Abstract
Background—
Prospective data on the medium-term safety and effectiveness of the AMPLATZER Septal Occluder in clinical practice are not available. The objective of this study was to prospectively evaluate the risk of hemodynamic compromise and obtain medium-term survival data on patients implanted with the AMPLATZER Septal Occluder for percutaneous closure of secundum atrial septal defects.
Methods and Results—
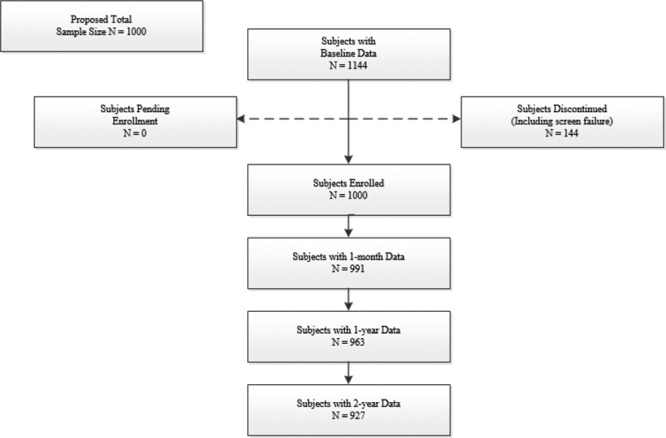
Subjects were enrolled prospectively at 50 US sites and followed for 2 years. Between 2008 and 2012, atrial septal defect closure with the AMPLATZER Septal Occluder was attempted in 1000 patients (aged 0.3–83.6 years, mean 21±22 years). Procedural closure occurred in 97.9%, with 1-month and 2-year closure 98.5% and 97.9%, respectively. Hemodynamic compromise occurred in 6 subjects (0.65%), because of dysrhythmia in 2, device embolization in 1, and cardiac erosion in 3. The rate of cardiac erosion was 0.3% (average 83, range 12–171 days from implant).
Conclusions—
Closure of atrial septal defect with the AMPLATZER Septal Occluder is safe and effective. The rate of hemodynamic compromise and cardiac erosion is rare. The risk factors for cardiac erosion after device closure are not yet clear.
Clinical Trial Registration—
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00650936.
Keywords: catheterization, echocardiography, heart, heart septal defects, risk factors
WHAT IS KNOWN
Prospective data on the medium-term safety and effectiveness of the ASO in clinical practice are not available.
There is concern about the rare occurrence of cardiac injury with hemodynamic compromise after ASO implantation.
WHAT THE STUDY ADDS
This is the largest prospective trial reporting the rate of hemodynamic compromise and medium-term survival data on patients after implantation of the ASO.
The rate of hemodynamic compromise after device placement is rare, lower in patients who received an appropriately sized device, and unrelated to implanting physician experience.
Identifying the risk factors for hemodynamic compromise and erosion requires further study.
The AMPLATZER Septal Occluder (ASO; St. Jude Medical [now Abbott], St. Paul, MN) is a self-expandable, double-disc, occlusion device made of Nitinol mesh, approved by the US Food and Drug Administration in 2001 for percutaneous closure of secundum atrial septal defect (ASD). Despite numerous studies reporting the overall safety and effectiveness of the ASO in small cohorts, there is concern about the rare occurrence of cardiac injury with hemodynamic compromise after ASO implantation.1 Initial study of these rare patients with hemodynamic compromise showed a potential association between the event and the sizing of the ASO at implant.2 Recommendations were made by an advisory panel in an attempt to decrease the rate of cardiac injury.3,4 Despite presumed adherence to these sizing recommendations, there is a persistent continued small risk of hemodynamic compromise after ASO implantation.1,5,6
As part of the Food and Drug Administration approval, a subset of the original cohort of ASO patients was to be followed for 5 years in an attempt to better understand the medium-term safety of the device. Follow-up of these patients was incomplete, and cases of hemodynamic compromise occurred outside of this patient subset, making the true rate of hemodynamic compromise unknown.5 This study is the largest prospective trial designed to evaluate the rate of hemodynamic compromise and obtain medium-term survival data on patients after implantation of the ASO.
Methods
The ASD PMS II (Closure of Atrial Septal Defects With the AMPLATZER Septal Occluder Post-Approval Study) is a prospective, nonrandomized, multicenter clinical study enrolling 1000 patients at 50 centers in the United States. Physicians previously certified to implant the ASO were considered for study participation. Patients were included in the study if they (1) had a secundum ASD that was indicated for closure, (2) were willing to perform the study follow-up requirements, and (3) signed the informed consent (self or legal representative). Patients were excluded from the study if they were known to have (1) complex congenital heart disease requiring surgical repair, (2) sepsis within 1 month before the implant procedure or any other systemic infection that could not be treated before the implant procedure, (3) a bleeding disorder, ulcer, or any contraindication to aspirin therapy, (4) echocardiographic evidence of intracardiac thrombi, (5) body size resulting in poor candidacy for cardiac catheterization, or (6) ASD rims of <5 mm from the coronary sinus, atrioventricular valves, or right upper pulmonary vein. Patients with fenestrated Fontan procedures or patent foramen ovale were not eligible. Study protocol and informed consent documents were approved by the Institutional Review Board of each study center.
Primary Objectives
To evaluate the risk of hemodynamic compromise in patients after receiving the ASO and to assess the safety and efficacy of the ASO and delivery system, patients were evaluated at baseline, implant, before discharge, and at 1 month, 1 year, and 2 years after implant.
Baseline Evaluation
A physical examination, ECG, and echocardiogram were completed no >6 months before device implant.
Device Implant Procedure
The device implant procedure was performed using standard technique as outlined in the ASO Instructions for Use (IFU). Balloon sizing using the stop-flow technique was required. Device size was recommended to be equal to the diameter of the defect determined by balloon sizing or 1 device size larger if identical device size was not available. Transthoracic echocardiography, transesophageal echocardiography (TEE), or intracardiac echocardiography (ICE) was performed to assess septal rims, balloon sizing, and stability of the device within the ASD. A physical examination, ECG, transthoracic echocardiogram, and adverse event assessment were performed before hospital discharge.
Clinical Follow-Up
At 1 month (25–60 days) post-implant, a physical examination, ECG, chest x-ray, and echocardiogram were performed. Complete echocardiographic closure was defined as a residual ASD shunt of ≤2 mm by color Doppler. At 1 (365±90 days) and 2 years (730±90 days) post-implant, an echocardiogram was required if successful ASD closure (≤2 mm shunt) was not documented on the 1-month study. If successful ASD closure was documented by the 1-month or 1-year follow-up, only an adverse event assessment was required at subsequent follow-up. Additional studies were performed if clinically indicated or if an adverse event was identified. Enrolled patients in whom a delivery system was placed in the body without ASO device implantation required only a physical examination and adverse event assessment at 1 month and an adverse event assessment at 1- and 2-year follow-up. Study center site monitoring was conducted by qualified research monitors to assure compliance with the protocol, data entry, and source documentation.
Adverse Event Assessment
Adverse events, if any, were reported at each of the follow-up visits. If an adverse event was reported by phone, patient evaluation at the study center was required with testing as indicated to allow for the investigator and independent medical advisor to evaluate the potential cause of the adverse event. Events were classified as (1) a procedure-related adverse event if related to the implant procedure; (2) a device-related adverse event if related to the presence or performance of the device; (3) a delivery system adverse event if caused by the presence or performance of any component of the delivery system; or (4) a cardiovascular adverse event if related to the cardiovascular system, including the heart, blood vessels, or circulation. Any events leading to hemodynamic compromise were reportable. Hemodynamic compromise was defined as any condition that resulted in shortness of breath and a decrease in systemic blood pressure that might result in patient collapse and death. Adverse events were adjudicated by an independent Medical Advisory Board.
Secondary Objectives
Device Size
To evaluate the association between device sizing and the risk of hemodynamic compromise, the size and location of the defect, the rim measurements, and the ASO size were collected in all patients. If an adverse event leading to hemodynamic compromise occurred, procedural device sizing was compared with the sizing criteria published in the IFU.
Physician Experience
The association between an implanter’s clinical volume and the risk of hemodynamic compromise was evaluated. Implanters were classified as high- or low-volume implanting physicians based on their historical number of implants performed before the study. If an adverse event leading to hemodynamic compromise occurred, the implanter’s clinical volume status was noted.
Statistics
The primary end point was the rate of hemodynamic compromise caused by the ASO device, delivery system, or implant procedure over the 2-year follow-up interval, which was determined by the number of patients with at least 1 event. In 2004, Amin et al2 published a rate of 0.1% for device erosion among patients receiving the ASO between 1998 and 2004. Because device erosion is only one of the causes of hemodynamic compromise, the primary end point in this study was tested against the performance goal (1.65%), derived from the sum of the erosion rate (0.1%) and a 1.55% noninferiority margin.2 Thus, the following null and alternative hypotheses were tested:
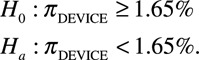 |
The event rate of hemodynamic compromise was estimated using exact binomial method. The null hypothesis was rejected if the upper bound of the 95% exact confidence interval of subjects who experienced a primary end point was less than the prespecified 1.65%. Patients were included in the analysis if an ASO delivery system entered the body. SAS statistical software package, Version 9.0 or higher, was used to provide all statistical analysis. A P value of <0.05 was considered statistically significant.
The study was powered based on the primary end point of hemodynamic compromise rate. Using a 1-sided type I error of 2.5%, and a conservative assumption of 0.5% for the rate of hemodynamic compromise, 800 patients were required to provide 80% power. The maximum of 1000 patients assumed that at least 80%, or 800 subjects, would be available for the entire follow-up period.
Device Size
Evaluation of procedural device sizing and compliance to sizing instructions in the IFU was performed. Rates of hemodynamic compromise were compared between patients with appropriately sized devices and those with under- or oversized devices. A survival analysis incorporating time-to-event data was performed.
Physician Experience
The relationship between physician experience with device placement and the rate of hemodynamic compromise was analyzed. Each implanting physician was classified based on the volume of ASO implants performed before the study. Physicians with ≥40 implants per year at the start of the trial were considered high-volume implanters, and those with 10 to 15 implants per year were grouped as low-volume implanters. The rates of hemodynamic compromise were compared between high- and low-volume implanter groups.
Results
Between March 2008 and May 2012, 1000 patients (aged 0.3–83.6 years, mean 21±22 years) were enrolled at 50 US sites and underwent attempted closure of secundum ASD with the ASO (Figure; Table 1). Defects were considered hemodynamically significant, with right ventricular volume overload in 97% and clinical symptoms of left-to-right shunt without right ventricular volume overload in 3% of patients. The average pulmonary to systemic flow ratio was 2.1±0.9. ASD device closure was always performed with echocardiographic guidance. Transesophageal echocardiography was used in 58% of total patients (68% of pediatric patients) and ICE in 36% of total patients (24% of pediatric patients). Transthoracic echocardiography was used in the remaining patients.
Table 1.
Baseline Clinical Characteristics
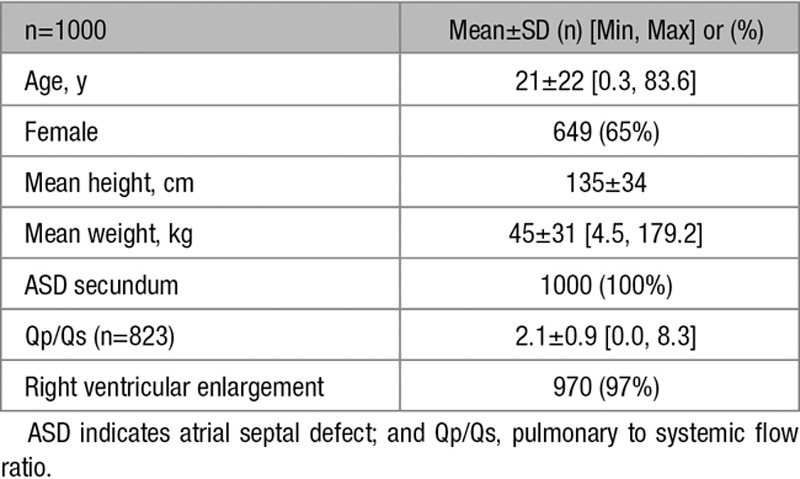
Figure.
Subject flow diagram.
Defect Characteristics
The average static ASD diameter was 14.5±6.2 mm (maximum 40 mm), and average stop-flow ASD diameter was 17.6±6.2 mm (maximum 41 mm). A deficient retroaortic rim (≤5 mm) was present in 11.5%, and deficient atrioventricular valve or caval rims were present in <3% of patients.
Primary End Points
Hemodynamic Compromise
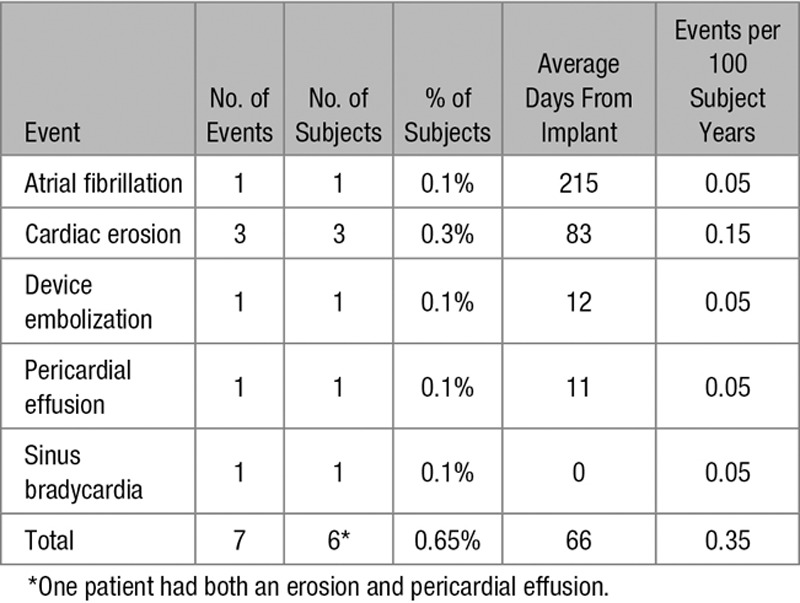
Seven episodes of hemodynamic compromise classified by the medical advisor as related to the ASO occurred in 6 of 928 evaluable subjects (0.65%) over the 2-year follow-up interval. The null hypothesis was rejected because the upper bound of the exact 95% confidence interval of the observed rate was 1.40%, less than the prespecified performance goal of 1.65%. A survival analysis was performed using Kaplan–Meier estimates, and similar results were found (0.65% with upper bound of the 95% confidence interval, 1.43%). The primary end point denominator of 928 includes 1 patient with a hemodynamic compromise event that was lost to follow-up at 415 days, before study completion (927 patients completed the 2-year follow-up). All of the hemodynamic compromise events occurred within 12 months of implant. Hemodynamic compromise was secondary to atrial fibrillation in 1 of 928 (0.1%), sinus bradycardia in 1 of 928 (0.1%), device embolization in 1 of 928 (0.1%), pericardial effusion in 1 of 928 (0.1%), and confirmed cardiac erosion in 3 of 928 (0.3%) patients (Table 2).
Table 2.
Hemodynamic Compromise Related to Device
Erosion
The rate of cardiac erosion was 0.3% and occurred at 12, 67, and 171 days from implant (average 83 days). The subjects with cardiac erosion were 14, 40, and 57 years old and had ASO sizes of 20, 22, and 26 mm. One patient with confirmed erosion had an ASD with deficient (<5 mm) aortic rim. The other 2 patients with erosion had sufficient aortic rim. No erosion events resulted in death.
Pericardial Effusion
An additional 31 patients were noted to have pericardial effusion treated by outpatient observation in 25 of 31 (81%), outpatient pharmacological therapy in 2 of 31 (6%), inpatient observation in 3 of 31 (10%), and inpatient pharmacological therapy plus pericardiocentesis in 1 of 31 (3%) patients. These cases of pericardial effusion were adjudicated by the Medical Advisory Board as not being caused by cardiac erosion. In 1 patient, a pericardial effusion was present on the precatheterization echocardiogram and was thought to be because of a preexisting condition.
Safety and Efficacy of the ASO and Delivery System
Closure was successful during the procedure in 97.9% of patients. Follow-up with transthoracic echocardiography was available in 99.1% at 1 month, 96.3% at 12 months, and 92.7% of patients at 24 months. A total of 927 patients completed 24-month follow-up. The percentage of patients with complete closure was 937 of 951 (98.5%) and 966 of 987 (97.9%) patients at 1-month and 2-year follow-up, respectively. The total number of patients with complete closure at 2-year follow-up included patients with complete closure noted at any time during the study.
A total of 56 device-related adverse events occurred in 46 of 1000 (4.6%) patients, including 11 of 1000 (1.1%) patients with atrial arrhythmia, 2 of 1000 (0.2%) patients with device embolization, and 19 of 1000 (1.9%) patients with migraine headaches (Table 3). No patient had pulmonary embolism or deep venous thrombosis. A total of 5 delivery system–related adverse events occurred in 5 of 1000 (0.5%) patients, including 2 of 1000 (0.2%) patients with atrial arrhythmias and 1 of 1000 (0.1%) patient each with device embolization, venous congestion, and thrombus (Table 4).
Table 3.
Device-Related Adverse Events
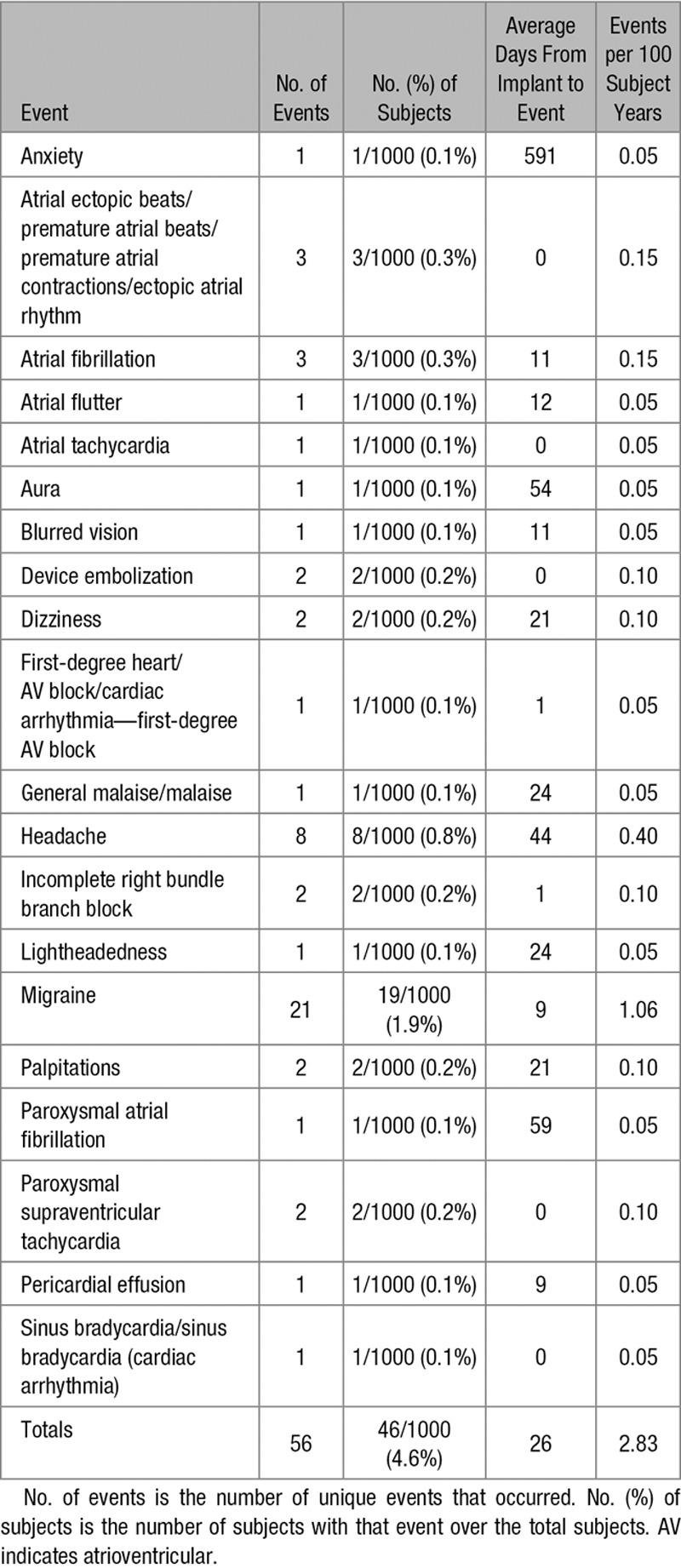
Table 4.
Delivery System–Related Adverse Events
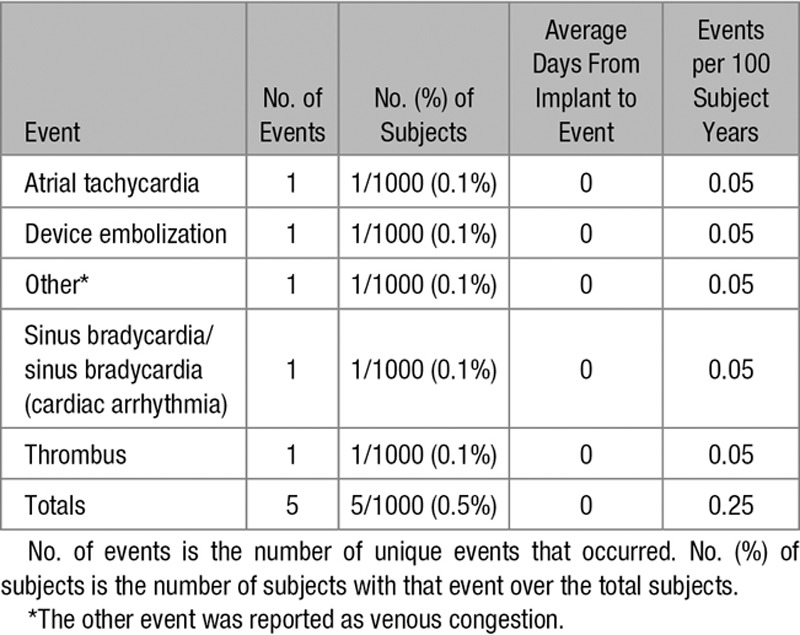
The total adverse event rate associated with the ASO or delivery system at 2-year follow-up was 6.56% (61/930; this denominator includes the 927 patients that completed the 2-year study follow-up and 3 that were lost to follow-up at 381, 415, and 767 days). There was no device, delivery system, or procedural related deaths at 2-year follow-up.
In addition to the device embolization at 12 days after implant resulting in hemodynamic compromise, there were 5 additional device embolizations. Embolization tended to occur with larger defects (stop-flow diameter, 30±7 mm) and larger devices (33±5, range 26–38 mm). The device size:stop-flow diameter ratio was 1.1±0.1 in the embolized devices, in accordance to the manufacturer’s recommendations. Three of the 5 devices were 38 mm, the largest device size available. All embolized devices were successfully recovered, 5 surgically with patch closure of the ASD and 1 during the implant procedure, with successful repositioning of the same device.
Secondary End Points
Device Sizing and Hemodynamic Compromise
Among the subjects who either completed the 2-year follow-up visit or had a hemodynamic compromise event within 2 years, a total of 553 patients received the ASO chosen according to the manufacturer’s sizing recommendations in the device IFU. Under- or oversized devices were used in 382 patients. In the 6 study patients with hemodynamic compromise, the static ASD measurement, stop-flow balloon diameter, and device size are listed in Table 5.
Table 5.
Device Sizing and Device-Related Hemodynamic Compromise
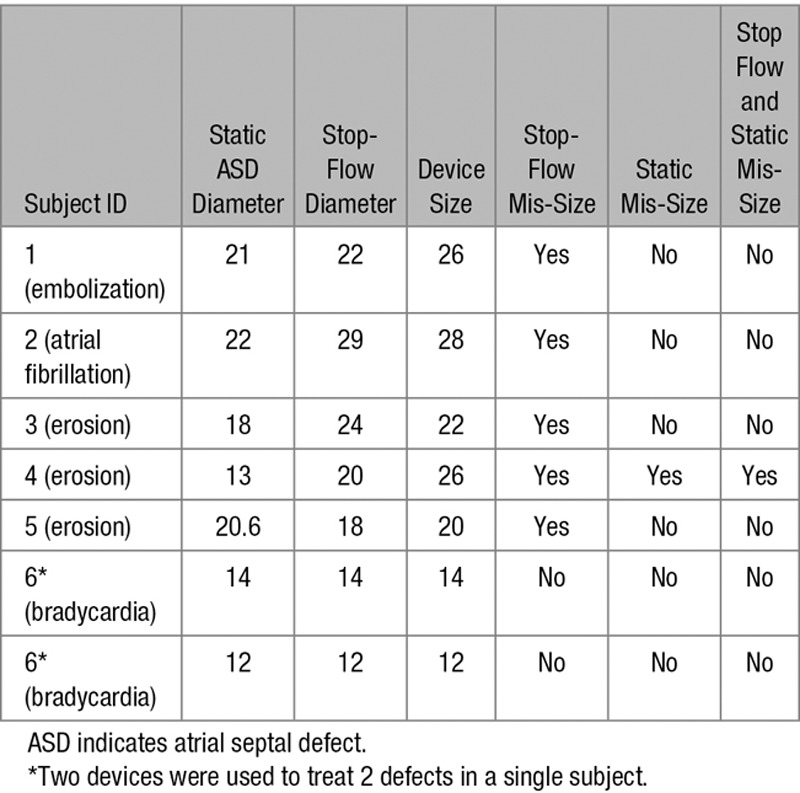
Oversizing occurred using the stop-flow balloon diameter, but not the static measurement in subjects 1 and 5 who experienced device embolization and cardiac erosion, respectively. Undersizing occurred using the stop-flow balloon diameter, but not the static measurement in subjects 2 and 3. Subject 2 experienced an event of atrial fibrillation, and subject 3 experienced events of pericardial effusion and erosion. Device mis-sizing occurred using both stop-flow balloon diameter and static measurement in subject 4 who experienced pericardial effusion and erosion. Device sizing was performed according to the IFU in subject 6, who experienced sinus bradycardia (Table 5).
Rates of hemodynamic compromise at 2-year follow-up were significantly lower in patients who received an appropriately sized ASO according to the IFU sizing recommendations compared with patients with under- or oversized devices (0.19% versus 1.40%). Using the log-rank test, there is a statistically significant difference in the time-to-event outcomes between the 2 sizing groups (P=0.03).
Physician Experience and Hemodynamic Compromise
Physicians participating in the study were classified as high-volume implanters if they had done ≥40 procedures per year before the study and as low-volume implanters if 10 to 15 procedures per year were previously performed. Among the subjects who either completed the 2-year visit or had a hemodynamic compromise event within 2 years, a total of 307 patients (33%) were treated by high-volume implanting physicians and 583 (63%) were treated by low-volume implanting physicians.
Rates of hemodynamic compromise at 2-year follow-up were lower in patients in the high-volume physician group compared with patients in the low-volume physician group (0.32% versus 0.69%). Using the log-rank test, there is no statistically significant difference in the time-to-event outcomes between the 2 physician experience groups (P=0.49).
Discussion
ASD is one of the most common congenital heart defects that requires intervention. Complications from untreated, hemodynamically significant ASD include right ventricular failure, atrial arrhythmia, paradoxical embolization, and pulmonary hypertension. The mortality rate from untreated, hemodynamically significant ASD can approach 25%.7 The American Heart Association recommends closure of ASD in patients with right heart volume overload.8,9
Transcatheter closure of secundum ASD has been shown to be safe and effective in patients with right heart volume overload, with similar success and complication rates compared with surgery.10,11 A 2012 observational study with 5 to 20 years of follow-up (mean 10 years) again showed similar overall complication rates between transcatheter and surgical closure.12 In fact, 2 recent studies showed that all-cause mortality rate for transcatheter ASD closure was less compared with surgical closure (0.09% versus 0.13% and 0.60% versus 0.88%).13,14
The most current large study with >10 000 children aged 1 to 17 years compared transcatheter and surgical closure of secundum ASD in pediatric hospitals. There was excellent short-term outcome in each group with no mortality. Children with surgical closure had a longer hospital stay, a higher risk of infection and procedural complications, and a higher total hospital cost compared with transcatheter closure.15
The ASO was approved for transcatheter closure of secundum ASD in the United States in 2001.10 Despite the numerous favorable reports discussed above, there is concern about the rare occurrence of cardiac injury with hemodynamic compromise after ASO implantation.1 Despite clear, published recommendations from an advisory panel formed in an attempt to decrease the incidence of cardiac injury, a small and largely unknown risk of cardiac injury persists after ASO implantation.1,5,6 This postapproval study was conducted to evaluate the real-world risk of cardiac injury with hemodynamic compromise and obtain medium-term survival data on patients after ASO implantation.
Hemodynamic compromise was rare, with 7 events occurring in 6 patients (0.65%). Device erosion as the mechanism of hemodynamic compromise occurred in only 3 patients (0.3%). These rates of hemodynamic compromise and erosion are similar to other reported series.1,2 The successful closure rate in this study was similar to the closure rate found at 12 months in the pivotal trial (98%).10 The total adverse event rate associated with the ASO or delivery system at 2-year follow-up was 6.56%. These data provide additional evidence for the overall safety and effectiveness of the ASO for transcatheter closure of secundum ASD.
Deficiency of the aortic rim is thought to be a risk factor for hemodynamic compromise caused by erosion after placement of the ASO.16 Therefore, the prevalence of deficient aortic rim in a meaningful study investigating hemodynamic compromise must closely match the actual prevalence. In this prospective study cohort, the prevalence of deficient aortic rim was 11.5%. This is less than in other nonprospective studies, where the prevalence of deficient aortic rim is as high as 60%.16–19 Although this difference may seem large, few studies, including this study, rigorously evaluate ASD rim size at the procedure or use a core laboratory for echocardiographic rim measurement. In fact, with the most rigorous evaluation, McElhinney et al16 reported a 24% prevalence of deficient aortic rim in their control patients, who were obtained from this postapproval study.
Device size was related to hemodynamic compromise. Both under- and oversized devices may increased the risk for erosion.3 Appropriate device diameter is defined by the manufacturer as <1.5× the echocardiography derived static ASD diameter and equal to or, if the identical size is not available, 1 device size larger than the stop-flow balloon diameter of the ASD. Rates of hemodynamic compromise at 2-year follow-up were lower in patients who received an appropriately sized ASO.
The experience of the implanting physician before the study did not influence the rate of hemodynamic compromise. The ASO and delivery system are easily used by both high- and low-volume implanters alike, with similar small rates of complications.
Study Limitations
This study was limited by the overall rarity of hemodynamic compromise and even smaller rate of erosion, increasing the potential of type II error. Although the study sample size is relatively large, it is not large enough to delineate the factors associated with these rare events. To obtain additional information and to evaluate the risk and predictive factors associated with hemodynamic compromise and device erosion, a larger study designed specifically to address this question is necessary.
An additional limitation is this study’s general inadequacy of septal rim echocardiographic data. A core laboratory to evaluate the echocardiograms was not used in this study. Although all studies were read by experienced echocardiographers at the study centers, there may be interobserver difference in how the studies were interpreted, most importantly with regard to the ASD rim measurements. Transesophageal echocardiography or ICE could be used during the procedure at the choice of the investigator. ICE accuracy for the assessment of aortic rim is dependent on the user’s commitment to full interrogation of this rim, the most challenging to image adequately. Perhaps even more important for consideration of erosion is the area directly above the aortic valve, an area infrequently evaluated carefully by ICE. Although few studies rigorously evaluate the aortic rim by echocardiography, the prevalence of deficient aortic rim in this study is thought to be similar to the true prevalence.
Conclusions
Transcatheter closure of ASDs using the ASO and delivery system is safe and effective at 2-year follow-up. Hemodynamic compromise after placement of the ASO is rare. The rate of hemodynamic compromise is lower in patients who received an appropriately sized device and in this trial, involving selected centers, was not associated with physician experience, although the cohort was underpowered to detect such differences in this rare outcome. Device erosion is rare, occurring in 0.3% of patients. In patients with hemodynamic compromise, device erosion must be considered. The associated risk factors for erosion are not yet clear. Further study is necessary to estimate the risk and hazard ratios of erosion after device placement for patients with and without prespecified risk factors.
Sources of Funding
This study was sponsored by St. Jude Medical (now Abbott), St. Paul, MN.
Disclosures
Dr Turner is a proctor for St. Jude Medical and received a consulting fee for article preparation and review. Wayne State University received per patient research funds from St. Jude Medical for this study. Dr Owada received a consulting fee for article preparation and review. Valley Children’s Hospital received per patient research funds from St. Jude Medical for this study. Dr Sang received a research grant from St. Jude Medical. The other authors report no conflicts.
References
- 1.Crawford GB, Brindis RG, Krucoff MW, Mansalis BP, Carroll JD. Percutaneous atrial septal occluder devices and cardiac erosion: a review of the literature. Catheter Cardiovasc Interv. 2012;80:157–167. doi: 10.1002/ccd.24347. doi: 10.1002/ccd.24347. [DOI] [PubMed] [Google Scholar]
- 2.Amin Z, Hijazi ZM, Bass JL, Cheatham JP, Hellenbrand WE, Kleinman CS. Erosion of Amplatzer septal occluder device after closure of secundum atrial septal defects: review of registry of complications and recommendations to minimize future risk. Catheter Cardiovasc Interv. 2004;63:496–502. doi: 10.1002/ccd.20211. doi: 10.1002/ccd.20211. [DOI] [PubMed] [Google Scholar]
- 3.Amin Z. Echocardiographic predictors of cardiac erosion after Amplatzer septal occluder placement. Catheter Cardiovasc Interv. 2014;83:84–92. doi: 10.1002/ccd.25175. doi: 10.1002/ccd.25175. [DOI] [PubMed] [Google Scholar]
- 4.US Food and Drug Administration. Rare serious erosion events associated with St. Jude AMPLATZER™ atrial septal occluder (ASO): FDA safety communication. 2013. https://www.fda.gov/MedicalDevices/Safety/AlertsandNotices/ucm371145.htm.
- 5.Roberts WT, Parmar J, Rajathurai T. Very late erosion of Amplatzer septal occluder device presenting as pericardial pain and effusion 8 years after placement. Catheter Cardiovasc Interv. 2013;82:E592–E594. doi: 10.1002/ccd.24755. doi: 10.1002/ccd.24755. [DOI] [PubMed] [Google Scholar]
- 6.Diab K, Kenny D, Hijazi ZM. Erosions, erosions, and erosions! Device closure of atrial septal defects: how safe is safe? Catheter Cardiovasc Interv. 2012;80:168–174. doi: 10.1002/ccd.24517. doi: 10.1002/ccd.24517. [DOI] [PubMed] [Google Scholar]
- 7.Kouchoukos NT, Blackstone EH, Hanley FL, Kirklin JK. Kirklin/Barratt-Boyes Cardiac Surgery. 4th ed. Elsevier Health Sciences; 2013. Atrial septal defect and partial anomalous pulmonary venous connection. pp. 1157–1163. [Google Scholar]
- 8.Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, del Nido P, Fasules JW, Graham TP, Jr, Hijazi ZM, Hunt SA, King ME, Landzberg MJ, Miner PD, Radford MJ, Walsh EP, Webb GD, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Antman EM, Buller CE, Creager MA, Ettinger SM, Halperin JL, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology; American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease); American Society of Echocardiography; Heart Rhythm Society; International Society for Adult Congenital Heart Disease; Society for Cardiovascular Angiography and Interventions; Society of Thoracic Surgeons. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–e263. doi: 10.1016/j.jacc.2008.10.001. doi: 10.1016/j.jacc.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 9.Webb G, Gatzoulis MA. Atrial septal defects in the adult: recent progress and overview. Circulation. 2006;114:1645–1653. doi: 10.1161/CIRCULATIONAHA.105.592055. doi: 10.1161/CIRCULATIONAHA.105.592055. [DOI] [PubMed] [Google Scholar]
- 10.Du ZD, Hijazi ZM, Kleinman CS, Silverman NH, Larntz K Amplatzer Investigators. Comparison between transcatheter and surgical closure of secundum atrial septal defect in children and adults: results of a multicenter nonrandomized trial. J Am Coll Cardiol. 2002;39:1836–1844. doi: 10.1016/s0735-1097(02)01862-4. doi: 10.1016/S0735-1097(02)01862-4. [DOI] [PubMed] [Google Scholar]
- 11.Butera G, Biondi-Zoccai G, Sangiorgi G, Abella R, Giamberti A, Bussadori C, Sheiban I, Saliba Z, Santoro T, Pelissero G, Carminati M, Frigiola A. Percutaneous versus surgical closure of secundum atrial septal defects: a systematic review and meta-analysis of currently available clinical evidence. EuroIntervention. 2011;7:377–385. doi: 10.4244/EIJV7I3A63. doi: 10.4244/EIJV7I3A63. [DOI] [PubMed] [Google Scholar]
- 12.Kutty S, Hazeem AA, Brown K, Danford CJ, Worley SE, Delaney JW, Danford DA, Latson LA. Long-term (5- to 20-year) outcomes after transcatheter or surgical treatment of hemodynamically significant isolated secundum atrial septal defect. Am J Cardiol. 2012;109:1348–1352. doi: 10.1016/j.amjcard.2011.12.031. doi: 10.1016/j.amjcard.2011.12.031. [DOI] [PubMed] [Google Scholar]
- 13.DiBardino DJ, McElhinney DB, Kaza AK, Mayer JE., Jr. Analysis of the US Food and Drug Administration Manufacturer and User Facility Device Experience database for adverse events involving Amplatzer septal occluder devices and comparison with the Society of Thoracic Surgery congenital cardiac surgery database. J Thorac Cardiovasc Surg. 2009;137:1334–1341. doi: 10.1016/j.jtcvs.2009.02.032. doi: 10.1016/j.jtcvs.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karamlou T, Diggs BS, Ungerleider RM, McCrindle BW, Welke KF. The rush to atrial septal defect closure: is the introduction of percutaneous closure driving utilization? Ann Thorac Surg. 2008;86:1584–1590. doi: 10.1016/j.athoracsur.2008.06.079. discussion 1590. doi: 10.1016/j.athoracsur.2008.06.079. [DOI] [PubMed] [Google Scholar]
- 15.Ooi YK, Kelleman M, Ehrlich A, Glanville M, Porter A, Kim D, Kogon B, Oster ME. Transcatheter versus surgical closure of atrial septal defects in children: a value comparison. JACC Cardiovasc Interv. 2016;9:79–86. doi: 10.1016/j.jcin.2015.09.028. doi: 10.1016/j.jcin.2015.09.028. [DOI] [PubMed] [Google Scholar]
- 16.McElhinney DB, Quartermain MD, Kenny D, Alboliras E, Amin Z. Relative risk factors for cardiac erosion following transcatheter closure of atrial septal defects: a case-control study. Circulation. 2016;133:1738–1746. doi: 10.1161/CIRCULATIONAHA.115.019987. doi: 10.1161/CIRCULATIONAHA.115.019987. [DOI] [PubMed] [Google Scholar]
- 17.O’Byrne ML, Glatz AC, Sunderji S, Mathew AE, Goldberg DJ, Dori Y, Rome JJ, Gillespie MJ. Prevalence of deficient retro-aortic rim and its effects on outcomes in device closure of atrial septal defects. Pediatr Cardiol. 2014;35:1181–1190. doi: 10.1007/s00246-014-0914-6. doi: 10.1007/s00246-014-0914-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Podnar T, Martanovic P, Gavora P, Masura J. Morphological variations of secundum-type atrial septal defects: feasibility for percutaneous closure using Amplatzer septal occluders. Catheter Cardiovasc Interv. 2001;53:386–391. doi: 10.1002/ccd.1187. doi: 10.1002/ccd.1187. [DOI] [PubMed] [Google Scholar]
- 19.O’Byrne ML, Gillespie MJ, Kennedy KF, Dori Y, Rome JJ, Glatz AC. The influence of deficient retro-aortic rim on technical success and early adverse events following device closure of secundum atrial septal defects: an Analysis of the IMPACT Registry(®). Catheter Cardiovasc Interv. 2017;89:102–111. doi: 10.1002/ccd.26585. doi: 10.1002/ccd.26585. [DOI] [PMC free article] [PubMed] [Google Scholar]


