Most bacteria do not live as single cells but form communities on surfaces called biofilms (1). Similar to human communities, some are fairly homogeneous, whereas others contain a diversity of microbes.
Biofilms are important for the growth and survival of all sorts of bacteria: bacteria that live in water or soil and also bacteria that live in or on animal hosts, including commensal bacteria, beneficial bacteria, and disease-causing bacteria (1). Biofilm communities are held together by a matrix containing a collection of molecules that are released by the bacterial cells within the biofilm (2). The composition of the biofilm matrix differs with different organisms but most often contains extracellular DNA (eDNA), polysaccharides, and proteins. The article in PNAS by Jurcisek et al. (3) demonstrates that nontypeable Haemophilus influenzae (Hflu) (4) uses components from two different secretion systems to release DNA and a biofilm-associated DNA-binding protein integration host factor (IHF) to allow efficient biofilm formation. This work shows that the secretion of DNA and DNABII proteins is an active process that uses components from two different apparatuses to enable robust biofilm formation.
Bacteria are single-cell organisms that have a complex envelope structure that provides essential functions for the bacteria, including protecting the cell’s interior (cytoplasm) from injury, providing energy to the cell, maintaining the characteristic shape of each bacterial species, and mediating cell division (5). Most bacteria fall into two major classes based on their envelope architecture, gram-negative and gram-positive. There are six well-defined secretion systems in gram-negative bacteria (type I to VI); all can transport proteins into or outside of a bacterial cell, and a few can also transport DNA, often complexed with proteins (6). How the molecules that form biofilm matrixes are released from bacterial cells is not always understood, but it has been assumed, and in a few instances shown, that intracellular material is released by selective cell disruption by bacterial factors or by phage-mediated lysis (7, 8). The Hflu biofilm matrix is known to have DNA complexed in a lattice-like structure with the IHF and histone-like protein (HU) chromosome-organizing proteins (DNABII proteins) bound at vertices of the lattice (ref. 9 and this work). Using a variety of clever microscopic techniques, it was conclusively shown that Hflu secretes DNA and IHF from a single location near the end of the bacterial cell, and that this secretion occurs without cell lysis. The main tool used in the study by Jurcisek et al. (3) is fluorescence microscopy, using DNA stains that either cannot access the cytoplasm of intact cells or can diffuse across the envelope. Using these dyes, they show that DNA is released from a subpopulation of cells within 3 h after the cells were deposited on a surface without any apparent cell lysis. Cells that extruded the DNA had lost their chromosomal contents, suggesting a process of programmed cell death. At later times, there is cell lysis, and it appears that the same type of biofilm may be formed by this alternative means of DNA/protein release. The DNABII protein IHF was also present in the same location with the secreted DNA, and these complexes were localized to a single spot on the secreting cells near one end of the cell. This localization of the secreted DNA was confirmed by a compelling scanning, transmission electron microscopy (STEM) image. These data show that the secretion of chromosomal DNA and associated proteins occurs by a process that is distinct from lysis.
The polar location where the DNA and DNABII proteins were secreted was similar to where the type IV pilus [not to be confused with type IV secretion, but produced by an apparatus related to the type II secretion apparatus (10)] has been observed to emerge from the Hflu cell (11). The pilus is a dynamic fiber involved in adherence and motility, and the apparatus that produces the pilus can also be responsible for internalizing DNA for genetic transformation in many gram-negative bacteria (12). Jurcisek et al. (3) asked whether the secretin protein that allows the pilus to transit the outer membrane of the envelope might also be the portal for DNA and DNABII protein secretion. Indeed, inactivating the comE gene encoding the secretin totally blocked the secretion of both DNA and IHF. Interestingly, mutation of the main pilus subunit, pilin, did not alter DNA/IHF secretion, showing that the ComE secretin can be used for more than one process. However, two conditions that induce competence for genetic transformation in Hflu also increased the frequency of cells undergoing DNA secretion. This result suggests that the secreted DNA may also be a substrate for genetic transfer in addition to its role in biofilm formation, but this possibility was not directly tested here.
Based on the observation that another host-restricted, gram-negative bacterium, Neisseria gonorrhoeae, secretes its chromosomal DNA out of the cell using a type IV secretion system (a conjugation-like system) (13), Jurcisek et al. (3) searched for genes encoding proteins similar to the proteins found in type IV secretion systems. They found orthologs of the type IV secretion TraC, TraG, TraI, ParA, ParB, and TraD proteins but no orthologs of the outer membrane type IV secretion components. When the traC and traG genes were inactivated together, which would be predicted to inactivate the inner membrane complex of the type IV secretion apparatus, the secretion of eDNA and IHF was lost. Differential staining of DNA by fluorescence microscopy and immuno-STEM showed that DNA was trapped in cytoplasm in the traCG mutant and, importantly, was trapped in the periplasm in the comE mutant. Similar analyses were not reported for the IHF protein, but because secretion of this protein also requires TraCG and ComE, it is likely that this protein would show the same signals if it were not degraded by the many proteases of the periplasm.
Finally, Jurcisek et al. (3) show that inactivation of traCG or comE prevents normal biofilm formation and that the amount of DNA and the IHF protein in the biofilms formed by the mutants was drastically reduced. The traCG mutant had reduced biofilm formation relative to the parent strain but was still more competent for biofilm formation than the comE mutant. This result is consistent with the established role of type IV pili in biofilm formation, because the comE mutant would be deficient for both pilus elaboration and DNA/DNABII secretion. Interestingly, addition of DNA and the DNABII protein IHF to the comE mutant partially restored the biofilm, albeit not to the levels seen with the parent strain.
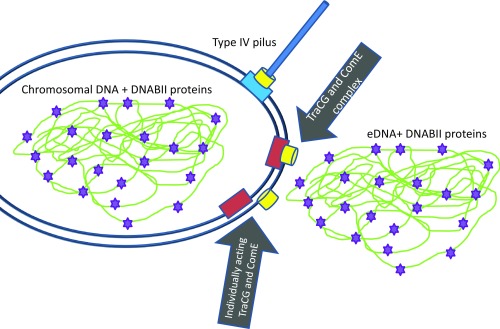
The study by Jurcisek et al. (3) shows that eDNA and at least one protein important for maintaining a biofilm are transported in a dedicated process that appears to use proteins normally associated with conjugation (ComC and ComG) and the ComE secretin that is involved in pilus expression and DNA transformation, but there are several next-step questions to be answered. It will be informative to determine which other known factors of the Tra system and the type IV pilus system are required for the secretion event and whether there are unrecognized factors unique to this secretion system. The identification of all of the factors required for eDNA and DNABII protein secretion will also help determine whether the transport through the inner membrane to the periplasm by TraCG and associated factors is coupled to the transport through the ComE secretin or whether these are individual steps by separate protein complexes (Fig. 1). Jurcisek et al. (3) prefer the model that the inner membrane complex formed by TraCG and the ComE secretin acts individually, although the micrographs presented in the paper clearly demonstrate that when the ComE secretin is inactivated, the DNA becomes trapped in the periplasm. However, this result might reflect a periplasmic state of the DNA that is only relevant to the comE mutant cells and does not exist in wild-type cells. It will need to be determined whether the TraCG, ComE, and other factors form a complex that spans the entire envelope to answer this question definitively. Because the ComE secretin is clearly involved in three processes: export of DNA and proteins for biofilm formation, formation of a type IV pilus, and the import of DNA for transformation, these data beg the question of how the ComE proteins involved in these processes are directed to the different roles/apparatuses. It is a reasonable hypothesis that the ComE secretin complexes interact with distinct partners to provide the alternative functions regardless of whether the secretin and inner membrane complex directly associate. Because the type IV pilus, eDNA, and DNABII proteins are all involved in biofilm formation and stability, there may be coordination between the systems, but the normal level of eDNA secretion in a pilus mutant and retained transformation competence in the TraCG mutant show that these processes are not intimately linked. Finally, it is not clear from the data presented whether the eDNA and IHF protein are exported together from the cytoplasm, associate in the periplasm, and are exported together, or are separately exported to the exterior and then associate. Because the DNABII proteins normally decorate the entire chromosome, the most simplistic model would have the DNA and protein exiting together. If the protein(s) and eDNA take separate paths, this system will have to assemble the lattices outside the cell, which would be much more complicated to accomplish. It is likely that the HU protein is also exported using the same mechanism as IHF, and if other proteins or molecules like polysaccharides also exit the cell using this secretion process, this finding would be quite exciting and broaden the importance of this secretion system to biofilm formation.
Fig. 1.
Single Hflu cell is depicted showing the chromosomal nucleoid (green line), which normally has the DNABII proteins IHF and HU bound (purple star). After eDNA secretion, this cell would no longer contain chromosomal DNA (not depicted). The type IV pilus is depicted using the ComE secretin (yellow cylinder) to pass the pilus through the outer membrane but otherwise uses a separate apparatus. This same apparatus is used for DNA transformation (not depicted). The two major possibilities for the arrangement of the ComE secretin pore and the TraCG inner membrane complex (red box) are shown. The coupled complex envisions the TraCG, ComE, and other unknown factors forming a distinct transport complex that spans the envelope. The individual component model is the one preferred by Jurcisek et al. (3), and suggests that the inner membrane complex and ComE secretin act separately. It is unlikely that the DNA and DNABII proteins in the chromosome and the eDNA are arranged identically as depicted.
There are many additional questions resulting from this work that might be answered in the future. Do many other bacterial species use dedicated secretion systems to secrete eDNA and specific proteins to promote the formation and stability of biofilms? Many other bacterial species have been suggested to rely on DNABII proteins to stabilize biofilms (9), and a similar secretion process could function in these organisms. The enhancement of eDNA secretion by conditions that enhance competence is also intriguing and may support a role for this system in prompting DNA transformation, similar to that shown for N. gonorrhoeae (13), in addition to biofilm formation. If a role for the DNABII proteins in DNA transformation were discovered, this finding would link these two processes more directly into the pathogenesis of Hflu infections. Alternatively, the secreted eDNA and remaining dead donor cells could be an important food source for intact cells within the complex structure of the biofilm. Lastly, how this process is regulated so that only a subset of cells die to produce eDNA and DNABII proteins will be an important aspect to understand the population dynamics underscoring biofilm formation.
Acknowledgments
My work is supported by NIH Grant R37 AI033493.
Footnotes
Conflict of interest statement: The author has received consulting fees from Pfizer, Inc., unrelated to this work.
See companion article on page E6632.
References
- 1.Flemming HC, et al. Biofilms: An emergent form of bacterial life. Nat Rev Microbiol. 2016;14:563–575. doi: 10.1038/nrmicro.2016.94. [DOI] [PubMed] [Google Scholar]
- 2.Dragoš A, Kovács AT. The peculiar functions of the bacterial extracellular matrix. Trends Microbiol. 2017;25:257–266. doi: 10.1016/j.tim.2016.12.010. [DOI] [PubMed] [Google Scholar]
- 3.Jurcisek JA, Brockman KL, Novotny LA, Goodman SD, Bakaletz LO. Nontypeable Haemophilus influenzae releases DNA and DNABII proteins via a T4SS-like complex and ComE of the type IV pilus machinery. Proc Natl Acad Sci USA. 2017;114:E6632–E6641. doi: 10.1073/pnas.1705508114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duell BL, Su YC, Riesbeck K. Host-pathogen interactions of nontypeable Haemophilus influenzae: From commensal to pathogen. FEBS Lett. 2016;590:3840–3853. doi: 10.1002/1873-3468.12351. [DOI] [PubMed] [Google Scholar]
- 5.Vollmer W, Blanot D, de Pedro MA. Peptidoglycan structure and architecture. FEMS Microbiol Rev. 2008;32:149–167. doi: 10.1111/j.1574-6976.2007.00094.x. [DOI] [PubMed] [Google Scholar]
- 6.Gold V, Kudryashev M. Recent progress in structure and dynamics of dual-membrane-spanning bacterial nanomachines. Curr Opin Struct Biol. 2016;39:1–7. doi: 10.1016/j.sbi.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 7.Okshevsky M, Meyer RL. The role of extracellular DNA in the establishment, maintenance and perpetuation of bacterial biofilms. Crit Rev Microbiol. 2015;41:341–352. doi: 10.3109/1040841X.2013.841639. [DOI] [PubMed] [Google Scholar]
- 8.Gödeke J, Paul K, Lassak J, Thormann KM. Phage-induced lysis enhances biofilm formation in Shewanella oneidensis MR-1. ISME J. 2011;5:613–626. doi: 10.1038/ismej.2010.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Goodman SD, et al. Biofilms can be dispersed by focusing the immune system on a common family of bacterial nucleoid-associated proteins. Mucosal Immunol. 2011;4:625–637. doi: 10.1038/mi.2011.27. [DOI] [PubMed] [Google Scholar]
- 10.Hansen JK, Forest KT. Type IV pilin structures: Insights on shared architecture, fiber assembly, receptor binding and type II secretion. J Mol Microbiol Biotechnol. 2006;11:192–207. doi: 10.1159/000094054. [DOI] [PubMed] [Google Scholar]
- 11.Bakaletz LO, et al. Demonstration of Type IV pilus expression and a twitching phenotype by Haemophilus influenzae. Infect Immun. 2005;73:1635–1643. doi: 10.1128/IAI.73.3.1635-1643.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Berry JL, Pelicic V. Exceptionally widespread nanomachines composed of type IV pilins: The prokaryotic Swiss Army knives. FEMS Microbiol Rev. 2015;39:134–154. doi: 10.1093/femsre/fuu001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hamilton HL, Dillard JP. Natural transformation of Neisseria gonorrhoeae: From DNA donation to homologous recombination. Mol Microbiol. 2006;59:376–385. doi: 10.1111/j.1365-2958.2005.04964.x. [DOI] [PubMed] [Google Scholar]