory signaling in innate immune cells such as macrophages and dendritic cells, leading to production of proinflammatory cytokines that display diversified immunological and inflammatory effects (1). Of these, IL-12 plays essential roles in inducing transcription factor (TF) STAT4-dependent production of IFN-γ in natural killer (NK) cells and T cells, contributing to development of Th1 cell-mediated protective immunity against intracellular pathogens such as Mycobacterium tuberculosis (TB) and Listeria monocytogenes (LM) (2). IL-12 expression is controlled by transcription activation of two separate genes, Il12a and Il12b, to form IL-12p70 with bioactivity. IL-12 deficiency results in defects in immune defense, causing increased susceptibility to infection by TB, Salmonella, or Candida both in humans and in mice (3).
IL-12 is also related to development of autoimmune and inflammatory disease, as demonstrated by resistance to experimental autoimmune disorders in mice lacking Il12b (4). Antagonists against IL-12p40 have been verified to be an effective treatment for a number of autoimmune diseases in clinical or preclinical trials (5). So the expression of IL-12 must be well balanced to achieve timely and effective protection against pathogenic infection. Further identification of new regulators of IL-12 production will contribute to a better understanding of the molecular basis for innate immunity against intracellular pathogens and also outline potential therapeutic strategies for control of infectious diseases.
Epigenetic mechanisms for gene expression during innate immunity and inflammation are attracting more and more attention now. Epigenetic regulations include DNA modifications, posttranslational modifications (PTMs) of histones, ATP-dependent chromatin remodeling, and noncoding RNA (ncRNA), which act in a coordinated manner to affect chromatin status and gene transcription and influence various immunological processes (6). Expression of IL-12 is mainly dependent on TLR-triggered nuclear factor-κB (NF-κB) and mitogen-activated protein kinase (MAPK) signaling pathways (2). C-Rel, which belongs to the NF-κB family, is a key TF in mediating TLR-stimulated expression of IL-12 (7). Histone methylation at the Il12b promoter is found to be important for IL-12 expression (8). LPS stimulation also induces rapid remodeling of a positioned nucleosome at the Il12b promoter, which might be important for accessibility of specific TFs to the promoter region (7). However, the detailed function of chromatin modifiers and transcription regulators in IL-12 expression and IL-12–mediated innate immunity in response to bacterial infections remains poorly understood.
Intracellular bacterial infection is one of the major health concerns today. For example, TB results in severe lung injury (9). Also, the widely existed Gram-positive intracellular pathogen LM causes severe listeriosis and even death. LM has been used as a model organism for the study of intracellular pathogens (10). LM mainly infects and induces IL-12 production in macrophages in vivo (11). E-selectin is a key adhesion molecule encoded by Sele-mediating recruitment of Th1 cells into inflamed tissues (12). To study LM-triggered innate response, we compared mRNA expression in macrophages from Sele+/+ and Sele−/− mice infected with LM. Interestingly, Car6, which encoded CA-VI, was significantly decreased in Sele−/− macrophages. Car6 has two transcripts: One is the secreted A type of CA-VI A (encoded by Car6-a), and the other is intracellular CA-VI B. CA-VI belongs to the carbonic anhydrase (CA) family (13). CA includes 16 members catalyzing the reversible hydration of CO2. CAs can be grouped into four kinds according to their different subcellular locations: CA-I, -II, -III, -VII, and -XIII are localized in cytoplasm; CA-IV, -IX, -XII, and -XIV are membrane-bound; CA-V is localized in mitochondria; and CA-VI is the only secreted CA (14). Car6 might be involved in cellular oxidative stress in the submandibular gland and tissue homeostasis in the respiratory system and brain (15). However, the roles of CAs in innate immunity remain unknown. Here, we demonstrated that Car6-b selectively promotes IL-12 expression by interaction with PRMT5 and consequently facilitates innate response against intracellular bacterial infection.
Results
Car6-b Is Preferentially Expressed in Macrophages and Inducible upon Innate Stimuli.
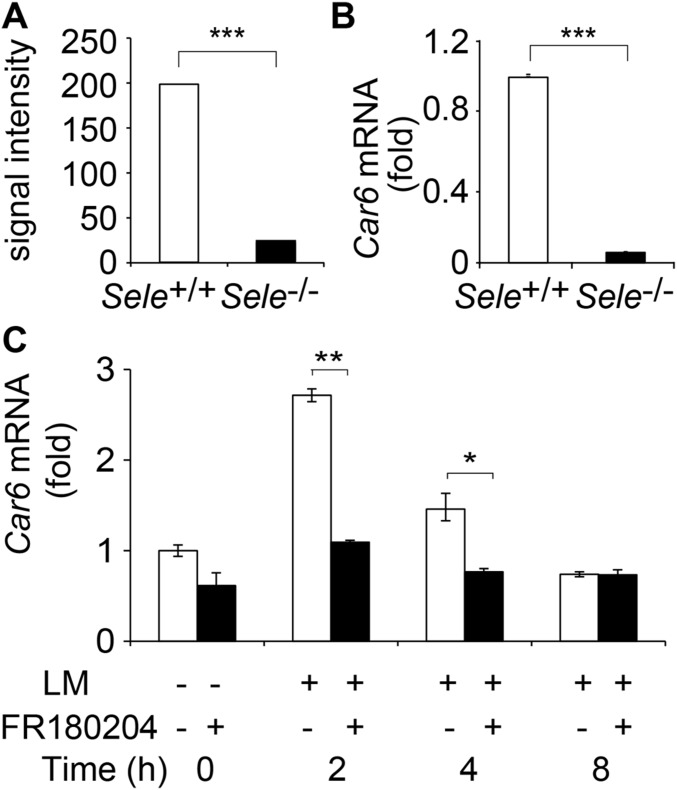
By cDNA microarray analysis of LM-infected peritoneal macrophages (PMs) from Sele−/− and Sele+/+ mice, we found mRNA expression of Car6 was significantly decreased in Sele−/− PMs (Fig. S1 A and B). We further analyzed the molecular mechanism of the impaired induction of Car6 mRNA in the absence of E-selectin. Given that E-selectin binds its ligands to activate the MAPK signaling pathway, to investigate the molecular mechanism behind how E-selectin engagement could up-regulate Car6 expression, we used ERK-specific inhibitor FR180204 to pretreat PMs before infection of LM. We found that Car6 expression was reduced in the presence of the ERK inhibitor (Fig. S1C), indicating that E-selectin engagement up-regulates Car6 expression via the ERK pathway.
Fig. S1.
Car6 expression in Sele−/− mice. (A) The signal intensity of Car6 in cDNA microarray of PMs from Sele−/− and Sele+/+ mice infected with LM in vivo for 72 h. (B) Examination of Car6 expression stimulated as in A by qPCR assay. (C) Car6 expression in PMs from Sele+/+ mice after being pretreated by ERK-specific inhibitor FR180204 for 2 h and then stimulated by LM for the indicated time. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
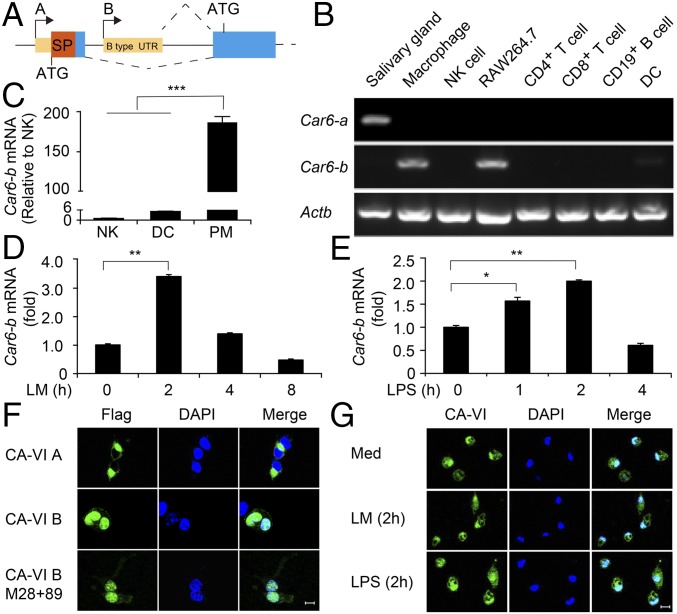
As shown in Fig. 1A, the two types of Car6 have different transcription start sites (TSSs) and use disparate start codon ATG. Therefore, CA-VI A has an additional 57-aa peptide on the N terminus than CA-VI B does (13). We next measured the mRNA expression profile of Car6-a and Car6-b in various immune cells, including NK cells, DCs, CD4+ T cells, CD8+ T cells, CD19+ B cells, PMs, and cell line RAW264.7. Although Car6-a was undetectable in all of the immune cells, except for the salivary gland, which served as a positive control, Car6-b was predominantly expressed in macrophages including PMs and RAW264.7 cells (Fig. 1B). We further confirmed that Car6-b expression in macrophages was higher than that in NKs and DCs by quantitative real-time PCR (qPCR) (Fig. 1C). Furthermore, Car6-b was significantly induced by LM and LPS stimulation (Fig. 1 D and E). Confocal analysis indicated that CA-VI B was located in the nucleus of 293T cells (Fig. 1F) and PMs (Fig. 1G) with or without innate stimuli, which was consistent with the observation that CA-VI B is located in the nucleus of mouse embryo fibroblast cells in response to stress (13). In addition, the enzyme catalytic mutant of Car6-b (M28+89) was also localized in the nucleus (Fig. 1F). Taken together, Car6-b is preferentially expressed in macrophages and inducible upon innate stimuli.
Fig. 1.
Car6-b is preferentially expressed in macrophages. (A) The structure of the A and B type of Car6 in genome. SP, signal peptide; UTR, untranslated region. (B) RT-PCR analysis of Car6-a and Car6-b in immune cells; Actb serves as a loading control. Car6-a expressed in the salivary gland serves as a positive control. (C) qPCR analysis of Car6-b in mouse immune cells; results were normalized to those of Actb. (D and E) Car6-b expression in PMs after stimulation by LM (D) or LPS (E) for indicated times. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01 ***P < 0.001. (F and G) Immunofluorescence assay of the location of CA-VI in 293T cells (F) and PMs (G) stimulated with LM or LPS for 2 h or left unstimulated (Med). (Scale bar for F and G, 10 µm.)
Car6-b Selectively Promotes IL-12 Expression in Macrophages upon Innate Stimuli.
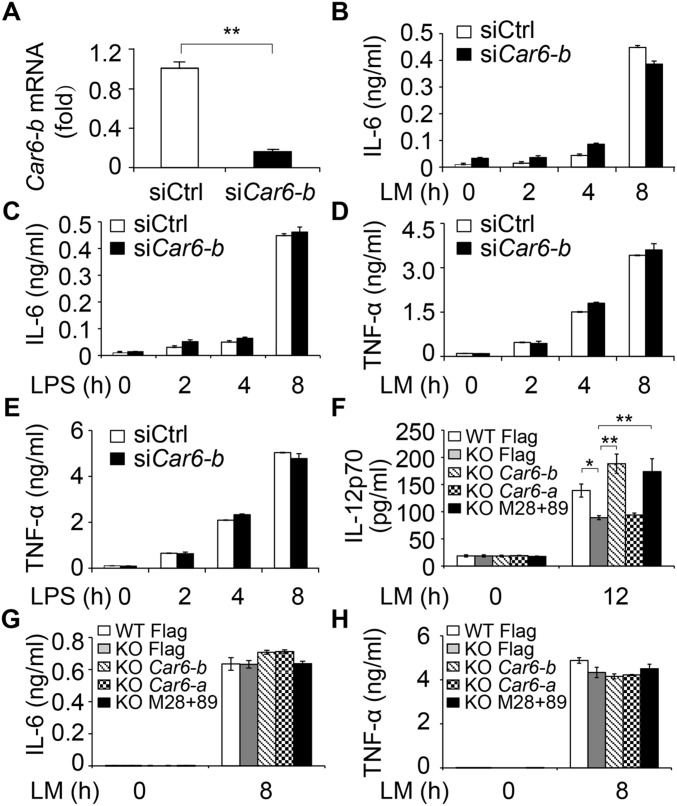
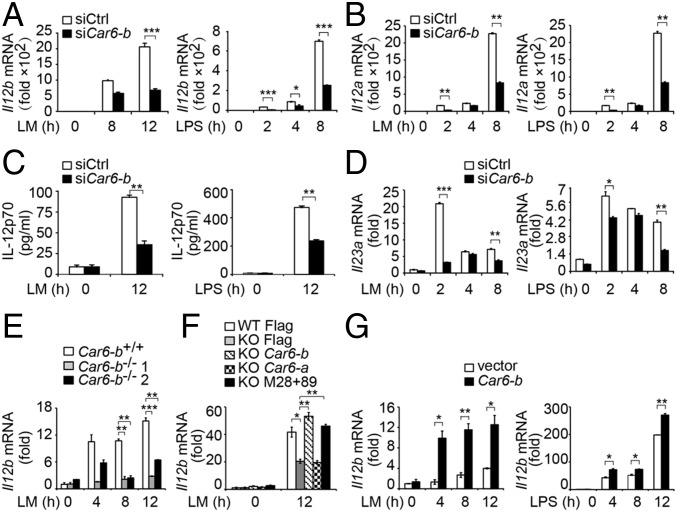
We next investigated the function of Car6-b in innate immune response. Given that only Car6-b but not Car6-a is expressed in macrophages, we silenced Car6-b in PMs (Fig. S2A) and found that LM- or LPS-induced Il12b, Il12a mRNA expression, and IL-12p70 production were dramatically reduced in Car6-b–silenced PMs (Fig. 2 A–C). IL-23 belongs to the IL-12 family and is composed of the IL-12p40 and IL-23p19 subunits. LM- and LPS-induced expression of Il23a (encoding IL-23p19) was also reduced by Car6-b knockdown (Fig. 2D). However, silencing of Car6-b did not affect the production of IL-6 and TNF-α by PMs stimulated with LM or LPS (Fig. S2 B–E).
Fig. S2.
Car6-b does not affect IL-6 and TNF-α production by macrophages upon innate stimuli. (A) Examination of silencing efficiency of Car6-b siRNA in PMs by qPCR assay. (B–E) ELISA of IL-6 (B and C) and TNF-α (D and E) in the supernatants of Car6-b–silenced cells stimulated with LM or LPS for the indicated time. (F–H) ELISA of IL-12p70 (F), IL-6 (G), and TNF-α (H) in the supernatants of Car6 knockout cells (KO) and control cells (WT) after transfection with the indicated plasmids and stimulation by LM. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01.
Fig. 2.
Car6-b promotes IL-12 expression in macrophages upon innate stimuli. (A and B) qPCR assay of Il12b (A) and Il12a (B) in PMs transfected with Car6-b–specific siRNA (siCar6-b) and scramble siRNA (siCtrl) and stimulated with LM or LPS for the indicated time. (C) ELISA in the supernatants of cells transfected and stimulated as in A. (D) qPCR assay of Il23a stimulated as in A. (E) Il12b expression in the RAW264.7 Car6-b−/− cells from two colonies (Car6-b−/− 1 and Car6-b−/− 2) and control cells (Car6-b+/+) stimulated by LM. (F) Il12b expression in Car6 knockout RAW264.7 cells (KO) and control cells (WT) after transfection with Flag-vector (Flag), Flag–Car6-b (Car6-b), Flag–Car6-a (Car6-a), or Flag–Car6-b–M28+89 (M28+89) stimulated by LM. (G) Il12b expression in Car6-b or control vector overexpressing RAW264.7 cells after stimulation by LM or LPS for indicated times. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
We next generated Car6-b−/− RAW264.7 cells by CRISPR/Cas9 technology (Fig. S3A). As expected, LPS or LM-induced Il12b expression was significantly decreased in Car6-b−/− RAW264.7 cells compared with wild-type RAW264.7 cells (Fig. 2E), whereas overexpression of Car6-b into Car6-b−/− RAW264.7 cells significantly rescued Il12b mRNA expression (Fig. 2F) and IL-12p70 production (Fig. S2F). However, IL-6 and TNF-α expressions were not affected by overexpression of Car6-b (Fig. S2 G and H). We also found that transfection of Car6-a did not rescue the expression of Il12b mRNA (Fig. 2F). In addition, overexpression of Car6-b remarkably promoted Il12b mRNA expression in RAW264.7 cells upon innate stimuli (Fig. 2G).
Fig. S3.
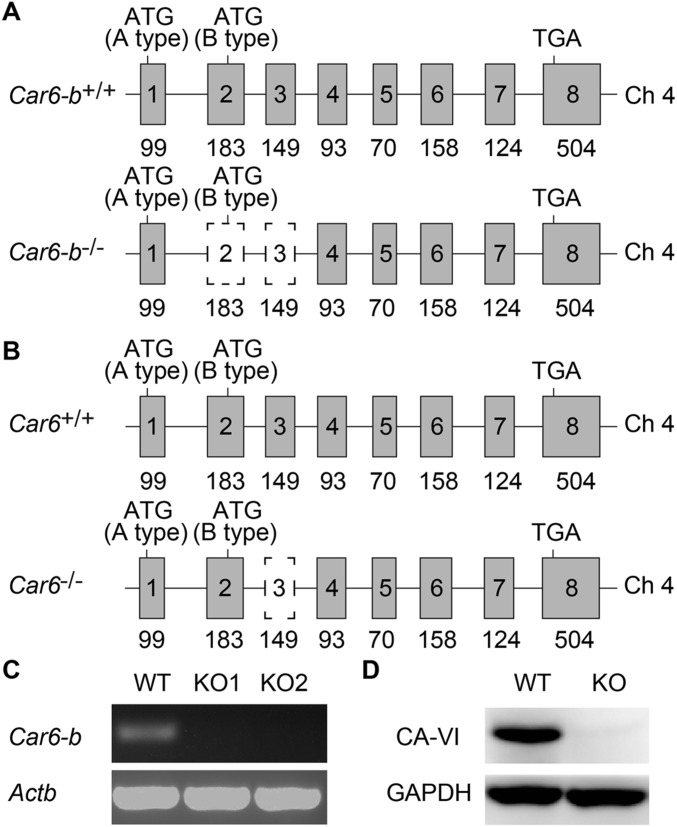
Generation and identification of Car6-b−/− cells and Car6−/− mice by CRISPR/Cas9 system. (A and B) Schematic illustration of the deletion sequences of Car6-b in Car6-b−/− RAW264.7 cells (A) or Car6 in Car6−/− mice (B). Car6 is located in chromosome 4 and contains eight exons. The targeting vector for the RAW264.7 cell line was designed to delete exons 2 and 3, and the targeting vector for mouse was designed to delete exon 3, resulting in a frame-shift of ORF and premature termination. The start codon of Car6-a and Car6-b and stop codon are indicated, respectively. (C) The efficiency of deletion was determined using PCR. (D) CA-VI protein in Car6+/+ or Car6−/− mouse salivary was detected using immunoblot analysis. Data shown are representative of three independent experiments.
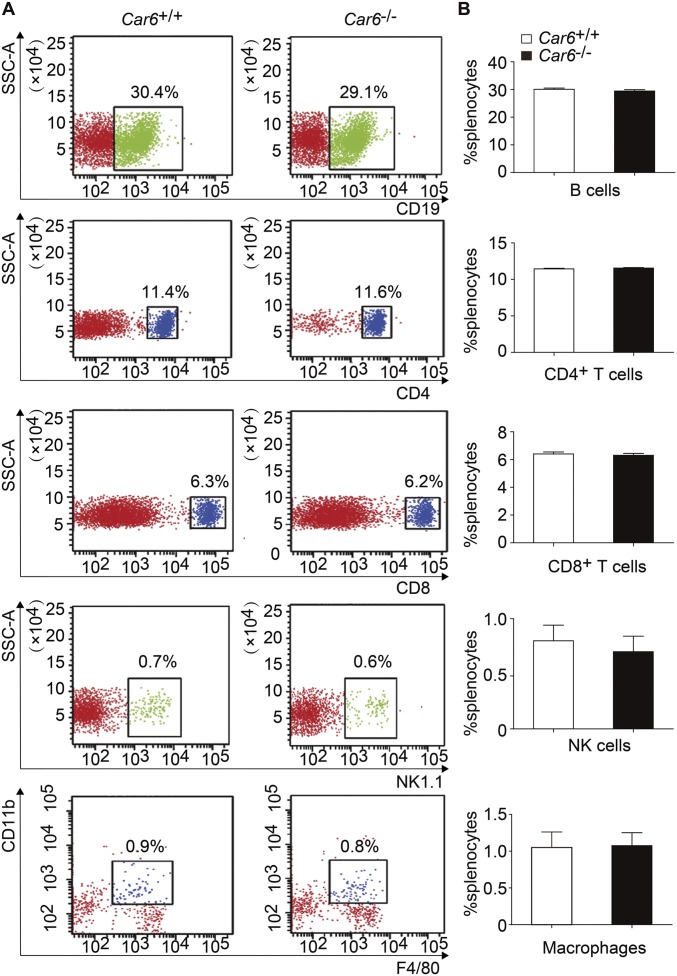
To further investigate the role of Car6-b, we tried to generate Car6-b−/− mice. Given the structure shown in Fig. 1A, we could not knock out Car6-b specifically without influencing Car6-a. On the other hand, Car6-a is not expressed in immune cells, and Car6-b is the only type expressed. Therefore, we generated Car6−/− mice in the C57BL/6J background using the CRISPR-Cas9 method (Fig. S3B) to represent knocking out Car6-b in immune cells. Car6−/− mice and Car6-sufficient littermate (Car6+/+) mice were fertile and normal in size. They were viable and normal, consistent with previous reports (16). FACS analysis showed no difference in immune cells including CD19+ B cells, CD4+ T cells, CD8+ T cells, NK cells, and macrophages between Car6+/+ and Car6−/− mice (Fig. S4); thus, Car6 deficiency does not affect the development of the immune system.
Fig. S4.
Normal development of immune cells in Car6−/− mice. (A) The percentages of CD19+ B cells, CD4+ T cells, CD8+ T cells, NK cells, and macrophages in the splenocytes from Car6+/+ and Car6−/− mice were analyzed by flow cytometry. Similar results were obtained in three independent experiments. The relevant statistical graphics are shown in B. Data are shown as mean ± SD (n = 3).
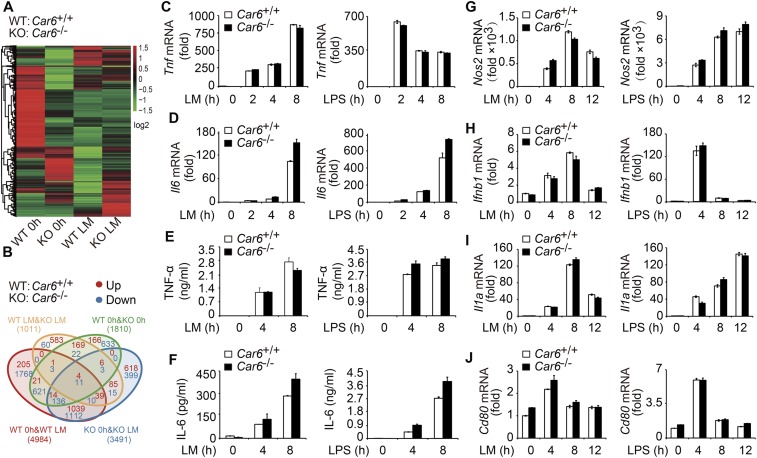
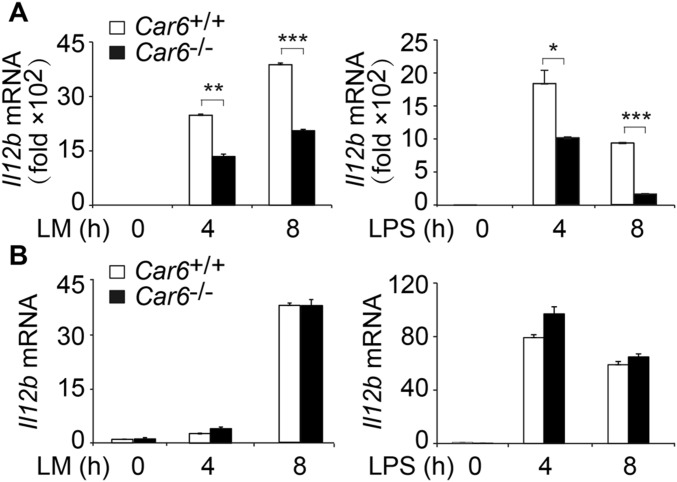
We collected PMs from Car6+/+ and Car6−/− mice to investigate the function of Car6-b in innate immune responses because there is no expression of Car6-a in macrophages. We performed RNA-sequencing (RNA-seq) of Car6+/+ and Car6−/− PMs with or without LM stimulation. The cluster heatmap of different samples was shown in Fig. S5A. There were 1,810 genes differentially expressed between Car6+/+ and Car6−/− PMs without LM stimulation. There were 1,011 genes differentially expressed between Car6+/+ and Car6−/− PMs stimulated with LM for 8 h (Fig. S5B). Car6−/− PMs had 887 genes up-regulated and 124 genes down-regulated compared with Car6+/+ PMs after stimulation with LM. We then analyzed the immune-related genes and found that II12b was down-regulated in Car6−/− PMs after stimulation by LM (Fig. 3A). However, there were no significantly different expressions of other cytokines, including Il6, Tnf, Il1, and Il10, between Car6+/+ and Car6−/− PMs, which was further confirmed by qPCR. Notably, LM- or LPS- induced Il12b, Il12a, and Il23a mRNA expression and IL-12p70 production were significantly decreased in Car6−/− PMs compared with Car6+/+ PMs (Fig. 3 B–E). In addition, Car6 deficiency did not affect innate signal-induced production of IL-6 and TNF-α (Fig. S5 C–F), and Car6-b deficiency did not affect the expression of Nos2, Ifnb1, Il1a, and Cd80 in Car6−/− PMs compared with that in Car6+/+ PMs after innate stimuli (Fig. S5 G–J). The innate signal-induced expression of Il12b mRNA was also significantly reduced in Car6−/− bone marrow-derived macrophages (BMMs) (Fig. S6A) but not in bone marrow-derived DCs (BMDCs) (Fig. S6B). Taken together, we demonstrate that Car6-b selectively promotes IL-12 expression in macrophages upon innate stimuli.
Fig. S5.
Car6-b deficiency does not affect the expression of TNF-α, IL-6, Nos2, Ifnb1, Il1a, and Cd80. (A and B) Hierarchical clustering of differentially expressed genes (A) and statistics for up-regulated (red) and down-regulated genes (blue) (B) in Car6+/+ (WT) and Car6−/− PMs (KO) after stimulation with LM for 8 h or without stimulation by RNA-seq. (C and D) qPCR assay of Tnf (C) and Il6 (D) expression in Car6+/+ and Car6−/− PMs after stimulation with LM or LPS for the indicated time. (E and F) ELISA of TNF-α (E) and IL-6 (F) in the supernatants of cells stimulated as in A. (G–J) qPCR assay of Nos2 (G), Ifnb1 (H), Il1a (I), and Cd80 (J) expression in Car6+/+ and Car6−/− PMs after stimulation with LM or LPS for the indicated time. Data were shown as mean ± SD (n = 3).
Fig. 3.
Car6 selectively promotes induction of IL-12 in macrophages upon innate stimuli. (A) Heatmap of gene expression in Car6+/+ and Car6−/− PMs with or without LM stimulation for 8 h. Log10-expression values were calculated from RNA-seq. (B–D) qPCR assay of Il12b (B), Il12a (C), and Il23a (D) expression in the Car6+/+ and Car6−/− PMs after stimulation by LM or LPS for the indicated time. (E) ELISA of IL-12p70 in the supernatants of cells stimulated as in B. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Fig. S6.
qPCR assay of Il12b expression in Car6−/− BMMs and BMDCs. (A and B) qPCR assay of Il12b expression in BMMs (A) or BMDCs (B) from Car6+/+ and Car6−/− mice after stimulation with LM or LPS for the indicated time. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Car6-b Protects Mice Against Infection with LM.
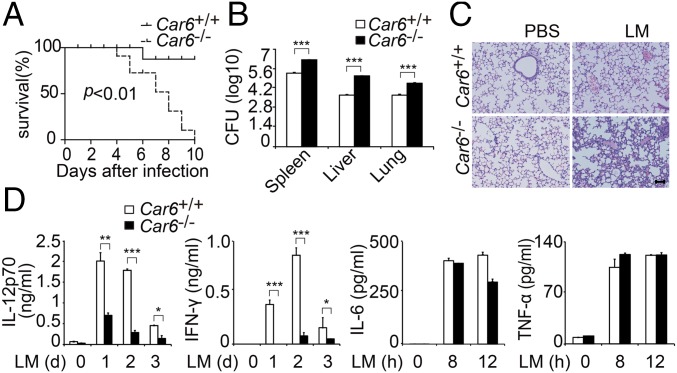
To further elucidate the significance of Car6 in innate responses in vivo, we challenged Car6+/+ and Car6−/− mice with the lethal load of LM by i.p. injection. The survival rate of Car6−/− mice was much lower than that of Car6+/+ mice after LM infection (Fig. 4A), consistent with the increased bacterial load in various organs and more obvious infiltration of mononuclear inflammatory cells in the lungs of Car6−/− mice (Fig. 4 B and C). Moreover, a much lower level of serum IL-12p70 and IFN-γ but similar levels of IL-6 and TNF-α production were detected in Car6−/− mice (Fig. 4D). Therefore, Car6-b significantly protected mice from LM infection by selectively promoting IL-12 production in vivo.
Fig. 4.
Car6 protects mice against infection with LM. (A) Survival rate of Car6+/+ and Car6−/− mice post-i.p. injection of LM (n = 10). (B) The cfus in the spleen/liver/lung were determined after 3 d of infection. (C) Pathology of Car6+/+ and Car6−/− mice after LM infection. Hematoxylin and eosin staining of lung sections from mice after 2 d of infection. (Scale bar, 100 µm.) (D) ELISA of indicated cytokine production in serum from Car6+/+ and Car6−/− mice stimulated as in A. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Car6-b Promotes IL-12 Production Independent of Its CA Activity and Not via NF-κB/MAPK Pathways.
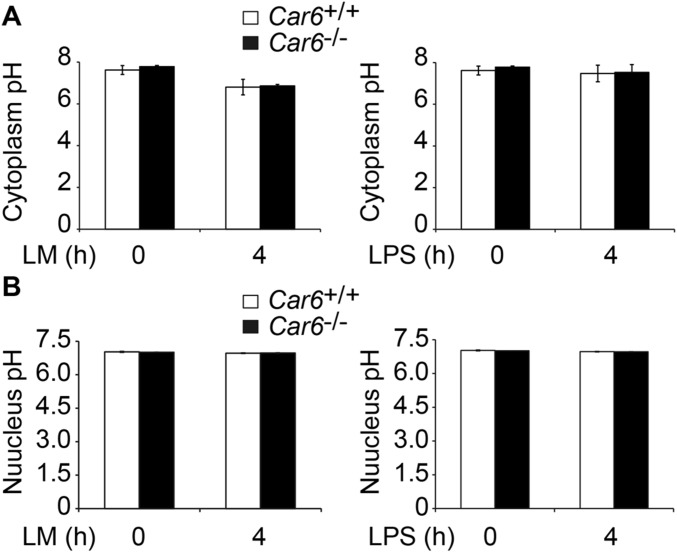
We wondered whether the underlying mechanism for Car6-b selectively promotes IL-12 expression in innate response. CA-VI A is secreted into saliva to maintain pH homeostasis by its CA enzyme activity (16), and CA-VI B also contains this enzyme domain. We wondered whether the pH of cytoplasm and the nucleus of PM might be involved in Car6-b–mediated promotion of IL-12 expression. However, there was no obvious difference in pH in either the cytoplasm or the nucleus of Car6+/+ and Car6−/− PMs with or without infection (Fig. S7). We also constructed catalytically inactive mutant Car6-b–M28+89 (H28A, Y89A) and found that Car6-b–M28+89 displayed a similar function as wild-type Car6-b in rescuing the Il12b mRNA expression (Fig. 2F) and IL-12p70 production (Fig. S2F) in Car6-b−/− RAW264.7 cells. However, the catalytically active Car6-a did not rescue the IL-12 expression. These results showed that Car6-b promotes IL-12 expression independent of its CA catalytic activity.
Fig. S7.
Comparative pH value between Car6+/+ and Car6−/− PMs. (A and B) The cytosolic pH (A) and nucleus pH (B) in PMs of Car6+/+ and Car6−/− mice after stimulation with LM or LPS for the indicated time. Data are shown as mean ± SD (n = 3).
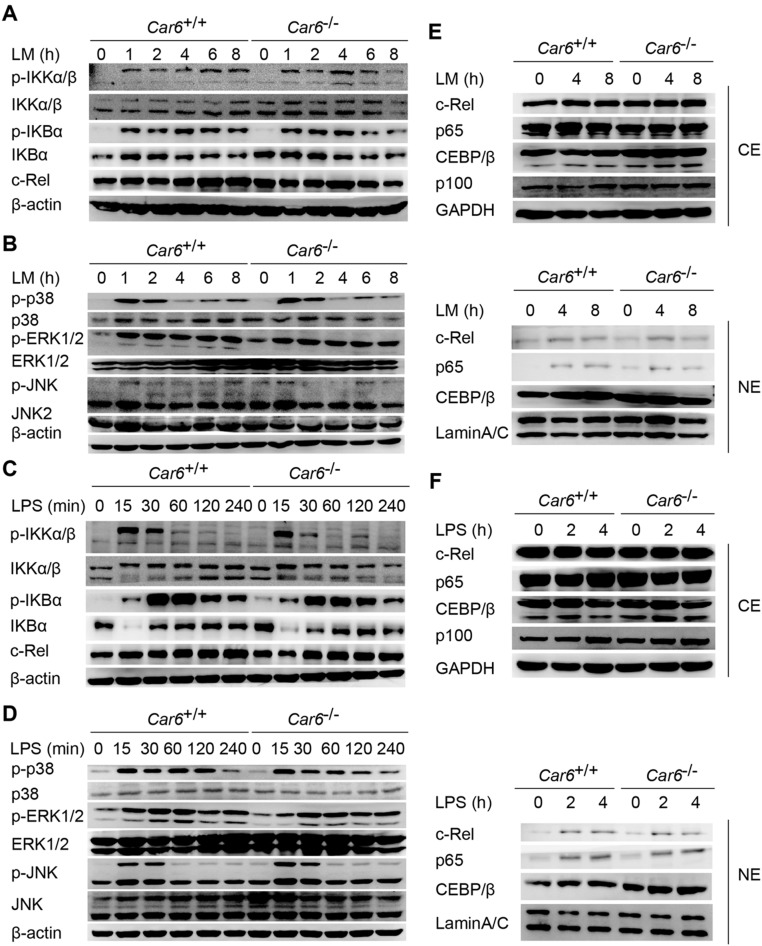
NF-κB and MAPK signaling pathways are known to induce the expression of IL-12 (2). However, no obvious difference was found in the activation of the inhibitor of NF-κB (IκB), kinase (IKK), TF (c-Rel), MAPKs (ERK, JNK, and p38) (Fig. S8 A–D), or the nucleus translocation of c-Rel, p65, and C/EBPβ (Fig. S8 E and F) between Car6−/− and Car6+/+ PMs upon LM or LPS stimuli. Therefore, Car6-b does not affect NF-κB and MAPK signaling pathways.
Fig. S8.
Car6-b does not affect NF-κB and MAPK pathways in macrophages. (A–D) Immunoblot analysis of NF-κB (A and C) and MAPK (B and D) signaling pathway proteins after infection with LM (A and B) or stimulation with LPS (C and D) in Car6+/+ and Car6−/− PMs for the indicated time. (E and F) Immunoblot analysis of the indicated proteins in cytoplasmic extracts (CEs) and nuclear extracts (NEs) of Car6+/+ and Car6−/− PMs stimulated for the indicated time with LM (E) or LPS (F). Similar results were obtained in three independent experiments.
Car6-b Promotes c-Rel Recruitment to the Il12 Promoter upon Innate Stimuli.
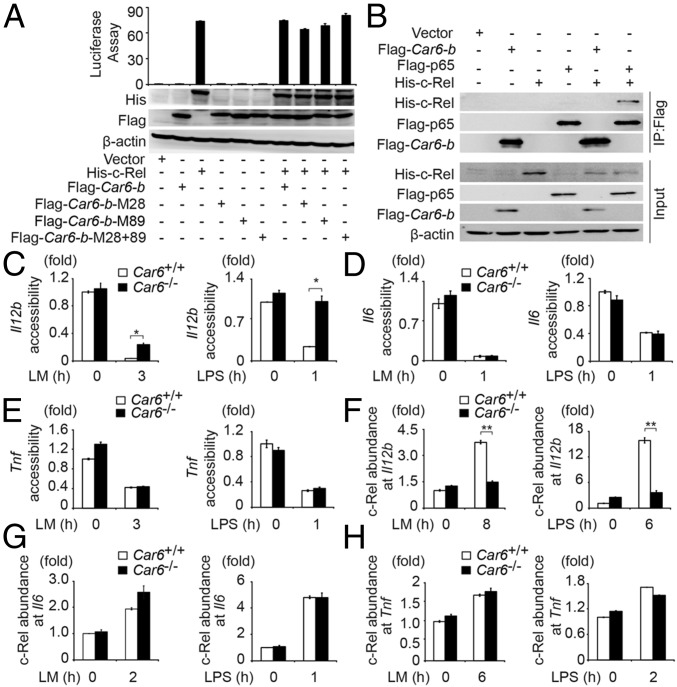
Based on the critical function of c-Rel in transcription of genes encoding IL-12 and other cytokines, we performed luciferase Il12b reporter assay to explore the detailed mechanism of Car6-b–promoted IL-12 production. Ectopic expression of Car6-b had no effect on transcriptional activity of the Il12b reporter that was induced by c-Rel, and Car6-b itself had no function on Il12b reporter (Fig. 5A), nor did CA-VI B interact with c-Rel in HEK293T cells as detected by co-immunoprecipitation (co-IP) assay (Fig. 5B). Thus, Car6-b might not directly affect c-Rel transcriptional activity.
Fig. 5.
Car6 promotes c-Rel recruitment to the Il12 promoter upon innate stimuli. (A) Dual luciferase reporter gene expression assay of the Il12b promoter and immunoblot assay in HEK293T cells transfected with plasmids as indicated. (B) HEK293T cells were transfected with indicated plasmids. Protein interactions were detected by co-IP experiments. (C–E) DNA accessibility assay at promoter sites of Il12b (C), Il6 (D), and Tnf (E) in PMs after stimulation by LM or LPS for the indicated time. (F–H) ChIP assay of c-Rel recruitment to the Il12b (F), Il6 (G), or Tnf (H) promoter sites in Car6+/+ and Car6−/− PMs after stimulation for the indicated time with LM or LPS. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01.
To clarify whether Car6-b affects chromatin conformation, we detected the DNA accessibility of the Il12b promoter by DNase I sensitivity analysis (17). Interestingly, DNA accessibility of the Il12b promoter was significantly decreased in Car6−/− PMs upon innate stimuli (Fig. 5C). We also found that the chromatin accessibility of promoter sites of Il6 and Tnf was not influenced by Car6 deficiency in PMs upon innate stimuli (Fig. 5 D and E). C-Rel particularly binds to the region containing an NF-κB binding site of the Il12b promoter (8). Therefore, we performed chromatin immunoprecipitation (ChIP) assay to examine c-Rel binding to the region between position –150 and –50 of the Il12b promoter, which contains an NF-κB binding site. We found that the recruitment of c-Rel to the promoter of Il12b was substantially decreased in Car6−/− PMs stimulated with LM or LPS (Fig. 5F). However, recruitment of c-Rel to the promoters of Il6 and Tnf was comparable between Car6+/+ and Car6−/− PMs upon innate stimuli (Fig. 5 G and H). Collectively, we demonstrate that Car6-b selectively promotes chromatin accessibility to facilitate c-Rel binding to the Il12b promoter.
CA-VI B Associates with PRMT5 to Promote c-Rel Recruitment to Il12 Promoters by Histone Modification.
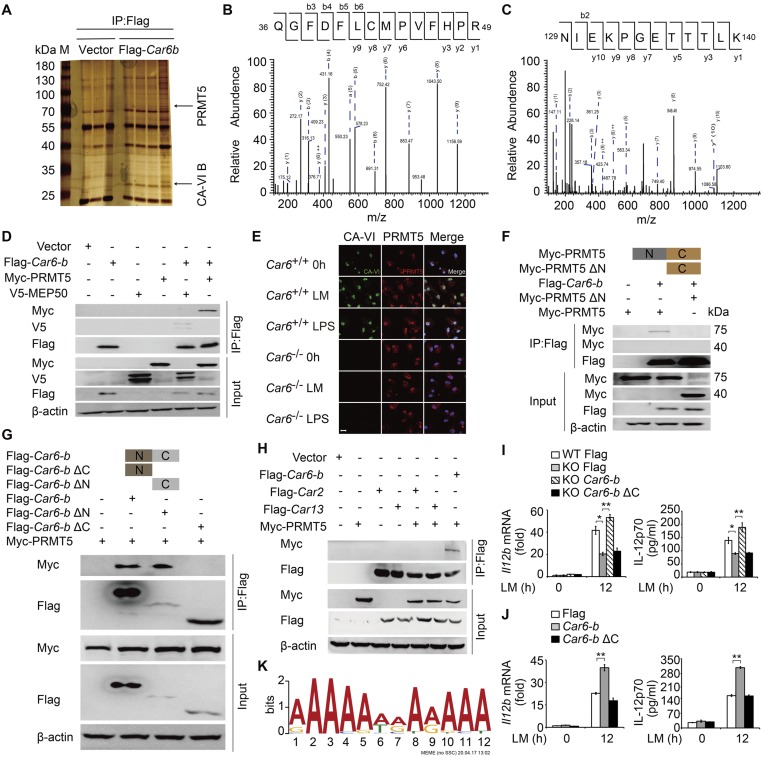
Because CA-VI B is located in the nucleus of macrophages and promotes c-Rel recruitment to the Il12b promoter, we speculated that Car6-b might be involved in histone modification to regulate chromatin accessibility. We performed mass spectrometry screening of the candidate proteins interacting with CA-VI B in HEK293T cells (Fig. S9 A–C). One potential candidate, PRMT5, attracted our attention. PRMT5, a type II arginine methyltransferase that symmetrically dimethylates arginine residues on histone H2AR3, H3R8, and H4R3, has been reported to regulate gene transcription through histone modification (18). Importantly, methylosome protein 50 (MEP50/WDR77) is required for PRMT5 function (19).
Fig. S9.
C-terminal domain of CA-VI B interacts with the N-terminal domain of PRMT5 to increase Il12b expression. (A) Silver staining of immunoprecipitation proteins in HEK293T cells transfected with Flag-vector or Flag–Car6-b plasmid. PRMT5 or CA-VI B confirmed by MS is indicated with arrows. (B and C) The peptides detected by MS are indicated as PRMT5 (B) and peptide refers to CA-VI B (C). (D) HEK293T cells were transfected with the indicated plasmids. Protein interactions were detected by co-IP. (E) Examination of colocalization between endogenous CA-VI B and PRMT5 in Car6+/+ and Car6−/− PMs with or without LM and LPS stimulation for 2 h. (Scale bar, 10 µm.) (F) HEK293T cells were transfected with deleted N-terminal PRMT5 (Myc-PRMT5 ΔN), Myc-PRMT5, and Flag–Car6-b to detect interaction by co-IP. (G) HEK293T cells were transfected with deleted N-terminal Car6-b (Flag–Car6-b ΔN) or deleted C-terminal Car6-b (Flag–Car6-b ΔC), Flag–Car6-b, and Myc-PRMT5 to detect the domain of CA-VI B to mediate the interaction with PRMT5 by co-IP experiments. (H) HEK293T cells were transfected with the indicated plasmids to detect Car2 or Car13 interaction with PRMT5 by co-IP experiments. (I) Il12b expression and IL-12p70 production in Car6 knockout RAW264.7 cells (KO) and control cells (WT) after transfection with Flag-vector (Flag), Flag–Car6-b (Car6-b), or Flag–Car6-b ΔC stimulated by LM. (J) Il12b expression and IL-12p70 production in RAW264.7 cells after transfection with Flag-vector (Flag), Flag–Car6-b (Car6-b), or Flag–Car6-b ΔC stimulated by LM for 12 h. (K) MEME analysis of PRMT5-bound regions defined by PRMT5 ChIP-seq. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01.
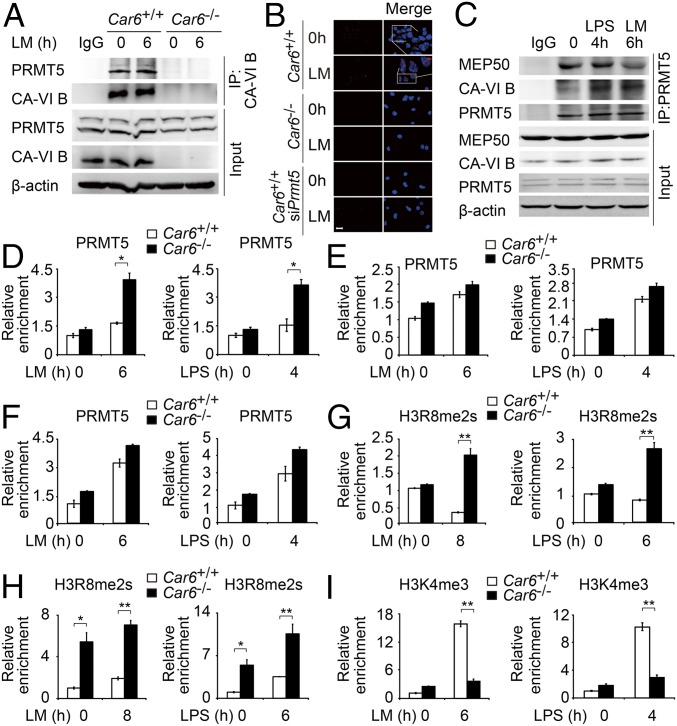
Co-IP experiments confirmed that Flag-tagged Car6-b interacted with both Myc-tagged PRMT5 and V5-tagged MEP50 (Fig. S9D). Moreover, we performed co-IP experiments to confirm the interaction between endogenous Car6-b and PRMT5 using CA-VI antibody in the lysates of Car6+/+ and Car6−/− PMs with or without LM stimulation (Fig. 6A). By immunofluorescence, we identified that CA-VI B and PRMT5 were colocalized in the nucleus of PMs after stimulation with LM or LPS (Fig. S9E). By proximal ligation assay (PLA), we further confirmed the interaction of CA-VI B and PRMT5 in the nucleus of Car6+/+ PMs but not in Car6−/− PMs or PRMT5-slienced Car6+/+ PMs (Fig. 6B). To further investigate the interaction site of CA-VI B and PRMT5, we constructed C-terminal deletion Car6-b (ΔC)-, N-terminal deletion Car6-b (ΔN)-, and N-terminal deletion PRMT5 (ΔN)-expressing plasmids. Our co-IP experiment showed that the C-terminal domain of Car6-b was indispensable for its interaction with the N-terminal domain of PRMT5 (Fig. S9 F and G). Furthermore, Car2 and Car13 were expressed in macrophages and located in the cytoplasm (14). So we constructed Car2- and Car13-expressing plasmids to detect the interaction with PRMT5. We found only Car6-b interacted with PRMT5 but not Car2 and Car13 (Fig. S9H). These data showed the specificity interaction between Car6-b and PRMT5, and overexpression of Car6-b ΔC did not promote IL-12 expression in RAW264.7 Car6-b KO cells upon LM stimulation (Fig. S9I). Furthermore, overexpression of Car6-b ΔC did not promote IL-12 expression in RAW264.7 and did not show dominant negative effects (Fig. S9J). These results indicate that the C-terminal domain is essential for Car6-b to interact with the N-terminal domain of PRMT5 and promotes IL-12 expression. Thus, Car6-b binds the same domain of PRMT5 as MEP50 (19). To further clarify whether Car6-b affected the activity of PRMT5, we used PRMT5 antibody for co-IP in RAW264.7 cells and found that PRMT5 interaction with Car6-b increased after innate stimuli. However, the interaction between PRMT5 and MEP50 was reduced simultaneously (Fig. 6C). As known, MEP50 is necessary for PRMT5 activity (19). Thus, Car6-b and MEP50 competitively interact with PRMT5, which leads to a decrease in the PRMT5 activity.
Fig. 6.
CA-VI B associates with PRMT5 to promote Il12 promoter accessibility by histone modification. (A and B) Interaction between CA-VI B and PRMT5 was detected by co-IP experiments in Car6+/+ and Car6−/− PMs with or without the indicated stimuli. (B) Interaction between CA-VI B and PRMT5 was detected by PLA in Car6+/+ PMs, Car6−/− PMs, and PRMT5-silenced Car6+/+ PMs (siPrmt5) with or without LM stimulation for 2 h. The interactions were visualized as red spots. (Scale bar, 10 µm.) (C) Co-IP experiments in RAW264.7 cells with or without the indicated stimuli. (D–F) ChIP assay of PRMT5 recruitment at promoter sites of Il12b (D), Il6 (E), or Tnf (F) in Car6+/+ and Car6−/− PMs after stimulation for the indicated time with LM or LPS. (G and H) ChIP assay of H3R8me2 modification at Il12b (G) or Il12a (H) promoter sites in Car6+/+ and Car6−/− PMs after stimulation for the indicated time with LM or LPS. (I) ChIP assay of H3K4me3 modification at the Il12b promoter site in Car6+/+ and Car6−/− PMs after stimulation for the indicated time with LM or LPS. Data were shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01.
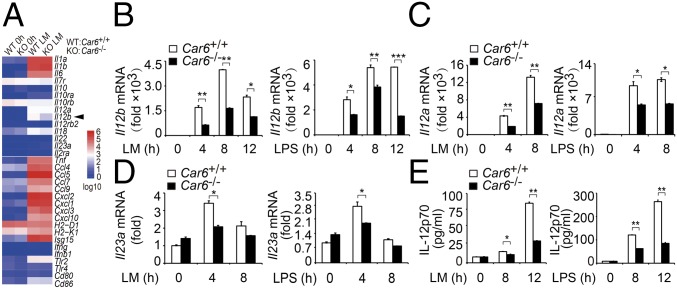
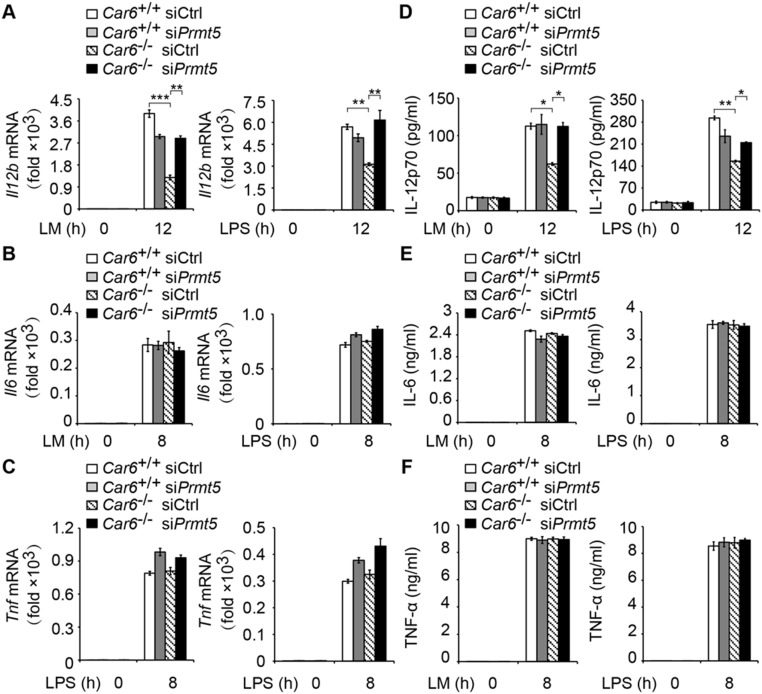
Furthermore, we performed ChIP assay to detect the recruitment of PRMT5 in promoters of Il12b, Il6, and Tnf. We found that the recruitment of PRMT5 to the Il12b promoter site was increased in Car6−/− PMs (Fig. 6D). However, the recruitment of PRMT5 to the promoters of Il6 and Tnf was not affected in Car6−/− PMs (Fig. 6 E and F). Furthermore, we used PRMT5 antibody for ChIP-seq of Car6+/+ and Car6−/− PMs with or without LM stimulation. We found PRMT5 consensus-binding element AAAAAAAAAAAA in Il12b promoter sites but not in Il6 and Tnf promoters (Fig. S9K). Additionally, we silenced Prmt5 in Car6+/+ and Car6−/− PMs and found that silenced Prmt5 in Car6−/− PMs selectively promoted IL-12 production and did not affect IL-6 and TNF-α production upon innate stimuli (Fig. S10). These results suggested Car6-b selectively regulated IL-12 expression by interaction with PRMT5, which is specifically recruited to the Il12b promoter. We further elucidate whether histone arginine methylation on promoters of Il12b and Il12a was regulated by CA-VI B and PRMT5. Indeed, H3R8me2s of Il12b and Il12a promoters were significantly increased in Car6−/− PMs upon innate stimuli (Fig. 6 G and H). In addition, we also found that trimethylation of histone H3 lysine 4 (H3K4me3) at the Il12b promoter was significantly decreased in Car6−/− PMs upon innate stimuli (Fig. 6I). Taken together, CA-VI B inhibits H3R8me2s modification at promoters of Il12 genes by interaction with PRMT5, which increases the chromatin accessibility upon innate stimuli, consequently enhancing c-Rel binding to the promoter of Il12b and promoting IL-12 expression.
Fig. S10.
Knockdown of Prmt5 promotes IL-12 production but does not affect IL-6 and TNF-α production in Car6−/− macrophages upon innate stimuli. (A–C) qPCR assay of Il12b (A), Il6 (B), and Tnf (C) in Prmt5-silenced Car6+/+ and Car6−/− PMs stimulated with LM or LPS for the indicated time. (D–F) ELISA of IL-12p70 (D), IL-6 (E), and TNF-α (F) in the supernatants of Prmt5-silenced Car6+/+ and Car6−/− PMs stimulated with LM or LPS for the indicated time. Data are shown as mean ± SD (n = 3). *P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
IL-12 is mainly produced by activated macrophages, monocytes, and DCs (2). Here we identify a previously unknown role of Car6-b in promoting host defense against bacterial infection by selectively enhancing IL-12 expression via interaction with PRMT5 to inhibit arginine methylation at Il12 promoter regions. This is a report about the function of CA members in innate immune response by selectively inducing IL-12 production independent of its CA activity.
Car6 has two types. In our study, we demonstrate that only Car6-b is expressed in the immune system and preferentially expressed in macrophages. A previous report showed that the mRNA expression of Car6 was up-regulated in Helicobacter pylori-infected RAW264.7 cells (20). Car6-b located in the nucleus has been discovered in mouse embryo fibroblasts in response to stress by unknown mechanisms, whereas its biological functions in the immune system remain unknown. Our study uncovered the function of Car6-b in innate immune response.
Car6-b deficiency particularly impairs IL-12 production in macrophages upon innate stimuli but not in DCs. This cell-type selectivity is consistent with the higher Car6-b expression in macrophages than that in DCs. DCs and macrophages show different developmental and functional properties in the regulation of innate and adaptive immunity (21). The mechanisms for IL-12 production in DCs and macrophages are different upon different TLR signaling (22). The selective role of Car6-b may thereby provide a new mechanism for the differential capacity of DC and macrophages in IL-12 production upon different innate stimuli.
The NF-κB complex contains RelA (p65), RelB, c-Rel, p100, and p105. C-Rel is expressed in hematopoietic cells (23), which might contribute to cell type-specific expression or function of many innate signaling regulators. C-Rel is necessary for Il12b transcription in macrophages, whereas Il12a is c-Rel–dependent in DCs (24, 25). Both Il6 and Tnf expression are mainly regulated by activation of p65 and p38 in NF-κB and MAPK signaling pathways, although c-Rel also has some functions in this process (23, 26). Both p50/p65 and p50/c-Rel bind to the promoter of Il12b, whereas c-Rel has a crucial role in induction of IL-12 expression in macrophages compared with p65 (7, 25). Consistently, deletion of Car6-b neither inhibits the production of TNF-α and IL-6 in macrophages upon LM or LPS stimulation nor affects c-Rel binding to the promoter region of Tnf and Il6. Indeed, the selectivity of regulating gene expression by epigenetic and transcriptional mechanisms has long been a challenging issue in the field. A complex cooperation is shown to be involved in the dynamic and spatial regulation of gene expression. For example, induction of de novo 5-hydroxymethylation upstream of the Il6 locus by methylcytosine dioxygenase TET2 recruits HDAC2 to specifically inhibit Il6 transcription via histone deacetylation and therefore is critical for termination of the high transcription of Il6 during the late phase of inflammatory response (27). In addition, there is a report showing that linc RNA-Cox2 can regulate Il12b expression (28). Our work showed that CA-VI B competitively binds PRMT5 with MEP50 to reduce the methylation activity of PRMT5 specifically recruited to the promoter of Il12b, leading it to selectively regulate Il12 production in innate immune response.
Lysine methylation modification on histone has been extensively investigated, whereas the role of arginine methylation on histone in regulation of immune response has not been clearly clarified. In the present study, we identified that CA-VI B inhibited H3R8me2s at the Il12 promotor region by interaction with PRMT5, thus adding insights into this histone modification type in regulation of innate immunity and inflammation. Increased activity of the PRMT5–MEP50 complex is found in many kinds of cancer cells and highly correlated with poor prognosis of human cancers, and it is the potential target for treatment of cancers (19). PRMT5 participates in inflammatory response by methylation modification of R35 or R30 of p65 to regulate interleukin-1α (IL-1α), TNF receptor-associated factor 1 (TRAF1), or CXCL10 expression (29). H3R8me2s and H4R3me2s repress gene expression, although the mechanisms mediated with PRMT5 remain to be elucidated (30). Our study reports PRMT5 in regulation of IL-12 expression by arginine modification of histone. Moreover, a crosstalk between arginine and lysine methylation exists to regulate gene expression (31). We found decreased H3K4me3 modification at the Il12b promoter in Car6-b−/− macrophages upon innate stimuli. Further investigations are required to uncover whether and how H3K4me3s and H3R8me2s, and possibly other types of histone modifications, may interact to regulate IL-12 expression.
According to published gene-profiling data, Car6 expression is up-regulated in inflammatory macrophages in an acute TB infection mouse model (1.3-fold higher than that of control cells; GEO accession no. GSE23014). In addition, Car6 is down-regulated in chronic TB infection (1.3-fold lower than that of acute infection period patients, GEO accession no. GSE54992). Moreover, IL-12 is induced in macrophages in response to Toxoplasma gondii infection (32). Car6 expression is also up-regulated in T. gondii-infected RAW 264.7 cell lines (1.4-fold higher than that of their control cells; GEO accession no. GSE55298) and in zymosan-induced active macrophages (1.2-fold higher than that of control cells; GEO accession no. GSE28621). These data indicate that Car6 can be up-regulated in acute infection, helping the host to defend against the invading pathogens, whereas down-regulation of Car6 expression may be one of the reasons for the host with chronic infection not to effectively eliminate the invading pathogens. These data, together with our results, suggest a potentially important connection between Car6 and human infection diseases and a possible clinical application of activating Car6 in treatment of chronic infectious disease with intracellular bacterial infection.
Materials and Methods
Mice.
C57BL/6J mice were purchased from Beijing Vital River Animal Center. Car6−/− mice were generated by using the CRISPR/Cas9 system. Car6−/− mice generation information is described in SI Materials and Methods.
All animal protocols were approved by the Animal Care and Use Committees of the Institute of Laboratory Animal Science of Chinese Academy of Medical Sciences (ILAS-GC-2015-002).
In Vivo Challenge with Listeria Monocytogenes.
LMs were obtained and cultured as described previously (33). Mice were infected with 5 × 104 virulent LMs intraperitoneally. Bacteria in the spleen, liver, and lung were quantified as described previously (33).
ChIP.
PMs were processed according to the protocol described in the ChIP Assay kit (Beyotime P2078). Antibody–chromatin complexes were pulled down using magnetic protein G beads (Invitrogen), washed, and then eluted. After cross-link reversal and protein K treatment, immunoprecipitated DNA was extracted with phenol-chloroform and ethanol-precipitated. ChIP primers used for Il12a were described previously (8), and for Il6, Tnfs were similar to ref. 27. Other mouse primers were as follows: Il12b pro (0-0.2K) forward, 5′-GGGGAGGGAGGAACTTCTTA-3′; Il12b pro (0-0.2K) reverse, 5′-TGATGGAAACCCAAAGTAGAAACTGAC-3′.
Chromatin Accessibility (DNase I Sensitivity) Assay.
Chromatin accessibility assay and analysis were performed as described previously (17). In brief, chromatin accessibility of the Il12b promoter region in Car6+/+ and Car6−/− macrophages stimulated with LPS for 1 h or LM for 3 h were assayed by qPCR.
Transfection.
Cells were seeded and kept overnight, and then RNAi MAX was used for gene-specific siRNA transfection for 36–48 h. Jetprime (Poly plus) and lipofectamine 3000 transfection reagent (Invitrogen) were used for transfection in 293T cells and RAW264.7 cells, respectively.
Statistical Analysis.
Statistical significance between two-group comparisons was analyzed by two-tailed Student t test. The statistical significance of survival curves was compared with the generalized Wilcoxon test. The value P < 0.05 was considered statistically significant.
Additional methods are described in SI Materials and Methods.
SI Materials and Methods
Generation of Car6-Deficient (Car6−/−) Mice.
Briefly, the microinjection of fertilized eggs with Cas9 mRNAs and sgRNAs were performed. The Cas9 mRNA was prepared by in vitro transcription using linearized Cas9 expression plasmid (Addgene, 44758) as template. The Car6 sgRNA expression plasmid was prepared by inserting the annealed paired synthesized oligonucleotides for sgRNAs into the pUC57-sgRNA expression vector (Addgene, 51132). The Car6 sgRNAs were prepared by in vitro transcription using linearized sgRNA expression plasmids. The Car6 sgRNA targeting sequences are as follows: target 1, GGGTTGCCACAGGTGAGGAG; target 2, GGAGCTGGCCATCGAGCCCC. Mouse genomic DNA of new pups was extracted from the tails of 7-d-old mice using phenol-chloroform and recovered by alcohol precipitation to detect the mutations. Car6-deficient mice were detected with primers for Car6 mouse forward (5′-GGCTTCAGTAAAGGGCGAC-3′) and Car6 mouse reverse (5′-CAACGCAATGACACAGATTCA-3′). The sequence information is as follows (deletion sequence is shown in bold and underlined): CTAGGCTGGCTTCAGTAAAGGGCGACCCTGAGCTCCTGACTTCCCCACTTAGGCTTACTGGGAGCTGGGATTACGGAGGTAACTACCACAGTTTACGCAGAGCTGAGGATCAAACCCAGGGCCCAGTGCTTGTTGGCCAAACACTCTACCTCAGCTCCATTGGTGGTCACCTAAGGGCAGCAAATATCTGGCGAGACACTGAACGTTCCCATTTTATAAGCCTAACAAGCTGGATCCGCCGCACTGTAGTCTTGCCCCTGACCGGTTGGTTCTGCATAGAGAGTTGTCCTGGCACCCATCTTGGCCCGCTCTGTGCACCACAGGTGCCCCAGTGTCACCTTCCCTCCGGGAACAGTGTTCTGATCCATCAGTAACACCTTGGGTTGCCACAGGTGAGGAGGGGTGGGGAGTGACCAGGGAGATACTTATGGGAAATCTGAGTCTCCCTGTGCTGTGTTCTCTCCTGCAGTATCGATCGATCTGCCGCCCTCCATGTACCTTGAAACCTCTGACGGCACTGAGTTCATCTCAAAGGCCTTTCACTTTCACTGGGGGGGTCGGGACTGGGAACTCAGCGGCTCTGAACACACCATTGATGGGATCAGGAGTATAATGGAGGTATGTGACAGCTCCCACAGGGTCTCCTTCCCTTCACGCTCCAGAGTCACTTTCTTTGTGTCAGTGTCTGCATGCTTGTTATACACATGTCATGTATGTGCGCATGCATGTGAATGTGCATGCACACATGTATGTGTACTCGTGGAGGCCAGAGATTGATGCTGTCTTCCTCTGTCACAATCTCTCTCACTGAGTCTGGATCTCATCAAGGAGCTGGCCATCGAGCCCCAGGGACCCTATTGCCTGCCATTGTCTCAGAGATTATAGATAAGCAATACTGTGCCCAGTTTTCCTTTGTGGGTGCAGGGAACTGAACTCAGTTTCTCATTCTCAATGACGAGTGCTTTACCAACTTAGCCATCTCTCCAGCTCTACCTTTCTTATTAAAATAGTCATTTTAATATTGGCTTATTCCCTTTTGGGGTGGTGGTGGTTTCGAGATATGGTTTCTCTGTATAGCCCTGGCTGTCCTGGAACTCACTCTGTAGACCAGGCTGGCCTCGAACTCAGAAATCGGCCTGCCTCTGTCTGCCTCCCGAGTGCTGGGATTAAAGGCGTGTGCCACCACTGCCCACCTTTGACTTATTCTTATTAAAGTAATCAATATGATTTTGTGCAGTGGTGATGGTTCAGTCACCCTTGTGTTTCCATCGGCAGTGAATCTGTGTCATTGCGTTGTGGG. All mice were bred in a specific pathogen-free animal facility, and 6- to 8-wk-old littermate mice were used in the experiment. All animal protocols were approved by the Animal Care and Use Committees of the Institute of Laboratory Animal Science of Chinese Academy of Medical Sciences (ILAS-GC-2015-002).
Cell Preparation and Reagents.
The RAW264.7 and HEK293T cell lines were purchased from American Type Culture Collection. All cells were maintained in DMEM (Corning) with 10% (vol/vol) FBS. PMs, BMMs, and BMDCs were prepared as described previously (27). Car6-b−/− cells were generated by using the CRISPR/Cas9 system in the RAW264.7 cell line. Briefly, RAW264.7 cells were transfected with sgRNAs and Cas9 expression plasmids by Lipofectamine 2000 (Invitrogen). The Cas9 expression plasmid was used as described before. The Car6 sgRNA expression plasmids were prepared by inserting the annealed paired synthesized oligonucleotides for sgRNAs into the pGL3-U6-sgRNA expression vector (Addgene, 51133). The sgRNA targeting sequences are as follows: target 1, GCCTTCTGGAAATAGAAGCA; target 2, GGTATGTGACAGCTCCCACA. At 24 h after transfection, blasticidin S hydrochloride (2 μg/mL; Life Technology) and puromycin (3 μg/mL; Life Technology) were added. Genomic DNA of cell clones was extracted using phenol-chloroform and recovered by alcohol precipitation to detect mutations. The sequence information is as follows (deletion sequence is shown in bold and underlined): CCACGTGACTACTGCAAGGCGGAGGAGACCCTTGGCTGTGAATCACCAGCTCTTGGTTCTTACTGCCAGTTGCCTCAGAGGAAGAACACCATCGCCACACCCCAAGTGCTGGGCTTAGTTTAGAGCTTTCCCGGCCTGCCTTCTGGAAGCCCTGCTTCTATTTCCAGAAGGCTGCCCCATGGGTCTTCCACCTAGAAGCCCCTGCGTGAGAAGCCTCAGAGAAGGCTCAGCGGGGCAGCTCTGAGTTGGGCCCCACTCTTTCCTGTTGCAGGGGATGATGGCGTGGGAGAAAGTCAGTGGTCTGAGCAGTATCCTTCCTGCGGCGGGGAAAGGCAGTCGCCCATCGACGTGAAGACGGAGGAGGTGATGTTCAATCCCTCCTTGAAGCCCCTGAGCTTGGTGAACTATGAGAAGGAGAACCTGGAGTTCACTATGACTAACAACGGACACACAGGTAGGGGGTACCGGTGGGGCTGGCAGTCTGCATGAGAGACAGGGCATGCACATGCGCTCAGGAGCAGCATGCACAGAGACCCCATTTTCTAGGACCTATGCATACGCTAGGCAACCCCGGGGAGGGCTGGCTATACTTGTCGACGTTCCTCCTTTTAAAGAGCCTAGGCTGGCTTCAGTAAAGGGCGACCCTGAGCTCCTGACTTCCCCACTTAGGCTTACTGGGAGCTGGGATTACGGAGGTAACTACCACAGTTTACGCAGAGCTGAGGATCAAACCCAGGGCCCAGTGCTTGTTGGCCAAACACTCTACCTCAGCTCCATTGGTGGTCACCTAAGGGCAGCAAATATCTGGCGAGACACTGAACGTTCCCATTTTATAAGCCTAACAAGCTGGATCCGCCGCACTGTAGTCTTGCCCCTGACCGGTTGGTTCTGCATAGAGAGTTGTCCTGGCACCCATCTTGGCCCGCTCTGTGCACCACAGGTGCCCCAGTGTCACCTTCCCTCCGGGAACAGTGTTCTGATCCATCAGTAACACCTTGGGTTGCCACAGGTGAGGAGGGGTGGGGAGTGACCAGGGAGATACTTATGGGAAATCTGAGTCTCCCTGTGCTGTGTTCTCTCCTGCAGTATCGATCGATCTGCCGCCCTCCATGTACCTTGAAACCTCTGACGGCACTGAGTTCATCTCAAAGGCCTTTCACTTTCACTGGGGGGGTCGGGACTGGGAACTCAGCGGCTCTGAACACACCATTGATGGGATCAGGAGTATAATGGAGGTATGTGACAGCTCCCACAGGGTCTCCTTCCCTTCACGCTCCAGAGTCACTTTCTTTGTGTCAGTGTCTGCATGCTTGTTATACACATGTCATGTATGTGCGCATGCATGTGAATGTGCATGCACACATGTATGTGTACTCGTGGAGGCCAGAGATTGATGCTGT. PCR primers were Car6-b cell forward (5′-GAGACCCTTGGCTGTGAATC-3′) and Car6-b cell reverse (5′-GAGAGATGGCTAAGTTGGTAAAG-3′). Cell clones with a wanted mutation were obtained for consequence experiments. Cells were stimulated with LPS (100 ng/mL) (Sigma) or LM (1 × 105 per mL) for indicated time periods.
Microarray Analysis.
Total RNA was isolated using RNA isolation kit (Ambion). Whole-genome wide expression analysis was performed with Affymetrix Mouse Genome 430 2.0 Arrays. Detailed procedures were described previously (17).
Plasmids and Antibodies.
The Flag-tagged Car6-b, mutant Car6-b M28+89, Car6-b M28, Car6-b M89, Car6-b ΔC, Car6-b ΔN, Car6-a plasmids, His-tagged c-Rel plasmid, Myc-tagged PRMT5, PRMT5 ΔN, and V5-tagged MEP50 plasmids were constructed by PCR-based amplification of plasmid (Invitrogen) and then subcloned in pcDNA3.1 eukaryotic expression vector and verified by sequencing. ERK inhibitor FR180204 (SML0320) was from Sigma. Antibodies specific for Myc tag (sc-40), ERK1/2 (sc-93), c-Rel (sc-70), C/EBPβ (sc-150), and CA-VI (sc-99172) were from Santa Cruz Biotechnology. Antibodies specific for His-tag (9991), p38 (9212), JNK (9252), p65 (8042), IκBα (4814), phosphorylated ERK1/2 (4370), phosphorylated p38 (9216), phosphorylated p65 (3033), phosphorylated JNK (9255), phosphorylated IKKαβ(2697), IKKα(2682), IKKβ(8943), phosphorylated IκBα (9246), and anti-Rabbit Alexa Fluor 488-conjugated secondary Ab (4412S) were from CST. Dylight 488 goat anti-mouse IgG Ab (405310) was from Biolegend. H3K4me3 (07-473) and normal IgG (12-370) were from Millipore. An antibody specific for H3R8me2s (A-3706-050) was bought from Epigentek. GAPDH (M171-3) and β-actin (M177-3) are from MBL. These antibodies were applied at a dilution of 1:1,000. Antibodies specific for Flag (F3040; Sigma) and V5 tag (ab1325; Abcam) were applied at a dilution of 1:5,000. Flow cytometer antibodies CD4-percp (553052), CD8a-FITC (553033), CD19-percp (551001), and NK1.1-PE (553165) were from BD Bioscience.
qPCR.
V7 (ABI) and SYBR RT-PCR kit (TOYOBO FSQ-101 and QPK-201) used for real-time PCR analysis and the analysis method for calculation of relative changes were as described previously (17). In ChIP assay, the analysis method was as described previously (27). The primers used for mouse Actb, Il6, and Tnf were the same as in ref. 27. Other mouse primer sequences used for qPCR were as follows: Car6 forward, 5′-GTGTTAGCCGTCTTGTTTA-3′; Car6 reverse, 5′-AGCCTGGGTAGGTGTAAT-3′; Car6-a forward, 5′-CAGGCCCACAGTGATTGGAGCTA-3′; Car6-a (Car6-b) reverse, 5′-CATCACCTCCTCCGTCTTCA-3′; Car6-b forward, 5′-AAGTGCTGGGCTTAGTTTAG-3′; Il12b forward, 5′-TGGTTTGCCATCGTTTTGCTG-3′; Il12b reverse, 5′-ACAGGT GAGGTTCACTGTTTCT-3′; Il12a forward, 5′-GCCAGGTGTCTTAGCCAGTC-3′; Il12a reverse, 5′-AGCTCCCTCTTGTTGTGGAA-3′; Il23a forward, 5′-TCCAGCCAGAGGATCAC CCC-3′; Il23a reverse, 5′-GCTGCTCCGTGGGCAAAGAC-3′; Ifnb1 forward, 5′-AGCTCCA AGAAAGGACGAACAT-3′; Ifnb1 reverse, 5′-GCCCTGTAGGTGAGGTTGATCT-3′; Il1a forward, 5′-CGAAGACTACAGTTCTGCCATT-3′; Il1a reverse, 5′-GACGTTTCAGAG GTTCTCAGAG-3′; Nos2 forward, 5′-GAGATGGTCCGCAAGAGAGT-3′; and Nos2 reverse, 5′-CTGCTCCTCGCTCAAGTTCAG-3′.
RNA Interference.
SiRNAs against Car6-b and negative control were from Invitrogen. PMs were transfected with siRNA by using RNAi MAX reagent (Invitrogen) by a transfection procedure. SiRNAs of Car6-b sequences are CGUACCACCUUUACCUAAATT, UUUAGGUAAAGGUGGUACGAA. SiRNAs of Prmt5 sequences are CCCUUAAUCAGGAAGAUAATT, UUAUCUUCCUGAUUAAGGGTT.
ELISA.
The concentration of IL-12p70, IFN-γ, TNF-α, and IL-6 in the supernatants or serum was measured by ELISA kits (R&D Systems).
Flow Cytometry.
The cell preparation and staining were as described previously (17). Samples were detected using BD FACS Aria II flow cytometer, and data were analyzed by BD FACS Diva software (BD Biosciences).
Immunoprecipitation and Immunoblot Analysis.
Cells were lysed with cell lysis buffer (CST) supplemented with an EDTA-free protease inhibitor “cocktail” (Roche). Immunoprecipitation experiments were performed as described previously (17). Cell extracts were subjected to SDS/PAGE. Immunoblotting was performed as described previously (17).
Immunofluorescence Assay and Confocal Microscopy.
PMs or 293T cells were plated on glass coverslips in 24-well plates for subsequent experiments. Then, cells were operated and labeled with specific antibodies as described previously (17). The fluorescence was analyzed using Olympus confocal microscope.
PLA.
Interaction between CA-VI B and PRMT5 in PMs was detected by in situ PLA. Cells for PLA were fixed, permeabilized, blocked, and incubated with the primary antibodies as for immunofluorescence. The next steps were followed according to the manufacturer’s instructions (DUO92101; Sigma-Aldrich). The fluorescence was analyzed using Olympus confocal microscope.
Measurement of Intracellular pH.
The pH-sensitive fluorescent dye BCECF-AM (B262-10; Dojindo) was used for measuring intracellular pH changes. For cytoplasm pH assay, cells were stained with BCECF-AM as described previously (34). For nucleus pH assay, the fluorescence was detected using ZEISS LSM 710 confocal microscope with excitation at 440 nm and 490 nm, respectively; emission was 535/20 nm. The data were analyzed using the ratio (Ex488/Ex440) as described previously (35).
RNA-Seq Analysis.
RNA samples were collected from Car6+/+ and Car6−/− PMs with or without LM stimulation. RNA isolation, library construction, and sequencing were performed on a BGISEQ-500 (Beijing Genomic Institution, www.genomics.org.cn, BGI). Clean-tags were mapped to the mm10 reference genome. For gene expression analysis, the significance of the differential expression genes was defined by the bioinformatics service of BGI according to the combination of the absolute value of log2-Ratio ≥ 1 and FDR ≤ 0.001. All original sequence datasets have been submitted to the database of NCBI Sequence Read Archive (SRA) under accession number SRP108814.
ChIP-Seq Analysis.
ChIP was performed using PRMT5 antibody. ChIP-seq libraries were prepared according to the NEBNext protocol and sequenced using BGISEQ-500 (Beijing Genomic Institution, www.genomics.org.cn, BGI). We used a tool named MEME (Multiple Expectation maximization for Motif Elicitation) to analyze the PRMT5 consensus-binding motif. All original sequence datasets have been submitted to the database of NCBI Sequence Read Archive (SRA) under accession number SRP108823.
Assays of Dual Luciferase Reporter Gene Expression.
For luciferase assays, HEK293T cells were cotransfected with Il12b luciferase reporter plasmid, Renilla luciferase reporter vector, and other indicated plasmids, respectively. At 24 h after transfection, the luciferase activity was measured by using the dual luciferase reporter assay kit (Promega) as described previously (33).
Acknowledgments
We thank Dr. Qian Zhang, Dr. Meng Xia, and Dr. Xia Li for helpful discussions and Professor Shaocong Sun (The University of Texas MD Anderson Cancer Center) for kindly providing c-Rel and Il12b promoter luciferase plasmids. This work was supported by National Nature Science Foundation of China Grants 31270945 and 31390431, National Key Basic Research Program of China Grant 2013CB530503, Mérieux Research Grant 2016, and Chinese Academy of Medical Sciences (CAMS) Innovation Fund for Medical Sciences Grant 2016-I2M-1-003.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The cDNA microarray data are available at Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo/ (accession no. GSE90808). All original RNA-sequencing and ChIP-sequencing datasets have been submitted to the NCBI Sequence Read Archive (SRA) (accession nos. SRP108814 and SRP108823, respectively).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1700917114/-/DCSupplemental.
References
- 1.Brubaker SW, Bonham KS, Zanoni I, Kagan JC. Innate immune pattern recognition: A cell biological perspective. Annu Rev Immunol. 2015;33:257–290. doi: 10.1146/annurev-immunol-032414-112240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 3.de Jong R, et al. Severe mycobacterial and Salmonella infections in interleukin-12 receptor-deficient patients. Science. 1998;280:1435–1438. doi: 10.1126/science.280.5368.1435. [DOI] [PubMed] [Google Scholar]
- 4.Kroenke MA, Carlson TJ, Andjelkovic AV, Segal BM. IL-12- and IL-23-modulated T cells induce distinct types of EAE based on histology, CNS chemokine profile, and response to cytokine inhibition. J Exp Med. 2008;205:1535–1541. doi: 10.1084/jem.20080159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Teng MW, et al. IL-12 and IL-23 cytokines: From discovery to targeted therapies for immune-mediated inflammatory diseases. Nat Med. 2015;21:719–729. doi: 10.1038/nm.3895. [DOI] [PubMed] [Google Scholar]
- 6.Portela A, Esteller M. Epigenetic modifications and human disease. Nat Biotechnol. 2010;28:1057–1068. doi: 10.1038/nbt.1685. [DOI] [PubMed] [Google Scholar]
- 7.Weinmann AS, et al. Nucleosome remodeling at the IL-12 p40 promoter is a TLR-dependent, Rel-independent event. Nat Immunol. 2001;2:51–57. doi: 10.1038/83168. [DOI] [PubMed] [Google Scholar]
- 8.Jin J, et al. Epigenetic regulation of the expression of Il12 and Il23 and autoimmune inflammation by the deubiquitinase Trabid. Nat Immunol. 2016;17:259–268. doi: 10.1038/ni.3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ernst JD. The immunological life cycle of tuberculosis. Nat Rev Immunol. 2012;12:581–591. doi: 10.1038/nri3259. [DOI] [PubMed] [Google Scholar]
- 10.Hsieh CS, et al. Development of TH1 CD4+ T cells through IL-12 produced by Listeria-induced macrophages. Science. 1993;260:547–549. doi: 10.1126/science.8097338. [DOI] [PubMed] [Google Scholar]
- 11.Regan T, et al. Identification of TLR10 as a key mediator of the inflammatory response to Listeria monocytogenes in intestinal epithelial cells and macrophages. J Immunol. 2013;191:6084–6092. doi: 10.4049/jimmunol.1203245. [DOI] [PubMed] [Google Scholar]
- 12.Austrup F, et al. P- and E-selectin mediate recruitment of T-helper-1 but not T-helper-2 cells into inflammed tissues. Nature. 1997;385:81–83. doi: 10.1038/385081a0. [DOI] [PubMed] [Google Scholar]
- 13.Sok J, et al. CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol Cell Biol. 1999;19:495–504. doi: 10.1128/mcb.19.1.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Supuran CT. Carbonic anhydrases: Novel therapeutic applications for inhibitors and activators. Nat Rev Drug Discov. 2008;7:168–181. doi: 10.1038/nrd2467. [DOI] [PubMed] [Google Scholar]
- 15.Patrikainen MS, Pan P, Barker HR, Parkkila S. Altered gene expression in the lower respiratory tract of Car6 (-/-) mice. Transgenic Res. 2016;25:649–664. doi: 10.1007/s11248-016-9961-5. [DOI] [PubMed] [Google Scholar]
- 16.Pan PW, et al. Gene expression profiling in the submandibular gland, stomach, and duodenum of CAVI-deficient mice. Transgenic Res. 2011;20:675–698. doi: 10.1007/s11248-010-9441-2. [DOI] [PubMed] [Google Scholar]
- 17.Wang P, et al. The STAT3-binding long noncoding RNA lnc-DC controls human dendritic cell differentiation. Science. 2014;344:310–313. doi: 10.1126/science.1251456. [DOI] [PubMed] [Google Scholar]
- 18.Pal S, et al. Low levels of miR-92b/96 induce PRMT5 translation and H3R8/H4R3 methylation in mantle cell lymphoma. EMBO J. 2007;26:3558–3569. doi: 10.1038/sj.emboj.7601794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stopa N, Krebs JE, Shechter D. The PRMT5 arginine methyltransferase: Many roles in development, cancer and beyond. Cell Mol Life Sci. 2015;72:2041–2059. doi: 10.1007/s00018-015-1847-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tan GM, et al. Suppression of cell division-associated genes by Helicobacter pylori attenuates proliferation of RAW264.7 monocytic macrophage cells. Sci Rep. 2015;5:11046. doi: 10.1038/srep11046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Varol C, Mildner A, Jung S. Macrophages: Development and tissue specialization. Annu Rev Immunol. 2015;33:643–675. doi: 10.1146/annurev-immunol-032414-112220. [DOI] [PubMed] [Google Scholar]
- 22.e Sousa CR, et al. In vivo microbial stimulation induces rapid CD40 ligand-independent production of interleukin 12 by dendritic cells and their redistribution to T cell areas. J Exp Med. 1997;186:1819–1829. doi: 10.1084/jem.186.11.1819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen LF, Greene WC. Shaping the nuclear action of NF-kappaB. Nat Rev Mol Cell Biol. 2004;5:392–401. doi: 10.1038/nrm1368. [DOI] [PubMed] [Google Scholar]
- 24.Sanjabi S, Hoffmann A, Liou HC, Baltimore D, Smale ST. Selective requirement for c-Rel during IL-12 P40 gene induction in macrophages. Proc Natl Acad Sci USA. 2000;97:12705–12710. doi: 10.1073/pnas.230436397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grumont R, et al. c-Rel regulates interleukin 12 p70 expression in CD8(+) dendritic cells by specifically inducing p35 gene transcription. J Exp Med. 2001;194:1021–1032. doi: 10.1084/jem.194.8.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasparakis M. Regulation of tissue homeostasis by NF-kappaB signalling: Implications for inflammatory diseases. Nat Rev Immunol. 2009;9:778–788. doi: 10.1038/nri2655. [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, et al. Tet2 is required to resolve inflammation by recruiting Hdac2 to specifically repress IL-6. Nature. 2015;525:389–393. doi: 10.1038/nature15252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tong Q, et al. LincRNA-Cox2 modulates TNF-α-induced transcription of Il12b gene in intestinal epithelial cells through regulation of Mi-2/NuRD-mediated epigenetic histone modifications. FASEB J. 2016;30:1187–1197. doi: 10.1096/fj.15-279166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei H, et al. PRMT5 dimethylates R30 of the p65 subunit to activate NF-κB. Proc Natl Acad Sci USA. 2013;110:13516–13521. doi: 10.1073/pnas.1311784110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yang Y, Bedford MT. Protein arginine methyltransferases and cancer. Nat Rev Cancer. 2013;13:37–50. doi: 10.1038/nrc3409. [DOI] [PubMed] [Google Scholar]
- 31.Pal S, Vishwanath SN, Erdjument-Bromage H, Tempst P, Sif S. Human SWI/SNF-associated PRMT5 methylates histone H3 arginine 8 and negatively regulates expression of ST7 and NM23 tumor suppressor genes. Mol Cell Biol. 2004;24:9630–9645. doi: 10.1128/MCB.24.21.9630-9645.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yap G, Pesin M, Sher A. Cutting edge: IL-12 is required for the maintenance of IFN-γ production in T cells mediating chronic resistance to the intracellular pathogen, Toxoplasma gondii. J Immunol. 2000;165:628–631. doi: 10.4049/jimmunol.165.2.628. [DOI] [PubMed] [Google Scholar]
- 33.Ma F, et al. The microRNA miR-29 controls innate and adaptive immune responses to intracellular bacterial infection by targeting interferon-γ. Nat Immunol. 2011;12:861–869. doi: 10.1038/ni.2073. [DOI] [PubMed] [Google Scholar]
- 34.Adamus G, Karren L. Autoimmunity against carbonic anhydrase II affects retinal cell functions in autoimmune retinopathy. J Autoimmun. 2009;32:133–139. doi: 10.1016/j.jaut.2009.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Masuda A, Oyamada M, Nagaoka T, Tateishi N, Takamatsu T. Regulation of cytosol-nucleus pH gradients by K+/H+ exchange mechanism in the nuclear envelope of neonatal rat astrocytes. Brain Res. 1998;807:70–77. doi: 10.1016/s0006-8993(98)00737-9. [DOI] [PubMed] [Google Scholar]