Abstract
Background: Diet is a potentially modifiable risk factor in the development and progression of many diseases, and there is evidence that diet plays a role in COPD.
Objective: Evaluate the relationship between dietary intake and clinical characteristics of COPD in a large and well-characterized population of COPD patients and controls who were part of the ECLIPSE study.
Methods: Limited diet records were available from 2,167 participants at 8 time points over a 3-year period. Participants reported the amount they had consumed over the last 24 hours for 8 food categories. Intake of each food group was handled as a dichotomous variable (Yes/last 24 hours at any of the 8 follow-up points vs. No at all 8 points). These 2 groups were then compared using clinical outcome measures at the last available follow-up that included lung function, emphysema, 6-minute walk, St. George’s Respiratory Questionaire (SGRQ) scores, the change in these scores over a 3-year period, and inflammatory biomarkers. Multivariate models for each food group and each outcome measure were run to adjust for confounding factors of age, sex, body mass index (BMI), and smoking.
Results: Participants who demonstrated recent consumption of foods associated with a healthful diet, including fish, fruit, tea, and dairy products, had greater lung function measures and less decline over time, less emphysema and emphysema progression, greater 6-minute walk and SGRQ scores, and lower levels of certain inflammatory markers. Increasing the number of diet record time points that were included in the analysis improved ability to detect significant associations.
Conclusion: Diet as a possible modifiable risk factor in COPD continues to warrant investigation.
Keywords: COPD, diet
Introduction
Chronic obstructive pulmonary disease (COPD) is a leading cause of morbidity and mortality in the United States, and results in an economic and social burden that is both substantial and increasing.1 Diet is a potentially modifiable risk factor in the development and progression of most diseases, including lung disease. As COPD and lung disease are major public health issues, identifying low-cost, non-invasive strategies to prevent these diseases is of primary importance.
Observational data suggest that nutritionally-based interventions may improve overall lung health. 2-11 While the hypothesis that nutrient status could influence lung function is strongly supported in studies of general populations, much less is known about the impact of nutrient intake on lung function in individuals with existing lung disease. This could in part be due to the difficulties associated with obtaining accurate estimates of dietary intake in the study of diet-disease relationships.
The Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints (ECLIPSE) study includes data on intake of specific food items at 8 time points over 3 years in a large and extremely well-characterized cohort of individuals with COPD and controls. Using this data set, we sought to determine if intake of certain foods was related to lung function measures, emphysema, inflammatory biomarkers, 6-minute walk distance, and St. George’s Respiratory Questionnaire (SGRQ) scores in a COPD patient population. We also assessed the ability of multiple sources of diet history information to more effectively elucidate these relationships.
Methods
This analysis is based on data from participants enrolled in the ECLIPSE study. Complete details of ECLIPSE study participants and study design have been previously described. 12 Limited diet records were available for 2,167 individuals with COPD who provided information on intake of specific food items at 8 time points over a 3-year period. The time points were: baseline, 3 months, 6 months, 1 year, 1.5 years, 2 years, 2.5 years, and 3 years. The food items had been chosen previously for inclusion in the study based on their potential impact on metabolomics parameters. Participants reported the amount they had consumed, over the last 24 hours, of 8 food categories that included grapefruit, fish, bananas, cheese, coffee, Earl Grey tea, other caffeinated beverages, and alcohol. For analysis, the intake of each food group was handled as an any/none dichotomous variable (Yes in the last 24 hours at any of the eight follow-up points vs. No at all eight points).
The authors hypothesized that more frequent sampling of diet histories would provide substantially more information regarding the relationships with the outcome variables than diet histories that are obtained less frequently. To test this hypothesis, models were created that used the information obtained from only four visits (baseline, 1 year, 2 years, and 3 years) and only two visits (baseline and 3 years) in addition to the models that tested the intake of the food items over 8 study visits. Outcome variables were identified and included post-bronchodilator forced expiratory volume in one second (FEV1), post-bronchodilator FEV1 percent predicted, post-bronchodilator forced vital capacity (FVC), emphysema score (defined as the percentage of lung voxels below -950 Hounsfield units [HU]), 6-minute walk distance, total SGRQ score, serum fibrinogen, club cell secretory protein, C-reactive protein (CRP), total neutrophil count, surfactant protein D, and white blood cell count (WBC). All outcome variables were measured at the final 3-year visit except for club cell secretory protein, CRP, and surfactant protein D, which were measured at the 1-year visit. Additionally, the longitudinal nature of ECLIPSE allowed for assessment of the relationship between dietary intake and changes over time in post-bronchodilator FEV1 (annual rate of change in milliliters13), and the total change over 3 years in post- bronchodilator FEV1, post- bronchodilator percent predicted FEV1, post- bronchodilator FEV1/FVC ratio, emphysema score, 6-minute walk distance, and SGRQ scores. Outcomes were compared between the two groups (any/no intake) using multivariate models for each food group and each outcome to adjust for confounding factors of age, sex, BMI, and smoking. Comparisons of SGRQ scores and 6-minute walk were also adjusted for FEV1.
Results
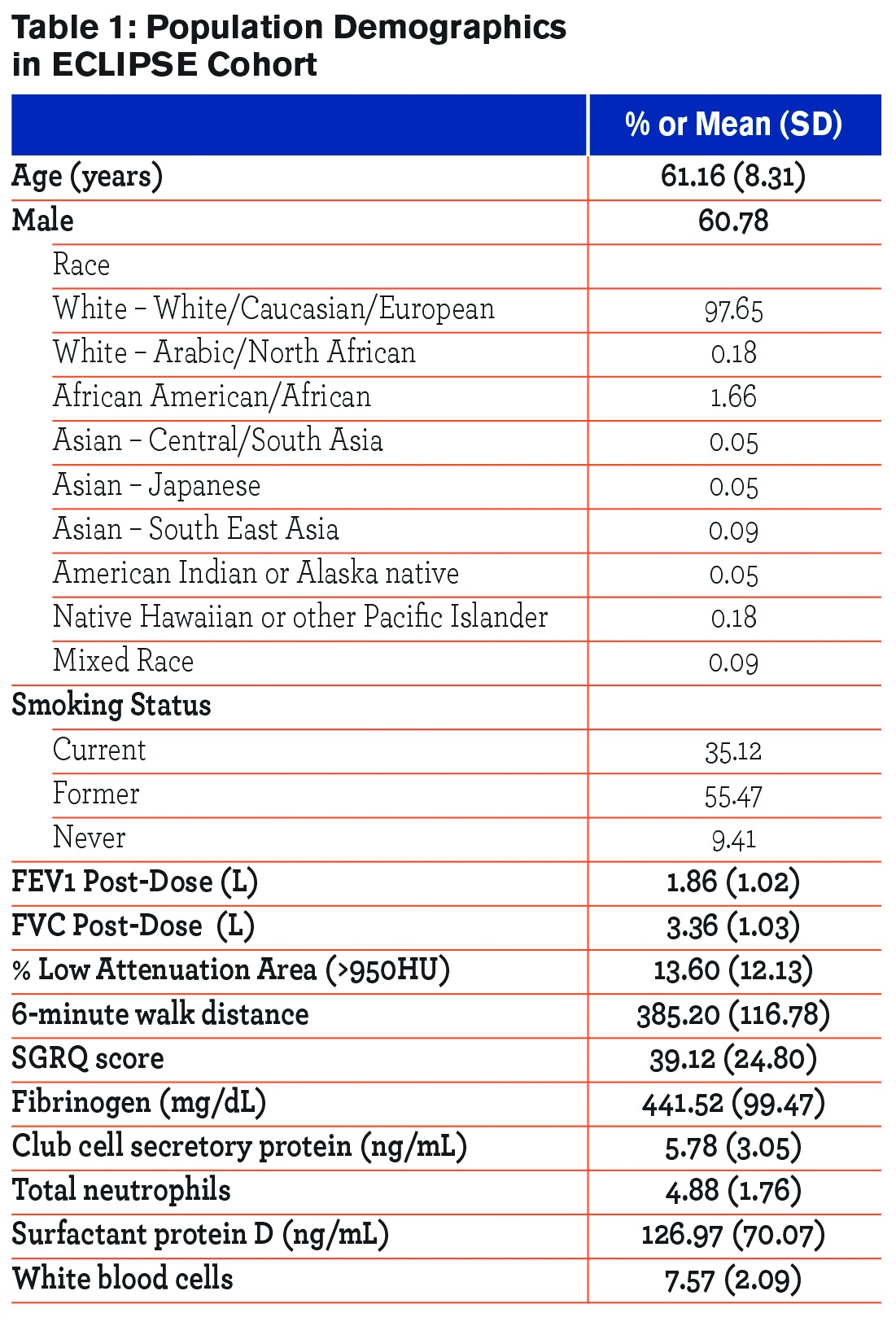
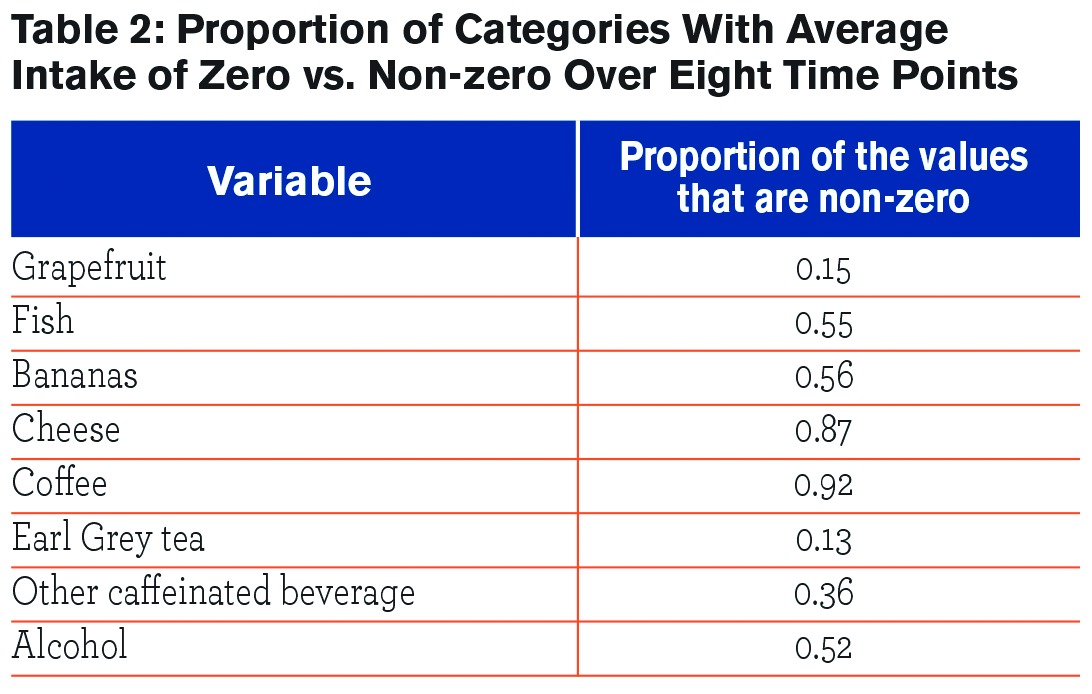
Demographic characteristics of the participants are given in Table 1. Table 2 provides the proportion of values for each food category that were non-zero, indicating the proportion of the population who consumed this food item at least once during the 8 time points that data were collected. Coffee had the highest proportion of non-zero values at 92%, meaning that 92% of participants had coffee intake for at least one of the time points. Earl Grey tea had the lowest proportion of non-zero values at 13%.
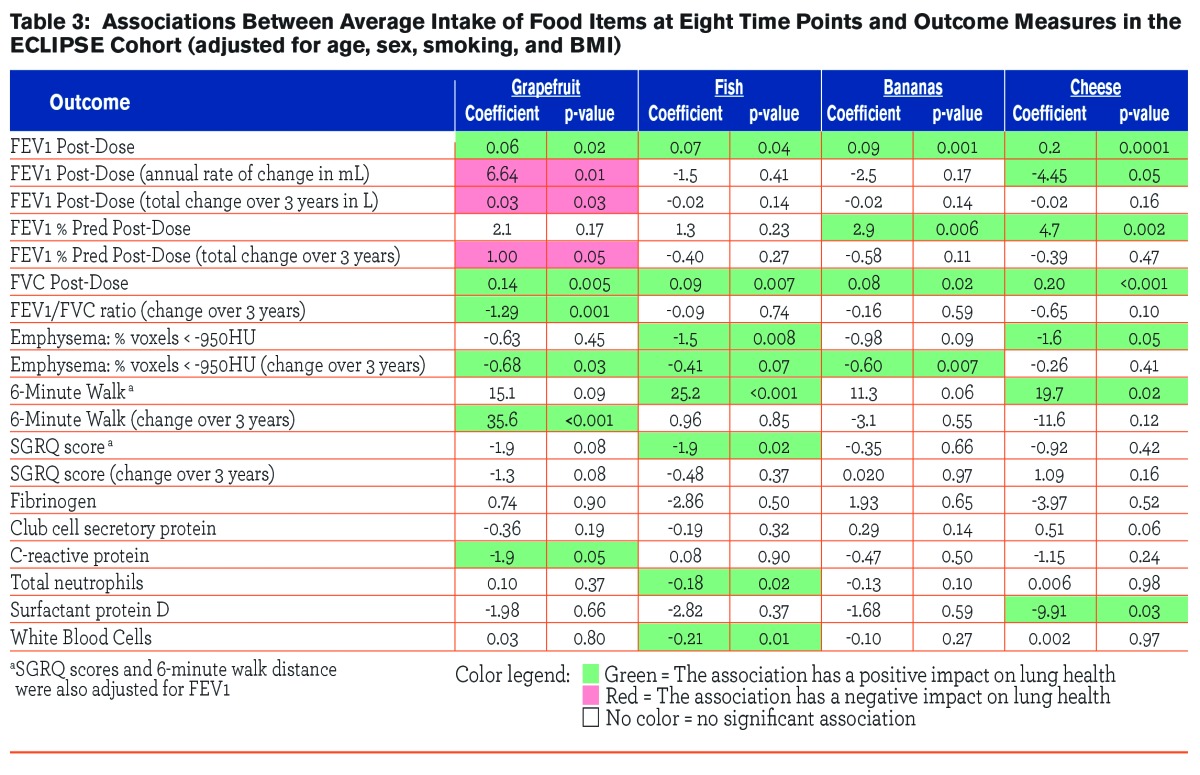
The observed associations between the outcome measures and intake of certain foods (grapefruit, fish, bananas and cheese) at 8 time points are given in Table 3. Intake of each of the food items showed significant and positive correlations with post-bronchodilator measurements of FEV1 and FVC. Bananas and cheese were correlated with post-bronchodilator FEV1 % predicted (β=2.9. p=0.02; β=0.20, p<0.001, respectively). Grapefruit was associated with the annual rate of decline in FEV1 (β=6.64, p=0.01), the total change in FEV1 over 3 years (β=0.03, p=0.03), and the change in % predicted FEV1 over 3 years (β=1.0, p=0.05). Grapefruit was also associated with the change over 3 years in post-bronchodilator FEV1/FVC ratio (β=-1.3, p=0.001). Fish (β=-1.5, p=0.008) and cheese (β=-1.6, p=0.05) were both inversely associated with emphysema score, meaning as intake of these food items increased, the percentage of voxels ˃950 HU decreased. Grapefruit and bananas were also inversely associated with the change in emphysema score over time (β=-0.68 and -0.60, p=0.03, and 0.007, respectively). Intake of fish and cheese were both also positively associated with 6-minute walk distance (β=25.2, p<0.001; β=19.7, p=0.02, respectively), while grapefruit intake was associated with the change in 6-minute walk distance over time (β=35.7, p˂0.001). Intake of fish was also associated with SGRQ score (β=-1.9, p=0.02). Intake of these food items demonstrated only minimal associations with the inflammatory biomarkers measured; intake of grapefruit was negatively associated with CRP levels (β=-1.9, p=0.05), fish intake was negatively associated with total neutrophils (β=-0.18, p=0.02) and WBC (β=-0.21, p=0.01), and cheese intake was negatively associated with surfactant protein D levels (β=-9.9, p=0.03).
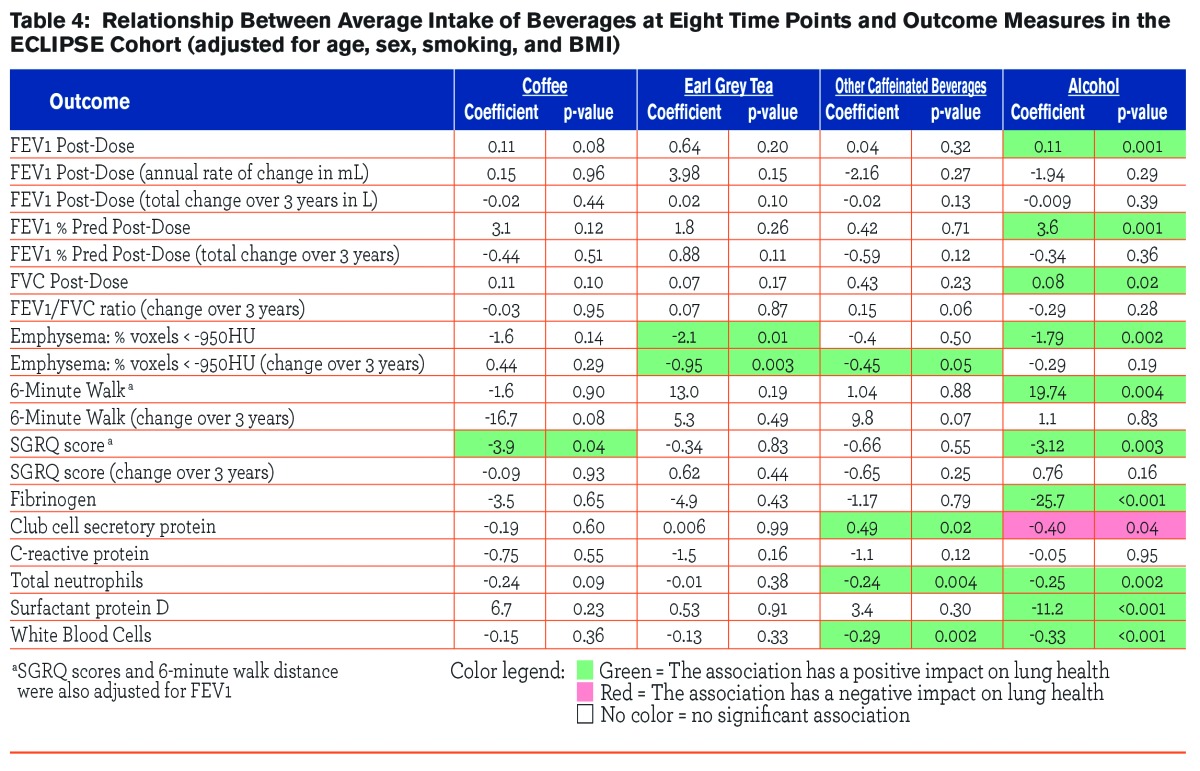
Observed associations between outcome measures and intake of beverages (coffee, tea, other caffeinated beverage, and alcohol) over 8 visits are given in Table 4. Intake of alcohol was associated with post-bronchodilator FEV1 (β=0.11, p=0.001), post-bronchodilator FVC (β=3.6, p=0.001), and post-bronchodilator FEV1 % predicted (β=0.08, p=0.02). Intake of tea and alcohol were both inversely associated with the emphysema score (β=-2.1, p=0.01, β=-1.79, p=0.002, respectively), and tea intake was also inversely associated with the change in emphysema score over time (β=-0.95, p=0.003). Coffee and alcohol intake were both inversely associated with the SGRQ score (β=-3.9, p=0.04, β=-3.1, p=0.003, respectively). Intake of alcohol demonstrated consistent, inverse associations with serum biomarkers, including fibrinogen (β=-25.7, p=<0.001), club cell secretory protein (β=-0.40, p=0.04), total neutrophils (β=-0.25, p=0.002), surfactant protein D (β=-11.2, p=<0.001), and WBC (β=-0.33, p=<0.001). Intake of other caffeinated beverages was also associated with serum biomarkers, specifically club cell secretory protein (β=0.49, p=0.02), total neutrophils (β=-0.024, p=0.004), and WBC (β=-0.29, p=0.002).
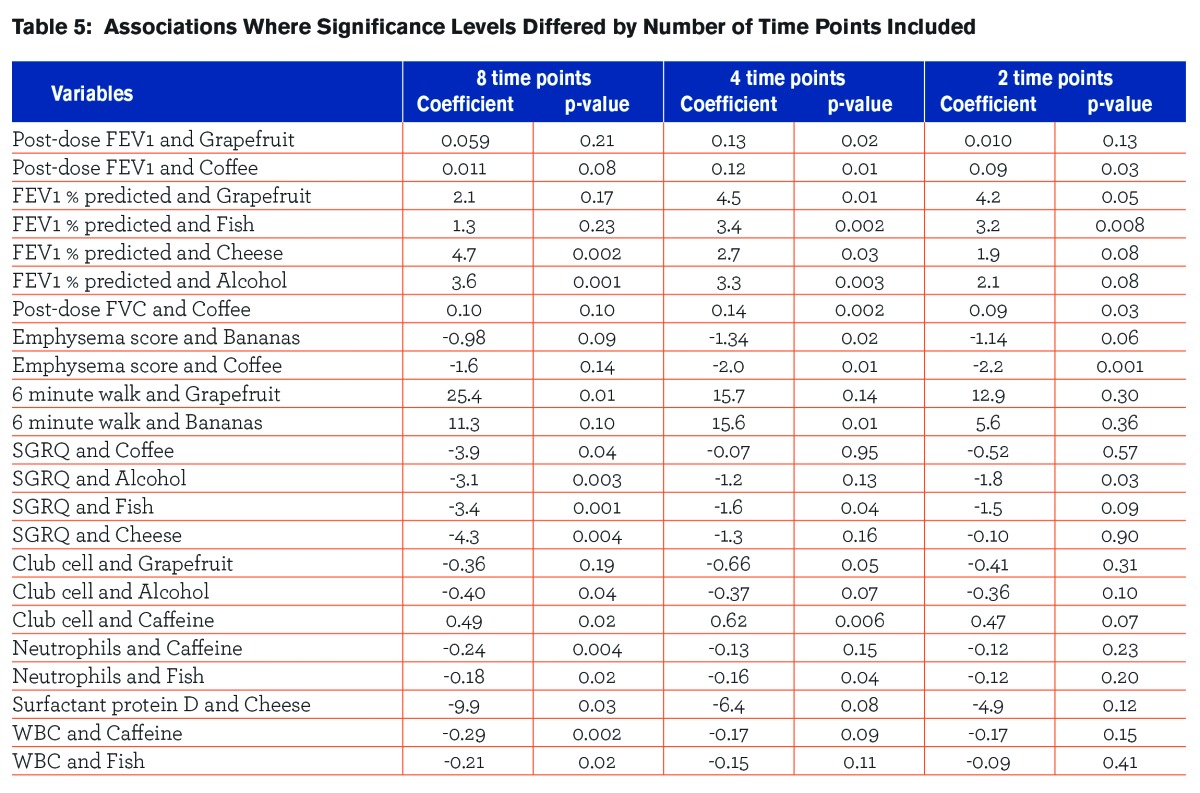
Table 5 shows the associations where the significance level of the relationships between dietary intake and outcomes changed when a different number of time points of dietary intake collection were used in the analysis.
Including an increasing number of time points of dietary intake collection in the analysis increased the number of statistically significant relationships that were found. There were a total of 14 significant associations observed when diet records collected at 8 time points were used in the analysis that were not seen when only 2 or 4 time points for diet intake collections were included. Conversely, there were 9 associations that were found to be significant when using either 2 or 4 time points for diet intake collections that were not seen when all 8 time points were included in the analysis. All 9 of these associations were significant when diet information was collected at 4 time points, but 4 lost significance when only 2 time points were included. Of the 14 associations that lost significance as less visits were included, 5 remained significant when 4 time points were included, but lost significance when only 2 visits were included. For all other associations, the attenuation of the measure of effect generally increased as the number of time points decreased. There was no association that held up across all 3 of the models (8,4, and 2 time points). The proportion of non-zero averages of intakes did not vary when different time points were included, suggesting that there was minimal seasonal variation in the intake of these specific food items.
Discussion
In this study, participants who demonstrated recent consumption of foods associated with a healthful diet, including fish, fruit, tea, and dairy products, had higher levels of lung function, less emphysema, greater 6-minute walk and SGRQ scores, and lower levels of certain inflammatory markers. An association between intake of certain foods and the progression of emphysema was also demonstrated.
Different strategies have been employed in various other studies to try to elucidate the relationship between diet and lung disease. Often the focus is on a single nutrient, and the relationship of either serum levels or intake levels of that nutrient are related to measures of lung function. However, nutrients do not occur in food in isolation; they occur simultaneously with many other compounds. It is possible that the effects associated with a certain nutrient are in fact due to another compound or group of compounds or the effects of multiple compounds could be additive, synergistic or interact in other ways. Similarly, food intake could be a marker for other associated behaviors. For this reason, studies investigating a dietary pattern including foods as part of a larger group have been undertaken. While the information available on the ECLIPSE participants for this analysis did not include information about overall diet pattern, it did capture what could be considered routine intake of certain food items that could be considered representative of habitual intake of foods of those types.
Among the strongest associations observed in this cohort were those between measures of lung function and intake of fruit, including either grapefruit or bananas. Intake of these food items was also associated with greater 6-minute walk and SGRQ scores. One of the most commonly reported associations in the epidemiological literature is the association between increased intake of fruit and improvement in lung function parameters. In one of the few randomized, controlled trials to evaluate this relationship, Keranis, et al, demonstrated that a shift to higher anti-oxidant foods, which was defined as an increased intake of fruits and vegetables, in a population of COPD patients followed for 3 years, showed an annual increase in % predicted FEV1, while those who were on a standard diet showed a decline.14 Prospective studies have also demonstrated a beneficial effect from fruit intake on COPD outcomes and lung function.15,16 In one such study, an inverse trend for 20-year COPD mortality was seen across tertiles of fruit intake15 with a 100 gram increase in fruit intake at baseline associated with a 24% lower COPD mortality risk. Intake of fruit has been shown to be lower in individuals with a diagnosis of COPD when compared to controls,17,18 and it has been speculated that intake of fruit may protect smokers from developing COPD.19 A unique study evaluating the impact of longitudinal change in fruit intake on lung function demonstrated fresh fruit consumption levels were positively associated with changes in FEV1, with a more marked fall in FEV1 in those individuals who had the greatest reduction in fruit intake.20 In cross-sectional studies, a high intake of fruit has consistently shown positive associations with lung function in multiple studies in different countries21-23,24 and in different age groups, including children.25 It is possible to speculate that the relationship between fruit intake and lung function has a dose-response relationship, given the results of a study in 2010 that showed a 132 ml difference in FEV1 between those eating fresh fruit less than once a month and those eating fruit daily.22
Another study has shown that while COPD patients were able to comply with an intervention to increase fruit intake, this increase had no effect on markers of airway or systemic oxidative stress and inflammation,26 however this intervention only took place over 12 weeks. In our study which collected data over 3 years, we were able to show inverse associations between grapefruit intake and CRP levels.
The intake of fish also demonstrated relationships with multiple outcome measures. Several other studies evaluating the impact of dietary patterns on lung function have found that a pattern including increased intakes of fruit and fish together have a beneficial effect on lung function. Analysis of the Seven Countries Study, a population-based cohort of 12,763 men, calculated that fruit and fish intake together explained about 67% of the variation in COPD mortality rates after 25 years.27 The beneficial effects of fish intake is thought to be primarily due to the anti-inflammatory effects of the types of oils present in fish, namely omega-3 fatty acids, and there have been several cross-sectional studies which show that increased fish intake as part of a diet pattern has beneficial effects on lung function.23,28 However, an equal number of studies have failed to find an association between fish intake and lung function. 2,16,22,24 In attempts to further elucidate this relationship, studies have been conducted investigating the individual fatty acid components thought to be partly responsible for the beneficial effects of fish intake. In one such study, an increased intake in omega-3 fatty acids did not appear to be protective against the development of COPD; however an increased intake of the more pro-inflammatory omega-6 fatty acids was associated with a reduction in FEV1, especially in smokers.3 In other studies, a higher intake of omega-3 fatty acids has been inversely associated with the risk of COPD in a quantity-dependent fashion.29
In our study, intake of cheese was associated with improved measures of FEV1, emphysema, and 6-minute walk distance. In this case, it is possible that some intake of cheese for at least 1 of these 8 time points serves as a surrogate marker for intake of dairy products. Dairy products contain vitamin D, which has been linked to lung function in some, 7,30 but not all studies,31 including a study by Shaheen, et al. In Shaheen’s study, intake of vitamin D was positively associated with FEV1, however, due to a lack of correlation between plasma 25(OH)D levels and lung function, the authors felt that the relationship between intake of vitamin D and lung function was likely explained by other highly correlated nutrients in the diet, such as antioxidants. Intake of dairy products, but not specifically vitamin D intake, has been associated with less severe measures of emphysema (defined by CT lung density) in 3,271 participants enrolled in the Multi-Ethnic Study of Atherosclerosis (MESA).32
Coffee intake in our study showed very little impact on parameters of COPD. The only positive association observed was an inverse association between coffee intake and SGRQ scores. Caffeine intake in other populations has been associated with increased measures of exercise endurance,33-37 and while it is possible that caffeine intake in the form of coffee could have increased perceived tolerance activity as measured by the SGRQ, we did not see any evidence of this in improvement in 6-minute walk scores. Similarly, intake of Earl Grey tea demonstrated an inverse association with the emphysema score as well as the change in emphysema score over 3 years. High intakes of black tea were shown in another study to be protective against the development of COPD in male smokers.19 Tea contains high quantities of antioxidants in the form of flavonoids, however, other studies which evaluated flavonoid intake have failed to find an association between tea intake and lung function.38 Additionally, our questionnaire addressed only Earl Grey tea, which only 13% of participants consumed at any time point, making tea intake a difficult topic for evaluation in this study.
Intake of alcoholic beverages showed the most significant associations of any of the food and beverage categories in our study. Intake of alcohol was positively associated with post-bronchodilator FEV1, FEV1 % predicted, FVC, and 6-minute walk distance. Alcohol intake was also inversely associated with emphysema score, SGRQ scores, fibrinogen, club cell secretory protein, total neutrophils, surfactant protein D, and WBC. It seems apparent from these results and others that alcohol intake has an effect on clinical parameters in individuals with COPD. The exact nature of this effect, however, remains undefined. Wine intake has been associated with better lung function in the general population, possibly due in part to resveratrol and other anti-inflammatory compounds found in wine.39 Our study did not specify the type of alcohol consumed as part of the intake collection. In direct contrast with our study, epidemiological evidence exists demonstrating that individuals with the lowest alcohol consumption had higher FEV1 values, lower risk of COPD, and a decreased prevalence of COPD symptoms.16,24 Alcohol intake can be extremely difficult to assess, as self-reported alcohol intake can be highly unreliable. A recent study using a blood marker of heavy alcohol consumption in addition to a validated alcohol questionnaire found that alcohol intake, especially heavy drinking, had an independent and additive negative effect on lung function in smokers.40
While there are many questionnaires to estimate food and nutrient intake, all have limitations. The method most commonly used in epidemiological studies is a food frequency questionnaire (FFQ). These questionnaires are considered representative of long-term intake, and can be useful in longitudinal studies as the ease of administration allows for repeated measurements. Limitations of FFQs include error in the estimated portion sizes of foods, inaccuracies resulting from incomplete listings of all possible foods, and the often complex estimation tasks required for analysis.41 Other diet intake assessment methods include 24-hour recalls and diet records. Twenty-four-hour recalls are relatively precise and can contain rich detail about the types and amounts of foods consumed. However, they may not reflect long-term intake and have been noted to have high variability.42 Diet records, such as 3-day diet dairies, can also be precise, however some bias is introduced due to the prospective nature, and high levels of motivation and literacy on the part of the participants are required for accurate data collection.41,43 In our study, only limited dietary intake information was collected as part of the parent study. However, information was collected repeatedly over 3 years, which allowed us to evaluate if increasing the number of times this type of dietary information was collected improved the ability of the tool to elucidate relationships with the disease outcomes. Our results show that this type of dietary intake information collected at 2 time points was not as effective at finding significant associations as when information was collected at either 4 or 8 time points; an outcome that is intuitively expected but worth confirming since fewer collection points would mean less work during data collection.
This study has several weaknesses that need to be acknowledged. The measuring instrument provided only limited information regarding nutrient intake. There are, however, repeated measures of intake of these food items over 3 years, which may represent habitual intake of certain food categories over time. Additionally, the data on diet intake was collected concurrently with clinical outcome measures. It is possible that results may not be truly representative of the participants’ usual or habitual intake and it is also possible that intake of certain foods may correlate with other lifestyle aspects, such as exercise, smoking, or the consumption of other food products. However, we were able to adjust for smoking and BMI in our regression models. This study does have a major strength in that objective measures of lung function, emphysema, functional and health status and biomarkers were available on participants, as opposed to self-reported symptoms or disease, which is often used in epidemiological literature.
Conclusion
The findings reported here suggest a role for diet in the prevention of lung disease and overall maintenance of lung function and health. Repeated diet collection intakes may be more likely to elucidate these relationships.
Abbreviations
St. George’s Respiratory Questionnaire, SGRQ; body mass index, BMI; Evaluation of COPD Longitudinally to Identify Predictive Surrogate Endpoints, ECLIPSE; forced expiratory volume in 1 second, FEV1; forced vital capacity, FVC; Hounsfield Units,<stronghu<>; C-reactive protein, CRP; white blood cell count, WBC, Multi-Ethnic Study of Atherosclerosis, MESA; Food Frequency Questionnaire, FFQ
Funding Statement
ECLIPSE study is funded by GlaxoSmithKline Clinical Trials.gov identifier NCT00292552 and GSK No. SCO104960
References
- 1. Vestbo J,Hurd SS,Agusti AG,et al. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease, GOLD executive summary. Am J Respir Crit Care Med. 2013;187(4):347-365. doi: http://dx.doi.org/10.1164/rccm.201204-0596PP [DOI] [PubMed] [Google Scholar]
- 2. McKeever TM,Lewis SA,Cassano PA,et al. Patterns of dietary intake and relation to respiratory disease, forced expiratory volume in 1 s, and decline in 5-y forced expiratory volume. Am J Clin Nutr. 2010;92(2):408-415. doi: http://dx.doi.org/10.3945/ajcn.2009.29021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKeever TM,Lewis SA,Cassano PA,et al. The relation between dietary intake of individual fatty acids, FEV1 and respiratory disease in dutch adults. Thorax. 2008;63(3):208-214. doi: http://dx.doi.org/10.1136/thx.2007.090399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. McKeever TM,Lewis SA,Smit HA,Burney P,Cassano PA,Britton J. A multivariate analysis of serum nutrient levels and lung function. Respir Res. 2008;967-967. doi: http://dx.doi.org/10.1186/1465-9921-9-67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McKeever TM,Scrivener S,Broadfield E,Jones Z,Britton J,Lewis SA. Prospective study of diet and decline in lung function in a general population. Am J Respir Crit Care Med. 2002;165(9):1299-1303. doi: http://dx.doi.org/10.1164/rccm.2109030 [DOI] [PubMed] [Google Scholar]
- 6. Hanson C,Rutten EP,Wouters EF,Rennard S. Diet and vitamin D as risk factors for lung impairment and COPD. Transl Res. 2013;162(4):219-236. doi: http://dx.doi.org/10.1016/j.trsl.2013.04.004 [DOI] [PubMed] [Google Scholar]
- 7. Berg I,Hanson C,Sayles H,et al. Vitamin D, vitamin D binding protein, lung function and structure in COPD. Respir Med. 2013;107(10):1578-1588. doi: http://dx.doi.org/10.1016/j.rmed.2013.05.010 [DOI] [PubMed] [Google Scholar]
- 8. Hu G,Cassano PA. Antioxidant nutrients and pulmonary function: The third National Health and Nutrition Examination Survey (NHANES III). Am J Epidemiol. 2000;151(10):975-981. doi: http://dx.doi.org/10.1093/oxfordjournals.aje.a010141 [DOI] [PubMed] [Google Scholar]
- 9. Hu G,Zhang X,Chen J,Peto R,Campbell TC,Cassano PA. Dietary vitamin C intake and lung function in rural China. Am J Epidemiol. 1998;148(6):594-599. doi: http://dx.doi.org/10.1093/oxfordjournals.aje.a009685 [DOI] [PubMed] [Google Scholar]
- 10. Schunemann HJ,Freudenheim JL,Grant BJ. Epidemiologic evidence linking antioxidant vitamins to pulmonary function and airway obstruction. Epidemiol Rev. 2001;23(2):248-267. doi: http://dx.doi.org/10.1093/oxfordjournals.epirev.a000805 [DOI] [PubMed] [Google Scholar]
- 11. Schunemann HJ,Grant BJ,Freudenheim JL,et al. The relation of serum levels of antioxidant vitamins C and E, retinol and carotenoids with pulmonary function in the general population. Am J Respir Crit Care Med. 2001;163(5):1246-1255. doi: http://dx.doi.org/10.1164/ajrccm.163.5.2007135 [DOI] [PubMed] [Google Scholar]
- 12. Agusti A,Calverley PM,Celli B,et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir Res. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Vestbo J,Edwards LD,Scanlon PD,et al. Changes in forced expiratory volume in 1 second over time in COPD. N Engl J Med. 2011;365(13):1184-1192. doi: http://dx.doi.org/10.1056/NEJMoa1105482 [DOI] [PubMed] [Google Scholar]
- 14. Keranis E,Makris D,Rodopoulou P,et al. Impact of dietary shift to higher-antioxidant foods in COPD: A randomised trial. Eur Respir J. 2010;36(4):774-780. doi: http://dx.doi.org/10.1183/09031936.00113809 [DOI] [PubMed] [Google Scholar]
- 15. Walda IC,Tabak C,Smit HA,et al. Diet and 20-year chronic obstructive pulmonary disease mortality in middle-aged men from three European countries. Eur J Clin Nutr. 2002;56(7):638-643. doi: http://dx.doi.org/10.1038/sj.ejcn.1601370 [DOI] [PubMed] [Google Scholar]
- 16. Miedema I,Feskens EJ,Heederik D,Kromhout D. Dietary determinants of long-term incidence of chronic nonspecific lung diseases. The Zutphen study. Am J Epidemiol. 1993;138(1):37-45. [DOI] [PubMed] [Google Scholar]
- 17. Hirayama F,Lee AH,Binns CW,et al. Do vegetables and fruits reduce the risk of chronic obstructive pulmonary disease? A case-control study in Japan. Prev Med. 2009;49(3):184-189. doi: http://dx.doi.org/10.1016/j.ypmed.2009.06.010 [DOI] [PubMed] [Google Scholar]
- 18. Watson L,Margetts B,Howarth P,Dorward M,Thompson R,Little P. The association between diet and chronic obstructive pulmonary disease in subjects selected from general practice. Eur Respir J. 2002;20(2):313-318. doi: http://dx.doi.org/10.1183/09031936.02.00256402 [DOI] [PubMed] [Google Scholar]
- 19. Celik F,Topcu F. Nutritional risk factors for the development of chronic obstructive pulmonary disease (COPD) in male smokers. Clin Nutr. 2006;25(6):955-961. doi: http://dx.doi.org/10.1016/j.clnu.2006.04.006 [DOI] [PubMed] [Google Scholar]
- 20. Carey IM,Strachan DP,Cook DG. Effects of changes in fresh fruit consumption on ventilatory function in healthy British adults. Am J Respir Crit Care Med. 1998;158(3):728-733. doi: http://dx.doi.org/10.1164/ajrccm.158.3.9712065 [DOI] [PubMed] [Google Scholar]
- 21. Tabak C,Smit HA,Rasanen L,et al. Dietary factors and pulmonary function: A cross sectional study in middle aged men from three European countries. Thorax. 1999;54(11):1021-1026. doi: http://dx.doi.org/10.1136/thx.54.11.1021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kelly Y,Sacker A,Marmot M. Nutrition and respiratory health in adults: Findings from the Health Survey for Scotland. Eur Respir J. 2003;21(4):664-671. doi: http://dx.doi.org/10.1183/09031936.03.00055702 [DOI] [PubMed] [Google Scholar]
- 23. Shaheen SO,Jameson KA,Syddall HE,et al. The relationship of dietary patterns with adult lung function and COPD. Eur Respir J. 2010;36(2):277-284. doi: http://dx.doi.org/10.1183/09031936.00114709 [DOI] [PubMed] [Google Scholar]
- 24. Tabak C,Smit HA,Heederik D,Ocke MC,Kromhout D. Diet and chronic obstructive pulmonary disease: Independent beneficial effects of fruits, whole grains, and alcohol (the MORGEN study). Clin Exp Allergy. 2001;31(5):747-755. doi: http://dx.doi.org/10.1046/j.1365-2222.2001.01064.x [DOI] [PubMed] [Google Scholar]
- 25. Cook DG,Carey IM,Whincup PH,et al. Effect of fresh fruit consumption on lung function and wheeze in children. Thorax. 1997;52(7):628-633. doi: http://dx.doi.org/10.1136/thx.52.7.628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Baldrick FR,Elborn JS,Woodside JV,et al. Effect of fruit and vegetable intake on oxidative stress and inflammation in COPD: A randomised controlled trial. Eur Respir J. 2012;39(6):1377-1384. doi: http://dx.doi.org/10.1183/09031936.00086011 [DOI] [PubMed] [Google Scholar]
- 27. Tabak C,Feskens EJ,Heederik D,Kromhout D,Menotti A,Blackburn HW. Fruit and fish consumption: A possible explanation for population differences in COPD mortality (the seven countries study). Eur J Clin Nutr. 1998;52(11):819-825. doi: http://dx.doi.org/10.1038/sj.ejcn.1600653 [DOI] [PubMed] [Google Scholar]
- 28. Schwartz J,Weiss ST. The relationship of dietary fish intake to level of pulmonary function in the first National Health and Nutrition Survey (NHANES I). Eur Respir J. 1994;7(10):1821-1824. doi: http://dx.doi.org/10.1183/09031936.94.07101821 [DOI] [PubMed] [Google Scholar]
- 29. Shahar E,Folsom AR,Melnick SL,et al. Dietary n-3 polyunsaturated fatty acids and smoking-related chronic obstructive pulmonary disease. N Engl J Med. 1994;331(4):228-233. doi: http://dx.doi.org/10.1056/NEJM199407283310403 [DOI] [PubMed] [Google Scholar]
- 30. Black PN,Scragg R. Relationship between serum 25-hydroxyvitamin d and pulmonary function in the third National Health and Nutrition Examination Survey. Chest. 2005;128(6):3792-3798. doi: http://dx.doi.org/10.1378/chest.128.6.3792 [DOI] [PubMed] [Google Scholar]
- 31. Shaheen SO,Jameson KA,Robinson SM,et al. Relationship of vitamin D status to adult lung function and COPD. Thorax. 2011;66(8):692-698. doi: http://dx.doi.org/10.1136/thx.2010.155234 [DOI] [PubMed] [Google Scholar]
- 32. Jiang R,Jacobs DR,He K,et al. Associations of dairy intake with CT lung density and lung function. J Am Coll Nutr. 2010;29(5):494-502. doi: http://dx.doi.org/10.1080/07315724.2010.10719886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Ryan EJ,Kim CH,Fickes EJ,et al. Caffeine gum and cycling performance: A timing study. J Strength Cond Res. 2013;27(1):259-264. doi: http://dx.doi.org/10.1519/JSC.0b013e3182541d03 [DOI] [PubMed] [Google Scholar]
- 34. Duncan MJ,Oxford SW. Acute caffeine ingestion enhances performance and dampens muscle pain following resistance exercise to failure. J Sports Med Phys Fitness. 2012;52(3):280-285. [PubMed] [Google Scholar]
- 35. Duncan MJ,Smith M,Cook K,James RS. The acute effect of a caffeine-containing energy drink on mood state, readiness to invest effort, and resistance exercise to failure. J Strength Cond Res. 2012;26(10):2858-2865. doi: http://dx.doi.org/10.1519/JSC.0b013e318241e124 [DOI] [PubMed] [Google Scholar]
- 36. Kilding AE,Overton C,Gleave J. Effects of caffeine, sodium bicarbonate, and their combined ingestion on high-intensity cycling performance. Int J Sport Nutr Exerc Metab. 2012;22(3):175-183. [DOI] [PubMed] [Google Scholar]
- 37. Laurence G,Wallman K,Guelfi K. Effects of caffeine on time trial performance in sedentary men. J Sports Sci. 2012;30(12):1235-1240. doi: http://dx.doi.org/10.1080/02640414.2012.693620 [DOI] [PubMed] [Google Scholar]
- 38. Tabak C,Arts IC,Smit HA,Heederik D,Kromhout D. Chronic obstructive pulmonary disease and intake of catechins, flavonols, and flavones: The MORGEN study. Am J Respir Crit Care Med. 2001;164(1):61-64. doi: http://dx.doi.org/10.1164/ajrccm.164.1.2010025 [DOI] [PubMed] [Google Scholar]
- 39. Siedlinski M,Boer JM,Smit HA,Postma DS,Boezen HM. Dietary factors and lung function in the general population: Wine and resveratrol intake. Eur Respir J. 2012;39(2):385-391. doi: http://dx.doi.org/10.1183/09031936.00184110 [DOI] [PubMed] [Google Scholar]
- 40. Frantz S,Wollmer P,Dencker M,Engstrom G,Nihlen U. Associations between lung function and alcohol consumption - assessed by both a questionnaire and a blood marker. Respir Med. 2014;108(1):114-121. doi: http://dx.doi.org/10.1016/j.rmed.2013.08.041 [DOI] [PubMed] [Google Scholar]
- 41. Willett W. Nutritional epidemiology. . Vol 15New York: (1990):396-396. [Google Scholar]
- 42. Dodd KW,Guenther PM,Freedman LS,et al. Statistical methods for estimating usual intake of nutrients and foods: a review of the theory. J Am Diet Assoc. 2006;106(10):1640-1650. doi: http://dx.doi.org/10.1016/j.jada.2006.07.011 [DOI] [PubMed] [Google Scholar]
- 43. Litonjua AA. Dietary factors and the development of asthma. Immunol Allergy Clin North Am. 2008;28(3):603-629, ix. doi: http://dx.doi.org/10.1016/j.iac.2008.03.005 [DOI] [PMC free article] [PubMed] [Google Scholar]