Abstract
Cochlear implants (CI) restore functional hearing in the majority of deaf patients. Despite the tremendous success of these devices, some limitations remain. The bottleneck for optimal electrical stimulation with CI is caused by the anatomical gap between the electrode array and the auditory neurons in the inner ear. As a consequence, current devices are limited through 1) low frequency resolution, hence sub-optimal sound quality and 2), large stimulation currents, hence high energy consumption (responsible for significant battery costs and for impeding the development of fully implantable systems). A recently completed, multinational and interdisciplinary project called NANOCI aimed at overcoming current limitations by creating a gapless interface between auditory nerve fibers and the cochlear implant electrode array. This ambitious goal was achieved in vivo by neurotrophin-induced attraction of neurites through an intracochlear gel-nanomatrix onto a modified nanoCI electrode array located in the scala tympani of deafened guinea pigs. Functionally, the gapless interface led to lower stimulation thresholds and a larger dynamic range in vivo, and to reduced stimulation energy requirement (up to fivefold) in an in vitro model using auditory neurons cultured on multi-electrode arrays. In conclusion, the NANOCI project yielded proof of concept that a gapless interface between auditory neurons and cochlear implant electrode arrays is feasible. These findings may be of relevance for the development of future CI systems with better sound quality and performance and lower energy consumption. The present overview/review paper summarizes the NANOCI project history and highlights achievements of the individual work packages.
Keywords: Auditory nerve regeneration, BDNF, Cochlear implant, Gapless interface, Guinea pig, Hearing loss, Hydrogel, Multi-electrode array, Neuron–electrode interface
Cochlear implants (CI) have become the gold standard treatment for acquired and congenital deafness, allowing the restoration of functional hearing in the majority of recipients. As of today over 300,000 devices are in use. Despite the tremendous success of current systems, there is a substantial variability of performance across CI-users. In some cases, sound quality is poor for music and tonal languages, and users may struggle in noisy environments. The major bottleneck for optimized stimulation is the anatomical gap between the implanted electrode array and the stimulated regions of the auditory nerve. As a consequence of the gap and the associated current spread during a stimulus pulse, thousands of auditory neurons are stimulated from devices featuring only between 12 and 22 electrodes (depending on the manufacturer). Whereas more than eight independent channels are needed in challenging listening situations (1), CI users typically perform as if they have only about eight channels of information (2). The main reason for this is spread of excitation from the electrodes and interference across channels. Any attempt to increase channel numbers is limited by this significant “cross talk” of electrodes or overlapping electrical fields. Hence, the resolution and specificity of neuronal stimulation is low, which is the main reason for suboptimal sound quality and variability of speech and music perception. Additionally, the high amplitudes contribute to a high energy consumption associated with significant maintenance costs for batteries. Moreover, high energy consumption challenges the development of fully implantable systems, which would have several advantages such as increased usability for small children, invisibility, hearing while sleeping and better acceptance by patients.
It is generally acknowledged that eliminating the gap between neurons and the electrode array, and increasing the number of active channels, should greatly improve the effectiveness of auditory nerve stimulation (3).
The NANOCI project, therefore, was conceived with the aim to create a gapless man:machine interface in the cochlea by attracting the peripheral processes of the auditory nerve towards the electrode array (Fig. 1). Such a gapless interface could open the door for a higher frequency resolution of the CI system, because smaller groups of auditory neurons could be stimulated from a higher number of separate electrodes, coming closer to the situation in the “normal” human inner ear, where around 3,500 inner hair cells stimulate around 30,000 auditory neurons. In acoustic simulation studies for normal hearing subjects, a higher frequency resolution is associated with improved speech and music perception. In addition to this, the gapless interface has a high chance of cutting down energy consumption.
FIG. 1.
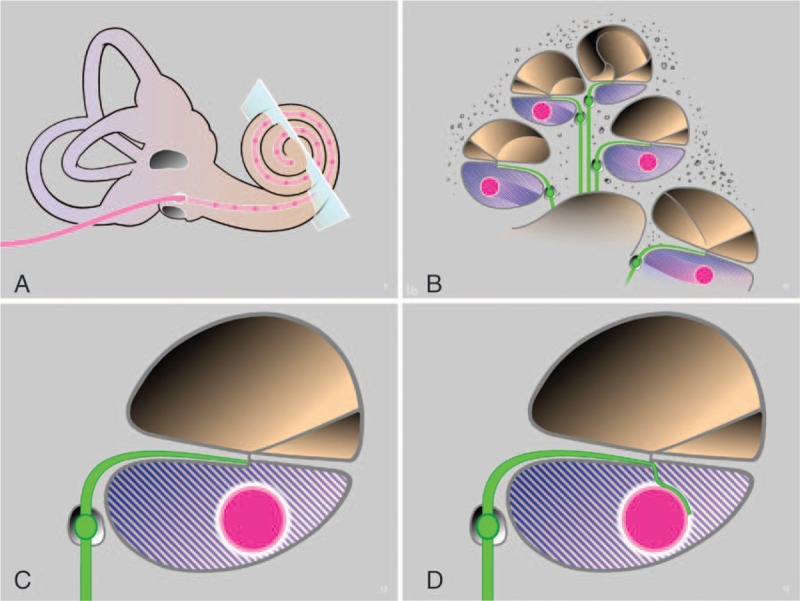
Schematic concept of the NANOCI project. Overview (A) and mid-modiolar cross-section through the cochlea with a CI electrode array inserted into the scala tympani (pink, B) at distance from the auditory neuron (green, B–D). By providing structural support, guidance, and attraction cues from an injected, functionalized nanomatrix (blue shaded fill, B–D) and from the modified electrode array surface (white coating of pink electrode array, C and D), a guided growth of the neurite onto the electrode surface is induced where a gapless nerve-CI interface is formed. CI indicates cochlear implants.
Supportive evidence for the feasibility of the NANOCI aim to recruit spiral ganglion neuron (SGN) processes to the CI can be found in the literature. Different neurotrophins have been reported to have prosurvival and guidance effects on SGNs. First, during embryonic development, auditory hair cells express the neurotrophin proteins brain-derived neurotrophic factor (BDNF) and neurotrophin-3, which direct growth of radial fiber bundles from the centrally localized spiral ganglion into the organ of Corti (4). Directed growth of nerve fibers can also be induced experimentally after infection of supporting cells with adenoviral vectors encoding for BDNF leading to its production in adult deafened guinea pigs (5). Similarly, BDNF in combination with fibroblast growth factor was capable of promoting survival and inducing re-sprouting of auditory neurons after experimentally induced deafness in adult guinea pigs (6). Moreover, these authors observed outgrowth of nerve fibers up to the border of the scala tympani, where the neurotrophins were released. The peripheral processes of the auditory neurons did not, however, enter the perilymph, possibly due to lack of structural support. In addition, the neurotrophic effect of BDNF can be enhanced if combined with electrical stimulation (7). Finally, in a recent animal study it was shown that ectopic nerve fibers observed in the cochlea in response to chronic neurotrophin delivery were associated with lower excitation thresholds and no increased spread of activation compared with deafened controls (8).
Importantly, specific neurotrophin receptors are expressed in the human cochlear neural tissue also in adulthood (9,10), making the treatment with exogenous neurotrophins such as BDNF by local intracochlear delivery system clinically highly relevant. Therefore, based on the literature it seemed clear that neurotrophins such as BDNF could be ideal candidates to induce and re-direct neurite outgrowth towards a modified CI electrode array acting as a source of delivery. Nevertheless, the fluid filled gap between the neurons and the surface of the CI electrode array would be likely to inhibit the regrowth. To overcome this limitation, we thought that a structural support should be offered, along with guidance and growth cues to recruit neurons to the array (Fig. 1).
For this challenging endeavor, expertize from different backgrounds was merged in an international and multicentric consortium (Table 1—overview of consortium partners and their key competences). The complex research project was subdivided into several smaller units (called work packages [WPs]) with defined goals and milestones, which are discussed along with the results obtained in more detail below. The main aim of the present review article is to summarize the historical background of the NANOCI project and to give an overview of its achievements.
TABLE 1.
List of NANOCI consortium partner institutions with key competences
| Role | Affiliation | Key Competences |
| Coordinator | University of Bern, Switzerland | Inner ear regeneration, human and murine bioassays, in vitro electrophysiology (multi-electrode arrays) |
| Partner 1 | University of Tübingen, Germany | In vivo electrophysiology, histology, murine bioassays |
| Partner 2 | University of Uppsala, Sweden | Human bioassays, time-lapse video |
| Partner 3 | University of Tampere, Finnland | Nanotoxicity assays in vitro, drug release studies, CB-CT and MRI imaging |
| Partner 4 | University of applied sciences, HES-SO, La Chaux-de-Fonds, Switzerland | Nanostructurization by solid on liquid deposition, irradiation; drug-elution solutions, modeling of release |
| Partner 5 | Bar-Ilan University, Ramat Gan, Israel | Nanostructurization by sonochemistry, bacteria bioassays, production of nanoparticles (e.g., carbon nanotubes) |
| Partner 6 | EMC Microcollections, GmbH, Tübingen, Germany | Production of BDNF mimetics, purification and functionalization of hydrogels |
| Partner 7 | MEDEL, Innsbruck, Austria | Production of animal- and human grade nanoCI prototypes with surface functionalizations; modelling |
| Partner 8 | Sciprom Sàrl, St-Sulpice, Switzerland | Project management, website design, organization of meetings |
BDNF, brain-derived neurotrophic factor; CB-CT, conebeam computed tomography; MRI, magnetic resonance imaging.
WP1—Nanomaterials and Bioactive Compounds for Improving the Interface
The main objectives of this work package were: 1) to develop a biocompatible and bio-functionalized, three-dimensional scaffold, or nanomatrix, to promote neural outgrowth of nerve fibers through the scala tympani onto the surface of the CI electrode pad, 2) to develop biomimetic analogues of BDNF, and 3) to introduce nano-surface modifications of the electrode contacts to stably lock neurons while improving the electrode conductive properties.
In the first place, commercially available fiber forming gel matrices were assessed. PuraMatrix™ was selected as the best candidate as it fulfilled the basic requirements of being injectable as solution, gelatinizing in response to ionic changes (i.e., contact with the perilymph) without swelling or mechanical stress to surrounding tissues. In addition, it had been shown to promote neurite growth (11).
Partner EMC microcollections GmbH (EMC) resynthesized the published gel forming PuraMatrix™ peptide sequence and additionally modified it with laminin-derived sequences for neuron adhesion. Additionally, linking strategies were included to biofunctionalize the nanomatrix with neurotrophic factors such as BDNF (12).
As outlined in the introduction, neurotrophic factors are important to mediate survival and sprouting of auditory neurons in vitro and in vivo. However, BDNF is not an ideal molecule for clinical inner ear applications because of its short in vivo half-life time. Small biomimetic compounds could offer an interesting way to solve this problem. However, to our knowledge, only few biomimetic analogues for BDNF have been published, and their effects on the inner ear neurons are as yet unknown (13). Partner EMC, therefore, set out to design and produce small molecule biomimetic compounds deduced from the different structural parts (loops) of the BDNF molecule with high stability, biocompatibility, and bioavailability in the inner ear. Promising candidates were tested in an in vitro bioassay using an auditory neuron explant assay. Results of this screen are currently prepared for publication.
For stable locking of auditory neurites on the platinum electrode surface and optimization of electrode conductivity, different nanofunctionalizations were developed and tested by partner Bar-Ilan University (BIU). From a pool of several candidates, hybrid nanocomposites of conductive polymers and multiwall carbon nanotubes were found to substantially roughen the platinum surface and thereby to improve conductivity of standard cochlear implant platinum pads in electrochemical measurements by partner MEDEL (14). In addition, these nanocomposites substantially reduced stimulation thresholds of auditory neuronal explant cultures on multi-electrode arrays compared with standard grey platinum surfaces.
WP2—Nanofunctionalization of the Electrode Array Surface
This work package was designed to structurally optimize the non-conductive silicone carrier surface of the CI for drug delivery and to implement antibacterial activity.
Partner HES-SO evaluated two different technologies for release of proteins from reservoirs on the surface. Firstly, the solid on liquid (SOLID) nanotechnology was evaluated. To this end, the silicone carrier of the electrode was further coated with a parylene layer containing microscale reservoirs with 60 μm holes created by laser (15,16). This allowed for release of fluorescent molecules (fluorescine), used as reporters in these studies. In addition, nanostructurization methods such as localized irradiations of Parylene-coated surfaces passed biocompatibility testing with an auditory neuron survival assay, suggesting that such technologies might be used for precise local etching of a parylene-coated surface to create wells or nanopores for controlled release of growth factors (17).
Finally, a simpler approach was evaluated, wherein fluorescein crystals, in powder form, were encapsulated in a thin layer of parylene, hence creating random nanometer-sized pinholes. This was found to be effective for controlled drug-release from the electrode surface (18). Therefore, the approach was selected, as it would facilitate the storage of proteins on the CI silicone surface in a solid form (powder), greatly improving handling and shipping properties.
To diminish the risk for infections and colonization of the electrode array, partner BIU further modified the non-conductive silicone parts with antimicrobial nanoparticles. ZnO-MgF2 and CuZnO were incorporated into the silicone array sonochemically. These nanoparticles proved to be stably locked on the surface with minimal or absent leaching of into artificial perilymph and with substantial inhibition of biofilm formation (19).
WP3—Modeling, Coding, and Information Transfer
This work package addressed the functional exploitation of the gapless electrode–auditory neuron interface and looked into optimized design of future cochlear implant systems with increased bi-directionality and higher channel numbers.
Interface-dependent stimulation patterns were studied using spiral ganglion neuron explant cultures on multi-electrode arrays (MEAs) by the University of Bern (20).
We were able to demonstrate a reduction in the energy needed to elicit a response as a function of the distance of the stimulating electrode to the neuronal culture. This proved the key hypothesis that the gapless interface allows for reduction of stimulation energy.
In addition, optimization of stimulation pulses using interphase gaps, trains of pulses, and long biphasic pulses was assessed. The latter were also shown to allow for a reduction of the energy needed for stimulation of SGNs (20). The close proximity of the electrodes to the neurons allows many electrodes to be active at the same time, thus imposing less strict temporal restriction on coding strategies compared with current CI systems. Based on this data, sound coding strategies for an implant featuring a gapless interface and more than 30 independent channels were modeled using a custom-made, multicompartment model of the auditory nerve by partner MEDEL (data not published).
To increase bi-directionality of future CI-systems, a Fabry–Perot fiber tip sensor was developed for measurement of temperature and pressure suitable for incorporation into the electrode array. The system includes a low-bending loss fiber equipped with a solid on liquid deposited terminal (21). The sensitivity of the system theoretically allows for the detection of contact of the tip with the surrounding structures in the cochlea, interesting for measuring insertion forces in robotic surgery, but also potentially for use as an implantable microphone or pressure sensor.
WP 4—Bioassays
A variety of bioassays were used in NANOCI to test growth factors, growth factor mimetics, nanomaterials, electrode surfaces, drug release, antibacterial activity, and stimulation patterns, among other entities. The partner University of Tübingen tested the injectable, peptide-based hydrogel developed by partner EMC for it's permissive properties to facilitate neurite growth towards the CI in a spiral ganglion explant assay (12). In addition, the partner University of Uppsala studied neural progenitor cell-derived spiral ganglion-like neurons (22). They also studied the regenerative capacity of human surgery-derived vestibular neurons (23) as well as the possibility of attracting and guiding these primary vestibular neurons in a 3-D nanomatrix (Li et al., Unpublished data, currently under revision). In addition, histological studies were undertaken to better understand the spiral ganglion anatomy and its relevance for cochlear implantation today as well as for potential future systems with a gapless interface to auditory neurons (24–26).
These results show, for the first time that the human auditory nerve has a regenerative capacity and that auditory neurons can grow through a 3-D nanomatrix. The histopathological studies also reveal potential underlying mechanisms that may allow human auditory neurons to, unlike their animal counterparts, persist after hair cell loss, making the use of cochlear implants possible.
WP5—Imaging, Biocompatibility, and Release Dynamics of the Modified CI Electrode
The aims of this work package were 1) to assess the neurotrophin release from hydrogels and from implant surfaces, 2) to measure and visualize the gap between the electrode array and the medial cochlear wall using cone-beam computed tomography (CBCT), and 3) to assess the biocompatibility and safety of the modified electrode array after coating with nanomaterials. The partner at the University of Tampere, in collaboration with others, mainly led this WP.
It was demonstrated that the release of neurotrophins or small molecules in a phantom model with artificial perilymph (data not published) corresponds to the in vivo distribution of labeled Gadolinium nanocomposites of TrkB-ligands in the rat cochlea after transtympanic injection, as measured by MRI (27,28). Based on these experiments, the inner ear distribution of neurotrophins released from the hydrogel and from the cochlear implant surface was predicted.
For the second aim, imaging parameters of cone-beam CT were optimized yielding higher resolution at lower irradiation doses. Cadaveric human temporal bones were implanted with different human grade cochlear implant electrode arrays (Fig. 2) and studied with the optimized CBCT settings for insertion depth, scalar location, and the distance of the array from the modiolar wall (29).
FIG. 2.
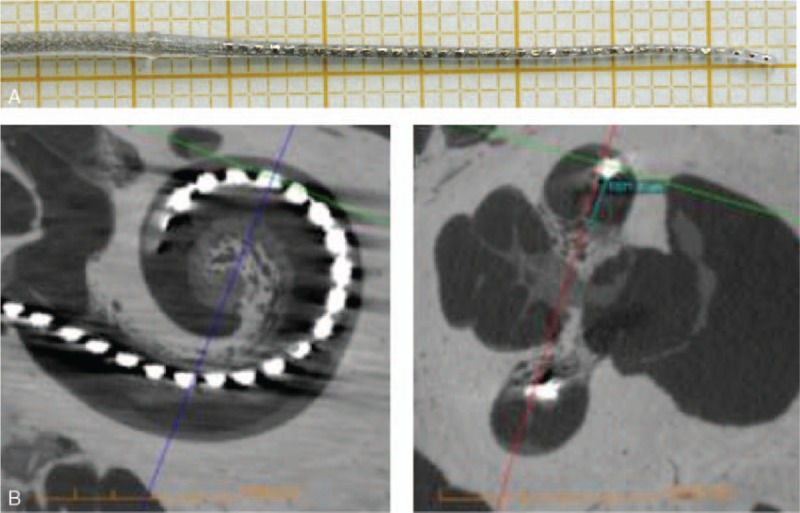
Prototype of the NANOCI electrode array featuring 36 electrodes on table (A) and after insertion into a cadaveric human temporal bone, as visualized through cone-beam computed tomography (B and C). This prototype yields proof of concept, that channel numbers can be tripled with today's manufacturing methods. No intracochlear damage and an intact basilar membrane were observed (D). However, due to increased stiffness of the device, a full insertion was not possible.
Finally, the biocompatibility and toxicity of nanotechnologically modified electrode components were evaluated in vitro using the BALB/c3T3 and NR8383 cell lines following the ISO 10993–5 and OECD GD-129 guidelines. No in vivo or specific ototoxicity tests were performed for the bulk of nanomaterial candidates and toxicity was predicted based on tissue equivalents at this stage. Some of the earlier candidates for antibacterial nanocoating in the project (ZnO and MgF2) were later abandoned due to toxicity in these assays. The nanocomposites used to coat the platinum electrode parts (multiwall carbon nanotubes) were found to be not toxic to BALB/c3T3 cells, while slightly decreasing viability of NR8383 cells (30).
WP6—Pilot, Animal Grade nanoCI Multicomponent Validation
The final goal of work package 6 was to develop an animal-grade prototype for in vivo testing incorporating the different nano- and bio-functionalization discussed above.
The partner MEDEL produced a 4-channel guinea pig electrode array (Fig. 3), which was then functionalized with different components. Namely 1) conductive polyTh copolymer (pEDOT)/oxidized multiwall carbon nanotubes bound on platinum contacts, 2) parylene-coated electrode array carrier doped with antifouling agent (CuZnO nanoparticles), and 3) 7,8,3-trihydroxyflavone, a BDNF analogue shown to act as a high affinity agonist for the TrkB receptor, deposited in solid state in the surface of the electrode array carrier.
FIG. 3.
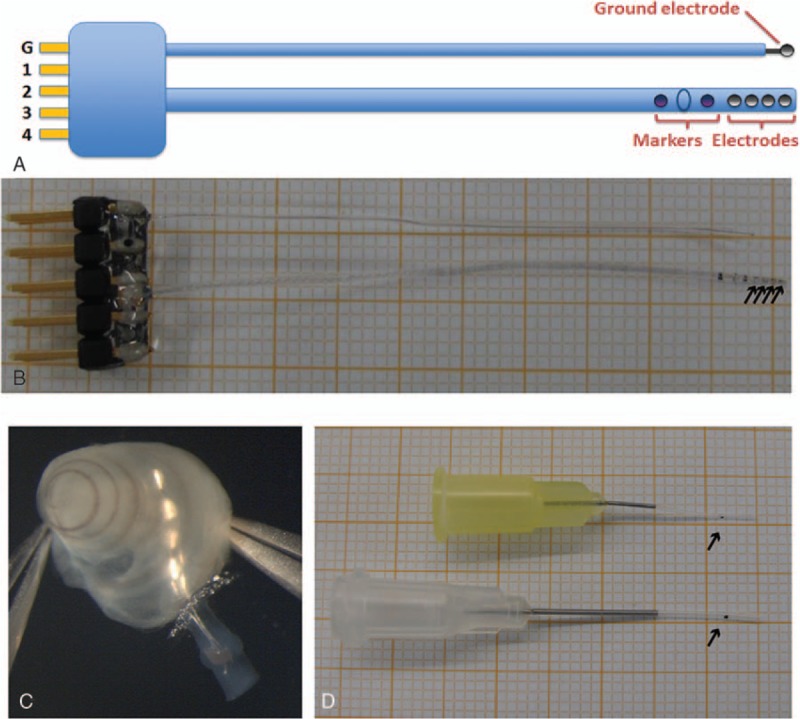
Specifications (A) and photographs of the prototype on table (B) and inserted into the cochlea (C) of the guinea pig cochlear implant with four electrodes (arrows in B). The length of the intracochlear array is 4 mm, as indicated with a stopper (blue oval) located between two dark markers at distance 3 and 5 mm from the tip for better estimate of achieved insertion depth. Catheters for intracochlear injection of the nanomatrix in fluidic form (D). The catheters were manufactured in two variants with different diameters of the silicone tubing (0.3 mm with yellow standard syringe needle and 0.6 mm with white standard syringe needle). The black dot indicates the 3-mm insertion depth as a reference point (arrows in D). CI indicates cochlear implants.
Furthermore, MEDEL produced a catheter for intracochlear application of the purified and laminin-loaded hydrogel (produced by EMC under WP2, Fig. 3D).
The multicomponent validation of these products included first the typical quality and performance testing at MEDEL, followed by in vivo experiments performed at the University of Tübingen using a deafened guinea pig animal model. Deafened guinea pigs were implanted with standard and modified guinea pig cochlear implant electrode arrays (Fig. 3) in combination with functionalized nanomatrix gels applied through the aforementioned catheter (Fig. 3D). The in vivo experiments yielded proof of principle that neurites can grow under neurotrophin stimulation and through structural support from the nanomatrix into the scala tympani and onto the surface of the electrode array, forming a gapless interface in the deafened and implanted cochlea (Fig. 4). Functionally, the gapless interface led to lower stimulation thresholds and a larger dynamic range in these animals in vivo and the detailed results are currently being prepared for publication.
FIG. 4.
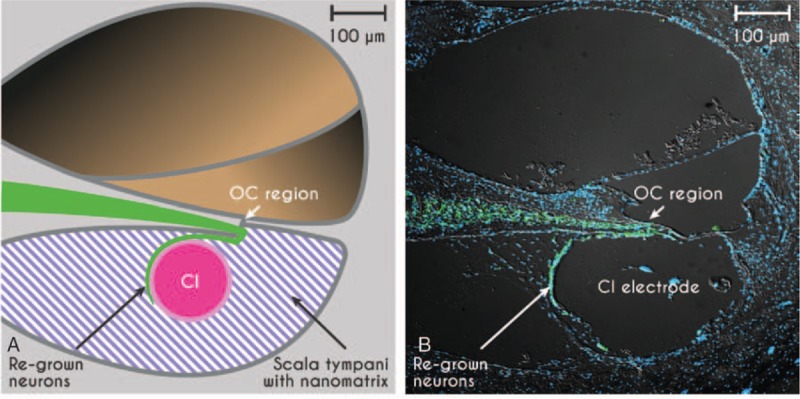
Proof of concept of the gapless interface between auditory neurons (green) and the CI-electrode array in scheme (A) and in the deafened guinea pig inner ear in vivo (B). Auditory neurons are stained with a neuron-specific marker for β-III tubulin (green, TUJ), cell nuclei are stained in blue (DAPI nuclear staining), photograph provided by Marcus Müller and Hubert Löwenheim. CI indicates cochlear implants.
Finally, MEDEL tested the limits of today's manufacturing technologies and built a human-grade prototype electrode array featuring 36 platinum contacts (Fig. 2A). The human-grade prototype electrode array was inserted into a cadaveric human temporal bone at the University of Tampere for imaging (and not used in vivo) (Fig. 2B). No intracochlear damage was observed (Fig. 2B and C), however due to increased stiffness related to the higher platinum contents of the array, it could not be fully inserted into the cochlea.
DISCUSSION AND CONCLUSION
In conclusion, the NANOCI project yielded proof of concept that a gapless interface between auditory neurons and cochlear implant electrode arrays is feasible in vivo. Functionally, the gapless interface led to lower stimulation thresholds and a larger dynamic range in vivo compared with untreated animals and to an up to fivefold reduction in required stimulation energy in an in vitro model using auditory neurons cultured on multi-electrode arrays. The relevance of this promising observation for potential future clinical applications remains unknown at this point in time. Particularly, it cannot be foreseen, how many regrown neurites will be functionally relevant and how many parallel channels of the cochlear implant can be effectively used for stimulation in the future. In addition, the total energy consumption of a future CI system that could be based on the NANOCI principle cannot be predicted based on our experiments, as many factors relevant to clinical CI systems were not taken into account in the experimental models. Several other issues such as safety and functional stability over time and beyond re-implantation procedures need to be seriously addressed. Nevertheless, we remain optimistic that some of the developments made during the NANOCI project may help to improve cochlear implant systems in the future in one way or another. It is important to note that all of the researchers experienced a substantial benefit from the interdisciplinary collaboration of the consortium partners involved in the project.
Acknowledgments
The authors would like to thank the following NANOCI collaborators for their valuable contributions: Emanuele Marconi, Anne Tscherter, Jürg Streit, Hans Ruedi Widmer, Stefano di Santo, Marija Boström, Sebastien Brun, Francois Feuvrier, Thierry Allen, Harry Withlow, Renate Spohn, Raphael Kiran, Roland Hessler, Frederik Klauser, Jochen Tillein, Giulia Mattini, Sören Schilp, Teresa Melchionna, Raimund Naschberger, Ingeborg Hochmair and Kirsten Leufgen. Further, they would like to thank Stefan Heller and Daniel Bodmer for being part of the advisory board and Silke Krol (project technical assistant) and Heico Frima (project officer) for their valuable work for the project at the EU. They would also like to thank Michal Natan for her contributions to this work.
Footnotes
M.M. and H.L. have equal contribution to the article.
Disclosure: The NANOCI-project (www.nanoci.org) was funded by the European Union under the grant agreement no. 281056 in the 7th frame work programme.
The authors disclose no conflicts of interest.
REFERENCES
- 1.Shannon RF, Fu QJ, Galvin J., 3rd The number of spectral channels required for speech recognition depends on the difficulty of the listening situation. Acta Otolaryngol Suppl 2004; 50–54. [DOI] [PubMed] [Google Scholar]
- 2.Friesen LS, Shannon RV, Baskent D, Wang X. Speech recognition in noise as a function of the number of spectral channels: comparison of acoustic hearing and cochlear implants. J Acoust Soc Am 2001; 110:1150–1163. [DOI] [PubMed] [Google Scholar]
- 3.Wilson BS, Dorman MF. Cochlear implants: current designs and future possibilities. J Rehab Res Dev 2008; 45:695–730. [DOI] [PubMed] [Google Scholar]
- 4.Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: their roles in sensory neuron survival and fiber guidance. Prog Brain Res 2004; 146:265–278. [DOI] [PubMed] [Google Scholar]
- 5.Shibata SB, Cortez SR, Beyer LA, et al. Transgenic BDNF induces nerve fiber regrowth into the auditory epithelium in deaf cochleae. Exp Neurol 2010; 223:464–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Glueckert R, Bitsche M, Miller JM, et al. Deafferentation-associated changes in afferent and efferent processes in the guinea pig cochlea and afferent regeneration with chronic intrascalar brain-derived neurotrophic factor and acidic fibroblast growth factor. J Comp Neurol 2008; 507:1602–1621. [DOI] [PubMed] [Google Scholar]
- 7.Shepherd RK, Coco A, Epp SB, Crook JM. Chronic depolarization enhances the trophic effects of brain-derived neurotrophic factor in rescuing auditory neurons following a sensorineural hearing loss. J Comp Neurol 2005; 486:145–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Landry TG, Fallon JB, Wise AK, Shepherd RK. Chronic neurotrophin delivery promotes ectopic neurite growth from the spiral ganglion of deafened cochleae without compromising the spatial selectivity of cochlear implants. J Comp Neurol 2013; 521:2818–2832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu W, Kinnefors A, Bostrom M, Rask-Andersen H. Expression of TrkB and BDNF in human cochlea-an immunohistochemical study. Cell Tissue Res 2011; 345:213–221. [DOI] [PubMed] [Google Scholar]
- 10.Liu W, Glueckert R, Kinnefors A, Schrott-Fischer A, Bitsche M, Rask-Andersen H. Distribution of P75 neurotrophin receptor in adult human cochlea--an immunohistochemical study. Cell Tissue Res 2012; 348:407–415. [DOI] [PubMed] [Google Scholar]
- 11.McGrath AM, Novikova LN, Novikov LN, Wiberg M. BD PuraMatrix peptide hydrogel seeded with Schwann cells for peripheral nerve regeneration. Brain Res Bull 2010; 83:207–213. [DOI] [PubMed] [Google Scholar]
- 12.Frick C, Muller M, Wank U, et al. Biofunctionalized peptide-based hydrogels provide permissive scaffolds to attract neurite outgrowth from spiral ganglion neurons. Colloids Surf B Biointerfaces 2016; 149:105–114. [DOI] [PubMed] [Google Scholar]
- 13.Massa SM, Yang T, Xie Y, et al. Small molecule BDNF mimetics activate TrkB signaling and prevent neuronal degeneration in rodents. J Clin Invest 2010; 120:1774–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ostrovsky S, Hahnewald S, Kiran R, et al. Conductive hybrid carbon nanotube (CNT)-polythiophene coatings for innovative auditory neuron-multi-electrode array interfacing. RCS Adv 2016; 6:41714–41723. [Google Scholar]
- 15.Charmet J, Banakh O, Laux E, et al. Solid on liquid deposition. Thin Solid Films 2010; 518:5061–5065. [Google Scholar]
- 16.Homsy A, Laux E, Jeandupeux L, et al. Solid on liquid deposition, a review of technological solutions. Microelectron Eng 2015; 141:267–279. [Google Scholar]
- 17.Whitlow H, Norarat R, Roccio M, et al. MeV ion beam lithography of biocompatible halogenated Parylenes using aperture masks. Nucl Instrum Methods Phys Res B 2015; 354:34–36. [Google Scholar]
- 18.Homsy A, Laux E, Brossard J, et al. Fine control of drug delivery for cochlear implant applications. Hear Balance Commun 2015; 13:153–159. [Google Scholar]
- 19.Natan M, Edin F, Perkas N, et al. Two are better than one: Combining ZnO and MgF2 nanoparticles reduces Streptococcus pneumoniae and Staphylococcus aureus biofilm formation on cochlear implants. Adv Funct Mater 2016; 26:2473–2481. [Google Scholar]
- 20.Hahnewald S, Marconi E, Streit J, et al. Response profiles of murine spiral ganglion neurons on multi-electrode arrays. J Neural Eng 2015; 13:016011. [DOI] [PubMed] [Google Scholar]
- 21.Llera M, Aellen T, Hervas J, et al. Liquid-air based Fabry-Perot cavity on fiber tip sensor. Optics Express 2016; 24:1–12. [DOI] [PubMed] [Google Scholar]
- 22.Edin F, Liu W, Bostrom M, Magnusson PU, Rask-Andersen H. Differentiation of human neural progenitor cell-derived spiral ganglion-like neurons: a time-lapse video study. Acta Otolaryngol 2014; 134:441–447. [DOI] [PubMed] [Google Scholar]
- 23.Edin F, Liu W, Li H, Atturo F, Magnusson PU, Rask-Andersen H. 3-D gel culture and time-lapse video microscopy of the human vestibular nerve. Acta Otolaryngol 2014; 134:1211–1218. [DOI] [PubMed] [Google Scholar]
- 24.Liu W, Edin F, Atturo F, et al. The pre- and post-somatic segments of the human type I spiral ganglion neurons—structural and functional considerations related to cochlear implantation. Neuroscience 2015; 284:470–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W, Glueckert R, Linthicum FH, et al. Possible role of gap junction intercellular channels and connexin 43 in satellite glial cells (SGCs) for preservation of human spiral ganglion neurons: A comparative study with clinical implications. Cell Tissue Res 2014; 355:267–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rask-Andersen H, Liu W. Auditory nerve preservation and regeneration in man: relevance for cochlear implantation. Neural Regen Res 2015; 10:710–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pyykko I, Zou J, Schrott-Fischer A, Glueckert R, Kinnunen P. An overview of nanoparticle based delivery for treatment of inner ear disorders. Methods Mol Biol 2016; 1427:363–415. [DOI] [PubMed] [Google Scholar]
- 28.Zou J, Ostrovsky S, Israel L, Lellouche J, Pyykkö I. Targeted delivery of contrast agents to the tympanic medial wall at minimum amount and the efficient uptake in the inner ear through oval and round windows. Austin J Radiol 2015; 2:1034. [Google Scholar]
- 29.Zou J, Hannula M, Lehto K, et al. X-ray microtomographic confirmation of the reliability of CBCT in identifying the scalar location of cochlear implant electrode after round window insertion. Hear Res 2015; 326:59–65. [DOI] [PubMed] [Google Scholar]
- 30.Mannerstrom M, Zou J, Toimela T, Pyykko I, Heinonen T. The applicability of conventional cytotoxicity assays to predict safety/toxicity of mesoporous silica nanoparticles, silver and gold nanoparticles and multi-walled carbon nanotubes. Toxicol In Vitro 2016; 37:113–120. [DOI] [PubMed] [Google Scholar]


