Abstract
Staphylococcus aureus (S. aureus) is one of the most common causes of biofilm infections in periprosthetic joint infections (PJIs). Accumulating evidence has shown that the immunosuppressive environment established by S. aureus biofilm infection in PJIs involves the presence of myeloid-derived suppressor cells (MDSCs) and M2-macrophages. Due to the diversity of MDSCs, little is known about whether S. aureus biofilm preferentially expands specific MDSC subsets or whether MDSCs can further differentiate into M2-macrophages during S. aureus biofilm infection. Here, we show that in agreement with the results from an established rat PJI model, S. aureus biofilm cocultured with freshly isolated bone marrow cells (BMCs) in vitro significantly increases the proportions of MDSCs, total macrophages and M2-macrophages. Interestingly, we find that treatment of the BMCs in vitro with S. aureus biofilm preferentially promotes the expansion of monocytic MDSCs but not granulocytic MDSCs. Biofilm treatment also substantially enhances the overall MDSC immunosuppressive activity in addition to the MDSC expansion in vitro. Importantly, we provide evidence that S. aureus biofilm is capable of further stimulating the conversion of monocytic MDSCs into M2-macrophages in vitro and in vivo. Collectively, our studies reveal a direct link between MDSCs and M2-macrophages occurring in S. aureus-associated PJIs.
Introduction
Prosthetic joint infections (PJIs) are a devastating complication after arthroplasty, which greatly affect the quality of a patient’s recovery [1–3]. The incidence of PJIs is about 1% to 2% for primary total joint arthroplasties, and approximately at 2%—6% for a revision joint arthroplasty [4]. Treatment strategies include radical debridement with retention of the implants, one-stage (or immediate) revision, two-stage (or delayed) revision, and excision arthroplasty [5]. However, the reinfection rate after re-implantation of the prosthesis is still high and more than 10% in the follow up [6, 7].
Staphylococcus aureus (S. aureus), a Gram-positive microorganism, is a major human pathogen and a major cause of community-associated and nosocomial infections. It also elicits biofilm infection on orthopedic implants or other internal medical devices. Biofilms are surface-associated communities of bacteria enclosed within a self-produced matrix, which is consisted of mainly polysaccharides, nucleic acids, proteins and lipids. S. aureus biofilm infection in PJIs often causes a serious health care concern based on their protection from antibiotic treatment and the host’s immune system. Currently, the methicillin-resistant S. aureus (MRSA) has gradually increased its presence in the human population causing this pathogen as a greater therapeutic problem [8]. The development of vaccines or immunotherapies to treat S. aureus-associated biofilm infection is an urgent need in the realm of public health [9, 10].
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of activated immature myeloid cells with the ability to suppress T cell responses. Intrinsically, MDSCs can be discriminated from mature myeloid cells (i.e., dentritic cells, macrophages, and neutrophils) by unique cell-surface marker signatures. For example, MDSCs in mice are commonly characterized as CD11b+Gr1+ cells, whereas MDSCs in rats are identified as CD11bc+His48+ cells. Due to the heterogeneity of these immature myeloid cells, MDSCs can be further classified into two subsets: monocytic MDSCs (M-MDSCs) and granulocytic MDSCs (G-MDSCs). Morphologically, M-MDSCs are immature mononuclear cells similar to monocytes, while G-MDSCs are immature polymorphonuclear cells similar to neutrophils. In mice, the Gr1 marker is composed of two components: Ly6G and Ly6C, which can serve as the subset markers for M-MDSCs (CD11b+Ly6ChighLy6G-) and G-MDSCs (CD11b+Ly6ClowLy6G+) [11]. In clinical investigations, MDSCs have been shown to accumulate during pathological situations, such as tumor development, acute or chronic inflammation and trauma, as well as bacterial infection [12, 13]. Although several studies have previously shown that MDSC expansion could occur during S. aureus biofilm infection [14], little is known about whether there is a preferential expansion of specific subsets within MDSCs in response to S. aureus biofilm.
In addition to MDSC expansion, S. aureus biofilm also exhibits the ability to polarize macrophages toward the alternatively activated (M2) macrophages [15], which are distinct from the classically activated (M1) macrophages [16]. M1-macrophages are considered as pro-inflammatory macrophages involved in host defense against bacterial infections, whereas M2-macrophages are responsible for anti-inflammation and bacterial persistence [17–23]. M2-macrophages also function to suppress T cell proliferation [24]. Activation of M2-macrophages is usually associated with abundant expression and secretion of anti-inflammatory factors such as Arginase-1, IL-10 and IL-12, and thereby restrains a pro-inflammatory immune response [25, 26]. Although both MDSCs and M2-macrophages play critical roles in the immune suppression during S. aureus biofilm infection, it remains unclear if MDSCs could further differentiate into M2-macrophages upon exposure to S. aureus biofilm.
In this report, both in vivo and in vitro model systems were designed to examine the production of both MDSCs and M2-macrophages during S. aureus biofilm stimulation. In a rat PJI model, we did find that circulating MDSCs and M2-macrophages were accumulated in the peripheral blood during S. aureus biofilm infection. Coculture of bone marrow precursor cells (BMCs) with S. aureus biofilm in vitro also caused a significant increase in the proportions of MDSCs and M2-macrophages. Specially, M-MDSCs, but not G-MDSCs, were preferentially expanded in the in vitro coculture system. Moreover, results from both in vitro and in vivo studies supported that MDSCs could turn into M2-macrophages in the presence of S. aureus biofilm. A detailed understanding of the development of MDSCs and M2-macrophages during S. aureus biofilm infection may allow us to develop better strategies in the treatment of S. aureus-associated PJIs or chronic osteomyelitis.
Materials and methods
Rats and mice
Male LEW/SsNNarl rats (12 weeks of age) with 350–400 gram and male C57BL/6J mice (12 weeks of age) with 22–25 gram obtained from National Laboratory Animal Center (Taiwan) were used for S. aureus biofilm infection. The Pgk1-EGFP transgenic mice (C57BL/6J-Tg[Pgk1-EGFP]03Narl) (National Laboratory Animal Center, Taiwan) were utilized to provide the CD11b-positive MDSCs that express EGFP. All animal experiments were approved by the Institutional Animal Care and Use Committee of the Chang Gung Memorial Hospital (IACUC permit number: 2011102502, 2012122508 and 2013122701), and were performed in accordance with the Animal Protection Law by the Council of Agriculture, Executive Yuan (R.O.C.) and the guideline of National Research Council (U.S.A) for the care and use of laboratory animals.
S. aureus strain and biofilm formation
S. aureus (ATCC43300) was purchased from Bioresource Collection and Research Center (BCRC), Taiwan. To prepare S. aureus biofilm, the bacteria were cultured in BHI media for 4 days as previously described [15, 27]. Biofilm communities were collected by centrifugation at 3900 xg for 15 min, and then sterilized by autoclaving. Biofilm pellets were resuspended with RPMI medium and total protein amounts of the lysates were measured using BCA protein assay kit (Pierce). For the in vivo study, S. aureus cells were pelleted, washed 3 times, and resuspended in normal saline. Bacterial concentrations were estimated spectrophotometrically by measuring the absorbance at 600 nm (A600). Colony forming units (CFU) were counted on the plates to verify the optical measurement.
S. aureus biofilm infection in rats and in mice
The rat model of the S. aureus orthopedic implant infection was performed as described previously with minor modifications [28]. Briefly, male LEW/SsNNarl rats were anesthetized with a 1:1 mixture of Zoletil 50 and Rompun 2% (1 ml/kg in rat and mice) and the surgical site was disinfected with povidone-iodine. A skin incision was made near the right knee. A burr hole was made in the femoral intercondylar notch extending into the intramedullary canal using a 26-gauge needle, and then a pre-cut 0.5 cm orthopedic grade Kirschner (K)-wire (0.6-mm diameter, Synthes GmbH, Switzerland) was implanted into the intramedullary canal. Following the insertion of K-wire implant, 2×103 CFU of S. aureus were inoculated. Subsequently, the burr hole was sealed with bone wax and then the surgical site was closed with Coated VICRYL* 4–0 sutures. Rats that received sterile implants served as the sham-operated group. For pain relief, rats were treated with Ketorolac (0.5~1 mg/kg, IM) immediately after the surgery and 24 hours later. For the PJI model in mice, all the procedures of S. aureus infection were the same as those in rats. Herein, after infection with S. aureus for 7 days in C57BL/6J mice, 1×107 CD11b-positive MDSCs isolated from Pgk1-EGFP transgenic mice were injected into the infected mice intravenously. Twenty-four hours later, the mice were sacrificed and the tissue near the S. aureus infected site were taken, embedded and frozen in -80°C.
Scanning electron microscopy
Rats were sacrificed at day 7 after S. aureus infection. The bone rougeur was used to cut the femur longitudinally and a piece of fragment (2×4 mm2) was picked. Femur samples were fixed in 0.1M cacodylate buffer (pH 7.4) containing 3% glutaraldehyde and 2% paraformaldehyde for 2 hr at 4°C. Subsequently, fixed specimens were cut longitudinally and washed three times in 0.1 M cacodylate buffer (pH 7.4) for 10 minutes at 4°C. In the fume hood, specimens were soaked in 0.1 M cacodylate buffer containing 1% Osmium Teteroxide for 1 hr at 4°C. After rinsing three times using 0.1M cacodylate buffer for 10 min at 4°C, specimens were dehydrated using a graded series of ethanol washes and critical-pointed-dried using a critical point dryer (CPD). Dried specimens were coated with gold-palladium. Samples were viewed using a HITACHI S-5000 scanning electron microscope.
Flow cytometry analysis
To measure the dynamic changes in the proportions of immune cell populations in the peripheral blood of rats, 50 μl of blood samples was taken for flow cytometry analysis. After lysing red blood cells, the remaining leukocytes were resuspended in PBS containing 2% FBS, and then were stained with HIS48-FITC (eBioscience), CD11bc-allophycocyanin (BioLegend), CD68-PE (Biolegend) or CD206-PerCP (Abcam) for 30 min at 4°C. On the other hand, mice cells were analyzed by staining with CD11b-FITC (BD Bioscience), Gr-1-PE, Ly6G-APC (BD Bioscience), Ly6C-PerCP-Cy5.5 (eBioscience), F4/80-PE (eBioscience) or CD206-PerCP (Biolegend, #141716). Acquisition of flow cytometry data from the BD FACSCantoII flow cytometer was performed using FACSDiva software (BD Biosciences). The number of events analyzed was 10,000 per sample. Analysis was performed using FlowJo software (Tree Star).
Isolation of specific immune cell populations from mice bone marrow
BMCs were collected from bone marrow of mouse femur. The CD11b-positive cell population was isolated using anti-CD11b antibody conjugating with biotin in combination along with anti-biotin magnetic beads (STEMCELL™) according to the manufacturer’s instructions. The purity of the isolated cell population was verified by flow cytometry. Two MDSC subsets, M-MDSCs (Ly6ChighLy6G-) and G-MDSCs (Ly6ClowLy6G+), were then sorted from the CD11b-positive population by using BD FACSAria Fusion cell sorter.
Coculture of the isolated cell populations with S. aureus biofilm in vitro
Mouse BMCs or the isolated cell populations were seeded in the 24-well plate and cocultured with different concentrations of S. aureus biofilm loaded in the transwell cell culture insert (0.4 μm pore size). All the cocultures of the indicated cell populations with S. aureus biofilm were maintained in complete medium (RPMI1640 with 10% FBS). After coculture for 48 or 72 hr, the biofilm-treated cells were analyzed by flow cytometry (BD FACSCanto II) using specific antibodies.
T cell proliferation assays
Mouse BMCs were seeded in 24-well plates and then left untreated or treated with S. aureus biofilm for 48 hr. After 48-hr treatment, the CD11b-positive cell population from the untreated or biofilm-treated BMCs was isolated using anti-CD11b antibody conjugating with biotin in combination along with anti-biotin magnetic beads (STEMCELL™). The untreated and biofilm-treated BMCs as well as their isolated CD11b-positive cell populations were subsequently cocultured with CFSE-labeled spleen T cells that were stimulated by Dynabeads Mouse T-Activator CD3/CD28 (Gibco). Different ratios of BMCs (or the isolated CD11b-positive cells) to activated T cells (2×105 cells/well in a 96-well round bottom plate in RPMI-1640 with 10% FBS) were examined in the study.
For isolation of spleen T cells, mouse spleens were gently pressed through a strainer using the plunger end of a syringe to get singlet cell. These cells were washed with PBS buffer and ACK lysing buffer (Gibco). Cells in warm RPMI (2 ml) were injected to nylon wool column, and washed with RPMI. The column was sealed and incubated at 37°C and 5% CO2 for 45 minutes. Cells were then eluted with 10 ml of warm RPMI.
Analysis of regulatory T (Treg) cells
Mouse BMCs were seeded in 24-well plates and then left untreated or treated with S. aureus biofilm. After 48-hr treatment, the CD11b-positive cell populations were isolated from the untreated or biofilm-treated BMCs. The isolated CD11b-positive cell populations were then cocultured with spleen T cells that were stimulated by Dynabeads Mouse T-Activator CD3/CD28 (Gibco). After 24- or 48-hr coculture, the proportions of Foxp3+CD25+CD4+ Tregs in the cell mixtures were analyzed by flow cytometry using a True-Nuclear One Step Staining Mouse Treg Flow kit (FOXP3 Alexa Fluor 488/CD25 PE/CD4 PerCP; Biolegend). Results were expressed as the percentages of Foxp3+CD25+CD4+ T cells in CD4+ cells.
Quantitative reverse transcription (RT)-PCR
Total RNAs were isolated from cells using an RNeasy Mini Kit (Qiagen, Valencia, CA) in combination with an RNase-free DNase set (Qiagen, Valencia, CA). RNA samples were first converted into cDNAs using RevertAid First Strand cDNA Synthesis Kit (Thermo Fisher, Waltham, MA). Real-time PCR was performed based on SYBR-Green fluorescence (Bio-Rad, #170-8882AP Hercules, CA) and using specific primer sets for Arginase-1, iNOS, IL-6 and IL-10. Relative gene expression levels were normalized to GAPDH expression. The primer sets were following: 5’-CACGGCAGTGGCTTTAACCT and 5’-TGGCGCATTCACAGTCACTT for Arginase-1; 5’-TGGCCACCTTGTTCAGCTACG and 5’-GCCAAGGCCAAACACAGCATAC for iNOS; 5’-TCCAGTTGCCTTCTTGGGAC and 5’-GTACTCCAGAAGACCAGAGG for IL-6; 5’-GGTTGCCAAGCCTTATCGGA and 5’-ACCTGCTCCACTGCCTTGCT for IL-10; 5’-CAAGGTCATCCATGACAACTTTG and 5’-GTCCACCACCCTGTTGCTGTAG for GAPDH.
Immunofluorescence staining and confocal microscopy
Tissue samples were embedded in optimal cutting temperature compound (OCT) and snap frozen in liquid nitrogen. Tissue sections (4 μm) were fixed in ethanol/acetic acid fixative solution for 2–10 min, and then were stained with anti-F4/80-PE (eBioscience), CD206-PerCP-Cy5.5 (Biolegend) overnight at 4°C in a humidified chamber. After three times of washes, slides were stained with DAPI and mounted with Prolong Gold mounting reagent (Invitrogen). Confocal imaging was performed using Leica SP5 II confocal microscope.
Statistical analyses
Statistical analyses were performed using Student’s t-test for independent samples, with significance determined at P<0.05. All data and standard deviation (SD) were calculated and graphed by Microsoft Excel software (Microsoft, Redmond, WA).
Results
Biofilm formation in an S. aureus-infected PJI model
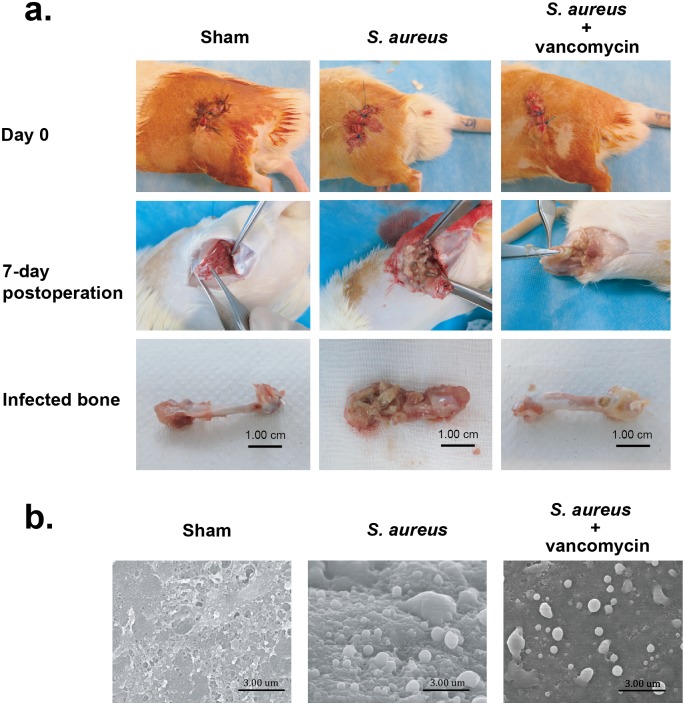
To mimic acute and chronic S. aureus biofilm infection in vivo, we initially established a PJI model in rats. In the PJI model, S. aureus (2×103 CFU) were inoculated at a metallic orthopedic implant (Kirschner wire, 0.6 mm diameter x 0.5 cm) in rat femurs. In addition to the sham-operated and S. aureus-infected groups, the S. aureus-infected group receiving vancomycin (2 mg/kg) was also included in the experiment. At 7 days after surgery, all rats were sacrificed and the treated femurs were taken (Fig 1a). Compared to the sham-operated femurs, severe osteolytic bony destruction and formation of abscesses were observed in the S. aureus-infected femurs; however, vancomycin treatment substantially reduced the bony destruction caused by S. aureus infection (Fig 1a). By using scanning electron microscopy, biofilm formation and S. aureus cocci in the lumen of the femur were evidently detected in both S. aureus-infected groups, despite a lower extent of bacterial dissemination for the vancomycin-treated group (Fig 1b). These results indicated that our established PJI model in rats shows persistent biofilm infection and may allow further investigation of host immune responses to S. aureus biofilm infection in vivo.
Fig 1. Surgical operation and S. aureus biofim formation in a rat PJI model.
(a) Rats were divided into three groups including the sham group, the S. aureus-infected group and the S. aureus-infected group receiving vancomycin treatment. All rats were sacrificed 7 days after surgery and the treated femurs were taken. Notably, severe bone osteolytic lesion with pus formation was observed in the S aureus-infected femur, but less osteolytic lesion was detected in the vancomycin-treated infection femur. (b) Scanning electron microscopy images of biofilm formation in femurs at day 7 post-infection. Biofilm formation and S. aureus cocci were more evident in the femur lumen of the S. aureus-infected group than those of the vanomycin-treated infection group (original magnification ×10,000).
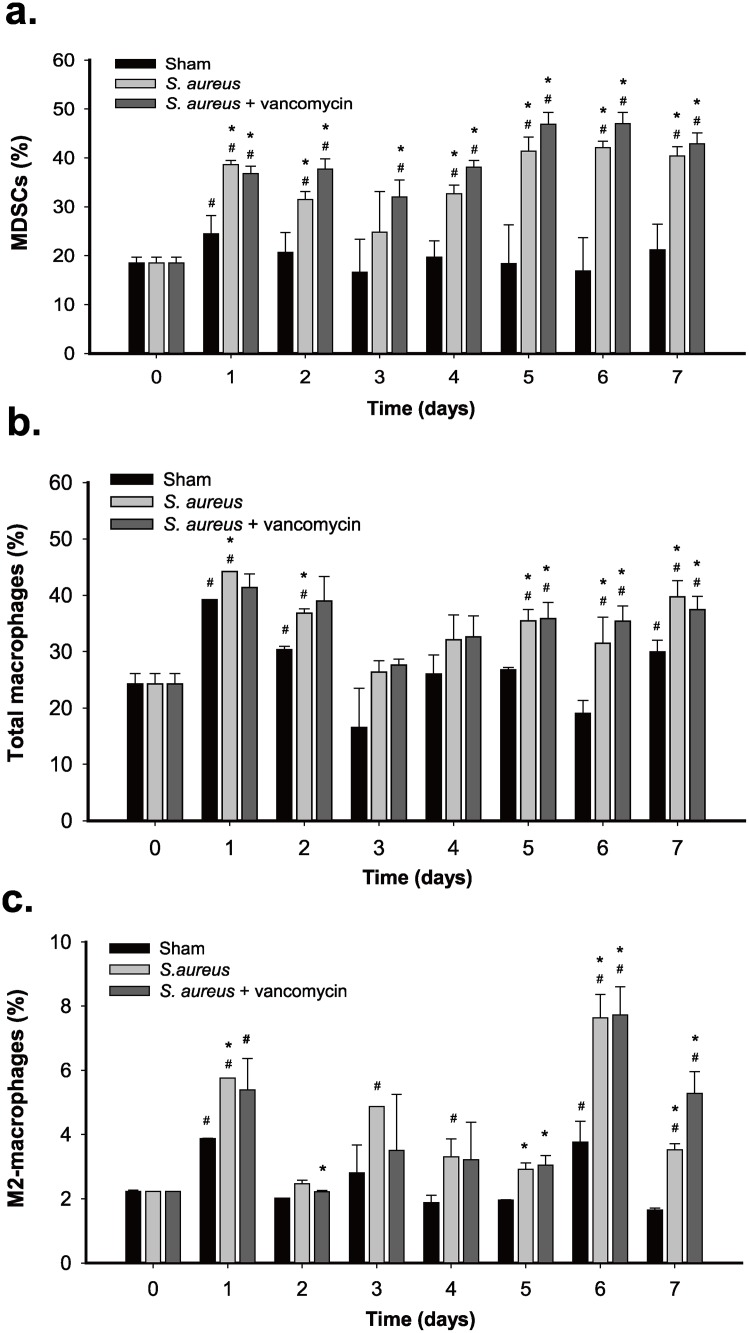
Significant expansion of MDSCs, total macrophages and M2-macrophages in the peripheral blood of rats during S. aureus biofilm infection
To investigate the possible immunosuppressive effect caused by S. aureus biofilm infection in vivo, we examined if the proportions of the circulating MDSCs, total macrophages and M2-macrophages were changed during S. aureus infection in our PJI model. Blood samples from the sham-operated group, the untreated infection group, and the vancomycin-treated infection group were then collected at different time points after operation. Compared to blood samples obtained immediately prior to surgery (designated as day 0), blood samples collected from day 1 after surgery (or surgery plus S. aureus infection) exhibited higher proportions of MDSCs (CD11bc+HIS48+), macrophages (CD68+) and M2-macrophages (CD68+CD206+) in all three operated groups (Fig 2a–2c and S1 Fig), suggesting that surgical injury might contribute to systemic expansion of these immune cells. In the sham-operated group, the increased proportions of these immune cells detected on day 1 after surgery could decline gradually to normal levels on days 2 or 3 after surgery (Fig 2). However, when these immune cells were examined in the untreated infection group or in the vancomycin-treated infection group, we found that the proportions of MDSCs, total macrophages and M2-macrophages in the two S. aureus-infected groups were regularly higher than those in the sham-operated group at all postoperative times (Fig 2a–2c). These results suggested that S. aureus biofilm infection substantially stimulated the expansion of MDSCs, total macrophages, and M2-macrophages in the PJI model. Noteworthily, there were no significant differences in the expansion rates of MDSCs, total macrophages, and M2-macrophages between the two infected groups that received no treatment or vancomycin treatment, implicating that vancomycin treatment could not completely eliminate S. aureus biofilm infections during the experimental period.
Fig 2. Expansion of MDSCs, total macrophages, and M2-macrophages during S. aureus infection in rats.
The sham group, the S. aureus-infected group, and the S. aureus-infected group receiving vancomycin were included in the experiments and each group contained 4 rats. Blood samples were collected at different time points after operation. After lysis of the red blood cells, the remaining leukocytes were analyzed for the proportions of CD11bc+His48+ MDSCs (a), CD68+ macrophages (b), and CD68+CD206+ M2-macrophages (c) by flow cytometry. Symbol # indicates significant difference vs. day 0 (p < 0.01) and symbol * indicates significant difference vs. the shame group (p < 0.01).
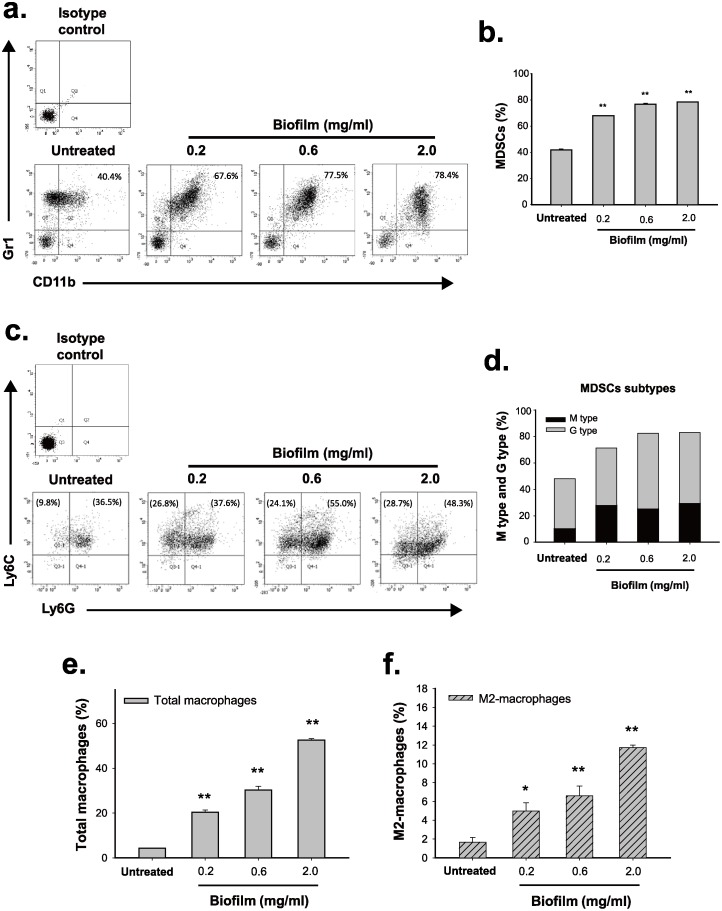
Treatment of mouse bone marrow cells with S. aureus biofilm increases the proportions of MDSCs, total macrophages and M2-macrophages in vitro
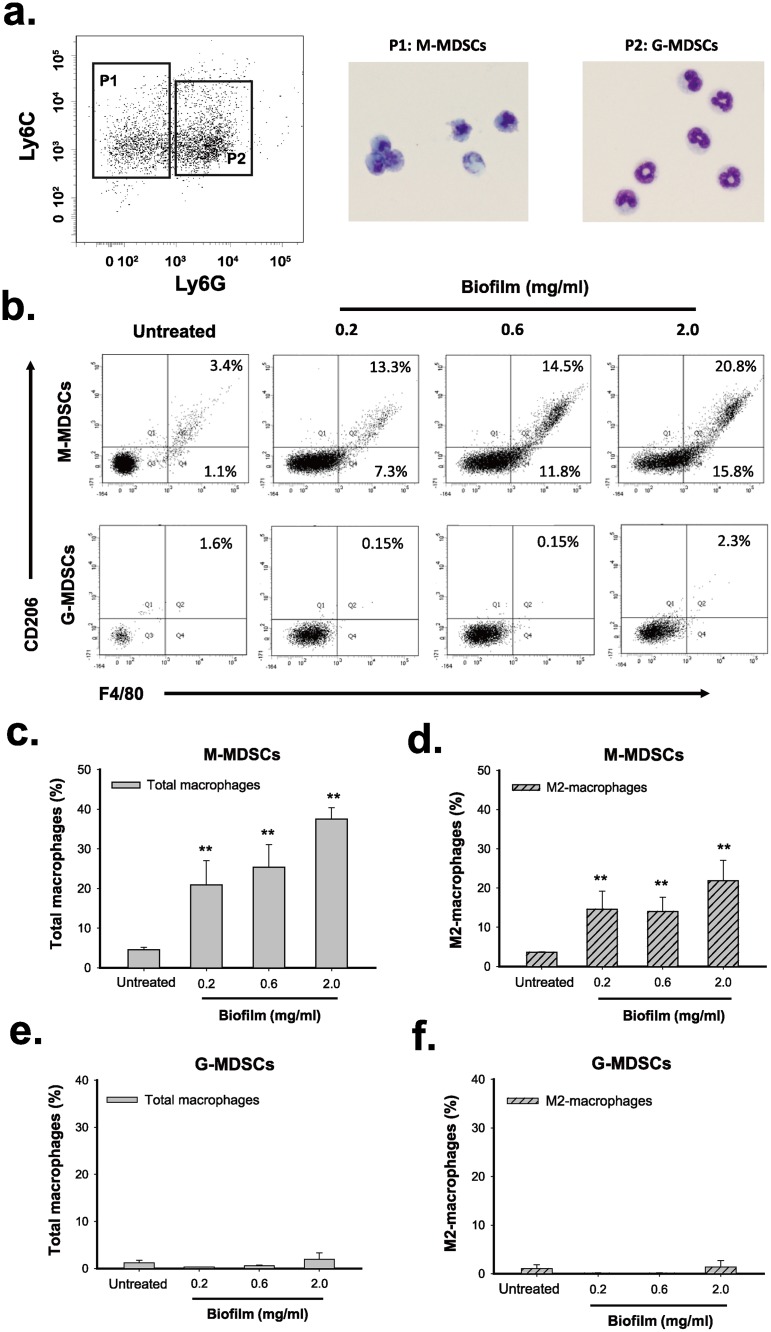
Since the circulating MDSCs, total macrophages, and M2-macrophages could be derived from bone marrow precursor cells (BMCs), we attempted to investigate whether S. aureus biofilm was able to stimulate the expansion of these immune cell types from BMCs in vitro. Here, we developed an in vitro coculture system in which the freshly isolated mouse BMCs were plated on the bottom of transwell cell culture system and then co-cultured with S. aureus biofilms loaded in the transwell cell culture insert (0.4 μm pore size). Notably, mouse BMCs (C57BL/6J) rather than rat BMCs were used in the testing because cell-surface markers for diverse immune cell types are better characterized in mouse. After treatment of mouse BMCs with increasing amounts (0.2, 0.6 and 2.0 mg/ml) of S. aureus biofilm for 48 hr, we found that the proportions of MDSCs (CD11b+Gr-1+) were significantly increased from 40% to about 67–80% along with the doses of S. aureus biofilm (Fig 3a and 3b). The MDSC subtypes, including M-MDSCs (CD11b+Ly6ChighLy6G-) and G-MDSCs (CD11b+Ly6ClowLy6G+), were then evaluated in the experiments. Interestingly, S. aureus biofilm at the low concentration (0.2 mg/ml) sufficiently elicited a marked increase in the proportion of M-MDSCs (from 9.8% to 26.8%), but not the proportion of G-MDSCs (from 3.6.5% to 37.6%), from the treated BMCs (Fig 3c and 3d). As compared to the untreated group, a mild increase in the proportion of G-MDSCs was detected only when BMCs were treated with S. aureus biofilm at high concentrations (0.6 and 2.0 mg/ml), resulting in an increase from 36.5% to 55% or 48.3% (Fig 3c and 3d). These results implicated that S. aureus biofilm differentially promotes the expansion of M-MDSCs and G-MDSCs.
Fig 3. S. aureus biofilm triggers expansion of different immune cell types from mouse bone marrow cells in vitro.
After coculture of mouse bone marrow cells (BMCs) with increasing concentrations (0.2, 0.6 and 2.0 mg/ml) of S. aureus biofilm for 48 hr, the proportions of CD11b+Gr1+ MDSCs (a and b), MDSC subsets including M-MDSCs (CD11b+Ly6ChighLy6G-) and G-MDSCs (CD11b+Ly6ClowLy6G+) (c and d), F4/80+ macrophages (e), as well as F4/80+CD206+ M2-macrophages (f) were evaluated by flow cytometry (N = 3). As noted, to examine the proportions of M-MDSCs and G-MDSCs, the CD11b+ cell populations were first gated from BMCs and then the proportions of Ly6C and Ly6G cells in the CD11b+ cell population were evaluated (c and d). Values in parentheses (c) represent the normalized percentages of individual MDSC subsets in BMCs. *p < 0.01, **p < 0.001.
In addition to MDSCs, the proportions of total macrophages and M2-macrophages were also measured in the experiments. We consistently found that S. aureus biofilm markedly increased the proportions of total macrophages (F4/80+) and M2-macrophages (F4/80+CD206+) in a dose-dependent manner (Fig 3e and 3f and S2 Fig). In agreement with the PJI model in rats, our in vitro coculture system reflected that S. aureus biofilm could elicit the expansion of MDSCs, total macrophages, and M2-macrophages from BMCs.
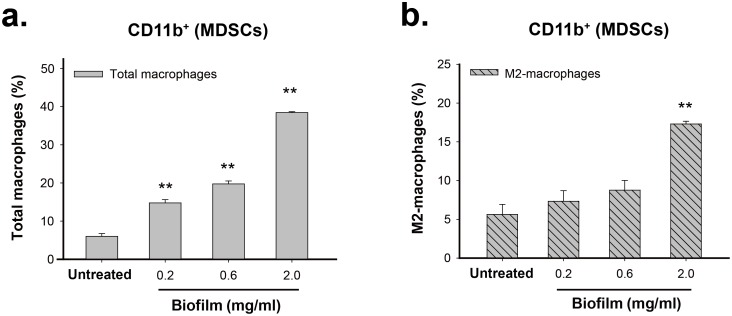
S. aureus biofilm promotes the differentiation of MDSCs into M2-macrophages in vitro
Since MDSCs are an immature population of myeloid cells, we then investigated whether MDSCs could be programmed toward M2-macrophages in response to S. aureus biofilms. To do the experiments, we first isolated the CD11b-positive cells from mouse BMCs using CD11b MicroBeads, which correspond to MDSC population (S3 Fig and see Discussion). We found that treatment of the isolated CD11b-positive MDSCs with increasing amounts of S. aureus biofilm caused significant increases in the proportions of total macrophages (F4/80+) and M2-macrophages (F4/80+CD206+) in a dose-dependent manner (Fig 4a and 4b). These results suggested that the CD11b-positive MDSCs had the potential to convert into M2-macrophages upon exposure to S. aureus biofilm. To further elucidate the conversion of these immune cells, two MDSC subsets (M-MDSCs and G-MDSCs) were sorted from the CD11b-positive MDSCs by cell sorter (Fig 5a). These two isolated MDSC subpopulations, M-MDSCs (CD11b+Ly6ChighLy6G-) and G-MDSCs (CD11b+Ly6ClowLy6G+), were then cocultured with S. aureus biofilm. Specially, like the CD11b-positive MDSCs, treatment of the sorted M-MDSC cell population (Fig 5a, P1) with S. aureus biofilm also exhibited increased proportions of total macrophages (F4/80+) and M2-macrophages (F4/80+CD206+) along with an increasing amount of biofilm (Fig 5b top, Fig 5c and 5d). In contrast, the coculture of the sorted G-MDSCs (Fig 5a, P2) with S. aureus biofilm did not cause expansion of macrophages (F4/80+) and M2-macrophages (F4/80+CD206+) (Fig 5b bottom, Fig 5e and 5f). These results indicated that S. aureus biofilm is capable of promoting the conversion of M-MDSCs (CD11b+Ly6ChighLy6G-) into M2-macrophages (F4/80+CD206+) in vitro.
Fig 4. S. aureus biofilm is capable of stimulating the conversion of the CD11b-positive MDSCs into macrophages or M2-macrophgates in vitro.
The isolated CD11b-positive MDSCs were cocultured with elevated amounts (0.2, 0.6 and 2.0 mg/ml) of S. aureus biofilm for 48 hr. The proportions of total macrophages (F4/80+) (a) and M2-macrophages (F4/80+CD206+) (b) in the CD11b-positive cell population were measured by flow cytometry. **p < 0.001.
Fig 5. S. aureus biofilm promotes differentiation of M-MDSCs but not G-MDSCs into macrophages or M2-macrophages in vitro.
(a) Morphological characterization of the sorted M-MDSCs (CD11b+Ly-6C+Ly-6G-) and G-MDSCs (CD11b+Ly-6ClowLy-6G+). Giemsa-stained M-MDSCs (P1) and G-MDSCs (P2) were examined by light microscopy. (b) M-MDSCs and G-MDSCs were individually co-cultured with S. aureus biofilms for 72 hr. The biofilm-treated cells were subsequently analyzed for the expression of F4/80 and CD206 by flow cytometry. (c) Changes in the proportion of F4/80-positive macrophages in the sorted M-MDSCs after treatment with S. aureus biofilm (N = 3). (d) Changes in the proportion of M2-macrophages (F4/80+CD206+) in the sorted M-MDSCs after biofilm treatment (N = 3). (e and f) Treatment of G-MDSCs with S. aureus biofilm failed to affect the proportions of F4/80+ cells (macrophages) and F4/80+CD206+ cells (M2-macrophages) (N = 3). **p < 0.001.
S. aureus biofilm enhances the immunosuppressive activity of BMCs and the CD11b-positive MDSCs in vitro
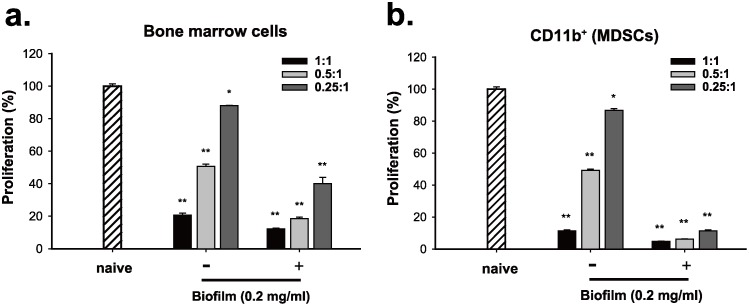
In addition to the expansion of the immunosuppressive cells induced by S. aureus biofilm, we next determined whether S. aureus biofilm substantially affected their immunosuppressive function in vitro. Herein, mouse BMCs were first left untreated or treated with S. aureus biofilm (0.2 mg/ml) for 48 hr. When the untreated or biofilm-treated BMCs were cocultured with activated CFSE-labeled T cells at different ratios (1:1, 0.5:1 and 0.25:1), we found that biofilm-treated BMCs evidently displayed greater suppressive activities on T cell proliferation as compared to the untreated BMCs (Fig 6a). Since biofilm treatment could promote MDSC expansion, equal numbers of the CD11b-positive MDSCs isolated from the untreated or biofilm-treated BMCs were used to compare their immunosuppressive function. In T cell suppression assays, we consistently found that the CD11b-positive MDSCs isolated from biofilm-treated BMCs had a much stronger immunosuppressive activity than those from untreated BMCs (Fig 6b).
Fig 6. S. aureus biofilm augments the immunosuppressive activity of the cultured BMCs and the CD11b-positive population in vitro.
(a) Mouse BMCs left untreated or treated with S. aureus biofilm (0.2 mg/ml) for 48 hr were cocultured with activated CFSE-labeled T cells at different ratios (1:1, 0.5:1 and 0.25:1). The proliferation of CFSE-labeled T cells was examined by flow cytometry (N = 3). Proliferation of T cell control stimulated by anti-CD3/CD28 beads only was set as 100% (naive) in the experiment. (b) The CD11b-positive cell populations isolated from the untreated or biofilm-treated BMCs were used to coculture with activated CFSE-labeled T cells at different ratios (1:1, 0.5:1 and 0.25:1). The proliferation of CFSE-labeled T cells was examined by flow cytometry (N = 3). *p < 0.01, **p < 0.001.
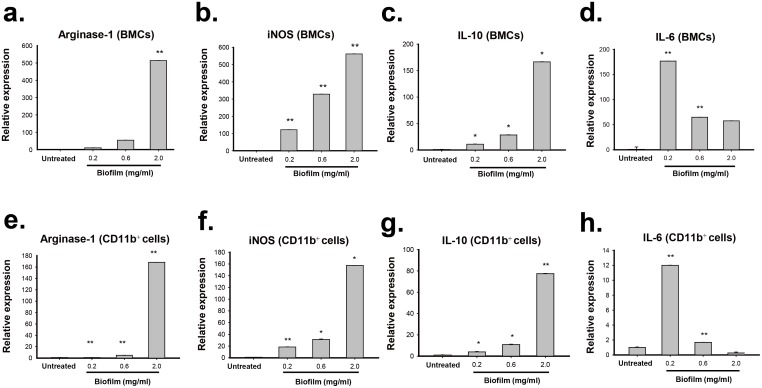
To further confirm the elevated immunosuppressive activity in these tested effector cells caused by biofilm, the expression patterns of various anti-inflammatory-related genes including Arginase-1, iNOS, IL-6 and IL-10 were measured in the untreated or biofilm-treated cells (Fig 7). Quantitative RT-PCR analysis showed that BMCs treated with increasing amounts of S. aureus biofilm substantially displayed enhanced expressions of Arginase-1, iNOS and IL-10 mRNAs (Fig 7a–7c), supporting that biofilm-treated BMCs have higher immunosuppressive activities than the untreated BMCs. Moreover, when gene expression patterns were compared between the CD11b-positive MDSCs isolated from the untreated and biofilm-treated BMCs, the levels of Arginase-1, iNOS and IL-10 mRNAs were also particularly higher in biofim-treated MDSC populations (Fig 7e–7g). Notably, we noticed that the levels of IL-6, which has both pro- and anti-inflammatory properties [29], could be abundantly induced in BMCs or in the CD11b-positive MDSCs when exposed to a low concentration of biofilm (0.2 mg/ml). However, treatment of these cell populations with biofilm at high concentrations (0.6 and 2.0 mg/ml) caused only a moderate increase in the levels of IL-6 as compared to the untreated controls (Fig 7d and 7h).
Fig 7. Expression of Arginase-1, iNOS, IL-10 and IL-6 in BMCs or in the CD11b-positive MDSCs after exposure to S. aureus biofilm.
(a-d) Mouse BMCs were treated with different amounts (0.2, 0.6 and 2.0 mg/ml) of S. aureus biofilm for 48 hr. RNA samples extracted from the untreated or biofilm-treated BMCs were used for analyzing the expression levels of Arginase-1, iNOS, IL-10 and IL-6 by quantitative RT-PCR. (e-h) The expression levels of Arginase-1, iNOS, IL-10 and IL-6 mRNAs were quantified among the CD11b-positive cell populations isolated from the biofilm-treated BMCs as described above. *p < 0.01, **p < 0.001.
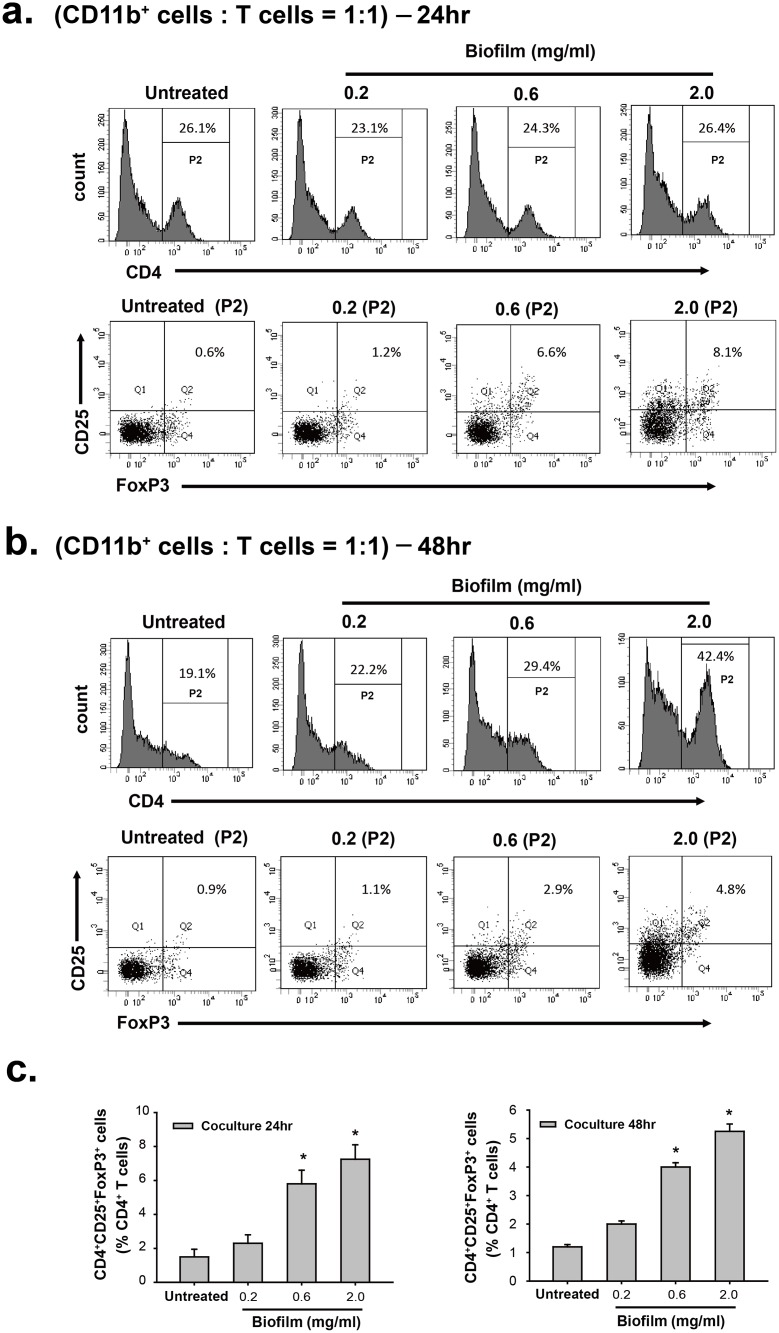
In addition to MDSCs and M2-macrophages, other immunosuppressive cells such as CD4+CD25+Foxp3+ regulatory T cells (Tregs) could possibly involve the immune modulation during S. aureus infection [30–32]. Since S. aureus biofilm significantly enhanced the immunosuppressive activity of MDSCs to inhibit T cell responses, we here attempted to determine whether the biofilm-treated MDSCs could modulate expansion of Tregs from spleen T cells in vitro. To do the experiments, the CD11b-positive cell populations (MDSCs) were isolated from mouse BMCs that were untreated or treated with increasing amounts of biofilm for 48 hr. The isolated MDSC populations were subsequently co-cultured at the 1:1 ratio with spleen T cells that were activated by anti-CD3/CD28 beads. As compared to the coculture of spleen T cells with the untreated MDSCs, we unexpectedly found that the percentages of CD4+CD25+Foxp3+ Tregs in CD4+ lymphocytes were significantly elevated in the cocultures of spleen T cells with the biofilm-treated MDSCs after 24- or 48-hr coculture (Fig 8). Furthermore, the isolated MDSCs undergoing treatment for biofilm at higher concentrations had higher capacity to increase the percentages of CD4+CD25+Foxp3+ Tregs in CD4+ lymphocytes (Fig 8). These results strongly suggested that biofilm-treated MDSCs could suppress immunity through multiple different mechanisms.
Fig 8. S. aureus biofilm is able to promote expansion of CD4+CD25+Foxp3+ Tregs from CD4+ lymphocytes through modulation of MDSC function in vitro.
(a and b) The CD11b-positive MDSC fractions were individually isolated from BMCs that were untreated or treated with different amounts (0.2, 0.6 and 2.0 mg/ml) of biofilm. The isolated CD11b-positive MDSCs were subsequently cocultured with spleen T cells (activated by anti-CD3/CD28 beads) at a 1:1 ratio. After 24- or 48-hr coculture, the frequency of CD4+CD25+Foxp3+ T cells was analyzed by flow cytometry using a Treg Flow kit. (c and d) Bars indicate the percentages of CD4+CD25+Foxp3+ T cells out of CD4+ lymphocytes in coculture experiments as described above (N = 2).
Recruitment of the transferred EGFP-labeled, CD11b-positive MDSCs into the site of S. aureus infection
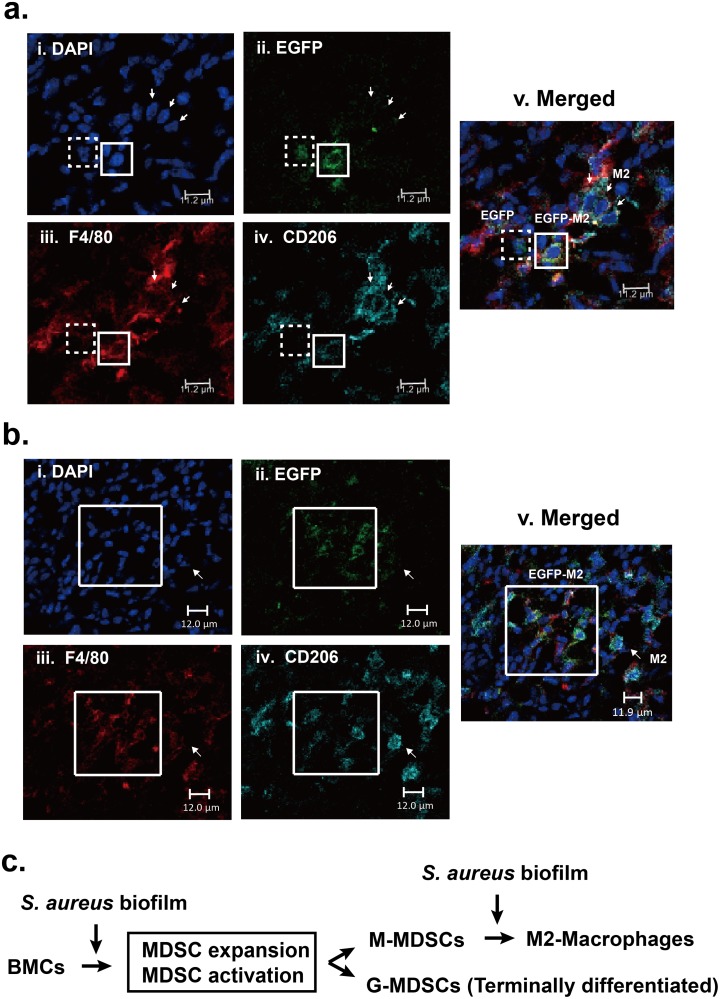
To study whether the circulating MDSCs could be recruited into the site of S. aureus infection in femurs and converted into M2-macrophages in vivo, we here employed a mouse model of PJIs to address these questions. In this model, C57BL/6J mice were infected with S. aureus for 7 days, and then 1×107 exogenously EGFP-labeled, CD11b-positive MDSCs were intravenously injected into the infected mice. Twenty-four hours later, tissues near the S. aureus-infected site were taken, embedded, frozen in -80°C, cryosectioned, and then stained with anti-F4/80 and anti-CD206 antibodies. Confocal microscopy analysis revealed that EGFP-positive cells were indeed recruited into the site of S. aureus infection (Fig 9a and 9b, i and ii). Especially, about 85% of EGFP-positive cells were positive for both F4/80 and CD206 (Fig 9b, iii and iv). These results strongly suggested that S. aureus biofilm infection could promote the conversion of MDSCs to M2-macrophages (EGFP+F4/80+CD206+) in vivo (Fig 9). Additionally, we here noticed that the overall number of M2-macrophages (F4/80+CD206+) was much higher than that of F4/80+CD206- cells (probably pro-inflammatory macrophages) present in S. aureus-infected sites (Fig 9a and 9b, iii and iv). A possible pathway for the development and polarization of MDSCs during S. aureus biofilm stimulation is proposed in Fig 9c.
Fig 9. Recruitment and polarization of exogenous EGFP-expressing, CD11b-positive cells during S. aureus biofilm infection in mice.
(a and b) Representative confocal microscopic images showing the recruitment and polarization of the EGFP-expressing cells in tissues near the S. aureus infection sites. Tissues near the S. aureus infected site were taken, frozen in -80°C, cryosectioned and stained with F4/80-PE (a and b, iii) and CD206-PerCP antibody (a and b, iv). White boxes indicate the EGFP+F4/80+CD206+ cells, and dashed boxes indicate the EGFP+F4/80-CD206- cells (probably MDSCs). Notably, only a very small proportion (<15%) of EGFP-positive cells were detected as the EGFP+F4/80-CD206- or EGFP+F4/80+CD206- phenotype in the infected sites. Moreover, cells double positive for F4/80 and CD206 (arrows; endogenous M2-macrophages) were commonly detected in the infected sites. (c) A model for the development and polarization of MDSCs during S. aureus biofilm stimulation.
Discussion
S. aureus biofilm infection in PJIs is the major complication in joint reconstruction. The formation of bacterial biofilm is important not only for protection against antibiotic treatment but also for evasion of host immune responses. In the latter case, S. aureus biofilm that interferes with the immune responses involves accumulation of both MDSCs and M2-macrophages in infected sites [12, 33–35]. In this report, we confirmed that S. aureus biofilm triggers the expansion and activation of MDSCs and M2-macrophages in our established in vitro and in vivo models. For MDSC expansion, we found that S. aureus biofilm preferentially induces the expansion of M-MDSCs but not G-MDSCs. Importantly, our results support that M-MDSCs have the potential to further differentiate into M2-macrophages in the presence of S. aureus biofilm. To our knowledge, this is the first study showing the relationship between MDSCs and M2-macrophages in S. aureus biofilm infection. Overall, our findings provide a better understanding of the development of these immunosuppressive cells during S. aureus biofilm infection.
Using our established PJI model in rats, two S. aureus-infected groups that received no treatment or vancomycin treatment were evaluated. Although vancomycin treatment greatly reduced bone infection caused by S. aureus (Fig 1a), S. aureus infection might persistently damage the infected femur even standard treatment with antibiotics. Particularly, we found that aggregates of S. aureus embedded with a self-produced extracellular matrix, which adhere to bone or prosthesis surface, were still observed in the vancomycin-treated group by scanning electron microscopy (Fig 1b). On the other hand, we did not detect significant differences in the expansion rates of the circulating MDSCs, macrophages, and M2-macrophages in the peripheral blood between the two S. aureus-infected groups that received no treatment or vancomycin treatment (Fig 2). Our results emphasize that S. aureus biofilm infection in PJIs is still a great challenge for orthopedic surgeons, and antibiotic treatment may be not so effective to eliminate the persistent bacterial infection.
In addition to a rat PJI model, we here successfully established an in vitro coculture system to study effects of S. aureus biofilm on the expansion of MDSCs, total macrophages and M2-macrophages from freshly isolated BMCs (Fig 3). Originally, we attempted to coculture BMCs with S. aureus biofilm directly. However, the direct contact between BMCs and S. aureus biofilm led to rapid cell death. To avoid the over-reaction of immune cells, S. aureus biofilms in our in vitro system were loaded in transwell cell culture inserts (0.4 μm pore size) and cocultured with BMCs. Consistent with the results found in our rat PJI model, we showed that treatment of BMCs with S. aureus biofilm in our in vitro system stimulated the expansions of MDSCs (CD11b+Gr1+), total macrophages (F4/80+) and M2-macrophages (F4/80+CD206+) (Fig 3). Most importantly, a dose-response relationship between S. aureus biofilm and the expansions of these immune cells could be established in the in vitro system.
MDSCs are composed of two subsets, M-MDSCs and G-MDSCs. Evidence has shown that these two subsets may manifest different functions under different circumstances [11]. Although earlier studies by Hem et al. [14] have reported that MDSCs function as a central contributor to mediate immunosuppression during S. aureus biofilm infection, the expansion and polarization of MDSC subsets in response to S. aureus biofilm remained poorly understood. Using our in vitro coculture system, we clearly showed that although both M-MDSCs and G-MDSCs could expand upon exposure to S. aureus biofilm, the expansion of M-MDSCs was much greater than G-MDSCs (Fig 3c and 3d). The maximal expansion of M-MDSC population in BMCs could be easily achieved after exposure to a low concentration (0.2 mg/ml) of S. aureus biofilm (Fig 3c and 3d; from 9.8% to 26.8%); however, higher concentrations (0.6 and 2.0 mg/ml) of S. aureus biofilm did not further elicit M-MDSC expansion. There are two possibilities that may explain these results. One possibility is that a low concentration of S. aureus biofilm may be already sufficient to confer the full induction of M-MDSC expansion from BMCs. The other possibility is that, in response to high concentrations of biofilm, the increased proportions of M-MDSCs may be able to further differentiate into another cell types, consequently maintaining a fixed level of M-MDSCs. In the study, we did find that M-MDSCs have the capacity to differentiate into M2-macrophages during S. aureus biofilm stimulation (Figs 5 and 9).
Although MDSCs in mice are commonly characterized as CD11b+Gr1+ cells, we noticed that almost all of CD11b-positive cells (up to 97%) freshly isolated from mouse bone marrow cells co-expressed Gr1 (or Ly6C/Ly6G) in our experiments (S3 Fig). This information would be helpful because the use of the CD11b-positive cells as a major MDSC cell population avoids tedious cell sorting processes that often result in cell membrane damage. Treatment of MDSCs (CD11b+) with S. aureus biofilm substantially increased the proportions of M2-macrophageswe in vitro. Furthermore, we revealed that M-MDSCs (CD11b+Ly6ChighLy6G-) but not G-MDSCs (Ly6ClowLy6G+) polarize toward M2-macrophges (F4/80+CD206+) in response to S. aureus biofilm (Fig 5b and 5d). Consistent with the in vitro studies, our in vivo mouse model also revealed that about 85% of exogenously injected EGFP-expressing CD11b-positive MDSCs were skewed toward M2-macrophages in surrounding soft tissue of infected bone (Fig 9).
To support the immunosuppressive function of these immune cells, both BMCs and MDSCs (CD11b+) untreated or treated with biofilm were co-cultured with activated T cells (Fig 6). Our results consistently showed that biofilm treatment significantly enhanced the suppressive activity of BMCs or the CD11b-positive MDSCs on T cell proliferation. Furthermore, quantitative RT-PCR analysis also revealed that the biofilm-treated cell populations abundantly expressed anti-inflammatory mediators, including Arginase-1, iNOS and IL-10 (Fig 7). In addition to these anti-inflammatory mediators, we noticed that IL-6 was markedly stimulated in BMCs or the MDSC (CD11b+) population after exposure to a low concentration of biofilm (0.2 mg/mL); however, at higher concentrations of biofilm the induction of IL-6 trended toward a modest increase (Fig 7d and 7h). It may be not so surprising because IL-6 is previously known to have both pro- and anti-inflammatory properties [29]. Since S. aureus biofilm enhanced the immunosuppressive activity of MDSCs to inhibit T cell responses, we additionally investigated whether the biofilm-treated MDSCs could modulate Treg cells, another cell type with immunosuppressive function. When compared to the coculture of spleen T cells with the untreated MDSCs, we unexpectedly found that the proportions of CD4+CD25+Foxp3+ Tregs in CD4+ lymphocytes could be significantly increased in the cocultures of spleen T cells with the biofilm-treated MDSCs (Fig 8). These results therefore suggested that biofilm-treated MDSCs could suppress immunity through multiple different ways. Taken together, our findings support that S. aureus biofilm mediate both MDSC expansion and activation.
In conclusion, since recalcitrant PJIs are still a difficult challenge for orthopaedic surgeons, a detailed understanding of the relationship between MDSCs, M2-macrophages and biofilms may further expand the possibility of immunotherapies in treating PJIs in the future.
Supporting information
Rat blood samples were collected from the sham group, the untreated infection group, and the vancomycin-treated infection group at different time points after operation. After lysing the red blood cells, the remaining leukocytes were analyzed by flow cytometry for the proportions of CD11bc+His48+ MDSCs (a), and the proportions of CD68+ macrophages and CD68+CD206+ M2-macrophages (b).
(TIF)
Mouse BMCs were cultured with increasing concentrations (0.2, 0.6 and 2.0 mg/ml) of S. aureus biofilm for 48 hr. Representative flow cytometry histograms show the expansion of F4/80+ macrophages and F4/80+CD206+ M2-macrophages from BMCs caused by S. aureus biofilm.
(TIF)
(a) The CD11b-positive cell population was sorted from bone marrow cells by BD FACSAria Fusion cell sorter, and then subjected to flow cytometric analysis. Up to 99% of the CD11b-positive cells co-expressed Gr1. (b) The CD11b-positive cell population was isolated from bone marrow cells using CD11b magnetic beads (STEMCELL™). Up to 97% of the CD11b-positive cells co-expressed either Ly6C or Ly6G (two components of Gr1).
(TIF)
Acknowledgments
The authors would like to thank the Expensive Advanced Instrument Core Laboratory in Department of Medical Research and Development, Chang Gung Memorial Hospital at Chiayi for offering the use of BD FACSCanto II flow cytometer, BD FACSAria Fusion cell sorter, and Leica SP5 II confocal microscope.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This study was supported by grants CMRPG6A0531, CMRPG6A0532 and CMRPG6F0341 from the Chang-Gung Memorial Hospital (Taiwan) and by grants NMRPG6C0081, NMRPG6D6031 and NMRPG6D6032 from the Ministry of Science and Technology (R.O.C.).
References
- 1.Kurtz SM, Lau E, Watson H, Schmier JK, Parvizi J. Economic burden of periprosthetic joint infection in the United States. J Arthroplasty. 2012;27(8 Suppl):61–5 e1. doi: 10.1016/j.arth.2012.02.022 . [DOI] [PubMed] [Google Scholar]
- 2.Peel TN, Dowsey MM, Buising KL, Liew D, Choong PF. Cost analysis of debridement and retention for management of prosthetic joint infection. Clin Microbiol Infect. 2013;19(2):181–6. doi: 10.1111/j.1469-0691.2011.03758.x . [DOI] [PubMed] [Google Scholar]
- 3.McConoughey SJ, Howlin R, Granger JF, Manring MM, Calhoun JH, Shirtliff M, et al. Biofilms in periprosthetic orthopedic infections. Future Microbiol. 2014;9(8):987–1007. doi: 10.2217/fmb.14.64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gallo J, Kolar M, Novotny R, Rihakova P, Ticha V. Pathogenesis of prosthesis-related infection. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2003;147(1):27–35. . [DOI] [PubMed] [Google Scholar]
- 5.Rao N, Ziran BH, Lipsky BA. Treating osteomyelitis: antibiotics and surgery. Plast Reconstr Surg. 2011;127 Suppl 1:177S–87S. doi: 10.1097/PRS.0b013e3182001f0f . [DOI] [PubMed] [Google Scholar]
- 6.Vilchez F, Martinez-Pastor JC, Garcia-Ramiro S, Bori G, Macule F, Sierra J, et al. Outcome and predictors of treatment failure in early post-surgical prosthetic joint infections due to Staphylococcus aureus treated with debridement. Clin Microbiol Infect. 2011;17(3):439–44. doi: 10.1111/j.1469-0691.2010.03244.x . [DOI] [PubMed] [Google Scholar]
- 7.Byren I, Bejon P, Atkins BL, Angus B, Masters S, McLardy-Smith P, et al. One hundred and twelve infected arthroplasties treated with 'DAIR' (debridement, antibiotics and implant retention): antibiotic duration and outcome. J Antimicrob Chemother. 2009;63(6):1264–71. doi: 10.1093/jac/dkp107 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drago L, De Vecchi E, Nicola L, Gismondo MR. In vitro evaluation of antibiotics' combinations for empirical therapy of suspected methicillin resistant Staphylococcus aureus severe respiratory infections. BMC Infect Dis. 2007;7:111 doi: 10.1186/1471-2334-7-111 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherr TD, Hanke ML, Huang O, James DB, Horswill AR, Bayles KW, et al. Staphylococcus aureus biofilms induce macrophage dysfunction through leukocidin AB and alpha-toxin. MBio. 2015;6(4):e01021–15. doi: 10.1128/mBio.01021-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanke ML, Heim CE, Angle A, Sanderson SD, Kielian T. Correction: Targeting Macrophage Activation for the Prevention and Treatment of Staphylococcus aureus Biofilm Infections. J Immunol. 2013;190(12):6709–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ost M, Singh A, Peschel A, Mehling R, Rieber N, Hartl D. Myeloid-derived suppressor cells in bacterial infections. Front Cell Infec Microbiol. 2016;6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9(3):162–74. doi: 10.1038/nri2506 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Peranzoni E, Zilio S, Marigo I, Dolcetti L, Zanovello P, Mandruzzato S, et al. Myeloid-derived suppressor cell heterogeneity and subset definition. Curr Opin Immunol. 2010;22(2):238–44. doi: 10.1016/j.coi.2010.01.021 . [DOI] [PubMed] [Google Scholar]
- 14.Heim CE, Vidlak D, Scherr TD, Kozel JA, Holzapfel M, Muirhead DE, et al. Myeloid-derived suppressor cells contribute to Staphylococcus aureus orthopedic biofilm infection. J Immunol. 2014;192(8):3778–92. doi: 10.4049/jimmunol.1303408 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thurlow LR, Hanke ML, Fritz T, Angle A, Aldrich A, Williams SH, et al. Staphylococcus aureus biofilms prevent macrophage phagocytosis and attenuate inflammation in vivo. J Immunol. 2011;186(11):6585–96. doi: 10.4049/jimmunol.1002794 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang W-C, Ma G, Chen S-H, Pan P-Y. Polarization and reprogramming of myeloid-derived suppressor cells. J Mol Cell Biol. 2013;5(3):207–9. doi: 10.1093/jmcb/mjt009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wang Y, Wang YP, Zheng G, Lee VW, Ouyang L, Chang DH, et al. Ex vivo programmed macrophages ameliorate experimental chronic inflammatory renal disease. Kidney Int. 2007;72(3):290–9. doi: 10.1038/sj.ki.5002275 . [DOI] [PubMed] [Google Scholar]
- 18.Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat Rev Immunol. 2004;4(8):583–94. doi: 10.1038/nri1412 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee S, Huen S, Nishio H, Nishio S, Lee HK, Choi BS, et al. Distinct macrophage phenotypes contribute to kidney injury and repair. J Am Soc Nephrol. 2011;22(2):317–26. doi: 10.1681/ASN.2009060615 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–83. doi: 10.1146/annurev.immunol.021908.132532 . [DOI] [PubMed] [Google Scholar]
- 21.Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3(1):23–35. doi: 10.1038/nri978 . [DOI] [PubMed] [Google Scholar]
- 22.Ka MB, Daumas A, Textoris J, Mege J-L. Phenotypic diversity and emerging new tools to study macrophage activation in bacterial infectious diseases. M1/M2 Macrophages: The Arginine Fork in the Road to Health and Disease. 2015;5(500):128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rőszer T. Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm. 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huber S, Hoffmann R, Muskens F, Voehringer D. Alternatively activated macrophages inhibit T-cell proliferation by Stat6-dependent expression of PD-L2. Blood. 2010;116(17):3311–20. doi: 10.1182/blood-2010-02-271981 . [DOI] [PubMed] [Google Scholar]
- 25.Heim CE, Vidlak D, Kielian T. Interleukin-10 production by myeloid-derived suppressor cells contributes to bacterial persistence during Staphylococcus aureus orthopedic biofilm infection. J Leukoc Biol. 2015;98(6):1003–13. doi: 10.1189/jlb.4VMA0315-125RR [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heim CE, Vidlak D, Scherr TD, Hartman CW, Garvin KL, Kielian T. IL-12 promotes myeloid-derived suppressor cell recruitment and bacterial persistence during Staphylococcus aureus orthopedic implant infection. J Immunol. 2015;194(8):3861–72. doi: 10.4049/jimmunol.1402689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cassat JE, Lee CY, Smeltzer MS. Investigation of biofilm formation in clinical isolates of Staphylococcus aureus. Methods Mol Biol. 2007;391:127–44. doi: 10.1007/978-1-59745-468-1_10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bernthal NM, Stavrakis AI, Billi F, Cho JS, Kremen TJ, Simon SI, et al. A mouse model of post-arthroplasty Staphylococcus aureus joint infection to evaluate in vivo the efficacy of antimicrobial implant coatings. PLoS One. 2010;5(9):e12580 doi: 10.1371/journal.pone.0012580 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Scheller J, Chalaris A, Schmidt-Arras D, Rose-John S. The pro- and anti-inflammatory properties of the cytokine interleukin-6. Biochim Biophys Acta. 2011;1813(5):878–88. doi: 10.1016/j.bbamcr.2011.01.034 . [DOI] [PubMed] [Google Scholar]
- 30.Prabhakara R, Harro JM, Leid JG, Harris M, Shirtliff ME. Murine immune response to a chronic Staphylococcus aureus biofilm infection. Infect Immun. 2011;79(4):1789–96. doi: 10.1128/IAI.01386-10 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Prabhakara R, Harro JM, Leid JG, Keegan AD, Prior ML, Shirtliff ME. Suppression of the inflammatory immune response prevents the development of chronic biofilm infection due to methicillin-resistant Staphylococcus aureus. Infect Immun. 2011;79(12):5010–8. doi: 10.1128/IAI.05571-11 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tebartz C, Horst SA, Sparwasser T, Huehn J, Beineke A, Peters G, et al. A major role for myeloid-derived suppressor cells and a minor role for regulatory T cells in immunosuppression during Staphylococcus aureus infection. J Immunol. 2015;194(3):1100–11. doi: 10.4049/jimmunol.1400196 . [DOI] [PubMed] [Google Scholar]
- 33.du Plessis N, Loebenberg L, Kriel M, von Groote-Bidlingmaier F, Ribechini E, Loxton AG, et al. Increased frequency of myeloid-derived suppressor cells during active tuberculosis and after recent mycobacterium tuberculosis infection suppresses T-cell function. Am J Respir Crit Care Med. 2013;188(6):724–32. doi: 10.1164/rccm.201302-0249OC . [DOI] [PubMed] [Google Scholar]
- 34.Obregon-Henao A, Henao-Tamayo M, Orme IM, Ordway DJ. Gr1(int)CD11b+ myeloid-derived suppressor cells in Mycobacterium tuberculosis infection. PLoS One. 2013;8(11):e80669 doi: 10.1371/journal.pone.0080669 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Poe SL, Arora M, Oriss TB, Yarlagadda M, Isse K, Khare A, et al. STAT1-regulated lung MDSC-like cells produce IL-10 and efferocytose apoptotic neutrophils with relevance in resolution of bacterial pneumonia. Mucosal Immunol. 2013;6(1):189–99. doi: 10.1038/mi.2012.62 . [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Rat blood samples were collected from the sham group, the untreated infection group, and the vancomycin-treated infection group at different time points after operation. After lysing the red blood cells, the remaining leukocytes were analyzed by flow cytometry for the proportions of CD11bc+His48+ MDSCs (a), and the proportions of CD68+ macrophages and CD68+CD206+ M2-macrophages (b).
(TIF)
Mouse BMCs were cultured with increasing concentrations (0.2, 0.6 and 2.0 mg/ml) of S. aureus biofilm for 48 hr. Representative flow cytometry histograms show the expansion of F4/80+ macrophages and F4/80+CD206+ M2-macrophages from BMCs caused by S. aureus biofilm.
(TIF)
(a) The CD11b-positive cell population was sorted from bone marrow cells by BD FACSAria Fusion cell sorter, and then subjected to flow cytometric analysis. Up to 99% of the CD11b-positive cells co-expressed Gr1. (b) The CD11b-positive cell population was isolated from bone marrow cells using CD11b magnetic beads (STEMCELL™). Up to 97% of the CD11b-positive cells co-expressed either Ly6C or Ly6G (two components of Gr1).
(TIF)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.