Abstract
Werner syndrome (WS) is a rare inheritable progeroid syndrome caused by a mutation in the WRN gene. Although WS has been described as a characteristic appearance of very slender extremities with a stocky trunk, few studies have investigated the loss of muscle mass, fat mass distribution (body composition), and mobility according to age and sex. Therefore, the aim of this study was to precisely describe the body composition in WS. Nine Japanese patients with WS (four males and five females; mean age 48±8.8 years) were recruited. Body composition was examined by dual-energy X-ray absorptiometry and computed tomography (CT). The hand grip strength and mobility were evaluated using the two-step test, stand-up test and 25-question geriatric locomotive function scale. The mean skeletal muscle index (SMI) was 4.0±0.6 kg/m. SMI of all patients met the criteria of sarcopenia, even though some patients were aged < 40 years. All patients also showed deceased mobility. In conclusion, these results indicate that all patients with WS, even those aged < 40 years, had already lost muscle mass to the level of sarcopenia. Continued research on sarcopenia in WS might facilitate the discovery of novel mechanisms and development of new treatment strategies for sarcopenia.
Keywords: sarcopenia, progeria, Werner syndrome
INTRODUCTION
Werner syndrome (WS) is a representative hereditary progeroid syndrome [1] with a high incidence in Japan [2]. It is frequently accompanied with metabolic disorders, such as diabetes and dyslipidemia [3, 4], vascular disease [5], and malignancy [6]. Patients with WS also show characteristic appearances of short stature, light body weight, and a so called bird-like face in which the nasal bridge appears to be pinched and the subcutaneous tissue is diminished [7]. Physicians with experience in treatment of WS may doubt the presence of the disease even if the patient presents with a characteristic bird-like face and a high pitched, squeaky, and/or hoarse voice. The skin of these patients is usually atrophic and tight. Clavus, callus, or intractable ulcers of the feet are also frequently observed [8]. Patients with WS have very slender extremities and have been described as a dried tree with a stocky trunk [7]. The appearance of these patients is sometimes referred to as Cushing or Klinefelter syndrome-like central obesity, although a few reports describe anomalies of muscle mass, fat mass distribution (body composition), and mobility, which differ according to age and sex. Therefore, the aim of this study was to precisely describe the body compositions of patients with WS.
RESULTS
Sarcopenia is a characteristic clinical feature of WS that appears before visceral fat accumulation
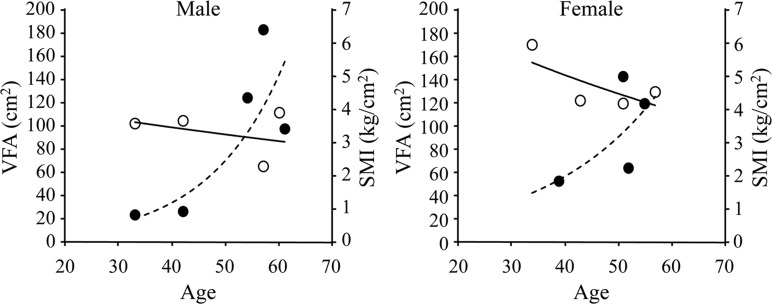
The basic characteristic of the patients is shown in Table 1. The body weight and body mass index (BMI) of all patients were low. According to the diagnostic criteria of the European Working Group on Sarcopenia in Older People [9], sarcopenia is diagnosed as hand grip strength and skeletal muscle index (SMI), as evaluated by dual-energy X-ray absorptiometry (DXA), of less than 26 kg and 7.0 kg/m2 for males and 18 kg and 5.4 kg/m2 for females, respectively. All patients were diagnosed with sarcopenia even though some were aged <40 years. SMI measurements were plotted against age (Figure 1). Although SMI usually decreases with age, patients with WS rapidly loose muscle mass and most of the participants in this study had already lost muscle mass to the level of sarcopenia. A graph of the accumulation of showed that sarcopenia seemed to appear before VFA accumulation.
Table 1. Basic patient characteristics.
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | Mean±SD |
|---|---|---|---|---|---|---|---|---|---|---|
| Age (year) | 56 | 60 | 40 | 60 | 39 | 51 | 42 | 43 | 41 | 48±8.8 |
| Sex | M | F | M | F | F | M | F | M | F | |
| mutation | 6/6 | 6/6 | 4/11 | 4/4 | 11/11 | 4/7 | 4/4 | 4/4 | 4/4 | |
| Height (cm) | 162.8 | 148 | 167.1 | 146.4 | 148.6 | 164 | 146.8 | 161.3 | 145.5 | 154.5±8.9 |
| BW (kg) | 46 | 40.8 | 48 | 43 | 33.6 | 51.8 | 31.4 | 43 | 36 | 41.5±6.8 |
| BMI (kg/m2) | 17.4 | 18.6 | 17.2 | 20.1 | 15.2 | 19.3 | 14.6 | 16.5 | 17.0 | 17.3±1.8 |
| SMI (kg/m2) | 4.5 | 3.9 | 5.1 | 3.4 | 3.3 | 4.2 | 3.7 | 4.3 | N/A | 4.0±0.6 |
| HGS (kg) | 26.3 | 11.4 | 29 | 6.9 | 13.2 | 19.3 | 14.4 | 17.8 | 12.6 | 16.6±7.2 |
| VFA (cm2) | 118.9 | 102.8 | 52.5 | 183.0 | 25.6 | 142.0 | 27.5 | N/A | N/A | 93.2±60.2 |
| BMD (L) (YAM) | 70 | 78 | 107 | 79 | 97 | 93 | 88 | 85 | N/A | 87.1±11.8 |
| BMD (F) (YAM) | 70 | 57 | 68 | 74 | 60 | 76 | 81 | N/A | 69.4±8.6 | |
| DM | - | + | - | + | - | + | - | - | + | |
| HT | - | + | + | - | - | + | - | - | - | |
| DL | + | + | + | - | - | + | - | - | - |
BW: body weight, BMI: body mass index, SMI: skeletal muscle index, HGS: hand grip strength, VFA: visceral fat area, BMD (L): bone mineral density (lumbar spine), BMD (F): bone mineral density, YAM: young adult mean, DM: diabetes mellitus, HT: hypertension, DL: dyslipidemia, SD: standard deviation
Figure 1. Correlations among skeletal muscle index (SMI), visceral fat area (VFA), and age.
Values for SMI and VFA were plotted against age. SMI is indicated by open circles and VFA is indicated by closed circles. Each line and the dotted line indicate an approximate curve.
WS patients exhibited lower mobility
According to the Japanese Orthopedic Association, any of the following criteria meet a diagnosis of decreased mobility [10]: two-step score < 1.1; difficulty in standing from a 20-cm-high seat using both legs in the stand-up test, or 25-question GLFS score ≥ 16. As shown in Table 2, scores of all patients with WS meet the criteria of impaired mobility. Correlation between body composi-tion and metabolic parameters was also investigated.
Table 2. The assessment of muscle strength and mobility in patients with WS.
| Patient number | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| Two-step test | 0.52 | 0.79 | 0.81 | 0.41 | 1.27 | N/A | 1.03 | N/A | 1.18 |
| Stand-up test | Bilateral 40cm |
Bilateral 30cm |
Bilateral 40cm |
N/A | One leg 40cm |
N/A | Bilateral 10cm |
N/A | One leg 40cm |
| 25-GLFS score | 73 | 22 | 29 | 63 | N/A | 54 | N/A | 57 | 53 |
For this purpose, patients were divided into two groups: those with and without diabetes. As shown in Table 3, patients with diabetes had a greater BMI and accumulation of VFA, while there was no difference in SMI between groups.
Table 3. Differences in clinical parameters among patients with WS with or without diabetes.
| Without diabetes | n | Diabetes | n | p value | |
|---|---|---|---|---|---|
| Age (year) | 44±6.9 | 5 | 53±9.1 | 4 | 0.16 |
| 25-question GLFS score | 40±31.7 | 4 | 43±18.8 | 4 | 0.88 |
| Two-step test value | 0.73±0.49 | 5 | 0.60±0.51 | 4 | 0.71 |
| Grip strength (kg) | 20.1±7.1 | 5 | 12.5±5.1 | 4 | 0.11 |
| VFA (cm2) | 56.1±43.6 | 4 | 142.6±40.1 | 3 | 0.04* |
| SMI (kg/m2) | 4.2±0.7 | 5 | 3.8±0.4 | 3 | 0.4 |
| BMD (L) (YAM) | 89.4±13.8 | 5 | 83.3±8.4 | 3 | 0.47 |
| BMD (F) (YAM) | 75.3±4.6 | 4 | 61.7±5.7 | 3 | 0.03* |
| BW (kg) | 40.4±7.5 | 5 | 42.9±6.6 | 4 | 0.61 |
| BMI (kg/m2) | 16.2±1.2 | 5 | 18.7±1.3 | 4 | 0.02* |
| Adiponectin (ng/mL) | 6.4±2.8 | 4 | 6.6±4.1 | 4 | 0.95 |
| TNF-α (pg/mL) | 1.4±0.6 | 4 | 3.0±4.3 | 4 | 0.51 |
| Leptin (ng/nL) | 7.2±3.6 | 4 | 30.0±16.9 | 4 | 0.07 |
GLFS: geriatric locomotive function scale, VFA: visceral fat area, SMI: skeletal muscle index, BMD (L): bone mineral density (lumbar spine), BMD (F): bone mineral density (femoral neck), YAM: young adult mean, BW: body weight BMI: body mass index, TNF: tumor necrosis factor, * p <0.05
DISCUSSION
This is the first report to precisely evaluate the body composition and mobility of patients with WS of different ages and both sexes. All patients met the criteria for sarcopenia even though some were aged <40 years and sarcopenia seemed to appear before the accumulation of VFA.
The dramatic increase in the number of elderly people is a significant public health concern, particularly in developed countries. As the number of elderly people continues to increase, sarcopenia, a progressive loss of muscle and strength, is certain to become a huge medical problem because the disease is associated with the deterioration of skeletal muscle function, which leads to disability, frailty, and increased morbidity and mortality [9, 11].
Although the underlying mechanism remains largely unknown, recent studies have revealed that sarcopenia is characterized by low physical activity [12]; poor nutrition, particularly with a low protein diet [13]; chronic low-grade systematic inflammation, known as “inflammaging” [14]; lipotoxicity [15]; dysfunction of neuromuscular junctions [16]; decreased blood concentrations of sex hormones [17] and 25(OH) vitamin D3 [18]; and stem cell aging [19].
WS, also known as adult progeria, is an autosomal recessive disorder caused by a mutation in the gene encoding RecQ DNA helicase [20]. Because some aging phenotypes accelerate WS, this disease has been used as a model of human aging. The results of this study confirmed that patients with WS aged <40 years have already lost skeletal muscle mass and fulfilled the diagnosis of sarcopenia. Loss of skeletal muscle mass usually begins in the fifth decade of life in the general population, while aging of the skeletal muscle is indeed accelerated in WS.
The underlying mechanism of sarcopenia in WS also remains incompletely understood. It has been reported that mammalian target of rapamycin (mTOR) signaling by fibroblasts is dysregulated in WS [21]. Because mTOR signaling is important for muscle hypertrophy, dysregulated mTOR signaling might play a role in sarcopenia development [22]. It has also been reported that mesenchymal stem cells, which are derived from pluripotent (iPS) cells, have a senescence phenotype in WS [23]. Therefore, it is also possible that satellite cells within the skeletal muscle, which are a type of stem cell, have an aging phenotype and low capacity for regeneration of the skeletal muscle. Using peripheral blood cells sample, it has recently been reported that WS is associated with intrinsic and extrinsic epigenetic age acceleration [24]. Therefore, accelerated epigenetic aging may result in accelerated sarcopenia seen in WS.
It is also intriguing that the sarcopenia seemed to appear before the accumulation of VFA. It has been reported that the accumulation of VFA seen in WS was related with metabolic disorders such as diabetes and dyslipidemia due to high insulin resistance [3, 25]. This is also true in our study, because WS with diabetes exhibited more VFA than WS without diabetes. Although we do not know the precise mechanisms by which accumulation of VFA was accelerated in WS, reduced physical activity due to the sarcopenia may affect accumulation of VFA.
Several interventions for sarcopenia have been reported, including diets rich in branched amino acids [26], exercise [27], and supplementation of vitamin D [28] as well as growth [29] and sex hormones [17]. Because WS is reportedly linked to a predisposition for malignancy, supplementation of growth and sex hormones might not be a good option for sarcopenia in WS. WS is highly associated with osteoporosis. It has recently been reported that femoral osteoporosis is more common in patients with WS [30]. Our results also indicated that the WS patients with diabetes seemed accelerated femoral osteoporosis than lumbar osteoporosis (Table 3). Although the mechanisms how osteoporosis is accelerated in WS have not been fully understood yet, it has been reported that osteoblastic bone formation was impaired in the patients [31]. Further analyses of the mechanism of osteoporosis seen in patients with WS may enable us to understand the mechanism of osteoporosis seen in normal aging. Nevertheless, patients with WS exhibit osteo-sarcopenic obesity; therefore, vitamin D supplementation may be a good option for treating osteoporosis and sarcopenia.
There were several limitations in this study that should be addressed. Although this study included patients of different ages and both sexes, this was cross-sectional study with a small number of patients. To overcome this problem, a registry of Japanese patients with WS was recently started by the Japan Agency for Medical Research and Development [http://www.m.chiba-u.jp/class/clin-cellbiol/werner/index.html]. A case of WS was also recently reported in a 17-year-old [32]. Hence, long-term follow-up in young patients with WS will allow further investigations of the precise natural history of WS.
In conclusion, WS is a human model of sarcopenia and continued research of sarcopenia in WS might facilitate the discovery of novel mechanisms and development of new treatment strategies for sarcopenia in WS as well as natural aging.
MATERIALS AND METHODS
Subjects
The study cohort included nine outpatients with WS who underwent treatment at the Chiba University Hospital (Chiba, Japan). The study protocol was approved by the ethics committee of Chiba University Hospital and was conducted in accordance with the ethical principles of the Declaration of Helsinki. All patients were informed of the study aims and methods and provided written, informed consent before participation in the study.
Genotyping
Genomic DNA was extracted from anonymized blood samples collected in tubes containing ethylene-diaminetetraacetic acid/disodium salt using the QIAamp DNA Blood Mini Kit. For genetic testing, exons were individually amplified as described elsewhere [20, 33] and then sequenced.
Measurement of body composition
Body composition was examined by dual-energy X-ray absorptiometry (DXA) [34]. The visceral fat area (VFA), as reference of abdominal obesity, was evaluated by computed tomography [35].
Assessment of muscle strength and mobility
Hand grip strength: Hand grip strength of the non-dominant hand was measured using a digital hand dynamometer (TKK5401; Takei Scientific Instruments, Niigata, Japan). The mean value of three measurements was used for analysis.
Two-step test: The two-step test was performed as previously described [36] as a measure of stride length to assess walking ability, muscle strength, balance, and flexibility. The two-step test score was calculated using the following formula: the length of two steps (cm)/height (cm).
Stand-up test: The stand-up test was also performed as previously described [37] as a measure of leg strength when standing up with one or both legs from a specified height of 10, 20, 30, and 40 cm. If the patient could stand without leaning back and maintain a standing posture for 3 s, then the patient was considered as having achieved that height level.
The 25-question geriatric locomotive function scale (GLFS): The 25-question GLFS was employed as described previously 38 and scored with a five-point scale, where a higher score indicated poorer locomotion.
Statistical analysis
Comparisons between two groups were conducted using Welch's t-test. A probability (p) value of <0.05 was considered statistically significant. All statistical analyses were performed using JMP pro12 software (SAS Institute Japan, Tokyo, Japan).
Acknowledgments
We wish to thank Mrs. Aki Watanabe and Mrs. Naoko Koizumi (Department of Clinical Cell Biology and Medicine, Chiba University Graduate School of Medicine) for their valuable technical assistance.
Abbreviations
- WS
Werner syndrome
- CT
computed tomography
- SMI
skeletal muscle index
- DXA
dual-energy X-ray absorptiometry
- mTOR
mammalian target of rapamycin
- VFA
visceral fat area
- GLFS
geriatric locomotive function scale.
Footnotes
CONFLICTS OF INTEREST
All authors have no conflict of interests.
FUNDING
This work was supported by the Health, Labour and Welfare Sciences Research Grants conducted by the Ministry of Health, Labour and Welfare for the Research on rare and intractable diseases and the Practical Research Project for Rare / Intractable Diseases from Japan Agency for Medical Research and development (AMED).
REFERENCES
- 1.Epstein CJ, Martin GM, Schultz AL, Motulsky AG. Werner's syndrome a review of its symptomatology, natural history, pathologic features, genetics and relationship to the natural aging process. Medicine (Baltimore) 1966;45:177–221. doi: 10.1097/00005792-196605000-00001. https://doi.org/10.1097/00005792-196605000-00001 [DOI] [PubMed] [Google Scholar]
- 2.Matsumoto T, Imamura O, Yamabe Y, Kuromitsu J, Tokutake Y, Shimamoto A, Suzuki N, Satoh M, Kitao S, Ichikawa K, Kataoka H, Sugawara K, Thomas W, et al. Mutation and haplotype analyses of the Werner's syndrome gene based on its genomic structure: genetic epidemiology in the Japanese population. Hum Genet. 1997;100:123–30. doi: 10.1007/s004390050477. https://doi.org/10.1007/s004390050477 [DOI] [PubMed] [Google Scholar]
- 3.Yokote K, Hara K, Mori S, Kadowaki T, Saito Y, Goto M. Dysadipocytokinemia in werner syndrome and its recovery by treatment with pioglitazone. Diabetes Care. 2004;27:2562–63. doi: 10.2337/diacare.27.10.2562. https://doi.org/10.2337/diacare.27.10.2562 [DOI] [PubMed] [Google Scholar]
- 4.Yokote K, Honjo S, Kobayashi K, Fujimoto M, Kawamura H, Mori S, Saito Y. Metabolic improvement and abdominal fat redistribution in Werner syndrome by pioglitazone. J Am Geriatr Soc. 2004;52:1582–83. doi: 10.1111/j.1532-5415.2004.52430_4.x. https://doi.org/10.1111/j.1532-5415.2004.52430_4.x [DOI] [PubMed] [Google Scholar]
- 5.Okabe E, Takemoto M, Onishi S, Ishikawa T, Ishibashi R, He P, Kobayashi K, Fujimoto M, Kawamura H, Yokote K. Incidence and characteristics of metabolic disorders and vascular complications in individuals with Werner syndrome in Japan. J Am Geriatr Soc. 2012;60:997–8. doi: 10.1111/j.1532-5415.2012.03944.x. https://doi.org/10.1111/j.1532-5415.2012.03944.x [DOI] [PubMed] [Google Scholar]
- 6.Ohnishi S, Fujimoto M, Oide T, Nakatani Y, Tsurutani Y, Koshizaka M, Mezawa M, Ishikawa T, Takemoto M, Yokote K. Primary lung cancer associated with Werner syndrome. Geriatr Gerontol Int. 2010;10:319–23. doi: 10.1111/j.1447-0594.2010.00638.x. https://doi.org/10.1111/j.1447-0594.2010.00638.x [DOI] [PubMed] [Google Scholar]
- 7.Takemoto M, Mori S, Kuzuya M, Yoshimoto S, Shimamoto A, Igarashi M, Tanaka Y, Miki T, Yokote K. Diagnostic criteria for Werner syndrome based on Japanese nationwide epidemiological survey. Geriatr Gerontol Int. 2013;13:475–81. doi: 10.1111/j.1447-0594.2012.00913.x. https://doi.org/10.1111/j.1447-0594.2012.00913.x [DOI] [PubMed] [Google Scholar]
- 8.Honjo S, Yokote K, Takada A, Maezawa Y, Kobayashi K, Tokuyama T, Sonezaki K, Saito Y. Etidronate ameliorates painful soft-tissue calcification in Werner syndrome. J Am Geriatr Soc. 2005;53:2038–39. doi: 10.1111/j.1532-5415.2005.00479_5.x. https://doi.org/10.1111/j.1532-5415.2005.00479_5.x [DOI] [PubMed] [Google Scholar]
- 9.Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinková E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older People Sarcopenia: European consensus on definition and diagnosis: Report of the European Working Group on Sarcopenia in Older People. Age Ageing. 2010;39:412–23. doi: 10.1093/ageing/afq034. https://doi.org/10.1093/ageing/afq034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yoshimura N, Muraki S, Oka H, Tanaka S, Ogata T, Kawaguchi H, Akune T, Nakamura K. Association between new indices in the locomotive syndrome risk test and decline in mobility: third survey of the ROAD study. J Orthop Sci. 2015;20:896–905. doi: 10.1007/s00776-015-0741-5. https://doi.org/10.1007/s00776-015-0741-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Beaudart C, McCloskey E, Bruyère O, Cesari M, Rolland Y, Rizzoli R, Araujo de Carvalho I, Amuthavalli Thiyagarajan J, Bautmans I, Bertière MC, Brandi ML, Al-Daghri NM, Burlet N, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr. 2016;16:170. doi: 10.1186/s12877-016-0349-4. https://doi.org/10.1186/s12877-016-0349-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kortebein P, Ferrando A, Lombeida J, Wolfe R, Evans WJ. Effect of 10 days of bed rest on skeletal muscle in healthy older adults. JAMA. 2007;297:1772–74. doi: 10.1001/jama.297.16.1772-b. https://doi.org/10.1001/jama.297.16.1772-b [DOI] [PubMed] [Google Scholar]
- 13.Deer RR, Volpi E. Protein intake and muscle function in older adults. Curr Opin Clin Nutr Metab Care. 2015;18:248–53. doi: 10.1097/MCO.0000000000000162. https://doi.org/10.1097/MCO.0000000000000162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franceschi C, Bonafè M, Valensin S, Olivieri F, De Luca M, Ottaviani E, De Benedictis G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann N Y Acad Sci. 2000;908:244–54. doi: 10.1111/j.1749-6632.2000.tb06651.x. https://doi.org/10.1111/j.1749-6632.2000.tb06651.x [DOI] [PubMed] [Google Scholar]
- 15.Addison O, Drummond MJ, LaStayo PC, Dibble LE, Wende AR, McClain DA, Marcus RL. Intramuscular fat and inflammation differ in older adults: the impact of frailty and inactivity. J Nutr Health Aging. 2014;18:532–38. doi: 10.1007/s12603-014-0019-1. https://doi.org/10.1007/s12603-014-0019-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pannérec A, Springer M, Migliavacca E, Ireland A, Piasecki M, Karaz S, Jacot G, Métairon S, Danenberg E, Raymond F, Descombes P, McPhee JS, Feige JN. A robust neuromuscular system protects rat and human skeletal muscle from sarcopenia. Aging (Albany NY) 2016;8:712–29. doi: 10.18632/aging.100926. https://doi.org/10.18632/aging.100926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morley JE, Malmstrom TK. Frailty, sarcopenia, and hormones. Endocrinol Metab Clin North Am. 2013;42:391–405. doi: 10.1016/j.ecl.2013.02.006. https://doi.org/10.1016/j.ecl.2013.02.006 [DOI] [PubMed] [Google Scholar]
- 18.Stein MS, Wark JD, Scherer SC, Walton SL, Chick P, Di Carlantonio M, Zajac JD, Flicker L. Falls relate to vitamin D and parathyroid hormone in an Australian nursing home and hostel. J Am Geriatr Soc. 1999;47:1195–201. doi: 10.1111/j.1532-5415.1999.tb05199.x. https://doi.org/10.1111/j.1532-5415.1999.tb05199.x [DOI] [PubMed] [Google Scholar]
- 19.Sousa-Victor P, Muñoz-Cánoves P. Regenerative decline of stem cells in sarcopenia. Mol Aspects Med. 2016;50:109–17. doi: 10.1016/j.mam.2016.02.002. https://doi.org/10.1016/j.mam.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 20.Yokote K, Chanprasert S, Lee L, Eirich K, Takemoto M, Watanabe A, Koizumi N, Lessel D, Mori T, Hisama FM, Ladd PD, Angle B, Baris H, et al. WRN Mutation Update: Mutation Spectrum, Patient Registries, and Translational Prospects. Hum Mutat. 2017;38:7–15. doi: 10.1002/humu.23128. https://doi.org/10.1002/humu.23128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Talaei F, van Praag VM, Henning RH. Hydrogen sulfide restores a normal morphological phenotype in Werner syndrome fibroblasts, attenuates oxidative damage and modulates mTOR pathway. Pharmacol Res. 2013;74:34–44. doi: 10.1016/j.phrs.2013.04.011. https://doi.org/10.1016/j.phrs.2013.04.011 [DOI] [PubMed] [Google Scholar]
- 22.von Walden F, Liu C, Aurigemma N, Nader GA. mTOR signaling regulates myotube hypertrophy by modulating protein synthesis, rDNA transcription, and chromatin remodeling. Am J Physiol Cell Physiol. 2016;311:C663–72. doi: 10.1152/ajpcell.00144.2016. https://doi.org/10.1152/ajpcell.00144.2016 [DOI] [PubMed] [Google Scholar]
- 23.Cheung HH, Liu X, Canterel-Thouennon L, Li L, Edmonson C, Rennert OM. Telomerase protects werner syndrome lineage-specific stem cells from premature aging. Stem Cell Reports. 2014;2:534–46. doi: 10.1016/j.stemcr.2014.02.006. https://doi.org/10.1016/j.stemcr.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maierhofer A, Flunkert J, Oshima J, Martin GM, Haaf T, Horvath S. Accelerated epigenetic aging in Werner syndrome. Aging (Albany NY) 2017;9:1143–52. doi: 10.18632/aging.101217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori S, Murano S, Yokote K, Takemoto M, Asaumi S, Take A, Saito Y. Enhanced intra-abdominal visceral fat accumulation in patients with Werner's syndrome. Int J Obes Relat Metab Disord. 2001;25:292–95. doi: 10.1038/sj.ijo.0801529. https://doi.org/10.1038/sj.ijo.0801529 [DOI] [PubMed] [Google Scholar]
- 26.Volpi E, Kobayashi H, Sheffield-Moore M, Mittendorfer B, Wolfe RR. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–58. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, Burggraf S, Pond A, Grim-Stieger M, Cvecka J, Sedliak M, Tirpáková V, Mayr W, Sarabon N, et al. Lifelong physical exercise delays age-associated skeletal muscle decline. J Gerontol A Biol Sci Med Sci. 2015;70:163–73. doi: 10.1093/gerona/glu006. https://doi.org/10.1093/gerona/glu006 [DOI] [PubMed] [Google Scholar]
- 28.Wintermeyer E, Ihle C, Ehnert S, Stöckle U, Ochs G, de Zwart P, Flesch I, Bahrs C, Nussler AK. Crucial Role of Vitamin D in the Musculoskeletal System. Nutrients. 2016;8:E319. doi: 10.3390/nu8060319. https://doi.org/10.3390/nu8060319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Giannoulis MG, Jackson N, Shojaee-Moradie F, Nair KS, Sonksen PH, Martin FC, Umpleby AM. The effects of growth hormone and/or testosterone on whole body protein kinetics and skeletal muscle gene expression in healthy elderly men: a randomized controlled trial. J Clin Endocrinol Metab. 2008;93:3066–74. doi: 10.1210/jc.2007-2695. https://doi.org/10.1210/jc.2007-2695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori S, Zhou H, Yamaga M, Takemoto M, Yokote K. Femoral osteoporosis is more common than lumbar osteoporosis in patients with Werner syndrome. Geriatr Gerontol Int. 2017;17:854–56. doi: 10.1111/ggi.12960. https://doi.org/10.1111/ggi.12960 [DOI] [PubMed] [Google Scholar]
- 31.Shiraki M, Aoki C, Goto M. Bone and calcium metabolism in Werner's syndrome. Endocr J. 1998;45:505–12. doi: 10.1507/endocrj.45.505. https://doi.org/10.1507/endocrj.45.505 [DOI] [PubMed] [Google Scholar]
- 32.Toda N, Ihara K, Takemoto M, Yokote K, Hara T. Endocrine and metabolic abnormalities in a girl with childhood Werner syndrome: case report. J Am Geriatr Soc. 2014;62:1404–05. doi: 10.1111/jgs.12897. https://doi.org/10.1111/jgs.12897 [DOI] [PubMed] [Google Scholar]
- 33.Yu CE, Oshima J, Wijsman EM, Nakura J, Miki T, Piussan C, Matthews S, Fu YH, Mulligan J, Martin GM, Schellenberg GD, Werner's Syndrome Collaborative Group Mutations in the consensus helicase domains of the Werner syndrome gene. Am J Hum Genet. 1997;60:330–41. [PMC free article] [PubMed] [Google Scholar]
- 34.Laskey MA. Dual-energy X-ray absorptiometry and body composition. Nutrition. 1996;12:45–51. doi: 10.1016/0899-9007(95)00017-8. https://doi.org/10.1016/0899-9007(95)00017-8 [DOI] [PubMed] [Google Scholar]
- 35.Tokunaga K, Matsuzawa Y, Ishikawa K, Tarui S. A novel technique for the determination of body fat by computed tomography. Int J Obes. 1983;7:437–45. [PubMed] [Google Scholar]
- 36.Ogata T, Muranaga S, Ishibashi H, Ohe T, Izumida R, Yoshimura N, Iwaya T, Nakamura K. Development of a screening program to assess motor function in the adult population: a cross‐sectional observational study. J Orthop Sci. 2015;20:888–95. doi: 10.1007/s00776-015-0737-1. https://doi.org/10.1007/s00776-015-0737-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Seichi A, Hoshino Y, Doi T, Akai M, Tobimatsu Y, Kita K, Iwaya T. Determination of the optimal cutoff time to use when screening elderly people for locomotive syndrome using the one‐leg standing test (with eyes open) J Orthop Sci. 2014;19:620–26. doi: 10.1007/s00776-014-0581-8. https://doi.org/10.1007/s00776-014-0581-8 [DOI] [PubMed] [Google Scholar]
- 38.Seichi A, Hoshino Y, Doi T, Akai M, Tobimatsu Y, Iwaya T. Development of a screening tool for risk of locomotive syndrome in the elderly: the 25‐question Geriatric Locomotive Function Scale. J Orthop Sci. 2012;17:163–72. doi: 10.1007/s00776-011-0193-5. https://doi.org/10.1007/s00776011-0193-5 [DOI] [PubMed] [Google Scholar]