Significance
The tuning of odorant receptors to their particular odorants is crucial for better understanding of how olfactory cues mediate ant social interactions. To help decode the olfactory system of ants, a selection of odorant receptors (ORs) from several phylogenetically distinct subfamilies from the ponerine ant Harpegnathos saltator were tested against a panel of ant semiochemicals. Responses were observed to both cuticular hydrocarbon components, some of which are known pheromones, and “general odorants,” demonstrating broad coverage of these odor spaces across several subfamilies of receptors. These results do not align with currently held hypotheses of OR subfamily odor coding and provide further insight into the evolution of pheromone perception within ant clades and the role this plays in complex social behaviors.
Keywords: ant, odorant receptor, odor coding, pheromone
Abstract
Animals use a variety of sensory modalities—including visual, acoustic, and chemical—to sense their environment and interact with both conspecifics and other species. Such communication is especially critical in eusocial insects such as honey bees and ants, where cooperation is critical for survival and reproductive success. Various classes of chemoreceptors have been hypothesized to play essential roles in the origin and evolution of eusociality in ants, through their functional roles in pheromone detection that characterizes reproductive status and colony membership. To better understand the molecular mechanisms by which chemoreceptors regulate social behaviors, we investigated the roles of a critical class of chemoreceptors, the odorant receptors (ORs), from the ponerine ant Harpegnathos saltator in detecting cuticular hydrocarbon pheromones. In light of the massive OR expansion in ants (∼400 genes per species), a representative survey based on phylogenetic and transcriptomic criteria was carried out across discrete odorant receptor subfamilies. Responses to several classes of semiochemicals are described, including cuticular hydrocarbons and mandibular gland components that act as H. saltator pheromones, and a range of more traditional general odorants. When viewed through the prism of caste-specific OR enrichment and distinctive OR subfamily odorant response profiles, our findings suggest that whereas individual HsOrs appear to be narrowly tuned, there is no apparent segregation of tuning responses within any discrete HsOr subfamily. Instead, the HsOR gene family as a whole responds to a broad array of compounds, including both cuticular hydrocarbons and general odorants that are likely to mediate distinct behaviors.
The detection of ecologically relevant chemosensory information is critical to the survival and propagation of all organisms. For example, sex pheromones allow members of the same species to locate and assess mates, and predators use volatile kairomones to locate prey. There is long-standing interest in understanding the pheromonal communication of insects and, in particular, exploring how semiochemicals govern the interactions of eusocial colonies. Ants are intriguing for the purposes of chemosensory studies, because of their diversity and exploitation of cuticular hydrocarbons (CHCs) for nest-mate recognition, and as signals of reproductive and caste status. Most ants live in closed societies within a shared colony or nest—with stereotypic social behaviors that involve a strict division of reproductive labor—in which multiple overlapping generations of sterile workers cooperate to nurture the progeny produced by the reproductives, which usually consist of single or small numbers of long-lived, highly fertile queens and short-lived male drones (1). Reproductive status within the colony is thought to be signaled primarily by a subset of the hydrocarbons secreted onto the external cuticle of insects and other arthropods (e.g. ref. 2) that also function to maintain water balance (3). In fact, colony identity is conveyed by a highly diverse set of CHCs, and intraspecific and interspecific invaders from other colonies are detected and defended against as a consequence of having a different CHC blend than the blend associated with a particular nest/colony (4). In addition, other non-CHC olfactory stimuli play important roles in ant chemical ecology as alarm, trail, or recognition pheromones and are often found in ant exocrine glands (5) and in the microbiota of the ant cuticle (6).
Although numerous ant species are being used as research models, the ponerine ant Harpegnathos saltator possesses several advantages that make it an ideal species for study. Notably, its basic social and chemosensory behaviors have been described in detail (7). Perhaps more critically, Harpegnathos workers can, under certain circumstances, convert into gamergates (from the Greek for “married worker”). As such, H. saltator represents a genetically tractable model system for studying social organization in an insect society.
Despite a rapidly developing body of knowledge on the phylogenetics of ant chemoreceptors (8, 9), the molecular elements that are responsible for the detection of ant pheromones remain largely uncharacterized. As is the case for other insects, the H. saltator genome contains three major classes of chemoreceptors—odorant receptors (ORs), gustatory receptors (GRs), and variant ionotropic receptors (IRs)—and several other receptor classes such as TRP channels, which also have been shown to have chemosensory roles, reviewed in ref. 10.
Within the ant clade, the highly expanded OR superfamily displays a striking degree of divergence (8, 9), suggesting that the detection of ant pheromones—and of CHCs in particular—is largely mediated by these diverse chemosensory receptors. In fact, the role of ORs in queen pheromone perception has already been confirmed in another eusocial hymenopteran— the honey bee Apis melifera (11). Functionally, insect OR complexes consist of an odorant coreceptor subunit (Orco), necessary for the trafficking and function of the complex, and a highly divergent “tuning OR” (ORx) that determines the odorant specificity of the complex (reviewed in ref. 10).
In Diptera, each odorant receptor neuron (ORN) is believed to generally express a single tuning Or gene, which determines the odorant specificity of the ORN, and each olfactory sensillum generally houses 1–4 ORNs (12). In contrast, ant sensilla are far more complex. In particular, female (worker) specific sensilla basiconica that have been shown to detect CHCs potentially contain in excess of 130 ORNs per sensillum (13).
Previous work has strongly implicated the nine-exon subfamily of ORs, which makes up nearly 30% of the 347 putative Or genes in H. saltator genome (HsOrs) and is highly expanded within the ant lineage (8, 9), in the detection of CHCs, based on their enrichment along the hydrocarbon-sensitive ventral portion of the worker antennae (14). Transgenic expression of a subset of these genes in Drosophila olfactory sensilla confers receptor-specific responses to a panel of CHCs. However, because other OR subfamilies are also expanded in ant lineages, there is the possibility that CHCs may also be detected by ORs outside of the nine-exon subfamily. To address this question, we have functionally characterized 25 distinct HsOrs spread across 9 OR subfamilies by using heterologous expression in Drosophila melanogaster antennal ORNs, which has proven to be amenable as an in vivo heterologous expression system for insect chemoreceptors. These receptors were further classified based on their enrichment in male versus worker antennae (9), because differentially abundant ORs are likely to underlie distinct pheromonal signaling pathways in ants. An understanding of the functional responses of these diverse receptors to multiple classes of compounds—CHC-associated hydrocarbons, mandibular gland components, and importantly, general odorants—provides significant insight into the chemical ecology of H. saltator that extends our understanding of the functionality of the expanded family of ant ORs.
Results and Discussion
To begin to understand the molecular components that facilitate the distinctive social interactions exhibited by different ant castes, we examined the responses of ORs to a variety of social and environmental stimuli. This endeavor was facilitated by the identification of the complete OR repertoire from several species of ants, which revealed that ants possess some of the largest tuning OR repertoires identified to date. The characterization of the odorant responses of these peripheral ORs represents the initial step in understanding the molecular processes that underlie the detection of CHCs and other semiochemicals by H. saltator. Although this report focuses on ORs, it is likely that additional non-OR chemosensory components may also play important roles in the perception of social pheromones.
We prioritized the characterization of HsOrs that showed enrichment in antennae of males and workers or which belong to OR subfamilies showing significant patterns of positive selection or gene birth and death in ants or eusocial hymenopterans (Fig. 1A) (8) because these subfamilies are potentially likely to encompass HsOrs with species-specific functionality often associated with pheromones. Within those parameters, preference was given to HsOrs that lie phylogenetically outside of the nine-exon subfamily in light of the functional characterization of 22 members of that HsOr subfamily in a parallel study (15). HsORs were tested against commercially available alkanes and other compounds known to be present on H. saltator cuticle or in exocrine glands and constituents of our in-house chemical screening library that encompass a selection of general odorants across diverse chemical classes that are commonly tested in insect olfactory systems. This base panel of ∼70 odorants spans a broad chemical space known to play a role in a diverse set of ant behaviors and would allow rapid identification of OR/ligand relationships with a high likelihood of biological relevance.
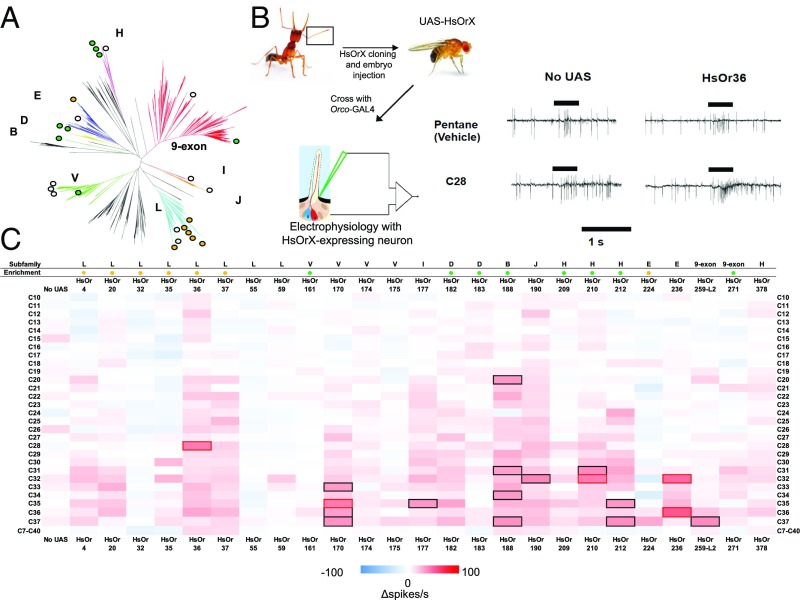
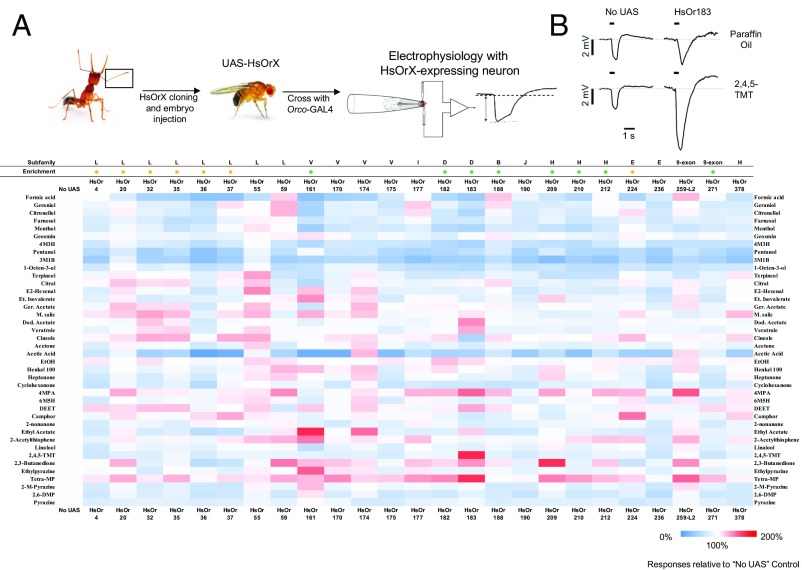
Fig. 1.
SSR responses of HsOR receptors to 28 cuticular hydrocarbons and a hydrocarbon mixture. (A) Summary of the phylogenetic relationship of the HsOr subfamilies and the receptors examined in this study. (B) Schematic summarizing SSR technique, along with sample traces comparing responses to heated control (pentane) with responses to heated cuticular hydrocarbons. (C) Heat map of responses in ab2A neurons to each hydrocarbon. Responses are calculated as the change in spike frequency induced by each stimulus, relative to the prestimulus spike frequency (after subtraction of the pentane control). The subfamily identity for each HsOr is indicated at the top, along with enhanced transcript abundance (9) for workers (orange dots) and males (green dots). Responses above 30 spikes per s are indicated by black boxes, and responses above 40 spikes per s are indicated by red boxes.
Responses to Cuticular Hydrocarbons in Single-Sensillum Drosophila Recordings.
We conducted an initial screen for hydrocarbon responses among our candidate HsOrs by assembling a stimulus panel of straight chain alkanes spanning C10 to C37 and testing them against transgenic flies expressing HsOrs of interest in ORNs where they can form functional heteromeric complexes with endogenous Orco coreceptors. Hydrocarbon stimuli were volatilized before application by using a brief heat pulse (Materials and Methods). We used single-sensillum recordings (SSRs) from individual antennal sensilla and found that the Drosophila ab2 sensillum displayed minimal background response to volatilized hydrocarbons or solvent (Fig. 1B), rendering this sensillum ideal for our investigations of HsOR-mediated hydrocarbon responses.
To sort potential CHC responses from nonresponses, we initially set a threshold of at least 30 spikes per s, which is six times higher than the spontaneous firing rate of ab2A (16). Using this response threshold, we identified 18 HsOR-ligand combinations across 9 HsORs that were responsive to alkane ligands (Fig. 1C). Further normalizing these responses by subtracting the “no-UAS” (i.e., from parental flies with only Orco-GAL4 containing chromosomes) control response for each hydrocarbon produced only minor changes in the overall results, with 17 of the 18 suprathreshold HsOR/hydrocarbon combinations exceeding the >30 spikes per s threshold (Dataset S1). Most strikingly, 17 of these 18 HsOR-hydrocarbon combinations were for hydrocarbons with a chain length of C28 or longer, suggesting a tuning bias toward longer-chain alkanes, consistent with our observation of odor coding within the nine-exon HsOr subfamily (15). The single exception was HsOr188, which showed a suprathreshold response to C20. It is noteworthy that this gene is the only known member of the ant OR subfamily B in H. saltator, which has relatively few members (1 to 2 genes) in all ant genomes examined thus far (8). The tight restriction of subfamily B members is maintained across species, suggesting they may have a highly conserved and relatively narrow role in ant chemosensory processes, although we can still only speculate as to whether that role is primarily as a detector for the shorter chain hydrocarbon C20. This sensitivity and others detailed in this report are interesting in light of the CHC biosynthetic pathways, which renders even-numbered straight-chain hydrocarbons generally much less abundant on insect cuticles than odd-numbered chains, although it must be stated that even low-abundance signals can function as powerful pheromones depending on the sensitivity of the corresponding receptor.
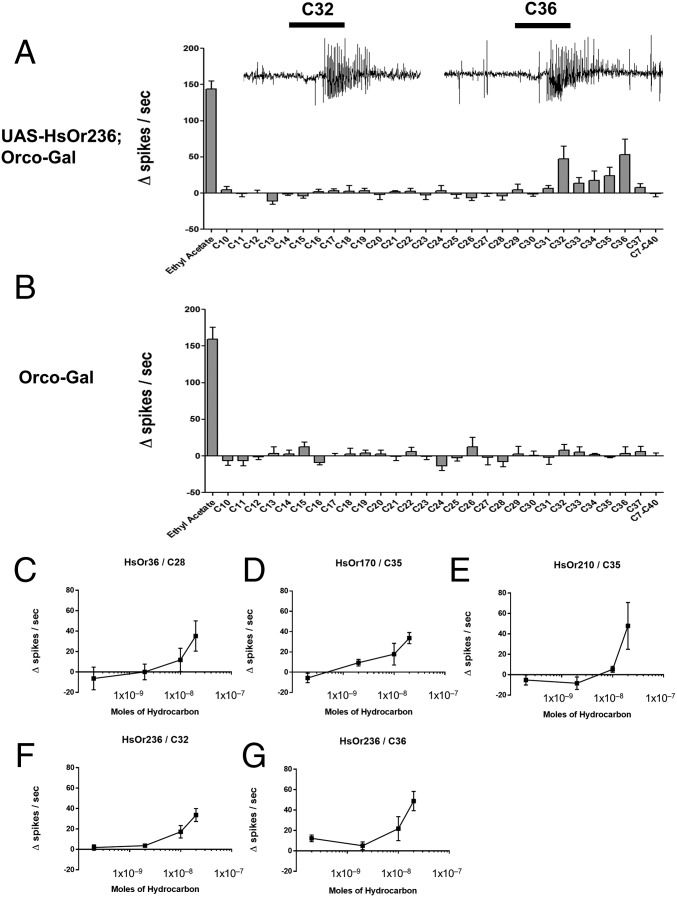
HsOr36, 210, 170, and 236 responded robustly to hydrocarbon stimuli with chain lengths of C28 or longer. HsOr36, a subfamily L receptor whose transcript shows an ∼6.4-fold enrichment in male antennae over worker antennae (9), was the most intriguing. HsOr36 responded strongly and specifically to C28 at >40 spikes per s, with no other responses that reached our 30 spikes per s threshold. In contrast, another subfamily H receptor, HsOr210, showed a highly significant, 46-fold enrichment in worker antennae compared with males and a suprathreshold response to C32. The third receptor in question, HsOr170, is a subfamily V receptor with low and equal mRNA levels in antennae of workers and males, which elicited a suprathreshold response to C35 along with responses slightly below the 30 spike per s cutoff to C33, C36, and C37. Finally, the fourth receptor, HsOr236, is a subfamily E receptor that also showed equal transcript abundance between antennae of workers and males. However, the response profile of HsOr236 was remarkable in having two distinct responses above 40 spikes per second to even-numbered alkanes—one to C32, and another to C36 (Fig. 2A). These responses were clearly absent in control lines without the UAS-HsOr236 transgene (Fig. 2B). As an additional validation, all receptor-ligand combinations above 40 spikes per s (including HsOr236 and C36) were retested within a dose–response paradigm, revealing a clear concentration dependency (Fig. 2 C–G).
Fig. 2.
Characteristic response of an HsOR receptor to cuticular hydrocarbons by SSR. (A) Example of an odorant receptor, HsOr236, which shows responses to specific CHCs by using the heated-puffing protocol. (B) Normal Drosophila ORNs show no response to CHCs. Ethyl acetate (the leftmost odorant shown) is a control odorant that activates the native Drosophila odorant receptor in the ab2A neuron (n = 5 for A and B, and error bars are SEM). (C–G) CHC responses shows dose dependency. The response of all receptor-hydrocarbon combinations >40 spikes per s in Fig. 1 were retested across four different doses: 0.2, 2, 10, and 20 nmol (n = 5, and error bars are SEM). In each case, the magnitude of response showed a clear correlation with the dose of hydrocarbon stimulus.
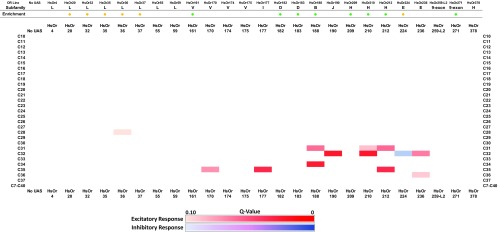
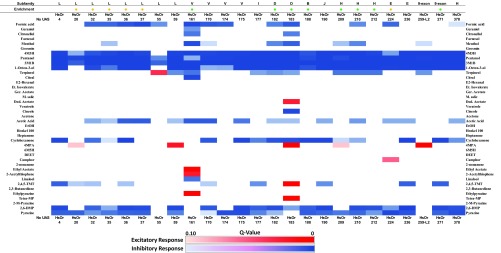
We next conducted a more quantitative analysis to assess significant differences in HsOR-mediated alkane responses compared with no-UAS controls by using a parametric one-way ANOVA with correction for multiple comparisons and a two-stage step-up method (17) at a 0.10 false discovery rate (FDR). Using this criteria, we identified nine HsOrs outside of the nine-exon subfamily that mediate significant excitatory (8) or inhibitory (1) responses to hydrocarbon stimuli (Fig. S1). A caveat to analyzing large electrophysiological datasets using strict statistical analysis that corrects for many comparisons is that potentially meaningful discoveries may be overlooked because of modest replication number. It is noteworthy that although no hydrocarbon responses were identified in the nine-exon subfamily through this quantitative analysis, we found that HsOr259-L2 had a sixfold higher response to C37 relative to controls.
Fig. S1.
Statistical analysis of SSR responses to CHCs. Analysis was conducted by using one-way ANOVA, followed by correction for multiple comparisons using a two-stage FDR method (17) with a threshold of q < 0.10. Significant excitatory responses are indicated in red, and inhibitory hits are indicated in blue.
Nevertheless, this broader analysis further supports and indeed extends our observation that HsOR-mediated responses to hydrocarbons are not, as previously hypothesized (8, 9, 14), restricted to the nine-exon HsOr gene subfamily. Furthermore, within the subset of statistically significant responses, we discovered a strong bias toward the longer chain alkanes commonly found in H. saltator CHCs (5, 18). Indeed, the majority of CHCs that have thus far been identified on cuticles of H. saltator workers and reproductives are between 28 and 37 carbons in length, although a CHC with 23 carbons has been reported (18), and antennal responses to hydrocarbons as small as decane (C10) have been observed from antennae of workers (19). This observation is consistent with the current paradigm that hydrocarbons play an essential role in signaling colony membership, social status, or other characteristics. If the long chain-sensitive HsORs characterized here function as biological detectors of CHC-based social pheromones, it would make sense that their sensitivity would mirror the narrow range of CHCs which H. saltator actually produces. Alternatively, it is possible that the molecular receptors for biologically salient short-chain hydrocarbons are among the HsOrs that remain functionally uncharacterized.
Another interesting aspect of our study is the significant sensitivity to hydrocarbons within a distinctive group of male-enriched HsOrs. This result would suggest that CHCs are not only used as pheromones to regulate social interactions between workers, gamergates, and queens in H. saltator, but may also be used to regulate social interactions between reproductive females and males (i.e., mating pheromones) or perhaps another class of semiochemicals with particular relevance to male biology. This finding is consistent with the recent report that CHCs are extensively used as sex pheromones throughout the Hymenoptera (20).
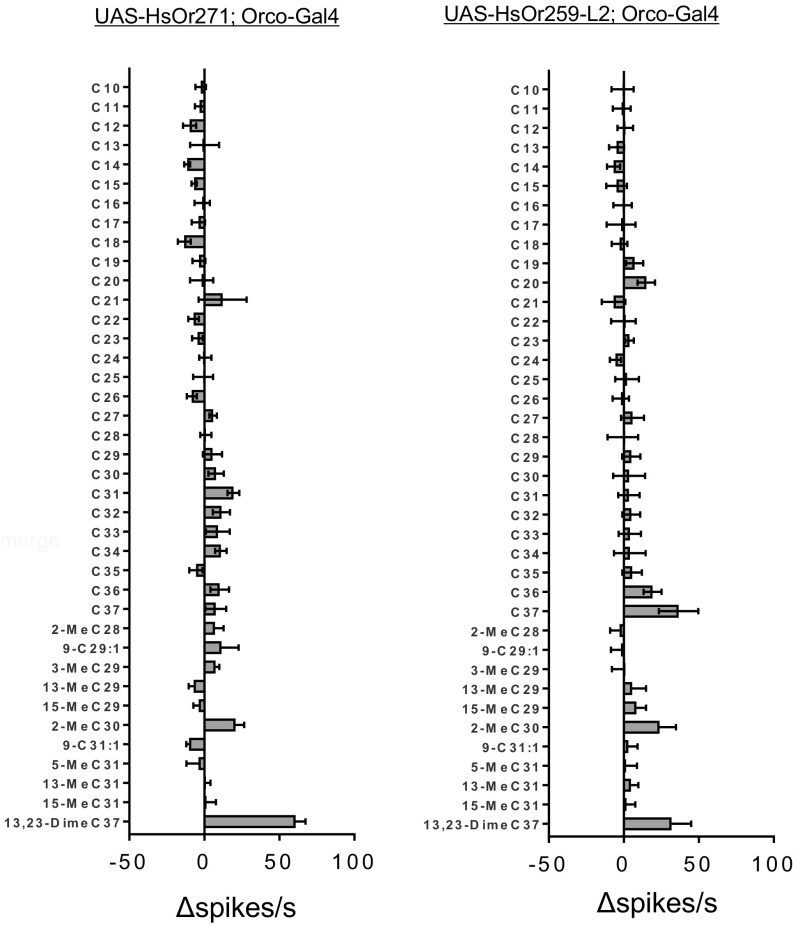
To expand the range of hydrocarbons in our odorant panel, we obtained 11 different alkenes and custom-synthesized methyl-branched hydrocarbons that are found among Harpegnathos CHCs (5, 18). These hydrocarbons were initially used to test responses from the two nine-exon HsORs in our receptor collection, which, based on phylogenetic and transcriptomic considerations, is hypothesized to be the HsOr gene subfamily most likely to detect CHC pheromones involved in eusociality (8, 9, 14). Of these receptors, HsOr271 displayed a strong response (60 spikes per s, Fig. 3) to 13,23-dimethyl-C37, which has been implicated as part of the fertility signal in H. saltator (i.e., the “queen pheromone”) (18). The expression of HsOr271, as is the case for many of the nine-exon receptors, is consistent with a role in the detection of reproductives by workers, as it is enriched ∼175-fold in the antennae of workers relative to antennae of males (with fragments per kilobase million values of 24.7404 and 0.14068, respectively) (9). In addition to HsOr271, a newly identified paralog of the nine-exon family member HsOr259, HsOr259-L2, also displayed a weaker response to the 13,23-dimethyl-C37 component of the fertility signal (31.8 spikes per second; Fig. 3), although it should be noted that this particular receptor showed a similarly strong response to C37 (36.6 spikes per second). These results suggest there may be multiple receptors with some level of tuning/sensitivity to this dimethyl queen pheromone, perhaps reflective of combinatorial interactions for gradient navigation and strong and redundant sensitivity to this important semiochemical.
Fig. 3.
Responses of two nine-exon HsOR receptors to a panel of branched-chain alkanes and alkenes. The 11 alkenes and branched-chain alkanes tested are known constituents on Harpegnathos worker and/or gamergate cuticle, including the queen pheromone 13,23-dimethylheptatriacontane. n = 5, and error bars are SEM.
General Odorant Responses in Drosophila Electroantennogram Recordings.
To expand our analysis beyond hydrocarbons, we next examined nine-exon and nonnine-exon HsOr-mediated responses to a stimulus panel comprising an additional 40 non-CHC volatiles across a broad range of general chemical space. To accomplish this survey, we used a whole-field electroantennogram (EAG) recording paradigm that provides high-throughput ability to broadly survey the whole antennae for physiological responses. Although both EAGs and SSRs reveal stimulation and inhibition of antennal ORNs (Dataset S1), it is important to note that our SSRs were narrowly focused on the ab2A ORN, which endogenously expresses DmOr59b. In Drosophila, DmOr59b is a broadly tuned receptor responding to many general odorants that, in this context, would mask the activity of exogenous HsOr transgenes (21). EAGs also allowed us to more fully exploit the ability to express HsOr transgenes throughout the antennae. Furthermore, the constituents of the general odor panel are much more volatile than CHCs, facilitating their delivery to the antennae as headspace volatiles. This feature removed the constraint of heat-assisted delivery that is required for CHCs and which generates significant whole antennal background activity.
As expected, the raw EAG responses were generally positive for all stimuli tested, likely due to the endogenous activity of the Drosophila chemosensory system that can be seen in the no-UAS parental background control antennae. To account for these responses, we used an additional level of normalization by subtracting the responses of the endogenous Drosophila receptors in the antenna in the Orco-Gal4 background from the stimulus responses (Fig. 4). After this normalization, the responses to most odorant stimuli were remarkably consistent across transgenic fly lines, with nearly all UAS-HsOr transgenes, notably including the two nine-exon HsORs in our test panel (HsOr259-L2 and HsOr271), facilitating odorant responses that were greater than (stimulatory) or, in many instances, less than (inhibitory) endogenous responses observed in the Orco-Gal4 background flies (within ±50%). That said, even with this treatment, several potential artifacts must be acknowledged. First, the simplest carboxylic acids—methanoic (formic) and ethanoic (acetic) acid—showed significantly reduced (inhibitory) responses relative to diluent alone control (paraffin oil), which given their intensity, potentially reflect recording artifacts induced in the antennae and/or recording electrodes by the chemical nature of these acids, a phenomenon that has been occasionally reported by other groups (22). This observation can likely be attributed to the high volatility of these acids that potentially could give rise to massive, nonbiologically relevant, odorant concentrations being delivered to the antenna. This effect may be exacerbated by the low solubility of such polar compounds in the paraffin oil diluent. Second, pentanol elicited the highest response observed to any odorant in most of the lines tested, that varied from two to five times the paraffin oil control response (before normalization to the Orco-Gal4 control). We attribute this response to the placement of the glass recording electrode proximate to the distal end of the Drosophila antenna, which contains high numbers of the pentanol-responsive at2 sensillum (23).
Fig. 4.
EAG responses of HsOR receptors to volatile odorants. (A) Schematic summarizing electroantennogram recording technique, along with sample traces comparing responses to diluent alone (paraffin oil) and the volatile odorant 2,4,5-trimethylthiazole. (B) Heat map of EAG responses. Data shown is the same as Fig. S2, with an additional round of normalization to the no-UAS control line. Responses are a percentage value relative to the no-UAS line.
In advance of quantitative analyses, several aspects of these odorant responses bear discussion. Most notably, four HsOrs displayed stimulatory responses >1.6 times greater than the Orco-Gal4 parental control flies (Fig. 4). HsOr59 (a subfamily L receptor), HsOr161 (a subfamily V receptor that is 5.6 times enriched in worker versus male antennae) (9), HsOr183 (subfamily D), and HsOr209 (subfamily H) exhibited the largest responses to this panel of 40 generic volatile odorants compared with Orco-Gal4 endogenous control flies. HsOr161 transgenes elicited robust responses to ethyl acetate that were ∼1.7 fold higher than those of Orco-Gal4 parental control flies, and significant inhibitory responses (<50% of the endogenous response) to the Harpegnathos mandibular gland pheromone 3-methyl-1-butanol (3M1B) (5) and the plant-based insect repellents menthol, citronellol, geraniol, and citral (Fig. 4). Inhibition by some ligands and activation by others is a common phenomenon among chemoreceptors and could indicate that HsOr161 is a relatively broadly tuned receptor. In contrast, transgenic flies expressing antennal HsOr59 were significantly stimulated by citronellol and geraniol as well as formic acid (a formicine ant alarm pheromone) and 2,3-butanedione, further demonstrating that our EAG system is not intrinsically biased against detecting stimulatory responses.
Similarly, HsOr183 exhibited strong responses to 2,3,5,6-tetramethylpyrazine (Tetra-MP or ligustrazine) and 2,4,5-trimethylthiazole, whereas 2,3-butanedione elicited strong responses from HsOr209. We also examined responses to the widely used insect repellent N,N-diethyl-meta-toluamide (DEET), which has been proposed to act as both an activator and an inhibitor of insect ORs. DEET elicited only modest responses from a subset of the HsOrs tested here. Of these receptors, HsOr183 showed the strongest response to the chemical (an increase of ∼28% relative to no-UAS control).
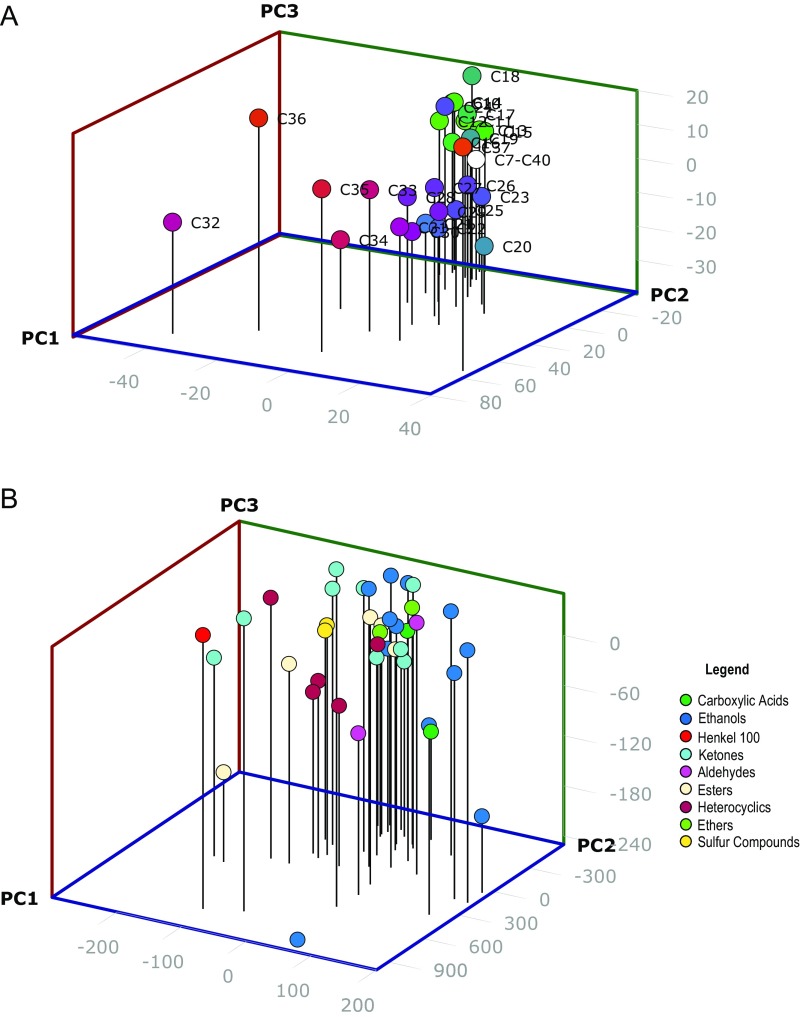
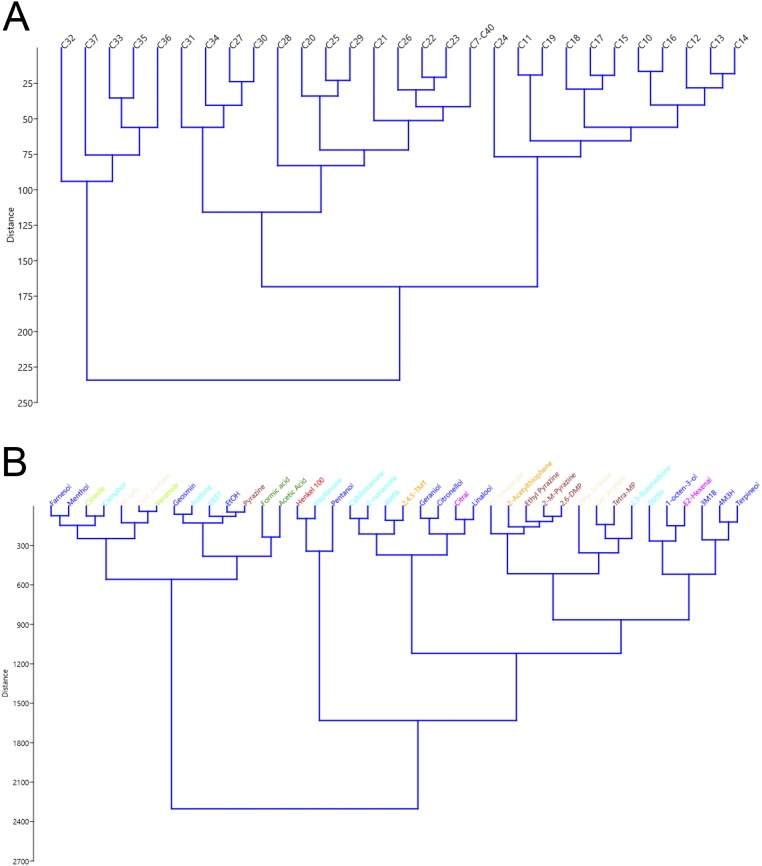
To examine general odorant response data more quantitatively, we tested for significantly different responses to general odorants between the no-UAS control and each HsOr. As before, we used a parametric one-way ANOVA with FDR correction. This analysis yielded far more significant inhibitory responses and a surprising number of excitatory responses (Fig. S2). Indeed, the broad range of significant inhibitory responses extended across all of the HsOrs tested. Whereas odor-evoked inhibitory responses have biological significance insofar as odor coding, it is also possible that a fraction of the widespread inhibition seen in this analysis is an artifact of the overexpression of HsOr transgenes across all antennal ORNs. To better understand the variance in our odorant response profiles, we also carried out a principal component analysis (PCA) of all of the HsOr-mediated responses to the different classes of ligands tested (SI Materials and Methods, Figs. S3 and S4, Table S1, and Dataset S2).
Fig. S2.
Statistical analysis of EAG responses to general odorants. Analysis was conducted by using one-way ANOVA, followed by correction for multiple comparisons using a two-stage FDR method (17) with a threshold of q < 0.10. Significant excitatory responses are indicated in red, and inhibitory hits are indicated in blue.
Fig. S3.
PCA of variance in ligand responses. The first three principal components are shown for the CHC response data (A) and the general odorant response data (B). The CHC responses show a particularly clear pattern of segregation along the principal component 1 (PC1) and principal component 2 (PC2) axes, with the longer-chain CHCs generally positioned toward the front-left part of the graph and the shorter-chain CHCs positioned more toward the rear-right section of the graph. For consistency, analyses in both A and B were performed on data before background subtraction of the Orco-Gal4 response.
Fig. S4.
Hierarchical cluster analysis of odorant responses to ligands. Analysis was performed in PAST 3, based on the diluent-adjusted data for the SSR responses to CHCs (A) and the EAG responses to general odorants (B). Distances are based on Euclidean distances between ligands.
Table S1.
Percentage of total variation accounted for by each principal component
| Principal component | Variance (SSR), % | Variance (EAG), % |
| 1 | 49.824 | 81.965 |
| 2 | 9.071 | 5.3733 |
| 3 | 7.8264 | 4.4292 |
| 4 | 6.5168 | 2.4759 |
| 5 | 4.9327 | 1.5678 |
| 6 | 3.9549 | 1.1542 |
| 7 | 3.3621 | 0.73217 |
| 8 | 3.075 | 0.38766 |
| 9 | 2.3536 | 0.35369 |
| 10 | 1.6465 | 0.3157 |
| 11 | 1.4048 | 0.26067 |
| 12 | 1.1818 | 0.18701 |
| 13 | 1.0174 | 0.1676 |
| 14 | 0.74656 | 0.13216 |
| 15 | 0.65659 | 0.095231 |
| 16 | 0.51317 | 0.087467 |
| 17 | 0.42462 | 0.07727 |
| 18 | 0.36747 | 0.07125 |
| 19 | 0.27962 | 0.051365 |
| 20 | 0.18266 | 0.031849 |
| 21 | 0.14629 | 0.025577 |
| 22 | 0.10462 | 0.019361 |
| 23 | 0.070552 | 0.016137 |
| 24 | 0.066399 | 0.011878 |
| 25 | 0.009485 | 0.006756 |
| 26 | 0.003646 | 0.004244 |
Overall, it is notable that the HsOrs showing the strongest EAG responses to general odorants are distinct from those that exhibited the strongest SSR responses to hydrocarbons. Thus, whereas HsOrs as a whole are broadly tuned to both volatile odorants and hydrocarbons, individual receptors appear to be relatively narrowly tuned to specific ligands. This observation is consistent with the lack of widespread EAG responses to the “Henkel 100,” a standardized mixture of 100 distinct general odorants that might be predicted to robustly activate broadly tuned receptors. In contrast, in our studies this blend elicited only a modest activation (never exceeding ∼20% increase over the native no-UAS response) for a single nine-exon family member (HsOr259L2) and five nonnine-exon HsOrs (HsOr59, 161, 174, 182, 209), none of which were quantitatively significant across the entire panel of HsOrs tested. Because the H. saltator genome contains almost 400 HsOr genes, the combinatorial capacity of this repertoire is likely to collectively provide broad coverage across the biologically relevant odor space although individual receptors, when viewed unilaterally, may be narrowly tuned.
Conclusions
This study provides additional support for the further development of H. saltator as an insect model for the study of the molecular basis of olfactory signaling, pheromone detection, and more broadly, the underlying mechanistic bases for social behavior. A significant aspect of those questions focuses on the role of peripheral chemosensory receptors, with particular emphasis on the rapidly evolving OR superfamily that is greatly expanded in the genomes of highly social insects. Among those HsOrs we interrogated with short and long-chain hydrocarbons, the response of the male-enriched odorant receptor HsOr36 is perhaps the most intriguing, given that male ants are generally thought to have little social interaction with the rest of the colony outside of mating. Octacosane (C28), a strong ligand for HsOr36, has no specifically defined role in Harpegnathos; although it is found on the cuticle of Harpegnathos workers and reproductives (18), and on the cuticles of distantly related ants such as Linepithema humile (24), the absolute abundance is likely quite low because of the biosynthetic constraints on even-numbered carbon chains.
Although it is unknown whether Harpegnathos female reproductives actually use octacosane or other CHCs as sex pheromones to attract males, it should be recognized that male ants are often promiscuous in their mating choices. In fact, males from some ant species will even mate with heterospecific queens—a fact that is often exploited by such queens to produce additional sterile workers (25). The response of the nine-exon receptor HsOr271 to the queen pheromone 13,23-dimethyl-C37 is also notable, although it is also possible that there are multiple, redundant receptors within the nine-exon subfamily tuned specifically for this critical compound.
Robust responses to CHC extracts and a panel of hydrocarbons found in H. saltator were observed among the majority of nine-exon HsOrs tested in a parallel study although only one (HsOr259-L2) of the two nine-exon receptors that were in our HsOr panel responded strongly to a CHC (to C37). In contrast, several of the other 23 HsOrs examined in this study, representing a diverse range of the other OR subfamilies of HsORs, also display significant responses to these CHC-associated ligands. Although responses to volatile nonhydrocarbon general odorants were also sparse and well-distributed phylogenetically across all of the OR subfamilies tested including the nine-exon ORs, they nevertheless encompassed different receptors from the ones that responded robustly to hydrocarbons.
In light of their complex phylogenetic structure and the sheer number of uncharacterized HsOrs, it is difficult to draw firm conclusions, but it nevertheless seems reasonable that absolute and inviolate odor-coding boundaries for ant OR subfamilies in relation to pheromonal and nonpheromonal stimuli do not exist. These questions are further complicated by the likelihood that additional membrane proteins and other factors may be required in order for pheromone ligands to elicit responses, as has been observed with the Drosophila pheromone receptor Or67d (26). The discriminatory power afforded by the combinatorial interactions of the large numbers of ant ORs, acting in concert with other chemosensory components, most notably the IR and GR gene families, seems more than capable of addressing the extraordinary challenges associated with the complex chemical ecology of eusocial colonies. That said, by analyzing members of distinct subfamilies of HsOrs beyond the highly expanded nine-exon subfamily and those with differential abundance among castes and genders, this study represents a quantum advance in the study of the molecular genetics of these critical peripheral chemoreceptors that are responsible for initiating many, if not all, of the distinct social behaviors that are the hallmark of these eusocial insects.
Materials and Methods
Odorant Receptor Cloning.
Full-length HsOr genes were subcloned or commercially synthesized (Genscript) for transgenic expression of HsOr genes in flies by insertion into a preexisting insertion site in the Drosophila genome, using the phiC31 integrase recombination system (27). See SI Materials and Methods for full details.
Drosophila Genetics.
For SSR and EAG experiments, experimental D. melanogaster genotypes were either w1118; w+, UAS-HsOr; w+, Orco-GAL4 or w1118; + ; w+, UAS-HsOr/w+, Orco-GAL4. Control flies were w1118; +; w+, Orco-GAL4.
Electrophysiology.
Flies were tested 2–10 d after eclosion for both single-sensillum and whole antennal EAG recordings, with an n = 4–8 per UAS-HsOrX line. We then manually normalized those responses to the Orco-GAL4 control. For SSRs, the ab2 sensillum-type was used for all recordings. Each compound (Table S2) was dissolved in pentane and 20 nmol of the compound was applied to each delivery cartridge. The cartridges were then heated for 1 s with a handheld butane torch, and then air was puffed through the heated cartridge into an airstream, and over the fly antenna for a 500-ms duration, using 3 mL of humidified air. See SI Materials and Methods for additional details.
Table S2.
List of odorants used in this study, their abbreviations, and Chemical Abstracts Service (CAS) registry numbers
| Abbreviation | Full name | CAS no. |
| Formic acid | Formic acid | 64-18-6 |
| Geraniol | Geraniol | 106-24-1 |
| Citronellol | Citronellol | 106-22-9 |
| Farnesol | Farnesol | 4602-84-0 |
| Menthol | Menthol | 89-78-1 |
| Geosmin | Geosmin | 16423-19-1 |
| 4M3H | 4-Methyl-3-heptanol | 14979-39-6 |
| Pentanol | Pentanol | 71-41-0 |
| 3M1B | 3-Methyl-1-butanol | 123-51-3 |
| 1-Octen-3-ol | 1-Octen-3-ol | 3391-86-4 |
| Terpineol | Terpineol | 98-55-5 |
| Citral | Citral | 5392-40-5 |
| E2-Hexenal | trans-2-Hexenal | 6728-26-3 |
| Et. isovalerate | Ethyl isovalerate | 108-64-5 |
| Ger. acetate | Geranyl acetate | 105-87-3 |
| M. salic | Methyl salicylate | 119-36-8 |
| Dod. acetate | Dodecyl acetate | 112-66-3 |
| Veratrole | Veratrole | 91-16-7 |
| Cineole | Cineole | 470-82-6 |
| Acetone | Acetone | 67-64-1 |
| Acetic acid | Acetic acid | 64-19-7. |
| EtOH | Ethanol | 64-17-5 |
| Henkel 100 | Henkel 100 | Not available |
| Heptanone | 2-Heptanone | 110-43-0 |
| Cyclohexanone | Cyclohexanone | 108-94-1 |
| 4MPA | 4-Methoxyphenylacetone | 122-84-9 |
| 6M5H | 6-Methyl-5-hepten-2-one | 110-93-0 |
| DEET | DEET | 134-62-3 |
| Camphor | Camphor | 76-22-2 |
| 2-Nonanone | 2-Nonanone | 821-55-6 |
| Ethyl acetate | Ethyl acetate | 141-78-6 |
| 2-Acetylthiophene | 2-Acetylthiophene | 88-15-3 |
| Linalool | Linalool | 78-70-6 |
| 2,4,5-TMT | 2,4,5-Trimethylthiazole | 13623-11-5 |
| 2,3-Butanedione | 2,3-Butanedione | 431-03-8 |
| Ethylpyrazine | Ethylpyrazine | 13925-00-3 |
| Tetra-MP | Tetramethylpyrazine | 1124-11-4 |
| 2-M-Pyrazine | 2-Methylpyrazine | 109-08-0 |
| 2,6-DMP | 2,6-Dimethylpyrazine | 108-50-9 |
| Pyrazine | Pyrazine | 290-37-9 |
| C5 | Pentane | 109-66-0 |
| C10 | n-Decane | 124-18-5 |
| C11 | n-Undecane | 1120-21-4 |
| C12 | n-Dodecane | 112-40-3 |
| C13 | n-Tridecane | 629-50-5 |
| C14 | n-Tetradecane | 629-59-4 |
| C15 | n-Pentadecane | 629-62-9 |
| C16 | n-Hexadecane | 544-76-3 |
| C17 | n-Heptadecane | 629-78-7 |
| C18 | n-Octadecane | 593-45-3 |
| C19 | n-Nonadecane | 629-92-5 |
| C20 | n-Icosane | 112-95-8 |
| C21 | n-Heneicosane | 629-94-7 |
| C22 | n-Docosane | 629-97-0 |
| C23 | n-Tricosane | 638-67-5 |
| C24 | n-Tetracosane | 646-31-1 |
| C25 | n-Pentacosane | 629-99-2 |
| C26 | n-Hexacosane | 630-01-3 |
| C27 | n-Heptacosane | 593-49-7 |
| C28 | n-Octacosane | 630-02-4 |
| C29 | n-Nonacosane | 630-03-5 |
| C30 | n-Triacontane | 638-68-6 |
| C31 | n-Hentriacontane | 630-04-6 |
| C32 | n-Dotriacontane | 544-85-4 |
| C33 | n-Tritriacontane | 630-05-7 |
| C34 | n-Tetratriacontane | 14167-59-0 |
| C35 | n-Pentatriacontane | 630-07-9 |
| C36 | n-Hexatriacontane | 630-06-8 |
| C37 | n-Heptatriacontane | 7194-84-5 |
| C7-C40 | C7 to C40 Mixture | Not available |
| 2-MeC28 | 2-Methyloctacosane | 1560-98-1 |
| 9-C29:1 | (Z)-9-Nonacosene | 36258-10-3 |
| 3-MeC29 | 3-Methylnonacosane | 14167-67-0 |
| 13-MeC29 | 13-Methylnonacosane | 7371-98-4 |
| 15-MeC29 | 15-Methylnonacosane | 65820-60-2 |
| 2-MeC30 | 2-Methyltriacontane | 1560-72-1 |
| 9-C31:1 | (Z)-9-Hentriacontene | 56987-72-5 |
| 5-MeC31 | 5-Methylhentriacontane | 71502-24-4 |
| 13-MeC31 | 13-Methylhentriacontane | 33116-06-2 |
| 15-MeC31 | 15-Methylhentriacontane | Not available |
| 13,23-DimeC37 | 13,23-Dimethylheptatriacontane | Not available |
SI Principal Component and Clustering Analysis of Receptor Responses
To better understand the variance in our odorant response profiles, we carried out a PCA of all of the HsOr-mediated responses to the different classes of ligands tested. Overall, for the SSR data with just the volatilized hydrocarbons, more than 66% of the variation in hydrocarbon responses is explained by just three components; of that, nearly 50% is explained by just the first principal component (Table S1). The first three components were even more critical for the EAG data with the non-CHC odorants, with just over 92% of the variation explained by the top three components and almost 82% explained by the first component alone (Table S1). Interestingly, both the PCA analysis (Fig. S3) and an additional hierarchical cluster analysis (Fig. S4) of the CHC data shows segregation of the hydrocarbons based on chain length, perhaps reflecting (in part) the overall lack of response to shorter chain hydrocarbons among the receptors tested. The non-CHC odorants, by contrast, show tight grouping of the strongest endogenous activators of the antenna (pentanol, heptanone, and Henkel 100) in the hierarchical clustering analysis (Fig. S4), and both analyses revealed distinct clusters for the carboxylic acids, sulfur compounds, and four of the five heterocyclics/pyrazines (Figs. S3 and S4). Similar to previous results in dipterans (23), however, we observed widespread intermingling of chemical classes by using both methods, consistent with the idea that chemical class is only one variable in the overall set of characteristics that determines the sensitivity of odorant receptors.
SI Materials and Methods
Odorant Receptor Cloning.
The cloning of the HsOr genes followed standard protocols. Full-length Or coding sequences were PCR-amplified from adult worker or male antennal cDNA and cloned by using the Gateway system (Life Technologies), or commercially synthesized (Genscript). For transgenic expression of HsOr genes in flies, we have subcloned our genes into a pUAS plasmid that allows for the insertion of our transgenes into a preexisting insertion site in the Drosophila genome, using the well-established phiC31 integrase recombination system (27).
Drosophila Genetics.
For SSR and EAG experiments, experimental D. melanogaster genotypes were either w1118; w+, UAS-HsOr; w+, Orco-GAL4 or w1118; + ; w+, UAS-HsOr/w+, Orco-GAL4. Control flies were w1118; +; w+, Orco-GAL4. Embryo injections to generate UAS-HsOr lines was outsourced to a commercial injection service (Rainbow Transgenic Flies).
Electrophysiology.
Flies were tested 2–10 d after eclosion for both single-sensillum and whole antennal EAG recordings, with an n = 4–8 per UAS-HsOrX line. For SSRs, odorants and volatilized hydrocarbons were puffed by using a Syntech CS-05 Stimulus Flow Controller (Ockenfels SYNTECH). The ab2 sensillum-type was used for all recordings and was confirmed by using a “diagnostic odorant” panel consisting of paraffin oil (negative control), ethyl acetate, geranyl acetate, 1-octen-3-ol, 2-heptanone, and (E)-2-hexenal. Responses were measured from the ab2A neuron, which can be separated from ab2B neurons based on spike amplitude (16). Each odorant was diluted to 0.01 M in paraffin oil, and 20 µL of the resulting solution was loaded into each delivery cartridge. For hydrocarbons, each compound was dissolved in pentane and 20 nmol of the compound was applied to each delivery cartridge, with the pentane solvent allowed to evaporate. The cartridges were then heated for 1 s with a handheld butane torch (BernzOmatic, Worthington Industries), then air was puffed through the heated cartridge into an airstream and over the fly antenna for a 500-ms duration of 3 mL of humidified air. There was an ∼300-ms delay between the initiation of the odorant puff and the odorant actually reaching the antenna.
Odorant responses were calculated by counting spike frequencies (from the ab2A neuron) in response to odorant stimulation, although there is mounting evidence that changes in spike amplitude and local field potentials can also carry olfactory information in certain cases (28). To maintain uniformity in analyses, the response was calculated by manually counting spikes in a 200-ms period between 400 and 600 ms after initiation of the stimulus, which (because of the 300-ms delay) lies within the stimulus window for the 500-ms puff. The response during the 200-ms period was multiplied by 5 to convert the response rate to spikes per s, and the prestimulus spiking rate during the 1,000 ms directly preceding the odorant puff was subtracted from the response rate to obtain the response relative to baseline.
EAG recordings were performed according to a protocol previously described by another group (21), with odorants diluted 1:100 by volume in paraffin oil. The EAG software (EAG2000, Ockenfels SYNTECH) initially normalizes the data to the paraffin oil control to account for day-to-day/prep-to-prep variation, responses to the solvent, and decay in the sensitivity of the antennae over the duration of the assay. We then manually normalized those responses to the Orco-GAL4 control.
Principal Component and Clustering Analysis of Receptor Responses.
PCA and hierarchical cluster analysis were performed in PAST 3 (29), based on the vehicle-adjusted data for each group of recordings. PCA was performed by using the variance-covariance matrix, and cluster analysis was performed by using Ward’s method to calculate Euclidean distance.
Statistical Analysis.
Statistical significance of HsOR-mediated ligand responses compared with no-UAS controls was calculated in GraphPad Prism (GraphPad Software) by using a parametric one-way ANOVA with correction for multiple comparisons using the two-stage step-up method of Benjamini, Krieger, and Yuketieli (17) at a 0.10 FDR.
Supplementary Material
Acknowledgments
We thank Zi Ye and Samuel Ochieng for technical support and members of the L.J.Z. laboratory and Roberto Bonasio, Claude Desplan, D. Patrick Abbot, and R. Jason Pitts for their thoughtful insights and help with statistics. We also thank other members of the Howard Hughes Medical Institute Collaborative Innovation Award (HCIA) Ant Epigenetics Group for valuable feedback during the design and implementation of these experiments, and for comments on this manuscript. Funding for this work was provided by HCIA 2009005 (to D.R., S.L.B., J.L., A.R., and L.J.Z.) and by Vanderbilt University (L.J.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1704647114/-/DCSupplemental.
References
- 1.Crespi BJ, Yanega D. The definition of eusociality. Behav Ecol. 1995;6:109–115. [Google Scholar]
- 2.Monnin T. Chemical recognition of reproductive status in social insects. Ann Zool Fenn. 2006;43:515–530. [Google Scholar]
- 3.Gibbs AG. Water-proofing properties of cuticular lipids. Am Zool. 1998;38:471–482. [Google Scholar]
- 4.van Zweden JS, Dreier S, d’Ettorre P. Disentangling environmental and heritable nestmate recognition cues in a carpenter ant. J Insect Physiol. 2009;55:158–163. doi: 10.1016/j.jinsphys.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.do Nascimento RR, Billen J, Morgan ED. The exocrine secretions of the jumping ant Harpegnathos saltator. Comparative Biochemistry and Physiology Part B: Comparative Biochemistry. 1993;104:505–508. [Google Scholar]
- 6.Dosmann A, Bahet N, Gordon DM. Experimental modulation of external microbiome affects nestmate recognition in harvester ants (Pogonomyrmex barbatus) PeerJ. 2016;4:e1566. doi: 10.7717/peerj.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peeters C, Hölldobler B. Reproductive cooperation between queens and their mated workers: The complex life history of an ant with a valuable nest. Proc Natl Acad Sci USA. 1995;92:10977–10979. doi: 10.1073/pnas.92.24.10977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou X, et al. Chemoreceptor evolution in hymenoptera and its implications for the evolution of eusociality. Genome Biol Evol. 2015;7:2407–2416. doi: 10.1093/gbe/evv149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhou X, et al. Phylogenetic and transcriptomic analysis of chemosensory receptors in a pair of divergent ant species reveals sex-specific signatures of odor coding. PLoS Genet. 2012;8:e1002930. doi: 10.1371/journal.pgen.1002930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suh E, Bohbot J, Zwiebel LJ. Peripheral olfactory signaling in insects. Curr Opin Insect Sci. 2014;6:86–92. doi: 10.1016/j.cois.2014.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wanner KW, et al. A honey bee odorant receptor for the queen substance 9-oxo-2-decenoic acid. Proc Natl Acad Sci USA. 2007;104:14383–14388. doi: 10.1073/pnas.0705459104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stocker RF. The organization of the chemosensory system in Drosophila melanogaster: A review. Cell Tissue Res. 1994;275:3–26. doi: 10.1007/BF00305372. [DOI] [PubMed] [Google Scholar]
- 13.Nakanishi A, Nishino H, Watanabe H, Yokohari F, Nishikawa M. Sex-specific antennal sensory system in the ant Camponotus japonicus: Structure and distribution of sensilla on the flagellum. Cell Tissue Res. 2009;338:79–97. doi: 10.1007/s00441-009-0863-1. [DOI] [PubMed] [Google Scholar]
- 14.McKenzie SK, Fetter-Pruneda I, Ruta V, Kronauer DJ. Transcriptomics and neuroanatomy of the clonal raider ant implicate an expanded clade of odorant receptors in chemical communication. Proc Natl Acad Sci USA. 2016;113:14091–14096. doi: 10.1073/pnas.1610800113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pask GM, et al. Specialized odorant receptors in social insects that detect cuticular hydrocarbon cues and candidate pheromones. Nat Commun. doi: 10.1038/s41467-017-00099-1. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.de Bruyne M, Foster K, Carlson JR. Odor coding in the Drosophila antenna. Neuron. 2001;30:537–552. doi: 10.1016/s0896-6273(01)00289-6. [DOI] [PubMed] [Google Scholar]
- 17.Benjamini Y, Krieger AM, Yekutieli D. Adaptive linear step-up procedures that control the false discovery rate. Biometrika. 2006;93:491–507. [Google Scholar]
- 18.Liebig J, Peeters C, Oldham NJ, Markstädter C, Hölldobler B. Are variations in cuticular hydrocarbons of queens and workers a reliable signal of fertility in the ant Harpegnathos saltator? Proc Natl Acad Sci USA. 2000;97:4124–4131. doi: 10.1073/pnas.97.8.4124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ghaninia M, et al. Chemosensory sensitivity reflects reproductive status in the ant Harpegnathos saltator. Sci Rep. 2017;7:3732. doi: 10.1038/s41598-017-03964-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kather R, Martin SJ. Evolution of cuticular hydrocarbons in the hymenoptera: A meta-analysis. J Chem Ecol. 2015;41:871–883. doi: 10.1007/s10886-015-0631-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ueira-Vieira C, Kimbrell DA, de Carvalho WJ, Leal WS. Facile functional analysis of insect odorant receptors expressed in the fruit fly: Validation with receptors from taxonomically distant and closely related species. CellV Mol Life Sci. 2014;71:4675–4680. doi: 10.1007/s00018-014-1639-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rollmann SM, Mackay TF, Anholt RR. Pinocchio, a novel protein expressed in the antenna, contributes to olfactory behavior in Drosophila melanogaster. J Neurobiol. 2005;63:146–158. doi: 10.1002/neu.20123. [DOI] [PubMed] [Google Scholar]
- 23.Hallem EA, Carlson JR. Coding of odors by a receptor repertoire. Cell. 2006;125:143–160. doi: 10.1016/j.cell.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 24.Liang D, Blomquist GJ, Silverman J. Hydrocarbon-released nestmate aggression in the Argentine ant, Linepithema humile, following encounters with insect prey. Comp Biochem Physiol B Biochem Mol Biol. 2001;129:871–882. doi: 10.1016/s1096-4959(01)00404-3. [DOI] [PubMed] [Google Scholar]
- 25.Umphrey GJ. Sperm parasitism in ants: Selection for interspecific mating and hybridization. Ecology. 2006;87:2148–2159. doi: 10.1890/0012-9658(2006)87[2148:spiasf]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 26.Benton R. Sensitivity and specificity in Drosophila pheromone perception. Trends Neurosci. 2007;30:512–519. doi: 10.1016/j.tins.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 27.Groth AC, Fish M, Nusse R, Calos MP. Construction of transgenic Drosophila by using the site-specific integrase from phage phiC31. Genetics. 2004;166:1775–1782. doi: 10.1534/genetics.166.4.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Martin F, Alcorta E. Measuring activity in olfactory receptor neurons in Drosophila: Focus on spike amplitude. J Insect Physiol. 2016;95:23–41. doi: 10.1016/j.jinsphys.2016.09.003. [DOI] [PubMed] [Google Scholar]
- 29.Hammer Ø, Harper DAT, Ryan PD. PAST: Paleontological statistics software package for education and data analysis. Palaeontol Electronica. 2001;4:4. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.