Abstract
Objective
Diabetic subjects are at higher risk of ischemic peripheral vascular disease (PVD). We tested the hypothesis that advanced glycation end products (AGEs) and their receptor (RAGE) block neoangiogenesis and blood flow recovery after hind limb ischemia induced by femoral artery ligation (FAL) through modulation of immune/inflammatory mechanisms.
Approach and Results
Wild type (WT) mice rendered diabetic with streptozotocin and subjected to unilateral FAL displayed increased accumulation and expression of AGEs and RAGE in ischemic muscle. In diabetic WT mice, FAL attenuated neoangiogenesis and impaired blood flow recovery, in parallel with reduced macrophage content in ischemic muscle and suppression of early inflammatory gene expression, including chemokine (C-C motif) ligand 2 (Ccl2) and early growth response gene 1 (Egr1) versus non-diabetic mice. Deletion of Ager or transgenic expression of Glo1 (reduces AGEs) restored adaptive inflammation, neoangiogenesis and blood flow recovery in diabetic mice. In diabetes, deletion of Ager increased circulating Ly6Chi monocytes and augmented macrophage infiltration into ischemic muscle tissue after FAL. In vitro, macrophages grown in high glucose display inflammation that is skewed to expression of tissue damage versus tissue repair gene expression. Further, macrophages grown in high versus low glucose demonstrate blunted macrophage-endothelial cell interactions. In both settings, these adverse effects of high glucose were reversed by Ager deletion in macrophages.
Conclusions
These findings indicate that RAGE attenuates adaptive inflammation in hind limb ischemia; underscore microenvironment-specific functions for RAGE in inflammation in tissue repair versus damage; and illustrate that AGE/RAGE antagonism may fill a critical gap in diabetic PVD.
Keywords: angiogenesis, diabetes, receptor, monocyte, inflammation
Introduction
Subjects with diabetes are at higher risk of developing ischemic vascular disease, including peripheral artery disease (PAD). PAD results from an obstruction of blood flow in the peripheral arteries. Compromised peripheral blood supply leads to tissue ischemia (lack of circulation) and tissue hypoxia (lack of oxygen). Neoangiogenesis, the formation of new capillaries from pre-existing vessels, occurs in response to an ischemic/hypoxic insult. Inflammation is a requisite process for ischemia/hypoxia-induced neoangiogenesis. However, the ability to develop functional new vessels is significantly lower in diabetic patients with PAD vs. age-matched control subjects.1–3 This may lead to the severe course of limb ischemia often observed in diabetic patients, in which PAD may result in foot ulceration, impaired wound healing, lower extremity amputation and mortality.4–7 Treatments to date have not been fully effective at improving blood flow through neoangiogenesis in PAD. Therefore, identification of new therapeutic targets is important for drug discovery for the treatment of PAD, especially in diabetes.
Hyperglycemia, a hallmark of diabetes, leads to the formation of advanced glycation end products (AGEs), a heterogeneous class of post-translationally modified proteins. AGEs form, in part, through the precursor methylglyoxal (MG) in subjects with diabetes.8 Glyoxalase 1 (GLO1) is a principal enzyme responsible for detoxifying MG and regulating AGE levels.9 However, in diabetes, elevated levels of glucose may render GLO1 insufficient to block AGE accumulation, thereby resulting in increased AGE levels. The receptor for AGE (RAGE) is a member of the immunoglobulin superfamily of cell surface molecules present on multiple cell types, usually expressed at low levels in homeostasis and to increased degrees in diabetic tissues and at sites of stress or injury. Increased accumulation of AGEs and enhanced activation of RAGE contribute to the pathogenesis of diabetic complications.10–15 In human subjects, RAGE is highly expressed in peripheral occlusive vascular disease lesions.16 In experimental models, earlier studies showed that RAGE plays central roles in the injury response to global hypoxia and ischemia/reperfusion (I/R) injury.17–20
Here, we hypothesized that deficiency of Ager (gene encoding RAGE) or overexpression of Glo1 in hind limb ischemia would restore blood flow recovery and neoangiogenesis in diabetic mice. To address this concept, we used a well-established mouse model of hind limb ischemia (a preclinical model of PAD)21 to assess the role of RAGE in both the immediate response to ischemic stress, and the long-term effects of this receptor on tissue remodeling and neoangiogenesis in both the non-diabetic and diabetic states. In addition, we explored the role of RAGE ligands, AGEs, by examining the impact of overexpression of Glo122 in mice undergoing hind limb ischemia and we probed the underlying mechanisms using in vivo, ex vivo, and in vitro analyses of peripheral blood and muscle tissues. Collectively, our data reveal that the AGE-RAGE axis blunts neoangiogenesis and blood flow recovery, at least in part through an impingement on adaptive immune and vascular cell responses consequent to hind limb ischemia, which may be restored by deletion of Ager or over-expression of Glo1. These data contribute to an emerging paradigm in which RAGE-condition-dependent pro- versus anti-inflammatory responses may prevail depending on the unique stress and distinct cues within the discrete microenvironment.
Materials and Methods
Materials and Methods are available in the online-only Supplement.
Results
RAGE impairs blood flow recovery and angiogenesis after hind limb ischemia
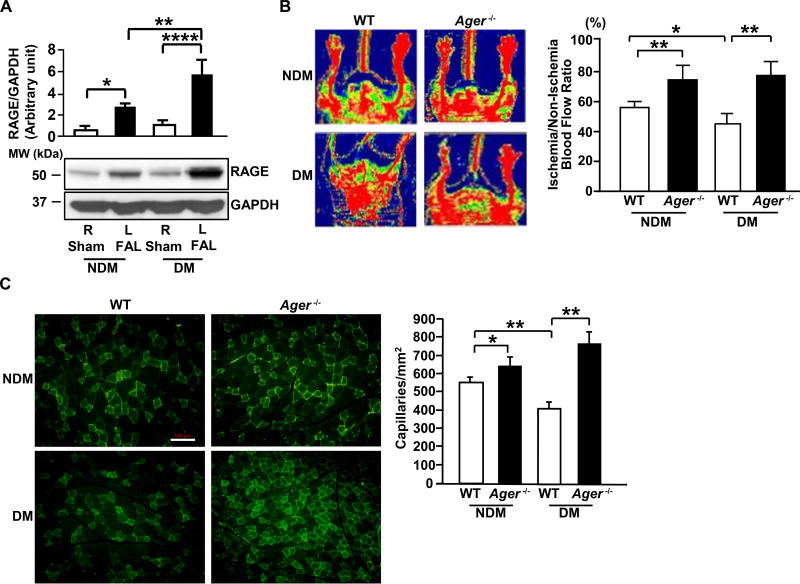
To establish if RAGE modulates responses to hind limb ischemia, we began by detecting RAGE expression in the leg muscles of streptozotocin-induced diabetic and non-diabetic WT mice on day 5 after femoral artery ligation (FAL). RAGE protein was significantly higher in the ischemic muscles of diabetic WT mice compared to non-ischemic muscles of diabetic WT mice (p<0.0001; Figure 1A). A significant increase in RAGE protein was also observed in the ischemic muscles of non-diabetic WT mice compared to the non-ischemic muscles of non-diabetic WT mice (p<0.05; Figure 1A). Furthermore, the expression of RAGE protein in the ischemic muscles of diabetic WT mice was significantly higher than that observed in the ischemic muscle of non-diabetic WT mice (p<0.01; Figure 1A). These observations indicate that hind limb ischemia induced increased expression of RAGE in ischemic muscles, especially in diabetic WT mice, and suggested potential roles for RAGE in the response to hind limb ischemia. To address this premise, we tested the effect of deletion of Ager on vascular repair responses in the presence or absence of diabetes.
Figure 1. Blood flow recovery and angiogenesis in hind limb ischemia: effect of RAGE.
(A) Proteins from the indicated skeletal muscle on day 5 after femoral artery ligation (FAL) were subjected to Western blotting for detection of RAGE and GAPDH (n=3 mice/group). (B) Hind limb blood flow was monitored by laser Doppler Perfusion Imaging on day 28 post-ligation (red indicates normal perfusion; blue, reduced blood flow) and quantitative evaluation of blood flow was expressed as a ratio of blood flow in ischemic to sham limb (n=10 mice/group). (C) Representative photomicrographs of ischemic muscles from the indicated mice stained with anti-CD31 IgG on day 28 post-ligation (scale bar=100 µm) and quantitative evaluation of capillary density was performed in muscle tissue sections of non-diabetic and diabetic WT vs. Ager−/− mice (n=10 mice/group). R denotes right leg/sham control and L denotes left leg/FAL. NDM denotes non-diabetes and DM denotes diabetes. Error bars represent ±SEM. *p<0.05, **p<0.01, ****p<0.0001.
We performed FAL and began by assessing the role of RAGE in blood flow recovery. Laser Doppler Perfusion Imaging revealed that restoration of perfusion was significantly lower in diabetic WT mice compared with non-diabetic WT mice on day 28 after FAL (p<0.05, Figure 1B). In contrast, significantly higher blood flow was observed in both diabetic and non-diabetic Ager−/− mice compared to their respective controls 28 days after FAL (p<0.01, Figure 1B). Of note, induction of diabetes by streptozotocin resulted in expected rises in levels of blood glucose and there were no differences based on Ager genotype (Table I in the online-only Data Supplement).
To assess the effects of diabetes and RAGE on neoangiogenesis in response to hind limb ischemia, histological assessment of capillary density was performed in WT mice and in mice devoid of Ager on day 28 post-FAL. As shown in Figure 1C, diabetic WT mice displayed significantly lower capillary density than non-diabetic mice on day 28 post-ligation (p<0.01). In contrast, mice devoid of Ager displayed significantly higher capillary density on day 28 in both the non-diabetic and diabetic states vs. respective WT mice (p<0.05 and p<0.01, Figure 1C).
RAGE impairs gene expression of inflammatory and angiogenic mediators and macrophage infiltration after hind limb ischemia, especially in diabetic mice
Prompted by these observations, we sought to identify the genes impacted by diabetes and RAGE in hind limb ischemia and we profiled genes essential to neoangiogenesis using a commercial angiogenesis RT2 profiler™ PCR array. Among the 84 genes tested, Table II, III and IV in the online-only Data Supplement illustrate some of the findings on skeletal muscle RNA of WT and Ager−/− mice on day 3, 5 and 7 after FAL in the absence and presence of diabetes compared to that of non-diabetic WT-sham controls. A significant theme that emerged from this analysis was that prominent gene expression changes related to inflammation were observed among the groups, and that these changes were driven, at least in part, by RAGE. After FAL, particularly in non-diabetic and diabetic Ager−/− mice, marked increases in chemokines, inflammatory and matrix metalloproteinase genes were observed compared to non-diabetic and diabetic WT mice. Levels of key chemokine [Ccl2, chemokine (C-X-C motif) ligand (Cxcl)2 (Gro-β), and Cxcl5 (Ena-78)] remain elevated in Ager−/− mice vs. respective WT mice in the non-diabetic and diabetic states on day 7, as well as on days 3 & 5. At the later time point, day 7, higher expression of growth and pro-angiogenic factors was observed in Ager−/− vs. WT mice in both the diabetic and non-diabetic states. Transcripts for Angpt2, hepatocyte growth factor (Hgf), midkine (Mdk), placental growth factor (Pgf), sphingosine kinase 1 (Sphk1), transforming growth factor beta (Tgfb) and thrombospondin-1 (Thbs1) were higher in Ager−/− ischemic tissues. Although we did not observe any differences in hypoxia-inducible factor-1 (Hif1)α mRNA transcripts on days 3 and 5, on day 7, the Ager−/− groups displayed higher levels of Hif1α mRNA transcripts vs. the WT groups. Levels of Integrin β chain, β3 precursor (Itgβ3) and platelet/endothelial cell adhesion molecule 1 (Pecam1/Cd31) transcripts were higher in both groups of Ager−/− mice on day 7. Note that no significant differences in Vegf a, b or c were observed by diabetes status or by the expression or not of Ager.
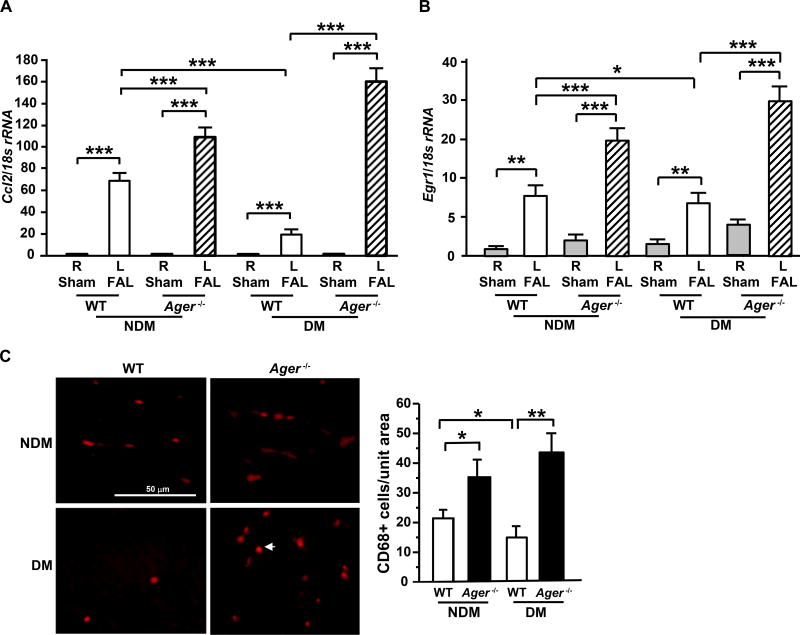
We performed real-time quantitative PCR to confirm the key findings and focused on inflammatory genes given the striking increase in the Ager-deficient tissues vs. the WT and the profound importance of inflammatory mechanisms in facilitating vascular and tissue repair after FAL. We found that in the ischemic muscle tissue, significantly lower levels of Ccl2 mRNA transcripts were observed in WT diabetic mice vs. non-diabetic WT mice on day 7 post-ligation (p<0.001, Figure 2A). In mice devoid of Ager, higher Ccl2 transcripts vs. WT mice on day 7 were observed in the skeletal muscle tissue in both the non-diabetic and diabetic state after FAL (p<0.001, Figure 2A). These data suggest that RAGE suppresses chemokine gene expression in the microenvironment of hind limb ischemia.
Figure 2. Gene expression and macrophage infiltration in hind limb ischemia: effect of RAGE.
(A–B) Total RNA was isolated from the indicated skeletal muscle and subjected to real-time PCR analysis. Ccl2 mRNA was detected on day 7 after FAL vs. sham (A) and Egr1 mRNA was detected on day 5 after FAL vs. sham (B), and normalized to 18s rRNA (n=5 mice/group). (C) Immunostaining with anti-CD68 IgG was performed on skeletal muscle tissue from the indicated mice on day 7 after FAL and the mean number of CD68+ cells/unit area is reported (n=3 mice/group). R denotes right leg/sham control and L denotes left leg/femoral artery ligation (FAL). NDM denotes non-diabetes and DM denotes diabetes. Error bars represent ±SEM. *p<0.05, **p<0.01, ***p<0.001.
Based on our previous findings that Egr1 is a principal regulator of Ccl2 in hypoxia/ischemia,17, 23 we assessed Egr1 mRNA in the ischemic muscles vs. sham. Compared to WT non-diabetic mice, levels of Egr1 mRNA transcripts were significantly lower in diabetic WT mice muscle tissues on day 5 post-ligation (p<0.05, Figure 2B). Moreover, Egr1 mRNA transcripts on day 5 post-ligation were significantly higher in Ager−/− vs. respective WT mice in the absence and presence of diabetes (p<0.001, Figure 2B). However, by day 28 post-ligation, mRNA levels of Egr1 and Ccl2 were overall much lower in both WT and Ager−/− mice, in the non-diabetic and diabetic state and in sham vs. ischemia, thereby indicating that the expression of mediators linked to the inflammatory response was significantly attenuated in all groups by this time point (Figure I in the online-only Data Supplement). Collectively, these data suggest that RAGE attenuates hypoxia-ischemia-dependent inflammation and mechanisms to recruit immune cells in the response to hind limb ischemia.
CCL2 has been recognized as one of the key chemokines that regulates migration and infiltration of monocytes macrophages,24 and macrophages have been recognized as critical regulators of neoangiogenesis.25–27 We thus evaluated the numbers of infiltrating macrophages into the ischemic muscle on day 7 post-FAL by quantifying CD68+ macrophages in this tissue. In line with gene expression data in the muscle tissue, diabetic ischemic muscle tissue displayed lower numbers of CD68+ macrophages per unit area compared to non-diabetic tissue in WT mice (p<0.05, Figure 2C). However, deficiency of Ager in the non-diabetic and especially diabetic ischemic muscles resulted in significantly higher numbers of CD68+ cells/unit area compared to that observed in respective WT mice (p<0.05 and p<0.01, respectively, Figure 2C). These data suggest that RAGE reduces macrophage content in ischemic muscle, in parallel with impaired angiogenesis and blood flow recovery.
AGEs are increased in diabetic, ischemic tissue
We next sought to identify putative RAGE ligands accounting for these effects in the ischemic muscles of diabetic and non-diabetic WT mice by using an antibody against carboxymethyl lysine (CML), one of the major structures of AGEs highly prevalent in vivo. Two key patterns were evident from the results of this experiment; first, a significant increase in CML modified protein was noted in WT diabetic muscle sham tissues at baseline compared to non-diabetic WT mice (p<0.001; Figure 3A). Second, in WT non-diabetic or diabetic mice, hind limb ischemia itself resulted in a significant increase in CML modified protein in muscle tissue vs. sham treatment (p<0.01 and p<0.05, respectively; Figure 3A). Since glyoxalase-1 (GLO1) is a principal detoxifying enzyme for AGE precursor methylglyoxal (MG) controlling AGE levels, we tested levels of Glo1 gene expression in hind limb muscle tissues. Levels of Glo1 mRNA were significantly reduced by FAL in hind limb ischemia vs. sham treatments in both the non-diabetic and diabetic state (p<0.05 in both cases; Figure 3B), but were significantly higher in respective Ager deficient tissue at baseline and on day 3 post-ligation compared to WT tissue in both the non-diabetic and diabetic state (p<0.05 in both cases, Figure 3B). Western blotting revealed no statistically significant differences in levels of GLO1 protein in the muscle tissues among these groups of mice (Figure II-A in the online-only Data Supplement).
Figure 3. Expression levels of CML-AGEs and Glo1 in skeletal muscle after hind limb ischemia.
(A) Proteins from the indicated skeletal muscles on day 5 after FAL were subjected to Western blotting for detection of carboxymethyl lysine (CML)-AGEs and normalized to GAPDH (n=3 mice/group). (B) Glo1 mRNA transcripts were detected in the indicated skeletal muscles of mice on day 3 after FAL vs. sham by real-time PCR analysis and normalized to 18s rRNA (n=3 mice/group). R denotes right leg/sham control and L denotes left leg/femoral artery ligation (FAL). NDM denotes non-diabetes and DM denotes diabetes. Error bars represent ±SEM. *p<0.05, **p<0.01, ***p<0.001, NS not statistically significant.
As it is established that AGEs are generated in diabetes, hypoxia/reoxygenation and ischemia/reperfusion injury,17, 20 we measured levels of CML protein in the ischemic and non-ischemic muscle tissues of diabetic and non-diabetic transgenic (Tg) mice overexpressing Glo1. We found that levels of CML modified protein were significantly lower in Tg(Glo1) mice compared to respective WT mice on day 5 post-ligation in diabetes (p<0.05, Figure 3A) and at baseline in the diabetic state (p<0.05, Figure 3A), although the levels of CML modified protein were undetectable at baseline in both non-diabetic WT and Tg(Glo1) mice (Figure 3A). These data validated that transgenic mice expressing Glo1 reduced AGE content in the muscle tissue, especially in diabetes and post-FAL and provided a key strategy to test the premise that AGEs contributed mechanistically to impaired neoangiogenesis and blood flow recovery in FAL.
Finally, we examined levels of a distinct RAGE ligand, S100B, in the skeletal muscle of the mice under study. No significant differences were observed among any of the mouse groups when considering surgical condition, diabetes vs. non-diabetes or genotype (Figure II – B in the online-only Data Supplement). These data suggested that the AGE ligands, not S100B, were more likely to contribute to the RAGE-dependent effects in both non-diabetic and diabetic hind limb ischemia.
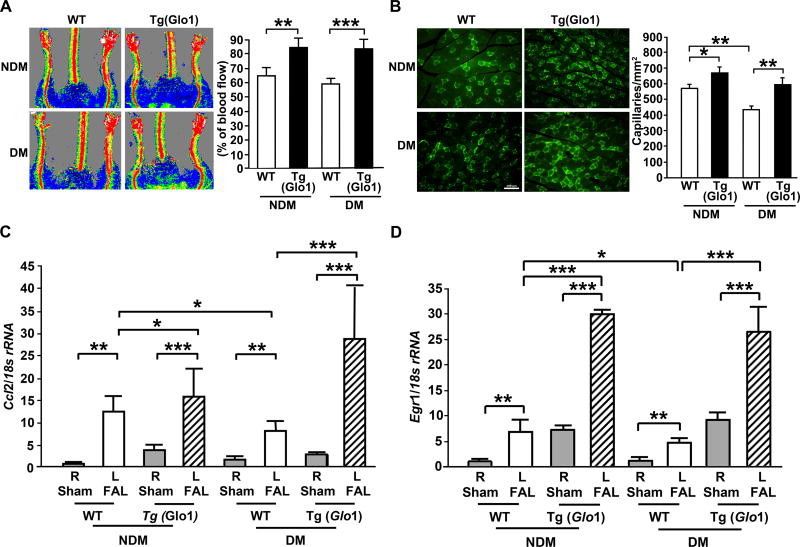
Overexpression of Glo1 restores diabetes- and ischemia-induced impairment of blood flow recovery, neoangiogenesis, macrophage infiltration and inflammatory mediator gene expression
As our data suggested that both diabetes and ischemia increased AGE levels, we tested the hypothesis that transgenic overexpression of Glo1 would rescue FAL-subjected mice from suppression of neoangiogenesis, blood flow recovery and inflammation in ischemic muscle tissue, especially in diabetes. Laser Doppler blood flow analyses showed significantly higher ischemic/sham limb blood flow ratio in both sets of Tg(Glo1) mice (non-diabetic and diabetic condition), compared to their respective WT controls on day 28 post-ligation (p<0.01 and p<0.001, respectively; Figure 4A). Consistent with this observation, Tg(Glo1) mice displayed significantly higher capillary density in both the non-diabetic and diabetic states compared to WT mice on day 28 post-ligation (p<0.05 and p<0.01, respectively, Figure 4B). We measured gene expression of inflammatory mediators in the muscle tissue using real-time PCR analysis. Our data reveal that significantly higher Ccl2 (p<0.05 and p<0.001, Figure 4C) and Egr1 (p<0.001 in both cases, Figure 4D) transcripts in the skeletal muscle tissue of Tg(Glo1) vs. WT mice on day 5 after FAL were noted in both the non-diabetic and diabetic state. These data indicate that reduction of RAGE ligands AGEs by overexpression of Glo1 restored adaptive inflammatory gene expression in the microenvironment of hind limb ischemia.
Figure 4. Impact of Glo1 overexpression on gene expression, inflammatory responses, angiogenesis and blood flow recovery in hind limb ischemia.
(A) Hind limb blood flow was monitored by Laser Doppler Perfusion Imaging on day 28 post-ligation (red indicates normal perfusion; blue, reduced blood flow). Quantitative evaluation of blood flow was expressed as a ratio of blood flow in ischemic to sham limb (n=10 mice/group). (B) Representative photomicrographs of ischemic muscles from the indicated mice stained with anti-CD31 IgG on day 28 post-ligation (scale bar=100 µm). Quantitative evaluation of capillary density is shown (n=10 mice/group). (C) Ccl2 and (D) Egr1 mRNA transcripts were detected in the indicated skeletal muscles of mice on day 5 after FAL vs. sham by real-time PCR analysis and normalized to 18s rRNA (n=5 mice/group). R denotes right leg/sham control and L denotes left leg/femoral artery ligation (FAL). NDM denotes non-diabetes and DM denotes diabetes. Error bars represent ±SEM. *p<0.05, **p<0.01, ***p<0.001.
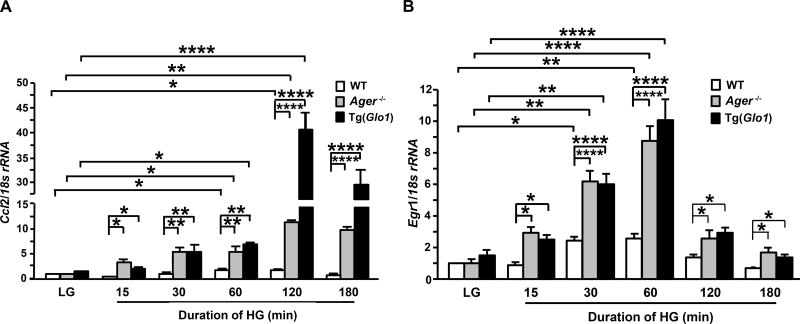
Effects of high glucose on gene expression of inflammatory mediators from Ager- or Glo1-modified macrophages
Based on the observation of increased macrophage content in the ischemic muscle tissues of Ager−/− mice compared to WT mice, especially in diabetes, the inflammatory gene expression of macrophages under high glucose conditions was studied using BMDMs from WT, Ager−/− and Tg(Glo1) mice as a model system. Following exposure to high glucose (HG, 25 mM D-glucose), BMDMs from all genotypes cultured in low glucose (LG, 5.5 mM D-glucose) displayed significant time-dependent induction of Ccl2 (Figure 5A) and Egr1 (Figure 5B) mRNA transcripts. Maximal induction of transcription of Ccl2 (Figure 5A) or Egr1 (Figure 5B) occurred within 2h or 1h of exposure to high glucose, respectively. The extent of high glucose-induced Ccl2 (p<0.0001, Figure 5A) and Egr1 (p<0.0001,, Figure 5B) transcription was significantly higher in Ager−/− or Glo1 overexpressing BMDMs compared to WT BMDMs.
Figure 5. High glucose induces gene expression in Ager- or Glo1-modified macrophages.
(A–B) Bone marrow derived macrophages (BMDMs) were isolated from mice of each genotype and cultured individually in 5.5 mM D-glucose (LG) for 7 days (n=6 WT/LG mice/group and n=4 TgGlo1 mice and n=4 Ager−/− mice/group) and exposed to 25 mM D-glucose (HG) (n=4 mice/group) for the indicated time periods. Total RNA was isolated from these cells and subjected to real-time PCR analysis. Ccl2 (A) and Egr1 (B) mRNA transcripts were detected and normalized to 18s rRNA. Error bars represent ±SEM. *p<0.05, **p<0.01, ****p<0.0001.
We extended the time course to 7 days to test if the effects of HG were sustained and if the impact of HG condition was RAGE-dependent. BMDMs were cultured in LG or HG and real-time quantitative PCR was performed to detect mRNA transcripts for pro- and anti-inflammatory genes. Levels of Egr1 mRNA were significantly higher in Ager−/− BMDMs grown in LG or HG vs. their respective WT controls (p<0.05 in both LG and HG; Figure III-A in the online-only Data Supplement). Levels of Ccl2 were significantly lower in WT BMDMs grown in HG vs. LG (p<0.01, Figure III-B in the online-only Data Supplement). However, in Ager−/− BMDMs, levels of Ccl2 were higher in the HG vs. LG state and were significantly higher when compared to their respective WT controls (p<0.05 and p<0.0001, respectively; Figure III-B in the online-only Data Supplement. We tested expression of pro-inflammatory mediators in BMDMs and found that levels of Il1b, Tnfa, Nos2 and Ccr7 were all higher in the WT HG vs. LG state and compared to WT HG, levels of these markers were all significantly lower in the Ager−/− HG state (Figure III-C-D-E-F the online-only Data Supplement). Finally, we tested two anti-inflammatory genes, Arg1 and Il10. Significantly lower levels of Arg1 and Il10 mRNA transcripts in HG vs. LG WT BMDMs were noted (p<0.05 and p<0.001, respectively, Figure III-G the online-only Data Supplement). However in Ager−/− BMDMs grown in HG, significantly higher levels of Arg1 and Il10 were noted compared to the WT HG state (p<0.0001, Figure III-G-H the online-only Data Supplement). Taken together, these data indicate that both HG and RAGE modulate macrophage inflammatory properties, with HG reducing expression of Egr1 and Ccl2 in WT but not Ager−/− BMDMs. Further, HG increases pro-inflammatory type markers and reduces anti-inflammatory markers, at least in part via RAGE.
Effect of RAGE on high glucose-induced macrophage/endothelial interaction and monocyte inflammation
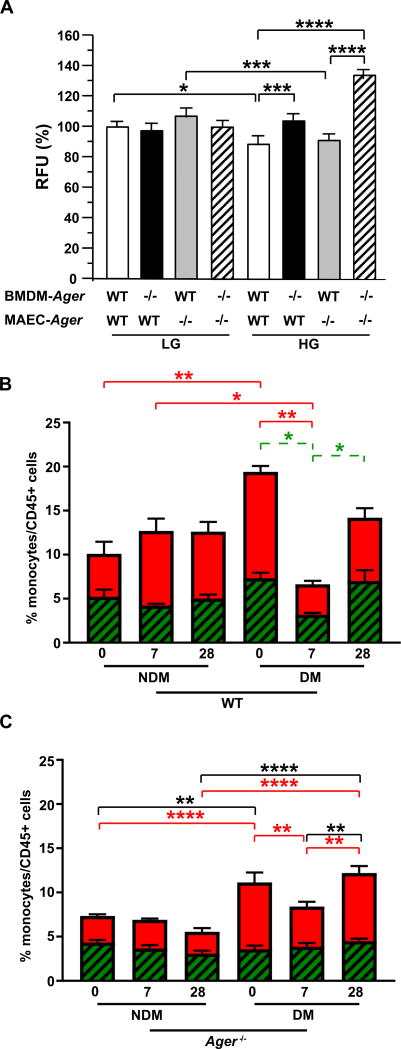
These data led us to probe the role of RAGE in macrophage-endothelial interactions and in monocyte inflammation, two key factors in the response to tissue injury. We began by investigating whether high glucose conditions modulated the interaction of macrophages with the endothelium and probed the potential role of RAGE by determining the adhesion of fluorescently-labeled WT or Ager−/− BMDMs to WT or Ager−/− MAEC monolayers, respectively, under LG or HG conditions. Under LG condition, no significant Ager genotype-dependent differences in the adhesion ability of BMDMs to MAEC monolayers were noted (Figure 6A). In contrast, when both cell types were cultured in HG, WT BMDM adhesion to WT MAECs was significantly lower compared to that observed in cells cultured in LG (p<0.05, Figure 6A). However, in HG, significantly higher adhesion of Ager−/− BMDMs to WT MAECs and to Ager−/− MAECs was observed compared with the adhesion of WT BMDMs to WT MAECs or Ager−/− MAECs (p<0.001 and p<0.0001, Figure 6A). Taken together, these data indicate that HG mediates reduced WT BMDM adhesion to MAECs and that deletion of Ager, particularly in BMDMs, or BMDMs and MAECS, but not MAECs alone, rescued the suppressive effects of HG on BMDM-MAEC adhesion. These data suggested that deletion of Ager, especially in the HG state, increased BMDM interaction with ECs.
Figure 6. Effect of RAGE on high glucose-induced macrophage/endothelial interaction and circulating monocyte populations.
(A) Mouse macrophage-endothelial cell adhesion assay, using BMDMs isolated from mice of n=4 mice per genotype was performed. Leuko Tracker™ labeled WT or Ager −/− BMDMs were allowed to attach to WT or Ager −/− murine aortic endothelial cell (MAEC) monolayer for 1 h. Adherent cells were lysed and quantified by reading fluorescence with a fluorescence plate reader at 480 nm/520 nm. (B–C) Ly6Chi and Ly6Clo monocyte populations were analyzed by flow cytometry in WT (B) and Ager −/− (C) mice at baseline (day 0), 7 and 28 days after FAL. NDM denotes non-diabetes and DM denotes diabetes. Red bars: Ly6Chi monocytes subpopulation; Green bars: Ly6Clo monocyte subpopulation. N=5–14 per group as follows: WT/NDM, 8; WT/DM, 5; Ager −/−/NDM, 14; and Ager −/−/DM, 6. Statistical analysis was performed from the total population (Ly6Chi + Ly6Clo/CD45+ cells) (black); Ly6Chi population (red) and Ly6Clo population (dashed green). Error bars represent ±SEM. *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001.
To address the underlying mechanisms, we first tested circulating levels of MCP1; MCP1 is the protein product of the Ccl2 gene. Although in diabetic WT mice, significantly higher baseline levels were observed compared to non-diabetic mice (p<0.01, Figure IV in the online-only Data Supplement), no increases were noted at day 7 or 28 days after FAL in the mice devoid of Ager (Figure IV in the online-only Data Supplement). In fact, no significant differences were noted at any time point or condition in the mice devoid of Ager. These findings suggested that cell-specific factors, not circulating mediators, might account for the significant functional differences observed in WT or mice devoid of Ager after hind limb ischemia. Hence, we examined the Ly6Chi and Ly6Clo monocyte and neutrophil populations.
Prompted by our finding that higher levels of CD68+ macrophages were noted in diabetic or non-diabetic ischemic tissues in mice devoid of Ager vs. the WT controls (Figure 2C), we obtained peripheral blood from the mice under study at baseline (day 0), 7 or 28 days after FAL and examined monocyte populations, including Ly6Chi and Ly6Clo subgroups normalized to the overall CD45+ cells (Figure V-A in the online-only Data Supplement). We first analyzed the baseline state and compared these cellular populations across WT vs. Ager−/− mice. In non-diabetes at baseline, there were no significant differences in total monocytes or monocyte subsets comparing WT vs. Ager−/− mice (Figure V-B in the online-only Data Supplement). In diabetes at baseline, significantly higher total monocytes, %Ly6Chi and %Ly6Clo monocyte subsets were observed in WT vs. Ager−/− mice (p<0.01, p<0.05 and p<0.05, respectively; Figure IV-B in the online-only Data Supplement).
We examined the total monocytes across the entire time course and within each genotype as well. In the WT mice over the time course and non-diabetic and diabetic conditions, there were no significant differences in total monocytes in any of the groups (Figure 6B). In contrast, in the Ager−/− mice, total monocytes were significantly higher in diabetic vs. non-diabetic mice at baseline (p<0.01) and total monocytes were significantly higher on day 28 in diabetic vs. non-diabetic mice devoid of Ager (p<0.0001). Finally, total monocytes were significantly higher in diabetic Ager−/− mice on day 28 vs. day 7 (p<0.01) (Figure 6C).
Next, we examined the monocyte subsets. First, in the WT mice at baseline, the %Ly6Chi cells was significantly higher in the diabetic vs. non-diabetic mice (p<0.01, Figure 6B). In non-diabetic WT mice, there were no significant differences in %Ly6Chi or Ly6Clo monocytes comparing baseline with day 7 or day 28 after FAL (Figure 6B). In the diabetic state, significantly lower %Ly6Chi and %Ly6Clo monocytes were observed at day 7 vs. baseline (p<0.01 and p<0.05, respectively, Figure 6B). By day 28 in the diabetic WT mice, only the %Ly6Clo not the %Ly6Chi population was significantly higher compared to the levels observed at day 7 (p<0.05, Figure 6B).
In Ager−/− mice, surprisingly, we found that significantly higher %Ly6Chi monocytes was observed at baseline in the diabetic vs. the non-diabetic mice devoid of Ager (p<0.0001, Figure 6C). The %Ly6Chi population was significantly lower in the diabetic Ager−/− mice on day 7 vs. diabetic baseline (p<0.01, Figure 6C) and unlike in the diabetic WT mice, this population had significantly recovered by day 28 (compared to day 7) in the diabetic Ager−/− mice (p<0.01, Figure 6C). In the Ager−/− mice, there were no statistically significant differences in the proportion of Ly6Clo cells at any time point or condition (Figure 6C).
Finally, we examined blood neutrophil content at baseline and found that the only significant difference was in the non-diabetic state, in which non-diabetic Ager−/− mice displayed significantly lower neutrophil content vs. the WT mice (p<0.01, Figure V-A, C in the online-only Data Supplement). In contrast, no genotype differences were observed in the diabetic state, thereby strongly suggesting that differences in neutrophils likely did not contribute to Ager-dependent effects in the response to hind limb ischemia.
Taken together, these data revealed that significant and surprising patterns emerged in the total monocytes and Ly6C monocyte subsets with respect to RAGE expression as follows: (1) total monocytes and Ly6Chi monocytes were significantly higher at baseline in diabetic vs. non-diabetic Ager−/− mice, indicating that deletion of Ager not only did not attenuate this inflammatory response, but enhanced it in diabetes; and (2) the %Ly6Chi cells significantly recovered by day 28 vs. day 7 post-FAL only in diabetic mice devoid of Ager but not in the WT mice, in parallel with improved blood flow recovery and neoangiogenesis on day 28.
Discussion
Enhancement of neoangiogenesis and the resulting improvement of limb blood flow are key restorative mechanisms in response to ischemia.28–30 However, the impairment of physiological neoangiogenesis has been implicated in exacerbation of peripheral limb ischemia, especially in diabetes, but the underlying mediating mechanisms have yet to be fully dissected, thereby mitigating the development of effective therapies.2, 3, 31 We demonstrate here that ischemia-induced neoangiogenesis was significantly impaired by the actions of RAGE ligand AGEs and RAGE, especially in diabetes, and the mechanism in WT mice likely involves multiple factors, including reduced expression of inflammatory mediators, such as Egr1 and Ccl2, that recruit myeloid cells into perturbed tissues; decreased inflammatory macrophage content in ischemic muscles; and downregulation of ischemic muscle matrix metalloproteinases and growth and repair factors. Compared to WT mice undergoing FAL, mice devoid of Ager or mice overexpressing Glo1 displayed restoration of adaptive inflammatory and angiogenic responses to hind limb ischemia.
Previous studies linked AGEs and RAGE to impairment of neoangiogenesis in diabetes but did not elucidate the underlying mechanisms.32–34 Inhibition of AGE formation by aminoguanidine, an inhibitor of AGE, improved angiographic score, capillary density, and laser Doppler skin-perfusion ratios and restored matrix degradation processes in diabetic mice subjected to hind limb ischemia.32 Blockade of AGE-RAGE by adenovirus-induced overexpression of soluble RAGE (sRAGE), a decoy receptor for AGE, as well as deficiency of Ager restored diabetes-induced impairment of angiogenesis in a matrigel patch model.33 In addition, intramuscular administration of sRAGE improved angiogenic responses to hind limb ischemia in diabetic mice.34 It is important to note that testing the effects of sRAGE does not directly point to RAGE-dependent mechanisms, as roles for non-RAGE receptors for the families of RAGE ligands are not ruled out solely by ligand sequestration. Therefore, studies using mice genetically deficient in Ager are essential to definitively assign a critical role for RAGE activation in diabetes- and ischemia-induced impaired neoangiogenesis and to pinpoint underlying mechanisms.
In the present study, we demonstrated the increased expression of CML-AGE and its receptor (RAGE) in the hind limb ischemic vs. sham muscles, in both the non-diabetic and diabetic state, five days post-FAL (Figure 3). This work adds to the body of evidence that even in the absence of diabetes, ischemia increases AGE formation, shown previously in the heart.35 Recently, it was reported that mice devoid of Ager displayed improved angiogenesis after hind limb ischemia and that 21 days post-FAL, levels of CML-AGE in the muscles were higher in diabetes but not lower in the Ager−/− mice (non-ischemic tissues were not tested), whereas levels of RAGE ligand HMGB1 were higher in ischemic vs. non-ischemic muscle, but not affected by deletion of Ager.36 In that work, however, genetically-modified mice or distinct ligand-directed strategies were not employed to probe the potential mechanistic roles of CML-AGE or HMGB1 in hind limb ischemia.
The present experiments, in contrast, addressed mechanistic roles for AGEs in this setting. Given their roles in AGE formation and accumulation, GLO1 and MG have been implicated in the pathogenesis of diabetic complications.8, 37–39 Several lines of experimental evidence indicate beneficial impact of overexpression of Glo1 in animal models in diabetes.40–43 Overexpression of Glo1 in Caenorhabditis elegans decreased hyperglycemia-induced accumulation of AGEs and oxidative stress and enhanced lifespan.40 Transgenic rats overexpressing human Glo1 decreased MG-derived AGE formation and reduced retinal, neuroglial, and vascular pathology in diabetes and were resistant to renal ischemia-reperfusion injury.41, 42 Overexpression of Glo1 in bone marrow cells in mice restored neovascularization to ischemic hind limbs in diabetes.43 In the present study, we identified the mechanism by which Glo1 exerted its beneficial effects. First, however, we confirmed that in our model, overexpression of Glo1 in mice prevented diabetes-induced increased levels of CML-AGE modified proteins (Figure 3). Further, we demonstrated that Glo1 prevented increases in CML-AGE modified proteins in ischemia, confirming that Glo1 plays an important role in the suppression of AGE formation in the vessel wall not only in diabetes, but also in ischemic injury. Second, consistent with our previous observation linking RAGE to down-regulation of Glo1 in the diabetic kidney,38 we discovered that Ager deficiency leads to higher levels of Glo1 mRNA in the skeletal muscle tissue after hind limb ischemia in both non-diabetic and diabetic mice, while levels of Glo1 mRNA were suppressed by RAGE in WT mice after hind limb ischemia. Although we did not find differences in GLO1 protein levels among WT and Ager modified mice, we did note overall trends to lower levels of GLO1 in the respective sham vs. FAL mice. Recent evidence suggests regulation of Glo1 by oxidative stress-related pathways such as NRF44; if and to what extent levels of GLO1 protein may be modulated in a high oxidative/inflammatory environment require further study. Third, we provided evidence that Tg(Glo1) mice reversed diabetes- and ischemia-induced impaired regulation of inflammatory mediators, Egr1 and Ccl2 and angiogenic responses to hind limb ischemia in both non-diabetic and diabetic states. Taken together, these data link RAGE ligand AGEs to impaired inflammatory and angiogenic responses in hind limb ischemia and suggest that upregulation of the Glo1 pathway may provide a complementary therapeutic target in the prevention of diabetic vascular damage.
Inflammatory cell infiltration early after ischemia is an important trigger for the angiogenic response to tissue ischemia. Impaired muscle regeneration in Ccl2−/− mice suggests an important role for macrophages and MCP1 in tissue reparative processes.45 Consistent with the implications of the current findings, Ccl2−/− mice displayed decreased angiogenesis and reduced blood flow recovery after hind limb ischemia vs. WT mice.45 Furthermore, it was earlier reported that mice devoid of Egr1 (non-diabetic state) displayed reduced numbers of regenerating arterioles after femoral artery ligation.46 In both studies, however, the effect of deletion of Egr1 or Ccl2 on inflammatory mechanisms in the ischemic tissue was not explored. Here, we demonstrate that RAGE-dependent mechanisms mediate regulation of these two key factors in the ischemic hind limb. In contrast to these findings in FAL and hind limb ischemia, in hyperlipidemic mice, deletion of either Egr1 or Ccl2 is atheroprotective.47, 48 What might begin to explain these apparent conflicting findings?
Our data unveil the intriguing finding that RAGE plays opposing roles in regulating Egr1 and Ccl2 expression in acute vs. chronic hypoxia/ischemia, as when Ager is deleted in hind limb ischemia, higher macrophage content in the ischemic muscle ensues – and is linked to repair – quite distinct from that observed in chronic atherosclerosis and acute hypoxia in mouse hearts in which Ager deletion significantly reduced Egr1 and/or Ccl2 transcripts and lesional macrophage content in vascular tissues.17, 49, 50 As differences in circulating levels of MCP1 did not explain the benefits of Ager deletion in hind limb ischemia, we turned to examination of peripheral monocytes and neutrophils in these mice, as our data revealed that muscle tissue levels of CD68+ macrophages were significantly higher in non-diabetic or diabetic Ager−/− mice vs. respective WT mice after FAL. These endeavors uncovered the surprising finding that higher Ly6Chi monocytes were found in diabetic vs. non-diabetic mice of both the WT and Ager−/− genotypes (Figure 6). In atherosclerosis, this subset of Ly6Chi monocytes facilitates atherosclerosis; in contrast, the present work implicates these cells in tissue repair.51, 52 Here, in hind limb ischemia, we surmise that the local environment, at least in part driven by RAGE, recruits tissue-reparative cells driven by early but transient upregulation of Egr1 and Ccl2. Critically, in all mouse groups, by day 28 post-FAL, muscle tissue levels of these factors were dramatically lower and did not greatly differ by genotype post-ischemia (Figure I in the online-only Data Supplement).
Examination of the PCR array data (Tables II, III and IV in the online-only Data Supplement) supports the paradigm that in hind limb ischemia, particularly in diabetes, RAGE blunts a pro-inflammatory microenvironment needed for tissue repair. In contrast, both non-diabetic or diabetic mice devoid of Ager display higher levels of a number of chemokines and their ligands, particularly Ccl2 in the ischemic muscle tissue, compared to their WT counterparts. CCL2/MCP1 are ligands for Ly6Chi cells53–55, thereby contributing to the higher number of macrophages infiltrating the ischemic muscle tissue on day 7 in the Ager−/− vs. WT mice (Figure 2C and Tables II, III, IV in the online-only Data Supplement). Interestingly, although levels of Il1b were significantly higher in non-diabetic or diabetic Ager−/− ischemic muscle vs. respective WT counterparts on day 5 post-FAL, these levels were much lower in the mice devoid of Ager by day 7, relative to the WT. In parallel, levels of Thbs1 (latent activator of Tgfb1)56 were significantly higher in Ager−/− ischemic tissue by day 7 post-FAL and by 7, significantly higher levels of tissue-reparative Tgfb1, as well as Angpt2, Hgf, Pgf, were evident in ischemic tissue devoid of Ager vs. the respective WT mice in the non-diabetic and diabetic states. These data mirror the improvement in neoangiogenesis and blood flow recovery observed in the Ager−/− vs. WT mice in both the diabetic and non-diabetic states and reflect the overall patterns that were observed in isolated BMDMs. In BMDMs devoid of Ager, lower levels of “M1” like pro-inflammatory cytokines and higher levels of “M2” like molecules linked to tissue repair (Arg1 and Il10) were observed in high glucose conditions (Figure III in the online-only Data Supplement). Interestingly, levels of Vegfa, b and c were not significantly modulated in FAL in the non-diabetic or diabetic ischemic muscles of WT or Ager−/− mice, but levels of other factors such as Thbs1 and Tgfb1 were significantly affected by diabetes and deletion of Ager. Our findings regarding lack of RAGE-dependent effects on Vegf are intriguing and warrant further analyses; it is notable, however, that studies of others in cultured bone marrow cells from 12 months diabetic or non-diabetic mice failed to demonstrate differences in the production of VEGF protein.57
In vitro studies also supported inflammatory roles for RAGE in macrophages, as we demonstrated that macrophages grown in high glucose displayed decreased functional potential, that is, these macrophages demonstrated reduced adhesion to WT murine endothelial cells compared to WT macrophages grown in physiological levels of glucose in a RAGE-dependent manner (Figure 6A). Critically, our study also uncovered that simply deleting Ager in murine endothelial cells did not rescue the suppressive effects of high glucose on impaired WT macrophage adherence. Rather, only when Ager was deleted either in macrophages alone, or in both macrophages and endothelial cells, was the reduced adhesion of macrophages to endothelial cells in high glucose rescued. In future studies, it would thus be useful to determine how conditions mimicking the hyperlipidemic environment might affect macrophage-endothelial adhesion vis-à-vis the RAGE axis. It is notable that Babu and colleagues tested the impact of hyperlipidemia vs. hyperglycemia (type 2 diabetes) stresses on ischemic muscle macrophages isolated 7 days post-FAL.58 Differential promoter methylation studies revealed that in both settings, significant promoter hypomethylation (increased transcription) of prototypic “M1” macrophages) and hypermethylation (reduced transcription) of anti-inflammatory and pro-angiogenic “M2” macrophages was evident when compared to the WT control mice. Of note, however, the genes altered by hyperlipidemia vs. hyperglycemia were not fully overlapping, suggesting that further in-depth probing of these datasets might uncover factors exhibiting differential responses to these metabolic perturbations.
It is not surprising that the immune/inflammatory system was designed to establish multiple checkpoints to protect against imbalances in pro- vs. anti-inflammatory forces. In the case of the toll receptor family, for example, it was shown that extensive cross-talk between Tlr4 and Tlr2 is required in mediating adaptive responses to hind limb ischemia, when tested in non-diabetic mice.59, 60 The present work adds RAGE to the cadre of genes that exhibit tissue microenvironment-dependent roles in inflammation and tissue regeneration. In this context, a burgeoning body of evidence links RAGE to opposing outcomes in murine models of infection challenge. For example, whereas deletion of Ager in Streptococcus pneumoniae pneumonia or Acinetobacter baumanii sepsis resulted in improved survival and diminished tissue damage,61, 62 Ager deletion in a distinct setting of Klebsiella pneumoniae was detrimental to survival and recovery63. These considerations underscore the complexities of RAGE in the immune and vascular response to imposed stresses.
Taken together, the present work suggests unique hypoxia/ischemia-dependent mechanisms in hind limb ischemia that may be rescued by deletion of Ager and by specific means to reduce AGE burden. This work underscores the premise that RAGE may dampen tissue-reparative inflammation in hind limb ischemia, thereby blocking adaptive neoangiogenesis and restoration of blood flow. We conclude that deletion of Ager re-sets adaptive inflammatory cues in a tissue microenvironment-sensitive manner and surmise that antagonism of RAGE might fill a key therapeutic gap in peripheral arterial disease, particularly in diabetes.
Supplementary Material
Highlights.
The present study demonstrates that ischemia-induced neoangiogenesis was significantly impaired by AGE-RAGE action in WT mice, especially in diabetes, and the mechanisms were traced to blockade of tissue reparative inflammatory responses, including reduction of gene expression of inflammatory/angiogenic mediators and decrease of pro-angiogenic inflammatory macrophage infiltration into ischemic muscles. In contrast, Ager deficiency or overexpression of Glo1 restored physiological inflammatory and angiogenic responses to hind limb ischemia.
These data challenge the paradigm that RAGE solely triggers tissue-damaging inflammation in stressed tissues and suggest that in hypoxia/ischemia in the hind limb, RAGE attenuates pro-repair inflammatory gene expression and macrophage content in ischemic muscle.
This work underscores the emerging concept that discrete microenvironment cues may stimulate unique RAGE-dependent responses and suggest that RAGE antagonism may fill a critical gap in the therapeutic arsenal targeting diabetic peripheral vascular disease.
Acknowledgments
The authors gratefully thank Dr. Abraham A. Palmer, University of California at San Diego School of Medicine, U.S, for providing transgenic mice overexpressing glyoxylase-1 (Glo1) for this study. The authors thank Latoya Woods, Division of Endocrinology, New York University School of Medicine, for assistance with manuscript preparation.
Sources of Funding
This study was supported by a grant from United States Public Health Service (PO1 HL60901).
Abbreviations
- AGEs
Advanced glycation end products
- RAGE
the receptor for AGE
- sRAGE
soluble RAGE
- Ager−/−
gene encoding RAGE
- MG
methylglyoxal
- GLO1
glyoxalase 1
- Tg
transgenic
- MG
methylglyoxal
- WT
wild type
- Egr1
early growth response gene-1
- Ccl2
chemokine (C-C motif) ligand 2
- MCP-1
formerly referred to as monocyte chemoattractant protein 1
- Vegf
vascular endothelial growth factor
- Angpt1
angiopoietin-1
- Ena78
chemokine (C-X-C motif) ligand (Cxcl)2 (Gro-β), and Cxcl5
- Hgf
hepatocyte growth factor
- Mdk
midkine
- Pgf
placental growth factor
- Sphk1
sphingosine kinase 1
- Tgfb
transforming growth factor beta
- Thbs1
thrombospondin-1
- Hif1
hypoxia-inducible factor-1
- Itgβ3
integrin β chain, β3 precursor
- Pecam1/Cd31
platelet/endothelial cell adhesion molecule 1
- FAL
femoral artery ligation
- CML
carboxymethyl lysine
- BMDMs
bone marrow derived macrophages
- MAEC
mouse aortic endothelial cell
- LG
low glucose
- HG
high glucose
- NDM
non-diabetes
- DM
diabetes
- PAD
peripheral artery disease
- PVD
peripheral vascular disease
Footnotes
Disclosures
None.
References
- 1.Abaci A, Oguzhan A, Kahraman S, Eryol NK, Unal S, Arinc H, Ergin A. Effect of diabetes mellitus on formation of coronary collateral vessels. Circulation. 1999;99:2239–42. doi: 10.1161/01.cir.99.17.2239. [DOI] [PubMed] [Google Scholar]
- 2.Rivard A, Silver M, Chen D, Kearney M, Magner M, Annex B, Peters K, Isner JM. Rescue of diabetes-related impairment of angiogenesis by intramuscular gene therapy with adeno-VEGF. Am J Pathol. 1999;154:355–63. doi: 10.1016/S0002-9440(10)65282-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin A, Komada MR, Sane DC. Abnormal angiogenesis in diabetes mellitus. Med Res Rev. 2003;23:117–45. doi: 10.1002/med.10024. [DOI] [PubMed] [Google Scholar]
- 4.Ouriel K. Peripheral arterial disease. Lancet. 2001;358:1257–64. doi: 10.1016/S0140-6736(01)06351-6. [DOI] [PubMed] [Google Scholar]
- 5.Waltenberger J. Impaired collateral vessel development in diabetes: potential cellular mechanisms and therapeutic implications. Cardiovasc Res. 2001;49:554–60. doi: 10.1016/s0008-6363(00)00228-5. [DOI] [PubMed] [Google Scholar]
- 6.Currie CJ, Morgan CL, Peters JR. The epidemiology and cost of inpatient care for peripheral vascular disease, infection, neuropathy, and ulceration in diabetes. Diabetes Care. 1998;21:42–8. doi: 10.2337/diacare.21.1.42. [DOI] [PubMed] [Google Scholar]
- 7.Olin JW, Sealove BA. Peripheral artery disease: current insight into the disease and its diagnosis and management. Mayo Clin Proc. 2010;85:678–92. doi: 10.4065/mcp.2010.0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 9.Thornalley PJ. Glyoxalase I--structure, function and a critical role in the enzymatic defence against glycation. Biochem Soc Trans. 2003;31:1343–8. doi: 10.1042/bst0311343. [DOI] [PubMed] [Google Scholar]
- 10.Schmidt AM, Yan SD, Stern DM. The dark side of glucose. Nat Med. 1995;1:1002–4. doi: 10.1038/nm1095-1002. [DOI] [PubMed] [Google Scholar]
- 11.Stirban A, Gawlowski T, Roden M. Vascular effects of advanced glycation endproducts: Clinical effects and molecular mechanisms. Mol Metab. 2014;3:94–108. doi: 10.1016/j.molmet.2013.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- 13.Yan SF, Ramasamy R, Schmidt AM. The RAGE axis: a fundamental mechanism signaling danger to the vulnerable vasculature. Circ Res. 2010;106:842–53. doi: 10.1161/CIRCRESAHA.109.212217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hammes HP, Weiss A, Hess S, Araki N, Horiuchi S, Brownlee M, Preissner KT. Modification of vitronectin by advanced glycation alters functional properties in vitro and in the diabetic retina. Lab Invest. 1996;75:325–38. [PubMed] [Google Scholar]
- 15.Ramasamy R, Yan SF, Schmidt AM. Receptor for AGE (RAGE): signaling mechanisms in the pathogenesis of diabetes and its complications. Ann N Y Acad Sci. 2011;1243:88–102. doi: 10.1111/j.1749-6632.2011.06320.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ritthaler U, Deng Y, Zhang Y, et al. Expression of receptors for advanced glycation end products in peripheral occlusive vascular disease. Am J Pathol. 1995;146:688–94. [PMC free article] [PubMed] [Google Scholar]
- 17.Chang JS, Wendt T, Qu W, Kong L, Zou YS, Schmidt AM, Yan SF. Oxygen deprivation triggers upregulation of early growth response-1 by the receptor for advanced glycation end products. Circ Res. 2008;102:905–13. doi: 10.1161/CIRCRESAHA.107.165308. [DOI] [PubMed] [Google Scholar]
- 18.Zeng S, Dun H, Ippagunta N, Rosario R, Zhang QY, Lefkowitch J, Yan SF, Schmidt AM, Emond JC. Receptor for advanced glycation end product (RAGE)-dependent modulation of early growth response-1 in hepatic ischemia/reperfusion injury. J Hepatol. 2009;50:929–36. doi: 10.1016/j.jhep.2008.11.022. [DOI] [PubMed] [Google Scholar]
- 19.Aleshin A, Ananthakrishnan R, Li Q, et al. RAGE modulates myocardial injury consequent to LAD infarction via impact on JNK and STAT signaling in a murine model. Am J Physiol Heart Circ Physiol. 2008;294:H1823–32. doi: 10.1152/ajpheart.01210.2007. [DOI] [PubMed] [Google Scholar]
- 20.Bucciarelli LG, Ananthakrishnan R, Hwang YC, Kaneko M, Song F, Sell DR, Strauch C, Monnier VM, Yan SF, Schmidt AM, Ramasamy R. RAGE and modulation of ischemic injury in the diabetic myocardium. Diabetes. 2008;57:1941–51. doi: 10.2337/db07-0326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Murohara T, Asahara T, Silver M, Bauters C, Masuda H, Kalka C, Kearney M, Chen D, Symes JF, Fishman MC, Huang PL, Isner JM. Nitric oxide synthase modulates angiogenesis in response to tissue ischemia. J Clin Invest. 1998;101:2567–78. doi: 10.1172/JCI1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Distler MG, Gorfinkle N, Papale LA, Wuenschell GE, Termini J, Escayg A, Winawer MR, Palmer AA. Glyoxalase 1 and its substrate methylglyoxal are novel regulators of seizure susceptibility. Epilepsia. 2013;54:649–57. doi: 10.1111/epi.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yan SF, Fujita T, Lu J, Okada K, Shan Zou Y, Mackman N, Pinsky DJ, Stern DM. Egr-1, a master switch coordinating upregulation of divergent gene families underlying ischemic stress. Nat Med. 2000;6:1355–61. doi: 10.1038/82168. [DOI] [PubMed] [Google Scholar]
- 24.Niu J, Azfer A, Zhelyabovska O, Fatma S, Kolattukudy PE. Monocyte chemotactic protein (MCP)-1 promotes angiogenesis via a novel transcription factor, MCP-1-induced protein (MCPIP) J Biol Chem. 2008;283:14542–51. doi: 10.1074/jbc.M802139200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–31. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 26.Okuno Y, Nakamura-Ishizu A, Kishi K, Suda T, Kubota Y. Bone marrow-derived cells serve as proangiogenic macrophages but not endothelial cells in wound healing. Blood. 2011;117:5264–72. doi: 10.1182/blood-2011-01-330720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pollard JW. Trophic macrophages in development and disease. Nat Rev Immunol. 2009;9:259–70. doi: 10.1038/nri2528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Isner JM. Tissue responses to ischemia: local and remote responses for preserving perfusion of ischemic muscle. J Clin Invest. 2000;106:615–9. doi: 10.1172/JCI10961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Banai S, Jaklitsch MT, Shou M, Lazarous DF, Scheinowitz M, Biro S, Epstein SE, Unger EF. Angiogenic-induced enhancement of collateral blood flow to ischemic myocardium by vascular endothelial growth factor in dogs. Circulation. 1994;89:2183–9. doi: 10.1161/01.cir.89.5.2183. [DOI] [PubMed] [Google Scholar]
- 30.Takeshita S, Zheng LP, Brogi E, Kearney M, Pu LQ, Bunting S, Ferrara N, Symes JF, Isner JM. Therapeutic angiogenesis. A single intraarterial bolus of vascular endothelial growth factor augments revascularization in a rabbit ischemic hind limb model. J Clin Invest. 1994;93:662–70. doi: 10.1172/JCI117018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Silvestre JS, Levy BI. Molecular basis of angiopathy in diabetes mellitus. Circ Res. 2006;98:4–6. doi: 10.1161/01.RES.0000200396.90220.41. [DOI] [PubMed] [Google Scholar]
- 32.Tamarat R, Silvestre JS, Huijberts M, Benessiano J, Ebrahimian TG, Duriez M, Wautier MP, Wautier JL, Levy BI. Blockade of advanced glycation end-product formation restores ischemia-induced angiogenesis in diabetic mice. Proc Natl Acad Sci U S A. 2003;100:8555–60. doi: 10.1073/pnas.1236929100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shoji T, Koyama H, Morioka T, et al. Receptor for advanced glycation end products is involved in impaired angiogenic response in diabetes. Diabetes. 2006;55:2245–55. doi: 10.2337/db05-1375. [DOI] [PubMed] [Google Scholar]
- 34.Kim BH, Ko Y-G, Kim SH, Chung JH, Hwang K-C, Choi D, Jang Y. Suppression of Receptor for Advanced Glycation End Products Improves Angiogenic Responses to Ischemia in Diabetic Mouse Hindlimb Ischemia Model. ISRN Vascular Medicine. 2013;2013:7. [Google Scholar]
- 35.Bucciarelli LG, Kaneko M, Ananthakrishnan R, et al. Receptor for advanced-glycation end products: key modulator of myocardial ischemic injury. Circulation. 2006;113:1226–34. doi: 10.1161/CIRCULATIONAHA.105.575993. [DOI] [PubMed] [Google Scholar]
- 36.Hansen LM, Gupta D, Joseph G, Weiss D, Taylor WR. The receptor for advanced glycation end products impairs collateral formation in both diabetic and non-diabetic mice. Lab Invest. 2017;97:34–42. doi: 10.1038/labinvest.2016.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ahmed N, Thornalley PJ. Advanced glycation endproducts: what is their relevance to diabetic complications? Diabetes Obes Metab. 2007;9:233–45. doi: 10.1111/j.1463-1326.2006.00595.x. [DOI] [PubMed] [Google Scholar]
- 38.Reiniger N, Lau K, McCalla D, et al. Deletion of the receptor for advanced glycation end products reduces glomerulosclerosis and preserves renal function in the diabetic OVE26 mouse. Diabetes. 2010;59:2043–54. doi: 10.2337/db09-1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rabbani N, Thornalley PJ. The critical role of methylglyoxal and glyoxalase 1 in diabetic nephropathy. Diabetes. 2014;63:50–2. doi: 10.2337/db13-1606. [DOI] [PubMed] [Google Scholar]
- 40.Schlotterer A, Kukudov G, Bozorgmehr F, et al. C. elegans as model for the study of high glucose- mediated life span reduction. Diabetes. 2009;58:2450–6. doi: 10.2337/db09-0567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Berner AK, Brouwers O, Pringle R, Klaassen I, Colhoun L, McVicar C, Brockbank S, Curry JW, Miyata T, Brownlee M, Schlingemann RO, Schalkwijk C, Stitt AW. Protection against methylglyoxal-derived AGEs by regulation of glyoxalase 1 prevents retinal neuroglial and vasodegenerative pathology. Diabetologia. 2012;55:845–54. doi: 10.1007/s00125-011-2393-0. [DOI] [PubMed] [Google Scholar]
- 42.Kumagai T, Nangaku M, Kojima I, Nagai R, Ingelfinger JR, Miyata T, Fujita T, Inagi R. Glyoxalase I overexpression ameliorates renal ischemia-reperfusion injury in rats. Am J Physiol Renal Physiol. 2009;296:F912–21. doi: 10.1152/ajprenal.90575.2008. [DOI] [PubMed] [Google Scholar]
- 43.Vulesevic B, McNeill B, Geoffrion M, Kuraitis D, McBane JE, Lochhead M, Vanderhyden BC, Korbutt GS, Milne RW, Suuronen EJ. Glyoxalase-1 overexpression in bone marrow cells reverses defective neovascularization in STZ-induced diabetic mice. Cardiovasc Res. 2014;101:306–16. doi: 10.1093/cvr/cvt259. [DOI] [PubMed] [Google Scholar]
- 44.Chaudhuri J, Bose N, Gong J, Hall D, Rifkind A, Bhaumik D, Peiris TH, Chamoli M, Le CH, Liu J, Lithgow GJ, Ramanathan A, Xu XZ, Kapahi P. A Caenorhabditis elegans Model Elucidates a Conserved Role for TRPA1-Nrf Signaling in Reactive alpha-Dicarbonyl Detoxification. Curr Biol. 2016;26:3014–25. doi: 10.1016/j.cub.2016.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Voskuil M, Hoefer IE, van Royen N, Hua J, de Graaf S, Bode C, Buschmann IR, Piek JJ. Abnormal monocyte recruitment and collateral artery formation in monocyte chemoattractant protein-1 deficient mice. Vasc Med. 2004;9:287–92. doi: 10.1191/1358863x04vm571oa. [DOI] [PubMed] [Google Scholar]
- 46.Sarateanu CS, Retuerto MA, Beckmann JT, McGregor L, Carbray J, Patejunas G, Nayak L, Milbrandt J, Rosengart TK. An Egr-1 master switch for arteriogenesis: studies in Egr-1 homozygous negative and wild-type animals. J Thorac Cardiovasc Surg. 2006;131:138–45. doi: 10.1016/j.jtcvs.2005.08.052. [DOI] [PubMed] [Google Scholar]
- 47.Harja E, Bucciarelli LG, Lu Y, Stern DM, Zou YS, Schmidt AM, Yan SF. Early growth response-1 promotes atherogenesis: mice deficient in early growth response-1 and apolipoprotein E display decreased atherosclerosis and vascular inflammation. Circ Res. 2004;94:333–9. doi: 10.1161/01.RES.0000112405.61577.95. [DOI] [PubMed] [Google Scholar]
- 48.Boring L, Gosling J, Cleary M, Charo IF. Decreased lesion formation in CCR2-/- mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–7. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- 49.Liu M, Yu Y, Jiang H, Zhang L, Zhang PP, Yu P, Jia JG, Chen RZ, Zou YZ, Ge JB. Simvastatin suppresses vascular inflammation and atherosclerosis in ApoE(-/-) mice by downregulating the HMGB1-RAGE axis. Acta Pharmacol Sin. 2013;34:830–6. doi: 10.1038/aps.2013.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Soro-Paavonen A, Watson AM, Li J, et al. Receptor for advanced glycation end products (RAGE) deficiency attenuates the development of atherosclerosis in diabetes. Diabetes. 2008;57:2461–9. doi: 10.2337/db07-1808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Swirski FK, Libby P, Aikawa E, Alcaide P, Luscinskas FW, Weissleder R, Pittet MJ. Ly-6Chi monocytes dominate hypercholesterolemia-associated monocytosis and give rise to macrophages in atheromata. J Clin Invest. 2007;117:195–205. doi: 10.1172/JCI29950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–94. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Morias Y, Abels C, Laoui D, Van Overmeire E, Guilliams M, Schouppe E, Tacke F, deVries CJ, De Baetselier P, Beschin A. Ly6C- Monocytes Regulate Parasite-Induced Liver Inflammation by Inducing the Differentiation of Pathogenic Ly6C+ Monocytes into Macrophages. PLoS Pathog. 2015;11:e1004873. doi: 10.1371/journal.ppat.1004873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Contreras-Shannon V, Ochoa O, Reyes-Reyna SM, Sun D, Michalek JE, Kuziel WA, McManus LM, Shireman PK. Fat accumulation with altered inflammation and regeneration in skeletal muscle of CCR2−/− mice following ischemic injury. Am J Physiol Cell Physiol. 2007;292:C953–67. doi: 10.1152/ajpcell.00154.2006. [DOI] [PubMed] [Google Scholar]
- 55.Shireman PK, Contreras-Shannon V, Ochoa O, Karia BP, Michalek JE, McManus LM. MCP-1 deficiency causes altered inflammation with impaired skeletal muscle regeneration. J Leukoc Biol. 2007;81:775–85. doi: 10.1189/jlb.0506356. [DOI] [PubMed] [Google Scholar]
- 56.Breitkopf K, Sawitza I, Westhoff JH, Wickert L, Dooley S, Gressner AM. Thrombospondin 1 acts as a strong promoter of transforming growth factor beta effects via two distinct mechanisms in hepatic stellate cells. Gut. 2005;54:673–81. doi: 10.1136/gut.2004.042911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hazra S, Jarajapu YP, Stepps V, et al. Long-term type 1 diabetes influences haematopoietic stem cells by reducing vascular repair potential and increasing inflammatory monocyte generation in a murine model. Diabetologia. 2013;56:644–53. doi: 10.1007/s00125-012-2781-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Babu M, Durga Devi T, Makinen P, Kaikkonen M, Lesch HP, Junttila S, Laiho A, Ghimire B, Gyenesei A, Yla-Herttuala S. Differential Promoter Methylation of Macrophage Genes Is Associated With Impaired Vascular Growth in Ischemic Muscles of Hyperlipidemic and Type 2 Diabetic Mice: Genome-Wide Promoter Methylation Study. Circ Res. 2015;117:289–99. doi: 10.1161/CIRCRESAHA.115.306424. [DOI] [PubMed] [Google Scholar]
- 59.Xu J, Benabou K, Cui X, Madia M, Tzeng E, Billiar T, Watkins S, Sachdev U. TLR4 Deters Perfusion Recovery and Upregulates Toll-like Receptor 2 (TLR2) in Ischemic Skeletal Muscle and Endothelial Cells. Mol Med. 2015;21:605–15. doi: 10.2119/molmed.2014.00260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Bastiaansen AJ, Karper JC, Wezel A, de Boer HC, Welten SM, de Jong RC, Peters EA, de Vries MR, van Oeveren-Rietdijk AM, van Zonneveld AJ, Hamming JF, Nossent AY, Quax PH. TLR4 accessory molecule RP105 (CD180) regulates monocyte-driven arteriogenesis in a murine hind limb ischemia model. PLoS One. 2014;9:e99882. doi: 10.1371/journal.pone.0099882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.van Zoelen MA, Schouten M, de Vos AF, Florquin S, Meijers JC, Nawroth PP, Bierhaus A, van der Poll T. The receptor for advanced glycation end products impairs host defense in pneumococcal pneumonia. J Immunol. 2009;182:4349–56. doi: 10.4049/jimmunol.0801199. [DOI] [PubMed] [Google Scholar]
- 62.Noto MJ, Becker KW, Boyd KL, Schmidt AM, Skaar EP. RAGE-Mediated Suppression of Interleukin-10 Results in Enhanced Mortality in a Murine Model of Acinetobacter baumannii Sepsis. Infect Immun. 2017:85. doi: 10.1128/IAI.00954-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Achouiti A, de Vos AF, van 't Veer C, Florquin S, Tanck MW, Nawroth PP, Bierhaus A, van der Poll T, van Zoelen MA. Receptor for Advanced Glycation End Products (RAGE) Serves a Protective Role during Klebsiella pneumoniae - Induced Pneumonia. PLoS One. 2016;11:e0141000. doi: 10.1371/journal.pone.0141000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.