Abstract
Background
Acidithiobacillus caldus, a Gram-negative, chemolithotrophic sulfur-oxidizing bacterium, is widely applied in bioleaching. The absence of an ideal selection marker has become a major obstacle to achieve high efficiency of the gene transfer system for A. caldus. Plasmid pJRD215, widely used in Acidithiobacillus spp., has severe drawbacks in molecular manipulations and potential biosafety issues due to its mobility. Therefore, finding a new selection marker and constructing new plasmids have become an urgent and fundamental work for A. caldus.
Results
Effective inhibitory effect of chloramphenicol on the growth of A. caldus was elucidated for the first time. The P2-cat gene cassette, including a chloramphenicol acetyltransferase gene (cat) from plasmid pACBSR and a promoter (P2) upstream of the tetracycline resistance gene on pBR322, was designed, chloramphenicol acetyltransferase was expressed in A. caldus, and the enzyme activity was assessed. A new vector pSDU1 carrying the replication and mobilization regions derived from pJRD215, the P2-cat gene cassette and a multiple cloning site from pUC19 was successfully constructed. Compared with pJRD215, pSDU1 had a 27-fold increase in electrotransformation efficiency (30.43±0.88×104 CFU/μg DNA for pSDU1 and 1.09±0.11×104 CFU/μg DNA for pJRD215), better carrying capacity and could offer more convenience for the restriction enzyme digestion. In addition, the generated plasmid pSDU1Δmob, a novel non-mobilizable derivative of pSDU1 lacking some DNA sequences involved in the mobilization process, had increased copy number in A. caldus and lost its mobility for biosafety considerations. Both pSDU1 and pSDU1Δmob exhibited stable maintenance in A. caldus within 50 passages. However, further deletion of orfEF region involved in regulating repAC operon resulted in a negative effect on transformation efficiency, copy number and stability of plasmid pSDU1ΔmobΔorfEF in A. caldus.
Conclusion
Chloramphenicol was proved to be an ideal selection marker for A. caldus. Novel plasmids carrying cat gene were constructed. The utilization of these vectors will undoubtedly facilitate efficient genetic manipulations and accelerate the research progress in A. caldus.
Introduction
Acidithiobacillus caldus is a Gram-negative, acidophilic, obligately chemolithotrophic, moderately thermophilic bacterium [1]. It generates energy by oxidizing reduced inorganic sulfur compounds (elemental sulfur, sulfide, sulfite, thiosulfate and tetrathionate) and fixing carbon dioxide from the air [2,3]. As one of the most abundant bacteria in bioleaching tanks of mineral ores, A. caldus can work cooperatively with other iron-oxidizing bacteria to facilitate bioleaching. In recent years, A. caldus has attracted researchers’ extensive attention because of its unique physiological characteristics and important applications in the bioleaching industry. Multiple A. caldus strains were isolated from the environment and the genome sequences were released [4,5], which provided a wealth of information on the genetic composition of this bacterium. However, the cellular functions of most of the putative proteins and the unique sulfur oxidation system of A. caldus remain unknown.
The gene transfer systems, more specifically, the conjugation system and electroporation system, are powerful techniques in genetic engineering for gene functional studies in vivo. Applications of conjugation techniques were reported in the representative species in Acidithiobacillus genus, including A. thiooxidans, A. ferrooxidans, and A. caldus [6–9]. In contrast, gene transfer by electrotransformation has been successful in A. ferrooxidans, and A. caldus only [10,11]. However, relatively low transfer efficiency has been observed in either conjugation or electrotransformation, which is an obstacle for functional studies in Acidithiobacillus genus.
The plasmid pJRD215 derived from RSF1010 has been widely employed in Acidithiobacillus genus as the shuttle vector for gene transfer because of its broad host range, the autonomous-replication capability in the host and the antibiotic resistance genes (kan and str) [12]. However, pJRD215 has several drawbacks when it is used in molecular biology research and genetic engineering in Acidithiobacillus spp.: (i) the instability of streptomycin and kanamycin in acidic environment leads to the low transformation efficiency and high false positive transformant yield; (ii) the multiple cloning site (MCS) on pJRD215 contains few recognition sites for restriction enzymes, most of which are not commonly used, resulting in troubles during molecular manipulations; (iii) the large size of pJRD215 (10.3 kbp) limits its carrying capacity and causes severe pressure on the growth of A. caldus cells; (ⅳ) the mobility of pJRD215 brings biological safety issues in industrial applications. However, the still acceptable transfer frequency and good maintenance of pJRD215 make it the best shuttle plasmid in Acidithiobacillus genus so far [13].
The essential replication (repA-repB-repC) and mobilization (mobA-mobB-mobC) regions of pJRD215 are derived from IncQ group plasmid RSF1010 [14,15]. RSF1010 is a broad host range vector that can be transferred by conjugation in many bacterial species [16,17]. The genes and loci involved in plasmid vegetative replication and mobilization have been identified in RSF1010. Among the four promoters in RSF1010, P1 and P3 initiate the transcription of mobA/repB, mobB, and, probably, orfE-orfF-repA-repC operon. The resulting MobA, together with MobC, auto-regulates the promoters P1, P2 and P3. Promoter P2 is in charge of the transcription of mobC in the opposite direction. P4 promoter controlled by OrfF repressor regulates the transcription of the orfE-orfF-repA-repC operon [18]. The essential replication proteins RepA, RepB and RepC and replication origin oriV control the initiation of DNA replication in RSF1010 [19], and this self-replication property extends the host range of RSF1010 [15]. Meanwhile, plasmid RSF1010 also carries the origin of conjugative DNA transfer (oriT) and the three genes (mobA, mobB and mobC) for mobilization purpose [20].
Electrotransformation, as an advanced gene transfer method, provides a convenient and efficient way for introducing exogenous DNA into mammalian cells, plants cells, yeasts, and bacteria [21–24]. Plasmid pJRD215 is the prior choice of shuttle vector for introducing foreign DNA sequences into A. caldus cells. However, for electroporation, the genes involved in mobilization on pJRD215 become unnecessary. Researchers have shown that the deletion of these mobilization genes in RSF1010 not only increases the copy number of the plasmid, but also ensures the antibiotic safety in applied biotechnology by decreasing the mobility rate of the plasmid [25,26]. Therefore, it is necessary to modify pJRD215 for applications. As A. caldus is acidophilic, an optimal antibiotic selection marker effective at low pH (pH<2.5) for A. caldus is required in order to improve the transfer efficiency. For years, kanamycin and streptomycin have been the only two antibiotics used for screening A. caldus recombinants. However, neither of them is quite effective in liquid Starkey-S0 medium (pH 2.0–2.5). Though kanamycin and streptomycin are used in solid Starkey-Na2S2O3 medium (pH 4.8) for selection purposes, the screening efficiency is too low to eliminate the false-positive cells. So, an acid stable selection marker other than kanamycin and streptomycin is needed to improve the gene transformation efficiency in A. caldus.
The aims of this study are to find the effective antibiotics marker to improve the conjugation and electroporation efficiencies and construct new plasmids for A. caldus, which will provide useful tools for molecular operations of A. caldus.
Results and discussion
Inhibitory effect of chloramphenicol on the growth of A. caldus cells
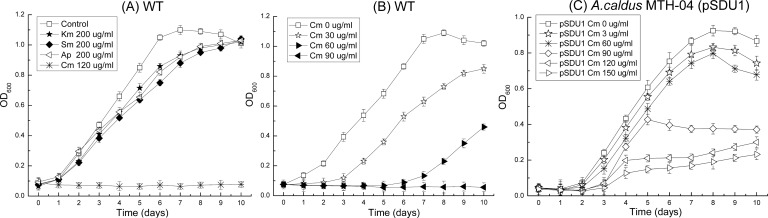
The inhibitory effect of antibiotics on the growth of A. caldus cells in Starkey-S0 liquid media was measured as shown in Fig 1A. Chloramphenicol at a concentration of 120 μg/ml in liquid Starkey-S0 media could inhibit the growth of A. caldus MTH-04 cells completely during the whole test period (data collected every 24 hours for 10 days), while the other antibiotics (ampicillin, kanamycin and streptomycin) had minor inhibitory effects on the growth of A. caldus MTH-04 cells after 1 day. Furthermore, the growth of A. caldus MTH-04 cells in liquid Starkey-S0 media containing different concentrations of chloramphenicol was tested. As shown in Fig 1B, chloramphenicol at a concentration of 30 μg/mL had a significant inhibitory effect on the growth of cells in the first 3 days; 60 μg/ml chloramphenicol could completely inhibit bacterial growth for 6 days and the inhibitory effect became weaker afterwards; 90 μg/ml chloramphenicol inhibited bacterial growth in the whole testing period (10 days). In addition, no colony was observed on the Starkey-Na2S2O3 solid agar plate containing chloramphenicol (60 μg/mL) within 15 days (data not shown). Taken together, these results suggested that chloramphenicol is suitable for antibiotic selection of A. caldus cells in both liquid and solid media.
Fig 1. Growth curves of A. caldus strains grown in liquid Starkey-S0 media with different antibiotics.
(A) A. caldus MTH-04 wild type in media with kanamycin, streptomycin, ampicillin and chloramphenicol, respectively. (B) A. caldus MTH-04 wild type in media with different concentrations of chloramphenicol. (C) A. caldus MTH-04 carrying pSDU1 in various concentrations of chloramphenicol.
The relatively low pH level suitable for the growth of A. caldus is an adverse factor for the effectiveness of most of the antibiotics. The optimal pH for the growth of A. caldus is 2.0–2.5, and the initial pH of liquid Starkey-S0 medium is 2.5. As the cells proliferate, elemental sulfur is oxidized to sulphuric acid, and the pH decreases to 0.8 in the stationary phase. Most antibiotics lose their functions in extremely low pH. Therefore, neither ampicillin, kanamycin nor streptomycin exhibits inhibitory effects on the growth of the cells (Fig 1A). Chloramphenicol is one of the most stable antibiotics and is effective in the pH range 0.4–10 [27]. In this study, we found that chloramphenicol at 90 μg/ml can successfully inhibit the growth of A. caldus in liquid Starkey-S0 medium (Fig 1B). Together with the fact that relatively low concentration of chloramphenicol (60 μg/mL) is needed to inhibit the growth of the cells on the solid agar plates, our results suggested that chloramphenicol is a suitable selection marker for molecular researches on A. caldus cells.
Construction of the plasmids carrying chloramphenicol acetyltransferase (cat) gene
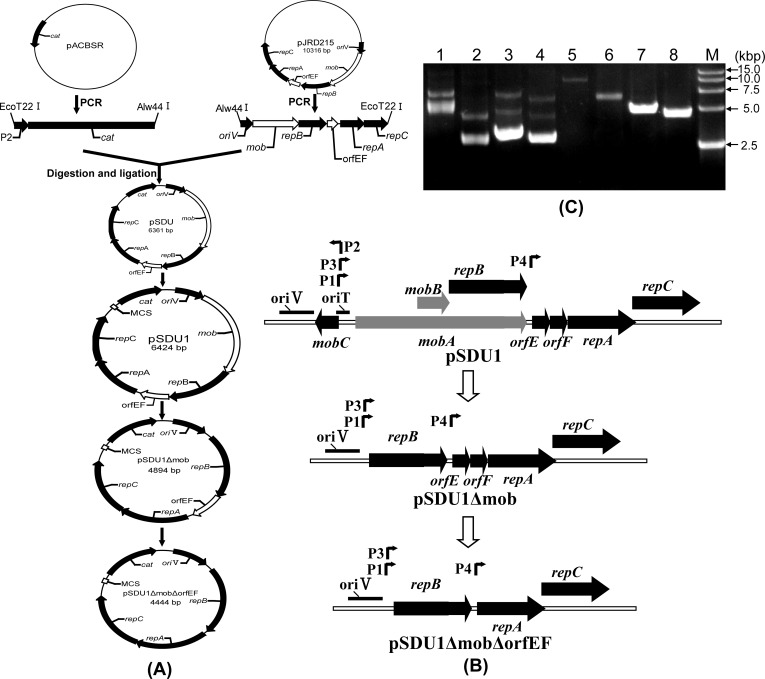
The completely inhibitory effect of chloramphenicol on the growth of A. caldus made the cat gene a suitable antibiotic selection marker for constructing novel plasmids. The detailed constructing processes of the vectors are described in the method section (Fig 2). To ensure the expression of the cat gene in A. caldus MTH-04, the promoter (P2) located upstream of the tetracycline resistance gene on the plasmid pBR322 was fused to the translational start site of the cat gene to generate P2-cat cassette [28]. The replication and mobilization regions of pJRD215 were used as the backbone of the generated plasmid pSDU1. In addition, pSDU1 also carried the MCS originated from pUC19. Based on the knowledge of the genetic loci responsible for the vegetative plasmid replication and mobilization of plasmid RSF1010 [18, 19], the loci including the mobB gene and a part of mobA gene on pSDU1 were removed to create a non-mobilizable plasmid pSDU1Δmob and the orfEF region was further eliminated from pSDU1Δmob to construct pSDU1ΔmobΔorfEF (Fig 2).
Fig 2. Construction of pJRD215-derived plasmids.
(A) Construction processes of pSDU1, pSDU1Δmob, and pSDU1ΔmobΔorfEF. A P2-cat cassette was constructed by the fusion of the promoter P2 from the plasmid pBR322 and the cat gene from plasmid pACBSR. The generated cassette and the backbone amplified from plasmid pJRD215 were ligated to generate plasmid pSDU. After that, the multiple cloning sites originated from pUC19 were introduced into pSDU to construct the plasmid pSDU1. To construct pSDU1Δmob, the mobB gene and a part of mobA gene on pSDU1 was removed. Finally, the orfEF region was further deleted from pSDU1Δmob, resulting in plasmid pSDU1ΔmobΔorfEF. (B) The detail diagram of the new pJRD215-derived plasmids. (C) Electrophoretic analysis of plasmids. Lane 1, 5: pJRD215; lane 2, 6: pSDU1; lane 3, 7: pSDU1Δmob; lane 4, 8: pSDU1ΔmobΔorfEF; lane 5–8, plasmids digested with Hind Ⅲ.
The key characteristics of the constructed plasmids (pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF) are as follows. (1) The chloramphenicol acetyltransferase gene was used as the selection marker in these plasmids, which improved the screening efficiency. (2) New multiple cloning sites were introduced into these plasmids, which facilitated efficient genetic manipulations. (3) The backbone of these plasmids was originated from the broad host range plasmid RSF1010, which introduced broad host range and autonomous replication capacity into the newly designed plasmids. (4) The sizes of pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF are only 6.4, 4.9 and 4.4 kbp, respectively. Compared with pJRD215 (10.3 kbp), the much smaller sizes of the three new plasmids allowed them to carry larger genetic inserts. (5) The non-mobilizable character of pSDU1Δmob and pSDU1ΔmobΔorfEF, caused by the deletion of mob region, enhances the biological safety in real applications.
Determination of cat gene function in A. caldus
To test the expression of the constructed P2-cat cassette, plasmid pSDU1 was electroporated into A. caldus MTH-04 [11]. Chloramphenicol was used for the screening of electrotransformants. Colonies were picked from the solid plates containing 68 μg/ml chloramphenicol and transferred into liquid Starkey-S0 medium containing different concentrations of chloramphenicol. In the end, the plasmid pSDU1 was recovered from the electrotransformants (data not shown). To investigate the inhibitory effect of chloramphenicol on the growth rate of A. caldus cells harboring pSDU1, the cells were cultured in liquid Starkey-S0 medium added by different concentrations of chloramphenicol and the growth was determined by measurement of the optical density (OD600). As shown in Fig 1C, with the final concentration of 0, 30, 60, 90, 120 and 150 μg/ml chloramphenicol in the culture broths, the maximum value of OD600 of A. caldus MTH-04 carrying pSDU1 could reach approximately 0.9, 0.8, 0.8, 0.4, 0.3, 0.2, respectively. Cells grew well when the concentration of chloramphenicol in the culture broth was less than 60 μg/ml; 90 μg/ml chloramphenicol obviously inhibited cell growth; and 120 and 150 μg/ml chloramphenicol had severe inhibition effects on the cell growth.
Since the autotrophic A. caldus is phylogenetically distant from other well-studied heterotrophic bacteria, e.g. E. coli, many known promoters that work in other bacteria can not initiate the transcription of genes in A. caldus. To ensure the transcription of chloramphenicol resistant gene in A. caldus, promoter P2 was used, which was from pBR322 and proved to have transcriptional capacity in A. caldus. The success of transformant selection on agar plates containing chloramphenicol indicated that the antibiotic resistant gene was expressed and worked well in A. caldus. Chloramphenicol functions as a bacteriostatic, by inhibiting protein chain elongation during protein synthesis. To protect the cells from being killed by chloramphenicol, the cat gene encoding a chloramphenicol acetyltransferase, was introduced into the cells to acetylate chloramphenicol and prevent it from binding to the ribosome. So, the transformants can grow well at 90 μg/ml chloramphenicol while the wildtype cells that do not carry any cat gene in their genome will be eliminated. With considerations of the inhibitory effect of chloramphenicol on the growth of both transformants and the wildtype cells, the suitable final concentration of chloramphenicol, 60–90 μg/ml, in broth culture was determined for antibiotic selection purpose.
Determination of electrotransformation efficiency and frequency
A significant improvement on electrotransformation efficiency and frequency was obtained when chloramphenicol was used as the selective marker. As shown in Table 1, the transformation efficiencies of plasmids pSDU1 and pSDU1Δmob were 30.43±0.88 ×104 and 38.67±0.79 ×104 CFU/μg DNA, respectively, while it was 1.09±0.11×104 CFU/μg DNA for plasmid pJRD215 using kanamycin as the selective marker. Compared with pJRD215, the transformation efficiencies of pSDU1 and pSDU1Δmob increased 28 and 34 times, respectively. Meanwhile, the transformation frequencies of pSDU1 and pSDU1Δmob increased approximately 27 and 34 times, respectively. The results indicated that addition of cat gene in the two new plasmids enhanced their transformation efficiencies and frequencies significantly (See Table 1 for detailed information on transformation efficiencies and frequencies). In contrast, plasmid pSDU1ΔmobΔorfEF had relatively lower transformation efficiency and frequency compared with those of pSDU1 and pSDU1Δmob (Table 1).
Table 1. Transformation efficiency and frequency of plasmids in electroporation of A. caldus MTH-04.
| Transformation efficiencya (×104 CFU/μg DNA) |
Transformation frequencyb (×10−6 CFU/cells added) |
|
|---|---|---|
| pJRD215 | 1.09±0.11 | 2.55±0.25 |
| pSDU1 | 30.43±0.88 | 71.00±2.05 |
| pSDU1Δmob | 38.67±0.79 | 90.22±1.85 |
| pSDU1ΔmobΔorfEF | 0.12±0.01 | 0.28±0.03 |
a Mean±Standard deviation.
b Mean±Standard deviation.
The increased transformation efficiencies and frequencies of pSDU1 and pSDU1Δmob likely result from the significant inhibitory effect of chloramphenicol on the growth of the wildtype A. caldus cells. After electroporation, the cells need some recovery time and antibiotics should be added after that time to inhibit the growth of negative transformants, which can increase the ratio of positive transformants to wildtype on selective agar plates. The protein encoded by orfF can autoregulate the repAC operon by inhibiting transcription from promoter P4 [18]. Mutagenesis of orfEF could weaken the feedback from orfF to repAC operon and in turn, affected the expression levels of repA and repC in pSDU1ΔmobΔorfEF, so this mutation made the plasmid unstable in A. caldus. This could possibly explain the significantly reduced transformation efficiency and frequency of pSDU1ΔmobΔorfEF.
Determination of conjugative transfer frequencies
The mobility of the series of plasmids in A. caldus was tested by the conjugation and reverse-conjugation as previously described [9]. As shown in Table 2, the transfer frequency of pSDU1 from E. coli SM10 to A. caldus MTH-04 had a 3-fold increase compared to that of pJRD215, while no transconjugant carrying pSDU1Δmob or pSDU1ΔmobΔorfEF was obtained. Utilization of chloramphenicol as the selection marker and the smaller size of pSDU1 (6.4 kbp) could contribute to the higher transfer frequency of pSDU1. In the reverse-conjugation, A. caldus MTH-04 transformants carrying plasmids pJRD215, pSDU1, pSDU1Δmob or pSDU1ΔmobΔorfEF, were used as the donor strains to conjugate with E. coli C600 recipient strain with the help E. coli C600 harboring plasmid RP4. The results are shown in Table 2. The transfer frequency of pSDU1 increased slightly compared with that of pJRD215 while the transfer frequencies of pSDU1Δmob and pSDU1ΔmobΔorfEF (less than 10−7) were undetectable. The results indicated that the deletion of a 1,636 bp fragment at the initial part of mobA gene (Fig 2B) made the plasmids lose their mobility, which improved the biological safety. These results were same with the previous report that RSF1010-derived plasmids with partially deleted mob-genes possessed undetectable mobilization frequencies under laboratory conditions [25,26].
Table 2. Transfer frequencies of plasmids between A. caldus MTH-04 and E. coli C600.
| Donor | Recipient | Selection marker | Transfer frequency a,b |
|---|---|---|---|
| E. coli | A. caldus | ||
| SM10 (pJRD215) | MTH-04 | Smr | (3.21±1.11) ×10−5 |
| SM10 (pSDU1) | MTH-04 | Cmr | (9.54±1.41) ×10−5 |
| SM10 (pSDU1Δmob) | MTH-04 | Cmr | — |
| SM10 (pSDU1ΔmobΔorfEF) | MTH-04 | Cmr | — |
| A. caldus | E.coli | ||
| MTH-04 (pJRD215) and E. coli C600 (RP4)c | C600 | Smr | (2.53±1.31) ×10−6 |
| MTH-04 (pSDU1) and E. coli C600 (RP4)c | C600 | Cmr | (3.62±1.28) ×10−6 |
| MTH-04 (pSDU1Δmob) and E. coli C600 (RP4)c |
C600 | Cmr | — |
| MTH-04 (pSDU1ΔmobΔorfEF) and E. coli C600 (RP4)c |
C600 | Cmr | — |
a Data are averages of at least three independent experiments.
b Mean±Standard deviation.
c E. coli C600 (RP4) was used as a helper strain.
—undetected.
Plasmid copy number of pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF in A. caldus
Two calculation methods (absolute quantification and relative quantification) based on real-time qPCR results were used to estimate the copy numbers of the pJRD215-derived plasmids in A. caldus.
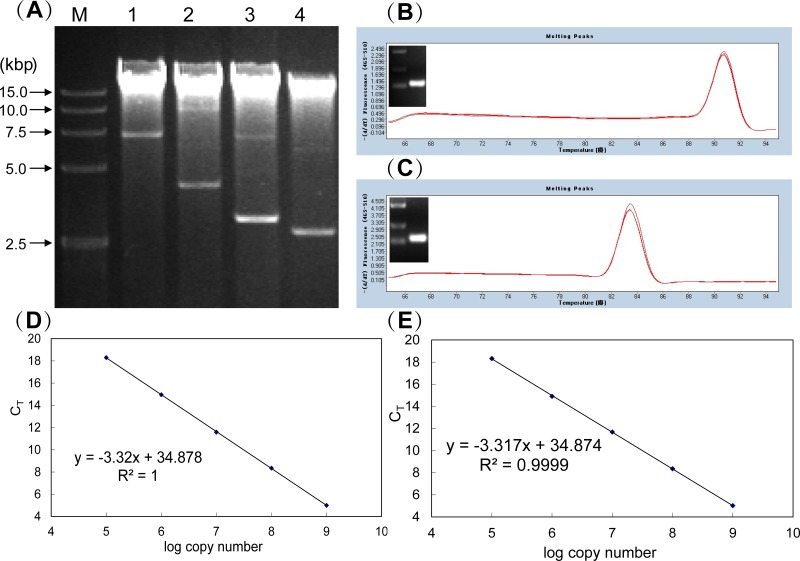
First, total DNA was purified from four different recombinants of A. caldus each carrying one of the plasmids (pJRD215, pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF) (Fig 3A). Then, two sets of primers were designed specific to the single-copy genes repA (from pJRD215) and tetH (from A. caldus chromosomal DNA). The amplification specificities of repA-set and the tetH-set primers were analysed by both melting curve analysis and gel electrophoresis. Sharp peaks were observed in the melting peak analysis on both sets of primers (Fig 3B), and prominent bands with expected sizes were found in the gel electrophoresis analysis for the real-time qPCR products (Fig 3C). The identities of amplified products were confirmed by sequencing analysis afterwards. These results indicated that non-specific PCR products amplified using these primer sets were undetectable in the analysed temperature ranges. The standard curves for repA and tetH genes, ranging from 0.5×105−0.5×109 copies/μL, were drawn in Fig 3D and 3E, respectively. Both curves were linear in the tested range (R2>0.999) based on the triplicate reactions. The slopes of the standard curves for repA and tetH were -3.32 and -3.317, respectively. From the slopes, the amplification efficiencies (E) of 1.00 for both repA and tetH primer sets were calculated in the investigated range and were used for relative quantification. Finally, these two sets of primers were used in real-time qPCR to obtain the CT (threshold cycle) values of repA and tetH genes in these total DNA samples, and the these CT values were used for further calculations.
Fig 3. Real-time qPCR experiments to determine the copy numbers of plasmids in A. caldus MTH-04.
(A) Electrophoretic analysis of the total DNA from A. caldus MTH-04 carrying pJRD215 (lane 1), A. caldus MTH-04 carrying pSDU1 (lane 2), A. caldus MTH-04 carrying pSDU1Δmob (lane 3), A. caldus MTH-04 carrying pSDU1ΔmobΔorfEF (lane 4), respectively. Confirmation of PCR amplification specificities of tetH-set (B) and repA-set (C) primers. Standard curves and amplification efficiencies of tetH-set (D) and the repA-set (E) primers.
Absolute quantification was carried out according to the method described earlier [29]. The absolute copy numbers of repA and tetH in A. caldus were determined based on the corresponding standard curves (Fig 3D & 3E), using the CT values. Because repA and tetH are single-copy genes on pJRD215 and A. caldus chromosomal DNA, respectively, the copy ratio of repA to tetH is equal to the plasmid copy number. Thus, the plasmid copy number was then calculated by dividing the copy number of repA by the copy number of tetH. As shown in Table 3, the copy numbers of pJRD215, pSDU1, pSDU1Δmob, pSDU1ΔmobΔorfEF in A. caldus were ~10, 11, 28 and 19, respectively (derived from absolute quantification).
Table 3. Estimated plasmid copy number (PCN) by absolute quantification.
| Plasmid | CT a | Copiesb (copies/μl) | PCNc | ||
|---|---|---|---|---|---|
| repA | tetH | repA | tetH | ||
| pJRD215 | 12.47±0.03 | 15.87±0.02 | 5.62×106 (1.8%) | 5.37×105 (1.4%) | 10.45 (3.3%) |
| pSDU1 | 12.04±0.03 | 15.48±0.02 | 7.55×106 (2.1%) | 7.04×105 (1.1%) | 10.72 (3.1%) |
| pSDU1Δmob | 9.48±0.03 | 14.32±0.02 | 4.46×107 (2.1%) | 1.57×106 (1.1%) | 28.42 (3.0%) |
| pSDU1ΔmobΔorfEF | 12.37±0.03 | 16.61±0.03 | 6.03×106 (1.7%) | 3.21×105 (1.8%) | 18.82 (3.5%) |
a Average±S.D. (n = 3).
b Average (coefficient of variation) (n = 3).
c Average (coefficient of variation) (n = 3).
For relative quantification, repA and tetH were used as the target and the reference genes, respectively. Because the standard plasmid pJRD215-tetH (i.e, calibrator) had one copy of each repA and tetH specific sequence, the copy ratio of repA to tetH in the calibrator should be 1 and the ΔCT of repA to tetH should be 0. In the experiment, ΔCT of the calibrator was -0.02 with acceptable experimental error compared to the theoretical value (Table 4). The ΔCT of the samples was obtained from the CT of repA and tetH shown in Table 4. Therefore, the ΔΔCT calculated from ΔCT of the sample and calibrator is equal to the copy ratio of repA to tetH in the sample. Then the copy numbers of the plasmids were obtained by the formula (1+E)-ΔΔCT [30]. Using the experimentally calculated amplification efficiency (E = 1.00), the plasmid copy numbers were determined using the 2−ΔΔCT calculation. The copy numbers of pJRD215, pSDU1, pSDU1Δmob, pSDU1ΔmobΔorfEF in A. caldus, calculated from relative quantification, were ~10, 11, 28 and 19, respectively (Table 4). The results of both absolute and relative quantification were identical and reproducible.
Table 4. Estimated plasmid copy number (PCN) by relative quantification method.
| Plasmid | ΔCT samplea | ΔCT calibratorb | ΔΔCTc | PCNd |
|---|---|---|---|---|
| 2-ΔΔCT | ||||
| pJRD215 | -3.40±0.05 | -0.02±0.05 | -3.38±0.05 | 10.39 (3.3%) |
| pSDU1 | -3.43±0.05 | -0.02±0.05 | -3.41±0.05 | 10.66 (3.1%) |
| pSDU1Δmob | -4.84±0.04 | -0.02±0.05 | -4.82±0.04 | 28.26 (3.0%) |
| pSDU1ΔmobΔorfEF | -4.24±0.05 | -0.02±0.05 | -4.22±0.05 | 18.69 (3.5%) |
a Average±S.D. (n = 3).
b Calculated from the serial dilutions of the quantitative standard sample used for standard curve construction. Average±S.D. (n = 10).
c Average±S.D. (n = 3).
d Average (coefficient of variation) (n = 3).
The copy numbers of pJRD215 and pSDU1 in A. caldus (10 per cell) were slightly lower than the reported value of RSF1010 in E. coli (12 per cell) [31]. The differences in the copy number in the two host bacteria is probably caused by their differences in growth characteristics: the chemolithotrophic growth of A. caldus and the heterotrophic growth of E. coli. The main structure of pSDU1 is similar to pJRD215, both carrying the replication and conjugation regions, thus the copy number of the two plasmids in A. caldus was basically the same.
The deletion of certain functional genes (mobB and part of mobA) on pSDU1 resulted in the obvious increase in the copy number of pSDU1Δmob in A. caldus. In pSDU1Δmob, the distance from promoters P1 and P2 to the replication gene repB is reduced compared with that of pSDU1. Meanwhile, the inhibitory effect of MobA on promoter P3 is eliminated in pSDU1Δmob. All of these possibly led to the increase in transcription rates and expression levels of repB and its following genes repA and repC, which would in turn increase the copy number of plasmid pSDU1Δmob. Our results are in accordance with the recent findings that the copy number of RSF1010-based plasmids increased significantly when the mob-genes are partially removed [25,26].
The copy number of pSDU1ΔmobΔorfEF decreased significantly in comparison to that of pSDU1Δmob in A. caldus. Further deletion of orfE and orfF genes from pSDU1Δmob probably got rid of the inhibitory effect of orfF gene product to P4 promoter or affected the transcription of repA, repC and repB so as to influence the replication and stability of the plasmid. This possibly explained the reduced copy number of pSDU1ΔmobΔorfEF in A. caldus.
Maintenance of the plasmids in A. caldus
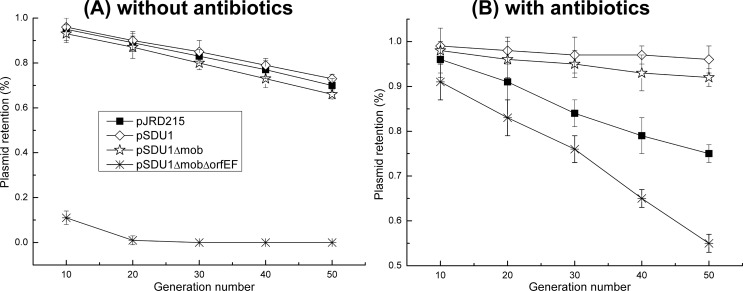
Plasmid stability analysis in A. caldus was carried out as described in the method section. After serial passages for approximately 50 times, the plasmid retention ratios were calculated and shown in Fig 4. When A. caldus cells passed 50 passages in culture broth without any antibiotics, the retention ratios of pJRD215, pSDU1, pSDU1Δmob were 70%, 73% and 66%, respectively. The plasmid retention rate of pSDU1ΔmobΔorfEF after 10-time-passage was approximately 11% and the plasmid was lost after 20 passages. As shown in Fig 4B, since chloramphenicol is stable in Starkey-S0 medium, the addition of chloramphenicol into the culture broth increased significantly the retention rates of pSDU1 and pSDU1Δmob to 90% after 50 passages, and the retention rate of pSDU1ΔmobΔorfEF to 50% after 50 passages. In contrast, kanamycin and streptomycin could not increase the retention rate of the plasmid pJRD215. Since these antibiotics are not stable at pH 2.5, they have no inhibitory effects on the growth of the cells.
Fig 4. Maintenance of the plasmids in A. caldus MTH-04 cultivated in liquid Starkey-S0 media during serial passages.
(A) without antibiotics; (B) with antibiotics, streptomycin 200 μg/ml, chloramphenicol 60μg/ml.
The deletion of replication and mobilization regions of pSDU1 had effects on the stabilities of the generated plasmids at various degrees. Without the antibiotic selection pressure, there were no significant differences in the retention rates among pJRD215, pSDU1 and pSDU1Δmob (Fig 4B), which indicated that the removed mobilization region was not essential for the plasmid stability in A. caldus. The significant decline in the retention rate of pSDU1ΔmobΔorfEF compared with that of pJRD215, pSDU1 and pSDU1Δmob in the culture broth with and without antibiotics, suggested the importance of orfEF region on the plasmid stability in A. caldus. Due to the regulatory role of OrfF on the expression of the repAC operon, the deletion of orfEF may have serious negative effects on the replication of the plasmid resulting in the instability of pSDU1ΔmobΔorfEF in A. caldus. Even with the addition of chloramphenicol to the culture, pSDU1ΔmobΔorfEF was still lost in most the cells.
Conclusions
The lack of a suitable selection marker for the gene transfer systems has been a major obstacle for the genetic manipulations in Acidithiobacillus genus. In this study, we discovered the significant inhibitory effect of chloramphenicol on the growth of A. caldus, and constructed a family of pJRD215-derived plasmids harboring the chloramphenicol acetyltransferase gene. Compared with pJRD215, these newly constructed plasmids, pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF, are smaller in sizes (≤ 6.4kbp) and carry the cat gene and the different multiple cloning sites. Due to the incorporation of the cat gene, the electroporation efficiency of pSDU1 had a progressive increase. Further deletion of certain sequences related to mobilization from pSDU1 resulted in the two new derivatives (pSDU1Δmob and pSDU1ΔmobΔorfEF) which were shown to be no longer mobilizable. Thus, this two plasmids could be considered as non-mobilizable vectors, which improved the biosafety in industrial applications. Moreover, there was an approximately 3-fold increase in the copy number of pSDU1Δmob in A. caldus compared with that of pJRD215. However, the removal of orfEF region had negative effects on the transformation efficiency, plasmid copy number and stability, suggesting that the absence of repressor OrfF would impair the plasmid replication.
To sum up, the miniaturized plasmid pSDU1, the higher copy number (in comparison to pSDU1) and non-mobilizable plasmid pSDU1Δmob, both carrying chloramphenicol selection marker, were considered as suitable vectors for A. caldus studies. In addition, the construction of these new plasmids and the development of electroporation and conjugation techniques together constitute a complete upgraded gene operating system for A. cladus, which will be useful tools for future functional studies and genetic engineering in A. caldus.
Methods
Bacterial strains and cultivation conditions
The bacterial strains and plasmids used in this study are listed in Table 5. E. coli was grown in Luria-Bertani broth or Luria-Bertani agar at 37°C [32], with the addition of ampicillin (100 μg/ml), kanamycin (100 μg/ml), streptomycin (100 μg/ml) and chloramphenicol (30 μg/ml) when needed. A. caldus MTH-04 was grown at 40°C in a liquid Starkey-S0 inorganic medium containing ampicillin (200 μg/ml), kanamycin (200 μg/ml), streptomycin (200 μg/ml) and chloramphenicol (60 μg/ml) or on the solid Starkey-Na2S2O3 medium containing kanamycin (100 μg/ml), streptomycin (100 μg/ml) and chloramphenicol (60 μg/ml) when needed [6].
Table 5. The bacterial strains and plasmids used in this work.
| Strain or plasmid | Phenotype or genotype | Source or reference |
|---|---|---|
| Strain | ||
| E. coli SM10 | Kmr thi-1 thr leu tonA lacY supE recA:: RP4-2-Tc::Mu | [34] |
| E. coli C600 | integrated thr, leu, hsd | Shandong University, China |
| E. coli DH5α | F-φ80d lacZΔM15Δ(lacZYA-argF) U169 end A1 recA1 hsdR17(rk-,mk+) supE44λ-thi-1 gyr96 relA1 phoA gyr96 relA1 phoA gyr96 relA1 phoA | TransGen Biotech Corp. China |
| A. caldus MTH-04 | Wild-type | [35] |
| Plasmid | ||
| RP4 | Apr, Tcr, Kmr, IncP, tra+ | Shandong University, China |
| pJRD215 | Smr, Kmr, IncQ, mob+ | [12] |
| pSDU1 | Cmr, IncQ, mob+ | this study |
| pSDU1Δmob | Cmr, IncQ | this study |
| pSDU1ΔmobΔorfEF | Cmr, IncQ | this study |
PCR and DNA recombination technique
Premix PrimeSTAR HS DNA polymerase, restriction enzymes and T4 DNA ligase were purchased from Takara Co. and used in accordance with the manufacturer's instructions. Oligonucleotides sequences of primers used in this work were listed in Table 6.
Table 6. Primers used in this work.
| Name | Sequence (5’ to 3’)a |
|---|---|
| Cm S1sen | GCGGTAGTTTATCACAGTTAAATTGCTAACGCAGTCAGGCACCGTGTATGGAGAAAAAAATCACTGG |
| CmS2 sen | CTGAGAATTCATGCATTCATGTTTGACAGCTTATCATCCATAAGGTTTAATGCGGTAGTTTATCACAG |
| Cm ant | CTTCGTGCACATTCATCCGCTTATTATCACTT |
| P215 sen | CTTCGTGCACTCCTTGCAATACTGTGTT |
| P215 ant | CTTCATGCATCCCAAGCTTAGAGCATACATCTGGAAGCA |
| MCS sen | CTAGTCTAGAGGATCCCCGGGTACCGAGCTCGAATTCATGCATTCATGTTTGACAGCT |
| MCS ant | CTAGTCTAGAGTCGACCTGCAGGCATGCAAGCTTAGAGCATACATCTGGAAGCA |
| RepB S1 sen | TGGAGGCACAGCATTGAGCCGAAAAGCAAAAGCAACAGCGAGGCAGCATGAAGAACGACAGGACT |
| RepB S2 sen | TCCCCTTAACCATCTTGACACCCCATTGTTAATGTGCTGTCTCGTAGGCTATCATGGAGGCACAGCATTG |
| Remove mob ant | GCTGAATGATCGACCGAGAC |
| orfEF sen | ATGGCTACCCATAAGCCTATCAAT |
| orfEF ant | AAAACCCCCTTCTGTGCGTGAGT |
| RepA sen | CGGGTGCTCTATCGTGTTCCTG |
| RepA ant | GCGGATGTTATCGACCAGTACC |
| tetH sen | ACGGCGTTAAGGAAGCACTG |
| tetH ant | GTCGTCACTTTCGGCATAGA |
| tetH Xba sen | CTAGTCTAGAACGGCGTTAAGGAAGCACTG |
| tetH Hind ant | TCCCAAGCTTGTCGTCACTTTCGGCATAGA |
a Artificial restriction sites are italic and underlined.
Plasmid construction
As shown in Fig 2, a P2-cat fragment (842 bp) was generated by two rounds of PCR. In the first round, PCR amplification was performed using pACBSR as the template and Cm S1sen and Cm ant primers. The generated PCR product (791 bp) was used as the template for the second round of PCR amplification using primers CmS2 sen and Cm ant. The P2 promoter sequence was introduced into the PCR fragments by primers CmS1 sen and CmS2 sen. The P2-cat fragment was digested with Alw44 I and EcoT22 I, ligated to the same enzyme treated OriV-mob-repB-orfEF-repA-repC fragment (5,539 bp) amplified from pJRD215 using primers P215 sen and P215ant, producing a new plasmid (6,361 bp). Then, the generated plasmid was amplified by PCR using primers MCS sen and MCS ant, and the generated fragment (6,438 bp) was digested with Xba I and self-ligated to produce the plasmid pSDU1 that had the MCS from pUC19. To delete a part of mob region on pSDU1, two round PCR were carried out to construct the non-mobilizable plasmid pSDU1Δmob. The first-round PCR amplification was carried out using plasmid pSDU1 as template and with the primers (RepB S1 sen and Remove mob ant). The generated PCR product (4,840 bp) was used as the template for the second round PCR amplification using 5’end phosphorylated primers RepB S2 sen and Remove mob ant. For the phosphorylation of primers, the PCR fragment (4,894 bp) could be self-ligated, generating the plasmid pSDU1Δmob. Finally, the 5’end phosphorylated primers orfEFsen and orfEF ant were used for PCR amplification from pSDU1Δmob and the amplified fragment (4,444 bp) were self-ligated to construct pSDU1ΔmobΔorfEF. All the sequences of the constructed plasmids were verified by DNA sequencing.
The nucleotide sequences of pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF have been deposited in NCBI GenBank database under accession number MF163397, MF163398, and MF163399, respectively.
Gene transfer manipulations
The detailed steps of electroporation in A. caldus MTH-04 and the methods for the determination of transformation efficiency and frequency of plasmids were described earlier [11]. Conjugation of plasmids from E. coli to A. caldus, the reverse-mobilization of plasmids from A. caldus MTH-04 to E. coli, and determination of their mobilization frequencies in A. caldus were carried out in accordance with the reference [9].
Quantification of plasmid copy number in A. caldus
The copy numbers of the plasmids in A. caldus MTH-04 cultivated in liquid Starkey-S0 medium were determined by SYBR-Green-based real-time qPCR according to the methods described in the references [29,33]. First, two primer sets specific to the tetrathionate hydrolase gene (tetH) and the repA gene were designed and denoted as the tetH- and repA- set, respectively. The sequences of the two sets of primers (RepA sen and RepA ant, tetH sen and tetH ant) are listed in Table 6. Second, a standard plasmid pJRD215-tetH, carrying part of tetH gene amplified from A. caldus MTH-04 chromosomal DNA using primers tetH Xba sen and tetH Hind ant, was constructed to confirm the amplification specificities and efficiencies of primers tetH-set and the repA-set. Third, real-time qPCR amplification and analysis were performed using a LightCycler instrument with software version 3.5 (Roche Diagnostics). SYBR Premix Ex Taq was purchased from Takara Co. The total DNA of A. caldus MTH-04 transformants was extracted using the QIAamp DNA Mini kit (Qiagen Co.). The thermal cycling protocol was as follows: initial denaturation for 2 min at 95°C followed by 40 cycles of 10 s at 95°C, 10 s at 55°C and 15 s at 72°C. The fluorescence signal was measured at the end of each extension step (72°C). After the amplification, a melting curve analysis with a temperature gradient of 0.5°C/s from 65 to 90°C was performed to confirm that only the specific products were amplified. Finally, the plasmid copy number in A. caldus was determined using absolute quantification and relative quantification methods [29,33].
Stability analysis of plasmids in A. caldus
Stability analysis of plasmids in A. caldus in the absence of antibiotic was determined as follows: A single colony of A. caldus transformant was inoculated into 20 ml of Starkey-S0 liquid medium in the absence of any antibiotic, incubated at 40°C for 6 days; then 10% (v/v) of the fully grown cultures was transferred to 20 ml of a fresh Starkey-S0 medium and incubated at 40°C for 6 days; the resulting culture was transferred additional four times and an aliquot was taken at the beginning of each cycle, diluted and plated onto solid Starkey-Na2S2O3 medium both with and without antibiotic. Plasmid stability was calculated as a ratio of the number of colonies on medium with or without antibiotic. Similarly, stability analysis of plasmids in A. caldus with the existence of antibiotic was tested according to the above manipulations except that the culturing media was replaced by the antibiotics containing liquid media.
Acknowledgments
We thanked Pro. Qingsheng Qi of Shandong University for providing the plasmid pACBSR. This work was supported by grants from the Natural Science Foundation (grant no. 31400093, 31370138, 31570036, 31070034), the National Basic Research Program (2010CB630902), the Natural Science Foundation (grant no. 31370084, 30800011, 61672329), and the China Postdoctoral Science Foundation (grant no. 2015M580585), People’s Republic of China.
Data Availability
All relevant data are within the paper. The nucleotide sequences of pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF have been deposited in NCBI GenBank database under accession numbers MF163397, MF163398, and MF163399, respectively.
Funding Statement
This work was supported by grants from the Natural Science Foundation (grant no. 31400093, 31370138, 31570036, 31070034)(http://www.nsfc.gov.cn/), the National Basic Research Program (2010CB630902)(http://program.most.gov.cn/), the Natural Science Foundation (grant no. 31370084, 30800011, 61672329)(http://www.nsfc.gov.cn/), and the China Postdoctoral Science Foundation (grant no. 2015M580585)(http://jj.chinapostdoctor.org.cn/V1/Program3/Default.aspx), People’s Republic of China.
References
- 1.Hallberg KB, Lindström EB. Characterization of Thiobacillus caldus sp. nov., a moderately thermophilic acidophile. Microbiology. 1994; 140: 3451–3456. doi: 10.1099/13500872-140-12-3451 [DOI] [PubMed] [Google Scholar]
- 2.Kamimura K, Okayama T, Murakami K, Sugio T. Isolation and characterization of a moderately thermophilic sulfur-oxidizing bacterium. Microbios. 1999; 99: 7–18. [Google Scholar]
- 3.Edwards KJ, Bond PL, Banfield JF. Characteristics of attachment and growth of Thiobacillus caldus on sulphide minerals: A chemotactic response to sulphur minerals? Environ Microbiol. 2000; 2: 324–332. [DOI] [PubMed] [Google Scholar]
- 4.Valdés J, Quatrini R, Hallberg K, Dopson M, Valenzuela PD, Holmes DS. Genome announcement: draft genome sequence of the extremely acidophilic bacterium Acidithiobacillus acldus ATCC 51756 reveals metabolic versatility in the genus Acidithiobacillus. J Bacteriol. 2009; 191: 5877–5878. doi: 10.1128/JB.00843-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.You XY, Guo X, Zheng HJ, Zhang MJ, Liu LJ, Zhu YQ, et al. Unraveling the Acidithiobacillus caldus complete genome and its central metabolisms for carbon assimilation. J Genet Genomics. 2011;38: 243–252. doi: 10.1016/j.jgg.2011.04.006 [DOI] [PubMed] [Google Scholar]
- 6.Jin SM, Yan WM, Wang ZN. Transfer of IncP plasmids to extremely acidophilic Thiobacillus thiooxidans. Appl Environ Microbiol. 1992; 58: 429–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Peng JB, Yan WM, Bao XZ. Plasmid and transposon transfer to Thiobacillus ferrooxidans. J Bacteriol. 1994; 176: 2892–2897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peng JB, Yan WM, Bao XZ. Expression of heterogenous arsenic resistance genes in the obligately autotrophic biomining bacterium Thiobacillus ferrooxidans. Appl Environ Microbiol. 1994; 60: 2653–2656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu XM, Lin JQ, Zhang Z, Bian J, Zhao Q, Liu Y, et al. Construction of conjugative gene transfer system between E. coli and moderately thermophilic, extremely acidophilic Acidithiobacillus caldus MTH-04. J Microbiol Biotechnol. 2007; 17: 162–167. [PubMed] [Google Scholar]
- 10.Kusano T, Sugawara K, Inoue C, Takeshima T, Numata M, Shiratori T. Electrotransformation of Thiobacillus ferrooxidans with plasmids containing a mer determinant. J Bacteriol. 1992; 174: 6617–6623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen LX, Lin JQ, Li B, Lin JQ, Liu XM. Method Development for electrotransformation of Acidithiobacillus caldus. J Microbiol Biotechnol. 2010; 20: 39–44. [PubMed] [Google Scholar]
- 12.Davison J, Heusterspreute M, Chevalier N, Ha-Thi V, Brunel F. Vectors with restriction site banks. V. pJRD215, a wide-host-range cosmid vector with multiple cloning sites. Gene. 1987; 51: 275–280. [DOI] [PubMed] [Google Scholar]
- 13.Liu Z, Borne F, Ratouchniak J, Bonnefoy V. Genetic transfer of IncP, IncQ and IncW plasmids to four Thiobacillus ferrooxidans strains by conjugation. Hydrometallurgy. 2001; 59: 339–345. [Google Scholar]
- 14.Sakai H, Komano T. DNA replication of IncQ broad-host-range plasmids in Gram-negative bacteria. Biosci Biotechnol Biochem. 1996; 60: 377–382. [DOI] [PubMed] [Google Scholar]
- 15.Rawlings DE, Tietze E. Comparative biology of IncQ and IncQ-like plasmids. Microbiol Mol Biol Rev. 2001; 65: 481–496. doi: 10.1128/MMBR.65.4.481-496.2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frey J, Bagdasarian M. The molecular biology of IncQ plasmids In: Thomas C, editor. Promiscuous plasmids of Gram-negative bacteria: New York: Academic Press; 1989; pp. 79–94. [Google Scholar]
- 17.Gormley EP, Davies J. Transfer of plasmid RSF1010 by conjugation from Escherichia coli to Streptomyces lividans and Mycobacterium smegmatis. J Bacteriol. 1991; 173: 6705–6708. 1657866 [Google Scholar]
- 18.Maeser S, Scholz P., Otto S, Scherzinger E.. Gene F of plasmid RSF1010 codes for a low-molecular-weight repressor protein that autoregulates expression of the repAC operon. Nucleic Acids Research. 1990; 18: 6215–6222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Honda Y, Sakai H, Hiasa H, Tanaka K, Komano T, Bagdasarian M. Functional division and reconstruction of a plasmid replication origin: Molecular dissection of the oriV of the broad-host-range plasmid RSF1010. Proc Nati Acad Sci. 1991; 88: 179–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scholz P, Haring V, Wittmann-Liebold B, Ashman K, Bagdasarian M, Scherzinger E. Complete nucleotide sequence and gene organization of the broad-host-range plasmid RSF1010. Gene. 1989; 75: 271–288. [DOI] [PubMed] [Google Scholar]
- 21.Chu G, Hayakawa H, Berg P. Electroporation for the efficient transfection of mammalian cells with DNA. Nucleic Acids Res. 1987; 15: 1311–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Calvin NM, Hanawalt PC. High-efficiency transformation of bacterial cells by electroporation. J Bacteriol. 1988; 170: 2796–2801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fromm M, Taylor L, Walbot V. Expression of genes transferred into monocot and dicot plant cells by electroporation. Proc Natl Acad Sci. 1985; 82: 5824–5828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simon JR, McEntee K. A rapid and efficient procedure for transformation of intact Saccharomyces cerevisiae by electroporation. Biochem Biophys Res Commun. 1989; 164: 1157–1164. [DOI] [PubMed] [Google Scholar]
- 25.Frey J, Bagdasarian MM, Bagdasarian M. Replication and copy number control of the broad-host-range plasmid RSF1010. Gene. 1992; 113: 101–106. [DOI] [PubMed] [Google Scholar]
- 26.Katashkina JI, Kuvaeva TM, Andreeva IG, Skorokhodova AY, Biryukova IV, Tokmakova IL, et al. Construction of stably maintained non-mobilizable derivatives of RSF1010 lacking all known elements essential for mobilization. BMC Biotechnol. 2007; 7:80 doi: 10.1186/1472-6750-7-80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bartz QR, Detroit, Mich. Process for obtaining chloramphenicol. Patent in United States. 1949.
- 28.Sutcliffe JG. Complete nucleotide sequence of the Escherichia coli plasmid pBR322. Cold Spring Harbor Symposia on Quantitative Biology. 1979; 43 Pt 1: 77–90. [DOI] [PubMed] [Google Scholar]
- 29.Lee C, Kim J, Shin SG, Hwang S. Absolute and relative QPCR quantification of plasmid copy number in Escherichia coli. J Biotechnol. 2006; 123(3):273–80. doi: 10.1016/j.jbiotec.2005.11.014 [DOI] [PubMed] [Google Scholar]
- 30.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 −ΔΔ C T method. Methods. 2001; 25: 402–408. doi: 10.1006/meth.2001.1262 [DOI] [PubMed] [Google Scholar]
- 31.Bagdasarian MM, Scholz P, Frey J, Bagdasarian M. Regulation of the rep operon expression of the broad-host-range plasmid RSF1010. Novick R, Levy S, editors: Cold Spring Harbor Laboratory; 1987. [Google Scholar]
- 32.Sambrook J, Fritsch EF, Maniatis T. Molecular cloning: a laboratory manual, 2nd ed.; Harbor CS, editor. New.York: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 33.Providenti MA, O'Brien JM, Ewing RJ, Paterson ES, Smith ML. The copy-number of plasmids and other genetic elements can be determined by SYBR-Green-based quantitative real-time PCR. J Microbiol Methods. 2006; 65 476–487. doi: 10.1016/j.mimet.2005.09.007 [DOI] [PubMed] [Google Scholar]
- 34.Simon R, Priefer U, Puhier A. A broad host range mobilization system for in vitro genetic engineering: transposon mutagenesis in Gram-negative bacteria. Nat Biotechnol. 1983; 1: 784–791. [Google Scholar]
- 35.Liu Y, Qi F, Lin J, Tian K, Yan W. Isolation and phylogenetic analysis of a moderately thermophilic acidophilic sulfur oxidizing bacterium. Acta Microbiol Sin. 2004; 44: 382–385. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper. The nucleotide sequences of pSDU1, pSDU1Δmob and pSDU1ΔmobΔorfEF have been deposited in NCBI GenBank database under accession numbers MF163397, MF163398, and MF163399, respectively.