Abstract
Rett syndrome (RTT), a leading cause of intellectual disability in girls, is predominantly caused by mutations in the X-linked gene MECP2. Disruption of Mecp2 in mice recapitulates major features of RTT, including neurobehavioral abnormalities, which can be reversed by re-expression of normal Mecp2. Thus, there is reason to believe that RTT could be amenable to therapeutic intervention throughout the lifespan of patients after the onset of symptoms. A common feature underlying neuropsychiatric disorders, including RTT, is altered synaptic function in the brain. Here, we show that Mecp2tm1.1Jae/y mice display lower presynaptic function as assessed by paired pulse ratio, as well as decreased long term potentiation (LTP) at hippocampal Schaffer–collateral-CA1 synapses. Treatment of Mecp2tm1.1Jae/y mice with D-cycloserine (DCS), an FDA-approved analog of the amino acid D-alanine with antibiotic and glycinergic activity, corrected the presynaptic but not LTP deficit without affecting deficient hippocampal BDNF levels. DCS treatment did, however, partially restore lower BDNF levels in the brain stem and striatum. Thus, treatment with DCS may mitigate the severity of some of the neurobehavioral symptoms experienced by patients with Rett syndrome.
Introduction
Rett syndrome (RTT) causes intellectual disability [1,2] in 1 out of every 10,000 females worldwide, and is caused ~95% of the time by mutations in the X chromosomal gene MECP2 that encodes for methyl-CpG-binding protein 2 (MeCP2) [3,4]. MeCP2 is a highly-abundant DNA binding protein that affects expression of multiple downstream genes and signaling pathways [5]. Most disease-causing mutations arise in the male germ line, resulting in almost all RTT patients being female heterozygotes with one normal and one mutated copy of Mecp2. Girls with RTT are born normally after full term pregnancy without perinatal complications, but from 6–18 months of age they experience regression that precludes attainment of normal developmental milestones and is associated with reduced head growth, seizures, intellectual disability, regressed motor function, emotional lability, autonomic dysregulation, anxiety, scoliosis, and potentially fatal aberrant respiratory function [6–8]. There is currently no effective treatment for patients with RTT.
Disruption of Mecp2 in mice recapitulates major features of RTT [9–13], and re-expression of normal Mecp2 reverses the deficits seen in mice [14]. In addition, post-mortem studies of brains of patients with RTT have demonstrated lack of neuronal cell loss or neurodegeneration, indicating that prolonged loss of MeCP2 protein does not result in major changes in brain structure. Thus, there is reason to believe that treatment of patients throughout their lifespan, well after the onset of developmental regression, could mitigate the severity of symptoms associated with RTT. Previously, aberrant synaptic neurotransmission thought to be related to the cognitive and functional deficits underlying RTT has been demonstrated in the brains of behaviorally-symptomatic Mecp2tm1.1Jae/y mice. Specifically, Mecp2tm1.1Jae/y mice display impaired excitatory glutamatergic neurotransmission, as indicated by deficient hippocampal long-term potentiation (LTP) associated with altered expression of N-methyl-D-aspartate (NMDA) receptor subunits [15]. Presynaptic function is also disrupted in Mecp2tm1.1Jae/y mice, as evidenced by significantly reduced paired-pulse facilitation in the brain [15,16]. Notably, altered patterns of expression of both the NMDA- and αgamma-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA)-type glutamate receptors have been observed in postmortem brain samples of patients with RTT [17,18], as well as elevated CSF glutamate levels in patients with RTT compared to subjects with other forms of autism-spectrum disorder [19,20]. These findings further support a role for dysfunctional glutamatergic signaling in RTT, suggesting possible therapeutic potential for pharmacologic modulation of this system in patients.
D-cycloserine (DCS) is an amino acid derivative sold under the brand name Seromycin. In addition to antibiotic efficacy against extensively drug-resistant strains of Mycobacterium tuberculosis, DCS is a partial agonist at the strychnine-insensitive glycine recognition site on the NR1 subunit of NMDA receptors in the brain. In this capacity, DCS increases calcium influx in response to NMDA-mediated glutamatergic signaling, without causing neurotoxicity [21]. In animal models, DCS enhances extinction of conditioned fear [22–27], a form of learning that represents a valid preclinical model of exposure-based therapy that is dependent on NMDA receptors for people with anxiety disorders [28–32]-. Treatment with DCS also shows efficacy in preclinical models of deficient fear extinction after stress [33,34], alcohol withdrawal [35], and REM sleep deprivation [36], as well as in mutant mice harboring the brain-derived neurotrophic factor (BDNF) Val66Met polymorphism associated with increased susceptibility to neuropsychiatric disease [37]. In humans, treatment with DCS enhances the efficacy of exposure therapy for various forms of maladaptive fear, including acrophobia [38], social anxiety [39,40], obsessive-compulsive disorder [41, 42], and panic disorder [43]. Because deficits in neurocognitive functioning are a prominent feature in patients with Rett, we applied the Mecp2tm1.1Jae/y mouse model of RTT to determine whether DCS might mitigate synaptic deficits associated with this condition.
Materials and methods
Animals
All animal procedures were performed in accordance with UT Southwestern Medical Center institutional animal care and use committee’s regulations. Male mice were housed in temperature controlled conditions, provided food and water ad libitum, and maintained on a 12-hr light/dark cycle (6 A.M. to 6 P.M.). The Mecp2tm1.1Jae/y mouse colony [44] was maintained on C57Bl/6J background. Animal protocols were approved by the ethics committees at both Ut Southwestern Medical Center and Weill Cornell Medicine.
D-cycloserine (DCS) administration
DCS was obtained from Sigma-Aldrich (C6880 SIGMA), dissolved in PBS at pH 7.4, and administered by intraperitoneal injection to animals at 20 mg/kg/day every day beginning at 3 weeks of age and continuing until death. Injection sites were alternated left and right every other day. WT and Mecp2tm1.1Jae/y mice were randomly assigned to either vehicle or DCS treatment, and subsequently coded unrelated to genotype or treatment group in order to blind experimenters to group conditions.
Behavioral analysis
Analysis was conducted blind to genotype and treatment at 18 weeks of age, according to established protocols [12].
Locomotor activity analysis
Locomotor activity was determined for 8 hours during the active phase of mice by automated counting of the number of beam breaks in an 18 cM wide X 12 cM high open field.
Apnea analysis
Apnea measurements were determined by placing mice in a whole body plethysmograph (Buxco) to measure breathing in an unrestrained state. Mice were acclimated for one half hour in the chamber before apneas were recorded for a 15 minute period. Apneas were defined as a pause in the breathing cycle lasting longer than 1.5 seconds.
Grip strength analysis
Grip strength was determined in mice suspended by their forelimbs on a standard 1 cm diameter bar positioned 20 cm above standard cage bedding, and time to fall was recorded.
Tremor analysis
Tremor score was determined through a 3 point observational scoring system while the mouse was standing on the flat palm of the observer’s hand. A score of 0 indicated no tremor, 1 indicated mild intermittent tremor, and 2 indicated constant tremor or intermittent severe tremor.
Walking gait analysis
Walking gait score was determined through a 3 point observational scoring system when the mouse was allowed to ambulate on a flat table top. A score of 0 indicated normal gait, 1 indicated hind legs spread wider than normal when ambulating, 2 indicated severe abnormality either with tremor when feet were lifted or with backwards walking by lifting both rear feet simultaneously.
Neurological function analysis
Neurological Function (NF) score was determined by summing the scores of general condition, hindlimb clasping, gait, and tremor. General condition score was determined by means of a 3 point observational scoring system that indicated coat condition, appearance of eyes, and body stance. A score of 0 meant clean shiny coat, clear eyes and normal stance. A score of 1 indicated dull coat or poorly-groomed appearance, dull eyes, and mildly hunched stance. A score of 2 indicated piloerection, crusted or narrowed eyes, and fully hunched stance. Hindlimb clasping was defined as when the hindlimbs were spread in the area less than their body width while the animal was suspended by its tail. No clasping received a score of 0, and clasping received a score of 1.
Hippocampal slice electrophysiology
18 week old mice that had been treated since 3 weeks of age with either vehicle or DCS (20 mg/kg/day IP), and not exposed to any behavioral testing, were used for electrophysiologic studies. The experimenter was blind to genotype and treatment during the recording and data analysis stages. Mice were anesthetized with Euthasol (30 mg/ml, 0.2 ml i.p.) before decapitation. Brains were removed and immersed for 2–3 minutes in ice-cold artificial cerebral spinal fluid (aCSF) containing (in mM): 119 NaCl, 2.5 KCl, 2.5 CaCl2, 1.3 MgSO4, 1 NaH2PO4, 26 NaHCO3 and 10 glucose, continuously bubbled with 95% O2 and 5% CO2, pH 7.4. The hippocampi were extracted and cut with a vibratome into 350 μm thick transverse sections in ice cold ACSF. Sections recovered in oxygenated ACSF for at least 1 hour at 32°C. Hippocampal slices were transferred into a recording chamber and superfused with ACSF at a constant rate of 2.5 ml/minutes at 30°C. Field excitatory post synaptic (fEPSP) potentials were recorded with glass recording electrodes filled with aCSF (Sutter Instruments, resistance, 1–2 MΩ). Extracellular stimuli were delivered by placing a bipolar platinum-tungsten stimulating electrode at the region of interest (A-M Systems Isolated Pulse Stimulator, Model 2100). The stimulating electrode was inserted to stimulate fibers of the Schaffer collateral pathway and the recording electrode was inserted into the CA1 just beneath the molecular layer. The stimulating and recording electrodes were separated by a distance of 300–350 μm. Electrical signals were amplified (A-M systems AC amplifier Model 1800), digitized, and stored on a PC for subsequent analysis using Labview 8.6 software (National Instruments). Input-output (I/O) relationship was determined by providing an ascending series of stimulus input intensities (range 4–24 μA) until the maximum amplitude response was reached. An input stimulus intensity that induces 40–60% of the maximum response was used for measuring paired-pulse ratios (PPR) and long-term potentiation (LTP). PPR was measured by giving 2 pulses at decreasing interstimulus intervals (500, 400, 200, 100, 50, 30, 20 ms) and analyzed by dividing the fEPSP slope of pulse 2 by pulse 1. Following 20 minutes of stable baseline, LTP was induced by high frequency stimulation using an input stimulus intensity that produces the maximum response (HFS, 2 100 Hz trains with 100 pulses with an interburst interval of 20 seconds). Slices that did not have 20 minutes of stable baseline were excluded from LTP analyses.
BDNF ELISA
Animals started treatment of either vehicle or DCS at 3 weeks old and it continued for 4 weeks. Euthanasia was performed by anesthesia overdose followed by microdissection of hippocampus, striatum and brainstem and immediate flash freeze in liquid nitrogen. Total BDNF (tBDNF) protein level was measured using the BDNF Emax ImmunoAssay (ELISA) system (Promega), with recombinant mature BDNF as a standard and samples were performed in duplicate, with each group containing 10–14 samples. Protein was extracted and quantified following the manufacturer’s protocol. Tissue samples were homogenized in lysis buffer (150 mM NaCl, 1% Triton X-100, 25 mM HEPES, 2 mM NaF) containing phosphatase and protease inhibitors, and then incubated by rotation at 4°C for 1 h. Homogenized tissue was centrifuged at maximum speed and the supernatant containing total protein was collected and quantified using the BCA protein assay kit (Thermo Fisher Scientific). Each sample was diluted 1:1 with block and sample buffer (BSB), and placed in designated wells of a 96-well plate previously coated with BDNF antibody in carbonate buffer (25 mM Na2CO3 and 25 mM Na2HCO3, pH 9.7, incubated at 4°C), followed by blocking with BSB. A second coating of primary anti-human BDNF antibody was added, followed by horseradish peroxidase-conjugated secondary antibody. The colorimetric reaction was initiated by tetramethylbenzidine. After 10 min, the reaction was stopped by addition of 1N HCl, and absorbance was read at 450 nm on a plate reader (iMark Absorbance Microplate Reader, Bio-Rad Laboratories).
BDNF western blot analysis
Forty micrograms of total protein lysate was separated on a 15% SDS protein gel with a Kaleidoscope-prestained protein standard (Bio-Rad). Blots were blocked in 5% non-fat dry milk and incubated in primary antibody (mature BDNF, Abcam, Catalog #ab108319 used at 1:5000 and secondary at 1:5000; proBDNF, Santa-Cruz Biotechnology, Catalog #sc-65514 used at 1:500 and secondary at 1:5000) for 24 hours at 4°degrees celsius. Blots were incubated in horseradish peroxidase-linked IgG-conjugated secondary antibody for 1 hour. Protein bands were visualized by chemiluminescence solution (Western Lightening, Perkin Elmer Life Sciences). Band at 15kDa was quantitated for mature BDNF, and band at 28kDa was quantitated for proBDNF. GAPDH (37 kDa; Abcam, Catalog# Ab22555 used at 1:20000 and secondary at 1:30000) was used as a loading control.
Statistics
For I/O relationships, slopes for each slice were calculated and a two way ANOVA was used to assess differences between groups. For PPR, paired pulse ratios were analyzed using a one-way ANOVA. If the overall F value was significant, Student Newman-Keuls post-hoc tests were used to determine significant differences between groups. LTP was analyzed using two way repeated measures ANOVA between vehicle groups. If the interaction effect was significant, Student Newman-Keuls was used as a post-hoc test. For the BDNF experiments, a two-way ANOVA was used followed by Bonferroni post-hoc test for factors with a main effect. Power analyses were conducted on PPR data to determine whether sample sizes were appropriate to achieve sufficient statistical power. Using GPower (Franz Faul, Universitat Kiel, Germany), we determined that the n numbers for the PPR experiments were sufficient to reject the null hypothesis, Power≥0.80 for 20, 30, and 100 ms interstimulus intervals and 0.7 for the 200 ms interstimulus interval.
Results and discussion
Treatment with DCS is safely tolerated by Mecp2tm1.1Jae/y mice
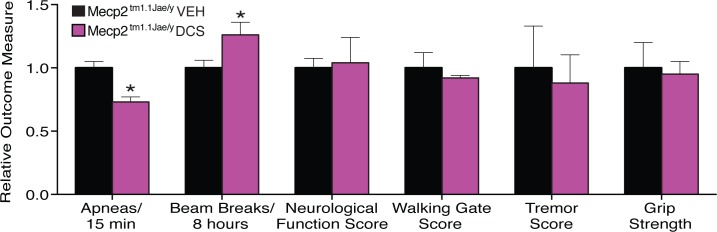
Before conducting electrophysiologic testing of the hypothesis that DCS might help treat synaptic deficits related to Rett syndrome, we examined whether Mecp2tm1.1Jae/y mice, a well-characterized animal model of RTT, would display obvious signs of detriment with chronic DCS exposure. Treatment with DCS (20 mg/kg/day) or vehicle was initiated in 3 week old Mecp2tm1.1Jae/y mice and continued every day until experimentation. This did not affect weight gain of Mecp2tm1.1Jae/y mice (Fig 1), indicating no obvious toxicity of the drug. Encouragingly, this treatment regimen moderately reduced breathing apneas in 18 week old Mecp2tm1.1Jae/y mice by 20% (t(8) = 4.55, p = .002), and also improved basal locomotor activity by about 20% (t(8) = 2.76, p = .025) (Fig 2). Vehicle-treated Mecp2tm1.1Jae/y mice showed the expected abnormalities in these parameters (Fig 2), as the raw numbers for these modalities were compared to previous published data from our laboratory and found to be comparable across all measures [12]. No changes were observed in overall neurologic function, walking gait, tremor, or grip strength between DCS- and vehicle-treated mice (Fig 2). Treatment with DCS also resulted in a modest enhancement of the 50% survival rate of Mecp2tm1.1Jae/y mice to 18.5 weeks, compared to 13.5 weeks in the vehicle-treated group (p = 0.0826, Gehan-Breslow-Wilcoxon test). Taken together, these results indicate that chronic treatment with DCS is not harmful to Mecp2tm1.1Jae/y mice, and in fact may possibly offer some measure of neurobehavioral benefit. More rigorously highly powered studies in the future will be needed to determine whether the behavioral benefits suggested here in our safety screen in fact manifest prominently in this model as a function of treatment with DCS.
Fig 1. Administration of DCS (20 mg/kg/day IP) to Mecp2tm1.1Jae/y mice did not alter body weight compared to Mecp2tm1.1Jae/y mice receiving vehicle (VEH) injection (n = 5 per group).
Mice were injected daily beginning at 3 weeks of age and continued throughout the life of the animal.
Fig 2. Administration of DCS (20 mg/kg/day IP) to Mecp2tm1.1Jae/y mice, beginning at 3 weeks of age, improved the breathing pattern and spontaneous locomotor activity, but did not affect neurological function, walking gate, tremors or grip strength compared to Mecp2tm1.1Jae/y mice receiving vehicle (VEH) injection (n = 5 per group), at 18 weeks of age.
Treatment of Mecp2tm1.1Jae/y mice with DCS restores normal hippocampal paired-pulse ratio
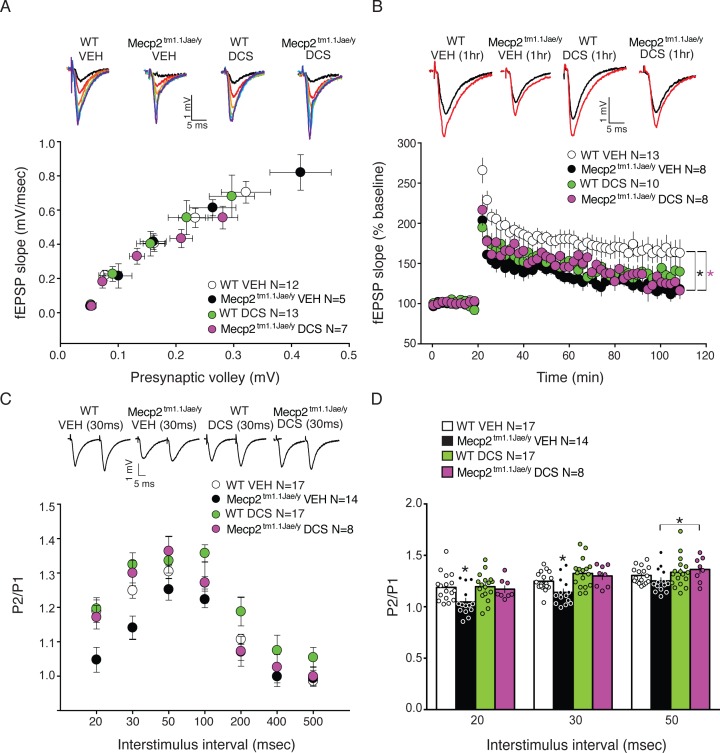
To examine electrophysiologic measures of synaptic function, ex vivo analysis was conducted on acute hippocampal brain slices derived from 18 week old behaviorally-naïve vehicle- and DCS-treated Mecp2tm1.1Jae/y and wild type littermate mice. I/O curves were generated by placing a stimulating electrode in the Schaffer collaterals and recording field potentials in the hippocampal CA1 region. The I/O slopes of presynaptic volley amplitude to the fEPSC slope were not significantly different between any of the four groups, suggesting that DCS treatment did not affect basal synaptic transmission in mice (Fig 3A).
Fig 3. PPR deficits but not LTP are ameliorated in Mecp2tm1.1Jae/y mice given chronic D-cycloserine treatment (20 mg/kg/day IP).
A, There were no significant differences in input output slopes in Mecp2tm1.1Jae/y and WT mice given vehicle (VEH) or drug, indicating no change in gross number of active synapses relative to WT VEH mice. B, LTP is substantially reduced in Mecp2tm1.1Jae/y mice compared to WT VEH controls. D-cycloserine had no effect on Mecp2tm1.1Jae/y mice compared to Mecp2tm1.1Jae/y mice given VEH. D-cycloserine significantly attenuated LTP responses in WT mice compared to WT VEH mice. C. PPR experiment. All groups are shown. D. Bar graph demonstrating that chronic D-cycloserine treatment reversed paired pulse ratios at 20, 30, and 50 msec interstimulus intervals in the Mecp2tm1.1Jae/y group compared to Mecp2tm1.1Jae/y mice given VEH treatment suggesting that D-cycloserine ameliorates PPR deficits at these intervals. Mecp2tm1.1Jae/y VEH group had significantly lower PPR compared to all groups at the 20 and 30 msec intervals. An independent t-test revealed a significant difference between the Mecp2tm1.1Jae/y VEH and Mecp2tm1.1Jae/y D-cycloserine groups at the 50 msec interstimulus interval. * p≤.05. Representative sample traces from each group are shown in top of respective graph.
To assess LTP, we stimulated the Schaffer-collateral pathway using high frequency stimulation (2 trains of enhanced pulses 100 Hz), a stimulation paradigm previously shown to induce LTP in a Mecp2 overexpression mouse line [45]. With respect to LTP, two-way repeated measures ANOVA revealed significant differences between vehicle-treated Mecp2tm1.1Jae/y and WT mice, demonstrating significant impairments in high frequency stimulation-induced LTP in hippocampal slices from Mecp2tm1.1Jae/y mice. A significant interaction effect was seen between vehicle groups (F(54, 1154) = 4.2, p = .001). Post-hoc analyses revealed a significant attenuation in LTP in Mecp2tm1.1Jae/y mice. A significant interaction effect was also seen between WT groups (F(54,1264) = 2.4, p = .001), and post-hoc analyses showed a significant attenuation in LTP in DCS-treated WT slices 35% of the time after HFS stimulation, compared to vehicle-treated WT hippocampal slices. In Mecp2tm1.1Jae/y mice, we did not observe any significant difference in LTP due to DCS compared to vehicle treatment (Fig 3B).
We next examined PPR, an index of presynaptic probability of release, by recording the response of two pulses separated by varying interstimulus intervals and then comparing the second and first responses. PPR was significantly different at 20 and 30 msec interstimulus intervals, with F(3,55) = 5.03, p = .004 and F(3,55) = 6.7, p = .001, respectively, with DCS-treatment reversing PPR deficits in Mecp2tm1.1Jae/y hippocampal slices (Fig 3C and 3D). Post-hoc analyses revealed differences between vehicle-treated Mecp2tm1.1Jae/y hippocampal slices and all other groups (p<0.05). We also included an effect size analysis using Cohen’s d and discovered that our Cohen’s d values were -1.1 for the 20 msec interstimulus interval (ISI) and -1.4 for the 30 msec ISI when comparing the effect size between the vehicle-treated Mecp2tm1.1Jae/y and DCS-treated Mecp2tm1.1Jae/y mice, indicating that over 80% of our DCS-treated Mecp2tm1.1Jae/y mice will be above the mean of the vehicle-treated Mecp2tm1.1Jae/y group. PPR was not statistically significant at 50 msec, but vehicle- and DCS-treated Mecp2tm1.1Jae/y slices showed significantly different PPRs, as determined by an independent t-test (2-tailed), t(20) = 2.1, p = .05. PPR was also significantly different between vehicle- and DCS-treated WT hippocampal slices at the 100 msec interstimulus interval (F(3,55) = 4.6, p = .006; Student-Newman-Keuls, p = .05). DCS-treated WT hippocampal slices showed a higher PPR at the 500 msec interstimulus interval compared to vehicle-treated WT hippocampal slices, as revealed by an independent t-test (t(32) = 2.2, p = .04).
DCS partially restores BDNF levels in brainstem and striatum of Mecp2tm1.1Jae/y mice
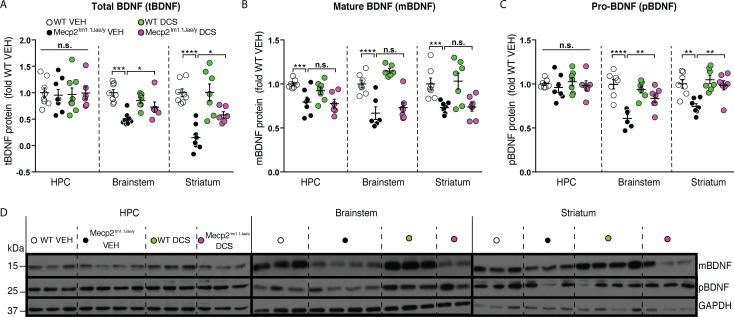
Brain-derived neurotrophic factor (BDNF) is a downstream target of MeCP2-mediated signaling [46,47], and reduced BDNF levels may contribute to the pathogenesis of RTT [48–50]. Stimulation of NMDA receptors increases release of neuronally-derived BDNF [51–54], and DCS treatment restores BDNF levels after neurologic injury in mice [55]. Accordingly, we wondered whether the improvement in behavior and synaptic transmission seen in Mecp2tm1.1Jae/y mice after treatment with DCS might be associated with augmented BDNF levels in the brain. First using ELISA, we found that total BDNF (tBDNF) protein levels in Mecp2tm1.1Jae/y mice were significantly lower in the brainstem (significant interaction, genotype x treatment, F1,25 = 14.04; p = 0.0067), and striatum (main effect of genotype F1,25 = 50.69; p < 0.0001; main effect of treatment, F1,25 = 5.97; p = 0.0425), compared to WT littermates (Fig 4A). DCS treatment significantly increased BDNF levels in both regions, without affecting levels in WT mice (Fig 4A). In the hippocampus, BDNF was unaltered in Mecp2tm1.1Jae/y mice compared to WT littermates, and DCS-treatment had no effect in either genotype (Fig 4A). Next we examined levels of matureBDNF (mBDNF) and its precursor protein proBDNF (pBDNF) using isoform-specific antibodies. We found that mBDNF levels were significantly lower in the hippocampus (main effect of genotype F1,23 = 16.57; p = 0.0005), brainstem (main effect of genotype F1,22 = 52.91; p < 0.0001) and striatum (main effect of genotype F1,24 = 4.453; p < 0.0454) of Mecp2tm1.1Jae/y mice compared to WT littermates. Surprisingly, DCS treatment had no effect in any of the brain regions (Fig 4B). By contrast, pBDNF was unaltered in the hippocampus but was significantly lower in the brainstem (significant interaction, genotype x treatment, F1,22 = 12.79; p = 0.0017) and striatum (significant interaction, genotype x treatment, F1,24 = 4.459; p = 0.0453). DCS treatment significantly increased pBDNF in both of these regions (Fig 4C).
Fig 4. DCS (20 mg/kg/day IP) partially restores proBDNF (pBDNF) in the brainstem and striatum of Mecp2tm1.1Jae/y mice.
A. The brainstem and striatum but not hippocampus (HPC) had lower levels of total BDNF (tBDNF) protein. DCS had no effect on BDNF protein levels in any brain region in WT mice. DCS increased BDNF in the brainstem and striatum of Mecp2tm1.1Jae/y mice. DCS had no effect on BDNF levels in the hippocampus. B. The HPC, brainstem and striatum had lower levels of mature BDNF (mBDNF) and DCS had no effect on protein levels in any of the brain regions. C. The brainstem and striatum but not HPC had lower levels of proBDNF (pBDNF) protein. DCS had no effect on pBDNF in any brain region in WT mice, however it increased pBDNF levels in the brainstem and striatum of Mecp2tm1.1Jae/y mice. WT VEH n = 8, WT DCS n = 7–8, Mecp2tm1.1Jae/y VEH n = 8, Mecp2tm1.1Jae/y DCS n = 6. *p < .05, ***p < .001, ****p < .0001. D. Representative western blot images showing mBDNF, pBDNF and GAPDH in HPC, brainstem and striatum of all groups.
Conclusions
DCS, a cyclic analog of D-alanine, is already FDA-approved for use in relatively high doses as an antibiotic treatment for multiple drug-resistant strains of M. tuberculosis by virtue of its ability to inhibit crucial enzymes involved in peptidoglycan synthesis [56,57]. The dose of DCS administered here to mice (20 mg/kg) corresponds to 1.62 mg/kg for a human, based solely on body surface area conversion [58]. Assuming an average child mass of 32 kg, this would correspond to a daily dosing of 52 mg, which is much lower than the maximum recommended pediatric dose of 1 gram of DCS for active tuberculosis. Thus, there is hope that a traditional dose of 500 mg– 1 gram a day of DCS could have beneficial effects in humans with RTT. Notably, side effects of DCS are relatively mild and rare, and patients have reported that they are generally unaware of whether they have ingested DCS or placebo [59]. This, in addition to the wide availability and established safety of DCS, has recently helped facilitate human trials for other potential applications of this agent as well. As DCS also safely increases calcium influx by acting as a partial agonist at the glycine recognition site on the NRI subunit of the NMDA-subtype glutamate receptor [21], applications for CNS disorders have been sought. Given the role of NMDA receptors in learning, one of the most heavily emphasized potential CNS applications of DCS has thus far been the possibility of facilitating exposure therapy for people suffering from fear and anxiety disorders. Our data presented here now also suggest that treatment with DCS might help mitigate some aspects of the synaptic deficits and symptoms experienced by patients suffering from RTT as well. Of note, we observed a small but significant decrease in LTP in mice treated with DCS, and it will be important to monitor humans for side effects that may correlate with LTP, such as cognitive decline. Thus far, this has not been reported in the extensive clinical literature on DCS.
Previous work has shown that mice with mutations in Mecp2 have significant cognitive impairments that may in part be mediated by long-term and short-term synaptic plasticity deficits [15,60,61]. In this study, we report significant decrements in hippocampal LTP, specifically in the Scaffer collateral pathway, as well as significant decreases in PPR in the Mecp2tm1.1Jae/y mouse model of RTT. Importantly, we demonstrate that chronic treatment with DCS reverses the short-term synaptic deficits seen in PPR at 20, 30 and 50 sec interstimulus intervals, indicating that presynaptic function may be restored by long-term treatment with DCS. Nelson et al [16]demonstrated significant alterations in short-term plasticity in hippocampal cell cultures deficient in MeCP2. This may be the result of imbalances in excitatory/inhibitory transmission, as mEPSC frequency was substantially decreased in MeCP2 KO hippocampal cell cultures [16]. Treatment of Mecp2tm1.1Jae/y mice with DCS may rectify this imbalance between excitatory and inhibitory tone, thus ameliorating the deficits in PPRs seen in Mecp2tm1.1Jae/y mice. Of note, synaptic inhibition was not measured in this study, and follow-up work should focus on this aspect of physiology. Past experiments in other mouse models of autism and Fragile X syndrome [62–64] have indicated that restoration of excitatory and inhibitory imbalance may provide a therapeutic target for individuals suffering from these neurodevelopmental disorders. Indeed, recent work has demonstrated that administration of picrotoxin, a GABAA antagonist, can reverse the synaptic impairments that arise as a function of MeCP2 overexpression [65]and may function to mitigate imbalances in excitatory/inhibitory neurotransmission.
DCS did not rescue the LTP deficits in the Mecp2tm1.1Jae/y mice used in this study. The lack of improvement in LTP might be due to the fact that DCS binds to the glycine-binding site on the NR1 subunit of NMDA receptors and not the NR2 subunits, which presumably are necessary for supporting LTP [66,67]. Given that we observe no significant differences in BDNF expression in the hippocampus of Mecp2tm1.1Jae/y mice compared to WT mice, the deficits in LTP in Mecp2tm1.1Jae/y mice are not necessarily due to alterations in hippocampal BDNF expression. Indeed, Mecp2308/y mice, another mouse model of RTT display deficient LTP with normal hippocampal Bdnf mRNA expression [60]. Thus, factors besides BDNF may be responsible for the deficits in hippocampal long-term synaptic plasticity observed in Mecp2 mutant mice. Other studies have indicated that DCS if given chronically may actually function as an NMDA antagonist, as prolonged exposure to DCS desensitizes the NMDA receptor complex [68,69]. Acute administration of DCS facilitates maze learning in mice, but chronic administration of DCS (15 days) significantly impairs consolidation and memory retrieval [70]. These data may explain the difference in LTP responses between the two WT groups. Future studies will need to focus on the mechanisms putatively involved in impairments in LTP in Mecp2 mutant mice.
The respiratory distress endured by patients with RTT is modeled in Mecp2tm1.1Jae/y mice as an increase in breathing apneas [71–73], (and has been linked to an abnormally highly excited default state of the brainstem respiratory network [72–76]. A crucial part of this respiratory network is the nucleus of the solitary tract (nTS), where BDNF normally modulates excitability at primary afferent synapses by inhibiting post-synaptic responses to excitatory glutamatergic transmission [77]. It has previously been shown that brief exposure of Mecp2-deficient brainstem slices to BDNF reduces synaptic hyperexcitability in the nTS [78]. Furthermore, administration of LM22A-4, a small molecule non-peptide BDNF loop-domain mimetic [79], to a mouse model of RTT decreases synaptic hyperexcitability in the brainstem and normalizes breathing apneas [80,81]. Here, we have observed lower levels of BDNF in the brainstem of Mecp2tm1.1Jae/y mice, and show that moderate mitigation of breathing apneas is associated with a DCS-mediated increase in brainstem proBDNF and not mature BDNF. We further observed deficient striatal mature BDNF and proBDNF in Mecp2tm1.1Jae/y mice, with proBDNF and not mature BDNF also elevated by treatment with DCS, which could relate to the improved motor function. This is supported by a recent finding of an increase in striatal proBDNF and not mature BDNF in the 6-hydroxydopamine model of Parkinson’s disease with improvement in motor function following treatment with the neuroprotective compound, Diphenyl diselenide [82]. Indeed, this also appears consistent with previous findings that administration of fingolimod, a sphingosine-1 phosphate receptor modulator that increases total BDNF in the striatum of Mecp2tm1.1Jae/y mice, improves locomotor activity in these mice as well [83]. It is intriguing that lower levels of proBDNF and not mature BDNF are elevated by DCS treatment in the brainstem and striatum, given that proBDNF is a negative regulator of synaptic plasticity [84], suggesting that yet unknown mechanisms could be attributed to the actions of proBDNF. Future studies will address the precise mechanism by which proBDNF may contribute to the improvement of irregular breathing and motor function. Additionally, it is hoped that future work in the field may focus on characterization of synapses in brainstem nuclei involved in the irregular breathing phenotype, as this is of paramount importance to patients with RTT.
Taken together, our data provide a novel therapeutic treatment for deficits in short-term synaptic plasticity in this mouse model of RTT. Future experiments will focus on developing and testing hippocampal-specific behavioral correlates of this deficit. Importantly, peripheral treatment with DCS also normalized BDNF expression in the brainstem, which may account for the improvement that we observe in sleep apneas. Given that we also see increases in BDNF expression in the thalamus and striatum, other behaviors may also be improved by DCS treatment. Importantly, since in RTT females are mostly affected, a test of the effects of DCS in female Mecp2 heterozygous mice is critical for determining the suitability of DCS as a possible treatment for patients. Future studies will focus on this as well as behaviors that may be markedly improved by DCS treatment, including striatal- and hippocampal-dependent behaviors, and also on evaluation of whether the beneficial effect of DCS is observed in additional preclinical models of RTT in other laboratories as well. If so, then clinical trials of DCS treatment of patients with RTT would be warranted. In addition, it is important to note that female RTT patients are a mosaic, and therefore mutated and phenotypically normal neurons and glial cells are intermixed in their brains. Thus, in future testing of the putative therapeutic efficacy of any drug for patients with RTT, including FDA-approved DCS, it will be essential to assess possible side effects on normal cells by rigorously assessing the behavioral effect in WT littermates as well.
Supporting information
(PDF)
Data Availability
All data files are available from the figshare database (https://doi.org/10.6084/m9.figshare.5114272.v1).
Funding Statement
This work was supported by research funds from the Rett Syndrome Research Trust to AAP, Weill Cornell Autism Research Program funding to AMR, funding from The Hartwell Foundation to AAP and AMR, and NIMH grant MH081060 to LMM.
References
- 1.Berger-Sweeney J. Cognitive deficits in Rett syndrome: What we know and what we need to know to treat them. Neurobiology of Learning and Memory 2011;96: 637–646. doi: 10.1016/j.nlm.2011.05.006 [DOI] [PubMed] [Google Scholar]
- 2.Naidu S. Rett syndrome: Natural history and underlying disease mechanisms. European Child and Adolescent Psychiatry 1997; 6(Suppl 1): 14–17. [PubMed] [Google Scholar]
- 3.Amir RE, Van der Veyver IB, Wan M, Tran CQ, Franke U, Zoghbi HY. Rett syndrome is caused by mutations in X-linked MECP2, encoding methyl-CpG-binding protein 2. Nat Genet 1999; 23: 185–188. doi: 10.1038/13810 [DOI] [PubMed] [Google Scholar]
- 4.Neul JL. The relationship of Rett syndrome and MECP2 disorders to autism. Dialogues Clin Neurosci 2012;14: 253–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Na ES, Nelson ED, Kavalali ET, Monteggia LM. The impact of MeCP2 Loss- or Gain- of-function on synaptic plasticity. Neuropsychopharmacology 2013;38: 212–219. doi: 10.1038/npp.2012.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hagberg B, Aicardi J, Dias K, Ramos O. A progressive syndrome of autism, dementia, ataxia, and loss of purposeful hand use in girls: Rett’s syndrome: Report of 35 cases. Annals of Neurology 1983;14: 471–479. doi: 10.1002/ana.410140412 [DOI] [PubMed] [Google Scholar]
- 7.Hagberg B, Witt-Engerstrom I. Rett syndrome: A suggested staging system for describing impairment profile with increasing age towards adolescence. American Journal of Medical Genetics 1986;Supplement 1: 47–59. [DOI] [PubMed] [Google Scholar]
- 8.Asthana JC, Sinha S, Haslam JS, Kingston HM. Survey of adolescents with severe intellectual handicap. Archives of Disease in Childhood 1990;65: 1133–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gemelli T, Berton O, Nelson ED, Perrotti LI, Jaenisch R, Monteggia LM. Postnatal loss of methyl-CpG binding protein 2 in the forebrain is sufficient to mediate behavioral aspects of Rett syndrome in mice. Biological Psychiatry 2006;59: 468–476. doi: 10.1016/j.biopsych.2005.07.025 [DOI] [PubMed] [Google Scholar]
- 10.Katz DM, Berger-Sweeney JE, Eubank JH, Justice MJ, Neul JL, Pozzo-Miller L, et al. Preclinical research in Rett syndrome: setting the foundation for translational success. Dis Model Mech 2012;5: 733–745. doi: 10.1242/dmm.011007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buchovecky CM, Turley SD, Brown HM, Kyle SM, McDonald JG, Liu B, et al. A suppressor screen in Mecp2 mutant mice implicates cholesterol metabolism in Rett syndrome. Nat Genet 2013;45: 1013–1020. doi: 10.1038/ng.2714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang J, Wegener JE, Huang TW, Sripathy S, De Jesus-Cortes H, Xu P, et al. Wild type-microglia do not reverse pathology in mouse models of Rett syndrome. Nature 2015;521L E1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahgoub M, Adachi M, Suzuki K, Liu X, Kavalali ET, Chahrour MH, et al. MeCP2 and histone deacetylases 1 and 2 in dorsal striatum collectively suppress repetitive behaviors. Nat Neurosci 2016. l19: 1506–1512. doi: 10.1038/nn.4395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guy J, Gan J, Selfridge J, Cobb S, Bird A. Reversal of neurological deficits in a mouse model of Rett syndrome. Science 2007;315: 1143–1147. doi: 10.1126/science.1138389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asaka Y, Jugloff DG, Zhang L, Eubanks JH, Fitzsimonds RM. Hippocampal synaptic plasticity is impaired in the Mecp2-null mouse model of Rett syndrome. Neurobiol Dis 2006;21: 217–227. doi: 10.1016/j.nbd.2005.07.005 [DOI] [PubMed] [Google Scholar]
- 16.Nelson ED, Kavalali ET, Monteggia LM. MeCP2-dependent transcriptional repression regulates excitatory neurotransmission. Curr Biol 2006;16: 710–716. doi: 10.1016/j.cub.2006.02.062 [DOI] [PubMed] [Google Scholar]
- 17.Blue ME, Naidu S, Johnston MV. Altered development of glutamate and GABA receptors in the basal ganglia of girls with Rett syndrome. Exp Neurol 1999;156: 345–352. doi: 10.1006/exnr.1999.7030 [DOI] [PubMed] [Google Scholar]
- 18.Blue ME, Naidu S, Johnston MV. Development of amino acid receptors in frontal cortex from girls with Rett syndrome. Ann Neurol 1999;45: 541–545. [DOI] [PubMed] [Google Scholar]
- 19.Hamberger A, Gillberg C, Palm A, Hagberg B. Elevated CSF glutamate in Rett syndrome. Neuropediatrics 1992;23: 212–213. doi: 10.1055/s-2008-1071344 [DOI] [PubMed] [Google Scholar]
- 20.Lappalainen R, Riikonen RS. High levels of cerebrospinal fluid glutamate in Rett syndrome. Pediatr Neurol 1996;15: 445–450. [DOI] [PubMed] [Google Scholar]
- 21.Scheinin A, Shavit S, Benveniste M. Subunit specificity and mechanism of action of NMDA partial agonist D-cycloserine. Neuropharmacology 2001;41: 151–158. [DOI] [PubMed] [Google Scholar]
- 22.Walker DL, Ressler KJ, Lu K-T, Davis M. Facilitation of conditioned fear extinction by systemic administration of intra-amygdala infusions of D-cycloserine as assessed with fear-potentiated startle in rats. The Journal of Neuroscience 2002;22: 2343–2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ledgerwood L, Richardson R, Cranney J. Effects of D-cycloserine on extinction of conditioned freezing. Behav Neurosci 2003;117: 341–349. [DOI] [PubMed] [Google Scholar]
- 24.Ledgerwood L, Richardson R, Cranney J. D-cycloserine and the facilitation of extinction of conditioned fear: Consequences for reinstatement. Behav Neurosci 2004;118: 505–513. doi: 10.1037/0735-7044.118.3.505 [DOI] [PubMed] [Google Scholar]
- 25.Ledgerwood L, Richardson R, Cranney J. D-cycloserine facilitates extinction of learned fear: Effects of reacquisition and generalized extinction. Biol Psychiatry 2005;57: 841–847. doi: 10.1016/j.biopsych.2005.01.023 [DOI] [PubMed] [Google Scholar]
- 26.Baker KD, McNally GP, Richardson R. D-cycloserine does not facilitate fear extinction by reducing conditioned stimulus processing or promoting conditioned inhibition to contextual clues. Learn Mem 2012;14: 461–469. [DOI] [PubMed] [Google Scholar]
- 27.Meyers KM, Carlezon WA, Davis M. Glutamate receptors in extinction and extinction-based therapies for psychiatric illness. Neuropsychopharmacology 2011;36: 274–293. doi: 10.1038/npp.2010.88 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meyers KM, David M, Behavioral and neural analysis of extinction. Neuron 2002;36: 567–584. [DOI] [PubMed] [Google Scholar]
- 29.Falls W, Miserendino M, Davis M. Extinction of fear-potentiated startle: Blockade by infusion of an NMDA antagonist into the amygdala. The Journal of Neuroscience 1992;12: 854–863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baker JD, Azorlosa JL. The NMDA antagonist MK-801 blocks the extinction of Pavlovian fear conditioning. Behav Neurosci 1996;110: 618–620. [DOI] [PubMed] [Google Scholar]
- 31.Sotres-Bayon F, Bush DEA, LeDoux JE. Acquisition of fear extinction requires activation of NR2B-containing NMDA receptors in the lateral amygdala. Neuropsychopharmacology 2007;32: 1929–1940. doi: 10.1038/sj.npp.1301316 [DOI] [PubMed] [Google Scholar]
- 32.Sortres-Bayon F, Diaz-Mataix L, Bush DEA, LeDoux JE. Dissociable roles for the ventromedial prefrontal cortex and amygdala in fear extinction: NR2B contribution. Cereb Cortex 2009;19: 474–482. doi: 10.1093/cercor/bhn099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akirav I. NMDA partial agonist reverses blocking of extinction of aversive memory by GABA(A) agonist in the amygdala. Neuropsychopharmacology 2007;32: 542–550. doi: 10.1038/sj.npp.1301050 [DOI] [PubMed] [Google Scholar]
- 34.Yamamoto S, Morinobu S, Fuchikami M, Kurata A, Kozuru T, Yamawaki S. Effects of single prolonged stress and D-cycloserine on contextual fear extinction and hippocampal NMDA receptor expression in a rat model of PTSD. Neuropsychopharmacology 2008;33: 2108–2116. doi: 10.1038/sj.npp.1301605 [DOI] [PubMed] [Google Scholar]
- 35.Bertotto ME, Bustos SG, Molina VA, Martijena ID. Influence of ethanol withdrawal on fear memory: effect of D-cycloserine. Neuroscience 2006;142: 979–990. doi: 10.1016/j.neuroscience.2006.07.013 [DOI] [PubMed] [Google Scholar]
- 36.Silvestri AJ, Root DH. Effects of REM deprivation and an NMDA agonist on the extinction of conditioned fear. Physiol Behav 2008;93: 274–281. doi: 10.1016/j.physbeh.2007.08.020 [DOI] [PubMed] [Google Scholar]
- 37.Yu H, Wang Y, Patwell S, Jing D, Liu T, Zhang Y, et al. Variant BDNF Val66Met polymorphism affects extinction of conditioned aversive memory. The Journal of Neuroscience 2009;29: 4056–4064. doi: 10.1523/JNEUROSCI.5539-08.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Grapp K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Arch Gen Psychiatry 2004;61: 1136–1144. doi: 10.1001/archpsyc.61.11.1136 [DOI] [PubMed] [Google Scholar]
- 39.Hofmann SG, Meuret AE, Smits JA, Simon NM, Pollack MH, Eisenmenger K, et al. Augmentation of exposure therapy with D-cycloserine for social anxiety disorder. Arch Gen Psychiatry 2006;63: 298–304. doi: 10.1001/archpsyc.63.3.298 [DOI] [PubMed] [Google Scholar]
- 40.Guastella AJ, Richardson R, Lovibond PF, Rapee RM, Gaston JE, Mitchell P, et al. A randomized controlled trial of D-cycloserine enhancement of exposure therapy for social anxiety disorder. Biol Psychiatry 2008;63: 544–549. doi: 10.1016/j.biopsych.2007.11.011 [DOI] [PubMed] [Google Scholar]
- 41.Kushner MG, Kim SW, Donahue C, Thuras P, Adson D, Kotlyar M, et al. D- cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 2007;62: 835–838. doi: 10.1016/j.biopsych.2006.12.020 [DOI] [PubMed] [Google Scholar]
- 42.Wilhelm S, Buhlmann U, Tolin DF, Meunier SA, Pearlson GD, Reese HE, et al. Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry 2008;165: 335–341. doi: 10.1176/appi.ajp.2007.07050776 [DOI] [PubMed] [Google Scholar]
- 43.Otto MW, Tolin DF, Simon NM, Pearlson GD, Basden S, Meunier SA, et al. Efficacy of D-cycloserine for enhancing response to cognitive-behavior therapy for panic disorder. Biol Psychiatry 2010;67: 365–370. doi: 10.1016/j.biopsych.2009.07.036 [DOI] [PubMed] [Google Scholar]
- 44.Chen RZ, Akbarian S, Tudor M, Jaenisch R. Deficiency of methyl-CpG binding protein-2 in CNS neurons results in a Rett-like phenotype in mice. Nat Genet 2001;27: 327–331. doi: 10.1038/85906 [DOI] [PubMed] [Google Scholar]
- 45.Na ES, Nelson ED, Adachi M, Autry AE, Mahgoub MA, Kavalali ET, et al. A mouse model for MeCP2 duplication syndrome: MeCP2 overexpression impairs learning and memory and synaptic transmission. Journal of Neuroscience 2012;32: 3109–3117. doi: 10.1523/JNEUROSCI.6000-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen WG, Chang Q, Meissner A, West AE, Griffith EC, Jaenisch R, et al. Derepression of BDNF transcription involves calcium-dependent phosphorylation of MeCP2. Science 2003;302: 885–889. doi: 10.1126/science.1086446 [DOI] [PubMed] [Google Scholar]
- 47.Martinowich K, Hattori D, Wu H, Fouse S, He F, Hu Y, et al. DNA methylation-related chromatin remodeling in activity-dependent BDNF gene regulation. Science 2003;302: 890–893. doi: 10.1126/science.1090842 [DOI] [PubMed] [Google Scholar]
- 48.Chang Q, Khare G, Dani V, Nelson S, Jaenisch R. The disease progression of Mecp2 mutant mice is affected by the level of BDNF expression. Neuron 2006;49: 341–346. doi: 10.1016/j.neuron.2005.12.027 [DOI] [PubMed] [Google Scholar]
- 49.Li W, Pozzo-Miller L. BDNF deregulation in Rett syndrome. Neuropharmacology 2014;76: 737–746. doi: 10.1016/j.neuropharm.2013.03.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Katz DM. Brain-derived neurotrophic factor and Rett syndrome. Handb Exp Pharmacol 2014;220: 481–495. doi: 10.1007/978-3-642-45106-5_18 [DOI] [PubMed] [Google Scholar]
- 51.Takasu MA, Dalva MB, Zigmond RE, Greenberg ME. Modulation of NMDA receptor- dependent calcium influx and gene expression through EphB receptors. Science 2002;295: 491–495. doi: 10.1126/science.1065983 [DOI] [PubMed] [Google Scholar]
- 52.Yaka R, He DY, Phamluong K, Ron D. Pituitary adenylate cyclase-activating polypeptide (PACAP(1–38)) enhances N-methyl-D-aspartate receptor function and brain-derived neurotrophic factor expression via RACK1. J Biol Chem 2003;278: 9630–9638. doi: 10.1074/jbc.M209141200 [DOI] [PubMed] [Google Scholar]
- 53.Zafra F, Castren E, Thoenen H, Lindholm D. Interplay between glutamate and gamma-aminobutyric acid transmitter systems in the physiological regulation of brain- derived neurotrophic factor and nerve growth factor synthesis in hippocampal neurons. Proc Natl Acad Sci USA 1991;88: 10037–10041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zafra F, Lindholm D, Castren E, Hartikka J, Thoenen H. Regulation of brain-derived neurotrophic factor and nerve growth factor mRNA in primary cultures of hippocampal neurons and astrocytes. J Neurosci 1992;12: 4793–4799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yaka R, Biegon A, Grigoriadis N, Simeonidou C, Grigoriadis S, Alexandrovich AG, et al. D-cycloserine improves functional recovery and reinstates long-term potentiation (LTP) in a mouse model of closed head injury. FASEB J 2007;21: 2033–2041. doi: 10.1096/fj.06-7856com [DOI] [PubMed] [Google Scholar]
- 56.Lambert MP, Neuhaus FC. Mechanism of D-Cycloserine action: alanine racemase from Escherichia col W. J Bacteriol 1972;110: 978–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Prosser GA, de Carvalho LPS. Kinetic mechanism and inhibition of Mycobacterium tuberculosis D-alanine:D-alanine ligase by the antibiotic D-cycloserine. The FEBS Journal 2013;280: 1150–1166. doi: 10.1111/febs.12108 [DOI] [PubMed] [Google Scholar]
- 58.Reagan-Shaw S, Nihal M, Ahmad N. Dose Translation from animal to human studies revisited. FASEB J 2007;22: 659–661. doi: 10.1096/fj.07-9574LSF [DOI] [PubMed] [Google Scholar]
- 59.Hofmann SG, Pollack MH, Otto MW. Augmentation treatment of psychotherapy for anxiety disorders with D-cycloserine. CNS Drug Rev 2006;12: 208–217. doi: 10.1111/j.1527-3458.2006.00208.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Moretti P, Levenson JM, Battaglia F, Atkinson R, Teague R, Antalffy B, et al. Learning and memory and synaptic plasticity are impaired in a mouse model of Rett syndrome. J Neurosci 2006;26: 310–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Weng SM, McLeod F., Bailey ME, Cobb SR. Synaptic plasticity deficits in an experimental model of rett syndrome: long term potentiation saturation and its pharmacological reversal. Neuroscience 2011;180: 314–321. doi: 10.1016/j.neuroscience.2011.01.061 [DOI] [PubMed] [Google Scholar]
- 62.Fernandez F, Morishita W, Zuniga E, Nguyen J, Blank M, Malenka RC, et al. Pharmacotherapy for cognitive impairment in a mouse model of Down syndrome. Nat Neurosci 2007;10: 411–413. doi: 10.1038/nn1860 [DOI] [PubMed] [Google Scholar]
- 63.Gibson JR, Bartley AF, Hays SA, Huber KM. Imbalance of neocortical excitation and inhibition and altered UP states reflect network hyperexcitability in the mouse model of fragile X syndrome. J Neurophysiol 2008;100: 2615–2626. doi: 10.1152/jn.90752.2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Tabuchi K, Blundell J, Etherton MR, Hammer RE, Liu X, Powell CM, et al. A neuroligin-3 mutation implicated in autism increases inhibitory synaptic transmission in mice. Science 2007;318: 71–376. doi: 10.1126/science.1146221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Na ES, Morris MJ, Nelson ED, Monteggia LM. GABAA receptor antagonism ameliorates behavioral and synaptic impairments associated with MeCP2 overexpression. Neuropsychopharmacology 2014;39: 1946–1954. doi: 10.1038/npp.2014.43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jin SX, Feig LA. Long-term potentiation in the CA1 hippocampus induced by NR2A subunit-containing NMDA glutamate receptors is mediated by Ras-GRF2/Erk map kinase signaling. PLoS One 2010;5: e11732 doi: 10.1371/journal.pone.0011732 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Yashiro K, Philpot BD. Regulation of NMDA receptor subunit expression and its implications for LTD, LTP, and metaplasticity. Neuropharmacology 2008;55: 1081–1094. doi: 10.1016/j.neuropharm.2008.07.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Boje KM, Wong G, Skolnick P. Desensitization of the NMDA receptor complex by glycinergic ligands in cerebellar granule cell cultures. Brain Res 1993; 603: 207–214. [DOI] [PubMed] [Google Scholar]
- 69.Hofmann SG, Wu JQ, Boettcher H. D-Cycloserine as an augmentation strategy for cognitive behavioral therapy of anxiety disorders. Biol Mood Anxiety Disord 2013;3: 11 doi: 10.1186/2045-5380-3-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Quartermain D, Mower J, Herting RL, Lanthorn TH. Acute but not chronic activation of the NMDA-coupled glycine receptor with D-cycloserine facilitates learning and retention. Eur J Pharmacol 1994;257: 7–12. [DOI] [PubMed] [Google Scholar]
- 71.Ren J, Ding X, Funk GD, Greer JJ. Anxiety-related mechanisms of respiratory dysfunction in a mouse model of Rett syndrome. J Neurosci 2012;32: 17230–17240. doi: 10.1523/JNEUROSCI.2951-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Katz DM, Dutschmann M, Ramirez JM, Hilaire G. Breathing disorders in Rett syndrome: progressive neurochemical dysfunction in the respiratory network after birth. Respir Physiol Neurobiol 2009;168: 101–108. doi: 10.1016/j.resp.2009.04.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramirez JM, Ward CS, Neul JL. Breathing challenges in Rett syndrome: lessons learned from humans and animal models. Respir Physiol Neurobiol 2013;189: 280–287. doi: 10.1016/j.resp.2013.06.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Stettner GM, Huppke P, Brendel C, Richter DW, Gartner J, Dutschmann M. Breathing dysfunctions associated with impaired control of postinspiratory activity in Mecp2-/y knockout mice. J Physiol 2007;579: 863–876. doi: 10.1113/jphysiol.2006.119966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Roux JC, Dura E, Villard L. Tyrosine hydroxylase deficit in the chemoafferent and the sympathoadrenergic pathways of the Mecp2 deficient mouse. Neurosci Lett 2008;447: 82–86. doi: 10.1016/j.neulet.2008.09.045 [DOI] [PubMed] [Google Scholar]
- 76.Voituron N, Zanella S, Menuet C, Dutschmann M, Hilaire G. Early breathing defects after moderate hypoxia or hypercapnia in a mouse model of Rett syndrome. Respir Physiol Neurobiol 2009;168: 109–118. doi: 10.1016/j.resp.2009.05.013 [DOI] [PubMed] [Google Scholar]
- 77.Balkowiec A, Katz DM. Cellular mechanisms regulating activity-dependent release of native brain-derived neurotrophic factor from hippocampal neurons. J Neurosci 2002;22: 10399–10407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kline DD, Ogier M, Kunze DL, Katz DM. Exogenous brain-derived neurotrophic factor rescues synaptic dysfunction in Mecp2-null mice. J Neurosci 2010;30: 5303–5310. doi: 10.1523/JNEUROSCI.5503-09.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Massa SM, Yang T, Xie Y, Shi J, Bilgen M, Joyce JN, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest 2010;120: 1774–1785. doi: 10.1172/JCI41356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Schmid DA, Yang T, Ogier A, Adams I, Mirakhur Y, Wang Q, et al. A TrkB small molecule partial agonist rescues TrkB phosphorylation deficits and improves respiratory function in a mouse model of Rett syndrome. J Neurosci 2012;32: 1803–1810. doi: 10.1523/JNEUROSCI.0865-11.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kron M, Lang M, Adams IT, Sceniak M, Longo F, Katz DM. A BDNF loop-mimetic acutely reverses spontaneous apneas and respiratory abnormalities during behavioral arousal in a mouse model of Rett syndrome. Dis Model Mech 2014;7: 1047–1055. doi: 10.1242/dmm.016030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sampaio TB, Pinton S, da Rocha JT, Gai BM, Noqueira CW. Involvement of BDNF/TrkB signaling in the effect of diphenyl diselenide on motor function in a Parkinson’s disease rat model. Eur J Pharmacol 2017;795: 28–35. doi: 10.1016/j.ejphar.2016.11.054 [DOI] [PubMed] [Google Scholar]
- 83.Deogracias R, Yazdani M, Dekkers MPJ, Guy J, Lonescu MCS, Vogt KE, et al. Fingolimod, a sphingosine-1 phosphate receptor modulator, increases BDNF levels and improves symptoms of a mouse model of Rett syndrome. Proc Natl Acad Sci USA 2012;109: 14230–14235. doi: 10.1073/pnas.1206093109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hempstead BL. Deciphering proneurotrophin actions. Handb Exp Pharmacol 2014;220: 17–32. doi: 10.1007/978-3-642-45106-5_2 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF)
Data Availability Statement
All data files are available from the figshare database (https://doi.org/10.6084/m9.figshare.5114272.v1).