Significance
Extracellular DNA and DNABII proteins are essential structural components of the extracellular polymeric substance, or matrix, of the nontypeable Haemophilus influenzae biofilm; however, the mechanisms by which these elements are released from the bacterial cell for incorporation into the biofilm matrix are not yet characterized. Here, we propose a mechanism for active DNA release during biofilm formation that involves an inner-membrane complex (TraCG) and the ComE pore through which the type IV pilus is typically expressed. Knowledge of how and when DNA and DNABII proteins are released into the extracellular milieu for integration into the biofilm matrix will further our understanding of biofilm formation and maturation and, in turn, guide development of directed therapies for diseases with a biofilm etiology.
Keywords: eDNA, Tra, secretin, IHF, HU
Abstract
Biofilms formed by nontypeable Haemophilus influenzae (NTHI) are central to the chronicity, recurrence, and resistance to treatment of multiple human respiratory tract diseases including otitis media, chronic rhinosinusitis, and exacerbations of both cystic fibrosis and chronic obstructive pulmonary disease. Extracellular DNA (eDNA) and associated DNABII proteins are essential to the overall architecture and structural integrity of biofilms formed by NTHI and all other bacterial pathogens tested to date. Although cell lysis and outer-membrane vesicle extrusion are possible means by which these canonically intracellular components might be released into the extracellular environment for incorporation into the biofilm matrix, we hypothesized that NTHI additionally used a mechanism of active DNA release. Herein, we describe a mechanism whereby DNA and associated DNABII proteins transit from the bacterial cytoplasm to the periplasm via an inner-membrane pore complex (TraC and TraG) with homology to type IV secretion-like systems. These components exit the bacterial cell through the ComE pore through which the NTHI type IV pilus is expressed. The described mechanism is independent of explosive cell lysis or cell death, and the release of DNA is confined to a discrete subpolar location, which suggests a novel form of DNA release from viable NTHI. Identification of the mechanisms and determination of the kinetics by which critical biofilm matrix-stabilizing components are released will aid in the design of novel biofilm-targeted therapeutic and preventative strategies for diseases caused by NTHI and many other human pathogens known to integrate eDNA and DNABII proteins into their biofilm matrix.
Biofilms contribute substantially to the chronic and recurrent nature of many bacterial diseases of humans and animals, including those of the airway, urogenital tract, skin, and oral cavity (1–3). Bacteria within a biofilm are protected from environmental stresses by a self-produced extracellular matrix, called the extrapolymeric substance (EPS), whose composition varies greatly depending upon the microbes that produce it and the environment in which it is formed. Despite vast compositional variability, the EPS is typically composed of a complex mixture of lipopolysaccharides, proteins, lipids, glycolipids, and nucleic acids (4). Extracellular DNA (eDNA) is a major constituent of the EPS formed by multiple human pathogens (5–7) and serves diverse roles within the bacterial biofilm. eDNA provides structural stability to the matrix, acts as a sink for antimicrobial peptides, protects resident bacteria from the host immune response, provides a source of “common goods” for the resident bacteria, acts as a universal structural material conducive to microbial community architecture, and facilitates the uptake of genetic material between bacterial species via a process known as horizontal gene transfer (5, 8–17). Members of the DNABII family of DNA-binding proteins, integration host factor (IHF) and histone-like protein (HU), are known for their strong structural influences on DNA intracellularly (18–24), and are also critical to the stability of the lattice-like 3D DNA structure found in biofilm matrices extracellularly (13, 25–31). To date, the presence of bacterial eDNA within biofilms has been attributed to release via various mechanisms, including cell lysis (autolysis or phage-mediated) (12, 32, 33), or active secretion through type IV secretion systems (T4SSs) (34, 35). Recently, DNA has been shown to be released from Pseudomonas aeruginosa by explosive cell lysis that is predicated on the formation of giant rounded cells that ultimately rapidly lyse, thereby releasing cell contents into the environment (36). However, a mechanism for the specific release of DNABII proteins into the extracellular space has been heretofore unknown.
Our laboratory demonstrated that DNABII proteins bind at the vertices of crossed eDNA strands and act as lynchpin-like molecules to stabilize the structure of eDNA within the biofilm matrix formed by nontypeable Haemophilus influenzae (NTHI) and multiple other human pathogens in vitro (25–31, 37, 38). We also show that biofilms formed within the chinchilla middle ear during experimental otitis media, as well as those within clinical specimens recovered from children with chronic posttympanostomy tube otorrhea and pediatric cystic fibrosis patients infected with Burkholderia cenocepacia, are stabilized by these proteins (13, 26, 38, 39). Further, we show that targeting the DNABII proteins with IHF- and/or HU-specific antibodies induces catastrophic collapse of the biofilm structure and subsequent release of resident bacteria that are now significantly more susceptible to the action of traditional antibiotics (13, 28, 39). Moreover, in a rat model of periodontitis (oral osteolytic infection) due to the oral pathogen Aggregatibacter actinomycetemcomitans, therapeutic treatment with antiserum against the DNABII family of proteins induced significant resolution of experimental periimplantitis (29). This same antiserum also disrupted multispecies biofilms in sputum solids recovered from pediatric cystic fibrosis patients (25). However, to date, the exact mechanism(s) by which NTHI releases DNA and DNABII proteins into the extracellular milieu has not yet been fully characterized.
NTHI is a predominant causative agent of multiple upper and lower respiratory tract diseases, which include otitis media, sinusitis, bronchitis, chronic cough, and exacerbations of both cystic fibrosis and chronic obstructive pulmonary disease (40, 41). Biofilm formation by NTHI contributes greatly to the chronicity of these diseases. Given the predominance and essential structural role of both extracellular DNA and DNABII proteins within an NTHI biofilm, we used this pathogen as a model organism with which to determine the mechanism(s) and kinetics by which these critical components are released by this bacterium. DNA and DNABII proteins are typically found within the bacterial cytoplasm, and must pass across both the inner and outer membranes for release into the extracellular environment. Whereas no active DNA secretion systems are characterized for NTHI, Neisseria, a closely related species, actively secretes single-stranded DNA via a T4SS (34, 35, 42, 43). The T4SS of Neisseria is composed of an inner-membrane complex, formed by TraC, TraD, and TraG; TraB, which spans both membranes; an outer-membrane complex, formed by TraK and TraV; and several cytoplasmic chaperone proteins (34, 35, 42, 43). Herein we investigated the possibility that NTHI used a similar mechanism for transit of DNA and associated DNABII proteins from the cytoplasm into the periplasm and, further, that this microbe used the ComE pore, through which the type IV pilus (Tfp) is typically expressed, to export the DNA into the extracellular environment. This mechanism for release of DNA and DNABII proteins from NTHI was independent of cell lysis and not preceded by changes in bacterial cell size or shape. We hypothesize that this unique mechanism of controlled DNA and DNABII protein release from NTHI contributes significantly to the structural stability and robust architecture of biofilms formed by NTHI as well as other pathogens that use a similar strategy for integration of eDNA and DNABII proteins into the biofilm matrix.
Results
NTHI Released DNA and DNABII Proteins Soon After Attachment to the Substratum.
Within 3 h of inoculation of NTHI onto a chambered coverglass, a lattice-like network of extracellular dsDNA was observed on the coverglass surface (Fig. 1A). In addition, DNABII proteins were detected bound with regular periodicity along these strands of eDNA and also positioned at the vertices of crossed strands of eDNA (Fig. 1B, red fluorescence). Because the inoculum consisted of NTHI in the mid-log phase of growth, we reasoned it unlikely that cell death was the primary mediator for the presence of the structured lattice of eDNA. For clarity, additional experiments were conducted with a viability stain to confirm this assertion. We next determined whether altruistic cell death by explosive cell lysis facilitated the observed deposition of DNA, as is described for Pseudomonas (36). Although a small number (<1 for every 1,000 cells) of NTHI did indeed form giant rounded cells similar in morphology to those described for Pseudomonas, these rounded cells did not form until much later in time [e.g., more than 3 h after inoculation compared with ≤1 h after incubation as reported for Pseudomonas (36)]. Moreover and importantly, these giant rounded cells were observed for up to 6 h of culture and never lysed. Conversely, once formed, 86% of large rounded Pseudomonas cells survive for <60 s before lysing (36). The limited (if any) bacterial cell death and absence of explosive cell lysis under the culture conditions we tested suggested that the observed release of eDNA and associated DNABII proteins from NTHI very early in biofilm formation likely occurred via an alternative nonlytic mechanism.
Fig. 1.
Detection of eDNA and DNABII proteins early in biofilm formation in vitro. (A) Three hours after inoculation, eDNA released from NTHI was arranged in a lattice-like structure (white). (B) Overlay image depicts DNABII proteins (red) associated with eDNA (white). DNABII proteins appeared as punctate red fluorescent signals situated at vertices of crossed strands of eDNA (yellow arrows) and also positioned at regular intervals along the eDNA strands. Whole bacterial cells also appear red, as the antiserum against NTHI IHF also labels the bacterial cell (blue arrow). (Scale bars, 10 μm.)
NTHI Released DNA and DNABII Proteins via a Non–Lysis-Dependent Mechanism.
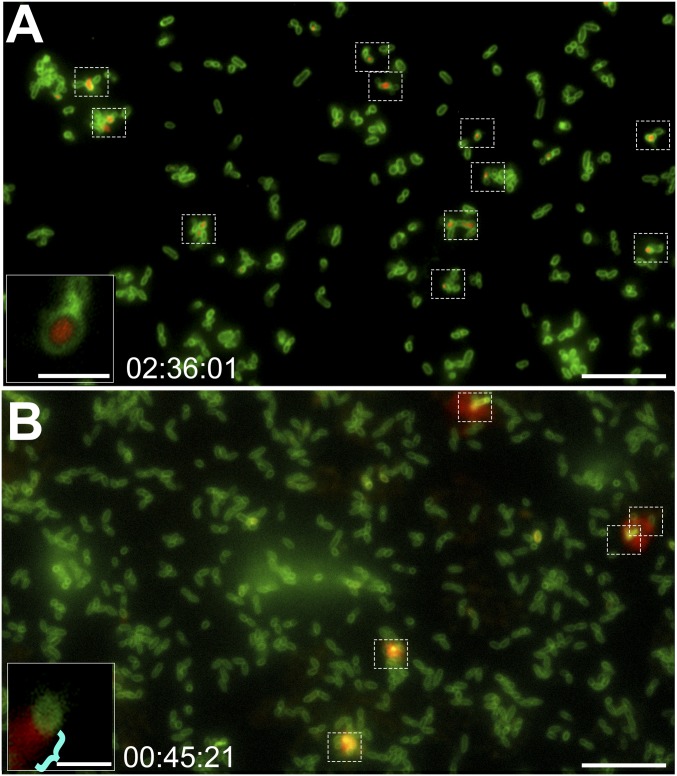
To visualize the release of DNA from NTHI during biofilm formation in vitro, we stained the NTHI outer membrane with FM1-43 membrane stain (Fig. 2, green fluorescence) and cultured the bacteria in the presence of the nucleic acid stain ethidium homodimer-2 (Fig. 2, red fluorescence), a membrane-impermeant stain that is only fluorescent when bound to DNA. As such, red fluorescence would indicate the presence of eDNA in the extracellular milieu or a bacterium with a compromised outer membrane. Via time-lapse fluorescence microscopy, we detected the release of DNA into the extracellular milieu by a subpopulation of NTHI within 10 to 120 min of inoculation, as evidenced by a “flare” of red fluorescence beyond the bacterial cell membrane (Fig. 2A and Movie S1). This release from a single site is more clearly viewed in Movie S2, which was captured by superresolution time-lapse microscopy. However, NTHI that released DNA by this mechanism did not first form giant rounded cells or exhibit explosive cell lysis before these events. This unique method of DNA release occurred on average from 17 of every 1,000 bacterial cells (Fig. 3, solid red bar) and within 2 h of inoculation.
Fig. 2.
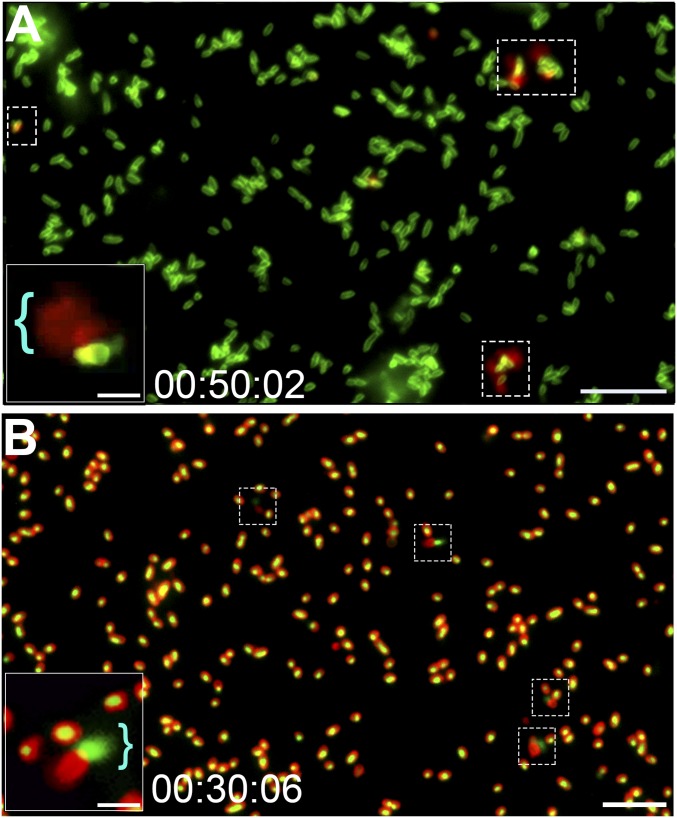
Subpopulation of NTHI released DNA from a single subpolar location along one long axis of the cell. (A) Time-lapse fluorescence video microscopy demonstrated DNA release from NTHI via a mechanism that did not involve development of large round cells or explosive cell lysis. Representative images show NTHI (green) cultured in medium that contained the membrane-impermeable dsDNA stain ethidium homodimer-2 (red), and thus a fluorescent red flare indicated DNA release from the bacterial cell (dashed boxes). Representative still frame was taken after 50 min of incubation. (Scale bar, 10 µm.) (A, Inset) Image of a cell with a red flare that indicated DNA release (blue bracket). (Scale bar, 2 μm.) (B) To validate that DNA was released from the bacterial cell, intracellular DNA was first stained with the cell-permeable stain Syto 9, which fluoresces green when bound to DNA, and the bacterial outer membrane was stained with FM4-64 (a red fluorescent membrane stain). Fluorescence time-lapse microscopy images confirmed that DNA was released from a subpolar location of a subset of cells. The presence of eDNA (now green) adjacent to a bacterial cell (now red) supported the proposed release mechanism (dashed boxes). (Scale bar, 10 μm.) (B, Inset) Magnified image of bacteria with a green flare that indicated DNA release (blue bracket). (Scale bar, 2 μm.)
Fig. 3.
Quantitation of DNA release events by NTHI. In rich medium (sBHI), we observed an average of 17 events per 1,000 parent cells after 2 h (solid red bar). There were no detectable events for either the ΔcomE or ΔtraCG mutant at this time point. Complementation of the mutations restored the average number of release events similar to that of the parent (compare solid red bar with green, blue, and yellow bars; no statistical differences). Induction of competence by incubation of NTHI in sBHI + cAMP (red striped bar) increased the number of DNA release events. This increase was statistically significant when NTHI was incubated in M-IV medium (red stippled bar). The number of DNA release events was calculated per 1,000 cells. Data are shown as the mean ± SEM from five separate sets of 1,000 cells (5,000 total cells evaluated). Statistical significance was calculated using one-way ANOVA with significance shown at P ≤ 0.001.
To both confirm that the observed DNA staining represented release of DNA from NTHI and not uptake of DNA from the environment (as had been clearly indicated by time-lapse microscopy) as well as validate the labeling of internal versus external DNA, we also reversed the use of the fluorochromes and now labeled intracellular DNA with the membrane-permeable stain Syto 9 and the bacterial outer membrane with the membrane stain FM4-64. Under the same culture conditions, intracellular DNA now appeared as a green fluorescent signal encircled by a red fluorescent ring that represented the bacterial outer membrane (Fig. 2B). As before, DNA was released (now seen as a green flare) from a subpopulation of NTHI without formation of a giant rounded cell (Fig. 2B). Intriguingly, regardless of the fluorochrome used to detect intra- versus extracellular DNA, the release appeared to originate from a single location along the long axis of the cell and at a single subpolar location (Fig. 2).
To reveal the approximate location of the DNA release from the bacterial cell, we immunolabeled cultures of whole unfixed NTHI for the presence of both eDNA and DNABII proteins. By scanning transmission electron microscopy (STEM), eDNA and DNABII proteins were observed associated with the bacterial membrane at a single subpolar location similar to that observed via each fluorescence time-lapse microscopy strategy used (Fig. 4A). These corroborating data confirmed that NTHI released DNA and DNABII proteins from a discrete location of the bacterial outer membrane. The subpolar region of this DNA release appeared highly similar to the location from which we have shown the NTHI Tfp is expressed and that coincides with the location of the ComE pore (Fig. 4B). Further evidence of the release of DNA and DNABII proteins from a single subpolar site on the bacterial cell and their assembly into a lattice-like structure is provided in Fig. 4C. The NTHI Tfp is an adhesive protein that is extruded through the outer-membrane pore formed by ComE (10, 44, 45). In addition to its role in adherence and twitching motility, expression of the ComE pore and Tfp is also required for the uptake of DNA from the extracellular environment (44, 45).
Fig. 4.
Imaging release of DNA and DNABII proteins via the ComE secretin of NTHI. (A) To confirm the conserved localization of DNA and DNABII release, immunoSTEM of NTHI cells imaged 3 h after inoculation onto the surface of a formvar-coated grid demonstrated the presence of both eDNA (15-nm gold spheres; arrows) and DNABII proteins (6-nm gold spheres) in close proximity to the bacterial cell and at a location that corresponded to the DNA flare seen in fluorescence microscopy. (Scale bar, 250 nm.) (B) E. coli that expressed NTHI ComE was immunolabeled to determine the localization of the ComE pore. ComE (green) was localized to a single subpolar location (red), similar to that of DNA and DNABII protein release events shown in A and C. (Scale bar, 2 μm.) (C) Low-magnification SEM image of an NTHI cell releasing DNA and DNABII proteins from a single subpolar site and the assembly of these components into a lattice-like matrix. (Scale bar, 500 nm.) These data indicated that the release of DNA and DNABII proteins occurred at a similar location as does expression of the ComE pore.
To determine whether the Tfp machinery was perhaps also involved in the release of DNA, we examined an isogenic ∆comE mutant via fluorescence time-lapse microscopy to assess whether this mutant would release DNA into the extracellular environment. In contrast to the parent, no extracellular fluorescence specific for DNA was observed with the ∆comE mutant, even after an extended incubation time of 6 h (compare Fig. 5A and Movie S3 with Fig. 2A and Movie S1). Interestingly, however, we did observe a subpopulation of cells that appeared to take up the ethidium homodimer-2 DNA stain, as indicated by red fluorescence within the borders of the green fluorescent bacterial outer membranes. The characteristic release of DNA seen with the parent as a red flare that extended beyond the cell membrane (as shown in Fig. 2A) was not observed. This intense red labeling of a subpopulation of ∆comE cells was observed at a later time point (>3 h) compared with the parent strain (∼1 h until the appearance of a flare) and suggested an accumulation of ethidium homodimer-2 within this subset of cells, likely due to the presence of DNA within the periplasm. Complementation of the comE mutation restored the ability of NTHI to release DNA as a flare and from a subpopulation of cells comparable to that observed for the parental isolate (Figs. 3, green bar and 5B). To confirm that release of DNA and DNABII proteins in this manner was dependent upon expression of the ComE secretin and not another component of the Tfp machinery, we repeated this study with a mutant that could not express the majority subunit of the Tfp, PilA (∆pilA). As shown in Figs. 3, yellow bar and 5C, the ∆pilA mutant released DNA into the environment as did the parental isolate. These results indicated that the subpolar release of DNA and DNABII proteins by NTHI required expression of the ComE pore.
Fig. 5.
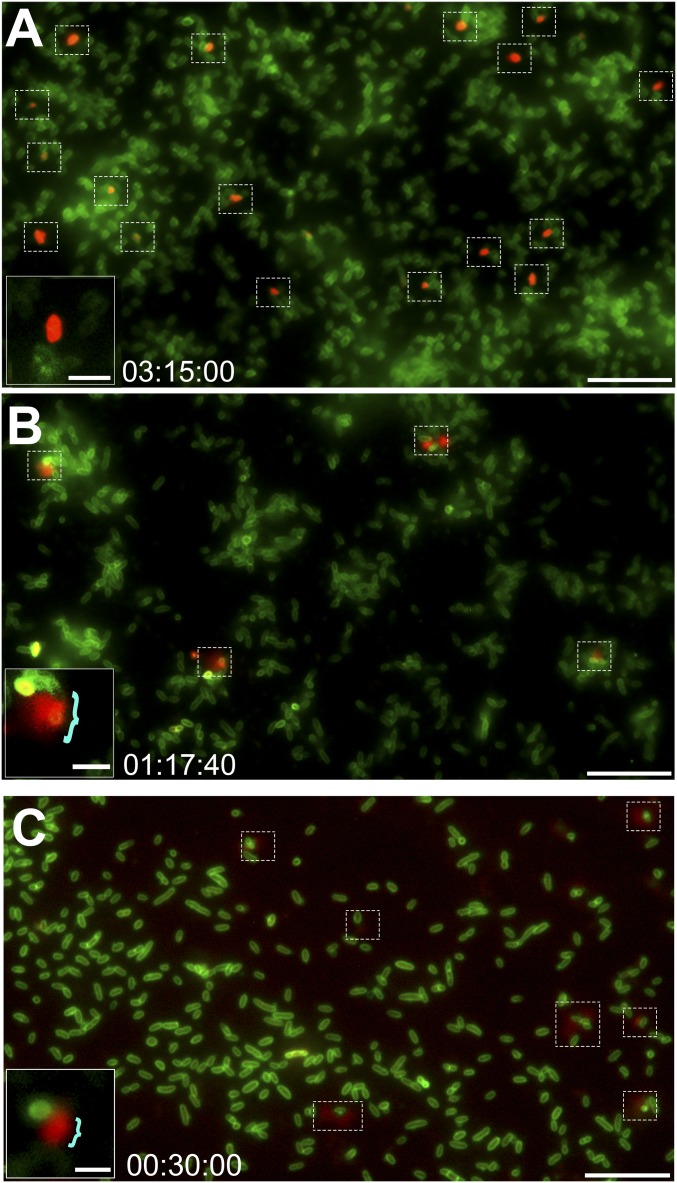
Expression of ComE, but not PilA, is required for the release of DNA by NTHI. Time-lapse fluorescence microscopy images of the ΔcomE mutant and the complemented ΔcomE mutant are shown. Time stamps indicate elapsed incubation time. (A) No DNA was released from the ΔcomE mutant at any time point; however, a subset of cells (dashed boxes) was uniformly red due to dye uptake. (Scale bar, 10 µm.) (A, Inset) Magnified image of a uniformly red fluorescent cell. (Scale bar, 2 μm.) (B) Complementation of the ΔcomE mutant restored the DNA flare release phenotype (dashed boxes). (Scale bar, 10 µm.) (B, Inset) Magnified image of a complemented ΔcomE mutant cell with the characteristic red flare (blue bracket). (Scale bar, 2 μm.) (C) DNA was released from ΔpilA cells via the characteristic red flare phenotype (dashed boxes), which indicated that deletion of this component of the Tfp machinery did not interfere with the described release mechanism. (Scale bar, 10 µm.) (C, Inset) Magnified image of a ΔpilA mutant cell with the characteristic red flare (blue bracket). (Scale bar, 2 μm.)
DNA Was Transported Across the NTHI Inner Membrane.
To this point, we have described a mechanism by which DNA and DNABII proteins appeared to be released from the bacterial cell into the environment through the ComE outer-membrane pore. However, DNA and DNABII proteins in the cytoplasm must first cross the inner membrane to access the periplasmic space before they can exit the cell. No inner-membrane complex specific for DNA transport has been described in NTHI. However, Neisseria sp. possesses an active T4SS composed of an inner-membrane complex, an outer-membrane complex, and several cytoplasmic chaperone proteins to release ssDNA into the environment for horizontal gene transfer (35). Neisseria shares multiple similarities with NTHI, including noted mosaicism between genomes, despite each organism failing to recognize each other’s uptake signal sequence (46–48). Bioinformatic analysis of the NTHI strain 86-028NP genome identified several genes that are homologous to those of the Neisseria T4SS. NTHI 86-028NP encodes orthologs of the Neisseria inner membrane-spanning proteins TraC and TraG, as well as the cytoplasmic chaperone proteins TraI, ParA, and ParB and the inner membrane-associated protein TraD. Surprisingly, no NTHI genes homologous to those that encode for the outer-membrane portion of the Neisseria T4SS were identified. Because NTHI has no known DNA secretion system and a relatively small conserved genome (1.9 Mb and 1,895 genes), we hypothesized that proteins similar to those of the inner-membrane complex in Neisseria were perhaps nonetheless sufficient to facilitate DNA translocation from the cytoplasm to the periplasm in NTHI. We also reasoned that once in the periplasm, DNA could then be released from the cell through the ComE pore situated within the outer membrane.
To test this hypothesis, we generated a ∆traCG mutant that lacked the predicted inner-membrane proteins TraC and TraG and assessed the ability of this mutant to release DNA into the extracellular environment. Similar to what we observed with the ∆comE mutant, the ∆traCG mutant did not release DNA via the described mechanism (Fig. 6A and Movie S4). Also similar to the ∆comE mutant, there was a minor subpopulation of cells that, over an extended time period, took up the ethidium homodimer-2 DNA stain but, unlike the ∆comE mutant, the stain was now localized to a central area of the cell with a clear demarcation (nonfluorescent, dark zone) between the green fluorescent outer membrane and the red fluorescence of the DNA, which suggested the presence of DNA within the cytoplasm but not within the periplasm. Complementation of the ∆traCG mutation restored the DNA release phenotype to similar to that of the parent (Figs. 3, blue bar and 6B). These data suggested that a Tra-like T4SS inner-membrane complex facilitated the translocation of cytoplasmic DNA across the inner membrane and into the periplasmic space of NTHI.
Fig. 6.
T4SS-like inner-membrane complex is required for the release of DNA from NTHI. Time-lapse fluorescence microscopy images of the ΔtraCG mutant and the complemented ΔtraCG mutant are shown. Time stamps indicate elapsed incubation time. (A) No DNA was released from the ΔtraCG mutant at any time point. However, a subset of cells (dashed boxes) had taken up the ethidium homodimer-2 DNA stain. (Scale bar, 10 µm.) (A, Inset) Magnified image of a ΔtraCG mutant cell showed a clear demarcation between red fluorescent DNA and green fluorescent outer membrane. (Scale bar, 2 μm.) (B) Complementation of the ΔtraCG mutant restored the characteristic DNA flare release phenotype (dashed boxes). (Scale bar, 10 µm.) (B, Inset) Magnified image of a complemented ΔtraCG mutant cell showed the characteristic red flare release phenotype (blue bracket). (Scale bar, 2 μm.)
Release of DNA from the Cytoplasm into the Extracellular Milieu Required Expression of a Tra-Like Inner-Membrane Complex and the ComE Outer-Membrane Pore.
Although no fluorescent flares indicative of a release of DNA to the extracellular milieu were observed by either the ΔcomE or ΔtraCG mutants, fluorescent staining specific for DNA was observed within a subpopulation of both mutants after extended incubation. As introduced above, examination of individual bacterial cells revealed a difference between the ΔcomE and ΔtraCG mutants in the pattern of fluorescent staining seen in the time-lapse videos. Whereas the ΔcomE cells exhibited red fluorescence that was uniformly distributed throughout the bacterial cell, similar fluorescent DNA labeling in the ΔtraCG cells was only present within the central portion of the cell, presumably the cytoplasm (compare Fig. 7 B and C). Interestingly, there was a distinct nonfluorescent area that separated the green fluorescent outer membrane and the red fluorescent DNA that was only observed in the ΔtraCG mutant. This nonfluorescent ring suggested an absence of DNA within the periplasm. Collectively, these observations support the hypothesis that TraC and TraG are part of an inner-membrane complex through which DNA is translocated from the cytoplasm to the periplasm for subsequent release into the extracellular environment via the ComE pore.
Fig. 7.
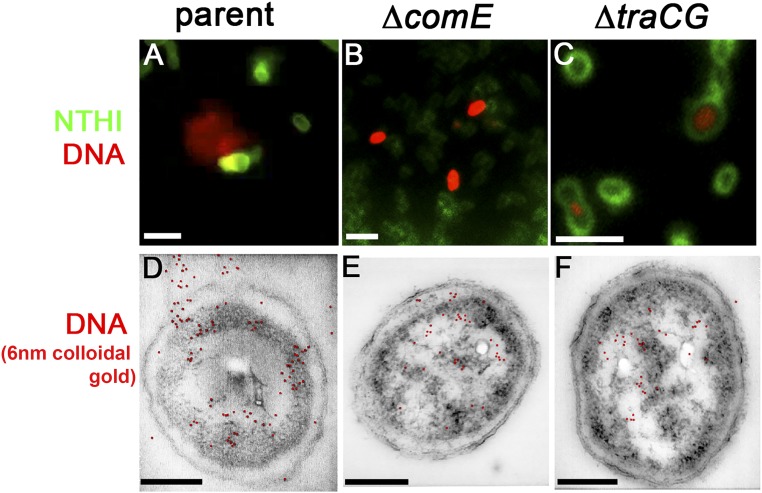
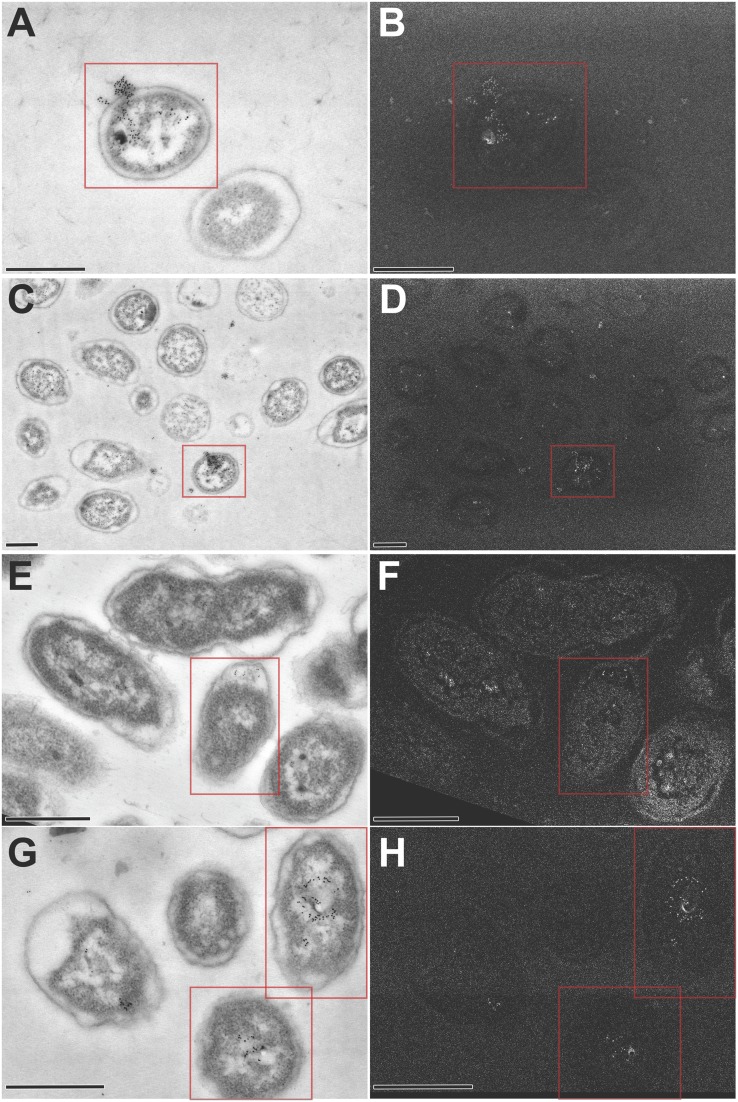
DNA was released from the parent but not from the ΔcomE or ΔtraCG mutants. (A–C) Duplicates of insets already presented in Figs. 2A, 5A, and 6A are repeated here for comparison (please see respective Fig. 2 legends for a detailed description). (D–F) ImmunoSTEM for the presence of DNA-confirmed fluorescent labeling seen in A–C. (D) A subset of NTHI parent cells positively labeled for DNA within the cytoplasm, periplasm, and extracellular environment as seen by 6-nm gold particles (pseudocolored red based on detection of secondary electrons; see Fig. S1 for additional images). (E) Subset of ΔcomE mutant cells positively labeled for DNA within the cytoplasm and in the periplasm, but no extracellular labeling was detected. (F) DNA was detected within the cytoplasm of the ΔtraCG mutant cells with no labeling within either the periplasm or extracellular space. (Scale bars, 200 nm.)
To confirm the compartmentalization of DNA as we had observed within fluorescently labeled cells (Figs. 7 A–C), we now immunolabeled ultrathin sections of parent, ΔcomE, and ΔtraCG cells for dsDNA (using 6-nm colloidal gold) that were then imaged by STEM (Figs. 7 D–F). Gold particles were identified by detection of secondary electrons and pseudocolored red in Fig. 7 for ease of interpretation; however, additional images that depict high-contrast secondary electron detection can be seen in Fig. S1. ImmunoSTEM of a 70-nm section of the parent strain showed a subpopulation of bacteria with positive labeling for DNA within the cytoplasm, periplasm, and extracellular milieu (Fig. 7D). Moreover, DNA release appeared to originate from a single location on the bacterial cell with both techniques (compare Fig. 7 A and D). The ΔcomE mutant exhibited positive labeling for DNA within the cytoplasm and periplasm; however, no labeling was noted beyond the bacterial outer membrane (Fig. 7E). This observation recapitulated the fluorescent localization observed in the time-lapse microscopy images (Fig. 7B). The distinct ring observed between the outer membrane and fluorescently stained DNA in the ΔtraCG mutant (Fig. 7C) strongly suggested that DNA was confined to the cytoplasm of these cells. This observation was confirmed by immunoSTEM, by which positive labeling for DNA was restricted to the cytoplasm of the ΔtraCG mutant (Fig. 7F). Collectively, these data further supported our hypothesis that DNA was transferred across the NTHI inner membrane via a T4SS-like Tra complex and across the outer membrane and into the extracellular environment through the ComE pore.
Fig. S1.
Representative STEM images of parent, ΔcomE, and ΔtraCG cells labeled for DNA. Ultrathin sections of bacterial cells were immunolabeled with monoclonal antibody against dsDNA and revealed with goat anti-mouse IgG conjugated to 6-nm gold. Sections were imaged by scanning transmission electron microscopy (A, C, E, and G), and secondary electron detection (B, D, F, and H) as an additional method to validate the presence and precise location of gold labeling. (A–D) A subset of NTHI parent cells (red boxes) positively labeled for DNA within the cytoplasm, periplasm, and extracellular environment as seen by 6-nm gold particles. (E and F) A subset of ΔcomE mutant cells (red boxes) positively labeled for DNA within the cytoplasm and in the periplasm, but no extracellular DNA was detected. (G and H) DNA was detected within the cytoplasm of the ΔtraCG mutant cells (red boxes) but there was no DNA labeled within either the periplasm or extracellular space. (Scale bars, 500 nm.)
The Frequency of DNA Release Increased with the Induction of Competence.
Natural competence is defined as the state in which bacteria are capable of being transformed via the uptake of extracellular DNA and is typically induced when bacterial cell densities are high or when bacteria are nutrient-deprived (49–51), as they are when resident within a biofilm (52–57). Because uptake of DNA is increased within biofilms, we hypothesized that this growth phenotype might also be associated with an increased release of DNA by biofilm-resident bacterial cells. To determine whether induction of competence augmented the relative proportion of NTHI that released DNA via the TraCG inner-membrane complex and ComE pore, the parental isolate as well as the ΔcomE and ΔtraCG mutants were exposed to 10 mM cAMP or grown in nutrient-depleted M-IV medium, methods commonly used to induce competence in NTHI (49, 58, 59). We observed an increase in the relative number of bacteria that released DNA when grown in the presence of cAMP (24 events per 1,000 cells) (Fig. 3, red striped bar) and an even greater and statistically significant increase in the number of release events when bacteria were incubated in M-IV medium (27 events per 1,000 cells) (Fig. 3, red stippled bar; P ≤ 0.001) compared with bacteria incubated in sBHI medium (brain heart infusion broth supplemented with β-NAD and heme, 2 μg each per mL) alone (17 events per 1,000 cells) (Fig. 3, solid red bar; P < 0.001). DNA release was not observed from either the ΔcomE or ΔtraCG mutant when grown in the presence of 10 mM cAMP or in M-IV medium, whereas their corresponding complemented mutants were restored in terms of the relative number of release events to that reported for the parent (Fig. 3, green and blue bars, respectively).
To determine whether TraCG was necessary for competence/uptake of DNA from the environment, we conducted transformation efficiency assays with the parental isolate, the ΔtraCG mutant, as well as the complemented ΔtraCG mutant. All isolates demonstrated equivalent competence to the parental isolate (the negative control, ΔcomE, was not competent, as expected) (Fig. S2). These data demonstrated that whereas TraCG facilitated export of DNA and DNABII proteins into the periplasm, it did not appear to be involved in transit of DNA across the inner membrane and into the cytoplasm. To rule out a specific nutritional limitation of the M-IV medium as a cause for the increase in the observed DNA release, given that H. influenzae has a strict growth requirement for iron, we assessed NTHI grown in M-IV medium supplemented with heme at the concentration typically used for culture in rich medium, sBHI (2 μg heme per mL). There was no difference in the relative number of bacteria that released DNA when NTHI was grown in M-IV medium supplemented with heme compared with unsupplemented M-IV medium. Therefore this avenue of investigation was not pursued further. These data thus confirmed that the observed increase in DNA release events under competence-inducing conditions was not due to increased cell death as a result of an iron deficiency nor was it likely due to increased biogenesis of outer-membrane vesicles known to occur in H. influenzae in response to iron starvation (60). Although competence has long been associated with the uptake of DNA, here we provide evidence that, in NTHI, induction of competence is also associated with the increased release of DNA and likely also the associated DNABII proteins.
Fig. S2.
Transformation frequency in M-IV nutrient-limited medium. Box and whiskers plot depicting mean transformation frequencies for the parent, ΔcomE mutant, ΔtraCG mutant, and complemented ΔtraCG mutant. No statistically significant differences in transformation frequency were noted among the parental isolate, ΔtraCG mutant, or complemented ΔtraCG mutant, whereas no transformants were observed for the ΔcomE mutant. These data suggested that the inner-membrane complex formed by TraCG did not play a role in the uptake of DNA from the environment. Mean efficiency for each strain was calculated from four replicate experiments. Whiskers indicate minimum to maximum.
eDNA and DNABII Proteins Released by TraCG and ComE Were Required for the Structure of NTHI Biofilms.
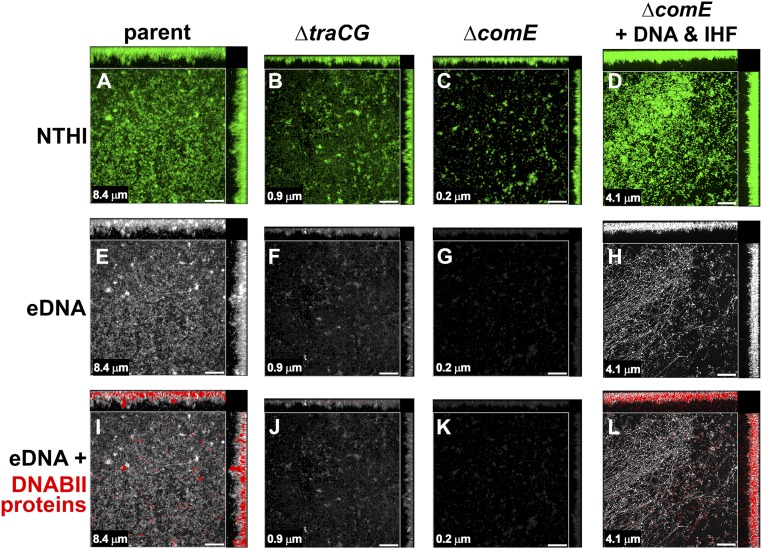
To determine the role of eDNA and DNABII proteins released via TraCG and ComE in the overall architecture of an NTHI biofilm, we assessed biofilms formed by the parent strain, the ΔcomE mutant, and the ΔtraCG mutant for relative biofilm structure as well as for the presence of eDNA and DNABII proteins in the biofilm matrix. Biofilms were grown for 24 h and then labeled for the presence of eDNA and DNABII proteins. The parent strain formed a robust, viable biofilm that contained eDNA and associated DNABII proteins, as expected for these culture conditions (Fig. 8 A, E, and I). In contrast, biofilms formed by both the ΔtraCG and ΔcomE mutants were of significantly reduced height (see each panel for mean biofilm height) and lacked the characteristic shape and architecture of the biofilm formed by the parent strain (Fig. 8 B and C, respectively). In addition, biofilms formed by either mutant had considerably less or no detectable eDNA or DNABII proteins within the biofilm matrix compared with what was observed with the parent strain (compare among Fig. 8 E–G and among Fig. 8 I–K). We attributed the apparent slight increase in labeling for DNA by the ΔtraCG mutant (Fig. 8F) over that by the ΔcomE mutant (Fig. 8G) to the increase in number of adherent cells along the glass surface. Fluorescent staining of the adherent cells resulted in the faint green haze seen in Fig. 8B and can cause some background fluorescence in the light path used to detect DNA. Because the ΔcomE mutant does not express Tfp, a major adhesin, there are fewer individual bacteria adherent to the glass surface to generate similar background labeling. The growth rates of both mutants were similar to that of the parent strain when grown in sBHI and, as such, a growth defect would not account for the observed reduction in biofilm biomass (Fig. S3). The absence of both eDNA and DNABII proteins within the biofilm matrix, both essential for biofilm structural stability, was reasoned to be responsible for the altered architecture of biofilms formed by the ΔtraCG and ΔcomE mutants.
Fig. 8.
eDNA and DNABII proteins were present in biofilms formed by the parental isolate but not the ΔcomE or ΔtraCG mutants. Biofilms were immunolabeled for DNA and DNABII proteins. (A–C) Bacteria within the biofilms were stained with FM1-43 outer-membrane stain (green fluorescence) with both top-down and orthogonal views of representative biofilms shown. (E–G) eDNA (white) is visible throughout the biofilm formed by the parental isolate with minimal labeling in biofilms formed by either mutant. (I–K) DNABII proteins (red) were visualized in addition to eDNA (white) throughout biofilms formed by the parental isolate. In comparison, minimal to no labeling for DNABII proteins (red) was observed in biofilms formed by either of the mutants. Addition of exogenous NTHI DNA and a DNABII protein (IHF) restored ΔcomE biofilm characteristics comparable to the parent (compare D, H, and L with A, E, and I). (Scale bars, 20 μm.) Note: Mean biofilm height as determined by COMSTAT2 analysis is indicated in the lower left-hand corner of each panel.
Fig. S3.
Twenty-four-hour growth curves for parent, isogenic ΔcomE and ΔtraCG mutants, and complemented ΔcomE and ΔtraCG mutants. Bacteria were grown in sBHI at 37 °C and 5% CO2 for 24 h. No differences in growth were observed among the strains; therefore the induced mutations did not confer a growth defect. Error bars indicate SEM.
To further address the importance of DNA and DNABII proteins in the biofilm matrix and provide substantiating evidence that a lack thereof was responsible for the altered architecture and reduced biomass formed by the ΔcomE mutant, we added back both exogenous NTHI DNA and DNABII proteins to the culture system at the time of seeding. This supplementation restored the biofilm height, biomass, and overall architecture to ∼50% of that observed with the parental isolate when grown in sBHI (compare Fig. 8D with Fig. 8A). Moreover, both DNA and DNABII proteins were observed within these reconstituted biofilms, as demonstrated by immunolabeling similar to what was observed in parent strain biofilms (compare Fig. 8 H and L with Fig. 8 E and I, respectively).
Discussion
Bacteria within a biofilm are significantly more protected from environmental stresses compared with their planktonic counterparts. The extracellular polymeric substance serves as a semipermeable barrier that protects resident bacteria from both immune effectors and therapeutics such as antibiotics (61, 62). The EPS is composed of a variety of cell-associated and secreted molecules, which include polysaccharides, proteins, lipids, and nucleic acids (63). Extracellular DNA protects bacteria within the biofilm from host defenses via the chelation of antimicrobial peptides and divalent cations (14, 64). In addition, we have shown that eDNA is an important structural component of the biofilm matrix and that the DNABII proteins IHF and HU are required to stabilize the eDNA scaffold that provides structural support to biofilms formed by NTHI and many additional human pathogens (7, 13, 25, 27, 28, 30, 37–39).
There are a number of possible mechanisms that may explain the presence of bacterial DNA in the extracellular environment. Some bacteria have a mechanism by which a subpopulation of cells will undergo programmed cell death and lysis to release all of their cellular contents into the environment (9). This type of cell lysis provides common goods for neighboring bacteria and releases DNA for horizontal gene transfer (4, 65). Staphylococcus aureus has been shown to undergo such an altruistic cell death (9). Recently, it was reported that a small proportion of Pseudomonas cells form very large rounded cells that then rapidly undergo explosive cell lysis and release their cellular contents, including DNA, into the extracellular environment (36). In addition, some species such as Neisseria have an active type IV secretion system that transports DNA from the cytoplasm to the extracellular space, presumably for gene transfer (35).
Herein, we identified a mechanism of release of both DNA and DNABII proteins into the environment by a subpopulation of NTHI. To first determine how DNA and DNABII proteins were able to get from within the bounds of the inner membrane and into the periplasm, we analyzed the NTHI genome for potential genes that encode for proteins known to be involved in DNA transport across the inner membrane. Although the genome of NTHI strain 86-028NP lacked orthologs of any of the periplasmic or outer-membrane components found in Neisseria, we did find orthologs of the Neisseria proteins that constitute the T4SS inner-membrane complex. Indeed, work by Juhas and colleagues (66–68) has shown that a novel T4SS, acquired through gene transfer of genomic islands of the ICEHin 1056 subfamily, is likely the origin of the traC- and traG-like genes found in strain 86-028NP, as well as other NTHI strains. In addition to traC and traG, the genomic island contains genes that encode homologs of the T4SS cytoplasmic chaperone proteins TraI, ParA, and ParB as well as the inner membrane-associated protein TraD. The genomic island of NTHI strain 86-028NP also includes genes involved in excision, integration, replication, and stabilization of integrative and conjugative elements (ICEs) (68).
In the current study, we found that the NTHI TraC and TraG proteins, predicted to form an inner-membrane complex of a T4SS, were required for the release of DNA during early biofilm formation. Fluorescence time-lapse microscopy provided evidence to suggest that this complex, similar to that of the Neisseria T4SS inner-membrane complex, actively translocated DNA across the NTHI inner membrane. ImmunoSTEM of cells that could not express either TraC or TraG further confirmed this finding, as no labeling for DNA was observed in the periplasm of any of the cells or in the extracellular space. Although a system similar to the Neisseria T4SS is likely involved in translocation of DNA across the inner membrane, NTHI lacks the components necessary to form a complete T4SS outer-membrane complex. As such, DNA or DNABII proteins transported into the periplasm via the Tra inner-membrane complex would require a heretofore uncharacterized additional mechanism to cross the outer membrane and be released into the extracellular environment.
In data presented here, we show how quickly NTHI releases DNA, with associated DNABII proteins into the extracellular environment, in a lattice-like array of DNA strands. This array is similar to what was observed by Barnes et al. (69) with Enterococcus faecalis, wherein they observed a network of strands of DNA in 4-h cultures by both immunofluorescence and scanning electron microscopy by an as-yet uncharacterized DNA export system. Here, by fluorescence imaging and electron microscopy, we revealed that DNA and DNABII proteins were released from NTHI at a single subpolar location on the cell. The location of this release is consistent with the location of Tfp expression through the ComE pore (44). Unlike the less well-understood mechanism(s) by which NTHI releases DNA into the extracellular milieu, uptake of DNA from the environment is well-studied. Many bacteria, including NTHI and Neisseria, take up DNA from the environment via a process that involves the machinery for expression of type IV pili. NTHI expresses Tfp from a single subpolar location along the long axis of the bacterial cell. These Tfp are essential for twitching motility, adherence to respiratory tract epithelial cells, colonization of the mammalian respiratory tract, and competence (10, 44, 45, 50, 51). The Tfp machinery in NTHI is encoded by two operons, pilABCD and comABCDEF. pilA, pilB, and pilD encode the Tfp major subunit, an ATPase, and the prepilin peptidase, respectively (44, 45). comE encodes a protein (ComE) that is homologous to the PilQ secretin of Pseudomonas, which complexes to form the outer-membrane pore through which the Tfp is expressed and foreign DNA is taken up during competence (44).
Collectively, these observations suggested to us that the ComE pore might be involved in the release of DNA and DNABII proteins from the periplasmic space into the extracellular environment, given that the secretin pore is just wide enough to accommodate a pair of DNA double helices (51). Therefore, we constructed an isogenic comE mutant and found that NTHI that could not express the ComE pore did not release DNA via the mechanism observed with the parent strain. These results suggested that, in addition to the Tra complex that was required for transport of DNA across the inner membrane, the ComE pore was required for the transfer of DNA across the outer membrane. In analysis of the completed genomes of 46 strains of H. influenzae available in the National Center for Biotechnology Information, we found that 25/46 (55%) possessed tra genes whereas 46/46 (100%) had the comE gene. This observation suggests that the majority of NTHI isolates are capable of using this mechanism to release DNA and DNABII proteins into the EPS of their biofilms. Other bacterial secretins are similarly known to function in dual processes (70–72).
A model of the predicted transfer of DNA from the cytoplasm into the periplasm via the Tra inner-membrane complex and then out of the cell via the ComE pore is presented in Fig. 9 (Left). Based on this model, we would predict that a ΔcomE mutant would be able to translocate DNA across the inner membrane via the Tra complex but would be unable to release DNA from the cell due to the absence of the ComE pore (Fig. 9, Center). In a ΔtraCG mutant, DNA would be unable to cross the inner membrane and, as such, no DNA would be present within the periplasmic space or be available to be released by the cell despite the presence of the ComE pore (Fig. 9, Right). This model is supported by our observations of both DNA release by fluorescently labeled whole cells and immunolabeled ultrathin sections of parent, ΔcomE and ΔtraCG cells. Whereas we cannot exclude the possibility that these inner-membrane (TraCG) and outer-membrane (ComE) components form a complex, given the canonical arrangement of the type IV pilus machinery, which does indeed require linkage of its own inner- and outer-membrane components both spatially and temporally, we do not currently favor this possibility. Further, we do not yet know whether the described method of release of both DNA and DNABII proteins into the extracellular milieu requires adherence of NTHI to a substratum or not; however, this is the subject of ongoing investigation.
Fig. 9.
Graphic representation of the proposed mechanism for DNA and DNABII protein release from NTHI. In the parental isolate, DNA and DNABII proteins cross the inner membrane (IM) via the Tra complex, and are released from the cell through the ComE pore. In the ΔcomE mutant, DNA and DNABII proteins can cross the IM via the Tra complex but are unable to cross the outer membrane (OM) in the absence of the ComE outer-membrane pore. Therefore, no DNA or DNABII proteins would be released outside of the cell via this mechanism. In the ΔtraCG mutant, wherein cells lack the T4SS-like inner-membrane Tra complex, DNA and DNABII proteins would be confined to the cytoplasm and unable to cross the IM.
Diseases caused by NTHI are often chronic and recurrent in nature due to the formation of highly recalcitrant biofilms. Extracellular DNA and DNABII proteins are required for biofilm structure and stability. The targeted removal of eDNA and/or DNABII proteins has been shown to cause catastrophic biofilm collapse in vitro and disruption of biofilms in three distinct animal models of disease induced by three important human pathogens of the middle ear, oral cavity, and lung (13, 26, 29). Here we show that release of DNA and DNABII proteins via a mechanism that involves both the Tra inner-membrane complex and the ComE pore is critical for biofilm formation in vitro. Biofilms formed in vitro by bacteria unable to release DNA and DNABII proteins via this mechanism lacked extracellular DNA and DNABII proteins in the biofilm matrix, which resulted in a compromised and atypical biofilm architecture. This mechanism of release of both DNA and DNABII proteins is therefore likely critical to biofilm stability in vivo as well.
Naturally competent bacteria can take up foreign DNA from the environment via a two-step process that involves both transfer of DNA from the bacterial surface to the cytoplasmic membrane and translocation of DNA across the cytoplasmic membrane that may or may not be spatially and temporally coupled (73, 74). There is a relationship between cell growth and development of competence in NTHI (58), wherein a greater cell density with slowed growth, as in a biofilm, increases competence. We have shown that induction of competence also modestly increased the number of NTHI within a population that released DNA and DNABII proteins. We surmise that this increase was limited due to the state of competence, which induced comE expression but not concomitant expression of traCG. This outcome resulted in the rate-limiting step being the transit of DNA through the inner membrane into the periplasm. Importantly, other genera demonstrate a similar DNA release phenotype induced by competence. Streptococcus pneumoniae can trigger cell lysis of neighboring bacteria of the same species (fratricide) and DNA release when competence is induced (75). A relationship between competence and eDNA has also been shown in Neisseria gonorrheae (76), Pseudomonas stutzeri (77), and Bacillus subtilis (78). However, to the best of our knowledge, the induction of competence has heretofore not been associated with the release of DNA by NTHI.
Herein, we described a mechanism by which NTHI releases DNA and DNABII proteins into the environment that is independent of explosive cell lysis. We have shown that DNA appeared to transit from the cytoplasmic space to the periplasm via a T4SS-like inner-membrane complex and was then released, along with DNABII proteins, into the environment through the ComE outer-membrane pore. Taken together, these data indicated that this TraCG- and ComE-dependent mechanism of release is critical for NTHI biofilm formation. These findings describe a mechanism by which a subset of NTHI released DNA and DNABII proteins into the extracellular milieu, where they are known to provide structural support to the biofilm, which protects resident bacteria. Knowledge of the mechanism and kinetics of release of DNA and DNABII proteins can be integrated into our development of biofilm-targeted disruption and prevention strategies.
Materials and Methods
NTHI strain 86-028NP is a low-passage clinical isolate. NTHI was maintained as frozen stocks and cultured on chocolate agar (Remel) at 37 °C with 5% CO2 for 18 to 20 h before use. A ComE deletion mutant (ΔcomE) and its complement (C′ ΔcomE) were described previously (44). A TraCG mutant (ΔtraCG) was generated by replacement of the traCG region of the chromosome with a kanamycin resistance cassette. Briefly, regions ∼1 kb upstream and downstream of the traCG region were amplified by PCR and digested with SphI and EcoRI, respectively. The flanking regions were then ligated to a kanamycin resistance cassette that had been amplified by PCR from pKMLN-1 (79) and digested with SphI and EcoRI. NTHI strain 86-028NP was transformed with the resultant DNA fragment using a modified M-IV transformation protocol (80, 81). Transformants were selected on chocolate agar supplemented with kanamycin (20 μg/mL) and sequenced to verify allelic exchange.
The ΔtraCG mutant was complemented via use of a derivative of pSPEC1 (44) that contained the traCG genes. Briefly, the traCG region of NTHI strain 86-028NP was amplified by PCR, digested with NcoI and PvuI, and ligated into pSPEC1 that had been digested with NcoI and PvuI. The resultant plasmid, pJAJ1, was confirmed by sequencing and used to transform the ΔtraCG mutant via electroporation. All PCR assays were performed with Q5 High-Fidelity DNA polymerase (New England Biolabs). All restriction enzymes were purchased from New England Biolabs.
To demonstrate the singular subpolar location of expression of ComE, the com locus from NTHI strain 86-028NP was amplified from genomic DNA by PCR using a forward primer containing a BglII site and a reverse primer that encodes a FLAG epitope tag and an EcoRI site. The product was digested with BglII and EcoRI and ligated with pCOLADuet-1 (EMD Millipore) previously digested with BamHI and EcoRI. The construct was transformed into ElectroMAX Escherichia coli DH5α (Thermo Fisher Scientific), and transformants were selected on Luria agar containing 20 µg kanamycin per mL. Isolated plasmids were confirmed by restriction analysis and sequencing and then transformed into E. coli BL21 Star (DE3) (Thermo Fisher Scientific). Transformants were again selected on Luria agar containing 20 µg kanamycin per mL.
Detailed materials and methods can be found in SI Materials and Methods.
SI Materials and Methods
Detection of DNA Early in Biofilm Formation.
For detection of eDNA at an early time point in biofilm formation, the parental isolate (NTHI strain 86-028NP) was collected from overnight culture on chocolate agar (Remel), suspended in brain heart infusion broth supplemented with β-NAD and heme (2 μg each per mL; sBHI), and seeded into wells of an eight-well chambered coverglass (Thermo Fisher Scientific). After a 3-h static incubation at 37 °C, 5% CO2, in a humidified atmosphere, bacteria were fixed for 2 h in 2.5% glutaraldehyde, 10 mM PBS (pH 7.4). Bacteria were washed with PBS, and then preparations were blocked (Super Block; ScyTek) for 10 min at room temperature. DNA was labeled with a murine monoclonal antibody to dsDNA (Abcam) and IHF-labeled with rabbit polyclonal anti-NTHI IHF protein by overnight incubation at 4 °C, and then washed twice with PBS. Primary antibodies were revealed with goat anti-mouse IgG-Alexa Fluor 594 and goat anti-rabbit IgG-Alexa Fluor 488 (Molecular Probes) for 30 min at room temperature, washed three times with PBS, and viewed in PBS; 10 mM PBS served as diluent. Images were captured with a Zeiss Axiovert 200M microscope and rendered with Zeiss AxioVision software (version 4.8.2.0).
In Vitro Biofilms.
NTHI biofilms were grown in eight-well chambered coverglasses as described previously (82). Briefly, NTHI strains cultured on chocolate agar were suspended in sBHI and adjusted to an OD490 of 0.65. Bacterial suspensions were further diluted 1:6 with sBHI and cultured statically at 37 °C, 5% CO2, in a humidified atmosphere for 3 h before seeding into wells of chambered coverglasses. To maintain bacterial viability, fresh sBHI was added after 16 h and biofilms were incubated an additional 8 h.
To detect eDNA and DNABII proteins within mature (24-h) NTHI biofilms formed by the parent, isogenic ΔcomE and ΔtraCG mutants, and complemented ΔcomE and ΔtraCG mutants, DNA was labeled with a murine monoclonal antibody to dsDNA and IHF-labeled with rabbit polyclonal anti-NTHI IHF protein and revealed with goat anti-mouse IgG-Alexa Fluor 594 and goat anti-rabbit IgG-Alexa Fluor 647 (Molecular Probes). Bacterial membranes were stained with FilmTracer FM1-43 (Life Technologies); 10 mM PBS served as diluent. Biofilms were imaged via a confocal scanning laser microscope attached to a Zeiss Axiovert 200M microscope, and images were rendered with Zeiss Zen software. Biofilm mean thickness was calculated with COMSTAT2 software (83, 84).
Time-Lapse Fluorescence Microscopy.
To detect release of DNA into the extracellular environment, 10 to 12 colonies of the parent, isogenic ΔcomE, ΔtraCG, and ΔpilA mutants, and the complemented ΔcomE and ΔtraCG mutants were collected from overnight growth on chocolate agar and suspended in 1 mL of sBHI. To stain bacterial membranes, the medium contained FilmTracer FM1-43 stain. An aliquot of 100 μL of each NTHI strain was applied to a 35-mm FluoroDish (World Precision Instruments) and incubated statically at 25 °C for 1 h to permit bacterial adherence. The medium was then replaced with 1 mL sBHI that contained the membrane-impermeant DNA stain ethidium homodimer-2 (Life Technologies). Images were captured every 12 to 15 min for up to 6 h from four to six locations within the FluoroDish using a Leica DMI6000 B inverted microscope with environmental enclosure (37 °C and 5% CO2) and visualized via LAS X software (Leica Microsystems).
To next discriminate between intra- and extracellular DNA and confirm that observed DNA “flares” represented release of DNA from NTHI and not uptake of DNA by NTHI, time-lapse video microscopy was performed wherein intracellular NTHI DNA was first stained with the membrane-permeant nuclear acid stain Syto 9 and bacterial membranes were stained with FilmTracer FM4-64 (FM lipophilic styryl dyes; Thermo Fisher Scientific) before imaging as described above.
ImmunoSTEM of Whole NTHI Bacterial Cells.
The parental isolate (NTHI strain 86-028NP) was collected from overnight culture on chocolate agar (Remel) and suspended in sBHI, and 20 μL of bacterial suspension was placed on top of carbon- and formvar-coated nickel grids (Electron Microscopy Sciences). Grids were incubated at room temperature for 1 h to allow for bacterial adherence, medium was replaced with fresh sBHI, and grids were incubated for 3 h at 37 °C, 5% CO2, in a humidified atmosphere. For detection of eDNA and DNABII proteins, grids were first washed in 1× Hanks’ balanced salt solution (HBSS) (Corning) for 5 min and then blocked with 0.5% BSA in HBSS for 2 h at room temperature. Grids were then washed in HBSS for 5 min at room temperature. DNA was labeled with a murine monoclonal antibody to dsDNA (Abcam) and IHF-labeled with rabbit polyclonal anti-E. coli IHF protein (both diluted 1:200 in HBSS). Grids were incubated with primary antibodies for 1 h at room temperature, washed three times for 5 min each in HBSS, incubated with goat anti-mouse IgG conjugated to 15-nm colloidal gold and goat anti-rabbit IgG conjugated to 6-nm colloidal gold (both diluted 1:200 in HBSS) for 1 h at room temperature, and washed three times for 5 min each in HBSS. Grids were negatively stained with 1.0% ammonium molybdate and 1.0% ammonium acetate (in diH2O) (Sigma) for 2 min at room temperature, and then air-dried overnight before viewing on a Hitachi S-4800 field emission scanning electron microscope.
ImmunoSTEM of Ultrathin Sections of NTHI.
To determine the location of DNA within bacterial cells, the parent and ΔtraCG and ΔcomE mutants were embedded in 2% agarose, fixed with 1% osmium tetroxide before dehydration through graded alcohols, and embedded in LR White resin (Electron Microscopy Sciences). Seventy-nanometer sections were placed on formvar-coated grids (Electron Microscopy Sciences) and air-dried before labeling DNA with a murine monoclonal antibody to dsDNA. Localization of DNA was revealed by incubation with goat anti-mouse IgG conjugated to 6-nm colloidal gold (Electron Microscopy Sciences). All images were collected on a Hitachi S-4800 field emission scanning electron microscope operating at 30 kV. Scanning transmission images were captured with a bottom-mounted bright-field transmitted electron detector. Secondary electron images were captured with an Everhart–Thornley secondary electron detector mounted above the specimen grid.
Induction of Competence.
Two well-established methods were used to induce competence of NTHI. First, NTHI was incubated in medium supplemented with 10 mM cAMP to stimulate expression of the cAMP receptor protein (CRP) (85). Second, NTHI was incubated in a nutrient-deficient medium, M-IV, to limit cell growth but not prevent protein synthesis (58, 59, 81).
To activate CRP, NTHI stained with FilmTracer FM1-43 as described above was suspended in sBHI supplemented with 10 mM cAMP (MP Biomedicals), aliquotted into a 35-mm FluoroDish, and incubated for 1 h to permit bacterial adherence. NTHI was maintained in sBHI supplemented with 10 mM cAMP and the membrane-impermeant stain ethidium homodimer-2 for the duration of time-lapse fluorescence microscopy.
Alternatively, NTHI stained with FilmTracer FM1-43 as described above was suspended in M-IV medium (with or without 2 μg heme per mL) and FilmTracer FM1-43 for 30 min. NTHI was maintained in sBHI supplemented with 1 mM cAMP and the membrane-impermeant stain ethidium homodimer-2 for the duration of time-lapse fluorescence microscopy.
After a 2-h incubation, the relative number of DNA release events per 1,000 cells was determined for the parent, isogenic ΔcomE, ΔtraCG, and ΔpilA mutants, and complemented ΔcomE and ΔtraCG mutants under each growth condition. A minimum of 5,000 cells from at least three different experiments performed on different days were enumerated.
Transformation Efficiency.
Transformation efficacy of the parent, isogenic ΔcomE and ΔtraCG mutants, and complemented ΔcomE and ΔtraCG mutants was determined as previously described via M-IV transformation (58, 59, 80). Briefly, bacteria were grown to mid-log phase in sBHI and then washed in M-IV medium before incubation in fresh M-IV medium for 70 min. cAMP was added to a final concentration of 1 mM, and bacterial cells were incubated for an additional 30 min. Linearized genomic NTHI 86-028NP DNA containing a chloramphenicol resistance cassette was then added to the medium. Cells were transferred to sBHI medium and incubated for 1.5 h before serial dilution and plating on both chocolate agar and chocolate agar supplemented with 1 μg chloramphenicol per mL. Transformation efficiency was determined as the ratio of CFUs of NTHI on selective medium compared with chocolate agar.
Add-Back Experiments.
To determine whether addition of exogenous DNA and IHF could restore the ability of the ΔcomE mutant to form a biofilm comparable in architecture to that of the parent, we performed add-back experiments. Bacteria were prepared as described above for inoculation into chambered coverglasses. At the time of bacterial seeding, 0.1 μg NTHI genomic DNA per mL plus 2 μg NTHI IHF protein per mL were also added. The concentrations of genomic DNA and IHF used herein were empirically determined using the parent strain such that the resultant biofilms were similar in viability and architecture to biofilms maintained in medium only. Biofilms were collected after 24 h and stained for the presence of eDNA and IHF as previously described.
OMX Time-Lapse Microscopy.
To visualize DNA release from the parent strain under greater microscopic resolution, time-lapse fluorescence microscopy was also performed using a GE OMX superresolution microscope. Briefly, NTHI was stained as described above for time-lapse fluorescence microscopy. Superresolution 3D-SIM images were acquired on a DeltaVision OMX SR (GE Healthcare) equipped with a 60×/1.42 N.A. Plan Apo oil-immersion lens (Olympus), 405-, 488-, 568-, and 640-nm solid-state lasers, and sCMOS cameras (pco.edge). Image stacks of 6.5 µm with 0.125-µm-thick z sections and 15 images per optical slice (three angles and five phases) were acquired using immersion oil with a refractive index of 1.516 every 15 min for 3 h. Images were reconstructed using Wiener filter settings of 0.003 and optical transfer functions measured specifically for each channel with SoftWoRx 6.5.2 (GE Healthcare) to obtain superresolution images with a twofold increase in resolution both axially and laterally. Images from different color channels were registered using parameters generated from a gold grid registration slide (GE Healthcare) and SoftWoRx 6.5.2.
Statistical Analysis.
Statistically significant differences were calculated using Prism version 7.00 (GraphPad Software). Differences in the frequency of release events were determined by one-way ANOVA and Tukey’s multiple-comparisons test using at least three replicate runs. A P value of ≤0.05 was considered significant.
Supplementary Material
Acknowledgments
We thank Jennifer Neelans for manuscript preparation. This work was funded by NIH R01 DC011818 (to L.O.B. and S.D.G.).
Footnotes
Conflict of interest statement: S.D.G. and L.O.B. are scientific advisors to and have equity in ProclaRx, LLC, to whom technology related to DNABII proteins has been licensed.
This article is a PNAS Direct Submission.
See Commentary on page 8444.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1705508114/-/DCSupplemental.
References
- 1.Murphy TF, Bakaletz LO, Smeesters PR. Microbial interactions in the respiratory tract. Pediatr Infect Dis J. 2009;28(Suppl)(10):S121–S126. doi: 10.1097/INF.0b013e3181b6d7ec. [DOI] [PubMed] [Google Scholar]
- 2.Hunstad DA, Justice SS. Intracellular lifestyles and immune evasion strategies of uropathogenic Escherichia coli. Annu Rev Microbiol. 2010;64:203–221. doi: 10.1146/annurev.micro.112408.134258. [DOI] [PubMed] [Google Scholar]
- 3.Bakaletz LO. Bacterial biofilms in the upper airway—Evidence for role in pathology and implications for treatment of otitis media. Paediatr Respir Rev. 2012;13:154–159. doi: 10.1016/j.prrv.2012.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Payne DE, Boles BR. Emerging interactions between matrix components during biofilm development. Curr Genet. 2016;62:137–141. doi: 10.1007/s00294-015-0527-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitchurch CB, Tolker-Nielsen T, Ragas PC, Mattick JS. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- 6.Hall-Stoodley L, et al. Characterization of biofilm matrix, degradation by DNase treatment and evidence of capsule downregulation in Streptococcus pneumoniae clinical isolates. BMC Microbiol. 2008;8:173. doi: 10.1186/1471-2180-8-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurcisek JA, Bakaletz LO. Biofilms formed by nontypeable Haemophilus influenzae in vivo contain both double-stranded DNA and type IV pilin protein. J Bacteriol. 2007;189:3868–3875. doi: 10.1128/JB.01935-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moscoso M, García E, López R. Biofilm formation by Streptococcus pneumoniae: Role of choline, extracellular DNA, and capsular polysaccharide in microbial accretion. J Bacteriol. 2006;188:7785–7795. doi: 10.1128/JB.00673-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qin Z, et al. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–2092. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- 10.Jurcisek JA, et al. The PilA protein of non-typeable Haemophilus influenzae plays a role in biofilm formation, adherence to epithelial cells and colonization of the mammalian upper respiratory tract. Mol Microbiol. 2007;65:1288–1299. doi: 10.1111/j.1365-2958.2007.05864.x. [DOI] [PubMed] [Google Scholar]
- 11.Izano EA, Amarante MA, Kher WB, Kaplan JB. Differential roles of poly-N-acetylglucosamine surface polysaccharide and extracellular DNA in Staphylococcus aureus and Staphylococcus epidermidis biofilms. Appl Environ Microbiol. 2008;74:470–476. doi: 10.1128/AEM.02073-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Thomas VC, Thurlow LR, Boyle D, Hancock LE. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190:5690–5698. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goodman SD, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 14.Jones EA, McGillivary G, Bakaletz LO. Extracellular DNA within a nontypeable Haemophilus influenzae-induced biofilm binds human beta defensin-3 and reduces its antimicrobial activity. J Innate Immun. 2013;5:24–38. doi: 10.1159/000339961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mulcahy H, Charron-Mazenod L, Lewenza S. Extracellular DNA chelates cations and induces antibiotic resistance in Pseudomonas aeruginosa biofilms. PLoS Pathog. 2008;4:e1000213. doi: 10.1371/journal.ppat.1000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Madsen JS, Burmølle M, Hansen LH, Sørensen SJ. The interconnection between biofilm formation and horizontal gene transfer. FEMS Immunol Med Microbiol. 2012;65:183–195. doi: 10.1111/j.1574-695X.2012.00960.x. [DOI] [PubMed] [Google Scholar]
- 17.Molin S, Tolker-Nielsen T. Gene transfer occurs with enhanced efficiency in biofilms and induces enhanced stabilisation of the biofilm structure. Curr Opin Biotechnol. 2003;14:255–261. doi: 10.1016/s0958-1669(03)00036-3. [DOI] [PubMed] [Google Scholar]
- 18.Goodman SD, Nash HA. Functional replacement of a protein-induced bend in a DNA recombination site. Nature. 1989;341:251–254. doi: 10.1038/341251a0. [DOI] [PubMed] [Google Scholar]
- 19.Goodman SD, Nicholson SC, Nash HA. Deformation of DNA during site-specific recombination of bacteriophage lambda: Replacement of IHF protein by HU protein or sequence-directed bends. Proc Natl Acad Sci USA. 1992;89:11910–11914. doi: 10.1073/pnas.89.24.11910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pontiggia A, Negri A, Beltrame M, Bianchi ME. Protein HU binds specifically to kinked DNA. Mol Microbiol. 1993;7:343–350. doi: 10.1111/j.1365-2958.1993.tb01126.x. [DOI] [PubMed] [Google Scholar]
- 21.Bonnefoy E, Takahashi M, Yaniv JR. DNA-binding parameters of the HU protein of Escherichia coli to cruciform DNA. J Mol Biol. 1994;242:116–129. doi: 10.1006/jmbi.1994.1563. [DOI] [PubMed] [Google Scholar]
- 22.Kamashev D, Rouviere-Yaniv J. The histone-like protein HU binds specifically to DNA recombination and repair intermediates. EMBO J. 2000;19:6527–6535. doi: 10.1093/emboj/19.23.6527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swinger KK, Rice PA. IHF and HU: Flexible architects of bent DNA. Curr Opin Struct Biol. 2004;14:28–35. doi: 10.1016/j.sbi.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 24.Rice PA, Yang S, Mizuuchi K, Nash HA. Crystal structure of an IHF-DNA complex: A protein-induced DNA U-turn. Cell. 1996;87:1295–1306. doi: 10.1016/s0092-8674(00)81824-3. [DOI] [PubMed] [Google Scholar]
- 25.Gustave JE, Jurcisek JA, McCoy KS, Goodman SD, Bakaletz LO. Targeting bacterial integration host factor to disrupt biofilms associated with cystic fibrosis. J Cyst Fibros. 2013;12:384–389. doi: 10.1016/j.jcf.2012.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Novotny LA, Amer AO, Brockson ME, Goodman SD, Bakaletz LO. Structural stability of Burkholderia cenocepacia biofilms is reliant on eDNA structure and presence of a bacterial nucleic acid binding protein. PLoS One. 2013;8:e67629. doi: 10.1371/journal.pone.0067629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Novotny LA, Jurcisek JA, Goodman SD, Bakaletz LO. Monoclonal antibodies against DNA-binding tips of DNABII proteins disrupt biofilms in vitro and induce bacterial clearance in vivo. EBioMedicine. 2016;10:33–44. doi: 10.1016/j.ebiom.2016.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Devaraj A, Justice SS, Bakaletz LO, Goodman SD. DNABII proteins play a central role in UPEC biofilm structure. Mol Microbiol. 2015;96:1119–1135. doi: 10.1111/mmi.12994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Freire MO, et al. A bacterial-biofilm-induced oral osteolytic infection can be successfully treated by immuno-targeting an extracellular nucleoid-associated protein. Mol Oral Microbiol. 2017;32:74–88. doi: 10.1111/omi.12155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rocco CJ, Davey ME, Bakaletz LO, Goodman SD. Natural antigenic differences in the functionally equivalent extracellular DNABII proteins of bacterial biofilms provide a means for targeted biofilm therapeutics. Mol Oral Microbiol. 2017;32:118–130. doi: 10.1111/omi.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunn JS, Bakaletz LO, Wozniak DJ. What’s on the outside matters: The role of the extracellular polymeric substance of gram-negative biofilms in evading host immunity and as a target for therapeutic intervention. J Biol Chem. 2016;291:12538–12546. doi: 10.1074/jbc.R115.707547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spoering AL, Gilmore MS. Quorum sensing and DNA release in bacterial biofilms. Curr Opin Microbiol. 2006;9:133–137. doi: 10.1016/j.mib.2006.02.004. [DOI] [PubMed] [Google Scholar]
- 33.Elbaz M, Ben-Yehuda S. Following the fate of bacterial cells experiencing sudden chromosome loss. MBio. 2015;6:e00092–15. doi: 10.1128/mBio.00092-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamilton HL, Domínguez NM, Schwartz KJ, Hackett KT, Dillard JP. Neisseria gonorrhoeae secretes chromosomal DNA via a novel type IV secretion system. Mol Microbiol. 2005;55:1704–1721. doi: 10.1111/j.1365-2958.2005.04521.x. [DOI] [PubMed] [Google Scholar]
- 35.Obergfell KP, Seifert HS. 2015. Mobile DNA in the pathogenic Neisseria. Microbiol Spectr 3:MDNA3-0015-2014.
- 36.Turnbull L, et al. Explosive cell lysis as a mechanism for the biogenesis of bacterial membrane vesicles and biofilms. Nat Commun. 2016;7:11220. doi: 10.1038/ncomms11220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Brandstetter KA, Jurcisek JA, Goodman SD, Bakaletz LO, Das S. Antibodies directed against integration host factor mediate biofilm clearance from Nasopore. Laryngoscope. 2013;123:2626–2632. doi: 10.1002/lary.24183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Idicula WK, et al. Identification of biofilms in post-tympanostomy tube otorrhea. Laryngoscope. 2016;126:1946–1951. doi: 10.1002/lary.25826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brockson ME, et al. Evaluation of the kinetics and mechanism of action of anti-integration host factor-mediated disruption of bacterial biofilms. Mol Microbiol. 2014;93:1246–1258. doi: 10.1111/mmi.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Murphy TF. Respiratory infections caused by non-typeable Haemophilus influenzae. Curr Opin Infect Dis. 2003;16:129–134. doi: 10.1097/00001432-200304000-00009. [DOI] [PubMed] [Google Scholar]
- 41.Starner TD, Zhang N, Kim G, Apicella MA, McCray PB., Jr Haemophilus influenzae forms biofilms on airway epithelia: Implications in cystic fibrosis. Am J Respir Crit Care Med. 2006;174:213–220. doi: 10.1164/rccm.200509-1459OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fronzes R, Christie PJ, Waksman G. The structural biology of type IV secretion systems. Nat Rev Microbiol. 2009;7:703–714. doi: 10.1038/nrmicro2218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fronzes R, et al. Structure of a type IV secretion system core complex. Science. 2009;323:266–268. doi: 10.1126/science.1166101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bakaletz LO, et al. Demonstration of type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun. 2005;73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Carruthers MD, et al. Biological roles of nontypeable Haemophilus influenzae type IV pilus proteins encoded by the pil and com operons. J Bacteriol. 2012;194:1927–1933. doi: 10.1128/JB.06540-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mandrell RE, Apicella MA. Lipo-oligosaccharides (LOS) of mucosal pathogens: Molecular mimicry and host-modification of LOS. Immunobiology. 1993;187:382–402. doi: 10.1016/S0171-2985(11)80352-9. [DOI] [PubMed] [Google Scholar]
- 47.Koomey JM, Falkow S. Nucleotide sequence homology between the immunoglobulin A1 protease genes of Neisseria gonorrhoeae, Neisseria meningitidis, and Haemophilus influenzae. Infect Immun. 1984;43:101–107. doi: 10.1128/iai.43.1.101-107.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kroll JS, Wilks KE, Farrant JL, Langford PR. Natural genetic exchange between Haemophilus and Neisseria: Intergeneric transfer of chromosomal genes between major human pathogens. Proc Natl Acad Sci USA. 1998;95:12381–12385. doi: 10.1073/pnas.95.21.12381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Herriott RM, Meyer EM, Vogt M. Defined nongrowth media for stage II development of competence in Haemophilus influenzae. J Bacteriol. 1970;101:517–524. doi: 10.1128/jb.101.2.517-524.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mell JC, Hall IM, Redfield RJ. Defining the DNA uptake specificity of naturally competent Haemophilus influenzae cells. Nucleic Acids Res. 2012;40:8536–8549. doi: 10.1093/nar/gks640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mell JC, Redfield RJ. Natural competence and the evolution of DNA uptake specificity. J Bacteriol. 2014;196:1471–1483. doi: 10.1128/JB.01293-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li YH, Lau PC, Lee JH, Ellen RP, Cvitkovitch DG. Natural genetic transformation of Streptococcus mutans growing in biofilms. J Bacteriol. 2001;183:897–908. doi: 10.1128/JB.183.3.897-908.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Marks LR, Reddinger RM, Hakansson AP. High levels of genetic recombination during nasopharyngeal carriage and biofilm formation in Streptococcus pneumoniae. MBio. 2012;3:e00200–12. doi: 10.1128/mBio.00200-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Oggioni MR, et al. Switch from planktonic to sessile life: A major event in pneumococcal pathogenesis. Mol Microbiol. 2006;61:1196–1210. doi: 10.1111/j.1365-2958.2006.05310.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Trappetti C, et al. The impact of the competence quorum sensing system on Streptococcus pneumoniae biofilms varies depending on the experimental model. BMC Microbiol. 2011;11:75. doi: 10.1186/1471-2180-11-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Solomon JM, Grossman AD. Who’s competent and when: Regulation of natural genetic competence in bacteria. Trends Genet. 1996;12:150–155. doi: 10.1016/0168-9525(96)10014-7. [DOI] [PubMed] [Google Scholar]
- 57.Johnsborg O, Eldholm V, Håvarstein LS. Natural genetic transformation: Prevalence, mechanisms and function. Res Microbiol. 2007;158:767–778. doi: 10.1016/j.resmic.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 58.Poje G, Redfield RJ. Transformation of Haemophilus influenzae. Methods Mol Med. 2003;71:57–70. doi: 10.1385/1-59259-321-6:57. [DOI] [PubMed] [Google Scholar]
- 59.Poje G, Redfield RJ. General methods for culturing Haemophilus influenzae. Methods Mol Med. 2003;71:51–56. doi: 10.1385/1-59259-321-6:51. [DOI] [PubMed] [Google Scholar]
- 60.Roier S, et al. A novel mechanism for the biogenesis of outer membrane vesicles in gram-negative bacteria. Nat Commun. 2016;7:10515. doi: 10.1038/ncomms10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Nickel JC, Ruseska I, Wright JB, Costerton JW. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob Agents Chemother. 1985;27:619–624. doi: 10.1128/aac.27.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costerton JW, Stewart PS, Greenberg EP. Bacterial biofilms: A common cause of persistent infections. Science. 1999;284:1318–1322. doi: 10.1126/science.284.5418.1318. [DOI] [PubMed] [Google Scholar]
- 63.Flemming HC, Neu TR, Wozniak DJ. The EPS matrix: The “house of biofilm cells.”. J Bacteriol. 2007;189:7945–7947. doi: 10.1128/JB.00858-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2015;41:341–352. doi: 10.3109/1040841X.2013.841639. [DOI] [PubMed] [Google Scholar]
- 65.Montanaro L, et al. Extracellular DNA in biofilms. Int J Artif Organs. 2011;34:824–831. doi: 10.5301/ijao.5000051. [DOI] [PubMed] [Google Scholar]
- 66.Juhas M, et al. Novel type IV secretion system involved in propagation of genomic islands. J Bacteriol. 2007;189:761–771. doi: 10.1128/JB.01327-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Juhas M, et al. Sequence and functional analyses of Haemophilus spp. genomic islands. Genome Biol. 2007;8:R237. doi: 10.1186/gb-2007-8-11-r237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Juhas M. Type IV secretion systems and genomic islands-mediated horizontal gene transfer in Pseudomonas and Haemophilus. Microbiol Res. 2015;170:10–17. doi: 10.1016/j.micres.2014.06.007. [DOI] [PubMed] [Google Scholar]
- 69.Barnes AM, Ballering KS, Leibman RS, Wells CL, Dunny GM. Enterococcus faecalis produces abundant extracellular structures containing DNA in the absence of cell lysis during early biofilm formation. MBio. 2012;3:e00193–12. doi: 10.1128/mBio.00193-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schmidt H, et al. Identification and characterization of a novel genomic island integrated at selC in locus of enterocyte effacement-negative, Shiga toxin-producing Escherichia coli. Infect Immun. 2001;69:6863–6873. doi: 10.1128/IAI.69.11.6863-6873.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Marciano DK, Russel M, Simon SM. An aqueous channel for filamentous phage export. Science. 1999;284:1516–1519. doi: 10.1126/science.284.5419.1516. [DOI] [PubMed] [Google Scholar]
- 72.Davis BM, et al. Convergence of the secretory pathways for cholera toxin and the filamentous phage, CTXphi. Science. 2000;288:333–335. doi: 10.1126/science.288.5464.333. [DOI] [PubMed] [Google Scholar]
- 73.Seitz P, Blokesch M. DNA transport across the outer and inner membranes of naturally transformable Vibrio cholerae is spatially but not temporally coupled. MBio. 2014;5:e01409–14. doi: 10.1128/mBio.01409-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Krüger NJ, Stingl K. Two steps away from novelty—Principles of bacterial DNA uptake. Mol Microbiol. 2011;80:860–867. doi: 10.1111/j.1365-2958.2011.07647.x. [DOI] [PubMed] [Google Scholar]
- 75.Steinmoen H, Knutsen E, Håvarstein LS. Induction of natural competence in Streptococcus pneumoniae triggers lysis and DNA release from a subfraction of the cell population. Proc Natl Acad Sci USA. 2002;99:7681–7686. doi: 10.1073/pnas.112464599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Dillard JP, Seifert HS. A variable genetic island specific for Neisseria gonorrhoeae is involved in providing DNA for natural transformation and is found more often in disseminated infection isolates. Mol Microbiol. 2001;41:263–277. doi: 10.1046/j.1365-2958.2001.02520.x. [DOI] [PubMed] [Google Scholar]
- 77.Stewart GJ, Carlson CA, Ingraham JL. Evidence for an active role of donor cells in natural transformation of Pseudomonas stutzeri. J Bacteriol. 1983;156:30–35. doi: 10.1128/jb.156.1.30-35.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zafra O, Lamprecht-Grandío M, de Figueras CG, González-Pastor JE. Extracellular DNA release by undomesticated Bacillus subtilis is regulated by early competence. PLoS One. 2012;7:e48716. doi: 10.1371/journal.pone.0048716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Novotny LA, Mason KM, Bakaletz LO. Development of a chinchilla model to allow direct, continuous, biophotonic imaging of bioluminescent nontypeable Haemophilus influenzae during experimental otitis media. Infect Immun. 2005;73:609–611. doi: 10.1128/IAI.73.1.609-611.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Barcak GJ, Chandler MS, Redfield RJ, Tomb JF. Genetic systems in Haemophilus influenzae. Methods Enzymol. 1991;204:321–342. doi: 10.1016/0076-6879(91)04016-h. [DOI] [PubMed] [Google Scholar]
- 81.Macfadyen LP, Ma C, Redfield RJ. A 3′,5′ cyclic AMP (cAMP) phosphodiesterase modulates cAMP levels and optimizes competence in Haemophilus influenzae Rd. J Bacteriol. 1998;180:4401–4405. doi: 10.1128/jb.180.17.4401-4405.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jurcisek JA, Dickson AC, Bruggeman ME, Bakaletz LO. In vitro biofilm formation in an 8-well chamber slide. J Vis Exp. 2011;(47):e2481. doi: 10.3791/2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Heydorn A, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–2407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- 84.Vorregaard M. 2008. Comstat2—A modern 3D image analysis environment for biofilms. Master’s thesis (Technical University of Denmark, Kongens Lyngby, Denmark)
- 85.Cameron AD, Redfield RJ. CRP binding and transcription activation at CRP-S sites. J Mol Biol. 2008;383:313–323. doi: 10.1016/j.jmb.2008.08.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.